- The University of Queensland Diamantina Institute, The University of Queensland, Translational Research Institute, Brisbane, QLD, Australia
Invariant natural killer T (iNKT) cells are a unique innate T lymphocyte population that possess cytolytic properties and profound immunoregulatory activities. iNKT cells play an important role in the immune surveillance of blood cancers. They predominantly recognize glycolipid antigens presented on CD1d, but their activation and cytolytic activities are not confined to CD1d expressing cells. iNKT cell stimulation and subsequent production of immunomodulatory cytokines serve to enhance the overall antitumor immune response. Crucially, the activation of iNKT cells in cancer often precedes the activation and priming of other immune effector cells, such as NK cells and T cells, thereby influencing the generation and outcome of the antitumor immune response. Blood cancers can evade or dampen iNKT cell responses by downregulating expression of recognition receptors or by actively suppressing or diverting iNKT cell functions. This review will discuss literature on iNKT cell activity and associated dysregulation in blood cancers as well as highlight some of the strategies designed to harness and enhance iNKT cell functions against blood cancers.
Introduction
Blood cancers are a heterogeneous group of malignancies broadly encompassing leukemia, myeloma, and lymphoma. As these cancers develop largely in lymphoid tissues, immune surveillance mechanisms are engaged, but inevitably fail due to changes in the microenvironment which are permissive to tumor growth but impede the development of antitumor immunity. Invariant natural killer T (iNKT) cells, an innate-like lymphocyte population defined by their semi-invariant T cell receptor (TCR)—Vα14Jα18 in mice and Vα24Jα18 in humans, have important roles in helping to regulate antitumor responses to cancer (1). These cells share similar properties to that of NK and T cells. The discovery of a potent prototypical NKT cell-activating glycolipid ligand known as α-galactosylceremide (αGalCer) (2, 3) prompted extensive attempts to manipulate this population to enhance antitumor immunity, both in solid and blood cancers. This review focuses on the activities of iNKT cells in blood malignancies and discusses the potential avenues for therapeutic targeting of iNKT cells in humans based on preclinical evidence (Table 1).
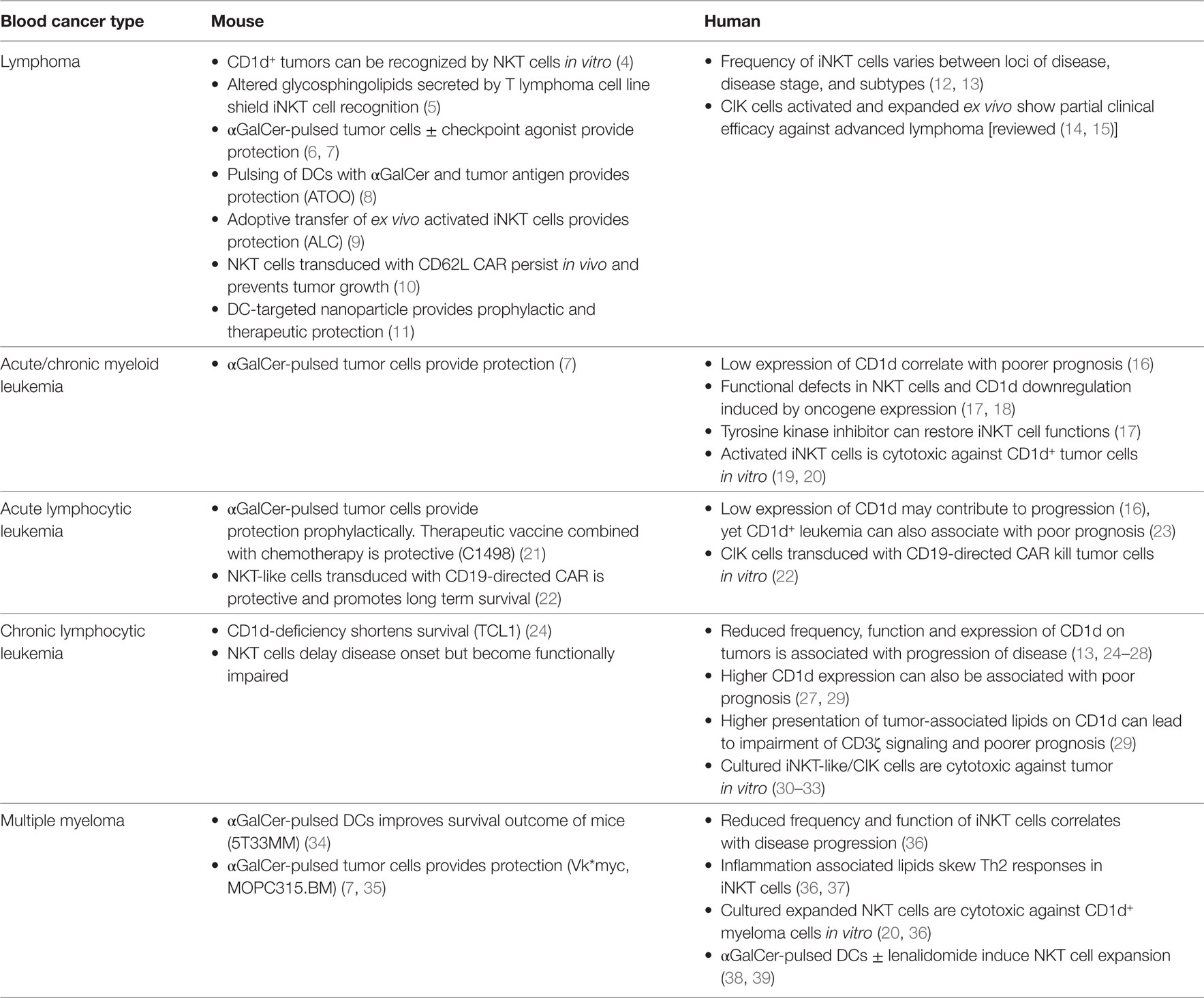
Table 1. Evidence for the involvement and effective targeting of iNKT cells for blood cancer control in mice and humans.
Immunoregulatory and Direct Cytotoxic Activities of iNKT Cells in Blood Cancers
Invariant natural killer T cells recognize glycolipid antigens presented on the MHC Class I-like molecule CD1d, which are expressed on many cell types, but most highly expressed on antigen-presenting cells (APCs) (40, 41). Both human and murine iNKT cells were found to recognize glycolipid antigens derived from components of bacteria (42, 43), as well as the synthetic molecule, αGalCer (44). However, iNKT cells have also been shown to recognize and respond to a variety of endogenous lipids including lysosomal glycosphingolipids such as isoglobotrihexosylceramide (iGb3) (45–48). iNKT cells were shown to directly recognize and kill various human tumor cell lines in vitro and murine tumors in vitro and in vivo through the recognition of endogenous lipids expressed on CD1d (36, 49, 50). The identities of these tumor-associated lipid antigens are mostly unknown. However, the tumor-associated ganglioside GD3 can be presented on CD1d for the activation of iNKT cells in vivo (45).
Early preclinical studies demonstrated that engagement of lipid antigen-CD1d complexes via the iNKT TCR results in the production of a diverse range of Th1/Th2 cytokines and chemokines (51–53), which can subsequently modulate both innate and adaptive immune cells. Notably, activation of iNKT cells leads to the downstream activation of NK cells and enhanced IFNγ production (54, 55), dendritic cell (DC) maturation and IL-12 production, and the induction of CD4 and CD8 T cell responses (56–59). Consequently, this cascade of events constitutes the indirect antitumor immunity imparted by activated iNKT cells (transactivation). Indeed, mice lacking iNKT cells (CD1d−/− and Jα18−/− mice) are more susceptible to tumor development in several spontaneous, oncogenic and carcinogenic models (60–63). In recent years, several studies have established the direct and spontaneous role of iNKT cells in the initiation of innate immune responses against blood cancers such as B/T cell lymphomas, chronic lymphocytic leukemia (CLL) and multiple myeloma (MM) (25, 36, 64–66). These studies show that iNKT cells have the potential to control or delay the progression of premalignant or early stage disease in a CD1d-dependent manner, as seen using murine models and iNKT cells derived from patients (4, 19, 49, 67–69). In addition, innate immune control of blood cancers was found to correlate to the functional ability of iNKT cells to produce inflammatory cytokines IFNγ, and TNFα and as well as the induction of IL-12 production in APCs (64, 70, 71) (Figure 1).
