- 1Laboratório de Imunoparasitologia, Instituto Oswaldo Cruz, FIOCRUZ, Rio de Janeiro, Brazil
- 2Department of Pathology, Division of Microbiology and Immunology, University of Utah School of Medicine, Salt Lake City, UT, USA
There have been exhaustive efforts to develop an efficient vaccine against leishmaniasis. Factors like host and parasite genetic characteristics, virulence, epidemiological scenarios, and, mainly, diverse immune responses triggered by Leishmania species make the achievement of this aim a complex task. It is already clear that the induction of a Th1, pro-inflammatory response, is important in the protection against Leishmania infection. However, many questions must still be answered to fully understand Leishmania immunopathology, especially regarding Leishmania-specific Th1 response induction, regulation, and persistence. A large number of Leishmania antigens able to induce pro-inflammatory response have been selected so far, but none of them demonstrated efficiency in protection assays. A possible explanation is that CD4 T cells display marked heterogeneity at a single-cell level especially regarding the production of Th1-defining cytokines and multifunctionality. It has been established in the literature that Th1 cells undergo a differentiation process, which can generate cells with diverse phenotypes and survival capabilities. Despite that, only a few studies evaluate this heterogenic response and the amount of multifunctional CD4 T cells induced by Leishmania vaccine candidates, missing what can be a crucial point in defining a correlate of protection after vaccination. Moreover, most of the knowledge involving the development of cutaneous leishmaniasis (CL) vaccines comes from the mouse model of infection with Leishmania major, which cannot be fully applied to New World Leishmaniasis. For this reason, the immune response triggered by infection with New World Leishmania species, as well as vaccine candidates, need further studies. In this review, we will reinforce the importance of evaluating the quality of immune response against Leishmania, using a multiparametric analysis in order to understand better this complex host-parasite interaction, discussing the differences in the responses triggered by different New World Leishmania species, as well as the impact on the development of an effective vaccine against CL.
Introduction
World Health Organization (WHO) has classified Leishmaniasis among the tropical neglected, emerging, and uncontrolled diseases that affect mainly poor regions around the Globe. The disease is endemic in 88 countries (72 are developing countries) with approximately 350 million individuals at risk of contracting the disease and an annual incidence of 1.5–2 million new cases (1). Its prevention has been based on control of vectors and animal reservoirs in countries where the disease has a zoonotic transmission, combined with chemotherapy of infected individuals where the disease possesses anthroponotic features. However, control of reservoir hosts and vectors is difficult due to operational issues, making the development of an effective and affordable vaccine against Leishmaniasis a highly desirable task.
The history of Leishmania vaccination dated from twentieth century, in which live, virulent parasites were inoculated in healthy individuals in a process called “Leishmanization.” The practice was banned because of safety concerns due to development of non-healing lesions and immunosuppression (2). First generation vaccines using whole-killed Leishmania promastigotes replaced Leishmanization and were tested as vaccines against cutaneous leishmaniasis (CL) and visceral leishmaniasis (VL) (3, 4). Second and third generation vaccines were also developed, based on the defined synthetic or recombinant subunits and DNA, respectively. Despite many years of efforts in identifying a great number of antigens (5) and advances in vaccine technologies, there does not yet appear to be a vaccine candidate capable of delivering the level of protection needed for disease control.
The localized form of CL, specially the one caused by the Old World specie Leishmania major, is a self-healing disease, usually characterized by a state of at least partial immunity against reinfection, demonstrating that prevention through prophylactic vaccination is feasible. On the other hand, although recovery from infection with the New World specie Leishmania braziliensis gives firm resistance to homologous challenge, Leishmania amazonensis infection does not provide protection against a subsequent challenge with L. braziliensis, or other Leishmania species from the subgenus Viannia (6, 7). Until now, there has been no consistent data, particularly in humans, indicating that recovery from a primary infection with L. amazonensis gives complete resistance to a homologous challenge.
The fact that there is not yet an efficient vaccine against Leishmaniasis, especially one that could protect against different species simultaneously, leads us to consider that a better understanding of immune response in Leishmania pathogenesis is still needed, taking into consideration the various species that cause different clinical manifestations of the disease. Among the reasons that can be pointed out to explain our failure in developing a vaccine against CL, particularly against American cutaneous leishmaniasis, is the fact that we are still far from fully understand the mechanisms of healing and of memory responses generated after Leishmania infections as well as how to evaluate this responses. Far from giving the answers, this review focuses on the current advances in T cell memory knowledge and the differences observed between the immune responses induced after infection with different Leishmania species, particularly between L. braziliensis and L. amazonensis.
American Tegumentary Leishmaniasis: Beyond the Th1/Th2 Paradigm
American tegumentary leishmaniasis (ATL) is endemic in Latin America and the most common species involved are: L. braziliensis, Leishmania guyanensis, Leishmania panamensis (all from the genus Viannia), L. amazonensis, and Leishmania mexicana (both from the subgenus Leishmania). Unlike Old World CL, usually characterized by subclinical or self-healing cutaneous lesions, the infection by ATL causing species can lead to uncontrolled parasite replication, producing non-healing cutaneous, mucosal, or even visceral disease (1) (Table 1).
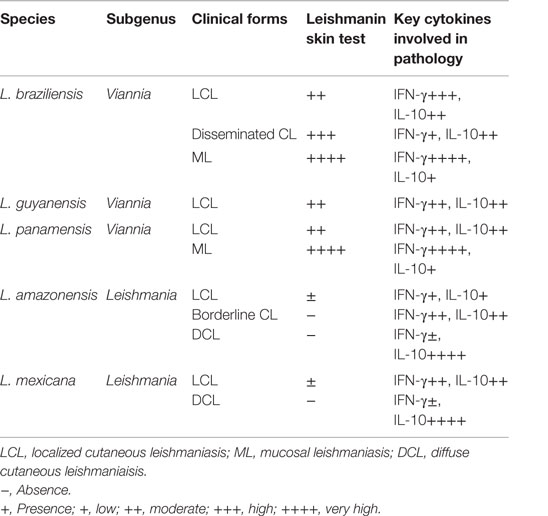
Table 1. Major human American cutaneous leishmaniasis causing species and their clinical manifestations.
