
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 28 June 2017
Sec. Primary Immunodeficiencies
Volume 8 - 2017 | https://doi.org/10.3389/fimmu.2017.00735
This article is part of the Research Topic Primary Immunodeficiencies Worldwide View all 23 articles
The global burden of fungal diseases has been increasing, as a result of the expanding number of susceptible individuals including people living with human immunodeficiency virus (HIV), hematopoietic stem cell or organ transplant recipients, patients with malignancies or immunological conditions receiving immunosuppressive treatment, premature neonates, and the elderly. Opportunistic fungal pathogens such as Aspergillus, Candida, Cryptococcus, Rhizopus, and Pneumocystis jiroveci are distributed worldwide and constitute the majority of invasive fungal infections (IFIs). Dimorphic fungi such as Histoplasma capsulatum, Coccidioides spp., Paracoccidioides spp., Blastomyces dermatiditis, Sporothrix schenckii, Talaromyces (Penicillium) marneffei, and Emmonsia spp. are geographically restricted to their respective habitats and cause endemic mycoses. Disseminated histoplasmosis, coccidioidomycosis, and T. marneffei infection are recognized as acquired immunodeficiency syndrome (AIDS)-defining conditions, while the rest also cause high rate of morbidities and mortalities in patients with HIV infection and other immunocompromised conditions. In the past decade, a growing number of monogenic immunodeficiency disorders causing increased susceptibility to fungal infections have been discovered. In particular, defects of the IL-12/IFN-γ pathway and T-helper 17-mediated response are associated with increased susceptibility to endemic mycoses. In this review, we put together the various forms of endemic mycoses on the map and take a journey around the world to examine how cellular and molecular defects of the immune system predispose to invasive endemic fungal infections, including primary immunodeficiencies, individuals with autoantibodies against interferon-γ, and those receiving biologic response modifiers. Though rare, these conditions provide importance insights to host defense mechanisms against endemic fungi, which can only be appreciated in unique climatic and geographical regions.
Endemic mycoses are infections caused by a diverse group of fungi that occupy specific ecologic niche in the environment (1). The major pathogenic fungi in this group, including Blastomyces dermatiditis, Coccidioides immitis and C. posadasii, Paracoccidioides brasiliensis and P. lutzii, Histoplasma capsulatum, Sporothrix schenckii, Talaromyces marneffei (formerly known as Penicillium marneffei) and Emmonsia spp., belong to the phylum Ascomycota and are evolutionary related (2) (Table 1). They share the common characteristic of thermal dimorphism—they grow as saprophytic molds in the environment at temperatures ranging from 25 to 30°C, and undergo morphological switch to the yeast form, or spherules in Coccidioides, at body temperatures of mammalian hosts. The yeast form serves to accommodate intracellular growth within host phagocytes (3). Majority of these organisms are primary pathogens that are able to cause disease in healthy human individuals. However, they may cause severe, disseminated infections in immunocompromised hosts, such as patients with human immunodeficiency virus (HIV) infection, organ transplant, or hematopoietic stem cell transplant (HSCT) recipients, and those with autoimmune disorders receiving immunosuppressants (4–7). In particular, T. marneffei and Emmonsia spp. more typically cause disease in HIV-infected individuals (5–8). The HIV pandemic and the increasing use of immunosuppressive medications, such as calcineurin and tumor necrosis factor (TNF) inhibitors, have resulted in a rising trend of histoplasmosis and coccidioidomycosis in endemic regions (9, 10). Exposure to the specific environmental niche, either residential, occupational, or travel precedes the development of disease.
Endemic mycoses occur predominantly in specific climate zones. Coccidioidomycosis is present in semidesert areas, histoplasmosis and paracoccidioidomycosis (PCM) are prevalent in tropical regions while T. marneffei is endemic in subtropical regions, and blastomycosis belongs to temperate climates (6–8). Coccidioioidomycosis (11–13) and histoplasmosis (14–18) are widely distributed in the American continent and some tropical regions, while PCM is limited to Central and South America (19–21). T. marneffei is unique to Southeast Asia (22–24), and blastomycosis is found in North America, and Central and East Africa (25–27). S. schenckii is distributed around the world, mainly reported in those tropical and temperate zones with high humidity and mild temperatures (22–27°C) (28). Recently, an emerging thermally dimorphic fungus within the genus Emmonsia that is most closely related to E. pasteuriana has been recognized to be uniquely associated with in HIV infection in South Africa (29–31). The geographical distribution of endemic mycoses is shown in Figure 1 (1, 12–14, 18–20, 27, 28, 32–39).
The biological niche is specific for each endemic fungus and knowledge of their natural habitat provides understanding about the risk factors for exposure to these pathogens (Table 2). C. immitis and C. posadasii are saprophytic fungi, which exist in their mycelial form in dry, alkaline soil in deserts with very low precipitation and extreme temperature variations (11–13). Coccidioidomycosis is most prevalent in Arizona and California in the United States (US) (40, 41). In contrast, H. capsulatum thrive in the tropical zones with high relative humidity, and its growth is favored by soil contaminated by bird and chicken excreta or bat guano, which creates an environment with high nitrogen content (14, 15). P. brasiliensis is also found in the tropical and very humid regions, especially in acidic soil where coffee and sugar canes are cultivated (20). PCM is prevalent in South America (Brazil, Columbia, Venezuela, Paraguay) and some regions of Central America and Mexico (19–21). B. dermatitidis exists in wet soils, and the most significant endemic epicenter is in Eastern US between the Ohio and Mississippi River valleys (25–27). T. marneffei is highly endemic in Thailand, Vietnam, Southern China, and other subtropical areas in Southeast Asia (22–24). Bamboo rats (Rhizomys spp. and Cannomys spp.) and soil from their burrows are important enzootic and environmental reservoirs of T. marneffei, respectively (39). S. schenckii is found in the soil containing decaying vegetation such as dead wood, mosses, hay, and cornstalks. Sporotrichosis is also widely prevalent in warm-blooded animals including cats, dogs, armadillos, birds, and parrots, which constitute a source of zoonotic transmission (28). To date, disseminated emmonsiosis associated with HIV infection caused by the new Emmonsia spp. has only been described in South Africa (29–31).
The acquisition of endemic fungi relates to human activities and climatic conditions that increase the risk of exposure to these organisms in susceptible individuals. Coccidioidomycosis, PCM, histoplasmosis, blastomycosis, and T. marneffei infection are acquired by the respiratory route. Occupational or recreational activities causing disturbance of the soil environment lead to aerosolization of the conidia, which could then be inhaled by exposed individuals to cause infection (1, 6–8, 14, 20, 22, 23, 27). In contrast, inhalation is not the major route by which S. schenkii is acquired. Instead, it typically occurs after traumatic inoculation or through microscopic breaks in the skin caused by pricks with plants, although the mode of transmission was not obvious in 60% of patients with sporotrichosis. Infection of the skin and subcutaneous tissues develops at the site of penetrating trauma and may spread to the muscles, fascia, cartilage, and bones (42, 43). The clinical features of these endemic mycoses are summarized in Table 3.
After entry to the body, inhaled conidia converts to the yeast form, which are taken up by tissue-resident macrophages. In most individuals, the pathogenic yeasts can be eliminated by the macrophages and the infection is usually asymptomatic or mild, and self-limiting in most cases. In patients whose immunity is compromised, the yeasts continue to proliferate in the macrophages, which if uncontained, systemic dissemination may occur via the reticuloendothelial system (12, 23, 44–46). HIV infection is the most important risk factor for disseminated endemic mycoses (5). Other risk factors include malignancy and immunosuppression, as listed in Table 3 (4–7, 9, 10).
Human immunodeficiency virus infection is the most common cause for disseminated or extrapulmonary forms of histoplasmosis, coccidioidomycosis, and T. marneffei infection, particularly in patients with profound T-cell lymphopenia (CD4+ lymphocytes <200/μL) (12, 47–50). They are considered as acquired immunodeficiency syndrome (AIDS)-defining conditions in the World Health Organization (WHO) clinical staging of HIV/AIDS for adults and adolescents (51). The association between PCM and AIDS is relatively rare in contrast to the higher incidence of other systemic mycosis. HIV coinfection has been detected in about 5% of patients with PCM (52, 53). Although PCM and sporotrichosis are not included as AIDS-defining conditions, they are increasingly recognized as an emerging neglected opportunistic infections in HIV patients in Latin America (7, 53, 54). Systemic sporotrichosis with organ involvement or widespread cutaneous lesions may occur in these patients, causing a mortality rate of up to 30% (54, 55). Blastomycosis infrequently develops in HIV patients, but disease tends to be more severe with increased risk of central nervous system (CNS) involvement with high mortality (46, 56). The new species of Emmonsia spp. discovered in South Africa was found to cause disseminated infection almost exclusively in patients with AIDS (29–31).
The first case series of histoplasmosis in HIV-infected patients was described in 1982 in the US (57). Extrapulmonary or disseminated histoplasmosis became an AIDS-defining disease in 1987 (58). The increase in morbidity and mortality from histoplasmosis has been largely contributed by the HIV pandemic. In the US, the availability of highly active anti-retroviral therapy (HAART) and lipid formulations of amphotericin B, the increased awareness of the disease, and the development of rapid, non-invasive diagnostic methods led to decrease in the incidence and mortality associated with histoplasmosis in patients with AIDS (16). On the other hand, extrapulmonary or disseminated histoplasmosis is becoming an important health issue in the increasing number of patients receiving chemotherapy, solid organ or HSCT, immunosuppressive treatment especially TNF-α blockade (59), as well as a rare group of patients with primary immunodeficiencies (PIDs). In contrast, in endemic areas of Latin America histoplasmosis occurs in up to 25% of HIV-infected patients and represents the first manifestation of AIDS in up to 50–75% of patients (60, 61). Due to the lack of diagnostic facilities and algorithm, histoplasmosis is undiagnosed in many HIV-infected patients and is considered as an “invisible burden” of AIDS in less resourced countries (62, 63). These phenomena illustrate how demographics of histoplasmosis could be shaped by HIV burden and socioeconomic forces in different endemic regions.
Shortly after the description of increased susceptibility to histoplasmosis in AIDS, Coccidioides infection emerged as an important form of mycosis in patients with HIV infection (64–66). A prospective study at an Arizona HIV clinic in 1988 showed a cumulative incidence of active coccidioidomycosis of 25% during 41 months of follow-up, corresponding to an average annual incidence of 7.3% (67). In contrast, in a retrospective review at the same clinic during 2003–2008, only 11.3% of HIV-infected patients (n = 257) had coccidioidomycosis, and the annual incidence was only 0.9% when compared to the previous study (47). Both studies showed that CD4 count was the only predictor for developing active coccidioidomycosis; factors such as a history of coccidioidomycosis and duration of residence in an endemic area or age, sex, race, ethnicity, plasma HIV RNA level, or receipt of HAART were not associated with increased risk for coccidioidomycosis (11, 47, 67).
While histoplasmosis and coccidioidomycosis are well recognized as human pathogens long before the HIV epidemic, the importance of T. marneffei as a human disease was not recognized until the outbreak of HIV in Asia (22, 68, 69). In 1973, the first naturally occurring human case of T. marneffei infection was reported in an American minister with Hodgkin’s disease who had been residing in Southeast Asia (70). No more than 50 cases were reported in the literature during the early 1980s (68–76). From 1988, T. marneffei infection was increasingly observed in patients with advanced HIV infection, initially in foreign visitors who have been to endemic regions, and later in local residents who were native to endemic parts of Thailand and Vietnam (22, 23, 77–79). In Northern Thailand, T. marneffei infection is the third most common opportunistic infection, accounting for 15–20% of all AIDS-related illness, after tuberculosis (TB) and cryptococcosis. T. marneffei infection is estimated to occur in 2.3% of new AIDS cases, compared with 0.39% for histoplasmosis (80). The trend of T. marneffei infection closely paralleled that of HIV, and in areas where reduction of HIV transmission and availability of HAART have improved, a decrease in the prevalence of T. marneffei infection has been observed (23, 81).
Together with Emmonsia spp., which causes disseminated infection almost exclusively in advanced HIV infection in South Africa (29–31), it is apparent that the epidemiology of coccidioidomycosis, histoplasmosis, and P. marneffei evolves with HIV epidemic. The close relationship between disease manifestation and severity with CD4+ cell count confirms the central importance of cell-mediated immunity against endemic fungi.
