- 1Bio Engineering LLC, Skopje, Macedonia
- 2Zan Mitrev Clinic, Skopje, Macedonia
- 3Neuromedica Hospital, Skopje, Macedonia
- 4Department of Pediatric Nephrology, Medical Faculty of Skopje, University Children's Hospital, Skopje, Macedonia
Exome sequencing can interrogate thousands of genes simultaneously and it is becoming a first line diagnostic tool in genomic medicine. Herein, we applied trio clinical exome sequencing (CES) in a patient presenting with undiagnosed skeletal disorder, minor facial abnormalities, and kidney hypoplasia; her parents were asymptomatic. Testing the proband and her parents led to the identification of a de novo mutation c.188C>T (p.Pro63Leu) in the MAFB gene, which is known to cause multicentric carpotarsal osteolysis syndrome (MCTO). The c.188C>T mutation lies in a hotspot amino acid stretch within the transactivation domain of MAFB, which is a negative regulator of RANKL-induced osteoclastogenesis. MCTO is an extremely rare autosomal dominant (AD) disorder that typically arises spontaneously and causes carpotarsal osteolysis, often followed by nephropathy. To the best of our knowledge, this is the first study reporting genetically diagnosed MCTO in the Balkans.
Case Presentation
A 13-year-old ethnic Macedonian girl and her parents attended the Zan Mitrev Clinic for pediatric examination, and concomitant genetic counseling. She presented with abnormal gait due to decreased joint mobility. Further investigations revealed destruction of carpal bones on both hands, olecranon bursitis, marfanoid habitus, cachexia, cutis laxa, mild facial abnormalities (triangular face, eye bulging, micrognathia). She did not show signs of intellectual disability; morphological abnormalities were absent in her parents (Figure 1; Supplementary Figures 1, 2).
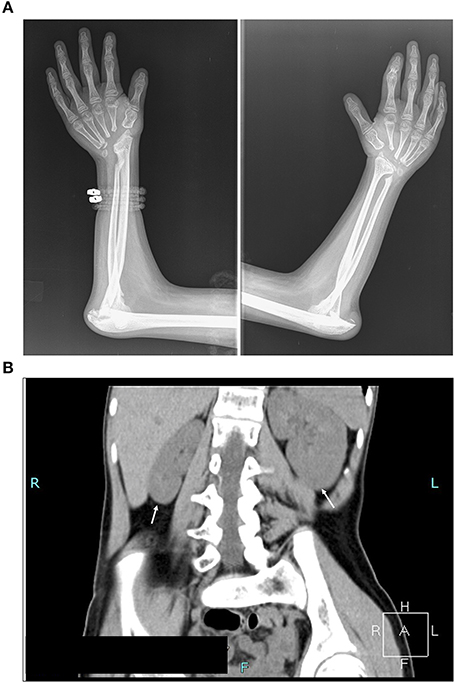
Figure 1. Assessment of the clinical symptoms of the patient. (A) X-ray image illustrating destruction of carpal bones. Absence of carpal bones and osteolysis of proximal end of metacarpal bones, and bones of the elbow joints with pathological dislocation of both elbow joints (B) Computed tomography scan (CT) illustrating kidney asymmetry. Please note the hypoplasia of the right kidney, and compensatory hypertrophy of the left kidney, and scoliosis dextroconvexa lumbalis and spina bifida of the lumbosacral vertebra (see also Supplementary Figure 1B).
The symptoms were first noted on her wrists and ankles when she was a toddler. At 13 years of age, her radiological examination showed osteolysis of carpal bones, destruction of the elbow joints with pathological dislocation of both elbows, osteolysis of the tarsal bones and first metatarsal with pathological dislocation of the first metatarsophalangeal joints, and cyst-like structures on the right femoral epiphysis (Figure 1A; Supplementary Figures 1, 2). Previous nephrology evaluation via ultrasound scanning revealed asymmetry in the size of the kidneys at 2 years of age. At that time, Tc99mDMSA scan showed 33% decreased uptake of the right kidney, however her blood work including renal function parameters were within reference limits. A recent ultrasound examination confirmed significant kidney hypoplasia of the right kidney (59 × 25 mm), in contrast, the left kidney was compensatorily hypertrophic with dimensions 107 × 45 mm. Computed tomography (CT) scan confirmed the ultrasound findings (Figure 1B). No audiometric and ophthalmological tests were carried out.
