- 1Jiangsu Key Laboratory for Biodiversity and Biotechnology, College of Life Sciences, Nanjing Normal University, Nanjing, China
- 2Comparative and Endocrine Biology Laboratory, Translational Research Institute–Institute of Health and Biomedical Innovation, School of Biomedical Sciences, Queensland University of Technology, Brisbane, QLD, Australia
Animals that are able to sustain life under hypoxic conditions have long captured the imagination of biologists and medical practitioners alike. Although the associated morphological modifications have been extensively described, the mechanisms underlying the evolution of hypoxia tolerance are not well understood. To provide such insights, we investigated genes in four major energy metabolism pathways, and provide evidence of distinct evolutionary paths to mammalian hypoxia-tolerance. Positive selection of genes in the oxidative phosphorylation pathway mainly occurred in terrestrial hypoxia-tolerant species; possible adaptations to chronically hypoxic environments. The strongest candidate for positive selection along cetacean lineages was the citrate cycle signaling pathway, suggestive of enhanced aerobic metabolism during and after a dive. Six genes with cetacean-specific amino acid changes are rate-limiting enzymes involved in the gluconeogenesis pathway, which would be expected to enhance the lactate removal after diving. Intriguingly, 38 parallel amino acid substitutions in 29 genes were observed between hypoxia-tolerant mammals. Of these, 76.3% were radical amino acid changes, suggesting that convergent molecular evolution drives the adaptation to hypoxic stress and similar phenotypic changes. This study provides further insights into life under low oxygen conditions and the evolutionary trajectories of hypoxia-tolerant species.
Introduction
Ever since Charles Darwin published his book ‘On the Origin of Species by Means of Natural Selection’ nearly 160 years ago (Darwin, 1859), adaptive evolution has remained a critical research question. Of particular interest are insights from hypoxia-tolerant animals – species adapted to oxygen (O2) poor aquatic or terrestrial environments (Nathaniel et al., 2015). Oxygen is vital to animals and hypoxia is associated with cell death and organ failure, as observed in stroke and ischemia reperfusion injury in humans (Buck and Pamenter, 2006).
Marine mammals include approximately 120 species, spanning three distinct mammalian orders which independently transitioned to an aquatic environment: Cetacea (whales, dolphins, and porpoises), Pinnipedia (walruses, sea lions, and other pinnipeds), and Sirenia (manatees and dugongs) (Hoelzel, 2002; Berta et al., 2006). Hypoxia-tolerance is one of the major features of marine mammals. Reduced metabolism, hypometabolism, in marine mammals is associated with an increased reliance on anaerobic (‘without oxygen’; nitrate) metabolism; the advantage of which is that energy in the form of adenosine triphosphate (ATP) can be produced in the absence of oxygen (Costa, 2007). Marine animals also display an enhanced capacity of glial cells to support anaerobic breakdown of glucose, glycolysis, coupled with an elevated number of astrocytes which store glycogen and provide lactate as an energy source (Ramirez et al., 2007). Higher concentrations or activity of key glycolytic enzymes that enhance the ability to process lactic acid, such as lactate dehydrogenase (LDH), may be associated with greater tolerance to hypoxia (Costa, 2007).
Mammals that live at high altitude are also exposed to hypoxic environments. To adapt to high-altitude hypoxia, for instance, Tibetan humans have a greatly reduced abundance of mitochondrial DNA in muscle (the number of mitochondrial genomes present), indicative of a reduced metabolic capacity (Storz et al., 2010). Moreover, alteration in enzyme kinetics, e.g., higher substrate affinity of cytochrome c oxidase enzyme (COX), may enhance hypoxia-tolerance by preventing oxidative damage in high-altitude species (Storz et al., 2010). In addition, exclusively subterranean species, such as the naked mole rat (Heterocephalus glaber), live in oxygen-poor burrows (Bennett and Faulkes, 2000). The naked mole rat has developed adaptive metabolic functions, such as a reduced metabolic rate and retarded somatic development (neoteny) (Fang et al., 2014; Park et al., 2017).
