- 1Department of Advanced Technology for Transplantation, Graduate School of Medicine, Osaka University, Osaka, Japan
- 2Department of Urology, Graduate School of Medicine, Osaka University, Osaka, Japan
The recent attention given to diseases associated with memory B-cell (mBC)-produced antibodies (Abs) suggests the need for a similar in vitro assay to evaluate the functions of mBCs. Here, we cultured peripheral blood mononuclear cells (PBMCs) with the intent to collect mBC-derived Abs in vitro and maintain their cell–cell contact-dependent interactions with helper T-cells. PBMCs were cultured with interleukin (IL)-21, CpG-oligodeoxynucleotides (ODN), phorbol myristate acetate (PMA), and phytohemagglutinin/leucoagglutinin (PHA-L) in 24-well flat-bottom plates (5 × 105 cells/well). A culture supernatant analysis of PBMCs from healthy donors (n = 10) indicated that antigen-specific IgM Ab levels in a PBMC culture supernatant might be better able to demonstrate the antigen sensitization status in a smaller peripheral blood sample, compared to IgG because Epstein–Barr virus-specific IgM mBCs circulate peripherally at a significantly higher frequency once antiviral humoral immunity has stabilized. Thus, our in vitro assay demonstrated the potential significance of antigen-specific IgM Ab production in the culture supernatants. Furthermore, an analysis of cultured PBMCs from allograft kidney recipients (n = 16) sensitized with de novo donor-specific human leukocyte antigen (HLA)-specific Abs (DSAs) showed that IgM-type HLA-specific Abs were detected mainly from the culture supernatants from PBMCs of patients with stable graft function, whereas IgG isotype HLA Abs were detectable only from patients with biopsy-proven antibody-mediated rejection. In other words, these IgG isotype Abs also represented an activated humoral immune response in vivo. Additionally, IgM- and IgG-expressing mBCs from healthy donors (n = 5) were cultured with IL-21, CpG-ODN, and a supernatant produced by stimulating CD19+ B-cell-depleted PBMCs with PHA-L and PMA in 24-well flat-bottom plates (1 × 105 cells/well), and the resulting in vitro analysis provided some information regarding the biological processes of IgG and IgM mBCs in peripheral blood. Taken together, our findings suggest that antigen-specific Ab subtype analyses of supernatants from cultured PBMCs might more effectively and accurately reflect a patient’s Ab-associated pathological condition vs. than serum IgG and IgM levels.
Introduction
Antigen-specific antibodies (Abs) are produced by memory B-cell (mBC)-derived plasma cells (PCs). Furthermore, some reports indicate that no available immunosuppressive agent can control PCs growth and survival. Therefore, an understanding of Ab-associated disease first requires an understanding of the biological processes that underlie the growth and survival of mBCs.
Briefly, B-cells initially develop in the bone marrow. Here, highly self-reactive immature B-cells are deleted, and the remaining cells exit the bone marrow to the peripheral circulation. During the transitional stage of B-cell differentiation, cells that express self-antigen-reactive B-cell receptors (BCRs) are subjected to clone deletion, BCR editing, anergy, and immunological ignorance (1).
The stimulation of BCRs on naïve B-cells in the peripheral lymphoid tissue via receptor cross-linking induces clonal B-cell expansion and antigen uptake. Subsequently, this antigen is presented in combination with a major histocompatibility complex class II molecule on the naïve B-cell surface for recognition by helper T-cells. Subsequently, activated naïve B-cells and accompanying T-cells migrate into primary lymphoid follicles and subsequently form germinal centers (GCs) in secondary lymphoid tissues (2).
Within GCs, activated naïve B-cells undergo somatic hypermutation (SHM) of the variable regions and class-switch recombination (CSR) of immunoglobulin-encoding genes and differentiate into mBCs or PCs. During this process, mBCs with higher affinities for non-self-antigens are selected and mBCs with low affinities are deleted. The remaining mBCs differentiate into PCs (3–6).
Previous research regarding Ab-associated diseases has mainly focused on antigen-specific IgGs as the etiologic agent. IgG-producing mBCs differentiate in GCs after undergoing SHM and CSR. These cells are localized in lymph nodes near the primary infection site and can more rapidly differentiate into PCs, compared with IgM-producing mBCs (7, 8). Furthermore, these mBCs-derived IgGs cause tissue injury by absorption into target antigen in context of Ab-associated diseases.
By contrast, the clinical significance of antigen-specific IgM with respect to Ab-associated diseases remains controversial.
Many reports have indicated that IgM mBCs can be subclassified as having either the IgD− or IgD+ phenotype. IgM (IgD−) mBCs, which do not develop in GCs (9), respond in an extra-follicular, thymus-independent manner and produce natural Abs with lower affinities for antigens (10). By contrast, IgM (IgD+) mBCs undergo SHM in GCs and differentiate into PCs that produce sufficient amounts of Abs specific for thymus-dependent antigens. These latter somatically mutated IgM mBCs have been reported to exhibit similar functional capacities to those of IgG mBCs (11).
Various types of Ab isotypes have elicited research interest. IgG-type DSAs have received considerable attention in the field of organ transplantation. Regarding autoimmune diseases, serum levels of self-antigen-specific IgM and IgG have been used to evaluate pathological conditions (12, 13). In the field of viral infection, both IgG and IgM viral antigen-specific Abs have been used to evaluate previous or current infection status, and IgM production has been recognized as an early diagnostic parameter (14, 15).
Accordingly, the role of mBC-derived antigen-specific IgM Abs in Ab-associated diseases should be elucidated further using in vitro assays of supernatants, similar to those used to study T-cells. In our study, we attempted to develop an in vitro assay method enabling us to collect mBC-derived Abs to possibly elucidate the biological processes of antigen-specific IgG and IgM mBCs in peripheral blood. We further aimed to establish a culture supernatant analysis to provide some information about the potential of each type of Ab associated with a pathological condition in context with Ab-associated diseases.
Materials and Methods
Participants and Samples
This study followed the principles of the Declaration of Helsinki, and all subjects provided informed consent to participate. Peripheral blood (8 ml) samples were collected from 10 healthy donors (3 men and 7 women; average age, 41.4 ± 8.8 years; range: 31–55 years) and 16 kidney-allograft recipients sensitized de novo to donor-specific antigens (8 men and 8 women; average age, 46.3 ± 17.0 years; age range: 17–77 years). Additionally, CD27+ mBCs and CD27− naïve B-cells, as well as IgM mBCs and IgG mBCs, were separated from the peripheral blood mononuclear cells (PBMCs) of the five healthy donors (four men and one woman; average age, 39.3 ± 9.7 years; age range: 24–49 years).
Isolation and Culture of PBMCs
Peripheral blood mononuclear cells were isolated over a Ficoll-Hypaque density gradient (Sigma-Aldrich, St. Louis, MO, USA) and cultured in 24-well flat-bottomed plates (5 × 105 cells/well) in basal B-cell culture medium [Iscove’s modified Dulbecco’s medium (Sigma-Aldrich) supplemented with 10% fetal calf serum (FCS; Thermo Scientific HyClone, Logan, UT, USA), 50 µg/ml human transferrin–selenium, and 5 µg/ml human insulin (Gibco/Invitrogen Co., Carlsbad, CA, USA)]. Cells were cultured in the presence of the following additives: 50 ng/ml interleukin (IL)-21, 2.5 µg/ml phosphorothioate CpG-oligodeoxynucleotides (ODN) 2006, 2.5 µg/ml phytohemagglutinin/leucoagglutinin (PHA-L), and 15 ng/ml phorbol myristate acetate (PMA). For IgM-BCR cross-linking, we cultured PBMCs (5 × 105 cells/well, 24-well flat-bottomed plate) in medium supplemented with Affini Pure F(ab)2 Fragment Goat antihuman IgM (5.2 µg/ml).
Isolation and Culture of CD27+ mBCs and CD27− Naïve B-Cells
To isolate mBCs from naïve B-cells, PBMCs were subjected to negative selection using a B-cell isolation kit (Miltenyi Biotech, Auburn, CA, USA). Unlabeled B-cells were passed through the column, washed with buffer, and subjected to magnetic activated cell sorting using CD27-conjugated microbeads according to the manufacturer’s instructions. The isolated mBCs were cultured in 24-well flat-bottom plates (1 × 105 cells/well) in basal B-cell culture medium supplemented as follows: 50 ng/ml IL-21, 2.5 µg/ml phosphorothioate CpG-ODN 2006, CD40 ligand, and 5 µg/ml anti-polyhistidine monoclonal antibody (mAb). The isolated B-cells were cultured without helper T-cells, which express CD40 ligand or humoral factors produced by these cells. Therefore, it was necessary to supplement the culture with soluble CD40 ligand to support B-cell growth and survival.
Isolation and Culture of IgG mBCs and IgM mBCs
IgG mBCs and IgM mBCs were further separated using IgG and IgM mBC isolation kits (Miltenyi Biotech) according to the manufacturer’s instructions. The isotype-specific mBCs were cultured in 24-well flat-bottom plates (1 × 105 cells/well) in basal B-cell culture medium supplemented as follows: 50 ng/ml IL-21, 2.5 µg/ml phosphorothioate CpG-ODN 2006, and 5% PBMC supernatant; the latter was produced by stimulating CD19+ B-cell-depleted PBMCs obtained from healthy male donors (n = 5) for 36 h in the presence of 15 ng/ml PMA and 2.5 µg/ml PHA-L in RPMI-1640 supplemented with 5% FCS. For IgM-BCR cross-linking, we cultured PBMCs (5 × 105 cells/well, 24-well flat-bottomed plate) in basal B-cell culture medium supplemented with Affini Pure F(ab)2 Fragment Goat antihuman IgM (5.2 µg/ml).
