- 1Oxford Vaccine Group, Department of Paediatrics, University of Oxford, The NIHR Oxford Biomedical Research Centre, Oxford, United Kingdom
- 2Statens Serum Institut, Copenhagen, Denmark
- 3Department of Infection, Immunity and Cardiovascular Disease, University of Sheffield, Sheffield, United Kingdom
- 4Nuffield Department of Primary Care Health Sciences, Clinical Trials Unit, University of Oxford, Oxford, United Kingdom
- 5Weatherall Institute of Molecular Medicine, University of Oxford, Oxford, United Kingdom
- 6The Hospital for Tropical Diseases, Wellcome Trust Major Overseas Programme, Oxford University Clinical Research Unit, Ho Chi Minh City, Vietnam
- 7Centre for Tropical Medicine and Global Health, Nuffield Department of Medicine, University of Oxford, Oxford, United Kingdom
- 8The Department of Medicine, University of Cambridge, Cambridge, United Kingdom
Effective vaccines against Salmonella Typhi, a major cause of febrile illness in tropical regions, can have a significant effect as a disease control measure. Earlier work has shown that immunization with either of two Salmonella Typhi vaccines, licensed Ty21a or candidate M01ZH09, did not provide full immunity in a controlled human infection model. Here, we describe the human humoral immune responses to these oral vaccines and their functional role in protection after challenge with S. Typhi. Serum, obtained from healthy volunteers before and after vaccination with Ty21a or M01ZH09 or placebo and before and after oral challenge with wild-type S. Typhi, was assessed for bactericidal activity. Single-dose vaccination with M01ZH09 induced an increase in serum bactericidal antibodies (p = 0.001) while three doses of Ty21a did not. No association between bactericidal activity and protection against typhoid after challenge was seen in either vaccine arm. Bactericidal activity after vaccination correlated significantly with delayed disease onset (p = 0.013), lower bacterial burden (p = 0.006), and decreased disease severity scores (p = 0.021). Depletion of antibodies directed against lipopolysaccharide significantly reduced bactericidal activity (p = 0.009). We conclude that antibodies induced after ingestion of oral live-attenuated typhoid vaccines or after challenge with wild-type S. Typhi exhibit bactericidal activity. This bactericidal activity is mediated by anti-O:LPS antibodies and significantly reduces clinical symptoms but does not provide sterile immunity. This directs future vaccine studies toward other antigens or mechanisms of protection against typhoid.
Introduction
Typhoid fever is a systemic infection caused by Salmonella enterica serovar Typhi (S. Typhi) and results in significant morbidity, with an estimated 11.9–26.9 million infections occurring each year (1, 2). The use of vaccination as a public health prevention measure may alleviate significant disease burden, especially as infection arises via human-to-human transmission and there is no known environmental reservoir (3). Currently, both subunit and live oral vaccines against S. Typhi are licensed and recommended for use in high-incidence settings by the WHO (4). The protective efficacy of the Vi (virulence) capsular polysaccharide vaccine in the first year following vaccination is ~69% (5, 6), whereas the live oral Ty21a vaccine offers ~35% protection (5, 7). Neither vaccine is licensed for use in young children and, as one-third of the cases occur under the age of five (1), the development of new vaccines is vital.
To enable the development and evaluation of next-generation typhoid vaccines, improved characterization of the nature of protection afforded by vaccination is required. While some protection against typhoid is thought to be conferred by cell-mediated immunity (8), evidence from serosurveillance and vaccine efficacy trials indicates that O lipopolysaccharide (O:LPS) and flagellin (H) antigens are immunogenic in a dose-dependent manner, and that anti-Vi IgG antibody is protective (6, 9). Further description of the functional role of these antibodies during infection has been evaluated in murine models of S. Typhimurium infection (10) and through in vitro studies after immunization (11, 12). While these studies suggest that antibodies against both Vi and O9:LPS have direct bactericidal activity in the presence of complement, or opsonize ahead of pathogen phagocytosis, extrapolation of such data to a human infection with S. Typhi has limitations (13).
Using a controlled human infection model (CHIM) of typhoid fever, the protective efficacy of two live oral attenuated typhoid vaccines (Ty21a and M01ZH09) was recently assessed relative to placebo (14, 15). Ty21a, a licensed vaccine, and M01ZH09, a candidate vaccine, are derived from the parent strain Ty2. M01ZH09 was constructed by defined, independently attenuated deletion of the ssaV and aroC genes and expresses Vi at negligible levels based on immunogenicity measurements (14, 16). Ty21a contains additional genetic attenuations and does not constitutively express Vi (17). Neither a single-dose M01ZH09 immunization nor three doses of Ty21a provided full immunity in a model where diagnosis of typhoid disease (TD) was defined as confirmed S. Typhi bacteremia or by ≥12 h of sustained fever ≥38°C (14, 15). Here, we explored the functionality of antibodies induced by oral vaccination and the association with protection against typhoid in the challenge model.
Materials and Methods
Ethics and Approvals
A randomized, double-blind, placebo-controlled trial was performed at the Centre for Clinical Vaccinology and Tropical Medicine, Churchill Hospital, Oxford, UK (clinicaltrials.gov NCT679172; EudraCT 2011-000381-35) (14). This study was carried out in accordance with the recommendations of the Oxford University Clinical Trials and Research Governance Department, and the protocol approved by NRES South Central––Oxford A (11/SC/0302) and conducted in accordance with the declaration of Helsinki (2008) and the International Conference of Harmonization of Good Clinical Practice guidelines. An independent Data Monitoring and Safety Committee monitored the trial. To perform the assays described, human complement was collected in a parallel, prospective observational study (clinicaltrials.gov NCT01945307; 13/SC/0375). All subjects gave written informed consent in accordance with the Declaration of Helsinki.
Study Design, Vaccination, and Challenge
The study design and results of the primary study objectives of the clinical trial have been reported previously (14). Briefly, 99 adult participants were randomly assigned in the ratio 1:1:1 to three study arms: (1) vaccine (a single oral dose of M01ZH09 vaccine containing 1 × 1010 CFU live attenuated S. Typhi M01ZH09 strain), (2) placebo, and (3) comparator (three doses of Ty21a capsules each containing 2 × 109 CFU). Participants were challenged with 104 CFU of wild-type S. Typhi Quailes strain 4 weeks after vaccination (D0) (15). Antibiotic treatment was initiated at typhoid diagnosis (TD) or 14 days after challenge in those participants who were not diagnosed with typhoid fever (nTD). Diagnosis of TD was defined as confirmed S. Typhi bacteremia or by ≥12 h of sustained fever ≥38°C (14, 15) and full protection was defined as not meeting the criteria for diagnosis (nTD).
Serum Bactericidal Antibody Assay
To prepare a source of human complement, fresh serum from a UK healthy adult donor was depleted of S. Typhi-binding antibodies that may otherwise influence the performance of complement in our assays, as modified from Hart et al. (11, 18). Following three rounds of adsorption with 1010 CFU of log phase S. Typhi Quailes per 1 mL of serum, significant depletion of anti-Vi and anti-O:LPS antibody titers was achieved and demonstrated by ELISA (Table S1 in Supplementary Material), without change in complement activity, as measured by CH100 and AP100 hemolytic assays (data not shown). To prepare aliquots of log-phase S. Typhi Quailes for the serum bactericidal antibody (SBA) assay, an overnight culture, set up from a single colony in Luria Bertani broth (LB), was diluted 33-fold in LB supplemented with 10% sucrose. This was grown at 37°C shaking to achieve 3.5 × 108–3.5 × 109 CFU/mL and frozen in aliquots ahead of use in SBA.
