Epiphytes Modulate Posidonia oceanica Photosynthetic Production, Energetic Balance, Antioxidant Mechanisms, and Oxidative Damage
- Centro de Ciencias do Mar (CCMAR), Universidade do Algarve, Gambelas, Faro, Portugal
Epiphytes impose physical barriers to light penetration into seagrass leaves causing shading, which may decrease the production of reactive oxygen species (ROS), but also constitute a physical aggression that may trigger the production of ROS, leading to oxidative damage. Here we investigate the effects of epiphytes on Posidonia oceanica under both interactive perspectives, light attenuation and oxidative stress. Specifically the role of epiphytes in net photosynthesis, chlorophyll a and b, photoprotection (Violaxanthin+Anteraxanthin+Zeaxanthin cycle), soluble sugar and starch contents, enzymatic [ascorbate peroxidase (APX) and dehydroascorbate reductase (DHAR)] and global [trolox equivalent antioxidant capacity (TEAC)] and [oxygen radical antioxidant capacity (ORAC)] antioxidant responses, phenolics and oxidative damage (malondialdehyde) are tested. Leaves with epiphytes showed higher chlorophyll b and lower content in VAZ cycle carotenoids. Epiphyte shading was the probable reason for the lower VAZ de-epoxidation-ratio of leaves with epiphytes. In spite of being shaded, leaves with epiphytes showed higher antioxidant levels, indicating that epiphytes trigger the production of ROS. Both ORAC and TEAC and also APX and DHAR activities were higher in leaves with epiphytes, indicating that this response was related with its presence. Malondialdehyde concentrations also suggest oxidative damage caused by epiphytes. We conclude that the epiphyte load causes oxidative stress in P. oceanica and the mechanisms to scavenge ROS were not completely effective to avoid cell damage.
Introduction
Seagrass epiphytes are a complex community composed by plants, bacteria, micro and macroalgae, heterotrophic organisms and organic and inorganic detritus, which use leaves as a physical support structure. The most commonly documented effect of epiphytes on seagrasses is shading and light attenuation (Van Montfrans et al., 1984; Brush and Nixon, 2002; Brodersen et al., 2015). This can be detrimental to photosynthesis of subtidal, light limited seagrasses (Oh et al., 2009). Shading may also decrease the activation of photoprotective mechanisms such as the xanthophyll (VAZ) cycle (Gilmore et al., 1995). High irradiances induce the de-epoxidation of violaxanthin (V) into antheraxanthin (A) and zeaxanthin (Z) and excess excitation energy is dissipated as heat when A and Z accumulate (Gilmore et al., 1995; Yamamoto and Bassi, 1996). The production of reactive oxygen species (ROS) leading to oxidative stress result when photoprotection mechanisms are not able to account for excess excitation energy (Mittler, 2002).
Epiphytes can either attach to the outer layers of the host tissue or anchor deeply in leaves (Ducker and Knox, 1984) in a parasitic relationship responsible by host cellular reactions similar to infections (Goff and Coleman, 1984). Even when attached in the outer layers, the presence of epiphytes may cause leaf damage such as sloughing of the surface layer and leaf chlorosis and necrosis (Ballesteros et al., 2007). A response of seagrasses to epiphyte wounding may be the production of ROS, as commonly observed in terrestrial plants and designated by oxidative burst (Wojtaszek, 1997). An antioxidant response to an epiphytic invasive alga has actually been described in the seagrass Posidonia oceanica (Sureda et al., 2008). The ROS upsurge probably acts as a protection mechanism, either by being directly toxic to epiphytes or by signaling and/or triggering other defense reactions. A recent report has also revealed that the physical aggression by herbivores also causes the production of ROS in marine algae (McDowell et al., 2014).
Under a moderate stress level, an increase of ROS triggers plant acclimation responses such as the modulation of gene expression, the upregulation of the antioxidant system and the downregulation of the photosystem II turnover rate in order to decrease the production of reactive species and protect the cell apparatus (Karuppanapandian et al., 2011), especially the photosynthetic system (Foyer and Shigeoka, 2011). Under high stress conditions, ROS concentration may exceed the plant antioxidant compensation capacity, leading to oxidative stress (Wojtaszek, 1997; Blokhina et al., 2003; Sureda et al., 2008), i.e., the accumulation of free radicals that result in chain reactions leading to the degradation of macromolecules and eventually to cell death. Malondialdehyde (MDA) results from the peroxidation of lipids, and can be used as a marker for oxidative stress (Valenzuela, 1991).
The antioxidant defense system includes enzymatic and non-enzymatic responses (Mittler, 2002; Blokhina et al., 2003). Enzymatic antioxidants are mainly composed by superoxide dismutases, peroxidases, catalases, and enzymes that use ascorbate as a reducing or oxidizing agent (Dabrowska et al., 2007). Non-enzymatic antioxidants include tocopherols, phenolic compounds, ascorbic acid, and carotenoids that quench the oxygen singlet (Telfer, 2005) and trap peroxyl radicals preventing lipid peroxidation (Stahl and Sies, 1996). Since different antioxidants have distinct mechanisms of action, general indicators of antioxidant scavenging capacity were developed, covering two different antioxidant pathways (Huang et al., 2005): the oxygen radical absorbance capacity (ORAC) based in hydrogen atoms transfer and the trolox® equivalents antioxidant capacity (TEAC), based on electron transfer, that measures the antioxidant capacity as compared to the commercial antioxidant standard trolox®.
P. oceanica (L.) Delile is a subtidal seagrass, endemic of the Mediterranean, characterized by slow growth (Duarte, 1991) and long-living (Arnaud-Haond et al., 2012). It is declared by the European Habitats Directive (92/43/CEE) as a habitat of priority interest (Boudouresque, 2004) in face of the valuable ecosystem services it provides (Vassallo et al., 2013; Campagne et al., 2015). The species leaves are frequently colonized by a distinct epiphytic community including foraminifera, encrusting coralline algae, diatoms, cyanobacteria, and bacteria (Gobert, 2002). Here we investigate the effects of epiphytes on P. oceanica under both interactive perspectives, light attenuation, which may decrease photoprotective mechanisms and ROS production and physical aggression, which may cause the production of ROS and oxidative stress. Both light attenuation and oxidative stress may decrease the production of P. oceanica and consequently the ecosystem services provided. Furthermore, ocean acidification may increase the epiphyte load on seagrasses as filamentous algae may take over calcareous epiphytes (Campbell and Fourqurean, 2014; Martinez-Crego et al., 2014) enhancing the relevance of understanding the effects of epiphytes on seagrasses. The role of epiphytes on the photosynthetic production, energetic balance, antioxidant mechanisms, and oxidative damage in P. oceanica was tested. The approach used involved the comparative analysis of the responses of shoots free of epiphytes with shoots loaded with epiphytes in terms of (1) net photosynthetic production (NPP), (2) chlorophyll a and chlorophyll b content, (3) photoprotection (VAZ cycle), (4) energetic balance (soluble sugar and starch contents), (5) enzymatic antioxidant responses [ascorbate peroxidase (APX) and dehydroascorbate reductase (DHAR)], (6) global enzymatic and non-enzymatic antioxidant responses (TEAC and ORAC), (7) phenolic compounds, and (8) oxidative stress damage (MDA).
