- 1Health Sciences Research Centre, Life Sciences Department, Whitelands College, University of Roehampton, London, United Kingdom
- 2Department of Food and Nutritional Sciences, University of Reading, Reading, United Kingdom
- 3Department of Veterinary Biosciences, University of Helsinki, Helsinki, Finland
- 4Immunobiology Research Program, Department of Bacteriology and Immunology, University of Helsinki, Helsinki, Finland
- 5Laboratory of Microbiology, Wageningen University, Wageningen, Netherlands
Background: The aging process leads to a potential decline in immune function and adversely affects the gut microbiota. To date, many in vitro and in vivo studies focused on the application of synbiotics (prebiotics combined with probiotics) as a promising dietary approach to affect gut microbiota composition and improved functioning of the immune system. However, studies using synbiotic preparations often have the limitation that it remains unclear whether any effect observed is a result of the prebiotic or probiotic or a synergistic effect of the combined supplement.
Objectives: We investigated the effects of a probiotic Lactobacillus rhamnosus GG and pilus-deficient L. rhamnosus GG-PB12 combined with Promitor™ Soluble Corn Fiber (SCF, a candidate prebiotic) on fecal microbiota, metabolism, immunity, and blood lipids in healthy elderly persons. A prospective, double-blind, placebo controlled, randomized, single-centered, crossover study in 40 healthy elderly subjects (aged 60–80 years) was carried out. Volunteers were randomized to consume either probiotic and prebiotic as synbiotic, prebiotic or placebo (maltodextrin) during 3 weeks. Three-week washout periods separated all the treatments. We assessed effects upon blood lipids, glucose, cytokines, natural killer (NK) cell activity, phenotype, and intestinal microbiota composition. SCF decreased IL-6, which was not observed with the synbiotics.
Results: Consumption of L. rhamnosus GG combined with SCF increased NK cell activity compared to baseline in females and the older group. In the fecal microbiota analyses, the strongest community shifts were due to L. rhamnosus GG combined with SCF and SCF treatments. L. rhamnosus GG combined with SCF and L. rhamnosus GG-PB12 combined with SCF significantly increased the genus Parabacteroides. L. rhamnosus GG combined with SCF and SCF increased concentrations of Ruminococcaceae Incertae Sedis. Oscillospira and Desulfovibrio slightly decreased in the L. rhamnosus GG combined with SCF group, whereas Desulfovibrio decreased also in the L. rhamnosus GG-PB12 combined with SCF group. L. rhamnosus GG combined with SCF reduced total cholesterol and LDL-cholesterol in volunteers with initially elevated concentrations. C-reactive protein significantly decreased during L. rhamnosus GG-PB12 combined with SCF intervention compared to baseline.
Conclusion: In conclusion, the synbiotic combination of L. rhamnosus GG with SCF showed a tendency to promote innate immunity by increasing NK cell activity in elderly women and in 70 to 80-year-old volunteers and decreased TC and LDL-c in hypercholesterolemic patients. In addition, L. rhamnosus GG-PB12 combined with SCF demonstrated an increase in NK cell activity compared to SCF alone in older volunteers. We also found significant positive effects on the immune response, evidenced by a decrease of the pro-inflammatory cytokine IL-6. Therefore, dietary intervention with L. rhamnosus GG combined with SCF could be of importance in elderly as an attractive option for enhancement of both the microbial and immune systems.
Introduction
The human gastrointestinal (GI) tract supports a rich and complex microbiota, whose composition and activities play important roles in nutrition, immunology, and certain disease processes. The composition of this microbial community is host specific, evolving throughout an individual’s lifetime and is susceptible to both exogenous and endogenous modifications. Recent interest in the function of this “organ” has illuminated a central position in health and disease. However, mechanisms by which the microbiota exerts its beneficial or detrimental influences remain largely undefined; thus, research in this area is key to future recommendations. A progressive increase in the proportion of elderly persons has led to heightened attention to their physiological needs. It is well known that immune function becomes compromised with age, an effect known as immune-senescence (1), which compromises the ability to respond to infections and develop increased protection after vaccination (2, 3). It may therefore contribute toward a higher mortality rate in older people and it has also been reported that in older people, the numbers and species diversity of putatively beneficial gut microbial groups, such as lactobacilli and bifidobacteria, as well as butyrate producers are reduced (4, 5). Appropriate dietary intervention is an attractive, safe, and non-invasive way to impact on gut bacteria and subsequent functioning of the immune system and has been proposed as an avenue to maintain microbiome homeostasis during aging (6). The use of probiotics, as live microbial food additions, is one such route (7). Experimental and clinical studies have suggested that probiotic supplementation may have beneficial effects on serum lipid profiles. Human studies have reported that certain probiotic strains boost natural killer (NK) cell (8) and phagocytic activities (8, 9) in healthy volunteers and may exert beneficial effects in inflammatory and atopic diseases, possibly by modulating pro- and anti-inflammatory cytokines (10). However, there are few studies examining the effect of probiotics on the immune function of elderly persons. Evidence exists on immune-stimulating effects of certain probiotics and the potential to use prebiotics to increase the levels of such bacteria, the latter being a selectively metabolized microbial substrate. Furthermore, by providing a probiotic at the same time as a prebiotic, conditions for survival of the live addition should be enhanced. Promitor™ Soluble Corn Fiber (SCF) is a well-known maize-derived source of dietary fiber with potential selective fermentation properties (11–14). Previous in vivo study has demonstrated the optimum prebiotic dose response of SCF (15). Previous in vitro work led to the selection of a probiotic bacterium, Lactobacillus rhamnosus GG, which combined with SCF, showed anti-pathogenic effects against E. coli and Campylobacter spp. (Costabile et al., unpublished data). Because of their strong mucus-binding properties, pili are thought to be major factors for binding and persistence of L. rhamnosus GG in the GI tract. Our purpose was to investigate the effects of L. rhamnosus GG combined with SCF (i.e., synbiotics) and SCF alone, on the fecal microbiota, metabolism, immunity, and blood lipids in healthy elderly subjects. Moreover, to address the essential role of the pili of L. rhamnosus GG, the pilus-less L. rhamnosus GG derivative LGG-PB12 was included into the study.
Materials and Methods
Intervention Study and Subjects
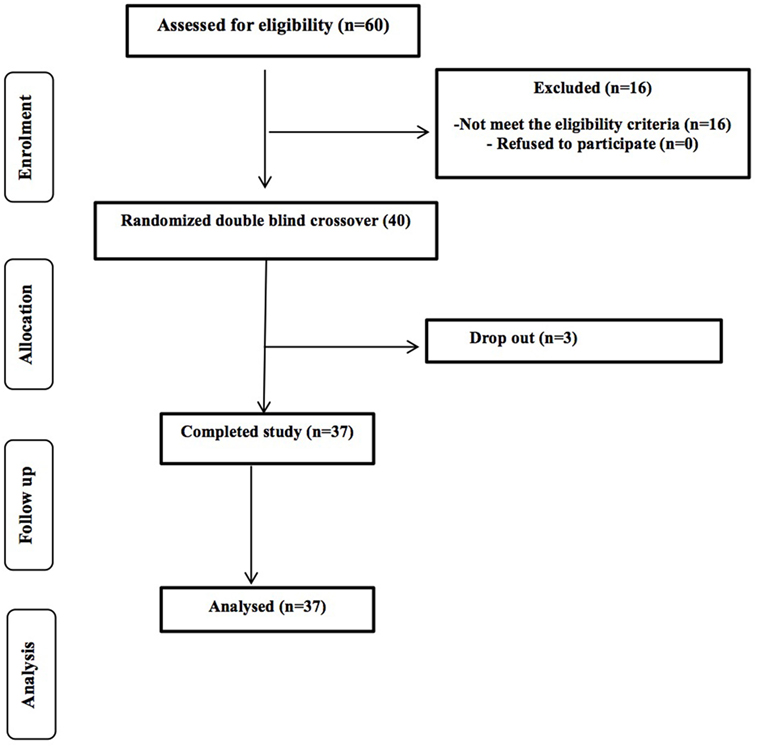
A total of 80 potential healthy volunteers (aged 60–80 years) were contacted from the University of Reading and surrounding area through the Hugh Sinclair Unit of Human Nutrition volunteer database, and through advertisements within the local community between September 2014 and January 2015. Of the first 75 responded, 60 were screened and we were recruited 40 volunteers; 37 participants completed the study with 3 drop-out due to personal circumstances (Figure 1). The study was powered to get 80% statistical power (Hedwig Harvard Software) on the basis of the data from previous intervention studies in human volunteers conducted on bacteriology. We obtained written consent from each person and selection took place following determinations of health status through a medical interview and adherence to the inclusion/exclusion criteria. Inclusion criteria were as follows: aged 60–80 years, good general health, and willing to participate in the entire study. We excluded volunteers with evidence of physical or mental disease, history of drug abuse, severe allergy, or a history of severe abnormal drug reaction and smokers. Intake of an experimental drug within 4 weeks before study, former participation in a prebiotic, probiotic, or laxative trial within 2 weeks, or use of antibiotics within 6 months before the study, chronic constipation, diarrhea or other chronic GI complaint (for example, irritable bowel syndrome), lactose intolerance, and gluten allergy were all exclusion criteria. Individuals with a clinically important renal, hepatic, endocrine (including diabetes mellitus), pulmonary, pancreatic, neurologic, dermatological, urogenital/rectal, dermatological, or lymphatic disorder and any major cardiovascular condition were also excluded, as were those with a recent history, or the presence, of cancer. Any intake of drugs active on GI motility, antibiotic treatment, or any class of laxative was not permitted. Volunteers recorded any medication taken throughout the duration of the study in diaries. We instructed volunteers not to alter their usual diet or fluid intake during the trial periods; however, they were asked to refrain from consuming prebiotics and probiotics, including daily fiber intake, live yogurts, and fermented milk drinks. All volunteers signed an informed consent form, and agreed to conform to the trial guidelines and provide notification of any non-compliance.