Human infection with L. braziliensis leads to a broad spectrum of clinical, immunological, and histopathological manifestations, varying from self-healing cutaneous lesions to the severe and destructive clinical form named mucocutaneous leishmaniasis (ML) (8–10). The localized cutaneous form (LCL) usually manifests as one or a few ulcers with elevated borders and sharp craters that increase rapidly in size and heal slowly without treatment (11). L. braziliensis can also cause disseminated leishmaniasis, in which up to hundreds of lesions erupt as a result of hematogenous spread of parasite (12, 13). L. amazonensis has also been isolated from patients with diverse clinical forms, such as simple CL lesions to diffuse cutaneous leishmaniasis (DCL) (14) and was also implicated in borderline disseminated CL, an intermediate form of disease (15). Patients with DCL are often resistant to chemotherapy, have negative leishmanin skin test (LST), and low or negative responses after Leishmania antigen-specific stimulation in vitro but remain responsive for other unrelated antigens, such as tuberculin (8).
For many years, murine CL models have been used to elucidate the cell types, cytokines, signal transduction cascades, and mechanisms needed for parasite control and clinical resolution of the disease. Since Leishmania is an obligate intracellular parasite, the protective immunity is associated with a cell-mediated immune response. Indeed, studies in the murine model have been helping to elucidate the immunological pathways that are responsible for resistance or susceptibility to Leishmania and were responsible for the description of the CD4 T cells Th1/Th2 dichotomy. It is well accepted that protective immunity against Leishmania parasites is mediated by a type 1, pro-inflammatory response, and most of the early studies, particularly on L. major infection, largely defined the Th1/Th2 paradigm of resistance/susceptibility to infection and the role of interleukin 12 (IL-12) and IL-4, respectively, in driving Th1 and Th2 cell development (16, 17). On the other hand, in human L braziliensis infection, some evidences suggest that higher percentage of activated IFNγ+ producing T CD4+ lymphocytes are associated with larger lesions (18), and an exacerbated Th1 response is observed in ML (19). The polarized CD4 lymphocyte response detected in the murine L. major model is not so evident in the human Leishmanasis, and the importance of IL-4 as a primary mediator of susceptibility to Leishmania infection is not corroborated by clinical trials (19). Indeed, in DCL (the most severe form of human ATL), the main cytokine associated with immunosuppression and pathology it is not IL-4 but IL-10 (20–22).
The vast majority of experimental CL studies come from the murine model of infection with L. major, although the disease outcome in inbred strains of mice differs among Leishmania species. While C57BL/6 and C3H mice are resistant to infection with L. major, they develop chronic lesions when infected with L. amazonensis while BALB/c mice are highly susceptible to L. major and L. amazonensis infection, but develop self-limited lesions when infected with L. braziliensis (16). Little information has been generated in the murine model regarding ATL causing Leishmania species. Although some data have been published with L. braziliensis and L. amazonensis infection, the protocols are heterogeneous with respect to the stage of parasite used (stationary phase or metacyclic promastigotes) and the inoculation route (subcutaneous or intradermal), making it difficult to compare the results obtained (23–26).
Even though we still lack a reliable, largely accepted, and utilized murine model for ATL, much progress was made in understanding the mechanisms involved in human pathology. However, many questions are still unanswered, especially those related to the immunological mechanisms leading to lesions healing and natural resistance to infection and cross-protection, as well as to the induction, regulation, and persistence of Leishmania-specific T cell response.
CD4 Immune Response and Memory
The goal of vaccination is the development of immunological memory, classically defined as the ability of the immune system to respond more effectively and faster to a pathogen previously encountered. In the late twentieth century, memory T cells were divided into central memory (TCM) and effector memory cell (TEM) populations, based on the expression of different cell surface markers (27). TCM cells constitutively express CCR7 and CD62L and are found in T cell areas of secondary lymphoid organs where they are able to proliferate and differentiate into effector cells in response to antigenic stimulation. TEM cells downregulate the expression of CCR7, have heterogeneous expression of CD62L, and are able to migrate to inflamed tissues, and have immediate effector functions (27, 28). One study in murine L. major infection demonstrated the importance of two populations of memory CD4 T cells in the protection against reinfection. While effector CD4+ T cells are lost in the absence of parasites, the central memory CD4+ T cells are kept and become tissue-homing effector T cells to mediate protection, suggesting that central memory T cells should be the targets for vaccines against Leishmania (29). The same group recently identified the presence of skin tissue Leishmania-specific resident memory T cells, and indicated the necessity of these cells, together with circulating memory T cells, for the success of a vaccine (30).
The induction of memory T cells was also evaluated in patients with CL. In patients healed form L. major infection both TEM IFN-γ producing cells (CD4+CD45RO+CD45RA−CCR7−) and Leishmania-reactive IL-2 producing TCM cells (CD4+CD45RO+CD45RA−CCR7+) were observed after “in vitro” stimulation with Leishmania soluble antigen (SLA), suggesting that both populations might play a role in protective recall immune response against reinfection (31). On the other hand, the majority of L. braziliensis-healed CL and ML patients did not produce IFN-γ “in vitro” after SLA stimulation, but are still responsive “in vivo” to LST. A positive LST was found in 87.5% of CL and 100% of ML cured individuals who did not produce IFN-γ, and in the individuals that maintains SLA-specific IFN-γ production, the main source of the cytokine was effector memory CD4+ T cell (32).
Usually, L. braziliensis patients healed from CL lesions should be monitored for approximately 5 years to rule out of the possibility of relapses or the development of metastatic mucosal lesions (33, 34). In one study where healed L. braziliensis CL patients were grouped according to the time elapsed since the end of therapy, a regulated leishmanial-specific response appeared to emerge only about 2 years after initial contact with the parasite. Ex vivo analyses showed a contraction for both CD4 and CD8 TEM compartments in patients with long-time elapsed after clinical cure (2–5 years). However, after “in vitro” SLA stimulation, they exhibit a recall response with expansion of TEM cells (35).
CD4 T cells also present different capacities to develop into memory cells based on their cytokine production (36), and the quality of a Th1 immune response has been related with a differentiation spectrum based on the production of three cytokines: IFN-γ, IL-2, and TNF-α (37). Cells that enter this differentiation processes are, at first, single producers of IL-2 or double producers of IL-2/TNF-α, but are negative for IFN-γ. They can be classified as central memory cells, since are long-lasting cells able to respond quickly to a second antigen encounter. Te other pole of this spectrum is IFN-γ single-positives cells that are short-lived, terminal effector cells (36, 37). From one pole to the other, a variety of phenotypes can be found, including multifunctional CD4 T that are triple positives for IFN-γ, IL-2, and TNF-α (37). Interestingly, the amount of IFN-γ produced by multifunctional cells is much higher than the amount produced by double- or single-positive cells (38, 39). The IL-2 produced by those cells together with the high production of IFN-γ and TNF-α give to multifunctional CD4 T cells the remarkable capacity to possess optimal effector functions and proliferation.