Primary immunodeficiencies are rare inborn errors of immunity. Defects of T-cell development and differentiation, phagocytic functions, and pathways involved in the innate recognition of pathogens and downstream signaling are associated with increased risk of fungal infections, the most common being candidiasis and aspergillosis. Endemic mycoses are rarely described in patients with PID, and little is known about the spectrum of PID associated with increased susceptibility to endemic fungi. On the other hand, as individuals who are apparently healthy can also develop disease caused by endemic fungi, recognition of those who may have an underlying PID could be a challenge.
Talaromyces marneffei infection is mostly seen in advanced HIV infection with CD4+ cell count <100/μL, and in fact, up to 80% or more of the cases have CD4+ count <50/μL (22, 81, 82). Only small proportion of disseminated T. marneffei infection occurs in patients with secondary immunodeficiencies (22, 23, 83, 84). It is otherwise rare in healthy persons, especially in children. The close epidemiological relationship between HIV and T. marneffei, and the fact that T. marneffei is an AIDS-defining illness (51) suggests that individuals who are HIV negative and without secondary immunodeficiencies may have underlying immune defects that are unrecognized. A systematic review by Lee et al (85) on more than 500 articles published in English and Chinese from 1970 to 2011 on penicilliosis revealed 32 patients aged 3 months to 16 years with T. marneffei infection but without known HIV infection. Twenty-four patients (75%) had disseminated disease, and 55% died of T. marneffei infection. Eight patients had PID or blood disorders, while four others had abnormal immune functions. Immune evaluations of the remaining patients were unstated. This observation highlights the knowledge gap in the immunological susceptibility to T. marneffei.
Two systematic reviews on PID in histoplasmosis and coccidioidomycoses were recently published. Lovell et al. (86) summarized all published cases of histoplasmosis in patients with PID up to August 2015 and revealed 47 patients with underlying PID, defined either molecularly or clinically. Together with the four patients described in their report, more than 50 PID patients have been documented to have Histoplasma infection. Disseminated histoplasmosis occurred in 68% of cases, and two deaths occurred because of progressive disease. Another systematic literature search on disseminated coccidioidomycosis yielded 370 case reports, and 8 cases of PID were identified (87). The frequency of PID underlying endemic mycoses is unknown. Given the rarity of PID, the proportion of PID accounting for disseminated endemic mycoses is likely to be small. However, the cellular and molecular defects of these PID can provide important mechanistic insights into host defense mechanisms against endemic fungi. More importantly, disseminated or extrapulmonary forms of endemic mycoses can be utilized as unique indicators for PIDs, which is of particular relevance to clinicians working in respective endemic areas (88).
Host immune response toward fungal pathogens is initiated by the recognition of invading fungi via pattern recognition receptors (PRRs) expressed on neutrophils, monocytes, macrophages, and dendritic cells (DCs). Receptor-mediated signaling induces downstream events such as cytokine and chemokine release, phagocytosis, and respiratory burst ultimately leading to fungal killing (89–93). In addition, the cytokine responses shape the induction of Th-1 and Th-17 adaptive immune response. IL-12 drives IFN-γ production by T-helper 1 (Th1) cells, which is crucial for phagocyte activation. On the other hand, IL-1β, IL-6, and IL-23 promotes Th17 differentiation (89–91). The various PRRs that recognize fungal pathogen-associated molecular patterns and the downstream signaling pathways leading to induction of Th1 and Th17 response are shown in Figure 2A.
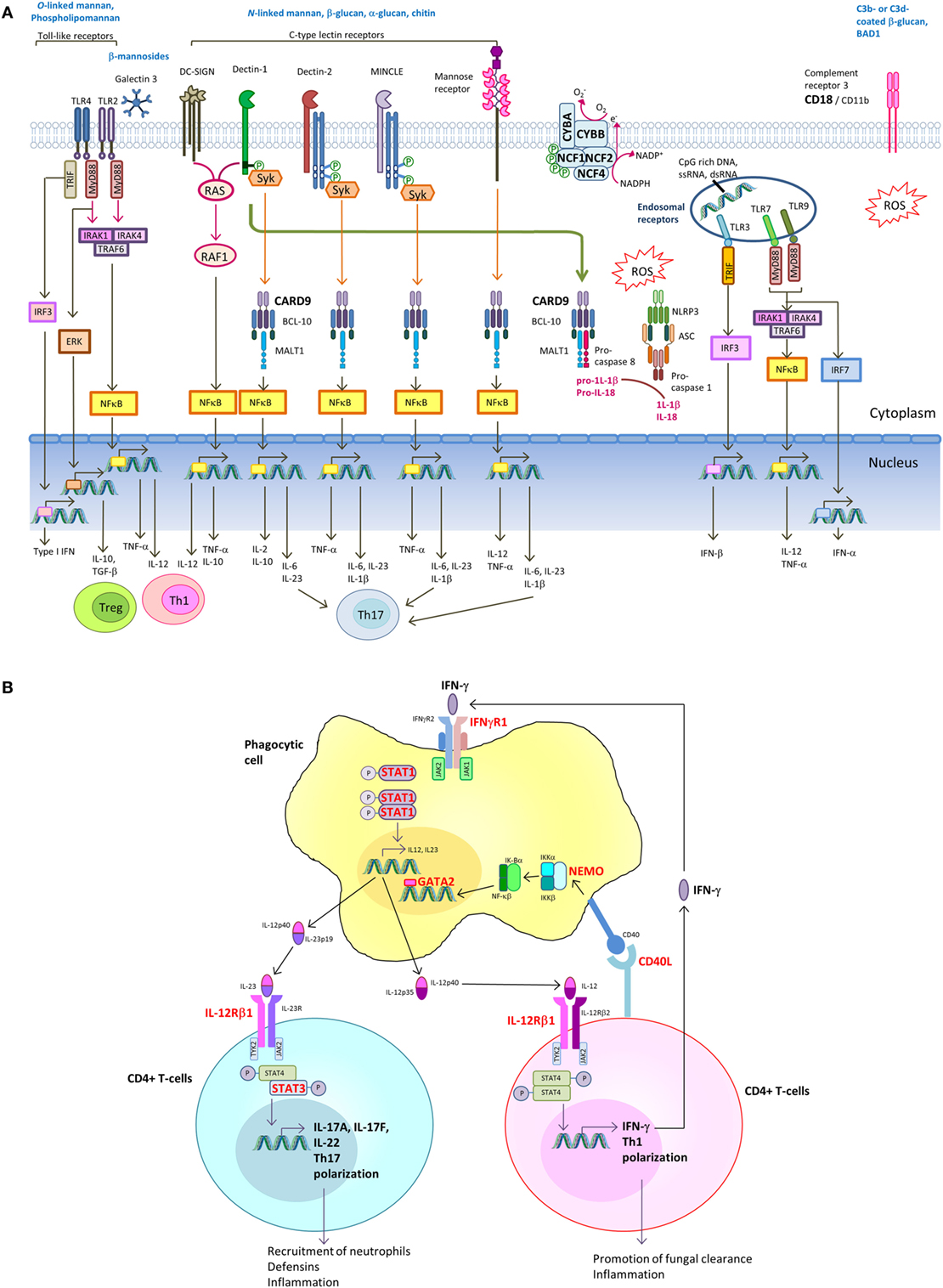
Figure 2. Signaling pathways in innate recognition of fungal pathogens and differentiation of CD4+ T helper cells. (A) Pathogen-associated molecular patterns (PAMPs) expressed by fungi are recognized by host pattern recognition receptors (PRRs), including toll-like receptors (TLRs), C-type lectin receptors (CLRs) [e.g., dendritic cell (DC)-specific ICAM3-grabbing non-integrin (DC-SIGN), Dectin-1, Dectin-2, MINCLE, and mannose receptor] and complement receptor 3 (CR3). TLRs and CLRs activate multiple intracellular signaling pathways upon binding to specific fungal PAMPs, including β-glucans, chitin, O-linked mannan and N-linked mannan, and nucleic acids. These signals activate canonical or non-canonical nuclear factor-κB and the NOD-, LRR- and pyrin domain-containing 3 (NLRP3) inflammasome. The integration of simultaneously activated PRRs occurs at the level of intracellular adaptors and transcription factors shared between overlapping pathways. The resulting cytokine responses shape the activation of adaptive immune response. Induction of IL-12 drives IFN-γ production by T-helper 1 (Th1) cells, which is crucial for phagocyte activation. Induction of IL-1β, IL-6, and IL-23 promotes Th17 differentiation. Regulatory T-cells (Treg) act as host-driven homeostatic response to keep inflammation under control. (B) Th1 and Th17 differentiation. Polarization of naive T cells into Th1 leads to IFNγ production, and its signaling is mediated through the Janus kinase (JAK)–signal transducer and activator of transcription 1 (STAT1) pathway, leading to transcription of IFNγ-inducible genes. IL-6 and IL-21 upregulate the expression of the retinoic acid-related orphan receptor RORγt and RORα, leading to expression of the inducible component of the IL-23 receptor (IL-23R) and further Th17 development. IL-17A and IL-17F produced by Th17 cells augments neutrophil production in the bone marrow and their recruitment to the site of infection. IL-17A, IL-17F, and IL-22 promote production of antimicrobial peptides in epithelial cells. Molecules in which genetic defects have been identified to be associated with increased susceptibility to endemic mycoses are marked in bold red.
Defects of the dectin-1/CARD9-MALT1-BCL10 signaling pathway are associated with chronic mucocutaneous candidiasis (CMC) (94). Patients with CARD9 deficiency have defects in Th17 differentiation and impaired neutrophil killing (95), and they are susceptible to CMC (96), deep dermatophytoses (97), and invasive fungal infection, particularly Candida meningitis. Other monogenic disorders causing CMC include autosomal recessive (AR) IL-17 receptor A (IL17RA), AR IL-17RC, AR ACT1, and autosomal-dominant (AD) IL-17F deficiencies (98). These patients display deficiency of IL-17F and IL-17A/F (IL-17F mutations) or dysfunctional responses to IL-17A, IL-17A/F, and IL-17F (IL17RA, IL-17RC, and ACT1 mutations). In patients with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy, high plasma titers of neutralizing autoantibodies against IL-17A, IL-17F, and IL-22 can be detected, as a result of the lack of AIRE expression in the thymus causing impaired T-cell tolerance (99). However, endemic mycoses have not been reported in patients with these genetic defects.
Opportunistic fungal infections are common in patients with severe combined immunodeficiencies (SCIDs) and phagocytic disorders. Infants with classical SCID often have recurrent or persistent mucosal and/or cutaneous candidiasis involving the orodigestive tract, genital area, nails, and skin. Pneumocystis jiroveci pneumonia (PCP) and invasive fungal infections (IFIs) including systemic candidiasis and aspergillosis are often life-threatening (100, 101). Interestingly, endemic mycoses have not been described in SCID babies, at least in the English literature. It may be due to the fact that it is uncommon for infants to be exposed to the natural habitats containing those endemic fungi, but further epidemiological data would be required to address this. Numerical and functional defects of phagocytes such as severe congenital neutropenia, chronic granulomatous disease (CGD), and leukocyte adhesion deficiency (LAD) are major groups of PIDs predisposing to systemic candidiasis and invasive aspergillosis (102–104). Filamentous fungi other than Aspergillus causing pulmonary infections in CGD include Geosmithia argillacea and Trichosporon inkin. Osteomyelitis can also be caused by rare non-Aspergillus filamentous fungi, including Cladophialophora arxii, Inonotus tropicalis, Scedospoium apiospermum, Penicillium piceum, and P. variotii. Cerebral abscesses caused by dematiaceous molds such as Exophiala spp., Phaeoacremonium spp., and Alternaria spp. have been reported (105–107). Interestingly, endemic mycoses have not been reported in CGD and LAD.
In the following sections, we highlight the spectrum of PIDs, which are associated with increased susceptibility to disseminated endemic mycoses. The genetic defects are summarized in Figure 2B.
