Experimental assessment of cumulative temperature and UV-B radiation effects on Mediterranean plankton metabolism
- 1The UWA Oceans Institute and School of Plant Biology, University of Western Australia, Perth, WA, Australia
- 2Biological and Environmental Sciences and Engineering, Red Sea Research Center, King Abdullah University of Science and Technology, Jeddah, Saudi Arabia
The Mediterranean Sea is a vulnerable region for climate change, warming at higher rates compare to the global ocean. Warming leads to increased stratification of the water column and enhanced the oligotrophic nature of the Mediterranean Sea. The oligotrophic waters are already highly transparent, however, exposure of Mediterranean plankton to ultraviolet radiation (UV-B and UV-A) may increase further if the waters become more oligotrophic, thereby, allowing a deeper UV radiation penetration and likely enhancing impacts to biota. Here we experimentally elucidate the cumulative effects of warming and natural UV-B radiation on the net community production (NCP) of plankton communities. We conducted five experiments at monthly intervals, from June to October 2013, and evaluated the responses of NCP to ambient UV-B radiation and warming (+3°C), alone and in combination, in a coastal area of the northwest Mediterranean Sea. UV-B radiation and warming lead to reduced NCP and resulted in a heterotrophic (NCP < 0) metabolic balance. Both UV-B radiation and temperature, showed a significant individual effect in NCP across treatments and time. However, their joint effect showed to be synergistic as the interaction between them (UV × Temp) was statistically significant in most of the experiments performed. Our results showed that both drivers, would affect the gas exchange of CO2−O2 from and to the atmosphere and the role of plankton communities in the Mediterranean carbon cycle.
Introduction
The Mediterranean Sea is highly vulnerable to climate change (Turley, 1999; Giorgi, 2006; Herrmann et al., 2014), because of the long water residence time and warm waters derived from its semi-enclosed nature (only a narrow connection with the Atlantic Ocean and an artificial connection with the Read Sea) and the high evaporation rates (Bethoux et al., 1999). Indeed, warming of the Mediterranean is proceeding at over three times the average rate for the global ocean, identifying the Mediterranean Sea as a region of rapid climate change (Vargas-Yañez et al., 2007) and is predicted to continue to warm rapidly in the future (Somot et al., 2006; Jordà et al., 2012). Models predict an increase of 1–4°C in water temperature for the Mediterranean area at the end of the XXI century as a consequence of global warming (IPCC, 2013) with an increased frequency and intensity of heat waves (Jordà et al., 2012). Warming leads to increased stratification and decreased primary production, enhancing the oligotrophic nature of the Mediterranean Sea (Estrada, 1996). During summer and fall (June–October) the Mediterranean Sea is already highly oligotrophic and plankton metabolism is dominated by heterotrophic processes (Duarte et al., 2004; Navarro et al., 2004; Longhurst, 2007; Regaudie-de-Gioux et al., 2010). Future warming may, therefore, enhance the oligotrophic nature of the Mediterranean Sea.
The oligotrophic waters of the Mediterranean Sea are already highly transparent and ultraviolet radiation penetrates deep in doses sufficient to impact on organisms. For instance, Llabrés et al. (2010) reported that lethal UV doses required to decrease the picocyanobacteria populations by half, can penetrated down to 26 m in the Mediterranean Sea. Exposure of Mediterranean plankton to UV-B and UV-A may increase further if the waters become more oligotrophic, thereby allowing a deeper UV radiation penetration, likely enhancing impacts to biota.
Plankton communities play an important role in the carbon cycle through their physiological and metabolic processes, by controlling the air-sea exchange of CO2 by affecting the partial pressure of CO2 in surface waters (Gazeau et al., 2005; Calleja et al., 2013). Net community production (NCP) is a community level process that integrates all of the processes affecting the balance between consumption and production (NCP = Gross Primary Production − Community Respiration). This rate includes physiological and metabolic processes (such as respiration or photosynthesis), but also processes such as viral lysis, microzooplankton (mesozooplankton are excluded from the incubations) grazing, nutrient recycling, DOC excretion and mortality. Mediterranean plankton communities are affected by temperature (Duarte et al., 2004; Regaudie-de-Gioux and Duarte, 2012) and by natural UV radiation (Häder et al., 2007; Llabrés et al., 2010; Bertoni et al., 2011; Agusti et al., 2014; Regaudie-de-Gioux et al., 2014). Increased temperature leads to increased metabolic rates, albeit community respiration (CR) rates increase faster with warming than gross primary production (GPP) does (Duarte et al., 2004; Harris et al., 2006; Regaudie-de-Gioux and Duarte, 2012), therefore leading to a decline in NCP with warming. UV-B radiation has been reported to impact NCP affecting both autotrophic and heterotrophic processes (Agusti et al., 2014). However, UV-B generally leads to reduced NCP (Regaudie-de-Gioux et al., 2014) by enhancing respiration over photosynthesis (Agusti et al., 2014), as warming does.
