Born to be free? Assessing the viability of releasing captive-bred wobbegongs to restock depleted populations
- 1Biological Sciences, Macquarie University, Sydney, NSW, Australia
- 2Threatened, Endangered, and Protected Species Subprogram, SARDI – Aquatic Sciences, Adelaide, SA, Australia
- 3School of Biological Sciences, Flinders University, Adelaide, SA, Australia
- 4Fisheries New South Wales, New South Wales Department of Prime Industries, Sydney Institute of Marine Science, Sydney, NSW, Australia
- 5Australian Animal Tagging and Monitoring Systems, Sydney Institute of Marine Science, Sydney, NSW, Australia
Large predatory fishes, such as sharks, play an important functional role within marine ecosystems. Restocking of depleted populations has been extensively studied for commercially or recreationally important teleost species; however, it has, to the best of our knowledge, never been successfully attempted and assessed on sharks. We evaluated whether 15 captive-bred wobbegongs (Orectolobus maculatus) released into a small bay inhabited by wild sharks would survive and remain within a small no-take marine reserve. The captive-bred sharks and 12 wild sharks were tagged with acoustic transmitters and their presence was monitored by an array of acoustic receivers. The detection rate of control tags was modeled against environmental variables to predict detection probabilities and account for days when environmental conditions hampered shark detections. The overall detection probability ranged from 28 to 38% and was most affected by wind direction. Wild wobbegongs showed clear seasonal patterns of attendance to the study site, with the highest probability of presence during the summer months. The captive-bred sharks did not display the same seasonal trend in occurrence. The age at which captive-bred sharks were released into the area affected residency periods. Four out of five adults remained in the area for up to three years post-release, while all the juveniles permanently left the area within a year post-release. Three of the juveniles were detected on receivers up to 12 km from the study site. Therefore, if restocking of depleted populations of sharks is to be used as a conservation strategy, the age at which sharks are released must be considered.
Introduction
Removal of large, predatory fish, such as sharks, can have substantial negative impacts for the health of marine ecosystems (Myers et al., 2007; Heithaus et al., 2008; Ferretti et al., 2010). This, coupled with many shark population declines (Botsford et al., 1997; Myers and Worm, 2003; Worm et al., 2013), makes the need for effective conservation strategies orientated toward predatory fish of the upmost importance. Many elasmobranchs (sharks, skates, and rays) have K-selected life history traits, such as late age-to-maturity, slow growing, or low fecundity (Holden, 1974, 1977), making these species particularly vulnerable to overexploitation (Bonfil, 1994; Smith et al., 1998; Walker, 1998). As a result and with harvesting rates increasing globally (Stevens et al., 2000; Field et al., 2009), a growing number of species are being driven toward extinction (Dulvy et al., 2014). For species that move over large distances, effective conservation strategies can be challenging, particularly when their movements cross multiple management jurisdictions (Dulvy et al., 2008). In the case of species that form predictable annual aggregations or exhibit strong site-fidelity (e.g., reef sharks—Speed et al., 2011; zebra sharks—Dudgeon et al., 2013), conservation strategies and management regulations can be informed through greater understanding of the environmental parameters driving spatio-temporal variations of these aggregations or residency patterns (Speed et al., 2011).
The ever-growing demand for fish has led fisheries scientists to consider several techniques to enhance productivity, two of which include restocking and stock enhancement (Bell et al., 2006). “Restocking” refers to restoring depleted populations to a level where they can once again provide regular fishing yields, whereas “stock enhancement” refers to releasing cultured animals to increase fishing yields beyond that supported by the natural environment of the species (Bell et al., 2006). Restocking is also a critical tool in the conservation of endangered terrestrial species (see reviews Jule et al., 2008; Williams and Hoffman, 2009; Champagnon et al., 2012). In the marine environment, studies have largely focused on commercially or recreationally targeted fish species (Hong and Zhang, 2003; Fraser, 2008). For example, a restocking program for Atlantic salmon (Salmo salar) was initiated in 1992 in the Malbaie River, Québec, Canada, in response to population declines. A pool of breeders is maintained at a provincial hatchery and each year 10 wild salmon are caught and added to the breeding population. The progeny are reared in indoor tanks until they are released as fry or smolt, respectively, at 4 or 15 months after hatching (Blanchet et al., 2008; Milot et al., 2013). Despite numerous examples of such of re-stocking initiatives being used worldwide, few have met expectations either due to a lack of consideration to wider fisheries management of the target species (Bell et al., 2006), reduced genetic diversity and fitness (Fraser, 2008), or different behavioral patterns of the captive bred fish (Salvanes and Braithwaite, 2006). However, when appropriate management initiatives are implemented, such as modeling of dispersal patterns and implementing no-take zones to protect the released animals (Purcell and Kirby, 2006) or maintenance of captive genetic diversity (Fraser, 2008), the primary aims of re-stocking may be accomplished.
To date, the potential role of restocking for elasmobranchs has not been investigated. However, increasing success in rearing captive-bred sharks and rays by aquaria may enable more restocking opportunities for threatened or protected species, in the same manner as with terrestrial animals. Internationally, aquaria, like zoos, have long argued their relevance in conservation through their potential to breed and maintain captive populations of threatened species (Maitland and Evans, 1986). To date, and to the best of our knowledge, their ability to successfully restock wild populations with captive-bred elasmobranchs has never been tested. While aquaria play an important role in educating the public on conservation issues (Gusset and Dick, 2011), their direct relevance to conservation efforts could be substantially enhanced if their captive-bred sharks and rays were successfully assimilated into wild populations.
Wobbegongs are demersal sharks found in temperate and tropical Western Pacific waters (Compagno, 2001; Last and Stevens, 2009). Three species of wobbegongs commonly occur in New South Wales (NSW), Australia, and all were heavily targeted for the sale of their flesh as “flake” (Huveneers et al., 2007a). Following a 50% decline in catches within a 10-year period, fishing regulations were introduced in 2008 restricting the commercial catch and banning recreation fishing of all wobbegong species (Pease and Grinberg, 1995; NSW DPI, 2006). However, given they are slow-growing and have low fecundity (Huveneers et al., 2007b, 2013), it will take a long time to evaluate the effectiveness of such regulations. Based on previous studies that have shown wobbegongs to have strong short- (days) to medium-term (months) site fidelity (Carraro and Gladstone, 2006; Huveneers et al., 2006) and seasonal variation in their abundance (Carraro and Gladstone, 2006; Lee et al., 2014), it has been implied that marine protected areas could only offer limited protection. However, wobbegongs can be successful bred in aquaria making them a good species for potential restocking of depleted populations.
Within this broader context, the aim of this study was to determine whether captive-bred wobbegong sharks would assimilate with wild populations following a release program by (1) comparing residency and seasonal movement patterns, (2) assessing environmental parameters affecting presence, and (3) identifying differences in inter-annual site fidelity between captive-bred and wild wobbegongs.
Methods
Study Site
The study was undertaken in Cabbage Tree Bay Aquatic Reserve (CTBAR, 33°47′57″S, 151°17′44″E), a small ~0.2 km2 no-take marine reserve off Sydney, NSW, Australia (Figure 1). Habitats within the reserve consist of barren boulders, areas of dense Ecklonia radiata and rocky reef covered with macroalgae and sponges, typical to subtidal inshore rocky reefs of temperate, south-eastern Australia (Underwood et al., 1991). There are two distinct reefs within the reserve that are separated by 120 m of sand (Figure 1). This site was chosen as it had a known population of wild wobbegongs that visited the site seasonally with up to a 100 sharks present during the summer months (Lee et al., 2014). In addition, if the captive-bred sharks remained in the no-take area fishers would not be able to catch them, thus the site fidelity and survival would not be affected by such anthropogenic causes.
