Variability in photosynthetic production of dissolved and particulate organic carbon in the North Pacific Subtropical Gyre
- 1Department of Oceanography, University of Hawaii at Manoa, Honolulu, HI, USA
- 2Daniel K. Inouye Center for Microbial Oceanography: Research and Education, University of Hawaii at Manoa, Honolulu, HI, USA
The partitioning of photosynthetically-derived organic carbon between particulate and dissolved phases has important implications for marine carbon cycling. In this study we utilized 14C-bicarbonate assimilation to quantify rates of photosynthetic production of both particulate and dissolved organic carbon (DOC) at Station ALOHA (22°45′N, 158°W) in the North Pacific Subtropical Gyre (NPSG). At near-monthly time scales over ~5 years, we examined retention of 14C-labeled organic matter by both glass fiber filters and 0.2 μm pore size polycarbonate membrane filters that are commonly used for measurements of 14C-based plankton productivity. Use of polycarbonate filters resulted in significantly lower (averaging 60%) estimates of 14C-production compared to glass fiber filters. Coincident measurements of chlorophyll a concentrations from both 0.2 μm polycarbonate and glass fiber filters were not significantly different, suggesting the differences in 14C-productivity between these filter types did not derive from differences in retention of photosynthetic biomass by these filters. Moreover, consistent with previous studies, results from experiments aimed at quantifying retention of organic matter by these filters suggested differences resulted from retention of DOC by glass fiber filters. We also quantified rates of 14C-DOC production to evaluate the partitioning of photosynthetic production between dissolved and particulate phases over daily to monthly time scales in this ecosystem. Unlike the strong depth dependence observed in measurements of particulate organic carbon production, measured rates of 14C-DOC demonstrated no clear depth dependence. On average, depth-integrated (0–75 m) rates of 14C-DOC production rates were equivalent to 18 ± 10% of the total (particulate and dissolved) productivity. Our findings indicate that in this oligotrophic ecosystem, rates of dissolved and particulate production can be temporally decoupled over daily to monthly time scales.
Introduction
Oceanic net primary production accounts for approximately 50 Pg C yr−1, and much of this productivity occurs in the vast, low nutrient subtropical ocean gyres (Behrenfeld and Falkowski, 1997; Field et al., 1998). Dissolved organic carbon (DOC), operationally defined as reduced carbon substrates passing through filters (typical pore sizes ranging 0.2–0.7 μm), constitutes >90% of total marine organic carbon inventories (Druffel et al., 1992; Hedges, 1992; Kaiser and Benner, 2009). Despite low inorganic nutrient concentrations throughout the upper euphotic zone of the subtropical gyres, concentrations of DOC are enriched in these ecosystems (Hansell et al., 2009). Hence, quantification of rates of DOC production and subsequent utilization are central to constraining carbon cycling in these systems.
A suite of processes can result in DOC production. These include direct release of organic material from phytoplankton cells either passively (Bjørnsen, 1988) or actively (Fogg, 1966; Lignell, 1990; Marañón et al., 2004). High light (Hellebust, 1965; Cherrier et al., 2014) and nutrient limitation (Lancelot, 1983; Conan et al., 2007; López-Sandoval et al., 2011) may also promote phytoplankton DOC release. Processes such as viral lysis and inefficient predation can also constitute major DOC production pathways (Lampert, 1978; Banse, 1995; Hygum et al., 1997; Wilhelm and Suttle, 1999; Møller et al., 2003; Møller, 2005; Suttle, 2005; Evans et al., 2009; Saba et al., 2011). Rates of DOC production are often measured by tracing phytoplankton assimilation of radiolabeled (14C) inorganic carbon and quantifying the subsequent accumulation of 14C-labeled DOC in seawater (Schindler et al., 1972; Baines and Pace, 1991; Carlson, 2002).
Measurement of 14C bicarbonate assimilation into autotrophic biomass (Steemann Nielsen, 1952) has proven a sensitive method for estimating primary productivity rates in aquatic ecosystems and is often reported as approximating net primary productivity (Peterson, 1980; Bender et al., 1987; Marra, 2009; Pei and Laws, 2013). However, studies utilizing this methodology often do not quantify rates of DOC production (hereafter termed 14C-DOC), or estimate respiratory losses during the incubation period; consequently, organic carbon production may be underestimated by this approach. Direct 14C-DOC measurements have been made in diverse aquatic ecosystems (Baines and Pace, 1991), with rates in the open oceans typically ranging between 10 and 40% of particulate carbon production (Karl et al., 1998; Carlson et al., 2000; Morán and Estrada, 2001; Teira et al., 2001, 2003; Marañón et al., 2004; Conan et al., 2007).
The choice of filters utilized for measurements of particulate carbon production is an important consideration. Several studies have compared the retention of organic matter by various types of filters commonly used in aquatic systems (Maske and Garcia-Mendoza, 1994; Chavez et al., 1995; Karl et al., 1998; Morán et al., 1999). Glass fiber filters commonly used for these measurements are known to retain both 14C-DOC and 14C-labeled particulate carbon (Karl et al., 1998). However, to date, there are relatively few reports describing how different filter types influence derived rates of 14C-productivity in the open ocean. In a comparative study of filter retention characteristics, Morán et al. (1999) reported greater retention of 14C-labeled organic material on glass fiber filters compared to polycarbonate filters. However, the study also observed differences in the retention efficiencies of these filters in different ecosystems, suggesting the structure of planktonic communities and the relative importance of DOC to total organic matter productivity by these communities influences the retention characteristics of these filters (Morán et al., 1999).
The North Pacific Subtropical Gyre (NPSG) is one of the largest open ocean habitats on the planet. Since 1988, the Hawaii Ocean Time-series (HOT) program has sustained near-monthly shipboard measurements at Station ALOHA (A Long-Term Oligotrophic Habitat Assessment; 22°45′N, 158°W) in the NPSG, where oligotrophic upper ocean waters exhibit seasonality in various biogeochemical processes and properties (Campbell et al., 1994; Winn et al., 1995; Karl et al., 2001; Landry et al., 2001; Letelier et al., 2004; Dore et al., 2008; Church et al., 2009). A number of previous studies indicate that rates of primary production at Station ALOHA demonstrate moderate seasonality, with rates higher in summer and lower in winter (Karl et al., 1996; Letelier et al., 1996; Quay et al., 2010; Church et al., 2013). A previous study quantifying 14C-DOC production rates at Station ALOHA revealed that 14C-DOC comprised a relatively large fraction (14–51%) of daily photosynthetic production (Karl et al., 1998). However, there is limited information on temporal variability associated with the partitioning of organic carbon production into dissolved and particulate phases in this ecosystem.
In the present study, we assess the magnitude and partitioning of primary production between particulate and dissolved pools at Station ALOHA. We evaluate retention characteristics of glass fiber and polycarbonate filters commonly used for measurements of 14C-based productivity and concentrations of chlorophyll a. Our results confirm that rates of 14C productivity were significantly greater when derived using glass fiber filters compared to polycarbonate filters, despite no significant differences in the retention of chlorophyll a by these filters. We also examined rates of 14C-DOC production to test the hypothesis that rates of 14C-DOC production would demonstrate similar time-varying patterns as rates of 14C-particulate carbon production. However, despite periods of moderate seasonality in photosynthetic production of particulate carbon, 14C-DOC production was more temporally variable than coincident rates of 14C-particulate carbon production.
Materials and Methods
Chlorophyll a and 14C-based Productivity Measurements
Sampling for this study was conducted at near-monthly time scales at Station ALOHA on HOT program cruises during two separate periods, October 2004 to October 2007 and April 2010 to October 2012. During the initial period of the study (2004–2007), we compared retention characteristics of 25 mm diameter 0.2 μm pore size polycarbonate membrane filters (Millipore) and 25 mm diameter glass fiber filters (Whatman GF/F) for subsequent analyses of both chlorophyll a concentrations and rates of 14C-productivity. To compare retention of chlorophyll a by these filter types, paired seawater samples were collected from pre-dawn hydrocasts using a conductivity-temperature-density (CTD) rosette sampler equipped with 12 L polyvinyl chloride bottles. Seawater from six discrete depths (5, 25, 45, 75, 100, and 125 m) was sampled from the CTD rosette bottles into 150 ml amber polyethylene bottles. The entire 150 ml sample was filtered onto either polycarbonate or glass fiber filters. Filters were immersed in 5 ml of 100% HPLC grade acetone in 7 ml glass culture tubes and placed at –20°C to passively extract. After 7 days (Letelier et al., 1996), tubes were removed from the freezer, filters were removed, and extracted chlorophyll in the acetone was quantified using a Turner Designs Model 10-AU fluorometer (Strickland and Parsons, 1972).
Sampling for 14C-based measurements of particulate production occurred on near-monthly HOT program cruises throughout the study period. We measured rates of 14C-assimilation into particulate carbon using polycarbonate filters to harvest plankton biomass (hereafter 14C-PC), and compared these rates to coincident (in both time and depth) core HOT program measurements of 14C-assimilation into plankton biomass (Letelier et al., 1996), based on use of glass fiber filters (hereafter 14C-GFF). During the latter period of observations (2010–2013) we also measured 14C-DOC production rates. Seawater for the 14C-based productivity measurements was collected from the same predawn CTD hydrocasts sampled for chlorophyll a concentrations. Water for the productivity measurements was sampled from the CTD rosette bottles into acid-cleaned 500-ml polycarbonate bottles. A total of four replicate 500 ml bottles were subsampled per depth and each bottle was spiked with ~1.85 MBq 14C-bicarbonate. One hundred milliliters from one replicate per depth was immediately vacuum filtered through a polycarbonate filter; these “time zero” filtrates provided a 14C-DOC blank and provided information on background adsorption of inorganic 14C to the filters. Time zero filters were placed in 20 ml glass scintillation vials (Kimble Chase) and stored at −20°C until shore-based laboratory processing. The remaining three bottles were hung on a free-drifting array, deployed before dawn, and incubated at their initial collection depths throughout the photoperiod (typically 11–13 h). After sunset the array was recovered, and 100 ml subsamples of all bottles were filtered under gentle vacuum (< 50 mm Hg) onto polycarbonate filters that were then placed in scintillation vials and frozen (−20°C). The total radioactivity added to each sample bottle was determined by subsampling 250 μl aliquots into scintillation vials containing 500 μl of β-phenylethylamine. At the shore-based laboratory, filters were acidified by the addition of 1 ml of 2 M hydrochloric acid (HCl) and allowed to passively vent (uncapped) for ~24 h to remove inorganic 14C. Ten milliliters of Ultima Gold LLT liquid scintillation cocktail was added to all vials (acidified filters and vials for determining total radioactivity) and the resulting radioactivity was determined using a Perkin Elmer 2600 liquid scintillation counter; glass fiber filters were recounted after 30 days (Karl et al., 1998).