However, the combined effect of warming and increased UV-B on plankton NCP has not been examined yet, although food web changes have been reported (Vidussi et al., 2011). Multiple stressors can interact amplifying or suppressing their effects. So, the combined effects can differ from the sum of the individual effects of each stressor (Vinebrooke et al., 2004; Christensen et al., 2006; Boyd and Hutchins, 2012). There is evidence that the effect of UV on aquatic organisms such as phytoplankton and zooplankon may be temperature dependent (Roos and Vincent, 1998; Williamson et al., 2002; MacFadyen et al., 2004; Halac et al., 2013).
However, plankton responses to warming and UVR appear to be rather idiosyncratic, as responses have been reported to vary among groups (e.g., Halac et al., 2013; Cabrerizo et al., 2014). NCP is an ecosystem-level property that integrates contrasting responses of different groups of eukaryotes, prokaryotes and protists, composing the microplankton communities and it also determines, the capacity to fix CO2 and the available production for the food web. A further complication, is that responses maybe dependent on the ambient temperature and UVR levels, so the responses may differ seasonally. Hence, evaluation of responses needs to consider the possible seasonal changes in the vulnerability of NCP to warming and UVR.
Here we experimentally assess the cumulative effects of warming and natural UV-B radiation on the NCP of plankton communities from the oligotrophic coastal waters of the NW Mediterranean Sea. We do so, by conducting experiments where at monthly intervals, from the late spring to the early fall, the responses to different levels of ambient temperature and natural incident UV-B radiation, alone and in combination, were evaluated.
Materials and Methods
Experiments were performed on a coastal area of Mallorca Island in the northwest Mediterranean Sea (Faro de Cap Ses Salines) during the stratified period, from June to October 2013 (39°15.52′N, 3°3.15′E). The study site is characterized by very oligotrophic conditions, reflected in deep underwater light penetration, (Secchi disk depth often >50 m), and very low chlorophyll a concentration, often <0.3 mg Chla m−3. Each month, surface water (1 m depth) was sampled using a 5 l Niskin bottle to evaluate community metabolism. Water was carefully siphoned into 10, 128 mL quartz bottles and into 21, 115 mL narrow-mouth glass Winkler bottles. Seven replicates of the glass bottles were used to determinate the initial oxygen concentration. Seven replicates of glass and five replicates of quartz bottles were incubated in natural light conditions and at the in situ temperature. The other set of replicates (seven glass and five quartz bottles) were incubated under natural light conditions and at 3°C above the in situ surface water temperature. The communities were incubated during 24 h in two 75-l tanks equipped with a cooling and heating system (Aquatic Nature micro Proccesor- 300W) to keep the in situ and the warmer water temperature. The incubation temperature for the “warming” treatment was set at 3°C above the ambient sea surface temperature to accommodate the central warming expected by year 2100 under the various scenarios of the IPCC (IPCC, 2013) and to allow the comparison between our results and those from others studies using a similar warming treatment (i.e., Vidussi et al., 2011). Moreover, as sea surface temperature shows diel fluctuations of 1°C or above, a warming treatment close to +1°C would not have represented a perturbation.
Quartz bottles allow the entire solar spectrum to pass through (photosynthetically active radiation- PAR- 400–700 nm, UV-A 400–320 nm and UV-B 320—80 nm), with more than 90% of UVB radiation passing through. In contrast, borosilicate glass Winkler bottles remove >90% of irradiance up to 315 nm (UV-B) and 50% of the radiation at 325 nm (UV-A). Hence, results derived from measurements conducted in quartz bottles represent the ambient light field, whereas those conducted in Winkler bottles test of the effect of removing UV-B and reducing partially UV-A at the shorter wavelengths off this band. Bottles were covered with neutral screens to reduce incident radiation by 20%, in addition to the 8–12% reduction in photosynthetically active radiation transmittance by the bottles themselves (Agusti et al., 2014), thereby representing the ambient irradiance at about 2–3 m depth.
Planktonic metabolism was evaluated from changes in dissolved oxygen concentrations along the incubations (Carpenter, 1965), which were determined by automated high-precision Winkler titration with a potentiometric end-point Metrohm 808 Titrando (Oudot et al., 1988). For both temperature treatments, NCP under full solar radiation and NCP when UV-B radiation was excluded (hereafter NCP and NCP−UV,respectively) were calculated from the difference between the oxygen concentration in the incubate borosilicate glass or quartz bottles and the initial fixed oxygen concentration (NCP = [O2] final glass/quartz bottles—[O2] initial bottles) after 24 h of incubation. Rates are expressed as mmol O2 m−3 d−1.
Chlorophyll a (Chl a) concentration was determined by filtering a 150 mL water sample through a Whatman GF/F (nominal pore size of 0.7 μm) filter, and extracting Chl a for 24 h with 90% acetone. Chl a concentration (μg Chl a L−1) was derived from the fluorescence of the extracts measured using a non-acidification module in a Trilogy fluorometer. Ultraviolet radiation values were obtained from a Kipp&Zonen UVA-B-T radiometer, which measures both UV-A and UV-B irradiance.