Materials and Methods
Sampling Design
The experiment was carried out in Cabo de Gata Natural Park (southern Spain, 36° 48′ 47.337″ N, 2° 3′ 0.875″ W) in two P. oceanica meadows developing at the same depth (4–5 m depth) on each side of the rocky point of Isleta del Moro. The plants developing in the Northeast side have almost no epiphytes compared with the plants developing in the sheltered Southwest side, that exhibit a high load of epiphytes, probably because of different hydrodynamic conditions.
Plants from each site, i.e., with low and high epiphyte load, from now on designated as NEp and Ep, respectively, were sampled at pre-dawn and solar noon. Leaf samples (n = 5) for biochemical analysis were collected and quickly cleaned of epiphytes (when necessary), rinsed with distilled water, blotted dry, and frozen in liquid nitrogen. Plants (whole shoots) of P. oceanica were incubated in situ for photosynthetic measurements along the day. The photosynthetic active radiation (PAR) was measured continuously along the incubations with an Odyssey light sensor (Dataflow Systems, New Zealand).
Photosynthesis and Respiration
NPP was measured as the oxygen evolution in closed incubation chambers. Sets of 5 incubations (n = 5) of NEp and Ep shoots were performed in parallel at different times along the day (at 13, 16, 18, and 22 h) and also at night, for dark respiration measurements. The incubation chambers were tightly closed at the base of the shoots so that only one shoot was incubated. An inner soft rubber band at the base of each chamber ensured sealing. They were built from a gas-tight polyethylene plastic bag (2.5 L approx.) with one sampling port to withdraw water samples. These were filled with ambient seawater, sealed tight with a plastic bag plastic sealer, and left incubating in situ for 2 h periods. Wave action promoted external movement of the chambers and consequent medium agitation. Oxygen evolution rates were quantified as the difference in dissolved oxygen concentration in seawater, between the beginning and the end of the incubations, normalized by the chamber volume and the incubated leaf area. Water sampling and oxygen fixation was performed according to Olivé et al. (2015). Dissolved oxygen was quantified by the Winkler method, as modified by Labasque et al. (2004). Sample absorbance was read at 466 nm in a Healthcare Novaspect plus visible spectrophotometer (Brea CA, USA). Oxygen concentrations were determined against potassium iodate standard solutions considering a 3:2 O2:KIO3 ratio.
Photosynthetic Pigments
Frozen leaf tissue (200 mg, n = 5) was powdered in liquid nitrogen and sodium ascorbate, and extracted under low light in 5 mL of acetone 100% neutralized with CaCO3 (Abadía and Abadía, 1993). Extracts were filtrated with 5.0 μm LS membrane filters followed by 0.2 μm hydrophobic Polytetrafluoroethylene (PTFE). Chlorophyll a and b were quantified spectrophotometrically (Beckman Coulter DU-650 spectrophotometer, Brea CA, USA) using the equations of Lichtenthaler and Buschmann (2001). Carotenoids were analyzed by isocratic high performance liquid chromatography (HPLC) (De Las Rivas et al., 1989; Larbi et al., 2004). HPLC calibration (De Las Rivas et al., 1989) was made with commercial pigments (CaroteNature, Lupsingen, Switzerland). Liquid chromatography analysis was performed in an Alliance Waters 2695 separation module (Milford MA, USA), with a Waters 2996 photodiode array detector and a Waters Novapak C18 radial 8 × 100 mm compression column (4 μm particle size). Twenty microliters of extract were injected via an auto-sampler. Samples were stored at 5°C prior to injection and the column was kept at 24°C during chromatographic analysis. All the eluents were prepared with HPLC grade solvents (VWR Hipersolv Chromanorm), filtered and sonicated prior to use. Peak areas were monitored at 450 nm and concentrations were calculated based on peak areas obtained for standards at known concentrations.
The de-epoxidation state of P. oceanica leaves (VAZ de-epoxidation ratio), i.e., the conversion of the violaxanthin (V) to antheraxanthin (A) and zeaxanthin (Z) in the de-epoxidation reaction of the carotenoids from the xanthophyll cycle was calculated as: (A+Z)/(V+A+Z).
Soluble Carbohydrates and Starch
Leaf soluble carbohydrates were determined in 60 mg of frozen leaf tissue (n = 5), powdered in liquid nitrogen and extracted in 10 mL of 80% ethanol at 80°C for 30 min. After extraction, extracts were centrifuged at 4700 × g for 10 min and the supernatant was cleaned with activated charcoal, centrifuged at 4700 × g for 8 min (Stitt et al., 1978, 1989) and reserved for analysis. The insoluble residue was washed in deionized water, heated at 100°C for 10 min and hydrolyzed to glucose using amyloglucosidase and α-amylase enzymatic solutions, as described in Smith and Zeeman (2006).
Soluble carbohydrates from the first extraction and glucose resulting from starch hydrolysis were quantified by a phenol-sulfuric assay (adapted from Dubois et al., 1956) using glucose standards. Results were expressed as glucose equivalents.
Activity of Antioxidant Enzymes
Frozen leaf tissue samples (1 g, n = 5) were powdered in liquid nitrogen, polyvinyl polypyrrolidone (PVPP) and sodium ascorbate and then extracted with 5 mL of 100 mM potassium phosphate buffer (pH 7.8) with 2% Triton-x and 10 mM ascorbate. Extracts were centrifuged at 4°C and 3500 × g for 30 min. The supernatant was purified by filtration in a sephadex PD-10 G-25 column (GE Healtcare), previously equilibrated with 20 mL of 100 mM potassium phosphate buffer (pH 7.0) with 1 mM ascorbate (Polle and Morawe, 1995). The purified enzymatic extracts were used to determine the activities of the enzymes APX (EC 1.11.1.11) and DHAR (EC 1.8.5.1), and also to determine the soluble protein content.
APX activity was measured as the decrease in absorbance at 290 nm (Beckman Coulter DU-650 spectrophotometer) of a reaction mixture containing 50 mM potassium phosphate buffer (pH 7.0), 8 mM ascorbate, 20 mM hydrogen peroxide, and 125 μL of extract. The reaction was followed during a 3 min period at 25°C (Nakano and Asada, 1987). APX activity was calculated after subtraction of the control rates, where the enzyme extract was replaced by the potassium phosphate buffer, using ε = 2.8 mM−1 cm−1. One unit (U) of APX is equivalent to the protein necessary to oxidize 1 μmol of ascorbate per min. Enzyme activity was expressed in U mg−1 soluble protein.
DHAR activity was measured as the increase in absorbance at 265 nm of a reaction mixture containing 60 mM potassium phosphate buffer (pH 6.1), 5 mM reduced glutathione, 800 μM dehydroascorbic acid, and 50 μL of extract. The reaction was followed during a 3 min period at 25°C (Polle and Morawe, 1995). DHAR activity was calculated after subtraction of the control rates, where the enzyme extract was replaced by the potassium phosphate buffer, using ε = 14 mM−1 cm−1. One unit (U) of DHAR is equivalent to the protein necessary to reduce 1 μmol of dehydroascorbate per min. Enzyme activity was expressed in U mg−1 soluble protein.
Soluble protein was quantified by a dye-binding assay (Bradford, 1976), using a commercial reagent (BioRad Protein Assay, Hercules, California, USA). Bovine serum albumin was used as standard.
Oxygen Radical Absorbance Capacity (ORAC), Trolox Equivalent Antioxidant Activity (TEAC), and Phenolic Compounds
Frozen leaf tissue samples (ca. 300 mg, n = 5), were powdered in liquid nitrogen, suspended in 9 mL 0.1 N hydrochloric acid (HCl), kept overnight under constant agitation at 4°C, and then centrifuged at 4700 × g for 30 min. The same supernatant was used for phenolic compounds quantification and ORAC and TEAC assays.