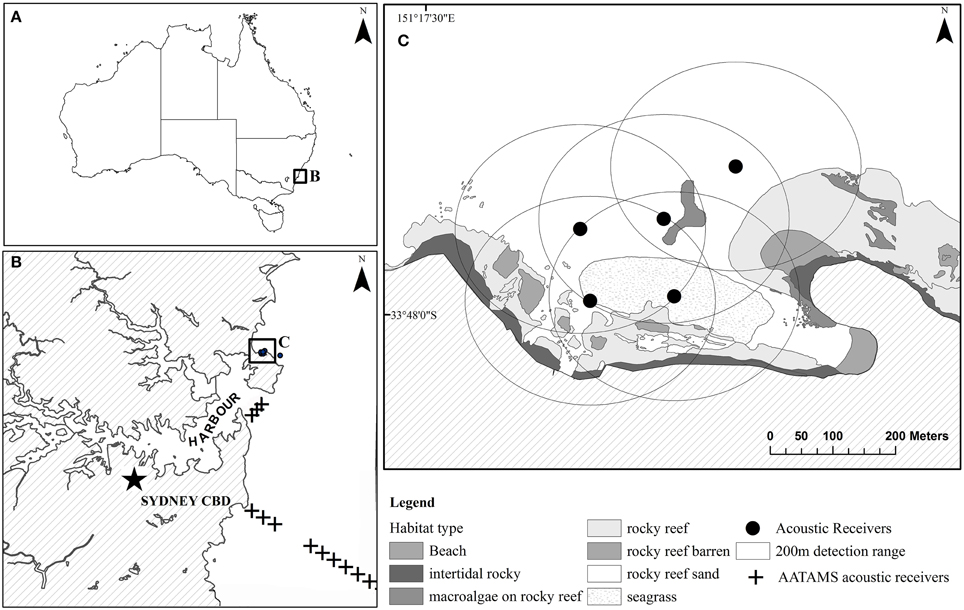
Figure 1. (A) Map of Cabbage Tree Bay Aquatic Reserve (CTBAR). Panel (B) shows CTBAR in relation to Sydney harbour and the AATAMS acoustic receivers wobbegongs were detected on. Panel (C) shows a close-up of the acoustic receivers at CTBAR and their detection ranges.
Acoustic Telemetry
Six VR2W acoustic receivers (Vemco Ltd, Nova Scotia, Canada) were deployed within CTBAR. Five were deployed up to 160 m apart within the bay forming an overlapping array and the sixth was deployed on the ocean side (Figure 1C). Range testing was conducted to determine the effective detection range of the receivers within the reserve (Heupel et al., 2006) and estimated at a minimum of 200 m radius for all oceanic conditions. This ensured that the entire protected area within the bay was acoustically covered (Figure 1C). Acoustic receivers were deployed on sand in 6–12 m depth, affixed to a 1.35-m-long steel post that was set in a concrete-filled tire. In addition, the Integrated Marine Observing System-Australian Animal Tagging and Monitoring System (IMOS-AATAMS, www.imos.org.au) maintains a line of four VR2Ws across the mouth of Sydney Harbour, 4 km south of CTBAR and 30 VR2Ws receivers perpendicular from the shore at Bondi, 12 km south of the study site, extending to the continental shelf 25 km offshore (Figure 1B).
Environmental variables are known to affect the probability of a transmitter being detected by the acoustic receivers and thus can influence the inference of animal behavior (Payne et al., 2010; Gjelland and Hedger, 2013). To determine the variability in detection probability three stationary transmitters (hereafter referred to as “control tags”) were deployed within the acoustic array detection range of 200 m to determine the level of environmental and/or biological noise affecting the detectability of the animal tags.
Twelve wild wobbegong sharks (four females and eight males Orectolobus maculatus) were captured and acoustically tagged within CTBAR. Nine adult wobbegongs were tagged in June to October 2008 (four females, five males), and another three in October 2009 (all males, two juveniles). Wobbegongs were sampled at random and were caught by diving inside CTBAR using a large hand net (diameter: 1 m; mesh size: 3 cm) that was held in front of the shark by one diver. A second diver used blunt ended poles (diameter: 2 cm; length: 1.5 m) to guide the shark into the net. The sharks were brought on board the research vessel and immediately placed in a 200 l tub containing oxygen-enriched seawater with a solution of 30 ppm eugenol (AQUI-S, AQUIS-S NZ, Wellington, New Zealand) for anesthetic induction. Once the sharks were fully anesthetized, a coded V13-1L acoustic transmitter (battery life ~1623 days; nominal interval 150–250 s) was inserted into the coelomic cavity using standard surgery practice (see Heupel and Hueter, 2001). All sharks were also fitted with an external identification tag, containing a unique number, which was inserted into the musculature below the first dorsal fin to facilitate reporting if commercial fishers caught any of the tagged wobbegongs.
Fifteen captive-bred wobbegongs (Orectolobus maculatus) were tagged with the same V13-1L acoustic transmitters and external identification tags as their wild counterparts prior to being released into CTBAR. All wobbegongs were first generation captive-bred sharks that had been bred from adults caught by commercial fishermen and surrendered to the local aquaria for unknown reasons. Seven sharks were released in September 2008, three in February 2009, and a further five in January 2010. The sharks released in 2008 and 2009 were classified as the “2008 release cohort.” In this cohort, all sharks were juveniles [mean 84 cm total length (TL); range: 77–87 cm TL] and consisted of five females and five males. In the “2010 release cohort,” all sharks were male, four were adults and one was a juvenile (mean 119 cm TL; range: 115–123 cm TL). Prior to release, all captive bred sharks were housed in aquaria located close to the release site. They were fed a combination of whiting, pilchards, yellowtails, trevally, squid, and octopus between two and six times a week. They were housed in indoor aquaria of 1000 to 2.2 million L capacity depending on their size. All aquaria were connected to a flow-through seawater system and were subjected to the same natural variation in water temperature (about 16–22°C) as found in Sydney Harbour. The photoperiod in the aquaria artificially mimicked the diel cycle at the study site.
Data Analysis
Environmental Effects on Detection Probability
The detection probability of a potential transmission from each control tag was calculated as the total number of detections (from all the receivers within a 200 m radius) divided by the expected number of transmissions (based on the average nominal interval of the tags). Generalized linear mixed modeling (GLMM) was used to determine if the detection probability was affected by the following environmental variables: time of day (day or night), hour of the day, rain, wind speed, wind direction, air pressure, and wind speed according to wind direction (i.e., an interaction between wind speed and direction). The time of day (day or night) was calculated from sunrise/sunset times from Australian Government Geoscience Australia (http://www.ga.gov.au/geodesy/astro/sunrise.jsp). The time was classified as “day” from sunrise to sunset and “night” from sunset to sunrise. Rain, wind speed, wind direction, and air pressure data were obtained from the Australian Bureau of Meteorology (BOM) from a weather station 10 km from the study site. All of the variables were recorded for each minute of each day. The total cumulative rain for each hour was calculated and the hourly average was calculated for wind speed, direction and air pressure. There was low collinearity between the variables (all have r2 < 0.7, Pearson's correlation) confirming that a GLMM was appropriate. The GLMM was implemented using the lme4 R package (Bates and Maechler, 2010). Since the aim of the GLMMs was to do model predictions, model selection for the best model was based on the Akaike Information Criterion (AIC). Any models with a difference in AIC (ΔAIC) of less than or equal to two had “strong support”; ΔAIC of four to seven showed “substantial support” and ΔAIC greater than 10 showed “no support” (Burnham and Anderson, 2002). If more than one model had ΔAIC of 10 or less, model averaging was used to calculate the variable coefficients (Bolker et al., 2009) using the MuMIn R package (Barton, 2012). Predicted detection probabilities were calculated from the model averaged coefficients and applied to the environmental data for the period of time the wobbegongs were monitored. For the following analyses, a shark was considered “present” within the array if it was detected at least twice within a 24-h period, eliminating the possibility of “false detections” (Pincock, 2011). Thus, to avoid false-absences of the wobbegongs due to limited detection probability, any environmental conditions whereby the detection probability of the control tags was less than 15% were removed from the study time. A 15% threshold was chosen in relation to the mean nominal interval of the wobbegong transmitters (i.e., 100% detection rate given an animal was present would yield 18 detections per hour, a 15% detection would yield 2.7 and be above the threshold used to indicate an animal was “present”).