Measurements of 14C-DOC Production
Over a ~2.5-year period (April 2010–October 2012), we measured 14C-DOC production from the same vertical profiles used for determination of 14C-PC production by utilizing filtrates derived from 14C-PC rate measurements. These 0.2 μm filtrates were collected from both time zero samples and triplicate bottles incubated in situ on the free-drifting array into 125 ml polyethylene amber bottles and stored frozen (−20°C) until subsequent processing for determination of 14C-DOC productivity. In the shore-based laboratory, these samples were processed following the 14C-DOC methodology described in Karl et al. (1998). Briefly, 100 ml of the 14C-PC filtrates were thawed, poured into 500 ml polyethylene separatory funnels, and acidified by the addition of 500 μl of 2 M sulfuric acid (H2SO4). Samples were vigorously bubbled with air in a fume hood to remove 14CO2. After at least 6 h of bubbling, a 70 ml subsample was removed from each separatory funnel and poured into a 100 ml glass serum bottle containing 1 ml of 2 M sodium hydroxide (NaOH) and 10 ml of 0.37 M potassium persulfate (K2S2O8) in 1 M NaOH. Bottles were sealed with rubber stoppers, crimp sealed with an aluminum cap, and autoclaved at 126°C for 200 min; oxidizing 14C-DOC to 14C-labeled dissolved inorganic carbon (14C-DIC) in an alkaline solution. Once cooled to room temperature, samples were uncapped and resealed using rubber sleeve stoppers holding plastic center wells containing ~2 × 2 cm pieces of fluted chromatographic filter paper (Whatman 2) soaked with 0.2 ml of β-phenylethylamine. A syringe was used to inject 4 ml of 9 N H2SO4 into the solution, converting the 14C-DIC to 14CO2. Samples were stored undisturbed at room temperature, passively trapping the 14CO2 on the β-phenylethylamine soaked wick. After 5 days, rubber sleeve stoppers were removed and center wells and wicks were placed in scintillation vials, followed by the addition of 10 ml of Ultima Gold LLT scintillation cocktail. Samples were subsequently counted on a Perkin Elmer Tri-Carb 2800TR liquid scintillation counter. Rates of 14C-DOC production were computed for each cruise as the mean of the triplicate bottles from each depth minus the average 14C-activity of the time zero (blank) samples. We defined a limit of detection for the 14C-DOC analyses per cruise as the value of the mean time zero “blank” samples for that cruise plus three times the standard deviation of those time zero “blank” samples (Skoog and Leary, 1992). Measurements falling below the detection limit were assigned a value of zero for subsequent analyses, including calculation of mean rates.
Diel Variability in Productivity
On three separate occasions (April 2013, May 2013, and June 2013), we conducted experiments to examine short-term (diel) variability in production and loss of 14C-PC, 14C-GFF, and 14C-DOC. Triplicate 500 ml samples were collected from 25 m depth from pre-dawn CTD hydrocasts, inoculated with ~1.85 MBq 14C-bicarbonate, and placed in a surface seawater cooled incubator shaded to 50% incident irradiance. Samples were incubated for varying lengths of time: predawn until noon (~6 h), full photoperiod (predawn to dusk, ~12 h), or over a full day (predawn to predawn, ~24 h). Following incubation, samples were filtered and processed as previously described for determination of 14C-PC, 14C-GFF, and 14C-DOC. Rates of 14C-DOC below the limit of detection were assigned a value of zero for these analyses.
Filter Retention Characteristics
We conducted an experiment designed to specifically evaluate retention characteristics of glass fiber and polycarbonate filters for measurements of 14C-productivity and chlorophyll a (October 2014). For this experiment, 500 ml seawater samples were collected from the near-surface (25 m) ocean, inoculated with ~1.85 MBq 14C-bicarbonate and incubated from dawn to dusk in temperature controlled, shaded (50% incident irradiance) incubators. After sunset, triplicate 100 ml subsamples were vacuum filtered onto both polycarbonate and glass fiber filters individually, and onto these filters in series (i.e., glass fiber filters underlain by polycarbonate filter or polycarbonate underlain by glass fiber filter). Filters were placed in scintillation vials, acidified, and processed as previously described for determination of 14C activities. From the same water sample, we also compared retention of planktonic chlorophyll a by glass fiber and polycarbonate filters; 125 ml was collected and filtered onto either a glass fiber filter, a polycarbonate filter, or onto these filters in series (as above). Chlorophyll concentrations were determined via fluorometric analysis as previously described.
An additional experiment (October 2014) was conducted to evaluate trapping of 14C-organic carbon by glass fiber filters. Replicate 500 ml polycarbonate bottles containing whole seawater from Station ALOHA were inoculated with ~1.85 MBq 14C-bicarbonate and incubated in a temperature controlled and shaded incubator for the duration of the photoperiod. After dusk, incubations were terminated by filtering the sample bottles onto polycarbonate filters. The resulting filtrates were collected and triplicate 100 ml volumes were sequentially filtered onto new glass fiber filters, resulting in a filtrate that had been filtered a total of five separate times through five different glass fiber filters. In addition, triplicate 500 ml samples of the original 0.2 μm filtrate were filtered onto glass fiber filters to evaluate possible volume-dependent differences in the trapping of 14C-DOC by these filters. The resulting 14C activities associated with each glass fiber filter were determined as previously described.
We also evaluated the effects of filtering different volumes of the 14C-productivity samples onto glass fiber and polycarbonate filters. For these comparisons, we examined paired primary production samples collected from the upper ocean (< 45 m) at Station ALOHA, where 100 ml of seawater was filtered onto a polycarbonate filter, while varying volumes of seawater (100, 150, 400, or 500 ml) were filtered onto glass fiber filters. These comparative measurements were used to calculate the difference between the derived rates of 14C-productivity on the glass fiber and polycarbonate filters (14C-delta =14C-GFF – 14C-PC). Results from this comparison provided information on whether differences in retention of 14C-organic carbon by glass fiber and polycarbonate filters in our time-series measurements might reflect differences in the volume of seawater filtered for these measurements (i.e., 500 ml onto glass fiber vs. 100 ml onto polycarbonate filters).
Contextual Biogeochemical Analyses
Seawater samples for measurements of nutrient concentrations (nitrate + nitrite, N + N; soluble reactive phosphorus, SRP) were collected in 125 or 500 ml acid-washed polyethylene bottles and stored upright in a freezer for analysis on shore. Concentrations of N + N were determined using the high sensitivity chemiluminescent technique (Garside, 1982; Dore and Karl, 1996); SRP concentrations were analyzed using the magnesium-induced co-precipitation (MAGIC) method (Karl and Tien, 1992). Daily fluxes of photosynthetically active radiation (PAR; 400 to 700 nm wavelength) were measured on HOT cruises using a deckboard LI-COR LI-192 cosine collector. Vertical profiles of downwelling PAR were measured daily at noon using a Satlantic HyperPro radiometer. Coincident measurements of incident PAR were collected using a deckboard radiometer (Satlantic); these measurements were used to derive diffuse attenuation coefficients (KPAR) for each cruise. Derived KPARvalues, together with daily integrated incident PAR measurements, were utilized to compute daily downwelling flux of PAR at discrete depths.
Data Analyses and Statistics
We evaluated seasonality in upper ocean properties and rates of 14C-productivity by binning our data into predefined seasons based on the solstices and equinoxes (i.e., Spring: March 20 to June 20; Summer: June 21 to September 22; Fall: September 23 to December 20; and Winter: December 21 to March 19). Analysis of variance (ANOVA) was used to examine possible seasonality in vertically-binned (0–45 m and 75–125 m) volumetric rates of 14C-GFF, 14C-PC, and 14C-DOC. We also examined temporal variability in rates of productivity using various time-series statistical models, including an optimized least squares monthly regression approach described in Llope et al. (2007), and the Lomb-Scargle periodogram for unevenly sampled time-series data (Scargle, 1982). We first used these techniques to test for seasonality in the near-monthly, depth-integrated (0–75 m) HOT 14C-GFF measurements collected between 1989 and 2013 (see http://hahana.soest.hawaii.edu/hot/hot-dogs/interface.html). We then examined the time-series of rate measurements (14C-GFF, 14C-PC, and 14C-DOC) from the two periods (i.e., October 2004 to October 2007 and April 2010 to October 2012) sampled in the current study using these statistical techniques. The Lomb-Scargle periodogram analysis was performed in the R statistical environment (R Development Core Team, 2008) using the “lomb” package (Ruf, 1999). The Llope et al. (2007) model was fit to the data using MATLAB (MathWorks). Depth-integrated rates and stocks were calculated using trapezoidal integration. Data were tested for normality, and if not normally distributed, were log10 transformed; when transformed data failed to conform to the assumption of normality, nonparametric statistical methods were utilized. For statistical analyses of ratios (e.g., 14C-DOC: 14C-PC), geometric rather than arithmetic means and standard deviations were used (Zar, 1999). For computing mean rates of 14C-DOC, measured rates falling below the limit of detection were designated as having a value of zero.
To evaluate the relationship between in situ PAR and measured rates of productivity, the derived daily PAR fluxes and measured rates of production were fitted to photosynthetic-irradiance (P-E) relationships using the equation of Platt et al. (1980):
where P is the rate of carbon fixation, Pmax is the maximum rate of photosynthesis without photoinhibition, E is the light flux (PAR), α is the initial slope of the curve (representing the rate of maximum light utilization), and β is the rate of photoinhibition. These relationships were examined for rates of 14C-GFF, 14C-PC, and 14C-DOC. From these relationships values for Ek (the irradiance necessary to saturate carbon fixation) were calculated as follows:
HOT program measurements utilized in this study (nutrients, PAR, chlorophyll a, and rates of 14C-GFF production) are available via the HOT program data website (http://hahana.soest.hawaii.edu/hot/hot-dogs/). Rates of 14C-DOC and 14C-PC are available via the Center for Microbial Oceanography: Research and Education (C-MORE) data system (http://cmore.soest.hawaii.edu/datasearch/data.php).
Results
Biogeochemical Context
Consistent with HOT program sampling of Station ALOHA, upper ocean concentrations of inorganic nutrients were very low throughout the period of this study, with near-surface (5 m) concentrations of N + N persistently < 3 nM and SRP averaging 66 nM. In the dimly-lit regions of the lower euphotic zone (100–125 m) concentrations of N + N increased and became more variable, ranging between 0.2 and 3.0 μM (Figure 1). The penetration of PAR decreased more than two orders of magnitude through the upper 125 m of the water, with fluxes at the sea surface ranging from ~11 to 57 mol quanta m−2 d−1 and decreasing to 0.02–0.8 mol quanta m−2 d−1 by 125 m. Incident PAR also demonstrated significant seasonal variability (One-Way ANOVA, p < 0.0001), with fluxes ranging between 11 and 42 mol quanta m−2 d−1 in the winter, increasing approximately 2-fold (on average) in the summer (ranging from 32 to 57 mol quanta m−2 d−1; Table 1). Concentrations of chlorophyll a were consistently elevated in the lower euphotic zone (70–140 m; Figure 1).
