Moving from Measuring to Predicting Bycatch Mortality: Predicting the Capture Condition of a Longline-Caught Pelagic Shark
- 1School of Biological Sciences, Monash University, Clayton, VIC, Australia
- 2School of Biological Sciences, Flinders University, Bedford Park, SA, Australia
Incidental fisheries capture has been identified as having a major effect on shark populations throughout the world. However, factors that contribute to the mortality of shark bycatch during fisheries capture are not fully understood. Here, we investigated the effects of capture duration, sea surface temperature, and shark total length (snout to the tip of the upper caudal lobe) on the physiology and condition of longline-caught bronze whalers, Carcharhinus brachyurus. Plasma lactate and potassium concentration had a positive linear relationship with capture duration, indicating that this species experiences increasing physiological challenges while on fishing gear. Additionally, we used stereotype logistic regression models to determine variables that could predict the capture condition of sharks (categorized as “healthy,” “sluggish,” or “moribund or dead”). In these models, elevated plasma lactate concentration, plasma potassium concentration, and capture duration increased the likelihood of C. brachyurus being captured in a “sluggish” condition or in a “moribund or dead” condition. After plasma lactate concentration exceeded 27.4 mmol/L, plasma potassium concentration exceeded 8.3 mmol/L, or capture durations exceeded 293 min, the majority of captured sharks (>50%) were predicted to be “moribund or dead.” We recommend that a reduction in the amount of time longlines are left fishing (soak time) will reduce immediate and post-release mortality in C. brachyurus bycatch and that our methods could be applied to identify causes of fisheries-induced mortality in future studies. The identification of operational, environmental, and biological variables contributing to poor condition will be necessary to implement conservation strategies that reduce mortality during capture.
Introduction
Commercial fisheries are one of the greatest threats to shark populations worldwide (Dulvy et al., 2014). Many sharks have slow life history strategies and their populations are inherently susceptible to the effects of fishing (Hoenig and Gruber, 1990; Smith et al., 1998). However, a large number of sharks caught by commercial fisheries are not retained, due to low commercial value, excessive physical damage, or fisheries regulations (Bonfil, 1994; Oliver et al., 2015). Sharks that are not retained may die before they are brought onto the vessel (immediate mortality) or after release (post-release mortality) due to physical injuries or physiological trauma caused by capture (Davis, 2002; Skomal, 2007; Dapp et al., 2015). Immediate and/or post-release mortality may be substantial in some species and can have detrimental effects on shark populations (Alverson et al., 1994; Dapp et al., 2015). Despite this, the variables influencing the likelihood of shark mortality during fisheries capture are poorly understood. Because longline fisheries constitute a large portion of the world's discarded shark catch (Bonfil, 1994; Oliver et al., 2015), additional investigation into variables contributing to mortality during longline capture is warranted.
Shark mortality studies should consider both immediate and post-release mortality (Carruthers et al., 2009; Dapp et al., 2015), but, post-release mortality studies require logistically challenging and/or expensive programs (e.g., tag and release, satellite tracking, or captive holding (Hueter et al., 2006; Campana et al., 2009; Frick et al., 2010). Consequently, these types of studies are limited to only a few shark species (Musyl et al., 2011; Dapp et al., 2015). In the absence of traditional post-release mortality estimation techniques, the externally visible condition (sometimes termed “vitality”) of the animal when removed from fishing gear (e.g., “healthy,” “sluggish,” and “moribund”) has been used as a proxy for post-release mortality (Hueter et al., 2006; Benoît et al., 2012; Braccini et al., 2012). The identification of variables that contribute to poor capture condition will allow researchers to better understand causes of post-release mortality during capture.
Ultimately, mortality studies should be used to design models that can predict species-specific mortality given operational, environmental, biological, and/or physiological measurements (Davis, 2002). Predictive models can then be used to make recommendations to fisheries managers that reduce the likelihood of immediate and post-release mortality in discarded species. For example, if the likelihood of mortality increases with increasing sea surface temperature, temporal restrictions on fishing during some months could be enacted to reduce bycatch mortality. Predictive models can also be used to identify physiological thresholds at which immediate and post-release mortality are likely to occur (Moyes et al., 2006; Hight et al., 2007; Frick et al., 2010).
The aim of the present study was to determine how shark capture condition is influenced by capture duration, sea surface temperature, total body length, and physiological variables. To achieve this aim, we took blood samples from longline-caught bronze whalers, Carcharhinus brachyurus, to measure hematological disruptions caused by capture. Hematological measurements (coupled with recordings of capture duration, sea surface temperature, and shark total length) were used to predict the capture condition of longline-caught C. brachyurus. Predictive models were applied to identify physiological thresholds that were likely to result in deteriorating shark condition when exceeded. The effect of non-physiological variables on capture condition was examined to make recommendations that can be used to reduce C. brachyurus mortality during and following longline capture.
Materials and Methods
Fishing Experiment
Longline fishing was conducted onboard research vessels within Gulf St Vincent, South Australia, Australia (−34.836801, 138.256073). The longline was set just before dusk and remained set until dawn, resulting in soak times of 12–14 h. Lines were checked approximately every 2 h throughout the night by slowly driving a boat parallel to the longline and checking for sharks caught on fishing gear. Moribund or dead sharks (defined in the capture condition indexing section) were removed from the line immediately after identification. In some instances, after a healthy or sluggish shark (defined in the capture condition indexing section) was identified on the main line, it was left there to reach a specific capture duration.
All hooks (16/0 circle hooks attached to 2 m stainless steel gangions; baited with Australian salmon, Arripis spp.) were fitted with a time-depth recorder (TDR; LAT 1100, Lotek Wireless, Newmarket, ON, Canada) or a hook timer (HT; 600, Lindgren-Pitman, Pompano Beach, FL, USA). Capture duration was determined by changes in depth recorded by the TDR or by triggered hook timers. However, small sharks (typically < 1000 mm total length) were usually not powerful enough to trigger hook timers, so capture duration could not be determined for those animals. All fieldwork conducted was approved by the Animal Ethics Committee of Flinders University (prior to October 2014; E360) or Monash University (during and after October 2014; BSCI/2014/22).
Sampling
Once captured, sharks were brought onboard the vessel and restrained. A bilge pump hose placed in the shark's mouth passed water over its gills to ensure continued oxygenation. Blood (~2.5 ml) was collected via the caudal vein with a heparinized 18-gauge × 1.5-inch needle and 3-ml syringe. The sample was immediately placed on crushed ice and stored for laboratory analysis. Shark total length (snout to the tip of the upper caudal lobe) and sea surface temperature were recorded. The entire sampling process, from the onset of aerial exposure until the shark was returned to the water, was completed in less than 5 min.