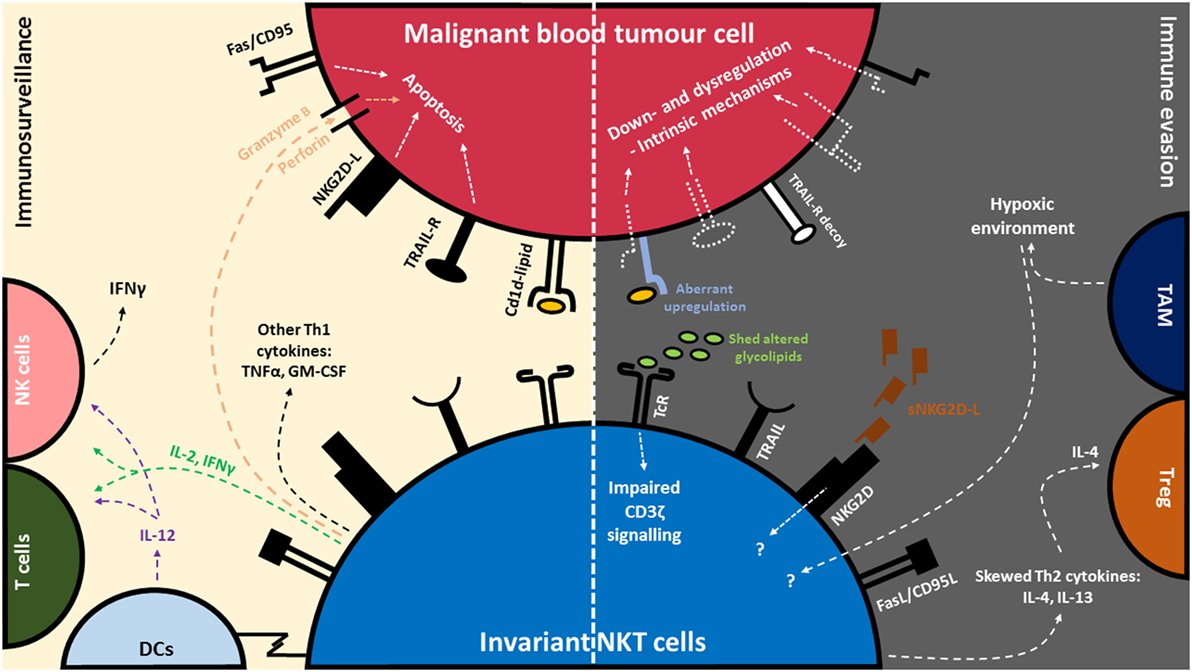
Figure 1. Invariant natural killer T (iNKT) cell-mediated immune surveillance of blood cancer and counteractive evasion strategies utilized by blood cancer cells. (Left) iNKT cells recognize glycolipid antigens presented on CD1d, commonly expressed by blood tumor cells. Recognition of glycolipid:CD1d complex via the invariant T cell receptor (TcR) leads to a cascade of events: the production of immunomodulatory cytokines such as interleukin-2 (IL-2), interferon-γ (IFNγ), tumor necrosis factor-α (TNFα), and granulocyte-macrophage colony-stimulating factor (GM-CSF), release of cytolytic mediators such as perforin/granzyme, activation of antigen-presenting cells (APCs) such as dendritic cells (DCs) and IL-12 production, as well as the rapid transactivation of NK cells and T cells. iNKT cells can also recognize tumor and degranulate in a CD1d-independent manner via Natural Killer Group 2D (NKG2D) receptors. (Right) In turn, tumor cells can evade recognition and killing by downregulating CD1d, NKG2D-L, TNF-related apoptosis-inducing ligand (TRAIL-L) and FAS/CD95. In addition, certain blood tumors can disrupt death signaling pathways to avoid killing. Some blood tumors express aberrant levels of glycolipids or shed soluble glycolipids and NKG2D-L which in turn dysregulate normal signaling pathway in iNKT cells. Blood tumors cells can also skew the production of Th2 cytokines (IL-4 and IL-13) in iNKT cells. IL-4 is associated with the activation of regulatory T cells (Treg) which are involved in dampening of antitumor responses. Dysfunction of iNKT cells have also been associated with tumor-associated macrophages (TAMs) and their ability to induce hypoxia in the tumor microenvironment.
In addition to their immunostimulatory effects, activated iNKT cells possess direct cytotoxic activity against blood cancers through the production of cytolytic molecules such as granzyme B and perforin, and through the interaction of death-inducing receptors such as Fas and TRAIL (19, 49, 72–75). More than half of all iNKT cells also express the NKG2D activating receptor enabling direct cytotoxicity against tumors expressing NKG2D ligands (76, 77). More broadly, NKG2D expression on immune effector cells is important for protection against hematological malignancy (78) (Figure 1). This was supported by two recent studies performed in NKG2D-deficient mice, which developed spontaneous lymphomas significantly faster than NKG2D-competent mice (79, 80). Similarly, the success of various inhibitors administered in mice that prevent the shedding of NKG2D ligands (NKG2D-L) or induce NKG2D-L expression on leukemic cells, and thereby enhancing cytotoxic killing, further demonstrates the significant role of NKG2D expression in immune surveillance of blood cancers (81, 82). In contrast, the functional role of NKG2D on human iNKT cells against tumors is less well defined. It has, however been demonstrated that human CD3+CD56+ NKT-like cells derived from the blood of healthy individuals are sensitive towards NKG2D-L-expressing cell lines including monocytic lymphoma (U937) and Burkitt’s lymphoma cell lines (Raji) (77, 83). More studies are required to understand the extent to which NKG2D expression on human iNKT cells is effective against blood cancers.
Invariant natural killer T cells have also been identified in the control of host response against allogenic donor cell rejection in leukemic patients receiving allogeneic HSCT. The suppression of graft-versus-host-disease (GvHD), while maintaining graft-versus-tumor effect has been shown to be highly dependent on the engraftment of donor iNKT cells, as failure to reconstitute iNKT cells after transplantation strongly correlated with disease relapse (84–87). Studies into the mechanisms of GvHD suppression show that iNKT cells modulate the overall immune response through production of Th2 cytokines such as IL-4, which in turn dampen inflammatory donor T cells, and promote Treg proliferation against both acute and chronic GvHD (88–91). These studies therefore highlight an importance function of the Th2 arm of activated iNKT cells in the facilitation of engraftment of allogenic donor cells against recurrence of leukemia.
iNKT Cell Dysfunction and Evasion of iNKT Cell Recognition in Blood Cancers
Tumor Cell Evasion of iNKT Cell Recognition and Killing
Blood tumor cells possess intricate methods of evading detection and elimination by the immune system (92–94). The downregulation of CD1d on malignant cells is one of the major contributing factors to the evasion of iNKT cell immunosurveillance in blood cancers (34, 95). In fact, lower expression levels of CD1d on a variety of blood cancers is associated with progressive and advanced stages of disease in both murine models and in humans (16, 25, 26, 64, 96). Various mechanisms have been associated with downregulation of CD1d expression in blood cancers. For example, surface CD1d downregulation in Epstein–Barr virus-transformed B cells is thought to be attributed to posttranscriptional mechanisms commonly employed by herpes viruses (97, 98). Downregulation of CD1d expression on CLL B cells is believed to be associated with the elevated levels of a transcriptional protein called lymphoid enhancer-binding factor-1 (26).
Aside from regulation of CD1d expression, blood cancers may also be able to evade recognition by NKG2D on iNKT cells. This assumption is derived from previous observations in solid tumors. In one particular study, serum samples taken from patients with ovarian and prostate cancer had elevated levels of tumor-derived soluble NKG2D ligands, namely MHC class I chain-related (MIC) proteins. When cocultured with freshly isolated iNKT-like CD3+CD56+ cells in vitro, the cytotoxic activity of these cells was compromised and NKG2D expression was downregulated (83). In a more recent study, Lu et al. (99) demonstrated that antibody blockade of soluble MIC in a model of adenocarcinoma could potentiate IFNγ production upon stimulation (100) As elevated levels of soluble NKG2D ligands in the plasma of patients with MM, acute lymphoblastic leukemia (ALL), chronic myeloid leukemia (CML), Hodgkin’s lymphoma (HL), and non-HL have been observed (101–105), it is predicted that NKG2D-expressing iNKT cells will be dysregulated in these tumor microenvironments. With evidence showing the capacity for iNKT cells to utilize TRAIL to kill leukemic cells in vitro (19), it is anticipated that blood tumors would be able to evade recognition by iNKT cells by altering TRAIL receptor expression. Indeed, myeloma and B cell lymphomas have been reported to resist TRAIL-induced killing (106), by downregulating TRAIL receptors—death receptor 4 (DR4) and DR5 (107, 108), or by dysregulating receptor signaling to evade killing (109, 110). Likewise, AML tumors have been observed to utilize decoy TRAIL receptors to resist apoptosis (111, 112).
Immunosuppressive Effects of Tumors on iNKT Cells
Blood cancer disease progression in humans is associated with a profound decrease in the frequency and function of circulating iNKT cells (12, 113–119). Although iNKT cell numbers have been shown to vary between subtypes and grade of B cell neoplasms in humans (13), this parameter has been used as an independent factor for predicting disease stage and progression in blood cancer patients (25, 36, 118). It is currently unclear how disease progression causes these defects in iNKT cells. Several studies have suggested that iNKT cell dysfunction caused by tumors are indirect, as iNKT cell function and expansion can be rescued upon administration of αGalCer-based treatments (36, 67, 120, 121), or lenalidomide treatment (122, 123). In studies in CML patients, aberrant tyrosine kinase expression and dysfunctional Rho-associated protein kinase (ROCK) expression have been suggested to exert suppressive effects on iNKT cells by regulating the transcription factor PLZF, expression of CD95L and perforin (17) as well as altering CD1d expression on myeloid DCs (mDCs) (18). Indeed, in CML patients who had undergone treatment using a tyrosine kinase inhibitor, iNKT cell functions could be restored (17). Likewise, in vitro treatment of CML mDCs with ROCK inhibitors was found to partially restore CD1d expression (18). iNKT cell dysfunction has also been associated with tumor-associated lipid antigen production, such as altered glycosphingolipids secreted by a murine T cell lymphoma cell line. The shedding of these lipid antigens were suggested to shield from iNKT cell recognition, as inhibition of the release of these lipid antigens could rescue iNKT cell functions (5). Interestingly, in certain patients with leukemia, higher CD1d levels have been detected on malignant cells that correlated with poorer prognosis and lower iNKT cell numbers (23, 27, 29). In this instance, higher presentation of tumor-associated lipids on CD1d by leukemic cells was suggested to cause iNKT cell hyporesponsiveness attributed to an impairment of CD3ζ signaling (29). In MM patients, inflammation-associated lysophospholipids and other glycolipids found to be elevated in the plasma were shown to induce iNKT cells to produce the Th2 cytokine IL-13 (36, 37), an anti-inflammatory cytokine associated with downregulation of tumor immunosurveillance (124). iNKT cell dysfunction has also been linked to hypoxia and tumor-associated macrophages (125), as well as interruptions in metabolic signaling caused by acidity of the tumor microenvironment (126) (Figure 1). These conditions have been implied to promote lymphoma tumor progression (127, 128). Better understanding of these immunosuppressive strategies of blood cancers will help with designing strategies that better harness the antitumor effects of iNKT cells.
Strategies to Modulate iNKT Cell Activity in Blood Cancers
Early Use of iNKT Cell Adjuvants
Over the past couple of decades, strategies to exploit iNKT cells have been explored to treat various types of cancer, including blood cancers. Early studies in preclinical models showed that direct injection of αGalCer or its derivatives could induce potent iNKT cell activation and subsequent innate and adaptive immune suppression of tumors, but was also associated with significant liver toxicity (63, 71, 129, 130). Unfortunately however, this antitumor effect was not recapitulated when tested against human cancers. A phase I clinical trial using αGalCer instead found limited value as a direct immunotherapeutic agent against advanced solid cancers, despite a relatively safe toxicity profile tested in dose-escalating studies (131, 132). Patients with a higher frequency of circulating iNKTs did however respond better to treatment and produce enhanced immunological responses (133). Yet, the induction of immunological activity in these patients did not result in any partial or complete responses, and only disease stabilization in some patients could be achieved (131, 132).