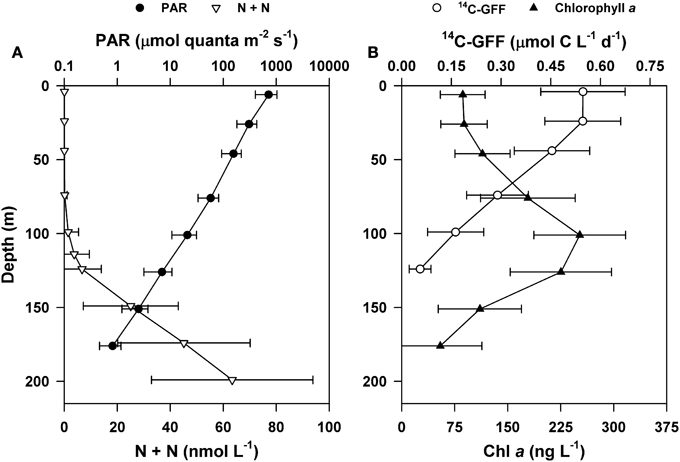
Figure 1. Depth profiles of mean upper ocean properties at Station ALOHA during the period of study (2004–2013). (A) Concentrations of nitrate + nitrite (N + N; open triangles) and flux of photosynthetically active radiation (PAR; closed circles). (B) Concentrations of chlorophyll a (Chl a; closed triangles) and rates of 14C-GFF based primary production (open circles). Error bars are ± 1 standard deviation of the time-averaged means.
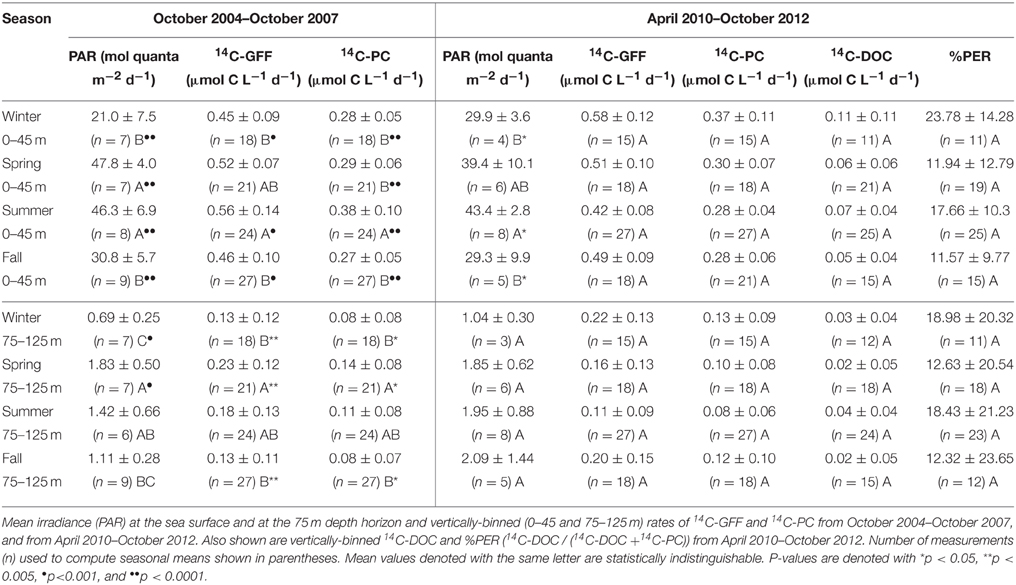
Table 1. Seasonally averaged (± standard deviations) rates of productivity and irradiance for the two time periods of this study.
Measurements of 14C-productivity and Chlorophyll a
We examined vertical variability associated with 14C- based productivity at Station ALOHA over the two time periods sampled as part of the current study (October 2004–October 2007 and April 2010–October 2012). Rates of 14C-PC and HOT program measurements of 14C-GFF demonstrated similar depth-dependent patterns and temporal variability. Average rates of 14C-PC and 14C-GFF decreased ~3-fold between the well-lit upper ocean waters (< 45 m; average PAR flux at 45 m of 4.1 ± 1.7 mol quanta m−2 d−1) and the lower euphotic zone (75–125 m; Figure 2). HOT program measurements of 14C-GFF averaged 0.52 ± 0.12 μmol C L−1 d−1 in the upper euphotic zone, decreasing and becoming more temporally variable (0.17 ± 0.13 μmol C L−1 d−1) in the light-limited regions of the lower euphotic zone (Figures 2, 3). Upper euphotic zone 14C-PC rates averaged 0.32 ± 0.08 μmol C L−1 d−1 and decreased to a mean value of 0.10 ± 0.07 μmol C L−1 d−1 near the base of the euphotic zone (Figures 2, 3). Both volumetric and depth-integrated (0–125 m) rates of 14C-GFF were significantly greater (by ~1.7-fold, on average) than coincident measurements of 14C-PC (Figure 2; Kruskal-Wallis, p < 0.0001).
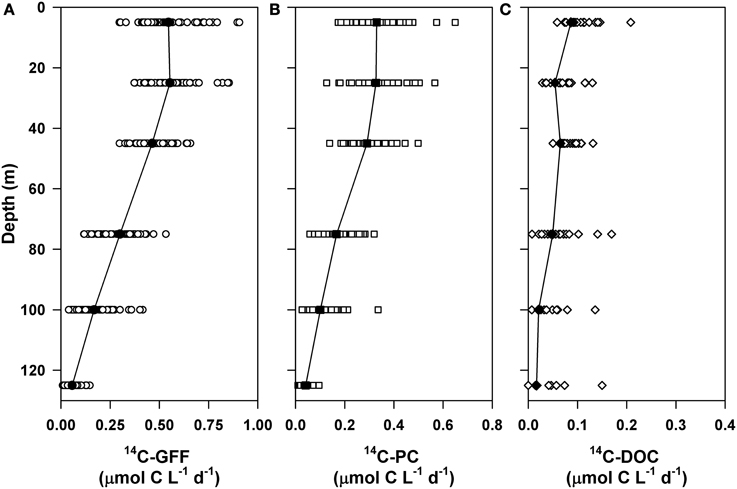
Figure 2. Vertical profiles of rates of 14C-based primary production filtered onto (A) glass fiber filters (14C-GFF) and (B) 0.2 μm polycarbonate membrane filters (14C-PC). Also shown are (C) rates of 14C-DOC production.
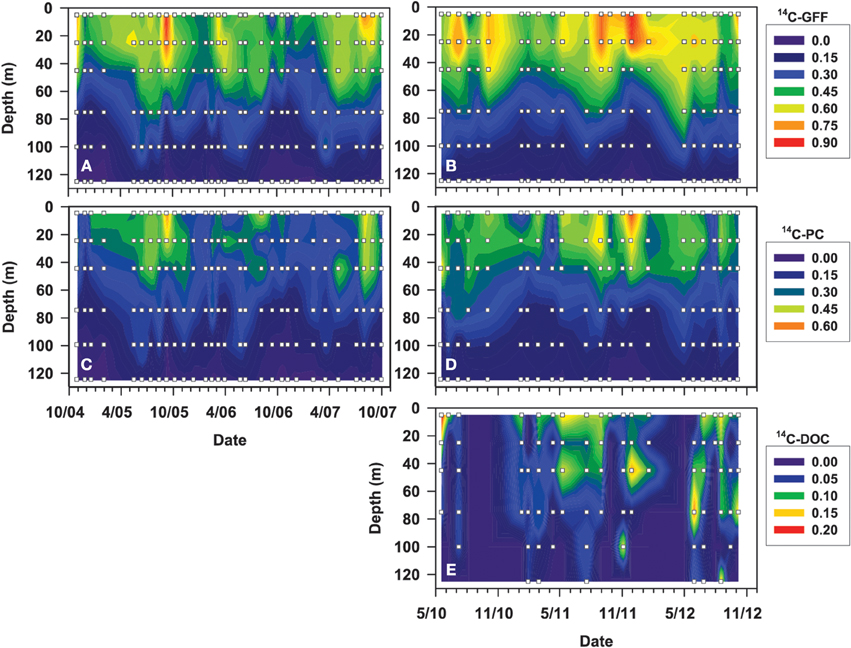
Figure 3. Contour plots depicting vertical distributions of rates of 14C-GFF (A,B), 14C-PC (C,D), and 14C-DOC (E), in μmol C L−1 d−1, over the time periods (October 2004–October 2007 and April 2010–October 2012) evaluated for this study. White squares depict depths and dates where detectable rates were measured.
By comparing concentrations of chlorophyll a and rates of 14C-productivity measured using polycarbonate filters to coincident HOT program measurements made using glass fiber filters we were able to examine differences in the retention characteristics of these two types of filters (Figure 4). This comparison revealed that volumetric concentrations and vertically integrated (0–125 m) inventories of chlorophyll a derived from both polycarbonate and glass fiber filters were statistically indistinguishable (Kruskal-Wallis, p > 0.05; Figure 4).
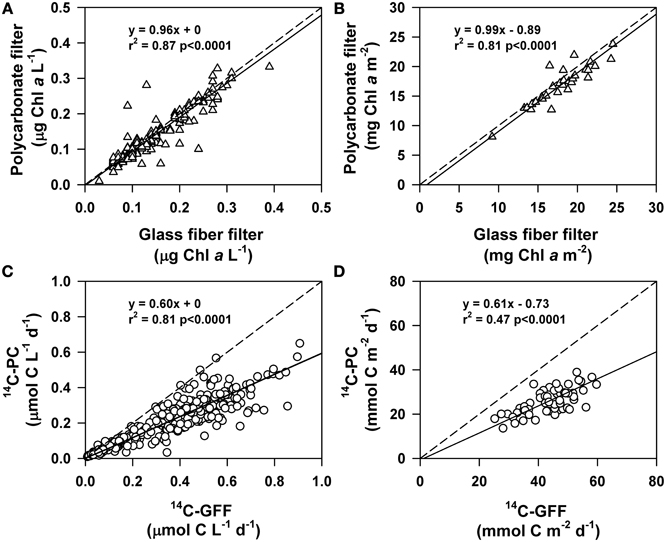
Figure 4. Comparison of measurements of chlorophyll a and 14C-primary production on either glass fiber or polycarbonate filters. Solid lines are Model II (geometric mean) linear regressions, while dashed lines depict the 1:1 ratio. Shown on each plot are the regression equation, the r2 of the relationship, and the p-value for (A) volumetric measurements of chlorophyll a, (B) depth-integrated (0–125 m) chlorophyll a, (C) volumetric measurements of 14C-primary production, and (D) depth-integrated (0–125 m) measurements of 14C-primary production.