Study Design and Treatments
The study was registered as a clinical trial (http://clinicaltrials.gov ID: NCT03168503) and was conducted according to the Declaration of Helsinki following Good Clinical Practice (GCP). The study was approved by the University of Reading Research Ethics Committee (Ethics reference number UREC14/06). The study was designed as a prospective, double-blind, randomized, placebo-controlled, single-centered crossover study with a 2-week run-in period prior to the beginning of the study, when volunteers were required to refrain from any probiotic or prebiotic containing food or drinks and record a 4-day food diary, including one weekend day, to assess habitual diet (Figure 2). An Excel-based covariate adaptive randomization program (16) to enter four intervention arms randomized the volunteers: L. rhamnosus GG-PB12 combined with SCF, L. rhamnosus GG combined with SCF, SCF alone, or placebo (maltodextrin) stratified by age, gender, and BMI. The SCF treatment was designed and confirmed by previous study to provide a total of 6 g from SCF (Promitor™ SCF) (equivalent to 8 g/fiber delivered) (15) and was provided by Tate & Lyle, France, as dry powders and were consumed as 250 mL beverages once daily. The probiotics strains were obtained from Valio Ltd and at the dose of 1 × 1010 CF g. The subjects consumed one sachet (12 g) every day during 3 weeks for each treatment, with 3-week washout periods between treatments. We collected overnight 10 h-fasting venous blood and one fecal sample in the morning at baseline and at the end of each intervention period for analyses. Fecal samples were collected weekly in fecal collection kit (FC 2040, Laboratories Ltd., UK) and volunteers were asked to keep them at −80°C until the visit day when they were transferred to the laboratory for all the analysis. Throughout the pre-treatment and treatment periods, volunteers recorded details of bowel habits including stool frequency and consistency (Bristol stool scale), stomach or intestinal bloating, abdominal pain, and incidence and frequency of flatulence and stomach noises. They also completed four-day diet records in the week before baseline and each end-of-treatment clinic visit. These were analyzed using the Food Processor SQL Nutrition Analysis and Fitness 8.6.0 software (ESHA Research, Salem, OR, USA). They completed a GI symptom survey at baseline and at the end of each treatment period.
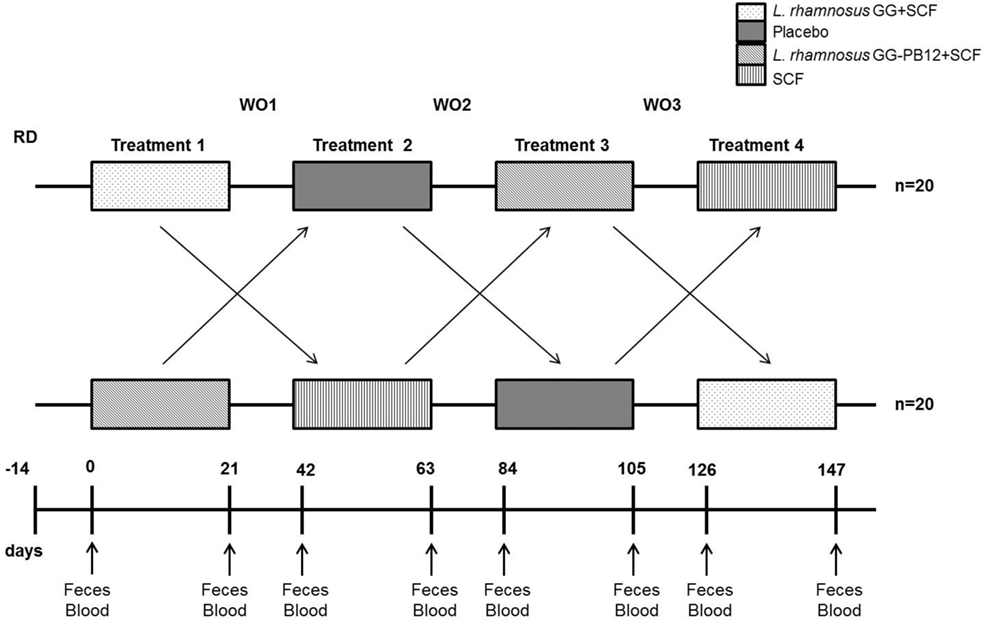
Figure 2. Saimes study design. The study was designed as a prospective, double-blind, randomized, placebo-controlled, single-centered crossover study with a 14 days run-in period prior to the beginning of the study, when volunteers were required to refrain from any probiotic or prebiotic containing food or drinks and control the fiber levels. The microbiota was analyzed from fecal samples taken prior to the intervention (time point 0) and at the end of the 3-week interventions (time points 21, 63, 105, and 147).
Preparation of Peripheral Blood Mononuclear Cells (PBMC)
Fasted blood samples (10 h-fasting) were taken from volunteers in sodium heparin vacutainer tubes (Greiner Bio-One Ltd., Gloucestershire, UK). Blood was layered over an equal volume of lympholyte (Cedarlane Laboratories Limited, Tyne & Wear, UK) and centrifuged at 930 × g for 15 min at room temperature. Plasma was removed into a sterile tube and stored at −80°C until further use. Cells were harvested from the interface, washed once, resuspended in RPMI 1640 medium (Autogen Bioclear, Wiltshire, UK) containing 0.75 mM glutamine (Autogen Bioclear, Wiltshire, UK), and the above steps were then repeated to achieve a lower degree of erythrocyte contamination. The pellet was finally resuspended in the same medium.
Preparation of Other Samples
Blood was collected by serum separator vacutainer tube (BD Biosciences, Oxford, UK) and centrifuged at 2,095 × g for 10 min. Aliquots of serum were stored at −20°C for later analysis of total cholesterol, HDL-cholesterol, triacylglycerol, non-esterified fatty acids (NEFA), glucose, and C-reactive protein (CRP).
Blood Lipids, Glucose, and Immune/Inflammatory Markers
Concentrations of serum total cholesterol, HDL-cholesterol, triacylglycerol, NEFA, glucose, and CRP were evaluated by iLab 600 (all kits and equipment from Instrumentation Laboratory, Warrington, UK).
Cytokine Analysis
We measured the production of IL-1β, IL-6, IL-8, IL-10, IL-12p70, and TNF using Cytometric Bead Array (CBA) multiplex kits (BD Biosciences, Oxford, UK) by flow cytometry according to the manufacturer’s instructions. BD™ CBA analysis software (BD Biosciences, Oxford, UK) was used to perform data analysis.
NK Cell Activity
Freshly prepared PBMC were adjusted to a concentration of 5 × 106 cells/mL. Viable target cells (K562 cell line) were enumerated by microscopy of trypan blue-stained cell preparations, and 5 × 106 cells collected and washed twice with PBS before incubation with carboxyfluorescein diacetate succinimidyl ester (CFDA-SE) (1 µg/mL) (Sigma, Dorset, UK) for 45 min at 37°C in an air/CO2 (19:1) atmosphere. Following incubation, target cells were washed twice and resuspended in 1 mL of complete medium composed of RPMI 1640 medium, 0.75 mM glutamine, and 10% (v/v) newborn calf serum (Sigma, Dorset, UK). PBMC were incubated with CFDA-SE-labeled target cells for 2 h at 37°C in an air/CO2 (19:1) atmosphere, at effector to target cell ratios of 100:1, 50:1, 25:1, and 12.5:1. Twenty microliters of PI at 1 mg/mL were added to the samples prior to analysis by flow cytometry. Samples were acquired using a FACSCanto II flow cytometer (BD Biosciences, San Jose, CA, USA), and data analyzed using FACSDiva v6 software (BD Biosciences, San Jose, CA, USA). The results were expressed as percent lysis of the target cells.