Serum samples were evaluated in the SBA from a subset of participants from whom serum was available and representing similar numbers across all study arms over multiple time points (Placebo n = 18, Ty21a n = 16, M01ZH09 n = 18, TD n = 30, nTD n = 22). Time points were at pre-vaccination (D28), at challenge (D0), and post-challenge days 28 (D28), 60 (D60), and 180 (D180). Briefly, serum samples were de-complemented by heat-inactivation and diluted before addition of 200 CFU of log phase S. Typhi Quailes in Hanks Buffered Saline Solution (HBSS; Life Technologies Ltd., UK) supplemented with 0.5% Bovine Serum Albumin (BSA; Sigma-Aldrich, UK). S. Typhi-specific antibody-depleted human complement serum was added to a final complement concentration of 25% and bacteria incubated for 1 h at 37°C with shaking before plating on tryptic soya agar (TSA) plates (Oxoid Ltd., UK). In each experiment, complement serum and heat-inactivated complement serum alone were included as controls for bacterial survival; test samples without complement were included to assess any complement-independent killing; and a positive control, from another UK healthy donor, to assess inter-plate variability. Only experimental plates in which the positive control was within twofold of its defined SBA titer and with >60% overlap in CFU counts between the complement only and heat-inactivated complement only controls were accepted. The SBA titer was defined as the reciprocal lowest dilution of test serum to achieve ≤50% bacterial killing relative to complement alone. The slide agglutination test using Salmonella Vi anti-sera (Oxoid Ltd., UK) confirmed that Vi was expressed in the bacterial working stock. In nTD participants bactericidal activity a month after challenge (D28) was not measured, as preliminary experiments confirmed complete bactericidal activity consistent with residual ciprofloxacin in the samples as expected based on its pharmacokinetic profile (D28 coincided with the last day of antibiotic treatment for this subset) (19).
Antigen Sources and Purification
Lipopolysaccharide (O9:LPS) was from Sigma-Aldrich, UK (L2387, >99% free of protein) and Vi capsular polysaccharide was from Sanofi Pasteur, UK (Typhim Vi®). Flagellin (H) was purified from a flagellin-deficient S. Enteritidis strain that expresses S. Typhi H:d from a plasmid (University of Maryland, USA) (14, 20). Cytolethal distending toxin B (CdtB) and pilus control protein L (PilL) coding sequences lacking transmembrane domains were PCR-amplified from S. Typhi CT18 strain genomic DNA, cloned into plasmid pET28b(+) (Novagen, UK) and expressed in Escherichia coli BL21(DE3)pLysS (Promega, WI, USA). Recombinant His-tagged CdtB and PilL were purified by tagging with nickel-coated agarose beads (Ni-NTA, Invitrogen) and elution from gravity flow columns (Qiagen, Germany). CdtB was renatured in 50-mM sodium phosphate solution and 500-mM NaCl. hlyE was PCR-amplified from Ty21a, cloned into pET21a vector (Novagen, UK), and expressed in an LPS-modified E. coli BL21 strain to produce endotoxin free proteins (Lucigen, ClearCoil BL21 cells). Recombinant His-tagged hemolysin E (HlyE) was purified using HisTrap nickel-affinity column (GE Healthcare) followed by desalting on a HiPrep 26/10 (GE Healthcare).
ELISA
Immunoglobulin G (IgG), IgA, and IgM isotype responses to O9:LPS, H, HlyE, CdtB, and PilL were measured in serum as previously described (15, 21), with some modifications regarding antigen source and controls. Nominal ELISA units were calculated based on standard curves derived from pooled sera collected from the highest responders to O-antigen following vaccination with M01ZH09 (Emergent BioSolutions, Reading, UK). In addition, Vi-specific IgG were measured at D28, D0, and D28 using a commercial ELISA kit (VaccZyme™ Human anti-S. Typhi Vi IgG Enzyme Immunoassay Kit, The Binding Site Ltd., Birmingham, UK) following the manufacturer’s instructions.
Antigen-Specific and Isotype-Specific Antibody-Depletion of Sera
In a subset of samples representing all study arms and both TD and nTD groups, antibodies specific to H or O9:LPS, or total IgA or IgG antibodies were depleted from the serum to assess the contributions of each of these antibodies to the bactericidal activity in sera. Antibodies specific to H or O9:LPS were adsorbed on to 96-well Maxisorp plates (Nunc, Copenhagen, Denmark) pre-coated with 1 µg/mL H or 15 µg/mL O9:LPS antigen in carbonate bicarbonate buffer (Sigma, UK), for eight rounds of 30-min incubations. A plate coated with BSA was used to create mock depleted controls. Total IgA and IgG antibodies were depleted using the Dynabeads Antibody coupling kit (Life Technologies Limited, UK). Briefly, each diluted serum sample was incubated for 1 h with 2-mg magnetic beads coated with either 10 µg of goat polyclonal IgG antibody raised against human IgA or IgG (AbD Serotec, UK), or an goat polyclonal IgG (Santa Cruz Biotechnology, USA). The depletions resulted in approximately 56% (H), 87% (O9:LPS), 72% (IgA), and 93% (IgG) median reduction in the antibody titer, as measured with ELISA.
ELISpot
Antibody-secreting cell (ASC) responses to LPS, H, and Vi were measured at D28, 7 days after vaccination (D21), before challenge (D0), Day 7 after challenge (D7), and 48 h after the typhoid diagnosis visit (TD + 48) by ELISpot assay, as described in Ref. (14). Spots were photographed using the AID ELISpot optical reader (AID ELR03m, Autoimmun Diagnostika GmbH, Germany), manually counted by two observers, and expressed as spots per 106 PBMCs. Zero ASC counts were given a nominal value of 0.1 and 0.1 added to all other observations.
Clinical and Microbiological Data Collection
Daily clinical review and temperature measurements were carried out for 7 days after vaccination and 28 days after challenge. In addition, participants collected symptom data daily (solicited for headache, feeling generally unwell, loss of appetite, abdominal pain, nausea/vomiting, myalgia, arthralgia, cough, diarrhea and constipation, and any unsolicited symptoms) (14). Symptom severity scores were calculated by summing maximum severity values assigned to each of up to 10 symptoms experienced daily during 14 days after challenge and deriving the mean (14).
Blood (10 mL) samples were collected for bacterial culture at each visit. Cultures were performed by the local hospital accredited pathology laboratories according to national standard operating procedures (22–24), and as previously described (14, 15). Quantitative blood culture samples were collected immediately prior to antibiotic treatment by inoculation of 10-mL blood into an ISOLATOR 10 tube (Alere, UK).
Cytokine Quantification
Cytokines were quantified in lithium-heparin plasma using a multiplex bead-based ELISA (Milliplex® Human cytokine/chemokine magnetic bead panel, Merck Millipore, UK), following the manufacturer’s protocol with dilution of beads by twofold. Analytes tested were as follows: EGF, Fractalkine (CX3CL1), GROα (CXCL1), IFNα2, IFNγ, IL1β, IL1RA, IL2, IL6, IL8 (CXCL8), IL10, IL12p40, IL15, IL17A, IP10 (CXCL10), sCD40L, TGFα, TNFα, and VEGF. All samples were run in duplicate and were measured on a Luminex MAGPIX instrument (Luminex, Netherlands). Values were calculated from the average of duplicates which were above cytokine sensitivity limits and whose %CV was <30%.
Statistical Analysis
SBA titers, ELISA antibody levels, and ASC counts were log10-transformed before statistical analysis. Within groups, the log10-transformed data were still skewed so non-parametric tests were used. All reported p-values are based on two-sided tests, with significance assumed at p < 0.05, without multiple testing corrections. Clinical, laboratory, and immunological data were collated using a clinical-trial database (OpenClinica, version 3.1). Data analysis was performed using R version 3.2.1 (25).
Results
Kinetics of Bactericidal Antibody Induction and Decay
Sera collected pre-vaccination in all study participants showed bactericidal activity against wild-type S. Typhi, with a 2–log10 range in titers but without significant differences between vaccine arms (Figure 1A; Table S2 in Supplementary Material). One month after vaccination, at D0, no increase in bactericidal activity was observed in either placebo or Ty21a recipients (Figure 1A). In contrast, a significant increase in bactericidal activity was measured 28 days after M01ZH09 vaccination (p = 0.001). This increase was principally attributable to the subset of M01ZH09 recipients who subsequently went on to be TD after challenge (p = 0.009, Figure 1B).