DC Vaccines
Subsequently, it was revealed that free-form αGalCer causes profound and enduring hyporesponsiveness in iNKT cells (134, 135). To overcome this treatment-induced anergy, various other delivery strategies have been designed, including the ex vivo stimulation and loading of autologous DCs with αGalCer. Initial studies in solid tumor preclinical models showed that administration of αGalCer-pulsed DCs could enhance the frequency of iNKT cells and circulating IFNγ-producing cells, as well as Th1 antitumor responses when compared to free-form αGalCer (38, 136, 137). In addition, αGalCer-pulsed DCs can also efficiently promote the infiltration of lymphocytes including iNKT cells into tumors, enhance circulating levels of IFNγ (138, 139), and promote iNKT cell-induced immune memory upon secondary administration (140). These properties are believed to contribute in part to the long-term survival of tumor-bearing mice receiving DC therapy. For example, αGalCer-pulsed DCs has been shown to improve overall survival of mice with MM (5T33MM model) (34). When tested in patients with advanced MM, administration of αGalCer-pulsed DCs was found to sufficiently induce iNKT cell expansion and persistence in the blood (38). However, this study did not observe any overall clinical improvement in these patients. In a Phase I/II study in six patients with asymptomatic myeloma, the combination therapy of αGalCer-pulsed monocyte-derived DCs with low-dose lenalidomide, resulted in improved modulation of both iNKT and NK cell responses, including the increased surface expression of NKG2D on NK cells. The addition of lenalidomide was intended to augment the effects of DC vaccination (39), as lenalidomide have been previously suggested to skew iNKT cell and cytokine induced killer (CIK) cell responses toward a protective Th1 profile in MM patients (123, 141, 142). Similarly, coloading of DCs with αGalCer and irradiated tumor cells has also been shown to be highly protective against B cell lymphoma in mice (4TOO model) (8). In this instance, the pulsing of DCs with tumor cells served to provide a source of undefined tumor antigens to initiate tumor-specific immune responses enhanced by the adjuvanting effects of αGalCer.
Tumor Cell-Based Vaccines
We and others have previously attempted to use autologous tumor cells as vaccine vehicles for αGalCer delivery in mice. Single administration of an αGalCer-loaded tumor cell vaccine could induce potent antitumor immunity and prolong overall survival in mice with various blood cancers, including B lymphoma (Eμ-myc), acute myeloid leukemia (AML-ETO9a), and myeloma (Vk*myc) (6, 7, 130, 143, 144). In addition, therapeutic effect of this vaccine approach was significantly enhanced when used in combination with immune checkpoint agonists, such as anti-4-1BB mAb (6). In other studies, the use of αGalCer-loaded tumor vaccines was also demonstrated to induce potent therapeutic responses against a murine model of MM (MOPC315.BM model) and found to generate long-term protection against tumor rechallenge (35). Interestingly, in a murine model of acute leukemia (C1498), the administration of αGalCer-loaded leukemic cells alone was found to be effective as a prophylactic vaccine but ineffective against established leukemia. The study found that while iNKT cells could be effectively activated, the downstream leukemia-specific T cell responses were suppressed. Instead, the benefit of vaccination became apparent following chemotherapy treatment, to prevent relapse of leukemia, and protect against rechallenge (21).
Adoptive Transfer of iNKT Cells and CIK Cells
While the use of autologous cell-based vaccines has proven to be effective in animal models, a potential limitation in human patients is the high variability of iNKT cell frequency. Also, the functionality of iNKT cells often diminishes with tumor progression. Therefore, to circumvent this issue, adoptive transfer of activated and expanded iNKT cells derived from patient peripheral blood mononuclear cells (PBMCs) have been explored. Notably, CD3+CD56+ CIK cells, which represent a mixture of NK cell-like T cells, and incorporate an iNKT population, possess non-MHC-dependent tumor activity mediated through perforin and NKG2D expression (14, 15). By culturing autologous PBMCs under various conditions (e.g., αGalCer in the presence of GM-CSF and/or IL-2, or with a combination of cytokines such as IFNγ, OKT3, IL-2, and IL-15), ex vivo expansion of autologous activated iNKT/CIK cells from patients can be achieved (20, 30, 145). Successful expansion of functional iNKT cells from adult hematopoietic stem-progenitor cells using artificial APCs coated with CD1d-immunoglobulin (146, 147) as well as iNKT cell generation from induced pluripotent stem cells have also been explored (148). Adoptive transfer of ex vivo expanded iNKT cells in conjunction with αGalCer administration is an effective treatment against CD1d+ leukemic cells implanted in immunodeficient NOD/SCID mice (67). Similarly, adoptive transfer of iNKT cells activated ex vivo with IL-12 and IL-18 could initiate protection against lymphoma (ALC model) in mice (9). In humans, cultured iNKT/CIK cells are able recognize autologous or allogenic blood tumor cells in vitro (20, 30–32, 149). However, therapeutic use of in vitro expanded iNKT cells against blood cancers in humans is limited. Thus far, three phase I trials and a phase II trial have looked into the safety profile and efficacy of expanded activated autologous iNKT cells in patients with solid tumors (150–153). All of these studies demonstrated safety and feasibility of treatment as well as induction of IFNγ in circulating iNKT in patients. In the phase II study, αGalCer-loaded APCs administered alongside activated iNKT cells led to iNKT cell accumulation at tumor sites and some clinical efficacy in 50% of patients enrolled (153).
Notably, the use of expanded CIK cells in association with other treatments has led to complete cancer remissions in patients with hematological malignancies [reviewed in Refs. (14, 15)]. CIK cells have also been used in combination with HSCT in a bid to potentiate the overall inhibitory effects of GvHD in blood cancer patients receiving transplants (154). In a phase I study published by Luo et al. (154), patients enrolled were refractory to chemotherapy or had relapsed after early allogenic HSCT treatment. While some patients displayed a response to engraftment of donor cells, and infusion of CIK cells appeared to contribute to the prolonged survival in these patients, the overall efficacy of the combination treatment remains limited for this small cohort of patients with highly aggressive hematological malignancies (154). The extent to which these responses can be attributed to iNKT-like cells specifically, is unknown.
Chimeric Antigen Receptor (CAR) Modified iNKT Cells and CIK Cells
Most recently, several studies have explored CAR engineering of iNKT/CIK cells (10, 22, 155). A summary of the proof of concept findings to date indicate that both CAR-NKT cells and CAR-CIK cells possess greater antitumor activity than their iNKT and CIK cell counterparts [recently reviewed in Ref. (156)]. In one example, donor CD62L+ iNKT cells that were identified to be highly proliferative in vitro were transduced with a CD19-specific CAR and tested for therapeutic activity against humanized mouse models of lymphoma and neuroblastoma. These CD62L+ CAR-NKT cells were demonstrated to persist long-term in vivo and were also highly effective at inhibiting tumor growth (10). The use of CAR-NKT cells was demonstrated to be safe and did not induce graft-versus-host disease (GvHD) in mice with neuroblastoma (155). In addition, the antitumor effects of CIK cells generated from donor PBMCs could also be further enhanced when transduced with CAR specific for CD19 and the CD28-CD3ζ signaling domain (22). These CAR-CIK cells were found to be highly effective against B-cell ALL (B-ALL) in vitro, including against CIK-resistant tumor cells. When tested in vivo, CAR-CIK cells were described to be more effective than non-CAR CIK cells in eliminating B-ALL tumors and promoting long-term survival in mice (22). We foresee that these studies will serve to accelerate research into modifying donor iNKT cells for adoptive therapies for blood cancers to complement other CAR-T cell-based therapies (157).
Nanoparticle-Based Delivery Systems for iNKT Cell Adjuvants
To overcome some of the limitations associated with adoptive NKT cell-based approaches and to provide less costly and time-consuming alternatives for NKT cell-targeting immunotherapy, research into the use of nanoparticle-based systems are emerging [reviewed in Ref. (158)]. Briefly, nanoparticle vectors are delivery vehicles less than 1 µM in size and have wide applications in various diagnostic and treatment settings, including tumor immunotherapy (159). Delivery of glycolipid adjuvants in suitable nanoparticles presents several advantages over delivery in soluble form, such as reduced toxicity profile (owing to the reduced amount required to elicit a biological response), the ability to overcome iNKT cell anergy (160) and the preferential targeted delivery to APCs in vivo (158). To date, there exists various published studies in preclinical models of solid cancers on the nanoparticulate delivery of αGalCer alone or co-delivered with tumor-associated antigens (11, 161–164). By comparison, few therapeutic applications of nanoparticle delivery of glycolipid adjuvants have been reported for blood cancers. One such study utilized a targeted PLGA nanoparticle to codeliver a model tumor antigen ovalbumin (OVA) and αGalCer to DEC205+ CD8α+ DCs. iNKT cells were rapidly activated using this approach and could drive the induction of cytolytic tumor-specific CD8 T cells. When assessed in prophylactic and therapeutic settings against a model of thymoma, administration of targeted nanoparticles could significantly suppress early tumor growth (11). Recently, a liposomal form of αGalCer (RGI-2001) has been designed to circumvent GvHD after HSCT. Initial preclinical studies show that RGI-2001 could aid in graft-versus-leukemia effect and significantly prevented acute GvHD in lethally irradiated leukemia-bearing mice given allele-mismatched donor bone marrow cells or spleen cells. This effect was believed to be largely due to the enhanced expansion of donor-derived CD4+ regulatory T (Treg) cells that could exert its effects in an antigen-specific manner (165). Although RGI-2001 was demonstrated to induce expansion of NKT cells as well as higher IL-4 levels early after treatment, the correlation between NKT cell expansion and Treg induction was not clearly demonstrated. In a Phase II study in blood cancer patients, RGI-2001 was administered as a single dose in combination with HSCT. Similar to findings in mice, this study showed that RGI-2001 was generally tolerable in most patients and suggested that immunosuppressive Treg cells could be efficiently induced in vivo in a small proportion of patients. However, due to limited patient recruitment and difficulties in the detection of NKT cells in the blood in this particular study, the extent to which NKT cells contributed to overall GvL response remained inconclusive (89).