We present the analysis for each experiment separately, using an ANOVA and post-hoc Tukey HSD test to compare NCP among treatments (+UV; −UV; +UV+T; −UV+T). Also, NCP was fitted to a general linear model using least-squares regression to evaluate the effect of the individual factors (removal of UV radiation or increase seawater temperature) and their combined effect.
Results
Sampled surface seawater temperature varied from 22.1°C in June to a maximum of 27.3°C in August, decreasing through September to reach 21.7°C in October (Table 1). The daily UV-B doses at surface decrease from June (40.56 kJ m−2) to October (9.69 kJ m−2, Table 1). Chlorophyll a concentrations varied across months, reaching a minimum in June with 0.03 mg Chl a m−3 and maximum values of 0.15 mg Chl a m−3 measured in August and October (Table 1).
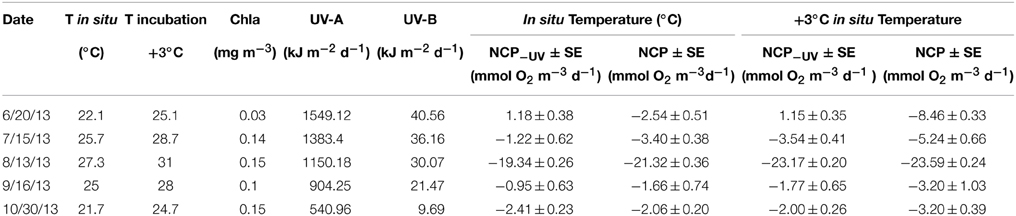
Table 1. In situ and warming incubation temperature (°C), chlorophyll a concentration (mg m−3), daily UV-A and B doses (kJ m−2 d−1), net community production (±SE) under full solar radiation (NCP) and when UV-B was removed (NCP−UV) in mmol O2 m−3 d−1 for each sampled month under both temperature treatments.
The communities tested were heterotrophic (NCP < 0) along the study, except in June when the communities showed an autotrophic metabolic balance (NCP > 0) when UV-B radiation was excluded and also at the warmer treatment (Table 1, Figure 1). NCP was enhanced when UV-B radiation was removed, by on average 2.15 ± 0.62 mmol O2 m−3 d−1 across months, except in October when the lowest doses of UV-B were measured, and NCP decreased 0.35 ± 0.30 mmol O2 m−3 d−1 when UV-B radiation was removed (Table 1, Figure 1). The lowest NCP rates were found during the month of August, when water temperature reached a maximum (Table 1, Figure 1).
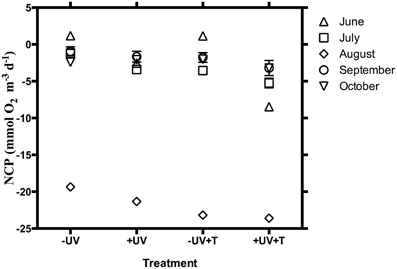
Figure 1. NCP ± SE (mmol O2 m−3 d−1) under the different treatments (−UV, +UV, −UV+T, +UV+T) for the 5 sampled months.
There were significant difference (paired t-test, p < 0.05, Table 2, Figure 2) between NCP at the in situ temperature and warming treatment when UV-B radiation was excluded (NCP−UV) except for June and September, while when the community received the full solar radiation, NCP decrease significantly at 3°C warming relative to that measured at the in situ temperature (paired t-test, p < 0.05) across all months except for September (Table 2, Figure 2).
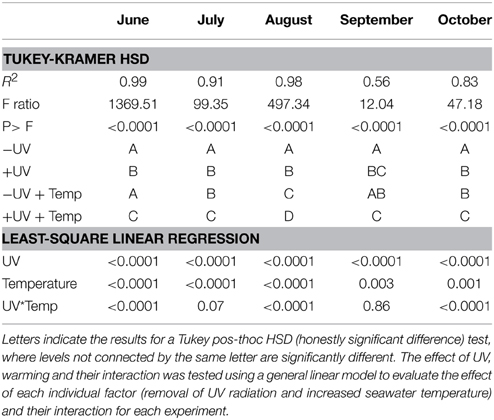
Table 2. ANOVA testing for the response of NCP among treatments (+UV, −UV; +UV+T; −UV + T) for each experiment performed.