Residency of Wobbegongs
Residency of the wild and captive bred sharks was measured using a residency index (RI) and the number of consecutive days sharks were “present” within CTBAR. Residency index was calculated by dividing the number of days a shark was “present” within the reserve by the duration of the study. A wobbegong was defined as “present” if it had been detected two or more times within a 24-h period. A value of 0 indicated no residency and a value of 1 permanent residency (Bryars et al., 2012; La Mesa et al., 2012). Residency index for the wild sharks was calculated from the day of tagging and for the captive-bred sharks it was calculated from the day of their release in CTBAR. Residency index was modeled against sex, age-class and whether the sharks were wild or captive bred, with each release representing a different factor within the model (hereafter referred to as “release cohort”). This was modeled using a beta regression model with a logit link function (Ferrari and Cribari-Neto, 2004; Cribari-Neto and Zeileis, 2010), using the “betareg” Package in R (version 15.3; R Core Development Team, 2009). This regression is the most suitable linear regression of response variables bounded by 0 and 1 (such as RI) and easily accommodates asymmetry (Ferrari and Cribari-Neto, 2004). Sharks were classified as juveniles if they were below minimum length at sexual maturity as reported in Huveneers et al. (2007b). The best model structure was found using a step-down approach with likelihood ratio tests (LRT). This involves dropping each model variable sequentially and testing its significance using a LRT. A variable was retained in the model if the p-value of the LRT was less than 0.05 (Zuur et al., 2009). The predicted mean for each group of interest was calculated from the best beta regression model using the “predict” function in the “betareg” package.
Presence-Absence
The presence of the wobbegongs at CTBAR was calculated for each hour of each day from 9th December 2008 to 13th November 2012. A wobbegong was defined as “present” if it had been detected two or more times within the hour. All the rest of the hourly bins where a wobbegongs was not detected were defined as “absent.”
A GLMM was used to examine how time of day (day or night), month, year, sex, age-class (adult or juvenile), breeding/non-breeding season, austral season, and environmental variables (moon illumination, length of day, and sea temperature) affected the wobbegongs presence. The unique shark tag code was used as the random effect. Additional environmental variables, such as rain and wind speed, were not included in the model to avoid over-parameterization given the limited number of sharks tagged. December to January was defined as the breeding season (Huveneers et al., 2007b) and all other months were defined as the non-breeding season. Summer was defined as December to February, autumn as March to May, winter as June to August, and spring as September to November. Again, the time of day (day or night) and length of day was calculated from sunrise/sunset times from the Australian Government Geoscience Australia. Daily moon illumination data was obtained from the United States Naval Observatory Astronomical Applications Department (http://aa.usno.navy.mil/data/docs/MoonPhase.php). The mean hourly sea temperature was calculated based on temperature recorded by an acoustic transmitter (V16T, nominal interval 300–900 s) deployed within the array. Given the low number of wild juveniles that were tagged (n = 2) and the lack of adult female captive bred sharks, no sex and age-class interaction was tested.
The response variable was coded as 1 if the sharks were “present” and 0 if they were “absent.” A binomial error structure using the complementary log-log link function was used for the GLMM analysis as there was an inflated number of zeros in the response variable (Zuur et al., 2009). Since the aim of the GLMMs was qualitative understanding, rather than model prediction, a step-down approach using LRTs were used to find the best model structure (Bolker et al., 2009; Zuur et al., 2009). The probability of “presence” was then calculated for all the model variables in the best model using back-transformations from the estimates of the model's fixed effects.
Results
A GLMM analysis on the proportion of detection from the control tags produced five models with a ΔAIC of two or less (see Table 1 in Supplementary Material). Using the MuMIn package in R and model averaging of all the models with a ΔAIC of 10 or less, it was determined that wind direction affected the detection probability the most, with a relative variable importance of 0.97. This was followed by time of day (relative importance of 0.93) and air pressure (0.92). Wind speed, total rain, water temperature, and wind speed depending on wind direction were all poor predictors of the detection probability (relative variable importance of 0.44, 0.37, 0.26, and 0.19 respectively). The probability of detection over the time period the wobbegongs were monitored only ranged between 28 and 38% (Figures 2A–F) and therefore did not fall below the 15% threshold set. Consequently, the chance of a false-absence of wobbegongs due to environmental conditions was low.
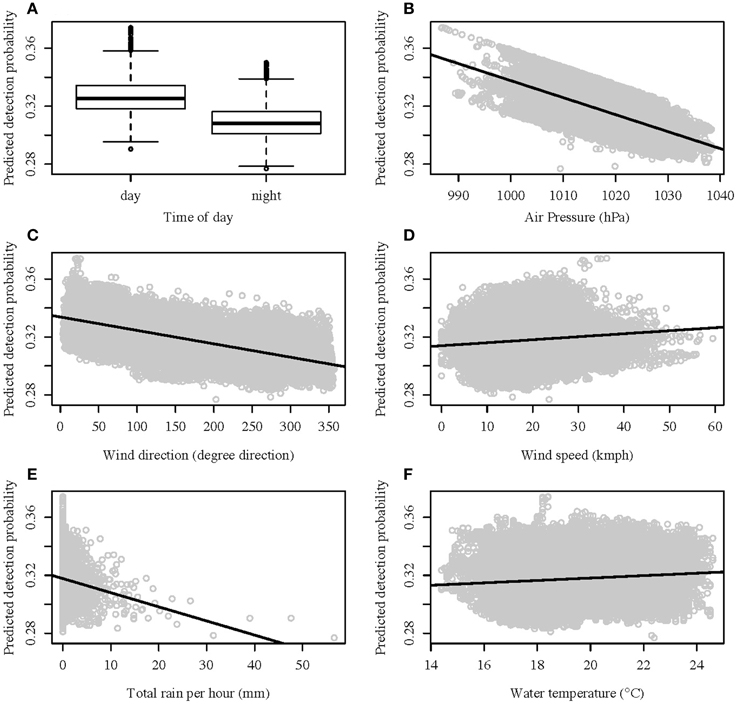
Figure 2. Predicted detection probability values against best predictors: (A) time of day; (B) air pressure; (C) wind direction; (D) wind speed; (E) rain; and (F) water temperature.
Acoustic data were recorded for 12 wild wobbegongs and 15 released captive-bred sharks from June 2008 to November 2012. A commercial fisher caught one of the wild wobbegongs on the 10th March 2010 15 km north of CTBAR. This individual, shark ID 1, was last detected in CTBAR on the 14th January 2009 and was not detected on any of AATAMS receivers after this time. It was only included in the analyses from the time of tagging to the time of reporting (i.e., the RI and presence-absence was only calculated from time of tagging to capture).
Both the wild and captive-bred sharks exhibited varying levels of low to medium residency, with a median RI of 0.13 (range: 0.00–0.53) and no permanent residency (Figure 3). Beta regression models showed that sex and age-class (juvenile or adult) did not affect the RI of the tagged sharks (beta regression p-values: sex = 0.34 and age-class = 0.78). However, there was a significant difference between the wild sharks and the different “release cohorts” of the captive-bred sharks (beta regression p-value = 0.02). The wild wobbegongs had the highest predicted RI value (predicted mean = 0.21) and the captive-bred sharks released in 2008 had the lowest predicted RI (predicted mean = 0.07). The predicted RI value for the adults was twice that of the juveniles (predicted means: adults = 0.20, juveniles = 0.10).