Phenolic compounds were quantified by the Folin-Ciocalteu method (Booker and Miller, 1998; Migliore et al., 2007). 0.25 N Folin-Ciocalteu reagent (0.4 mL) and 7.5% Na2CO3(0.4 mL) were added to the supernatant (42 μL). Absorbance was read at 724 nm in a Beckman Coulter DU-650 spectrophotometer, against a blank. Chlorogenic acid was used as standard and the assay results were expressed as chlorogenic acid equivalents.
ORAC was quantified according to Huang et al. (2002). 8.16 × 10−5 mM fluorescein dissolved in 75 mM phosphate buffer (150 μL) were added to the extract (25 μL). This mixture was heated at 37°C and read in a Synergy TM 4 multi-detection microplate reader with a 485 nm excitation filter (20 nm bandpass) and a 528 nm emission filter (20 nm bandpass). The reaction was initiated by the addition of 153 mM ABAP (25 μL) [2,2′-azobis (2-methylpropionamidine) dihydrochloride]. Results were expressed as Trolox® equivalents (6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid).
For TEAC assay, the cation radical ABTS•+ was produced by the reaction of 7 mM ABTS with potassium persulfate in a final concentration of 2.45 mM according with Re et al. (1999). Nine hundred microliters of diluted ABTS•+ (A734 nm = 0.800 ± 0.020) was added to 10 μL of extract and, after an incubation period of 6 min, absorbance was read at 734 nm in a Beckman Coulter DU-650 spectrophotometer, against a blank sample. Results were expressed as Trolox® equivalents.
Malondialdehyde (MDA) Quantification
MDA extraction and quantification was performed according to Hodges et al. (1999). Three hundred milligram of frozen leaf tissue (n = 5) was powdered in liquid nitrogen and suspended in 5 mL of 80% ethanol. The extract was homogenized and centrifuged (4°C, 3000 × g, 10 min) and each sample was divided in two reading-replicates. 1.0 mL of the supernatant was added to 1.0 mL of 20% trichloroacetic acid (TCA) with 0.65% thiobarbituric acid (TBA), and 0.01% butylated hydroxytoluene (BHT) solution.
Two blanks were prepared in which the sample was replaced by 80% ethanol. All the samples and blanks were first heated (90°C, 25 min), then cooled (ice bath, 15 min), and again centrifuged (4°C, 3000 × g, 10 min). Absorbance of the supernatants was read at 440, 532, and 600 nm (Beckman Coulter DU-650 spectrophotometer, Brea CA, USA) and MDA equivalents were calculated as in Hodges et al. (1999).
Statistical Analysis
All results are presented as mean values ± standard error. The effects of epiphytes and time of the day on the variables measured were tested using Two-way analyses of variance (ANOVA). The Student-Newman-Keuls post-hoc test was used to test for significant differences between factor levels. All data treatment and statistical analysis was performed using the SigmaStat/SigmaPlot (SPSS Inc., v.11) software package.
Results
Under most irradiance levels, epiphytes did not affect the total NPP of P. oceanica shoots (including the epiphyte contribution), except in the period of the day where maximum production was observed, 16–18 h at an irradiance of 768 μmolquanta m−2s−1 (Figure 1). The diel cycle of NPP was similar for NEp and Ep shoots. Both did not peak at the maximum irradiance of 1086 μmolquanta m−2s−1 from 13 to 15 h, but in the following period of 16–18 h, decreasing afterwards to 18–20 h. As well, there were no significant differences between NEp and Ep shoots in dark respiration (Figure 1, 22–24 h).
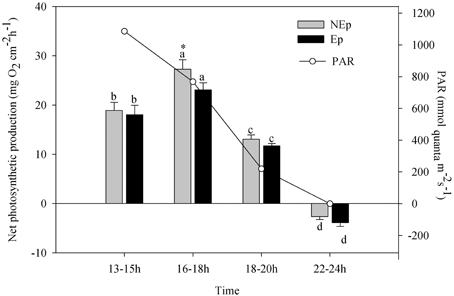
Figure 1. Net photosynthetic rates of Posidonia oceanica along the day (local time, UTC+1). Different letters indicate significant differences with time of the day (p < 0.05); *indicates significant differences between shoots with (Ep) and without (NEp) epiphytes.
Chlorophyll b content was always lower in leaves with epiphytes, resulting in significantly higher chlorophyll a/b ratios in NEp than Ep leaves (Figure 2A). There were no significant differences between pre-dawn and noon times. The foliar concentration of the VAZ pool pigments [violaxanthin (V) + anteraxanthin (A) + zeaxanthin (Z)] was significantly higher in NEp than in Ep leafs at pre-dawn, but no differences were detected at noon. Foliar VAZ pool did not change significantly from pre-dawn to noon in neither epiphyte loads (Figure 2B). However, the de-epoxidation state of the VAZ-cycle pigments, (A+Z)/(V+A+Z), significantly increased from pre-dawn to noon in both cases, but particularly in leaves without epiphytes where it increased 2.6 fold (Figure 2C).
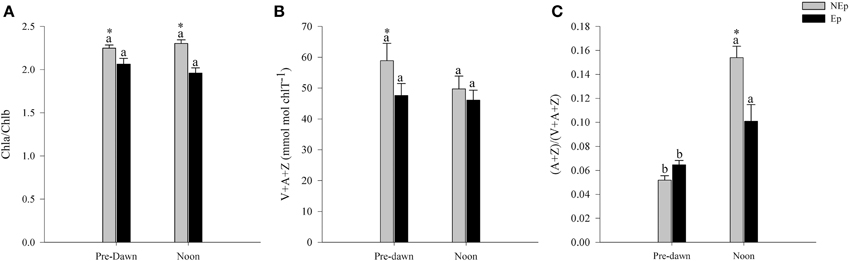
Figure 2. Chlorophyll a/b ratio (A), xanthophyll cycle pool [violaxanthin (V) + anteraxanthin (A) + zeaxanthin (Z)] (B), and de-epoxidation index (A+Z/V+A+Z) (C) in Posidonia oceanica leaves with (Ep) and without (NEp) epiphytes at pre-dawn and noon. Different letters indicate significant differences between pre-dawn and noon; *indicates significant differences between Ep and NEp leaves (n = 5, p < 0.05).
The soluble carbohydrates content was significantly higher in Ep leaves at pre-dawn, but not at noon (Figure 3A), as opposed to starch, which was significantly lower (Figure 3B). The soluble carbohydrates content did not vary significantly with the time of the day (Figure 3A), whereas the starch content decreased significantly from pre-dawn to noon in NEp leaves. At noon there were no significant differences in starch content between NEp and Ep leaves.
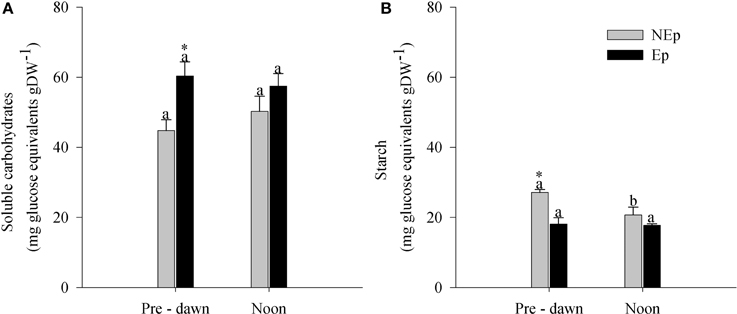
Figure 3. Leaf soluble sugars (A) and starch (B) in Posidonia oceanica leaves with (Ep) and without (NEp) epiphytes, at pre-dawn and noon. Different letters indicate significant differences between pre-dawn and noon; *indicates significant differences between Ep and NEp leaves (n = 5, p < 0.05).