Molecular evolution of genes associated with energy metabolism has attracted much interest in recent decades. For example, evidence of positive selection was found in genes in the mitochondrial oxidative phosphorylation (OXPHOS) pathway (the final step in the production of ATP from the oxidation of nutrients) in two snakes and bats-burrowing and flying animals (Castoe et al., 2008; Shen et al., 2010; Castoe et al., 2013); suggesting that evolution of energy metabolism genes are critical for acclimation to novel environments. Here, to provide additional evidence on the evolution of energy metabolism genes in hypoxia-tolerant mammals, we investigated coding sequences of 194 energy metabolism-related genes across diverse mammalian taxa, aquatic and terrestrial hypoxia-tolerant species.
Materials and Methods
Sequences Retrieval and Alignments
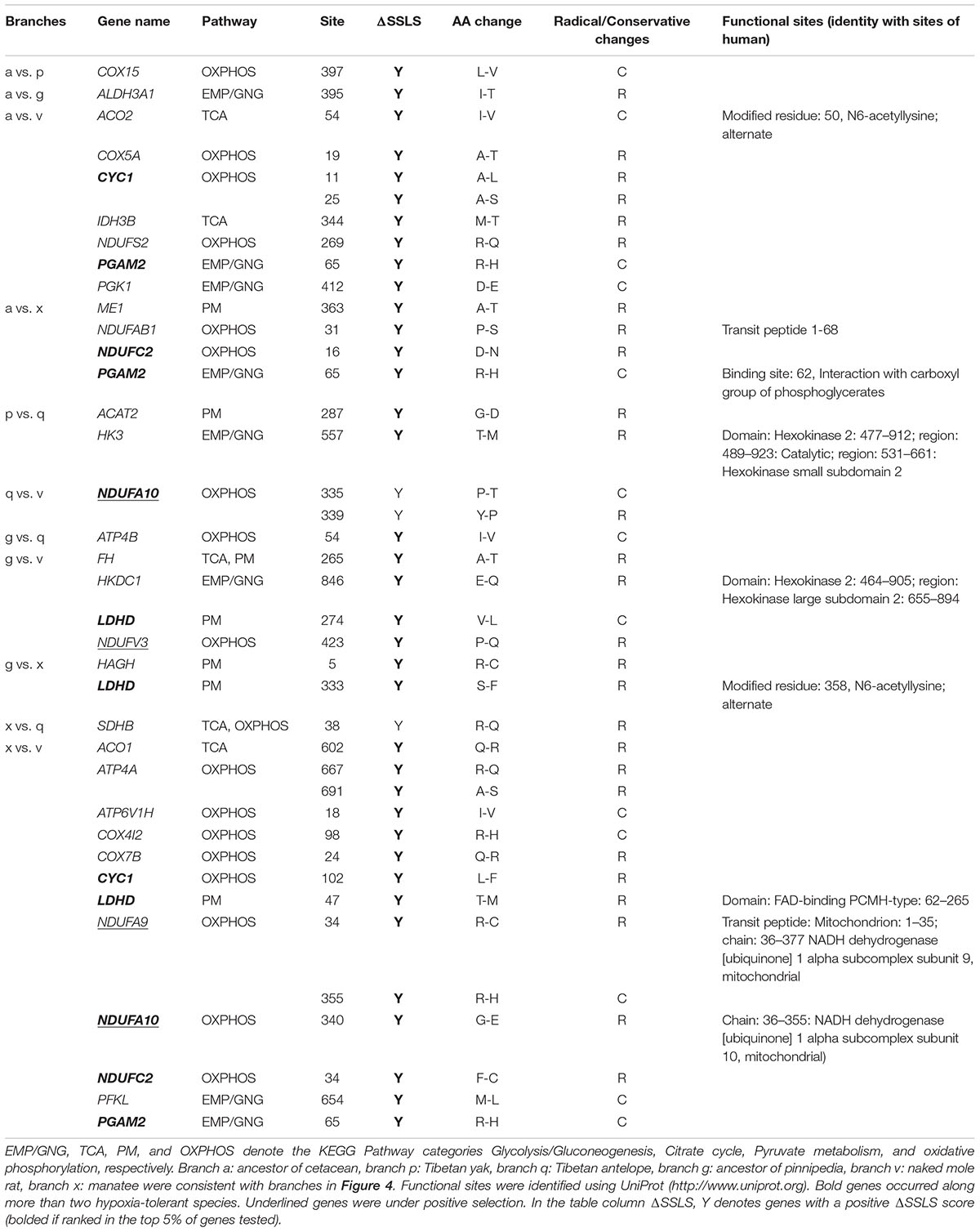
A list of human energy metabolism-related genes was retrieved from the KEGG (Kyoto Encyclopedia of Genes and Genomes) database (Kanehisa and Goto, 2000). The following pathways were retrieved: Glycolysis/Gluconeogenesis (EMP/GNG): ko00010; Citrate cycle: ko00020 (TCA); Pyruvate metabolism (PM): ko00620; and Oxidative phosphorylation (OXPHOS): ko00190 (Figure 1). The resulting gene list was used to query Ensembl BioMart and retrieve non-human Ensembl gene identificators (Kinsella et al., 2011). The gene IDs were used to search annotated coding sequences (CDS) of mammals in the NCBI1 and Ensembl databases2 (Supplementary Table S1). Additionally, BLASTn and tBLASTn searches (Johnson et al., 2008) were performed to identify one-to-one orthologs in the genomes of 12 hypoxia-tolerant species. This included 9 marine mammals, cetaceans: bottlenose dolphin (Tursiops truncatus), baiji (Lipotes vexillifer), killer whale (Orcinus orca), sperm whale (Physeter macrocephalus), bowhead whale (Balaena mysticetus), minke whale (Balaenoptera acutorostrata); pinnipeds: Weddell seal (Leptonychotes weddellii), Pacific walrus (Odobenus rosmarus divergens), sirenians: West Indian manatee (Trichechus manatus latirostris); 2 highland mammals: Tibetan yak (Bos mutus), Tibetan antelope (Pantholops hodgsonii); and 1 subterranean mammals: naked mole rat (Heterocephalus glaber) (Supplementary Figure S1). For genes with multiple splice variants, the longest transcript was retained. The resulting dataset, derived from 54 mammalian genomes, included 181 nuclear-encoded energy metabolism-related genes (Supplementary Table S1). In addition, nucleotide sequences of 13 mitochondrial protein-coding genes from 101 species were downloaded from NCBI and the MitoZoa database3 (Supplementary Figure S2). A total of 194 energy metabolism-related (nuclear and mitochondrial) genes were obtained. The EMP/GNG, PM, TCA, and OXPHOS metabolic pathways were composed of 49, 34, 29, and 111 genes, respectively (Supplementary Figure S3). Genes shared by different pathways are depicted in Supplementary Figure S3.
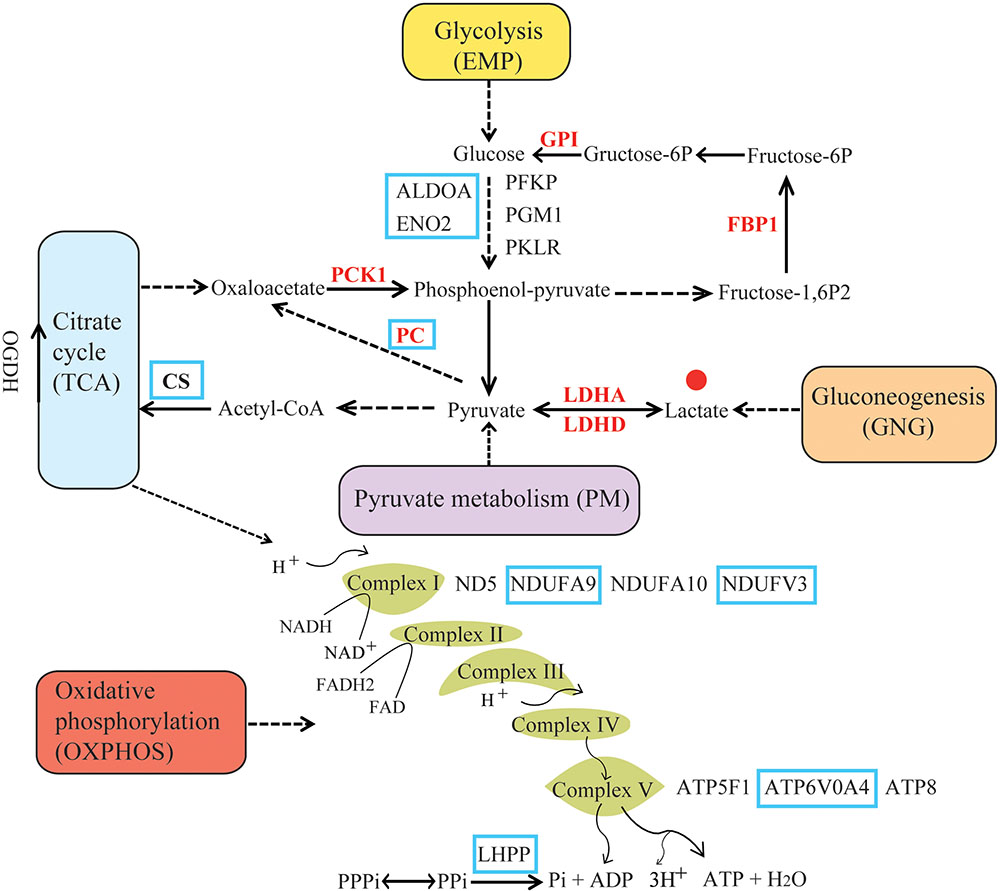
FIGURE 1. Summary diagram of energy metabolism pathway. Pathway categories were obtained from KEGG. Positively selected genes (PSGs) identified in hypoxia-tolerant mammals are shown in red. The solid lines indicate direct relationships between enzymes and metabolites. The dashed lines indicate that more than one step is involved in a process. Glycolysis (EMP) metabolizes glucose to pyruvate, which provides the cell with ATP under anaerobic conditions (Fromm and Hargrove, 2012). In contrast, gluconeogenesis (GNG) drives the synthesis of D-glucose from non-carbohydrate sources (e.g., lactate), and some glycolytic enzymes are part of the gluconeogenesis pathway. In the pyruvate metabolism (PM) pathway, pyruvate is processed anaerobically (fermented) to lactate (lactic acid fermentation) or ethanol (alcoholic fermentation), and can be aerobically oxidized to CO2. Of note, lactate and pyruvate is either the product of EMP or the major precursor for GNG; moreover, the former can be converted back to pyruvate when conditions return to normal, or excreted in the urine (Pelley, 2007). The citrate cycle (TCA) contains genes responsible for the conversion of pyruvate to acetyl CoA under aerobic conditions. Oxidative phosphorylation (OXPHOS) is composed of multi-subunit complexes, encoded by both nuclear and mitochondrial genes, which catalyzes the synthesis of ATP (Berg et al., 2002). In the absence of sufficient oxygen, electron transport ceases and the energy demands of the cell are not maintained (Giordano, 2005).