Lymphocyte/NK Cells Phenotype Analysis
Peripheral blood mononuclear cells were stained with appropriate combinations of fluorescently labeled mouse anti-human monoclonal antibodies for discrimination between different lymphocyte subsets. Monoclonal antibodies were conjugated to fluorescein isothiocyanate, phycoerythrin (PE), or (Cy-7 PE). PBMC were incubated with fluorescently labeled monoclonal antibodies (all from BD Biosciences, Oxford, UK) for 20 min at room temperature in the dark and washed by FACS Wash solution [1% (w/v) BSA, 0.1% (w/v) sodium azide in PBS] twice before fixing with FACS Fix solution [2% (w/v) paraformaldehyde, pH 7.4]. The fixed cells were analyzed by flow cytometry within 24 h. At least 50,000 lymphocytes were acquired using a FACSCanto II flow cytometer (BD Biosciences, San Jose, CA, USA), and data were analyzed using FlowJo V 10.0.8 software (BD Biosciences, San Jose, CA, USA). Isototypic controls were used to minimize electronic noise. Using side scatter and forward scatter, a gate was drawn in lymphocyte populations. Anti-CD3 and anti-CD56 mAb (present in all tubes) were used to define three cells populations: conventional T cells (CD3+CD56−), conventional NK cells (CD3−CD56+), and NKT cells (CD3+CD56+). Anti-CD56 and anti-CD16 mAb (present in all tubes) were used to further divide NK cells based in CD56 expression into two subsets: CD56dim and CD56bright. Gates strategies were designed based on Almeida-Oliveira et al. (17). Results of phenotypes were expressed as percentage of the subsets or within the cell group specified.
DNA Extraction and Quantitative PCR Analysis
DNA extraction from feces was carried out using the RBB method as described by Salonen et al. (18). Following cell lysis and precipitation of nucleic acids, QIAamp DNA Minikit was used for further purification of the DNA. Afterward, compliance of the study subjects to the trial was verified using quantitative PCR assays. The assays were designed to target the position of one of the mutations, 443187, in srtC1 gene in L. rhamnosus GG-PB12 genome (19) (Figure S1 in Supplementary Material). Primers for both L. rhamnosus GG and GG-PB12 assays were the same (for 5′-AGTGCGACTATTAGCTTTA-3′ and Rev 5′-GGATCTTGTGACCTTAATG-3′), while the probes contained one nucleotide difference (L. rhamnosus GG: 5′-(FAM) TTGTTCCACCAAACC (MGB-NFQ)-3′; L. rhamnosus GG-PB12: 5′-(FAM) TTGTTTCACCAAACC (MGB-NFQ)-3′, nucleotide difference marked in bold). Probes were non-fluorescent quenched minor groove binder (NFQ-MGB) hydrolysis probes with 6-fluorescein amidite (6-FAM) as the fluorophore. Nucleotide oligo (5′-CGACTATTAGCTTTAATTTGTTC-3, Tm 48°C) was used in the L. rhamnosus GG-PB12 assay to reduce potential non-specific signal from strain GG. The oligo was designed so that it anneals to the binding site of the L. rhamnosus GG-specific probe before the temperature reaches the probe annealing temperature and blocks the binding site of the probe. HOT FIREPol® Probe qPCR Mix Plus with ROX (Solis BioDyne Ltd.) was used in the qPCRs with program 15 min at 95°C, 20 s at 95°C, and 1 min at 60°C, for 35 cycles. 25 ng of DNA was used per reaction in a total volume of 20 µL. In addition, total bacteria were quantitated using an qPCR assay described by Nadkarni et al. (20), with total DNA quantity of 0.5 ng per reaction and using master mix HOT FIREPol® EvaGreen® qPCR Mix Plus (Solis BioDyne Ltd.). Measurements were performed with Stratagene MX3005P real-time PCR equipment and analysis of the data was performed using MxPro qPCR Software version 4.1 (Stratagene).
Intestinal Microbiota Profiling Analyses
Comprehensive intestinal microbiota profiling and phylogenetic analysis was carried out with 16S rRNA amplicon sequencing by Illumina Miseq. In the paired-end mode, the variable region V3–V4 was sequenced, using primers (CTACGGGNGGCWGCAG and GACTACHVGGGTATCTAATCC). However, we only kept the forward reads, truncated to 150 bases, as we have observed, using artificial microbial communities, that longer reads result in distorted microbiota compositions. Pre-processing and statistical analyses of the reads were done using the R package mare (Korpela 2016 mare: Microbiota Analysis in R Easily. R package version 1.0). To eliminate sequencing errors, we discarded all unique reads occurring <100 times in the total dataset. Taxonomic annotation of the reads was conducted using USEARCH (Edgar 2010 Bioinformatics).
Statistics
Data on immunology and metabolism were analyzed using SPSS version 21.0. Significant differences between baseline and end of intervention were evaluated by paired t-test, while significant differences between treatments were evaluated by two-way analysis of variance (ANOVA) using the general linear model with Bonferroni correction. All data are shown as mean ± SE. The statistical significance level was defined as p < 0.05 with p values represented in the figures as ***p < 0.001, **p < 0.01, and *p < 0.05. Effect of the different treatments on genus-level microbiota composition was assessed using ANOVA. The change in the relative abundance of each genus during each of the synbiotic and SCF treatments was compared to changes during the placebo treatment, keeping baseline abundance of the focal genus as a covariate in the model. p values were corrected for multiple testing using the false discovery rate method (21). The effect of treatments on overall microbiota was assessed using permutational multivariate ANOVA (function adonis in R-package vegan) and principal coordinates analysis, using the Bray–Curtis dissimilarities (function capscale in R-package vegan).
Results
Blood Lipids, Glucose, Cytokines, NK Cell Activity, Phenotype, and Intestinal Microbiota Composition
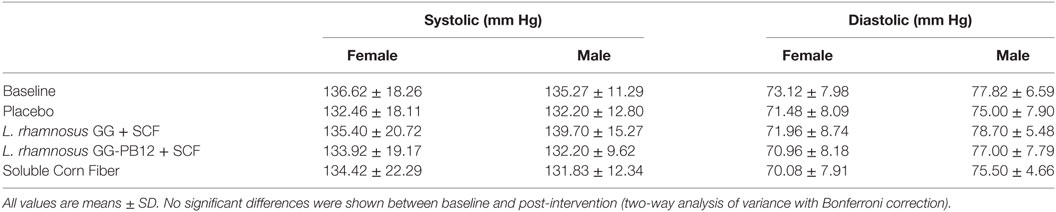
A total of 37 volunteers completed the study. Intestinal symptoms, anthropometric values and blood pressure of the volunteers are shown in Tables 1–3, respectively. Overall, no statistically significant differences were found. There were no significant differences between treatments for serum TC, HDL-cholesterol, LDL-cholesterol, triacylglycerol, NEFA, or glucose (Table 4). However, after analyzing blood lipid results from volunteers with raised cholesterol (TC > 5 mmol/L) at baseline (n = 26), a statistically significant decrease in TC and LDL-c was observed after synbiotic intervention with L. rhamnosus GG combined with SCF, but not with the other treatments (Figure 3). Concentrations of IL-1β, IL-6, IL-8, IL-10, IL-12p70, TNF, and CRP were measured in serum. The cytokines IL-1β, IL-10, IL-12p70, and TNF were undetectable or only very low levels were found in the samples from elderly people (data not shown). Therefore, only IL-6, IL-8, and CRP results were analyzed (Table 5). Even though CRP serum concentrations of the subjects treated with L. rhamnosus GG-PB12 combined with SCF and SCF alone were higher than that with L. rhamnosus GG combined with SCF and placebo treatments, there were no significant differences between these. The use of SCF alone led to a decrease in the pro-inflammatory cytokine IL-6, which was not observed with the synbiotics. Although highest numbers for NK cell activity were obtained after synbiotic treatments for all ratios, no significant differences were found (Figure 4A). Therefore, there was no effect of synbiotic on NK cell activities compared to other treatments. When NK cell activity was expressed on a per cell basis (divided by NK cell percentage in lymphocytes), similar results were found (data not shown). When gender was considered, NK cell activity after L. rhamnosus GG combined with SCF intervention almost reached significance for the E/T of 100 (p = 0.064) compared to baseline in females but not in males (Figure 4B). Furthermore, NK cell activity at the beginning of the study was significantly higher in males than in females (Figure 4C). Interestingly, when dividing volunteers by age, i.e., 60–69 and 70–80 years old, NK cell activity after L. rhamnosus GG combined with SCF intervention almost reached significance in the latter group (Figure 4D). Finally, L. rhamnosus GG-PB12 combined with SCF increased NK cell activity compared to SCF alone in older volunteers, showing tendency in E/T = 100 (p = 0.079) and significance when expressed as log E/T = 100 (p = 0.042). There were no significant effects of synbiotics on lymphocyte and NK cell phenotypes (data not shown). Quantities of the L. rhamnosus GG strains and total bacteria, in feces, were measured using quantitative PCR (Table 6). Not surprisingly, increased numbers of L. rhamnosus GG or L. rhamnosus GG-PB12 were detected during intervention with the synbiotic in question, but not during placebo or SCF intervention. Impact of the interventions on total microbial communities was addressed by phylogenetic analysis of the fecal microbiota (Figure 5). L. rhamnosus GG combined with SCF, L. rhamnosus GG-PB12 combined with SCF, and SCF treatments had significant effects on overall microbiota composition when compared to baseline, each treatment explaining 2% of the variation in microbiota compositions (p = 0.001). All active treatments caused a similar microbiota shift along principal components 4 and 5, toward higher Parabacteroides and Ruminococcaceae abundance; the strongest shift was seen in L. rhamnosus GG combined with SCF and L. rhamnosus GG-PB12 combined with SCF treatments. The placebo treatment did not have a significant impact compared to baseline levels on overall microbiota composition (R2 = 0.03, p = 0.95) compared to the baseline. Significant changes were detected in four bacterial genera in the different treatments. Parabacteroides was significantly increased during both synbiotic treatments (L. rhamnosus GG: 4.3% increase compared to placebo, p = 0.0004; L. rhamnosus GG-PB12: 3.4% increase, p = 0.0075). Ruminococcaceae Incertae sedis was increased significantly L. rhamnosus GG synbiotic treatment and treatment with SCF only (L. rhamnosus GG: 2.4% increase, p = 0.014; SCF: 2.4% increase, p = 0.015). A subtle, but significant, decrease in Oscillospira was detected following L. rhamnosus GG combined with SCF treatment (0.04%, p = 0.012), while a significant decrease in Desulfovibrio was detected during both synbiotic treatments (L. rhamnosus GG: 0.09% p = 0.011; L. rhamnosus GG-PB12: 0.1%, p = 0.0075).
