- 1National Key Laboratory of Agricultural Microbiology, Huazhong Agricultural University, Wuhan, China
- 2College of Veterinary Medicine, Huazhong Agricultural University, Wuhan, China
- 3Key Laboratory of Development of Veterinary Diagnostic Products, Ministry of Agriculture, Wuhan, China
Streptococcus suis 2 (SS2) has evolved into a highly invasive pathogen responsible for two large-scale outbreaks of streptococcal toxic shock-like syndrome (STSLS) in China. Excessive inflammation stimulated by SS2 is considered a hallmark of STSLS, even it also plays important roles in other clinical symptoms of SS2-related disease, including meningitis, septicemia, and sudden death. However, the mechanism of SS2-caused excessive inflammation remains poorly understood. Here, a novel pro-inflammatory protein was identified (HP1330), which could induce robust expression of pro-inflammatory cytokines (TNF-α, MCP-1, and IL-1β) in RAW264.7 macrophages. To evaluate the role of HP1330 in SS2 virulence, an hp1330-deletion mutant (Δhp1330) was constructed. In vitro, hp1330 disruption led to a decreased pro-inflammatory ability of SS2 in RAW 264.7 macrophages. In vivo, Δhp1330 showed reduced lethality, pro-inflammatory activity, and bacterial loads in mice. To further elucidate the mechanism of HP1330-induced pro-inflammatory cytokine production, antibody blocking and gene-deletion experiments with macrophages were performed. The results revealed that the pro-inflammatory activity of HP1330 depended on the recognition of toll-like receptor 2 (TLR2). Furthermore, a specific inhibitor of the extracellular signal-regulated kinase 1/2 (ERK1/2) pathways could significantly decrease HP1330-induced pro-inflammatory cytokine production, and western blot analysis showed that HP1330 could induce activation of the ERK1/2 pathway. Taken together, our findings demonstrate that HP1330 contributes to SS2 virulence by inducing TLR2- and ERK1/2-dependent pro-inflammatory cytokine production and influencing in vivo bacterial loads, implying that HP1330 may be associated with STSLS caused by SS2.
Introduction
“Streptococcus suis is responsible for severe economic losses in the worldwide swine industry and poses serious threats to human health” (1). In general, “of the 29 described serotypes, serotype 2 (SS2) is the most prevalent in humans” (2), but human infections with other serotypes also occur sporadically (3). Since “the first human case was reported in Denmark in 1968” (4), to date, >1,500 S. suis infections in humans have been documented worldwide (5). Although most reports concerned sporadic cases of infection, two recent large-scale outbreaks of human SS2 occurred in China (6, 7). In addition, “a large series of 151 S. suis meningitis cases was also reported in southern Vietnam” (8). “SS2 has evolved into a severe pathogen, particularly in light of patients presenting with streptococcal toxic shock-like syndrome (STSLS), indicating that new, highly virulent bacterial variants have emerged recently in Asia” (9).
In previous studies, several virulence-related factors of SS2 were identified, such as capsular polysaccharide, muramidase-released protein, suilysin, subtilisin-like protease, and IgA1 protease (10–12). However, current knowledge regarding the pathogenesis of SS2 infection remains limited, particularly for STSLS (13). In general, streptococcal toxic-shock syndrome (STSS) is toxin-mediated and associated primarily with superantigens. However, no putative superantigen or homologous gene was identified in the genomes of SS2 isolates associated with STSLS, indicating that several unique mechanisms could be involved (14). Excessive inflammation, as a hallmark of SS2 infection, is responsible for most clinical signs of SS2-related pathology leading to meningitis, septicemia, STSLS, and sudden death (7, 15–17). Therefore, explaining the mechanisms of excessive inflammatory responses induced by SS2 could help understand the pathogenesis, even of STSLS caused by SS2. As a Gram-positive bacterium, SS2 produces some common pathogen-associated molecular pattern (PAMP) molecules, including peptidoglycan (PGN), lipoteichoic acid, and lipoproteins, which can induce the release of cytokines and chemokines (18). Indeed, several previous studies have shown that PGN, LTA, and some lipoproteins are associated with SS2 virulence (19–22). However, little evidence indicates that these PAMPs are responsible for excessive inflammatory responses, even STSLS caused by SS2. At present, the mechanism whereby SS2 causes excessive inflammation remains poorly understood.
To explore the mechanisms of excessive inflammation stimulated by SS2, we investigated novel pro-inflammatory mediators of SS2. In our previous study, over 50 extracellular SS2 proteins were expressed in Escherichia coli and purified using a His-tag (18), and these proteins had been previously described as secreted proteins, cell wall proteins, and membrane proteins (23–25). Several novel pro-inflammatory proteins were identified (data not shown), of which HP1330 (encoded by SSUSC84_1330) displayed rather potent pro-inflammatory activity. In present study, we sought to evaluate the role of HP1330 in SS2 infection and elucidate the mechanism through which it induces pro-inflammatory responses.
Materials and Methods
Bacterial Strains, Plasmids, and Growth Conditions
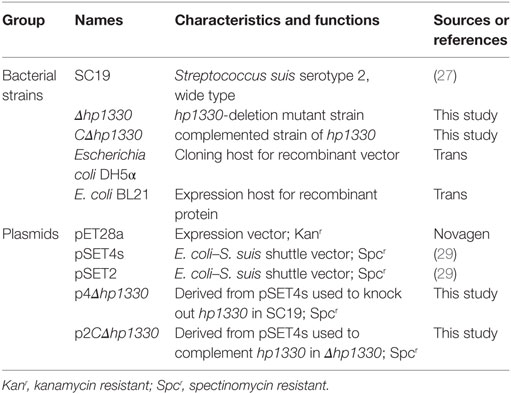
In Table 1, we showed the information of bacterial strains and plasmids used in this study. SS2 strain SC19 was selected as the wild-type (WT) strain, which “was isolated from the brain of a dead pig during the epidemic outbreak in the Sichuan Province of China in 2005” (26). SC19 is highly pathogenic to mice and pigs and can cause STSLS (27). SC19, Δhp1330, and CΔhp1330 were cultured in tryptic soy broth (TSB) or on tryptic soy agar (TSA) plates (Difco, MI, USA) with 10% newborn bovine serum (Sijiqing Biological Engineering Materials Co., Ltd., Hangzhou, China) at 37°C (28).
Preparation of the Recombinant HP1330 Protein
The HP1330 protein was prepared according to published methods (30). Briefly, the hp1330 gene was amplified by PCR using the primers listed in Table 2, and then inserted into the pET28a vector. After the recombinant vector was transformed into E. coli BL21 (DE3) cells, 0.5 mM isopropyl-b-d-thiogalactopyranoside was added to induce expression. Then HP1330 was purified by ultrasonication and Ni-NTA agarose chromatography. Before being used to stimulate RAW264.7 macrophages, HP1330 was confirmed to contain low levels of endotoxin, using the Endotoxin Removal Kit (Genmed Scientifics Inc., USA) and Quantitative Chromogenic Tachypleus Amebocyte Lysate for Endotoxin Detection Kit (Chinese Horseshoe Crab Reagent Manufactory Co., Ltd., Xiamen, China) (31). After passage through a 0.22-µm filter, the HP1330 protein was stored at −80°C.
Cell Culture
“RAW 264.7 macrophages were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (Gibco, USA) at 37°C in a 5% CO2 atmosphere” (32). “Primary mouse macrophages were prepared as described previously” (18). Toll-like receptor 2 (TLR2)-deficient, TLR4-deficient, and WT mice were injected intraperitoneally (i.p.) with 4% thioglycolate (TLR2-deficient and TLR4-deficient mice were obtained from the Collaborative Innovation Center of Model Animal, Wuhan University). Peritoneal exudate cells were harvested 4 days later and identified by microscopic analysis and non-specific esterase staining (33). When >90% of the exudate cells were identified as macrophages, the cells were plated at a density of 106 cells per well in 12-well plates.
Stimulation of RAW 264.7 Cells with HP1330
RAW 264.7 cells were exposed to 10 µg⋅ml−1 HP1330 protein, lipopolysaccharide (LPS, 100 ng⋅ml−1, Sigma), and LPS inhibitor polymyxin B (Poly.B, 10 µg⋅ml−1, Sigma) for 6 h, as described (34).
RNA Extraction and qRT-PCR
After RAW 264.7 cells were stimulated, the expressions of TNF-α, MCP-1, and IL-1β were measured by qRT-PCR as reported previously (35). Briefly, the total RNA of cells was extracted using the TRIzol® reagent (Invitrogen, Paisley, UK). Then, complementary DNA was synthesized from 4 µg of the total RNA by using AMV reverse transcriptase (Takara, Japan), as previously described (36). qRT-PCR was performed using ViiA™ 7 Software (Applied Biosystems) with SYBR green PCR Kit (Roche). All of the primers used in qRT-PCR were listed in Table 2. The relative amounts of target gene expression were normalized with GAPDH housekeeping gene, using 2−ΔΔCt method (37).
Enzyme-Linked Immunosorbent Assays (ELISAs) for Cytokines
“The concentrations of TNF-α, MCP-1, and IL-1β in the cell culture supernatants or serums were determined using commercially available ELISA kits (BioLegend), following the manufacturer’s instructions” (18).