Upon taking written and signed informed consent from the proband's legal guardians, she underwent clinical exome sequencing (CES) (TruSight One panel) from Illumina. In brief, DNA was extracted from 400 μl of whole blood on a SaMag-12 automatic nucleic acid extraction system (Sacace Biotechnologies, Como, Italy). Libraries were pooled together and underwent two rounds of hybridization and capture, and additional quantification and size assessment. Between 19 and 26 million reads were obtained with a NextSeq machine (Illumina, San Diego, USA), with a coverage of at least 50x for 80–90% of all sequences. Sequence quality control was done with FastQC (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/), and sequences were mapped to Hg19 with BWA (Li and Durbin, 2009). SNP and INDEL calling, together with advanced variant annotation were done with proprietary technologies such as PEPPER™ and MOKA™, respectively, both integrated within the Sophia DDM platform (Sophia Genetics, Saint-Sulpice, Switzerland). Later on, mapping, SNP and INDEL calling, annotation, and in-depth analyses were carried out with the Genoox platform (https://www.genoox.com/).
The analysis focused on virtual gene panels composed of genes causing skeletal dysplasia, and connective tissue disorders using the Sophia DDM platform (Sophia Genetics). The analysis failed to identify known Clinvar mutations. We detected several (>10) heterozygous candidate variants based on ACMG computational criteria (Richards et al., 2015; Supplementary Table 1).
In order to accurately pin down the causal variant, we performed additional CES of her parents. Upon analysis of high confidence de novo mutations (present in the proband, but absent in the parents), using an in-built tool in the Genoox platform we identified the c.188C>T (p.Pro63Leu) variant in the MAFB gene (Figure 2; Supplementary Table 2). This finding was confirmed by Sanger sequencing (Supplementary Figure 3). This variant has been reported to cause multicentric carpotarsal osteolysis syndrome (MCTO), OMIM # 166300, (Zankl et al., 2012; Mehawej et al., 2013). The mutation lies in a hotspot amino acid stretch within the transactivation domain of MAFB, which is a negative regulator of RANKL-induced osteoclastogenesis (Mumm et al., 2014). The mutation also meets computational criteria of pathogenicity such as low population frequency (g1000, ExAC), damaging effect (SIFT, Mutation Taster, FATHMM), and evolutionary conservation (GERP) (Supplementary Table 2).
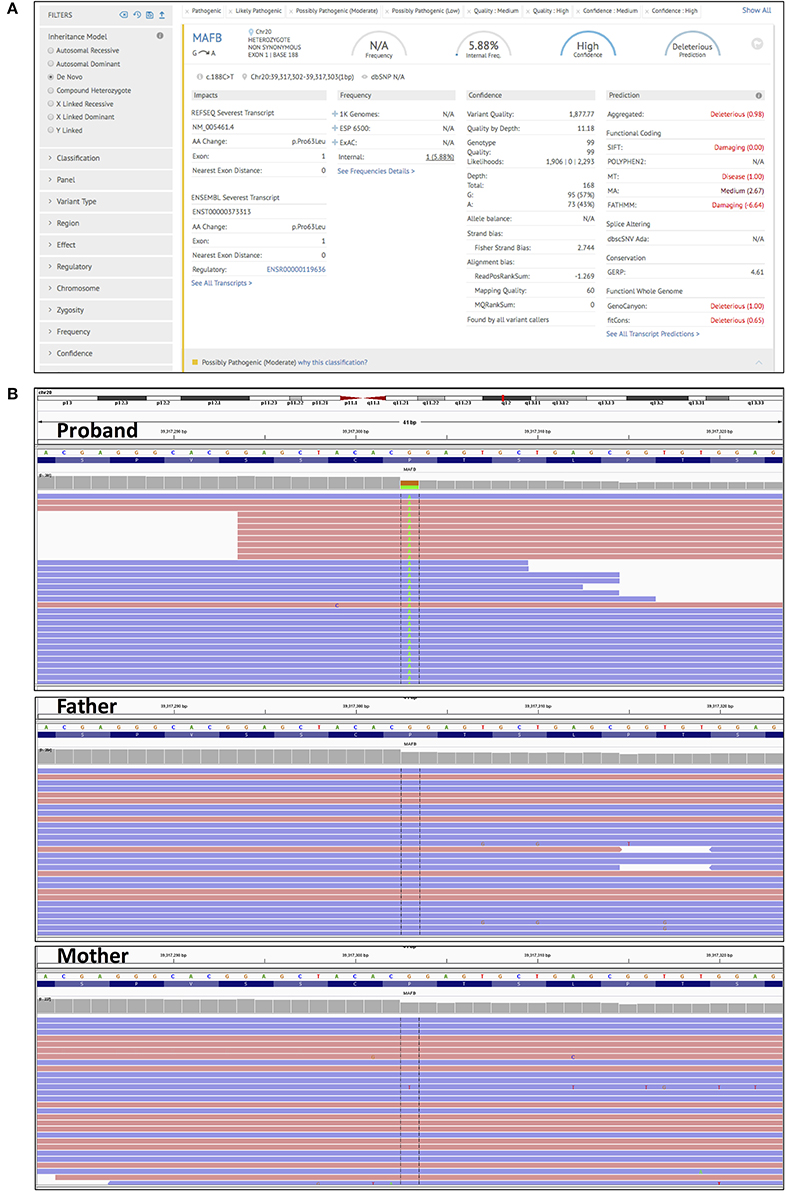
Figure 2. Trio clinical exome sequencing. (A) A screen shot taken from the Genoox platform, showing the detection and annotation of de novo c.188C>T (p.Pro63Leu) mutation in the MAFB gene. (B) IGV genome browser screenshot of reads obtained by NGS. Detection of the c.188C>T (p.Pro63Leu) in the MAFB gene in the proband (upper panel), and its absence in the father, and mother (middle and lower panel).