APX and DHAR activities were significantly higher in Ep than NEp leaves at noon but not at pre-dawn (Figure 4). APX activity in Ep leaves increased significantly from pre-dawn to noon as opposed to NEp leaves where it did not change with time. DHAR activity showed the opposite trend: NEp DHAR activity in Nep leaves decreased from pre-dawn to noon, while it did not change in Ep leaves.
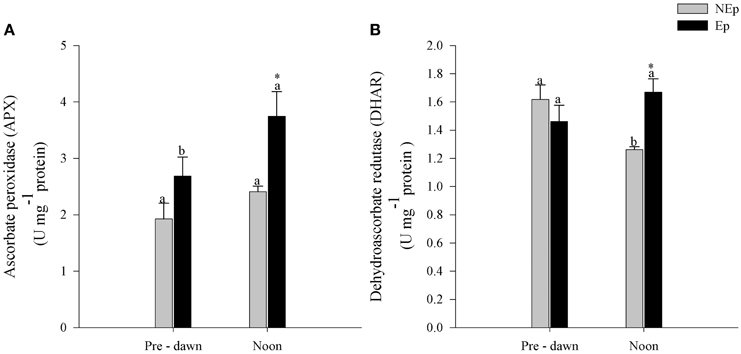
Figure 4. Ascorbate peroxidase (APX) activity (A) and dehydroascorbate (DHAR) activity (B) in Posidonia oceanica leaves with (Ep) and without (NEp) epiphytes, at pre-dawn and noon. Different letters indicate significant differences between pre-dawn and noon; *indicates significant differences between Ep and NEp leaves (n = 5, p < 0.05).
The antioxidant response (TEAC and ORAC) of P. oceanica leaves was significantly higher (1.4 to 2-fold) in Ep leaves, both at pre-dawn and noon (Figures 5A,B). The antioxidant capacity (TEAC) and the ORAC of both Ep and NEp decreased significantly from pre-dawn to noon, except in the case of ORAC of NEp leaves. Phenolic compounds decreased significantly from pre-dawn to noon in both Ep and NEp leaves (Figure 6).
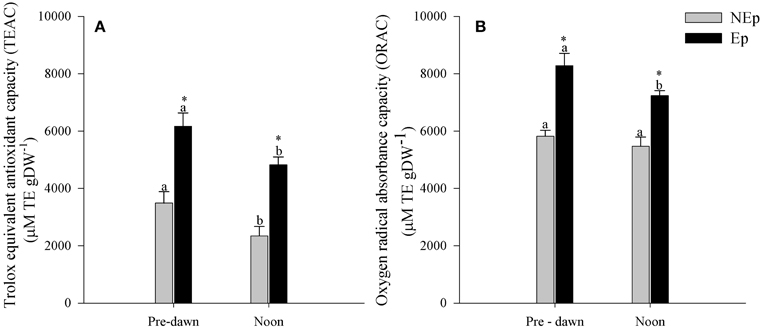
Figure 5. Antioxidant capacity (TEAC) (A) and oxygen radical absorbance capacity (ORAC) (B) in Posidonia oceanica leaves with (Ep) and without (NEp) epiphytes, at pre-dawn and noon. Different letters indicate significant differences between pre-dawn and noon; *indicates significant differences between Ep and NEp leaves (n = 5, p < 0.05).
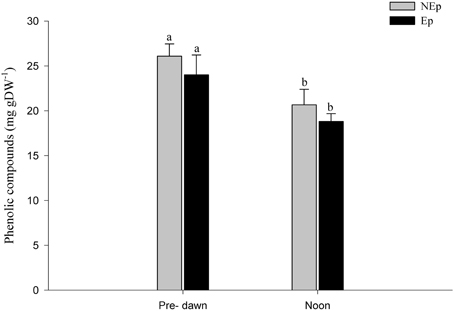
Figure 6. Phenolic compounds concentration in Posidonia oceanica leaves with (Ep) and without (NEp) epiphytes, at pre-dawn and noon. Different letters indicate significant differences between pre-dawn and noon.
Epiphytes had a positive effect on the level of peroxidation of membrane lipids (MDA) at pre-dawn at p = 0.055, but not at noon, because NEp levels of MDA increased to Ep levels (Figure 7). MDA remained high and unaltered in Ep leaves.
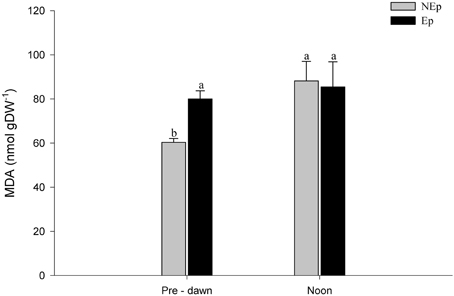
Figure 7. Malondialdehyde (MDA) concentration in Posidonia oceanica leaves with (Ep) and without (NEp) epiphytes, at pre-dawn and noon. Different letters indicate significant differences between pre-dawn and noon.
Discussion
Our results suggest that leaf epiphytes were responsible for high levels of oxidative stress in P. oceanica even though other uncontrolled factors such as hydrodynamics, nutrients, etc. may contribute to the observed differences between sites. The role of epiphytes on modulating the physiological acclimation of P. oceanica to irradiance (between pre-dawn and noon) is also highlighted in our work. Table 1 shows the significant differences found between the responses of P. oceanica shoots with contrasting loads of epiphytes. The shading effect of epiphytes is the probable cause for the observed increase of chlorophyll b concentration in Ep leaves, to improve light harvesting. As well, the observed decrease of the xanthophylls involved in the VAZ cycle in Ep leaves is in line with this hypothesis, as their photoprotective role is less necessary at low light levels. Epiphyte shading was also the probable reason for the lower VAZ de-epoxidation ratio of Ep leaves at noontime in relation to the leaves exposed to full sunlight. In NEp leaves the photoprotective VAZ cycle was highly active at noontime (Figure 2), concurrently with the low values of NPP (Figure 1, 13–15 h period). The maximum photosynthetic production of P. oceanica shoots did not coincide with maximum light intensity (13–16 h), probably due to photoinhibition, which may occur in P. oceanica above 540 μmol m−2s−1 (Figueroa et al., 2002), but rather during the afternoon (16–18 h) when saturating but not inhibiting light levels were recorded. No effects of epiphytes were observed on the dark respiration of P. oceanica shoots.
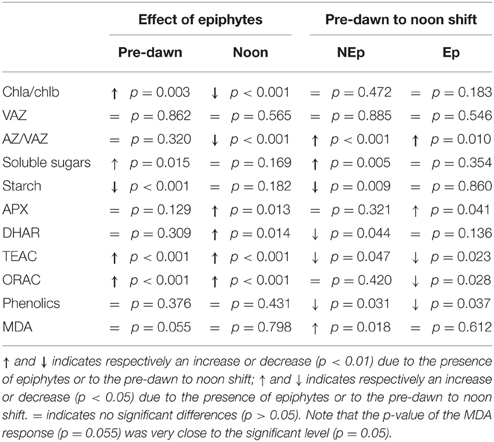
Table 1. Responses of Posidonia oceanica to the presence of epiphytes and to the pre-dawn to noon shift.