Knockout and Complement of hp1330
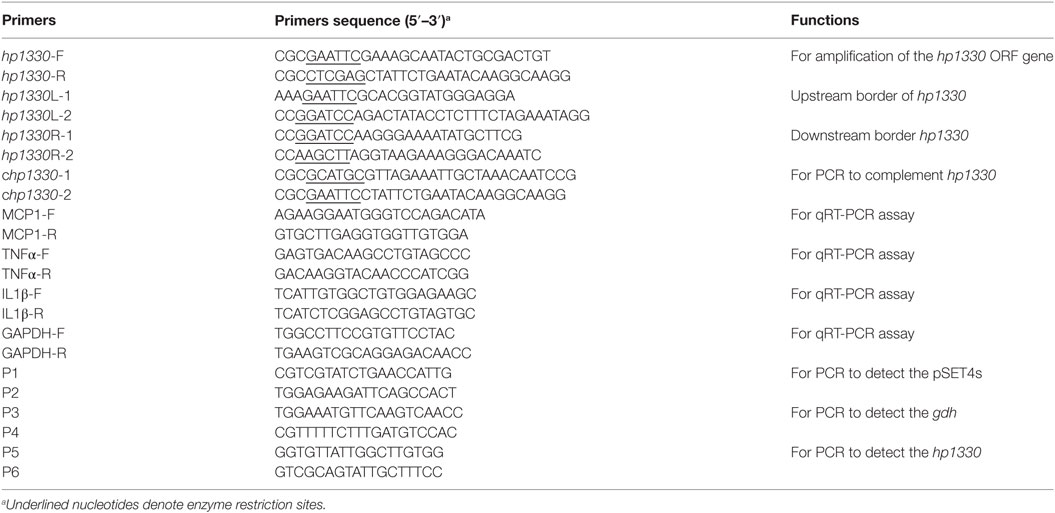
The Δhp1330 mutant strain was constructed as previously described (29). The left (714 bp) and right (688 bp) DNA fragments of hp1330 were prepared from the SC19 genome using PCR with the primers hp1330L-1/2 and hp1330R-1/2, respectively. The products were inserted into the pSET4s vector to generate plasmid p4Δhp1330. Next, the recombinant vector was electrotransformed into SC19 competent cells. The mutant strain was screened based on spectinomycin resistance and thermosensitive suicide of the pSET4s vector. The suspected mutant was verified using three pairs of primers: P1/P2 (to detect the pSET4s vector), P3/P4 (to detect gdh), and P5/P6 (to detect hp1330).
The complemented strain of hp1330 was obtained according to a previous procedure (27). A DNA fragment covering the hp1330 ORF region and its promoter region was prepared by PCR using the primers chp1330-1 and chp1330-2. This fragment was then cloned into pSET2 to generate plasmid p2CΔhp1330. To obtain the complemented strain CΔhp1330, the recombinant plasmid was electrotransformed into Δhp1330.
Experimental Infections In Vitro and In Vivo
In vitro, the WT (SC19), Δhp1330, and CΔhp1330 strains were used to infect RAW 264.7 cells at a dose of 5 × 106 colony-forming units (CFUs). After 6 h, culture supernatants and RAW 264.7 cells were collected for ELISA and qRT-PCR analysis, respectively.
This study was carried out in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals Monitoring Committee of Hubei Province, China, and the protocol was approved by the Committee on the Ethics of Animal Experiments at the College of Veterinary Medicine, Huazhong Agricultural University. For virulence studies, 6-week-old female C57BL/6 mice (10 mice/group) were challenged i.p. with 6 × 108 CFUs of the SC19, Δhp1330, or CΔhp1330 strain. The infected mice were monitored for clinical signs and survival times for 7 days. In addition, another batch of 60 6-week-old female C57BL/6 mice was randomly assigned to three groups with 20 mice/group and challenged i.p. with a non-lethal dose (2 × 108 CFUs per mouse) of the SC19, Δhp1330, or CΔhp1330 strain. At 3, 6, 9, and 12 h postinfection, an equal number of mice in each group were sacrificed to collect blood, which was used for bacteria counts and ELISAs to measure TNF-α, MCP-1, and IL-1β production (26, 38).
Investigating the Recognition Receptor of HP1330
It is reported that “TLR2 is the major immune receptor involved in S. suis recognition” (39, 40). To investigate which receptor was specifically responsible for HP1330-mediated cytokine upregulation, we first detected TLR2 changes after HP1330 stimulation by qRT-PCR, with TLR4 as a control. Second, antibody blocking assays were performed as previously described (41). After pretreatment with 8 µg of an anti-TLR2 (BioLegend) or anti-TLR4 (BioLegend) antibody for 30 min, RAW264.7 cells were incubated with 10 µg⋅ml−1 HP1330 for 6 h. The concentrations of TNF-α, MCP-1, and IL-1β in the culture supernatants were determined by ELISA. On the basis of these experiments, we identified the recognition receptor of HP1330. Finally, TLR2−/− and TLR4−/− macrophages were isolated from TLR2−/− and TLR4−/− mice to verify the above results.
Analysis of HP1330-Induced Cellular Signal-Transduction Pathways
For cell-signaling analysis, RAW 264.7 cells were incubated with the following specific inhibitors 30 min prior to the addition of HP1330, including SB203580 (for p38 MAPK, 10 µM; Cayman Chemical), SP600125 (for JNK, 10 µM; Cayman Chemical), pyrrolidine dithiocarbamate (PDTC; for NF-κB, 20 µM; Sigma), LY294002 (for PI3K, 20 µM; Cayman Chemical), and U0126 (for ERK1/2, 10 µM; Cayman Chemical) (42). After HP1330 stimulation for 6 h, culture supernatants were collected for ELISAs to measure TNF-α, MCP-1, and IL-1β production. According to the conditions used for cytokine activation, we screened for the signal-transduction molecule induced by HP1330.
To verify the above analysis results, the phosphorylation of HP1330-induced signal-transduction molecules was confirmed by western blotting (43). Briefly, after stimulation with 10 µg⋅ml−1 HP1330 for 6 h, RAW 264.7 cells were washed once with cold PBS and incubated on ice for 15 min using radioimmunoprecipitation assay lysis buffer with phosphatase inhibitors (Roche). The supernatants were collected, and their protein concentrations were quantified by Bradford protein assay. Then 40 µg proteins were resolved on a 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel, followed by electrotransfer to a 0.22-µm nitrocellulose membrane. Activation of ERK1/2 was assessed using a specific antibody against phosphorylated ERK1/2 (Cell Signaling Technology). In addition, β-actin was detected by an anti-β-actin antibody (Wuhan PMK Biotechnology Co., Ltd.), as an internal control. Protein bands were visualized by incubation with a horseradish peroxidase-conjugated secondary antibody and then detected using the ECL System (Amersham Life Science, Arlington Heights, IL, USA).
Statistical Analysis
Statistical analyses were performed by an unpaired Student’s t-test. All assays were repeated at least three times, and a P value < 0.05 was considered significant. In the figures, * and ** represent P values < 0.05 and <0.01, respectively.
Results
HP1330-Induced Expression of Pro-inflammatory Cytokines in RAW 264.7 Macrophages
The purity of the recombinant protein HP1330 was analyzed by SDS-PAGE (Figure 1A) and western blotting (Figure 1B), revealing that HP1330 was successfully purified. After endotoxin removal, the protein concentration and endotoxin level of HP1330 were approximately 1.3 mg⋅ml−1 and 0.01 endotoxin unit⋅ml−1, respectively. Next, we investigated the pro-inflammatory activity of HP1330 in RAW 264.7 cells. As shown in Figure 2, HP1330 significantly induced TNF-α, MCP-1, and IL-1β expression, and the cytokine response induced by HP1330 was not reduced by Poly.B, an inhibitor of negatively charged molecules like LPS that is normally used to exclude the effects of contaminating endotoxins (44, 45). These results showed that HP1330 has robust pro-inflammatory activity in RAW 264.7 cells, and that residual bacterial endotoxins were not responsible for the effect.
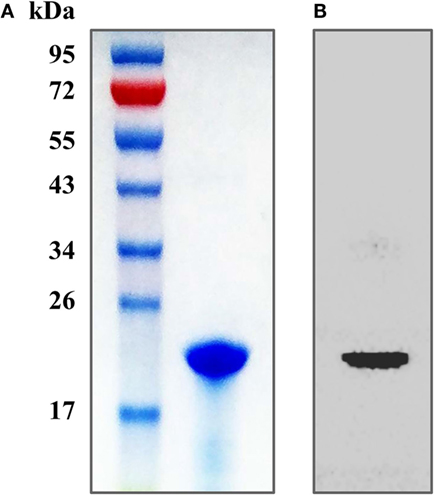
Figure 1. Purification of the recombinant HP1330 protein. (A) SDS-PAGE analysis. (B) Western blot analysis, the blot was probed with an anti-His tag monoclonal antibody (Cali-Bio).