Concluding Remarks
Increasing knowledge of how different blood cancers modulate their environment to avoid or suppress antitumor immunity has advanced the development of counteractive measures with immunotherapies. The fortuitous discovery of the potent NKT cell-stimulatory properties of αGalCer has enabled us to better understand how iNKT cells function to transactivate both the innate and adaptive immune system, and importantly, their unique role in antitumor immunity. However, encouraging findings in preclinical studies have not yet convincingly translated to similar outcomes in human cancers. In fact, the number of human trials testing the therapeutic use of various glycolipid compounds against cancer is limited, perhaps not only due to interindividual variability between patients but also due to the lack of understanding on the effects of tumors on decreasing iNKT frequencies and function. This is also true in harnessing the functions of NKT cells against GvHD after HSCT. In general, there still exists an uncertainty on the proper manipulation of iNKT cells and their different responses to a variety of glycolipids. We should continue to fully utilize preclinical models to understand how to best influence the functions of iNKT cells through synthetic glycolipid ligands, but also place more emphasis on the translation of these findings into the clinical setting, with the goal to rescue or enhance iNKT cell functions in different human blood cancer settings.
Author Contributions
PL undertook critical review of the literature, wrote the manuscript, and designed the figure. MN contributed to the writing and editing of the manuscript. SM designed the scope of the manuscript and assisted with writing and editing of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Mr. Takumi Kobayashi for critical review of the manuscript. PL was supported by a University of Queensland International Scholarship. MN was supported by an Australian Government Research Training Program (RTP) Scholarship. SM was supported by a National Health and Medical Research Council Career Development Fellowship (APP1061429).
References
1. McEwen-Smith RM, Salio M, Cerundolo V. The regulatory role of invariant NKT cells in tumor immunity. Cancer Immunol Res (2015) 3(5):425–35. doi:10.1158/2326-6066.CIR-15-0062
2. Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, et al. CD1d-restricted and TCR-mediated activation of Valpha14 NKT cells by glycosylceramides. Science (1997) 278(5343):1626–9. doi:10.1126/science.278.5343.1626
3. Kronenberg M, Gapin L. The unconventional lifestyle of NKT cells. Nat Rev Immunol (2002) 2(8):557–68. doi:10.1038/nri854
4. Haraguchi K, Takahashi T, Nakahara F, Matsumoto A, Kurokawa M, Ogawa S, et al. CD1d expression level in tumor cells is an important determinant for anti-tumor immunity by natural killer T cells. Leuk Lymphoma (2006) 47(10):2218–23. doi:10.1080/10428190600682688
5. Sriram V, Cho S, Li P, O’Donnell PW, Dunn C, Hayakawa K, et al. Inhibition of glycolipid shedding rescues recognition of a CD1+ T cell lymphoma by natural killer T (NKT) cells. Proc Natl Acad Sci U S A (2002) 99(12):8197–202. doi:10.1073/pnas.122636199
6. Kobayashi T, Doff BL, Rearden RC, Leggatt GR, Mattarollo SR. NKT cell-targeted vaccination plus anti-4-1BB antibody generates persistent CD8 T cell immunity against B cell lymphoma. Oncoimmunology (2015) 4(3):e990793. doi:10.4161/2162402X.2014.990793
7. Mattarollo SR, West AC, Steegh K, Duret H, Paget C, Martin B, et al. NKT cell adjuvant-based tumor vaccine for treatment of myc oncogene-driven mouse B-cell lymphoma. Blood (2012) 120(15):3019–29. doi:10.1182/blood-2012-04-426643
8. Escribà-Garcia L, Alvarez-Fernández C, Tellez-Gabriel M, Sierra J, Briones J. Dendritic cells combined with tumor cells and α-galactosylceramide induce a potent, therapeutic and NK-cell dependent antitumor immunity in B cell lymphoma. J Transl Med (2017) 15(1):115. doi:10.1186/s12967-017-1219-3
9. Baxevanis CN, Gritzapis AD, Papamichail M. In vivo antitumor activity of NKT cells activated by the combination of IL-12 and IL-18. J Immunol (2003) 171(6):2953–9. doi:10.4049/jimmunol.171.6.2953
10. Tian G, Courtney AN, Jena B, Heczey A, Liu D, Marinova E, et al. CD62L+ NKT cells have prolonged persistence and antitumor activity in vivo. J Clin Invest (2016) 126(6):2341–55. doi:10.1172/JCI83476
11. Macho-Fernandez E, Cruz LJ, Ghinnagow R, Fontaine J, Bialecki E, Frisch B, et al. Targeted delivery of alpha-galactosylceramide to CD8alpha+ dendritic cells optimizes type I NKT cell-based antitumor responses. J Immunol (2014) 193(2):961–9. doi:10.4049/jimmunol.1303029
12. Hus I, Staroslawska E, Bojarska-Junak A, Dobrzynska-Rutkowska A, Surdacka A, Wdowiak P, et al. CD3+/CD16+CD56+ cell numbers in peripheral blood are correlated with higher tumor burden in patients with diffuse large B-cell lymphoma. Folia Histochem Cytobiol (2011) 49(1):183–7. doi:10.5603/FHC.2011.0025
13. Gibson SE, Swerdlow SH, Felgar RE. Natural killer cell subsets and natural killer-like T-cell populations in benign and neoplastic B-cell proliferations vary based on clinicopathologic features. Hum Pathol (2011) 42(5):679–87. doi:10.1016/j.humpath.2010.07.023
14. Schmeel LC, Schmeel FC, Coch C, Schmidt-Wolf IG. Cytokine-induced killer (CIK) cells in cancer immunotherapy: report of the international registry on CIK cells (IRCC). J Cancer Res Clin Oncol (2015) 141(5):839–49. doi:10.1007/s00432-014-1864-3
15. Schmeel FC, Schmeel LC, Gast SM, Schmidt-Wolf IG. Adoptive immunotherapy strategies with cytokine-induced killer (CIK) cells in the treatment of hematological malignancies. Int J Mol Sci (2014) 15(8):14632–48. doi:10.3390/ijms150814632
16. Guo W, Dong A, Xing C, Lin X, Pan X, Lin Y, et al. CD1d levels in peripheral blood of patients with acute myeloid leukemia and acute lymphoblastic leukemia. Oncol Lett (2014) 8(2):825–30. doi:10.3892/ol.2014.2208
17. Rossignol A, Levescot A, Jacomet F, Robin A, Basbous S, Giraud C, et al. Evidence for BCR-ABL-dependent dysfunctions of iNKT cells from chronic myeloid leukemia patients. Eur J Immunol (2012) 42(7):1870–5. doi:10.1002/eji.201142043
18. Basbous S, Levescot A, Piccirilli N, Brizard F, Guilhot F, Roy L, et al. The Rho-ROCK pathway as a new pathological mechanism of innate immune subversion in chronic myeloid leukaemia. J Pathol (2016) 240(3):262–8. doi:10.1002/path.4779
19. Metelitsa LS, Weinberg KI, Emanuel PD, Seeger RC. Expression of CD1d by myelomonocytic leukemias provides a target for cytotoxic NKT cells. Leukemia (2003) 17(6):1068–77. doi:10.1038/sj.leu.2402943
20. Rogers PR, Emerson M, Nguyen E, Ball E, Bashey A, Kato S. Function and therapeutic potential of in vitro-expanded natural killer T (NKT) cells from patients with hematopoietic malignancies. Blood (2007) 110(11):805a–6a.
21. Gibbins JD, Ancelet LR, Weinkove R, Compton BJ, Painter GF, Petersen TR, et al. An autologous leukemia cell vaccine prevents murine acute leukemia relapse after cytarabine treatment. Blood (2014) 124(19):2953–63. doi:10.1182/blood-2014-04-568956
22. Oelsner S, Wagner J, Friede ME, Pfirrmann V, Genssler S, Rettinger E, et al. Chimeric antigen receptor-engineered cytokine-induced killer cells overcome treatment resistance of pre-B-cell acute lymphoblastic leukemia and enhance survival. Int J Cancer (2016) 139(8):1799–809. doi:10.1002/ijc.30217
23. Fais F, Tenca C, Cimino G, Coletti V, Zanardi S, Bagnara D, et al. CD1d expression on B-precursor acute lymphoblastic leukemia subsets with poor prognosis. Leukemia (2005) 19(4):551–6. doi:10.1038/sj.leu.2403671
24. Zaborsky N, Gassner FJ, Asslaber D, Reinthaler P, Denk U, Flenady S, et al. CD1d expression on chronic lymphocytic leukemia B cells affects disease progression and induces T cell skewing in CD8 positive and CD4CD8 double negative T cells. Oncotarget (2016) 7(31):49459–69. doi:10.18632/oncotarget.10372
25. Gorini F, Azzimonti L, Delfanti G, Scarfo L, Scielzo C, Bertilaccio MT, et al. Invariant NKT cells contribute to chronic lymphocytic leukemia surveillance and prognosis. Blood (2017) 129(26):3440–51. doi:10.1182/blood-2016-11-751065
26. Kotsianidis I, Nakou E, Spanoudakis E, Bouchliou I, Moustakidis E, Miltiades P, et al. The diagnostic value of CD1d expression in a large cohort of patients with B-cell chronic lymphoproliferative disorders. Am J Clin Pathol (2011) 136(3):400–8. doi:10.1309/AJCP2F2DOXOTXHZA
27. Anastasiadis A, Kotsianidis I, Papadopoulos V, Spanoudakis E, Margaritis D, Christoforidou A, et al. CD1d expression as a prognostic marker for chronic lymphocytic leukemia. Leuk Lymphoma (2014) 55(2):320–5. doi:10.3109/10428194.2013.803222
28. Weinkove R, Brooks CR, Carter JM, Hermans IF, Ronchese F. Functional invariant natural killer T-cell and CD1d axis in chronic lymphocytic leukemia: implications for immunotherapy. Haematologica (2013) 98(3):376–84. doi:10.3324/haematol.2012.072835
29. Bojarska-Junak A, Hus I, Chocholska S, Tomczak W, Wos J, Czubak P, et al. CD1d expression is higher in chronic lymphocytic leukemia patients with unfavorable prognosis. Leuk Res (2014) 38(4):435–42. doi:10.1016/j.leukres.2013.12.015
30. Guven H, Gilljam M, Chambers BJ, Ljunggren HG, Christensson B, Kimby E, et al. Expansion of natural killer (NK) and natural killer-like T (NKT)-cell populations derived from patients with B-chronic lymphocytic leukemia (B-CLL): a potential source for cellular immunotherapy. Leukemia (2003) 17(10):1973–80. doi:10.1038/sj.leu.2403083
31. Lefterova P, Schakowski F, Buttgereit P, Scheffold C, Huhn D, Schmidt-Wolf IG. Expansion of CD3+CD56+ cytotoxic cells from patients with chronic lymphocytic leukemia: in vitro efficacy. Haematologica (2000) 85(10):1108–9.