To examine temporal variability in rates of 14C-DOC, we utilized the methodology described by Karl et al. (1998). The resulting precision of the derived rates, based on the coefficient of variation of triplicate 14C-DOC samples, ranged from 2 to 74% (averaging 29%). In comparison, the precision associated with the 14C-PC and 14C-GFF measurements ranged 0.3–70% (averaging 19%) and 0.5–50% (averaging 10%), respectively. More than half of 14C-DOC samples were above the calculated detection limit (defined as three times the standard deviation values of the mean time zero “blanks” for each cruise) in the three uppermost depths (5, 25, 45 m), but by 125 m less than 20% of 14C-DOC measurements were above the detection limit (Figure 3, Table 2). In contrast, measurements of 14C-PC and 14C-GFF were consistently above detection limits, irrespective of the depth sampled.
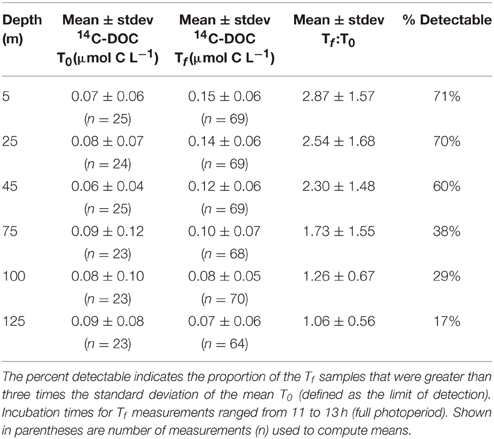
Table 2. Mean (± standard deviation; stdev) of 14C-DOC concentrations measured at the beginning (time zero; T0) and end of incubation period (time final; Tf) at six euphotic zone depths evaluated in this study.
Rates of 14C-DOC production were consistently lower than either 14C-PC or 14C-GFF (Figure 2). Upper ocean rates of 14C-DOC production averaged 0.07 ± 0.05 μmol C L−1 d−1 and decreased to 0.03 ± 0.04 μmol C L−1 d−1 in the lower euphotic zone; however, rates of 14C-DOC in the lower euphotic were frequently below detection (Table 2). The resulting depth-dependent decreases in rates of 14C-DOC were slightly less (~2.4-fold) than observed for either 14C-PC or 14C-GFF. Rates of 14C-DOC in the upper euphotic zone averaged ~21% of 14C-PC, while mean rates of 14C-DOC in the lower euphotic zone were equivalent to ~33% of 14C-PC (Figure 2). The resulting sum of the 14C-PC and 14C-DOC was consistently lower than the HOT program measurements of 14C-GFF; on average, the 14C-GFF rates were 1.4-fold greater than the sum of the coincident 14C-PC and 14C-DOC measurements.
We evaluated potential relationships between depth-dependent changes in PAR and the various 14C-based measurements of productivity using a hyperbolic photosynthesis-irradiance model (Platt et al., 1980). Although the model provided information on vertical relationships between 14C-GFF, 14C-PC and the downwelling light field, the relationship between light intensity and rates of 14C-DOC productivity was poorly described using this model (Table 3, Figure 5). Rates of both 14C-PC and 14C-GFF demonstrated similar patterns as a function of irradiance, increasing linearly with increasing light intensity in the lower euphotic zone, and saturating at light intensities (EK) ~1.5 mol quanta m−2 d−1 (Table 3). Throughout the study, the 1.5 mol quanta m−2 d−1 isolume varied between 35 and 97 m. Neither 14C-GFF nor 14C-PC demonstrated significant photoinhibition (Table 3). The initial slope (α) derived from the relationship between 14C-GFF and PAR was significantly greater than that derived from the relationship of 14C-PC to PAR (Table 3).
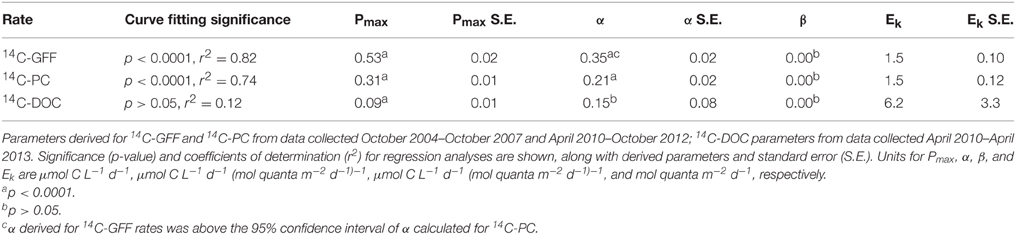
Table 3. Descriptive characteristics of production-irradiance curve fitting results based on the Platt et al. (1980) model.
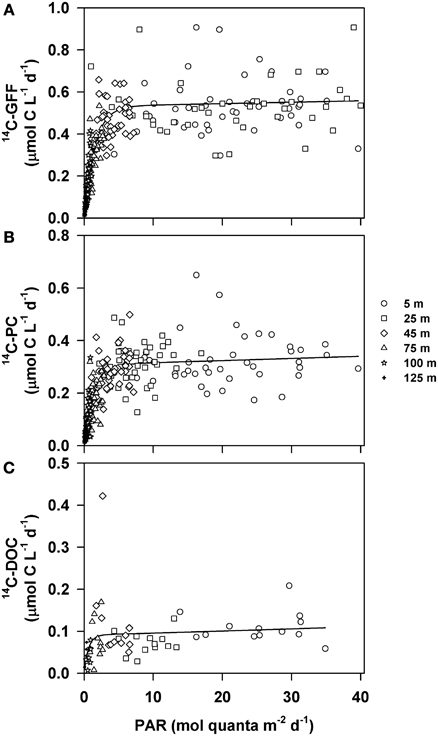
Figure 5. Relationships between downwelling photosynthetically active radiation (PAR) and rates of (A) 14C-GFF; (B) 14C-PC; and (C) 14C-DOC. Circles represent data points collected from 5 m, squares from 25 m, diamonds from 45 m, triangles from 75 m, stars 100 m and crosses from 125 m. Lines depict least-squares regression fits to the measured production rates and PAR using the Platt et al. (1980) formulation. Parameters describing the regression fits are provided in Table 2.
Temporal Variability in Rates of 14C-based Productivity
We examined temporal variability in the resulting time-series measurements of 14C-GFF, 14C-PC, and 14C-DOC productivity. As a result of the low detectability of 14C-DOC production rates in the lower euphotic zone, we confined our analysis of time variability in productivity to the upper 75 m. Depth-integrated (0–75 m) rates of 14C-GFF ranged between 21.8 and 48.7 mmol C m−2 d−1 (Figure 6) throughout the study, while rates (0–75 m) of 14C-PC production ranged between 11.4 and 31.5 mmol C m−2 d−1 (Figure 6). Rates of 14C-DOC productivity ranged from undetectable to 12.6 mmol C m−2 d−1 (Figure 6), and did not vary significantly with time-varying changes in 14C-GFF (Model II linear regression; r2 = 0.01, p > 0.2), rates of 14C-PC (Model II linear regression; r = 0.00, p > 0.4), or with the resulting differences in derived rates of 14C-production (14C-delta =14C-GFF −14C-PC) (Model II linear regression; r = 0.06, p > 0.15). The resulting depth-integrated rates of 14C-DOC were temporally variable, with rates varying ~9-fold (1.4 to 12.6 mmol C m−2 d−1) over the period of study, while rates of 14C-delta varied ~11-fold (2.6 to 27.8 mmol C m−2 d−1). In contrast, rates of 14C-GFF and 14C-PC varied by ~2 and ~3-fold, respectively (Figure 6).
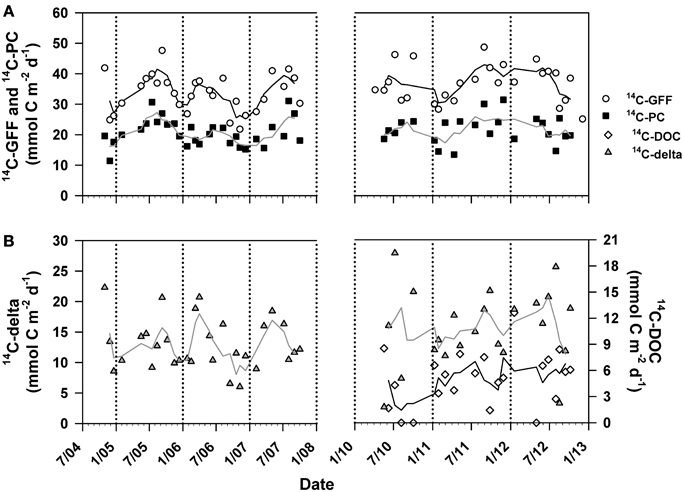
Figure 6. Time-series measurements of depth-integrated (0–75 m) rates of (A) 14C-GFF (open circles) and 14C-PC production (closed squares). Also depicted are depth-integrated (0–75 m) rates of (B) 14C-delta production (14C-GFF—14C-PC; gray triangles) and 14C-DOC production (open diamonds). Lines are 3-point running means. Break in time-series indicate period where measurements were not conducted. Rates of 14C-DOC below detection were considered to be equal to zero.
Binning our measurements by predefined seasons and examining possible seasonality in volumetric rates of 14C-DOC, 14C-PC, and 14C-GFF in both the well-lit, upper ocean (0–45 m) and dimly-lit, lower euphotic zone (75–125 m) highlighted apparent seasonal differences among the measures of productivity. When combining all the data collected for this study (October 2004–October 2007 and April 2010–October 2012), rates of both 14C-PC and 14C-GFF in the upper euphotic zone were significantly greater during the summer than during the winter (One-Way ANOVA; p < 0.01 and p < 0.05, respectively), while rates of 14C-DOC demonstrated no significant seasonality (One-Way ANOVA; p > 0.05). In the lower euphotic zone, rates of 14C-GFF were greater in the spring than during fall and winter (One-Way ANOVA; p < 0.0005), while rates in the summer were greater than rates measured in the fall (One-Way ANOVA; p < 0.0005). Lower euphotic zone 14C-PC rates were greater during the spring than during fall and winter, while rates of 14C-DOC were not significantly different among seasons (One-Way ANOVA; p < 0.005). However, when we considered the two periods measured during this study (Table 1), seasonal differences were only observed during the first period of this study. These differences were similar to those observed when both periods were considered together. In contrast, no significant differences were seen for any rates measured during the second period of observations (One-Way ANOVA; p > 0.05).