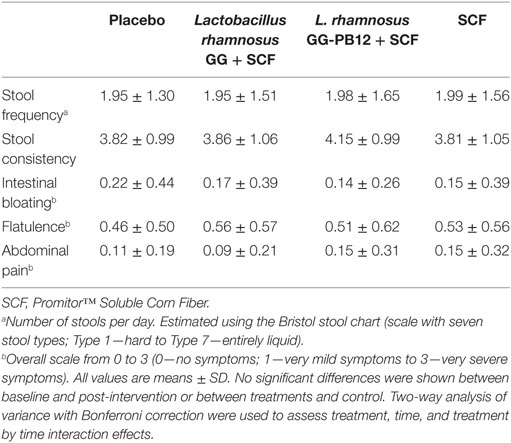
Table 1. Average, self-reported, gastrointestinal symptom scores for stool frequency, consistency, bloating, flatulence, and abdominal pain, over the 3-week intervention period for the placebo, synbiotics, and SCF for each treatment group (n = 37).
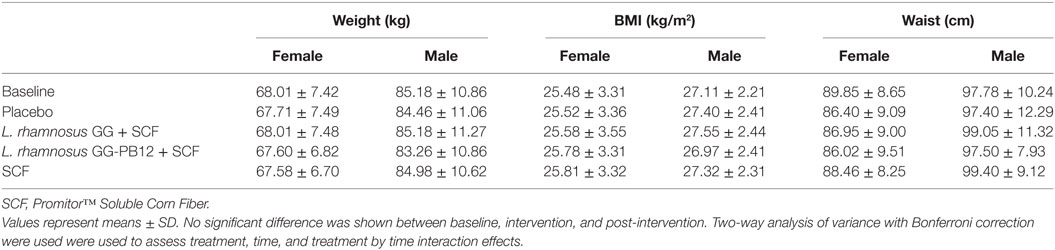
Table 2. Change in anthropometric measurements in all study participants (n = 37) between baseline and 3 weeks intervention period with placebo (maltodextrin), Lactobacillus rhamnosus GG + SCF, L. rhamnosus GG-PB12 + SCF, and SCF only.
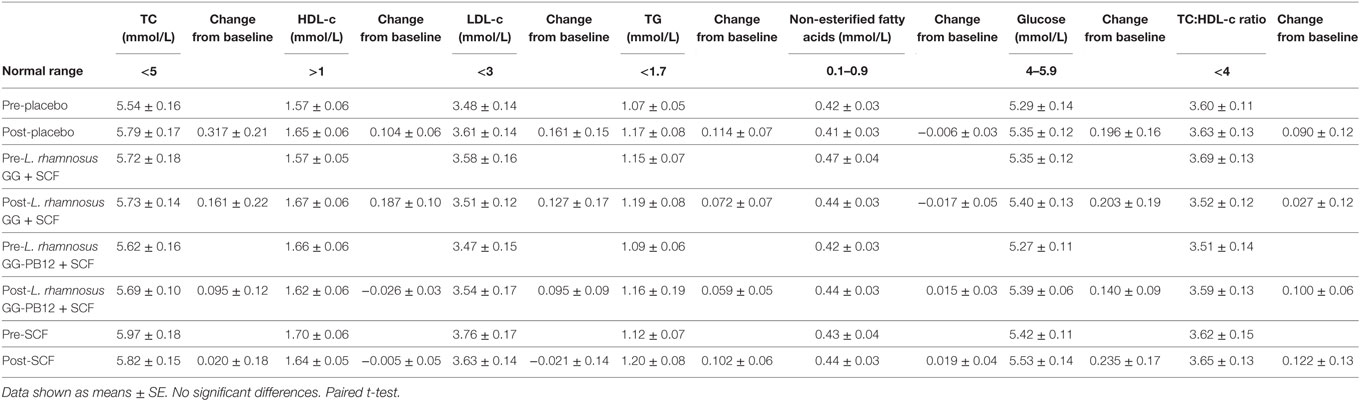
Table 4. Lipid parameters baseline and 3 weeks intervention study in the active [Lactobacillus rhamnosus GG + Soluble Corn Fiber (SCF), L. rhamnosus GG-PB12 + SCF, and only SCF] and placebo treatment groups (n = 37).
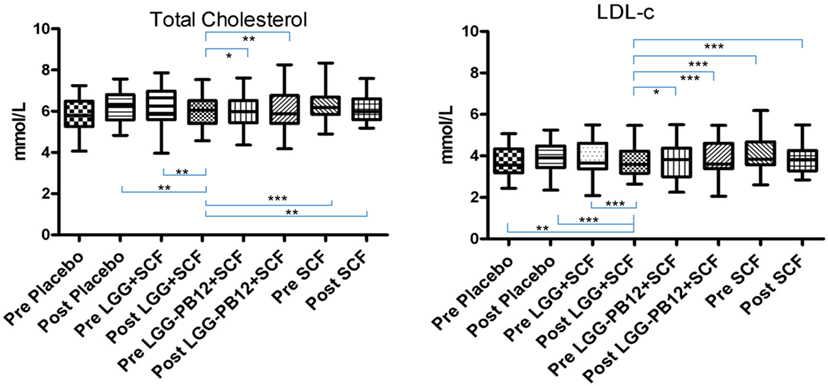
Figure 3. Total cholesterol (A) and LDL-cholesterol concentrations (B) in each group after the treatment, in volunteers with raised cholesterol (total cholesterol >5 mmol/L) at baseline (n = 26) (*p < 0.05, **p < 0.01, and ***p < 0.001).
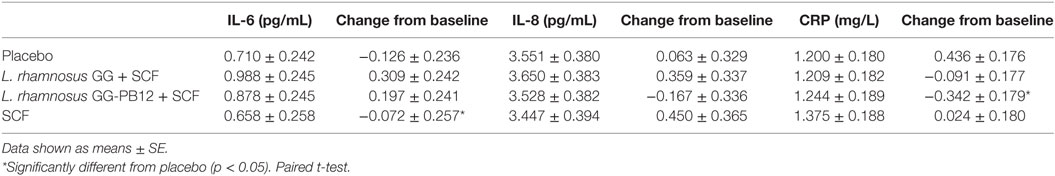
Table 5. Cytokine production and C-reactive protein (CRP) concentrations expressed in millimolar as change from baseline and 3 weeks intervention study in the active [Lactobacillus rhamnosus GG + SCF, L. rhamnosus GG-PB12 + Soluble Corn Fiber (SCF), and only SCF] and placebo treatment groups (n = 37).
