
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 13 January 2016
Sec. Immunological Memory
Volume 6 - 2015 | https://doi.org/10.3389/fimmu.2015.00660
This article is part of the Research Topic Bone marrow T cells at the center stage in immunological memory View all 11 articles
Bone marrow (BM) plays an important role in the long-term maintenance of memory T cells. Yet, BM is found in numerous bones throughout the body, which are not equal in structure, as they differ in their ratio of cortical and trabecular bone. This implies that BM cells within different bones are subjected to different microenvironments, possibly leading to differences in their frequencies and function. To address this, we examined BM from murine tibia, femur, pelvis, sternum, radius, humerus, calvarium, and the vertebrae and analyzed the presence of effector memory (TEM), central memory (TCM), and naïve (TNV) CD8+ T cells. During steady-state conditions, the frequency of the total CD8+ T cell population was comparable between all bones. Interestingly, most CD8+ T cells were located in the vertebrae, as it contained the highest amount of BM cells. Furthermore, the frequencies of TEM, TCM, and TNV cells were similar between all bones, with a majority of TNV cells. Additionally, CD8+ T cells collected from different bones similarly expressed the key survival receptors IL-7Rα and IL-15Rβ. We also examined BM for memory CD8+ T cells with a tissue-resident memory phenotype and observed that approximately half of all TEM cells expressed the retention marker CD69. Remarkably, in the memory phase of acute infection with the lymphocytic choriomeningitis virus (LCMV), we found a massive compositional change in the BM CD8+ T cell population, as the TEM cells became the dominant subset at the cost of TNV cells. Analysis of Ki-67 expression established that these TEM cells were in a quiescent state. Finally, we detected higher frequencies of LCMV-specific CD8+ T cells in BM compared to spleen and found that BM in its entirety contained fivefold more LCMV-specific CD8+ T cells. In conclusion, although infection with LCMV caused a dramatic change in the BM CD8+ T cell population, this did not result in noticeable differences between BM collected from different bones. Our findings suggest that in respect to CD8+ T cells, BM harvested from a single bone is a fair reflection of the rest of the BM present in the murine body.
The bone marrow (BM) acts as the primary site for the formation of all mature blood cells through the process of hematopoiesis. The complex hematopoietic process that gives rise to these cells takes place in the red (hematopoietic) part of the BM. At birth, BM primarily consists of red marrow, but with age, the red marrow decreases and is replaced by yellow (adipocytic) marrow (1). In adults (>25 years of age), red marrow is predominantly located in the tips (epiphysis), whereas yellow marrow is mostly found in the shafts (diaphysis) of the long bones. The epiphysis primarily consists of trabecular (spongy) bone, whereas the diaphysis consists of cortical (compact) bone (2). These differences in the composition of bone have been shown to influence the function of the BM. Human hematopoietic stem cells (HSCs) isolated from trabecular marrow of long bones have superior regenerative and self-renewal capacity compared to HSCs from the cortical marrow harvested from the shaft area and have also been shown to be molecularly distinct (3). Farrell et al. (4) found similar numbers of human HSCs and myeloid progenitor cells (GM-CFCs) in trochanter marrow (region between the epiphysis and diaphysis of the femur) compared to marrow in the femoral epiphysis. However, they observed a decline in numbers of femoral marrow-derived GM-CFCs in aged individuals, while the numbers for GM-CFCs derived from trochanter marrow did not change. In mice, substantial heterogeneity has been found in bone remodeling activity, blood volume fraction, and hypoxia between epiphysis, diaphysis, and calvarium, which were also shown to affect HSC function (5). These data indicate that distinct compartments within the BM are different, leading to functional differences for the cells that reside in these specific niches.
Next to its important function as a primary lymphoid organ, the BM has also gained recognition for its role as a secondary lymphoid organ. Dendritic cells in the BM can take up and present blood-borne antigens and thereby activate local naïve T cells (6). Neutrophils can capture and transport virus from the dermis into the BM, leading to priming of virus-specific CD8+ T cells by local antigen presenting cells (7). Additionally, the BM is also actively involved in immunological memory. Effector T cells that survive antigen clearance develop into memory T cells and home to the BM. Here, they provide life-long protection against reinfection (8–10). Studies in mice lacking IL-7 and IL-15 or their receptors IL-7Rα (CD127) and IL-15Rβ (CD122) have shown that these two cytokines are vital for the maintenance of memory CD8+ T cells, as they affect both their generation and survival (11–13). These effects could be direct, but they could also be mediated indirectly through the induction of costimulatory molecules that control memory T cell survival (14, 15). It has recently been shown that BM memory CD8+ T cells acquire IL-7 by docking to IL-7-producing reticular stromal cells (16). Additionally, human memory CD8+ T cells have been shown to be in close contact with a variety of IL-15-producing BM cells. These BM resident cells displayed morphological characteristics of stromal cells, dendritic cells, and monocytes (17).
Thus, it is now clear that BM is important for long-term memory maintenance and is therefore more frequently included in studies of (adaptive) immune responses [reviewed in Ref. (18, 19)]. However, little is known about quantitative and qualitative differences between various bones regarding T cell maintenance. Most information on BM T cells has been obtained from single cell suspensions prepared from crushed or flushed tibia and/or femurs. Hence, the BM has been conceptually and also practically regarded as a single organ. However, this view may not be justified, as bones throughout the body are diverse in their composition of cortical and trabecular bone depending on their mechanical or organ protection function (2), already leading to functional differences at the level of HSCs. Here, we examined if anatomical differences exist in BM, by assessing the CD8+ T cell population in BM harvested from murine tibia, femur, pelvis, sternum, radius, humerus, calvarium, and vertebrae and compared this to the spleen. We found that both in steady state and after infection with acute lymphocytic choriomeningitis virus (LCMV), BM located in distinct bones have comparable frequencies of CD8+ T cell subsets. Furthermore, by calculating the total number of BM CD8+ T cells found in the entire body, we demonstrate that BM is superior to spleen in harboring memory CD8+ T cells, and that this is attributed to the major contribution of the memory CD8+ T cells present in the vertebrae.
Wild-type (WT) C57BL/6J mice were kept under specific pathogen-free conditions in the animal facility of the Academic Medical Center (Amsterdam, The Netherlands) or Netherlands Cancer institute (Amsterdam, The Netherlands). Female or male mice between the age of 13 and 17 weeks were used for steady-state experiments. For LCMV experiments, mice that were 9–16 weeks old were injected intraperitoneally with 2.0 × 105 PFU of the Armstrong strain, kindly provided by Dr. Ramon Arens (LUMC, Leiden, The Netherlands) in 200 μl PBS. Mice were sacrificed during the memory phase (>42 days post injection). Mice received chow and acidified drinking water ad libitum. Animal experiments were performed in accordance with the institutional and national guidelines and approved by the Experimental Animal Committees of both animal facilities.