Next, we focused our attention to additional two heterozygous genetic variants as well. The first one, c.1851C>A (p.Asp617Glu) in the COL9A3 gene is a variant of unknown significance (VUS) and it was detected in the proband and her father. The second one, c.908C>T (p.Thr303Met) in the MATN3 gene was detected in the proband and her mother; it has been reported to be associated with osteoarthritis (Supplementary Table 1; Stefánsson et al., 2003; Pullig et al., 2007). The COL9A3 and MATN3 proteins are known to co-interact in their native state (Fresquet et al., 2007). In addition, known pathogenic variants in the COL9A3 and MATN3 genes have been reported to cause multiple epiphyseal dysplasia; Thus it is conceivable that these two variants might have an additional modifier effect on the disease. Further functional studies are necessary to substantiate this claim.
Background
Multicentric carpotarsal osteolysis syndrome (MCTO), OMIM # 166300 is an extremely rare skeletal disorder (the frequency is not known), usually presenting in early childhood characterized by symptoms mimicking juvenile rheumatoid arthritis, and progressive bone loss of hands and feet, typically carpal and tarsal bones. Other symptoms include peculiar facial manifestations such as triangular shape of the face, micrognatia, protruding eyes, as well as nephropathy and eventual renal failure (Kohler et al., 1973; Tyler and Rosenbaum, 1976; Whyte et al., 1978; Lemaitre et al., 1983; Hardegger et al., 1985; Carnevale et al., 1987; Pai and Macpherson, 1988; Thomas et al., 2006).
According to https://rarediseases.info.nih.gov between 80 and 99% of all patients with MCTO have the following symptoms: cachexia, carpal osteolysis, EMG abnormality, abnormal gait, decreased joint mobility, metacarpal osteolysis, micrognathia, proptosis, proteinuria, slender long bone, triangular face, wrist swelling. Furthermore, 30–79% of patients have camptodactyly of finger, and nephropathy. Nephropathy in majority of cases is recognized lately during the course of the disease and leads to end stage renal failure. It manifests with proteinuria (Mehawej et al., 2013; Mumm et al., 2014); there are only few cases documented with renal biopsy, which disclosed focal segmental glomerulosclerosis (Hirooka and Hirota, 1985). In the French series two out of eight patients presented with bilateral kidney hypoplasia (Mehawej et al., 2013). Our patient had hypoplastic right kidney, but no significant proteinuria was detected at the last check-up.
Multicentric carpotarsal osteolysis syndrome is inherited in an autosomal dominant manner with a complete penetrance, and in most cases it occurs sporadically. It is caused by mutations clustering in the N-terminal activation of MAFB (Zankl et al., 2012; Mehawej et al., 2013; Mumm et al., 2014). MAFB is thought to cause imbalance in bone remodeling followed by osteolysis, likely resulting in suppression of RANKL/RANK signaling induced genes, which are crucial for osteoclastogenesis (Kim et al., 2007). Therefore, defects in the MAFB protein regulate RANKL-induced osteoclastogenesis in a negative manner, and collectively manifest as the abnormalities seen in MCTO patients. MAFB gene is involved in the process of nephrogenesis as well, and it is expressed in podocytes; hence it might account for the structural and functional changes in the kidneys. According to current literature, all MCTO patients are genetically homogeneous (mutations are found in the MAFB gene), with moderate clinical variability, possibly due to modifier effects (Mehawej et al., 2013).