Results of the seasonal comparisons led us to analyze the resulting time-series measurements of 14C-DOC, 14C-delta, 14C-PC, and 14C-GFF using two different time-series statistical models. Application of the Lomb-Scargle periodogram (Ruf, 1999) to the full 14C-GFF time-series (1989–2013) revealed a significant periodicity at ~12 months (p < 0.0000005; data not shown), consistent with previously described annual cycle of primary productivity at Station ALOHA, where rates increase in summer compared to winter (Karl et al., 1996; Letelier et al., 1996; Church et al., 2013). However, use of the Lomb-Scargle periodogram to analyze the time-series rates (14C-DOC, 14C-PC, and 14C-GFF) either as a combined record (i.e., October 2004–October 2007 and April 2010–October 2012) or either record alone identified no significant periodicity (p > 0.05). Use of the model described in Llope et al. (2007) revealed similar results. Results from both the seasonal regression and the Lomb-Scargle periodogram suggested that the measurement records made during this study were of insufficient length to identify recurring temporal patterns.
Diel Variability in Rates of Productivity
We conducted three experiments designed to evaluate short-term (daily scale) variability in the various measures of 14C-productivity. For these experiments, we varied the incubation period for the 14C measurements, including samples incubated during the morning hours only (predawn to noon), the full photoperiod (predawn to dusk), and over a full 24 h period (predawn to the following predawn). These experiments demonstrated significant overnight losses of particulate 14C-labeled carbon relative to incubations conducted throughout the photoperiod (Figure 7). Hourly rates measured during morning hours only were not significantly different from hourly rates measured during the entire photoperiod for 14C-PC (One-Way ANOVA; p > 0.5). In contrast, hourly rates measured during the photoperiod were greater than those measured over the full 24 h for both 14C-GFF and 14C-PC (One-Way ANOVA; p < 0.001). The resulting hourly rates of 14C-PC and 14C-GFF production measured over a 24 h period were 37 ± 16% and 43 ± 17% of rates measured over the photoperiod, hence the amount of carbon fixed over 24 h averaged 75 ± 15% and 87 ± 8% of photoperiod carbon fixation for 14C-PC and 14C-GFF, respectively. In contrast, hourly rates of 14C-DOC and 14C-GFF were significantly greater for incubations during the morning hours (One-Way ANOVA; p < 0.05) than for incubations lasting the full photoperiod; photoperiod rates of 14C-DOC were 43 ± 17% of hourly rates measured during morning hours only. Rates of 14C-DOC measured over a 24 h period were not significantly different from rates measured over the photoperiod (One-Way ANOVA; p > 0.10).
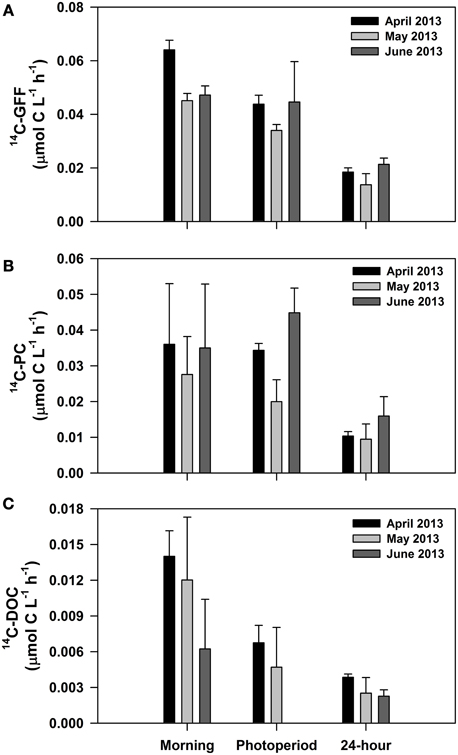
Figure 7. Results from experiments conducted in April, May, and June 2013 examining daytime and nighttime changes in hourly rates of carbon fixation. Depicted are average hourly rates of (A) 14C-GFF; (B) 14C-PC; and (C) 14C-DOC. All rates represent incubations initiated before dawn and terminated at different periods of the day (“morning” samples terminated at noon, “photoperiod” samples terminated at sunset, and “24-hour” samples terminated before dawn the following day).
14C-DOC Retention by Filter Type
We conducted several experiments designed to evaluate possible reasons for the greater retention of 14C-organic carbon on glass fiber filters relative to polycarbonate filters. The first set of experiments involved stacking polycarbonate and glass fiber filters in series for subsequent filtration of 14C-labeled whole seawater samples. When a glass fiber filter was stacked on top of a polycarbonate filter, the polycarbonate filter retained a small fraction (< 5%) of the total 14C activity associated with both filters (Supplementary Figure 1). In contrast, when a polycarbonate filter was overlaid on a glass fiber filter, the glass fiber filter retained >33% of the resulting total 14C-activity. Similar experiments conducted to examine filter retention of chlorophyll a through stacked glass fiber and polycarbonate filters revealed that irrespective of which filter type was on top, the bottom filter retained < 5% of the measured chlorophyll a (Supplementary Figure 1) of the top filter. Another experiment was conducted to evaluate successive retention of 14C-organic carbon by glass fiber filters. We refiltered 100 ml volumes of 0.2 μm 14C-PC filtrate onto a succession of glass fiber filters and found that retention of 14C labeled organic carbon by these filters decreased ~5-fold (to < 20% of the first 100 ml filtration) by the second filtration and ~8-fold (to 13% of the first 100 ml filtration) by the fourth filtration (Supplementary Figure 1). Additionally, the amount of 14C-DOC adsorbed onto the glass fiber filters following filtration of the first 100 ml of sample was not significantly different than the amount of 14C-DOC adsorbed after filtration of 500 ml (One-Way ANOVA; p > 0.05; Supplementary Figure 1) onto one filter. We also conducted an experiment to evaluate the effects of filtering different volumes of seawater onto both glass fiber and 0.2 μm polycarbonate filters (Supplementary Figure 2); the resulting differences in derived rates of 14C-delta did not vary with increasing volume filtered onto glass fiber filters, suggesting that the observed differences between the time-series based rates of 14C-GFF and 14C-PC was not an artifact of differences in filtration volumes used for these filters (500 vs. 100 ml, respectively; Supplementary Figure 2).
Discussion
The partitioning of organic carbon production between dissolved and particulate phases has important biogeochemical and ecological implications, and numerous hypotheses have been proposed to explain processes regulating the magnitude and variability of this partitioning (see review by Carlson, 2002). While photosynthetic production of particulate organic carbon (i.e., cellular material) can fuel numerous food web pathways (e.g., predation, viral lysis) and constitute a pathway for organic carbon export from the upper ocean (via gravitational settling or zooplankton migration), production of DOC subsidizes the energetic and nutritional demands of heterotrophic bacteria, fueling a grazing intensive microbial loop (Azam et al., 1983). Moreover, DOC can constitute an important component of carbon export, via removal through physical processes such as mixing and eddy diffusivity (Carlson et al., 1994; Emerson, 2014). Hence, quantifying the partitioning of organic carbon productivity through these distinct pathways is important for insight into the fate of recently fixed carbon through aquatic ecosystems.
In the current study, we examined rates of dissolved and particulate 14C-based measures of primary production at Station ALOHA. The resulting time-series measurements yielded insight into methodological considerations underlying application of the 14C-based production assays, and highlighted vertical and temporal variability in the partitioning of recently fixed carbon between particulate and dissolved pools in this ecosystem. Consistent with previous reports (Maske and Garcia-Mendoza, 1994; Karl et al., 1998; Morán et al., 1999), our study demonstrated greater (1.6-fold on average) 14C-productivity derived from samples filtered onto glass fiber filters (with a nominal pore size of 0.7 μm) relative to productivity rates derived from 0.2 μm polycarbonate membrane filters. In contrast, but consistent with a previous study (Chavez et al., 1995), simultaneous fluorometric determinations of chlorophyll a concentrations filtered onto polycarbonate and glass fiber filters revealed no consistent difference between these filter types for retention of chlorophyll a, suggesting the observed differences between 14C-GFF and 14C-PC did not derive from differences in the efficiency with which these filters trap photosynthetic organisms.
Various studies have documented adsorption of DOC by glass fiber filters (Abdel-Moati, 1990; Maske and Garcia-Mendoza, 1994), and our findings largely support the hypothesis that the greater rates of 14C-GFF relative to 14C-PC derive from adsorption of 14C-DOC produced during the incubation to the glass fiber filters (Karl et al., 1998; Morán et al., 1999). Notably, on average, rates of 14C-GFF productivity exceeded the sum of the independent measurements of 14C-PC and 14C-DOC production by ~42%. Such differences between rates of 14C-PC + 14C-DOC and 14C-GFF may reflect incomplete recovery of 14C-DOC by the oxidation and trapping procedure, loss of volatile 14C-DOC during the active bubbling procedure in the 14C-DOC assay, incomplete oxidation of 14C-DOC by the persulfate oxidation procedure, or incomplete removal of 14C-DIC from the glass fiber filters (Mague et al., 1980). Moreover, the relatively large methodological uncertainty of the 14C-DOC assay (coefficient of variation of triplicate measurements averaged 29%) further complicates this comparison. Time zero blanks indicated that the amount of 14C remaining on glass fiber filters post-acidification was always less than 5% of total 14C retained on the these filters post-incubation. These results, together with our experiments examining retention of 14C organic matter onto glass fiber filters using particle-free seawater, suggest the differences between measured 14C-GFF and 14C-PC reflect adsorption of 14C-DOC by glass fiber filters. Hence we computed the difference between the 14C-GFF and 14C-PC rate measurements (14C-delta) as an additional proxy for 14C-DOC production. Throughout our study, there was no relationship between depth-integrated rates of 14C-DOC and 14C-delta, with 14C-delta rates greater than 14C-DOC rates on all but one cruise.
The observed differences between the 14C-PC and 14C-GFF rates could derive from differences in the volumes filtered for these analyses (see Huete-Ortega et al., 2012). For the 14C-PC filtrations, we concentrated 100 ml of seawater sample onto polycarbonate filters, while the HOT program 14C-GFF measurements rely on filtration of 500 ml volumes of seawater. This difference in volume filtered could result in greater trapping of particles or 14C-DOC by the glass fiber filters, which could account for the observed discrepancy between 14C-GFF and 14C-DOC + 14C-PC. However, results from experiments we conducted examining adsorption characteristics of glass fiber filters suggest that the majority of 14C-DOC is adsorbed during filtration of the first 100 ml of sample. Moreover, we observed no significant increases in the volume corrected differences between 14C-GFF and 14C-PC (14C-delta) with increasing filtration volume above 100 ml. Such results suggest the differences in filtered volumes for the 14C-PC and 14C-GFF determinations likely would not account for the observed differences in 14C-delta and direct quantification of 14C-DOC. Regardless of the mechanism responsible for the apparent offset between these measurements, the coincident measurements of 14C-productivity using both glass fiber and polycarbonate filters provided a useful constraint on the partitioning of primary production between dissolved and particulate phases in our study.