Coding (amino acid) sequence for each gene was aligned using MUSCLE v3.2 (Edgar, 2004) with default settings, manually corrected, and used to align nucleotide sequences in MEGA 6 (Tamura et al., 2013). Because the quality of sequence alignments dramatically affects the estimation of evolutionary parameters, we deleted all gaps and undetermined bases ‘N’ in alignments to reduce the rate of false-positive prediction, and only high-quality sequences were used in downstream analyses. The species used for each gene, and associated accession numbers, are presented in Supplementary Table S1.
Molecular Evolution Analyses
The impact of natural selection on energy metabolism related genes was determined by estimating the ratio of non-synonymous (dN) and synonymous (dS) mutations (ω = dN/dS) implemented in CodeML (implemented in PAML v4.8) (Yang, 2007). Briefly, ω > 1, ω = 1, and ω < 1 indicate positive selection, neutral, and negative selection, respectively. To determine whether codon positions in hypoxia-tolerant branches were under positive selection, we employed the PAML branch-site model, which assesses selective pressure (ω) at sites in foreground lineages of interest, specified a priori, and remaining background lineages (Zhang et al., 2005). In separate tests, branches leading to cetaceans, pinnipeds, sirenians, highland (high-altitude) species, or subterranean (burrowing) mammals were specified as foreground branches, with the remaining of the tree as the background branch (Supplementary Figures S1, S2). Additionally, we also analyzed the combined branches, all hypoxia-tolerant mammals, independently. Compared with the corresponding null hypothesis of neutral evolution, foreground branches in the modified model A (test 2) that have a class of sites with the ratio ω2 > 1 are candidates for positive selection. Likelihood ratio tests (LRTs) with a chi-squared test (df = 2) were conducted on nested models. Such sites under positive selection were detected by the Bayes empirical Bayes (BEB) method, with posterior probabilities of ≥0.80 (Yang et al., 2005). Resulting P-values were corrected by employing a false discovery rate (FDR) cutoff of 0.05 (Storey and Tibshirani, 2003). A well-accepted species tree among mammals (Supplementary Figures S1, S2) was used as the input tree in all analyses (Murphy et al., 2001).
Parallel/Convergent Site Detection
In order to identify parallel/convergent amino acid changes in hypoxia-tolerant mammals, the sequences of the ancestral nodes were reconstructed with the help of the CodeML program implemented in PAML (Yang, 2007). Convergent/parallel amino acid replacements, from the last common ancestor-state to present-species along each hypoxia-tolerant branch, were detected by an in-house Python script (Supplementary Data Sheet 1). Amino acid residue changes were classified into convergent or parallel changes. Convergent changes: any amino acid at the same position changes to the same amino acid in two or more distinct lineages (for example, Ala10Glu in cetaceans; His10Glu in pinnipeds). Parallel changes: an identical amino acid at the same position changes to the same amino acid in two or more distinct lineages (for example, Ala10Glu in cetaceans; Ala10Glu in pinnipeds). To test whether observed parallel/convergent substitutions in focal hypoxia-tolerant branches were fixed by random chance or by natural selection, the statistical significance of amino acid changes was tested by the method developed by Zhang and Kumar (1997). To carry out in-depth investigation of convergence between hypoxia-tolerant species, we measured the fit (site-wise log-likelihood support; SSLS) (Parker et al., 2013) of each amino acid along the orthologous CDS alignment to the generally accepted species tree (H0) and a convergent tree (HA) that constrained hypoxia-tolerant species into monophyletic clades. The difference in SSLS for a single site under different tree topologies was calculated as follows: ΔSSLS = SSLS (HA) - SSLS (H0) (Supplementary Figure S4), where a negative ΔSSLS implies support for convergence. SSLS for each gene alignment was calculated by RAxML v8.0.26 (Stamatakis, 2014).
Liver Transcriptome Analyses
The expression of the 194 energy metabolism-related genes were examined in de novo assembled transcriptomes (Seim et al., 2014). We contrasted cetacean (bowhead whale and minke whale) and terrestrial (naked mole rat, domestic yak, Brandt’s bat, Chinese tree shrew, rhesus monkey, rat, and mouse) liver transcriptomes. Briefly, library size-normalized read counts were subjected to the voom function (variance modeling at the observation-level) function in the R package ‘limma’ v3.18.13 (Law et al., 2014) with trend = TRUE for the eBayes function and correction for multiple testing (Benjamini–Hochberg false discovery P ≤ 0.05). Following limma analysis, genes with a log2 1.2-fold-change differences between cetaceans (bowhead whale, and minke whale) and other mammals were considered differentially expressed. RNA-seq gives very accurate measurements of relative expression across a broad dynamic range (Wang et al., 2008). However, in cross-species analyses (here, two cetaceans versus seven terrestrial mammals), one would expect more heterogeneity than when comparing within a species (where typically a log2 2.0-fold-change cutoff is employed). Thus, a gene could be modestly upregulated, but still be biologically relevant.
Results
Selective Regimes on Energy Metabolism Related Genes Imposed by Hypoxia-Tolerant Species
To estimate the selective pressure acting on genes in energy metabolism pathways in mammals, we ran separate branch-site tests on the ancestral and terminal branches leading to cetaceans (bottlenose dolphin, baiji, killer whale, sperm whale, minke whale, and bowhead whale), pinnipeds (Weddell seal and Pacific walrus), sirenian (West Indian manatee), highland species (Tibetan yak, and Tibetan antelope), and a subterranean species (naked mole rat) (Figure 2 and Supplementary Table S2). In cetaceans, eight genes showed evidence of positive selection (positively selected genes; PSGs) (test 2, Supplementary Table S2) along the lineages leading to bottlenose dolphin (Figure 2: branch i, ALDOA, CS, and PC), killer whale (Figure 2: branch j, NDUFV3), sperm whale (Figure 2: branch l, LHPP, and NDUFA9), minke whale (Figure 2: branch m, LHPP), bowhead whale (Figure 2: branch n, ATP6V0A4, ENO2, and PC), and the last common ancestor of baleen whales (mysticetes; Figure 2: branch e, ENO2) (Table 1) after correcting for multiple testing. For the remaining aquatic mammals, the LRT test showed evidence for positive selection on the genes PC and OGDH, respectively, in the lineages leading to Weddell seal (Figure 2: branch t) and West Indian manatee (Figure 2: branch x). When considering highland species, one gene (NDUFA10) was under positive selection in the Tibetan antelope (Figure 2: branch q), whereas in Tibetan yak four genes (PGM1, PKLR, ATP5F1, and NDUFA10) were under positive selection (Figure 2: branch o). When we set the subterranean (naked mole rat) as the foreground branch (Figure 2: branch v), positive selection was detected in two mitochondrial genes: ATP8 and ND5. Notably, the PSGs identified in hypoxia-tolerant species were not found in their sister taxa, although some genes in the sister taxa were also positively selected (Supplementary Table S2). These finding suggests that the PSGs identified are robust candidate hypoxia adaptation genes.
