- Department of Pathobiology, Ontario Veterinary College, University of Guelph, Guelph, ON, Canada
During early Mycobacterium avium subspecies paratuberculosis (Map) infection, complex interactions occur between the bacteria, cells from the mononuclear phagocyte system (MPS) including both resident (macrophages and dendritic cells) and recruited (monocytes) cells, and other mucosal sentinel cells such as γδ T lymphocytes. Though the details of early host–pathogen interactions in cattle remain largely underexplored, our hypothesis is that these significantly influence development of host immunity and ultimate success or failure of the host to respond to Map infection. The aims of the present study were to first characterize monocyte-derived MPS cells from young calves with respect to their immunophenotype and function. Then, we set out to investigate the effects of WC1+ and WC1neg γδ T lymphocytes on (1) the differentiation of autologous monocytes and (2) the maturation of autologous monocyte-derived dendritic cells (MDDCs). To achieve this, peripheral blood WC1+ or WC1neg γδ T lymphocytes were cocultured with either autologous freshly isolated peripheral blood-derived monocytes or autologous immature MDDCs (iMDDCs). We began by measuring several markers of interest on MPS cells. Useful markers to distinguish monocyte-derived macrophages (MDMs) from MDDCs include CD11b, CD163, and CD172a, which are expressed significantly higher on MDMs compared with MDDCs. Function, but not phenotype, was influenced by WC1neg γδ T lymphocytes: viability of Map harvested from monocytes differentiated in the presence of WC1neg γδ T lymphocytes (dMonWC1neg) was significantly lower compared to MDMs and MDDCs. With respect to DC maturation, we first showed that mature MDDCs (mMDDCs) have significantly higher expression of CD11c, CD80, and CD86 compared with iMDDCs, and the phagocytic capacity of mMDDCs is significantly reduced compared to iMDDCs. We then showed that γδ T lymphocyte subsets induce functional (reduced phagocytosis) but not phenotypic (surface marker expression) iMDDC maturation. These data collectively show that γδ T lymphocytes influence differentiation, maturation, and ultimately the function of monocytes during Map infection, which has significant implications on survival of Map and success of host defense during early Map infection.
Introduction
The mononuclear phagocyte system (MPS) comprises monocytes, macrophages, dendritic cells (DCs), and their precursors in the bone marrow (1). Myeloid progenitor cells give rise to circulating monocytes which migrate into various tissues where they function as resident tissue macrophages or DCs (2–4). A primary function of cells from the MPS under normal physiologic conditions is to maintain homeostasis in peripheral tissues (5). During inflammatory processes, they play a crucial role initiating and regulating immune responses by processing and presenting antigens to naïve T lymphocytes (6). Cells from the MPS share several surface markers and functions, making it difficult to clearly define the distinction between them (6). The phenotype of monocytes, macrophages, and DCs of humans and mice has been extensively studied and these cells have been classified according to the expression of specific markers [reviewed in Ref. (7)]. Classification of bovine DCs, including monocyte-derived dendritic cells (MDDCs) based on phenotype and function has been described [reviewed in Ref. (8)]; however, little is known about the phenotype and function of bovine monocytes, macrophages, and the expression of phenotypic surface markers after monocyte in vitro differentiation.
During initial exposure to pathogens at mucosal surfaces, cells from the MPS including tissue-resident macrophages and DCs interact with other immune cells, such as γδ T lymphocytes at mucosal surfaces. γδ T lymphocytes are considered to be a bridge between innate and adaptive immune systems. In cattle, γδ T lymphocytes are classified broadly as WC1+ and WC1neg according to their expression of the workshop cluster 1 (WC1) molecule, which is a transmembrane glycoprotein belonging to the scavenger receptor cysteine-rich family (CD163) (9). WC1+ γδ T lymphocytes are considered pro-inflammatory (9) and less is known about the function of WC1neg γδ T lymphocytes; however, it is believed that they are mucosal sentinel cells, given their presence at mucosal surfaces (10). Human and murine γδ T lymphocytes have been the most widely studied. In these species, γδ T lymphocytes recognize pathogen-associated molecular patterns (PAMPs) through pattern-recognition receptors (11), execute their effector functions without clonal expansion because they are not major histocompatibility complex (MHC)-restricted (12, 13), and present antigens to naïve αβ T lymphocytes (14). During adaptive immune responses, γδ T lymphocytes develop memory responses (15, 16), induce DC maturation (17), and polarize into TH1-, TH2-, TH17-, TFH-, or TREG-effector functions based on the cytokine milieu in which γδ T lymphocytes encounter the antigen (17–20). In cattle, γδ T lymphocytes have shown to produce pro-inflammatory cytokines, such as IFN-γ and IL-17A (18–21), regulate granuloma development (22), have regulatory effects (23, 24), and modulate macrophage-effector functions (25, 26).
This work focuses on studying the specific interactions of bovine γδ T lymphocyte subsets with cells from the MPS in the context of Mycobacterium avium subspecies paratuberculosis (Map) infection in vitro. Macrophages and DCs are the primary host cells for Map (27, 28), an intracellular bacterium causing paratuberculosis, which is an important mycobacterial infection of ruminants. The disease is characterized by a long subclinical phase (>2 years) (27), followed by a clinical phase in which animals show diarrhea and weight loss caused by inadequate nutrient absorption as a result of progressive granulomatous enteritis (29).
Both γδ T lymphocytes and the MPS play critical roles during the early pathogenesis of Map infection in cattle: (1) macrophages are the preferred cell host and the main effector cell during Map infection (30); (2) Map also infects DCs (28); and (3) monocytes migrate into the intestinal tract during infection and differentiate into effector cells, presumably in the presence of both WC1+ and WC1neg γδ T lymphocyte subsets (9, 31). Furthermore, we have previously shown that γδ T lymphocytes influence autologous monocyte-derived macrophage (MDM) effector functions of young calves and heifers during Map infection in vitro (25, 26). Therefore, the hypothesis for this study was that WC1+ and WC1neg γδ T lymphocytes of young calves influence (1) monocyte differentiation and (2) DC maturation during Map infection in vitro. The specific aims of this study were to first characterize cells from MPS of young calves and then to understand how bovine WC1+ or WC1neg γδ T lymphocytes influence autologous monocyte differentiation and DC maturation during in vitro Map infection.
Materials and Methods
Animals and Blood Collection
All animal procedures in this study were approved by the Institutional Committee on Animal Care at the University of Guelph (Animal Utilization Protocol # 3373). All animals were randomly selected from the Elora Dairy Research Centre, where there is no official paratuberculosis herd certification program; however, the estimated prevalence is near zero in this herd, because it is under continual surveillance for Map infection by regular screening for Map-specific antibodies using ELISA. No positive antibody tests, clinical or suspect paratuberculosis cases have been diagnosed on this farm for several years. Approximately 120 mL of blood were collected via jugular venipuncture using EDTA vacutainer tubes (BD Biosciences, Mississauga, ON, Canada) from seven healthy Holstein calves between 30 and 40 days of age. Number of animals was selected based on sample power calculations. Additional 60 mL of blood from the same calves were collected in serum separator vacutainer tubes (BD Biosciences). Blood samples were stored at 4°C and promptly transferred to the laboratory.