Tibia, femur, pelvis, radius together with humerus were collected from every mouse. Additionally, we harvested sternum, calvarium, and the vertebrae (cervical vertebrae C1–sacral vertebrae S5). Bones were cleaned and crushed in MACS buffer (PBS + 1% FCS + 2 mM EDTA) using a mortar and pestle. BM cell suspensions were filtered through a 40-μm cell strainer to remove bone debris. Single splenocyte suspensions were prepared by crushing the spleen through a 40-μm cell strainer with the plunger of a syringe. For several LCMV experiments, whole spleen and BM cells were enriched for CD8+ T cells with CD8α microbeads (Miltenyi Biotec) and MACS LS columns (Miltenyi Biotec). Erythrocytes were lysed with red blood cell lysis buffer (155 mM NH4Cl, 10 mM KHCO3, 127 mM EDTA). White blood cells were counted with CASY Cell Counter and Analyzer (Roche).
The following antibodies were used in this study: CD3ε-eFlour 450 (145-2C11, eBioscience), CD3ε-APC-eFluor 780 (17A2, eBioscience), CD8α-APC-eFluor 780 (53-6.7, eBioscience), CD8α-BV605 (53-6.7, Biolegend), CD8α-PerCP-Cy5.5 (53-6.7, eBioscience), CD44-PE-Cy7 (IM7, Biolegend), CD62L-APC (MEL-14, eBioscience), CD62L-BV510 (MEL-14, Biolegend), CD69eFluor 450 (H1.2F3, eBioscience) CD69-biotin (H1.2F3, eBioscience), CD122-FITC (TM-B1, BD Biosciences), CD127-BV605 (A7R34, Biolegend), and Streptavidin PerCP-Cy5.5 (BD Biosciences). The MHC class I tetramers H2-DbGP33–41 APC and H2-DbNP396–404 PE were kind gifts from Dr. Ramon Arens (LUMC, Leiden, The Netherlands). Cells were fixed with Foxp3/Transcription Factor Staining buffer set (eBioscience) and stained with Ki-67 PE or Ki-67 FITC (B56, BD Biosciences). Samples were acquired with the LSR Fortessa (BD) and analyzed with FlowJo software (Tree Star, Inc.).
Statistical analyses were performed with Prism (GraphPad Software, Inc.) using an unpaired t test followed by Welch’s correction or a one-way ANOVA followed by Tukey’s correction. Significance is indicated by *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, and #p < 0.05 between spleen and all the different bones.
To examine whether the composition of the CD8+ T cell population in a single bone is representative of all other bones, we examined the CD8+ T cells in BM from tibia, femur, pelvis, sternum, radius, humerus, calvarium, and vertebrae and compared this to the spleen. We observed that the frequencies of CD3+ cells, and also of CD8+ T cells, were significantly lower in all bones compared to the spleen (Figures 1A,B). Regarding the presence of the classical CD8+ T cell subsets, i.e., effector memory (TEM; CD44+CD62L−), central memory (TCM; CD44+CD62L+), and naïve (TNV; CD44−CD62L+) T cells (20, 21), we found that all bones consisted primarily (~65%) of TNV cells (Figures 1C,D). Strikingly, the frequencies of TEM, TCM, and TNV cells between the different bones were highly comparable. We observed that in comparison to spleen, all bones had higher frequencies of CD8+ TEM cells (Figure 1E). A similar pattern was observed for CD8+ TCM cells (Figure 1H). In absolute numbers, the majority of BM memory CD8+ T cells was located in the vertebrae (Figures 1F,I), as these contained the highest amount of BM cells, almost equivalent to the spleen (Table 1). BM, in its totality, contained ~2.8-fold more TEM and ~1.5 more TCM cells compared to spleen (Figures 1G,J). Taken together, we conclude that in the steady state, BM is quite distinct from spleen regarding frequencies of CD3+ and CD8+ T cells, but comparable between the different bones. Additionally, we show that BM accumulates, also in absolute numbers, more memory CD8+ T cells compared to spleen.