Upon reaching the laboratory, blood samples were mixed using a vortex mixer (model SVM1, Selby Biolab, Clayton, VIC, Australia). Whole blood (~75 μl) was drawn into a microcapillary tube and centrifuged at 10,000 rpm for 10 min to determine hematocrit. The remaining blood sample was centrifuged for 10 min at 10,000 rpms to separate the plasma and red blood cells. Once separated, plasma was extracted and stored at −20°C for chemical analysis at a later date. Concentrations of plasma glucose, lactate, potassium, chloride, and calcium were subsequently measured using blood gas analyzers (ABL 800 and ABL 80 Flex, Radiometer Pacific, Mt Waverley, VIC, Australia).
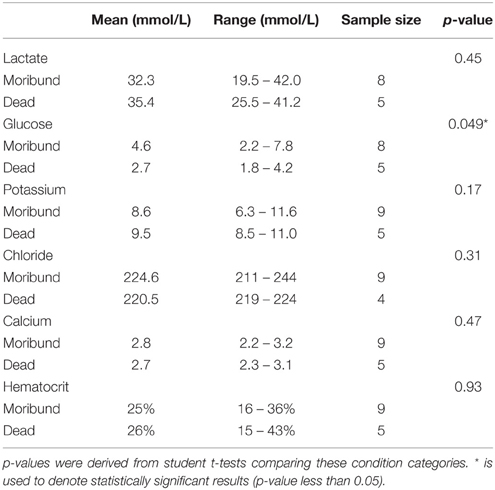
There was some variation in the number of blood variable measurement records. Because ABL 80 Flex analyzers are designed to operate on human blood, seven plasma chloride and three plasma calcium concentrations were out of measurement range. After initial tests, not enough sample remained to dilute and retest plasma chloride and calcium concentrations. Logistical difficulties caused two samples to be destroyed prior to glucose and lactate analysis. Although, 52 sharks were captured in total, blood was only acquired from 51 sharks, because needles used were to small to collect blood from one captured shark that was approximately 3 m in total length.
Capture Condition Indexing
We used a qualitative capture condition indexing system to categorize shark condition upon capture (Manire et al., 2001; Enever et al., 2009; Benoît et al., 2010; Braccini et al., 2012; Mandelman et al., 2013). Condition categories were adapted from Benoît et al. (2010), by combining moribund and dead sharks into a single category to strengthen statistical analyses (Table 1). This is a valid approach because obligate ram-ventilating sharks, such as C. brachyurus, released in a “very poor” condition (defined as limited or no swimming observed when released) have high percentages of post-release mortality (e.g., Carcharhinus limbatus and Sphyrna tiburo, 82% and 79% post-release mortality, Hueter et al., 2006). C. brachyurus released in a moribund condition required long revival periods alongside the boat (>15 min), could not effectively swim after revival, and were observed sinking with no tail movements upon release. Although, stationary respiring elasmobranchs have been able to effectively recover from a moribund condition (Laptikhovsky, 2004), it is unlikely that an obligate ram-ventilating shark would have the same capacity to physiologically recover if unable to move.

Table 1. Capture condition descriptions adapted from Benoît et al. (2010).
Additionally, combination of these categories was warranted because continuous line checks throughout the night ensured that most “moribund” and “dead” sharks were identified within 2 h. Those sharks that did progress from a “sluggish” to a “dead” category within the 2 h checking interval were likely to have died shortly before sampling.
Lastly, we performed t-tests to ensure that physiological measurements examined did not significantly differ between sharks categorized as moribund and dead. No significant differences were noted in concentrations of plasma lactate, potassium, chloride, calcium, or hematocrit percentage (Table 2). Plasma glucose concentrations in dead sharks were significantly lower than moribund sharks (Table 2), so glucose could not be examined across three categories. Glucose was not used to predict capture condition in subsequent analyses because combining moribund and dead sharks was necessary to achieve an adequate category sample size for regression models.
Statistical Analysis
We used regression analysis to investigate how each physiological variable was affected by capture duration (chloride, n = 35; calcium, n = 38; lactate and glucose, n = 39; hematocrit and potassium, n = 40; only sharks with a known capture duration included). For variables which conformed to a normal distribution, we fitted first (linear) order equations to the data (statistical significance designated as p < 0.05). Plasma glucose was not normally distributed, so capture duration was assigned to 100 min bins and its effect on glucose concentration was analyzed using non-parametric Kruskal-Wallis tests (statistical significance designated as p < 0.05).
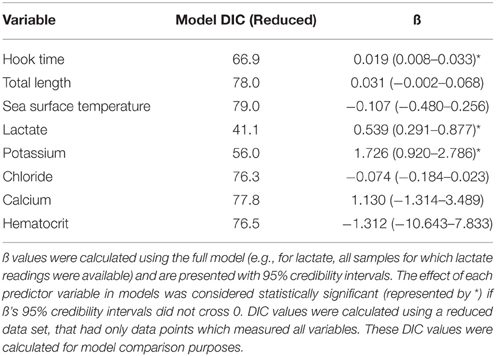
To relate capture condition to capture duration (n = 40), sea surface temperature (n = 52), total length of shark (n = 52), and physiological variables (lactate and glucose, n = 49; potassium and hematocrit, n = 51; chloride, n = 44; calcium, n = 48), we used stereotype logistic regression models (Anderson, 1984). These models were chosen over ordered logistic regression models because our data violated the proportional odds ratio assumption (Long and Freese, 2006). Models were run using a Bayesian statistical philosophy (Markov chain Monte Carlo estimation—three chains with 500,000 iterations per chain; burn-in length = 1000, thinning rate = 100, uninformative prior distribution). DIC (deviance information criterion) values were used to determine the most appropriate models and variables to predict capture condition and 95% credibility intervals were used to determine statistically significant predictor variables. The final models used (including derived coefficients) can be found in the Supplementary Material Section.
Statistical data analysis was conducted using the “R Project for Statistical Computing” version 2.14.2 (R Development Core Team, 2013) and “Just Another Gibbs Sampler” version 3.4.0 (http://mcmc-jags.sourceforge.net/).