Peripheral Blood Mononuclear Cells (PBMCs), MDMs, and MDDCs
Under sterile conditions, whole blood was diluted (1:1) with PBS containing 0.5% BSA. PBMCs were isolated from whole blood using Histopaque 1077 (Sigma Aldrich, Oakville, ON, Canada) density gradient centrifugation, counted using a Moxi Z cell counter (Orflo, Hailey, ID, USA), and resuspended in complete medium RPMI 1640 containing 2 mM of l-glutamine and 25 mM of HEPES (Gibco, Carlsbad, CA, USA) supplemented with 5 × 10−5 M 2-mercaptoethanol (Sigma Aldrich, Oakville, ON, Canada), with penicillin (1,000 U/mL), streptomycin sulfate (10 mg/mL), and amphotericin B (0.25 µg/mL) (Sigma Aldrich). Our source of serum was 10% autologous serum based on our previous findings that show that cells are not self-reactive to cytokines or other soluble mediators present in autologous serum (26). Cell suspensions were transferred to 175 cm2 flasks (Corning, Tewksbury, MA, USA) at a concentration of 7.5 × 106/mL per flask. After 1 h of incubation at 37°C in 5% CO2, non-adherent cells were collected by washing each flask 3× with PBS prior to lymphocyte staining and sorting. Adherent cells (monocytes) were detached from the flasks using TrypLE Express (Gibco), washed, counted, and resuspended in complete RPMI. 2 × 105 monocytes/well were cultured in 24-well flat-bottomed plates (Corning). To obtain MDMs, monocytes were incubated for 6 days in complete RPMI. To obtain iMDDCs, complete RPMI was supplemented with 200 ng/mL of recombinant bovine interleukin-4 (Kingfisher Biotech, MN, USA) and 100 ng/mL of recombinant bovine GM-CSF (Kingfisher Biotech). After 6 days of differentiation, cells were used in coculture assays as iMDDCs; some wells of iMDDCs were induced to maturity (mMDDCs) by adding 1 µL/mL of Escherichia coli LPS (Sigma Aldrich) to the cell culture for 48 h prior to use in coculture assays as previously described (32, 33).
γδ T Lymphocyte Sorting
Non-adherent cells collected from the original flasks were resuspended in PBS containing 0.5% BSA, incubated in the dark at 4°C for 15 min with WC1 γδ T lymphocyte monoclonal antibody (BAQ4A, N2 epitope, IgG1, Monoclonal Antibody Center, Washington State University) and γδ T cell receptor monoclonal antibody (GB21A, TCR1-N24 δ chain-specific, IgG2b, Washington State University), washed, and incubated in the dark at 4°C for 15 min with the secondary antibody PE-Cy7 (IgG1, Biolegend, San Diego, CA, USA) and DyLight 405 (IgG2b, Jackson ImmunoResearch, Suffolk, UK). After washing, stained cells were sorted by FACS (Aria IIu, BD Biosciences, Mississauga, ON, Canada). After sorting WC1+ and WC1neg γδ T cell populations, cells were resuspended in complete RPMI. The purity of each subset was verified by FACS to be 85–95% and confirmation of a viability over 85% was assessed using the trypan blue exclusion assay described previously (34).
Cocultures
For monocyte differentiation assays, 1 × 106 sorted WC1+ or WC1neg γδ T lymphocytes were added directly to wells containing 2 × 105 monocytes the same day of PBMC isolation (day 0). After 6 days, cultures of MDMs, iMDDC, monocytes differentiated in presence of WC1+ (dMonWC1+), or WC1neg (dMonWC1neg) γδ T lymphocytes were obtained (Figure 1). For MDDC maturation assays, 1 × 106 sorted WC1+ or WC1neg γδ T lymphocytes were added to wells containing 2 × 105 iMDDCs (day 6) for 48 h (iMDDC + WC1+ and iMDDC + WC1neg) (Figure 4).
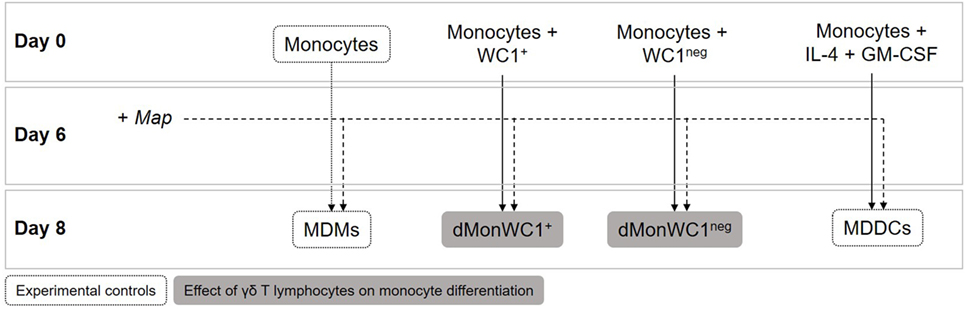
Figure 1. Monocyte differentiation assays. 1 × 106 WC1+ or WC1neg γδ T lymphocytes were added directly to wells containing 2 × 105 monocytes the same day of peripheral blood mononuclear cell isolation (day 0). After 6 days of differentiation, cultures of monocyte-derived macrophages (MDMs), monocyte-derived dendritic cells (MDDCs), monocytes differentiated in presence of WC1+ (dMonWC1+) or WC1neg (dMonWC1neg) γδ T lymphocytes were obtained. On day 6, live Map was added at a multiplicity of infection of 10:1 to evaluate how it affected phenotype of MDMs, dMonWC1+ and dMonWC1neg; and MDDC after 48 h.
Infection with Map
For monocyte differentiation assays on day six MDMs, iMDDC, dMonWC1+, and dMonWC1neg were infected. For MDDC maturation assays, γδ T lymphocyte subsets and bacterial suspensions of Map were added on day 6 to iMDDCs. Cell cultures were infected at a multiplicity of infection (MOI) of 10:1 for 48 h with an Ontario-derived clinical bovine Map strain (gc86). The Map strain was cultured in Middlebrook 7H9 broth supplemented with 10% OADC (oleic acid, albumin, dextrose, catalase) enrichment (BD Biosciences), 0.05% Tween 80 (Sigma Aldrich), and 2 mg/L of mycobactin J (Allied Monitor, Inc., Fayette, MO, USA) referred to below as 7H9-OADC-MJ-T. Optical density (OD) was measured with a spectrophotometer (Genesys 10S VIS, ThermoFisher Scientific, Waltham, MA, USA) at 540 nm wavelength and quantification of bacteria was performed using a standard growth curve. The bacterial suspension was briefly sonicated with a sonic dismembrator (Model 120, Fisher Scientific) at 60% amplitude during 2 s pulses to disperse bacterial clumps. Aliquots of Map with viability of 97.4% measured by fluorescein diacetate (Sigma Aldrich) as described previously (25) were kept at −80°C in saline to ensure that the same Map passage was used throughout this study.
Antibodies and Flow Cytometry
Antibodies used in this study are shown in Table 1. Cells were collected 48 h after Map infection into serum-free media before staining and assessment (FACSAria IIu, BD Biosciences). Viability was assessed using Zombie NIR fixable viability kit (Biolegend, CA, USA). The acquisition software used was FACS Diva II, and data were analyzed using FlowJo software (Treestar, Inc., San Carlos, CA, USA) (Figure S2 in Supplementary Material shows gating strategy).
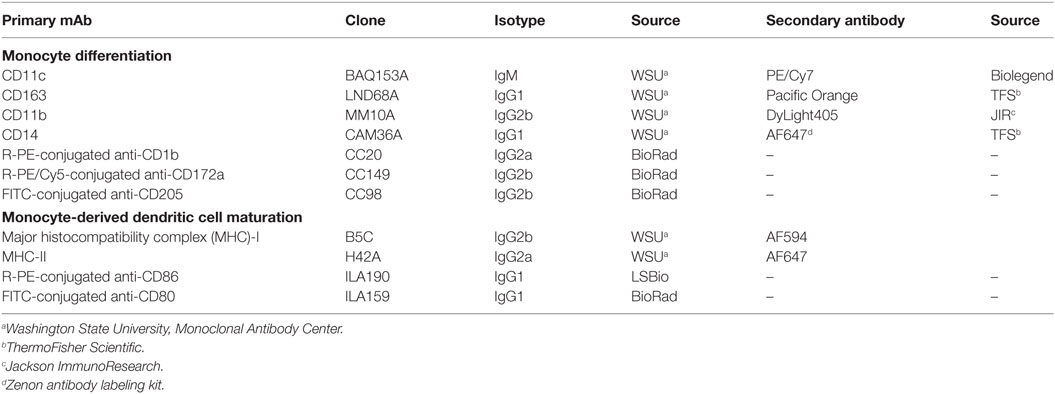
Table 1. Anti-bovine monoclonal antibodies used for peripheral blood mononuclear cell immunophenotyping and coculture experiments.