Mucosal candidiasis is common in combined immunodeficiencies e.g. CD40 ligand (CD40L) deficiency, NEMO (IKBG) deficiency, IKBA gain-of-function (GOF) mutation and DOCK8 deficiency, in addition to a broad range of viral, bacterial, and IFI (102, 103). CD40L is expressed on activated T-cells and signals through NEMO/NF-κB to induce IL-12 production. CD40L deficiency, also known as hyper-IgM syndrome, is inherited in an X-linked recessive manner. Patients are susceptible to opportunistic infections including PCP, cryptosporidiosis, and mycobacterial infections due to impaired interaction between T-cells and antigen-presenting cells (APCs). In addition, the failure of B-cell immunoglobulin (Ig) isotype switching results in markedly low serum IgG and IgA, while IgM is elevated (108, 109). Disseminated and cutaneous forms of histoplasmosis have been reported in eight patients in patients with X-linked hyper-IgM disorder (Table 4). Five cases had disseminated histoplasmosis, while two had lymphadenitis and one had cutaneous involvement only (86, 110–115). All of them responded well to antifungal therapy, and only two patients had recurrent histoplasmosis (86, 111). One case of PCM was reported in Brazil (116). It was shown that mature DCs from patients with CD40L deficiency exhibited markedly reduced IL-12 and increased IL-10 production in response to P. brasiliensis and C. albicans compared with normal controls, and T cells had significantly reduced IFN-γ production when cocultured with their DCs, whereas IL-4 and IL-5 production was increased. In contrast, T-cell proliferation and generation of TGF-β and IL-17 were comparable with normal controls. These findings suggested that the absence of CD40L during monocyte/DC differentiation leads to functional DC abnormalities, which may contribute to the susceptibility to fungal infections in patients with CD40L deficiency (117). Four cases of T. marneffei infection were reported in CD40L deficiency (118–120). One patient who had disseminated T. marneffei infection had rapid deterioration due to late diagnosis and died, and CD40L deficiency was diagnosed after he passed away (120).
One patient with NEMO deficiency was reported to have persistent nodal histoplamosis at the age of 52 years. Symptoms including worsening dyspnea and intermittent night sweats that lasted for 1 year, and imaging studies revealed perihilar mass and mediastinal lymphadenopathy. Positive culture of H. capsulatum was obtained from paratracheal lymph node biopsy. He responded well to posaconazole (86). A patient with DOCK8 deficiency had miliary pneumonia caused by H. capsulatum. She also had numerous infectious caused by viruses (molluscum contagiosum, recurrent herpes zoster, cutaneous human papilloma virus infection), and dermatitis with Staphylococcus aureus superinfection (86, 121).
A syndrome of monocytopenia with susceptibility to non-tuberculous mycobacterial (NTM) infections, often termed “MonoMAC,” is caused by haploinsufficiency of the hematopoietic transcription factor GATA2 (122). Majority of the patients have monocytopenia, natural killer (NK), and B lymphocytopenia, while CD4 lymphocytopenia and neutropenia are also common but less marked. Affected individuals are susceptible to a broad range of viral (human herpes virus and human papillomavirus), disseminated NTM, bacterial, and fungal infections (123, 124). In a cohort of 57 patients with GATA2 mutations evaluated at the National Institutes of Health in the US (124), severe fungal infections were observed in 16%, including invasive aspergillosis (9%), disseminated histoplasmosis (5%), and recalcitrant mucosal candidiasis (5%). Another patient with GATA2 deficiency and disseminated histoplasmosis was reported by Lovell et al. (86). All the patients diagnosed to have GATA deficiency with disseminated histoplamosis were adults (86, 124). Apart from infections, other clinical features of GATA2 deficiency include congenital lymphedema, pulmonary alveolar proteinosis, and predisposition to myelodysplastic syndrome or acute myeloid leukemia, but considerable clinical heterogeneity exists.
Host defense against intracellular bacterial and fungal pathogens depends on effective cell-mediated immunity, which is coordinated by APC and T-lymphocytes (89, 90, 125). Following phagocytosis, macrophages, monocytes, and DCs secrete IL-12p70, a heterodimer of IL-12p40 (IL12B) and IL-12p35 (IL-12A) that stimulates T and NK cells through its receptor IL-12R, a heterodimer of IL-12Rβ1 and IL-12Rβ2. IL-12Rβ1 is bound to tyrosine kinase 2 (TYK2), and IL-12Rβ2 is bound to Janus kinase-2 (JAK2). IL-12 receptor signaling induces phosphorylation, dimerization, and nuclear translocation of signal transducer and activator of transcription-4 (STAT4) to induce IFN-γ production in T-cells and NK cells, and drives Th1 polarization of CD4+ T-cells. Binding of IFN-γ to its heterodimeric receptor consisting of IFN-γR1 and IFN-γR2 leads to signal transducer and activator of transcription 1 (STAT1) phosphorylation by Janus kinase 1 (JAK1) and JAK2. The phospho-STAT1 (p-STAT1) homodimer translocates to the nucleus and modifies gene expression regulated by the γ-regulated sequencing, resulting in phagocyte activation including production of bactericidal ROS by NADPH oxidase, further IL-12 production, and killing of intracellular pathogens (126, 127). IL-12 production is augmented by a T-cell dependent pathway through interaction of CD40 on the surface of APC with CD40L expressed on activated T-cells (128). The signaling pathway is shown in Figure 2B.
IL-23 shares the p40 component with IL-12p70, and IL-12Rβ1 combines with IL-23 receptor (IL-23R) to form the IL-23R complex (129). IL-23R signaling leads to STAT3/STAT4 heterodimer phosphorylation by TYK2 and JAK2, supporting the proliferation of Th17 cells, which are critical mediators of immunity at the mucosal surface (130). Activated Th17 cells produce IL-17 and IL-22, which induce antimicrobial peptide production in epithelial cells, and the recruitment and activation of inflammatory cells, especially neutrophils (131). These effector functions are critical in the control of mycobacteria, fungi, and bacterial pathogens such as salmonella (131–133).
Genetic defects of the IFN-γ-dependent immunity are collectively known as the Mendelian susceptibility to mycobacterial disease (MSMD) (126, 127). These disorders encompass defects of IFN-γ production or response to IFN-γ, caused by mutations in IL12B, IL12RB1, ISG15, NEMO, IFNGR1, IFNGR2, STAT1, NEMO, IRF8, and CYBB. Altogether, they constitute 18 genetic etiologies of MSMD based on the mode of inheritance, complete or partial defect, expression of the mutant allele, and the functional aberrations. Mycobacterial infection is the sole infectious phenotype in some of these disorders (AD IRF8 deficiency, AR ISG15 deficiency), while others have increased susceptibility to a broader range of pathogens (126, 127). Endemic fungal infections have been reported in AR IL12Rβ1, AR IFN-γR1, and AD GOF STAT1 defects.
Autosomal recessive IL12Rβ1 deficiency is the most common form of MSMD, accounting for approximately half of the cases in which a genetic cause has been identified (126, 127, 134). Patients with IL12Rβ1 deficiency are recognized by their susceptibility to mycobacterial infections (M. bovis BCG, NTM, and M. tuberculosis) and non-typhoidal salmonellosis of unusual severity or frequency, but some patients are also susceptible to Candida, Klebsiella, Nocardia, Leishmania, Histoplasma, Coccicioides, and Paracoccidioides (134). Peripheral blood mononuclear cells of these patients do not respond to IL-12 and IL-23, resulting in impaired IFN-γ production by T and NK cells. The development of IL-17-producing T-cells is also impaired, due to defect of the IL-23R complex, which is composed of IL-12Rβ1 (135–137). This accounts for the susceptibility to develop CMC observed in 23% of patients (134–137).
The seven reported cases of coccidioidomycosis, PCM, and histoplasmosis in patients with IL12Rβ1 deficiency are summarized in Table 5 (134, 138–141). The age at which disseminated mycoses developed varied from childhood to adulthood, some with past history of mycobacterial infection and salmonellosis. Two cases had recurrence disease, but could be controlled by antifungal treatment.
IFN-γR1 and IFN-γ2 are the ligand-binding and transducing receptor chains of the INF-γ receptor, respectively. Biallelic null mutations in the IFNGR1 gene result in AR complete IFNγR1 deficiency, which is characterized by high plasma concentration of IFN-γ and a lack of response to IFNγ in vitro (142, 143). These patients have early onset, life-threatening disseminated mycobacterial infections and the overall prognosis is poor with high rate of fatality. Other infections caused by viruses (cytomegalovirus, respiratory syncytial virus, varicellar zoster virus, human herpes virus 8) and bacteria (Listeria monocytogenes) have been described. In AR partial IFN-γR1 deficiency, the clinical phenotype is less severe (144). Apart from mycobacterial infections, bacterial, viral, and parasitic organisms have been reported (126, 127, 142, 144).
Autosomal dominant partial IFN-γR1 deficiency is caused by mono-allelic mutations affecting exon 6 and exon 7. A hotspot mutation, 818del4, accounts for over 80% of patients with AD IFN-γR1 deficiency (126, 127). In contrast with AR IFN-γR1 deficiency, these is an increase in IFN-γR1 protein expression on the cell surface, due to the accumulation of truncated IFN-γR1 receptors lacking the recycling domain. Despite the presence of receptors encoded by the wild-type IFNGR1 allele, the non-functioning IFN-γR1 protein impedes the normal function of IFN-γR1 dimers by negative dominance and impairs the response to IFN-γ in vitro (145). The clinical features are less severe than those seen in patients in AR complete IFN-γR1 deficiency. Most patients have BCG or NTM infections, and salmonella infection was reported in only 5% of cases (126, 127, 142). Disseminated histoplasmosis (146) and coccidioidomycosis (147) were reported in two patients with IFN-γR1 deficiency and both had a refractory or relapsing course (Table 5). Our group diagnosed AR IFN-γ receptor 1 deficiency in a Burmese infant suffering from disseminated T. marneffei infection, and she eventually died of salmonellosis (manuscript in preparation).
IFN-γR2 deficiency is less common than IFN-γR1 deficiency. Similarly, AR complete or partial IFN-γR2 deficiency cause increased susceptibility to mycobacterial infections (148–150); other infections are rare and include salmonellosis in one patient and cytomegalovirus disease in three patients (126), but mycosis has not been reported. AD form of partial IFN-γR2 deficiency was diagnosed in a patient with mild BCG disease, and clinical penetrance is very low (151).
Signal transducer and activator of transcription 1 (STAT1) is a transcription factor involved in cellular responses mediated by type I (IFNα/β), type II (IFN-γ), and type III (IFN-λ) IFNs (152). AR complete STAT1 deficiency is characterized by the absence of STAT1 expression and abolished cellular response to IFN-γ as well as IFN-α/β and IFN-λ, resulting in severely impaired antimycobacterial and antiviral immunity (153, 154). Patients with complete STAT1 deficiency caused by null mutations have increased susceptibility to mycobacterial, viral, and bacterial infections, whereas biallelic hypomorphic mutations in AR partial STAT1 deficiency are associated with milder clinical severity (153–155). AD partial STAT1 deficiency with mono-allelic loss-of-function (LOF) STAT1 mutation predispose to mycobacterial infection (156); in contrast, AD GOF STAT1 mutation is recognized as the most common cause of CMC disease, accounting for half of the cases (157–159). Majority of the mutations affect the coiled-coil domain or DNA-binding domain of STAT1 (159). They increase STAT1 phosphorylation by impairing nuclear dephosphorylation. They are GOF for the STAT1-dependent cytokines including IFN-α, IFN-β, IFN-γ, and IL-27, which repress Th17 development, accounting for the low numbers of IL-17-producing T-cells in these patients (160).
In addition to CMC, invasive mycoses caused by a variety of yeasts (e.g., Cryptococcus), molds (Aspergillus, Fusarium), and endemic fungi (Histoplasma, Coccidioides, T. marneffei) have been reported in patients with GOF STAT1 mutations, as summarized in Table 6 (161, 162). The two patients who developed disseminated coccidioidomycosis during childhood had progressive disease, which persisted into teenage despite intensive treatment; one had spinal cord compression and one died of overwhelming coccidioidomycosis at 17 years. Of note, the latter patient did not have CMC or other unusual infections, implying that coccidioidomycosis could be the sole infection in patients with GOF STAT1 defect. Three patients had disseminated histoplasmosis and two of them had recurrent disease. All of them responded well to antifungal treatment (161).
In Hong Kong, five pediatric patients were diagnosed to have T. marneffei infection from 1983 to 2009 in a single center (74, 85, 162, 163). One patient was lost to follow-up after complete recovery from T. marneffei infection (163), while the remaining four patients underwent thorough immunological investigations and genetic studies, and all were found to have GOF STAT1 defect (162). They all had CMC, and two had recurrent sinopulmonary infections, herpes virus infections (cytomegalovirus, Epstein–Barr virus and varicella zoster), TB, and disseminated aspergillosis (85, 162). The patient with chronic relapsing T. marneffei infection first reported by Yuen et al. in 1986 was subsequently investigated when his son was referred for CMC, and both were confirmed to have AD GOF STAT1 defect in 2015.