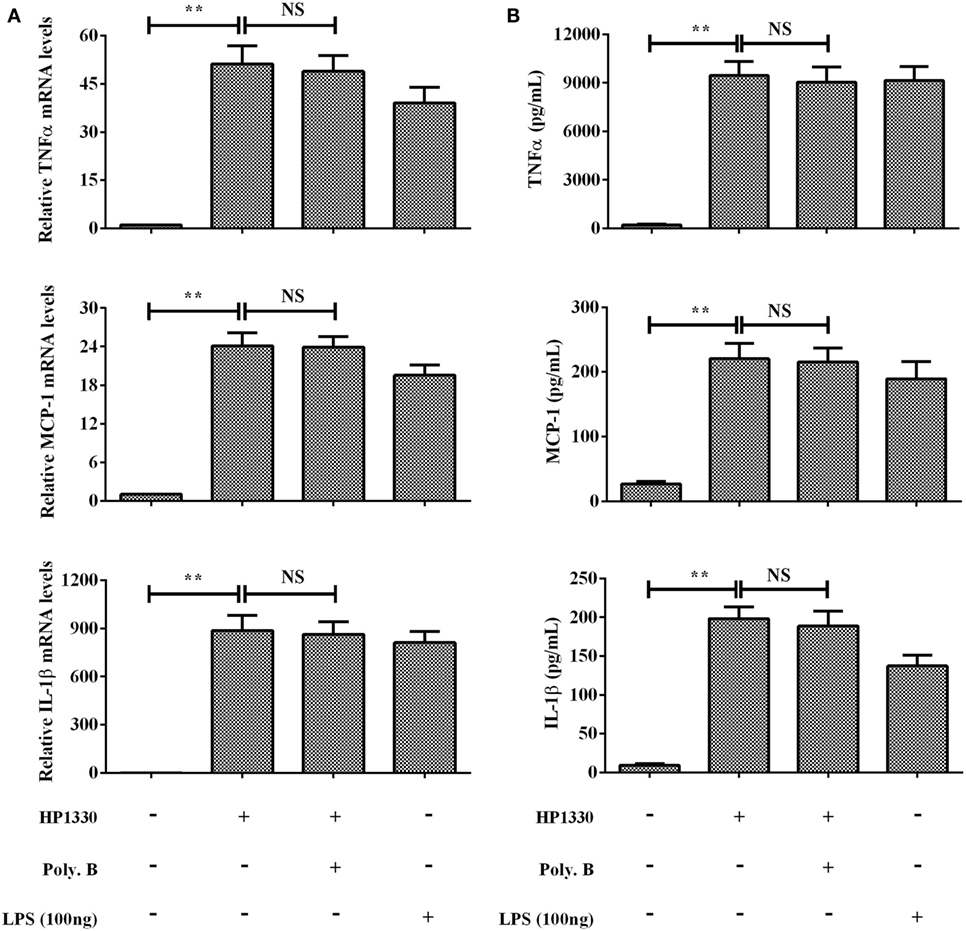
Figure 2. Induction of cytokine mRNA and protein expression in RAW 264.7 macrophages by stimulation with recombinant HP1330. RAW 264.7 macrophages were treated with 100 ng⋅ml−1 lipopolysaccharide (LPS) (positive control) or 10 µg⋅ml−1 HP1330 protein in the absence or presence of 10 µg⋅ml−1 Poly.B for 6 h, or with culture medium (negative control). (A) The cytokine mRNA levels were then determined by qRT-PCR, (B) and the protein levels of TNF-α, MCP-1, and IL-1β in the culture supernatants were determined by enzyme-linked immunosorbent assay. The bars represent the SEMs, based on three independent experiments. **P < 0.01.
Heat Inactivation of HP1330 Blocked Cytokine Induction
To determine whether heat treatment influences the pro-inflammatory activity of HP1330, we next pretreated HP1330 using previously reported conditions [100°C for 10 min (42)] before adding it to RAW264.7 cells. Heat-treated HP1330 failed to induce TNF-α, MCP-1, and IL-1β production in RAW264.7 cells after a 6-h stimulation (Figure 3). These results indicated that the pro-inflammatory activity of HP1330 in RAW 264.7 cells is heat-sensitive.
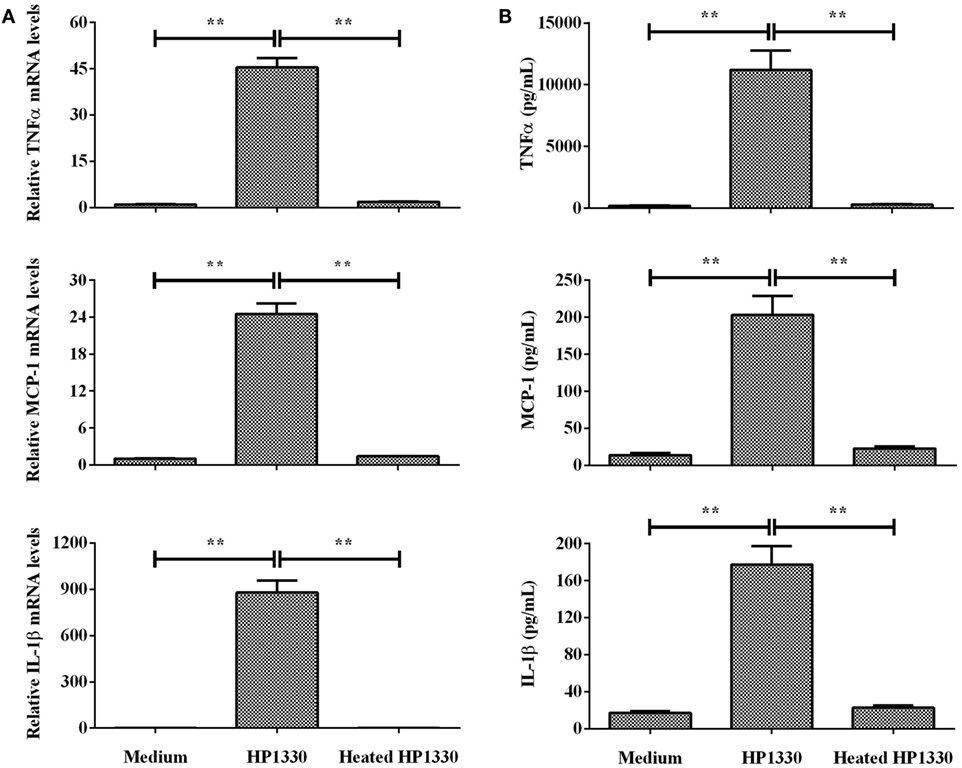
Figure 3. Effect of heat inactivating HP1330 on pro-inflammatory cytokine induction. RAW 264.7 macrophages were stimulated with HP1330 (10 µg⋅ml−1) and pretreated HP1330 (10 µg⋅ml−1, 100°C for 10 min). (A) The mRNA levels of TNF-α, MCP-1, and IL-1β were examined by qRT-PCR, (B) and the cytokine protein levels in the culture supernatants were examined by enzyme-linked immunosorbent assay. The bars represent the SEMs, based on three independent experiments. **P < 0.01.
Construction and Characterization of Δhp1330
To confirm the deletion of hp1330, PCR analysis was performed with three pairs of primers (P1/P2, P3/P4, and P5/P6). hp1330 could be detected in the SC19 strain, but not in Δhp1330. As a control, gdh could be detected in both SC19 and Δhp1330. In addition, the pSET4s vector could not be detected in Δhp1330 (Figure 4A). These results indicated that the hp1330 gene was deleted from the bacterial chromosome. To further examine the influence of the hp1330 deletion, the growth curves and Gram staining of the SC19, Δhp1330, and CΔhp1330 strains were determined by culturing to logarithmic growth phase in TSB. As shown in Figure 4B, no significant difference was found among the growth curves of the three strains, while the chain of Δhp1330 strain was obvious longer than SC19 or CΔhp1330 (Figure 4C). To avoid the influence of chain length differences on the accuracy of bacterial counting, we then examined the morphologies of the SC19, Δhp1330, and CΔhp1330 strains cultured on TSA via Gram staining. The result showed that no marked difference occurred among these three strains (Figure 4D). Thus, in subsequent cellular and animal experiments, we cultured SC19, Δhp1330, and CΔhp1330 on TSA. In addition, transmission electron microscopy was used to detect the capsules of SC19, Δhp1330, and CΔhp1330 strains, respectively. There was no obvious difference in capsular thickness and morphology among these three strains (Figure 4E). This indicated that HP1330 does not regulate the capsule of S. suis.