DQ-Ovalbumin (DQ-OVA) Endocytosis Assay
DQ-ovalbumin was added at a concentration of 10 µg/mL to 60 µL of cell suspension (2 × 105 cells) of monocytes, MDMs, iMDDCs, mMDDCs, dMonWC1+, dMonWC1neg, iMDDC + WC1+, or iMDDC + WC1neg. Cells were incubated at 37°C for 45 min. After incubation, cells were washed with cold PBS and immediately analyzed by flow cytometry (FACSAria IIu, BD Biosciences) to measure the bright green fluorescence exhibited by the ovalbumin labeled with the pH-insensitive fluorescent dye, boron-dipyrromethene, upon proteolytic degradation after phagocytosis.
Map Viability
Forty-eight hours after Map infection of MDMs, iMDDCs, dMonWC1+, or dMonWC1neg, culture supernatants were collected and wells containing Map-infected cells were washed twice with warm PBS to remove free Map. Cells were then detached from the flasks using TrypLE Express (Gibco), centrifuged at 400 × g for 2 min, and resuspended in sterile saline solution. Cell suspensions were stored at −80°C until further analysis. After thawing, cell suspensions were vortexed vigorously for 10 s to lyse cells, centrifuged at 400 × g for 2 min, resuspended in 7H9-OADC-MJ-T, and incubated in 24-well plates for 24 h at 37°C in 5% CO2. Contents of each well were centrifuged at 400 × g for 10 min and pellets were resuspended in 100 µL of saline solution in 5 mL conical tubes. One microliter of fluorescein diacetate at a concentration of 2 mg/mL (Sigma Aldrich) was added to each tube. After 30 min of incubation at 37°C, samples were analyzed by flow cytometry (Accuri C6, BD Biosciences). Standardization of the procedure and determination of gates were performed using a standard curve generated from known proportions of live and heat-killed Map as described previously (25).
Statistical Analysis
Statistical comparisons were performed using analysis of variance with SAS 9.4 software (SAS Institute, Cary, NC, USA) and GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA, USA). The mean and SEM were calculated in experiments containing multiple data points. A P value of ≤0.05 was considered statistically significant.
Results
Phenotypic and Functional Characterization of Bovine Cells from the MPS Show Clear Distinctions between MDMs and MDDCs
To determine the expression of MPS cell markers in this study, several cell types were defined: monocytes were freshly isolated by adherence; MDMs were collected from flasks after fresh adherent monocytes were cultured for 6 days; MDDCs were generated by adding IL-4 and GM-CSF to fresh adherent monocyte cultures (Figure 1). Monocytes display significantly lower expression (p < 0.0001) of CD14, CD11b, CD11c, and CD172a compared with MDMs or MDDCs (Figure 2). Fresh monocytes lack expression of CD163, CD1b, and CD205. The markers that help to differentiate MDMs from MDDCs in our system are CD11b, CD163, and CD172a, which are each significantly higher on MDMs compared to MDDCs with p values of <0.0001, <0.0001, and 0.0333, respectively (Figures 2B–D). To establish the phagocytic capacity of MPS cells in our study, monocytes, MDMs, and MDDCs were assessed with the DQ-OVA endocytosis assay. As expected, our data show that MDMs had the highest phagocytic capacity followed by MDDC (p = 0.0418), while monocytes have minimal phagocytic activity compared with MDMs and MDDCs (p < 0.0001 and 0.0028, respectively) (Figure 3A). To compare the ability of MDMs and MDDCs to alter Map viability, the viability of Map recovered from MDMs and MDDCs 48 h after in vitro infection with live Map was evaluated by flow cytometry. No significant differences were found between the viability of Map harvested from MDMs and MDDCs (p = 0.2929) (Figure 3B).
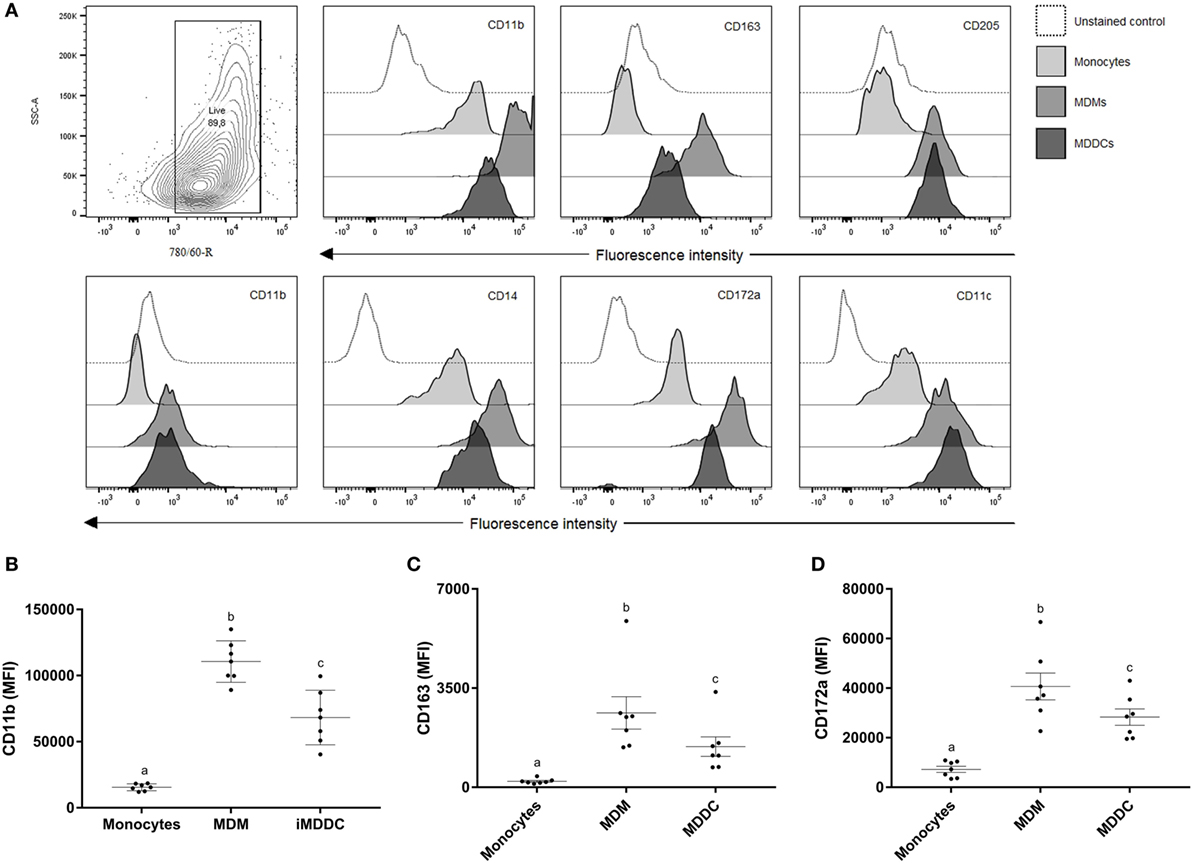
Figure 2. Surface marker expression of cells from the MPS is heterogeneous. (A) Contour plot of live cell population and flow cytometry analysis of surface expression of CD11b, CD163, CD205, CD1b, CD14, CD172a, and CD11c on monocytes, monocyte-derived macrophages (MDMs), and monocyte-derived dendritic cells (MDDCs). CD11b, CD163, and CD172a are useful surface markers to distinguish MDMs from MDDCs (B–D). Useful markers that distinguish MDMs from MDDCs include CD11b, CD163, and CD172a. Histograms of a representative animal are shown (n = 7). Different letters indicate statistically significant difference (p < 0.05).