CD4+ T cells not always go through each possible stage of differentiation and after antigen recognition, an IL-2 single-positive cell can go straight to the IFN-γ single-positive effector phenotype, particularly if the stimulus is strong (36). Thus, it is possible that a vaccine candidate can elicit an immune response predominantly composed by effector cells, and fail to induce long, last protection against infection. A promising vaccine candidate should be able to induce multifunctional T cells that are able to proliferate and generate memory and effector cells.
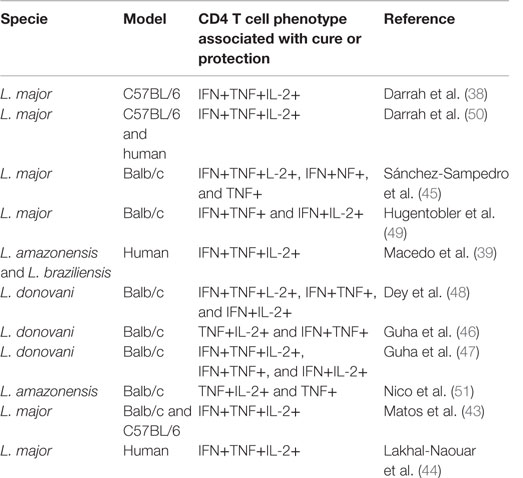
In past years, the majority of studies designed to evaluate possible immunogens against Leishmania infection utilized the production of IFN-γ by antigen-specific T cells as the main factor to predict protection. However, it is clear that the quality and the magnitude of a T-cell response measured by a single parameter do not reflect its full functional potential which may be the reason why vaccines that reached phase III trials failed to protect against Leishmania infection (40–42). In 2007, the first compelling evidence for the importance of multifunctional Th1 cells in mediating protection against Leishmaniasis revealed, after immunization with various vaccine formulations encoding specific L. major antigens, a strong correlation between the generation of multifunctional CD4 T cells and the degree of protection observed after a subsequent challenge (38). Intriguingly, the best degree of protection and the higher percentage of multifunctional T cells were observed in animals that healed primary lesions and were reinfected (“live vaccination”). Afterward, this approach started to be utilized by many other research groups to characterize immune correlates of protection after infection or after immunization against CL and VL (Table 2) (39, 43–51), but only two concerned ATL causing species (39, 51). In all of them, protection was demonstrated to be associated with the induction of multifunctional T cells among other double producers or with TNF-α producing cells (either TNF-α single-positives or TNF-α/IL-2 and TNF-α/IFN-γ double positive cells) (45–47, 51).
L. Amazonensis Versus L. Braziliensis: Differences on Quality of Immune Response
It has already been reported that patients infected with parasites from the subgenus Viannia (as L. braziliensis) display higher T cell responses (evaluated by proliferation and IFN-γ production) to Leishmania crude antigens than L. amazonnsis-infected patients, and that L. amazonensis-infected patients also have stronger responses to L. braziliensis than to L. amazonensis antigens in vitro, before and after therapy (52).
Vaccine candidates formulated with L. braziliensis total extract have been tested against Canine VL (LBSap and LBSApSal) with promising results in phase I and II trials (53–55). LBSap induced both humoral and cellular immune responses against Leishmania infantum, with high levels of total IgG and its subtypes (IgG1 and IgG2), expansion of circulating CD5+, CD4+, and CD8+ T lymphocytes as well as reduction of splenic parasite load (55).
One previous study designed to evaluate the quality of the Th1 response induced by L. amazonensis and L. braziliensis promastigotes extracts in PBMC from healed CL patients demonstrated that L. amazonensis response is associated with a low contribution of multifunctional T cells and a high number of IFN-γ single-positive effector cells, while L. braziliensis induces a Th1 response with high proportion of multifunctional T cells and low proportion of IFN-γ single-positive cells (39). As IFN-γ single-positive CD4+ T cells are short-lived, this can offer a possible explanation for the contrasting results observed in prophylaxis and immunotherapy studies with L. amazonensis whole-cell extract vaccine (Leishvacin®) (40, 56–58). The substantial amount of IFN-γ single-positive effector CD4+ T cells induced by this antigen may not be sufficient to induce long-term and good-quality protection against infection, but could be effective when a rapid and transient Th1 response is needed, as in the case of immunotherapeutic interventions. In addition, the capacity of L. amazonensis promastigotes extract to induce IL-10 secretion (59, 60), together with the generation of short-lived IFN-γ producing CD4+ T cells, could result in equilibrium between inflammatory and anti-inflammatory responses, allowing parasite killing and lesion resolution, as observed in the immunotherapeutic protocols tested so far.
If we combine the information that mice healed from a primary infection with L. major present the highest proportion of multifunctional CD4+ T cells and protection after a homologous challenge (38), together with the results obtained in healed CL patients after stimulation with L. braziliensis and L. amazonensis promastigotes extracts (39), we can consider the possibility that patients healed from L. braziliensis infection should display better protection to reinfection than L. amazonensis healed patients.
It has never been reported that individuals that were infected with L. braziliensis or any other Leishmania specie are more susceptible to infection with L. amazonensis, but L. amazonensis infection does not give protection against a subsequent challenge with L. braziliensis or other Leishmania specie from the subgenus Viannia. On the other hand, recovery from L. braziliensis infection confers resistance to homologous challenge as well as to infection with L. amazonensis or L. mexicana parasites (6, 7). Interestingly, cells from DCL patients infected with L. amazonensis are able to differentiate into multifunctional T cells in vitro only after simulation with L. braziliensis promastigotes extract, while L. amazonensis stimulates high proportions of IFN-γ single-positive, terminal differentiated cells (Figure 1). This finding indicates that something intrinsic to L. amazonensis parasite antigens is responsible for the weak specific Th1 immune response observed during L. amazonensis infection (52, 61).