Discussion
Multicentric Carpo-Tarsal Osteolysis—Treatment Options
Multicentric carpo-tarsal osteolysis is a very rare disorder, with tens of cases reported in literature worldwide. Herein, by applying trio CES we solved a case, which had been left undiagnosed for more than a decade. To the best of our knowledge this is the first genetically diagnosed and reported case of MCTO in the Balkans. The case described in this report illustrates the power of CES to interrupt the diagnostic odyssey of this family. Although the establishment of the correct diagnosis did not result in a direct causal treatment, some preventive measurements should be implemented in this patient such as: corrective bone surgery (Sun et al., 2016), regular monitoring of proteinuria and renoprotective treatment, as well as avoiding or careful administration of non-steroidal anti-inflammatory and other nephrotoxic drugs. In the same vein, clinicians should take into account the therapeutic success reported in a couple of studies for the spectrum of osteolytic diseases with bisphosphonates, cyclosporine A, tocilizumab (Connor et al., 2007; Lee et al., 2010; Nishikomori et al., 2015; Pichler et al., 2016).
Multicentric Carpo-Tarsal Osteolysis—Larger Cohorts Are Needed
Multicentric carpotarsal osteolysis syndrome is a disorder, where mutations cluster in a hotspot region of the MAFB protein. MCTO has been shown to be always caused by aberrant MAFB function albeit with clinical variability. This variability can emanate from numerous sources. Firstly, MAFB is a basic leucine zipper transcription factor that plays a role in the regulation of lineage specific hematopoiesis, and osteoclastogenesis. It is therefore conceivable that minor changes, even in the same hotspot regions of the protein can lead to nuanced but nonetheless differential regulatory, and consequently transcriptional effects that can explain the clinical variability. Secondly, additional genetic or epigenetic variations can have a modifier effect and lead to clinical variability in a seemingly well-defined “genetic homogeneity.” In this context, application of broad DNA tests in lieu of focused gene panels, in a sufficiently high cohort of patients can provide more insights about modifier effects, even in the cases where the penetrance seems to be complete. Finally, the functional role of MAFB and its pathogenic variants should be further studied via biochemical, genomic, transcriptomic and epigenetic approaches in cell culture, and model organisms.
In our opinion, in order to sneak a peek into the molecular peculiarities of the disease, a more centralized MCTO institutional body should be established. This primary task of this body should be to find, select and study already diagnosed or candidate MCTO patients under one umbrella in a controlled manner. Such an effort could aid the discovery of candidate targets in the RANKL-associated pathway for development of precision therapies.
Concluding Remarks
The plummeting prices of next-generation sequencing (NGS) technologies, have led to broad availability of comprehensive DNA tests such as whole human genome sequencing (WGS), whole exome sequencing (WES), and CES in everyday clinical settings (Shendure and Lieberman Aiden, 2012; Katsanis and Katsanis, 2013). These approaches of massively parallel sequencing, coupled with new and easy-to-use platforms for NGS data processing, analysis, filtering, and interpretation are setting the foundation for a new wave of quick and cheaper clinical diagnostics, and accumulation of knowledge, particularly valuable in the context of rare diseases (Stark et al., 2017). Singleton exome/genome sequencing can lead to a definitive diagnosis in many cases, but the modus operandi of preference in genetic diagnostics, in our opinion should be trio or family cohorts, whenever possible.
Clinicians should embrace these new technologies and join efforts with geneticists in tackling and solving numerous rare disease cases. Ultimately, comprehensive DNA testing can be used to provide new insights of molecular pathways and switches and aid the development of new therapies.
Ethics Statement
Written and signed informed consent was obtained from all subjects or their legal guardians of this case study.
Data Access
Fastq data have been deposited to the SRA under the accession number SRP136097.
Author Contributions
GK: conceived and designed this case study; GK: processed, analyzed, and interpreted the clinical exome data with the help of AS, SM, MS, KJ; GK and SM: contributed to genetic counseling; VT, NN, and GZ: contributed in the assessment and interpretation of the clinical symptoms and data; IK and ZM: contributed in the recruitment of patients in the hospital and contributed intellectually; GK: wrote the manuscript with help in the clinical part from VT. All authors contributed to improvement of the manuscript and read the final version of the manuscript.
Funding
Privately funded by the Zan Mitrev Clinic.
Conflict of Interest Statement
GK, IK, and AS are employees of Bio Engineering LLC.