Lower photosynthetic rates in leaves with epiphytes has long been related with the shading created by epiphytes on the leaf surface (Sand-Jensen, 1977; Brush and Nixon, 2002), which decreases light quantity and alters light quality reaching the leaf surface (Brodersen et al., 2015). Recently, Oh et al. (2009) showed that epiphytes induce a permanent loss of chlorophyll a fluorescence signal in the leaf portion where they are located, causing a long term disruption of photosynthesis.
The lower chlorophyll a/b ratio observed in leaves with epiphytes (due to increased chlorophyll b) agrees with the expected adaptation of plants to lower light intensities (Lichtenthaler and Burkart, 1999). The lower chlorophyll a/b ratio corresponds to an additional enlargement of the PSII antennae system (Lichtenthaler and Buschmann, 2001) due to a proportionally higher content in chlorophyll b. This can be a strategy that allows the P. oceanica shaded by epiphytes to enhance the light capture in the chlorophyll b absorbance windows of the electromagnetic spectrum, and that way the photosynthetic production is maintained at high levels.
If light limitation results in a specific modulation of pigments, the excess of light may be highly stressful, able to create oxidative stress, photoinhibition, and permanent photo-damage (Demmig-Adams and Adams, 1992). The xanthophyll VAZ [violaxanthin (V) + anteraxanthin (A) + zeaxanthin (Z)] cycle is an important mechanism to protect plants from the excess of light. The de-epoxidation of violaxanthin into anteraxanthin and zeaxanthin is a dynamic and quick response to light changes (Demmig-Adams and Adams, 1992; Jahns, 1995; Han et al., 2004). In terrestrial plants, the VAZ-pool size depends upon light intensity and varies according to species (Walters and Horton, 1994; Niinemets et al., 2003). Usually, plants adapted to high irradiance have higher concentrations of VAZ-cycle pigments than the ones adapted to low irradiances (Han et al., 2004). Our observations suggest that epiphytes do not influence significantly the foliar VAZ pool of P. oceanica but, on the other hand, the de-epoxidation ratio of this species decrease significantly in leaves with epiphytes (Table 1). The observed compensatory increase in the de-epoxidation ratio of P. oceanica leaves exposed to full sunlight probably caused a great midday depression of PSII efficiency, responsible for the observed lower photosynthetic production at midday, called dynamic photoinhibition (Adams et al., 2006). It is noteworthy to highlight that Ep leaves presented a significantly lower VAZ de-epoxidation ratio at noon than NEp leaves (Figure 2), which was probably related with the fact that the plants were shaded.
P. oceanica leaves with epiphytes presented higher soluble sugar and lower starch concentrations than leaves without epiphytes, at pre-dawn. This could indicate that the presence of epiphytes lead to higher energetic requirements for some physiological process, which were obtained at the expense of starch reserves during the night period. Nonetheless, during this period, the higher amount of soluble sugars in Ep leaves in relation to NEp leaves is balanced by the lower amount of starch. The allocation of the two carbohydrate forms to fulfill energetic requirements from growth to stress response has been well described (Andersen, 2003). Processes that increase the demand for carbohydrates, such as antioxidant defense, lead to the depletion of storage in the form of starch (Andersen, 2003). The allocation of energetic resources is done according to the major physiological needs, and there is an inverse relationship between allocation to growth and to non-growth processes (Lattanzio et al., 2009), being plant defense the priority energy sink (Close and McArthur, 2002). Our observations indicate that the energy required to control ROS bursts in P. oceanica leaves with epiphytes may be derived from sources other than the carbohydrate reserves, such as photosynthesis, since the activity of enzymatic antioxidant defenses increases with light.
The integrated response of P. oceanica to high levels of irradiance concurs with the energy dissipation and photoinhibition mechanisms described elsewhere for terrestrial plants (Adams et al., 2006). Under excessive light levels, ROS are produced and dynamic photoinhibition is induced (Adams et al., 2006). In order to control ROS bursts and avoid oxidative stress, plant defenses comprise a complex set of enzymatic and non-enzymatic antioxidants and secondary metabolites. Our results indicate that the epiphytes of P. oceanica triggered a burst of ROS because a significantly higher antioxidant scavenging capacity was found in leaves with epiphytes. Both the antioxidant radical scavenging capacity (TEAC) and the absorbance of oxygen species capacity (ORAC) corroborate this (Table 1). These assays reflect the antioxidant reducing capacity and the hydrogen atom donation capacity, respectively (Huang et al., 2005). Moreover, both ORAC and TEAC indexes were significantly higher at pre-dawn than at noon in both Ep and NEp leaves and independently from the time of the day, always significantly higher in Ep (Table 1), showing that this response was more related with the presence of epiphytes than with the effect of light.
The antioxidant defense enzymes APX and DHAR are main scavengers of hydrogen peroxide, H2O2, which has been shown to be overproduced by plants as a response to stress (Cheeseman, 2007). Hydrogen peroxide is a potentially ROS, as in the presence of transition metals gives rise to the highly reactive hydroxyl radical (Cheeseman, 2007), but also acts as a signaling molecule for the antioxidant system to respond to bursts of oxidative species (Forman and Torres, 2002; Cheeseman, 2007). APX and DHAR are both part of the plants antioxidant enzymatic system that prevents the H2O2 related oxidative stress. APX is an H2O2-peroxidase that uses ascorbate as an electron-donor in plants and algae, to convert H2O2 into water and DHAR is part of the ascorbate recycling system (Mittler and Poulos, 2005). Here we found that both APX and DHAR activities were significantly higher in leaves with epiphytes at noontime (Table 1), showing that H2O2 was being produced and scavenged.
Phenolic compounds can have multiple biological functions related with the reproductive strategy (Ning et al., 2010), adaptation and survival to environmental disturbances (Ning et al., 2010; Cheynier et al., 2013), antimicrobial and anti-fouling properties (Maddox et al., 2010). Phenolic compounds can be polymerized by peroxidases during pathogen attacks to create morphological barriers against possible infections (Close and McArthur, 2002). Their deposition as lignin in cell walls increases their mechanical strength and improves plants response against pathogens and wounding (Booker and Miller, 1998). Some, but not all, phenolic species can have a direct effect in herbivores (Close and McArthur, 2002), but in general they are mostly linked to photodamage prevention (Close and McArthur, 2002; Lattanzio et al., 2009). Phenolic compounds also have antioxidant activity (Kähkönen et al., 1999; Ahn et al., 2007), reducing free radical formation and scavenging ROS (Rösch et al., 2003; Cheynier et al., 2013). Phenolic compounds decreased significantly from pre-dawn to noontime in both Ep and NEp leaves (Table 1), suggesting a biological rhythm independent of light, but we did not observe any effects of epiphytes on the phenolic compounds content of P. oceanica leaves. Being a complex group of compounds with diverse functions, total phenolic concentrations do not necessarily reflect antioxidant capacity (Kähkönen et al., 1999; Miguel et al., 2010). Plants submitted to different environmental conditions and with different physiological requirements will most likely have a qualitatively distinct phenolic composition. Consequently, the fact that Ep and NEp leaves of P. oceanica did not differ in terms of total phenolic compounds does not suggest that epiphytes have no effect on particular phenolic molecules, but rather emphasizes the need for a detailed scrutiny to understand the phenolics diversity, specific dynamics, and functions.