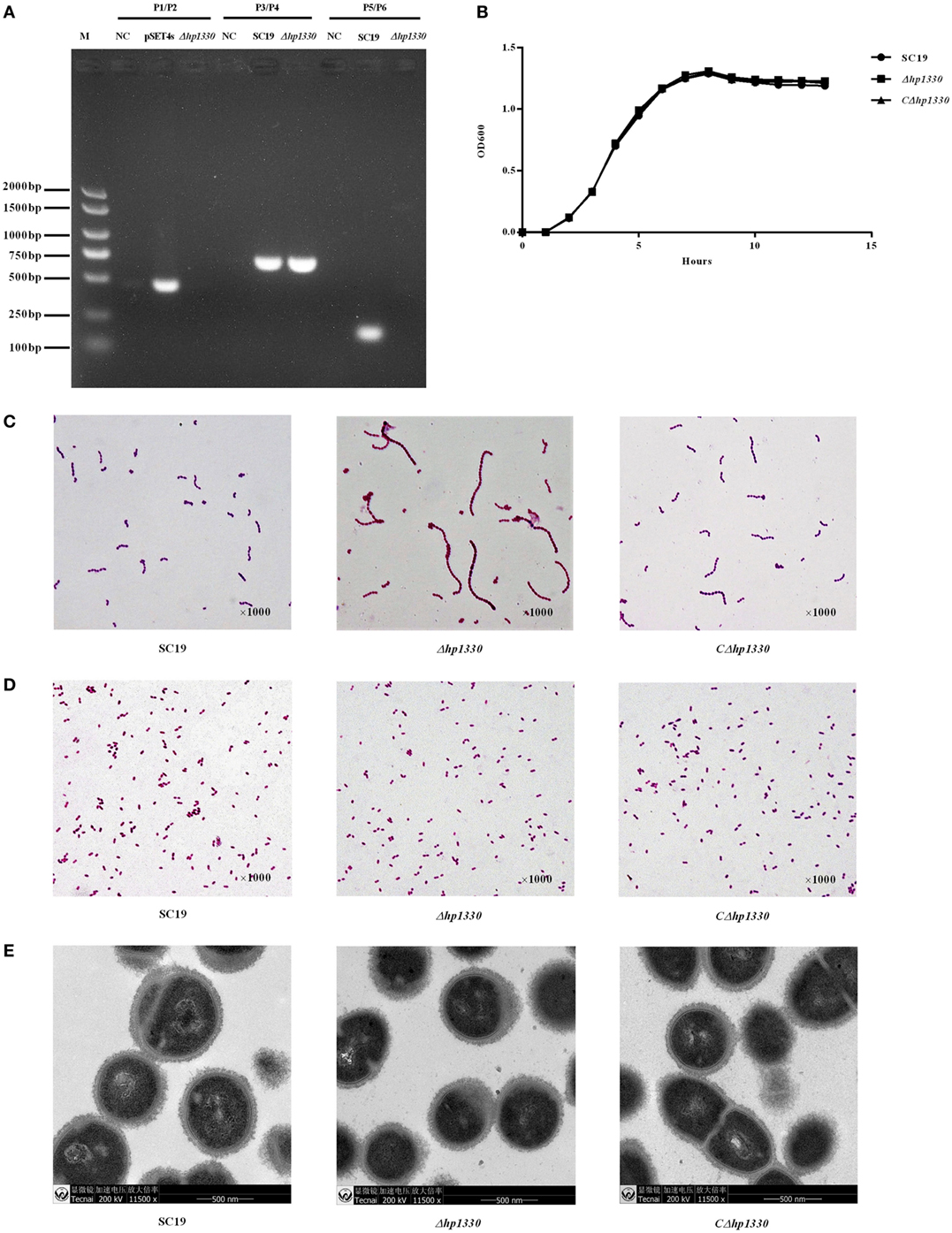
Figure 4. Construction and confirmation of the Δhp1330 mutant. (A) Confirmation of the Δhp1330 mutant by PCR using the primers pairs P1/P2 (to detect the pSET4s vector), P3/P4 (to detect the gdh gene), and P5/P6 (to detect the hp1330 gene). (B) Growth curves of the SC19, Δhp1330, and CΔhp1330 strains. The bacteria were cultured in tryptic soy broth (TSB) containing 5% newborn bovine serum at 37°C. The absorbance at 600 nm was measured at intervals of 1 h. Results shown are representative of three independent experiments. Light microscope morphology of the SC19, Δhp1330, and CΔhp1330 strains were observed by Gram staining (×1,000) following culture in panel (C) TSB or in panel (D) tryptic soy agar. (E) The capsules of SC19, Δhp1330, and CΔhp1330 strains were detected by transmission electron microscopy (×11,500).
Δhp1330 Inhibited Pro-inflammatory Responses in RAW264.7 Cells
After RAW 264.7 cells were incubated with the SC19, Δhp1330, or CΔhp1330 strain, the levels of TNF-α, MCP-1, and IL-1β were measured by ELISA and qRT-PCR. Deleting the hp1330 gene significantly reduced the pro-inflammatory ability of SS2 (Figure 5). These results indicated that HP1330 plays an important role in SS2-induced, pro-inflammatory responses in vitro.
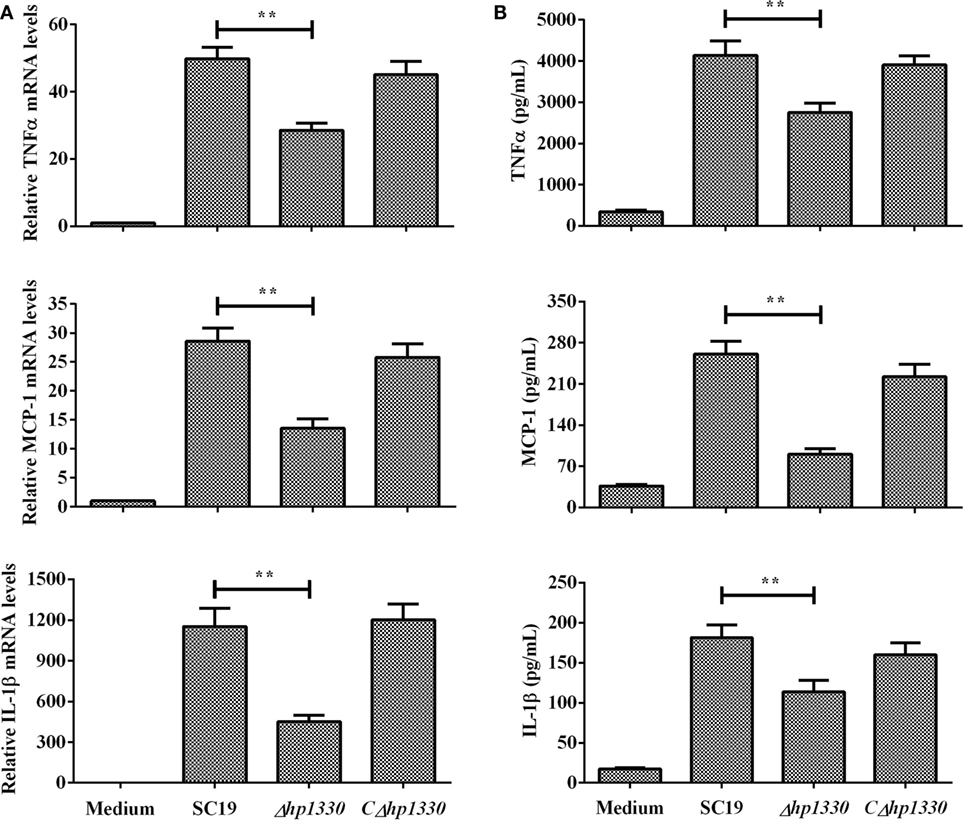
Figure 5. Induction of cytokine mRNA and protein expression in RAW 264.7 macrophages by SS2 strains. RAW 264.7 macrophages were incubated with the SC19, Δhp1330, and CΔhp1330 strains for 6 h. (A) The mRNA levels of TNF-α, MCP-1, and IL-1β were examined by qRT-PCR, (B) and the cytokine protein levels in the supernatants were examined by enzyme-linked immunosorbent assay. The bars represent the SEMs, based on three independent experiments. **P < 0.01.
Δhp1330 Displayed Attenuated Virulence, Decreased Pro-inflammatory Ability, and Reduced Bacterial Loads in Mice
To examine the role of HP1330 in SS2 infection, C57BL/6 mice were used as an experimental infection model. Firstly, for virulence testing, mice were inoculated with the SC19, Δhp1330, or CΔhp1330 strain at a dose of 6 × 108 CFUs, which was a lethal dose of SC19 for C57BL/6 mice. The group of mice infected with SC19 developed obvious clinical signs of SS2 infection, including a rough hair coat, weight loss, depression, shivering, and suppuration of the eyes during the first day postinfection. Only 20% of the mice survived to 7 days postinfection. However, mice in the Δhp1330 group showed an overall survival rate of 90%, with no obvious symptoms (Figure 6). These results indicated that deleting the hp1330 gene significantly decreased the virulence of SS2 to mice (P < 0.01). Second, to further analyze why the Δhp1330 deletion resulted in attenuated virulence, mice were challenged i.p. with a non-lethal dose of the SC19, Δhp1330, or CΔhp1330 strain, and then cytokine concentrations and bacterial loads in the blood were determined. As a result, the blood of mice infected with Δhp1330 contained lower cytokine concentrations at 6 and 12 h postinfection, and lower bacterial loads at 6 h postinfection (Figures 7A,C), whereas the chain length of the SC19, Δhp1330, and CΔhp1330 strains showed no obvious differences (Figure 7B). Overall, our data suggested that HP1330 may contribute to SS2 virulence by inducing high-level pro-inflammatory responses and influencing in vivo bacterial loads.
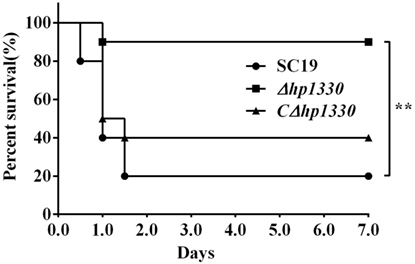
Figure 6. Survival curves of mice infected with SS2 strains. Female C57BL/6 mice in different groups were challenged i.p. with 6 × 108 colony-forming units SC19, Δhp1330, or CΔhp1330 strains cultured on tryptic soy agar. The mortality of mice was recorded for 1 week. The results shown are representative of three independent experiments.