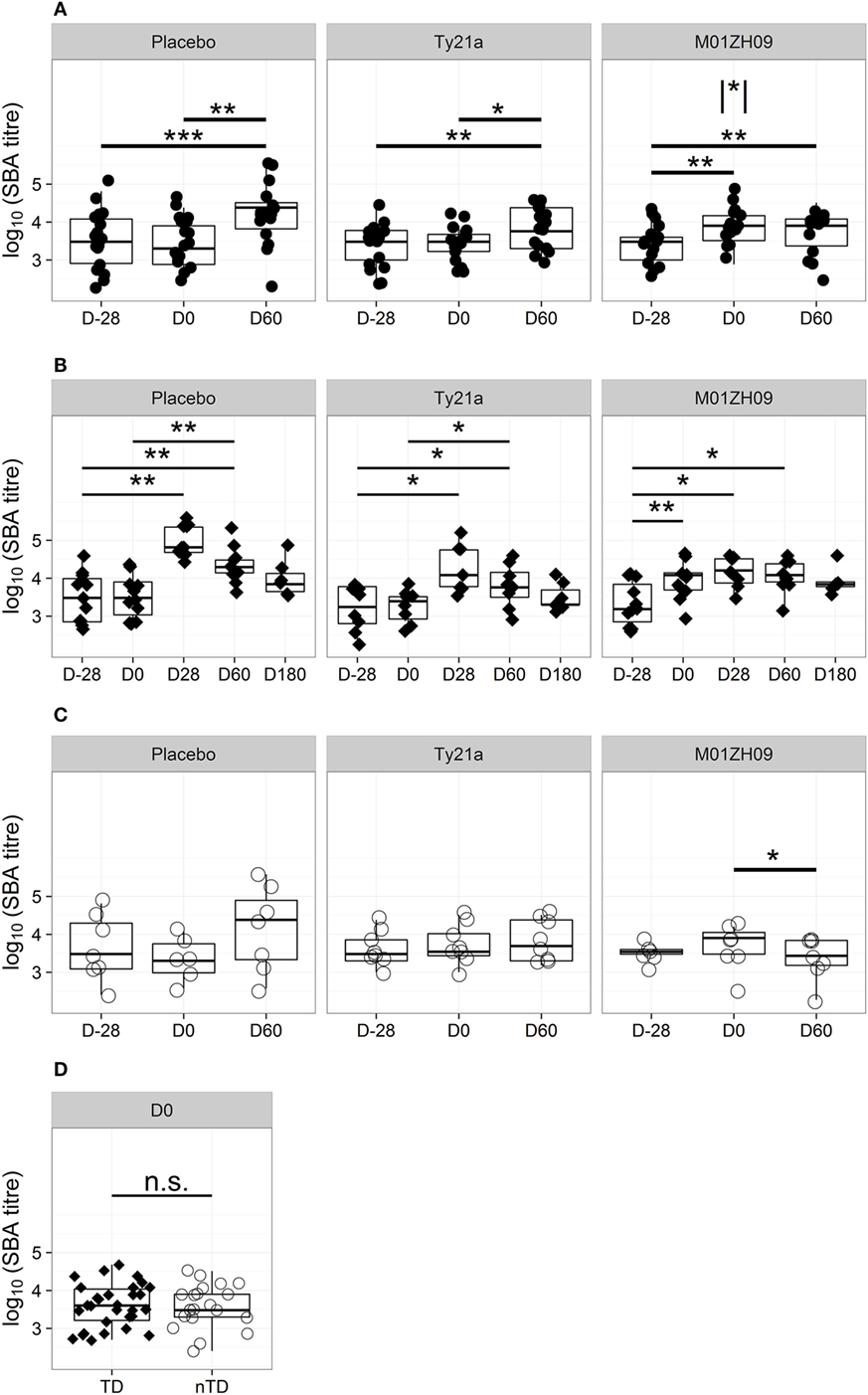
Figure 1. Kinetics of bactericidal antibody induction and decay. (A) Serum bactericidal antibody (SBA) titers in each study arm at D28 (pre-vaccination baseline), D0 (day of challenge), and D60 after challenge. (B) SBA titer kinetics after challenge in TD participants (filled diamonds) from each study arm. (C) SBA titers on D28, D0, and D60 after challenge in each study arm, shown for those with no typhoid diagnosis (nTD, empty circles). (D) SBA titers on the day of challenge, in those with typhoid diagnosis (TD) and nTD across all study arms. n.s., not significant, * p < 0.05; ** p < 0.01; *** p < 0.001. Asterisk within vertical lines indicates significance of pair-wise comparison between M01ZH09 and Ty21a vaccine arms for D0. Significance was determined by Wilcoxon signed rank test for paired value comparisons and by Mann–Whitney U-tests between groups.
To identify differences in the immune responses induced by attenuated vaccines and wild-type S. Typhi, the effect of exposure to the challenge agent on levels of bactericidal antibody activity was examined. Sixty days after challenge bactericidal antibody activity was significantly higher than on D0 in the placebo and Ty21a arms in participants with TD after challenge (p = 0.009 and p = 0.035, respectively; Figure 1B). The bactericidal activity at D60 in sera from nTD placebo and nTD Ty21a recipients showed marked variability in inter-individual responses. In contrast, D60 titers in M01ZH09 recipients remained unchanged from D0 in TD participants and waned in nTD participants (p = 0.036; Figure 1C).
Evaluation of bactericidal activity in sera from TD individuals over a longer time course confirmed that exposure to the challenge strain-induced bactericidal antibodies only in placebo and Ty21a recipients (Figure 1B). In both groups, titers peaked at D28 after challenge and returned to near pre-vaccination baseline levels by D180. M01ZH09-vaccinated TD participants had bactericidal titers persistently higher than baseline for at least 60 days after challenge, despite the apparent absence of any boosting effect by exposure to/infection with wild-type challenge.
The Impact of Bactericidal Antibody Activity on Challenge Outcome
We hypothesized that the presence of circulating bactericidal antibodies on the day of challenge may have a protective effect on challenge outcome. However, comparison of the bactericidal antibody titers between TD and nTD participants at D0 demonstrated no significant difference in protection (Figure 1D), even when assessed by vaccine allocation (Figure S1A in Supplementary Material). The similar titer ranges between TD and nTD groups indicated that serum bactericidal activity at D0, resulting either from preexisting exposure or vaccine induction, did not protect against TD.
The D0 bactericidal antibody titers in TD participants were positively correlated, however, with time to typhoid diagnosis after challenge, when vaccination arms were combined (Figure 2A). Furthermore, D0 bactericidal antibody titer in these participants negatively correlated with quantitative bacterial blood count at diagnosis and with cumulative symptom severity score following challenge, but not with temperature (Figures 2B,C; Table 1). We found no significant correlations when placebo TD was similarly analyzed (Table 1). Taken together, these observations suggest that higher bactericidal activity delayed the onset of infection and reduced disease severity in vaccinated participants.