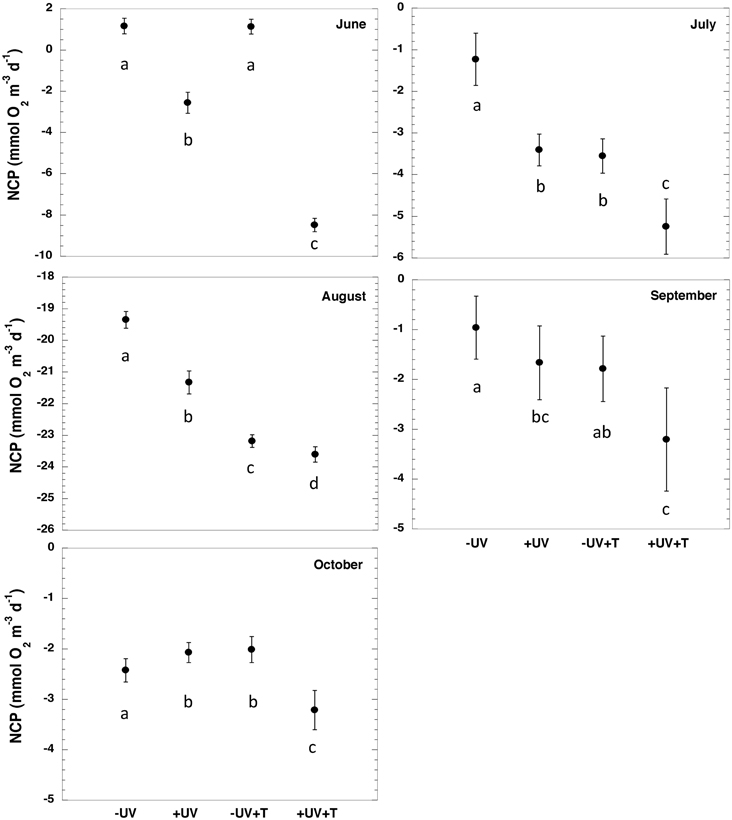
Figure 2. NCP ± SE (mmol O2 m−3 d−1) for the different treatments evaluated (−UV, +UV, −UV+T, +UV+T) for each sampled month. Letters indicate the results of the ANOVA and post-hoc Tukey HSD test where levels not connected by the same letter are significantly different.
The effect of UV-B on NCP, as the difference between NCP and NCP−UV, was strongly dependent on the UV-B dose received (R2 = 0.94, p = 0.006), with the magnitude of the suppression of NCP increasing at higher daily UV-B doses (Figure 3). However, this strong correlation is lost when the communities were exposed to warming (R2 = 0.35, p > 0.05).
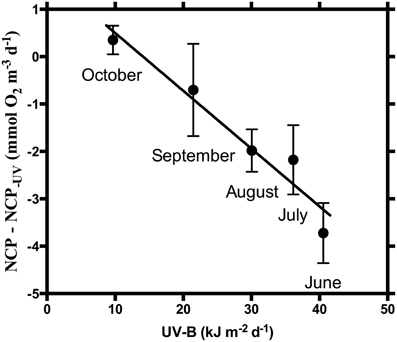
Figure 3. Difference between NCP and NCP−UV (mmol O2 m−3 d−1) at in situ temperature vs. daily incident UV-B radiation (kJ m−2). NCP-NCP−UV = 1.72 (±0.53) − 0.12 (±0.02) UV-B, R2 = 0.94, p = 0.006.
The removal of UV-B radiation showed a strong significant effect (paired t-test p < 0.0001) on NCP (Table 2). Warming also showed a significant effect across all months where experiments were performed (Table 2). The combination of both factors, UV-B radiation (presence/removal) and temperature (in situ/warmed), showed a significant synergistic effect on NCP (t-test UV × Temp, p < 0.05), in three of the five months when experiments were conducted (Table 2). Hence, the average NCP for all the experiments performed decreased when seawater temperature was warmed up +3°C and communities received UV-B radiation (Figure 4).
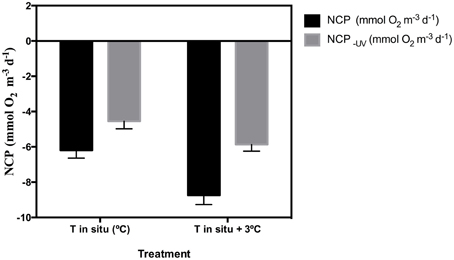
Figure 4. Mean (±SE) for the five sampled months of NCP under full solar radiation and NCP−UV where UVR is excluded (mmol O2 m−3 d−1) for the in situ temperature (control) and the warming treatment (in situ plus 3°C).
Discussion
The results showed a clear negative trend in NCP with warming and incident UV-B. Negative NCP characterized the plankton community in the oligotrophic Mediterranean waters as heterotrophic (NCP < 0) during the summer and fall period, consistent with previous reports of the prevalence of heterotrophic communities elsewhere in the Mediterranean in summer and fall (Satta et al., 1996; Duarte et al., 2004; Navarro et al., 2004; Regaudie-de-Gioux et al., 2010). In addition to anthropogenic inputs, riverine and atmospheric inputs (Guerzoni et al., 1999) can act as an important source of organic carbon into the Mediterranean waters (Dachs et al., 2005; Jurado et al., 2008; Bonilla-Findji et al., 2010), providing the allochtonous carbon required to support net heterotrophic communities. Whereas there is no surface runoff in Mallorca Island, its karstic nature leads to significant groundwater input (Basterretxea et al., 2010), which could also provide organic carbon supporting excess respiration.