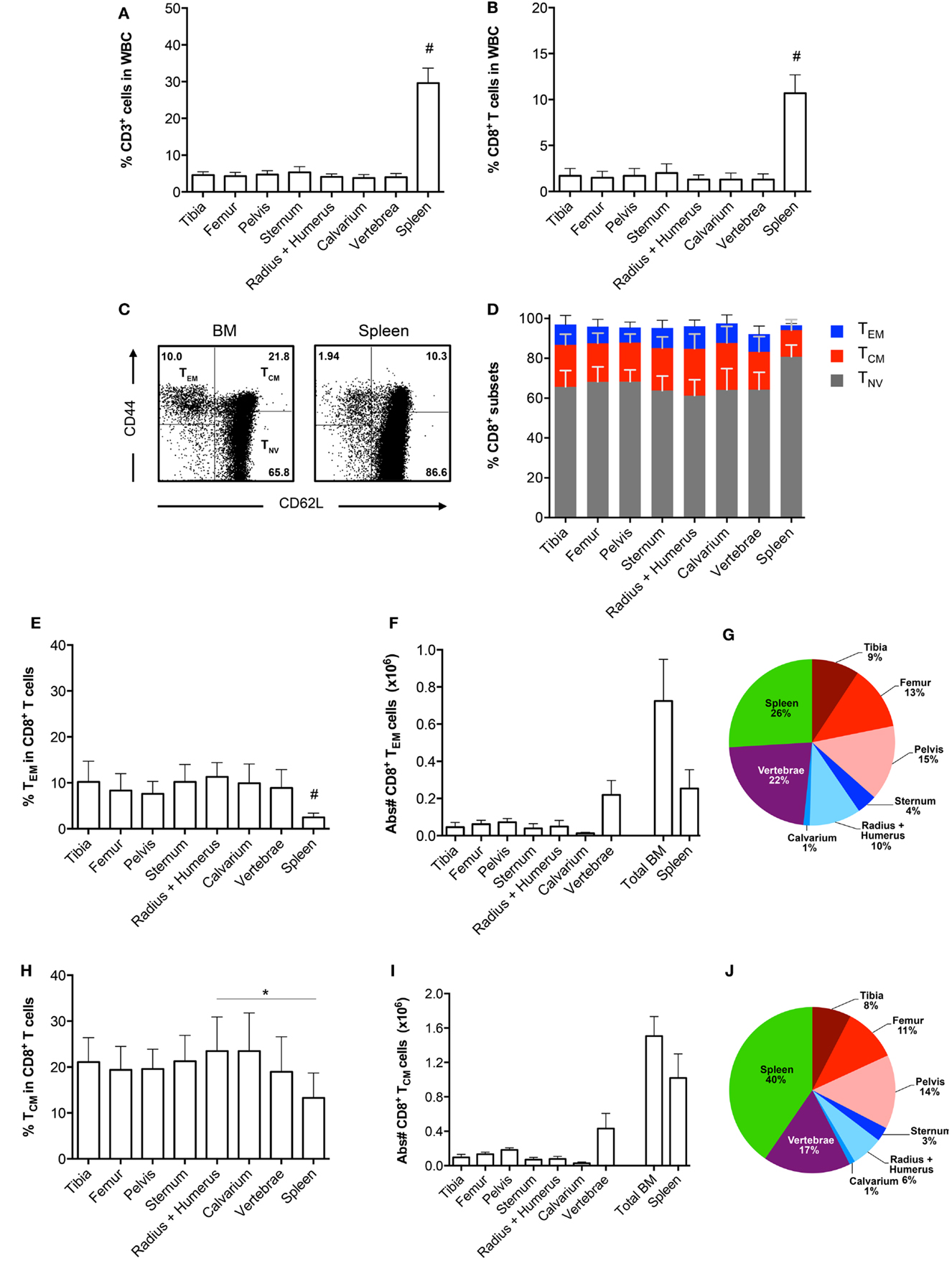
Figure 1. Comparable CD8+ T cell frequencies in BM obtained from different bones. (A) Frequency of CD3+ and (B) CD8+ T cells in total white blood cells (WBC). (C) Representative FACS plots showing expression of CD44 and CD62L in CD8+ T cells for BM (radius + humerus) and spleen. (D) Frequency of TEM, TCM, and TNV cells within the total CD8+ T cell population. (E) Frequency and (F) absolute numbers of TEM cells within the total CD8+ T cell population. (G) Distribution of TEM cells (based on absolute numbers). (H) Frequency and (I) absolute numbers of TCM cells within the total CD8+ T cell population. (J) Distribution of TCM cells (based on absolute numbers). The double amount of cells was taken into account for estimating the contribution of tibia, femur, pelvis, and radius + humerus for the calculation of “Total BM” in (F,I) and for their contribution in (G,J). Graphs show mean ± SD of each bone (n = 4–8), pooled from the three independent experiments. Statistical analysis was performed with a one-way ANOVA followed by Tukey’s correction. Significance is indicated by *p < 0.05 or #p < 0.05 between spleen and all bones.
The cytokines IL-7 and IL-15 are important for the development and maintenance of BM CD8+ T cells (11–13). In the murine BM, the expression of these cytokines is primarily confined to stromal cells (22, 23). As bones differ in structure, and possibly also in composition of IL-7 and IL-15 producing stromal cells, we tested if BM CD8+ T cells regulate the expression of the IL-7 and IL-15 receptors, according to their current location. This is particularly interesting, as IL-15 can regulate the expression of the receptor for IL-7 (24). Therefore, we compared the presence of CD122 and CD127 on CD8+ T cells collected from different bones. The highest expression of CD122 was found on the TCM cells, whereas TNV cells had the lowest expression of CD122 (Figure 2A). In contrast, TNV cells expressed the highest levels of CD127, while TEM cells expressed the lowest levels of CD127 (Figure 2E). Despite these marked differences in expression levels between the CD8+ T cell subsets, we observed similar expression of CD122 (Figures 2B–D) and CD127 (Figures 2F–H) by CD8+ T cells harvested from different bones. It remains to be investigated whether the lack of anatomical differences in respect to receptor levels also reflects similar concentrations of IL-7 and IL-15 in BM in different bones.
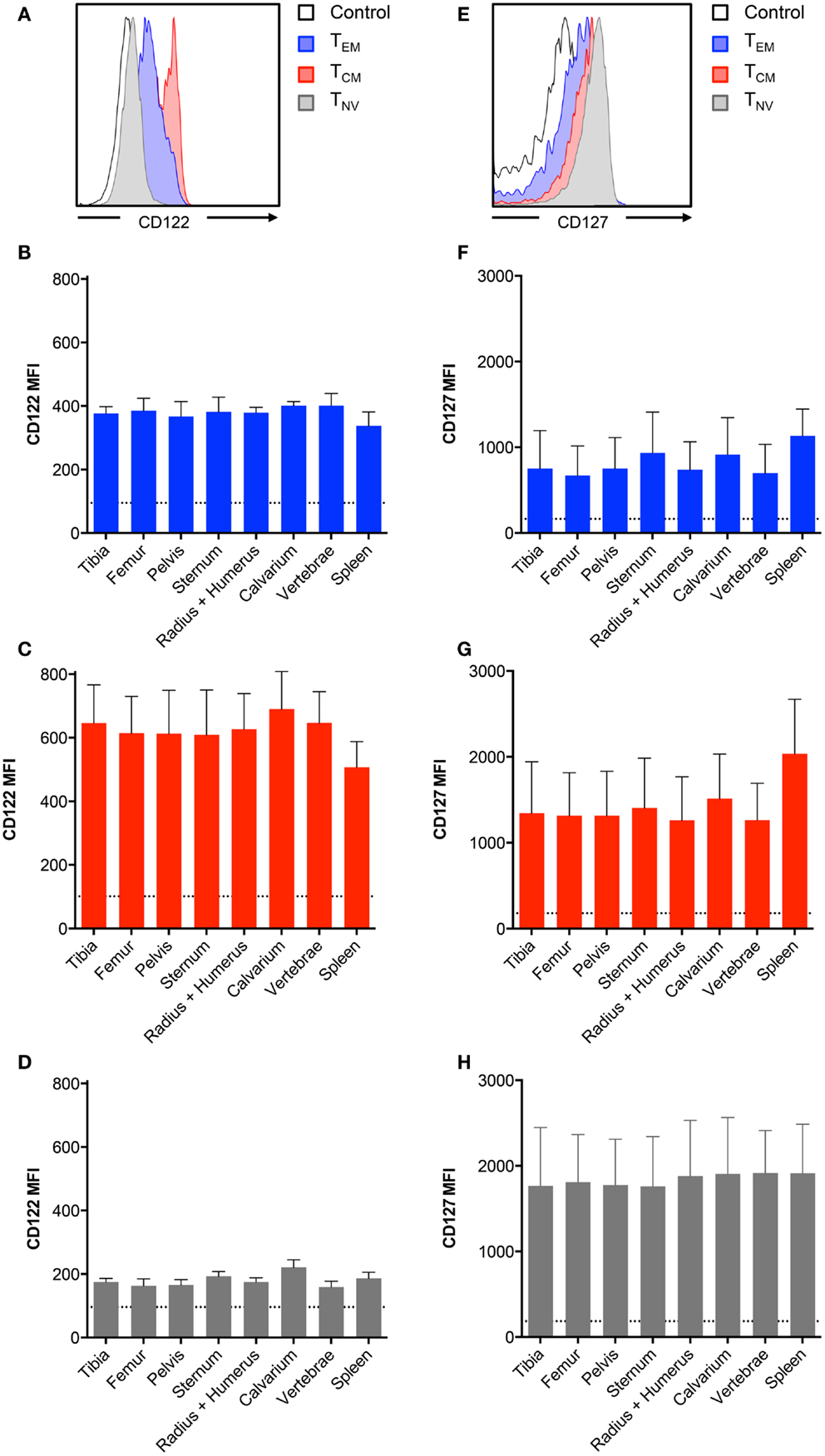
Figure 2. BM memory CD8+ T cell subsets differ in their expression of survival receptors. (A,E) Representative histograms showing expression of CD122 and CD127 in CD8+ TEM, TCM, and TNV subsets. Black line indicates intensity of unstained cells. (B–D) MFI of CD122 or (F–H) MFI of CD127 on TEM, TCM, and TNV subsets. Dotted line indicates MFI of unstained cells. Graphs show mean ± SD for each bone (n = 5–8).