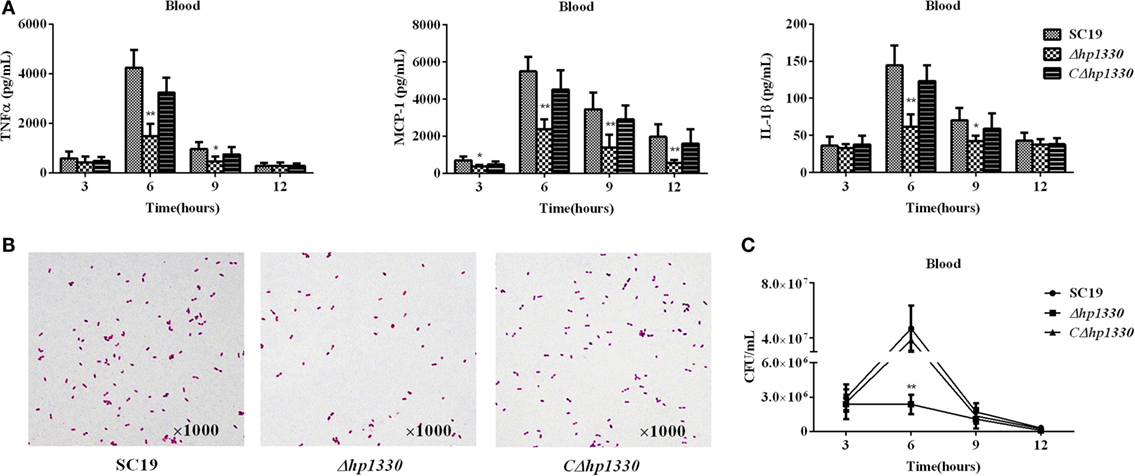
Figure 7. Cytokine concentrations and bacterial loads in blood. Female C57BL/6 mice were challenged with 2 × 108 colony-forming units of the SC19, Δhp1330, or CΔhp1330 strain cultured on tryptic soy agar. After infection for 3, 6, 9, or 12 h, an equal number of mice in each group were sacrificed to collect blood. (A) The blood cytokine concentrations were determined by enzyme-linked immunosorbent assay. (B) The morphologies of the SC19, Δhp1330, and CΔhp1330 strains from blood were observed under a light microscope by Gram staining (×1,000). (C) Bacteria loads in the blood were examined by determining colony-forming unit counts. The results shown are representative of three independent experiments. *P < 0.05, **P < 0.01.
HP1330-Triggered Pro-inflammatory Cytokine Production Dependent on Recognition of TLR2
The qRT-PCR assay results showed that TLR2 could be obviously upregulated in RAW264.7 cells by HP1330 stimulation, but TLR4 not (Figure 8A). This implied that TLR2 may be the inflammatory recognition receptor of HP1330. An antibody blocking assay was performed to test this possibility. Compared with the positive control, the anti-TLR2 antibody significantly reduced the expression levels of TNF-α, MCP-1, and IL-1β (Figure 8B). In addition, TLR2−/− macrophages were isolated from TLR2−/− mice and then used to evaluate the pro-inflammatory activity of HP1330. The results showed that HP1330 could stimulate obvious pro-inflammatory responses in WT macrophages, but not in TLR2−/− macrophages (Figure 8C). All of the above experiments demonstrated that HP1330-induced cytokine secretion depends on TLR2.
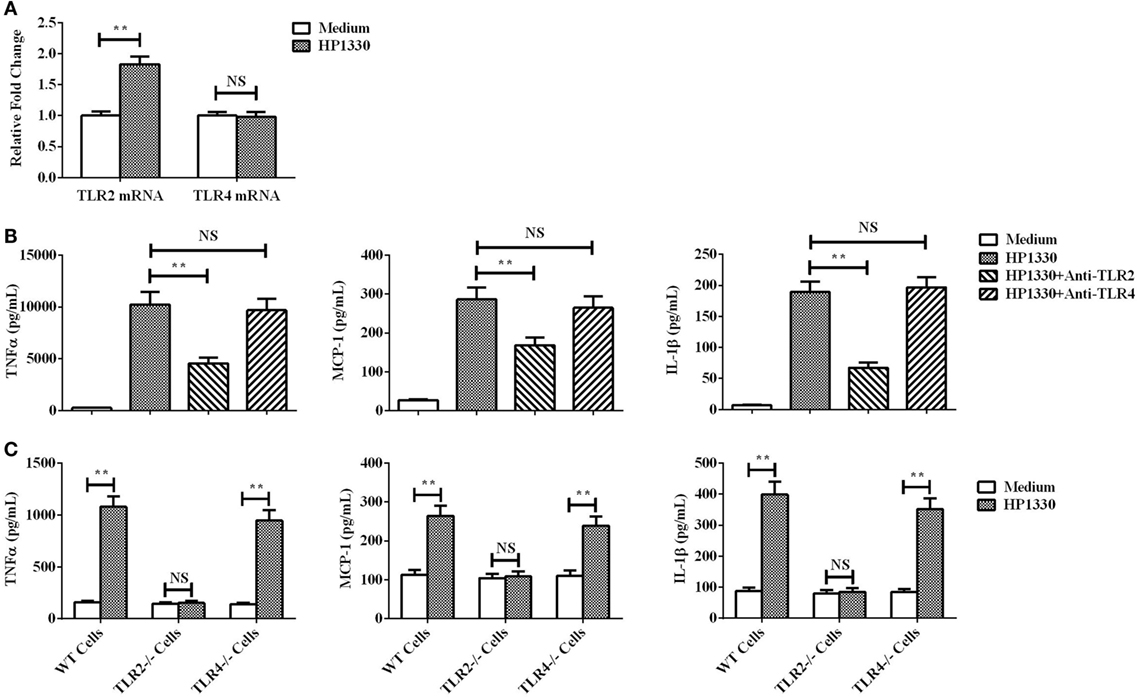
Figure 8. Recognition receptor of the HP1330-stimulated pro-inflammatory response. (A) After stimulation with HP1330, RAW264.7 cells were collected to analyze the mRNA levels of toll-like receptor 2 (TLR2) or TLR4 by qRT-PCR. (B) Antibody blocking assays. After pretreatment with 8 µg of an anti-TLR2 or anti-TLR4 antibody for 30 min, RAW 264.7 cells were incubated with 10 µg⋅ml−1 HP1330 for 6 h. The concentrations of TNF-α, MCP-1, and IL-1β were determined by enzyme-linked immunosorbent assay (ELISA). (C) Primary peritoneal macrophages were isolated from TLR2−/−, TLR4−/−, and wild-type (WT) mice, after which they were incubated with 10 µg⋅ml−1 HP1330 for 6 h. The cytokine concentrations in the supernatants were determined by ELISA. The error bars represent the SEMs, based on three independent experiments. **P < 0.01.
HP1330 Activates Pro-inflammatory Responses Dependent on ERK1/2 Phosphorylation
To further elucidate the mechanisms through which HP1330-induced pro-inflammatory responses, we investigated HP1330-dependent signal-transduction pathways in RAW264.7 cells. Inhibitors of p38 MAPK, JNK, NF-κB, PI3K, and ERK1/2 were used to analyze which signaling pathway was responsible for HP1330-induced pro-inflammatory responses. As shown in Figure 9A, the ERK 1/2 MAPK inhibitor (U0126) significantly suppressed cytokine production induced by HP1330, whereas the other four inhibitors (SB203580, SP600125, PDTC, and LY294002) did not. This indicated that HP1330-induced pro-inflammatory responses likely depended on ERK 1/2 MAPK phosphorylation. To test this hypothesis, the phosphorylation of ERK 1/2 MAPK was measured by western blot analysis in HP1330-stimulated RAW264.7 cells. Compared with that in the control group, ERK 1/2 MAPK phosphorylation was significantly enhanced in the HP1330-stimulated group, detecting actin as a loading control (Figure 9B). These results indicated that HP1330 activated pro-inflammatory responses in an ERK1/2 phosphorylation-dependent manner.
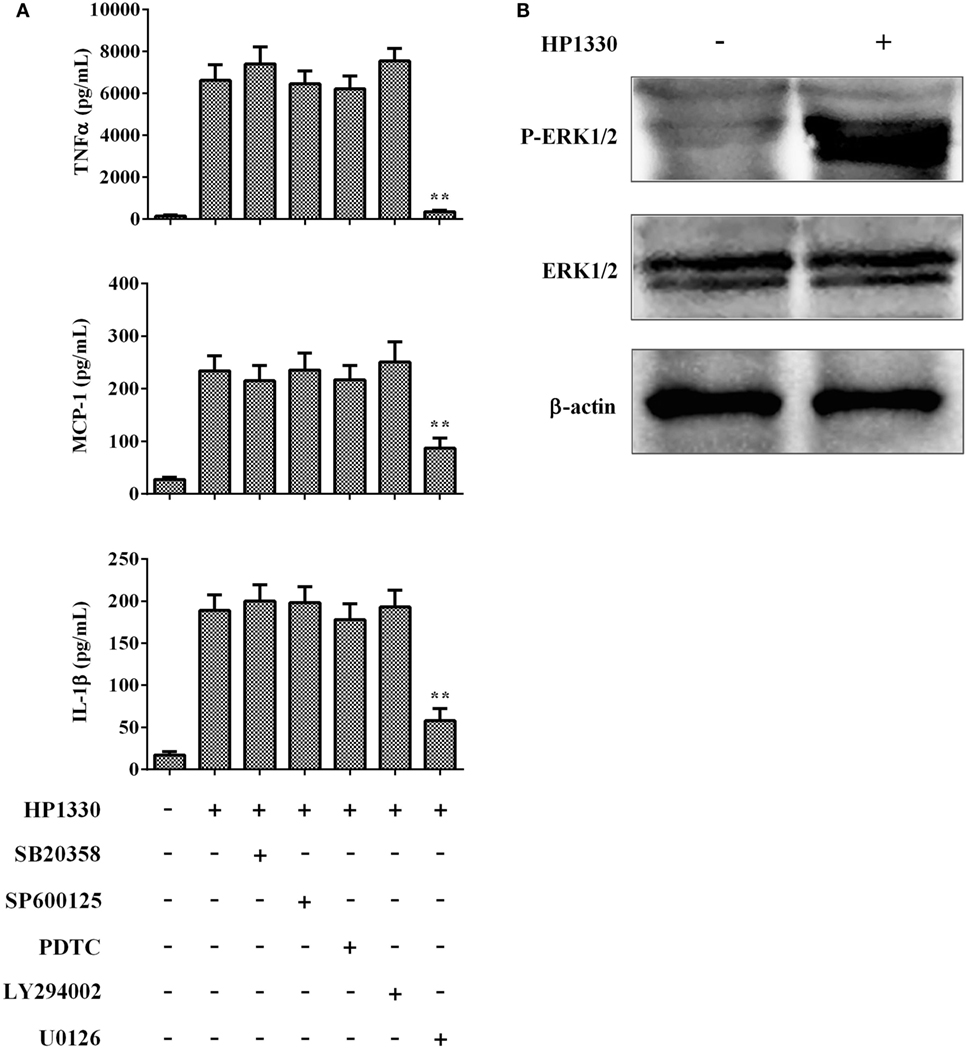
Figure 9. Signal-transduction pathways of the HP1330-stimulated, pro-inflammatory response in RAW 264.7 macrophages. (A) After pretreatment with inhibitors of p38 MAPK (SB203580), JNK (SP600125), NF-κB (PDTC), PI3K (LY294002), or ERK1/2 (U0126) for 30 min, RAW 264.7 macrophages were stimulated with 10 µg⋅ml−1 HP1330 for 6 h. The cytokine levels were then determined by enzyme-linked immunosorbent assay. Data are expressed as the mean ± SD of three independent experiments. **P < 0.01. (B) HP1330-induced phosphorylation of ERK 1/2 MAPK in RAW264.7 macrophages. RAW264.7 macrophages were stimulated with HP1330 (10 µg⋅ml−1) for 6 h. The cell lysates were analyzed by western blotting using specific antibodies against ERK 1/2 MAPK and phospho-ERK 1/2 MAPK. β-Actin was detected as an internal control using an anti-β-actin antibody. The results shown are representative of three independent experiments.
