- 1Cancer Genetics and Cancer Stem Cell Laboratory, Centro de Investigaciones Biologicas, Department of Cellular and Molecular Medicine, Spanish National Research Council (CSIC), Madrid, Spain
- 2Centro Nacional de Biotecnologia, Department of Immunology and Oncology, Spanish National Research Council (CSIC), Madrid, Spain
The cancer stem cell (CSC) hypothesis suggests that within a tumor, there is a small subpopulation of cells with stem cell properties responsible for tumor maintenance and metastasis generation. This hypothesis also implies that new antitumor drugs, rather than targeting the bulk of the tumor mass, would be more effective if they directly targeted the CSC subpopulation. The CSCs from several types of tumors have been identified with mAbs recognizing surface antigens in these cells; however, antigens specifically or exclusively expressed in the CSC population have not yet been identified. Thus, questioning the possibility of using therapeutic antibodies directed against the CSCs. Here, we review the possibilities of using antibodies directly targeting the CSCs as therapeutic agents in the form of naked antibodies, antibodies conjugated to nanoparticles, or antibody cocktails.
Introduction
Although the most frequently used anticancer treatments still are chemotherapy and radiotherapy, it is clear by now that monoclonal antibodies have emerged on the last 20 years as the most relevant new type of anticancer drugs with clinically proven therapeutic value. Concomitantly, this has generated an enormous interest, which has led to a burst of new approaches and clinical trials, where monoclonal antibodies represent the key element (1). However, most of the current anticancer treatments, including antibodies or other molecular interventions, increase the survival and improve the quality of life on patients, but do not necessarily cure.
It is obvious that antibodies against HER2, CD20, VEGF, EGFR, or CD52 have shown their clinical therapeutic value as anticancer drugs (1). In addition, antibodies that enhance the immune response by either blocking the PD-1/PD-L1 axis (2); antibodies anti-CTLA-4 (3, 4); or antibodies that block inhibitory receptors of NK cells (5, 6); or even CAR T cells (variable antibody regions engineered TCR-carrying T cells) (7), have proven also very useful. Indeed, they are able to redirect the antitumor immune response and allow envisaging the possibility of a cure for cancer patients. Obviously, the cure for cancer patients might come from the use of more or less complex combinations of antibodies that will include other drugs or cells (8).
Thus, the remaining questions are as follows: Is this the best we can do to cure cancer patients? Are we hitting the right targets? In this review, we would like to discuss the characteristics of the cancer stem cells (CSCs) that make them ideal targets, and the possible strategies of using antibodies to directly target the CSC population as the best option to cure cancer patients.
Adult Stem Cells and CSCs
One of the concepts that have largely changed our understanding about tumor biology was the CSC hypothesis (9). Stem cells are defined as cells with the ability of self-renew (perpetuate themselves) and to differentiate, generating mature cells of a particular tissue. Adult (or tissue-specific) stem cells are rare cells that have been identified in many tissues, including the hematopoietic stem cells (HSCs) in the bone marrow (10, 11), the mammary stem cells in the mammary gland (12, 13), neural stem cells in the nervous system (14, 15), and the intestine stem cells in the intestine (16), among others. In several cases, a hierarchical structure has been demonstrated, where adult stem cells generate the appropriate cells from that tissue and maintain its homeostasis. The adult stem cell is able to undergo either symmetric cell divisions, generating two daughter stem cells, or asymmetrically, where the stem cell gives rise to a daughter stem cell and another cell committed for differentiation (17). From the committed cell, a common progenitor will be generated lacking self-renewal ability, but able to generate all the cell types of the differentiated tissue. The common progenitor will in turn generate more committed progenitors; each one of them will be able to generate one or two differentiated cell types from the tissue (Figure 1). This differentiation process is concomitant with cell expansion, explaining the reason why in many cases the frequency of adult stem cells is below 1% (18).
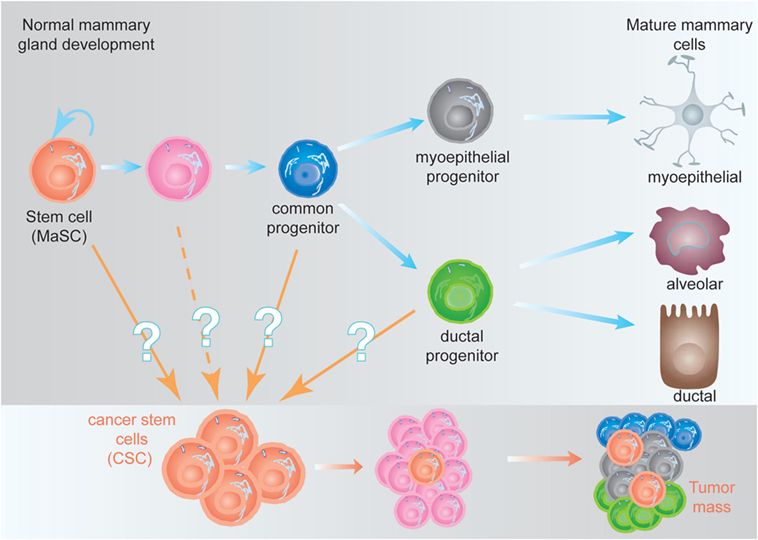
Figure 1. Hypothetical model of the mammary epithelial hierarchy and its relationship with cancer stem cells (CSCs). (Top) The mammary stem cell (MaSC) differentiates through a common progenitor into either a myoepithelial or a ductal progenitor, which are committed to generate mature myoepithelial or ductal and alveolar cells, respectively. During this process, the MaSC and its progeny undergo at least nine cell divisions to generate the fully differentiated cells (not represented here), giving a ratio 1:500 MaSC:differentiated cells (18). (Bottom) CSCs, independent of their origin, are malignant-transformed cells with stem cell characteristics. They are able to generate a tumor (or metastases), although they represent a small fraction of the tumor mass (9).