We did not observe differences in CD8+ T cell frequencies in BM collected from different bones in the steady state. Thus, we questioned whether this would change after an infection that elicits a large influx of memory T cells. Therefore, we infected mice with the Armstrong strain of LCMV. This acute systemic infection is cleared from the BM within 8 days due to a strong CD8+ T cell response (25, 26). We analyzed the BM in the memory phase (>42 days) and found that the frequencies of CD3+ cells and CD8+ T cells were lower when compared to the spleen, but still similar between bones (data not shown). Interestingly, we found that the frequency of the CD8+ TEM subset strongly increased, ranging from ~10% in the steady state to ~60% after LCMV (Figures 3A,B). The increase in TEM cells corresponded with a decrease in TNV cells, whereas the TCM subset was largely unaffected (Figures 3C–E). This was comparable between all bones. The TEM subset also increased in the spleen, although here the majority (~55%) of the CD8+ T cells still exhibited a naïve phenotype. Both BM and spleen TEM cells were primarily Ki-67−, indicating that they are in the G0 phase of cell cycle, and are thus resting memory CD8+ T cells (Figure 3F). This was similar for all the different bones (data not shown). In summary, we show that even after resolved infection with LCMV, no anatomical differences occur in the BM regarding CD8+ T cell frequencies.
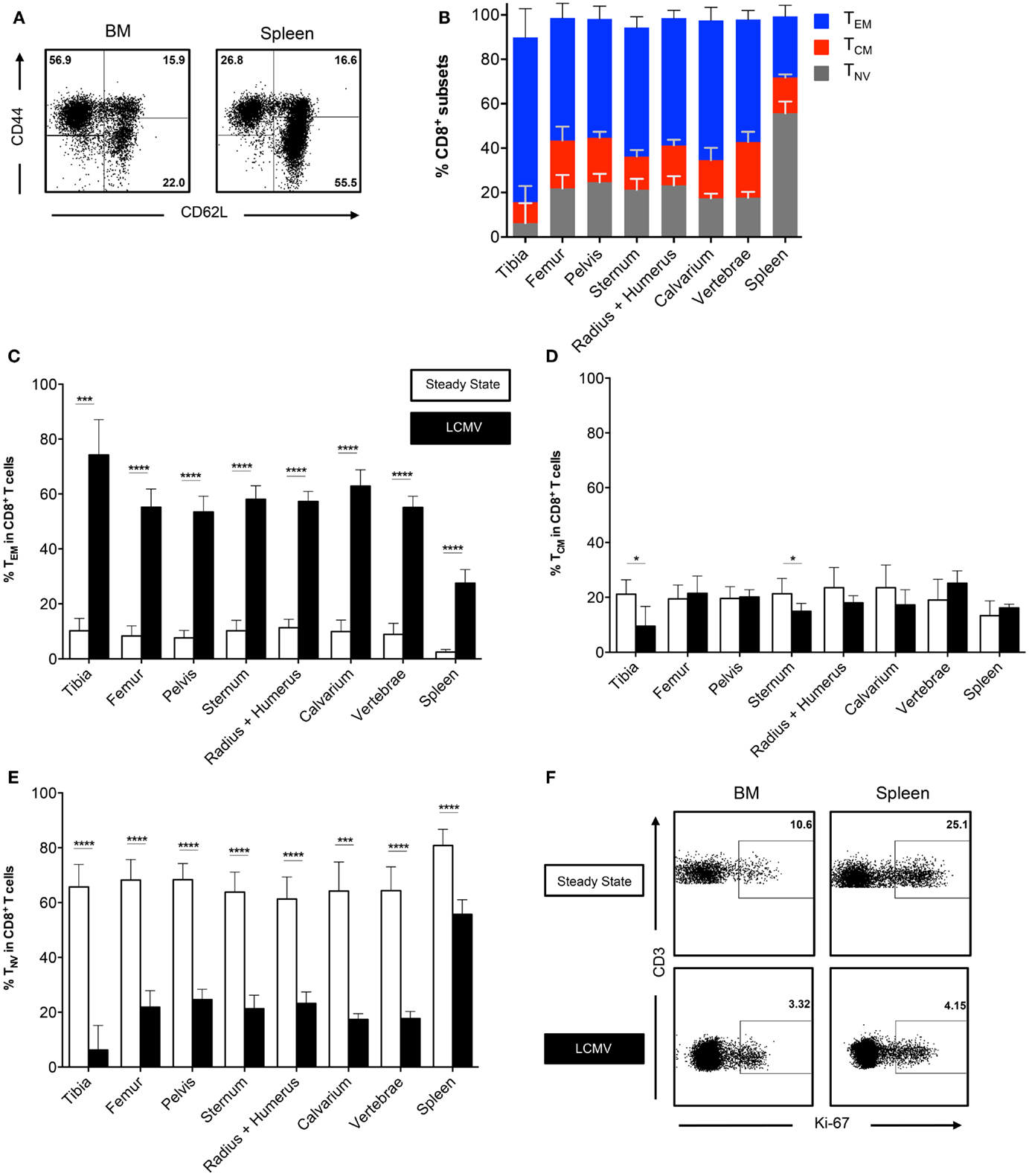
Figure 3. The CD8+ T cell population consists primarily of memory cells after infection with LCMV. (A) Representative FACS plots showing expression of CD44 and CD62L in CD8+ T cells for BM (tibia) and spleen. (B) Frequency of TEM, TCM, and TNV subsets in CD8+ T cells. (C–E) Frequency of TEM, TCM, and TNV subsets in the CD8+ T cell population during steady state and after infection with LCMV. White bars = steady state and black bars = LCMV. Data for (C–E) are identical to that for Figure 1D and (B). (F) Representative FACS plots showing expression of Ki-67 on TEM cells in spleen and BM during steady state (femur) and after infection with LCMV (vertebrae). Graphs show mean ± SD of each bone (n = 4–8), pooled from the three independent steady states and the two independent LCMV experiments. Statistical analysis was performed with unpaired t test followed by Welch’s correction. Significance is indicated by *p < 0.05, ***p < 0.001, and ****p < 0.0001.