MDA formation is associated with strong oxidative stress conditions that result in cell damage, namely in the degradation of polyunsaturated lipids (Valenzuela, 1991). At pre-dawn, MDA concentrations were higher (at p = 0.055) in leaves with epiphytes than in leaves without epiphytes suggesting oxidative damage caused by epiphytes. Light did not have an effect on MDA of Ep leaves due to shading, but had a significant effect on NEp leaves (Table 1), whose MDA levels increased from pre-dawn to noon to the same level of Ep leaves (Figure 7). The ORAC and TEAC results support this interpretation as the antioxidant capacity of Ep leaves was higher at pre-dawn and consequently it was not caused by light. We conclude that both the epiphyte load and light caused comparable oxidative damage levels in P. oceanica. Even though the mechanisms to scavenge ROS were active, as indicated by the activities of the enzymes APX and DHAR and the TEAC and ORAC indices, they were not completely effective to avoid the occurrence of cell damage.
Most of the epiphytes found in P. oceanica leaves were calcareous. As carbon dioxide (CO2) concentration in the ocean is increasing, the inherent changes in the carbonate balance are leading to a concomitant decrease in seawater pH. Calcifying marine organisms are among the most threatened by ocean acidification since the predicted pH decrease will affect their ability to form and maintain calcium carbonate skeletons (Büdenbender et al., 2011; Barry et al., 2013) and ultimately compromise the survival of many species. The existence of boundary layers allows plants to alter pH at leaf surfaces (Hurd et al., 2011) and seagrass photosynthesis is known to increase pH at leaf surface (Hendriks et al., 2014), somehow mitigating ocean acidification impacts in calcareous algae and other calcareous epiphytes normally associated to seagrasses (Hendriks et al., 2014). Nevertheless, a significant reduction in epiphytic coralline algae cover was found on seagrasses growing in natural acidified seawater, in CO2 vents with pH bellow 7.9 (Martin et al., 2008). This reduction could benefit P. oceanica meadows, decreasing epiphyte related oxidative stress and allowing higher photosynthetic rates and hence more resources could be allocated to processes such as growth and reproduction. However, it is not clear that the total load of epiphytes would decrease, as it has been reported that other forms of epiphytes such as filamentous algae may take over calcareous epiphytes (Campbell and Fourqurean, 2014; Martinez-Crego et al., 2014). On the other hand, the excess light resulting from a putative decrease of epiphytes may also contribute to increase the oxidative stress of P. oceanica, as we showed here.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The present work was developed in the context of the 9th International Workshop of the Group for Aquatic Primary Productivity (GAP) in Malaga-Spain. MC was supported by Fundação para a Ciência e a Tecnologia PhD grant (SFRH/BD/64590/2009) from the Portuguese Government. IO was supported by Fundação para a Ciência e Tecnologia post-doctoral fellowship (SFRH/BPD/71129/2010) from the Portuguese Government. This paper is also a contribution to the project HighGrass (PTDC/MAR-EST/3687/2012). This study received national funds from FCT - Foundation for Science and Technology through project UID/Multi/04326/2013.
References
Abadía, J., and Abadía, A. (1993). “Iron and plant pigments,” in Iron Chelation in Plants and Soil Microorganisms, eds L. L. Barton and B. Hemming (New York, NY: Academic Press, Inc), 327–343.
Adams, W. III, Zarter, C. R., Mueh, K. E., and Amiard, V. (2006). “Energy dissipation and photoinhibition: a continuum of photoprotection,” in Photoprotection, Photoinhibition, Gene Regulation, and Environment, eds B. Demmig-Adams, W. Adams III, and A. K. Mattoo (Amsterdam: Springer), 49–64.
Ahn, M.-R., Kumazawa, S., Usui, Y., Nakamura, J., Matsuka, M., Zhu, F., et al. (2007). Antioxidant activity and constituents of propolis collected in various areas of China. Food Chem. 101, 1383–1392. doi: 10.1016/j.foodchem.2006.03.045
Andersen, C. P. (2003). Source–sink balance and carbon allocation below ground in plants exposed to ozone. New Phytol. 157, 213–228. doi: 10.1046/j.1469-8137.2003.00674.x
Arnaud-Haond, S., Duarte, C. M., Diaz-Almela, E., Marbà, N., Sintes, T., and Serrão, E. A. (2012). Implications of extreme life span in clonal organisms: millenary clones in meadows of the threatened seagrass Posidonia oceanica. PLos ONE 7:e30454. doi: 10.1371/journal.pone.0030454
Ballesteros, E., Cebrian, E., and Alcoverro, T. (2007). Mortality of shoots of Posidonia oceanica following meadow invasion by the red alga Lophocladia lallemandii. Bot. Mar. 50, 8–13. doi: 10.1515/BOT.2007.002
Barry, S. C., Frazer, T. K., and Jacoby, C. A. (2013). Production and carbonate dynamics of Halimeda incrassata (Ellis) Lamouroux altered by Thalassia testudinum Banks and Soland ex König. J. Exp. Mar. Biol. Ecol. 444, 73–80. doi: 10.1016/j.jembe.2013.03.012
Blokhina, O., Virolainen, E., and Fagerstedt, K. V. (2003). Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann. Bot. 91, 179–194. doi: 10.1093/aob/mcf118
Booker, F. L., and Miller, J. E. (1998). Phenylpropanoid metabolism and phenolic composition of soybean [Glycine max (L.) Merr.] leaves following exposure to ozone. J. Exp. Bot. 49, 1191–1202. doi: 10.1093/jxb/49.324.1191
Boudouresque, C.-F. (2004). Marine biodiversity in the mediterranean: status of spicies, populations and communities. Sci. Rep. Port Cros Natl. Park Fr. 20, 97–146.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254. doi: 10.1016/0003-2697(76)90527-3
Brodersen, K. E., Lichtenberg, M., Paz, L.-C., and Kühl, M. (2015). Epiphyte-cover on seagrass (Zostera marina L.) leaves impedes plant performance and radial O2 loss from the below-ground tissue. Front. Mar Sci. 2:58. doi: 10.3389/fmars.2015.00058
Brush, M. J., and Nixon, S. W. (2002). Direct measurements of light attenuation by epiphytes on eelgrass Zostera marina. Mar. Ecol. Prog. Ser. 238, 73–79. doi: 10.3354/meps238073
Büdenbender, J., Riebesell, U., and Form, A. (2011). Calcification of the Arctic coralline red algae Lithothamnion glaciale in response to elevated CO2. Mar. Ecol. Prog. Ser. 441, 79–87. doi: 10.3354/meps09405
Campagne, C. S., Salles, J.-M., Boissery, P., and Deter, J. (2015). The seagrass Posidonia oceanica: ecosystem services identification and economic evaluation of goods and benefits. Mar. Poll. Bull. 97, 391–400. doi: 10.1016/j.marpolbul.2015.05.061
Campbell, J. E., and Fourqurean, J. W. (2014). Ocean acidification outweighs nutrient effects in structuring seagrass epiphyte communities. J. Ecol. 102, 730–737. doi: 10.1111/1365-2745.12233
Cheeseman, J. M. (2007). “Hydrogen peroxide and plant stress: a challenging relationship,” in Plant Stress, eds J. A. T. Silva and K. Shima (London, UK: Global Science Books, Ltd.), 4–15.