Discussion
Currently, S. suis remains a major pathogen that causes severe annual economic losses in the global swine industry, and seriously threatens to human health (46). Especially, “two human large-scale outbreaks caused by SS2 in China in 1998 and 2005 have provoked considerable public health concerns worldwide” (12, 47, 48). Although some insights have been gained (49, 50), many aspects of the pathogenesis of the bacteria remain uncertain. For example, the mechanism whereby SS2 causes STSLS still needs to be elucidated. STSLS was first found during the 2005 Sichuan S. suis outbreak, with a high (62%) mortality rate (51). Focusing on this novel symptom, some findings indicated that high serum pro-inflammatory cytokine levels and acute bacteremia are responsible for STSLS (7, 52); even several new putative virulence factors were found to be likely associated with STSLS (53–55). However, the exact mechanism whereby SS2 causes STSLS remains unclear. In this study, we found that the Δhp1330 mutant strain showed clear reductions in lethality, pro-inflammatory ability, and bacterial loads in mice. These results strongly suggested that HP1330 could contribute to SS2-induced STSLS.
When a pathogen invades a host, its PAMP molecules are recognized by the innate immune system of the host via pattern-recognition receptors. Subsequently, inflammatory responses are activated to eliminate the pathogen (56). Thus, inflammatory responses are usually beneficial to host (57). However, excessive inflammation is harmful and can lead to shock and organ failure (58). For example, the superantigen secreted by Streptococcus pyogenes can induce high levels of inflammatory cytokines and cause STSS (59). Previous findings demonstrated that SS2 has evolved to acquire the ability to stimulate the host immune system to produce massive amounts of pro-inflammatory cytokines, such as TNF-α, IFN-γ, IL-1β, IL-6, IL-12, and MCP-1 (7). Even inflammation has been considered a hallmark of SS2 infection, which plays an important role in most clinical symptoms of S. suis disease, including meningitis, septicemia, sudden death, and STSLS (60). Thus, we investigated the mechanisms whereby SS2-induced excessive inflammation contributes to SS2 pathogenesis. In the present study, HP1330 showed potent pro-inflammatory activity in vitro and played an important role during SS2-induced excessive pro-inflammatory responses in vivo. Through further analyzing the inflammatory signaling pathways activated by HP1330, we found that the pro-inflammatory activity of HP1330 depends on TLR2 recognition and ERK 1/2 MAPK phosphorylation. These results not only demonstrated that HP1330 contributes to SS2 virulence by inducing robust pro-inflammatory responses but they also laid the foundation for attaining a more comprehensive understanding of the excessive inflammation stimulated by SS2.
In this study, we found that when bacteria were cultured in liquid medium (TSB), the chain of Δhp1330 mutant was longer than WT strain SC19. Because the difference of chain length will influence the accuracy of bacterial counting, to ensure that SC19, Δhp1330, and CΔhp1330 are equally challenged in subsequent cellular and animal experiments, these three strains were cultured on solid medium (TSA), of which the morphologies does not show marked differences. It has been widely reported that PGN hydrolysis is required to promote septal PGN splitting and eventual daughter cell separation (61). Thus, to explore the mechanism whereby HP1330 influences the chain length of SS2, zymogram analysis was performed to analyze PGN hydrolysis induced by HP1330, as described previously (62). Using S. suis PGN as the substrate for zymogram analysis, we noticed that HP1330 exhibited apparent enzymatic activity, as did positive control lysozyme, while the negative control protein (bovine serum albumin) did not (Figure S1 in Supplementary Material). These results suggested that HP1330 may influence the chain length of SS2 through PGN hydrolysis. In addition, sequence analysis showed that HP1330 contains a potential zinc-binding site, implying a possible matrix metalloproteinases (MMPs) activity. MMPs contribute to the degradation of the extracellular matrix, which have been extensively recognized in eukaryotes (63). However, the function and mechanism of MMPs in bacteria remain poorly understood, and need further study.
Based on that the deletion of hp1330 could influence the chain length of SS2 cultured in liquid, in mice experiment, we analyzed the chain length of SC19, Δhp1330 and CΔhp1330 strains by Gram staining before bacterial counting. As shown in Figure 7B, no obvious change was observed, excluding the influence of chain length change to bacterial loads. It is known that high pathogenic bacterial loads in vivo play an important role in disease (64). Our data showed that disruption of the hp1330 gene led to a clear decrease of the blood bacterial load during SS2 infection. Thus, HP1330 may contribute to SS2 virulence not only through its potent pro-inflammatory activity but also by influencing the blood bacterial load of SS2. Since the loss of individual PGN hydrolase factors usually has little effect on growth and division (65), we speculate that the PGN hydrolysis of HP1330 may be not responsible for the reduced bacterial load of Δhp1330 mutant in blood. A moderate inflammatory response maintains a relative immunological balance between pro- and anti-inflammatory actions, which is advantageous for host defense against and clearance of bacterial infections. However, cytokine overexpression can break this balance and contribute to promote organ injury and exacerbate disease progression (66). Infection with the highly virulent SS2 strain can cause excessive inflammation, while the Δhp1330 mutant strain showed significantly reduced pro-inflammatory ability. Thus, we speculate that deletion of the hp1330 gene may help in the complete or partial recovery of inflammatory response functions against bacteria, in turn helping to reduce the blood bacterial load during SS2 infection.
In conclusion, our data demonstrated that HP1330 is a novel virulence-related protein of SS2, which shows potent pro-inflammatory activity and influences the bacterial load in vivo. Furthermore, through further analyzing the inflammatory signaling pathways induced by HP1330, we found that TLR2 recognition and ERK 1/2 MAPK activation mediate the pro-inflammatory responses of mouse macrophages to HP1330 exposure. These findings increase our understanding of the excessive inflammation and STSLS caused by SS2.
Ethics Statement
This study was carried out in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals Monitoring Committee of Hubei Province, China, and the protocol was approved by the Committee on the Ethics of Animal Experiments at the College of Veterinary Medicine, Huazhong Agricultural University.
Author Contributions
The experiments were performed mainly by QZ and JH, and some experiments were performed with the assistance of JY, ZX, LL, YS, and XS. QZ and JH analyzed the data. The study was conceived and designed by AZ and MJ. QZ, JH, AZ, and MJ wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31672557), the Special Fund for Agro-Scientific Research in the Public Interest (201303041), and the China Postdoctoral Science Foundation funded project (2015M580654). The authors thank Pei Zhang and Du Anna from The Core Facility and Technical Support, Wuhan Institute of Virology, for their help with producing TEM micrographs. The authors also thank Collaborative Innovation Center of Model Animal, Wuhan University for TLR2-deficient and TLR4-deficient mice.
Supplementary Material
The Supplementary Material for this article can be found online at http://journal.frontiersin.org/article/10.3389/fimmu.2017.00869/full#supplementary-material.
Figure S1. Detecting the peptidoglycan (PGN) hydrolase activity of HP1330. As a substrate for zymogram analysis, SS2 PGN was uniformly added to two protein gels. Following SDS-PAGE: (A) one gel was stained with Coomassie blue to observe the positive control lysozyme, the negative control bovine serum albumin (BSA) protein, and HP1330, and (B) another gel was stained with methylene blue to detect the PGN hydrolase activity of lysozyme, BSA, and HP1330.