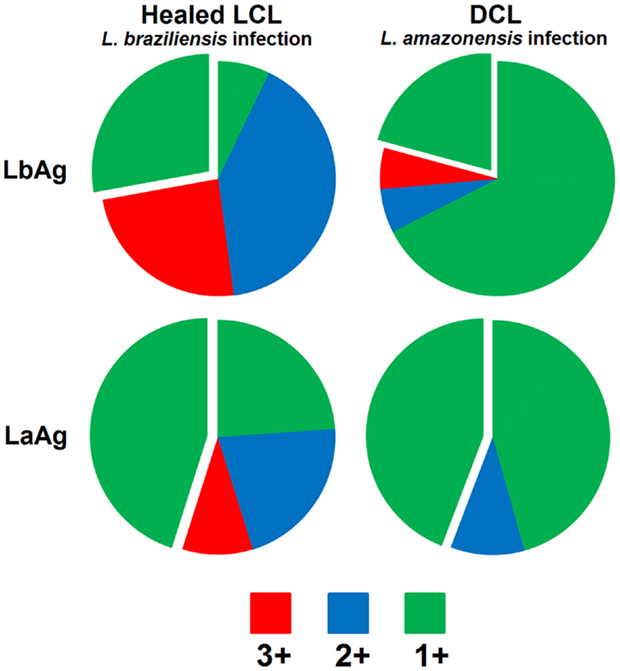
Figure 1. Demonstrative figure of the CD4 T cell response induce in PBMC cultures of one patient healed from localized cutaneous Leishmaniasis caused by L. braziliensis (Healed LCL) in comparison to the response induced in PBMC cultures obtained from one patient with diffuse cutaneous Leishmaniasis caused by L. amazonensis, during remission of symptoms (DCL). Cells were stimulated “in vitro” with total promastigotes extracts form L. braziliensis (LbAg) or L. amazonensis (LaAg) and stained with monoclonal antibodies to determine the frequency of CD4 T cells expressing IFN-γ, IL-2, and TNF-α by multiparametric flow cytometry. Combination gates were applied to determine the percentage of cells that were able to produce any combination of these three cytokines. To determine the contribution of each phenotype to the total Th1 immune response analyzed the results are represented in the pie charts comprising cells expressing all three cytokines (in red – IFN-γ+TNF-α+IL-2+), any two cytokines (in blue – IFN-γ+TNF-α+IL-2−, IFN-γ+TNF-α−IL-2+, and IFN-γ−TNF-α+IL-2+), or any one cytokine (in green – IFN-γ+TNF-α−IL-2−, IFN-γ−TNF-α+IL-2−, and IFN-γ−TNF-α−IL-2+). Detached is the contribution of the IFN-γ+TNF-α−IL-2− single-positive cells phenotype. Data showed in this figure are part of a published study (39) approved by the National Ethical Clearance Committee of Brazil (CONEP), as well as by the Ethical Committee for Human Research from IPEC/FIOCRUZ, all of which adhere to the principles laid out in the Declaration of Helsinki. Informed consent was obtained from all participants.
Even though parasites from the Viannia and Leishmania subgenera show highly conserved gene sequences with very few genes restricted to a given species (62–64), these similarities did not prevent different species from evolving some particularities related to the expression of virulence factors and the development of particular evasion mechanisms. Recently, the genome of L. amazonensis was sequenced and compared with other human pathogenic Leishmania spp. indicating that L. amazonensis and L. mexicana share groups of amastin surface proteins unique to the genus that could be related to specific disease outcomes. Additionally, a hypothetical interactome model of host protein and secreted L. (L.) amazonensis proteins revealed a possible interaction between an L. (L.) amazonensis heat-shock protein and mammalian Toll-like receptor 9 (65).
The low generation of multifunctional T cells induced by L. amazonensis can be one more factor, or, and most likely, can be a consequence of many others already described in the literature, implicated with the susceptibility to this Leishmania specie (23, 59, 65–73).
Concluding Remarks
Prophylactic immunization is accepted as the most efficient and low-cost/benefit alternative to control infectious diseases. An ideal vaccine against Leishmaniasis must have several attributes: (1) safety, (2) accessibility for populations at risk, (3) induce long-lasting CD4 and CD8 specific T cell response, (4) be effective against Leishmania species responsible for visceral and tegumentary forms, (5) stability at room temperature to be used in the field, and (6) have prophylactic and therapeutic potential (74). Although it is possible to fulfill the attributes related to cost/benefit and safety, the development of a Leishmaniasis vaccine has proven a difficult goal to achieve. Not because of the discovery of candidate molecules, especially after the sequencing of the genome of different species of parasite, but rather because of the difficulties related to the still incomplete knowledge involving pathogenesis, the complex immune response needed for induction of protection, the lack of suitable experimental models, and the still fragmented knowledge about the development of immunological memory mechanisms.
Currently more than 30 Leishmania antigens have been or are being tested as candidate vaccines against visceral or tegumentary leishmaniasis. Many of them are very well conserved among different species of the parasite, but were not capable of inducing protection in clinical trials or are unable to protect against all species of the parasite. However, one study has demonstrated that heterologous protection is feasible, and associated with the presence of a “multifunctional Th1 response.” BALB/c mice immunized with a non-pathogenic Leishmania donovani parasite showed cross-protection against the challenge with L. major or L. braziliensis, and the immunization induced a long-term immune response characterized by high levels of multifunctional CD4 and CD8 T cells (48). Additionally, other authors observed a reduction in the frequency of parasitism in the bone marrow (54), as well as a reduction in splenic parasite loads (55) in dogs vaccinated against VL with LbSAP (a preparation of killed L. braziliensis promastigotes together with saponin), after L. infantum infection, although multifunctionality were not evaluated in those studies.
A point that also needs to be emphasized is that in natural infection, all the Leishmania species are co-deposited into the skin together with the vector saliva, and that saliva contains factors able to modulate the immune response (75–77). Studies have demonstrated that pre-exposure of sand fly saliva lead to either disease exacerbation (78, 79) or protection (80–83) upon Leishmania infectious challenge. Carregaro et al. (84) demonstrated that different inocula of Lutzomyia longipalpis salivary gland extract could modify the cellular immune response, reflecting in the pattern of susceptibility or resistance to L. braziliensis infection. It would be interesting to investigate whether a combination of saliva proteins with Leishmania proteins or extracts can shape the immune responses against infection, altering the quality of the immune responses by increasing the frequencies of multifunctional T cells. Moreover, the use of components that participate in the initial phase of infection could improve vaccine efficiency at the earlier stages of infection.
Certainly, there is still a long road ahead of us until an ideal Leishmaniasis vaccine be developed, but it is also undoubtable that multiparametric flow cytometry gave us a powerful tool to better evaluate correlates of protection and the development of memory T cell responses after infection and immunization. Since the crude and synthetic antigens tested so far were not able to induce consistent protection against Leishmania infections, it may be time to turn away our efforts from finding new candidate molecules, and focus on evaluating new presentation approaches of existing conserved molecules, specially the design of safe new adjuvants, that could direct the T cell-specific response toward long-lasting memory and multifunctional T cell phenotypes.