An increased incidence of herpes virus infections as well as TB and NTM infections (e.g., M. bovis BCG) has been observed in patients with GOF STAT1 defect, and it was thought that the enhancement of signaling downstream to IFN-α/IFN-β and IFN-γ caused by GOF STAT1 mutations could lead to exhaustion of virus-specific T-cells and refractory response to IFN-γ. Other clinical manifestations of GOF STAT1 mutations include autoimmunity (e.g., type 1 diabetes mellitus, thyroid disease, autoimmune cytopenia, and hepatitis), vascular aneurysms, and malignancies, particularly squamous cell carcinoma of the esophagus (159, 160, 164).
Autosomal-dominant hyper-IgE syndrome caused by LOF STAT3 mutation, also known as Job’s syndrome, is characterized by recurrent staphylococcal cold abscesses, pneumonia, and eczema. In addition, patients often display joint hyperextensibility, skeletal abnormalities and pathological fractures, delayed dental deciduation, coronary artery aneurysms, brain lesions, and Chiari’s malformation (165, 166). Pneumonia is typically caused by S. aureus, Haemophilus influenzae, or Streptococcus pneumoniae, and is often complicated by pneumoatocele formation. Approximately 20% of patients with AD hyper-IgE syndrome develop invasive infection caused by Aspergillus, which has angioinvasive properties with tendency to cause hematogenous dissemination (167–170). STAT3 promotes the expression of the gene encoding the retinoic acid receptor-related orphan receptor-γt (RORγt), and important transcription factor that drives differentiation of naïve CD4+ T-cells to Th17 cells. Dominant negative STAT3 mutation leads to impaired RORγt induction in response to IL-1β, IL-6, and IL-23, causing severe deficiency of IL-17-producing effector cells (171, 172).
Three cases of coccidioidomycosis, 10 cases of histoplasmosis and two cases of T. marneffei infection were reported in patients with hyper-IgE syndrome, as summarized in Table 7 (85, 87, 173–183). The three cases of hyper-IgE syndrome with coccidioidomycosis all had CNS involvement (87, 173, 174), while those with IL-12Rβ1 deficiency (139), IFN-γR1 deficiency (147), and GOF STAT1 defect (161) mainly had lymphadenopathy and osteomyelitis, so it appears that there is a predilection for Coccidioides to disseminate to the CNS in hyper-IgE syndrome. In contrast, disseminated histoplasmosis occurred in IL-12Rβ1 deficiency (141), IFN-γR1 deficiency (146), and GOF STAT1 defect (161), but 7 out of 10 cases of histoplasmosis in hyper-IgE syndrome involved the aerodigestive tract only (175–182). Two cases of T. marneffei infection were reported in hyper-IgE syndrome (85, 183).
To summarize, the susceptibility to endemic mycoses in CD40L deficiency, IL12Rβ1 deficiency, and IFN-γR1 deficiency highlights the critical role of the IL-12/IFN-γ crosstalk in macrophage activation and killing of these endemic fungi, while the deficiency of Th17 cells in patients with GOF STAT1 defect and AD hyper-IgE syndrome puts them at risk for both CMC and IFIs, and they frequently have CMC due to impaired mucosal immunity against C. albicans. Defective oxidative burst alone, as in CGD, is not sufficient to cause increased risk to endemic mycoses, suggestive that other mechanisms of phagosomal killing may compensate for the lack of NADPH oxidase activity to control these endemic fungi, distinguishing them from many other invasive fungi to which CGD patients are susceptible.
The development of biologic response modifiers (BRMs), such as monoclonal antibodies and receptor antagonists that target pro-inflammatory cytokines and their receptors has led to major advances in the treatment of autoimmune and malignant disorders. However, they have the potential to suppress host immune response and increase the risk of infections. The use of biologics is associated with a small but important risk of IFI. Histoplasmosis is the most common IFI associated with TNF-α inhibitors (10, 59, 184, 185). In a survey of infectious disease specialists, histoplasmosis was second only to S. aureus as the cause of serious infection complicating anti-TNF and other BRM (184). In most cases, patients reside in areas where the fungus is endemic and have received other immunosuppressants concurrently. Up to 2% of patients receiving BRMs will develop coccidioidomycosis if they reside in an endemic region (186). The American Academy of Pediatrics (AAP) Committee on Infectious Diseases recommends that patients on BRM should be enquired about epidemiologic risk factors and possible exposures to histoplasmosis and coccidioidomycosis, which have symptoms and signs that significantly overlap with TB. If there is suspicion of signs or symptoms compatible with acute histoplasmosis or coccidioidomycosis, BRM should be discontinued immediately and patients will require evaluation with a combination of chest radiography and serologic, antigen detection, and culture tests, which are best conducted in consultation with an infectious diseases expert (186, 187).
Other targeted therapies have also been implicated as risk factors for endemic mycoses. In Hong Kong, four cases of disseminated T. marneffei infection were diagnosed in adult hematology patients receiving anti-CD20 monoclonal antibodies (rituximab and obinutuzumab) and kinase inhibitors (84, 188, 189). The observation is revealing, as the importance of B-lymphocytes and humoral immune response against fungus is not well defined, and T. marneffei infection has so far not been reported in patients with congenital agammaglobulinemia. Depletion of B-lymphocytes may lead to profound deficiency in the production of neutralizing antibodies against key virulence factors of T. marneffei. Kinase inhibitors such as ruxolitinib and sorafenib are increasingly used in treating hematological malignancies, solid tumors, psoriasis, and alopecia areata. Ruxolitinib is a selective JAK1 and JAK2 inhibitor that interferes with the IFN-γ and its downstream JAK-STAT signaling. Sorafenib is a multi-kinase inhibitor that exhibits immunomodulatory effect by impairing T-lymphocyte proliferation, production of IFN-γ and other pro-inflammatory cytokines, NK cell, and DC functions. The suppression of IFN-γ signaling pathway poses risk to develop T. marneffei infection (84). It would be important for clinicians to have a high index of suspicion on T. marneffei infection in patients receiving these targeted therapies to avoid delay in diagnosis and treatment.
Autoantibody against IFN-γ has been reported to be associated with adult-onset immunodeficiency in patients from Asian countries (190–195). Disseminated NTM is the most common clinical presentation. In a cross-sectional, case-control study conducted in Chiang Mai, Thailand showed that patients with opportunistic infections including disseminated NTM, disseminated T. marneffei infection, melioidosis and non-typhoidal Salmonellosis had anti-IFN-γ autoantibody level above 99th percentile of cut-off for healthy individuals, and the level of autoantibody in patients who had active opportunistic infection was relatively higher than those without active infection (193). Similar observations were also reported in Hong Kong and Taiwan (190, 192, 194, 195). HLA class II molecules HLA-DRB1*15:02–HLA-DQB1*05:01 and HLA-DRB1*16:02–HLA-DQB1*05:02 are specifically associated with anti-IFN-γ autoantibodies and NTM (196, 197). The high frequency of such alleles in Southeast Asia might account for the relatively high prevalence this condition in the Asian population. The study by Lin et al. showed that anti-IFN-γ autoantibody from patients recognizes an epitope at the C terminus of IFN-γ, and binding of the autoantibody neutralizes IFNγ-induced signaling. This epitope displays a high degree of sequence homology to the Aspergillus Noc2 protein. It was postulated that in the warm and humid environment of Southeast Asia where exposure to Aspergillus species is common in everyday life, some individuals might develop anti-IFN-γ autoantibodies due to molecular mimicry (198). The co-evolution of anti-IFN-γ autoantibody production as the susceptibility trait amongst Southeast Asians, and the high prevalence of T. marneffei, NTM, and mellioidosis in this region is a unique combination not observed in the rest of the world, and it is so interesting that exposure to a common environmental fungal agent could indirectly induce susceptibility to other pathogens.
The understanding about inborn errors of immunity predisposing to endemic mycoses is limited. First, exposure to these environmental fungal pathogens is often a chance event that depends on the natural habitat and climate, as well as the circumstances and activities in which the individual is engaged. Thus, the number of cases of endemic mycoses associating with PID is likely to be low. The proportion of patients with PID amongst those with disseminated endemic mycoses is unknown, due to the lack of information about the population incidence (i.e., the “denominator”). Second, some forms of endemic mycoses, particularly T. marneffei are prevalent in less resourced countries where well-developed clinical service for PID is lacking, and diagnosis is often delayed or missed. Third, the global health impact of these geographically restricted endemic fungi is probably less than those opportunistic fungal pathogens of worldwide distribution causing high disease burden in immunocompromised patients (e.g., Candida, Aspergillus, P. jiroveci, Cryptococcus, and Rhizopus), and they probably generate less attractions and interests in the global public and scientific community (7, 199, 200). Regional and global effort in establishing registries on disseminated endemic mycoses is crucial, in order to collect patient demographic data and determine their true population incidence.
While endemic mycoses are geographically restricted to certain regions, clinicians looking after patients with PID or acquired immunodeficiencies should gain knowledge about these rare fungal infections so that appropriate advice can be given to their patients when planning for travels, and to have heightened awareness of such diagnostic possibility when they return from endemic areas. Climate, environment, and exposure (behavior) are the “triad” that determine the risk of endemic fungal infection in susceptible hosts. High-risk activities that increase the chance of exposure to fungal conidia should be avoided, otherwise, precautionary measures should be taken.
The discovery of PID predisposing to endemic mycoses is a fascinating journey, as it illuminates the key molecules and signaling pathways that are crucial in host defense against this group of dimorphic fungi, which are closely related in phylogeny. As disseminated coccidioidomycosis, histoplasmosis, PCM, and T. marneffei infection are recognized as AIDS-defining illness, they should also be regarded as indicator diseases for PID in individuals who are HIV-negative, and without known risk factors and secondary immunosuppression, particularly in children. There is a need to design an algorithm to evaluate such patients, with stepwise immunological investigations. A detailed history on previous infections, CMC, autoimmune manifestations, and family history should be taken. We recommend a basic panel of immunological evaluations including Ig pattern (IgG, IgA, IgM, and IgE), lymphocyte subset, and nitroblue tetrazolium, or dihydrorhodamine tests to assess oxidative burst activity. These patients should be assessed by immunologists. Abnormal results obtained during acute illness should be repeated upon full recovery. A systematic approach will facilitate clinicians to identify patients who warrant candidate gene studies or functional delineation of the pathways involved in immune recognition, T-cell activation and differentiation, cytokine signaling, and phagocytic killing. The presence of anti-IFN-γ autoantibody should be excluded. Functional evaluation of the IL-12/IFN-γ axis, STAT1 phosphorylation studies, and Th17 enumeration will be particularly relevant in this context. The utilization of next-generation sequencing techniques may lead to discoveries of novel monogenic disorders causing unique susceptibility to endemic mycoses.
PL wrote the article. Y-LL provided the conceptual framework and reviewed the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer, PR-J, and handling editor declared their shared affiliation and the handling editor states that the process nevertheless met the standards of a fair and objective review.
The authors would like to thank the Hong Kong Society for the Relief of Disabled Children for funding the molecular testing of primary immunodeficiency disorders for patients in Hong Kong and others referred to the Asian Primary Immunodeficiency Network.