Reagents
Recombinant human IL-21 was purchased from Miltenyi Biotech. Histidine-tagged soluble recombinant human CD40 ligand, anti-polyhistidine mAb, and human recombinant a proliferation-inducing ligand (APRIL) were obtained from R&D Systems (Minneapolis, MN, USA). Phosphorothioate CpG-ODN 2006 was purchased from Invivogen (San Diego, CA, USA). PHA-L and PMA were purchased from Sigma-Aldrich (St. Louis, MO, USA). The Affini Pure F (ab)2 fragment goat antihuman IgM used for IgM-BCR cross-linking was purchased from Jackson Immunoresearch Laboratories (West Grove, PA, USA). A Mem-PER™ Plus Membrane Protein Extraction Kit from Thermo Scientific (Waltham, MA, USA) was used for membrane extraction.
Flow Cytometric Analysis
To evaluate differentiation, cells were labeled with fluorescein isothiocyanate (FITC)-tagged anti-CD38 (eBioscience, San Diego, CA, USA), phycoerythrin (PE)-tagged, anti-138 and allophycocyanin-tagged anti-CD19 mAbs (BD Biosciences, San Jose, CA, USA). Dead cells were stained with propidium iodide (Sigma-Aldrich). All flow cytometric analyzes were performed on a FACS Calibur dual-laser flow cytometer, and data were analyzed using Cell Quest acquisition/analysis software (BD Biosciences).
Enzyme-Linked Immunosorbent Assay (ELISA)
For ELISAs, 96-well plates were coated with a recombinant Epstein–Barr virus (HHV-4) p18, GST tag (ProSpec, East Brunswick, NJ, USA) to generate a standard curve and polyclonal F(ab)2 goat antihuman IgG heavy chain Abs (Biosource, Camarillo, CA, USA) or affinipure F(ab′)2 goat antihuman IgM Fc5μ fragment-specific Abs (Jackson Immunoresearch Laboratories). Coated plates were incubated at 4°C overnight.
Each plate was washed five times with phosphate-buffered saline (PBS; Sigma-Aldrich) containing 0.1% Tween-20. Next, 100 µl aliquots of supernatant from each culture condition, media alone, and IgG standards (calibration curve) were diluted with PBS in the range of 1:200–1:10,000 (IgM standards, 1:1,600–1:80,000), added to the plates, and incubated overnight at 4°C. The plates were then washed five times with PBS containing 0.1% Tween-20 and incubated with horseradish peroxidase-conjugated antihuman IgG or antihuman IgM (Jackson Immunoresearch Laboratories) for 2 h at room temperature. The plates were again washed five times with PBS containing 0.1% Tween-20. After adding tetramethylbenzidine substrate (Thermo Scientific, Rockford, IL, USA) to each well for color development, the optical density in each well was measured at 630 nm using a PowerScan4 Microplate Reader (DS Pharma Medical Co., Ltd., Osaka, Japan); the calibration curve was subsequently generated and used to determine the total IgG/IgM EBV antibody titers and IgG/IgM levels. To determine the IgG and IgM concentrations in the samples, a Human IgG total Ready-SET-GO! and Human IgM total Ready-SET-GO! were purchased from eBioscience.
Detection of Human Leukocyte Antigen (HLA) Abs in the Culture Supernatants
We collected additional culture supernatant samples and concentrated these by fivefold prior to an Ab screening evaluation based on Flow PRA 60 kits (One Lambda, Canoga Park, CA, USA). Positive cases were subjected to a further Luminex technology-based analysis involving HLA LAB Screen class 1 and/or class 2 single-antigen beads (One Lambda, Canoga Park, CA, USA).
Statistical Analysis
All data are presented as median values and ranges unless otherwise indicated. An analysis of variance or paired or unpaired Student’s t-test was used to evaluate the significance of differences between variables. Prism 7 software (GraphPad Inc., San Diego, CA, USA) was used for statistical analyzes. A two-tailed p-value < 0.05 was considered statistically significant.
Results
Compared with CD27−Naïve B-Cells, CD27+ mBCs Differentiate More Rapidly into PCs
In vitro-cultured CD27+ mBCs and CD27− naïve B-cells were collected after 3, 7, and 10 days and analyzed by flow cytometry analysis. B-cells were identified as CD19+ cells. PCs were identified as CD19+CD38++CD138+ cells. Stimulated CD27+ mBCs were found to differentiate into PCs significantly earlier compared with CD27− naïve B-cells, as determined by the ratio (%) of PCs/total B-cells on day 7 (15.4 ± 6.2 vs. 4.1 ± 0.6%: p < 0.01) (Figure 1A).
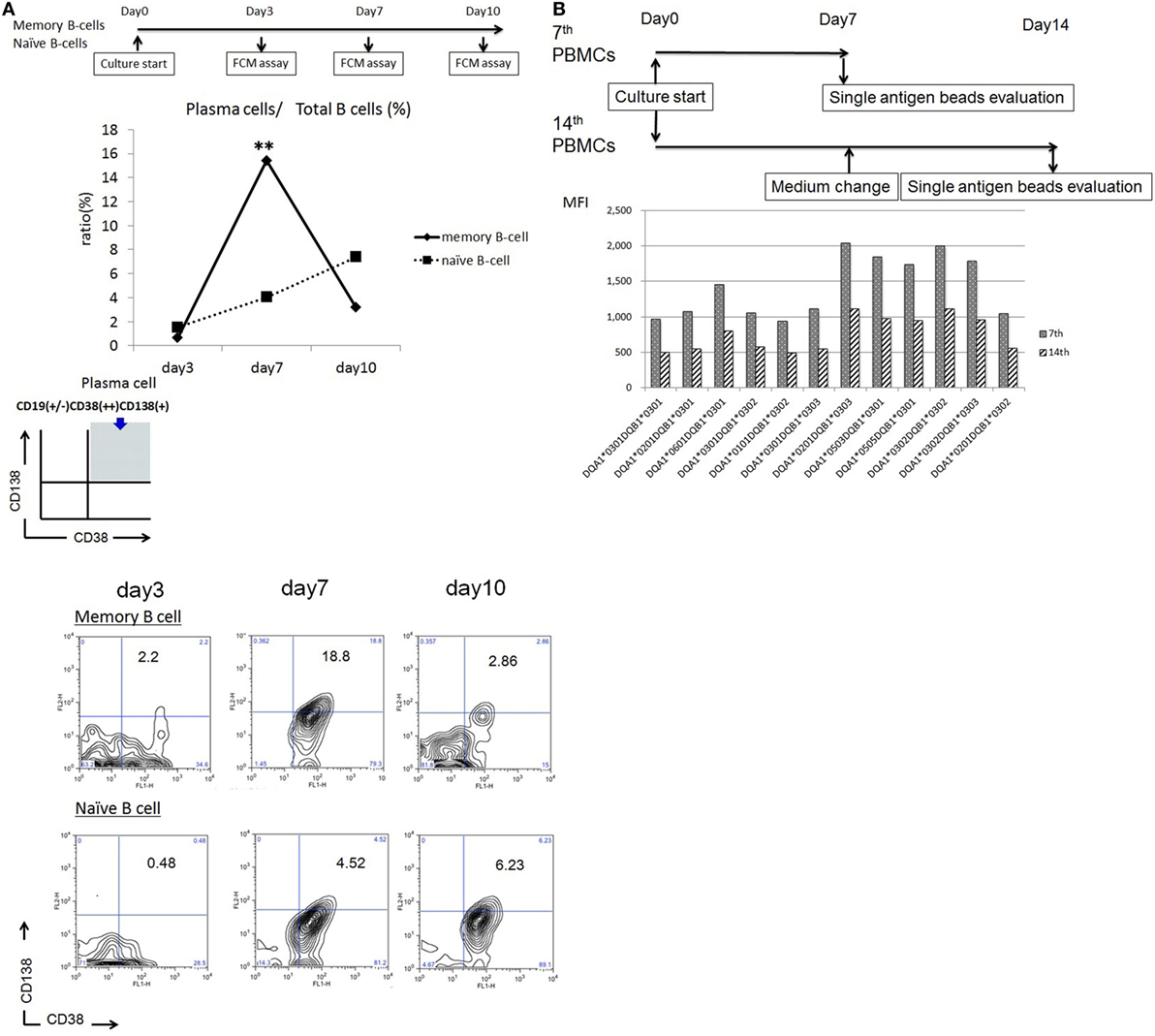
Figure 1. (A) Memory (CD27+) and naïve (CD27−) B cells isolated from single donors were cultured in 24-well flat-bottomed plates (1 × 105 cells/well) in basal B-cell culture medium supplemented as follows: 50 ng/ml interleukin (IL)-21, 2.5 µg/ml phosphorothioate CpG-oligodeoxynucleotide (ODN) 2006, CD40 ligand, and 5 µg/ml anti-polyhistidine monoclonal antibody (mAb) in vitro; the cultured cells were stained with fluorescein isothiocyanate -tagged anti-CD38, phycoerythrin -tagged anti-CD138, and allophycocyanin -tagged anti-CD19 mAbs on days 3, 7, and 10; and subjected to flow cytometry analysis. Total B cells were identified as CD19+ cells. Plasma cells (PCs) were identified as CD19 +/−CD38++CD138+ cells. Differentiation of stimulated CD27+ memory B-cell into PCs was compared with that of CD27− naïve B cells, as determined by the ratio (%) of PCs to total B cells. Memory (CD27+) and naïve (CD27−) B cells were collected from five independent healthy donors, and the data represent five independent experiments. (B) Peripheral blood mononuclear cells obtained from an allograft recipient sensitized to donor human leukocyte antigen were cultured using 50 ng/ml IL-21 and 2.5 µg/ml phosphorothioate CpG-ODN 2006 in the presence of 2.5 µg/ml phytohemagglutinin/leucoagglutinin and 15 ng/ml phorbol myristate acetate in vitro for 1 week, after which the culture medium was replaced under the same conditions, and cells were cultured for another week. Culture supernatants were collected on days 7 and 14 and subjected to evaluation with Luminex single-antigen beads.