The CSC hypothesis proposes for tumors a hierarchical structure similar to the described for adult tissues. A small fraction of cells within the tumor harbor stem-cell like characteristics (referred to as CSCs), with an indefinite self-renewal potential and able to drive tumorigenesis, being able to develop into a heterogeneous, more differentiated population, which constitutes the tumor mass (9). The CSCs were initially identified in acute myeloid leukemia (19) and prospectively identified in solid tumors including the mammary gland (20), the brain (21), and many others. The existence of CSC has been unequivocally demonstrated in vivo in glioblastomas, intestine, melanomas, and mammary tumors (22–25). One of the predictions of the CSC hypothesis was that more effective cancer therapies would target the CSC, instead of the bulk of the tumor (9). This is supported by the finding that CSC, such as normal stem cells, are more resistant to conventional chemotherapy and radiotherapy than more differentiated tumor cells (26), suggesting that effective therapies against the CSC would target self-renewal and/or differentiation of these cells (27).
Interestingly, it has been demonstrated in glioblastomas that therapies directly targeting the CSC are more effective than the ones targeting the tumor mass. In fact, standard chemotherapy was able to kill the bulk of the glioblastoma, but not the CSC, and the tumors quickly returned. When, in addition to chemotherapy, the CSC population was depleted in mouse glioblastoma models using a genetic trick, the tumors shrank back into “residual vestiges” that did not resemble glioblastomas (22). Thus, these data suggest that the predictions of the CSC hypothesis are true and that therapies directed to the CSC will turn out to be more effective.
CSC Markers
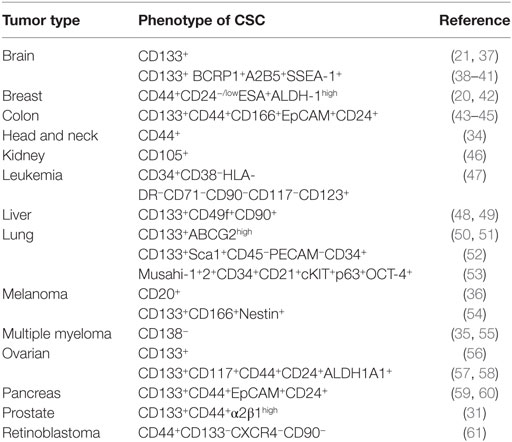
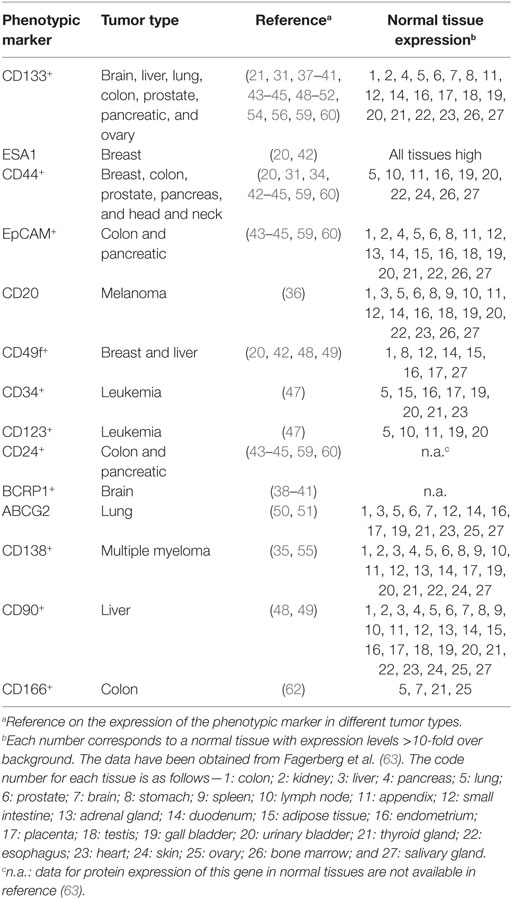
Once established that the CSC represents a distinct tumor cell population, involved in tumor formation and maintenance, the identification of their specific markers has been a priority. First, for the isolation of the CSC and a more detailed analysis on their biology, but also for the possibility of using some of these markers as putative therapeutic targets. In many cases, the combination of positive and negative expression of surface markers allowed the identification of CSC populations. For example, on the identification of CSC in acute myelocytic leukemia (AML), where the cells were fractionated on the basis of CD34 and CD38 expression, demonstrating that only the CD34+CD38− cells, but not the CD34+CD38+ or CD34− cells, were able to engraft immunocompromised mice, replicating many aspects of human AML (19). Similarly, combinations of other surface markers, such as CD24, CD44, ESA, and CD133, allowed the identification of CSC in tumors from breast (20), liver (28), brain (21), lung (29), colon (30), prostate (31), pancreas (32, 33), head and neck squamous carcinoma (34), multiple myeloma (35), melanoma (36), among others (Table 1). It should be noted that in many cases, the surface markers used to identify CSC also identify adult stem cells on the corresponding normal tissues, or are surface markers shared by other cell types (Table 2).
The available data allow raising the question of whether there are specific CSC markers. Although at this time we cannot formally exclude their existence, since the CSC possess the same genetic information as the rest of the tumor (there are no additional mutations in the CSC as compared to the tumor mass), it is more likely that the phenotypic differences on CSC are due to differential gene expression. Indeed, both phenotypic and genetic analyses have failed so far, to pinpoint a single marker specific of any CSC population. In this context, genetic analyses aiming to understand self-renewal, a hallmark of stem cells and cancer, allowed pinpointing two genetic programs, one of them expressed by embryonic stem cells (ESC), and the other by adult tissue stem cells. When analyzing expression of these programs in human cancers, it was observed that in tumors where the ESC-like transcriptional program was activated, strongly predicted metastasis and death, whereas expression of the adult tissue stem cells program led to a better prognosis (64). These types of analyses might allow to identify differentially expressed genes in the CSC as compared with the tumor mass and consequently be highly relevant for the identification of new CSC markers (cell surface markers, secreted proteins, intracellular proteins, or transcription factors). It should be noted that therapeutic antibodies can be generated, in addition to surface marker proteins, also against intracellular proteins, including transcription factors (65).