Apart from being a primary and secondary lymphoid organ, BM, like any other tissue, is susceptible to viral infections (27, 28). Interestingly, over the past few years, it has been reported that following an infection, a subset of memory CD8+ T cells has the ability to take up residence in a particular tissue and provide tissue-specific immunity. These tissue-resident memory T cells (TRM) have been identified in skin, female genital tract, intestinal mucosa, kidney, pancreas, stomach, heart, salivary glands, and the brain (29–34). These cells are characterized by the expression of the C-type lectin CD69, which inhibits the expression of the egress receptor sphingosine-1-phosphate receptor 1 (S1PR1) (35, 36). Moreover, TRM cells are identified by the absence of CD62L, making them a subgroup of the TEM subset (37). Here, we examined the expression of CD69 by BM TEM cells and compared these frequencies with the CD69 expression in other BM CD8+ T cell subsets. We found that during the steady state, approximately half (~47%) of BM TEM cells and ~20% of BM TCM expressed CD69 (Figures 4A,B). The TNV subset barely expressed CD69 (data not shown). The frequencies of CD69 in each CD8+ T cell subset were similar for all bones (data not shown). Furthermore, the frequencies in the BM memory CD8+ T cells subsets were much higher than in the equivalent memory CD8+ T cell subsets located in the spleen (Figures 4A–D). Of specific note, after the infection with LCMV, the frequency of BM and spleen CD69+ cells decreased in the TEM subset, while the frequency of BM CD69+ cells in the TCM subset remained the same. However, we did not observe differences in absolute numbers of CD69+ TEM cells before and after infection (data not shown). This indicates that the decreased frequency of CD69+ TEM cells after infection results from a massive influx of CD69− TEM cells. In conclusion, BM contains a significant number of CD8+ T cells with a TRM phenotype, though an LCMV infection elicits an influx of mostly conventional TEM cells.
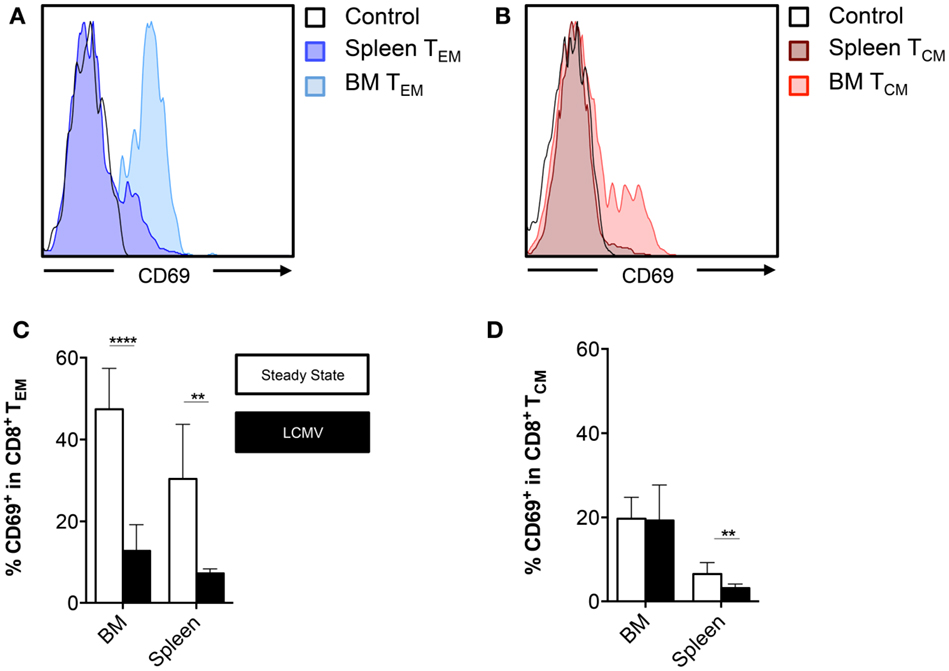
Figure 4. BM memory CD8+ T cell subsets contain cells that express CD69. (A,B) Representative histograms showing expression of CD69 on TEM or TCM cells in BM (femur) and spleen. (C,D) Frequency of CD69+ cells in the CD8+ TEM or TCM subsets in BM (vertebrae) and spleen. Graphs show mean ± SD of the vertebrae (n = 4–8), pooled from the three independent steady states and the two independent LCMV experiments. White bars = steady state and black bars = LCMV. Statistical analysis was performed with unpaired t test followed by Welch’s correction. Significance is indicated by **p < 0.01 and ****p < 0.0001.
In order to analyze CD8+ T cells generated specifically against LCMV, we pooled BM from different bones to obtain sufficient cell numbers (26). BM from the vertebrae was analyzed separately because of its high cellularity. We enriched for CD8+ T cells and stained with MHC-I tetramers loaded with the LCMV epitopes GP33–41 and NP396–404. We observed that the spleen had lower frequencies of LCMV-specific CD8+ T cells compared to either BM compartment (Figures 5A,D). We did not observe differences in frequencies of LCMV-specific CD8+ T cells between the BM from vertebrae and the other bones (Figures 5B,E). Based on the absolute numbers, the vertebrae itself contained ~35% of all GP33-tetramer+ and 37% of all NP396-tetramer+ CD8+ T cells present in the total BM. Furthermore, as we harvested the majority of the BM from the body, we could calculate that BM harbored 1.5 × 105 GP33-tetramer+ and 1.4 × 105 NP396-tetramer+ CD8+ T cells, which was fivefold to sixfold more than the spleen (Figures 5C,F), thereby emphasizing the role of the BM as memory T cell organ. LCMV-specific CD8+ T cells in BM primarily (~75%) had a TEM phenotype, while the remainder exhibited a TCM phenotype (Figure 5G). Additionally, LCMV-specific CD8+ T cells barely expressed CD69 or Ki-67, indicating that they were not actively cycling, but also not TRM cells (Figure 5H). Our results suggest that the distribution of LCMV-specific CD8+ T cells is comparable between BM in the vertebrae and the rest of the bones, and that these cells are phenotypically similar to the rest of the memory CD8+ T cells. Our data also suggest that infection with LCMV does not result in substantial generation of LCMV-specific CD8+ T cells with a TRM phenotype.