Antigen-Specific mBCs Could Be Stimulated to Differentiate into PCs by Day 7 under Immunosuppression
Peripheral blood mononuclear cells obtained from an allograft recipient sensitized to donor HLA were cultured in vitro for 1 week. Following this, the culture medium was replaced and cells were cultured for another week. Culture supernatants were collected on day 14. As a result, HLA Abs produced in the PBMC culture supernatants from days 0 to 7 had a higher concentration than those produced from days 7 to 14 (Figure 1B).
In Vitro Assay Evaluation of IgG/IgM Production in PBMC Culture Supernatant
Peripheral blood mononuclear cells were cultured as described above in the presence or absence of 2.5 µg/ml PHA-L and 15 ng/ml PMA. A comparison of IgG/IgM levels in the culture supernatants revealed significant differences in both IgG and IgM levels in the presence and absence of PHA-L and PMA (1,367.8 ± 443.1 vs. 314.6 ± 198.6 ng/ml, p < 0.01 and 4,540.5 ± 1,953.1 vs. 1,537.9 ± 879.4 ng/ml, p < 0.05, respectively) (Figure 2A).
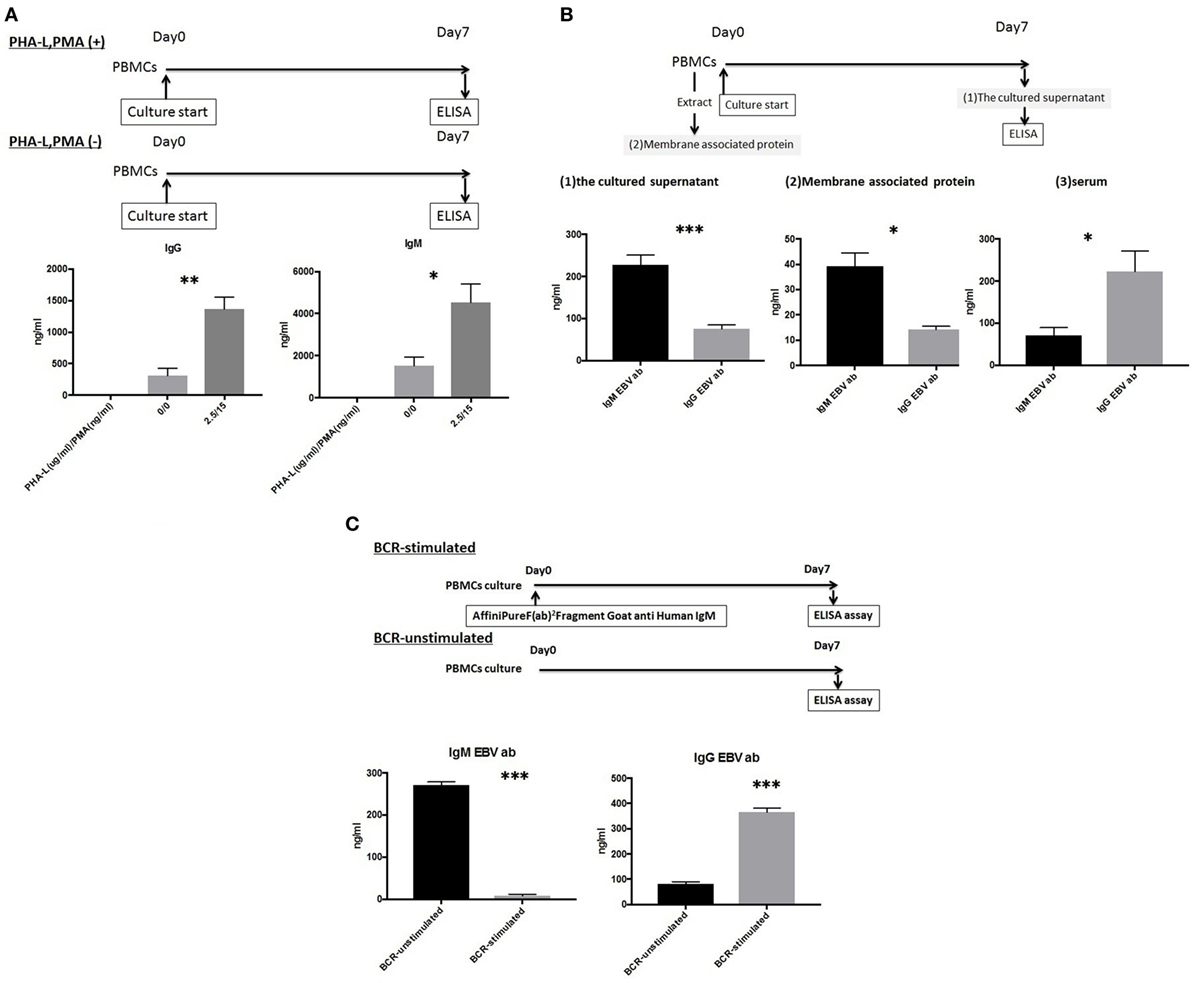
Figure 2. (A) Peripheral blood mononuclear cell (PBMCs) from 10 healthy donors were cultured using 50 ng/ml interleukin (IL)-21 and 2.5 µg/ml phosphorothioate CpG-oligodeoxynucleotide (ODN) 2006 and in the presence or absence of 2.5 µg/ml phytohemagglutinin/leucoagglutinin (PHA-L) and 15 ng/ml phorbol myristate acetate (PMA) in vitro for 1 week. IgG and IgM levels in the supernatants were quantified using enzyme-linked immunosorbent assay (ELISA). (B) PBMCs from 10 healthy donors were cultured using 50 ng/ml IL-21 and 2.5 µg/ml phosphorothioate CpG-ODN 2006 in the presence of 2.5 µg/ml PHA-L and 15 ng/ml PMA in vitro for 1 week. EBV-specific IgG and IgM antibodies (Abs) in cell supernatants were quantified using ELISA (1), extracted membrane-associated proteins. Membrane-associated proteins were extracted from non-cultured PBMCs (5 × 106 cells). EBV-specific IgG and IgM Abs in extracted membrane-associated proteins were quantified using ELISA (2), and sera. EBV-specific IgG and IgM Abs in serum were quantified using ELISA (3). (C) PBMCs from five healthy donors were cultured using 50 ng/ml IL-21 and 2.5 µg/ml phosphorothioate CpG-ODN 2006 and in the presence or absence of the IgM-B-cell receptor (BCR) in vitro for 1 week, and EBV-specific IgG and IgM Ab levels in supernatants were quantified using ELISA. For IgM-BCR cross-linking, we cultured PBMCs (5 × 105 cells/well, 24-well flat-bottomed plate) with IL-21 and phosphorothioate CpG-ODN 2006 supplemented with Affini Pure F(ab)2 Fragment Goat antihuman IgM (5.2 µg/ml) in vitro for 1 week, and IgG and IgM levels in supernatant were quantified using ELISA. The data represent five independent experiments, and graphs indicate the means ± SEMs. Significant changes from baseline were evaluated using an analysis of variance and are indicated with asterisks (*p < 0.05, **p < 0.01, ***p < 0.001).
Evaluation of IgG/IgM EBV-Specific Abs in Culture Supernatant Using an Established In Vitro Assay
Both IgG and IgM EBV-specific Abs were detectable in the cultures, and significant differences in the levels of these isotypes were observed (74.99 ± 30.33 vs. 228.11 ± 70.80; p < 0.001). Significant differences in the serum levels of IgG and IgM isotypes were also observed (222.48 ± 154.16 vs. 70.33 ± 59.54; p < 0.05). We further evaluated the levels of membrane-associated Abs on non-cultured PBMCs and observed a significant difference between IgG and IgM (14.16 ± 4.18 vs. 39.22 ± 16.6; p < 0.05) (Figure 2B).
Cross-Linking-Induced IgM-BCR Signaling Mediates the Class-Switching of EBV-Specific IgM mBCs and IgM Naïve B-Cells
We next investigated the effect of cross-linking-induced IgM-BCR signaling on the differentiation of EBV-specific IgM mBCs in an in vitro PBMC culture and not in selected mBCs.
An ELISA was used to evaluate supernatants from PBMCs cultured in the presence or absence of 5.2 µg/ml Affini Pure F(ab)2 Fragment Goat antihuman IgM. Significant differences in the levels of EBV-specific IgG and IgM Abs were observed between BCR-stimulated and unstimulated cultures (364.89 ± 23.27 vs. 81.57 ± 12.76 ng/ml, p < 0.001 and 8.3 ± 5.40 vs. 271.18 ± 10.51 ng/ml, p < 0.001, respectively) (Figure 2C).
The Presence of Growth Factors and Cytokines Produced by Activated T-Cells, Macrophages, and Dendritic Cells Promoted the Differentiation of IgG and IgM mBCs into PCs
IgG and IgM mBCs were cultured as described above for 1 week in the presence or absence of 5% PBMCs supernatant. An ELISA analysis of culture supernatants revealed significant differences in the levels of IgG or IgM Abs produced in the presence and absence of 5% PBMC supernatant, with IgG levels (1,334.5 ± 122.0 vs.264.5 ± 89.9 ng/ml, p < 0.001) detected in IgG mBC culture supernatant and IgG levels (82.0 ± 5.7 vs. 76.3 ± 2.7 ng/ml, p > 0.05)/IgM levels (1,244.2 ± 674.0 vs. 336.3 ± 125.0 ng/ml, p < 0.05) detected in IgM mBC culture supernatant (Figure 3A).