The next question that can be raised is whether the markers used for the identification of the CSC can also be used as therapeutic antibody targets. There is no straight answer to this question. Obviously, only mAbs that positively identify the CSC population could be used for therapeutic purposes. Returning to the example of the myelocytic leukemia, the combination of the CD34 and CD38 markers has been useful for the identification and isolation of the CSC (19). But since the CSCs are CD34+CD38− cells, the CD38 antibody cannot be used for therapeutic purposes (the CSCs are negative for this marker), although the CD34 mAb could.
Possible Strategies to Target CSC
A priori, the strategies to directly target the CSC population would tackle (i) differences in surface marker expression; (ii) interfere with signaling pathways relevant for their function; (iii) inhibit their function; (iv) interfere with metastasis formation; or (v) a combination of the above. In the following paragraphs, we will try to dissect these strategies (see Figure 2).
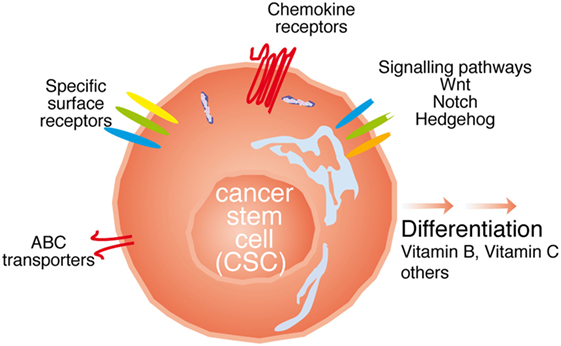
Figure 2. Possibilities to target cancer stem cell (CSC). The CSC can be targeted with either antibodies against specific surface receptors, interfering with signaling molecules relevant for CSC function such as Wnt, Notch, and Hedgehog, or the ATP-binding cassette (ABC) transporters, through chemokine receptor antibodies, or inducing the differentiation of thee cells.
Therapies Targeting CSC Surface Markers
It turns out, from the data on Table 1, that CD133 (prominin-1) has been established as a marker of CSC on many solid tumors including brain, colon, liver, lung, ovarian, pancreatic, and prostate tumors. The role of CD133 as a CSC marker has, however, been questioned, for example, using the lung cancer cell lines A549 and H446, where more than 45% of the cells represent bona-fide CSC, it has been reported that both CD133+ and CD133− cells are able to form tumors with the same efficiency (29). In addition, CD133 exhibits several splice variants and different poorly characterized glycosylated isoforms (38), and as shown on Table 2, this antigen is broadly expressed on normal tissues (63). Thus, making it questionable whether CD133 represents a specific CSC marker and a therapeutic target for antibody-mediated elimination of CSC.
Targeting the adhesion molecule CD44 with monoclonal antibodies in xenografts of AML allowed to demonstrate that this treatment eradicated the leukemic CSC (66). Similarly, an antibody specific for the membrane IL-3Ra receptor (CD123) overexpressed in leukemia CSC (see Table 1) has been used to specifically target leukemia CSC in human AML. The treatments decreased leukemogenicity and eradicated CSC in mice (67, 68). In addition, an antibody targeting CD47 has demonstrated its ability to eliminate human acute lymphoblastic leukemia in xenograft transplants (69). The T cell immunoglobulin mucin-3 (TIM-3) was also identified as a surface molecule expressed on leukemia stem cells in most types of AML except for acute promyelocytic leukemia, but not on normal HSCs. TIM-3+ but not TIM-3− AML cells engrafted, replicating in immunodeficient mice, many of the aspect on human AML. Furthermore, antibodies specific for TIM-3 dramatically diminished their leukemic burden (69). It should be noted that these experiments were carried out in xenotransplants, where the only cells expressing CD44, CD123, CD47, or TIM-3 were the transplanted tumor cells, thus any putative toxic effects on other body cells expressing these markers CD44+, CD123+, CD47+, or TIM-3+ could not be assessed in these models. However, a possibility, discussed in details in another review from this issue is to use combinations of antibodies (8), where even if the antibodies mentioned earlier for the treatment of AML used separately could be also toxic for the normal tissues, their combination (CD44, CD123, CD47, and TIM-3) could use smaller doses of each one of them, avoiding the concentrations required to induce toxicity in normal cells, but still be effective killing the CSC on AML. This is one of the possibilities that should be investigated for the treatment of AML and other types of cancer.
Another possibility of combination of antibodies against surface marker that can be investigated from the data on Table 1 deal with liver tumor CSCs, which are CD133+CD49f+CD90+ (48, 49). Each one of these markers is broadly expressed in normal tissues (63) as seen in Table 2. The use of antibodies against any of these markers as therapeutic tools might not be sufficiently selective for CSC and be toxic to healthy tissues. However, it might turn out that a strategy combining antibodies against the three molecules, using lower doses of each one of them, may still be effective while avoiding the unwanted toxicity with these lower doses.
Therapies targeting CSC surface markers can be exemplified by a clinical trial on untreated multiple myeloma using the anti-CD19 mAb MEDI-551 in combination with dexamethasone and lenalidomide. The rational of the trial is to determine whether the treatment with MEDI-551 decreases the number of CSC in these multiple myeloma patients (NCT01861340) (70).
In some cases, although the mAb identifies a target present on both adult stem cells and CSC, the antibody could be used to target the CSC. This would be the case for the mAb Nilo1, identifying mouse embryonic radial glia, adult neural stem cells, and also a subpopulation of mouse and human glioblastoma cells (71, 72), allowing to suggest that it might identify the CSC population (73). If Nilo1 indeed identifies the CSC, it could be envisaged that this antibody conjugated, for example, to gold nanoparticles would be able to photo-ablate Nilo1+ cells after these targeted cells absorb near infrared light. This would result in increased local temperature at the selected location, destroying the target cells (74). This approach would be feasible since the adult neural stem cells are restricted to their niche (subventricular zone), an expected different location from the tumor. However, in other tumor types, such as hematopoietic tumors or tumors of the mammary gland, this approach would be much more difficult to apply.