Results
In total, 52 C. brachyurus were captured and 51 were blood sampled. Of those caught, 40 C. brachyurus had known capture durations, because small sharks (mean: 883 mm, range: 740–1170 mm) were not physically strong enough to trigger hook timers. Capture duration ranged from 5 to 402 min (mean = 167 min ± 17.2 SE). Of sharks with known capture durations, 16, 14, and 10 C. brachyurus were in “healthy,” “sluggish,” and “moribund or dead” conditions, respectively.
Regression models showed that capture duration had a significant effect on plasma lactate (p < 0.05, r2 = 0.27, Figure 1A) and plasma potassium (p < 0.05, r2 = 0.49, Figure 1D). Plasma lactate and potassium increased as capture duration increased. There was no significant effect of capture duration on hematocrit (Figure 1C), glucose concentration (Figure 1B), calcium concentration (Figure 1E), or chloride concentration (Figure 1F).
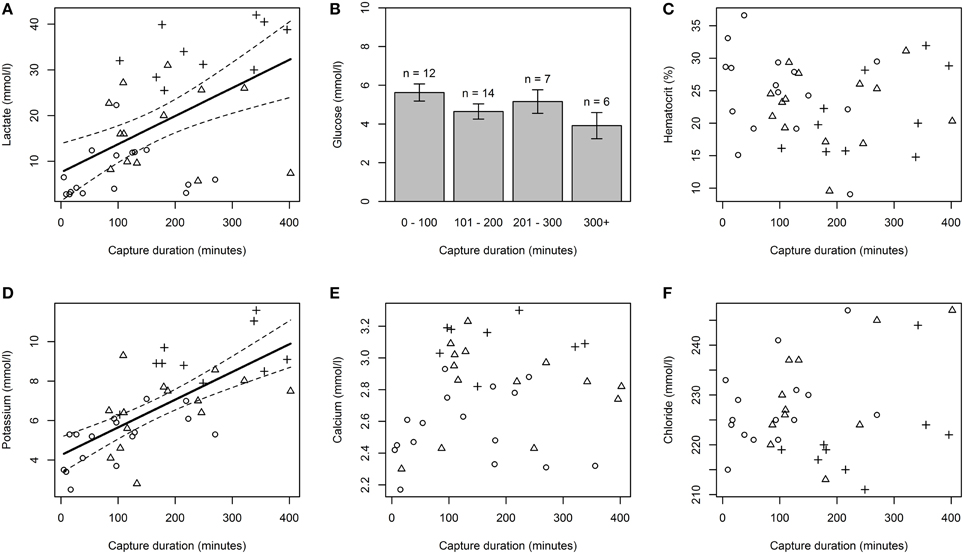
Figure 1. The effect of capture duration on physiological response variables. Physiological variables examined include plasma lactate (A), plasma glucose (B), hematocrit (C), plasma potassium (D), plasma calcium (E), and plasma chloride (F). Solid lines in plasma lactate and potassium plots represent a statistically significant linear relationship between capture duration and the physiological variable examined. Dashed lines represent 95% confidence intervals. Glucose was binned using 100 min intervals because the variable deviated significantly from a normal distribution. Circles, triangles, and crosses symbolize sharks caught in a “healthy,” “sluggish,” and “moribund or dead” condition respectively.
Predictive models incorporating plasma lactate had the lowest DIC values and this variable significantly effected capture condition (Table 3). Increasing lactate resulted in a higher probability of sharks being caught in a “sluggish” condition or in a “moribund or dead” condition. When plasma lactate concentration in C. brachyurus was < 18 mmol/L, nearly all sharks boated (removed from fishing gear) were predicted to be in a “healthy” (32%) or “sluggish” (62%) condition (Figure 2). After plasma lactate concentrations exceeded 18 mmol/L, there was a sharp increase in the probability of sharks boated “moribund or dead” (Figure 2). From 18 to 23 mmol/L an increase in 1 mmol/L of plasma lactate resulted in a mean 2.9% increase in sharks boated in a “moribund or dead” condition (Figure 2). From 23 to 28 mmol/L, 1 mmol/L lactate increment increases enhanced chances of boating a shark “moribund or dead” by an average of 7.1% (Figure 2). Once plasma lactate concentrations exceeded 28 mmol/L, the majority of sharks captured were predicted to be “moribund or dead” (55%) (Figure 2). Our lactate-only models predicted that 50, 75, and 90% of longline-caught C. brachyurus were “moribund or dead” at plasma lactate concentrations of 27.3, 30.8, and 34.8 mmol/L respectively (Figure 2). No single or additive combination of variables was significantly better at predicting capture condition than a model incorporating only plasma lactate.
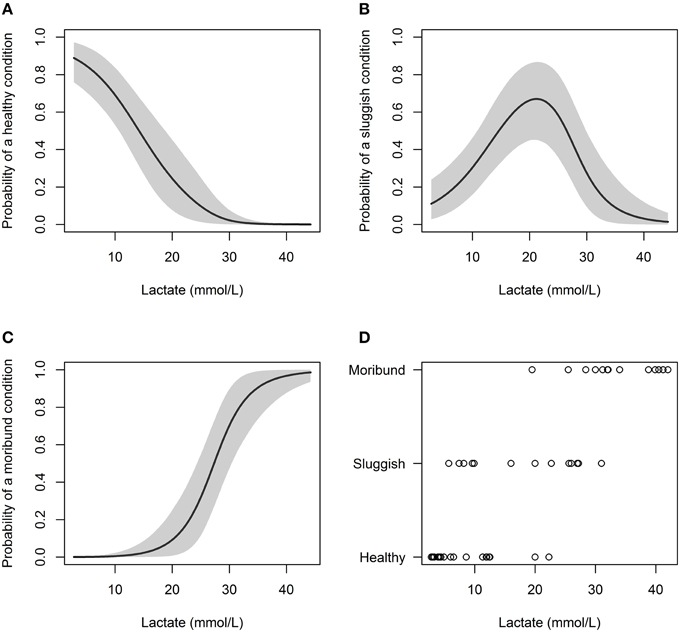
Figure 2. Probability of C. brachyurus being boated in a certain condition (A – “healthy,” B – “sluggish,” C – “moribund or dead”) given a plasma lactate concentration. Gray shading in (A–C) represents 95% credibility intervals. (D) Plots the raw data vs. lactate concentration, sorted by category (“healthy,” “sluggish,” “moribund or dead”). The mean plasma lactate concentrations for captured sharks in “healthy,” “sluggish,” and “moribund or dead” conditions were 7.7, 18.0, and 33.5 mmol/L, respectively.