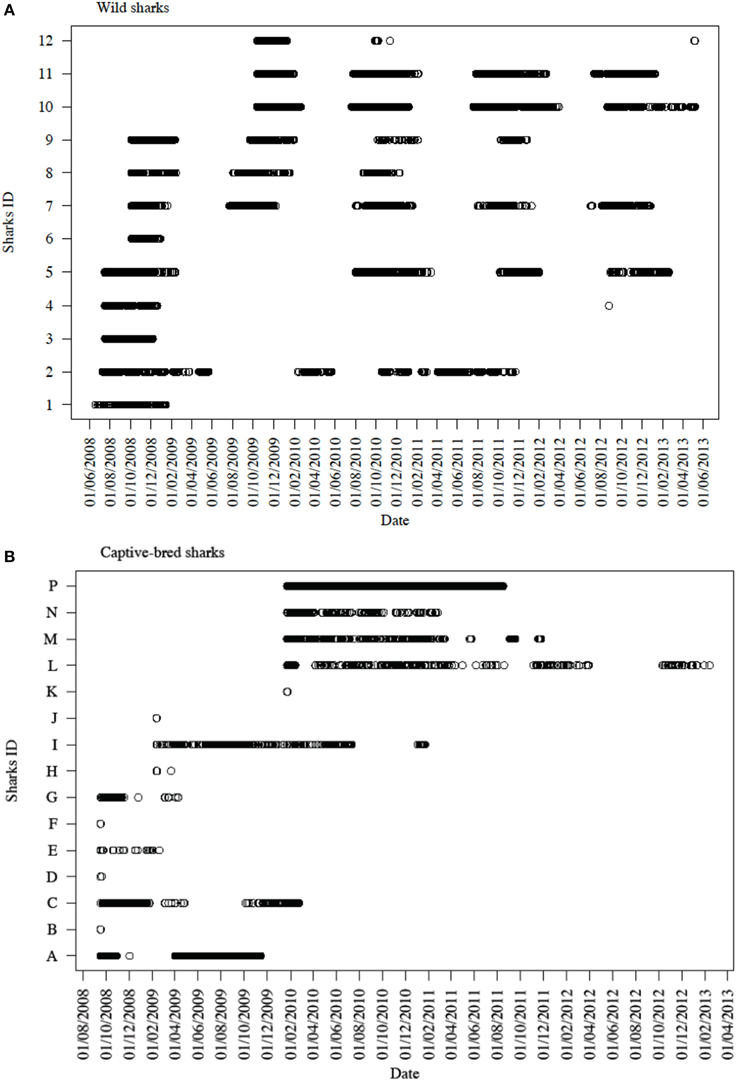
Figure 3. Time series of detections across all six VR2W receivers in CTBAR for (A) all 12 tagged wild wobbegongs and (B) all 15 captive-bred sharks released into the area.
Sharks were only detected in CTBAR for a median of 10 consecutive days (range: 1–206 days), although there was high individual variation in how long each shark spent within CTBAR (Figure 3). Four of the wild wobbegongs tagged in 2008 (44% of tagged sharks), three males and one female, remained in the area for the following summer and then did not return to the area again (#1, 3, 4, and 6) (Figure 3A). A similar pattern was observed from one of the wild sharks tagged in 2009 (33%), however, this shark returned to the area for a total of 11 days nine months later (#12) (Figure 3A). The remaining sharks showed a clear seasonal presence at CTBAR (Figure 3A). In contrast, the captive-bred sharks exhibited a range of responses after release, with residency ranging from 1 to 206 consecutive days across seasons (Figure 3B). This cross-seasonal residency was not displayed by wild-caught sharks (except possibly #2). However, once the captive-bred sharks left CTBAR, only one animal returned in a similar cycle to that displayed by free-ranging sharks (#C). Departure date did not correspond with that of wild-caught sharks (Figure 3B).
Two of the captive-bred sharks released in 2008 were only detected in CTBAR for 1 day following their release (#B and F). However, both of these sharks were later detected on IMOS-AATMAS receivers to the south of CTBAR. One (#F) was detected on receivers deployed across the mouth of Sydney Harbour for 9 days in October 2011 and the other (#B) was periodically detected on receivers deployed near-shore off Bondi between August 2011 and April 2012. Similarly, another of the sharks from the same release cohort was detected in CTBAR for 18 months after its release, left CTBAR for 6 months, before returning for 15 days (#I). However, during its absence from CTBAR it was detected on the Bondi receivers. Five of the wild tagged wobbegongs were also detected on the Bondi receivers for periods of up to 5 days (#2, 7, 8, 9, and 11). The majority of the detections were from near-shore receivers, however, one shark (#11) was detected 23 km offshore.
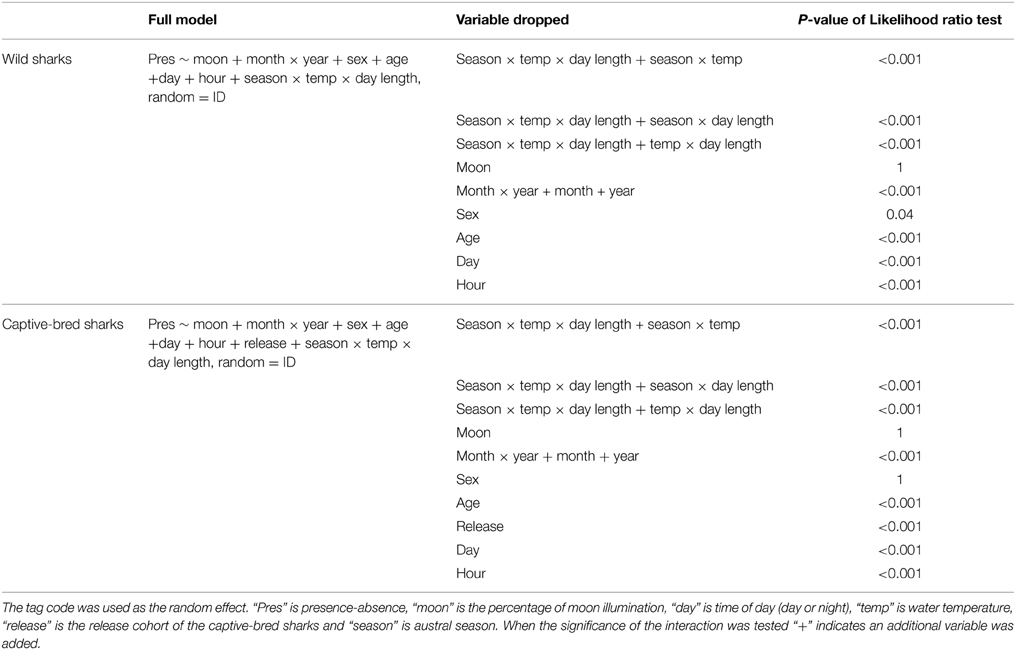
The GLMMs for both the wild and captive-bred sharks showed that austral season was a better predictor for presence-absence than breeding/non-breeding season (both ΔAICs > 10, LRT p-value < 0.001). Furthermore, the three-way interaction between austral season, water temperature, and day length was a better predictor than the variables as main effects or combinations of two-way interactions (Table 1). Moon illumination was found to be a poor predictor of presence-absence of both wild and captive-bred sharks and was not included in either of the best models (Table 1).
Presence-absence of wild wobbegongs was found to be dependent on the hour of the day, day/night, sex, age-class, month depending on the year, and austral season depending on water temperature and day length (Table 1). Males and juvenile sharks had the highest probability of being present (Figures 4A,C). Although hour of the day, day/night, and a month-year interaction were included in the best model, there was little variation in the probability of a wobbegong being present across the levels of these variables but high variation within each level (Figures 4E, 5A). Again, the seasonal attendance of the wobbegongs was evident, with a higher probability of presence between August and February in each of the years monitored (Figure 5A). This was reflected in the differences in the probability of presence between the four austral seasons depending on the water temperature and day length (Figure 6A).