The resulting time-series measurements of 14C-DOC, 14C-GFF, and 14C-PC productivity provided insight into vertical- and time-dependent variations in the partitioning of primary production among dissolved and particulate phases at Station ALOHA. Over ~2.5 years (2010–2012), rates of 14C-DOC production averaged 18% (± 10%) of the daytime photosynthetic production (sum of 14C-DOC and 14C-PC). Integrated rates (0–75 m) of 14C-DOC production ranged approximately 8.8-fold over the period of study (1.4 to 12.6 mmol C m−2 d−1) and did not appear to co-vary with changes in 14C-GFF or 14C-PC primary productivity. Similar to studies in the oligotrophic Atlantic Ocean (Teira et al., 2001, 2003), rates of 14C-DOC in the current study were not well correlated (either in depth or in time) with estimates of photosynthetic particulate matter production (based on rates of 14C-GFF or 14C-PC). When data from both time periods of this study were combined, in both the upper and lower regions of the euphotic zone, rates of 14C-GFF and 14C-PC varied seasonally, with elevated production in the upper euphotic zone during the summer, while rates in the lower euphotic zone were greatest in the spring. We utilized various statistical models (the Lomb-Scargle periodogram and an optimized least squares monthly regression) to evaluate possible recurring temporal patterns in our datasets. However, the relatively limited duration of our near-monthly observations (~2.5 years for 14C-DOC, and ~5 years for 14C-GFF and 14C-PC) proved insufficient to derive statistically robust patterns based on these analyses. Use of comparative statistical models (ANOVA) did highlight apparent seasonality in the longer duration time-series measurements of 14C-PC and 14C-GFF; however, rates of 14C-DOC did not demonstrate similar seasonal fluctuations. These analyses confirmed that the latter period of our observations (2010–2012) coincided with a period of time where anomalous patterns in productivity were observed (Wilson et al., 2015). Unlike the seasonal climatology observed in the HOT program record of productivity, where rates of 14C-GFF increase 2-3-fold during the summer months (Karl and Church, 2014), rates of 14C-GFF were greatest during the winter months of 2011 and 2012. Such anomalous seasonal patterns in productivity likely contributed to the relatively poor fit of the various statistical models we applied to our measurements of 14C-GFF, 14C-PC, and 14C-DOC.
The weak relationship observed between measurements of 14C-DOC and rates of 14C-PC and 14C-GFF may reflect the complex suite of processes that control net production of DOC in this ecosystem, many of which may not be directly coupled in time to photosynthetic production of particulate carbon and likely vary with depth. Ecosystems dominated by picoplankton such as the NPSG often appear to partition a greater fraction of photosynthetic production toward DOC than do larger phytoplankton (Legendre and Rassoulzadegan, 1995; Malinsky-Rushansky and Legrand, 1996; Teira et al., 2001). The greater partitioning of fixed carbon into the DOC pool is hypothesized to derive from tightly coupled trophodynamic processes, including those linked to the functioning of the microbial loop (predation and/or viral lysis). Several studies have observed that nutrient-limited and light replete systems tend to partition a greater fraction of recently produced photosyntheate into the dissolved pool relative to the particulate pool; conversely nutrient-enriched ecosystems appear to partition a greater fraction of the daily net productivity toward cellular (particulate) production (Carlson et al., 1998; Biddanda et al., 2001). Over the course of our study we observed significant production of 14C-DOC in the persistently oligotrophic, well-lit upper euphotic zone (0–45 m); in contrast, in the light-limited but nutrient-enriched lower euphotic zone (75–125 m), rates of 14C-DOC were often below our detection limit. Unlike rates of 14C-DOC, rates of particulate carbon production (14C-PC and 14C-GFF) exhibited strong depth dependence, with rates in the dimly-lit lower euphotic zone averaging ~33 and 34% (14C-PC and 14C-GFF, respectively) of rates measured in the well-lit upper ocean. As a result, the vertical distribution of PER [Percent Extracellular Release; 14C-DOC / (14C-DOC +14C-PC)] did not demonstrate clear depth dependence, a finding consistent with previous work in more eutrophic marine systems (e.g., Marañón et al., 2004).
Application of a photosynthesis-irradiance model further emphasized the differences between vertical changes in 14C-DOC compared to particulate production. Rates of 14C-DOC production demonstrated no significant relationships with downwelling PAR. The observation that rates of 14C-DOC varied less with depth than rates of 14C-PC or 14C-GFF suggests that unlike cellular production (as estimated from measurements of 14C-PC and 14C-GFF), light does not appear to be as strong a control on net photosynthetic production of DOC at Station ALOHA. Such results are consistent with other environmental studies conducted in ocean ecosystems (Lancelot, 1983; Marañón et al., 2004), although studies in the Gulf of Mexico and the Eastern tropical North Pacific revealed a dependence of 14C-DOC production with vertical changes in irradiance (Cherrier et al., 2014). While we did not evaluate mechanisms that underlie the partitioning of photosynthetically fixed carbon into DOC, such results suggest the potential importance of processes that are light independent.
The time-series rate measurements conducted as part of this study derived from incubations lasting over the full daily photoperiod (11–13 h); during that time period, a significant fraction of the 14C-DOC produced during the incubation was likely consumed. Hence our measurements of 14C-DOC presumably approximate net rates of production. On three occasions, we compared 14C-productivity during three time periods: morning (predawn to noon), photoperiod (predawn to dusk), and 24-h (predawn to predawn). These experiments were conducted to provide insight into nighttime removal of the recently fixed 14C, and to investigate possible differences in partitioning of recently fixed 14C throughout the daylight period. Rates of 14C-DOC production were significantly greater during the morning compared to the full photoperiod, suggesting a larger fraction of recently fixed photosyntheate is partitioned to DOC in the morning. Alternatively, these results could reflect changes in the coupling between production and consumption throughout the day, and hence the longer duration (photoperiod) rate measurements may approximate net production while the shorter incubation may be more similar to gross 14C-DOC production (e.g., Lancelot, 1979).
Our experiments demonstrated no significant difference between the total amount of 14C-DOC produced during 24 h and photoperiod incubations, compared to significant nighttime losses of fixed 14C-particulate carbon (14C-PC and 14C-GFF, respectively). Such results suggest that 14C-DOC produced during the photoperiod was consumed at night at lower rates than contemporaneous particulate production; alternatively, there may be sources of 14C-DOC production at night that offset simultaneous consumption. Previous results from a coastal upwelling system (Marañón et al., 2004) found no significant DOC production at night, and that study attributed DOC production to phytoplankton exudation rather than trophic processes. In contrast, in the oligotrophic NPSG, lack of significant change in 14C-DOC produced at night could reflect a combination of reinforcing processes. Rapid coupling between production and consumption of 14C-DOC during daylight hours could leave a relatively less reactive pool of 14C-DOC to persist through the nighttime. In addition, the diverse pathways that create DOC during the day (grazing, viral lysis, direct exudation/excretion) likely continue during the night (Christaki et al., 2002; Tsai et al., 2005). In contrast, photoperiod photosynthetic production of particulate material presumably reflects net cellular production, and subsequent nighttime losses of the fixed carbon likely reflect phytoplankton respiration together with cellular removal processes (grazing, viral lysis) (Marra and Barber, 2004). Recent studies have reported diel cycle fluctuations in the physiological and transcriptional activities of both phytoplankton and heterotrophic bacteria, that appear tightly coupled (Vaulot et al., 1995; Binder and DuRand, 2002; Poretsky et al., 2009; Ottesen et al., 2013, 2014; Aylward et al., 2015). Results from the short-term productivity experiments in the present study indicate that in addition to the lack of a significant relationship between rates of particulate and dissolved primary productivity observed during our monthly scale sampling, these rates are also decoupled over daily time scales, which may have important implications for the diel activity cycles of the heterotrophic bacteria that rely on recently produced photosyntheate.
The time-series measurements of 14C-primary production reported here underscore several important features of the NPSG ecosystem. Rates of net 14C-DOC production appear both vertically and temporally decoupled from variations in rates of 14C-GFF or 14C-PC. In particular, over a ~2.5 year period of near-monthly observations, rates of particulate matter productivity were decoupled from 14C-DOC production over diel to seasonal time scales. Moreover, consistent with a prior report (Karl et al., 1998), we find that photosynthetic production of DOC can be an important, but variable pathway for organic carbon production in the NPSG, accounting for nearly one fifth of the net daytime 14C-based estimates of productivity. Our results also provide indications of the complexity of interacting processes that control net production and consumption of organic matter in this ecosystem, highlighting the need for future studies quantifying the magnitude and variability of such processes.
Author Contributions
All authors contributed substantially to the design of this study, interpretation of results, and preparation of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Funding for this study derived from the National Science Foundation, including grants OCE-0850827 (MJC), OCE-1260164 (MJC and DMK), and EF-0424599 (DMK). Additional support derived from the Simons Foundation via the Simons Collaboration on Ocean Processes and Ecology (SCOPE; DMK and MJC) and the Gordon and Betty Moore Foundation Marine Microbiology Investigator grant 3794 (DMK). We thank the various scientists and staff of the HOT program for their assistance at sea and in the laboratory. We thank Benedetto Barone for his thoughtful comments that improved this manuscript. We extend our gratitude to the officers and crew of the R/V Kilo Moana and the R/V Kaimikai-o-Kanaloa. Comments by two anonymous reviewers improved the presentation of this work.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/article/10.3389/fmars.2015.00073
Supplementary Figure 1. Results from experiments comparing retention of 14C organic matter onto either glass fiber or polycarbonate membrane filters. (A) 14C-labeled primary production samples filtered either onto glass fiber filters alone, polycarbonate filters alone, glass fiber filters placed on top of polycarbonate filters (GFF/PC), or polycarbonate filters placed on top of glass fiber filters (PC/GFF); (B) chlorophyll a samples filtered across the same configuration of filters. (C) Sequential 100 ml re-filtrations of 14C-PC filtrate onto successive new glass fiber filters (in triplicate). (D) Comparison of average (from triplicates) 14C-DOC adsorbed to glass fiber filters from initial 100 ml filtration, sum of all five 100 ml re-filtrations (for a total volume of 500 ml, 100 ml per filter) onto five separate filters, and filtration of 500 ml onto single glass fiber filters.
Supplementary Figure 2. Differences between 14C-activity on glass fiber and polycarbonate filters (14C-delta =14C-GFF - 14C-PC) where different volumes of seawater were filtered. 14C-delta derived from paired samples where 100 ml of seawater was filtered onto polycarbonate filters, while varying volumes (100, 150, 400, and 500 ml) of seawater were filtered onto glass fiber filters. Midline of box plots indicates median value, while the upper and lower borders of the box represent the 75th and 25th percentiles, respectively.