Capture condition also deteriorated with increasing plasma potassium concentration (Table 3; Figure 3). When plasma potassium concentration was < 6.0 mmol/L, only 5% of C. brachyurus were predicted to be in a “moribund or dead” condition (Figure 3). When plasma potassium concentration exceeded 6.0 mmol/L, minor increases in potassium were indicative of greatly increased chances of capturing sharks “moribund or dead” (Figure 3). From concentrations of 6.0–7.5 mmol/L of plasma potassium, a 0.5 mmol/L increase in plasma potassium concentration resulted in a 6.9% increase in C. brachyurus being boated in a “moribund or dead” condition (Figure 3). Models predicted that the probability of being boated in a “moribund or dead” condition increases by 14.7% per 0.5 mmol/L increase in plasma potassium at plasma potassium concentrations between 7.5 and 9.0 mmol/L (Figure 3). Other physiological variables examined (hematocrit, plasma chloride concentration, and plasma calcium concentration) had no significant effect on capture condition (Table 3).
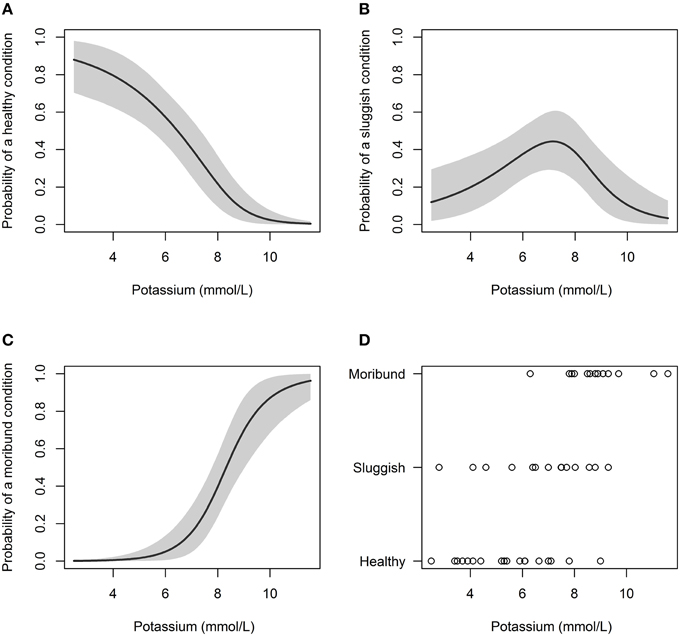
Figure 3. Probability of a C. brachyurus being boated in a certain condition (A – “healthy,” B – “sluggish,” C – “moribund or dead”) given a plasma potassium concentration. Gray shading in (A–C) represents 95% credibility intervals. (D) Plots the raw data vs. potassium concentration, sorted by category (“healthy,” “sluggish,” “moribund or dead”). The mean plasma potassium concentrations for captured sharks in “healthy,” “sluggish,” and “moribund or dead” conditions were 5.4, 6.7, and 8.9 mmol/L, respectively.
Of the non-physiological variables examined, capture duration significantly affected capture condition, while, sea surface temperature and total body length did not (Table 3). After 180 min of capture, only 31% of C. brachyurus were predicted to be boated in a “healthy” condition (Figure 4). By 266 min of capture, our model predicted that it is more likely to catch C. brachyurus in a “moribund or dead” condition than a “sluggish” condition (Figure 4). When capture durations exceed 270 min, only 12% of C. brachyurus captured are predicted to be in a “healthy” condition (Figure 4). Between 230 and 270 min capture durations, an increase in 5 min on fishing gear resulted in a mean of 1.1% increased probability of sharks being boated “moribund or dead.”
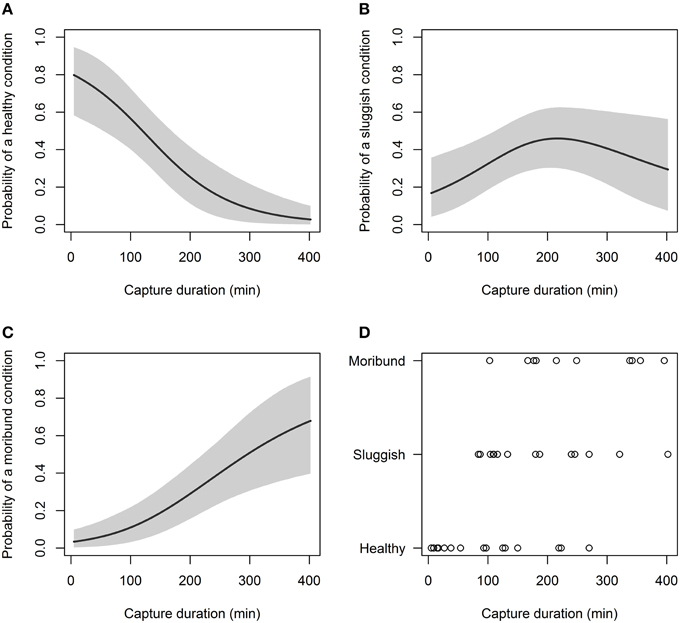
Figure 4. Probability of a C. brachyurus being boated in a certain condition (A – “healthy,” B – “sluggish,” C – “moribund or dead”) given capture duration. Gray shading in (A–C) represents 95% credibility intervals. (D) Plots the raw data vs. capture duration, sorted by category (“healthy,” “sluggish,” and “moribund or dead”). The mean capture durations for captured sharks in “healthy,” “sluggish,” and “moribund or dead” conditions were 98, 185, and 252 min, respectively.
Discussion
Our results demonstrated that the physiology of sharks during capture is correlated to capture duration and that plasma lactate concentration, plasma potassium concentration, and capture duration can influence a shark's capture condition. Plasma lactate and potassium concentrations in C. brachyurus had a positive linear relationship with capture duration. Some species capable of stationary respiration (e.g., Mustelus antarticus, Heterodontus portusjacksoni, and Carcharhinus perezi) exhibit a physiological response characterized by plasma lactate concentration peaking at 0–6 h after longline capture (Frick et al., 2010; Brooks et al., 2012). Following this peak, plasma lactate concentration decreases as physiological recovery begins while captured on fishing gear (Frick et al., 2010; Brooks et al., 2012). In our study, obligate ram-ventilating C. brachyurus did not begin to recover by 402 min of capture. Instead, plasma lactate and potassium concentrations increased with increasing capture duration. Due to the movement-restrictive nature of longline capture (Morgan and Burgess, 2007), obligate ram-ventilating sharks would be unlikely to achieve the minimal swimming speed needed to pass water over the gills to oxygenate their tissues (Gruber and Keyes, 1981; Carlson et al., 2004). Dapp et al. (2015) estimated that post-release mortality is more likely in obligate ram-ventilating sharks than stationary respiring sharks, and our results indicate that obligate ram-ventilating sharks may have a diminished ability to recover from physiological disturbance and other fishery-induced stress compared to species capable of stationary respiration.