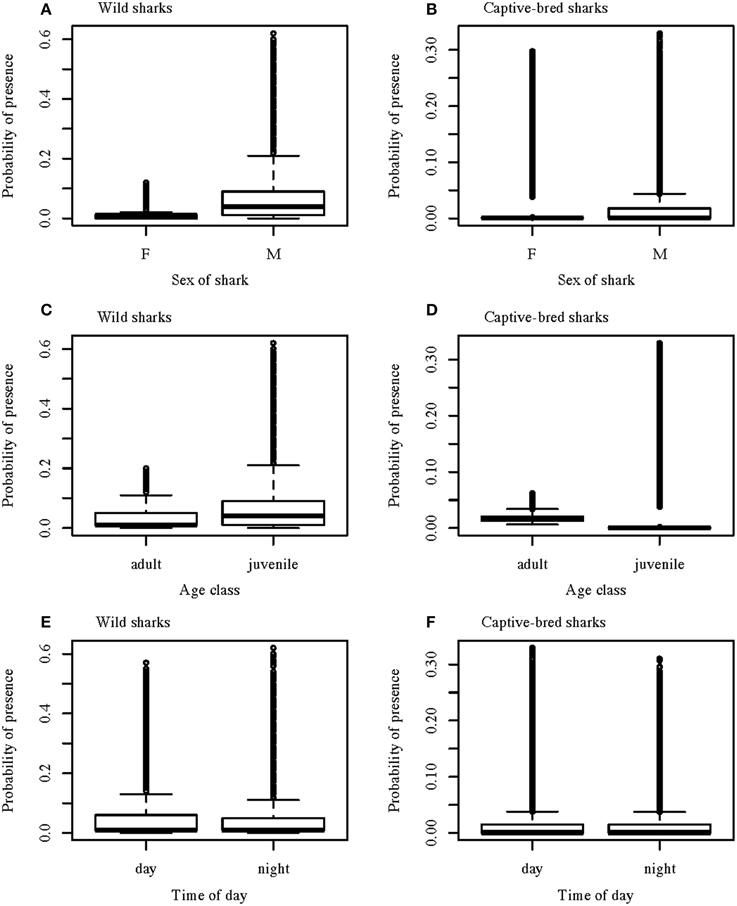
Figure 4. Boxplots of the predicted values from the best GLMM models for the probability of wild and captive-bred wobbegongs being present at CTBAR against sex of the shark (A,B); age class (C,D); and time of day (E,F). The box indicates the interquartile range; the line indicates the median and the whiskers show the 95% confidence interval.
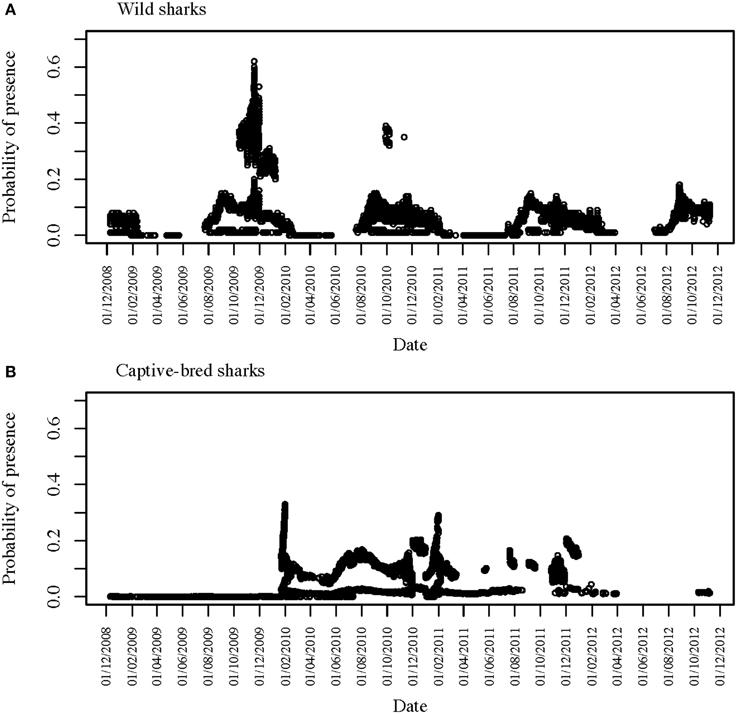
Figure 5. Predicted values from the best GLMM models for the probability of (A) wild and (B) captive-bred wobbegongs being present at CTBAR against month by year.
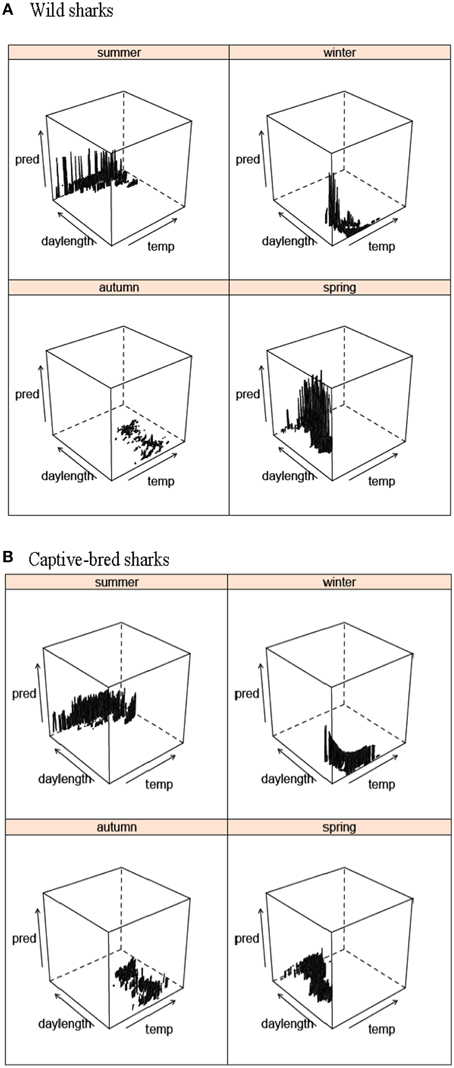
Figure 6. Predicted values from the best GLMM models for the probability of (A) wild and (B) captive-bred wobbegongs being present at CTBAR against austral season depending on water temperature and day length.
The best GLMM model structure for the captive-bred sharks had all the same variables as the best GLMM for wild sharks, for the exclusion of sex and the inclusion of “release cohort” (Table 1). The sharks released in 2010 had higher probability of presence in CTBAR than the sharks released in 2008 (Figure 7). As with the wild wobbegongs, there was no substantial difference in probability of presence between the day and night, and between adults and juveniles (Figures 4B,D,F). Males had a slightly higher probability of presence than females (Figure 4B), however this was likely biased by all the sharks released in 2010 being males. There was a less obvious seasonal pattern across the year for captive than the wild wobbegongs (Figure 5B). In addition, the probability of presence between the different austral seasons depending on water temperature and day length was less pronounced than the wild sharks (Figure 6B).
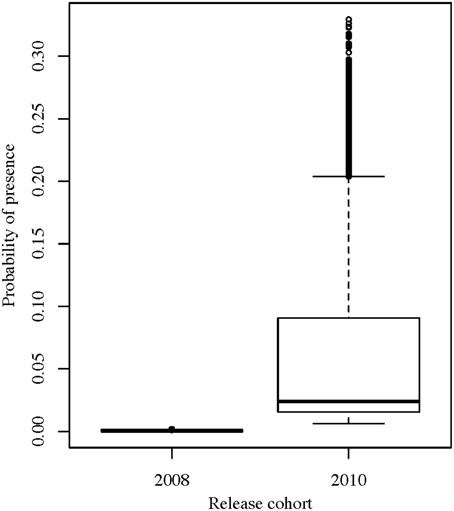
Figure 7. Predicted values from the best GLMM model for the probability of captive-bred wobbegongs from the 2008 and 2010 cohorts being present at CTBAR.
Discussion
Despite global declines in abundance of many elasmobranch species, restocking of depleted populations with captive-bred animals has, to the best of our knowledge, never been successfully attempted and evaluated for any shark species. This study shows that captive-bred wobbegong sharks were able to assimilate into the wild, with some individuals exhibiting medium- to long-term residency at the release site (up to 206 days), prior to permanent emigration from the area. Some of the sharks that left the area were subsequently detected 12 km south of the release site, with one shark being detected there two and a half years after being released. Five of the wild wobbegongs were also detected at this same site. Therefore, although their long-term survival could not be determined, this shows that the released individuals had similar geographical movements as wild wobbegongs and demonstrates their successful introduction into, the wild for extended periods. Importantly, these individuals were able to successfully forage, avoid predators, and survive in a natural environment following release.
Residency of Wild vs. Captive-Bred Wobbegongs
The tagged wild wobbegongs exhibited seasonal movement and many returned to the area even after long absences. A few of the sharks exhibited a degree of transience and were only detected for the summer following their tagging and were not detected again. Overall, the captive-bred sharks had a significantly lower long-term site-fidelity to the study area than their wild counterparts and did not display the same level of seasonality. Only four of the captive-bred sharks returned to the study after temporary absences.