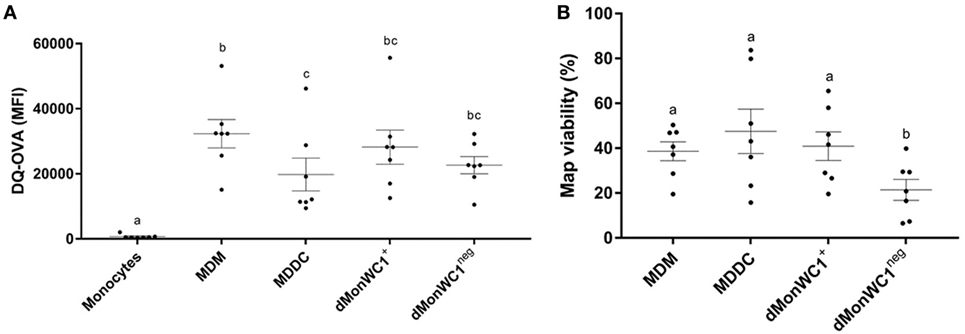
Figure 3. Cells from the MPS have a heterogeneous phagocytic capacity and the presence of WC1neg γδ T lymphocytes increase the ability of differentiated monocytes to alter Mycobacterium avium subspecies paratuberculosis (Map) viability. (A) Monocyte-derived macrophages (MDMs) had the highest phagocytic capacity, followed by monocyte-derived dendritic cell (MDDC). dMonWC1+ and dMonWC1neg showed an intermediate phagocytic activity between MDMs and MDDC. (B) Map viability measured by flow cytometry after 48 h of infection of MDMs, MDDC, dMonWC1+, and dMonWC1neg. The viability of Map harvested from dMonWC1neg was significantly lower compared to MDMs, MDDC, and dMonWC1+. Results are individual animal data points and means ± SEM of seven animals. Different letters indicate statistically significant difference (p < 0.05).
The Presence of γδ T Lymphocytes during Monocyte Differentiation Does Not Affect Phenotype or Phagocytic Capacity; However, Viability of Map Recovered from dMonWC1neg Was Significantly Lower
To analyze the effect of WC1+ or WC1neg γδ T lymphocyte subsets on monocytes during differentiation, sorted WC1+ or WC1neg γδ T lymphocytes were added to freshly isolated autologous monocytes, and the cells were left in direct contact for 6 days prior to assessment of MPS surface-marker expression (Figure 1). Our data show that the phenotype of monocytes differentiated after 6 days in presence of either WC1+ or WC1neg γδ T lymphocytes (dMonWC1+ and dMonWC1neg) was not significantly different from the phenotype of monocytes differentiated without γδ T lymphocytes (MDMs) in our system. The mean and SD median fluorescence intensity (MFI) of surface markers of MPS in this study are shown in Table S1 in Supplementary Material. The phagocytic capacity of dMonWC1+ and dMonWC1neg was assessed with the DQ-OVA endocytosis assay. dMonWC1+ and dMonWC1neg showed an intermediate phagocytic activity between MDMs (p = 0.5403 and 0.1582, respectively) and MDDC (p = 0.3165 and 0.8772, respectively) (Figure 3A). To determine the ability of dMonWC1+ and dMonWC1neg to alter Map viability, the viability of Map recovered from these cells 48 h after live Map infection was evaluated by flow cytometry. The viability of Map harvested from dMonWC1neg was significantly lower compared with the viability of Map harvested from either MDMs (p = 0.0492), MDDC (p = 0.0044), or dMonWC1+ (p = 0.0277) (Figure 3B).
Presence of Live Map Does Not Alter Phenotype or Functions of Cells from the MPS
To determine the effect of the presence of Map on the phenotype of MPS cells in this system, Map was added to cultures of MDMs, MDDC, dMonWC1+, and dMonWC1neg for 48 h (Figure 1). The effect of the presence of Map was examined by comparing the same cell type (i.e., uninfected MDMs vs. Map-infected MDMs). Our data show that infection of MDMs, MDDCs, dMonWC1+, or dMonWC1neg with Map did not significantly alter surface expression of CD163, CD1b, CD205, CD14, CD172a, CD11b, or CD11c at 48 h post infection (Table S1 in Supplementary Material).
MDDC Maturation Is Characterized by Upregulation of Surface Expression of MHC-I, CD80, and CD86, and Significant Reduction in Phagocytic Capacity
To examine the DC maturation process, iMDDCs were generated by adding IL-4 and GM-CSF to fresh peripheral blood-derived adherent monocyte cultures and their maturation was then induced by adding LPS for 48 h (Figure 4). As expected, our data showed that expression of MHC-I, CD80, and CD86 were significantly higher on mMDDCs compared with iMMDCs (Figure 5, p = 0.0105, <0.0001, and 0.0002, respectively); however, expression of MHC-II on mMDDCs was not significantly different from iMDDCs (p = 0.0640) (Figure 5). To study the effect of maturation of MDDCs on their phagocytic capacity, a DQ-OVA phagocytosis assay was performed on iMDDCs and mMDDCs using flow cytometry. Our data showed that the phagocytic capacity of mMDDCs was significantly reduced compared with iMDDCs (p ≤ 0.0001, Figure 6).
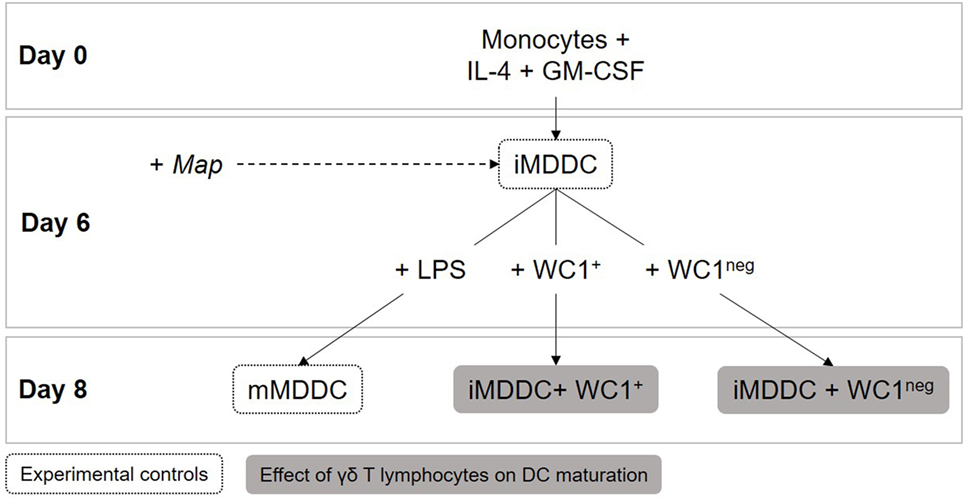
Figure 4. Dendritic cell maturation assays. Immature monocyte-derived dendritic cells (iMDDCs) were generated by adding IL-4 and GM-CSF to fresh monocyte cultures. After 6 days of differentiation, 1 × 106 sorted γδ T lymphocytes were added to wells containing 2 × 105 iMDDCs for 48 h (iMDDC + WC1+ and iMDDC + WC1neg). On day 6, live Mycobacterium avium subspecies paratuberculosis was added at a multiplicity of infection of 10:1 to evaluate if it affected iMDDC maturation after 48 h.