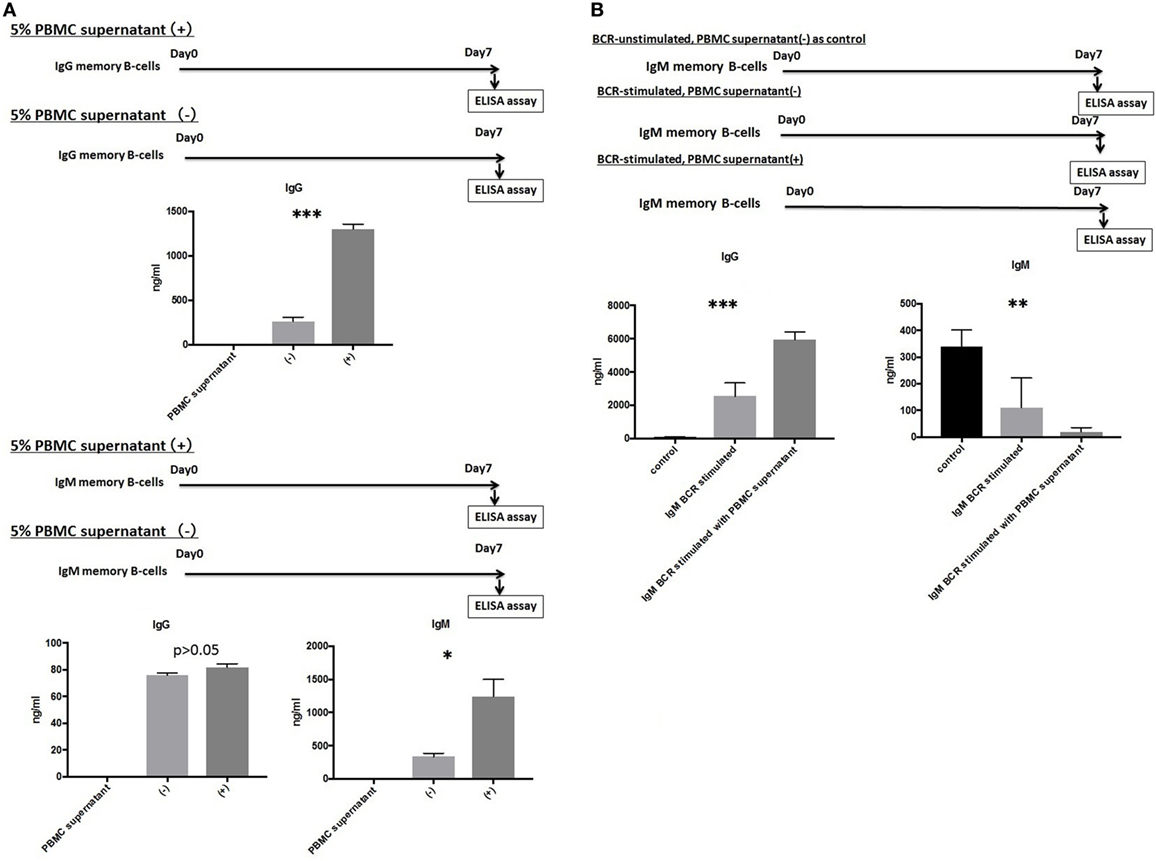
Figure 3. (A) IgG and IgM memory B-cell (mBCs) were separated from peripheral blood mononuclear cells (PBMCs) from five healthy donors and cultured with interleukin (IL)-21 and phosphorothioate CpG-oligodeoxynucleotide (ODN) 2006 and in the presence or absence of 5% PBMC supernatant in vitro for 1 week. IgG and IgM levels in supernatant were quantified using enzyme-linked immunosorbent assay (ELISA). (B) IgM mBCs separated from the PBMCs of five healthy donors were cultured in vitro for 1 week with IL-21, phosphorothioate CpG-ODN 2006, and one of the following conditions: IgM-B-cell receptor (BCR) stimulated with 5% PBMC supernatant; IgM-BCR stimulated without 5% PBMC supernatant; and IgM-BCR unstimulated without 5% PBMC supernatant (control). For IgM-BCR cross-linking, we cultured PBMCs (1 × 105 cells/well, 24-well flat-bottomed plate) with IL-21 and phosphorothioate CpG-ODN 2006 supplemented with Affini Pure F(ab)2 fragment goat antihuman IgM (5.2 µg/ml) in vitro for 1 week. IgG and IgM levels in supernatant were quantified using ELISA. The data represent five independent experiments, and graphs indicate the means ± SEMs. Significant changes from baseline were evaluated using an analysis of variance and are indicated with asterisks (*p < 0.05, **p < 0.01, ***p < 0.001).
IgM-BCR Cross-Linking Is Indispensable for IgM mBC Class-Switching and Is Promoted by Growth Factors and Cytokines Produced by Activated T-Cells, Macrophages, and Dendritic Cells
We next investigated the effect of IgM-BCR cross-linking and PBMCs supernatant supplementation on the biological processes of IgM mBCs cultured in vitro in the presence of IL-21 and CpG-ODN. Cells were IgM-BCR stimulated or not, and cultured in the presence or absence of 5% PBMC supernatant (control: unstimulated, no PBMC supernatant). Significant intergroup differences in the levels of IgG (p < 0.001) and IgM (p < 0.01) were observed (Figure 3B).
Detectable IgM and IgG HLA Class I and II-Specific Abs in Supernatants of Cultured PBMCs from Kidney Allograft Recipients Varied by Graft Function Status
Peripheral blood mononuclear cells from allograft kidney recipients with stable graft function or biopsy-proven Ab-mediated rejection (ABMR) (see Table 1 for patient characteristics) and de novo sensitization to donor-specific antigens were cultured in vitro for 1 week. Subsequently, the cultured supernatants were analyzed using a Flow PRA screening test. A comparison of IgG and IgM HLA Abs levels (%) in the supernatants of these cultures revealed significant differences related to graft function stability (class I: p < 0.05, p < 0.05, respectively; class II: p < 0.05, p < 0.001, respectively; Figure 4).
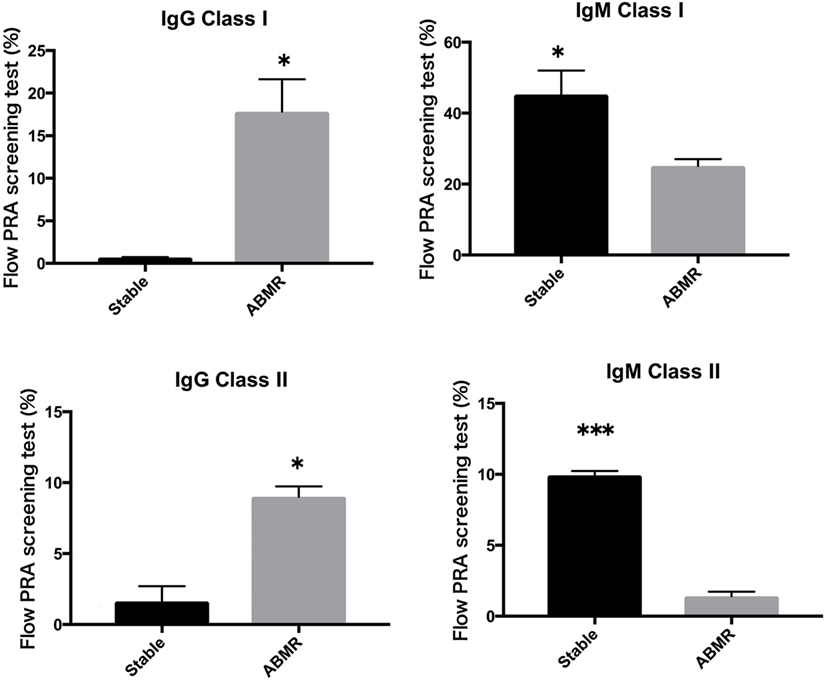
Figure 4. Peripheral blood mononuclear cells were collected from 16 kidney allograft recipients who were sensitized de novo to donor-specific antigens and cultured PBMCs from 10 healthy donors who were not sensitized to human leukocyte antigen. These were then cultured in the presence of the following additives: 50 ng/ml interleukin-21 and 2.5 µg/ml phosphorothioate CpG-oligodeoxynucleotide (ODN) 2006 in the presence of 2.5 µg/ml phytohemagglutinin/leucoagglutinin and 15 ng/ml phorbol myristate acetate in vitro for 1 week. The supernatants were subjected to Flow PRA screening. Stable graft function and biopsy-confirmed antibody-mediated rejection were identified using Banff scores. The data represent 16 independent experiments, and graphs indicate the means ± SEMs. Significant changes from baseline were evaluated using an analysis of variance and are indicated by asterisks (*p < 0.05, **p < 0.01, ***p < 0.001).
Higher Frequencies of DSA-Specific IgM mBCs Relative to IgG mBCs Are Usually Observed in the Peripheral Blood
Peripheral blood mononuclear cells from allograft kidney recipients with de novo donor-specific antigen sensitization and stable graft function were cultured in vitro for 1 week. A Luminex single-antigen bead analysis mainly detected IgM DSAs the culture supernatants (Figure 5).