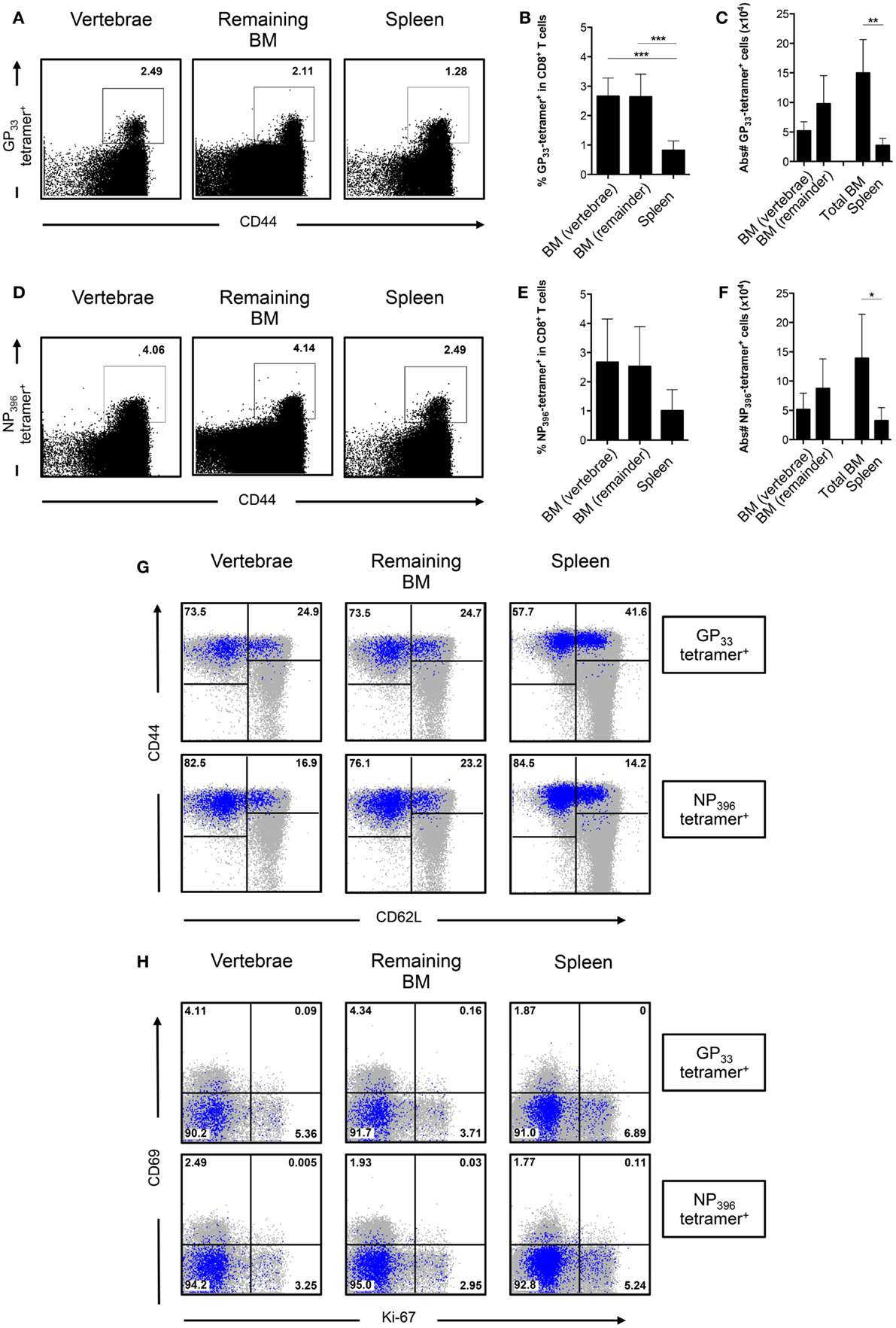
Figure 5. The frequency of LCMV-specific CD8+ T cells is similar between BM in vertebrae and the remaining bones. (A) Representative FACS plots showing staining of GP33-tetramer in CD8+ T cells. (B) Frequency and (C) absolute numbers of GP33-tetramer+ cells in CD8+ T cells. (D) Representative FACS plots showing staining of NP396-tetramer in CD8+ T cells. (E) Frequency and (F) absolute numbers of NP396-tetramer+ cells in CD8+ T cells. (G) Representative FACS plots showing expression of CD44 and CD62L of GP33-tetramer+ or NP396-tetramer+ CD8+ T cells. (H) Representative FACS plots showing expression of CD69 and Ki-67 of GP33-tetramer+ or NP396-tetramer+ CD8+ T cells. For (G,H), tetramer+ cells (blue) are superimposed on all CD8+ T cells (gray). Percentages shown in (G,H) reflect the frequencies of tetramer+ CD8+ T cells. Graphs show mean ± SD of vertebrae or remaining BM (n = 3–6), pooled from the two independent experiments. Statistical analysis was performed with one-way ANOVA followed by Tukey’s correction or with unpaired t test followed by Welch’s correction. Significance is indicated by *p < 0.05, **p < 0.01, and ***p < 0.001.
In the present study, we examined BM from murine tibia, femur, pelvis, sternum, radius, humerus, calvarium, and vertebrae and addressed if anatomical differences exist at the level of BM CD8+ T cells. Here, we show that during the steady state, BM derived from different bones had similar CD8+ T cell frequencies. Furthermore, the frequencies of the TEM, TCM, and TNV subsets were also comparable between all the bones. We also examined BM during the memory phase of a LCMV infection. This virus is cleared primarily by CD8+ T cells and results in the generation of virus-specific memory CD8+ T cells, which remain detectable long after the initial infection (25, 26). Similarly to the steady state, we did not observe anatomical differences in BM after infection with LCMV. To date, only a limited number of studies have addressed the possible anatomical differences in the BM. These studies primarily focused on the functional differences within different regions inside a bone, but not necessarily between different bones. The majority of the studies found functional, but limited differences in frequencies of HSCs (3–5). It remains to be determined if BM T cells derived from different bones are also functionally distinct. Results obtained from a study performed with human BM suggest that this might not be the case. Pritz et al. (38) compared the phenotype and function of T cells derived from iliac crest and the femoral shaft and found no differences between the distribution of T cell populations and their cytokine production. Interestingly, although we found no differences between bones, we did observe that both during the steady state and after infection with LCMV, the majority of CD8+ T cells were located in the vertebrae, a collection of bones that has not been well studied and is not frequently included during sample preparation. From both a practical and ethical point of view, inclusion of the vertebrae can limit the amount of mice required for any given experiment, as it holds more than a third of all BM present in the murine body.