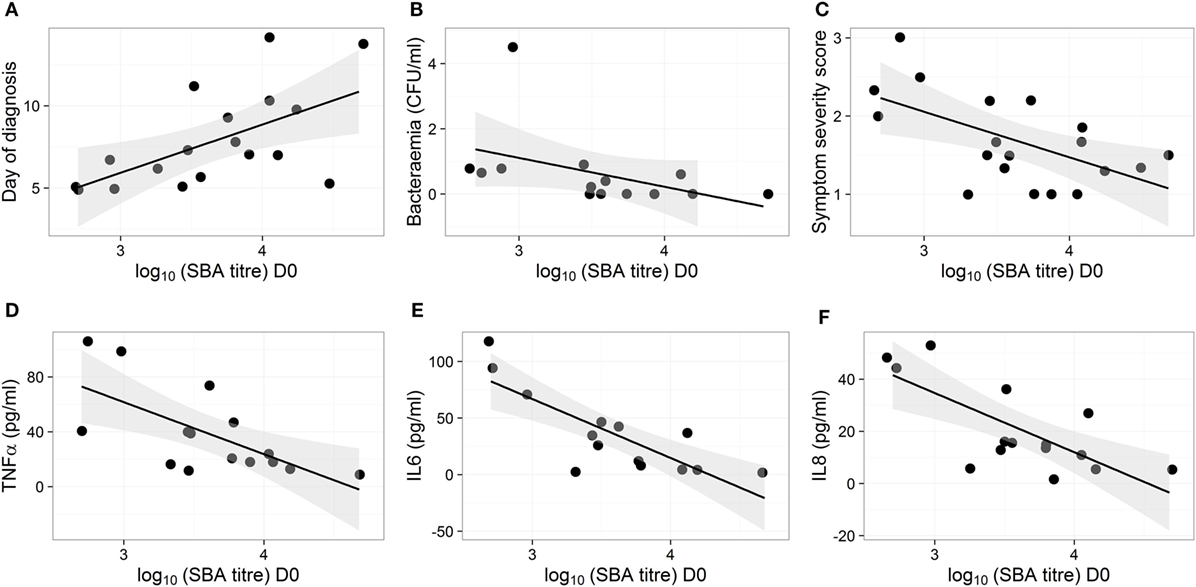
Figure 2. Bactericidal antibodies do not protect against typhoid infection but high bactericidal antibody activity reduces disease severity and cytokine response in vaccinated participants. (A–F) Correlation of disease parameters and plasma cytokine levels after diagnosis with SBA titers on D0 in participants from the Ty21a and M01ZH09 vaccine arms. Shown are day of diagnosis (A), bacterial load in blood on the day of diagnosis (B), symptom severity score (C), and maximum concentrations of cytokines TNFα, IL6, and IL8 [(D–F), respectively], in plasma samples collected within 48 h from diagnosis.
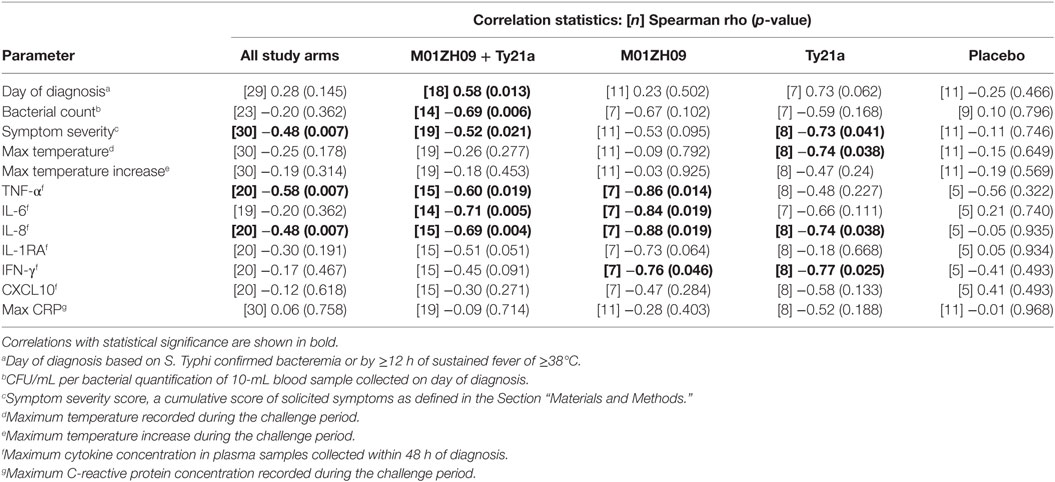
Table 1. Statistical output following correlations of D0 bactericidal titers with clinical and immunological data in TD groups.
The concentrations of various plasma cytokines associated with the acute phase of infection after S. Typhi challenge (26) were measured and analyzed to evaluate the impact of bactericidal activity on other events in disease pathogenesis. TNFα, IL6 and IL8 were negatively correlated with the D0 bactericidal titers in the vaccinated participants (Figures 2D–F; Table 1). However, CRP did not correlate with D0 titers (Table 1). There was no association between D0 titers and these cytokines for the placebo group (Table 1). These data corroborate an effect of bactericidal antibody activity in reducing the inflammatory response during infection in live oral vaccine recipients. This was further supported by a vaccine subgroup analysis, which demonstrated stronger correlations in the M01ZH09 arm in whom the highest D0 bactericidal titers were observed (Table 1).
Anti-LPS Antibodies Are the Key Mediators of Bactericidal Activity
The concentrations of IgG and IgA to assorted S. Typhi antigens were measured to elucidate the antigen specificity of functional antibodies. Other than O9:LPS, flagellin, and Vi polysaccharide that are known key S. Typhi surface epitopes, we also tested responses to hemolysin E (HlyE), cytolethal distending protein subunit B (CdtB) and pilus control protein L (PilL). In antigen array experiments these antigens were previously found to be the dominant targets of circulating antibodies in patients with acute typhoid fever (27, 28). Negligible anti-Vi titers were observed in almost all tested samples (Figure S2A in Supplementary Material). In a mixed-effects model, we identified O9:LPS as being the most likely antigen targeted by bactericidal antibody with some activity also shown to flagellin (Table 2).
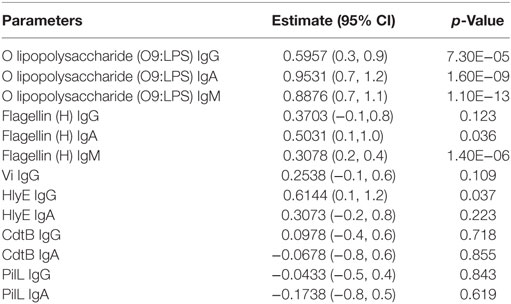
Table 2. Estimates and p-values from linear mixed-effects models of SBA data from all arms and time points to ELISA titers against specific antigens, taking into account random effects for grouping multiple time points from the same participants.
For confirmation, we depleted sera of antigen-specific antibodies prior to assessing bactericidal activity. As shown in Figure 3, depletion of O9:LPS antibodies resulted in significant reduction in bactericidal titers from samples at baseline or at D28 (both p = 0.009), independent of subset. In contrast, depleting antibodies specific to flagellin, or total IgA antibodies, did not affect activity (Figure 3).
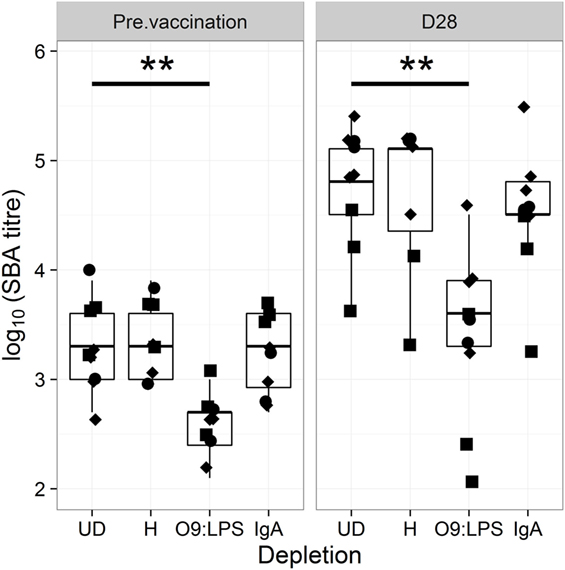
Figure 3. Bactericidal antibodies are specific to O9:LPS. SBA titer pre-vaccination (D28) and at D28 after challenge of samples undepleted (UD), or depleted of anti-H, anti-O9:LPS or total IgA antibodies. Diamonds indicate placebo, circles indicate Ty21a and squares indicate M01ZH09 samples. Significance was determined by Wilcoxon signed rank tests.