References
Abdel-Moati, A. R. (1990). Adsorption of dissolved organic carbon (DOC) on glass fibre filters during particulate organic carbon (POC) determination. Water Res. 24, 763–764. doi: 10.1016/0043-1354(90)90033-3
Aylward, F. O., Eppley, J. M., Smith, J. M., Chavez, F. P., Scholin, C. A., and DeLong, E. F. (2015). Microbial community transcriptional networks are conserved in three domains at ocean basin scales. Proc. Natl. Acad. Sci. U.S.A. 112, 5443–5448. doi: 10.1073/pnas.1502883112
Azam, F., Fenchel, T., Field, J., Gray, J., Meyer-Reil, L., and Thingstad, F. (1983). The ecological role of water-column microbes in the sea. Mar. Ecol. Prog. Ser. 10, 257–263. doi: 10.3354/meps010257
Baines, S., and Pace, M. (1991). The production of dissolved organic matter by phytoplankton and its importance to bacteria: patterns across marine and freshwater systems. Limnol. Oceanogr. 36, 1078–1090. doi: 10.4319/lo.1991.36.6.1078
Banse, K. (1995). Zooplankton: pivotal role in the control of ocean production. ICES J. Mar. Sci. 52, 265–277. doi: 10.1016/1054-3139(95)80043-3
Behrenfeld, M. J., and Falkowski, P. G. (1997). Photosynthetic rates derived from satellite-based chlorophyll concentration. Limnol. Oceanogr. 42, 1–20. doi: 10.4319/lo.1997.42.1.0001
Bender, M., Grande, K., Johnson, K., Marra, J., Williams, P., Sieburth, J., et al. (1987). A comparison of four methods for determining planktonic community production. Limnol. Oceanogr. 32, 1085–1098. doi: 10.4319/lo.1987.32.5.1085
Biddanda, B., Ogdahl, M., and Cotner, J. (2001). Dominance of bacterial metabolism in oligotrophic relative to eutrophic waters. Limnol. Oceanogr. 46, 730–739. doi: 10.4319/lo.2001.46.3.0730
Binder, B. J., and DuRand, M. D. (2002). Diel cycles in surface waters of the equatorial Pacific. Deep Sea Res. II 49, 2601–2617. doi: 10.1016/S0967-0645(02)00050-4
Bjørnsen, P. K. (1988). Phytoplankton exudation of organic matter: why do healthy cells do it? Limnol. Oceanogr. 33, 151–154. doi: 10.4319/lo.1988.33.1.0151
Campbell, L., Nolla, H. A., and Vaulot, D. (1994). The importance of Prochlorococcus to community structure in the central North Pacific Ocean. Limnol. Oceanogr. 39, 954–961. doi: 10.4319/lo.1994.39.4.0954
Carlson, C. A. (2002). “Production and removal processes,” in Biogeochemistry of Marine Dissolved Organic Matter, eds D. Hansell and C. A. Carlson (Amsterdam: Academic), 91–151.
Carlson, C. A., Ducklow, H. W., Hansell, D. A., and Smith, W. O. (1998). Organic carbon partitioning during spring phytoplankton blooms in the Ross Sea polynya and the Sargasso Sea. Limnol. Oceanogr. 43, 375–386. doi: 10.4319/lo.1998.43.3.0375
Carlson, C. A., Hansell, D. A., Peltzer, E. T., and Smith, W. O. Jr. (2000). Stocks and dynamics of dissolved and particulate organic matter in the southern Ross Sea, Antarctica. Deep Sea Res. II 47, 3201–3225. doi: 10.1016/S0967-0645(00)00065-5
Carlson, C., Ducklow, H., and Michaels, A. (1994). Annual flux of dissolved organic carbon from the euphotic zone in the northwestern Sargasso Sea. Nature 371, 405–408. doi: 10.1038/371405a0
Chavez, F. P., Buck, K. R., Bidigare, R. R., Karl, D. M., Hebel, D., Latasa, M., et al. (1995). On the chlorophyll a retention properties of glass-fiber GF/F filters. Limnol. Oceanogr. 40, 428–433. doi: 10.4319/lo.1995.40.2.0428
Cherrier, J., Valentine, S., Hamill, B., Jeffrey, W. H., and Marra, J. F. (2014). Light-mediated release of dissolved organic carbon by phytoplankton. J. Marine Syst. 147, 45–51. doi: 10.1016/j.jmarsys.2014.02.008
Christaki, U., Courties, C., Karayanni, H., Giannakourou, A., Maravelias, C., Kormas, K. A., et al. (2002). Dynamic characteristics of Prochlorococcus and Synechococcus consumption by bacterivorous nanoflagellates. Microb. Ecol. 43, 341–352. doi: 10.1007/s00248-002-2002-3
Church, M. J., Lomas, M. W., and Muller-Karger, F. (2013). Sea change: charting the course for biogeochemical ocean time-series research in a new millennium. Deep Sea Res., Part II 93, 2–15. doi: 10.1016/j.dsr2.2013.01.035
Church, M. J., Mahaffey, C., Letelier, R. M., Lukas, R., Zehr, J. P., and Karl, D. M. (2009). Physical forcing of nitrogen fixation and diazotroph community structure in the North Pacific subtropical gyre. Global Biogeochem. Cycles 23, GB2020. doi: 10.1029/2008GB003418
Conan, P., Sondergaard, M., Kragh, T., Thingstad, F., Pujo-Pay, M., Williams, P. J., et al. (2007). Partitioning of organic production in marine plankton communities: the effects of inorganic nutrient ratios and community composition on new dissolved organic matter. Limnol. Oceanogr. 52, 753–765. doi: 10.4319/lo.2007.52.2.0753
Dore, J., Letelier, R., Church, M., Lukas, R., and Karl, D. (2008). Summer phytoplankton blooms in the oligotrophic North Pacific Subtropical Gyre: historical perspective and recent observations. Prog. Oceanogr. 76, 2–38. doi: 10.1016/j.pocean.2007.10.002
Dore, J. E., and Karl, D. M. (1996). Nitrite distributions and dynamics at Station ALOHA. Deep Sea Res. II 43, 385–402. doi: 10.1016/0967-0645(95)00105-0
Druffel, E., Williams, P., Bauer, J., and Eretl, J. (1992). Cycling of dissolved and particulate organic matter in the open ocean. J. Geophys. Res. 97, 15639–15659. doi: 10.1029/92JC01511
Emerson, S. (2014). Annual net community production and the biological carbon flux in the ocean. Global Biogeochem. Cycles 28, 2013GB004680. doi: 10.1002/2013GB004680
Evans, C., Pearce, I., and Brussaard, C. P. D. (2009). Viral−mediated lysis of microbes and carbon release in the sub−Antarctic and Polar Frontal zones of the Australian Southern Ocean. Environ. Microbiol. 11, 2924–2934. doi: 10.1111/j.1462-2920.2009.02050.x
Field, C. B., Behrenfeld, M. J., Randerson, J. T., and Falkowski, P. (1998). Primary production of the biosphere: integrating terrestrial and oceanic components. Science 281, 237–240. doi: 10.1126/science.281.5374.237
Garside, C. (1982). A chemiluminescent technique for the determination of nanomolar concentrations of nitrate and nitrite in seawater. Mar. Chem. 11, 159–167. doi: 10.1016/0304-4203(82)90039-1
Hansell, D. A., Carlson, C. A., Repeta, D. J., and Schlitzer, R. (2009). Dissolved organic matter in the ocean: a controversy stimulates new insights. Oceanography 22, 202–211. doi: 10.5670/oceanog.2009.109
Hedges, J. I. (1992). Global biogeochemical cycles: progress and problems. Mar. Chem. 39, 67–93. doi: 10.1016/0304-4203(92)90096-S
Hellebust, J. A. (1965). Excretion of some organic compounds by marine phytoplankton. Limnol. Oceanogr. 10, 192–206. doi: 10.4319/lo.1965.10.2.0192
Huete-Ortega, M., Cermeño, P., Calvo-Díaz, A., and Marañón, E. (2012). Isometric size-scaling of metabolic rate and the size abundance distribution of phytoplankton. P. Roy. Soc. Lond. B Biol. Sci. 279, 1815–1823. doi: 10.1098/rspb.2011.2257
Hygum, B. H., Petersen, J. W., and Søndergaard, M. (1997). Dissolved organic carbon released by zooplankton grazing activity-a high-quality substrate pool for bacteria. J. Plankton Res. 19, 97–111. doi: 10.1093/plankt/19.1.97
Kaiser, K., and Benner, R. (2009). Biochemical composition and size distribution of organic matter at the Pacific and Atlantic time-series stations. Mar. Chem. 113, 63–77. doi: 10.1016/j.marchem.2008.12.004
Karl, D., Bidigare, R., and Letelier, R. (2001). Long-term changes in plankton community structure and productivity in the North Pacific Subtropical Gyre: the domain shift hypothesis. Deep Sea Res. II 48, 1449–1470. doi: 10.1016/S0967-0645(00)00149-1
Karl, D., Christian, J., Dore, J., Hebel, D., Letelier, R., Tupas, L., et al. (1996). Seasonal and interannual variability in primary production and particle flux at Station ALOHA. Deep Sea Res. II 43, 539–568. doi: 10.1016/0967-0645(96)00002-1
Karl, D., Hebel, D., Björkman, K., and Letelier, R. (1998). The role of dissolved organic matter release in the productivity of the oligotrophic North Pacific Ocean. Limnol. Oceanogr. 43, 1270–1286. doi: 10.4319/lo.1998.43.6.1270
Karl, D. M., and Church, M. J. (2014). Microbial oceanography and the Hawaii Ocean Time-series programme. Nat. Rev. Microbiol. 12, 699–713. doi: 10.1038/nrmicro3333
Karl, D. M., and Tien, G. (1992). MAGIC: a sensitive and precise method for measuring dissolved phosphorus in aquatic environments. Limnol. Oceanogr. 37, 105–116. doi: 10.4319/lo.1992.37.1.0105
Lampert, W. (1978). Release of dissolved organic carbon by grazing zooplankton. Limnol. Oceanogr. 23, 831–834. doi: 10.4319/lo.1978.23.4.0831
Lancelot, C. (1979). Gross excretion rates of natural marine phytoplankton and heterotrophic uptake of excreted products in the Southern North Sea, as determined by short-term kinetics. Mar. Ecol. Prog. Ser. 1, 179–186. doi: 10.3354/meps001179
Lancelot, C. (1983). Factors affecting phytoplankton extracellular release in the Southern Bight of the North Sea. Mar. Ecol. Prog. Ser. 12, 115–121. doi: 10.3354/meps012115
Landry, M., Al-Mutairi, H., Selph, K., Christensen, S., and Nunnery, S. (2001). Seasonal patterns of mesozooplankton abundance and biomass at Station ALOHA. Deep Sea Res. II 48, 2037–2061. doi: 10.1016/S0967-0645(00)00172-7