The other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to Zvonko Atanasov, M.D for helping us with the CT data. We also thank Rodney Rosalia, Ph.D. for his critical assessment of the manuscript, and his constructive suggestions.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2018.00113/full#supplementary-material
References
Carnevale, A., Canún, S., Mendoza, L., and del Castillo, V. (1987). Idiopathic multicentric osteolysis with facial anomalies and nephropathy. Am. J. Med. Genet. 26, 877–886. doi: 10.1002/ajmg.1320260415
Connor, A., Highton, J., Hung, N. A., Dunbar, J., MacGinley, R., and Walker, R. (2007). Multicentric carpal-tarsal osteolysis with nephropathy treated successfully with cyclosporine A: a case report and literature review. Am. J. Kidney Dis. 50, 649–654. doi: 10.1053/j.ajkd.2007.06.014
Fresquet, M., Jowitt, T. A., Ylöstalo, J., Coffey, P., Meadows, R. S., Ala-Kokko, L., et al. (2007). Structural and functional characterization of recombinant matrilin-3 A-domain and implications for human genetic bone diseases. J. Biol. Chem. 282, 34634–34643. doi: 10.1074/jbc.M705301200
Hardegger, F., Simpson, L. A., and Segmueller, G. (1985). The syndrome of idiopathic osteolysis. Classification, review, and case report. J. Bone Joint Surg. Br. 67, 88–93. doi: 10.1302/0301-620X.67B1.3968152
Hirooka, M., and Hirota, M. (1985). Chronic nephropathy in idiopathic multicentric osteolysis. Int. J. Pediatr. Nephrol. 6, 145–150.
Katsanis, S. H., and Katsanis, N. (2013). Molecular genetic testing and the future of clinical genomics. Nat. Rev. Genet. 14, 415–426. doi: 10.1038/nrg3493
Kim, J. G., Kim, J. H., Kim, J. Y., Ku, S. Y., Jee, B. C., Suh, C. S., et al. (2007). Association between osteoprotegerin (OPG), receptor activator of nuclear factor-kappaB (RANK), and RANK ligand (RANKL) gene polymorphisms and circulating OPG, soluble RANKL levels, and bone mineral density in Korean postmenopausal women. Menopause 14, 913–918. doi: 10.1097/gme.0b013e31802d976f
Kohler, E., Babbitt, D., Huizenga, B., and Good, T. A. (1973). Hereditary osteolysis. A clinical, radiological and chemical study. Radiology 108, 99–105. doi: 10.1148/108.1.99
Lee, S. J., Whitewood, C., and Murray, K. J. (2010). Inherited multicentric osteolysis: case report of three siblings treated with bisphosphonate. Pediatr. Rheumatol. Online J. 8:12. doi: 10.1186/1546-0096-8-12
Lemaitre, L., Remy, J., Smith, M., Nuyts, J. P., Cousin, J., Farine, M. O., et al. (1983). Carpal and tarsal osteolysis. Pediatr. Radiol. 13, 219–226. doi: 10.1007/BF00973160
Li, H., and Durbin, R. (2009). Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760. doi: 10.1093/bioinformatics/btp324
Mehawej, C., Courcet, J. B., Baujat, G., Mouy, R., Gérard, M., Landru, I., et al. (2013). The identification of MAFB mutations in eight patients with multicentric carpo-tarsal osteolysis supports genetic homogeneity but clinical variability. Am. J. Med. Genet. Part A 161A, 3023–3029. doi: 10.1002/ajmg.a.36151
Mumm, S., Huskey, M., Duan, S., Wenkert, D., Madson, K. L., Gottesman, G. S., et al. (2014). Multicentric carpotarsal osteolysis syndrome is caused by only a few domain-specific mutations in MAFB, a negative regulator of RANKL-induced osteoclastogenesis. Am. J. Med. Genet. Part A 164A, 2287–2293. doi: 10.1002/ajmg.a.36641
Nishikomori, R., Kawai, T., Toshiyuki, K., Oda, H., Yasumi, T., Izawa, K., et al. (2015). Remarkable improvement of articular pain by biologics in a Multicentric carpotarsal osteolysis patient with a mutation of MAFB gene. Pediatr. Rheumatol. 13(Suppl. 1):P152. doi: 10.1186/1546-0096-13-S1-P152