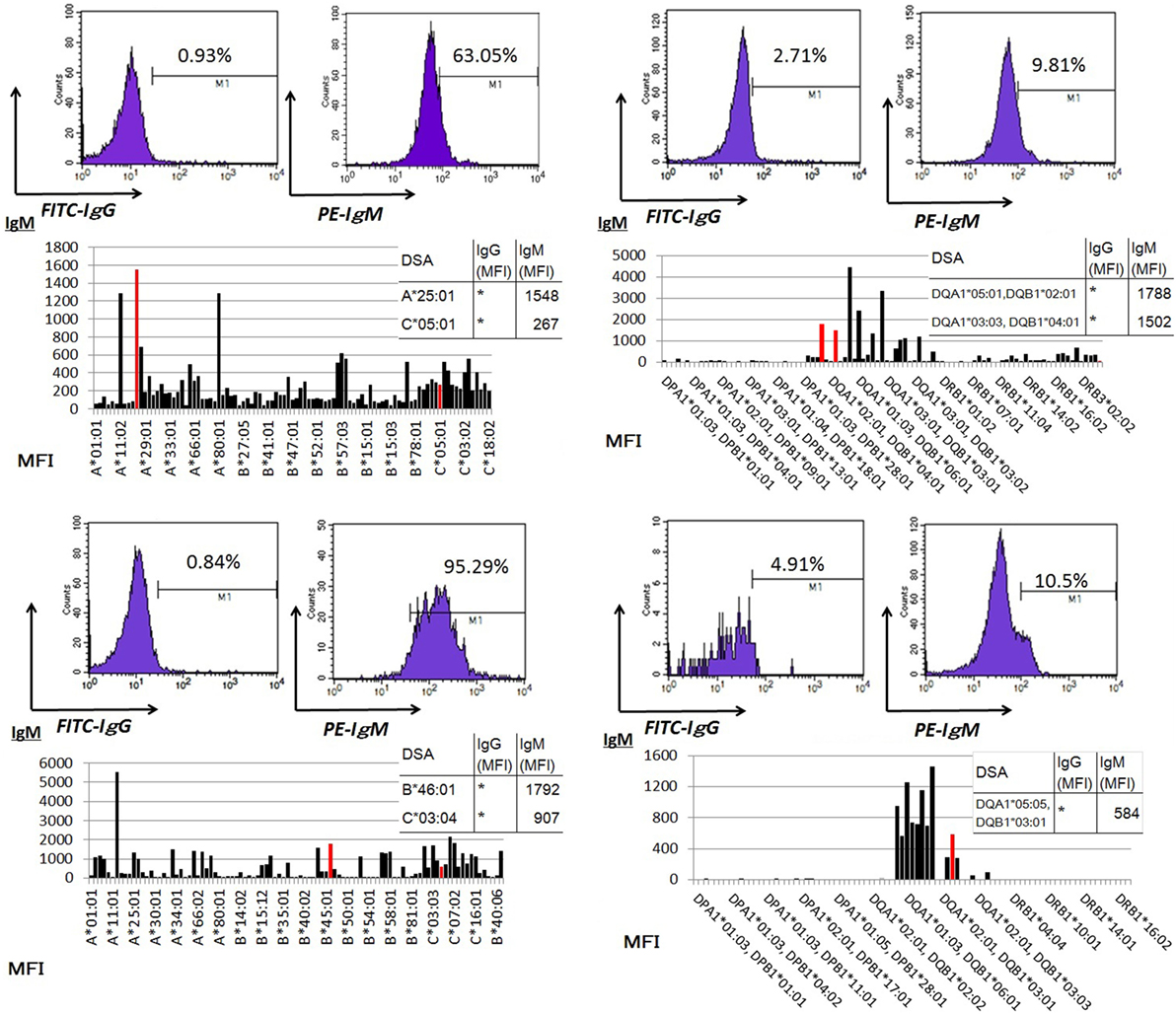
Figure 5. Peripheral blood mononuclear cells were cultured using 50 ng/ml interleukin-21 and 2.5 µg/ml phosphorothioate CpG-oligodeoxynucleotide 2006 in the presence of 2.5 µg/ml phytohemagglutinin/leucoagglutinin and 15 ng/ml phorbol myristate acetate in vitro for 1 week. The supernatants were subjected to Flow PRA screening, and positive cases underwent an additional Luminex single-antigen beads evaluation. PBMCs were collected from four kidney allograft recipients with stable graft function who were sensitized de novo to donor-specific antigens and cultured under in vitro assay conditions. The data represent four independent experiments. The red column indicates DSAs. * indicates that human leukocyte antigen antibodies were not detected by Flow PRA screening and the supernatant was not analyzed by Luminex single-antigen bead evaluation.
IgG DSA Production in PBMC Culture Increases following the In Vivo Activation of Humoral Immunity against a Donor-Specific Antigen
Peripheral blood mononuclear cells from allograft kidney recipients with de novo donor-specific antigen sensitization and biopsy-confirmed ABMR were cultured in vitro for 1 week. A Luminex analysis of culture supernatants mainly detected IgG DSAs (Figure 6).
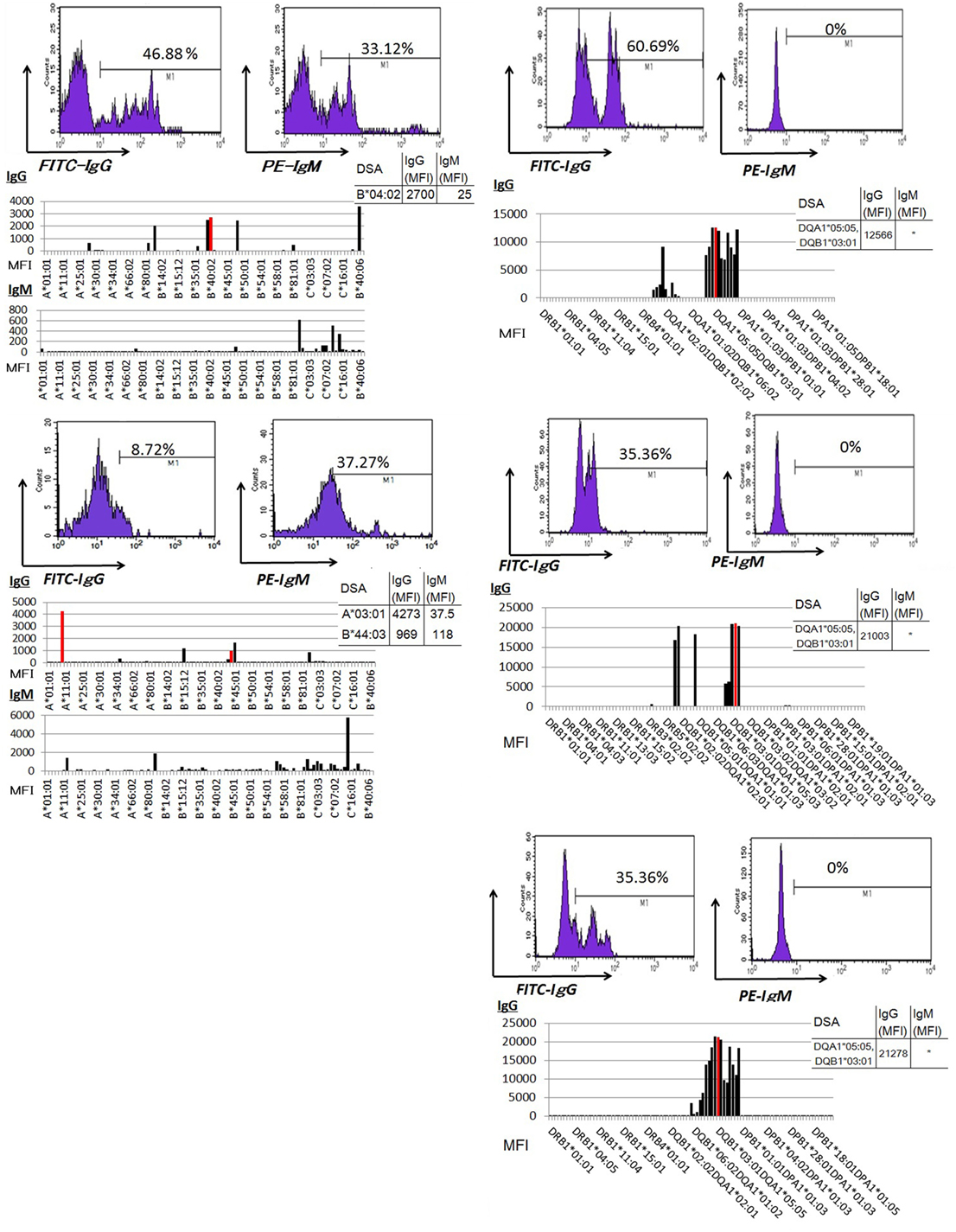
Figure 6. Peripheral blood mononuclear cells were cultured using 50 ng/ml interleukin-21 and 2.5 µg/ml phosphorothioate CpG-oligodeoxynucleotide 2006 in the presence of 2.5 µg/ml phytohemagglutinin/leucoagglutinin and 15 ng/ml phorbol myristate acetate in vitro for 1 week. The supernatants were subjected to Flow PRA screening, and positive cases underwent an additional Luminex single-antigen beads evaluation. PBMCs from five kidney allograft recipients with biopsy-confirmed antibody-mediated rejection were cultured under in vitro assay. The data represent five independent experiments. The red column indicates DSAs. * indicates that human leukocyte antigen antibodies were not detected by Flow PRA screening and the supernatant was not analyzed by Luminex single-antigen bead evaluation.
IgG-Type DSAs Were Detectable in the Serum Regardless of Humoral Immune Activity against Donor-Specific Antigens, Whereas IgM Type DSA Detection Was Unreliable
Sera from donor-specific antigen-sensitized and non-sensitized healthy donors were subjected to a Luminex analysis. IgG DSAs were detectable in sera from one allograft kidney recipient with stable graft function and two allograft kidney recipients with biopsy-proven antibody-mediated rejection (Figure 7A). By contrast, the level of IgM type DSAs was significantly lower compared to that of IgG- and many IgM type HLA Abs were detected broadly in the sera. In addition, IgM HLAs were detected in the sera from non-sensitized healthy donors; these were not detected in PBMC culture supernatants (Figure 7B).