The heterotrophic nature of Mediterranean plankton communities examined was enhanced by both ambient UV-B and warming, with the effect of UV-B (and partial UV-A) on NCP being dependent of the incident UV-B radiation. The effect of UV-B on NCP is, thus, strongest in June, when UV-B reaches a maximum and declines along the summer, as NCP becomes more negative with increasing temperatures as well.
Here we cannot elucidate whether NCP decreases with UV-B or temperature due to a decline in gross primary production (GPP) or to an increase in community respiration (CR). Previous works have demonstrate that both, impacts on GPP and CR can occur, as Bertoni et al. (2011) measured photoinhibition of 32–42% in primary production and a 50–70% inhibition in bacterial production due to solar radiation (UVR+PAR) in surface waters. The responses of NCP to temperature and UV radiation reported do not necessarily reflect physiologic or metabolic responses, but integrate also responses involving interactions within the microbial community, such as microzooplankton grazing of bacteria or phytoplankton, virus lysis and nutrient release and uptake. For instance, Agusti et al. (2014) showed that the responses of NCP to UV-B radiation integrated a complex suite of responses including suppression of bacterial growth by UV-B radiation and enhancement of bacterial growth in the dark due to increased liability of photoproducts. Likewise warming led to changes in the size structure of the phytoplankton community, leading to an increase in small-size phytoplankton species in detriment of larger ones (Moline et al., 2004). Moreover, the nutrients status of the community has been showed to be an important factor determining the variability in the ratio of microzooplankton herbivore to phytoplankton growth. Chen et al. (2012) reported that warming may enhance phytoplankton losses to microzooplankton herbivore in eutrophic but not in oligotrophic regions, such as the Mediterranean waters investigated here.
The Metabolic Theory of Ecology (Brown et al., 2004) predicts an increase in metabolic rates when temperature increases, although not at the same kinetics, as CR increases quicker and higher than GPP rates (Harris et al., 2006; López-Urrutia et al., 2006; Regaudie-de-Gioux and Duarte, 2012; Garcia-Corral et al., 2014). Regaudie-de-Gioux and Duarte (2012), derived an activation energy for the GPP/CR ratio of plankton communities of 0.52 ± 0.09 eV, indicating that they are expected to become heterotrophic (GPP/CR < 1) at temperatures >21°C. This is consistent with the prevalence of heterotrophic communities throughout our study, where ambient temperature were always >21°C. Our experimental results suggest that increased UV-B and warming, further suppress NCP, leading to more heterotrophic metabolism in Mediterranean plankton communities, although their effects are synergistic (Boyd and Hutchins, 2012) in most of the experiments performed. Changes in UVR could be an important factor influencing the dynamics and distribution of cyanobacteria populations in the surface waters of this oligotrophic sea (Llabrés et al., 2010). Changes in community structure have been reported by Mostajir et al. (1999) were UV-B radiation reduces large plankton species resulting in accumulation of small microorganisms, so changes in the carbon transfer cycles can be derived.
These stresses are connected, as warming is likely to lead to increased underwater UVR doses in the future (Häder et al., 2007). However, the interaction between these two drivers (UVR and temperature) is not straightforward as more factors affects, as for instance, the nutrient input (Häder et al., 2014). Moreover, contrary results showing opposite interaction between UVR and temperature have been observed in the microbial foodweb structure of freshwater plankton communities (Rae and Vincent, 1998; Persaud and Williamson, 2005). Our results showed that UVR and warming have relatively simple effects on NCP, possibly because NCP is an integrative property that brings together impacts at multiple levels, which may be individually complex, onto a more simple higher-level outcome.
The combined, an independent effect, of both drivers, temperature increase and UV-B radiation, to shift net plankton metabolism toward more heterotrophic rates portends more heterotrophic plankton communities in the future, as the Mediterranean Sea is expected to warm further with climate change (Jordà et al., 2012; IPCC, 2013) possibly resulting in oligotrophication and further penetration of already elevated UV-B radiation in the European regions as a result of stratospheric ozone depletion (Reuder et al., 2001; Weatherhead and Andersen, 2006). Mediterranean plankton communities already tend to act as sources of CO2 to the atmosphere (Satta et al., 1996; Duarte et al., 2004; Navarro et al., 2004; Regaudie-de-Gioux et al., 2010). Hence, our results suggest that the role of Mediterranean plankton communities as CO2 from to the atmosphere may increase further in the future, also reducing the flux of organic carbon flowing up the food web.
Author Contributions
SA, CD, and LG designed the study, JM conducted the measurements, SA, CD, and LG analyzed the results and all authors contributed to writing the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research is a contribution to the ESTRESX project, funded by the Spanish Ministry of Economy and Competitiveness (ref. CTM2012-32603). LG was funded by a JAE-Predoc Malaspina fellowship (CSIC-FBBVA, Spain).