Another possible approach tackles the observation that both adult stem cell and CSC express higher levels of the ATP-binding cassette (ABC) transporters on their cell membranes. The ABC transporters have been proposed to contribute to multidrug resistance, because they allow to pump out of the cytoplasm many antitumor drugs, resulting in lower intracellular drug concentrations (35, 50), allowing the CSC to become more resistant to chemotherapeutic drugs (50, 75). However, some experiments using inhibitors of the ABC transporter have been successfully carried out (76). It seems that the generation of ABC transporter-blocking antibodies might inhibit ABC-transporter functions, without many of the negative toxic effects of the inhibitors, and therefore this will make the CSC more sensitive to chemotherapeutic drugs.
Finally, a clinical trial aims to determine the CSC load of HER2+ breast cancer tumors treated with the anti-HER2 antibody trastuzumab, in combination with adjuvant, doxorubicin hydrochloride, or cyclophosphamide followed by paclitaxel (NCT01424865).
Targeting Signal Pathways
The signaling pathways involved in stemness, both in adult stem cells and CSC, including Notch, Hedgehog, and Wnt representing relevant therapeutic targets for CSC (9). Indeed, monoclonal antibodies against Notch are able to reduce the CSC population in colorectal tumors (77) and also in breast cancer cell lines (78). Similarly, antibodies against the Wnt-1 signaling pathway induce apoptosis in human colorectal cancer cells (79).
Small molecule Hedgehog antagonists have also been successfully used to inhibit systemic metastases in xenografts with tumors derived from human pancreas (80), but in this case, as far as the authors are aware, blocking antibodies have not yet been used. In fact, inhibitors of Wnt, Notch, and Hedgehog activities are being investigated in a clinical trial on esophageal cancer patients (NCT02221245). Other clinical trials use therapeutic antibodies against DLL4 to inhibit Notch signaling (presumably targeting Notch expressed on the CSC) in combination with paclitaxel in ovarian, peritoneal, and fallopian tube cancer (NCT03030287); in combination with FOLFIRI (irinotecan, folic acid, leucovorin, and fluorouracil), in metastatic colorectal cancer (NCT01189942); or the anti-DLL4 antibody demcizumab in combination with Gemcitabine Abraxane on metastatic pancreatic cancer (NCT01189929). Other examples use either a bispecific DLL4/VEGF antibody (OMP-305B83) in metastatic colorectal cancer, combined with the chemotherapeutic agents FOLFIRI (NCT03035253); or in combination with the chemotherapeutics carboplatin and pemetrexed for lung cancer (NCT01189968). Finally, another clinical trial uses the anti-DLL4 antibody demcizumab, in combination with the anti-PD-1 antibody pembrolizumab (immune checkpoint) in metastatic solid tumors (NCT02722954), aiming to inhibit Notch and simultaneously busting the antitumor immune response by inhibiting the PD-1/PD-L1 immune checkpoint.
A different approach used was to combine the Hedgehog inhibitor IPI-926 with the anti-EGFR antibody cetuximab in head and neck cancer patients (NCT01255800).
Other signaling pathways relevant in oncology include the tyrosine kinase family. The tyrosine kinase inhibitor lapatinib has been combined with the anti-HER2 antibody trastuzumab in a clinical trial in breast cancer patients (NCT00524303), where the authors want to analyze changes in CSC load.
Trigger Differentiation
An additional possibility is to trigger the differentiation of the CSC. This will imply that they are not able to self-renew anymore, and therefore they would be more sensitive to regular chemotherapy and radiotherapy. In fact, several agents, such as retinoic acid (RA) [i.e., 13-cis RA (isotretinoin)], are used to modify cell expression patterns inhibiting proliferation and inducing cell differentiation and apoptosis (81–83). In addition, vitamin C has also been shown to trigger differentiation of CSC on leukemia, enhancing their sensitivity to PARP inhibition (84). It seems clear that these compounds will be used in combinations with antibodies and/or other drugs.
An example of therapeutic interest on triggering CSC differentiation is shown by a current clinical trial, aiming to analyze the role of the vitamin B derivate Fursultiamine on the differentiation of CSC in squamous cell carcinomas (NCT02423811) (76, 85).
Others
The effects of any anti-CSC antibody can be potentiated if it is used in combination with antibodies inhibiting immune-checkpoints negative signals. These include antibodies binding to the PD-1 receptor on the T cells (nivolumab and pembrolizumab), to PD-L1 on the tumor cells (atezolizumab, durvalumab, and avelumab) or to CTLA-4 on T cells (ipilimumab) (86). This strategy will be relevant since the anti-checkpoint antibodies are able to switch the antitumor response from an immunosuppressed status, to another that allows to attack the tumor.
Since the CSCs are, in addition to the tumor-initiating cells, the unique cells that can form metastasis, as they are only cells within a tumor with a strong proliferation potential, able to generate the more differentiated tumor cells, which form the tumor mass, and at the same time a strong self-renewal potential through symmetric cell divisions (9). The use of any antibody or drug against the CSC, in combination with anti-chemokine receptor antibodies such as CXCR4, CCR7, and CCR9 (85, 87–96), would inhibit the migration of the CSC, their migration, invasion, and seeding of the metastatic cells, therefore improving the patient’s health.
Another possibility is to combine any antibody or drug specific for CSC with antibodies inhibiting tumor neo-vascularization, such as VEGF or VEGFR. In this context, there is a clinical trial that combines the preoperative treatment with the anti-VEGF antibody bevacizumab and chemotherapy in patients with breast cancer (NCT01190345), where they aim to determine the CSC activity (measured by the amount of aldehyde dehydrogenase 1/ALDH1+ cells before and after treatment).
Here, we have pinpointed some of the ongoing trials and preclinical experiments being carried out aiming to directly target CSC; however, there are many more possibilities to be carefully analyzed.