Cheynier, V., Comte, G., Davies, K. M., Lattanzio, V., and Martens, S. (2013). Plant phenolics: recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 72, 1–20. doi: 10.1016/j.plaphy.2013.05.009
Close, D. C., and McArthur, C. (2002). Rethinking the role of many plant phenolics – protection from photodamage not herbivores? Oikos 99, 166–172. doi: 10.1034/j.1600-0706.2002.990117.x
Dabrowska, G., Kata, A., Goc, A., Szechynska-Hebda, M., and Skrzypek, E. (2007). Characteristics of the plant ascorbate peroxidase family. Acta Bio. Crac. Ser. Bot. 49, 7–17.
De Las Rivas, J., Abadía, A., and Abadía, J. (1989). A new reversed phase-HPLC method resolving all major higher plant photosynthetic pigments. Plant Physiol. 91, 190–192. doi: 10.1104/pp.91.1.190
Demmig-Adams, B., and Adams, W. W. (1992). Photoprotection and other responses of plants to high light stress. Annu. Rev. Plant. Physiol. Plant Mol. Biol. 43, 599–626. doi: 10.1146/annurev.pp.43.060192.003123
Duarte, C. M. (1991). Allometric scaling of seagrass form and productivity. Mar. Ecol. Prog. Ser. 77, 289–300. doi: 10.3354/meps077289
Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A., and Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356. doi: 10.1021/ac60111a017
Ducker, S. C., and Knox, R. B. (1984). “Epiphytism at the cellular level with special reference to algal epiphytes,” in Cellular Interactions, Encyclopedia of Plant Physiology, New Series, eds H. F. Linskens and J. Heslop-Harrison (Berlin: Springer-Verlag), 113–133.
Figueroa, F. L., Jiménez, C., Viñegla, B., Pérez-Rodríguez, E., Aguilera, J., Flores-Moya, A., et al. (2002). Effects of solar UV radiation on photosynthesis of the marine angiosperm Posidonia oceanica from southern Spain. Mar. Ecol. Prog. Ser. 230, 59–70. doi: 10.3354/meps230059
Forman, H. J., and Torres, M. (2002). Reactive oxygen species and cell signaling. Respiratory burst in macrophages. Am. J. Respir. Crit. Care Med. 166, S4–S8. doi: 10.1164/rccm.2206007
Foyer, C. H., and Shigeoka, S. (2011). Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 155, 93–100. doi: 10.1104/pp.110.166181
Gilmore, A. M., Hazlett, T. L., and Govindjee (1995). Xanthophyll cycle-dependent quenching of photosystem II chlorophyll a fluorescence: formation of a quenching complex with a short fluorescence lifetime. Proc. Natl. Acad. Sci. U.S.A. 92, 2273–2277. doi: 10.1073/pnas.92.6.2273
Gobert, S. (2002). Variations Spatiale et Temporelle de l'Herbier à Posidonia oceanica (L.) Delile. Dissertation présentée en vue de l'obtention du grade de Docteur en Océanologie, Docteur, Université de Lièrge.
Goff, L. J., and Coleman, A. W. (1984). Transfer of nuclei from a parasite to its host. Proc. Natl. Acad. Sci. U.S.A. 81, 5420–5424. doi: 10.1073/pnas.81.17.5420
Han, Q., Katahata, S., Kakubari, Y., and Mukai, Y. (2004). Seasonal changes in the xanthophyll cycle and antioxidants in sun-exposed and shaded parts of the crown of Cryptomeria japonica in relation to rhodoxanthin accumulation during cold acclimation. Tree Physiol. 24, 609–616. doi: 10.1093/treephys/24.6.609
Hendriks, I. E., Olsen, Y. S., Ramajo, L., Basso, L., Steckbauer, A., Moore, T. S., et al. (2014). Photosynthetic activity buffers ocean acidification in seagrass meadows. Biogeosciences 11, 333–346. doi: 10.5194/bg-11-333-2014
Hodges, D. M., Delong, J. M., Forney, C. F., and Prange, R. K. (1999). Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207, 604–611. doi: 10.1007/s004250050524
Huang, D., Ou, B., Hampsch-Woodill, M., Flanagan, J. A., and Prior, R. L. (2002). High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 50, 4437–4444. doi: 10.1021/jf0201529
Huang, D., Ou, B., and Prior, R. L. (2005). The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 53, 1841–1856. doi: 10.1021/jf030723c
Hurd, C. L., Cornwall, C. E., Currie, K., Hepburn, C. D., McGraw, C. M., Hunter, K. A., et al. (2011). Metabolically induced pH fluctuations by some coastal calcifiers exceed projected 22nd century ocean acidification: a mechanism for differential susceptibility? Glob. Change Biol. 17, 3254–3262. doi: 10.1111/j.1365-2486.2011.02473.x
Jahns, P. (1995). The xanthophyll cycle in intermittent light-grown pea plants. Possible functions of chlorophyll a/b-binding proteins. Plant Physiol. 108, 149–156.
Kähkönen, M. P., Hopia, A. I., Vuorela, H. J., Rauha, J.-P., Pihlaja, K., Kujala, T. S., et al. (1999). Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 47, 3954–3962. doi: 10.1021/jf990146l
Karuppanapandian, T., Moon, J.-C., Kim, C., Manoharan, K., and Kim, W. (2011). Reactive oxygen species in plants: their generation, signal transduction, and scavenging mechanisms. AJCS 5, 709–725.
Labasque, T., Chaumery, C., Aminot, A., and Kergoat, G. (2004). Spectrophotometric Winkler determination of dissolved oxygen: re-examination of critical factors and reliability. Mar. Chem. 88, 53–60. doi: 10.1016/j.marchem.2004.03.004
Larbi, A., Abadía, A., Morales, F., and Abadía, J. (2004). Fe resupply to Fe-deficient sugar beet plants leads to rapid changes in the violaxanthin cycle and other photosynthetic characteristics without significant de novo chlorophyll synthesis. Photosynth. Res. 79, 59–69. doi: 10.1023/B:PRES.0000011919.35309.5e
Lattanzio, V., Cardinali, A., Ruta, C., Fortunato, I. M., Lattanzio, V. M. T., Linsalata, V., et al. (2009). Relationship of secondary metabolism to growth in oregano (Origanum vulgare L.) shoot cultures under nutritional stress. Environ. Exp. Bot. 65, 54–62. doi: 10.1016/j.envexpbot.2008.09.002
Lichtenthaler, H. K., and Burkart, S. (1999). Photosynthesis and high light stress. Bulg. J. Plant Physiol. 25, 3–16.
Lichtenthaler, H. K., and Buschmann, C. (2001). “Chlorophylls and carotenoids: measurement and characterization by UV-VIS spectroscopy,” in Current Protocols in Food Analytical Chemistry, eds R. E. Wrolstad, T. E. Acree, H. An, E. A. Decker, M. H. Penner, D. S. Reid, S. J. Schwartz, C. F. Shoemaker, and P. Sporns (London; New York, NY: John Wiley and Sons, Inc.), F4.3.1–F4.3.8.