Author Contributions
All authors listed have made substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to Erin Larragoite and Dr. Steve M. Skoumal for carefully revising the manuscript.
References
1. World Health Organization. Leishmaniasis Situation and Trends. Global Health Observatory (2016). Available from: http://www.who.int/gho/neglected_diseases/leishmaniasis/en/
2. Nadim A, Javadian E, Tahvildar-Biruni G, Ghorbani M. Effectiveness of leishmanization in the control of cutaneous leishmaniasis. Bull Soc Pathol Exot Filiales (1983) 76:377–83.
3. Noazin S, Modabber F, Khamesipour A, Smith PG, Moulton LH, Nasseri K, et al. First generation leishmaniasis vaccines: a review of field efficacy trials. Vaccine (2008) 26:6759–67. doi: 10.1016/j.vaccine.2008.09.085
4. Mayrink W, Williams P, da Costa CA, Magalhães PA, Melo MN, Dias M, et al. An experimental vaccine against American dermal leishmaniasis: experience in the State of Espírito Santo, Brazil. Ann Trop Med Parasitol (1985) 79:259–69.
5. Nagill R, Kaur S. Vaccine candidates for leishmaniasis: a review. Int Immunopharmacol (2011) 11:1464–88. doi:10.1016/j.intimp.2011.05.008
6. Lainson R, Shaw JJ. Leishmaniasis in Brazil: XII. Observations on cross-immunity in monkeys and man infected with Leishmania mexicana mexicana, L. m. amazonensis, L. braziliensis braziliensis, L. b. guyanensis and L. b. panamensis. J Trop Med Hyg (1977) 80:29–35.
7. Porrozzi R, Teva A, Amaral VF, Santos da Costa MV, Grimaldi G. Cross-immunity experiments between different species or strains of Leishmania in rhesus macaques (Macaca mulatta). Am J Trop Med Hyg (2004) 71:297–305.
8. Grimaldi G, Tesh RB. Leishmaniases of the New World: current concepts and implications for future research. Clin Microbiol Rev (1993) 6:230–50.
9. de Oliveira-Neto MP, Mattos MS, Perez MA, Da-Cruz AM, Fernandes O, Moreira J, et al. American tegumentary leishmaniasis (ATL) in Rio de Janeiro State, Brazil: main clinical and epidemiologic characteristics. Int J Dermatol (2000) 39:506–14. doi:10.1046/j.1365-4362.2000.00969.x
10. de Oliveira Guerra JA, Prestes SR, Silveira H, Coelho LI, Gama P, Moura A, et al. Mucosal leishmaniasis caused by Leishmania (Viannia) braziliensis and Leishmania (Viannia) guyanensis in the Brazilian Amazon. PLoS Negl Trop Dis (2011) 5:e980. doi:10.1371/journal.pntd.0000980
11. Bittencourt A, Silva N, Straatmann A, Nunes VLC, Follador I, Badaró R. Post-kala-azar dermal leishmaniasis associated with AIDS. Braz J Infect Dis (2003) 7:229–33. doi:10.1590/S1413-86702003000300009
12. Costa JM, Marsden PD, Llanos-Cuentas EA, Netto EM, Carvalho EM, Barral A, et al. Disseminated cutaneous leishmaniasis in a field clinic in Bahia, Brazil: a report of eight cases. J Trop Med Hyg (1986) 89:319–23.
13. Turetz ML, Machado PR, Ko AI, Alves F, Bittencourt A, Almeida RP, et al. Disseminated leishmaniasis: a new and emerging form of leishmaniasis observed in northeastern Brazil. J Infect Dis (2002) 186:1829–34. doi:10.1086/345772
14. Almeida RP, Barral-Netto M, De Jesus AMR, De Freitas LAR, Carvalho EM, Barral A. Biological behavior of Leishmania amazonensis isolated from humans with cutaneous, mucosal, or visceral leishmaniasis in BALB/c mice. Am J Trop Med Hyg (1996) 54:178–84.
15. Silveira FT, Lainson R, Corbett CEP. Further observations on clinical, histopathological, and immunological features of borderline disseminated cutaneous leishmaniasis caused by Leishmania (Leishmania) amazonensis. Mem Inst Oswaldo Cruz (2005) 100:525–34. doi:10.1590/S0074-02762005000500013
16. McMahon-Pratt D, Alexander J. Does the Leishmania major paradigm of pathogenesis and protection hold for New World cutaneous leishmaniases or the visceral disease? Immunol Rev (2004) 201:206–24. doi:10.1111/j.0105-2896.2004.00190.x
17. Reiner SL, Locksley RM. The regulation of immunity to Leishmania major. Annu Rev Immunol (1995) 13:151–77. doi:10.1146/annurev.iy.13.040195.001055
18. Antonelli LRV, Dutra WO, Almeida RP, Bacellar O, Carvalho EM, Gollob KJ. Activated inflammatory T cells correlate with lesion size in human cutaneous leishmaniasis. Immunol Lett (2005) 101:226–30. doi:10.1016/j.imlet.2005.06.004
19. Oliveira WN, Ribeiro LE, Schrieffer A, Machado P, Carvalho EM, Bacellar O. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of human tegumentary leishmaniasis. Cytokine (2014) 66:127–32. doi:10.1016/j.cyto.2013.12.016
20. Akuffo H, Maasho K, Blostedt M, Hojeberg B, Britton S, Bakhiet M. Leishmania aethiopica derived from diffuse leishmaniasis patients preferentially induce mRNA for interleukin-10 while those from localized leishmaniasis patients induce interferon-gamma. J Infect Dis (1997) 175:737–41. doi:10.1093/infdis/175.3.737
21. Gollob KJ, Viana AG, Dutra WO. Immunoregulation in human American leishmaniasis: balancing pathology and protection. Parasite Immunol (2014) 36:367–76. doi:10.1111/pim.12100
22. Soong L, Henard CA, Melby PC. Immunopathogenesis of non-healing American cutaneous leishmaniasis and progressive visceral leishmaniasis. Semin Immunopathol (2012) 34:735–51. doi:10.1007/s00281-012-0350-8
23. Jones DE, Buxbaum LU, Scott P. IL-4-independent inhibition of IL-12 responsiveness during Leishmania amazonensis infection. J Immunol (2000) 165:364–72. doi:10.4049/jimmunol.165.1.364