Conclusion
The existence on many tumors of a subpopulation of cells with stem cell characteristics (the CSC population) it is clear by now. Furthermore, the concept that new anticancer treatments will be more effective if they directly target the CSC population, seems settled in the scientific community. The number of clinical trials targeting the CSC is, however, relatively small. Furthermore, from the 86 clinical trials found with the keywords “cancer stem cells,” only 12 of them use monoclonal antibodies as therapeutic agents. This is due, at least in part, to the lack of CSC-specific markers. We are optimistic, however, and believe that in the near future, this number will greatly increase. The new clinical trials will involve several combinations of antibodies, antibodies and chemotherapeutic drugs, small drug molecules, or the discovery of molecules able to differentiate the CSC. These will make a large advance in oncologic treatments specifically designed to destroy or kill CSCs.
Taken together, this does not mean that the work ahead will be easy, in particular since examples have been described where not only the CSC give rise to daughter CSC and non-CSC but also where the non-CSC population can, in some situations, give rise to some CSC (97). Thus, advances in the field of antibody immunotherapy directly targeting the CSC will require combinations of genetic analyses to identify differentially expressed genes in the CSC population, and an improved knowledge on the biology of the CSC (98), together with the use of complex algorithms to determine effective concentrations of different antibodies and drugs, to avoid adult stem cells harm. Thus, strategies using antibodies directly targeting the CSC population, while bursting the antitumor immune response and inhibiting neo-vascularization may represent an unparalleled opportunity to cure cancer.
Author Contributions
All authors contributed to drafting, revising, and approving the final article.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank all the members of their laboratories for critical reading of the manuscript and helpful suggestions.
Funding
The work in the author’s laboratories was partially supported by grants from the CSIC (PIE-201420E109, to LK), the PN2014-A from the ISCIII (PI14/00703, cofinanced by FEDER funds from the EU, Operative program on Intelligent Growth 2014–2020, to LK), and from the Spanish Ministry of Economy, Industry and Competitiveness (RTC-2015-3786-1 to LK and JG-S and RTC-2015-3846-1 to JG-S), both cofinanced by FEDER funds from the EU.
References
1. Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer (2012) 12(4):278–87. doi:10.1038/nrc3236
2. Xu-Monette ZY, Youg KH. PD-1, PD-L1, and PD-1/PD-L1 blockade: have we found the key to unleash the antitumor immune response? Front Immunol (2017).
3. Baksh K, Weber J. Immune checkpoint protein inhibition for cancer: preclinical justification for CTLA-4 and PD-1 blockade and new combinations. Semin Oncol (2015) 42(3):363–77. doi:10.1053/j.seminoncol.2015.02.015
4. Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol (2015) 33(17):1974–82. doi:10.1200/JCO.2014.59.4358
5. Muntasell A, Ochoa MC, Cordeiro L, Berraondo P, Lopez-Diaz de Cerio A, Cabo M, et al. Targeting NK-cell checkpoints for cancer immunotherapy. Curr Opin Immunol (2017) 45:73–81. doi:10.1016/j.coi.2017.01.003
6. Kohrt HE, Thielens A, Marabelle A, Sagiv-Barfi I, Sola C, Chanuc F, et al. Anti-KIR antibody enhancement of anti-lymphoma activity of natural killer cells as monotherapy and in combination with anti-CD20 antibodies. Blood (2014) 123(5):678–86. doi:10.1182/blood-2013-08-519199
7. Majzner RG, Heitzeneder S, Mackall CL. Harnessing the immunotherapy revolution for the treatment of childhood cancers. Cancer Cell (2017) 31(4):476–85. doi:10.1016/j.ccell.2017.03.002
8. Corraliza I, Somovilla-Crespo B, Santamaria S, Garcia-Sanz JA, Kremer L. New strategies using antibody combinations to increase cancer treatment effectiveness. Front Immunol (2017).
9. Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature (2001) 414(6859):105–11. doi:10.1038/35102167
10. Morrison SJ, Weissman IL. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity (1994) 1(8):661–73. doi:10.1016/1074-7613(94)90037-X
11. Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science (1996) 273(5272):242–5. doi:10.1126/science.273.5272.242
12. Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, et al. Generation of a functional mammary gland from a single stem cell. Nature (2006) 439(7072):84–8. doi:10.1038/nature04372
13. Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, et al. Purification and unique properties of mammary epithelial stem cells. Nature (2006) 439:993–7. doi:10.1038/nature04496
14. Fuentealba LC, Rompani SB, Parraguez JI, Obernier K, Romero R, Cepko CL, et al. Embryonic origin of postnatal neural stem cells. Cell (2015) 161(7):1644–55. doi:10.1016/j.cell.2015.05.041
15. Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci (2009) 32:149–84. doi:10.1146/annurev.neuro.051508.135600
16. Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature (2007) 449(7165):1003–7. doi:10.1038/nature06196
17. Morrison SJ, Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature (2006) 441(7097):1068–74. doi:10.1038/nature04956
18. Diaz-Guerra E, Lillo MA, Santamaria S, Garcia-Sanz JA. Intrinsic cues and hormones control mouse mammary epithelial tree size. FASEB J (2012) 26(9):3844–53. doi:10.1096/fj.11-200782
19. Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature (1994) 367(6464):645–8. doi:10.1038/367645a0
20. Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A (2003) 100(7):3983–8. doi:10.1073/pnas.0530291100
21. Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature (2004) 432(7015):396–401. doi:10.1038/nature03128
22. Chen J, Li Y, Yu TS, McKay RM, Burns DK, Kernie SG, et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature (2012) 488(7412):522–6. doi:10.1038/nature11287
23. Driessens G, Beck B, Caauwe A, Simons BD, Blanpain C. Defining the mode of tumour growth by clonal analysis. Nature (2012) 488(7412):527–30. doi:10.1038/nature11344
24. Schepers AG, Snippert HJ, Stange DE, van den Born M, van Es JH, van de Wetering M, et al. Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science (2012) 337(6095):730–5. doi:10.1126/science.1224676
25. Zomer A, Inge Johanna Ellenbroek S, Ritsma L, Beerling E, Vrisekoop N, van Rheenen J. Intravital imaging of cancer stem cell plasticity in mammary tumors. Stem Cells (2013) 31:602–6. doi:10.1002/stem.1296
26. Massard C, Deutsch E, Soria JC. Tumour stem cell-targeted treatment: elimination or differentiation. Ann Oncol (2006) 17(11):1620–4. doi:10.1093/annonc/mdl074
27. Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer (2003) 3(12):895–902. doi:10.1038/nrc1232
28. Feng D, Wang N, Hu J, Li W. Surface markers of hepatocellular cancer stem cells and their clinical potential. Neoplasma (2014) 61(5):505–13. doi:10.4149/neo_2014_061
29. Meng X, Wang X, Wang Y. More than 45% of A549 and H446 cells are cancer initiating cells: evidence from cloning and tumorigenic analyses. Oncol Rep (2009) 21(4):995–1000. doi:10.3892/or_00000314
30. Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, et al. Identification and expansion of human colon-cancer-initiating cells. Nature (2007) 445(7123):111–5. doi:10.1038/nature05384
31. Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res (2005) 65(23):10946–51. doi:10.1158/0008-5472.CAN-05-2018
32. Li X, Zhao H, Gu J, Zheng L. Prognostic value of cancer stem cell marker CD133 expression in pancreatic ductal adenocarcinoma (PDAC): a systematic review and meta-analysis. Int J Clin Exp Pathol (2015) 8(10):12084–92.