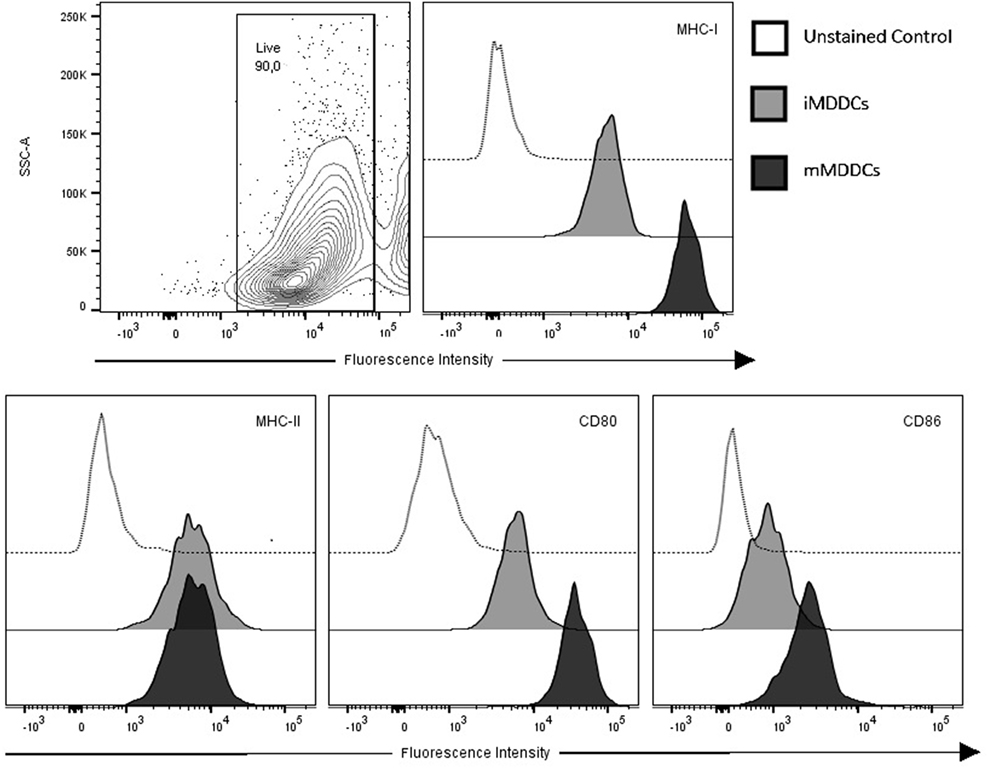
Figure 5. Monocyte-derived dendritic cells (MDDCs) upregulate surface expression of major histocompatibility complex (MHC)-I, CD80, and CD86 during maturation. Contour plot of live cell population and flow cytometry analysis of surface expressions of MHC-I, MHC-II, CD80, and CD86 on immature MDDCs and mature MDDCs. Histograms of a representative animal are shown (n = 7).
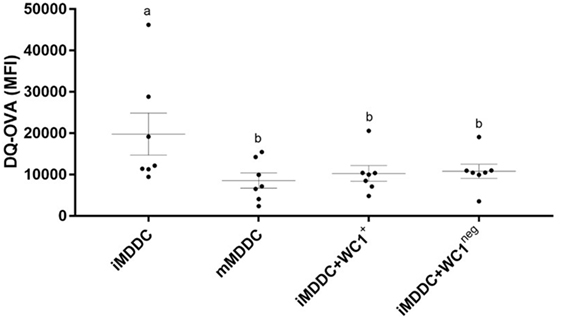
Figure 6. γδ T lymphocytes induce monocyte-derived dendritic cell (MDDC) maturation. DQ-ovalbumin endocytosis assay on immature MDDC (iMDDC), mature MDDC (mMDDC), iMDDC + WC1+, and iMDDC + WC1neg. iMDDC + WC1+ and iMDDC + WC1neg had a reduction in their phagocytic ability in the same proportion as mMDDC. Results are individual animal data points and means ± SEM of seven animals. Different letters indicate statistically significant difference (p < 0.05).
WC1neg γδ T Lymphocytes Increase MHC-II Expression on iMDDCs and Both γδ T Lymphocyte Subsets Reduce Phagocytic Capacity of iMDDCs
To determine the effect of WC1+ and WC1neg γδ T lymphocytes on maturation of MDDCs, sorted γδ T lymphocyte subsets were added to iMDDCs for 48 h (Figure 4). iMDDC + WC1neg had significantly increased expression of MHC-II compared with both iMDDC (p = 0.0013) and mMDDC (p = 0.0046) (Figure 7). Presence of γδ T lymphocytes did not affect the expression of CD80, CD86, and MHC-I on mMDDCs at 48 h post Map infection (data not shown). An interesting finding was increased individual variation of expression of MHC-II on mMDDCs compared with all iMDDCs, iMDDC + WC1+, and iMDDC + WC1neg, which may suggest that the DC maturation process varies widely between animals. To study the effects of WC1+ and WC1neg γδ T lymphocytes on the maturation of MDDCs, γδ T lymphocytes were added to cultures of iMDDC for 48 h and then the DQ-OVA phagocytosis assay was performed using flow cytometry to measure changes in phagocytic ability. iMDDCs cultured in the presence of either WC1+ or WC1neg γδ T lymphocytes had significantly reduced phagocytic ability compared to iMDDCs cultured without γδ T lymphocytes (p = 0.0111 and 0.0174, respectively) (Figure 6). These findings indicate that both γδ T lymphocyte subsets reduce phagocytosis by iMDDCs in our model.
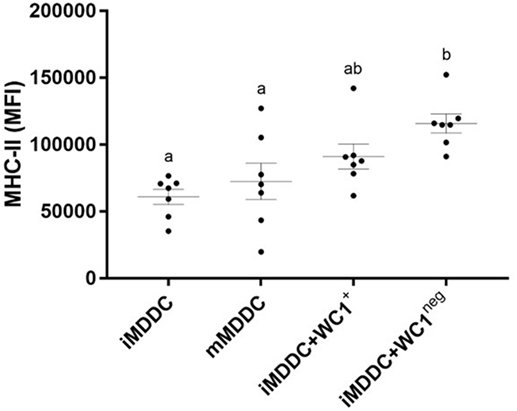
Figure 7. WC1neg γδ T lymphocytes increase the antigen presenting ability of immature monocyte-derived dendritic cells (iMDDCs). Median fluorescence intensity of major histocompatibility complex-II on iMDDCs, mature MDDCs, iMDDCs + WC1+, and iMDDCs + WC1neg. Results are individual animal data points and means ± SEM of seven animals. Different letters indicate statistically significant difference (p < 0.05).
Live Map Was Associated with Significantly Increased Expression of MHC-I on iMDDC + WC1neg
To determine the effect of Map on maturation of MDDCs, live Map was added to cultures of iMDDC, iMDDC + WC1+, and iMDDC + WC1neg for 48 h (Figure 4). The presence of live Map was associated with significantly increased expression of MHC-I on iMDDC + WC1neg compared to iMDDC + WC1neg unexposed to Map (p = 0.0090) (Figure 8). The presence of live Map did not affect the expression of CD80, CD86, and MHC-II on MDDCs at 48 h post Map infection (data not shown).
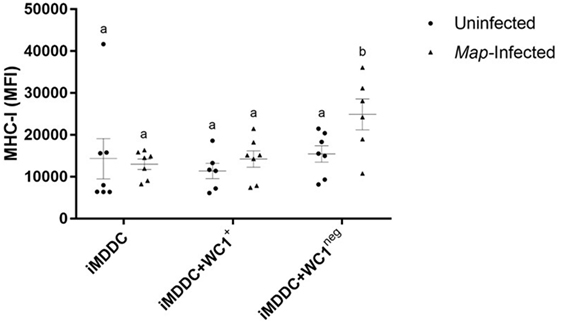
Figure 8. Live Mycobacterium avium subspecies paratuberculosis (Map) was associated with significantly increased expression of major histocompatibility complex (MHC)-I on immature monocyte-derived dendritic cell (iMDDC) + WC1neg. Infection with live Map was associated with significantly increased expression of MHC-I on iMDDC + WC1neg. Median fluorescence intensity of MHC-I on iMDDCs, iMDDCs + WC1+, and iMDDCs + WC1neg. Results are individual animal data points and means ± SEM of seven animals. Different letters indicate statistically significant difference (p < 0.05).
Discussion
During the development of host responses against pathogens, monocytes are recruited to the site of infection where they differentiate into effector cells amidst crosstalk with resident tissue immune cells (35, 36). After encountering an antigen, immature DCs begin their maturation process, migrate to local draining lymph nodes where they present antigen to naïve T lymphocytes to initiate, or perpetuate antigen-specific immune responses (37, 38). γδ T lymphocytes are resident sentinel cells in a variety of mucosal surfaces but especially in the ileum, where infection with Map is generally assumed to initially occur (39). In this study, we sought to first define and characterize MPS cells from young calves; using that information, we then set out to determine how WC1+ and WC1neg γδ T lymphocytes affect (1) monocyte differentiation and (2) DC maturation, both processes important to initiation and propagation of effective immune responses during infection by Map and other pathogens. For monocyte differentiation experiments, sorted peripheral blood derived WC1+ or WC1neg γδ T lymphocytes were cocultured with freshly isolated autologous monocytes for 6 days so that their phenotype and function could be compared with the experimental controls previously defined: monocytes, MDMs, and MDDCs. For DC maturation experiments, sorted peripheral blood WC1+ or WC1neg γδ T lymphocytes were cocultured for 48 h with iMDDCs and then compared with iMDDCs (without γδ T lymphocytes) and mMDDCs (obtained after stimulation of iMDDCs with LPS for 48 h).