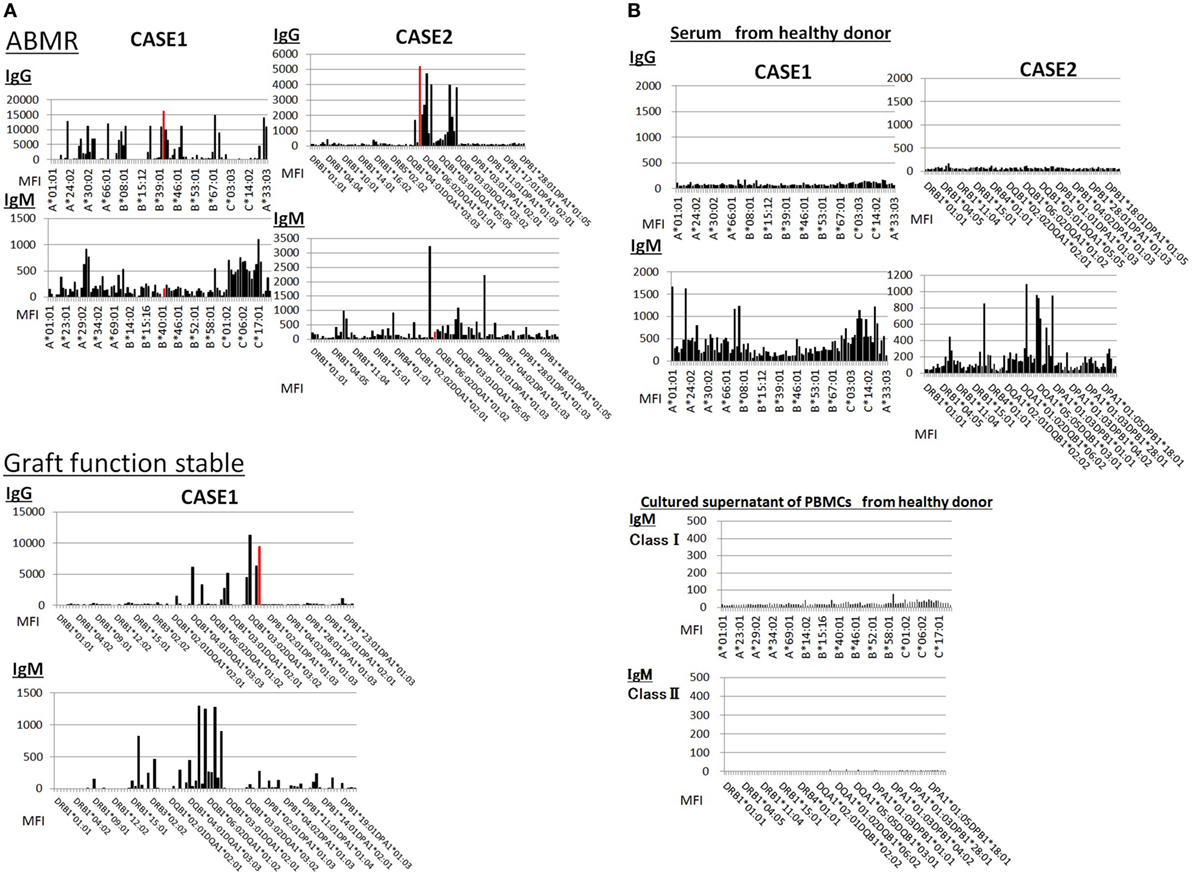
Figure 7. (A) Serum samples were collected from one kidney allograft recipients with stable graft function and two kidney allograft recipients with biopsy-proven Ab-mediated rejection (ABMR) who were sensitized de novo to donor-specific antigens and subjected to a Luminex single-antigen evaluation. The data represent six independent experiments. The red column indicates DSAs. (B) Serum samples were collected from two healthy donors who were not sensitized to human leukocyte antigens and subjected to a Luminex single-antigen evaluation. Peripheral blood mononuclear cells (PBMCs) obtained from healthy donors who were not sensitized by human leukocyte antigen were cultured using 50 ng/ml interleukin -21 and 2.5 µg/ml phosphorothioate CpG-oligodeoxynucleotide 2006 in the presence of 2.5 µg/ml phytohemagglutinin/leucoagglutinin and 15 ng/ml phorbol myristate acetate in vitro for 1 week, and culture supernatants were analyzed by Luminex single-antigen evaluation. The data represent six independent experiments.
Discussion
In context of transplantation, the development of an in vitro T-cell assay for drug sensitivity has facilitated better control of T-cell-mediated rejection, which occurs during the early post-procedural stage (16, 17). By contrast, antibody-mediated rejection has not been well addressed, and effective early diagnostic and treatment methods have not been developed (18–21). Currently, no available humoral immunosuppressive agent can control PC growth and survival, as these cells can survive independently of helper T-cells (22). Furthermore, Abs produced by these PCs might be absorbed into target tissues or form complexes with target antigens (23, 24), thus causing irreversible disease progression (25, 26).
Therefore, a method to detect mBCs prior to PC differentiation is urgently needed to ensure the timely initiation of essential treatment. Fortunately, the identification and clarification of the target antigens of Ab-associated diseases should theoretically allow the detection of specific Ab-producing mBCs in the periphery using minimally invasive diagnostic methods.
Some reports suggest that enzyme-linked immunospot assays can be used to detect antigen-specific mBCs (27, 28). However, we further expect that a useful in vitro assay for the analysis of mBCs-derived Abs in the supernatants will allow drug sensitivity tests, evaluate the differentiation of mBCs into PCs, and contribute to the development of more effective treatments for controlling Ab-associated disease.
Previously, Haneda et al. successfully demonstrated the evaluation of immunosuppressive sensitivity in an in vitro B-cell assay originally developed by Jourdan et al. (29) to quantitate IgG in culture supernatants (30). We note, however, that the total B-cell population includes both naïve B-cells and mBCs (31, 32). Because IL-21, CD40 ligand, and CpG-ODN have been reported to induce IgG class-switching in vitro, IgG Abs from a total B-cell population might also include naïve B-cell-derived IgG Abs (33–37). We, therefore, urge the use of selective assays to evaluate specifically mBCs-derived Abs in culture supernatants.
Some researchers have attempted to induce the in vitro differentiation of CD19+ B-cells into PCs during analyses of targeted antigen-specific Abs in culture supernatants. However, we found many reports stating that mBCs require assistance from antigen-specific helper T-cells in a cell–cell contact-dependent manner (38–42). Therefore, we attempted to more efficiently culture PBMCs in vitro to collect mBC-derived Abs from culture supernatants.
In our PBMC cultures, PHA-L was used to activate helper T-cells, which subsequently produced growth factors and cytokines (including CD40 ligand). PMA, a tyrosine-protein kinase activator, was added to promote TNF-α production from macrophages (43). CpG-ODN was added to induce B-cell proliferation and promote dendritic cell and macrophage maturation by binding to TLR-7, ultimately leading to helper T-cell activation. IL-21 was added to promote the differentiation of mBCs into PCs after stimulation through the BCR and CD40; this cytokine also activates helper T-cells through IL-21 receptor (44, 45). In summary, we expect that our in vitro culture conditions will be more suitable for mBC growth and survival, compared to previously reported conditions.
We further note that mBC-derived Abs should be collected selectively to address the possibility that natural Abs, including naïve B-cell-derived IgM Abs might inhibit the detection of targeted antigen-specific Abs in form of pentamers (46, 47). Despite a lack of previous stimulation, naïve B-cells possess the ability to produce IgM Abs against foreign antigens within context of a primary immune response. We, therefore, focused on differences in the periods required for the differentiation of naïve B-cells and mBCs into PCs. As mBCs have already been sensitized to antigen, they would be expected to differentiate more rapidly (48, 49). Our in vitro experiments demonstrated that mBCs were stimulated to differentiate into PCs by day 7, or earlier than naïve B-cells.
We further note that our cultures might have contained the TNF ligand superfamily member, APRIL, which is produced by activated macrophages and dendritic cells (50). According to many reports, APRIL supports B-cell proliferation and differentiation, especially during later stage differentiation (51–56). Consequently, the proportions of naïve B-cell-derived Abs in our supernatants may have increased as the culture period increased. Therefore, we focused on the IgG and IgM levels in supernatants from culture day 7 as the most suitable parameter and time point for mBC-derived Abs evaluations after confirming that stimulated CD27+ mBCs were found to differentiate into PCs significantly earlier, compared with CD27− naïve B-cells at that time (Figure 1A).
In this study, we further confirmed our ability to detect both IgG and IgM EBV-specific Abs in culture supernatants using our established in vitro assay, an important point given the high rate of EBV infection history in the population (>90% of healthy adult donors). For this analysis, we took care to enroll only healthy donors who did not exhibit signs of active EBV infection (e.g., fever, swollen lymph nodes, and hepatitis) (14, 57, 58). According to our results, a stable humoral immune reaction against EBV correlated with a higher ratio of peripheral blood IgM mBCs relative to IgG mBCs, suggesting that antigen-specific IgM might be more easily detectable when using a smaller volume of blood (8 ml). By contrast, some reports have indicated that a large quantity of peripheral blood (>20 ml) (12, 14, 26) might be needed to reliably detect antigen-specific IgG in culture supernatants because the frequency of antigen-specific IgG mBCs might vary greatly according to the activation status of an antigen-specific humoral immune response (27).
In addition, our in vitro IgM mBC assay demonstrated that cross-linking-induced IgM-BCR stimulation is indispensable for the class-switching of IgM to IgG, and that growth factors and cytokines produced by activated T-cells, macrophages, and dendritic cells promoted this process. These results suggest that circulating IgM mBCs can undergo class-switching to become IgG mBCs and could thereby cause tissue injury following an antigen–BCR reaction, as BCR cross-linking-mediated stimulation increases the generation of antigen-specific IgG mBCs in the peripheral blood (Figure S1 in Supplementary Material).