References
Agusti, S., Regaudie-de-Gioux, A., Arrieta, J. M., and Duarte, C. M. (2014). Consequences of UV-enhanced community respiration for plankton metabolic balance. Limnol. Ocean. 59, 223–232. doi: 10.4319/lo.2014.59.1.0223
Basterretxea, G., Tovar-Sanchez, A., Beck, A. J., Masqué, P., Bokuniewicz, H. J., Coffey, R., et al. (2010). Submarine groundwater discharge to the coastal environment of a Mediterranean Island (Majorca, Spain): ecosystem and Biogeochemical Significance. Ecosystems 13, 629–643. doi: 10.1007/s10021-010-9334-5
Bertoni, R., Jeffrey, W. H., Pujo-Pay, M., Oriol, L., Conan, P., and Joux, F. (2011). Influence of water mixing on the inhibitory effect of UV radiation on primary and bacterial production in Mediterranean coastal water. Aquat. Sci. 73, 377–387. doi: 10.1007/s00027-011-0185-8
Bethoux, J. P., Gentili, B., Morin, P., Nicolas, E., Pierre, C., and Ruiz-Pino, D. (1999). The Mediterranean Sea: a miniature ocean for climatic and environmental studies and a key for the climatic functioning of the North Atlantic. Prog. Oceanogr. 44, 131–146. doi: 10.1016/S0079-6611(99)00023-3
Bonilla-Findji, O., Gattuso, J.-P., Pizay, M.-D., and Weinbauer, M. G. (2010). Autotrophic and heterotrophic metabolism of microbial planktonic communities in an oligotrophic coastal marine ecosystem: seasonal dynamics and episodic events. Biogeosciences 7, 3491–3503. doi: 10.5194/bg-7-3491-2010
Boyd, P., and Hutchins, D. (2012). Understanding the responses of ocean biota to a complex matrix of cumulative anthropogenic change. Mar. Ecol. Prog. Ser. 470, 125–135. doi: 10.3354/meps10121
Brown, J. H., Gillooly, J. F., Allen, A. P., Savage, V. M., and West, G. B. (2004). Toward a metabolic theory of ecology. Ecology 85, 1771–1789. doi: 10.1890/03-9000
Cabrerizo, M. J., Carrillo, P., Villafañe, V. E., and Walter Helbling, E. (2014). Current and predicted global change impacts of UVR, temperature and nutrient inputs on photosynthesis and respiration of key marine phytoplankton groups. J. Exp. Mar. Biol. Ecol. 461, 371–380. doi: 10.1016/j.jembe.2014.08.022
Calleja, M. L., Duarte, C. M., Álvarez, M., Vaquer-Sunyer, R., Agusti, S., and Herndl, G. J. (2013). Prevalence of strong vertical CO2 and O2 variability in the top meters of the ocean. Glob. Biogeochem. Cycles 27, 941–949. doi: 10.1002/gbc.20081
Carpenter, J. H. (1965). The accuracy of the winkler method for dissolved oxygen analysis. Limnol. Oceanogr. 10, 135–140. doi: 10.4319/lo.1965.10.1.0135
Chen, B., Landry, M. R., Huang, B., and Liu, H. (2012). Does warming enhance the effect of microzooplankton grazing on marine phytoplankton in the ocean? Limnol. Oceanogr. 57, 519–526. doi: 10.4319/lo.2012.57.2.0519
Christensen, M. R., Graham, M. D., Vinebrooke, R. D., Findlay, D. L., Paterson, M. J., and Turner, M. A. (2006). Multiple anthropogenic stressors cause ecological surprises in boreal lakes. Glob. Change Biol. 12, 2316–2322. doi: 10.1111/j.1365-2486.2006.01257.x
Dachs, J., Calleja, M. L., Duarte, C. M., del Vento, S., Turpin, B., Polidori, A., et al. (2005). High atmosphere-ocean exchange of organic carbon in the NE subtropical Atlantic. Geophys. Res. Lett. 32, L21807. doi: 10.1029/2005gl023799
Duarte, C. M., Agustí, S., and Vaqué, D. (2004). Controls on planktonic metabolism in the Bay of Blanes, northwestern Mediterranean littoral. Limnol. Oceanogr. 49, 2162–2170. doi: 10.4319/lo.2004.49.6.2162
Garcia-Corral, L. S., Barber, E., Regaudie-de-Gioux, A., Sal, S., Holding, J. M., Agusti, S., et al. (2014). Temperature dependence of planktonic metabolism in the subtropical North Atlantic Ocean. Biogeosciences 11, 4529–4540. doi: 10.5194/bg-11-4529-2014
Gazeau, F., Duarte, C. M., Gattuso, J.-P., Barrón, C., Navarro, N., Ruiz, S., et al. (2005). Whole-system metabolism and CO2 fluxes in a Mediterranean Bay dominated by seagrass beds (Palma Bay, NW Mediterranean). Biogeosciences 2, 43–60. doi: 10.5194/bg-2-43-2005