32. Linn YC, Lau LC, Hui KM. Generation of cytokine-induced killer cells from leukaemic samples with in vitro cytotoxicity against autologous and allogeneic leukaemic blasts. Br J Haematol (2002) 116(1):78–86. doi:10.1046/j.1365-2141.2002.03247.x
33. Linn YC, Hui KM. Cytokine-induced killer cells: NK-like T cells with cytotolytic specificity against leukemia. Leuk Lymphoma (2003) 44(9):1457–62. doi:10.3109/10428190309178764
34. Nur H, Fostier K, Aspeslagh S, Renmans W, Bertrand E, Leleu X, et al. Preclinical evaluation of invariant natural killer T cells in the 5T33 multiple myeloma model. PLoS One (2013) 8(5):e65075. doi:10.1371/journal.pone.0065075
35. Hong S, Lee H, Jung K, Lee SM, Lee SJ, Jun HJ, et al. Tumor cells loaded with α-galactosylceramide promote therapeutic NKT-dependent anti-tumor immunity in multiple myeloma. Immunol Lett (2013) 156(1–2):132–9. doi:10.1016/j.imlet.2013.10.002
36. Dhodapkar MV, Geller MD, Chang DH, Shimizu K, Fujii S, Dhodapkar KM, et al. A reversible defect in natural killer T cell function characterizes the progression of premalignant to malignant multiple myeloma. J Exp Med (2003) 197(12):1667–76. doi:10.1084/jem.20021650
37. Chang DH, Deng H, Matthews P, Krasovsky J, Ragupathi G, Spisek R, et al. Inflammation-associated lysophospholipids as ligands for CD1d-restricted T cells in human cancer. Blood (2008) 112(4):1308–16. doi:10.1182/blood-2008-04-149831
38. Chang DH, Osman K, Connolly J, Kukreja A, Krasovsky J, Pack M, et al. Sustained expansion of NKT cells and antigen-specific T cells after injection of alpha-galactosyl-ceramide loaded mature dendritic cells in cancer patients. J Exp Med (2005) 201(9):1503–17. doi:10.1084/jem.20042592
39. Richter J, Neparidze N, Zhang L, Nair S, Monesmith T, Sundaram R, et al. Clinical regressions and broad immune activation following combination therapy targeting human NKT cells in myeloma. Blood (2013) 121(3):423–30. doi:10.1182/blood-2012-06-435503
40. Dougan SK, Kaser A, Blumberg RS. CD1 expression on antigen-presenting cells. In: Moody DB, editor. T Cell Activation by CD1 and Lipid Antigens. Berlin, Heidelberg: Springer (2007). p. 113–41.
41. Brigl M, Brenner MB. CD1: antigen presentation and T cell function. Annu Rev Immunol (2004) 22:817–90. doi:10.1146/annurev.immunol.22.012703.104608
42. Mattner J, Debord KL, Ismail N, Goff RD, Cantu C III, Zhou D, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature (2005) 434(7032):525–9. doi:10.1038/nature03408
43. Zajonc DM, Girardi E. Recognition of microbial glycolipids by natural killer T cells. Front Immunol (2015) 6:400. doi:10.3389/fimmu.2015.00400
44. Banchet-Cadeddu A, Henon E, Dauchez M, Renault JH, Monneaux F, Haudrechy A. The stimulating adventure of KRN 7000. Org Biomol Chem (2011) 9(9):3080–104. doi:10.1039/c0ob00975j
45. Wu DY, Segal NH, Sidobre S, Kronenberg M, Chapman PB. Cross-presentation of disialoganglioside GD3 to natural killer T cells. J Exp Med (2003) 198(1):173–81. doi:10.1084/jem.20030446
46. Dias BR, Rodrigues EG, Nimrichter L, Nakayasu ES, Almeida IC, Travassos LR. Identification of iGb3 and iGb4 in melanoma B16F10-Nex2 cells and the iNKT cell-mediated antitumor effect of dendritic cells primed with iGb3. Mol Cancer (2009) 8:116. doi:10.1186/1476-4598-8-116
47. Zhou D, Mattner J, Cantu C III, Schrantz N, Yin N, Gao Y, et al. Lysosomal glycosphingolipid recognition by NKT cells. Science (2004) 306(5702):1786–9. doi:10.1126/science.1103440
48. Dowds CM, Kornell SC, Blumberg RS, Zeissig S. Lipid antigens in immunity. Biol Chem (2014) 395(1):61–81. doi:10.1515/hsz-2013-0220
49. Bassiri H, Das R, Nichols KE. Invariant NKT cells: killers and conspirators against cancer. Oncoimmunology (2013) 2(12):e27440. doi:10.4161/onci.27440
50. Metelitsa LS, Naidenko OV, Kant A, Wu HW, Loza MJ, Perussia B, et al. Human NKT cells mediate antitumor cytotoxicity directly by recognizing target cell CD1d with bound ligand or indirectly by producing IL-2 to activate NK cells. J Immunol (2001) 167(6):3114–22. doi:10.4049/jimmunol.167.6.3114
51. Crowe NY, Coquet JM, Berzins SP, Kyparissoudis K, Keating R, Pellicci DG, et al. Differential antitumor immunity mediated by NKT cell subsets in vivo. J Exp Med (2005) 202(9):1279–88. doi:10.1084/jem.20050953
52. Coquet JM, Chakravarti S, Kyparissoudis K, McNab FW, Pitt LA, McKenzie BS, et al. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4-NK1.1- NKT cell population. Proc Natl Acad Sci U S A (2008) 105(32):11287–92. doi:10.1073/pnas.0801631105
53. Smyth MJ, Godfrey DI. NKT cells and tumor immunity – a double-edged sword. Nat Immunol (2000) 1(6):459–60. doi:10.1038/82698
54. Carnaud C, Lee D, Donnars O, Park SH, Beavis A, Koezuka Y, et al. Cutting edge: cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J Immunol (1999) 163(9):4647–50.
55. Smyth MJ, Crowe NY, Pellicci DG, Kyparissoudis K, Kelly JM, Takeda K, et al. Sequential production of interferon-gamma by NK1.1(+) T cells and natural killer cells is essential for the antimetastatic effect of alpha-galactosylceramide. Blood (2002) 99(4):1259–66. doi:10.1182/blood.V99.4.1259
56. Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature (1998) 392(6673):245–52. doi:10.1038/32588
57. Fujii S, Shimizu K, Hemmi H, Steinman RM. Innate Valpha14(+) natural killer T cells mature dendritic cells, leading to strong adaptive immunity. Immunol Rev (2007) 220:183–98. doi:10.1111/j.1600-065X.2007.00561.x
58. Fujii S, Liu K, Smith C, Bonito AJ, Steinman RM. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J Exp Med (2004) 199(12):1607–18. doi:10.1084/jem.20040317
59. Hermans IF, Silk JD, Gileadi U, Salio M, Mathew B, Ritter G, et al. NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J Immunol (2003) 171(10):5140–7. doi:10.4049/jimmunol.171.10.5140
60. Swann JB, Uldrich AP, van Dommelen S, Sharkey J, Murray WK, Godfrey DI, et al. Type I natural killer T cells suppress tumors caused by p53 loss in mice. Blood (2009) 113(25):6382–5. doi:10.1182/blood-2009-01-198564
61. Smyth MJ, Thia KY, Street SE, Cretney E, Trapani JA, Taniguchi M, et al. Differential tumor surveillance by natural killer (NK) and NKT cells. J Exp Med (2000) 191(4):661–8. doi:10.1084/jem.191.4.661
62. Crowe NY, Smyth MJ, Godfrey DI. A critical role for natural killer T cells in immunosurveillance of methylcholanthrene-induced sarcomas. J Exp Med (2002) 196(1):119–27. doi:10.1084/jem.20020092
63. Hayakawa Y, Rovero S, Forni G, Smyth MJ. Alpha-galactosylceramide (KRN7000) suppression of chemical- and oncogene-dependent carcinogenesis. Proc Natl Acad Sci U S A (2003) 100(16):9464–9. doi:10.1073/pnas.1630663100
64. Renukaradhya GJ, Khan MA, Vieira M, Du W, Gervay-Hague J, Brutkiewicz RR. Type I NKT cells protect (and type II NKT cells suppress) the host’s innate antitumor immune response to a B-cell lymphoma. Blood (2008) 111(12):5637–45. doi:10.1182/blood-2007-05-092866
65. Li J, Sun W, Subrahmanyam PB, Page C, Younger KM, Tiper IV, et al. NKT cell responses to B cell lymphoma. Med Sci (Basel) (2014) 2(2):82–97. doi:10.3390/medsci2020082
66. Renukaradhya GJ, Sriram V, Du W, Gervay-Hague J, Van Kaer L, Brutkiewicz RR. Inhibition of antitumor immunity by invariant natural killer T cells in a T-cell lymphoma model in vivo. Int J Cancer (2006) 118(12):3045–53. doi:10.1002/ijc.21764
67. Bagnara D, Ibatici A, Corselli M, Sessarego N, Tenca C, De Santanna A, et al. Adoptive immunotherapy mediated by ex vivo expanded natural killer T cells against CD1d-expressing lymphoid neoplasms. Haematologica (2009) 94(7):967–74. doi:10.3324/haematol.2008.001339
68. Xu C, de Vries R, Visser L, Diepstra A, Gadola SD, Poppema S, et al. Expression of CD1d and presence of invariant NKT cells in classical Hodgkin lymphoma. Am J Hematol (2010) 85(7):539–41. doi:10.1002/ajh.21743
69. Fais F, Morabito F, Stelitano C, Callea V, Zanardi S, Scudeletti M, et al. CD1d is expressed on B-chronic lymphocytic leukemia cells and mediates alpha-galactosylceramide presentation to natural killer T lymphocytes. Int J Cancer (2004) 109(3):402–11. doi:10.1002/ijc.11723
70. Chan AC, Neeson P, Leeansyah E, Tainton K, Quach H, Prince HM, et al. Natural killer T cell defects in multiple myeloma and the impact of lenalidomide therapy. Clin Exp Immunol (2013) 175(1):49–58. doi:10.1111/cei.12196
71. Hayakawa Y, Takeda K, Yagita H, Smyth MJ, Van Kaer L, Okumura K, et al. IFN-gamma-mediated inhibition of tumor angiogenesis by natural killer T-cell ligand, alpha-galactosylceramide. Blood (2002) 100(5):1728–33.