Capture duration elicits varying responses in several of the variables examined. Captured sharks can experience significant changes in plasma chloride concentration, plasma glucose concentration, plasma calcium concentration, and hematocrit percentage (Cliff and Thurman, 1984; McDonald and Milligan, 1992; Heberer et al., 2010). However, these measurements are unaffected by capture duration in some species (Brooks et al., 2012; Kneebone et al., 2013; Butcher et al., 2015). In the present study, we observed no significant effect of capture duration on C. brachyurus plasma chloride concentration, plasma calcium concentration, plasma glucose concentration, and hematocrit percentage.
The probability that C. brachyurus were caught in a “sluggish” condition or a “moribund or dead” condition increased with increasing potassium. Several studies have suggested that hyperkalemia (elevated plasma potassium) can cause neuromuscular interference and muscle tetany in physiologically stressed sharks (Cliff and Thurman, 1984; Frick et al., 2010; Skomal and Mandelman, 2012). These conditions are thought to be one of the primary causes of shark mortality during and following fisheries capture (Martini, 1974; Cliff and Thurman, 1984; Frick et al., 2010). Several C. brachyurus captured in a “moribund or dead” condition during our study had stiffened muscles, indicating tetany. This symptom was not observed in any sharks in a “healthy” or “sluggish” condition (pers. obs). Given the importance of potassium as a predictor of condition and qualitative observations in the field, our results provide further evidence for the role of hyperkalemia in fisheries-induced mortality.
It is unlikely that increased plasma lactate is the proximate cause of death in physiologically stressed sharks because, in isolation, the lactate ion is relatively benign to fish (Jonas et al., 1962). However, in previous shark stress studies, plasma lactate has been used as an indicator of metabolic acidosis (Frick et al., 2010). Along with respiratory acidosis, metabolic acidosis can cause depressions in blood pH (Skomal and Mandelman, 2012). Metabolic acidosis can then compromise the integrity of cellular membranes, causing a leakage of inorganic ions (e.g., potassium, magnesium, and calcium) into the blood stream, resulting in intracellular acidosis (Cliff and Thurman, 1984). Intracellular acidosis is thought to be the primary cause of hyperkalemia in sharks during capture (Cliff and Thurman, 1984). Consequently, lactate was a good predictor of mortality in our study, not because it is toxic, but probably because it is an indicator of physiological processes that are detrimental to the health of sharks.
In addition to predicting when C. brachyurus are likely to be boated in a “moribund or dead” condition, it is crucial to estimate when sharks are more likely to be boated in a “sluggish” condition than a “healthy” condition. Forty-two percent (22 of 52) captured sharks were deemed to be in a “healthy” condition in our study. Previous studies have estimated that “healthy” sharks have low post-release mortality percentages (e.g., Prionace glauca–0%, Campana et al., 2009), so these sharks were likely to survive subsequent release. In contrast, sharks released “sluggish” are estimated to have post-release mortality percentages between 19% (Sphyrna tiburo) and 34% (Carcharhinus limbatus) (Hueter et al., 2006). Twenty-nine percent (15 of 52) of C. brachyurus we captured were in a “sluggish” condition and it is likely that some of these animals may have experienced post-release mortality.
Our models predicted that the percentage of C. brachyurus boated in a “sluggish” condition begins to exceed the percentage of C. brachyurus boated in a “healthy” condition at a plasma lactate concentration of 14.4 mmol/L, plasma potassium concentration of 6.9 mmol/L, and capture duration of 148 min. Peak percentages when C. brachyurus are most likely to be captured in a “sluggish” condition occur at a plasma lactate concentration of 21.2 mmol/L (67% of C. brachyurus captured “sluggish”), plasma potassium concentration of 7.2 mmol/L (44% of C. brachyurus captured “sluggish”), and capture duration of 216 min (46% of C. brachyurus captured “sluggish”). At these peaks, only 20%, 37%, 22% of C. brachyurus caught are predicted to be caught in a “healthy” condition, respectively, and are susceptible to immediate or post-release mortality. Future, studies are necessary to determine the likelihood of post-release mortality for C. brachyurus released in a “sluggish” condition.
Given our results, we consider plasma lactate concentrations of about 18 mmol/L and 23 mmol/L to be threshold values in C. brachyurus. These threshold values were established because C. brachyurus below an 18 mmol/L plasma lactate concentration are unlikely to be boated in a “moribund or dead” condition and sharks exceeding a 23 mmol/L concentration are likely to die with minor further plasma lactate concentration increases. Similarly, threshold values can be established for plasma potassium concentrations. Few C. brachyurus with a plasma potassium concentration < 6.0 mmol/L were predicted to be in a “moribund or dead” condition and the likelihood of sharks being boated in this condition greatly increased after plasma potassium concentration exceeded 7.5 mmol/L. Thus, captured animals with plasma lactate or potassium approaching these threshold values are at risk of tipping over into a condition with a significantly increased risk of an adverse outcome.
The likelihood of boating a shark in a “moribund or dead” condition increases gradually immediately after capture starts and, unlike physiological variables, there is no range of capture durations that result in a rapid increase in the likelihood this condition. Accordingly, we consider capture duration threshold values in C. brachyurus to be 94, 205, 293, and 402 min. At these capture durations 10, 30, 50, and 68% are predicted to be in a “moribund or dead” condition, respectively. It is also important to consider that by 293 min, few (9%) of sharks are captured in a “healthy” condition.
Ideally, maximum soak times of 94 min could be implemented to ensure that the majority of C. brachyurus caught are “healthy” and that few (predicted 10%) are caught “moribund or dead.” However, regulations reducing soak time to 94 min may be unrealistic because it can take up to 3 h to haul longline gear (although haul times can be reduced by reducing the number of hooks set) and may not be economically viable for fishers (Butcher et al., 2015). Butcher et al. (2015) recommended that a reduction in allowable longline soak time to 300 min would reduce the mortality of shark bycatch. Such regulations would benefit C. brachyurus, because few sharks would be caught dead, if we assume an average capture duration of 150 min, resulting in 40% predicted “healthy,” 41% predicted “sluggish,” and 19% predicted “moribund or dead.”