Consistent with these bactericidal activity data, the numbers of IgM, IgA, or IgG antibody-secreting cells specific to O9:LPS present in circulation 7 days after vaccination were not associated with protection from typhoid fever (Figures S1B–D in Supplementary Material). Similarly, the D0 IgM, IgA, or IgG antibody titers against O9:LPS or flagellin were not associated with protection against development of disease (Figures S1E–G and S2B–D in Supplementary Material). Anti-O9:LPS antibody titers showed a similar longitudinal profile to bactericidal activity (Figure 4). A notable exception was that anti-O9:LPS IgG significantly increased in Ty21a TD participants following vaccination (p = 0.009 at D0) but not in response to challenge.
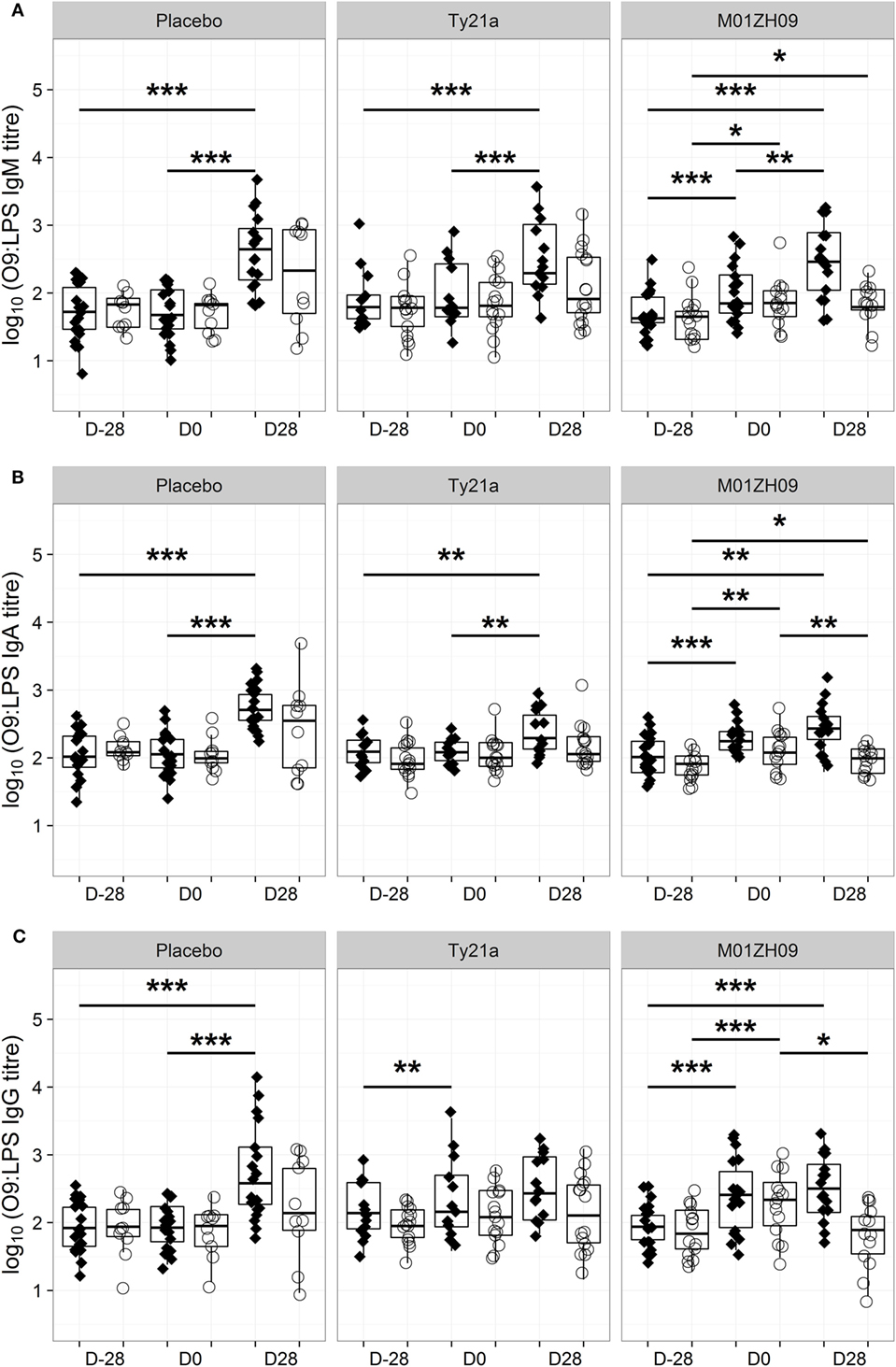
Figure 4. Kinetics of O9:LPS antibody induction. Anti-O9:LPS IgM (A), IgA (B), and IgG (C) antibodies at baseline (D28), on the day of challenge (D0) and at D28, in those with typhoid diagnosis (TD, diamonds), and no typhoid diagnosis (nTD, empty circles) within each study arm. *p < 0.05, **p < 0.01, ***p < 0.001. Significance was determined by Wilcoxon signed rank tests for paired value comparisons.
Discussion
This is the first direct investigation of the association between bactericidal activity and outcome of S. Typhi infection in humans, despite multiple in vivo studies of murine challenge with Salmonella species (10, 29, 30) and typhoid vaccine clinical trials (6, 7). Alongside recent investigations of Shigella spp. and pseudomonas infection (31, 32) this study points to a moderating role for bactericidal antibodies common to multiple bacterial diseases.
We reported previously that vaccination with Ty21a or with M01ZH09-induced IgG antibodies against O9:LPS (14). Further analyses here show that in the Ty21a group this rise in anti-LPS IgG is limited to TD participants. Earlier studies found that vaccination with Ty21a induces anti-LPS IgG antibodies in serum (9) that opsonize ahead of phagocytic killing (12), but their role in complement-mediated bactericidal killing had not been investigated. It is noteworthy that levels of serum bactericidal activity did not significantly change after Ty21a vaccination, even in TD participants, perhaps reflecting the contribution of additional uncharacterized mutations in Ty21a (17). M01ZH09 induced significant rises in anti-O9:LPS IgG, IgA, and IgM titers and in bactericidal activity, consistent with previous immunogenicity studies (16, 33). We observed that these rises were highest in the group with TD after challenge.
Bactericidal activity is an established correlate of protection for Neisseria meningitidis and used in vaccine licensure (34, 35). Bactericidal antibodies have been associated with age-related decrease in incidence of invasive non-typhoidal Salmonella (NTS) disease in African children and an age-dependent increase in bactericidal activity titer has also been documented in areas of high typhoid incidence (36–38). However, in our study the depletion of antibodies against O9:LPS abrogated bactericidal activity so it does not seem likely that either the presence, or bactericidal activity, of anti-LPS antibodies confers protection against invasion by S. Typhi. The data presented here do not support the assumption that these functional antibodies have an important role in sterile immunity against S. Typhi in adults.
Consistent with an inability to prevent disease oral challenge with wild-type S. Typhi in naïve volunteers induced anti-LPS IgG, IgA, and IgM and bactericidal antibodies but the responses were limited to those who developed typhoid infection, as shown in previous studies of anti-LPS antibodies (15, 21). Similarly, Ty21a vaccine recipients who developed typhoid generated anti-LPS IgG and bactericidal antibodies in response to oral challenge. In contrast, in both Ty21a vaccinated and naïve individuals the exposure to LPS during invasive infection did not induce a bactericidal response among those who are challenged, and presumably have at least mucosal exposure to the pathogen, but do not succumb to infection.
The participants who received M01ZH09 vaccine also developed a rise in anti-LPS IgM antibodies following infection but no further rise in anti-LPS IgG, IgA, or bactericidal activity was observed. Repeat vaccination in a relatively short period of time can induce hyporesponsiveness to polysaccharide antigens (39, 40) and anergy to protein superantigens (41), both associated with saturation of the immune response and presumably a negative feedback control to avoid overstimulation of B cells. However, bactericidal antibody titers in the M01ZH09 group were lower than those eventually attained in naive volunteers and Ty21a vaccine recipients. It seems more likely that the reduced bacterial load due to increased bactericidal activity from vaccination resulted in reduced exposure to LPS in this group, thus blunting any further response. This is supported by significantly higher bactericidal titers in the M01ZH09 group than in the Ty21a volunteers at D0.