33. Zhang SN, Huang FT, Huang YJ, Zhong W, Yu Z. Characterization of a cancer stem cell-like side population derived from human pancreatic adenocarcinoma cells. Tumori (2010) 96(6):985–92.
34. Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A (2007) 104(3):973–8. doi:10.1073/pnas.0610117104
35. Matsui W, Huff CA, Wang Q, Malehorn MT, Barber J, Tanhehco Y, et al. Characterization of clonogenic multiple myeloma cells. Blood (2004) 103(6):2332–6. doi:10.1182/blood-2003-09-3064
36. Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S, et al. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res (2005) 65(20):9328–37. doi:10.1158/0008-5472.CAN-05-1343
37. Gilbert CA, Ross AH. Cancer stem cells: cell culture, markers, and targets for new therapies. J Cell Biochem (2009) 108(5):1031–8. doi:10.1002/jcb.22350
38. Cheng JX, Liu BL, Zhang X. How powerful is CD133 as a cancer stem cell marker in brain tumors? Cancer Treat Rev (2009) 35(5):403–8. doi:10.1016/j.ctrv.2009.03.002
39. Gal H, Pandi G, Kanner AA, Ram Z, Lithwick-Yanai G, Amariglio N, et al. MIR-451 and Imatinib mesylate inhibit tumor growth of glioblastoma stem cells. Biochem Biophys Res Commun (2008) 376(1):86–90. doi:10.1016/j.bbrc.2008.08.107
40. Niu CS, Li MW, Ni YF, Chen JM, Mei JM, Li J, et al. Effect of all-trans retinoic acid on the proliferation and differentiation of brain tumor stem cells. J Exp Clin Cancer Res (2010) 29:113. doi:10.1186/1756-9966-29-113
41. Oka N, Soeda A, Noda S, Iwama T. Brain tumor stem cells from an adenoid glioblastoma multiforme. Neurol Med Chir (Tokyo) (2009) 49(4):146–50; discussion 50–1. doi:10.2176/nmc.49.146
42. Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell (2007) 1(5):555–67. doi:10.1016/j.stem.2007.08.014
43. O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature (2007) 445(7123):106–10. doi:10.1038/nature05372
44. Yeung TM, Gandhi SC, Wilding JL, Muschel R, Bodmer WF. Cancer stem cells from colorectal cancer-derived cell lines. Proc Natl Acad Sci U S A (2010) 107(8):3722–7. doi:10.1073/pnas.0915135107
45. Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A (2007) 104(24):10158–63. doi:10.1073/pnas.0703478104
46. Grange C, Tapparo M, Collino F, Vitillo L, Damasco C, Deregibus MC, et al. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res (2011) 71(15):5346–56. doi:10.1158/0008-5472.CAN-11-0241
47. Guzman ML, Jordan CT. Considerations for targeting malignant stem cells in leukemia. Cancer Control (2004) 11(2):97–104. doi:10.1177/107327480401100216
48. Rountree CB, Senadheera S, Mato JM, Crooks GM, Lu SC. Expansion of liver cancer stem cells during aging in methionine adenosyltransferase 1A-deficient mice. Hepatology (2008) 47(4):1288–97. doi:10.1002/hep.22141
49. Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai P, et al. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell (2008) 13(2):153–66. doi:10.1016/j.ccr.2008.01.013
50. Ho MM, Ng AV, Lam S, Hung JY. Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res (2007) 67(10):4827–33. doi:10.1158/0008-5472.CAN-06-3557
51. Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A, et al. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ (2008) 15(3):504–14. doi:10.1038/sj.cdd.4402283
52. Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell (2005) 121(6):823–35. doi:10.1016/j.cell.2005.03.032
53. Moreira AL, Gonen M, Rekhtman N, Downey RJ. Progenitor stem cell marker expression by pulmonary carcinomas. Mod Pathol (2010) 23(6):889–95. doi:10.1038/modpathol.2010.68
54. Klein WM, Wu BP, Zhao S, Wu H, Klein-Szanto AJ, Tahan SR. Increased expression of stem cell markers in malignant melanoma. Mod Pathol (2007) 20(1):102–7. doi:10.1038/modpathol.3800720
55. Matsui W, Wang Q, Barber JP, Brennan S, Smith BD, Borrello I, et al. Clonogenic multiple myeloma progenitors, stem cell properties, and drug resistance. Cancer Res (2008) 68(1):190–7. doi:10.1158/0008-5472.CAN-07-3096
56. Suzuki S, Terauchi M, Umezu T, Kajiyama H, Shibata K, Nawa A, et al. Identification and characterization of cancer stem cells in ovarian yolk sac tumors. Cancer Sci (2010) 101(10):2179–85. doi:10.1111/j.1349-7006.2010.01672.x
57. Gao MQ, Choi YP, Kang S, Youn JH, Cho NH. CD24+ cells from hierarchically organized ovarian cancer are enriched in cancer stem cells. Oncogene (2010) 29(18):2672–80. doi:10.1038/onc.2010.35
58. Steg AD, Bevis KS, Katre AA, Ziebarth A, Dobbin ZC, Alvarez RD, et al. Stem cell pathways contribute to clinical chemoresistance in ovarian cancer. Clin Cancer Res (2012) 18(3):869–81. doi:10.1158/1078-0432.CCR-11-2188