24. Jones DE, Ackermann MR, Wille U, Hunter CA, Scott P. Early enhanced Th1 response after Leishmania amazonensis infection of C57BL/6 interleukin-10-deficient mice does not lead to resolution of infection. Infect Immun (2002) 70:2151–8. doi:10.1128/IAI.70.4.2151-2158.2002
25. de Moura TR, Novais FO, Oliveira F, Clarencio J, Noronha A, Barral A, et al. Toward a novel experimental model of infection to study American cutaneous leishmaniasis caused by Leishmania braziliensis. Infect Immun (2005) 73:5827–34. doi:10.1128/IAI.73.9.5827
26. Giudice A, Camada I, Leopoldo PTG, Pereira JMB, Riley LW, Wilson ME, et al. Resistance of Leishmania (Leishmania) amazonensis and Leishmania (Viannia) braziliensis to nitric oxide correlates with disease severity in tegumentary leishmaniasis. BMC Infect Dis (2007) 7:7. doi:10.1186/1471-2334-7-7
27. Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature (1999) 401:708–12. doi:10.1038/44385
28. Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol (2004) 22:745–63. doi:10.1146/annurev.immunol.22.012703.104702
29. Zaph C, Uzonna J, Beverley SM, Scott P. Central memory T cells mediate long-term immunity to Leishmania major in the absence of persistent parasites. Nat Med (2004) 10:1104–10. doi:10.1038/nm1108
30. Glennie ND, Yeramilli VA, Beiting DP, Volk SW, Weaver CT, Scott P. Skin-resident memory CD4+ T cells enhance protection against Leishmania major infection. J Exp Med (2015) 212:1405–14. doi:10.1084/jem.20142101
31. Keshavarz Valian H, Nateghi Rostami M, Tasbihi M, Miramin Mohammadi A, Eskandari SE, Sarrafnejad A, et al. CCR7+ central and CCR7 effector memory CD4+ T cells in human cutaneous leishmaniasis. J Clin Immunol (2013) 33:220–34. doi:10.1007/s10875-012-9788-7
32. Carvalho AM, Magalhães A, Carvalho LP, Bacellar O, Scott P, Carvalho EM. Immunologic response and memory T cells in subjects cured of tegumentary leishmaniasis. BMC Infect Dis (2013) 13:529. doi:10.1186/1471-2334-13-529
33. Zajtchuk JT, Casler JD, Netto EM, Grogl M, Neafie RC, Hessel CR, et al. Mucosal leishmaniasis in Brazil. Laryngoscope (1989) 99:925–39. doi:10.1288/00005537-198909000-00006
34. Netto EM, Marsden PD, Llanos-Cuentas EA, Costa JML, Cuba CC, Barreto AC, et al. Long-term follow-up of patients with Leishmania (Viannia) braziliensis infection and treated with Glucantime®. Trans R Soc Trop Med Hyg (1990) 84:367–70. doi:10.1016/0035-9203(90)90321-5
35. Pereira-Carvalho R, Mendes-Aguiar CO, Oliveira-Neto MP, Covas CJF, Bertho ÁL, Da-Cruz AM, et al. Leishmania braziliensis-reactive T cells are down-regulated in long-term cured cutaneous leishmaniasis, but the renewal capacity of T effector memory compartments is preserved. PLoS One (2013) 8:e81529. doi:10.1371/journal.pone.0081529
36. Wu C-Y, Kirman JR, Rotte MJ, Davey DF, Perfetto SP, Rhee EG, et al. Distinct lineages of T(H)1 cells have differential capacities for memory cell generation in vivo. Nat Immunol (2002) 3:852–8. doi:10.1038/ni832
37. Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol (2008) 8:247–58. doi:10.1038/nri2355
38. Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med (2007) 13:843–50. doi:10.1038/nm1592
39. Macedo AB, Sánchez-Arcila JC, Schubach AO, Mendonça SC, Marins-Dos-Santos A, de Fatima Madeira M, et al. Multifunctional CD4 +T cells in patients with American cutaneous leishmaniasis. Clin Exp Immunol (2012) 167:505–13. doi:10.1111/j.1365-2249.2011.04536.x
40. Vélez ID, Gilchrist K, Arbelaez MP, Rojas CA, Puerta JA, Antunes CMF, et al. Failure of a killed Leishmania amazonensis vaccine against American cutaneous leishmaniasis in Colombia. Trans R Soc Trop Med Hyg (2005) 99:593–8. doi:10.1016/j.trstmh.2005.04.002
41. Armijos RX, Weigel MM, Calvopina M, Hidalgo A, Cevallos W, Correa J. Safety, immunogenecity, and efficacy of an autoclaved Leishmania amazonensis vaccine plus BCG adjuvant against New World cutaneous leishmaniasis. Vaccine (2004) 22:1320–6. doi:10.1016/j.vaccine.2003.06.002
42. Marques-Da-Silva EA, Coelho EA, Gomes DC, Vilela MC, Masioli CZ, Tavares CA, et al. Intramuscular immunization with p36(LACK) DNA vaccine induces IFN-γ production but does not protect BALB/c mice against Leishmania chagasi intravenous challenge. Parasitol Res (2005) 98:67–74. doi:10.1007/s00436-005-0008-8
43. Matos I, Mizenina O, Lubkin A, Steinman RM, Idoyaga J. Targeting Leishmania major antigens to dendritic cells in vivo induces protective immunity. PLoS One (2013) 8:e67453. doi:10.1371/journal.pone.0067453
44. Lakhal-Naouar I, Slike BM, Aronson NE, Marovich MA. The immunology of a healing response in cutaneous leishmaniasis treated with localized heat or systemic antimonial therapy. PLoS Negl Trop Dis (2015) 9:e0004178. doi:10.1371/journal.pntd.0004178
45. Sánchez-Sampedro L, Gómez CE, Mejías-Pérez E, Sorzano CO, Esteban M. High quality long-term CD4 + and CD8 + effector memory populations stimulated by DNA-LACK/MVA-LACK regimen in Leishmania major BALB/C model of infection. PLoS One (2012) 7:e38859. doi:10.1371/journal.pone.0038859
46. Guha R, Das S, Ghosh J, Naskar K, Mandala A, Sundar S, et al. Heterologous priming-boosting with DNA and vaccinia virus expressing kinetoplastid membrane protein-11 induces potent cellular immune response and confers protection against infection with antimony resistant and sensitive strains of Leishmania (Leishmania) donovani. Vaccine (2013) 31:1905–15. doi:10.1016/j.vaccine.2013.02.025