Variants of O9:LPS antigen are abundant in the Enterobacteriaceae, including gut commensals, which may explain the presence of baseline bactericidal activity and the strong boost upon challenge in the placebo and even the Ty21a subgroups. Consistent with our data, others have shown bactericidal activity resulting from anti-LPS antibodies in sera from mice immunized with S. Paratyphi A ΔguaBA ΔclpX or S. Typhi ΔguaBA ΔhtrA strains or invasive NTS isolates (10, 11, 29, 33). The serum bactericidal activity after vaccination or infection with S. Typhi is likely associated with anti-O9:LPS IgM or IgG complement-binding antibodies (42), as also observed with anti-O:LPS-mediated bactericidal activity against NTS. Although secretory IgA activates the mannan-binding lectin pathway (43), depletion of IgA from our samples did not significantly affect bactericidal activity. In studies examining the role of bactericidal activity against NTS in African adults with or without HIV infection, bactericidal activity was not seen with anti-O:LPS IgA and the bactericidal properties of sera depended on the relative titers of anti-O:LPS isotypes so that high titers of IgA antibodies correlated with absence of bactericidal activity (37, 44). Other mechanisms may be at play for the absence of protection from typhoid challenge we observed.
Despite the absence of protection, among vaccinated participants the delay in onset of infection associated with higher bactericidal antibody titer suggests that these antibodies may have an important role in reducing the bacterial load during infection, either at the point of invasion or during multiplication after the primary bacteremia. Additionally, the observed association with a reduction in disease manifestations and plasma cytokines suggests a role for these antibodies in reducing inflammation and symptom severity. In previous CHIM studies of both typhoid and paratyphoid infection (15, 21), we have shown a relationship between challenge dose and duration of the incubation period as well as a challenge dose-dependent association with blood bacterial load at diagnosis and anti-O:LPS titers. It is thus possible that the bactericidal activity of antibodies produced by vaccination reduced the bacterial load in the initial stages of infection and this in turn may have delayed disease onset or reduced other disease symptoms. This is consistent with a very recent investigation of shigellosis in a human challenge model and studies on patients with chronic Pseudomonas aeruginosa respiratory infections (31, 32). In these studies reduced SBA activity was associated with increased disease severity using parameters such as stool symptomatology and temperature (32) or lung forced expiratory volume (31). Bactericidal activity may also result in lower bacterial burden in these cases.
Our analysis, which relies on gaining multiple samples from the same individuals, is limited by low statistical power. We were not able to determine the role of anti-Vi antibodies since the immune response to the capsular antigen was negligible. Nevertheless, the data imply that O9:LPS might not be the ideal antigen for new typhoid vaccines as neither the concentration of anti-O9:LPS antibody, frequency of anti-O9:LPS ASC nor bactericidal antibody titers at time of challenge correlated with protection in our model. Recent vaccine development for typhoid fever has focused on Vi-conjugate vaccines, but efforts to develop vaccines against invasive NTS strains (30, 45) and against S. Paratyphi have focused on O:LPS antigens (46). In contrast to S. Typhi, none of these pathogens are encapsulated with polysaccharide. For S. Paratyphi B there is evidence from field trials that Ty21a provides cross-protection (47) and in our CHIM there is also evidence that anti-O2:LPS IgG titers may correlate with protection against S. Paratyphi A (21), thus examination of O polysaccharide-conjugate vaccines currently in development (46) in a CHIM is warranted. No such model for invasive NTS is available, and would raise some ethical concerns (13), to evaluate the role of bactericidal activity mediated by anti-O:LPS antibodies observed in vitro and in vivo (29, 37, 44). Future studies examining other antibody properties and cell-mediated mechanisms would enhance understanding of immunity to invasive Salmonellae.
We sought to identify the role of bactericidal antibodies in serum following challenge with S. Typhi in a human challenge model. We identified that anti-LPS antibodies mediate a bactericidal effect that delays onset of infection and reduces disease severity and inflammation. This directs future vaccine studies toward other antigens or mechanisms of protection against typhoid.
Ethics Statement
A randomized, double-blind, placebo-controlled trial was performed at the Centre for Clinical Vaccinology and Tropical Medicine, Churchill Hospital, Oxford, UK (clinicaltrials.gov NCT679172; EudraCT 2011-000381-35) (14). This study was carried out in accordance with the recommendations of the Oxford University Clinical Trials and Research Governance Department, and the protocol approved by NRES South Central––Oxford A (11/SC/0302) and conducted in accordance with the declaration of Helsinki (2008) and the International Conference of Harmonization of Good Clinical Practice guidelines. An independent Data Monitoring and Safety Committee monitored the trial. To perform the assays described, human complement was collected in a parallel, prospective observational study (clinicaltrials.gov NCT01945307; 13/SC/0375). All subjects gave written informed consent in accordance with the Declaration of Helsinki.
Author Contributions
HT-B and HJ contributed to the drafting of the manuscript. CB, TD, and AP conceived and designed the work. HJ, HT-B, CJ, EJ, SS, RS, AE, PK, TV, and NT acquired data for the work. HJ, HT-B, and UG analyzed the work. HJ, HT-B, CJ, TD, SB, and AP interpreted data for the work. All authors were involved in article revision and approved the final published version. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of Interest Statement
AP has previously undertaken clinical studies on behalf of the University of Oxford, which were funded by vaccine manufacturers, but no longer does so. His department has received unrestricted educational grants from vaccine manufacturers to support delivery of a course on childhood infection. AP is chair of the UK Department of Health’s (DH) Joint Committee on Vaccination and Immunization (JCVI), and a member of WHOs Strategic Advisory Group of Experts, but the views expressed in this manuscript do not necessarily represent the views of JCVI or DH or WHO. All other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors wish to acknowledge Myron Levine and Raphael Simon (University of Maryland) for provision of antigens and the S. Typhi Quailes strain, Stephen Lockhart and Zoe Hindle (Emergent BioSolutions) for funding of challenge strain manufacture and provision of M01ZH09 vaccine and standard sera, Giorgio Napolitani (Weatherall Institute of Molecular Medicine, University of Oxford) for provision of antigens and Elizabeth Bateman (Department of Clinical Immunology, Churchill Hospital, Oxford) for performance of the sheep erythrocyte lysis assay. The authors also wish to acknowledge Professor David Lalloo (Liverpool School of Tropical Medicine), Professor David Hill (Quinnipiac University), Dr Philip Monk (Public Health England), and Professor Andrew Nunn (MRC Clinical Trials Unit) for study oversight as part of the Data Safety Monitoring Committee. The authors also wish to acknowledge the assistance of Leanne Marsay (Oxford Vaccine Group) and of Matthew Siggins, Peter Hart, and Calman MacLennan (School of Immunity and Infection, College of Medicine and Dental Sciences, University of Birmingham) for optimization of the SBA. The authors wish to acknowledge the contribution of study participants and the assistance of Public Health England, Oxfordshire, of Oxford University Hospital Laboratories and of clinical and laboratory staff at Oxford Vaccine Group.
Funding
This work was supported by a Wellcome Trust Strategic Translational Award (grant number 092661 to University of Oxford; www.wellcome.ac.uk). Additional support was provided by the Oxford Biomedical Research Centre (Clinical Research Fellowship to TCD; www.oxfordbrc.nihr.ac.uk), the European Commission (Marie Curie IIF Fellowship to CJB), Jenner Institute (www.jenner.ac.uk) and the Oxford Martin School (www.oxfordmartin.ox.ac.uk). The study funders are independent of the study design, study management, analyses, and interpretation of the study results. The Oxford Vaccine Group, University of Oxford, is supported by the National Institute for Health Research Clinical Research Network (CRN).
Supplementary Material
The Supplementary Material for this article can be found online at http://www.frontiersin.org/articles/10.3389/fimmu.2017.01916/full#supplementary-material.