Pai, G. S., and Macpherson, R. I. (1988). Idiopathic multicentric osteolysis: report of two new cases and a review of the literature. Am. J. Med. Genet. 29, 929–936. doi: 10.1002/ajmg.1320290425
Pichler, K., Karall, D., Kotzot, D., Steichen-Gersdorf, E., Rümmele-Waibel, A., Mittaz-Crettol, L., et al. (2016). Bisphosphonates in multicentric osteolysis, nodulosis and arthropathy (MONA) spectrum disorder - an alternative therapeutic approach. Sci. Rep. 6:34017. doi: 10.1038/srep34017
Pullig, O., Tagariello, A., Schweizer, A., Swoboda, B., Schaller, P., and Winterpacht, A. (2007). MATN3 (matrilin-3) sequence variation (pT303M) is a risk factor for osteoarthritis of the CMC1 joint of the hand, but not for knee osteoarthritis. Ann. Rheum. Dis. 66, 279–280. doi: 10.1136/ard.2006.058263
Richards, S., Aziz, N., Bale, S., Bick, D., Das, S., Gastier-Foster, J., et al. (2015). Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 17, 405–424. doi: 10.1038/gim.2015.30
Shendure, J., and Lieberman Aiden, E. (2012). The expanding scope of DNA sequencing. Nat. Biotechnol. 30, 1084–1094. doi: 10.1038/nbt.2421
Stark, Z., Schofield, D., Alam, K., Wilson, W., Mupfeki, N., Macciocca, I., et al. (2017). Prospective comparison of the cost-effectiveness of clinical whole-exome sequencing with that of usual care overwhelmingly supports early use and reimbursement. Genet. Med. 19, 867–874. doi: 10.1038/gim.2016.221
Stefánsson, S. E., Jónsson, H., Ingvarsson, T., Manolescu, I., Jónsson, H. H., Olafsdóttir, G., et al. (2003). Genomewide scan for hand osteoarthritis: a novel mutation in matrilin-3. Am. J. Hum. Genet. 72, 1448–1459. doi: 10.1086/375556
Sun, K., Barlow, B., Malik, F., Inglis, A., Figgie, M., and Goodman, S. (2016). Total hip arthroplasty in a patient with multicentric carpotarsal osteolysis: a case report. HSS J. 12, 177–181. doi: 10.1007/s11420-015-9478-0
Thomas, C. W., Bisset, A. J., Sampson, M. A., and Armstrong, R. D. (2006). Case report: multicentric carpal/tarsal osteolysis: imaging review and 25-year follow-up. Clin. Radiol. 61, 892–895. doi: 10.1016/j.crad.2006.03.018
Tyler, T., and Rosenbaum, H. D. (1976). Idiopathic multicentric osteolysis. Am. J. Roentgenol. 126, 23–31. doi: 10.2214/ajr.126.1.23
Whyte, M. P., Murphy, W. A., Kleerekoper, M., Teitelbaum, S. L., and Avioli, L. V. (1978). Idiopathic multicentric osteolysis. Report of an affected father and son. Arthritis Rheum. 21, 367–376. doi: 10.1002/art.1780210313
Keywords: next-generation sequencing, exome sequencing, case report, multicentric carpotarsal osteolysis syndrome, Balkan
Citation: Stajkovska A, Mehandziska S, Stavrevska M, Jakovleva K, Nikchevska N, Mitrev Z, Kungulovski I, Zafiroski G, Tasic V and Kungulovski G (2018) Trio Clinical Exome Sequencing in a Patient With Multicentric Carpotarsal Osteolysis Syndrome: First Case Report in the Balkans. Front. Genet. 9:113. doi: 10.3389/fgene.2018.00113
Received: 04 January 2018; Accepted: 21 March 2018;
Published: 05 April 2018.
Edited by:
Enrico Baruffini, Università degli Studi di Parma, ItalyReviewed by:
Alexandre Rezende Vieira, University of Pittsburgh, United StatesMuhammad Jawad Hassan, National University of Sciences and Technology, Pakistan
Copyright © 2018 Stajkovska, Mehandziska, Stavrevska, Jakovleva, Nikchevska, Mitrev, Kungulovski, Zafiroski, Tasic and Kungulovski. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Goran Kungulovski, goran@bioengineering.mk; goran.kungulovski@zmc.mk
 Aleksandra Stajkovska
Aleksandra Stajkovska Sanja Mehandziska
Sanja Mehandziska Margarita Stavrevska
Margarita Stavrevska Kristina Jakovleva
Kristina Jakovleva Natasha Nikchevska2
Natasha Nikchevska2 Velibor Tasic
Velibor Tasic Goran Kungulovski
Goran Kungulovski