For in vivo analysis, we used PBMCs from allograft kidney recipients sensitized de novo to donor-specific antigens because the activation of a humoral immune reaction against these antigens can be evaluated accurately through a kidney biopsy. We found that IgG DSAs were only detectable in the culture supernatants from the cells of kidney allograft recipients with biopsy-proven ABMR, whereas IgM Abs were generally detectable in supernatants from the cells of patients with stable graft function.
On the contrary, PCs in the bone marrow maintain long-term production of antigen-specific IgG Abs in the serum regardless of the antigen–antibody reaction status.
Regarding IgM Abs, we examined the effect of natural IgM Abs on the detection of antigen-specific IgM and found that some IgM HLA Abs were detectable in the sera from healthy donors who had not been sensitized to HLA (59, 60). These false positives indicated the possibility that the patients’ natural Abs could have reacted non-specifically with the Luminex single-antigen beads (Figure 7B).
In addition, the level of IgM-type DSAs was significantly lower than that of IgG type, and many IgM-type HLA Abs were broadly detected in the sera from kidney recipients sensitized with de novo DSAs; it was very difficult to determine whether DSA was produced in serum. Thus, it might indicate that natural IgM Abs might inhibit the detection of IgM DSAs or specific in vivo conditions might be necessary for DSA-specific IgM mBCs differentiation into PCs.
By contrast, we could not detect IgM HLA Abs in the culture supernatants of PBMCs from these healthy donors (Figure 7B). Therefore, our culture condition might not be ideal for the production of natural Abs, whereas IgM (IgD+) mBCs differentiate selectively into PCs through a GC -dependent process and produce antigen-specific Abs.
In conclusion, the simultaneous detection of antigen-specific IgG and IgM from PBMC culture supernatant may provide a more effective and accurate minimally invasive evaluation of Ab-associated pathological conditions, compared to the detection of each type of antigen-specific Ab from the serum.
Furthermore, the establishment of an in vitro assay to collect both antigen-specific IgG and IgM Abs has a possibility of allowing immunosuppressive agent susceptibility tests targeting the differentiation of each type of mBC into PCs. Thus, the combination of detected Abs in the culture supernatant may contribute in introducing a more individualized immunosuppressive therapy.
Additionally, we confirmed the class-switching of IgM mBCs in vitro, and showed that immunosuppressive agent susceptibility tests targeting class-switching from IgM mBCs to IgG are also possible.
In other words, there is a possibility that this study may also be applied to the development of more effective immunosuppressive therapies corresponding to the level of progression of the disease state in context of Ab-associated diseases.
Ethics Statement
This study was carried out in accordance with the ethics committee of Osaka University. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the ethics committee of Osaka University.
Author Contributions
YM designed and wrote paper, did all experiments. RI and ST provided excellent advice.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial of financial relationship that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Atsumi Takayama for supporting us with sample analyzing by Luminex single-antigen beads.
Funding
Department of Advanced Technology for Transplantation, Osaka University Graduate School of Medicine is an endowment department, supported with an unrestricted grant from Miz Company Limited, Chugai Pharmaceutical Co., Ltd., Napajen Pharma, Inc., CSL Behring, Japan Blood Products Organization, Mitsubishi Tanabe Pharma, Takeda Pharmaceutical Company Limited, and Astellas Pharma Inc.
Supplementary Material
The Supplementary Material for this article can be found online at http://journal.frontiersin.org/article/10.3389/fimmu.2017.00794/full#supplementary-material.
Figure S1. Peripheral blood mononuclear cell (PBMCs) collected from two kidney allograft recipients sensitized to de novo DSAs were cultured with interleukin-21 and phosphorothioate CpG-oligodeoxynucleotide 2006 and in the presence or absence of Affini Pure F(ab)2 fragment goat antihuman IgM (5.2 µg/ml) in vitro for 1 week. IgM cross-linking was performed on PBMC culture and not on selected memory B-cells. Culture supernatants were subjected to Flow PRA and Luminex single-antigen beads. The mean fluorescence intensity (MFI) of each human leukocyte antigen antibody from the PBMC-cultured supernatant was compared between B-cell receptor (BCR)-stimulated and -unstimulated culture conditions, and the MFI of the latter was divided by that of the former. The ratios are shown in the graph. Data represent two independent experiments. Red column shows DSAs.
References
1. Hippen KL, Schram BR, Tze LE, Pape KA, Jenkins MK, Behrens TW. In vivo assessment of the relative contributions of deletion, anergy, and editing to B cell self-tolerance. J Immunol (2005) 175(2):909–16. doi:10.4049/jimmunol.175.2.909
2. Moens L, Kane A, Tangye SG. Naïve and memory B cells exhibit distinct biochemical responses following BCR engagement. Immunol Cell Biol (2016) 94(8):774–86. doi:10.1038/icb.2016.41
3. De Silva NS, Klein U. Dynamics of B cells in germinal centres. Nat Rev Immunol (2015) 15(3):137–48. doi:10.1038/nri3804
4. MacLennan IC, Gulbranson-Judge A, Toellner KM, Casamayor-Palleja M, Chan E, Sze DM, et al. The changing preference of T and B cells for partners as T-dependent antibody responses develop. Immunol Rev (1997) 156:53–66. doi:10.1111/j.1600-065X.1997.tb00958.x
5. McHeyzer-Williams LJ, Malherbe LP, McHeyzer-Williams MG. Helper T cell-regulated B cells immunity. Curr Top Microbiol Immunol (2006) 311:59–83.
6. Li Z, Woo CJ, Iglesias-Ussel MD, Ronai D, Scharff MD. The generation of antibody diversity through somatic hypermutation and class switch recombination. Genes Dev (2004) 18(1):1–11. doi:10.1101/gad.1161904
7. Onodera T, Takahashi Y, Yokoi Y, Ato M, Kodama Y, Hachimura S, et al. Memory B cells in the lung participate in protective humoral immune responses to pulmonary influenza virus reinfection. Proc Natl Acad Sci U S A (2012) 109(7):2485–90. doi:10.1073/pnas.1115369109
8. Aiba Y, Kometani K, Hamadate M, Moriyama S, Sakaue-Sawano A, Tomura M, et al. Preferential localization of IgG memory B cells adjacent to contracted germinal centers. Proc Natl Acad Sci U S A (2010) 107(27):12192–7. doi:10.1073/pnas.1005443107
9. Stuart GT, Kim LG. Human IgM+CD27+ B cells: memory B cells or “memory” B cells? J Immunol (2007) 179(1):13–9. doi:10.4049/jimmunol.179.1.13
10. Capolunghi F, Rosado MM, Sinibaldi M, Aranburu A, Carsetti R. Why do we need IgM memory B cells? Immunol Lett (2013) 152(2):114–20. doi:10.1016/j.imlet.2013.04.007
11. Seifert M, Przekopowitz M, Taudien S, Lollies A, Ronge V, Drees B, et al. Functional capacities of human IgM memory B cells in early inflammatory responses and secondary germinal center reactions. Proc Natl Acad Sci U S A (2015) 112(6):E546–55. doi:10.1073/pnas.1416276112
12. Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived PCs. Immunity (1998) 8:363–72. doi:10.1016/j.clim.2012.01.002
13. Han M, Rogers JA, Lavingia B, Stastny P. Peripheral blood B cells producing donor-specific HLA antibodies in vitro. Hum Immunol (2009) 70(1):29–34. doi:10.1016/j.humimm.2008.10.013
14. Sokal EM, Hoppenbrouwers K, Vandermeulen C, Moutschen M, Léonard P, Moreels A, et al. Recombinant gp350 vaccine for infectious mononucleosis: a phase 2, randomized, double- blind, placebo-controlled trial to evaluate the safety, immunogenicity, and efficacy of an Epstein-Barr virus vaccine in healthy young adults. J Infect Dis (2007) 196(12):1749–53. doi:10.1086/523813
15. Bellatin MF, Han M, Fallena M, Fan L, Xia D, Olsen N, et al. Production of autoantibodies against citrullinated antigens/peptides by human B cells. J Immunol (2012) 188(7):3542–50. doi:10.4049/jimmunol.1100577
16. Cerottini JC, Brunner KT. Cell-mediated cytotoxicity, allograft rejection, and tumor immunity. Adv Immunol (1974) 18:67–132. doi:10.1016/S0065-2776(08)60308-9
17. Bunjes D, Hardt C, Röllinghoff M, Wagner H. Cyclosporin A mediates immunosuppression of primary cytotoxic T cell responses by impairing the release of interleukin 1 and interleukin 2. Eur J Immunol (1981) 11(8):657–61. doi:10.1002/eji.1830110812
18. Sellarés J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant (2012) 12(2):388–99. doi:10.1111/j.1600-6143.2011.03840.x
19. Einecke G, Sis B, Reeve J, Mengel M, Campbell PM, Hidalgo LG, et al. Antibody-mediated microcirculation injury is the major cause of late kidney transplant failure. Am J Transplant (2009) 9(11):2520–31. doi:10.1111/j.1600-6143.2009.02799.x