Legendre, L., and Rassoulzadegan, F. (1995). Plankton and nutrient dynamics in marine waters. Ophelia 41, 153–172. doi: 10.1080/00785236.1995.10422042
Letelier, R., Dore, J., Winn, C., and Karl, D. (1996). Seasonal and interannual variations in photosynthetic carbon assimilation at Station ALOHA. Deep Sea Res. II 43, 467–490. doi: 10.1016/0967-0645(96)00006-9
Letelier, R., Karl, D., Abbott, M., and Bidigare, R. (2004). Light driven seasonal patterns of chlorophyll and nitrate in the lower euphotic zone of the North Pacific Subtropical Gyre. Limnol. Oceanogr. 49, 508–519. doi: 10.4319/lo.2004.49.2.0508
Lignell, R. (1990). Excretion of organic carbon by phytoplankton: its relation to algal biomass, primary productivity and bacterial secondary production in the Baltic Sea. Mar. Ecol. Prog. Ser. 68, 85–99. doi: 10.3354/meps068085
Llope, M., Anadón, R., Sostres, J. Á., and Viesca, L. (2007). Nutrients dynamics in the southern Bay of Biscay (1993-2003): winter supply, stoichiometry, long-term trends, and their effects on the phytoplankton community. J. Geophys. Res. 112, C07029. doi: 10.1029/2006jc003573
López-Sandoval, D. C., Fernández, A., and Marañón, E. (2011). Dissolved and particulate primary production along a longitudinal gradient in the Mediterranean Sea. Biogeosciences 8, 815–825. doi: 10.5194/bg-8-815-2011
Mague, T. H., Friberg, E., Hughes, D. J., and Morris, I. (1980). Extracellular release of carbon by marine phytoplankton; a physiological approach. Limnol. Oceanogr. 25, 262–279. doi: 10.4319/lo.1980.25.2.0262
Malinsky-Rushansky, N., and Legrand, C. (1996). Excretion of dissolved organic carbon by phytoplankton of different sizes and subsequent bacterial uptake. Mar. Ecol. Prog. Ser. 132, 249–255. doi: 10.3354/meps132249
Marañón, E., Cermeño, P., Fernández, E., Rodríguez, J., and Zabala, L. (2004). Significance and mechanisms of photosynthetic production of dissolved organic carbon in a coastal eutrophic ecosystem. Limnol. Oceanogr. 49, 1652–1666. doi: 10.4319/lo.2004.49.5.1652
Marra, J. (2009). Net and gross productivity: weighing in with 14C. Aquat. Microb. Ecol. 56, 123–131. doi: 10.3354/ame01306
Marra, J., and Barber, R. T. (2004). Phytoplankton and heterotrophic respiration in the surface layer of the ocean. Geophys. Res. Lett. 31, L09314. doi: 10.1029/2004GL019664
Maske, H., and Garcia-Mendoza, E. (1994). Adsorption of dissolved organic matter to the inorganic filter substrate and its implications for 14C uptake measurements. Appl. Environ. Microbiol. 60, 3887–3889.
Møller, E., Thor, P., and Nielsen, T. (2003). Production of DOC by Calanus finmarchicus, C. glacialis and C. hyperboreus through sloppy feeding and leakage from fecal pellets. Mar. Ecol. Prog. Ser. 262, 185–191. doi: 10.3354/meps262185
Møller, E. F. (2005). Sloppy feeding in marine copepods: prey-size-dependent production of dissolved organic carbon. J. Plankton Res. 27, 27–35. doi: 10.1093/plankt/fbh147
Morán, X. A. G., and Estrada, M. (2001). Short-term variability of photosynthetic parameters and particulate and dissolved primary production in the Alboran Sea (SW Mediterranean). Mar. Ecol. Prog. Ser. 212, 53–67. doi: 10.3354/meps212053
Morán, X. A. G., Gasol, J. M., Arin, L., and Estrada, M. (1999). A comparison between glass fiber and membrane filters for the estimation of phytoplankton POC and DOC production. Mar. Ecol. Prog. Ser. 187, 31–41. doi: 10.3354/meps187031
Ottesen, E. A., Young, C. R., Eppley, J. M., Ryan, J. P., Chavez, F. P., Scholin, C. A., et al. (2013). Pattern and synchrony of gene expression among sympatric marine microbial populations. Proc. Natl. Acad. Sci. U.S.A. 110, E488–E497. doi: 10.1073/pnas.1222099110
Ottesen, E. A., Young, C. R., Gifford, S. M., Eppley, J. M., Marin, R. III., Schuster, S. C., et al. (2014). Multispecies diel transcriptional oscillations in open ocean heterotrophic bacterial assemblages. Science 345, 207–212. doi: 10.1126/science.1252476
Pei, S., and Laws, E. A. (2013). Does the 14C method estimate net photosynthesis? Implications from batch and continuous culture studies of marine phytoplankton. Deep Sea Res. I 82, 1–9. doi: 10.1016/j.dsr.2013.07.011
Peterson, B. J. (1980). Aquatic primary productivity and the 14C-CO2 method: a history of the productivity problem. Annu. Rev. Ecol. Syst. 11, 359–385. doi: 10.1146/annurev.es.11.110180.002043
Platt, T., Gallegos, C., and Harrison, W. (1980). Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J. Mar. Res. 38, 687–701.
Poretsky, R. S., Hewson, I., Sun, S., Allen, A. E., Zehr, J. P., and Moran, M. A. (2009). Comparative day/night metatranscriptomic analysis of microbial communities in the North Pacific subtropical gyre. Environ. Microbiol. 11, 1358–1375. doi: 10.1111/j.1462-2920.2008.01863.x
Quay, P. D., Peacock, C., Björkman, K., and Karl, D. M. (2010). Measuring primary production rates in the ocean: enigmatic results between incubation and non-incubation methods at Station ALOHA. Global Biogeochem. Cycles 24, 1–14. doi: 10.1029/2009G.B.003665
R Development Core Team (2008). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: http://www.R-project.org
Ruf, T. (1999). The Lomb-Scargle periodogram in biological rhythm research: analysis of incomplete and unequally spaced time-series. Biol. Rhythm Res. 30, 178–201. doi: 10.1076/brhm.30.2.178.1422
Saba, G. K., Steinberg, D. K., and Bronk, D. A. (2011). The relative importance of sloppy feeding, excretion, and fecal pellet leaching in the release of dissolved carbon and nitrogen by Acartia tonsa copepods. J. Exper. Mar. Biol. Ecol 404, 47–56. doi: 10.1016/j.jembe.2011.04.013
Scargle, J. D. (1982). Studies in astronomical time series analysis. II - Statistical aspects of spectral analysis of unevenly spaced data. Astrophys. J. 263, 835–853. doi: 10.1086/160554
Schindler, D. W., Schmidt, R. V., and Reid, R. A. (1972). Acidification and bubbling as an alternative to filtration in determining phytoplankton production by the 14C method. J. Fish. Res. Board. Can. 29, 1627–1631. doi: 10.1139/f72-250
Skoog, D. A., and Leary, J. J. (1992). Principles of Instrumental Analysis. Fort Worth, TX: Saunders College Pub.
Steemann Nielsen, E. (1952). The use of radio-active carbon (C14) for measuring organic production in the sea. J. Conseil Int. Explor. Mer 18, 117–140. doi: 10.1093/icesjms/18.2.117
Strickland, J. D. H., and Parsons, T. R. (1972). A Practical Handbook of Seawater Analysis. Ottawa, ON: Fisheries Research Board of Canada.
Teira, E., Pazo, M. J., Quevedo, M., Fuentes, M. V., Niell, F. X., and Fernandez, E. (2003). Rates of dissolved organic carbon production and bacterial activity in the eastern North Atlantic Subtropical Gyre during summer. Mar. Ecol. Prog. Ser. 249, 53–67. doi: 10.3354/meps249053
Teira, E., Pazo, M. J., Serret, P., and Fernandez, E. (2001). Dissolved organic carbon production by microbial populations in the Atlantic Ocean. Limnol. Oceanogr. 46, 1370–1377. doi: 10.4319/lo.2001.46.6.1370
Tsai, A.-Y., Chiang, K.-P., Chang, J., and Gong, G.-C. (2005). Seasonal diel variations of picoplankton and nanoplankton in a subtropical western Pacific coastal ecosystem. Limnol. Oceanogr. 50, 1221–1231. doi: 10.4319/lo.2005.50.4.1221
Vaulot, D., Marie, D., Olson, R. J., and Chisholm, S. (1995). Growth of Prochlorococcus, a photosynthetic prokaryote, in the Equatorial Pacific Ocean. Science 268, 1480–1482. doi: 10.1126/science.268.5216.1480
Wilhelm, S., and Suttle, C. (1999). Viruses and nutrient cycles in the sea - Viruses play critical roles in the structure and function of aquatic food webs. Bioscience 49, 781–788. doi: 10.2307/1313569
Wilson, S. T., Barone, B., Ascani, F., Bidigare, R. R., Church, M. J., del Valle, D. A., et al. (2015). Short-term variability in euphotic zone biogeochemistry and primary productivity at Station ALOHA: a case study of summer 2012. Global Biogeochem. Cycles 29:2015GB005141. doi: 10.1002/2015GB005141
Winn, C., Campbell, L., Christian, J., Letelier, R., Hebel, D., Dore, J., et al. (1995). Seasonal variability in the phytoplankton community of the North Pacific Subtropical gyre. Global Biogeochem. Cycles 9, 605–620. doi: 10.1029/95GB02149
Keywords: primary productivity, dissolved organic carbon, North Pacific Ocean, Station ALOHA
Citation: Viviani DA, Karl DM and Church MJ (2015) Variability in photosynthetic production of dissolved and particulate organic carbon in the North Pacific Subtropical Gyre. Front. Mar. Sci. 2:73. doi: 10.3389/fmars.2015.00073
Received: 23 May 2015; Accepted: 10 September 2015;
Published: 01 October 2015.
Edited by:
Cecile Guieu, Centre National de la Recherche Scientifique, FranceReviewed by:
Antonio Bode, Instituto Español de Oceanografía, SpainEva Teira, Universidade de Vigo, Spain
Copyright © 2015 Viviani, Karl and Church. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Donn A. Viviani, Department of Oceanography, University of Hawaii at Manoa, 1950 East-West Road, Honolulu, HI 96822, USA, viviani@hawaii.edu
 Donn A. Viviani
Donn A. Viviani David M. Karl
David M. Karl Matthew J. Church
Matthew J. Church