Here, we also demonstrated that BM substantially changes after infection with LCMV. The decline in frequency of TNV cells coincided with the increased frequency of TEM cells. As we did not observe differences in absolute numbers of total CD8+ T cells between steady state and LCMV-infected mice, our results suggest that the space in the BM is limited, resulting in one subset being replaced by another. Sercan Alp et al. (16) demonstrated that memory CD8+ T cells colocalize with IL-7-producing reticular stromal cells, in a 1:1 ratio in the BM. This, combined with our results and the fact that CD8+ TNV cells primarily depend on IL-7 for survival [reviewed in Ref. (39)], suggests that CD8+ TNV cells were outcompeted or blocked from entering these IL-7-rich niches, as these became occupied by memory CD8+ T cells. Yet, we show that BM memory CD8+ T cells express the receptors to respond to both IL-7 and IL-15, which could indicate that naïve and memory CD8+ T cells reside in different niches. If this is indeed the case, our results suggest that after infection with LCMV, the BM microenvironment changed and became less favorable for naïve CD8+ T cells and/or a more advantageous for memory CD8+ T cells. As LCMV has been shown to infect BM stromal fibroblast and endothelial cells (27), it could well be that the cellular sources of IL-7 and IL-15 in the BM are severely affected by the infection. Alternatively, there could also be a role for hematopoietic cells, as dendritic cells can increase their IL-15 production in response to inflammatory signals (40). Further studies are required to show how the BM niches that maintain CD8+ T cells adapt in order to accommodate the substantial amount of memory CD8+ T cells generated after infection with LCMV and how this relates to the spleen. Moreover, it remains unclear why the BM harbors so many TEM cells long after the infection has been resolved and whether their presence affects the hematopoietic function of the BM, as activated immune cells have been shown to directly influence HSC function and hematopoiesis [reviewed in Ref. (41)].
Furthermore, we demonstrated that BM contained CD8+ T cells with a TRM (CD44+CD62L−CD69+) phenotype. In the past, the surface molecule CD69 was associated with the “recently activated” status of T cells. More recently, this surface molecule has become important for its role in tissue retention, as it downregulates S1PR1 and thereby blocks the egress of lymphocytes from tissues (35). Currently, it is unclear if CD69 alone is sufficient for identification of TRM cells in BM, as it has not been unequivocally demonstrated that CD69+ BM memory CD8+ T cells are non-circulating cells. Additionally, it has been postulated that TRM cells may reside in the CD69− fraction of memory T cells (42). Nonetheless, BM memory CD8+ T cells, which express CD69, resemble TRM cells in other tissues, as they have low expression of S1PR1 (16). Furthermore, in accordance with our findings, BM memory CD8+ T cells are not activated, but rather quiescent in terms of proliferation and gene expression (16). It is thus highly likely that the CD69+ memory CD8+ T cells that we identified in BM are resident, rather than recently activated T cells. Currently, it is understood that TRM cells reside in tissues where the initial infection took place and are positioned as the first line of defense, in order to accelerate pathogen elimination during secondary encounters (37). Whether TRM cells in the BM are also strategically positioned to fulfill a similar function and protect the BM from invading pathogens remains to be investigated.
In summary, our findings suggest that in respect to the frequency of CD8+ T cells, BM harvested from one bone is representative of the BM found in all bones located throughout the body. Our results reinforce the notion that BM is a major immunological organ, as it is quantitatively superior to the spleen in accumulation and accommodation of memory CD8+ T cells, and should therefore be included when studying (adaptive) immune responses and memory T cell maintenance.
SG, SH, GB, and MP conducted the experiments; SG analyzed data; MP and MN supervised the project; and SG and MN wrote the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Dr. Brenda Olivier for critical reading of the manuscript and the staff of the animal facility of the AMC and NKI for excellent animal care.
SG, SH, GB, and MP were financially supported by a Fellowship obtained by MN from the Landsteiner Foundation for Blood Transfusion Research, grant nr #1014 www.lsbr.nl. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
1. Bianco P. Bone and the hematopoietic niche: a tale of two stem cells. Blood (2011) 117:5281–9. doi: 10.1182/blood-2011-01-315069
2. Gurkan UA, Akkus O. The mechanical environment of bone marrow: a review. Ann Biomed Eng (2008) 36:1978–91. doi:10.1007/s10439-008-9577-x
3. Guezguez B, Campbell CJV, Boyd AL, Karanu F, Casado FL, Di Cresce C, et al. Regional localization within the bone marrow influences the functional capacity of human HSCs. Cell Stem Cell (2013) 13:175–89. doi:10.1016/j.stem.2013.06.015
4. Farrell TL, McGuire TR, Bilek LD, Brusnahan SK, Jackson JD, Lane JT, et al. Changes in the frequencies of human hematopoietic stem and progenitor cells with age and site. Exp Hematol (2014) 42:146–54. doi:10.1016/j.exphem.2013.11.003
5. Lassailly F, Foster K, Lopez-onieva L, Currie E, Bonnet D. Multimodal imaging reveals structural and functional heterogeneity in different bone marrow compartments: functional implications on hematopoietic stem cells. Blood (2013) 122:1730–40. doi:10.1182/blood-2012-11-467498
6. Feuerer M, Beckhove P, Garbi N, Mahnke Y, Limmer A, Hommel M, et al. Bone marrow as a priming site for T-cell responses to blood-borne antigen. Nat Med (2003) 9:1151–7. doi:10.1038/nm914
7. Duffy D, Perrin H, Abadie V, Benhabiles N, Boissonnas A, Liard C, et al. Neutrophils transport antigen from the dermis to the bone marrow, initiating a source of memory CD8+ T cells. Immunity (2012) 37:917–29. doi:10.1016/j.immuni.2012.07.015
8. Di Rosa F, Santoni A. Memory T-cell competition for bone marrow seeding. Immunology (2003) 108:296–304. doi:10.1046/j.1365-2567.2003.01593.x
9. Mazo IB, Honczarenko M, Leung H, Cavanagh LL, Bonasio R, Weninger W, et al. Bone marrow is a major reservoir and site of recruitment for central memory CD8+ T cells. Immunity (2005) 22:259–70. doi:10.1016/j.immuni.2005.01.008
10. Tokoyoda K, Zehentmeier S, Hegazy AN, Albrecht I, Grün JR, Löhning M, et al. Professional memory CD4+ T lymphocytes preferentially reside and rest in the bone marrow. Immunity (2009) 30:721–30. doi:10.1016/j.immuni.2009.03.015
11. Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity (1998) 9:669–76. doi:10.1016/S1074-7613(00)80664-0
12. Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med (2000) 191:771–80. doi:10.1084/jem.191.5.771
13. Schluns KS, Kieper WC, Jameson SC, Lefrançois L. Interleukin-7 mediates the homeostasis of naïve and memory CD8 T cells in vivo. Nat Immunol (2000) 1:426–32. doi:10.1038/80868
14. Pulle G, Vidric M, Watts TH. IL-15-dependent induction of 4-1BB promotes antigen-independent CD8 memory T cell survival. J Immunol (2006) 176:2739–48. doi:10.4049/jimmunol.176.5.2739
15. Snell LM, Lin GHY, Watts TH. IL-15-dependent upregulation of GITR on CD8 memory phenotype T cells in the bone marrow relative to spleen and lymph node suggests the bone marrow as a site of superior bioavailability of IL-15. J Immunol (2012) 188:5915–23. doi:10.4049/jimmunol.1103270
16. Sercan Alp Ö, Durlanik S, Schulz D, McGrath M, Grün JR, Bardua M, et al. Memory CD8+ T cells colocalize with IL-7+ stromal cells in bone marrow and rest in terms of proliferation and transcription. Eur J Immunol (2015) 45(4):975–87. doi:10.1002/eji.201445295
17. Herndler-Brandstetter D, Landgraf K, Jenewein B, Tzankov A, Brunauer R, Brunner S, et al. Human bone marrow hosts polyfunctional memory CD4+ and CD8+ T cells with close contact to IL-15-producing cells. J Immunol (2011) 186:6965–71. doi:10.4049/jimmunol.1100243
18. Di Rosa F, Pabst R. The bone marrow: a nest for migratory memory T cells. Trends Immunol (2005) 26:360–6. doi:10.1016/j.it.2005.04.011
19. Tokoyoda K, Radbruch A. Signals controlling rest and reactivation of T helper memory lymphocytes in bone marrow. Cell Mol Life Sci (2012) 69:1609–13. doi:10.1007/s00018-012-0969-6
20. Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature (1999) 401:708–12. doi:10.1038/44385
21. Weninger W, Crowley MA, Manjunath N, von Andrian UH. Migratory properties of naive, effector, and memory CD8(+) T cells. J Exp Med (2001) 194:953–66. doi:10.1084/jem.194.7.953
22. Cui G, Hara T, Simmons S, Wagatsuma K, Abe A, Miyachi H, et al. Characterization of the IL-15 niche in primary and secondary lymphoid organs in vivo. Proc Natl Acad Sci U S A (2014) 111:1915–20. doi:10.1073/pnas.1318281111
23. Hara T, Shitara S, Imai K, Miyachi H, Kitano S, Yao H, et al. Identification of IL-7-producing cells in primary and secondary lymphoid organs using IL-7-GFP knock-in mice. J Immunol (2012) 189:1577–84. doi:10.4049/jimmunol.1200586
24. Quinci AC, Vitale S, Parretta E, Soriani A, Iannitto ML, Cippitelli M, et al. IL-15 inhibits IL-7Rα expression by memory-phenotype CD8+ T cells in the bone marrow. Eur J Immunol (2012) 42:1129–39. doi:10.1002/eji.201142019
25. Slifka MK, Matloubian M, Ahmed R. Bone marrow is a major site of long-term antibody production after acute viral infection. J Virol (1995) 69:1895–902.
26. Slifka MK, Whitmire JK, Ahmed R. Bone marrow contains virus-specific cytotoxic T lymphocytes. Blood (1997) 90:2103–8.
27. Binder BD, Fehr J, Hengartner H, Zinkernagel RM. Virus-induced transient bone marrow aplasia: major role of interferon-alpha/beta during acute infection with the noncytopathic lymphocytic choriomeningitis virus. J Exp Med (1997) 1997(185):517–30. doi:10.1084/jem.185.3.517
28. Subramanian A, Hegde S, Porayette P, Yon M, Hankey P, Paulson RF. Friend virus utilizes the BMP4-dependent stress erythropoiesis pathway to induce erythroleukemia. J Virol (2008) 82:382–93. doi:10.1128/JVI.02487-06
29. Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol (2009) 10:524–30. doi:10.1038/ni.1718
30. Tang VA, Rosenthal KL. Intravaginal infection with herpes simplex virus type-2 (HSV-2) generates a functional effector memory T cell population that persists in the murine genital tract. J Reprod Immunol (2010) 87:39–44. doi:10.1016/j.jri.2010.06.155
31. Masopust D, Choo D, Vezys V, Wherry EJ, Duraiswamy J, Akondy R, et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med (2010) 207:553–64. doi:10.1084/jem.20090858
32. Casey KA, Fraser KA, Schenkel JM, Moran A, Abt MC, Beura LK, et al. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J Immunol (2012) 188:4866–75. doi:10.4049/jimmunol.1200402
33. Hofmann M, Pircher H. E-cadherin promotes accumulation of a unique memory CD8 T-cell population in murine salivary glands. Proc Natl Acad Sci U S A (2011) 108:16741–6. doi:10.1073/pnas.1107200108
34. Wakim LM, Woodward-Davis A, Bevan MJ. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc Natl Acad Sci U S A (2010) 107:17872–9. doi:10.1073/pnas.1010201107
35. Shiow LR, Rosen DB, Brdicková N, Xu Y, An J, Lanier LL, et al. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature (2006) 440:540–4. doi:10.1038/nature04606
36. Mackay LK, Braun A, Macleod BL, Collins N, Tebartz C, Bedoui S, et al. Cutting edge: CD69 interference with sphingosine-1-phosphate receptor function regulates peripheral T cell retention. J Immunol (2015) 194(5):2059–63. doi:10.4049/jimmunol.1402256
37. Schenkel JM, Masopust D. Tissue-resident memory T cells. Immunity (2014) 41:886–97. doi:10.1016/j.immuni.2014.12.007
38. Pritz T, Landgraf-Rauf K, Herndler-Brandstetter D, Rauf R, Lair J, Gassner R, et al. Bone marrow T cells from the femur are similar to iliac crest derived cells in old age and represent a useful tool for studying the aged immune system. Immun Ageing (2013) 10:17. doi:10.1186/1742-4933-10-17
39. Cox MA, Kahan SM, Zajac AJ. Anti-viral CD8 T cells and the cytokines that they love. Virology (2013) 435:157–69. doi:10.1016/j.virol.2012.09.012
40. Mattei F, Schiavoni G, Belardelli F, Tough DF. IL-15 is expressed by dendritic cells in response to type I IFN, double-stranded RNA, or lipopolysaccharide and promotes dendritic cell activation. J Immunol (2001) 167:1179–87. doi:10.4049/jimmunol.167.3.1179
41. Libregts SFWM, Nolte MA. Parallels between immune driven-hematopoiesis and T cell activation: 3 signals that relay inflammatory stress to the bone marrow. Exp Cell Res (2014) 329(2):239–47. doi:10.1016/j.yexcr.2014.09.016
Keywords: vertebrae, bone marrow, CD8+ T cells, memory, LCMV, CD69, tissue-resident, Ki-67
Citation: Geerman S, Hickson S, Brasser G, Pascutti MF and Nolte MA (2016) Quantitative and Qualitative Analysis of Bone Marrow CD8+ T Cells from Different Bones Uncovers a Major Contribution of the Bone Marrow in the Vertebrae. Front. Immunol. 6:660. doi: 10.3389/fimmu.2015.00660
Received: 24 September 2015; Accepted: 24 December 2015;
Published: 13 January 2016
Edited by:
Francesca Di Rosa, Italian National Research Council, ItalyReviewed by:
Ulrich Kalinke, TWINCORE – Centre for Experimental and Clinical Infection Research, GermanyCopyright: © 2016 Geerman, Hickson, Brasser, Pascutti and Nolte. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Martijn A. Nolte, m.nolte@sanquin.nl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.