Figure S1. Bactericidal antibodies do not protect against typhoid infection. (A) SBA titer on the day of challenge, in those with typhoid diagnosis (TD) and no typhoid diagnosis (nTD) within each study arm. Significance was determined by Mann–Whitney tests. (B–D) Numbers of cells secreting IgM (B), IgA (C), or IgG (D) antibodies against O9:LPS 7 days after vaccination, in TD and nTD groups across all study arms. Significance was determined by Mann–Whitney tests. (E–G) Antibody titers against O9:LPS IgM (E), O9:LPS IgA (F), and O9:LPS IgG (G) on the day of challenge (D0), in TD and nTD groups across all study arms. Significance was determined by Mann–Whitney tests.
Figure S2. Anti-Vi and anti-flagellin antibodies do not protect against typhoid infection. Antibody titers against Vi IgG (A), flagellin (H) IgM (B), H IgA (C), and H IgG (D) on the day of challenge (D0), in TD and nTD groups across all study arms. Significance was determined by Mann–Whitney tests.
References
1. Antillón M, Warren JL, Crawford FW, Weinberger DM, Kürüm E, Pak GD, et al. The burden of typhoid fever in low- and middle-income countries: a meta-regression approach. PLoS Negl Trop Dis (2017) 11:e0005376. doi:10.1371/journal.pntd.0005376
2. Mogasale V, Maskery B, Ochiai RL, Lee JS, Mogasale VV, Ramani E, et al. Burden of typhoid fever in low-income and middle-income countries: a systematic, literature-based update with risk-factor adjustment. Lancet Glob Health (2014) 2:e570–80. doi:10.1016/S2214-109X(14)70301-8
3. Gunn JS, Marshall JM, Baker S, Dongol S, Charles RC, Ryan ET. Salmonella chronic carriage: epidemiology, diagnosis and gallbladder persistence. Trends Microbiol (2014) 22:648–55. doi:10.1016/j.tim.2014.06.007
5. Anwar E, Goldberg E, Fraser A, Acosta CJ, Paul M, Leibovici L. Vaccines for preventing typhoid fever. Cochrane Database Syst Rev (2014) 1:CD001261. doi:10.1002/14651858.CD001261.pub3
6. Klugman K, Gilbertson IT, Koornhof HJ, Robbins JB, Schneerson R, Schulz D, et al. Protective activity of Vi capsular polysaccharide vaccine against typhoid fever. Lancet (1987) 330:1165–9. doi:10.1016/S0140-6736(87)91316-X
7. Levine MM, Ferreccio C, Abrego P, Martin OS, Ortiz E, Cryz S. Duration of efficacy of Ty21a, attenuated Salmonella typhi live oral vaccine. Vaccine (1999) 17(Suppl 2):S22–7. doi:10.1016/S0264-410X(99)00231-5
8. Fresnay S, McArthur MA, Magder L, Darton TC, Jones C, Waddington CS, et al. Salmonella Typhi-specific multifunctional CD8+ T cells play a dominant role in protection from typhoid fever in humans. J Transl Med (2016) 14:62. doi:10.1186/s12967-016-0819-7
9. D’Amelio R, Tagliabue A, Nencioni L, Di Addario A, Villa L, Manganaro M, et al. Comparative analysis of immunological responses to oral (Ty21a) and parenteral (TAB) typhoid vaccines. Infect Immun (1988) 56:2731–5.
10. Boyd MA, Tennant SM, Saague VA, Simon R, Muhsen K, Ramachandran G, et al. Serum bactericidal assays to evaluate typhoidal and nontyphoidal Salmonella vaccines. Clin Vaccine Immunol (2014) 21:712–21. doi:10.1128/CVI.00115-14
11. Hart PJ, O’Shaughnessy CM, Siggins MK, Bobat S, Kingsley RA, Goulding DA, et al. Differential killing of Salmonella enterica serovar Typhi by antibodies targeting Vi and lipopolysaccharide O:9 antigen. PLoS One (2016) 11:e0145945. doi:10.1371/journal.pone.0145945
12. Wahid R, Zafar SJ, McArthur MA, Pasetti MF, Levine MM, Sztein MB. Live oral Salmonella enterica serovar Typhi vaccines Ty21a and CVD 909 induce opsonophagocytic functional antibodies in humans that cross-react with S. Paratyphi A and S. Paratyphi B. Clin Vaccine Immunol (2014) 21:427–34. doi:10.1128/CVI.00786-13
13. Gibani MM, Jin C, Darton TC, Pollard AJ. Control of invasive Salmonella disease in Africa: is there a role for human challenge models? Clin Infect Dis (2015) 61:S266–71. doi:10.1093/cid/civ673
14. Darton TC, Jones C, Blohmke CJ, Waddington CS, Zhou L, Peters A, et al. Using a human challenge model of infection to measure vaccine efficacy: a randomised, controlled trial comparing the typhoid vaccines M01ZH09 with placebo and Ty21a. PLoS Negl Trop Dis (2016) 10:e0004926. doi:10.1371/journal.pntd.0004926
15. Waddington CS, Darton TC, Jones C, Haworth K, Peters A, John T, et al. An outpatient, ambulant-design, controlled human infection model using escalating doses of Salmonella Typhi challenge delivered in sodium bicarbonate solution. Clin Infect Dis (2014) 58:1230–40. doi:10.1093/cid/ciu078
16. Hindle Z, Chatfield SN, Phillimore J, Bentley M, Johnson J, Cosgrove CA, et al. Characterization of Salmonella enterica derivatives harboring defined aroC and Salmonella pathogenicity island 2 type III secretion system (ssaV) mutations by immunization of healthy volunteers. Infect Immun (2002) 70:3457–67. doi:10.1128/IAI.70.7.3457-3467.2002
17. Gilman RH, Hornick RB, Woodard WE, DuPont HL, Snyder MJ, Levine MM, et al. Evaluation of a UDP-glucose-4-epimeraseless mutant of Salmonella typhi as a live oral vaccine. J Infect Dis (1977) 136:717–23. doi:10.1093/infdis/136.6.717
18. Mackie TJ, Finkelstein MH. Complement-fixation by the interaction of normal serum and bacterial suspensions––a contribution to the study of natural immunity phenomena. J Hyg (1930) 30:1–24. doi:10.1017/S002217240001024X
19. Lubasch A, Keller I, Borner K, Koeppe P, Lode H. Comparative pharmacokinetics of ciprofloxacin, gatifloxacin, grepafloxacin, levofloxacin, trovafloxacin, and moxifloxacin after single oral administration in healthy volunteers. Antimicrob Agents Chemother (2000) 44:2600–3. doi:10.1128/AAC.44.10.2600-2603.2000
20. Simon R, Curtis B, Deumic V, Nicki J, Tennant SM, Pasetti MF, et al. A scalable method for biochemical purification of Salmonella flagellin. Protein Expr Purif (2014) 102:1–7. doi:10.1016/j.pep.2014.07.005
21. Dobinson HC, Gibani MM, Jones C, Thomaides-Brears HB, Voysey M, Darton TC, et al. Evaluation of the clinical and microbiological response to Salmonella paratyphi A infection in the first paratyphoid human challenge model. Clin Infect Dis (2017) 64:1066–73. doi:10.1093/cid/cix042
22. Bale JA. Salmonella Identification: Serotypes and Antigenic Formulae: Kauffmann-White Scheme. Great Britain: Centre for Infections, Health Protection Agency (2007).
23. Health Protection Agency. Identification of Salmonella Species. London: UK Standards for Microbiology Investigations (2011).
24. Health Protection Agency. Investigation of Blood Cultures (for Organisms Other Than Mycobacterium Species). London: UK Standards for Microbiological Investigations (2013).