72. Wingender G, Krebs P, Beutler B, Kronenberg M. Antigen-specific cytotoxicity by invariant NKT cells in vivo is CD95/CD178-dependent and is correlated with antigenic potency. J Immunol (2010) 185(5):2721–9. doi:10.4049/jimmunol.1001018
73. Nieda M, Nicol A, Koezuka Y, Kikuchi A, Lapteva N, Tanaka Y, et al. TRAIL expression by activated human CD4(+)V alpha 24NKT cells induces in vitro and in vivo apoptosis of human acute myeloid leukemia cells. Blood (2001) 97(7):2067–74. doi:10.1182/blood.V97.7.2067
74. Matsuda JL, Zhang Q, Ndonye R, Richardson SK, Howell AR, Gapin L. T-bet concomitantly controls migration, survival, and effector functions during the development of Valpha14i NKT cells. Blood (2006) 107(7):2797–805. doi:10.1182/blood-2005-08-3103
75. Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Sato H, et al. Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated Valpha14 NKT cells. Proc Natl Acad Sci U S A (1998) 95(10):5690–3. doi:10.1073/pnas.95.10.5690
76. Gumperz JE, Miyake S, Yamamura T, Brenner MB. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J Exp Med (2002) 195(5):625–36. doi:10.1084/jem.20011786
77. Kuylenstierna C, Bjorkstrom NK, Andersson SK, Sahlstrom P, Bosnjak L, Paquin-Proulx D, et al. NKG2D performs two functions in invariant NKT cells: direct TCR-independent activation of NK-like cytolysis and co-stimulation of activation by CD1d. Eur J Immunol (2011) 41(7):1913–23. doi:10.1002/eji.200940278
78. Guerra N, Tan YX, Joncker NT, Choy A, Gallardo F, Xiong N, et al. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity (2008) 28(4):571–80. doi:10.1016/j.immuni.2008.02.016
79. Belting L, Homberg N, Przewoznik M, Brenner C, Riedel T, Flatley A, et al. Critical role of the NKG2D receptor for NK cell-mediated control and immune escape of B-cell lymphoma. Eur J Immunol (2015) 45(9):2593–601. doi:10.1002/eji.201445375
80. Raju S, Kretzmer LZ, Koues OI, Payton JE, Oltz EM, Cashen A, et al. NKG2D-NKG2D ligand interaction inhibits the outgrowth of naturally arising low-grade B cell lymphoma in vivo. J Immunol (2016) 196(11):4805–13. doi:10.4049/jimmunol.1501982
81. Poggi A, Catellani S, Garuti A, Pierri I, Gobbi M, Zocchi MR. Effective in vivo induction of NKG2D ligands in acute myeloid leukaemias by all-trans-retinoic acid or sodium valproate. Leukemia (2009) 23(4):641–8. doi:10.1038/leu.2008.354
82. Diermayr S, Himmelreich H, Durovic B, Mathys-Schneeberger A, Siegler U, Langenkamp U, et al. NKG2D ligand expression in AML increases in response to HDAC inhibitor valproic acid and contributes to allorecognition by NK-cell lines with single KIR-HLA class I specificities. Blood (2008) 111(3):1428–36. doi:10.1182/blood-2007-07-101311
83. Wang H, Yang D, Xu W, Wang Y, Ruan Z, Zhao T, et al. Tumor-derived soluble MICs impair CD3(+)CD56(+) NKT-like cell cytotoxicity in cancer patients. Immunol Lett (2008) 120(1–2):65–71. doi:10.1016/j.imlet.2008.07.001
84. Pillai AB, George TI, Dutt S, Teo P, Strober S. Host NKT cells can prevent graft-versus-host disease and permit graft antitumor activity after bone marrow transplantation. J Immunol (2007) 178(10):6242–51. doi:10.4049/jimmunol.178.10.6242
85. Casorati G, de Lalla C, Dellabona P. Invariant natural killer T cells reconstitution and the control of leukemia relapse in pediatric haploidentical hematopoietic stem cell transplantation. Oncoimmunology (2012) 1(3):355–7. doi:10.4161/onci.18399
86. Dellabona P, Casorati G, de Lalla C, Montagna D, Locatelli F. On the use of donor-derived iNKT cells for adoptive immunotherapy to prevent leukemia recurrence in pediatric recipients of HLA haploidentical HSCT for hematological malignancies. Clin Immunol (2011) 140(2):152–9. doi:10.1016/j.clim.2010.11.015
87. de Lalla C, Rinaldi A, Montagna D, Azzimonti L, Bernardo ME, Sangalli LM, et al. Invariant NKT cell reconstitution in pediatric leukemia patients given HLA-haploidentical stem cell transplantation defines distinct CD4+ and CD4- subset dynamics and correlates with remission state. J Immunol (2011) 186(7):4490–9. doi:10.4049/jimmunol.1003748
88. Guan P, Bassiri H, Patel NP, Nichols KE, Das R. Invariant natural killer T cells in hematopoietic stem cell transplantation: killer choice for natural suppression. Bone Marrow Transplant (2016) 51(5):629–37. doi:10.1038/bmt.2015.335
89. Chen YB, Efebera YA, Johnston L, Ball ED, Avigan D, Lekakis LJ, et al. Increased Foxp3+helios+ regulatory t cells and decreased acute graft-versus-host disease after allogeneic bone marrow transplantation in patients receiving sirolimus and RGI-2001, an activator of invariant natural killer T cells. Biol Blood Marrow Transplant (2017) 23(4):625–34. doi:10.1016/j.bbmt.2017.01.069
90. Hongo D, Tang XB, Dutt S, Nador RG, Strober S. Interactions between NKT cells and Tregs are required for tolerance to combined bone marrow and organ transplants. Blood (2012) 119(6):1581–9. doi:10.1182/blood-2011-08-371948
91. Du J, Paz K, Thangavelu G, Schneidawind D, Baker J, Flynn R, et al. Invariant natural killer T cells ameliorate murine chronic GVHD by expanding donor regulatory T cells. Blood (2017) 129(23):3121–5. doi:10.1182/blood-2016-11-752444
92. Upadhyay R, Hammerich L, Peng P, Brown B, Merad M, Brody JD. Lymphoma: immune evasion strategies. Cancers (Basel) (2015) 7(2):736–62. doi:10.3390/cancers7020736
93. Teague RM, Kline J. Immune evasion in acute myeloid leukemia: current concepts and future directions. J Immunother Cancer (2013) 1(13). doi:10.1186/2051-1426-1-13
94. Zheng Z, Venkatapathy S, Rao G, Harrington CA. Expression profiling of B cell chronic lymphocytic leukemia suggests deficient CD1-mediated immunity, polarized cytokine response, altered adhesion and increased intracellular protein transport and processing of leukemic cells. Leukemia (2002) 16(12):2429–37. doi:10.1038/sj.leu.2402711
95. Hix LM, Shi YH, Brutkiewicz RR, Stein PL, Wang CR, Zhang M. CD1d-expressing breast cancer cells modulate NKT cell-mediated antitumor immunity in a murine model of breast cancer metastasis. PLoS One (2011) 6(6):e20702. doi:10.1371/journal.pone.0020702
96. Fiedler T, Walter W, Reichert TE, Maeurer MJ. Regulation of CD1d expression by murine tumor cells: escape from immunosurveillance or alternate target molecules? Int J Cancer (2002) 98(3):389–97. doi:10.1002/ijc.10141
97. Chaudhry MS, Karadimitris A. Role and regulation of CD1d in normal and pathological B cells. J Immunol (2014) 193(10):4761–8. doi:10.4049/jimmunol.1401805
98. Yuan W, Dasgupta A, Cresswell P. Herpes simplex virus evades natural killer T cell recognition by suppressing CD1d recycling. Nat Immunol (2006) 7(8):835–42. doi:10.1038/ni1364
99. Lu S, Zhang J, Liu D, Li G, Staveley-O’Carroll KF, Li Z, et al. Nonblocking monoclonal antibody targeting soluble MIC revamps endogenous innate and adaptive antitumor responses and eliminates primary and metastatic tumors. Clin Cancer Res (2015) 21(21):4819–30. doi:10.1158/1078-0432.CCR-15-0845
100. Bezu L, Gomes-de-Silva LC, Dewitte H, Breckpot K, Fucikova J, Spisek R, et al. Combinatorial strategies for the induction of immunogenic cell death. Front Immunol (2015) 6:187. doi:10.3389/fimmu.2015.00187
101. Salih HR, Antropius H, Gieseke F, Lutz SZ, Kanz L, Rammensee HG, et al. Functional expression and release of ligands for the activating immunoreceptor NKG2D in leukemia. Blood (2003) 102(4):1389–96. doi:10.1182/blood-2003-01-0019
102. Nuckel H, Switala M, Sellmann L, Horn PA, Durig J, Duhrsen U, et al. The prognostic significance of soluble NKG2D ligands in B-cell chronic lymphocytic leukemia. Leukemia (2010) 24(6):1152–9. doi:10.1038/leu.2010.74
103. Hilpert J, Grosse-Hovest L, Grunebach F, Buechele C, Nuebling T, Raum T, et al. Comprehensive analysis of NKG2D ligand expression and release in leukemia: implications for NKG2D-mediated NK cell responses. J Immunol (2012) 189(3):1360–71. doi:10.4049/jimmunol.1200796
104. Zocchi MR, Catellani S, Canevali P, Tavella S, Garuti A, Villaggio B, et al. High ERp5/ADAM10 expression in lymph node microenvironment and impaired NKG2D ligands recognition in Hodgkin lymphomas. Blood (2012) 119(6):1479–89. doi:10.1182/blood-2011-07-370841
105. Jinushi M, Vanneman M, Munshi NC, Tai YT, Prabhala RH, Ritz J, et al. MHC class I chain-related protein A antibodies and shedding are associated with the progression of multiple myeloma. Proc Natl Acad Sci U S A (2008) 105(4):1285–90. doi:10.1073/pnas.0711293105
106. Testa U. TRAIL/TRAIL-R in hematologic malignancies. J Cell Biochem (2010) 110(1):21–34. doi:10.1002/jcb.22549
107. Kagawa K, Nakano A, Miki H, Oda A, Amou H, Takeuchi K, et al. Inhibition of TACE activity enhances the susceptibility of myeloma cells to TRAIL. PLoS One (2012) 7(2):e31594. doi:10.1371/journal.pone.0031594
108. Rubio-Moscardo F, Blesa D, Mestre C, Siebert R, Balasas T, Benito A, et al. Characterization of 8p21.3 chromosomal deletions in B-cell lymphoma: TRAIL-R1 and TRAIL-R2 as candidate dosage-dependent tumor suppressor genes. Blood (2005) 106(9):3214–22. doi:10.1182/blood-2005-05-2013
109. Plumas J, Jacob MC, Chaperot L, Molens JP, Sotto JJ, Bensa JC. Tumor B cells from non-Hodgkin’s lymphoma are resistant to CD95 (Fas/Apo-1)-mediated apoptosis. Blood (1998) 91(8):2875–85.