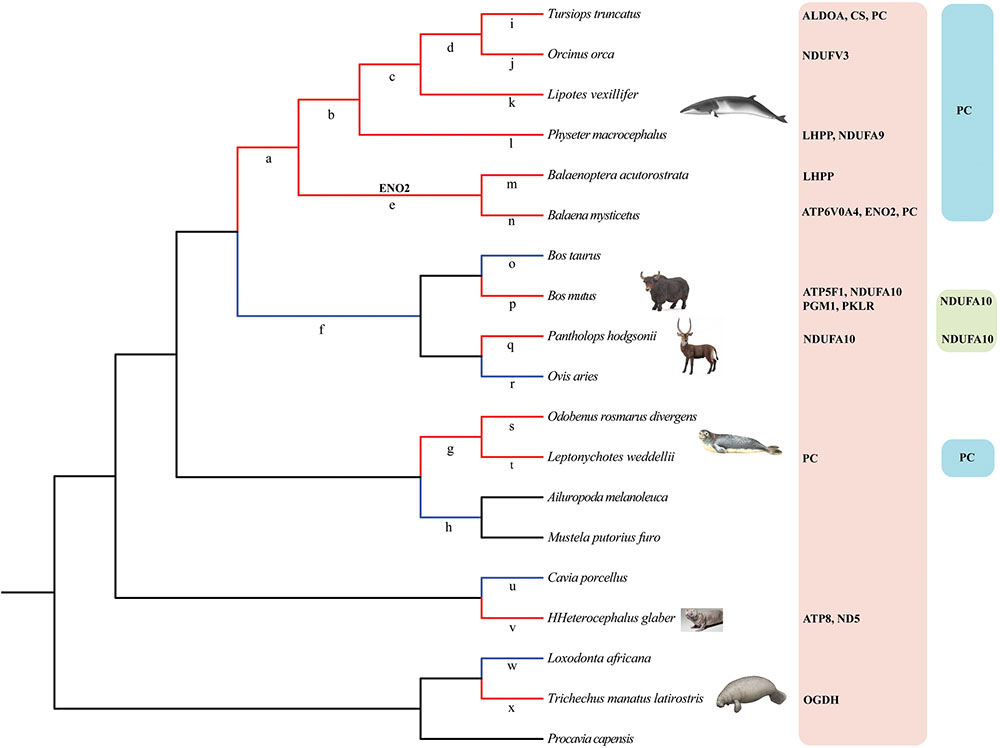
FIGURE 2. Phylogenetic tree representation of energy metabolism genes under positive selection in hypoxia-tolerant mammals. Red and blue color branches in the tree denote hypoxia-tolerant mammals and their sister taxa, respectively. Branches a-x in the tree were used in branch-site tests. PSGs (PC, NDUFA10) shared by two or more hypoxia-tolerant species were also listed in the rightmost part of the Figure 2.
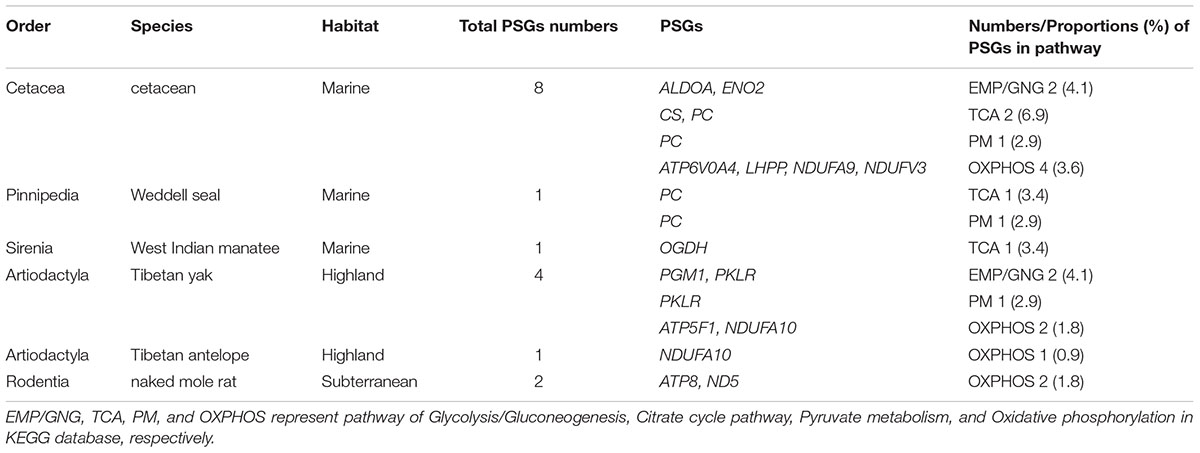
TABLE 1. Positively selected genes (PSGs) in hypoxia-tolerant mammals after multiple-testing correction (FDR).
Energy Metabolism Pathway Clustering of PSGs in Hypoxia-Tolerant Species
To assess the potential function of PSGs in hypoxia-tolerant mammals, we categorized them at the pathway level. In cetaceans, PSGs were found in all four energy metabolism pathways of interest: 6.9% (2/29 genes) in TCA, 4.1% (2/49 genes) in EMP/GNG, 3.6% (4/111 genes) in OXPHOS, and 2.9% (1/34 genes) in PM (Figure 3 and Table 1). Notably, PSGs were enriched in TCA signaling pathway in the cetacean lineages, with approximately twice as many PSGs as the PM pathway (Figure 3 and Table 1). Similarly, PSGs were also enriched in TCA signaling pathway in the pinniped and sirenian branches (Figure 3). In contrast, PSGs in the Tibetan antelope (0.9%, 1/111 genes) and naked mole rat (1.8%, 2/111) were exclusively found in the OXPHOS pathway. Tibetan yak showed positive selection in EMP/GNG (4.1%, 2/49 genes), PM (2.9%, 1/34 genes) pathways, and OXPHOS (1.8%, 2/111 genes) pathways.
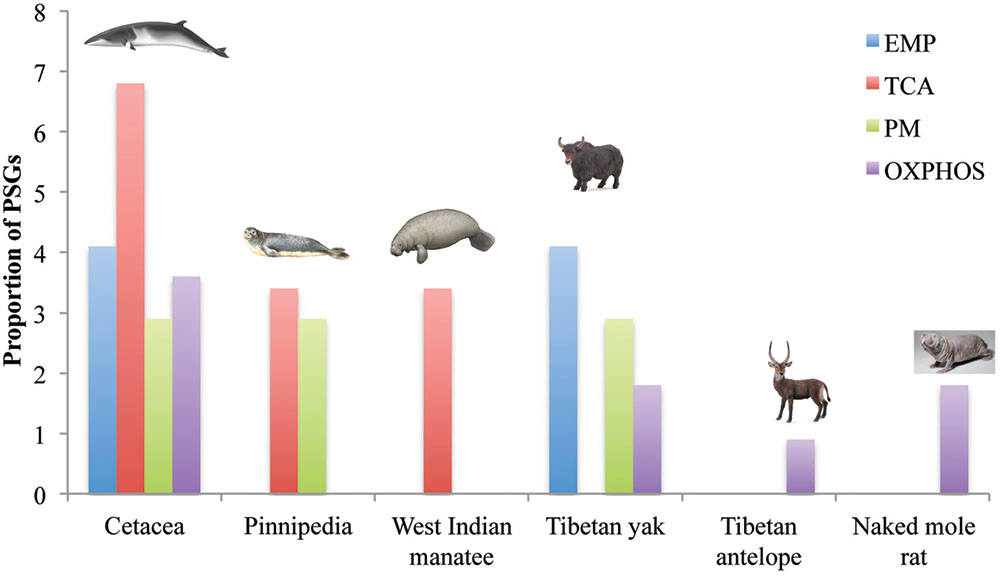
FIGURE 3. Positively selected genes of four energy metabolism pathways in hypoxia-tolerant mammals. EMP/GNG, TCA, PM, and OXPHOS denote the KEGG Pathway categories Glycolysis/Gluconeogenesis, Citrate cycle (TCA cycle), Pyruvate metabolism, and Oxidative phosphorylation, respectively.