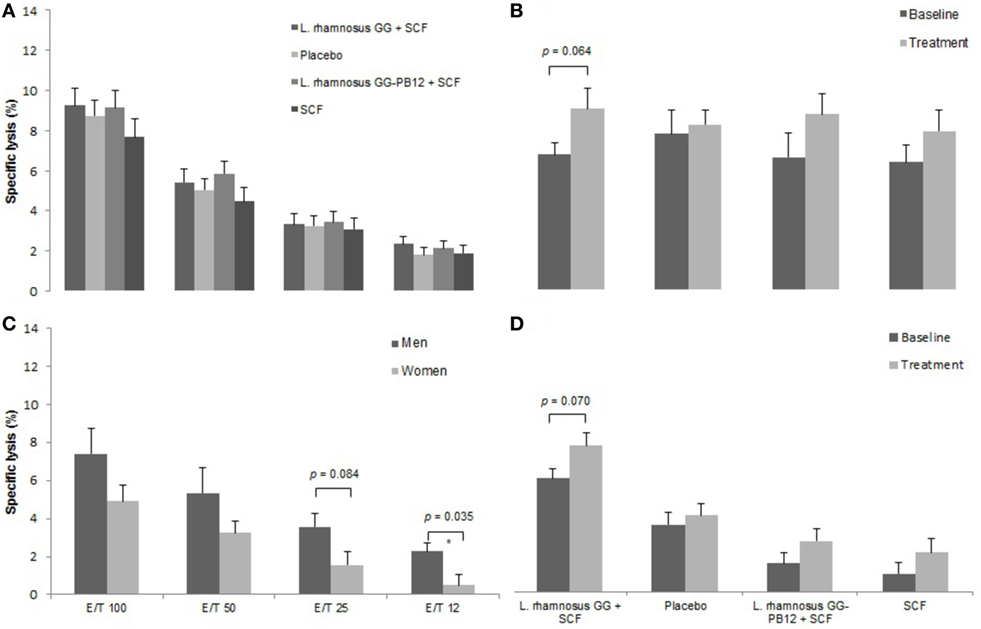
Figure 4. Natural killer cell activity in each group after the treatment. Data are mean ± SE. (A) Specific lysis after all treatments. (B) Specific lysis after LGG + Soluble Corn Fiber (SCF) intervention in females. E/T = 100/1. (C) Specific lysis at the beginning of the study in females and males. (D) Specific lysis after all treatments in the older group (70–80 years old) (n = 37).
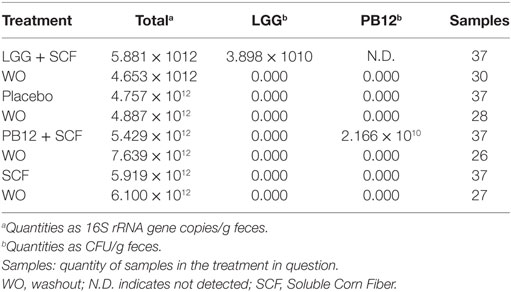
Table 6. Median quantities of total bacteria, Lactobacillus rhamnosus GG, and L. rhamnosus GG-PB12 in 23 fecal samples.
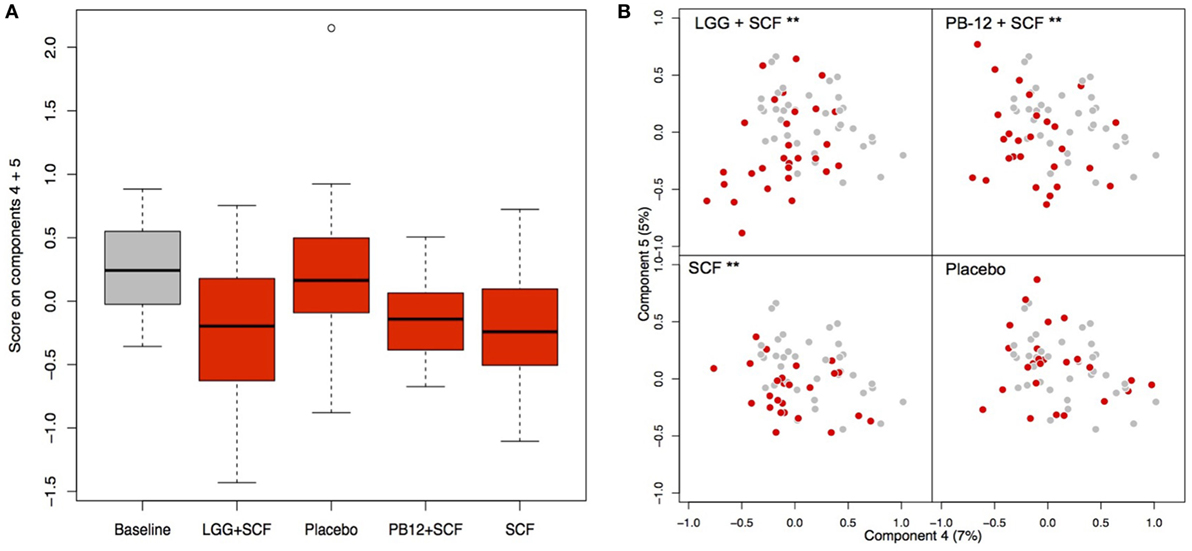
Figure 5. Principal coordinate analysis (PCoA) of the fecal microbiota composition in each group after the treatments. (A) Score on components 4 and 5 in the PCoA plots shown as a box plot; (B) PCoA plots indicating the shift between baseline samples (gray) and treatment samples (red) of each participant arm: LGG + Soluble Corn Fiber (SCF)**, PB12 + SCF**, SCF** (**p = 0.001), and placebo (p = 0.95).
Discussion
The health-promoting properties of probiotics are suggested to be strain dependent; therefore, strain identification and characterization are essential (22). Probiotics elicit their functions in different ways, among which immunomodulation is suggested to be one mechanism (23). Current US national guidelines for CVD risk reduction are primarily focused on strategies to reduce concentrations of LDL-c, with the most recent focus being “lower is better” (23). Some components of the immune response, including phagocytosis, NK cell activity, and mucosal immunoglobulin A production, have been shown to be improved by certain probiotic bacterial strains, while other components, including lymphocyte proliferation, production of cytokines, and elevation of antibodies other than immunoglobulin A, appear to be less sensitive to probiotic modulation (24). NK cells are critical for the removal of intracellular pathogens and also possess vital tumoricidal activities, but their activity declines with aging (25), even though numbers of NK cells in the circulation increase with age (26). NK cell activity has been demonstrated to be enhanced by different probiotic strains studied in healthy adults (8, 9, 27) and in healthy older subjects (28, 29), Dong et al. (30) showed that six different probiotic strains enhanced activation of all NK cell subsets and elicited similar capacity to stimulate NK cell activities, without showing any strain-specific properties. This property of probiotic strains has been confirmed by human studies, in which oral consumption of Lactobacillus casei Shirota (LcS) (31), Lactobacillus fermentum CECT5716 (32), Lactobacillus paracasei NCC 2461 (33), Bifidobacterium lactis HN019 (34, 35), or L. rhamnosus HN001 (36, 37), enhanced NK cell activity in healthy adult or elderly populations. However, some human studies did not show any effect of probiotic strains (L. rhamnosus, B. lactis, LcS, Lactobacillus gasseri PA 16/8, Bifidobacterium longum SP 07/9, Bifidobacterium bifidum MF 20/5) on NK cell activity (38, 39), perhaps due to differences in sample size, probiotic dose, consumption period, study design, and volunteers. In our study, the L. rhamnosus GG combined with SCF intervention showed a tendency to increase NK cell activity compared to baseline in women. We found higher baseline NK activity values in males compared to females, which has been reported by other authors (7, 40), who also demonstrated that LcS consumption had a greater effect on female compared to male volunteers, and this effect might relate to an initially lower NK cell activity in females. Nagao et al. (41) have shown that daily intake of fermented milk containing LcS-enhanced NK cell activity in healthy young subjects with relatively lower NK cell activity. In the current study, after randomizing the volunteers by age, the L. rhamnosus GG combined with SCF intervention also showed a tendency to increase NK cell activity compared to baseline in elderly whose average age was 70–80 years. A possible lower baseline NK cell activity in this population could be related to the increase. It may then be suggested that the synbiotic does not necessarily enhance immune function above “normal” levels in healthy individuals, but rather modulates it back to normal levels in situations where immunity is impaired and thus plays a role in homeostasis, as previously reported for LcS (7). Flow cytometry analysis showed no difference in the relative proportion of NK cells and subsets in groups consuming the different treatments, suggesting that these synbiotics demonstrated a tendency to enhance NK cell activity but did not induce the expansion of NK cells. Overall, we have shown that synbiotic supplementation could slow down the reduction of NK cell activity without changing the relative proportion of NK cells, as previously found by Morimoto et al. (42) for LcS. In fact, these authors reported that NK cells increasing after supplementation of probiotics may be dependent on the species of lactic acid bacteria and/or the condition of the host (such as healthy subjects versus either cancer patients, or younger versus aged subjects, etc.). NK cell activity is important for immune surveillance against cancer and pathogenic infections (43, 44). On the other hand, environmental and psychological condition and NK cell activity are closely related (42). The reported effects of probiotics on cytokine production are complex and suggest strain-specific differences (31, 33). A range of cytokines was examined in the current study, but there were no significant changes in cytokine production by the synbiotics. Other human studies with probiotic interventions showed no changes in stimulated cytokine production (7, 45). In the present study, a significant decrease of pro-inflammatory IL-6 was only observed during the intervention with SCF and not in synbiotic combination. It is well known that appropriate dietary intervention is an important way to impact on gut bacteria and subsequent functioning of the immune system (6), and prebiotics have demonstrable benefits. Shukla et al. (46) reported that prebiotic supplementation modulated gut morphology and improved immune status in infected mice, while Marciano et al. (47) found that oligofructose decreased the concentration of IL-6 in rats. In the same way, Aliasgharzadeh et al. (48) observed that patients supplemented with resistant dextrin exhibited a significant decrease in IL-6 concentrations than those supplemented with maltodextrin. Childs et al. (49) demonstrated that XOS induced bifidogenesis, improved aspects of the plasma lipid profile, and modulated markers of immune function in healthy adults. A possible limitation associated with the study was that we did not specifically select subjects who were immunocompromised. The hypothesis of this study was that synbiotic intervention would at least partially reverse some of the decline in immune parameters caused by aging, although it cannot be ascertained whether the subjects in the current study were immunocompromised. Immune senescence is observed in healthy aging, and the purpose of this study was to investigate an older group, which was expected to have some degree of immune-senescence. It could be argued that the results from this study could also be applied to younger subjects and to those who are not necessarily immunocompromised. Finally, a limitation of all studies employing immune biomarkers in the absence of clinical outcomes is that it is difficult to interpret the biological significance of minor immunomodulatory effects. It is of specific interest to note that the reduction of these risk factors was only obtained with the synbiotic intervention L. rhamnosus GG combined with SCF but not the isogenic L. rhamnosus GG-PB12 that only differ in the production of mucus-binding pili (19). Furthermore, in regard to the fecal microbiota changes, an increased amount in the quantities of L. rhamnosus GG strains and L. rhamnosus GG-PB12 were detected during the intervention with the synbiotic in question, but not during placebo or SCF intervention. There are indications that L. rhamnosus GG persists longer in the intestinal tract of healthy adult volunteers than L. rhamnosus GG-PB12 (in