59. Simeone DM. Pancreatic cancer stem cells: implications for the treatment of pancreatic cancer. Clin Cancer Res (2008) 14(18):5646–8. doi:10.1158/1078-0432.CCR-08-0584
60. Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, et al. Identification of pancreatic cancer stem cells. Cancer Res (2007) 67(3):1030–7. doi:10.1158/0008-5472.CAN-06-2030
61. Balla MM, Vemuganti GK, Kannabiran C, Honavar SG, Murthy R. Phenotypic characterization of retinoblastoma for the presence of putative cancer stem-like cell markers by flow cytometry. Invest Ophthalmol Vis Sci (2009) 50(4):1506–14. doi:10.1167/iovs.08-2356
62. Patel GK, Yee CL, Terunuma A, Telford WG, Voong N, Yuspa SH, et al. Identification and characterization of tumor-initiating cells in human primary cutaneous squamous cell carcinoma. J Invest Dermatol (2012) 132(2):401–9. doi:10.1038/jid.2011.317
63. Fagerberg L, Hallstrom BM, Oksvold P, Kampf C, Djureinovic D, Odeberg J, et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics (2014) 13(2):397–406. doi:10.1074/mcp.M113.035600
64. Wong DJ, Liu H, Ridky TW, Cassarino D, Segal E, Chang HY. Module map of stem cell genes guides creation of epithelial cancer stem cells. Cell Stem Cell (2008) 2(4):333–44. doi:10.1016/j.stem.2008.02.009
65. Trenevska I, Li D, Banham AH. Therapeutic antibodies against intracellular tumor antigens. Front Immunol (2017) 8:1001. doi:10.3389/fimmu.2017.01001
66. Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med (2006) 12(10):1167–74. doi:10.1038/nm1483
67. Liu K, Zhu M, Huang Y, Wei S, Xie J, Xiao Y. CD123 and its potential clinical application in leukemias. Life Sci (2015) 122:59–64. doi:10.1016/j.lfs.2014.10.013
68. Jin L, Lee EM, Ramshaw HS, Busfield SJ, Peoppl AG, Wilkinson L, et al. Monoclonal antibody-mediated targeting of CD123, IL-3 receptor alpha chain, eliminates human acute myeloid leukemic stem cells. Cell Stem Cell (2009) 5(1):31–42. doi:10.1016/j.stem.2009.04.018
69. Kikushige Y, Shima T, Takayanagi S, Urata S, Miyamoto T, Iwasaki H, et al. TIM-3 is a promising target to selectively kill acute myeloid leukemia stem cells. Cell Stem Cell (2010) 7(6):708–17. doi:10.1016/j.stem.2010.11.014
70. NCI. (2017). Available from: https://clinicaltrials.gov/ct2/home
71. Del Valle I, Elvira G, Garcia-Benzaquen L, Armesilla-Diaz A, Kremer L, Garcia-Sanz JA, et al. Characterization of novel monoclonal antibodies able to identify neurogenic niches and arrest neurosphere proliferation and differentiation. Neuroscience (2010) 169(3):1473–85. doi:10.1016/j.neuroscience.2010.04.058
72. Elvira G, Garcia I, Gallo J, Benito M, Montesinos P, Holgado-Martin E, et al. Detection of mouse endogenous type B astrocytes migrating towards brain lesions. Stem Cell Res (2015) 14(1):114–29. doi:10.1016/j.scr.2014.11.006
73. Santamaria S, Garcia-Sanz JA. Insights of the brain damage response using antibodies identifying surface antigens on neural stem cells and neuroblasts. Neural Regen Res (2015) 10(10):1574–5. doi:10.4103/1673-5374.165266
74. Alkilany AM, Thompson LB, Boulos SP, Sisco PN, Murphy CJ. Gold nanorods: their potential for photothermal therapeutics and drug delivery, tempered by the complexity of their biological interactions. Adv Drug Deliv Rev (2012) 64(2):190–9. doi:10.1016/j.addr.2011.03.005
75. Deeley RG, Westlake C, Cole SP. Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol Rev (2006) 86(3):849–99. doi:10.1152/physrev.00035.2005
76. Li Y, Atkinson K, Zhang T. Combination of chemotherapy and cancer stem cell targeting agents: preclinical and clinical studies. Cancer Lett (2017) 396:103–9. doi:10.1016/j.canlet.2017.03.008
77. Fischer M, Yen WC, Kapoun AM, Wang M, O’Young G, Lewicki J, et al. Anti-DLL4 inhibits growth and reduces tumor-initiating cell frequency in colorectal tumors with oncogenic KRAS mutations. Cancer Res (2011) 71(5):1520–5. doi:10.1158/0008-5472.CAN-10-2817
78. McGowan PM, Simedrea C, Ribot EJ, Foster PJ, Palmieri D, Steeg PS, et al. Notch1 inhibition alters the CD44hi/CD24lo population and reduces the formation of brain metastases from breast cancer. Mol Cancer Res (2011) 9(7):834–44. doi:10.1158/1541-7786.MCR-10-0457
79. He B, Reguart N, You L, Mazieres J, Xu Z, Lee AY, et al. Blockade of Wnt-1 signaling induces apoptosis in human colorectal cancer cells containing downstream mutations. Oncogene (2005) 24(18):3054–8. doi:10.1038/sj.onc.1208511
80. Feldmann G, Fendrich V, McGovern K, Bedja D, Bisht S, Alvarez H, et al. An orally bioavailable small-molecule inhibitor of Hedgehog signaling inhibits tumor initiation and metastasis in pancreatic cancer. Mol Cancer Ther (2008) 7(9):2725–35. doi:10.1158/1535-7163.MCT-08-0573