Evidence for Species-Specific Adaptations in Cetacean Energy Metabolism Related Genes
To gather additional evidence on the adaptive evolution of energy metabolism related genes in cetaceans, we reconstructed ancestral sequences and mapped species-specific amino acid changes along cetacean lineages. Compared with other mammals, a total of 55 unique amino acid substitutions in 44 genes were observed in all cetaceans; three of which (ATP6V0A4, ENO2, and PC) were inferred to have evolved under positive selection (Supplementary Table S3). Given that differential gene expression could conceivably also contribute to hypoxia-tolerance (Böhne et al., 2013; Fang et al., 2014), we contrasted liver (a key metabolic organ) transcriptomes from two cetaceans (bowhead whale, and minke whale) and seven terrestrial mammals, a liberal cutoff (log2 fold-change ≥ 1.2) revealed no significant differential expression of the 194 energy metabolism genes interrogated in this study (Supplementary Figure S5 and Supplementary Table S4).
Identification of Parallel/Convergent Substitutions in Hypoxia-Tolerant Mammals
To identify convergent evolution of energy metabolism related genes in hypoxia-tolerant mammals from different habitats, we reconstructed ancestral gene sequences for the internal nodes of the species tree and identified shared amino acid substitutions along lineages leading to hypoxia-tolerant species (Figure 4). In total, 7 parallel amino acid residue substitutions in 7 genes were identified in the marine group (branch a, g, x), as well as 2 parallel substitutions in 2 genes in high-altitude species (branch p, q). We identified 26, 3 and 2 parallel substitutions in 23, 3, and 1 genes in marine + subterranean mammals (branch v), marine + highland mammals, and highland + subterranean mammals, respectively (Table 2). Taken together, 38 parallel non-synonymous amino acid substitutions in 29 genes occurred along hypoxia-tolerant species. The number of substitutions was not random (P ≤ 0.05; data not shown). Three of these genes (NDUFA9, NDUFA10, and NDUFV3) were inferred to have evolved under positive selection in the hypoxia-tolerant lineages. Remarkably, parallel non-synonymous amino acid substitutions in 5 genes (CYC1, LDHD, NDUFA10, NDUFC2, and PGAM2) occurred along more than two hypoxia-tolerant species lineages (Table 2). Moreover, all 38 substitutions also ranked highly in the convergence signal distribution, and 92.1% (35/38) loci in the top 5% supporting convergent tree by ΔSSLS test (Table 2). Additionally, 76.3% (29/38) of substitutions were radical amino acid changes.
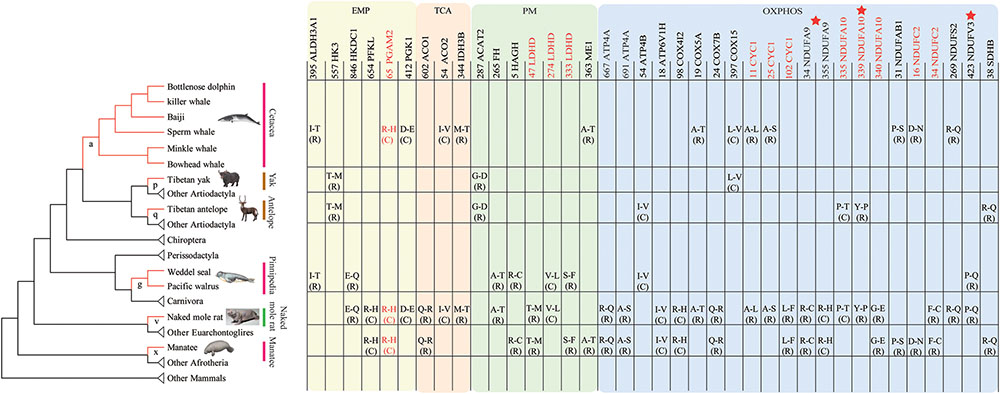
FIGURE 4. Parallel/convergent amino acid substitutions specifically occurred along hypoxia-tolerant species. Red sites represent parallel non-synonymous amino acid substitutions occurred along more than 3 hypoxia-tolerant lineages; Stars represent PSGs. Branch a represented the last common ancestor (LCA) of cetaceans, g of the LCA of pinnipeds, x of West Indian manatee, p of the Tibetan yak, q of the Tibetan antelope, and v of the naked mole rat, respectively.
Discussion
Genetic Discrepancy between Hypoxia-Tolerant Species
We found evidence of positive selection acting on energy metabolism related genes in diverse hypoxia-tolerant species. PSGs related to the OXPHOS pathway mainly occurred along hypoxia-tolerant lineages with a terrestrial lifestyle (such as Tibetan yak, Tibetan antelope, and naked mole rat), whereas PSGs associated with the TCA pathway appeared to have a stronger signal of positive selection in marine mammals. Species-specific PSGs were examined in the hypoxia-tolerant mammals, providing useful pointers to the evolution of unique traits. For example, ALDOA, ENO2, CS, ATP6V0A4, LHPP, NDUFA9, and NDUFV3 PSGs were unique to cetaceans.
Another PSG (PC) found in two cetaceans and the Weddell seal encodes pyruvate carboxylase, an enzyme which converts pyruvate to oxalacetic acid - the first control step of gluconeogenesis (Söling and Willms, 2013). Adaptive evolution of this gene suggests that a hypoxia adaptation of marine mammals involves the Cori cycle and improved lactic acid metabolism during recovery from dives (Cori, 1981; Castellini, 2012). Similarly, PKLR, which encodes pyruvate kinase, a rate-limiting enzyme in glycolysis, was unique to Tibetan yak. This finding is consistent with a previous study on this species (Ge et al., 2013), suggesting that glycolysis is essential for survival in high-altitude hypoxic environments. Two mitochondrial genes (ND5 and ATP8) in the OXPHOS pathway were exclusively identified to be under positive selection in the naked mole rat, suggesting that mitochondria play a role in subterranean hypoxia-tolerance. Various studies have investigated hypoxia-tolerance in the naked mole rat (Fang et al., 2014; Park et al., 2017), but its mitochondrial energetics remains largely unexplored, which are likely critical to its hypoxia-tolerance and extraordinary lifespan (Stoll et al., 2016).
The above observations are indicative of species- or lineage-specific adaptations to hypoxia. There are a several, non-mutually exclusive explanations for this deduction. It is well established that physiological features supporting a hypoxic lifestyle have evolved independently. Marine mammals routinely experience acute hypoxia, which causes them to depend on a finite supply of intrinsic oxygen stores (Ramirez et al., 2007). A dive is manifested by progressive asphyxia – concomitant with increased hypoxia, hypercapnia, and acidosis (Elsner and Gooden, 1983) – yet marine mammals exhibit an extraordinary ability to circumvent the deleterious effects of hypoxia. These include apnea (cease of breath), bradycardia, and subsequent decreased cardiac output, as well as peripheral vasoconstriction (selective shutdown of blood flow in non-essential, peripheral tissues) (Panneton, 2013). In contrast to marine mammals, terrestrial animals that live at high-altitudes or in burrows cope with a different form of hypoxic stress, chronic hypoxia due to a lower partial oxygen pressure (pO2), by improving O2 affinity and increasing ventilation and cardiac output (Ramirez et al., 2007). For example, the genomes of the Tibetan antelope and Tibetan yak show independent signals of adaptive evolution and gene-family expansion. Genes associated with oxygen transmission and mitochondrial membranes expanded in Tibetan antelope (Ge et al., 2013), whereas genes related to sensory perception and olfactory receptor activity expanded in Tibetan yak (Qiu et al., 2012), respectively. A series of genome scans of high-altitude mammals have identified genes under positive selection: EGLN1 and EPAS1 in Tibetan humans (Simonson et al., 2010; Yi et al., 2010); and ADAM17, ARG2, and MMP3 in Tibetan yak (Qiu et al., 2012). Moreover, candidate high-altitude gene loci in snub-nosed monkey populations are manifested by habitat-specific molecular evolution (Yu et al., 2016). Taken together, ecological and/or biogeographic differences, as well as distinct physiological traits, may have imposed divergent selective pressures on the evolution of hypoxia-tolerance.