Cells from the MPS share precursors as well as several surface markers and functions which makes it difficult to clearly distinguish between them (6). We show that freshly isolated peripheral blood monocytes express low levels of CD14, CD11b, CD11c, and CD172a and lack expression of CD163, CD1b, and CD205. These data are consistent with a recent review showing that bovine monocytes express CD172a but lack expression of CD1b and CD205 [reviewed in Ref. (8)]. Other studies have defined three distinct phenotypic bovine monocyte subsets based on their variable surface expression of CD14 and CD16 among CD172a+ cells (40). Expression of CD11c and CD172a is considered constitutive in bovine monocyte subsets, while expression of CD11b is variable (41). We did not find significant expression of CD163 on monocytes in our study; however, expression of CD163 has been described by others in bovine monocytes (41). A possible explanation for these contradictory findings is the utilization of different clones of the CD163 monoclonal antibody. We used a murine anti-bovine clone (LND68A) while Corripio-Miyar et al. (41) used human clone (EDHu-1); potential concerns regarding interspecies cross-reactivity of monoclonal antibodies have been published (42).
In our model CD1b, CD11c, CD14, and CD205 were all upregulated following in vitro differentiation of monocytes; however, these particular markers do not reliably distinguish MDMs from MDDCs. Our data do suggest that MDMs can be phenotypically distinguished from MDDCs because of significantly higher expression of CD11b, CD163, and CD172a on MDMs compared to MDDCs. Bovine MDDCs have been described as CD172a+ while the expression of CD1b, CD11b, CD14, and CD205 vary depending on the subset of MDDC [reviewed in Ref. (8)]. MDMs have historically been classified by phenotype and function using surface-marker expression and cytokine-secretion profiles, respectively, but more recently classification of MDMs as either classically (M1) and alternatively activated (M2) macrophages using expression of CD163 has been described. M1 macrophages are CD163− and secrete pro-inflammatory cytokines while M2 macrophages are CD163+ and secrete low levels of pro-inflammatory cytokines and high levels of IL-10 (43). In our in vitro model, we neither identified CD163− populations of MDMs nor assessed cytokine concentration in supernatants. Thus, further research is required to characterize and classify cells of the bovine MPS under different isolation (a.k.a. magnetic beads, FACS, adherence), culture conditions in vitro and evaluating other relevant surface markers such as CD209 (DC-SIGN) (44), CD16 (41), CD68 (45), and CD11a (46). Regardless, our data support the basic hypothesis that MPS cells comprise a complex network of distinct cell subsets that though they share some overlapping phenotypic and functional characteristics, they polarize depending on the local microenvironment for specific functions (47).
After coculturing freshly isolated blood monocytes with sorted WC1+ and WC1neg γδ T lymphocytes (dMonWC1+ and dMonWC1neg, respectively) during the differentiation process, our data indicate that the presence of γδ T lymphocytes has no phenotype-altering effect on monocyte differentiation. Viability of Map recovered from dMonWC1neg, however, was significantly reduced suggesting that the presence of WC1neg γδ T lymphocytes improves the ability of differentiated monocytes (dMonWC1neg) to limit Map viability. In a previous study, we showed that the presence of either WC1+ or WC1neg γδ T lymphocytes cocultured with autologous Map-infected MDMs from 30- to 40-day-old calves was associated with reduced viability of Map recovered from MDMs (26). Taking into account that (1) in the previous study, MDMs were considered fully differentiated prior to the addition of γδ T lymphocytes into cocultures, (2) in the current study, significant differences were observed between the viability of Map recovered from dMonWC1+ and dMonWC1neg, and (3) when Map was introduced in the current study, WC1+ and WC1neg γδ T lymphocytes had been removed; we hypothesize that dMonWC1neg are distinct from classical MDMs, and that WC1neg γδ T lymphocytes alter the functional differentiation of peripheral blood monocytes. Based on these data, our hypothesis is that WC1neg γδ T lymphocytes have a direct effect on transcription factor expression during monocyte differentiation resulting in a monocyte-derived cell with increased ability to limit Map viability. Because we have observed that the number of γδ T lymphocyte subsets within tissues of mucosal surfaces is variable between calves (unpublished data), we hypothesize that inter-animal variability also influences the different immune responses and disease outcomes that can be commonly observed within groups of calves (i.e., a herd with endemic Map infection). This inter-animal variability might explain why some animals (i.e., those with more WC1neg γδ T lymphocytes or those that have more cognate γδ T lymphocyte/MPS cell interactions in the ileum) clear Map infection and do not progress to later stages of bovine paratuberculosis. Further studies are required to characterize the distribution and function of bovine γδ T lymphocytes in different mucosal and non-mucosal tissues.
To determine how γδ T lymphocyte subsets affect DC maturation, we first defined the characteristics of both iMDDC and mMDDC in our system by evaluating expression of specific maturation markers and co-stimulatory molecules. As expected and based on the current literature, our data confirm that MHC-I, CD80, and CD86 are useful markers to differentiate mMDDCs from iMDDCS because they are upregulated during the maturation process. Other bovine models have shown that Salmonella typhimurium-infected DCs had significantly increased expression of MHC-I, MHC-II, CD40, CD80, and CD86 (48). In our system, MHC-II was not upregulated on mMDDCs at 48 h post Map infection; however, the expression of MHC-II was highly variable between animals in our study. Inter-animal immune cell phenotypic variability has been well established in bovine studies of our lab and others (25, 26, 49), and the distinct pattern of high/low-effector functions and specific phenotypes in cattle is complex but probably explains an individual’s unique ability to respond (or not) to infection. Timing may also have influenced our ability to detect early and transient changes in MHC-II expression. It is known that after stimulation of murine DC PAMPs receptors, MHC-II expression increases transiently but then decreases (50), though this phenomenon has not been shown in bovine DCs. Furthermore, it is known that MHC-II expression on bovine MDMs is downregulated between 24 and 48 h post Map infection (51) and because cells in this study were analyzed at only a single time point (48 h after Map infection), we may have thus been unable to detect early differences in the expression of MHC-II due to timing.
The presence of WC1neg γδ T lymphocytes in our coculture experiment was associated with significantly increased expression of MHC-II on iMDDC + WC1neg cells compared with iMDDCs and mMDDCs. Upregulation of MHC-II, along with CD86 and CD83 has also been induced by human Vγ9Vδ2 T lymphocytes on DCs, suggesting the ability of γδ T lymphocytes to specifically promote DC maturation (17). Furthermore, human iMDDCs induce Vγ9Vδ2 T lymphocytes to secrete pro-inflammatory cytokines required for their own maturation (52). This reciprocal effect has not been definitively demonstrated in cattle, and further research is required to determine if bovine DCs could induce this effect on either WC1+ and/or WC1neg γδ T lymphocytes.
A major functional change during MDDC maturation is reduced phagocytic capacity of MDDCs; this finding is supported by studies in adult cows (28). In our study, a reduced phagocytic capacity was observed in iMMDCs cocultured with either WC1+ or WC1neg γδ T lymphocyte subsets, which suggests that WC1+ or WC1neg γδ T lymphocytes induce “functional” MDDC maturation with respect to phagocytic capacity.
Our overall hypothesis was that early γδ T lymphocytes/MPS/Map interactions influence the initiation of early local host immunity and potentially the induction of adaptive immunity and progression or eventual outcome of Map infection. To test our hypothesis, we first needed to define the phenotype and effector functions of MPS cells specifically under in vitro conditions in our laboratory. We have shown that cells from the MPS can be distinguished by collective examination of phenotype and function: (1) monocytes lack the expression of CD1b, CD205, and CD163; (2) MDMs express higher levels of CD11b, CD163, and CD172a compared to MDDCs; (3) mMDDCs express higher levels of CD11c, CD80, and CD86 compared to iMDDC; mMDDCs have reduced phagocytic capacity compared to iMDDCs. In this study, the most significant findings related to the effect of γδ T lymphocytes include: (1) the presence of WC1neg γδ T lymphocytes contributes to differentiation of monocytes into cells with increased ability to limit Map viability and (2) both γδ T lymphocyte subsets induce functional MDDC maturation (reduced phagocytosis).