Models used could not predict a capture duration that caused all sharks caught to be “moribund or dead” and the maximum capture duration (402 min) used in our study only predicted that 68.3% of C. brachyurus captured were in this condition. This result may be because capture durations used in our study were shorter than commercial fishing operations. Only two of the sharks we captured were on fishing gear for more than 6 h and 73% of the sharks had capture durations less than 4 h. Morgan and Carlson (2010) noted that peak increases in immediate mortality percentage occur at capture durations of 6 and 10 h in blacktip, Carcharhinus limbatus, and sandbar, Carcharhinus plumbeus, sharks respectively. Although, our models predicted that sharks may still be alive after 402 min, only 1% of sharks captured at this duration were in a “healthy” condition and most sharks caught live at this duration are predicted to be “sluggish.”
One caveat in our study is that, ideally, blood lactate concentrations should be measured immediately following capture using a portable analyzer (e.g., i-Stat – Frick et al., 2012; Accutrend Plus – Butcher et al., 2015) or blood samples should be centrifuged immediately to separate plasma (Frick et al., 2010). Although, we had access to an i-Stat portable blood gas analyzer, it was consistently out of temperature range and, thus, unreliable for our purposes. Blood samples were not immediately centrifuged due to logistical difficulties onboard a rocking vessel. In humans, lactate concentrations in whole blood stored on ice increase minimally (approximately 0.1 mmol/L after 1 h; Toffaletti et al., 1992); unknown effect in shark blood) and the maximum time before centrifuging in our study was approximately 12 h. Although, several previous elasmobranch stress studies have measured and reported plasma lactate concentrations after whole blood was left on ice or frozen prior to centrifuging (Hoffmayer and Parsons, 2001; Manire et al., 2001; Brill et al., 2008; Hoffmayer et al., 2012), we recommend that this caveat should be taken into consideration when interpreting our lactate results.
To our knowledge, ours is the first study to predict capture condition under varying capture duration, sea surface temperature, shark total length, and physiological measurements. Previous studies have shown that assessing condition upon capture is valuable because it can be a reliable predictor of post-release mortality (Hueter et al., 2006; Benoît et al., 2012). In the absence of tagging studies, capture condition indexing can be applied on a broad scale and to multiple species to estimate post-release mortality. The methods used in our study can be readily applied to future research examining operational, biological, physiological, and environmental variables such as gangion length, capture depth, air temperature, wounding, and time spent struggling on fishing gear. Methods used are especially well-suited to observer program data sets because observers can be trained to correctly categorize capture condition and could collect data across a broad spatial and temporal range.
Future, physiological studies could include additional physiological measurements, such as intramuscular lactate and plasma catecholamine, to predict shark capture condition. Although, measuring these two variables was out of the scope of the present study, intramuscular lactate and plasma catecholamine may be good predictors of shark mortality following capture (Hight et al., 2007; Frick et al., 2012). Additional information on how these variables can influence capture condition can be used to determine the biological systems that are most detrimentally effected by fisheries capture and can help to design effective management strategies. For example, if high intramuscular lactate concentrations (indicative of struggling and/or insufficient oxygenation) result in decreasing capture condition, then it can be determined that intense struggling behavior and/or insufficient oxygenation during capture likely have a detrimental effect on sharks. In this scenario, management measures that reduce movement restriction (e.g., longer gangions) may reduce struggling, enhance oxygenation ability, and reduce mortality percentages in sharks.
Conclusion
Plasma lactate and potassium concentrations had a positive linear relationship with capture duration in C. brachyurus, indicating that obligate ram-ventilating sharks may not have the same ability to recover during capture as stationary respiring species. The best physiological predictors of C. brachyurus capture condition we examined were plasma lactate and potassium. Previous studies have suggested that elevations in plasma lactate and potassium are indicative of metabolic and intracellular acidosis, and our results support the notion that these conditions are associated with deteriorating capture condition in sharks. It is possible that the extent of these conditions can be decreased during capture by reducing aerial exposure times (Cicia et al., 2012; Heard et al., 2014), avoiding fishing during hot seasons or sea surface temperatures (Hoffmayer et al., 2012), and/or reducing gear soak times (present study). We recommend that regulations reducing maximum allowable soak time to 300 min will decrease immediate and post-release mortality in longline-caught C. brachyurus bycatch.
Our study used capture condition indexing and stereotype logistic regression models to predict the capture condition of sharks. In the absence of logistically challenging and expensive tagging studies, we recommend that these methods could be applied to immediate mortality studies to predict causes of immediate mortality and to estimate post-release mortality. Although, we focused on C. brachyurus, these methods can be applied to any elasmobranch, bony teleost, turtle, or marine mammal during fisheries capture. Use of these methods will allow future studies to go beyond simply recording mortality and will allow fisheries scientists to predict the best management strategies to reduce the mortality of bycatch.
Author Contributions
Experimental design: DD, RR, CH, and TW, Data collection: DD, CH, and MD, Data processing: DD, Data analysis: DD, Manuscript writing: DD, RR, TW, CH, and MD, Final draft approval: DD, CH, TW, MD, and RR.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Paul Rogers, Matthew Lloyd, Leonardo Guida, Crystal Beckmann, Jens Neiser, and volunteers for helping with shark collection. We thank Alison Moxham, David Walker, David Stone, and The Monash Institute of Medical Research for access to necessary equipment for sample analysis. Statistical advice was provided by Jonathan Keith and Murray Logan. Financial support for this work was provided by the Australian Research Council Grant LP110200572 to RR & TW and LP120100652 to CH, the Holsworth Wildlife Research Endowment—Equity Trustees Charitable Foundation, Monash University, Flinders University, the Neiser Foundation, the Australian Fisheries Management Authority, the Department of Primary Industries (Victoria), and Melbourne Sea Life Aquarium.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/article/10.3389/fmars.2015.00126
References
Alverson, D. L., Freeberg, M. H., Murawski, S. A., and Pope, J. G. (1994). A Global Assessment of Fisheries Bycatch and Discards. FAO Fisheries Technical Paper 339, Food and Agriculture Organization of the United Nations, Rome.
Anderson, J. A. (1984). Regression and ordered categorical variables. J. Roy. Stat. Soc. B Met. 46, 1–30.