Further Insights into Lineage-Specific Evolution of Hypoxia-Tolerance from Cetaceans
Cetaceans, one of three extant marine mammal orders descended from terrestrial ancestors that recolonized the sea, offer an ideal case study. During the habitat transition of cetacean ancestors, dramatic changes in anatomy and physiology occurred, e.g., streamlined body, paddle-shaped fore-flippers, degenerated hindlimbs, and lack of hair (Berta et al., 2006; Muizon, 2009). Cetaceans are also further challenged by acute episodes of hypoxia during diving. Cetacean adaptations to hypoxia are manifested as increased oxygen storage in blood and muscle, a reduced metabolic rate, and selective vasoconstriction (Costa, 2007; Ramirez et al., 2007). Molecular mechanisms underlying these innovations have only begun to be explored (Mirceta et al., 2013; McGowen et al., 2014; Tian et al., 2016).
In this study, we also identified genes under positive selection in cetaceans and investigated their functional enrichment, at the energy metabolism pathway level. We found a signature of positive selection across cetaceans in a total of eight genes, and in all four energy metabolism pathways (EMP/GNG: 2; TCA: 2; PM: 1; and OXPHOS: 4). Interestingly, the highest proportion of PSGs was in the TCA cycle. The TCA cycle is a central part of aerobic metabolism and a chief source of electrons for ATP generation from the chemical breakdown of carbohydrates, fats, and proteins (Weitzman, 1987). Of note, this includes the citrate synthase (CS) gene, which encodes a key rate-limiting enzyme in energy production via the TCA cycle. We speculate that these changes mediate an enhanced capability for aerobic metabolism by cetaceans during dives (Castellini, 2012). Of particular interest, six genes (LDHA, LDHD, PC, PCK1, FBP1, and GPI) with cetacean-specific amino acid changes are rate-limiting enzymes in the gluconeogenesis pathway (GNG) (Cori, 1981) (Figure 1 and Supplementary Figure S6). Changes in these genes may result in improved conversion of lactate to glucose or glycogen; clearing lactate from the blood and restoring circulating levels of glucose. Two genes (ALDOA and ENO2) associated with anaerobic respiration adaptively evolved in cetaceans, further supporting the role of glycolysis (EMP/GNG pathway) as a critical energy supply during prolonged, deep dives (Butler and Jones, 1997).
It is appreciated, however, that stratifying hypoxia-resistance and other unique adaptations at the genetic level is not straightforward. Cetaceans have large, energy-demanding brains and previous genome-wide studies of mammals revealed that genes associated with energy metabolism are targets of natural selection in cetaceans (McGowen et al., 2012; Shen et al., 2012; Sun et al., 2013). It then follows that selection acting on energy metabolism-related genes may be in response to the evolution of hypoxia adaptation, large brains, as well as aquatic locomotion (reflecting the historic shift from running to swimming). Similarly, flying insects and mammals (bats) also require significant energy for locomotion. Indeed, studies on bats (Shen et al., 2010, 2012) revealed a critical role for genes in the OXPHOS pathway in flight. Previous molecular studies on primates also showed evidence of adaptive evolution in energy metabolism-related genes, suggesting that these genes played a major role in overcoming elevated energy demands during the expansion of the neocortex (Grossman et al., 2001). Taken together, positive selection of energy metabolism-related genes in non-hypoxia-tolerant species is expected, and not mutually exclusive to a role in hypoxia-tolerance, given that such genes had multiple advantageous phenotypic effects (pleiotropy) during mammalian evolution.
Convergent Evolution of Energy Metabolism-Related Genes in Hypoxia-Tolerant Species
Conceptually, hypoxia-tolerance provides a classical example of convergent evolution. Even though there are clearly environmental differences, inherent similarities may drive recurrent evolution at the sequence level, manifested as convergent or parallel amino acid changes. Recent work, relying on whole-genome sequences of marine mammals (Foote et al., 2015), revealed that convergent amino acid substitutions in genes associated with phenotypic adaptations can be identified. It is appreciated that hypoxia-tolerance is a very complex phenomenon that involves a suite of genes. Although the morphologies and physiological characteristics of hypoxia-tolerance in marine and terrestrial mammals differ greatly, we reveal sequence convergence in energy metabolism-related genes. These genes could reflect core hypoxia-tolerance associated functions. Interestingly, one PSG (PFKP) was detected in the ‘combined’ hypoxia-tolerant mammal branches (Supplementary Table S1). Five genes (NDUFA9, NDUFA10, NDUFAB1, NDUFC2, and NDUFV3) encodes the nuclear-encoded subunits of complex I in the OXPHOS pathway (Figure 1). Parallel/convergent evolution of these gene may reflect the importance of oxidative phosphorylation for survival in hypoxic environments. Hypoxia also induces oxidative stress, manifested by lipid peroxidation, protein oxidation, and DNA damage (Ramirez et al., 2007). ALDH3A1 (encodes cytosolic aldehyde dehydrogenase 3A1; Figure 1) protects against oxidative stress in various ways, including enhanced DNA and cell repair (Estey et al., 2007). Another gene, PFKP (phosphofructokinase platelet type; Figure 1), catalyzes the glycolytic pathway by converting fructose-6-phosphate to fructose-1, 6-bisphosphate (Webb et al., 2015). Mutations inhibiting phosphofructokinase activity cause glycogen storage disease (Webb et al., 2015). Taken together, we reveal several convergent genes in hypoxia-tolerant species.
Conclusion
In summary, in this study we investigated genes in four major, interconnected energy metabolism pathways in hypoxia-tolerant marine and terrestrial mammals. We reveal distinct selection of a 194-gene set in hypoxia-tolerant species. Moreover, we report cetacean-specific amino acid changes in the rate-limiting enzymes of the gluconeogenesis (GNG) pathway that likely enhance lactate removal following hypoxic dives. Surprisingly, we found evidence of convergent evolution of a subset of genes in hypoxia-tolerant mammals, a phenomenon which could reflect phenotypic convergence. Our work reveals a number of candidate hypoxia-tolerance genes which can be further explored by experimental and computational analyses.
Ethics Statement
All methods used to collect observational data were non-invasive and in compliance with the regulations and guidelines of the Nanjing Normal University Institutional Animal Care and Use Committee.