47. Guha R, Gupta D, Rastogi R, Vikram R, Krishnamurthy G, Bimal S, et al. Vaccination with Leishmania hemoglobin receptor-encoding DNA protects against visceral leishmaniasis. Sci Transl Med (2013) 5:202ra121. doi:10.1126/scitranslmed.3006406
48. Dey R, Dagur PK, Selvapandiyan A, McCoy JP, Salotra P, Duncan R, et al. Live attenuated Leishmania donovani p27 gene knockout parasites are nonpathogenic and elicit long-term protective immunity in BALB/c mice. J Immunol (2013) 190:2138–49. doi:10.4049/jimmunol.1202801
49. Hugentobler F, Yam KK, Gillard J, Mahbuba R, Olivier M, Cousineau B. Immunization against Leishmania major infection using LACK- and IL-12-expressing Lactococcus lactis induces delay in footpad swelling. PLoS One (2012) 7:e30945. doi:10.1371/journal.pone.0030945
50. Darrah PA, Hegde ST, Patel DT, Lindsay RWB, Chen L, Roederer M, et al. IL-10 production differentially influences the magnitude, quality, and protective capacity of Th1 responses depending on the vaccine platform. J Exp Med (2010) 207:1421–33. doi:10.1084/jem.20092532
51. Nico D, Gomes DC, Palatnik-de-Sousa I, Morrot A, Palatnik M, Palatnik-de-Sousa CB. Leishmania donovani nucleoside hydrolase terminal domains in cross-protective immunotherapy against Leishmania amazonensis murine infection. Front Immunol (2014) 5:273. doi:10.3389/fimmu.2014.00273
52. Silveira FT, Lainson R, De Castro Gomes CM, Laurenti MD, Corbett CEP. Immunopathogenic competences of Leishmania (V.) braziliensis and L. (L.) amazonensis in American cutaneous leishmaniasis. Parasite Immunol (2009) 31:423–31. doi:10.1111/j.1365-3024.2009.01116.x
53. Vitoriano-Souza J, Moreira Nd, Menezes-Souza D, Roatt BM, de Oliveira Aguiar-Soares RD, Siqueira-Mathias FA, et al. Dogs immunized with LBSap vaccine displayed high levels of IL-12 and IL-10 cytokines and CCL4, CCL5 and CXCL8 chemokines in the dermis. Mol Immunol (2013) 56:540–8. doi:10.1016/j.molimm.2013.05.231
54. Resende LA, Roatt BM, Aguiar-Soares RD, Viana KF, Mendonça LZ, Lanna MF, et al. Cytokine and nitric oxide patterns in dogs immunized with LBSap vaccine, before and after experimental challenge with Leishmania chagasi plus saliva of Lutzomyia longipalpis. Vet Parasitol (2013) 198:371–81. doi:10.1016/j.vetpar.2013.09.011
55. Aguiar-Soares R, Roatt B, Ker H, Moreira N, Mathias F, Cardoso J, et al. LBSapSal-vaccinated dogs exhibit increased circulating T-lymphocyte subsets (CD4+ and CD8+) as well as a reduction of parasitism after challenge with Leishmania infantum plus salivary gland of Lutzomyia longipalpis. Parasit Vectors (2014) 7:61. doi:10.1186/1756-3305-7-61
56. Mayrink W, Cristina A, Botelho DC, Magalhães PA, Batista SM, Lima ADO, et al. Immunotherapy, immunochemotherapy and chemotherapy for American cutaneous leishmaniasis treatment. Rev Soc Bras Med Trop (2006) 39:14–21.
57. Convit J, Ulrich M, Zerpa O, Borges R, Aranzazu N, Valera M, et al. Immunotherapy of American cutaneous leishmaniasis in Venezuela during the period 1990-99. Trans R Soc Trop Med Hyg (2003) 97:469–72. doi:10.1016/S0035-9203(03)90093-9
58. Carneiro MB, de Andrade e Sousa LM, Vaz LG, Dos Santos LM, Vilela L, de Souza CC, et al. Short-term protection conferred by Leishvacin® against experimental Leishmania amazonensis infection in C57BL/6 mice. Parasitol Int (2014) 63:826–34. doi:10.1016/j.parint.2014.07.010
59. Veras PST, Welby-Borges M, de Santana CD, Nihei J, Cardillo F, de Freitas LAR. Leishmania amazonensis: participation of regulatory T and B cells in the in vitro priming (PIV) of CBA/J spleen cells susceptible response. Exp Parasitol (2006) 113:201–5. doi:10.1016/j.exppara.2006.01.008
60. Telino E, De Luca PM, Matos DC, Azeredo-Coutinho RB, Meirelles MN, Conceição-Silva F, et al. In vitro responses of human peripheral blood mononuclear cells to whole-cell, particulate and soluble extracts of Leishmania promastigotes. Clin Exp Immunol (2006) 143:338–44. doi:10.1111/j.1365-2249.2006.02995.x
61. Afonso LC, Scott DF. Immune responses associated with susceptibility of C57BL/10 mice to Leishmania amazonensis. Infect Immun (1993) 61:2952–9. doi:10.1007/s00436-004-1193-6
62. Peacock CS, Seeger K, Harris D, Murphy L, Ruiz JC, Quail MA, et al. Comparative genomic analysis of three Leishmania species that cause diverse human disease. Nat Genet (2007) 39:839–47. doi:10.1038/ng2053
63. Raymond F, Boisvert S, Roy G, Ritt J-F, Legare D, Isnard A, et al. Genome sequencing of the lizard parasite Leishmania tarentolae reveals loss of genes associated to the intracellular stage of human pathogenic species. Nucleic Acids Res (2012) 40:1131–47. doi:10.1093/nar/gkr834
64. Downing T, Imamura H, Decuypere S, Clark TG, Coombs GH, Cotton JA, et al. Whole genome sequencing of multiple Leishmania donovani clinical isolates provides insights into population structure and mechanisms of drug resistance. Genome Res (2011) 21:2143–56. doi:10.1101/gr.123430.111
65. Real F, Vidal RO, Carazzolle MF, Mondego JMC, Costa GGL, Herai RH, et al. The genome sequence of Leishmania (Leishmania) amazonensis: functional annotation and extended analysis of gene models. DNA Res (2013) 20:567–81. doi:10.1093/dnares/dst031
66. Maioli TU, Takane E, Arantes RME, Fietto JLR, Afonso LCC. Immune response induced by New World Leishmania species in C57BL/6 mice. Parasitol Res (2004) 94:207–12. doi:10.1007/s00436-004-1193-6