Ethics Statement
All animal procedures in this study were approved by the Institutional Committee on Animal Care at the University of Guelph (Animal Utilization Protocol # 3373).
Author Contributions
MMB and BLP performed the experiments; designed the experiments; interpreted the data; drafted the manuscript; reviewed and approved the final version of the manuscript; agreed to be accountable for the content of the work.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank John F. Prescott, Lucy Mutharia, Stefan Keller and Shayan Sharif for their scientific advice and Laura Wright for her collaboration during the blood collection.
Funding
This work was funded by The National Science and Engineering Research Council (NSERC) of Canada. We acknowledge The Vanier Canada Graduate Scholarship and the Colombian Administrative Department of Science, Technology and Innovation (Colciencias) for their financial assistance.
Supplementary Material
The Supplementary Material for this article can be found online at http://journal.frontiersin.org/article/10.3389/fimmu.2017.00534/full#supplementary-material.
References
1. Hume DA. The mononuclear phagocyte system. Curr Opin Immunol (2006) 18:49–53. doi: 10.1016/j.coi.2005.11.008
2. Hume DA, Ross IL, Himes SR, Sasmono RT, Wells CA, Ravasi T. The mononuclear phagocyte system revisited. J Leukoc Biol (2002) 72:621–7.
3. Wu L, Li CL, Shortman K. Thymic dendritic cell precursors: relationship to the T lymphocyte lineage and phenotype of the dendritic cell progeny. J Exp Med (1996) 184:903–11. doi:10.1084/jem.184.3.903
4. Manz MG, Traver D, Akashi K, Merad M, Miyamoto T, Engleman EG, et al. Dendritic cell development from common myeloid progenitors. Ann N Y Acad Sci (2001) 938:167–73; discussion 173–4. doi:10.1111/j.1749-6632.2001.tb03586.x
5. Jenkins SJ, Hume DA. Homeostasis in the mononuclear phagocyte system. Trends Immunol (2014) 35:358–67. doi:10.1016/j.it.2014.06.006
6. Strauss O, Dunbar PR, Bartlett A, Phillips A. The immunophenotype of antigen presenting cells of the mononuclear phagocyte system in normal human liver—a systematic review. J Hepatol (2015) 62:458–68. doi:10.1016/j.jhep.2014.10.006
7. Haniffa M, Bigley V, Collin M. Human mononuclear phagocyte system reunited. Semin Cell Dev Biol (2015) 41:59–69. doi:10.1016/j.semcdb.2015.05.004
8. Summerfield A, Auray G, Ricklin M. Comparative dendritic cell biology of veterinary mammals. Annu Rev Anim Biosci (2015) 3:533–57. doi:10.1146/annurev-animal-022114-111009
9. Rogers AN, Vanburen DG, Hedblom EE, Tilahun ME, Telfer JC, Baldwin CL.Gammadelta T cell function varies with the expressed WC1 coreceptor. J Immunol (2005) 174:3386–93. doi:10.4049/jimmunol.174.6.3386
10. Hedges JF, Cockrell D, Jackiw L, Meissner N, Jutila MA. Differential mRNA expression in circulating gammadelta T lymphocyte subsets defines unique tissue-specific functions. J Leukoc Biol (2003) 73:306–14. doi:10.1189/jlb.0902453
11. Hedges JF, Lubick KJ, Jutila MA. γδ T cells respond directly to pathogen-associated molecular patterns. J Immunol (2005) 174:6045–53. doi:10.4049/jimmunol.174.10.6045
12. Vantourout P, Hayday A. Six-of-the-best: unique contributions of γδ T cells to immunology. Nat Rev Immunol (2013) 13:88–100. doi:10.1038/nri3384
13. Tanaka Y, Morita CT, Tanaka Y, Nieves E, Brenner MB, Bloom BR. Natural and synthetic non-peptide antigens recognized by human γδ T cells. Nature (1995) 375:155–8. doi:10.1038/375155a0
14. Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human gammadelta T cells. Science (2005) 309:264–8. doi:10.1126/science.1110267
15. Hartwig T, Pantelyushin S, Croxford AL, Kulig P, Becher B. Dermal IL-17-producing γδ T cells establish long-lived memory in the skin. Eur J Immunol (2015) 45:3022–33. doi:10.1002/eji.201545883
16. Murphy AG, O’Keeffe KM, Lalor SJ, Maher BM, Mills KHG, McLoughlin RM. Staphylococcus aureus infection of mice expands a population of memory γδ T cells that are protective against subsequent infection. J Immunol (2014) 192:3697–708. doi:10.4049/jimmunol.1303420
17. Ismaili J, Olislagers V, Poupot R, Fournié J-J, Goldman M. Human gamma delta T cells induce dendritic cell maturation. Clin Immunol (2002) 103:296–302. doi:10.1006/clim.2002.5218
18. Rhodes SG, Hewinson RG, Vordermeier HM. Antigen recognition and immunomodulation by gamma delta T cells in bovine tuberculosis. J Immunol (2001) 166:5604–10. doi:10.4049/JIMMUNOL.166.9.5604
19. Baldwin CL, Sathiyaseelan T, Rocchi M, McKeever D. Rapid changes occur in the percentage of circulating bovine WC1(+)gamma delta Th1 cells. Res Vet Sci (2000) 69:175–80. doi:10.1053/rvsc.2000.0410
20. Plattner BL, Huffman E, Jones DE, Hostetter JM. T lymphocyte responses during early enteric Mycobacterium avium subspecies paratuberculosis infection in cattle. Vet Immunol Immunopathol (2014) 157:12–9. doi:10.1016/j.vetimm.2013.11.001
21. Zarin P, Chen ELY, In TSH, Anderson MK, Zúñiga-Pflücker JC. Gamma delta T-cell differentiation and effector function programming, TCR signal strength, when and how much? Cell Immunol (2015) 296:70–5. doi:10.1016/j.cellimm.2015.03.007
22. Plattner BL, Doyle RT, Hostetter JM. Gamma–delta T cell subsets are differentially associated with granuloma development and organization in a bovine model of mycobacterial disease. Int J Exp Pathol (2009) 90:587–97. doi:10.1111/j.1365-2613.2009.00679.x
23. Hoek A, Rutten VP, Kool J, Arkesteijn GJ, Bouwstra RJ, Van Rhijn I, et al. Subpopulations of bovine WC1(+) gammadelta T cells rather than CD4(+)CD25(high) Foxp3(+) T cells act as immune regulatory cells ex vivo. Vet Res (2009) 40:6. doi:10.1051/vetres:2008044
24. Guzman E, Hope J, Taylor G, Smith AL, Cubillos-Zapata C, Charleston B. Bovine γδ T cells are a major regulatory T cell subset. J Immunol (2014) 193:208–22. doi:10.4049/jimmunol.1303398
25. Baquero MM, Plattner BL. Bovine WC1(+) γδ T lymphocytes modify monocyte-derived macrophage responses during early Mycobacterium avium subspecies paratuberculosis infection. Vet Immunol Immunopathol (2016) 170:65–72. doi:10.1016/j.vetimm.2015.12.002
26. Baquero MM, Plattner BL. Bovine peripheral blood WC1+ and WC1neg γδ T lymphocytes modulate monocyte-derived macrophage effector functions during in vitro Mycobacterium avium subspecies paratuberculosis infection. Cell Immunol (2017) 315:34–44. doi:10.1016/j.cellimm.2017.01.009
27. Subharat S, Shu D, Wedlock DN, Price-Carter M, de Lisle GW, Luo D, et al. Immune responses associated with progression and control of infection in calves experimentally challenged with Mycobacterium avium subsp. paratuberculosis. Vet Immunol Immunopathol (2012) 149:225–36. doi:10.1016/j.vetimm.2012.07.005