Author Contributions
GY and SX conceived and designed the study. RT, DY, and YL collected the data. RT and IS conducted the bioinformatics analyses. RT wrote the manuscript, and GY, SX, and IS revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the National Science Fund for Distinguished Young Scholars (grant number 31325025 to GY); the State Key Program of National Natural Science of China (grant number 31630071 to GY); the National Natural Science Foundation of China (NSFC) (grant numbers 31570379; 31772448 to SX), the National Key Research and Development Programme, Ministry of Science and Technology (Grant number 2016YFC0503200 to GY and SX; the Priority Academic Program Development of Jiangsu Higher Education Institutions to GY and SX); the Natural Science Foundation of Jiangsu Province of China (grant number BK20141449 to SX); the Cultivation Plan for Excellent Doctorial Dissertations of Nanjing Normal University (to RT); and a Queensland University of Technology Vice-Chancellor’s Senior Research Fellowship (to IS).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Mr. Xinrong Xu for help with collecting samples for many years. They also thank Dr. Harold H. Zakon and Dr. Mary Lynne McAnelly for valuable comments on the manuscript. Special thanks are also due from Dr. Zhengfei Wang for technical support.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2017.00205/full#supplementary-material
Footnotes
References
Bennett, N. C., and Faulkes, C. G. (2000). African Mole-Rats: Ecology and Eusociality. Cambridge: Cambridge University Press.
Berg, J. M., Tymoczko, J. L., and Stryer, L. (2002). Biochemistry, 5th Edn. New York, NY: WH Freeman.
Berta, A., Sumich, J. L., and Kovacs, K. M. (2006). Marine Mammals: Evolutionary Biology. San Diego, CA: Academic Press, doi: 10.1111/j.1439-0469.2006.00402.x
Böhne, A., Heule, C., Boileau, N., and Salzburger, W. (2013). Expression and sequence evolution of aromatase cyp19a1 and other sexual development genes in east african cichlid fishes. Mol. Biol. Evol. 30, 2268–2285. doi: 10.1093/molbev/mst124
Buck, L. T., and Pamenter, M. E. (2006). Adaptive responses of vertebrate neurons to anoxia-matching supply to demand. Respir. Physiol. Neurobiol. 154, 226–240. doi: 10.1016/j.resp.2006.03.004
Butler, P. J., and Jones, D. R. (1997). Physiology of diving of birds and mammals. Physiol. Rev. 77, 837–899.
Castellini, M. (2012). Life under water: physiological adaptations to diving and living at sea. Compr. Physiol. 2, 1889–1919. doi: 10.1002/cphy.c110013
Castoe, T. A., de Koning, A. P., Hall, K. T., Card, D. C., Schield, D. R., Fujita, M. K., et al. (2013). The Burmese python genome reveals the molecular basis for extreme adaptation in snakes. Proc. Natl. Acad. Sci. U.S.A. 110, 20645–20650. doi: 10.1073/pnas.1314475110
Castoe, T. A., Jiang, Z. J., Gu, W., Wang, Z. O., and Pollock, D. D. (2008). Adaptive evolution and functional redesign of core metabolic proteins in snakes. PLOS ONE 3:e2201. doi: 10.1371/journal.pone.0002201
Cori, C. F. (1981). The glucose-lactic acid cycle and gluconeogenesis. Curr. Top. Cell. Regul. 18, 377–387. doi: 10.1016/B978-0-12-152818-8.50028-1
Costa, D. P. (2007). “Diving physiology of marine vertebrates,” in Encyclopedia of Life Sciences, ed. Clavier (Hoboken, NJ: John Wiley & Sons). doi: 10.1002/9780470015902.a0004230
Darwin, C. (1859). On the Origin of Species Based on Natural Selection, or the Preservation of Favoured Races in the Struggle for Life, 6th Edn. London: John Murray, doi: 10.5962/bhl.title.59991
Edgar, R. C. (2004). MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC. Bioinformatics. 5:113. doi: 10.1186/1471-2105-5-113
Elsner, R., and Gooden, B. (1983). Diving and Asphyxia. Cambridge: Cambridge University Press. doi: 10.1017/CBO9780511735714
Estey, T., Piatigorsky, J., Lassen, N., and Vasiliou, V. (2007). ALDH3A1: a corneal crystallin with diverse functions. Exp. Eye Res. 84, 3–12. doi: 10.1016/j.exer.2006.04.010
Fang, X., Seim, I., Huang, Z., Gerashchenko, M. V., Xiong, Z., Turanov, A. A., et al. (2014). Adaptations to a subterranean environment and longevity revealed by the analysis of mole rat genomes. Cell Rep. 8, 1354–1364. doi: 10.1016/j.celrep.2014.07.030
Foote, A. D., Liu, Y., Thomas, G. W., Vinař, T., Alföldi, J., Deng, J., et al. (2015). Convergent evolution of the genomes of marine mammals. Nat. Genet. 47, 272–275. doi: 10.1038/ng.3198
Fromm, H. J., and Hargrove, M. S. (2012). “Carbohydrate metabolism a: glycolysis and gluconeogenesis,” in Essentials of Biochemistry, eds H. J. Fromm and M. Hargrove (Berlin: Springer), 163–204.
Ge, R. L., Cai, Q., Shen, Y. Y., San, A., Ma, L., Zhang, Y., et al. (2013). Draft genome sequence of the Tibetan antelope. Nat. Commun. 4:1858. doi: 10.1038/ncomms2860
Giordano, F. J. (2005). Oxygen, oxidative stress, hypoxia, and heart failure. J. Clin. Invest. 115, 500–508. doi: 10.1172/JCI24408
Grossman, L. I., Schmidt, T. R., Wildman, D. E., and Goodman, M. (2001). Molecular evolution of aerobic energy metabolism in primates. Mol. Phylogenet. Evol. 18, 26–36. doi: 10.1006/mpev.2000.0890
Hoelzel, A. R. (2002). Marine Mammal Biology, an Evolutionary Approach. Oxford: Blackwell Publishing.