Benoît, H. P., Hurlbut, T., and Chasse, J. (2010). Assessing the factors influencing discard mortality of demersal fishes using a semi-quantitative indicator of survival potential. Fish. Res. 106, 436–447. doi: 10.1016/j.fishres.2010.09.018
Benoît, H. P., Hurlbut, T., Chasse, J., and Jonsen, I. D. (2012). Estimating fishery-scale rates of discard mortality using conditional reasoning. Fish. Res. 125, 318–330. doi: 10.1016/j.fishres.2011.12.004
Bonfil, R. (1994). Overview of World Elasmobranch Fisheries. FAO Fisheries Technical Paper 341, Food and Agriculture Organization of the United Nations, Rome.
Braccini, M., Van Rijn, J., and Frick, L. (2012). High post-capture survival for sharks, rays and chimaeras discarded in the main shark fishery of Australia? PLoS ONE 7:e32547. doi: 10.1371/journal.pone.0032547
Brill, R., Bushnell, P., Schroff, S., Seifert, R., and Galvin, M. (2008). Effects of anaerobic exercise accompanying catch-and-release fishing on blood-oxygen affinity of the sandbar shark (Carcharhinus plumbeus, Nardo). J. Exp. Mar. Biol. Ecol. 354, 132–143. doi: 10.1016/j.jembe.2007.10.011
Brooks, E. J., Mandelman, J. W., Sloman, K. A., Liss, S., Danylchuk, A. J., Cooke, S. J., et al. (2012). The physiological response of the Caribbean reef shark (Carcharhinus perezi) to longline capture. Comp. Biochem. Phys. A 162, 94–100. doi: 10.1016/j.cbpa.2011.04.012
Butcher, P. A., Peddemors, V. M., Mandelman, J. W., McGrath, S. P., and Cullis, B. R. (2015). At-vessel mortality and blood biochemical status of elasmobranchs caught in an Australian commercial longline fishery. Glob. Ecol. Conserv. 3, 878–889. doi: 10.1016/j.gecco.2015.04.012
Campana, S. E., Joyce, W., and Manning, M. J. (2009). Bycatch and discard mortality in commercially caught blue sharks Prionace glauca assessed using archival satellite pop-up tags. Mar. Ecol.-Prog. Ser. 387, 241–253. doi: 10.3354/meps08109
Carlson, J. K., Goldman, K. J., and Lowe, C. G. (2004). “Metabolism, energetic demand, and endothermy,” in Biology of Sharks and their Relatives, eds. J. Carrier, J. Musick, and M. Heithaus (Boca Raton, FL: CRC Press), 203–225.
Carruthers, E. H., Schneider, D. C., and Neilson, J. D. (2009). Estimating the odds of survival and identifying mitigation opportunities for common bycatch in pelagic longline fisheries. Biol. Conserv. 142, 2620–2630. doi: 10.1016/j.biocon.2009.06.010
Cicia, A. M., Schlenker, L. S., Sulikowski, J. A., and Mandelman, J. W. (2012). Seasonal variations in the physiological stress response to discrete bouts of aerial exposure in the little skate, Leucoraja erinacea. Comp. Biochem. Phys. A 162, 130–138. doi: 10.1016/j.cbpa.2011.06.003
Cliff, G., and Thurman, G. D. (1984). Pathological and physiological effects of stress during capture and transport in the juvenile dusky shark, Carcharhinus obscurus. Comp. Biochem. Phys. A 78, 167–173. doi: 10.1016/0300-9629(84)90111-7
Dapp, D., Walker, T., Huveneers, C., and Reina, R. (2015). Respiratory mode and gear type are important determinants of elasmobranch immediate and post-release mortality. Fish and Fisheries. doi: 10.1111/faf.12124. [Epub ahead of print].
Davis, M. W. (2002). Key principles for understanding fish bycatch discard mortality. Can. J. Fish. Aquat. Sci. 59, 1834–1843. doi: 10.1139/f02-139
Dulvy, N. K., Fowler, S. L., Musick, J. A., Cavanagh, R. D., Kyne, P. M., Harrison, L. R., et al. (2014). Extinction risk and conservation of the world's sharks and rays. eLife 3, e00590–e00624. doi: 10.7554/eLife.00590
Enever, R., Catchpole, T. L., Ellis, J. R., and Grant, A. (2009). The survival of skates (Rajidae) caught by demersal trawlers fishing in UK waters. Fish. Res. 97, 72–76. doi: 10.1016/j.fishres.2009.01.001
Frick, L. H., Reina, R. D., and Walker, T. I. (2010). Stress related physiological changes and post-release survival of Port Jackson sharks (Heterodontus portusjacksoni) and gummy sharks (Mustelus antarcticus) following gill-net and longline capture in captivity. J. Exp. Mar. Biol. Ecol. 385, 29–37. doi: 10.1016/j.jembe.2010.01.013
Frick, L. H., Walker, T. I., and Reina, R. D. (2012). Immediate and delayed effects of gill-net capture on acid-base balance and intramuscular lactate concentration of gummy sharks, Mustelus antarcticus. Comp. Biochem. Phys. A 162, 88–93. doi: 10.1016/j.cbpa.2011.02.023
Gruber, S. H., and Keyes, R. S. (1981). “Keeping sharks for research,” in Aquarium Systems, eds A. Hawkings (London: Academic Press), 373–402.
Heard, M., Van Rijn, J. A., Reina, R. D., and Huveneers, C. (2014). Impacts of crowding, trawl duration and air exposure on the physiology of stingarees (family: Urolophidae). Conserv. Physiol. 2:cou040. doi: 10.1093/conphys/cou040. Available online at: http://conphys.oxfordjournals.org/content/2/1/cou040.full
Heberer, C., Aalbers, S. A., Bernal, D., Kohin, S., DiFiore, B., and Sepulveda, C. A. (2010). Insights into catch-and-release survivorship and stress-induced blood biochemistry of common thresher sharks (Alopias vulpinus) captured in the southern California recreational fishery. Fish. Res. 106, 495–500. doi: 10.1016/j.fishres.2010.09.024
Hight, B. V., Holts, D., Graham, J. B., Kennedy, B. P., Taylor, V., Sepulveda, C. A., et al. (2007). Plasma catecholamine levels as indicators of the post-release survivorship of juvenile pelagic sharks caught on experimental drift longlines in the Southern California Bight. Mar. Freshwater Res. 58, 145–151. doi: 10.1071/mf05260
Hoenig, J. M., and Gruber, S. H. (1990). “Life-history patterns in the elasmobranchs: implications for fisheries management,” in Elasmobranchs as Living Resources: Advances in the Biology, Ecology, Systematics, and the Status of the Fisheries, eds H. Pratt, S. Gruber, and T. Taniuchi. (Springfield, VA: National Marine Fisheries Service), 1–6.