1. Hsu LY, Ng ES, Koh LP. Common and emerging fungal pulmonary infections. Infect Dis Clin North Am (2010) 24:557–77. doi:10.1016/j.idc.2010.04.003
2. Sil A, Andrianopoulos A. Thermally dimorphic human fungal pathogens – polyphyletic pathogens with a convergent pathogenicity trait. Cold Spring Harb Perspect Med (2014) 5:a019794. doi:10.1101/cshperspect.a019794
3. Boyce KJ, Andrianopoulos A. Fungal dimorphism: the switch from hyphae to yeast is a specialized morphogenetic adaptation allowing colonization of a host. FEMS Microbiol Rev (2015) 39:797–811. doi:10.1093/femsre/fuv035
4. Kauffman CA, Freifeld AG, Andes DR, Baddley JW, Herwaldt L, Walker RC, et al. Endemic fungal infections in solid organ and hematopoietic cell transplant recipients enrolled in the transplant-associated infection surveillance network (TRANSNET). Transpl Infect Dis (2014) 16:213–24. doi:10.1111/tid.12186
5. Ramos-e-Silva M, Lima CM, Schechtman RC, Trope BM, Carneiro S. Systemic mycoses in immunodepressed patients (AIDS). Clin Dermatol (2012) 30:616–27. doi:10.1016/j.clindermatol.2012.01.008
6. Bonifaz A, Vázquez-González D, Perusquía-Ortiz AM. Endemic systemic mycoses: coccidioidomycosis, histoplasmosis, paracoccidioidomycosis and blastomycosis. J Dtsch Dermatol Ges (2011) 9:705–14. doi:10.1111/j.1610-0387.2011.07731.x
7. Seyedmousavi S, Guillot J, Tolooe A, Verweij PE, de Hoog GS. Neglected fungal zoonoses: hidden threats to man and animals. Clin Microbiol Infect (2015) 21:416–25. doi:10.1016/j.cmi.2015.02.031
8. Chakrabarti A, Slavin MA. Endemic fungal infections in the Asia-Pacific region. Med Mycol (2011) 49:337–44. doi:10.3109/13693786.2010.551426
9. Bryant PA, Baddley JW. Opportunistic infections in biological therapy, risk and prevention. Rheum Dis Clin North Am (2017) 43:27–41. doi:10.1016/j.rdc.2016.09.005
10. Vallabhaneni S, Chiller TM. Fungal infections and new biologic therapies. Curr Rheumatol Rep (2016) 18:29. doi:10.1007/s11926-016-0572-1
11. Brown J, Benedict K, Park BJ, Thompson GR III. Coccidioidomycosis: epidemiology. Clin Epidemiol (2013) 5:185–97. doi:10.2147/CLEP.S34434
12. Nguyen C, Barker BM, Hoover S, Nix DE, Ampel NM, Frelinger JA, et al. Recent advances in our understanding of the environmental, epidemiological, immunological, and clinical dimensions of coccidioidomycosis. Clin Microbiol Rev (2013) 26:505–25. doi:10.1128/CMR.00005-13
13. Malo J, Luraschi-Monjagatta C, Wolk DM, Thompson R, Hage CA, Knox KS. Update on the diagnosis of pulmonary coccidioidomycosis. Ann Am Thorac Soc (2014) 11:243–53. doi:10.1513/AnnalsATS.201308-286FR
14. Wheat LJ, Azar MM, Bahr NC, Spec A, Relich RF, Hage C. Histoplasmosis. Infect Dis Clin North Am (2016) 30:207–27. doi:10.1016/j.idc.2015.10.009
15. Teixeira Mde M, Patané JS, Taylor ML, Gómez BL, Theodoro RC, de Hoog S, et al. Worldwide phylogenetic distributions and population dynamics of the genus Histoplasma. PLoS Negl Trop Dis (2016) 10:e0004732. doi:10.1371/journal.pntd.0004732
16. Bahr NC, Antinori S, Wheat LJ, Sarosi GA. Histoplasmosis infections worldwide: thinking outside of the Ohio River valley. Curr Trop Med Rep (2015) 2:70–80. doi:10.1007/s40475-015-0044-0
17. Benedict K, Mody RK. Epidemiology of histoplasmosis outbreaks, United States, 1938–2013. Emerg Infect Dis (2016) 22:370–8. doi:10.3201/eid2203.151117
18. Pan B, Chen M, Pan W, Liao W. Histoplasmosis: a new endemic fungal infection in China? Review and analysis of cases. Mycoses (2013) 56:212–21. doi:10.1111/myc.12029
19. Theodoro RC, Teixeira Mde M, Felipe MS, Paduan Kdos S, Ribolla PM, San-Blas G, et al. Genus Paracoccidioides: species recognition and biogeographic aspects. PLoS One (2012) 7:e37694. doi:10.1371/journal.pone.0037694
20. Martinez R. New trends in paracoccidioidomycosis epidemiology. J Fungi (2017) 3:1. doi:10.3390/jof3010001
21. Arantes TD, Theodoro RC, Teixeira Mde M, Bosco Sde M, Bagagli E. Environmental mapping of Paracoccidioides spp. in Brazil reveals new clues into genetic diversity, biogeography and wild host association. PLoS Negl Trop Dis (2016) 10:e0004606. doi:10.1371/journal.pntd.0004606
22. Supparatpinyo K, Khamwan C, Baosoung V, Nelson KE, Sirisanthana T. Disseminated Penicillium marneffei infection in southeast Asia. Lancet (1994) 344:110–3. doi:10.1016/S0140-6736(94)91287-4
23. Vanittanakom N, Cooper CR Jr, Fisher MC, Sirisanthana T. Penicillium marneffei infection and recent advances in the epidemiology and molecular biology aspects. Clin Microbiol Rev (2006) 19:95–110. doi:10.1128/CMR.19.1.95-110.2006
24. Hu Y, Zhang J, Li X, Yang Y, Zhang Y, Ma J, et al. Penicillium marneffei infection: an emerging disease in mainland China. Mycopathologia (2013) 175:57–67. doi:10.1007/s11046-012-9577-0
25. Seitz AE, Adjemian J, Steiner CA, Prevots DR. Spatial epidemiology of blastomycosis hospitalizations: detecting clusters and identifying environmental risk factors. Med Mycol (2015) 53:447–54. doi:10.1093/mmy/myv014
26. Bradsher RW Jr. The endemic mimic: blastomycosis an illness often misdiagnosed. Trans Am Clin Climatol Assoc (2014) 125:188–202.
27. Castillo CG, Kauffman CA, Miceli MH. Blastomycosis. Infect Dis Clin North Am (2016) 30:247–64. doi:10.1016/j.idc.2015.10.002
28. Chakrabarti A, Bonifaz A, Gutierrez-Galhardo MC, Mochizuki T, Li S. Global epidemiology of sporotrichosis. Med Mycol (2015) 53:3–14. doi:10.1093/mmy/myu062
29. Kenyon C, Bonorchis K, Corcoran C, Meintjes G, Locketz M, Lehloenya R, et al. A dimorphic fungus causing disseminated infection in South Africa. N Engl J Med (2013) 369:1416–24. doi:10.1056/NEJMoa1215460
30. van Hougenhouck-Tulleken WG, Papavarnavas NS, Nel JS, Blackburn LY, Govender NP, Spencer DC, et al. HIV-associated disseminated emmonsiosis, Johannesburg, South Africa. Emerg Infect Dis (2014) 20:2164–6. doi:10.3201/eid2012.140902
31. Schwartz IS, Govender NP, Corcoran C, Dlamini S, Prozesky H, Burton R, et al. Clinical characteristics, diagnosis, management, and outcomes of disseminated emmonsiosis: a retrospective case series. Clin Infect Dis (2015) 61:1004–12. doi:10.1093/cid/civ439
32. Centers for Disease Control and Prevention. Environmental Fungal Diseases in the United States. (2016). Available from: https://www.cdc.gov/features/fungalinfections/
33. Centers for Disease Control and Prevention. Areas Endemic for Coccidioidomycosis. (2016). Available from: https://www.cdc.gov/fungal/diseases/coccidioidomycosis/causes.html
34. Centers for Disease Control and Prevention. Areas Endemic for Histoplasmosis. (2016). Available from: https://www.cdc.gov/fungal/diseases/histoplasmosis/causes.html
35. Leading International Fungal Education (LIFE). Paracoccidioidomycosis. (2016). Available from: http://www.life-worldwide.org/fungal-diseases/paracoccidioidomycosis
36. Leading International Fungal Education (LIFE). Blastomycosis. (2016). Available from: http://www.life-worldwide.org/fungal-diseases/blastomyces
37. Leading International Fungal Education (LIFE). Histoplasmosis. (2016). Available from: http://www.life-worldwide.org/fungal-diseases/histoplasmosis-chronic-cavitary-pulmonary
38. Leading International Fungal Education (LIFE). Talaromyces marneffei Infectiton. (2016). Available from: http://www.life-worldwide.org/fungal-diseases/talaromyces-marneffei-infection
39. Cao C, Liang L, Wang W, Luo H, Huang S, Liu D, et al. Common reservoirs for Penicillium marneffei infection in humans and rodents, China. Emerg Infect Dis (2011) 17:209–14. doi:10.3201/eid1702.100718
40. Centers for Disease Control and Prevention (CDC). Increase in reported coccidioidomycosis – United States, 1998–2011. MMWR Morb Mortal Wkly Rep (2013) 62:217–21.
41. Hector RF, Rutherford GW, Tsang CA, Erhart LM, McCotter O, Anderson SM, et al. The public health impact of coccidioidomycosis in Arizona and California. Int J Environ Res Public Health (2011) 8:1150–73. doi:10.3390/ijerph8041150
42. Téllez MD, Batista-Duharte A, Portuondo D, Quinello C, Bonne-Hernández R, Carlos IZ. Sporothrix schenckii complex biology: environment and fungal pathogenicity. Microbiology (2014) 160:2352–65. doi:10.1099/mic.0.081794-0
43. Mahajan VK. Sporotrichosis: an overview and therapeutic options. Dermatol Res Pract (2014) 2014:272376. doi:10.1155/2014/272376
44. Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev (2007) 20:115–32. doi:10.1128/CMR.00027-06
45. de Oliveira HC, Assato PA, Marcos CM, Scorzoni L, de Paula E Silva AC, Da Silva Jde F, et al. Paracoccidioides-host interaction: an overview on recent advances in the paracoccidioidomycosis. Front Microbiol (2015) 6:1319. doi:10.3389/fmicb.2015.01319
46. Saccente M, Woods GL. Clinical and laboratory update on blastomycosis. Clin Microbiol Rev (2010) 23:367–81. doi:10.1128/CMR.00056-09
47. Masannat FY, Ampel NM. Coccidioidomycosis in patients with HIV-1 infection in the era of potent antiretroviral therapy. Clin Infect Dis (2010) 50:1–7. doi:10.1086/648719
48. Adenis A, Nacher M, Hanf M, Vantilcke V, Boukhari R, Blachet D, et al. HIV-associated histoplasmosis early mortality and incidence trends: from neglect to priority. PLoS Negl Trop Dis (2014) 8:e3100. doi:10.1371/journal.pntd.0003100
49. Zheng J, Gui X, Cao Q, Yang R, Yan Y, Deng L, et al. A clinical study of acquired immunodeficiency syndrome associated Penicillium marneffei infection from a non-endemic area in China. PLoS One (2015) 10:e0130376. doi:10.1371/journal.pone.0130376
50. Kawila R, Chaiwarith R, Supparatpinyo K. Clinical and laboratory characteristics of penicilliosis marneffei among patients with and without HIV infection in Northern Thailand: a retrospective study. BMC Infect Dis (2013) 13:464. doi:10.1186/1471-2334-13-464
51. World Health Organization (WHO). WHO Case Definitions of HIV for Surveillance and Revised Clinical Staging and Immunological Classification of HIV-Related Disease in Adults and Children. Geneva: WHO (2007).
52. Morejón KM, Machado AA, Martinez R. Paracoccidioidomycosis in patients infected with and not infected with human immunodeficiency virus: a case-control study. Am J Trop Med Hyg (2009) 80:359–66.
53. Sarti EC, de Oliveira SM, dos Santos LF, de Camargo ZP, Paniago AM. Paracoccidioidal infection in HIV patients at an endemic area of paracoccidioidomycosis in Brazil. Mycopathologia (2012) 173:145–9. doi:10.1007/s11046-011-9495-6
54. Freitas DF, Valle AC, da Silva MB, Campos DP, Lyra MR, de Souza RV, et al. Sporotrichosis: an emerging neglected opportunistic infection in HIV-infected patients in Rio de Janeiro, Brazil. PLoS Negl Trop Dis (2014) 8:e3110. doi:10.1371/journal.pntd.0003110
55. Moreira JA, Freitas DF, Lamas CC. The impact of sporotrichosis in HIV-infected patients: a systematic review. Infection (2015) 43:267–76. doi:10.1007/s15010-015-0746-1
56. Witzig RS, Hoadley DJ, Greer DL, Abriola KP, Hernandez RL. Blastomycosis and human immunodeficiency virus: three new cases and review. South Med J (1994) 87:715–9. doi:10.1097/00007611-199407000-00008
57. Jones PG, Cohen RL, Batts DH, Silva J Jr. Disseminated histoplasmosis, invasive pulmonary aspergillosis, and other opportunistic infections in a homosexual patient with acquired immune deficiency syndrome. Sex Transm Dis (1983) 10:202–4. doi:10.1097/00007435-198311000-00010
58. Centers for Disease Control (CDC). Revision of the CDC surveillance case definition for acquired immunodeficiency syndrome. Council of State and Territorial Epidemiologists; AIDS program, Center for Infectious diseases. MMWR Suppl (1987) 36(Suppl 1):1S–15S.