References
1. Zhao J, Lin L, Fu L, Han L, Zhang A. Neutrophil extracellular taps play an important role in clearance of Streptococcus suis in vivo. Microbiol Immunol (2016) 60:228–33. doi:10.1111/1348-0421.12367
2. Haas B, Grenier D. Impact of sub-inhibitory concentrations of amoxicillin on Streptococcus suis capsule gene expression and inflammatory potential. Pathogens (2016) 5:37. doi:10.3390/pathogens5020037
3. Kerdsin A, Gottschalk M, Hatrongjit R, Hamada S, Akeda Y, Oishi K. Fatal septic meningitis in child caused by Streptococcus suis serotype 24. Emerg Infect Dis (2016) 22:1519–20. doi:10.3201/eid2208.160452
4. Perch B, Kristjansen P, Skadhauge K. Group R streptococci pathogenic for man. Two cases of meningitis and one fatal case of sepsis. Acta Pathol Microbiol Scand (1968) 74:69–76. doi:10.1111/j.1699-0463.1968.tb03456.x
5. Huong VT, Ha N, Huy NT, Horby P, Nghia HD, Thiem VD, et al. Epidemiology, clinical manifestations, and outcomes of Streptococcus suis infection in humans. Emerg Infect Dis (2014) 20:1105–14. doi:10.3201/eid2007.131594
6. Segura M. Streptococcus suis: an emerging human threat. J Infect Dis (2009) 199:4–6. doi:10.1086/594371
7. Ye CY, Zheng H, Zhang J, Jing HQ, Wang L, Xiong YW, et al. Clinical, experimental, and genomic differences between intermediately pathogenic, highly pathogenic, and epidemic Streptococcus suis. J Infect Dis (2009) 199:97–107. doi:10.1086/594370
8. Mai NT, Hoa NT, Nga TV, Linh Le D, Chau TT, Sinh DX, et al. Streptococcus suis meningitis in adults in Vietnam. Clin Infect Dis (2008) 46:659–67. doi:10.1086/527385
9. Zhao J, Pan S, Lin L, Fu L, Yang C, Xu Z, et al. Streptococcus suis serotype 2 strains can induce the formation of neutrophil extracellular traps and evade trapping. FEMS Microbiol Lett (2015) 362(6):fnv022. doi:10.1093/femsle/fnv022
10. Segura M, Gottschalk M, Olivier M. Encapsulated Streptococcus suis inhibits activation of signaling pathways involved in phagocytosis. Infect Immun (2004) 72:5322–30. doi:10.1128/IAI.72.9.5322-5330.2004
11. Zhang A, Mu X, Chen B, Han L, Chen H, Jin M. IgA1 protease contributes to the virulence of Streptococcus suis. Vet Microbiol (2011) 148:436–9. doi:10.1016/j.vetmic.2010.09.027
12. Fittipaldi N, Segura M, Grenier D, Gottschalk M. Virulence factors involved in the pathogenesis of the infection caused by the swine pathogen and zoonotic agent Streptococcus suis. Future Microbiol (2012) 7:259–79. doi:10.2217/fmb.11.149
13. Baums CG, Valentin-Weigand P. Surface-associated and secreted factors of Streptococcus suis in epidemiology, pathogenesis and vaccine development. Anim Health Res Rev (2009) 10:65–83. doi:10.1017/S146625230999003X
14. Chen C, Tang J, Dong W, Wang C, Feng Y, Wang J, et al. A glimpse of streptococcal toxic shock syndrome from comparative genomics of S. suis 2 Chinese isolates. PLoS One (2007) 2:e315. doi:10.1371/journal.pone.0000315
15. Segura M, Vanier G, Al-Numani D, Lacouture S, Olivier M, Gottschalk M. Proinflammatory cytokine and chemokine modulation by Streptococcus suis in a whole-blood culture system. FEMS Immunol Med Microbiol (2006) 47:92–106. doi:10.1111/j.1574-695X.2006.00067.x
16. Zheng H, Ye C, Segura M, Gottschalk M, Xu J. Mitogenic effect contributes to increased virulence of Streptococcus suis sequence type 7 to cause streptococcal toxic shock-like syndrome. Clin Exp Immunol (2008) 153:385–91. doi:10.1111/j.1365-2249.2008.03722.x
17. Yang C, Zhao J, Lin L, Pan S, Fu L, Han L, et al. Targeting TREM-1 signaling in the presence of antibiotics is effective against streptococcal toxic-shock-like syndrome (STSLS) caused by Streptococcus suis. Front Cell Infect Microbiol (2015) 5:79. doi:10.3389/fcimb.2015.00079
18. Zhang Q, Yang Y, Yan S, Liu J, Xu Z, Yu J, et al. A novel pro-inflammatory protein of Streptococcus suis 2 induces the toll-like receptor 2-dependent expression of pro-inflammatory cytokines in RAW 264.7 macrophages via activation of ERK1/2 pathway. Front Microbiol (2015) 6:178. doi:10.3389/fmicb.2015.00178
19. Fittipaldi N, Sekizaki T, Takamatsu D, Harel J, Dominguez-Punaro Mde L, Von Aulock S, et al. d-Alanylation of lipoteichoic acid contributes to the virulence of Streptococcus suis. Infect Immun (2008) 76:3587–94. doi:10.1128/IAI.01568-07
20. Sun L. [Putative lipoproteins of Streptococcus suis type 2 identified by bioinformatic genome analysis]. Wei Sheng Wu Xue Bao (2008) 48:1104–9.
21. Lecours MP, Gottschalk M, Houde M, Lemire P, Fittipaldi N, Segura M. Critical role for Streptococcus suis cell wall modifications and suilysin in resistance to complement-dependent killing by dendritic cells. J Infect Dis (2011) 204:919–29. doi:10.1093/infdis/jir415
22. Wichgers Schreur PJ, Rebel JM, Smits MA, Van Putten JP, Smith HE. TroA of Streptococcus suis is required for manganese acquisition and full virulence. J Bacteriol (2011) 193:5073–80. doi:10.1128/JB.05305-11
23. Jiang H, Fan HJ, Lu CP. Identification and distribution of putative virulent genes in strains of Streptococcus suis serotype 2. Vet Microbiol (2009) 133:309–16. doi:10.1016/j.vetmic.2008.07.014
24. Liu L, Cheng G, Wang C, Pan X, Cong Y, Pan Q, et al. Identification and experimental verification of protective antigens against Streptococcus suis serotype 2 based on genome sequence analysis. Curr Microbiol (2009) 58:11–7. doi:10.1007/s00284-008-9258-x
25. Mandanici F, Gomez-Gascon L, Garibaldi M, Olaya-Abril A, Luque I, Tarradas C, et al. A surface protein of Streptococcus suis serotype 2 identified by proteomics protects mice against infection. J Proteomics (2010) 73:2365–9. doi:10.1016/j.jprot.2010.07.009
26. Li W, Wan Y, Tao Z, Chen H, Zhou R. A novel fibronectin-binding protein of Streptococcus suis serotype 2 contributes to epithelial cell invasion and in vivo dissemination. Vet Microbiol (2013) 162:186–94. doi:10.1016/j.vetmic.2012.09.004
27. Zhang A, Chen B, Yuan Z, Li R, Liu C, Zhou H, et al. HP0197 contributes to CPS synthesis and the virulence of Streptococcus suis via CcpA. PLoS One (2012) 7:e50987. doi:10.1371/journal.pone.0050987
28. Gao T, Tan M, Liu W, Zhang C, Zhang T, Zheng L, et al. GidA, a tRNA modification enzyme, contributes to the growth, and virulence of Streptococcus suis serotype 2. Front Cell Infect Microbiol (2016) 6:44. doi:10.3389/fcimb.2016.00044
29. Takamatsu D, Osaki M, Sekizaki T. Thermosensitive suicide vectors for gene replacement in Streptococcus suis. Plasmid (2001) 46:140–8. doi:10.1006/plas.2001.1532
30. Liu B, Teng D, Wang X, Yang Y, Wang J. Expression of the soybean allergenic protein P34 in Escherichia coli and its indirect ELISA detection method. Appl Microbiol Biotechnol (2012) 94:1337–45. doi:10.1007/s00253-012-4006-3
31. Liu N, Liu JT, Ji YY, Lu PP. Rosiglitazone regulates c-reactive protein-induced inflammatory responses via glucocorticoid receptor-mediated inhibition of p38 mitogen-activated protein kinase-toll-like receptor 4 signal pathway in vascular smooth muscle cells. J Cardiovasc Pharmacol (2011) 57:348–56. doi:10.1097/FJC.0b013e31820a0e67
32. Kang EH, Gebru E, Kim MH, Cheng H, Park SC. EstA protein, a novel virulence factor of Streptococcus pneumoniae, induces nitric oxide and pro-inflammatory cytokine production in RAW 264.7 macrophages through NF-kappaB/MAPK. Microb Pathog (2009) 47:196–201. doi:10.1016/j.micpath.2009.07.002
33. Sodhi A, Sharma RK, Batra HV. Yersinia rLcrV and rYopB inhibits the activation of murine peritoneal macrophages in vitro. Immunol Lett (2005) 99:146–52. doi:10.1016/j.imlet.2005.02.009
34. Yang X, Izadi H, Coleman AS, Wang P, Ma Y, Fikrig E, et al. Borrelia burgdorferi lipoprotein BmpA activates pro-inflammatory responses in human synovial cells through a protein moiety. Microbes Infect (2008) 10:1300–8. doi:10.1016/j.micinf.2008.07.029