A previous study, using mark-recapture techniques, also showed a high rate of transience in this wild wobbegong population, with over 60% of the sharks captured only once during the 2-year study (Lee et al., 2014). The current study found a lower level of transience in the wild population, with two-thirds of the sharks being detected for two or more austral seasons. This lower level of transiency may be a result of tagging the sharks in the spring and summer months, seasons which appeared to lead to a higher probability of them being present the following summer (Lee et al., 2014). However, the captive-bred sharks did not display the same level of seasonality as the wild ones and the captive-bred sharks had a lower probability of remaining resident in the release area. This could be biased by the different ages at which the sharks were released as some adults remained in the area for up to 3 years post-release, whereas all juveniles had left within a year. Lee et al. (2014) sighted fewer juvenile male and female wobbegongs than adults and Huveneers et al. (2007a) found that commercial fisherman rarely caught neonate or juvenile wobbegongs along the NSW coast. This suggests that neonate and juveniles wobbegongs may inhabit different habitats from the adults, i.e., nursery areas.
Many different shark species utilize nursery areas in the developmental stages of life. For example, Taylor and Bennett (2013) found that neonates of five shark species, including dusky sharks (Carcharhinus obscurus), nervous sharks (C. cautus) and blacktip sharks (C. limbatus–C. tilstoni), were abundant in shallow waters of Moreton Bay, Queensland, Australia, that were not utilized by their adult counterparts. Similarly, Simpfendorfer et al. (2010) found that adult smalltooth sawfish (Pristis pectinata) inhabited deeper water than juveniles. Therefore, if restocking is used as a conservation strategy for elasmobranch species, the age of the animals and the habitat use of each age group must be taken into consideration, especially if the release area is meant to take advantage of a marine reserve.
Conservation of Wobbegong Sharks
This study suggests that small marine reserves are likely to play a limited role in wobbegong conservation given the low overall residency and the extent of movement out of the reserve. This is further demonstrated by one of the study individuals having been caught by a commercial fisher only 15 km out of the reserve. Previous studies on other wobbegong species found that the sharks exhibited low site fidelity and were only present at localized study sites for a limited number of consecutive days (Carraro and Gladstone, 2006; Huveneers et al., 2006). The results of this study indicate there was clear seasonality in the movement of the sharks, with a higher probability of presence in the spring and summer months. This supports findings from a mark-recapture study conducted in the same study area (Lee et al., 2014), that found temporary emigration was based on austral season rather than breeding and non-breeding seasons. These results are supported by previous studies on sympatric species of wobbegong that also found stronger seasonal correlation with abundance than mating-related periods, per se (Carraro and Gladstone, 2006; Huveneers et al., 2009).
The wobbegong sharks tracked in this study display inter-annual site fidelity, returning to the same area every year. Exploitation of such aggregation areas could have high impacts for the local population. This suggests that fishing closures during the summer months in areas where wobbegongs are known to aggregate may contribute toward the conservation of, at least, part of the population.
Understanding the environmental drivers of coastal shark aggregations is critical to developing effective conservation and management plans. Being able to predict when they may occur would allow fisheries managers to implement a spatio-temporal system of management options, including temporal fishing closures during periods of peak abundance. We found that the primary drivers for determining wobbegong abundance and distribution were day length and water temperature. Many shark species use photoperiod (day length) and water temperature as migratory cues (Milner-Gulland and Fryxell, 2011; Dudgeon et al., 2013), and use environmental signals to form mating aggregations (Pratt and Carrier, 2001). The probability of a wobbegong being present was lowest in the autumn, a time when the water temperature peaks and abundance of temperate fish species is highest (e.g., Irigoyen et al., 2013), suggesting that other factors such as mating may be driving movements in these animals. Ideally, future studies should assess predator and prey abundance and movements concurrently to model and differentiate effects from environmental and prey fluctuations.
Effects of Environmental Variables on Detection Rate
By utilizing passive acoustic telemetry in this study, we were able to track the tagged wild and captive-bred sharks over a large spatial and temporal scale. However, environmental variables can affect the detection probability of transmissions by acoustic tags, and subsequently influence the inferences made from animal detections (Payne et al., 2010) and must be considered when designing a study. We found that for this presence-absence study the effects of changing detection probabilities may not be as dramatic as in analyses based on the total count of detections, e.g., changes in diel behavior. A minimum threshold by which an animal is considered present can be set to allow for a reduced detection probability, while still accounting for the chance of false detections. This study found that day/night, wind direction, and air pressure were the strongest influences on the detection probability of control tags. The strong effect of time of day is unsurprising given the results of previous studies that have showed the presence of nocturnally active crustaceans have been shown to reduce detection frequency (Heupel et al., 2006; Payne et al., 2010). However, the remaining results must be extrapolated to other studies with caution. The study site was a bay that is very sheltered from strong winds from all directions except north-westerlies, which explains the reduced detectability observed with wind from this direction as any winds would cause localized surface currents and increase the amount of background noise. The effect of wind speed may therefore be more apparent in more open, exposed environments and subsequently may influence the detectability of tags more than wind direction, such as found by Gjelland and Hedger (2013). However, it was not possible to incorporate wave height or ocean current into the analyses used in this study, as there were no local data although the strong effect from air pressure may indicate that sea swell would affect the detectability. Water temperature may also play an important role in studies conducted in the open ocean, especially in areas with high isothermals. The presence of thermoclines has previously been found to affect the detection range of receivers (Singh et al., 2009) and thus would affect the probability of detections. However, this study was conducted in a shallow, near-shore area where the receivers were above thermocline depth (Gray and Kingsford, 2003).
Future Research
While the use of acoustic telemetry allowed us to meet the aims of this study, the use of a more spatially detailed biologging method, such as GPS tracking or satellite tags, may be more appropriate to address such questions as the survival rate of animals released. To further investigate the affect that age at release has on the behavior and survival of captive-bred elasmobranchs, studies should also endeavor to release both groups at the same time or under the same environmental conditions to remove the potential for seasonal movements affecting the behavior of the released animals. This was not possible in this study due to the novelty of this research, which meant that the options for further releases were dependent on the success of the first one.
This study suggests that well managed re-stocking programs may enhance wild elasmobranch populations, however, lessons learnt from similar research on teleost should be assessed and quantified. First, the genetic diversity of the captive bred populations would need to be carefully maintained to ensure the fitness of the released individuals (Fraser, 2008). Second, an assessment of the genetic diversity of the wild populations before and after the releases should occur (Ward, 2006). Finally, rearing conditions of the captive individuals should enhance natural behavior to enhance their likelihood of post-release survival (Salvanes and Braithwaite, 2006). These conditions should form the basis of further research into restocking of elasmobranch populations.
Conclusions
In this study, we have shown that released captive-bred sharks can successfully forage, avoid predators, and inhabit areas populated by wild wobbegongs. Although the slow life history characteristics for most elasmobranchs may preclude large-scale restocking, we have demonstrated that for some species successfully kept in captivity there may be the opportunity to contribute to the conservation measures. While restocking of sharks per se is unlikely to be effective in the absence of other measures, as has been shown in critically endangered terrestrial species, the release of captive-bred elasmobranchs has the potential to be a valuable conservation tool.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This project was funded by grants from SeaWorld Research and Rescue Foundation, PADI Aware, SEA LIFE Conservation Fund, Oceania Chondrichthyan Society/Passions of Paradise and co-investment from the Office of Environment NSW. Thanks to the Integrated Marine Observing system Animal Tagging and Monitoring System (IMOS-AATAMS) for in-kind contributions and the AATAMS community for sharing their data on the AATAMS database. IMOS is supported by the Australian Government through the National Collaborative Research Infrastructure Strategy and the Super Science Initiative. KL was supported by a Macquarie University Research of Excellence Scholarship. Sea Life and Manly OceanWorld staff are thanked for their support and provision of the captive-bred sharks. Thanks are also extended to all the volunteers who helped with the collection of the data. This project was approved by the NSW Fisheries Animal Care and Ethics Committee (ACEC ref: 07/08) and NSW Scientific Collection permit (P08/0039). Thank you to the Sydney Institute of Marine Science for technical support. This is a SIMS contribution (number 0098).