25. R Core Team. R Foundation for Statistical Computing. Vienna, Austria (2015). Available from: https://www.R-project.org/
26. Blohmke CJ, Darton TC, Jones C, Suarez NM, Waddington CS, Angus B, et al. Interferon-driven alterations of the host’s amino acid metabolism in the pathogenesis of typhoid fever. J Exp Med (2016) 213:1061–77. doi:10.1084/jem.20151025
27. Liang L, Juarez S, Nga TV, Dunstan S, Nakajima-Sasaki R, Davies DH, et al. Immune profiling with a Salmonella Typhi antigen microarray identifies new diagnostic biomarkers of human typhoid. Sci Rep (2013) 3:1043. doi:10.1038/srep01043
28. Charles RC, Liang L, Khanam F, Sayeed MA, Hung C, Leung DT, et al. Immunoproteomic analysis of antibody in lymphocyte supernatant in patients with typhoid fever in Bangladesh. Clin Vaccine Immunol (2014) 21:280–5. doi:10.1128/CVI.00661-13
29. Rondini S, Lanzilao L, Necchi F, O’Shaughnessy CM, Micoli F, Saul A, et al. Invasive African Salmonella typhimurium induces bactericidal antibodies against O-antigens. Microb Pathog (2013) 63:19–23. doi:10.1016/j.micpath.2013.05.014
30. Baliban SM, Yang M, Ramachandran G, Curtis B, Shridhar S, Laufer RS, et al. Development of a glycoconjugate vaccine to prevent invasive Salmonella Typhimurium infections in sub-Saharan Africa. PLoS Negl Trop Dis (2017) 11:e0005493. doi:10.1371/journal.pntd.0005493
31. Wells TJ, Whitters D, Sevastsyanovich YR, Heath JN, Pravin J, Goodall M, et al. Increased severity of respiratory infections associated with elevated anti-LPS IgG2 which inhibits serum bactericidal killing. J Exp Med (2014) 211:1893–904. doi:10.1084/jem.20132444
32. Shimanovich AA, Buskirk AD, Heine SJ, Blackwelder WC, Wahid R, Kotloff KL, et al. Functional and antigen-specific serum antibody levels as correlates of protection against shigellosis in a controlled human challenge study. Clin Vaccine Immunol (2017) 24:e00412–6. doi:10.1128/CVI.00412-16
33. Lindow JC, Fimlaid KA, Bunn JY, Kirkpatrick BD. Antibodies in action: role of human opsonins in killing Salmonella enterica serovar Typhi. Infect Immun (2011) 79:3188–94. doi:10.1128/IAI.05081-11
34. Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus: I. The role of humoral antibodies. J Exp Med (1969) 129:1307–26. doi:10.1084/jem.129.6.1307
35. Marsay L, Dold C, Green CA, Rollier CS, Norheim G, Sadarangani M, et al. A novel meningococcal outer membrane vesicle vaccine with constitutive expression of FetA: a phase I clinical trial. J Infect (2015) 71:326–37. doi:10.1016/j.jinf.2015.05.006
36. MacLennan CA, Gondwe EN, Msefula CL, Kingsley RA, Thomson NR, White SA, et al. The neglected role of antibody in protection against bacteremia caused by nontyphoidal strains of Salmonella in African children. J Clin Invest (2008) 118:1553–62. doi:10.1172/JCI33998
37. MacLennan CA, Gilchrist JJ, Gordon MA, Cunningham AF, Cobbold M, Goodall M, et al. Dysregulated humoral immunity to nontyphoidal Salmonella in HIV-infected African adults. Science (2010) 328:508–12. doi:10.1126/science.1180346
38. Pulickal AS, Gautam S, Clutterbuck EA, Thorson S, Basynat B, Adhikari N, et al. Kinetics of the natural, humoral immune response to Salmonella enterica serovar Typhi in Kathmandu, Nepal. Clin Vaccine Immunol (2009) 16:1413–9. doi:10.1128/CVI.00245-09
39. Clutterbuck EA, Lazarus R, Yu LM, Bowman J, Bateman EA, Diggle L, et al. Pneumococcal conjugate and plain polysaccharide vaccines have divergent effects on antigen-specific B cells. J Infect Dis (2012) 205:1408–16. doi:10.1093/infdis/jis212
40. Richmond P, Kaczmarski E, Borrow R, Findlow J, Clark S, McCann R, et al. Meningococcal C polysaccharide vaccine induces immunologic hyporesponsiveness in adults that is overcome by meningococcal C conjugate vaccine. J Infect Dis (2000) 181:761–4. doi:10.1086/315284
41. Janik DK, Lee WT. Staphylococcal enterotoxin B (SEB) induces memory CD4 T cell anergy in vivo and impairs recall immunity to unrelated antigens. J Clin Cell Immunol (2015) 6:1–8. doi:10.4172/2155-9899.1000346
42. Lucisano Valim YM, Lachmann PJ. The effect of antibody isotype and antigenic epitope density on the complement-fixing activity of immune complexes: a systematic study using chimaeric anti-NIP antibodies with human Fc regions. Clin Exp Immunol (1991) 84:1–8. doi:10.1111/j.1365-2249.1991.tb08115.x
43. Roos A, Bouwman LH, van Gijlswijk-Janssen DJ, Faber-Krol MC, Stahl GL, Daha MR. Human IgA activates the complement system via the mannan-binding lectin pathway. J Immunol (2001) 167:2861. doi:10.4049/jimmunol.167.5.2861
44. Goh YS, Necchi F, O’Shaughnessy CM, Micoli F, Gavini M, Young SP, et al. Bactericidal immunity to Salmonella in Africans and mechanisms causing its failure in HIV infection. PLoS Negl Trop Dis (2016) 10:e0004604. doi:10.1371/journal.pntd.0004604
45. Fiorino F, Rondini S, Micoli F, Lanzilao L, Alfini R, Mancini F, et al. Immunogenicity of a bivalent adjuvanted glycoconjugate vaccine against Salmonella Typhimurium and Salmonella Enteritidis. Front Immunol (2017) 8:168. doi:10.3389/fimmu.2017.00168
46. Martin LB, Simon R, MacLennan CA, Tennant SM, Sahastrabuddhe S, Khan MI. Status of paratyphoid fever vaccine research and development. Vaccine (2016) 34:2900–2. doi:10.1016/j.vaccine.2016.03.106
Keywords: typhoid infection, bactericidal activity, human challenge model, Salmonella enterica Typhi, immune responses
Citation: Juel HB, Thomaides-Brears HB, Darton TC, Jones C, Jones E, Shrestha S, Sie R, Eustace A, Galal U, Kurupati P, Van TT, Thieu NTV, Baker S, Blohmke CJ and Pollard AJ (2018) Salmonella Typhi Bactericidal Antibodies Reduce Disease Severity but Do Not Protect against Typhoid Fever in a Controlled Human Infection Model. Front. Immunol. 8:1916. doi: 10.3389/fimmu.2017.01916
Received: 16 August 2017; Accepted: 14 December 2017;
Published: 17 January 2018
Edited by:
Pere-Joan Cardona, Universitat Autònoma de Barcelona, SpainReviewed by:
Elisabetta Adelaide Pia Soldaini, GlaxoSmithKline, ItalyNianshuang Wang, Dartmouth College, United States
Adam Cunningham, University of Birmingham, United Kingdom
Copyright: © 2018 Juel, Thomaides-Brears, Darton, Jones, Jones, Shrestha, Sie, Eustace, Galal, Kurupati, Van, Thieu, Baker, Blohmke and Pollard. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Helena B. Thomaides-Brears, helena.thomaides-brears@paediatrics.ox.ac.uk
†These authors have contributed equally to this work.
 Helene B. Juel
Helene B. Juel Helena B. Thomaides-Brears
Helena B. Thomaides-Brears Thomas C. Darton
Thomas C. Darton Claire Jones
Claire Jones Elizabeth Jones
Elizabeth Jones Sonu Shrestha
Sonu Shrestha Rebecca Sie1
Rebecca Sie1 Ushma Galal
Ushma Galal Tan T. Van
Tan T. Van Stephen Baker
Stephen Baker Christoph J. Blohmke
Christoph J. Blohmke Andrew J. Pollard
Andrew J. Pollard