110. Mathas S, Lietz A, Anagnostopoulos I, Hummel F, Wiesner B, Janz M, et al. c-FLIP mediates resistance of Hodgkin/Reed-Sternberg cells to death receptor-induced apoptosis. J Exp Med (2004) 199(8):1041–52. doi:10.1084/jem.20031080
111. Riccioni R, Pasquini L, Mariani G, Saulle E, Rossini A, Diverio D, et al. TRAIL decoy receptors mediate resistance of acute myeloid leukemia cells to TRAIL. Haematologica (2005) 90(5):612–24.
112. Chamuleau MED, Ossenkoppele GJ, van Rhenen A, van Dreunen L, Jirka SMG, Zevenbergen A, et al. High TRAIL-R3 expression on leukemic blasts is associated with poor outcome and induces apoptosis-resistance which can be overcome by targeting TRAIL-R2. Leuk Res (2011) 35(6):741–9. doi:10.1016/j.leukres.2010.12.032
113. Zeng W, Maciejewski JP, Chen G, Risitano AM, Kirby M, Kajigaya S, et al. Selective reduction of natural killer T cells in the bone marrow of aplastic anaemia. Br J Haematol (2002) 119(3):803–9. doi:10.1046/j.1365-2141.2002.03875.x
114. Yoneda K, Morii T, Nieda M, Tsukaguchi N, Amano I, Tanaka H, et al. The peripheral blood Valpha24+ NKT cell numbers decrease in patients with haematopoietic malignancy. Leuk Res (2005) 29(2):147–52. doi:10.1016/j.leukres.2004.06.005
115. Tahir SM, Cheng O, Shaulov A, Koezuka Y, Bubley GJ, Wilson SB, et al. Loss of IFN-gamma production by invariant NK T cells in advanced cancer. J Immunol (2001) 167(7):4046–50. doi:10.4049/jimmunol.167.7.4046
116. Jadidi-Niaragh F, Jeddi-Tehrani M, Ansaripour B, Razavi SM, Sharifian RA, Shokri F. Reduced frequency of NKT-like cells in patients with progressive chronic lymphocytic leukemia. Med Oncol (2012) 29(5):3561–9. doi:10.1007/s12032-012-0262-4
117. Nowak M, Arredouani MS, Tun-Kyi A, Schmidt-Wolf I, Sanda MG, Balk SP, et al. Defective NKT cell activation by CD1d+ TRAMP prostate tumor cells is corrected by interleukin-12 with alpha-galactosylceramide. PLoS One (2010) 5(6):e11311. doi:10.1371/journal.pone.0011311
118. Fujii S, Shimizu K, Klimek V, Geller MD, Nimer SD, Dhodapkar MV. Severe and selective deficiency of interferon-gamma-producing invariant natural killer T cells in patients with myelodysplastic syndromes. Br J Haematol (2003) 122(4):617–22. doi:10.1046/j.1365-2141.2003.04465.x
119. Chuc AEN, Cervantes LAM, Retiguin FP, Ojeda JV, Maldonado ER. Low number of invariant NKT cells is associated with poor survival in acute myeloid leukemia. J Cancer Res Clin Oncol (2012) 138(8):1427–32. doi:10.1007/s00432-012-1251-x
120. Toura I, Kawano T, Akutsu Y, Nakayama T, Ochiai T, Taniguchi M. Cutting edge: inhibition of experimental tumor metastasis by dendritic cells pulsed with alpha-galactosylceramide. J Immunol (1999) 163(5):2387–91.
121. van der Vliet HJ, Molling JW, Nishi N, Masterson AJ, Kolgen W, Porcelli SA, et al. Polarization of Valpha24+ Vbeta11+ natural killer T cells of healthy volunteers and cancer patients using alpha-galactosylceramide-loaded and environmentally instructed dendritic cells. Cancer Res (2003) 63(14):4101–6.
122. Chang DH, Liu N, Klimek V, Hassoun H, Mazumder A, Nimer SD, et al. Enhancement of ligand-dependent activation of human natural killer T cells by lenalidomide: therapeutic implications. Blood (2006) 108(2):618–21. doi:10.1182/blood-2005-10-4184
123. Song W, van der Vliet HJ, Tai YT, Prabhala R, Wang R, Podar K, et al. Generation of antitumor invariant natural killer T cell lines in multiple myeloma and promotion of their functions via lenalidomide: a strategy for immunotherapy. Clin Cancer Res (2008) 14(21):6955–62. doi:10.1158/1078-0432.CCR-07-5290
124. Terabe M, Matsui S, Noben-Trauth N, Chen H, Watson C, Donaldson DD, et al. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat Immunol (2000) 1(6):515–20. doi:10.1038/82771
125. Liu D, Song L, Wei J, Courtney AN, Gao X, Marinova E, et al. IL-15 protects NKT cells from inhibition by tumor-associated macrophages and enhances antimetastatic activity. J Clin Invest (2012) 122(6):2221–33. doi:10.1172/JCI59535
126. Xie D, Zhu S, Bai L. Lactic acid in tumor microenvironments causes dysfunction of NKT cells by interfering with mTOR signaling. Sci China Life Sci (2016) 59(12):1290–6. doi:10.1007/s11427-016-0348-7
127. Vishvakarma NK, Singh SM. Mechanisms of tumor growth retardation by modulation of pH regulation in the tumor-microenvironment of a murine T cell lymphoma. Biomed Pharmacother (2011) 65(1):27–39. doi:10.1016/j.biopha.2010.06.012
128. Wein F, Otto T, Lambertz P, Fandrey J, Hansmann ML, Kuppers R. Potential role of hypoxia in early stages of Hodgkin lymphoma pathogenesis. Haematologica (2015) 100(10):1320–6. doi:10.3324/haematol.2015.127498
129. Nakagawa R, Motoki K, Ueno H, Iijima R, Nakamura H, Kobayashi E, et al. Treatment of hepatic metastasis of the colon26 adenocarcinoma with an alpha-galactosylceramide, KRN7000. Cancer Res (1998) 58(6):1202–7.
130. Fujii S, Shimizu K, Hemmi H, Fukui M, Bonito AJ, Chen G, et al. Glycolipid alpha-C-galactosylceramide is a distinct inducer of dendritic cell function during innate and adaptive immune responses of mice. Proc Natl Acad Sci U S A (2006) 103(30):11252–7. doi:10.1073/pnas.0604812103
131. Giaccone G, Punt CJ, Ando Y, Ruijter R, Nishi N, Peters M, et al. A phase I study of the natural killer T-cell ligand alpha-galactosylceramide (KRN7000) in patients with solid tumors. Clin Cancer Res (2002) 8(12):3702–9.