59. Luckett K, Dummer JS, Miller G, Hester S, Thomas L. Histoplasmosis in patients with cell-mediated immunodeficiency: human immunodeficiency virus infection, organ transplantation, and tumor necrosis factor-α inhibition. Open Forum Infect Dis (2015) 2:ofu116. doi:10.1093/ofid/ofu116
61. Adenis AA, Aznar C, Couppié P. Histoplasmosis in HIV-infected patients: a review of new developments and remaining gaps. Curr Trop Med Rep (2014) 1:119–28. doi:10.1007/s40475-014-0017-8
62. Nacher M, Adenis A, Mc Donald S, Do Socorro Mendonca Gomes M, Singh S, Lopes Lima I, et al. Disseminated histoplasmosis in HIV-infected patients in South America: a neglected killer continues on its rampage. PLoS Negl Trop Dis (2013) 7:e2319. doi:10.1371/journal.pntd.0002319
63. The Neglected Histoplasmosis in Latin America Group. Disseminated histoplasmosis in Central and South America, the invisible elephant: the lethal blind spot of international health organizations. AIDS (2016) 30:167–70. doi:10.1097/QAD.0000000000000961
64. Abrams DI, Robia M, Blumenfeld W, Simonson J, Cohen MB, Hadley WK. Disseminated coccidioidomycosis in AIDS. N Engl J Med (1984) 310:986–7. doi:10.1056/NEJM198404123101511
65. Roberts CJ. Coccidioidomycosis in acquired immune deficiency syndrome: depressed humoral as well as cellular immunity. Am J Med (1984) 76:734–6. doi:10.1016/0002-9343(84)90304-8
66. Bronnimann DA, Adam RD, Galgiani JN, Habib MP, Petersen EA, Porter B, et al. Coccidioidomycosis in the acquired immunodeficiency syndrome. Ann Intern Med (1987) 106:372–9. doi:10.7326/0003-4819-106-3-372
67. Ampel NM, Dols CL, Galgiani JN. Coccidioidomycosis during human immunodeficiency virus infection: results of a prospective study in a coccidioidal endemic area. Am J Med (1993) 94:235–40. doi:10.1016/0002-9343(93)90054-S
68. Li PC, Yeoh EK. Current epidemiological trends of HIV infection in Asia. AIDS Clin Rev (1992):1–23.
69. Kaldor JM, Sittitrai W, John TJ, Kitamura T. The emerging epidemic of HIV infection and AIDS in Asia and the Pacific. AIDS (1994) 8:S1–2.
70. DiSalvo AF, Fickling AM, Ajello L. Infection caused by Penicillium marneffei: description of first natural infection in man. Am J Clin Pathol (1973) 60:259–63. doi:10.1093/ajcp/60.2.259
71. Pautler KB, Padhye AA, Ajello L. Imported penicilliosis marneffei in the United States: report of a second human infection. Sabouraudia (1984) 22:433–8. doi:10.1080/00362178485380691
72. Jayanetra P, Nitiyanant P, Ajello L, Padhye AA, Lolekha S, Atichartakarn V, et al. Penicilliosis marneffei in Thailand: report of five human cases. Am J Trop Med Hyg (1984) 33:637–44. doi:10.4269/ajtmh.1984.33.637
73. Deng ZL, Connor DH. Progressive disseminated penicilliosis caused by Penicillium marneffei. Report of eight cases and differentiation of the causative organism from Histoplasma capsulatum. Am J Clin Pathol (1985) 84:323–7. doi:10.1093/ajcp/84.3.323
74. Yuen WC, Chan YF, Loke SL, Seto WH, Poon GP, Wong KK. Chronic lymphadenopathy caused by Penicillium marneffei: a condition mimicking tuberculous lymphadenopathy. Br J Surg (1986) 73:1007–8. doi:10.1002/bjs.1800731224
76. Deng Z, Ribas JL, Gibson DW, Connor DH. Infections caused by Penicillium marneffei in China and Southeast Asia: review of eighteen published cases and report of four more Chinese cases. Rev Infect Dis (1988) 10:640–52. doi:10.1093/clinids/10.3.640
77. Peto TE, Bull R, Millard PR, Mackenzie DW, Campbell CK, Haines ME, et al. Systemic mycosis due to Penicillium marneffei in a patient with antibody to human immunodeficiency virus. J Infect (1988) 16:285–90. doi:10.1016/S0163-4453(88)97700-6
78. Chierakul W, Rajanuwong A, Wuthiekanun V, Teerawattanasook N, Gasiprong M, Simpson A, et al. The changing pattern of bloodstream infections associated with the rise in HIV prevalence in northeastern Thailand. Trans R Soc Trop Med Hyg (2004) 98:678–86. doi:10.1016/j.trstmh.2004.01.011
79. Nga TV, Parry CM, Le T, Lan NP, Diep TS, Campbell JI, et al. The decline of typhoid and the rise of non-typhoid salmonellae and fungal infections in a changing HIV landscape: bloodstream infection trends over 15 years in southern Vietnam. Trans R Soc Trop Med Hyg (2012) 106:26–34. doi:10.1016/j.trstmh.2011.10.004
80. Chayakulkeeree M, Denning DW. Serious fungal infections in Thailand. Eur J Clin Microbiol Infect Dis (2017) 36:931–35. doi:10.1007/s10096-017-2927-6
81. Le T, Wolbers M, Chi NH, Quang VM, Chinh NT, Lan NP, et al. Epidemiology, seasonality, and predictors of outcome of AIDS-associated Penicillium marneffei infection in Ho Chi Minh City, Viet Nam. Clin Infect Dis (2011) 52:945–52. doi:10.1093/cid/cir028
82. Wu TC, Chan JW, Ng CK, Tsang DN, Lee MP, Li PC. Clinical presentations and outcomes of Penicillium marneffei infections: a series from 1994 to 2004. Hong Kong Med J (2008) 14:103–9.
83. Wong SS, Wong KH, Hui WT, Lee SS, Lo JY, Cao L, et al. Differences in clinical and laboratory diagnostic characteristics of penicilliosis marneffei in human immunodeficiency virus (HIV)- and non-HIV-infected patients. J Clin Microbiol (2001) 39:4535–40. doi:10.1128/JCM.39.12.4535-4540.2001
84. Chan JF, Lau SK, Yuen KY, Woo PC. Talaromyces (Penicillium) marneffei infection in non-HIV-infected patients. Emerg Microbes Infect (2016) 5:e19. doi:10.1038/emi.2016.18
85. Lee PP, Chan KW, Lee TL, Ho MH, Chen XY, Li CH, et al. Penicilliosis in children without HIV infection – are they immunodeficient? Clin Infect Dis (2012) 54:e8–19. doi:10.1093/cid/cir754
86. Lovell JP, Foruraghi L, Freeman AF, Uzel G, Zerbe CS, Su H, et al. Persistent nodal histoplasmosis in nuclear factor kappa B essential modulator deficiency: report of a case and review of infection in primary immunodeficiencies. J Allergy Clin Immunol (2016) 138:903–5. doi:10.1016/j.jaci.2016.02.040
87. Odio CD, Marciano BE, Galgiani JN, Holland SM. Risk factors for disseminated coccidioidomycosis, United States. Emerg Infect Dis (2017) 23:308–11. doi:10.3201/eid2302.160505
88. Lee PP, Lau YL. Endemic infections in Southeast Asia provide new insights to the phenotypic spectrum of primary immunodeficiency disorders. Asian Pac J Allergy Immunol (2013) 31:217–26.
90. Drummond RA, Gaffen SL, Hise AG, Brown GD. Innate defense against fungal pathogens. Cold Spring Harb Perspect Med (2014) 5:a019620. doi:10.1101/cshperspect.a019620
91. Plato A, Hardison SE, Brown GD. Pattern recognition receptors in antifungal immunity. Semin Immunopathol (2015) 37:97–106. doi:10.1007/s00281-014-0462-4
92. Sukhithasri V, Nisha N, Biswas L, Anil Kumar V, Biswas R. Innate immune recognition of microbial cell wall components and microbial strategies to evade such recognitions. Microbiol Res (2013) 168:396–406. doi:10.1016/j.micres.2013.02.005
93. Barreto-Bergter E, Figueiredo RT. Fungal glycans and the innate immune recognition. Front Cell Infect Microbiol (2014) 4:145. doi:10.3389/fcimb.2014.00145
94. Turvey SE, Durandy A, Fischer A, Fung SY, Geha RS, Gewies A, et al. The CARD11-BCL10-MALT1 (CBM) signalosome complex: stepping into the limelight of human primary immunodeficiency. J Allergy Clin Immunol (2014) 134:276–84. doi:10.1016/j.jaci.2014.06.015
95. Drewniak A, Gazendam RP, Tool AT, van Houdt M, Jansen MH, van Hamme JL, et al. Invasive fungal infection and impaired neutrophil killing in human CARD9 deficiency. Blood (2013) 121:2385–92. doi:10.1182/blood-2012-08-450551
96. Glocker EO, Hennigs A, Nabavi M, Schäffer AA, Woellner C, Salzer U, et al. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N Engl J Med (2009) 361:1727–35. doi:10.1056/NEJMoa0810719
97. Lanternier F, Pathan S, Vincent QB, Liu L, Cypowyj S, Prando C, et al. Deep dermatophytosis and inherited CARD9 deficiency. N Engl J Med (2013) 369:1704–14. doi:10.1056/NEJMoa1208487
98. Okada S, Puel A, Casanova JL, Kobayashi M. Chronic mucocutaneous candidiasis disease associated with inborn errors of IL-17 immunity. Clin Transl Immunol (2016) 5:e114. doi:10.1038/cti.2016.71
99. Puel A, Döffinger R, Natividad A, Chrabieh M, Barcenas-Morales G, Picard C, et al. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med (2010) 207:291–7. doi:10.1084/jem.20091983
100. Chinn IK, Shearer WT. Severe combined immunodeficiency disorders. Immunol Allergy Clin North Am (2015) 35:671–94. doi:10.1016/j.iac.2015.07.002
101. Cirillo E, Giardino G, Gallo V, D’Assante R, Grasso F, Romano R, et al. Severe combined immunodeficiency – an update. Ann N Y Acad Sci (2015) 1356:90–106. doi:10.1111/nyas.12849
102. Vinh DC. Insights into human antifungal immunity from primary immunodeficiencies. Lancet Infect Dis (2011) 11:780–92. doi:10.1016/S1473-3099(11)70217-1
103. Lanternier F, Cypowyj S, Picard C, Bustamante J, Lortholary O, Casanova JL, et al. Primary immunodeficiencies underlying fungal infections. Curr Opin Pediatr (2013) 25:736–47. doi:10.1097/MOP.0000000000000031
104. Dinauer MC. Primary immune deficiencies with defects in neutrophil function. Hematology Am Soc Hematol Educ Program (2016) 2016:43–50. doi:10.1182/asheducation-2016.1.43
105. Falcone EL, Holland SM. Invasive fungal infection in chronic granulomatous disease: insights into pathogenesis and management. Curr Opin Infect Dis (2012) 25:658–69. doi:10.1097/QCO.0b013e328358b0a4
106. Henriet S, Verweij PE, Holland SM, Warris A. Invasive fungal infections in patients with chronic granulomatous disease. Adv Exp Med Biol (2013) 764:27–55. doi:10.1007/978-1-4614-4726-9_3
107. Haidar G, Zerbe CS, Cheng M, Zelazny AM, Holland SM, Sheridan KR. Phellinus species: an emerging cause of refractory fungal infections in patients with X-linked chronic granulomatous disease. Mycoses (2017) 60:155–60. doi:10.1111/myc.12573
108. Davies EG, Thrasher AJ. Update on the hyper immunoglobulin M syndromes. Br J Haematol (2010) 149:167–80. doi:10.1111/j.1365-2141.2010.08077.x
109. de la Morena MT. Clinical phenotypes of hyper-IgM syndromes. J Allergy Clin Immunol Pract (2016) 4:1023–36. doi:10.1016/j.jaip.2016.09.013
110. Tu RK, Peters ME, Gourley GR, Hong R. Esophageal histoplasmosis in a child with immunodeficiency with hyper-IgM. AJR Am J Roentgenol (1991) 157:381–2. doi:10.2214/ajr.157.2.1853826
111. Hostoffer RW, Berger M, Clark HT, Schreiber JR. Disseminated Histoplasma capsulatum in a patient with hyper IgM immunodeficiency. Pediatrics (1994) 94:234–6.