67. Yang Z, Mosser DM, Zhang X. Activation of the MAPK, ERK, following Leishmania amazonensis infection of macrophages. J Immunol (2007) 178:1077–85. doi:10.4049/jimmunol.178.2.1077
68. Hernández-Ruiz J, Salaiza-Suazo N, Carrada G, Escoto S, Ruiz-Remigio A, Rosenstein Y, et al. CD8 cells of patients with diffuse cutaneous leishmaniasis display functional exhaustion: the latter is reversed, in vitro, by TLR2 agonists. PLoS Negl Trop Dis (2010) 4:e871. doi:10.1371/journal.pntd.0000871
69. Figueiredo AB, Serafim TD, Marques-da-Silva EA, Meyer-Fernandes JR, Afonso LCC. Leishmania amazonensis impairs DC function by inhibiting CD40 expression via A2B adenosine receptor activation. Eur J Immunol (2012) 42:1203–15. doi:10.1002/eji.201141926
70. Soong L. Subversion and utilization of host innate defense by Leishmania amazonensis. Front Immunol (2012) 3:58. doi:10.3389/fimmu.2012.00058
71. Carlsen ED, Hay C, Henard C, Popov V, Garg NJ, Soong L. Leishmania amazonensis amastigotes trigger neutrophil activation but resist neutrophil microbicidal mechanisms. Infect Immun (2013) 81:3966–74. doi:10.1128/IAI.00770-13
72. Henard CA, Carlsen ED, Hay C, Kima PE, Soong L. Leishmania amazonensis amastigotes highly express a tryparedoxin peroxidase isoform that increases parasite resistance to macrophage antimicrobial defenses and fosters parasite virulence. PLoS Negl Trop Dis (2014) 8:e3000. doi:10.1371/journal.pntd.0003000
73. Lacerda DI, Cysne-Finkelstein L, Nunes MP, De-Luca PM, Genestra MDS, Leon LLP, et al. Kinetoplastid membrane protein-11 exacerbates infection with Leishmania amazonensis in murine macrophages. Mem Inst Oswaldo Cruz (2012) 107:238–45. doi:10.1590/S0074-02762012000200014
74. Kedzierski L, Zhu Y, Handman E. Leishmania vaccines: progress and problems. Parasitology (2006) 133:S87. doi:10.1017/S0031182006001831
75. Morris RV, Shoemaker CB, David JR, Lanzaro GC, Titus RG. Sandfly maxadilan exacerbates infection with Leishmania major and vaccinating against it protects against L. major infection. J Immunol (2001) 167:5226–30. doi:10.4049/jimmunol.167.9.5226
76. Mbow ML, Bleyenberg JA, Hall LR, Titus RG. Phlebotomus papatasi sand fly salivary gland lysate down-regulates a Th1, but up-regulates a Th2, response in mice infected with Leishmania major. J Immunol (1998) 161:5571–7.
77. de Moura TR, Oliveira F, Rodrigues GC, Carneiro MW, Fukutani KF, Novais FO, et al. Immunity to Lutzomyia intermedia saliva modulates the inflammatory environment induced by Leishmania braziliensis. PLoS Negl Trop Dis (2010) 6:e712. doi:10.1371/journal.pntd.0000712
78. Norsworthy NB, Sun J, Elnaiem D, Lanzaro G, Soong L. Sand fly saliva enhances Leishmania amazonensis infection by modulating interleukin-10 production. Infect Immun (2004) 72:1240–7. doi:10.1128/IAI.72.3.1240-1247.2004
79. Weinkopff T, de Oliveira CI, de Carvalho AM, Hauyon-La Torre Y, Muniz AC, Miranda JC, et al. Repeated exposure to Lutzomyia intermedia sand fly saliva induces local expression of interferon-inducible genes both at the site of injection in mice and in human blood. PLoS Negl Trop Dis (2014) 8:e2627. doi:10.1371/journal.pntd.0002627
80. Kamhawi S, Belkaid Y, Modi G, Rowton E, Sacks D. Protection against cutaneous leishmaniasis resulting from bites of uninfected sand flies. Science (2000) 290:1351–4. doi:10.1126/science.290.5495.1351
81. de Moura TR, Oliveira F, Carneiro MW, Miranda JC, Clarêncio J, Barral-Netto M, et al. Functional transcriptomics of wild-caught Lutzomyia intermedia salivary glands: identification of a protective salivary protein against Leishmania braziliensis infection. PLoS Negl Trop Dis (2013) 7:e2242. doi:10.1371/journal.pntd.0002242
82. Teixeira C, Gomes R, Oliveira F, Meneses C, Gilmore DC, Elnaiem DE, et al. Characterization of the early inflammatory infiltrate at the feeding site of infected sand flies in mice protected from vector-transmitted Leishmania major by exposure to uninfected bites. PLoS Negl Trop Dis (2014) 8:e2781. doi:10.1371/journal.pntd.0002781
83. Oliveira F, Rowton E, Aslan H, Gomes R, Castrovinci PA, Alvarenga PH, et al. A sand fly salivary protein vaccine shows efficacy against vector-transmitted cutaneous leishmaniasis in nonhuman primates. Sci Transl Med (2015) 7:290ra90. doi:10.1126/scitranslmed.aaa3043
84. Carregaro V, Costa DL, Brodskyn C, Barral AM, Barral-Netto M, Cunha FQ, et al. Dual effect of Lutzomyia longipalpis saliva on Leishmania braziliensis infection is mediated by distinct saliva-induced cellular recruitment into BALB/c mice ear. BMC Microbiol (2013) 13:102. doi:10.1186/1471-2180-13-102
Keywords: vaccines, multifunctionality, T cell response, Leishmania amazonensis, Leishmania braziliensis
Citation: De Luca PM and Macedo ABB (2016) Cutaneous Leishmaniasis Vaccination: A Matter of Quality. Front. Immunol. 7:151. doi: 10.3389/fimmu.2016.00151
Received: 29 January 2016; Accepted: 07 April 2016;
Published: 21 April 2016
Edited by:
Wanderley De Souza, Universidade Federal do Rio de Janeiro, BrazilReviewed by:
Sukanya Narasimhan, Yale University School of Medicine, USAAdriana Gruppi, National University of Córdoba, Argentina
Copyright: © 2016 De Luca and Macedo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paula Mello De Luca, pmdeluca@ioc.fiocruz.br
 Paula Mello De Luca
Paula Mello De Luca Amanda Beatriz Barreto Macedo
Amanda Beatriz Barreto Macedo