Maddox, C., Laur, L., and Tian, L. (2010). Antibacterial activity of phenolic compounds against the phytopathogen Xylella fastidiosa. Curr. Microbiol. 60, 53–58. doi: 10.1007/s00284-009-9501-0
Martin, S., Rodolfo-Metalpa, R., Ransome, E., Rowley, S., Buia, M.-C., Gattuso, J.-P., et al. (2008). Effects of naturally acidified seawater on seagrass calcareous epibionts. Biol. Lett. 4, 689–692. doi: 10.1098/rsbl.2008.0412
Martinez-Crego, B., Olivé, I., and Santos, R. (2014). CO2 and nutrient-driven changes across multiple levels of organization in Zostera noltii ecosystems. Biogeosciences 11, 7237–7249. doi: 10.5194/bg-11-7237-2014
McDowell, R. E., Amsler, C. D., Dickinson, D. A., McClintock, J. B., and Baker, B. J. (2014). Reactive oxygen species and the Antarctic macroalgal wound response. J. Phycol. 50, 71–80. doi: 10.1111/jpy.12127
Migliore, L., Rotini, A., Randazzo, D., Albanese, N., and Giallongo, A. (2007). Phenols content and 2-D electrophoresis protein pattern: a promising tool to monitor Posidonia meadows health state. BMC Ecol. 7:6. doi: 10.1186/1472-6785-7-6
Miguel, M. G., Nunes, S., Dandlen, S. A., Cavaco, A. M., and Antunes, M. D. (2010). Phenols and antioxidant activity of hydro-alcoholic extracts of propolis from Algarve, South of Portugal. Food Chem. Toxicol. 48, 3418–3423. doi: 10.1016/j.fct.2010.09.014
Mittler, R. (2002). Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 7, 405–410. doi: 10.1016/S1360-1385(02)02312-9
Mittler, R., and Poulos, T. L. (2005). “Ascorbate peroxidase,” in Antioxidants and Reactive Oxygen Species in Plants, ed N. Smirnoff (London, UK: Blackwell Publishing Ltd.), 87–100.
Nakano, Y., and Asada, K. (1987). Purification of ascorbate peroxidase in spinach chloroplasts; its inactivation in ascorbate-depleted medium and reactivation by monodehydroascorbate radical. Plant Cell Physiol. 28, 131–140.
Niinemets, Ü., Kollist, H., García-Plazaola, J. I., Hernández, A., and Becerril, J. M. (2003). Do the capacity and kinetics for modification of xanthophyll cycle pool size depend on growth irradiance in temperate trees? Plant Cell Environ. 26, 1787–1801. doi: 10.1046/j.1365-3040.2003.01096.x
Ning, J., Li, X., Hicks, L. M., and Xiong, L. (2010). A RAF-like MAPKKK gene DSM1 mediates drought resistance through reactive oxygen species scavenging in rice. Plant Physiol. 152, 876–890. doi: 10.1104/pp.109.149856
Oh, M.-H., Kang, D., Kim, T., Moon, Y.-H., Moon, B., Chung, I., et al. (2009). Effects of epiphytic load on the photosynthetic performance of a seagrass, Zostera marina, monitored in vivo by chlorophyll fluorescence imaging. J. Plant Biol. 52, 171–175. doi: 10.1007/s12374-009-9010-5
Olivé, I., Silva, J., Costa, M. M., and Santos, R. (2015). Estimating seagrass community metabolism using benthic chambers: the effect of incubation time. Estuar. Coast. doi: 10.1007/s12237-015-9973-z. [Epub ahead of print].
Polle, A., and Morawe, B. (1995). Properties of ascorbate-related enzymes in foliar extracts from beech (Fagus sylvatica L.). Phyton 35, 117–129.
Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., and Rice-Evans, C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 26, 1231–1237. doi: 10.1016/S0891-5849(98)00315-3
Rösch, D., Bergmann, M., Knorr, D., and Kroh, L. W. (2003). Structure-antioxidant efficiency relationships of phenolic compounds and their contribution to the antioxidant activity of Sea Buckthorn Juice. J. Agric. Food Chem. 51, 4233–4239. doi: 10.1021/jf0300339
Sand-Jensen, K. (1977). Effect of epiphytes on eelgrass photosynthesis. Aquat. Bot. 3, 55–63. doi: 10.1016/0304-3770(77)90004-3
Smith, A. M., and Zeeman, S. C. (2006). Quantification of starch in plant tissues. Nat. Protoc. 1, 1342–1345. doi: 10.1038/nprot.2006.232
Stahl, W., and Sies, H. (1996). Lycopene: a biologically important carotenoid for humans? Arch. Biochem. Biophys. 336, 1–9. doi: 10.1006/abbi.1996.0525
Stitt, M., Bulpin, P. V., and Rees, T. A. (1978). Pathway of starch breakdown in photosynthetic tissues of Pisum sativum. Biochim. Biophys. Acta 544, 200–214. doi: 10.1016/0304-4165(78)90223-4
Stitt, M., Lilley, R. M., Gerhardt, R., Heldt, H. W., and Sidney Fleischer, B. F. (1989). “Metabolite levels in specific cells and subcellular compartments of plant leaves,” in Methods in Enzymology, eds J. N. Abelson and M. I. Simon (San Diego, CA: Academic Press), 518–552.
Sureda, A., Box, A., Terrados, J., Deudero, S., and Pons, A. (2008). Antioxidant response of the seagrass Posidonia oceanica when epiphytized by the invasive macroalgae Lophocladia lallemandii. Mar. Environ. Res. 66, 359–363. doi: 10.1016/j.marenvres.2008.05.009
Telfer, A. (2005). Too much light? How β-carotene protects the photosystem II reaction centre. Photochem. Photobiol. Sci. 4, 950–956. doi: 10.1039/b507888c
Valenzuela, A. (1991). The biological significance of malondialdehyde determination in the assessment of tissue oxidative stress. Life Sci. 48, 301–309. doi: 10.1016/0024-3205(91)90550-U
Van Montfrans, J., Wetzel, R., and Orth, R. (1984). Epiphyte-grazer relationships in seagrass meadows: consequences for seagrass growth and production. Estuaries 7, 289–309. doi: 10.2307/1351615
Vassallo, P., Paoli, C., Rovere, A., Montefalcone, M., Morri, C., and Bianchi, C. N. (2013). The value of the seagrass Posidonia oceanica: A natural capital assessment. Mar. Poll. Bull. 75, 157–167. doi: 10.1016/j.marpolbul.2013.07.044
Walters, R., and Horton, P. (1994). Acclimation of Arabidopsis thaliana to the light environment: changes in composition of the photosynthetic apparatus. Planta 195, 248–256. doi: 10.1007/BF00199685
Wojtaszek, P. (1997). Oxidative burst: an early plant response to pathogen infection. Biochem. J. 322, 681–692. doi: 10.1042/bj3220681
Keywords: seagrass, epiphytes, antioxidants, photosynthesis, oxidative stress, VAZ cycle, Posidonia oceanica
Citation: Costa MM, Barrote I, Silva J, Olivé I, Alexandre A, Albano S and Santos R (2015) Epiphytes Modulate Posidonia oceanica Photosynthetic Production, Energetic Balance, Antioxidant Mechanisms, and Oxidative Damage. Front. Mar. Sci. 2:111. doi: 10.3389/fmars.2015.00111
Received: 24 July 2015; Accepted: 01 December 2015;
Published: 21 December 2015.
Edited by:
Angel Borja, AZTI-Tecnalia, SpainReviewed by:
Elva G. Escobar-Briones, Universidad Nacional Autonoma de Mexico, MexicoRaquel Vaquer-Sunyer, Universitat de les Illes Balears, Spain
Copyright © 2015 Costa, Barrote, Silva, Olivé, Alexandre, Albano and Santos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Monya M. Costa, mmcosta@ualg.pt
 Monya M. Costa
Monya M. Costa Isabel Barrote
Isabel Barrote João Silva
João Silva Irene Olivé
Irene Olivé Ana Alexandre
Ana Alexandre Sílvia Albano
Sílvia Albano  Rui Santos
Rui Santos