preparation, Rasinkangas et al.). We could not address this in the present study as assessment of persistence requires several samplings during the washout period. The 2-week washout was sufficient to eliminate effectively either of the L. rhamnosus strains. Both isogenic strains showed differential effects on the intestinal microbiota analysis and significant changes were detected in four bacterial genera. Parabacteroides was significantly increased during both synbiotic treatments, whereas a significant decrease in Oscillospira was observed. We also observed a significant decrease in numbers of Desulfovibrio spp. for both symbiotic treatments. This group of sulfate-reducing bacteria has been implied in as potential players in the etiology of various intestinal disorders, including inflammatory bowel disease and colon cancer (50). Hence, their reduction by the synbiotic treatment with either of the L. rhamnosus GG strains may be a relevant example of their probiotic activity. As reported by Nakamura et al. (51), the acetogens, methanogenic archaea, and sulfate-reducing bacteria, which dispose the colonic hydrogen gas generated during fermentation, are low in abundance, but critical for functioning of the gut ecosystem. It is very likely that the organisms we found may be indicator species, particularly sensitive to the environment and therefore informative of important structural or functional differences between ecosystems, which lead to the differential responses. Not surprisingly, Ruminococcaceae Incertae sedis was increased significantly during L. rhamnosus GG combined with SCF treatment and SCF treatment alone, but the increase in SCF was more pronounced, indicating a possible role for this group as active plant degraders. The Ruminococcaceae are one of the most abundant families from the order Clostridiales found in the mammalian gut environment, and have been associated with maintenance of gut health. Abundance estimates, based on 16S rRNA surveys, suggest that Ruminococcaceae are the most abundant Firmicute families in gut environments, accounting for roughly 50 and 30% of phylotypes, respectively (52, 53). One key point is that in gut environments the ability to degrade cellulose and hemicellulose components of plant material enables members of the Ruminococcaceae to decompose substrates that are indigestible by the host. Declines in Ruminococcaceae and Parabacteroides have been identified as main microbial shifts associated with aging in mice (54, 55). The fact that the L. rhamnosus GG combined with SCF treatment caused an increase specifically in these groups suggests that the treatment may have positive effects on the microbiota of elderly people. Elevated serum TC, LDL-c, and TAG concentrations and low HDL-c concentrations are well-established risk factors for CVD (56–58). Current US national guidelines for CVD risk reduction are primarily focused on strategies to reduce concentrations of LDL-c, with the most recent focus being “lower is better” (57). Based on meta-regression analysis of randomized trials of statins, Delahoy et al. (59) concluded that there was a significant positive relationship between reductions in LDL-c and reductions in the risk for major cardiovascular events. To date, experimental and clinical studies have suggested that probiotic supplementation may have beneficial effects on serum lipid profiles. However, there are conflicting results on the efficacy of probiotic preparations in reducing serum cholesterol. Recently, Shimizu et al. (60) evaluated the effects of probiotics on human serum lipid concentrations in a meta-analysis of interventional studies. Sadrzadeh-Yeganeh et al. (61) have reported that probiotic interventions (including fermented milk products and probiotics) produced changes in TC and LDL-c, while HDL-c and TAG concentrations did not differ significantly between probiotic and control groups. Moreover, they concluded that long-term (>4-week) probiotic intervention was statistically more effective in decreasing TC and LDL-c than short-term (<4-week) intervention. Interestingly, Shimizu et al. (60) found that decreases in TC and LDL-c concentrations during probiotic intervention were greater in mildly hypercholesterolemic than in normocholesterolemic individuals. These results have been confirmed in our findings, since L. rhamnosus GG combined with SCF decreased TC and LDL-c compared to the other treatments in volunteers with raised cholesterol at baseline. Therefore, synbiotic intervention will have more benefit in patients with hypercholesterolemia than in individuals with normal lipid concentrations. In addition, reductions in TC and LDL-c in the elderly have been reported to be greater than those in younger individuals. This could be considered to be due to differences in baseline values, as in the elderly they were higher (2,010). Delahoy et al. (62) suggested that a reduction of 1 mmol/L in LDL-c was associated with a 21% proportional reduction in the incidence of vascular events. Therefore, based on our present results, it is expected that synbiotic intervention in hypercholesterolemic patients would lead to an approximately 18% reduction in major cardiovascular events if a linear relationship between reduced LDL-c concentrations and reduced incidence of vascular events is assumed.
Conclusion
We could conclude that L. rhamnosus GG combined with SCF intervention could be useful in the primary intervention of hypercholesterolemia and may lead to reductions in risk factors for CVD. The observed reduction of TC and LDL-c by L. rhamnosus GG combined with SCF and not L. rhamnosus GG-PB12 is of interest and may have various mechanistic explanations. First, L. rhamnosus GG showed a much higher mucus-binding capacity than L. rhamnosus GG-PB12 (19). Hence, L. rhamnosus GG is expected to reside in close proximity to intestinal cells, allowing for increased interactions. Second, the increased persistence of L. rhamnosus GG may also impact the extent of host interactions. Both of these explanations imply that L. rhamnosus GG has beneficial host signaling capacity that affects TC and LDL-c concentrations. Third, L. rhamnosus GG has a differential effect on the total intestinal community and hence it cannot be excluded that the affected microbial community is involved in reduction of hypercholesteremia. Whatever the exact mechanism would be, the observation that TC and LDL-c concentrations were only reduced by an intervention with L. rhamnosus GG and not L. rhamnosus GG-PB12, provided evidence that the mucus-binding pili may confer a health influence. In conclusion, the synbiotic containing L. rhamnosus GG combined with SCF showed a tendency to promote innate immunity by increasing NK cell activity in elderly women and in 70- to 80-year-old volunteers and decreased TC and LDL-c in hypercholesterolemic patients. In addition, L. rhamnosus LGG-PB12 combined with SCF demonstrated an increase in NK cell activity compared to SCF alone in older volunteers. We also found significant positive effects on the immune response, evidenced by a decrease of the pro-inflammatory cytokine IL-6. Therefore, dietary intervention with L. rhamnosus GG combined with SCF could be of importance in elderly as an attractive option for enhancement of both the microbial and immune systems.
Ethics Statement
The study was registered as a clinical trial (http://clinicaltrials.gov ID: NCT03168503) and was conducted according to the Declaration of Helsinki following GCP. The study was approved by the University of Reading Research Ethics Committee (Ethics reference number UREC14/06).
Author Contributions
AC was the principal investigator of the study; AC and GG designed research; TB-M, AC, PR, KK, and WV conducted research, analyzed data, and performed statistical analysis; TB-M, AC, and GG wrote the paper; AC had primary responsibility for the final content. All authors read and approved the final manuscript.
Conflict of Interest Statement
The funders of this study had no input on the design, implementation, analysis, or interpretation of the data. The authors have no other conflicts of interest to declare.
Acknowledgments
Special acknowledgment goes to Mr. Alex Zheng and Mr. Ivan Buttarazzi for their technical support during the intervention study.