Supplementary Material
The Supplementary Material for this article can be found online at: http://www.frontiersin.org/journal/10.3389/fmars.2015.00018/abstract
References
Barton, K. (2012). MuMIn: Multi-Model Inference [Online]. R Package Version 1.7.7. Available online at: http://CRAN.R-project.org/package=MuMIn
Bates, D., and Maechler, M. (2010). lme 4. Linear Mixed-Effects Models using S4 Classes. Available online at: http://CRAN.R-project.org/package=lme4
Bell, J. D., Bartley, D. M., Lorenzen, K., and Loneragan, N. R. (2006). Restocking and stock enhancement of coastal fisheries: potential, problems and progress. Fish. Res. 80, 1–8. doi: 10.1016/j.fishres.2006.03.008
Blanchet, S., Páez, D. J., Bernatchez, L., and Dodson, J. J. (2008). An integrated comparison of captive-bred and wild Atlantic salmon (Salmo salar): implications for supportive breeding programs. Biol. Conserv. 141, 1989–1999. doi: 10.1016/j.biocon.2008.05.014
Bolker, B. M., Brooks, M. E., Clark, C. J., Geange, S. W., Poulsen, J. R., Stevens, M. H. H., et al. (2009). Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol. Evol. 24, 127–135. doi: 10.1016/j.tree.2008.10.008
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bonfil, R. (1994). Overview of World Elasmobranch Fisheries. Rome: Food and Agriculture Organization of the United Nations.
Botsford, L. W., Castilla, J. C., and Peterson, C. H. (1997). The management of fisheries and marine ecosystems. Science 277, 509–515. doi: 10.1126/science.277.5325.509
Bryars, S., Rogers, P., Huveneers, C., Payne, N., Smith, I., and McDonald, B. (2012). Small home range in southern Australia's largest resident reef fish, the western blue groper (Achoerodus gouldii): implications for adequacy of no-take marine protected areas. Mar. Freshw. Res. 63, 552–563. doi: 10.1071/MF12016
Burnham, K. P., and Anderson, D. R. (2002). Model Selection and Multimodal Inference: A Practical Information-Theoretic Approach. New York, NY: Springer-Verlag.
Carraro, R., and Gladstone, W. (2006). Habitat preferences and site fidelity of the ornate Wobbegong Shark (Orectolobus ornatus) on rocky reefs of New South Wales. Pac. Sci. 60, 207–217. doi: 10.1353/psc.2006.0003
Champagnon, J., Elmberg, J., Guillemain, M., Gauthier-Clerc, M., and Lebreton, J.-D. (2012). Conspecifics can be aliens too: a review of effects of restocking practices in vertebrates. J. Nat. Conserv. 20, 231–241. doi: 10.1016/j.jnc.2012.02.002
Compagno, L. J. V. (2001). Sharks of the World. An Annotated and Illustrated Catalogue of Shark Species Known to Date. Volume 2. Bullhead, Mackerel and Carpet Sharks (Heterodontiformes, Lamniformes and Orectolobiformes). Rome: FAO.
Cribari-Neto, F., and Zeileis, A. (2010). Beta regression in R. J. Stat. Softw. 32, 1–24. Available online at: http://www.jstatsoft.org/v34/i02/
Dudgeon, C. L., Lanyon, J. M., and Semmens, J. M. (2013). Seasonality and site fidelity of the zebra shark, Stegostoma fasciatum, in southeast Queensland, Australia. Animal Behav. 85, 471–481. doi: 10.1016/j.anbehav.2012.12.013
Dulvy, N. K., Baum, J. K., Clarke, S., Compagno, L. J. V., Cortes, E., Domingo, A., et al. (2008). You can swim but you can't hide: the global status and conservation of oceanic pelagic sharks and rays. Aquat. Conserv. 18, 459–482. doi: 10.1002/aqc.975
Dulvy, N. K., Fowler, S. L., Musick, J. A., Cavanagh, R. D., Kyne, P. M., Harrison, L. R., et al. (2014). Extinction risk and conservation of the world's sharks and rays. Elife 3:e00590. doi: 10.7554/eLife.00590
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ferrari, S. L. P., and Cribari-Neto, F. (2004). Beta regression for modeling rates and proportions. J. Appl. Stat. 31, 799–815. doi: 10.1080/0266476042000214501
Ferretti, F., Worm, B., Britten, G. L., Heithaus, M. R., and Lotze, H. K. (2010). Patterns and ecosystem consequences of shark declines in the ocean. Ecol. Lett. 13, 1055–1071. doi: 10.1111/j.1461-0248.2010.01489.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Field, I. C., Meekan, M. G., Buckworth, R. C., and Bradshaw, C. J. A. (2009). Susceptibility of sharks, rays and chimaeras to global extinction. Adv. Mar. Biol. 56, 275–363. doi: 10.1016/S0065-2881(09)56004-X
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fraser, D. J. (2008). How well can captive breeding programs conserve biodiversity? A review of salmonids. Evol. Appl. 1, 535–586. doi: 10.1111/j.1752-4571.2008.00036.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gjelland, K. Ø., and Hedger, R. D. (2013). Environmental influence on transmitter detection probability in biotelemetry: developing a general model of acoustic transmission. Methods Ecol. Evol. 4, 665–674 doi: 10.1111/2041-210X.12057
Gray, C. A., and Kingsford, M. J. (2003). Variability in thermocline depth and strength, and relationships with vertical distributions of fish larvae and mesozooplankton in dynamic coastal waters. Mar. Ecol. Prog. Ser. 247, 211–224. doi: 10.3354/meps247211
Gusset, M., and Dick, G. (2011). The global reach of zoos and aquariums in visitor numbers and conservation expenditures. Zoo Biol. 30, 566–569. doi: 10.1002/zoo.20369
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Heithaus, M. R., Frid, A., Wirsing, A. J., and Worm, B. (2008). Predicting ecological consequences of marine top predator declines. Trends Ecol. Evol. 23, 202–210. doi: 10.1016/j.tree.2008.01.003
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Heupel, M. R., and Hueter, R. E. (2001). “Use of an automated acoustic telemetry system to passively track juvenile blacktip shark movements,” in Electronic Tagging and Tracking in Marine Fisheries, eds J. R. Sibert and J. L. Nielsen (Dordrecht: Kluwer Academic Publishers), 217–236. doi: 10.1007/978-94-017-1402-0_10
Heupel, M. R., Semmens, J. M., and Hobday, A. J. (2006). Automated acoustic tracking of aquatic animals: scales, design and deployment of listening station arrays. Mar. Freshw. Res. 57, 1–13. doi: 10.1071/MF05091
Holden, M. J. (1974). “Problem in the rational exploitation of elasmobranch populations and suggested solutions,” in Sea Fisheries Research, ed F. R. H. Jones (New York, NY: John Wiley & Sons), 117–137.
Holden, M. J. (1977). “Elasmobranchs,” in Fish Population Dynamics, ed J. A. Gulland (New York, NY: John Wiley & Sons), 187–215.
Hong, W., and Zhang, Q. (2003). Review of captive bred species and fry production of marine fish in China. Aquaculture 227, 305–318. doi: 10.1016/S0044-8486(03)00511-8
Huveneers, C., Harcourt, R. G., and Otway, N. M. (2006). Observation of localised movements and residence times of the wobbegong shark Orectolobus halei at Fish Rock, NSW, Australia. Cybium 30, 103–111.
Huveneers, C., Luo, K., Otway, N. M., and Harcourt, R. G. (2009). Assessing the distribution and relative abundance of wobbegong sharks (Orectolobidae) in New South Wales, Australia, using recreational scuba-divers. Aquat. Living Resour. 22, 255–264. doi: 10.1051/alr/2009046
Huveneers, C., Otway, N. M., and Harcourt, R. G. (2007a). Morphometric relationships and catch composition of wobbegong sharks (Chondrichthyes: Orectolobus) commercially fished in New South Wales, Australia. Proc. Linn. Soc. N. S. W. 23, 243–250.