112. Yilmaz GG, Yilmaz E, Coşkun M, Karpuzoğlu G, Gelen T, Yeğin O. Cutaneous histoplasmosis in a child with hyper-IgM. Pediatr Dermatol (1995) 12:235–8. doi:10.1111/j.1525-1470.1995.tb00166.x
113. Danielian S, Oleastro M, Eva Rivas M, Cantisano C, Zelazko M. Clinical follow-up of 11 Argentinian CD40L-deficient patients with 7 unique mutations including the so-called “milder” mutants. J Clin Immunol (2007) 27:455–9. doi:10.1007/s10875-007-9089-8
115. Pedroza LA, Guerrero N, Stray-Pedersen A, Tafur C, Macias R, Muñoz G, et al. First case of CD40LG deficiency in Ecuador, diagnosed after whole exome sequencing in a patient with severe cutaneous histoplasmosis. Front Pediatr (2017) 5:17. doi:10.3389/fped.2017.00017
116. Cabral-Marques O, Schimke LF, Pereira PV, Falcai A, de Oliveira JB, Hackett MJ, et al. Expanding the clinical and genetic spectrum of human CD40L deficiency: the occurrence of paracoccidioidomycosis and other unusual infections in Brazilian patients. J Clin Immunol (2012) 32:212–20. doi:10.1007/s10875-011-9623-6
117. Cabral-Marques O, Arslanian C, Ramos RN, Morato M, Schimke L, Soeiro Pereira PV, et al. Dendritic cells from X-linked hyper-IgM patients present impaired responses to Candida albicans and Paracoccidioides brasiliensis. J Allergy Clin Immunol (2012) 129:778–86. doi:10.1016/j.jaci.2011.10.026
118. Kamchaisatian W, Kosalaraksa P, Benjaponpitak S, Hongeng S, Direkwattanachai C, Lumbiganon P, et al. Penicilliois in patients with X-linked hyperimmunoglobulin M syndrome (XHIGM), case reports from Thailand. J Allergy Clin Immunol (2006) 117:S282. doi:10.1016/j.jaci.2005.12.1166
119. Sripa C, Mitchai J, Thongsri W, Sripa B. Diagnostic cytology and morphometry of Penicillium marneffei in the sputum of a hypogammaglobulinemia with hyper-IgM patient. J Med Assoc Thai (2010) 93:S69–72.
120. Liu D, Zhong LL, Li Y, Chen M. Recurrent fever, hepatosplenomegaly and eosinophilia in a boy [article in Chinese]. Zhongguo Dang Dai Er Ke Za Zhi (2016) 18:1145–9.
121. Chu EY, Freeman AF, Jing H, Cowen EW, Davis J, Su HC, et al. Cutaneous manifestations of DOCK8 deficiency syndrome. Arch Dermatol (2012) 148:79–84. doi:10.1001/archdermatol.2011.262
122. Hsu AP, McReynolds LJ, Holland SM. GATA2 deficiency. Curr Opin Allergy Clin Immunol (2015) 15:104–9. doi:10.1097/ACI.0000000000000126
123. Collin M, Dickinson R, Bigley V. Haematopoietic and immune defects associated with GATA2 mutation. Br J Haematol (2015) 169:173–87. doi:10.1111/bjh.13317
124. Spinner MA, Sanchez LA, Hsu AP, Shaw PA, Zerbe CS, Calvo KR, et al. GATA2 deficiency: a protean disorder of hematopoiesis, lymphatics, and immunity. Blood (2014) 123:809–21. doi:10.1182/blood-2013-07-515528
125. Erwig LP, Gow NA. Interactions of fungal pathogens with phagocytes. Nat Rev Microbiol (2016) 14:163–76. doi:10.1038/nrmicro.2015.21
126. Bustamante J, Boisson-Dupuis S, Abel L, Casanova JL. Mendelian susceptibility to mycobacterial disease: genetic, immunological, and clinical features of inborn errors of IFN-γ immunity. Semin Immunol (2014) 26:454–70. doi:10.1016/j.smim.2014.09.008
127. Boisson-Dupuis S, Bustamante J, El-Baghdadi J, Camcioglu Y, Parvaneh N, El Azbaoui S, et al. Inherited and acquired immunodeficiencies underlying tuberculosis in childhood. Immunol Rev (2015) 264:103–20. doi:10.1111/imr.12272
128. Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol (2003) 3:133–46. doi:10.1038/nri1001
129. Floss DM, Schröder J, Franke M, Scheller J. Insights into IL-23 biology: from structure to function. Cytokine Growth Factor Rev (2015) 26:569–78. doi:10.1016/j.cytogfr.2015.07.005
130. Boniface K, Blom B, Liu YJ, de Waal Malefyt R. From interleukin-23 to T-helper 17 cells: human T-helper cell differentiation revisited. Immunol Rev (2008) 226:132–46. doi:10.1111/j.1600-065X.2008.00714.x
131. Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol (2014) 14:585–600. doi:10.1038/nri3707
132. Espinosa V, Rivera A. Cytokines and the regulation of fungus-specific CD4 T cell differentiation. Cytokine (2012) 58:100–6. doi:10.1016/j.cyto.2011.11.005
133. Jin W, Chen D. IL-17 cytokines in immunity and inflammation. Emerg Microbes Infect (2013) 2:e60. doi:10.1038/emi.2013.58
134. de Beaucoudrey L, Samarina A, Bustamante J, Cobat A, Boisson-Dupuis S, Feinberg J, et al. Revisiting human IL-12Rβ1 deficiency: a survey of 141 patients from 30 countries. Medicine (Baltimore) (2010) 89:381–402. doi:10.1097/MD.0b013e3181fdd832
135. Ouederni M, Sanal O, Ikinciogullari A, Tezcan I, Dogu F, Sologuren I, et al. Clinical features of candidiasis in patients with inherited interleukin 12 receptor β1 deficiency. Clin Infect Dis (2014) 58:204–13. doi:10.1093/cid/cit722
136. Fieschi C, Bosticardo M, de Beaucoudrey L, Boisson-Dupuis S, Feinberg J, Santos OF, et al. A novel form of complete IL-12/IL-23 receptor {beta}1 deficiency with cell surface-expressed nonfunctional receptors. Blood (2004) 104:2095–101. doi:10.1182/blood-2004-02-0584
137. de Beaucoudrey L, Puel A, Filipe-Santos O, Cobat A, Ghandil P, Chrabieh M, et al. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J Exp Med (2008) 205:1543–50. doi:10.1084/jem.20080321
138. Moraes-Vasconcelos DD, Grumach AS, Yamaguti A, Andrade ME, Fieschi C, de Beaucoudrey L, et al. Paracoccidioides brasiliensis disseminated disease in a patient with inherited deficiency in the beta1 subunit of the interleukin (IL)-12/IL-23 receptor. Clin Infect Dis (2005) 41:e31–7. doi:10.1086/432119
139. Vinh DC. Coccidioidal meningitis: disseminated disease in patients without HIV/AIDS. Medicine (Baltimore) (2011) 90:87. doi:10.1097/MD.0b013e3182073ae3
140. Hwangpo TA, Harriw WT, Atkinson P, Cassady K, Kankirawatana S. IL-12 receptor defect predisposes to histoplasmosis. Ann Allergy Asthma Immunol (2012) 109:A80.
141. Falcão ACAM, Marques PTL, Santos AR, Oliveira JB. Disseminated histoplasmosis caused by IL12RB1 gene mutations in two Brazilian siblings. J Clin Immunol (2012) 32:405.
142. Dorman SE, Picard C, Lammas D, Heyne K, van Dissel JT, Baretto R, et al. Clinical features of dominant and recessive interferon gamma receptor 1 deficiencies. Lancet (2004) 364:2113–21. doi:10.1016/S0140-6736(04)17552-1
143. Fieschi C, Dupuis S, Picard C, Smith CI, Holland SM, Casanova JL. High levels of interferon gamma in the plasma of children with complete interferon gamma receptor deficiency. Pediatrics (2001) 107:E48. doi:10.1542/peds.107.4.e48
144. Sologuren I, Boisson-Dupuis S, Pestano J, Vincent QB, Fernández-Pérez L, Chapgier A, et al. Partial recessive IFN-γR1 deficiency: genetic, immunological and clinical features of 14 patients from 11 kindreds. Hum Mol Genet (2011) 20:1509–23. doi:10.1093/hmg/ddr029
145. Jouanguy E, Lamhamedi-Cherradi S, Lammas D, Dorman SE, Fondanèche MC, Dupuis S, et al. A human IFNGR1 small deletion hotspot associated with dominant susceptibility to mycobacterial infection. Nat Genet (1999) 21:370–8. doi:10.1038/7701
146. Zerbe CS, Holland SM. Disseminated histoplasmosis in persons with interferon-gamma receptor 1 deficiency. Clin Infect Dis (2005) 41:e38–41. doi:10.1086/432120
147. Vinh DC, Masannat F, Dzioba RB, Galgiani JN, Holland SM. Refractory disseminated coccidioidomycosis and mycobacteriosis in interferon-gamma receptor 1 deficiency. Clin Infect Dis (2009) 49:e62–5. doi:10.1086/605532
148. Dorman SE, Holland SM. Mutation in the signal-transducing chain of the interferon-gamma receptor and susceptibility to mycobacterial infection. J Clin Invest (1998) 101:2364–9. doi:10.1172/JCI2901
149. Döffinger R, Jouanguy E, Dupuis S, Fondanèche MC, Stephan JL, Emile JF, et al. Partial interferon-gamma receptor signaling chain deficiency in a patient with bacille Calmette-Guérin and Mycobacterium abscessus infection. J Infect Dis (2000) 181:379–84. doi:10.1086/315197
150. Moncada-Vélez M, Martinez-Barricarte R, Bogunovic D, Kong XF, Blancas-Galicia L, Tirpan C, et al. Partial IFN-γR2 deficiency is due to protein misfolding and can be rescued by inhibitors of glycosylation. Blood (2013) 122:2390–401. doi:10.1182/blood-2013-01-480814
151. Kong XF, Vogt G, Itan Y, Macura-Biegun A, Szaflarska A, Kowalczyk D, et al. Haploinsufficiency at the human IFNGR2 locus contributes to mycobacterial disease. Hum Mol Genet (2013) 22:769–81. doi:10.1093/hmg/dds484
152. Schneider WM, Chevillotte MD, Rice CM. Interferon-stimulated genes: a complex web of host defenses. Annu Rev Immunol (2014) 32:513–45. doi:10.1146/annurev-immunol-032713-120231
153. Dupuis S, Jouanguy E, Al-Hajjar S, Fieschi C, Al-Mohsen IZ, Al-Jumaah S, et al. Impaired response to interferon-alpha/beta and lethal viral disease in human STAT1 deficiency. Nat Genet (2003) 33:388–91. doi:10.1038/ng1097
154. Chapgier A, Wynn RF, Jouanguy E, Filipe-Santos O, Zhang S, Feinberg J, et al. Human complete Stat-1 deficiency is associated with defective type I and II IFN responses in vitro but immunity to some low virulence viruses in vivo. J Immunol (2006) 176:5078–83. doi:10.4049/jimmunol.176.8.5078
155. Chapgier A, Kong XF, Boisson-Dupuis S, Jouanguy E, Averbuch D, Feinberg J, et al. A partial form of recessive STAT1 deficiency in humans. J Clin Invest (2009) 119:1502–14. doi:10.1172/JCI37083
156. Dupuis S, Dargemont C, Fieschi C, Thomassin N, Rosenzweig S, Harris J, et al. Impairment of mycobacterial but not viral immunity by a germline human STAT1 mutation. Science (2001) 293:300–3. doi:10.1126/science.1061154
157. Smeekens SP, Plantinga TS, van de Veerdonk FL, Heinhuis B, Hoischen A, Joosten LA, et al. T1 hyperphosphorylation and defective IL12R/IL23R signaling underlie defective immunity in autosomal dominant chronic mucocutaneous candidiasis. PLoS One (2011) 6:e29248. doi:10.1371/journal.pone.0029248
158. van de Veerdonk FL, Plantinga TS, Hoischen A, Smeekens SP, Joosten LA, Gilissen C, et al. STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. N Engl J Med (2011) 365:54–61. doi:10.1056/NEJMoa1100102