Funding
The study was supported by Tate & Lyle Ingredients Americas LLC, which claims proprietary ownership of data it provides for such study, if any, and “exclusive right of reference for any data generated from the study to possibly be used for health claim petitions” in accordance with Regulation (European Community) no. 1924/2006 of the European Parliament and of the Council on Nutrition and Health Claims Made on Foods. The “exclusive right of reference” does not prohibit the referencing or quotation of the present article or study, provided any such reference or quotation does not undermine Tate & Lyle ownership of data provided for the study, if any.
Supplementary Material
The Supplementary Material for this article can be found online at http://www.frontiersin.org/article/10.3389/fimmu.2017.01443/full#supplementary-material.
References
1. Weiskopf D, Weinberger B, Grubeck-Loebenstein B. The aging of the immune system. Transpl Int (2009) 22(11):1041–50. doi:10.1111/j.1432-2277.2009.00927.x
2. Nichol KL, Nordin JD, Nelson DB, Mullooly JP, Hak E. Effectiveness of influenza vaccine in the community-dwelling elderly. N Engl J Med (2007) 357:1373–81. doi:10.1056/NEJMoa070844
3. Goronzy JJ, Fulbright JW, Crowson CS, Poland GA, O’Fallon WM, Weyand CM. Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. J Virol (2001) 75:12182–7. doi:10.1128/JVI.75.24.12182-12187.2001
4. Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E, et al. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One (2010) 5(5):e10667. doi:10.1371/journal.pone.0010667
5. O’Toole PW, Claesson MJ. Gut microbiota: changes throughout the lifespan from infancy to elderly. Int Dairy (2010) 20(4):281–91. doi:10.1016/j.idairyj.2009.11.010
6. Candela M, Biagi E, Brigidi P, O’Toole PW, De Vos WM. Maintenance of a healthy trajectory of the intestinal microbiome during aging: a dietary approach. Mech Ageing Dev (2014) 13(6–137):70–5. doi:10.1016/j.mad.2013.12.004
7. Dong H, Rowland I, Thomas LV, Yaqoob P. Immunomodulatory effects of a probiotic drink containing Lactobacillus casei Shirota in healthy older volunteers. Eur J Nutr (2013) 52:1853–63. doi:10.1007/s00394-012-0487-1
8. Zanini K, Marzotto M, Castellazzi A, Borsari A, Dellaglio F, Torriani S. The effects of fermented milks with simple and complex probiotic mixtures on the intestinal microbiota and immune response of healthy adults and children. Int Dairy J (2007) 17(11):1332–43. doi:10.1016/j.idairyj.2007.01.017
9. Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, et al. Complement factor H polymorphism in age-related macular degeneration. Sci (2005) 308:385–9. doi:10.1126/science.1109557
10. Wichers H. Immunomodulation by food: promising concept for mitigating allergic disease? Anal Bioanal Chem (2009) 395(1):37–45. doi:10.1007/s00216-009-2838-1
11. Maathuis A, Hoffman A, Evans A, Sanders L, Venema K. The effect of maize based fibers on the activity and composition of the microbiota determined in a dynamic in vitro model of the human large intestine. J Am Coll Nutr (2009) 28(6):657–66. doi:10.1080/07315724.2009.10719798
12. Vester Boler BM, Rossoni Serao MC, Bauer LL, Staeger MA, Boileau TW, Swanson KS, et al. Digestive physiological outcomes related to polydextrose and soluble maize fibre consumption by healthy adult men. Br J Nutr (2011) 106(12):1864–71. doi:10.1017/S0007114511002388
13. Konings E, Schoffelen PF, Stegen J, Blaak EE. Effect of polydextrose and soluble maize fibre on energy metabolism, metabolic profile and appetite control in overweight men and women. Br J Nutr (2014) 111:111–21. doi:10.1017/S0007114513002183
14. Stewart ML, Nikhanj SD, Timm DA, Thomas W, Slavin JL. Evaluation of the effect of four fibers on laxation, gastrointestinal tolerance and serum markers in healthy humans. Ann Nutr Metab (2010) 56:91–8. doi:10.1159/000275962
15. Costabile A, Deaville ER, Morales AM, Gibson GR. Prebiotic potential of a maize-based soluble fibre and impact of dose on the human gut microbiota. PLoS One (2016) 11(1):e0144457. doi:10.1371/journal.pone.0144457
16. Kang M, Ragan BG, Park JH. Issues in outcomes research: an overview of randomization techniques for clinical trials. J Athl Train (2008) 43(2):215–21. doi:10.4085/1062-6050-43.2.215
17. Almeida-Oliveira A, Smith-Carvalho M, Porto LC, Cardoso-Oliveira J, Ribeiro Ados S, Falcao RR, et al. Age-related changes in natural killer cell receptors from childhood through old age. Hum Immunol (2011) 72(4):319–29. doi:10.1016/j.humimm.2011.01.009
18. Salonen A, Nikkila J, Jalanka-Tuovinen J, Immonen O, Rajilic-Stojanovic M, Kekkonen RA, et al. Comparative analysis of faecal DNA extraction methods with phylogenetic microarray: effective recovery of bacterial and archaeal DNA using mechanical cell lysis. J Microbiol Methods (2010) 81:127–34. doi:10.1016/j.mimet.2010.02.007
19. Rasinkangas P, Reunanen J, Douillard FP, Ritari J, Uotinen V, Palva A, et al. Genomic characterization of non-mucus-adherent derivatives of Lactobacillus rhamnosus GG reveals genes affecting pilus biogenesis. Appl Environ Microbiol (2014) 80(22):7001–9. doi:10.1128/AEM.02006-14
20. Nadkarni MA, Martin FE, Jacques NA, Hunter N. Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology (2002) 148(1):257–66. doi:10.1099/00221287-148-1-257
21. Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Stat (2001) 29(4):1165–88. doi:10.1214/aos/1013699998
22. Salminen S, Collado MC, Isolauri E, Gueimonde M. Microbial–host interactions: selecting the right probiotics and prebiotics for infants. Nestle Nutr Workshop Ser Pediatr Program (2009) 64:201–17. doi:10.1159/000235792
23. López P, Gueimonde M, Margolles A, Suárez A. Distinct Bifidobacterium strains drive different immune responses in vitro. Int J Food Microbiol (2010) 138:157–65. doi:10.1016/j.ijfoodmicro.2009.12.023
24. Lomax AR, Calder PC. Probiotics, immune function, infection and inflammation: a review of the evidence from studies conducted in humans. Curr Pharm Des (2009) 15(13):1428–518. doi:10.2174/138161209788168155
25. Plackett TP, Boehmer ED, Faunce DE, Kovacs EJ. Aging and innate immune cells. J Leukoc Biol (2004) 76(2):291–9. doi:10.1189/jlb.1103592
26. Solana R, Alonso MC, Pena J. Natural killer cells in healthy aging. Exp Gerontol (1999) 34(3):435–43. doi:10.1016/S0531-5565(99)00008-X
27. Berman SH, Eichelsdoerfer P, Yim D, Elmer GW, Wenner CA. Daily ingestion of a nutritional probiotic supplement enhances innate immune function in healthy adults. Nutr Res (2006) 26(9):454–9. doi:10.1016/j.nutres.2006.08.002
28. Elmadfa I, Klein P, Meyer AL. Immune-stimulating effects of lactic acid bacteria in vivo and in vitro. Proc Nutr Soc (2010) 69(3):416–20. doi:10.1017/S0029665110001710
29. Gill HS, Rutherfurd KJ, Cross ML. Dietary probiotic supplementation enhances natural killer cell activity in the elderly: an investigation of age-related immunological changes. J Clin Immunol (2001) 21:264–71. doi:10.1023/A:1010979225018
30. Dong H, Rowland I, Yaqoob P. Comparative effects of six probiotic strains on immune function in vitro. Br J Nutr (2012) 108(3):459–70. doi:10.1017/S0007114511005824
31. Takeda K, Okumura K. Effects of a fermented milk drink containing Lactobacillus casei strain Shirota on the human NK-cell activity. J Nutr (2007) 137(3 Suppl 2):791S–3S.