20. Piazza A, Poggi E, Borrelli L, Servetti S, Monaco PI, Buonomo O, et al. Impact of donor-specific antibodies on chronic rejection occurrence and graft loss in renal transplantation: posttransplant analysis using flow cytometric techniques. Transplantation (2001) 71(8):1106–12. doi:10.1097/00007890-200104270-00017
21. Lefaucheur C, Suberbielle-Boissel C, Hill GS, Nochy D, Andrade J, Antoine C, et al. Clinical relevance of preformed HLA donor-specific antibodies in kidney transplantation. Am J Transplant (2008) 8(2):324–31. doi:10.1111/j.1600-6143.2007.02072.x
22. Stuart GT. Staying alive: regulation of plasma cell survival. Trends Immunol (2011) 32(12):595–602. doi:10.1016/j.it.2011.09.001
23. Perrey C, Brenchley PE, Johnson RW, Martin S. An association between antibodies specific for endothelial cells and renal transplant failure. Transpl Immunol (1998) 6(2):101–6. doi:10.1016/S0966-3274(98)80024-5
24. Fedrigo M, Abate D, Sgarabotto D, Feltrin G, Castellani C, Gambino A, et al. P761Antibody mediated rejection related with CMV and EBV infection in heart transplants recipients: a possible relation with infection and complement activation. Cardiovasc Res (2014) 103(Suppl 1):139. doi:10.1093/cvr/cvu098.180
25. McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annu Rev Immunol (2005) 23:487–513. doi:10.1146/annurev.immunol.23.021704.115732
26. Markiewski MM, Lambris JD. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am J Pathol (2007) 171(3):715–27. doi:10.2353/ajpath.2007.070166
27. Lúcia M, Luque S, Crespo E, Melilli E, Cruzado JM, Martorell J, et al. Preformed circulating HLA-specific memory B cells predict high risk of humoral rejection in kidney transplantation. Kidney Int (2015) 88(4):874–87. doi:10.1038/ki.2015.205
28. Heidt S, Roelen DL, de Vaal YJ, Kester MG, Eijsink C, Thomas S, et al. A NOVel ELISPOT assay to quantify HLA-specific B cells in HLA-immunized individuals. Am J Transplant (2012) 12(6):1469–78. doi:10.1111/j.1600-6143.2011.03982.x
29. Jourdan M, Caraux A, De Vos J, Fiol G, Larroque M, Cognot C, et al. An in vitro model of differentiation of memory B cells into plasmablasts and plasma cells including detailed phenotypic and molecular characterization. Blood (2009) 114:5173–81. doi:10.1182/blood-2009-07-235960
30. Haneda M, Owaki M, Kuzuya T, Iwasaki K, Miwa Y, Kobayashi T. Comparative analysis of drug action on B-cell proliferation and differentiation for mycophenolic acid, everolimus, and prednisolone. Transplantation (2014) 97(4):405–12. doi:10.1097/01.TP.0000441826.70687.f6
32. Wu YC, Kipling D, Dunn-Walters DK. The relationship between CD27 negative and positive B cell populations in human peripheral blood. Front Immunol (2011) 2:81. doi:10.3389/fimmu.2011.00081
33. Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature (1998) 393:480–3. doi:10.1038/31002
34. Huggins J, Pellegrin T, Felgar RE, Wei C, Brown M, Zheng B, et al. CpG DNA activation and plasma-cell differentiation of CD27- naive human B cells. Blood (2007) 109:1611–9. doi:10.1182/blood-2006-03-008441
35. He B, Qiao X, Cerutti A. CpG DNA induces IgG class switch DNA recombination by activating human B cells through an innate pathway that requires TLR9 and cooperates with IL-10. J Immunol (2004) 173:4479–91. doi:10.4049/jimmunol.173.7.4479
36. Avery DT, Bryant VL, Ma CS, de Waal Malefyt R, Tangye SG. IL-21-induced isotype switching to IgG and IgA by human naive B cells is differentially regulated by IL-4. J Immunol (2008) 181:1767–79. doi:10.4049/jimmunol.181.3.1767
37. Ström L, Laurencikiené J, Miskiniené A, Severinson E. Characterization of CD40-dependent immunoglobulin class switching. Scand J Immunol (1999) 49:523–32. doi:10.1046/j.1365-3083.1999.00539.x
39. Cahalan MD, Parker I. Close encounters of the first and second kind; T-DC and T-B interactions in the lymph nodes. Semin Immunol (2005) 6:442–51. doi:10.1016/j.smim.2005.09.001
40. Okada T, Cyster JG. B cell migration and interactions in the early phase of antibody responses. Curr Opin Immunol (2006) 3:278–85. doi:10.1016/j.coi.2006.02.005
41. Pape KA, Kouskoff V, Nemazee D, Tang HL, Cyster JG, Tze LE, et al. Visualization of the genesis and fate of isotype-switched B cells during a primary immune responses. J Exp Med (2003) 197(12):1677–87. doi:10.1084/jem.20012065
42. Rau FC, Dieter J, Luo Z, Priest SO, Baumgarth N. B7-1/2 (CD80/CD86) direct signaling to B cells enhances IgG secretion. J Immunol (2009) 183(12):7661–71. doi:10.4049/jimmunol.0803783
43. Craxton A, Magaletti D, Ryan EJ, Clark EA. Macrophage- and dendritic cell – dependent regulation of human B-cell proliferation requires the TNFfamily ligand BAFF. Blood (2003) 101(11):4464–71. doi:10.1182/blood-2002-10-3123
44. Pani G, Kozlowski M, Cambier JC, Mills GB, Siminovitch KA. Identification of the tyrosine phosphatase PTP1C as a B cell antigen receptor-associated protein involved in the regulation of B cell signaling. J Exp Med (1995) 181(6):2077–84.
45. Bryant VL, Ma CS, Avery DT, Li Y, Good KL, Corcoran LM, et al. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J Immunol (2007) 179(12):8180–90. doi:10.4049/jimmunol.179.12.8180
46. Visentin J, Guidicelli G, Couzi L, Merville P, Lee JH, Di Primo C, et al. Deciphering IgM interference in IgG anti-HLA antibody detection with flow beads assays. Hum Immunol (2016) 77(11):1048–54. doi:10.1016/j.humimm.2016.02.008
47. Konvalinka A, Tinckam K. Utility of HLA antibody testing in kidney transplantation. J Am Soc Nephrol (2015) 26(7):1489–502. doi:10.1681/ASN.2014080837
48. Omori SA, Cato MH, Anzelon-Mills A, Puri KD, Shapiro-Shelef M, Calame K, et al. Regulation of class-switch recombination and plasma cell differentiation by phosphatidylinositol 3-kinase signaling. Immunity (2006) 4:545–57. doi:10.1016/j.immuni.2006.08.015
49. Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KG, Dörner T, et al. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat Rev Immunol (2006) 10:741–50. doi:10.1038/nri1886
50. Nadra I, Mason JC, Philippidis P, Florey O, Smythe CD, McCarthy GM, et al. Proinflammatory activation of macrophages by basic calcium phosphate crystals via protein kinase C and MAP kinase pathways: a vicious cycle of inflammation and arterial calcification? Circ Res (2005) 96(12):1248–56. doi:10.1161/01.RES.0000171451.88616.c2
51. Rickert RC, Jellusova J, Miletic AV. Signaling by the TNFR superfamily in B-cell biology and disease. Immunol Rev (2011) 244(1):115–33. doi:10.1111/j.1600-065X.2011.01067.x
52. Matsuda Y, Haneda M, Kadomatsu K, Kobayashi T. A proliferation-inducing ligand sustains the proliferation of human naïve (CD27–) B cells and mediates their differentiation into long-lived plasma cells in vitro via transmembrane activator and calcium modulator and cyclophilin ligand interactor and B-cell mature antigen. Cell Immunol (2015) 295(2):127–36. doi:10.1016/j.cellimm.2015.02.011
53. Bossen C, Schneider P. BAFF, APRIL and their receptors: structure, function and signaling. Semin Immunol (2006) 18:263–75. doi:10.1016/j.smim.2006.04.006
54. Vincent FB, Saulep-Easton D, Figgett WA, Fairfax KA, Mackay F. The BAFF/APRIL system: emerging functions beyond B cell biology and autoimmunity. Cytokine Growth Factor Rev (2013) 24:203–15. doi:10.1016/j.cytogfr.2013.04.003
55. Coquery CM, Erickson LD. Regulatory roles of the tumor necrosis factor receptor BCMA. Crit Rev Immunol (2012) 32:287–305. doi:10.1615/CritRevImmunol.v32.i4.10
56. Lee JJ, Ozcan E, Rauter I, Geha RS. Transmembrane activator and calcium-modulator and cyclophilin ligand interactor mutations in common variable immunodeficiency. Curr Opin Allergy Clin Immunol (2008) 8:520–6. doi:10.1097/ACI.0b013e3283141200
57. Bailey RE. Diagnosis and treatment of infectious mononucleosis. Am Fam Physician (1994) 49(4):879–88.
58. Buchwald DS, Rea TD, Katon WJ, Russo JE, Ashley RL. Acute infectious mononucleosis: characteristics of patients who report failure to recover. Am J Med (2000) 109(7):531–7. doi:10.1016/S0002-9343(00)00560-X
59. Clarke SH, Arnold LW. B-1 cell development: evidence for an uncommitted immunoglobulin (Ig)M+ B cell precursor in B-1 cell differentiation. J Exp Med (1998) 187(8):1325–34. doi:10.1084/jem.187.8.1325
Keywords: in vitro assay, antibody-associated disease, IgM memory B cells, IgG memory B cells, germinal centers
Citation: Matsuda Y, Imamura R and Takahara S (2017) Evaluation of Antigen-Specific IgM and IgG Production during an In Vitro Peripheral Blood Mononuclear Cell Culture Assay. Front. Immunol. 8:794. doi: 10.3389/fimmu.2017.00794
Received: 28 February 2017; Accepted: 22 June 2017;
Published: 10 July 2017
Edited by:
Gilles Blancho, University of Nantes, FranceReviewed by:
Federica Casiraghi, Istituto Di Ricerche Farmacologiche Mario Negri, ItalyLuiza Guilherme, University of São Paulo, Brazil
Paolo Casali, The University of Texas Health Science Center San Antonio, United States
Copyright: © 2017 Matsuda, Imamura and Takahara. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yoshiko Matsuda, yoshikomatsuday@gmail.com
 Yoshiko Matsuda
Yoshiko Matsuda Ryoichi Imamura2
Ryoichi Imamura2