132. Schneiders FL, Scheper RJ, von Blomberg BM, Woltman AM, Janssen HL, van den Eertwegh AJ, et al. Clinical experience with alpha-galactosylceramide (KRN7000) in patients with advanced cancer and chronic hepatitis B/C infection. Clin Immunol (2011) 140(2):130–41. doi:10.1016/j.clim.2010.11.010
133. Schneiders FL, de Bruin RC, van den Eertwegh AJ, Scheper RJ, Leemans CR, Brakenhoff RH, et al. Circulating invariant natural killer T-cell numbers predict outcome in head and neck squamous cell carcinoma: updated analysis with 10-year follow-up. J Clin Oncol (2012) 30(5):567–70. doi:10.1200/JCO.2011.38.8819
134. Parekh VV, Wilson MT, Olivares-Villagomez D, Singh AK, Wu L, Wang CR, et al. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J Clin Invest (2005) 115(9):2572–83. doi:10.1172/JCI24762
135. Sullivan BA, Kronenberg M. Activation or anergy: NKT cells are stunned by alpha-galactosylceramide. J Clin Invest (2005) 115(9):2328–9. doi:10.1172/JCI26297
136. Fujii S, Shimizu K, Kronenberg M, Steinman RM. Prolonged IFN-gamma-producing NKT response induced with alpha-galactosylceramide-loaded DCs. Nat Immunol (2002) 3(9):867–74. doi:10.1038/ni827
137. Motohashi S, Okamoto Y, Yoshino I, Nakayama T. Anti-tumor immune responses induced by iNKT cell-based immunotherapy for lung cancer and head and neck cancer. Clin Immunol (2011) 140(2):167–76. doi:10.1016/j.clim.2011.01.009
138. Nagato K, Motohashi S, Ishibashi F, Okita K, Yamasaki K, Moriya Y, et al. Accumulation of activated invariant natural killer T cells in the tumor microenvironment after alpha-galactosylceramide-pulsed antigen presenting cells. J Clin Immunol (2012) 32(5):1071–81. doi:10.1007/s10875-012-9697-9
139. Tatsumi T, Takehara T, Yamaguchi S, Sasakawa A, Sakamori R, Ohkawa K, et al. Intrahepatic delivery of alpha-galactosylceramide-pulsed dendritic cells suppresses liver tumor. Hepatology (2007) 45(1):22–30. doi:10.1002/hep.21447
140. Nieda M, Okai M, Tazbirkova A, Lin H, Yamaura A, Ide K, et al. Therapeutic activation of Valpha24+Vbeta11+ NKT cells in human subjects results in highly coordinated secondary activation of acquired and innate immunity. Blood (2004) 103(2):383–9. doi:10.1182/blood-2003-04-1155
141. Handa H, Saitoh T, Murakami H. [Immunomodulatory effects of lenalidomide]. Nihon Rinsho (2015) 73(1):156–61.
142. Handa H, Kamiya A, Nagai K, Saitoh T, Alkebsi L, Mawatari M, et al. Lenalidomide expand CD3+CD56+ T cell cytokine induced killer (CIK) cells in vivo in multiple myeloma patients. Clin Lymphoma Myeloma Leuk (2015) 15:e296. doi:10.1016/j.clml.2015.07.603
143. Chung Y, Qin H, Kang CY, Kim S, Kwak LW, Dong C. An NKT-mediated autologous vaccine generates CD4 T-cell dependent potent antilymphoma immunity. Blood (2007) 110(6):2013–9. doi:10.1182/blood-2006-12-061309
144. Shimizu K, Kurosawa Y, Taniguchi M, Steinman RM, Fujii S. Cross-presentation of glycolipid from tumor cells loaded with alpha-galactosylceramide leads to potent and long-lived T cell mediated immunity via dendritic cells. J Exp Med (2007) 204(11):2641–53. doi:10.1084/jem.20070458
145. Leemhuis T, Wells S, Scheffold C, Edinger M, Negrin RS. A phase I trial of autologous cytokine-induced killer cells for the treatment of relapsed Hodgkin disease and non-Hodgkin lymphoma. Biol Blood Marrow Transplant (2005) 11(3):181–7. doi:10.1016/j.bbmt.2004.11.019
146. Webb TJ, Bieler JG, Schneck JP, Oelke M. Ex vivo induction and expansion of natural killer T cells by CD1d1-Ig coated artificial antigen presenting cells. J Immunol Methods (2009) 346(1–2):38–44. doi:10.1016/j.jim.2009.05.003
147. Sun W, Wang Y, East JE, Kimball AS, Tkaczuk K, Kesmodel S, et al. Invariant natural killer T cells generated from human adult hematopoietic stem-progenitor cells are poly-functional. Cytokine (2015) 72(1):48–57. doi:10.1016/j.cyto.2014.12.009
148. Yamada D, Iyoda T, Vizcardo R, Shimizu K, Sato Y, Endo TA, et al. Efficient regeneration of human Valpha24+ invariant natural killer T cells and their anti-tumor activity in vivo. Stem Cells (2016) 34(12):2852–60. doi:10.1002/stem.2465
149. Mattarollo SR, Kenna T, Nieda M, Nicol AJ. Chemotherapy pretreatment sensitizes solid tumor-derived cell lines to Valpha24+ NKT cell-mediated cytotoxicity. Int J Cancer (2006) 119(7):1630–7. doi:10.1002/ijc.22019
150. Exley MA, Friedlander P, Alatrakchi N, Vriend L, Yue S, Sasada T, et al. Adoptive transfer of invariant NKT cells as immunotherapy for advanced melanoma: a phase I clinical trial. Clin Cancer Res (2017) 23(14):3510–9. doi:10.1158/1078-0432.Ccr-16-0600
151. Motohashi S, Ishikawa A, Ishikawa E, Otsuji M, Iizasa T, Hanaoka H, et al. A phase I study of in vitro expanded natural killer T cells in patients with advanced and recurrent non-small cell lung cancer. Clin Cancer Res (2006) 12(20 Pt 1):6079–86. doi:10.1158/1078-0432.CCR-06-0114
152. Kunii N, Horiguchi S, Motohashi S, Yamamoto H, Ueno N, Yamamoto S, et al. Combination therapy of in vitro-expanded natural killer T cells and alpha-galactosylceramide-pulsed antigen-presenting cells in patients with recurrent head and neck carcinoma. Cancer Sci (2009) 100(6):1092–8. doi:10.1111/j.1349-7006.2009.01135.x
153. Yamasaki K, Horiguchi S, Kurosaki M, Kunii N, Nagato K, Hanaoka H, et al. Induction of NKT cell-specific immune responses in cancer tissues after NKT cell-targeted adoptive immunotherapy. Clin Immunol (2011) 138(3):255–65. doi:10.1016/j.clim.2010.11.014
154. Luo Y, Zeng HQ, Shen Y, Zhang P, Lou SF, Chen L, et al. Allogeneic hematopoietic stem cell transplantation following donor CIK cell infusion: a phase I study in patients with relapsed/refractory hematologic malignancies. Leuk Res (2016) 48:6–10. doi:10.1016/j.leukres.2016.06.006
155. Heczey A, Liu D, Tian G, Courtney AN, Wei J, Marinova E, et al. Invariant NKT cells with chimeric antigen receptor provide a novel platform for safe and effective cancer immunotherapy. Blood (2014) 124(18):2824–33. doi:10.1182/blood-2013-11-541235
156. Bollino D, Webb TJ. Chimeric antigen receptor-engineered natural killer and natural killer T cells for cancer immunotherapy. Transl Res (2017) 187:32–43. doi:10.1016/j.trsl.2017.06.003
157. Wang Z, Wu Z, Liu Y, Han W. New development in CAR-T cell therapy. J Hematol Oncol (2017) 10(1):53. doi:10.1186/s13045-017-0423-1
158. Ghinnagow R, Cruz LJ, Macho-Fernandez E, Faveeuw C, Trottein F. Enhancement of adjuvant functions of natural killer T cells using nanovector delivery systems: application in anticancer immune therapy. Front Immunol (2017) 8:879. doi:10.3389/fimmu.2017.00879
159. Baetke SC, Lammers T, Kiessling F. Applications of nanoparticles for diagnosis and therapy of cancer. Br J Radiol (2015) 88(1054):20150207. doi:10.1259/bjr.20150207
160. Thapa P, Zhang G, Xia C, Gelbard A, Overwijk WW, Liu C, et al. Nanoparticle formulated alpha-galactosylceramide activates NKT cells without inducing anergy. Vaccine (2009) 27(25–26):3484–8. doi:10.1016/j.vaccine.2009.01.047
161. Dolen Y, Kreutz M, Gileadi U, Tel J, Vasaturo A, van Dinther EA, et al. Co-delivery of PLGA encapsulated invariant NKT cell agonist with antigenic protein induce strong T cell-mediated antitumor immune responses. Oncoimmunology (2016) 5(1):e1068493. doi:10.1080/2162402X.2015.1068493
162. McKee SJ, Young VL, Clow F, Hayman CM, Baird MA, Hermans IF, et al. Virus-like particles and alpha-galactosylceramide form a self-adjuvanting composite particle that elicits anti-tumor responses. J Control Release (2012) 159(3):338–45. doi:10.1016/j.jconrel.2012.02.015
163. Nakamura T, Yamazaki D, Yamauchi J, Harashima H. The nanoparticulation by octaarginine-modified liposome improves alpha-galactosylceramide-mediated antitumor therapy via systemic administration. J Control Release (2013) 171(2):216–24. doi:10.1016/j.jconrel.2013.07.004
164. Gehrmann U, Hiltbrunner S, Georgoudaki AM, Karlsson MC, Naslund TI, Gabrielsson S. Synergistic induction of adaptive antitumor immunity by codelivery of antigen with alpha-galactosylceramide on exosomes. Cancer Res (2013) 73(13):3865–76. doi:10.1158/0008-5472.CAN-12-3918
165. Duramad O, Laysang A, Li J, Ishii Y, Namikawa R. Pharmacologic expansion of donor-derived, naturally occurring CD4(+)Foxp3(+) regulatory T cells reduces acute graft-versus-host disease lethality without abrogating the graft-versus-leukemia effect in murine models. Biol Blood Marrow Transplant (2011) 17(8):1154–68. doi:10.1016/j.bbmt.2010.11.022
Keywords: invariant natural killer T, natural killer T cells, blood cancer, immunosurveillance, immunotherapy, tumor immune evasion
Citation: Lam PY, Nissen MD and Mattarollo SR (2017) Invariant Natural Killer T Cells in Immune Regulation of Blood Cancers: Harnessing Their Potential in Immunotherapies. Front. Immunol. 8:1355. doi: 10.3389/fimmu.2017.01355
Received: 31 August 2017; Accepted: 03 October 2017;
Published: 23 October 2017
Edited by:
Paolo Dellabona, San Raffaele Hospital (IRCCS), ItalyReviewed by:
Maria Leite-de-Moraes, UMR8253 Institut Necker Enfants Malades Centre de médecine moléculaire (INEM), FranceKarl O. A. Yu, Rochester General Hospital, United States
Copyright: © 2017 Lam, Nissen and Mattarollo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stephen R. Mattarollo, s.mattarollo@uq.edu.au
 Pui Yeng Lam
Pui Yeng Lam Michael D. Nissen
Michael D. Nissen Stephen R. Mattarollo
Stephen R. Mattarollo