Giorgi, F. (2006). Climate change hot-spots. Geophys. Res. Lett. 33, L08707. doi: 10.1029/2006GL025734
Guerzoni, S., Chester, R., Dulac, F., Herut, B., Loÿe-Pilot, M.-D., Measures, C., et al. (1999). The role of atmospheric deposition in the biogeochemistry of the Mediterranean Sea. Prog. Oceanogr. 44, 147–190. doi: 10.1016/S0079-6611(99)00024-5
Häder, D. P., Villafañe, V. E., and Helbling, E. W. (2014). Productivity of aquatic primary producers under global climate change. Photochem. Photobiol. Sci. 13, 1370–1392. doi: 10.1039/C3PP50418B
Häder, D.-P., Kumar, H. D., Smith, R. C., and Worrest, R. C. (2007). Effects of solar UV radiation on aquatic ecosystems and interactions with climate change. Photochem. Photobiol. Sci. 6, 267. doi: 10.1039/b700020k
Halac, S. R., Guendulain-García, S. D., Villafañe, V. E., Helbling, E. W., and Banaszak, A. T. (2013). Responses of tropical plankton communities from the Mexican Caribbean to solar ultraviolet radiation exposure and increased temperature. J. Exp. Mar. Biol. Ecol. 445, 99–107. doi: 10.1016/j.jembe.2013.04.011
Harris, L. A., Duarte, C. M., and Nixon, S. W. (2006). Allometric laws and prediction in estuarine and coastal ecology. Estuaries Coasts 29, 343–347. doi: 10.1007/BF02782002
Herrmann, M., Estournel, C., Adloff, F., and Diaz, F. (2014). Impact of climate change on the northwestern Mediterranean Sea pelagic planktonic ecosystem and associated carbon cycle. J. Geophys. Res. Oceans 119, 5815–5836. doi: 10.1002/2014jc010016
IPCC. (2013). Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, eds T. F. Stocker, D. Qin, G.-K. Plattner, M. Tignor, S. K. Allen, J. Boschung, et al. (Cambridge; New York, NY: Cambridge University Press), 1535.
Jordà, G., Marbà, N., and Duarte, C. M. (2012). Mediterranean seagrass vulnerable to regional climate warming. Nat. Clim. Change 2, 821–824. doi: 10.1038/nclimate1533
Jurado, E., Dachs, J., Duarte, C. M., and Simó, R. (2008). Atmospheric deposition of organic and black carbon to the global oceans. Atmos. Environ. 42, 7931–7939. doi: 10.1016/j.atmosenv.2008.07.029
Llabrés, M., Agusti, S., Alonso-Laita, P., and Herndl, G. (2010). Synechococcus and Prochlorococcus cell death induced by UV radiation and the penetration of lethal UVR in the Mediterranean Sea. Mar. Ecol. Prog. Ser. 399, 27–37. doi: 10.3354/meps08332
Longhurst, A. R. (2007). Ecological Geography of the Sea. Amsterdam; Boston, MA: Academic Press. Available online At: http://search.ebscohost.com/login.aspx?direct=true&scope=site&db=nlebk&db=nlabk&AN=182190 [Accessed August 4, 2014].
López-Urrutia, Á., San Martin, E., Harris, R. P., and Irigoien, X. (2006). Scaling the metabolic balance of the oceans. Proc. Natl. Acad. Sci. U.S.A. 103, 8739–8744. doi: 10.1073/pnas.0601137103
MacFadyen, E. J., Williamson, C. E., Grad, G., Lowery, M., Jeffrey, W. H., and Mitchell, D. L. (2004). Molecular response to climate change: temperature dependence of UV-induced DNA damage and repair in the freshwater crustacean Daphnia pulicaria. Glob. Change Biol. 10, 408–416. doi: 10.1111/j.1529-8817.2003.00750.x
Moline, M. A., Claustre, H., Frazer, T. K., Schofield, O., and Vernet, M. (2004). Alteration of the food web along the Antarctic Peninsula in response to a regional warming trend. Glob. Change Biol. 10, 1973–1980. doi: 10.1111/j.1365-2486.2004.00825.x
Mostajir, B., Demers, S., de Mora, S., Belzile, C., Chanut, J.-P., Gosselin, M., et al. (1999). Experimental test of the effect of ultraviolet-B radiation in a planktonic community. Limnol. Oceanogr. 44, 586–596. doi: 10.4319/lo.1999.44.3.0586
Navarro, N., Agustí, S., and Duarte, C. M. (2004). Plankton metabolism and dissolved organic carbon use in the Bay of Palma, NW Mediterranean Sea. Aquat. Microb. Ecol. 37, 47–54. doi: 10.3354/ame037047
Oudot, C., Gerard, R., Morin, P., and Gningue, I. (1988). Precise shipboard determination of dissolved oxygen (Winkler Procedure) for productivity studies with a commercial system. Limnol. Oceanogr. 33, 146–150. doi: 10.4319/lo.1988.33.1.0146