35. Liu SJ, Shi Y, Liu C, Zhang M, Zuo ZC, Zeng CJ, et al. The upregulation of pro-inflammatory cytokines in the rabbit uterus under the lipopolysaccaride-induced reversible immunoresponse state. Anim Reprod Sci (2017) 176:70–7. doi:10.1016/j.anireprosci.2016.11.012
36. Moore LJ, Pridmore AC, Lee ME, Read RC. Induction of pro-inflammatory cytokine release by human macrophages during exposure of Streptococcus pneumoniae to penicillin is influenced by minimum inhibitory concentration ratio. Int J Antimicrob Agents (2005) 26:188–96. doi:10.1016/j.ijantimicag.2005.06.006
37. Abdelsalam M, Isobe N, Yoshimura Y. Effects of lipopolysaccharide on the expression of proinflammatory cytokines and chemokines and influx of leukocytes in the hen ovary. Poult Sci (2011) 90:2054–62. doi:10.3382/ps.2011-01394
38. Zhao P, Wang Y, Zeng S, Lu J, Jiang TM, Li YM. Protective effect of astragaloside IV on lipopolysaccharide-induced cardiac dysfunction via downregulation of inflammatory signaling in mice. Immunopharmacol Immunotoxicol (2015) 37:428–33. doi:10.3109/08923973.2015.1080266
39. Graveline R, Segura M, Radzioch D, Gottschalk M. TLR2-dependent recognition of Streptococcus suis is modulated by the presence of capsular polysaccharide which modifies macrophage responsiveness. Int Immunol (2007) 19:375–89. doi:10.1093/intimm/dxm003
40. Lecours MP, Segura M, Fittipaldi N, Rivest S, Gottschalk M. Immune receptors involved in Streptococcus suis recognition by dendritic cells. PLoS One (2012) 7:e44746. doi:10.1371/journal.pone.0044746
41. Li X, Wang S, Zhu R, Li H, Han Q, Zhao RC. Lung tumor exosomes induce a pro-inflammatory phenotype in mesenchymal stem cells via NFkappaB-TLR signaling pathway. J Hematol Oncol (2016) 9:42. doi:10.1186/s13045-016-0269-y
42. Liu D, Yumoto H, Hirota K, Murakami K, Takahashi K, Hirao K, et al. Histone-like DNA binding protein of Streptococcus intermedius induces the expression of pro-inflammatory cytokines in human monocytes via activation of ERK1/2 and JNK pathways. Cell Microbiol (2008) 10:262–76. doi:10.1111/j.1462-5822.2007.01040.x
43. Bolden A, Bernard L, Jones D, Akinyeke T, Stewart LV. The PPAR gamma agonist troglitazone regulates Erk 1/2 phosphorylation via a PPARgamma-independent, MEK-dependent pathway in human prostate cancer cells. PPAR Res (2012) 2012:929052. doi:10.1155/2012/929052
44. Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J Biol Chem (1999) 274:17406–9. doi:10.1074/jbc.274.25.17406
45. Draing C, Pfitzenmaier M, Zummo S, Mancuso G, Geyer A, Hartung T, et al. Comparison of lipoteichoic acid from different serotypes of Streptococcus pneumoniae. J Biol Chem (2006) 281:33849–59. doi:10.1074/jbc.M602676200
46. Huang K, Yuan Z, Li J, Zhang Q, Xu Z, Yan S, et al. Identification and characterisation a surface-associated arginine peptidase in Streptococcus suis serotype 2. Microbiol Res (2015) 170:168–76. doi:10.1016/j.micres.2014.08.001
47. Normile D. Infectious diseases. WHO probes deadliness of China’s pig-borne disease. Science (2005) 309:1308–9. doi:10.1126/science.309.5739.1308a
48. Tang J, Wang C, Feng Y, Yang W, Song H, Chen Z, et al. Streptococcal toxic shock syndrome caused by Streptococcus suis serotype 2. PLoS Med (2006) 3:e151. doi:10.1371/journal.pmed.0030151
49. Feng Y, Zhang H, Ma Y, Gao GF. Uncovering newly emerging variants of Streptococcus suis, an important zoonotic agent. Trends Microbiol (2010) 18:124–31. doi:10.1016/j.tim.2009.12.003
50. Gottschalk M, Xu J, Calzas C, Segura M. Streptococcus suis: a new emerging or an old neglected zoonotic pathogen? Future Microbiol (2010) 5:371–91. doi:10.2217/fmb.10.2
51. Yu H, Jing H, Chen Z, Zheng H, Zhu X, Wang H, et al. Human Streptococcus suis outbreak, Sichuan, China. Emerg Infect Dis (2006) 12:914–20. doi:10.3201/eid1206.051194
52. Bi Y, Li J, Yang L, Zhang S, Li Y, Jia X, et al. Assessment of the pathogenesis of Streptococcus suis type 2 infection in piglets for understanding streptococcal toxic shock-like syndrome, meningitis, and sequelae. Vet Microbiol (2014) 173:299–309. doi:10.1016/j.vetmic.2014.08.010
53. Ge J, Feng Y, Ji H, Zhang H, Zheng F, Wang C, et al. Inactivation of dipeptidyl peptidase IV attenuates the virulence of Streptococcus suis serotype 2 that causes streptococcal toxic shock syndrome. Curr Microbiol (2009) 59:248–55. doi:10.1007/s00284-009-9425-8
54. Wang C, Li M, Feng Y, Zheng F, Dong Y, Pan X, et al. The involvement of sortase A in high virulence of STSS-causing Streptococcus suis serotype 2. Arch Microbiol (2009) 191:23–33. doi:10.1007/s00203-008-0425-z
55. Zhao Y, Liu G, Li S, Wang M, Song J, Wang J, et al. Role of a type IV-like secretion system of Streptococcus suis 2 in the development of streptococcal toxic shock syndrome. J Infect Dis (2011) 204:274–81. doi:10.1093/infdis/jir261
56. Martinon F, Tschopp J. NLRs join TLRs as innate sensors of pathogens. Trends Immunol (2005) 26:447–54. doi:10.1016/j.it.2005.06.004
57. Hersh D, Weiss J, Zychlinsky A. How bacteria initiate inflammation: aspects of the emerging story. Curr Opin Microbiol (1998) 1:43–8. doi:10.1016/S1369-5274(98)80141-0
58. Kahn F, Morgelin M, Shannon O, Norrby-Teglund A, Herwald H, Olin AI, et al. Antibodies against a surface protein of Streptococcus pyogenes promote a pathological inflammatory response. PLoS Pathog (2008) 4:e1000149. doi:10.1371/journal.ppat.1000149
59. Norrby-Teglund A, Chatellier S, Low DE, Mcgeer A, Green K, Kotb M. Host variation in cytokine responses to superantigens determine the severity of invasive group A streptococcal infection. Eur J Immunol (2000) 30:3247–55. doi:10.1002/1521-4141(200011)30:11<3247::AID-IMMU3247>3.0.CO;2-D
60. Dominguez-Punaro Mde L, Segura M, Radzioch D, Rivest S, Gottschalk M. Comparison of the susceptibilities of C57BL/6 and A/J mouse strains to Streptococcus suis serotype 2 infection. Infect Immun (2008) 76:3901–10. doi:10.1128/IAI.00350-08
61. Vollmer W, Joris B, Charlier P, Foster S. Bacterial peptidoglycan (murein) hydrolases. FEMS Microbiol Rev (2008) 32:259–86. doi:10.1111/j.1574-6976.2007.00099.x
62. Siewering K, Jain S, Friedrich C, Webber-Birungi MT, Semchonok DA, Binzen I, et al. Peptidoglycan-binding protein TSAP functions in surface assembly of type IV pili. Proc Natl Acad Sci U S A (2014) 111:E953–61. doi:10.1073/pnas.1322889111
63. Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol (2004) 4:617–29. doi:10.1038/nri1418
64. Sullivan TD, Lascolea LJ Jr, Neter E. Relationship between the magnitude of bacteremia in children and the clinical disease. Pediatrics (1982) 69:699–702.
65. Uehara T, Dinh T, Bernhardt TG. LytM-domain factors are required for daughter cell separation and rapid ampicillin-induced lysis in Escherichia coli. J Bacteriol (2009) 191:5094–107. doi:10.1128/JB.00505-09
Keywords: Streptococcus suis 2, streptococcal toxic shock-like syndrome, excessive inflammation, signaling pathway, recognition receptor
Citation: Zhang Q, Huang J, Yu J, Xu Z, Liu L, Song Y, Sun X, Zhang A and Jin M (2017) HP1330 Contributes to Streptococcus suis Virulence by Inducing Toll-Like Receptor 2- and ERK1/2-Dependent Pro-inflammatory Responses and Influencing In Vivo S. suis Loads. Front. Immunol. 8:869. doi: 10.3389/fimmu.2017.00869
Received: 04 May 2017; Accepted: 10 July 2017;
Published: 31 July 2017
Edited by:
Tobias Schuerholz, Universitätsmedizin Rostock, GermanyReviewed by:
Zsuzsa Szondy, University of Debrecen, HungaryTakato Takenouchi, National Agriculture and Food Research Organization, Japan
Copyright: © 2017 Zhang, Huang, Yu, Xu, Liu, Song, Sun, Zhang and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anding Zhang, andye8019@mail.hzau.edu.cn;
Meilin Jin, jinmeilin@mail.hzau.edu.cn
†These authors have contributed equally to this work.
 Qiang Zhang
Qiang Zhang Jingjing Huang1†
Jingjing Huang1† Junping Yu
Junping Yu Zhongmin Xu
Zhongmin Xu Liang Liu
Liang Liu Yajing Song
Yajing Song Xiaomei Sun
Xiaomei Sun Anding Zhang
Anding Zhang Meilin Jin
Meilin Jin