28. Lei L, Hostetter JM. Limited phenotypic and functional maturation of bovine monocyte-derived dendritic cells following Mycobacterium avium subspecies paratuberculosis infection in vitro. Vet Immunol Immunopathol (2007) 120:177–86. doi:10.1016/j.vetimm.2007.06.031
29. Fernández-Silva JA, Abdulmawjood A, Akineden Ö, Dräger K, Klawonn W, Bülte M. Molecular epidemiology of Mycobacterium avium subsp. paratuberculosis at a regional scale in Germany. Res Vet Sci (2012) 93:776–82. doi:10.1016/j.rvsc.2011.12.005
30. Mitchell RM, Gollnick NS, Sreevatsan S, Russell DG, Schukken YH. Quantification of Mycobacterium avium subsp. paratuberculosis (MAP) survival in monocyte-derived macrophages. Vet Immunol Immunopathol (2011) 139(1):73–8. doi:10.1016/j.vetimm.2010.08.003
31. Machugh ND, Mburu JK, Carol MJ, Wyatt CR, Orden JA, Davis WC. Identification of two distinct subsets of bovine gamma delta T cells with unique cell surface phenotype and tissue distribution. Immunology (1997) 92:340–5. doi:10.1046/j.1365-2567.1997.00350.x
32. Pinchuk L, Boyd B, Kruger E, Roditi I, Furger A. Bovine dendritic cells generated from monocytes and bone marrow progenitors regulate immunoglobulin production in peripheral blood B cells. Comp Immunol Microbiol Infect Dis (2003) 26:233–49. doi:10.1016/S0147-9571(02)00061-9
33. De Smedt T, Pajak B, Muraille E, Lespagnard L, Heinen E, De Baetselier P, et al. Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo. J Exp Med (1996) 184:1413–24. doi:10.1084/jem.184.4.1413
34. Strober W. Trypan blue exclusion test of cell viability. Curr Protoc Immunol (2001) Appendix 3:Appendix 3B. doi:10.1002/0471142735.ima03bs21
35. Yoshihara K, Nagata R, Muneta Y, Inumaru S, Yokomizo Y, Mori Y. Generation of multinucleated giant cells in vitro from bovine monocytes and macrophages. J Vet Med Sci (2004) 66:1065–9. doi:10.1292/jvms.66.1065
36. Ingersoll MA, Platt AM, Potteaux S, Randolph GJ. Monocyte trafficking in acute and chronic inflammation. Trends Immunol (2011) 32:470–7. doi:10.1016/j.it.2011.05.001
37. Hope JC, Whelan AO, Hewinson R, Vordermeier M, Howard CJ. Maturation of bovine dendritic cells by lipopeptides. Vet Immunol Immunopathol (2003) 95:21–31. doi:10.1016/S0165-2427(03)00104-1
38. Alexis NE, Lay JC, Almond M, Bromberg PA, Patel DD, Peden DB. Acute LPS inhalation in healthy volunteers induces dendritic cell maturation in vivo. J Allergy Clin Immunol (2005) 115:345–50. doi:10.1016/j.jaci.2004.11.040
39. Koets A, Rutten V, Hoek A, van Mil F, Müller K, Bakker D, et al. Progressive bovine paratuberculosis is associated with local loss of CD4(+) T cells, increased frequency of gamma delta T cells, and related changes in T-cell function. Infect Immun (2002) 70:3856–64. doi:10.1128/IAI.70.7.3856-3864.2002
40. Hussen J, Düvel A, Sandra O, Smith D, Sheldon IM, Zieger P, et al. Phenotypic and functional heterogeneity of bovine blood monocytes. PLoS One (2013) 8:e71502. doi:10.1371/journal.pone.0071502
41. Corripio-Miyar Y, Hope J, McInnes CJ, Wattegedera SR, Jensen K, Pang Y, et al. Phenotypic and functional analysis of monocyte populations in cattle peripheral blood identifies a subset with high endocytic and allogeneic T-cell stimulatory capacity. Vet Res (2015) 46:112. doi:10.1186/s13567-015-0246-4
42. Cummings HS, Ploplis VA, Beals JM, Castellino FJ. Interspecies cross-reactivity of monoclonal antibodies to various epitopes of human plasminogen. Arch Biochem Biophys (1984) 230:306–15. doi:10.1016/0003-9861(84)90112-7
43. Verreck FAW, de Boer T, Langenberg DML, van der Zanden L, Ottenhoff THM. Phenotypic and functional profiling of human proinflammatory type-1 and anti-inflammatory type-2 macrophages in response to microbial antigens and IFN-gamma- and CD40L-mediated costimulation. J Leukoc Biol (2006) 79:285–93. doi:10.1189/jlb.0105015
44. Park KT, Burnett S, Davis WC. Development and characterization of a monoclonal antibody specific for bovine CD209. Vet Immunol Immunopathol (2015) 163(3):216–20. doi:10.1016/j.vetimm.2014.12.008
45. Kitani H, Yoshioka M, Takenouchi T, Sato M, Yamanaka N. Isolation and characterization of macrophages from a mixed primary culture of bovine liver cells. Vet Immunol Immunopathol (2011) 140(3):341–5. doi:10.1016/j.vetimm.2011.01.011
46. Hussen J, Koy M, Petzl W, Schuberth H-J. Neutrophil degranulation differentially modulates phenotype and function of bovine monocyte subsets. Innate Immun (2016) 22:124–37. doi:10.1177/1753425915620911
47. Mansouri-Attia N, Oliveira LJ, Forde N, Fahey AG, Browne JA, Roche JF, et al. Pivotal role for monocytes/macrophages and dendritic cells in maternal immune response to the developing embryo in cattle. Biol Reprod (2012) 87:123. doi:10.1095/biolreprod.112.101121
48. Norimatsu M, Harris J, Chance V, Dougan G, Howard CJ, Villarreal-Ramos B. Differential response of bovine monocyte-derived macrophages and dendritic cells to infection with Salmonella typhimurium in a low-dose model in vitro. Immunology (2003) 108:55–61. doi:10.1046/j.1365-2567.2003.01557.x
49. Umemura M, Yahagi A, Hamada S, Begum MD, Watanabe H, Kawakami K, et al. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J Immunol (2007) 178:3786–96. doi:10.4049/jimmunol.178.6.3786
50. Casals C, Barrachina M, Serra M, Lloberas J, Celada A. Lipopolysaccharide up-regulates MHC class II expression on dendritic cells through an AP-1 enhancer without affecting the levels of CIITA. J Immunol (2007) 178:6307–15. doi:10.4049/JIMMUNOL.178.10.6307
51. Weiss DJ, Evanson OA, Mcclenahan DJ, Abrahamsen MS, Walcheck BK. Regulation of expression of major histocompatibility antigens by bovine macrophages infected with Mycobacterium avium subsp. paratuberculosis or Mycobacterium avium subsp. avium. Infect Immun (2001) 69:1002–8. doi:10.1128/IAI.69.2.1002-1008.2001
Keywords: Mycobacterium avium subspecies paratuberculosis, γδ T lymphocytes, WC1, macrophages, monocytes, dendritic cells, mononuclear phagocyte system
Citation: Baquero MM and Plattner BL (2017) Bovine WC1+ and WC1neg γδ T Lymphocytes Influence Monocyte Differentiation and Monocyte-Derived Dendritic Cell Maturation during In Vitro Mycobacterium avium Subspecies paratuberculosis Infection. Front. Immunol. 8:534. doi: 10.3389/fimmu.2017.00534
Received: 09 March 2017; Accepted: 21 April 2017;
Published: 22 May 2017
Edited by:
Geanncarlo Lugo-Villarino, UMR5089 Institut de Pharmacologie et de Biologie Structurale (IPBS), FranceReviewed by:
Paul M. Coussens, Michigan State University, USAJayne Hope, University of Edinburgh, UK
Copyright: © 2017 Baquero and Plattner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Monica M. Baquero, mbaquero@uoguelph.ca
 Monica M. Baquero
Monica M. Baquero Brandon L. Plattner
Brandon L. Plattner