81. Moreb JS, Ucar-Bilyeu DA, Khan A. Use of retinoic acid/aldehyde dehydrogenase pathway as potential targeted therapy against cancer stem cells. Cancer Chemother Pharmacol (2017) 79(2):295–301. doi:10.1007/s00280-016-3213-5
82. van Gils N, Verhagen H, Smit L. Reprogramming acute myeloid leukemia into sensitivity for retinoic-acid-driven differentiation. Exp Hematol (2017) 52:12–23. doi:10.1016/j.exphem.2017.04.007
83. Wu MJ, Kim MR, Chen YS, Yang JY, Chang CJ. Retinoic acid directs breast cancer cell state changes through regulation of TET2-PKCzeta pathway. Oncogene (2017) 36(22):3193–206. doi:10.1038/onc.2016.467
84. Cimmino L, Dolgalev I, Wang Y, Yoshimi A, Martin GH, Wang J, et al. Restoration of TET2 function blocks aberrant self-renewal and leukemia progression. Cell (2017) 170:1079–95.e20. doi:10.1016/j.cell.2017.07.032
85. Xu L. Cancer stem cell in the progression and therapy of pancreatic cancer. Front Biosci (Landmark Ed) (2013) 18:795–802. doi:10.2741/4143
86. Philips GK, Atkins M. Therapeutic uses of anti-PD-1 and anti-PD-L1 antibodies. Int Immunol (2015) 27(1):39–46. doi:10.1093/intimm/dxu095
87. Vela M, Aris M, Llorente M, Garcia-Sanz JA, Kremer L. Chemokine receptor-specific antibodies in cancer immunotherapy: achievements and challenges. Front Immunol (2015) 6:12. doi:10.3389/fimmu.2015.00012
88. Pang MF, Georgoudaki AM, Lambut L, Johansson J, Tabor V, Hagikura K, et al. TGF-beta1-induced EMT promotes targeted migration of breast cancer cells through the lymphatic system by the activation of CCR7/CCL21-mediated chemotaxis. Oncogene (2016) 35(6):748–60. doi:10.1038/onc.2015.133
89. Zhong G, Chen L, Yin R, Qu Y, Bao Y, Xiao Q, et al. Chemokine (CC motif) ligand 21/CC chemokine receptor type 7 triggers migration and invasion of human lung cancer cells by epithelial-mesenchymal transition via the extracellular signal-regulated kinase signaling pathway. Mol Med Rep (2017) 15(6):4100–8. doi:10.3892/mmr.2017.6534
90. Zhou M, Wang S, Hu L, Liu F, Zhang Q, Zhang D. miR-199a-5p suppresses human bladder cancer cell metastasis by targeting CCR7. BMC Urol (2016) 16(1):64. doi:10.1186/s12894-016-0181-3
91. Boyle ST, Gieniec KA, Gregor CE, Faulkner JW, McColl SR, Kochetkova M. Interplay between CCR7 and Notch1 axes promotes stemness in MMTV-PyMT mammary cancer cells. Mol Cancer (2017) 16(1):19. doi:10.1186/s12943-017-0592-0
92. Lun SW, Cheung ST, Cheung PF, To KF, Woo JK, Choy KW, et al. CD44+ cancer stem-like cells in EBV-associated nasopharyngeal carcinoma. PLoS One (2012) 7(12):e52426. doi:10.1371/journal.pone.0052426
93. Lu L, Tao H, Chang AE, Hu Y, Shu G, Chen Q, et al. Cancer stem cell vaccine inhibits metastases of primary tumors and induces humoral immune responses against cancer stem cells. Oncoimmunology (2015) 4(3):e990767. doi:10.4161/2162402X.2014.990767
94. Tu Z, Xie S, Xiong M, Liu Y, Yang X, Tembo KM, et al. CXCR4 is involved in CD133-induced EMT in non-small cell lung cancer. Int J Oncol (2017) 50(2):505–14. doi:10.3892/ijo.2016.3812
95. Fujita T, Chiwaki F, Takahashi RU, Aoyagi K, Yanagihara K, Nishimura T, et al. Identification and characterization of CXCR4-positive gastric cancer stem cells. PLoS One (2015) 10(6):e0130808. doi:10.1371/journal.pone.0130808
96. Gassenmaier M, Chen D, Buchner A, Henkel L, Schiemann M, Mack B, et al. CXC chemokine receptor 4 is essential for maintenance of renal cell carcinoma-initiating cells and predicts metastasis. Stem Cells (2013) 31(8):1467–76. doi:10.1002/stem.1407
97. Chaffer CL, Marjanovic ND, Lee T, Bell G, Kleer CG, Reinhardt F, et al. Poised chromatin at the ZEB1 promoter enables breast cancer cell plasticity and enhances tumorigenicity. Cell (2013) 154(1):61–74. doi:10.1016/j.cell.2013.06.005
Keywords: cancer stem cells, therapeutic antibodies, immunotherapy, effective cancer therapies, cancer genetics
Citation: Santamaria S, Delgado M, Kremer L and Garcia-Sanz JA (2017) Will a mAb-Based Immunotherapy Directed against Cancer Stem Cells Be Feasible? Front. Immunol. 8:1509. doi: 10.3389/fimmu.2017.01509
Received: 31 August 2017; Accepted: 25 October 2017;
Published: 09 November 2017
Edited by:
Sherven Sharma, VA Greater Los Angeles Healthcare System (VHA), United StatesReviewed by:
Kawaljit Kaur, University of California, Los Angeles, United StatesDaniel Olive, Institut national de la santé et de la recherche médicale, France
Copyright: © 2017 Santamaria, Delgado, Kremer and Garcia-Sanz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jose A. Garcia-Sanz, jasanz@cib.csic.es
 Silvia Santamaria
Silvia Santamaria Marisa Delgado
Marisa Delgado Leonor Kremer
Leonor Kremer Jose A. Garcia-Sanz
Jose A. Garcia-Sanz