Huveneers, C., Stead, J., Bennett, M. B., Lee, K. A., and Harcourt, R. G. (2013). Age and growth determination of three sympatric wobbegong sharks: how reliable is growth band periodicity in Orectolobidae? Fish. Res. 147, 413–425. doi: 10.1016/j.fishres.2013.03.014
Huveneers, C., Walker, T. I., Otway, N. M., and Harcourt, R. G. (2007b). Reproductive synchrony of three sympatric species of wobbegong shark (genus Orectolobus) in New South Wales, Australia: reproductive parameter estimates necessary for population modelling. Mar. Freshw. Res. 58, 765–777. doi: 10.1071/MF06187
Irigoyen, A. J., Galvan, D. E., Venerus, L. A., and Parma, A. M. (2013). Variability in abundance of temperate reef fishes estimated by visual census. PLoS ONE 8:e61072. doi: 10.1371/journal.pone.0061072
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Jule, K. R., Leaver, L. A., and Lea, S. E. G. (2008). The effects of captive experience on reintroduction survival in carnivores: a review and analysis. Biol. Conserv. 141, 355–363. doi: 10.1016/j.biocon.2007.11.007
La Mesa, G., Consalvo, I., Annunziatellis, A., and Canese, S. (2012). Movement patterns of the parrotfish Sparisoma cretense in a Mediterranean marine protected area. Mar. Environ. Res. 82, 59–68. doi: 10.1016/j.marenvres.2012.09.006
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lee, K., Huveneers, C., Gimenez, O., Peddemors, V., and Harcourt, R. (2014). To catch or to sight? A comparison of demographic parameter estimates obtained from mark-recapture and mark-resight models. Biodivers. Conserv. 23, 2781–2800. doi: 10.1007/s10531-014-0748-9
Maitland, P. S., and Evans, D. (1986). The role of captive breeding in the conservation of fish species. Int. Zoo Yearb. 24, 66–74. doi: 10.1111/j.1748-1090.1985.tb02521.x
Milner-Gulland, E. J., and Fryxell, J. M. (2011). Animal Migration: a Synthesis. Oxford: Oxford University Press. doi: 10.1093/acprof:oso/9780199568994.001.0001
Milot, E., Perrier, C., Papillon, L., Dodson, J. J., and Bernatchez, L. (2013). Reduced fitness of Atlantic salmon released in the wild after one generation of captive breeding. Evol. Appl. 6, 472–485. doi: 10.1111/eva.12028
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Myers, R. A., Baum, J. K., Shepherd, T. D., Powers, S. P., and Peterson, C. H. (2007). Cascading effects of the loss of apex predatory sharks from a coastal ocean. Science 315, 1846–1850. doi: 10.1126/science.1138657
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Myers, R. A., and Worm, B. (2003). Rapid worldwide depletion of predatory fish communities. Nature 423, 280–283. doi: 10.1038/nature01610
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
NSW DPI. (2006). Ocean Trap and Line Fishery Environmental Impact Statement. Cronulla, NSW: Public consultation document, NSW Department of Primary Industries.
Payne, N. L., Gillanders, B., Webber, D. M., and Semmens, J. M. (2010). Interpreting diel activity patterns from acoustic telemetry: the need for controls. Mar. Ecol. Prog. Ser. 419, 295–301. doi: 10.3354/meps08864
Pease, B. C., and Grinberg, A. (1995). New South Wales Commercial Fisheries Statistics 1940 to 1992. Cronulla, NSW: NSW Fisheries.
Pincock, D. G. (2011). False Detections: What They are and How to Remove them from Detection Data [Online]. Vemco. Available online at: http://www.vemco.com/pdf/false_detections.pdf. Document #: DOC-004691 Version 02 April 13, 2011.
Pratt, H. L. J., and Carrier, J. C. (2001). A review of elasmobranch reproductive behavior with a case study on the nurse shark, Ginglymostoma cirratum. Environ. Biol. Fish. 60, 157–188. doi: 10.1023/A:1007656126281
Purcell, S. W., and Kirby, D. S. (2006). Restocking the sea cucumber Holothuria scabra: sizing no-take zones through individual-based movement modelling. Fish. Res. 80, 53–61. doi: 10.1016/j.fishres.2006.03.020
R Core Development Team. (2009). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Salvanes, A. G. V., and Braithwaite, V. (2006). The need to understand the behaviour of fish reared for mariculture or restocking. ICES J. Mar. Sci. 63, 345–354. doi: 10.1016/j.icesjms.2005.11.010
Simpfendorfer, C. A., Wiley, T. R., and Yeiser, B. G. (2010). Improving conservation planning for an endangered sawfish using data from acoustic telemetry. Biol. Conserv. 143, 1460–1469. doi: 10.1016/j.biocon.2010.03.021
Singh, L., Downey, N. J., Roberts, M. J., Webber, D. M., Smale, M. J., Van Den Berg, M. A., et al. (2009). Design and calibration of an acoustic telemetry system subject to upwelling events. Afr. J. Mar. Sci. 31, 355–364. doi: 10.2989/AJMS.2009.31.3.8.996
Smith, S. E., Au, D. W., and Show, C. (1998). Intrinsic rebound potentials of 26 species of Pacific sharks. Mar. Freshw. Res. 49, 663–678. doi: 10.1071/MF97135
Speed, C. W., Meekan, M. G., Field, I. C., McMahon, C. R., Stevens, J. D., McGregor, F., et al. (2011). Spatial and temporal movement patterns of a multi-species coastal reef shark aggregation. Mar. Ecol. Prog. Ser. 429, 261–275. doi: 10.3354/meps09080
Stevens, J. D., Bonfil, R., Dulvy, N. K., and Walker, P. A. (2000). The effects of fishing on sharks, rays, and chimaeras (chondrichthyans), and the implications for marine ecosystems. ICES J. Mar. Sci. 57, 476–494. doi: 10.1006/jmsc.2000.0724
Taylor, S. M., and Bennett, M. B. (2013). Size, sex and seasonal patterns in the assemblage of Carcharhiniformes in a sub-tropical bay. J. Fish Biol. 82, 228–241. doi: 10.1111/jfb.12003
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Underwood, A. J., Kingsford, M. J., and Andrew, N. L. (1991). Patterns in shallow marine assemblages along the coast of New South Wales. Aust. J. Ecol. 6, 231–249. doi: 10.1111/j.1442-9993.1991.tb01050.x
Walker, T. I. (1998). Can shark resources be harvested sustainably? A question revisited with a review of shark fisheries. Mar. Freshw. Res. 49, 553–572. doi: 10.1071/MF98017
Ward, R. D. (2006). The importance of identifying spatial population structure in restocking and stock enhancement programmes. Fish. Res. 80, 9–18. doi: 10.1016/j.fishres.2006.03.009
Williams, S. E., and Hoffman, E. A. (2009). Minimizing genetic adaptation in captive breeding programs: a review. Biol. Conserv. 142, 2388–2400. doi: 10.1016/j.biocon.2009.05.034
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Worm, B., Davis, B., Kettemer, L., Ward-Paige, C. A., Chapman, D., Heithaus, M. R., et al. (2013). Global catches, exploitation rates, and rebuilding options for sharks. Mar. Policy 40, 194–204. doi: 10.1016/j.marpol.2012.12.034
Keywords: detection rate, IMOS, movement ecology, Orectolobus maculatus, passive acoustic telemetry
Citation: Lee KA, Huveneers C, Peddemors V, Boomer A and Harcourt RG (2015) Born to be free? Assessing the viability of releasing captive-bred wobbegongs to restock depleted populations. Front. Mar. Sci. 2:18. doi: 10.3389/fmars.2015.00018
Received: 15 December 2014; Accepted: 13 March 2015;
Published: 09 April 2015.
Edited by:
Graeme Clive Hays, Deakin University, AustraliaReviewed by:
Jonathan D. R. Houghton, Queen's University Belfast, UKLucy Alice Hawkes, University of Exeter, UK
Copyright © 2015 Lee, Huveneers, Peddemors, Boomer and Harcourt. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kathryn A. Lee, Biological Sciences, Macquarie University, North Ryde, Sydney, NSW 2109, Australia kate.asha.lee@gmail.com
 Kathryn A. Lee
Kathryn A. Lee Charlie Huveneers
Charlie Huveneers Victor Peddemors
Victor Peddemors Andrew Boomer5
Andrew Boomer5  Robert G. Harcourt
Robert G. Harcourt