Persaud, A. D., and Williamson, C. E. (2005). Ultraviolet and temperature effects on planktonic rotifers and crustaceans in northern temperate lakes. Freshw. Biol. 50, 467–476. doi: 10.1111/j.1365-2427.2005.01334.x
Rae, R., and Vincent, W. F. (1998). Effects of temperature and ultraviolet radiation on microbial foodweb structure:potential responses to global change. Freshw. Biol. 40, 747–758. doi: 10.1046/j.1365-2427.1998.00361.x
Regaudie-de-Gioux, A., Agusti, S., and Duarte, C. M. (2014). UV sensitivity of planktonic net community production in ocean surface waters. J. Geophys. Res. Biogeosci. 119, 929–936. doi: 10.1002/2013JG002566
Regaudie-de-Gioux, A., and Duarte, C. M. (2012). Temperature dependence of planktonic metabolism in the ocean. Glob. Biogeochem. Cycles 26, GB1015. doi: 10.1029/2010gb003907
Regaudie-de-Gioux, A., Vaquer-Sunyer, R., and Duarte, C. M. (2010). Patterns in planktonic metabolism in the Mediterranean Sea. Biogeosciences 6, 3081–3089. doi: 10.5194/bg-6-3081-2009
Reuder, J., Dameris, M., and Koepke, P. (2001). Future UV radiation in Central Europe modelled from ozone scenarios. J. Photochem. Photobiol. B 61, 94–105. doi: 10.1016/S1011-1344(01)00143-9
Roos, J. C., and Vincent, W. F. (1998). Temperature dependence of UV radiation effects on Antarctic cyanobacteria. J. Phycol. 34, 118–125. doi: 10.1046/j.1529-8817.1998.340118.x
Satta, M. P., Agustí, S., Mura, M. P., Vaqué, and D., Duarte, C. M. (1996). Microplankton respiration and net community metabolism in a bay on the NW Mediterranean coast. Aquat. Microb. Ecol. 10, 165–172. doi: 10.3354/ame010165
Somot, S., Sevault, F., and Déqué, M. (2006). Transient climate change scenario simulation of the Mediterranean Sea for the twenty-first century using a high-resolution ocean circulation model. Clim. Dyn. 27, 851–879. doi: 10.1007/s00382-006-0167-z
Turley, C. M. (1999). The changing Mediterranean Sea—a sensitive ecosystem? Prog. Oceanogr. 44, 387–400. doi: 10.1016/S0079-6611(99)00033-6
Vargas-Yañnez, M., Martinez, M. C., Moya-Ruiz, F., Tel, E., Parrilla, G., Plaza, F., et al. (2007). Cambio Climaìtico en el Mediterraìneo Español. Madrid: Instituto Español de Oceanografiìa.
Vidussi, F., Mostajir, B., Fouilland, E., Le Floch, E., Nouguier, J., Roques, C., et al. (2011). Effects of experimental warming and increased ultraviolet B radiation on the Mediterranean plankton food web. Limnol. Oceanogr. 56, 206–218. doi: 10.4319/lo.2011.56.1.0206
Vinebrooke, R. D., Cottingham, K. L., Norberg, J., Martin, S., Dodson, S. I, Maberley, S. C., et al (2004). Impacts of multiple stressors on biodiversity and ecosystem functioning: the role of species co-tolerance. Oikos 104, 451–457. doi: 10.1111/j.0030-1299.2004.13255.x
Weatherhead, E. C., and Andersen, S. B. (2006). The search for signs of recovery of the ozone layer. Nature 441, 39–45. doi: 10.1038/nature04746
Keywords: UV-B radiation, temperature, combined effects, plankton, net community production, Mediterranean Sea
Citation: Garcia-Corral LS, Martinez-Ayala J, Duarte CM and Agusti S (2015) Experimental assessment of cumulative temperature and UV-B radiation effects on Mediterranean plankton metabolism. Front. Mar. Sci. 2:48. doi: 10.3389/fmars.2015.00048
Received: 24 March 2015; Accepted: 23 June 2015;
Published: 07 July 2015.
Edited by:
Peng Xiu, Chinese Academy of Sciences, ChinaReviewed by:
Aleksandra M. Lewandowska, Carl von Ossietzky University Oldenburg, GermanyPengfei Lin, Chinese Acadamic of Science, China
Copyright © 2015 Garcia-Corral, Martinez-Ayala, Duarte and Agusti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lara S. Garcia-Corral, The UWA Oceans Institute and School of Plant Biology, University of Western Australia, 39 Fairway Street, Crawley, Perth, WA 6009, Australia, lara.garcia-corral@uwa.edu.au
 Lara S. Garcia-Corral
Lara S. Garcia-Corral Juan Martinez-Ayala
Juan Martinez-Ayala Carlos M. Duarte
Carlos M. Duarte Susana Agusti
Susana Agusti