Johnson, M. Z. I., Raytselis, Y., Merezhuk, Y., Mcginnis, S., and Madden, T. L. (2008). Ncbi blast: a better web interface. Nucleic Acids Res. 36, W5–W9. doi: 10.1093/nar/gkn201
Kanehisa, M., and Goto, S. (2000). KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30. doi: 10.1093/nar/28.1.27
Kinsella, R. J., Kähäri, A., Haider, S., Zamora, J., Proctor, G., Spudich, G., et al. (2011). Ensembl BioMarts: a hub for data retrieval across taxonomic space. Database 2011:bar030. doi: 10.1093/database/bar030
Law, C. W., Chen, Y., Shi, W., and Smyth, G. K. (2014). Voom: precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 15:R29. doi: 10.1186/gb-2014-15-2-r29
McGowen, M. R., Gatesy, J., and Wildman, D. E. (2014). Molecular evolution tracks macroevolutionary transitions in Cetacea. Trends Ecol. Evol. 29, 336–346. doi: 10.1016/j.tree.2014.04.001
McGowen, M. R., Grossman, L. I., and Wildman, D. E. (2012). Dolphin genome provides evidence for adaptive evolution of nervous system genes and a molecular rate slowdown. Proc. Biol. Sci. 279, 3643–3651. doi: 10.1098/rspb.2012.0869
Mirceta, S., Signore, A. V., Burns, J. M., Cossins, A. R., KCampbell, K. L., and Berenbrink, M. (2013). Evolution of mammalian diving capacity traced by myoglobin net surface charge. Science 340:1234192. doi: 10.1126/science.1234192
Muizon, C. (2009). Origin and evolutionary history of cetaceans. C. R. Palevol. 8, 295–309. doi: 10.1016/j.crpv.2008.07.002
Murphy, W. J., Eizirik, E., Johnson, W. E., Zhang, Y. P., Ryder, O. A., and O’Brien, S. J. (2001). Molecular phylogenetics and the origins of placental mammals. Nature 409, 614–618. doi: 10.1038/35054550
Nathaniel, T. I., Williams-Hernandez, A., Hunter, A. L., Liddy, C., Peffley, D. M., Umesiri, F. E., et al. (2015). Tissue hypoxia during ischemic stroke: adaptive clues from hypoxia-tolerant animal models. Brain Res. Bull. 114, 1–12. doi: 10.1016/j.brainresbull.2015.02.006
Panneton, W. M. (2013). The mammalian diving response: an enigmatic reflex to preserve life? Physiology 28, 284–297. doi: 10.1152/physiol.00020.2013
Park, T. J., Reznick, J., Peterson, B. L., Blass, G., Omerbašić, D., Bennett, N. C., et al. (2017). Fructose-driven glycolysis supports anoxia resistance in the naked mole-rat. Science 356, 307–311. doi: 10.1126/science.aab3896
Parker, J., Tsagkogeorga, G., Cotton, J. A., Liu, Y., Provero, P., Stupka, E., et al. (2013). Genome-wide signatures of convergent evolution in echolocating mammals. Nature 502, 228–231. doi: 10.1038/nature12511
Qiu, Q., Zhang, G., Ma, T., Qian, W., Wang, J., Ye, Z., et al. (2012). The yak genome and adaptation to life at high altitude. Nat. Genet. 44, 946–949. doi: 10.1038/ng.2343
Ramirez, J. M., Folkow, L. P., and Blix, A. S. (2007). Hypoxia tolerance in mammals and birds: from the wilderness to the clinic. Annu. Rev. Physiol. 69, 113–143. doi: 10.1146/annurev.physiol.69.031905.163111
Seim, I., Ma, S., Zhou, X., Gerashchenko, M. V., Lee, S. G., Suydam, R., et al. (2014). The transcriptome of the bowhead whale Balaena mysticetus reveals adaptations of the longest-lived mammal. Aging 6, 879–899. doi: 10.18632/aging.100699
Shen, Y. Y., Liang, L., Zhu, Z. H., Zhou, W. P., Irwin, D. M., and Zhang, Y. P. (2010). Adaptive evolution of energy metabolism genes and the origin of flight in bats. Proc. Natl. Acad. Sci. U.S.A. 107, 8666–8671. doi: 10.1073/pnas.0912613107
Shen, Y. Y., Zhou, W. P., Zhou, T. C., Zeng, Y. N., Li, G. M., Irwin, D. M., et al. (2012). Genome-wide scan for bats and dolphin to detect their genetic basis for new locomotive styles. PLOS ONE 7:e46455. doi: 10.1371/journal.pone.0046455
Simonson, T. S., Yang, Y., Huff, C. D., Yun, H., Qin, G., Witherspoon, D. J., et al. (2010). Genetic evidence for high-altitude adaptation in Tibet. Science 329, 72–75. doi: 10.1126/science.1189406
Söling, H. D., and Willms, B. (eds) (2013). Regulation of Gluconeogenesis: 9th Conference of the Gesellschaft Für Biologische Chemie. New York, NY: Elsevier.
Stamatakis, A. (2014). RAxML Version 8: a tool for phylogenetic analysis and Post-analysis of large phylogenies. Bioinformatics 30, 1312–1313. doi: 10.1093/bioinformatics/btu033
Stoll, E. A., Karapavlovic, N., Rosa, H., Woodmass, M., Rygiel, K., White, K., et al. (2016). Naked mole-rats maintain healthy skeletal muscle and Complex IV mitochondrial enzyme function into old age. Aging 8, 3468–3485. doi: 10.18632/aging.101140
Storey, J. D., and Tibshirani, R. (2003). Statistical significance for genomewide studies. Proc. Natl. Acad. Sci. U.S.A. 100, 9440–9445. doi: 10.1073/pnas.1530509100
Storz, J. F., Scott, G. R., and Cheviron, Z. A. (2010). Phenotypic plasticity and genetic adaptation to high-altitude hypoxia in vertebrates. J. Exp. Biol. 213, 4125–4136. doi: 10.1242/jeb.048181
Sun, Y. B., Zhou, W. P., Liu, H. Q., Irwin, D. M., Shen, Y. Y., and Zhang, Y. P. (2013). Genome-wide scans for candidate genes involved in the aquatic adaptation of dolphins. Genome Biol. Evol. 5, 130–139. doi: 10.1093/gbe/evs123
Tamura, K., Stecher, G., Peterson, D., Filipski, A., and Kumar, S. (2013). MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729. doi: 10.1093/molbev/mst197
Tian, R., Wang, Z. F., Niu, X., Zhou, K. Y., Xu, S. X., and Yang, G. (2016). Evolutionary genetics of hypoxia tolerance in cetaceans during diving. Genome Biol. Evol. 8, 827–839. doi: 10.1093/gbe/evw037
Wang, E. T., Sandberg, R., Luo, S., Khrebtukova, I., Zhang, L., Mayr, C., et al. (2008). Alternative isoform regulation in human tissue transcriptomes. Nature 456, 470–476. doi: 10.1038/nature07509
Webb, B. A., Forouhar, F., Szu, F. E., Seetharaman, J., Tong, L., and Barber, D. L. (2015). Structures of human phosphofructokinase-1 and atomic basis of cancer-associated mutations. Nature 523, 111–114. doi: 10.1038/nature14405
Weitzman, P. D. J. (1987). “Patterns of diversity of citric acid cycle enzymes,” in Krebs’ Citric Acid Cycle - Half A Century and Still Turning, eds J. Kay and P. D. J. Weitzman (London: Biochemical Society), 33–43.
Yang, Z. (2007). PAML 4: phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 24, 1586–1591. doi: 10.1093/molbev/msm088
Yang, Z., Wong, W. S., and Nielsen, R. (2005). Bayes empirical Bayes inference of amino acid sites under positive selection. Mol. Biol. Evol. 22, 1107–1118. doi: 10.1093/molbev/msi097
Yi, X., Liang, Y., Huerta-Sanchez, E., Jin, X., Cuo, Z. X., Pool, J. E., et al. (2010). Sequencing of 50 human exomes reveals adaptation to high altitude. Science 329, 75–78. doi: 10.1126/science.1190371
Yu, L., Wang, G. D., Ruan, J., Chen, Y. B., Yang, C. P., Cao, X., et al. (2016). Genomic analysis of snub-nosed monkeys (Rhinopithecus) identifies genes and processes related to high-altitude adaptation. Nat. Genet. 48, 947–952. doi: 10.1038/ng.3615
Zhang, J., and Kumar, S. (1997). Detection of convergent and parallel evolution at the amino acid sequence level. Mol. Biol. Evol. 14, 527–536. doi: 10.1093/oxfordjournals.molbev.a025789
Keywords: hypoxia-tolerance, energy metabolism, adaptive evolution, positive selection, convergent evolution
Citation: Tian R, Yin D, Liu Y, Seim I, Xu S and Yang G (2017) Adaptive Evolution of Energy Metabolism-Related Genes in Hypoxia-Tolerant Mammals. Front. Genet. 8:205. doi: 10.3389/fgene.2017.00205
Received: 08 September 2017; Accepted: 24 November 2017;
Published: 07 December 2017.
Edited by:
Juan Caballero, Universidad Autónoma de Querétaro, MexicoReviewed by:
Xiyin Wang, North China University of Science and Technology, ChinaAdam Michael Reitzel, University of North Carolina at Charlotte, United States
Copyright © 2017 Tian, Yin, Liu, Seim, Xu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shixia Xu, xushixia78@163.com Guang Yang, gyang@njnu.edu.cn
 Ran Tian
Ran Tian Daiqing Yin
Daiqing Yin Yanzhi Liu
Yanzhi Liu Inge Seim
Inge Seim Shixia Xu
Shixia Xu Guang Yang
Guang Yang