32. Olivares M, Díaz-Ropero MP, Sierra S, Lara-Villoslada F, Fonollá J, Navas M, et al. Oral intake of Lactobacillus fermentum CECT5716 enhances the effects of influenza vaccination. Nutrition (2007) 23:254–60. doi:10.1016/j.nut.2007.01.004
33. Bunout D, Barrera G, Hirsch S, Gattas V, de la Maza MP, Haschke F, et al. Effects of a nutritional supplement on the immune response and cytokine production in free-living Chilean elderly. J Parenter Enteral Nutr (2004) 28:348–54. doi:10.1177/0148607104028005348
34. Chiang BL, Sheih YH, Wang LH, Liao CK, Gill HS. Enhancing immunity by dietary consumption of a probiotic lactic acid bacterium (Bifidobacterium lactis HN019): optimization and definition of cellular immune responses. Eur J Clin Nutr (2000) 54:849–55. doi:10.1038/sj.ejcn.1601093
35. Gill HS, Rutherfurd KJ, Cross ML, Gopal PK. Enhancement of immunity in the elderly by dietary supplementation with the probiotic Bifidobacterium lactis HN019. Am J Clin Nutr (2001) 74:833–9. doi:10.3390/nu3060637
36. Sheih YH, Chiang BL, Wang LH, Liao CK, Gill HS. Systemic immunity-enhancing effects in healthy subjects following dietary consumption of the lactic acid bacterium Lactobacillus rhamnosus HN001. J Am Coll Nutr (2001) 20:149–56. doi:10.1080/07315724.2001.10719027
37. Gill HS, Rutherfurd KJ. Probiotic supplementation to enhance natural immunity in the elderly: effects of a newly characterized immunostimulatory strain Lactobacillus rhamnosus HN001 (DR20 (TM)) on leucocyte phagocytosis. Nutr Res (2001) 21(1–2):183–9. doi:10.1016/S0271-5317(00)00294-3
38. Roller M, Clune Y, Collins K, Rechkemmer G, Watzl B. Consumption of prebiotic inulin enriched with oligofructose in combination with the probiotics Lactobacillus rhamnosus and Bifidobacterium lactis has minor effects on selected immune parameters in polypectomised and colon cancer patients. Br J Nutr (2007) 97:676–84. doi:10.1017/S0007114507450292
39. De Vrese M, Winkler P, Rautenberg P, Harder T, Noah C, Laue C, et al. Effect of Lactobacillus gasseri PA 16/8, Bifidobacterium longum SP 07/3, B-bifidum MF 20/5 on common cold episodes: a double blind, randomized, controlled trial. Clin Nutr (2005) 24(4):481–91. doi:10.1016/j.clnu.2005.02.006
40. Oshimi K, Gonda N, Sumiya M, Kano S. Effects of corticosteroids on natural-killer cell activity in systemic lupus-erythematosus. Clin Exp Immunol (1980) 40(1):83–8.
41. Nagao F, Nakayama M, Muto T, Okumura K. Effects of a fermented milk drink containing Lactobacillus casei strain Shirota on the immune system in healthy subjects. Biosci Biotechnol Biochem (2000) 64:2706–8. doi:10.1271/bbb.64.2706
42. Morimoto K, Takeshita T, Nanno M, Tokudome S, Nakayama K. Modulation of natural killer cell activity by supplementation of fermented milk containing Lactobacillus casei in habitual smokers. Prev Med (2005) 40(5):589–94. doi:10.1016/j.ypmed.2004.07.019
43. Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet (2000) 356:1795–9. doi:10.1016/S0140-6736(00)03231-1
44. Ogata K, An E, Shioi Y, Nakamura K, Luo S, Yokose N, et al. Association between natural killer cell activity and infection in immunologically normal elderly people. Clin Exp Immunol (2001) 124:392–7. doi:10.1046/j.1365-2249.2001.01571.x
45. Gleeson M, Bishop NC, Oliveira M, Tauler P. Daily probiotic’s (Lactobacillus casei Shirota) reduction of infection incidence in athletes. Int J Sport Nutr Exerc Metab (2011) 21(1):55–64. doi:10.1123/ijsnem.21.1.55
46. Shukla G, Bhatia R, Sharma A. Prebiotic inulin supplementation modulates the immune response and restores gut morphology in Giardia duodenalis-infected malnourished mice. Parasitol Res (2016) 115(11):4189–98. doi:10.1007/s00436-016-5196-x
47. Marciano R, Santamarina AB, de Santana AA, Silva Mde L, Amancio OM, do Nascimento CM, et al. Effects of prebiotic supplementation on the expression of proteins regulating iron absorption in anaemic growing rats. Br J Nutr (2015) 113(6):901–8. doi:10.1017/S0007114514004334
48. Aliasgharzadeh A, Dehghan P, Gargari BP, Asghari-Jafarabadi M. Resistant dextrin, as a prebiotic, improves insulin resistance and inflammation in women with type 2 diabetes: a randomised controlled clinical trial. Br J Nutr (2015) 113(2):321–30. doi:10.1017/S0007114514003675
49. Childs CE, Röytiö H, Alhoniemi E, Fekete AA, Forssten SD, Hudjec N, et al. Xylo-oligosaccharides alone or in synbiotic combination with Bifidobacterium animalis subsp. lactis induce bifidogenesis and modulate markers of immune function in healthy adults: a double-blind, placebo-controlled, randomised, factorial cross-over study. Br J Nutr (2014) 111(11):1945–56. doi:10.1017/S0007114513004261
50. Carbonero F, Benefiel AC, Alizadeh-Ghamsari AH, Gaskins HR. Microbial pathways in colonic sulfur metabolism and links with health and disease. Front Physiol (2012) 3:448. doi:10.3389/fphys.2012.00448
51. Nakamura N, Lin HC, McSweeney CS, Mackie RI, Gaskins HR. Mechanisms of microbial hydrogen disposal in the human colon and implications for health and disease. Annu Rev Food Sci Technol (2010) 1:363–95. doi:10.1146/annurev.food.102308.124101
52. Peris-Bondia F, Latorre A, Artacho A, Moya A, D’Auria G. The active human gut microbiota differs from the total microbiota. PLoS One (2011) 6(7):e22448. doi:10.1371/journal.pone.0022448
53. Tap J, Mondot S, Levenez F, Pelletier E, Caron C, Furet J, et al. Towards the human intestinal microbiota phylogenetic core. Environ Microbiol (2009) 11:2574–84. doi:10.1111/j.1462-2920.2009.01982.x
54. Langille MG, Meehan CJ, Koenig JE, Dhanani AS, Rose RA, Howlestt SE, et al. Microbial shifts in the aging mouse gut. Microbiome (2014) 2(1):50. doi:10.1186/s40168-014-0050-9
55. Conley MN, Wong CP, Duyck KM, Hord N, Ho E, Sharpton TJ. Aging and serum MCP-1 are associated with gut microbiome composition in a murine model. PeerJ (2016) 4:e1854. doi:10.7717/peerj.1854
56. Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ (2003) 326:1423–9. doi:10.1136/bmj.326.7404.1423
57. Grundy SM, Cleeman JI, Merz CN, Brewer HBJR, Clark LT, Hunninghake DB, et al. Implications of recent clinical trials for the national cholesterol education program adult treatment panel III guidelines. Circulation (2004) 110:227–39. doi:10.1161/01.CIR.0000133317.49796.0E
58. De Caterina R, Scarano M, Marfisi R, Lucisano G, Palma F, Tatasciore A, et al. Cholesterol-lowering interventions and stroke: insights from a meta-analysis of randomized controlled trials. J Am Coll Cardiol (2010) 55:198–211. doi:10.1016/j.jacc.2009.07.062
59. Delahoy P, Thompson S, Marschner IC. Pregabalin versus gabapentin in partial epilepsy: a meta-analysis of dose-response relationships. BMC Neurol (2010) 10:104. doi:10.1186/1471-2377-10-104
60. Shimizu M, Hashiquchi M, Shiga T, Tamura HO, Mochizuki M. Meta-analysis: effects of probiotic supplementation on lipid profiles in normal to mildly hypercholesterolemic individuals. PLoS One (2015) 10(10):e0139795. doi:10.1371/journal.pone.0139795
61. Sadrzadeh-Yeganeh H, Elmadfa I, Djazayery A, Jalali M, Heshmat R, Chamary M. The effects of probiotic and conventional yoghurt on lipid profile in women. Br J Nutr (2010) 103:1778–83. doi:10.1017/S0007114509993801
Keywords: Lactobacillus rhamnosus GG, Lactobacillus rhamnosus GG-PB12, pilus-deficient, Soluble Corn Fiber, fecal microbiota, metabolism, immunity
Citation: Costabile A, Bergillos-Meca T, Rasinkangas P, Korpela K, de Vos WM and Gibson GR (2017) Effects of Soluble Corn Fiber Alone or in Synbiotic Combination with Lactobacillus rhamnosus GG and the Pilus-Deficient Derivative GG-PB12 on Fecal Microbiota, Metabolism, and Markers of Immune Function: A Randomized, Double-Blind, Placebo-Controlled, Crossover Study in Healthy Elderly (Saimes Study). Front. Immunol. 8:1443. doi: 10.3389/fimmu.2017.01443
Received: 02 August 2017; Accepted: 17 October 2017;
Published: 12 December 2017
Edited by:
Nadiya V. Boyko, Uzhhorod National University, UkraineReviewed by:
Brandt D. Pence, University of Memphis, United StatesDiego Mora, Università degli Studi di Milano, Italy
Copyright: © 2017 Costabile, Bergillos-Meca, Rasinkangas, Korpela, de Vos and Gibson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adele Costabile, adele.costabile@roehampton.ac.uk
 Adele Costabile
Adele Costabile Triana Bergillos-Meca1
Triana Bergillos-Meca1