Hoffmayer, E. R., Hendon, J. M., and Parsons, G. R. (2012). Seasonal modulation in the secondary stress response of a carcharhinid shark, Rhizoprionodon terraenovae. Comp. Biochem. Phys. A 162, 81–87. doi: 10.1016/j.cbpa.2011.05.002
Hoffmayer, E. R., and Parsons, G. R. (2001). The physiological response to capture and handling stress in the Atlantic sharpnose shark, Rhizoprionodon terraenovae. Fish Physiol. Biochem. 25, 277–285. doi: 10.1023/A:1023210620904
Hueter, R. E., Manire, C. A., Tyminski, J. P., Hoenig, J. M., and Hepworth, D. A. (2006). Assessing mortality of released or discarded fish using a logistic model of relative survival derived from tagging data. T. Am. Fish. Soc. 135, 500–508. doi: 10.1577/T05-065.1
Jonas, R. E. E., Sehdev, H. S., and Tomlinson, N. (1962). Blood pH and mortality in rainbow trout (Salmo gairdnerii) and sockeye salmon (Oncorhynchus nerka). J. Fish. Res. Board Can. 19, 619–624. doi: 10.1139/f62-041
Kneebone, J., Chisholm, J., Bernal, D., and Skomal, G. (2013). The physiological effects of capture stress, recovery, and post-release survivorship of juvenile sand tigers (Carcharias taurus) caught on rod and reel. Fish. Res. 147, 103–114. doi: 10.1016/j.fishres.2013.04.009
Laptikhovsky, V. V. (2004). Survival rates for rays discarded by the bottom trawl squid fishery off the Falkland Islands. Fish. B NOAA 102, 757–759.
Long, J. S., and Freese, J. (2006). Regression Models for Categorical Dependent Variables Using Stata. College Station, TX: StataCorp.
Mandelman, J. W., Cicia, A. M., Ingram, G. W., Driggers, W. B., Coutre, K. M., and Sulikowski, J. A. (2013). Short-term post-release mortality of skates (family Rajidae) discarded in a western North Atlantic commercial otter trawl fishery. Fish. Res. 139, 76–84. doi: 10.1016/j.fishres.2012.09.020
Manire, C., Hueter, R., Hull, E., and Spieler, R. (2001). Serological changes associated with gill-net capture and restraint in three species of sharks. T. Am. Fish. Soc. 130, 1038–1048. doi: 10.1577/1548-8659(2001)130<1038:scawgn>2.0.co;2
Martini, F. (1974). Effects of Capture and Fasting Confinement on an Elasmobranch, Squalus acanthias. Doctoral Thesis, Cornell University, Ithaca, NY.
McDonald, D. G., and Milligan, C. L. (1992). “Chemical properties of the blood,” in Fish Physiology, eds D. Randall, W. Hoar, and A. Farrell (San Diego, CA: Academic Press), 55–133.
Morgan, A., and Burgess, G. H. (2007). At-vessel fishing mortality for six species of sharks caught in the northwest Atlantic and Gulf of Mexico. Gulf Caribb. Res. 19, 1–7. doi: 10.18785/gcr.1902.15
Morgan, A., and Carlson, J. K. (2010). Capture time, size and hooking mortality of bottom longline-caught sharks. Fish. Res. 101, 32–37. doi: 10.1016/j.fishres.2009.09.004
Moyes, C. D., Fragoso, N., Musyl, M. K., and Brill, R. W. (2006). Predicting postrelease survival in large pelagic fish. T. Am. Fish. Soc. 135, 1389–1397. doi: 10.1577/t05-224.1
Musyl, M. K., Brill, R. W., Curran, D. S., Fragoso, N. M., McNaughton, L. M., Nielsen, A., et al. (2011). Postrelease survival, vertical and horizontal movements, and thermal habitats of five species of pelagic sharks in the central Pacific Ocean. Fish. B NOAA 109, 341–368. Available online at: http://fishbull.noaa.gov/1094/1094toc.htm
Oliver, S., Braccini, M., Newman, S. J., and Harvey, E. S. (2015). Global patterns in the bycatch of sharks and rays. Mar. Policy 54, 86–97. doi: 10.1016/j.marpol.2014.12.017
R Development Core Team (2013). R: A Language for Environment and Statistical Computing. (version 2.14.2). Available online at: http://www.r-project.org/
Skomal, G. B. (2007). Evaluating the physiological and physical consequences of capture on post-release survivorship in large pelagic fishes. Fish. Manag. Ecol. 14, 81–89. doi: 10.1111/j.1365-2400.2007.00528.x
Skomal, G. B., and Mandelman, J. W. (2012). The physiological response to anthropogenic stressors in marine elasmobranch fishes: a review with a focus on the secondary response. Comp. Biochem. Phys. A 162, 146–155. doi: 10.1016/j.cbpa.2011.10.002
Smith, S. E., Au, D. W., and Show, C. (1998). Intrinsic rebound potentials of 26 species of Pacific sharks. Mar. Freshwater Res. 49, 663–678. doi: 10.1071/MF97135
Keywords: shark, longline, biochemistry, post-release mortality, capture duration, capture condition
Citation: Dapp DR, Huveneers C, Walker TI, Drew M and Reina RD (2016) Moving from Measuring to Predicting Bycatch Mortality: Predicting the Capture Condition of a Longline-Caught Pelagic Shark. Front. Mar. Sci. 2:126. doi: 10.3389/fmars.2015.00126
Received: 12 November 2015; Accepted: 24 December 2015;
Published: 19 January 2016.
Edited by:
Rob Harcourt, Macquarie University, AustraliaReviewed by:
Gail Schofield, Deakin University, AustraliaMichael Brian Bennett, The University of Queensland, Australia
Copyright © 2016 Dapp, Huveneers, Walker, Drew and Reina. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Derek R. Dapp, derek.dapp@monash.edu;
Richard D. Reina, richard.reina@monash.edu
 Derek R. Dapp
Derek R. Dapp Charlie Huveneers
Charlie Huveneers Terence I. Walker1
Terence I. Walker1  Richard D. Reina
Richard D. Reina