159. Toubiana J, Okada S, Hiller J, Oleastro M, Lagos Gomez M, Aldave Becerra JC, et al. Heterozygous STAT1 gain-of-function mutations underlie an unexpectedly broad clinical phenotype. Blood (2016) 127:3154–64. doi:10.1182/blood-2015-11-679902
160. Liu L, Okada S, Kong XF, Kreins AY, Cypowyj S, Abhyankar A, et al. Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J Exp Med (2011) 208:1635–48. doi:10.1084/jem.20110958
161. Sampaio EP, Hsu AP, Pechacek J, Bax HI, Dias DL, Paulson ML, et al. Signal transducer and activator of transcription 1 (STAT1) gain-of-function mutations and disseminated coccidioidomycosis and histoplasmosis. J Allergy Clin Immunol (2013) 131:1624–34. doi:10.1016/j.jaci.2013.01.052
162. Lee PP, Mao H, Yang W, Chan KW, Ho MH, Lee TL, et al. Penicillium marneffei infection and impaired IFN-γ immunity in humans with autosomal-dominant gain-of-phosphorylation STAT1 mutations. J Allergy Clin Immunol (2014) 133:894–6.e5. doi:10.1016/j.jaci.2013.08.051
163. Kwan EY, Lau YL, Yuen KY, Jones BM, Low LC. Penicillium marneffei infection in a non-HIV infected child. J Paediatr Child Health (1997) 33:267–71. doi:10.1111/j.1440-1754.1997.tb01596.x
164. Uzel G, Sampaio EP, Lawrence MG, Hsu AP, Hackett M, Dorsey MJ, et al. Dominant gain-of-function STAT1 mutations in FOXP3 wild-type immune dysregulation-polyendocrinopathy-enteropathy-X-linked-like syndrome. J Allergy Clin Immunol (2013) 131:1611–23. doi:10.1016/j.jaci.2012.11.054
165. Farmand S, Sundin M. Hyper-IgE syndromes: recent advances in pathogenesis, diagnostics and clinical care. Curr Opin Hematol (2015) 22:12–22. doi:10.1097/MOH.0000000000000104
166. Woellner C, Gertz EM, Schäffer AA, Lagos M, Perro M, Glocker EO, et al. Mutations in STAT3 and diagnostic guidelines for hyper-IgE syndrome. J Allergy Clin Immunol (2010) 125:424–32.e8. doi:10.1016/j.jaci.2009.10.059
167. Freeman AF, Holland SM. The hyper-IgE syndromes. Immunol Allergy Clin North Am (2008) 28:277–91, viii. doi:10.1016/j.iac.2008.01.005
168. Vinh DC, Sugui JA, Hsu AP, Freeman AF, Holland SM. Invasive fungal disease in autosomal-dominant hyper-IgE syndrome. J Allergy Clin Immunol (2010) 125:1389–90. doi:10.1016/j.jaci.2010.01.047
169. Chandesris MO, Melki I, Natividad A, Puel A, Fieschi C, Yun L, et al. Autosomal dominant STAT3 deficiency and hyper-IgE syndrome: molecular, cellular, and clinical features from a French national survey. Medicine (Baltimore) (2012) 91:e1–19. doi:10.1097/MD.0b013e31825f95b9
170. Roxo P, Menezes UP, Tucci S Jr, Andrade MF, Silva GE, Melo JM. Renal abscess in hyper-IgE syndrome. Urology (2013) 81:414–6. doi:10.1016/j.urology.2012.10.035
171. Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature (2008) 452:773–6. doi:10.1038/nature06764
172. Al Khatib S, Keles S, Garcia-Lloret M, Karakoc-Aydiner E, Reisli I, Artac H, et al. Defects along the T(H)17 differentiation pathway underlie genetically distinct forms of the hyper IgE syndrome. J Allergy Clin Immunol (2009) 124:342–8, 348.e1–5. doi:10.1016/j.jaci.2009.05.004
173. Stanga SD, Dajud MV. Visual changes in a 4-year-old. Clin Pediatr (Phila) (2008) 47:959–61. doi:10.1177/0009922808319788
174. Powers AE, Bender JM, Kumánovics A, Ampofo K, Augustine N, Pavia AT, et al. Coccidioides immitis meningitis in a patient with hyperimmunoglobulin E syndrome due to a novel mutation in signal transducer and activator of transcription. Pediatr Infect Dis J (2009) 28:664–6. doi:10.1097/INF.0b013e31819866ec
175. Alberti-Flor JJ, Granda A. Ileocecal histoplasmosis mimicking Crohn’s disease in a patient with Job’s syndrome. Digestion (1986) 33:176–80. doi:10.1159/000199290
176. Cappell MS, Manzione NC. Recurrent colonic histoplasmosis after standard therapy with amphotericin B in a patient with Job’s syndrome. Am J Gastroenterol (1991) 86:119–20.
177. Desai K, Huston DP, Harriman GR. Previously undiagnosed hyper-IgE syndrome in an adult with multiple systemic fungal infections. J Allergy Clin Immunol (1996) 98:1123–4. doi:10.1016/S0091-6749(96)80202-8
178. Steiner SJ, Kleiman MB, Corkins MR, Christenson JC, Wheat LJ. Ileocecal histoplasmosis simulating Crohn disease in a patient with hyperimmunoglobulin E syndrome. Pediatr Infect Dis J (2009) 28:744–6. doi:10.1097/INF.0b013e31819b65e0
179. Robinson WS, Arnold SR, Michael CF, Vickery JD, Schoumacher RA, Pivnick EK, et al. Case report of a young child with disseminated histoplasmosis and review of hyper immunoglobulin e syndrome (HIES). Clin Mol Allergy (2011) 9:14. doi:10.1186/1476-7961-9-14
180. Rana C, Krishnani N, Kumari N, Shastri C, Poddar U. Rectal histoplasmosis in Job’s syndrome. Indian J Gastroenterol (2013) 32:64–5. doi:10.1007/s12664-012-0275-0
181. Jiao J, Horner CC, Kau AL. Terminal ileum perforation in a patient with hyper-IgE syndrome. Ann Allergy Asthma Immunol (2014) 113:S61.
182. Odio CD, Milligan KL, McGowan K, Rudman Spergel AK, Bishop R, Boris L, et al. Endemic mycoses in patients with STAT3-mutated hyper-IgE (Job) syndrome. J Allergy Clin Immunol (2015) 136:1411–3.e1–2. doi:10.1016/j.jaci.2015.07.003
183. Ma BH, Ng CS, Lam R, Wan S, Wan IY, Lee TW, et al. Recurrent hemoptysis with Penicillium marneffei and Stenotrophomonas maltophilia in Job’s syndrome. Can Respir J (2009) 16:e50–2. doi:10.1155/2009/586919
184. Salvana EM, Salata RA. Infectious complications associated with monoclonal antibodies and related small molecules. Clin Microbiol Rev (2009) 22:274–90. doi:10.1128/CMR.00040-08
185. Ordonez ME, Farraye FA, Di Palma JA. Endemic fungal infections in inflammatory bowel disease associated with anti-TNF antibody therapy. Inflamm Bowel Dis (2013) 19:2490–500. doi:10.1097/MIB.0b013e31828f1fba
186. Davies HD; Committee on Infectious Diseases. Infectious complications with the use of biologic response modifiers in infants and children. Pediatrics (2016) 138:e20161209. doi:10.1542/peds.2016-1209
187. Galgiani JN, Ampel NM, Blair JE, Catanzaro A, Geertsma F, Hoover SE, et al. Executive summary: 2016 Infectious Diseases Society of America (IDSA) clinical practice guideline for the treatment of coccidioidomycosis. Clin Infect Dis (2016) 63:717–22. doi:10.1093/cid/ciw538
188. Chan JF, Chan TS, Gill H, Lam FY, Trendell-Smith NJ, Sridhar S, et al. Disseminated infections with Talaromyces marneffei in non-AIDS patients given monoclonal antibodies against CD20 and kinase inhibitors. Emerg Infect Dis (2015) 21:1101–6. doi:10.3201/eid2107.150138
189. Tse E, Leung RY, Kwong YL. Invasive fungal infections after obinutuzumab monotherapy for refractory chronic lymphocytic leukemia. Ann Hematol (2015) 94:165–7. doi:10.1007/s00277-014-2120-2
190. Tang BS, Chan JF, Chen M, Tsang OT, Mok MY, Lai RW, et al. Disseminated penicilliosis, recurrent bacteremic nontyphoidal salmonellosis, and burkholderiosis associated with acquired immunodeficiency due to autoantibody against gamma interferon. Clin Vaccine Immunol (2010) 17:1132–8. doi:10.1128/CVI.00053-10
191. Kampitak T, Suwanpimolkul G, Browne S, Suankratay C. Anti-interferon-γ autoantibody and opportunistic infections: case series and review of the literature. Infection (2011) 39:65–71. doi:10.1007/s15010-010-0067-3
192. Browne SK, Burbelo PD, Chetchotisakd P, Suputtamongkol Y, Kiertiburanakul S, Shaw PA, et al. Adult-onset immunodeficiency in Thailand and Taiwan. N Engl J Med (2012) 367:725–34. doi:10.1056/NEJMoa1111160
193. Wongkulab P, Wipasa J, Chaiwarith R, Supparatpinyo K. Autoantibody to interferon-gamma associated with adult-onset immunodeficiency in non-HIV individuals in Northern Thailand. PLoS One (2013) 8:e76371. doi:10.1371/journal.pone.0076371
194. Chan JF, Trendell-Smith NJ, Chan JC, Hung IF, Tang BS, Cheng VC, et al. Reactive and infective dermatoses associated with adult-onset immunodeficiency due to anti-interferon-gamma autoantibody: Sweet’s syndrome and beyond. Dermatology (2013) 226:157–66. doi:10.1159/000347112
195. Chi CY, Lin CH, Ho MW, Ding JY, Huang WC, Shih HP, et al. Clinical manifestations, course, and outcome of patients with neutralizing anti-interferon-γ autoantibodies and disseminated nontuberculous mycobacterial infections. Medicine (Baltimore) (2016) 95:e3927. doi:10.1097/MD.0000000000003927
196. Chi CY, Chu CC, Liu JP, Lin CH, Ho MW, Lo WJ, et al. Anti-IFN-γ autoantibodies in adults with disseminated nontuberculous mycobacterial infections are associated with HLA-DRB1*16:02 and HLA-DQB1*05:02 and the reactivation of latent varicella-zoster virus infection. Blood (2013) 121:1357–66. doi:10.1182/blood-2012-08-452482
197. Ku CL, Lin CH, Chang SW, Chu CC, Chan JF, Kong XF, et al. Anti-IFN-γ autoantibodies are strongly associated with HLA-DR*15:02/16:02 and HLA-DQ*05:01/05:02 across Southeast Asia. J Allergy Clin Immunol (2016) 137:945–8.e8. doi:10.1016/j.jaci.2015.09.018
198. Lin CH, Chi CY, Shih HP, Ding JY, Lo CC, Wang SY, et al. Identification of a major epitope by anti-interferon-γ autoantibodies in patients with mycobacterial disease. Nat Med (2016) 22:994–1001. doi:10.1038/nm.4158
199. Dujardin JC, Herrera S, do Rosario V, Arevalo J, Boelaert M, Carrasco HJ, et al. Research priorities for neglected infectious diseases in Latin America and the Caribbean region. PLoS Negl Trop Dis (2010) 4:e780. doi:10.1371/journal.pntd.0000780
Keywords: endemic mycoses, primary immunodeficiencies, human immunodeficiency virus, histoplasmosis, coccidioidomycosis, paracoccidioidomycosis, blastomycosis, Taloromyces marneffei
Citation: Lee PP and Lau Y-L (2017) Cellular and Molecular Defects Underlying Invasive Fungal Infections—Revelations from Endemic Mycoses. Front. Immunol. 8:735. doi: 10.3389/fimmu.2017.00735
Received: 15 March 2017; Accepted: 09 June 2017;
Published: 28 June 2017
Edited by:
Antonio Condino-Neto, University of São Paulo, BrazilReviewed by:
Pérsio Roxo-Junior, University of São Paulo, BrazilCopyright: © 2017 Lee and Lau. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu-Lung Lau, lauylung@hku.hk
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.