Quorum Sensing Plays a Complex Role in Regulating the Enzyme Hydrolysis Activity of Microbes Associated with Sinking Particles in the Ocean
- 1Department of Marine Chemistry and Geochemistry, Woods Hole Oceanographic Institution, Woods Hole, MA, USA
- 2School of Oceanography, University of Washington, Seattle, WA, USA
The concentration of atmospheric carbon dioxide is directly linked to the sinking of photosynthetically derived particulate organic carbon (POC) from surface waters to deep waters. This process, known as the marine biological carbon pump, removes carbon from exchange with the atmosphere, thus regulating global climate. Recent evidence suggests that microbial chemical communication systems (e.g., quorum sensing) amongst heterotrophic bacteria associated with sinking POC, significantly influence their hydrolytic enzyme activity and, as such, may affect the efficiency of the biological carbon pump. Here, we present data showing that a class of quorum sensing molecules, acylated homeserine lactones (AHLs) substantially impact hydrolytic phosphatase, aminopeptidase, and lipase activity in samples of sinking particles collected from the Atlantic and Pacific Ocean. Incubations of sinking particles amended with exogenous AHLs showed both stimulated and inhibited rates of activity after 24 h of incubation, suggesting a critical link between bacterial AHL signaling mechanisms and the rate of POC degradation. Further experiments reveal that hydrolytic pathways could be affected within a few hours of amendment with AHLs, suggesting that microbial communities are able to dynamically modify their metabolic pathways in response to perceived quorum sensing. Finally, the concentration of the AHL amendment also affected hydrolytic activity. AHL-based quorum sensing may be thought of as a global language among marine bacteria, but it is highly complex.
Introduction
Oceanic carbon export via sinking particles plays a pivotal role in regulating Earth's climate. Photosynthetic microorganisms (e.g., phytoplankton) in the marine environment convert atmospheric CO2 into biomass; the majority of this organic carbon is recycled in surface waters by diverse microbial processes. However, some of this biomass sinks as particulate organic carbon (POC) into deeper regions of the ocean via the biological carbon pump, where bacterial cells continue to play a critical role in regulating the efficiency of carbon export through the colonization and enzymatic hydrolysis of sinking particles (Turley and Mackie, 1994; Simon et al., 2002). Understanding the link between vertical changes in POC degradation and hydrolytic activity by particle-associated bacteria is critical for predicting carbon flux through the water column across a wide range of oceanic regimes.
A study by Hmelo et al. (2011) found that hydrolytic enzymes excreted by some particle-associated bacteria may be affected by quorum sensing mechanisms. Quorum sensing is a cell–density dependent form of communication between microorganisms that involves the secretion of distinct chemical signals in order to coordinate directed behaviors ranging from biofilm formation to antibiotic resistance (Whiteley et al., 1999; Ng and Bassler, 2009). Thus, far, our knowledge about the potential importance of bacterial quorum sensing on sinking particles is limited because only a few cultivation–independent studies have been carried out in the marine environment (Decho et al., 2009; Van Mooy et al., 2012; Ransome et al., 2014; Zimmer et al., 2014; Certner and Vollmer, 2015).
Acylated homoserine lactones (AHLs) represent the most studied and best characterized class of bacterial quorum sensing signaling molecules (Eberhard et al., 1981; Pearson et al., 1994; Fuqua et al., 2001). These so-called autoinducers are constitutively excreted extracellularly and may contain chemically diverse acyl chains; chain lengths from C4 to C18 and additional modifications to the side chain (e.g., substitutions at the 3-carbon position or unsaturated double bonds) allow signal specificity (Parsek and Greenberg, 2000; Fuqua et al., 2001). The usage and production of AHLs are common among many bacterial species, particularly Gram-negative bacteria, but the corresponding biological functions that are regulated by AHLs remain poorly understood, particularly in marine systems.
Quorum sensing processes are of particular interest on sinking particles due to the taxonomic composition of the associated bacterial communities and the high population density of those communities. Previous investigations have shown that Gram-negative bacteria such as Gammaproteobacteria can be the dominant clades attached to sinking particles (DeLong et al., 1993; Rath, 1998; Moeseneder et al., 2001), and these clades contain a broad range of known quorum sensing species (Doberva et al., 2015). In addition, abundances of particle-associated bacteria can be several magnitudes higher than free-living bacteria in the surrounding seawater (Caron et al., 1986; Turley and Mackie, 1994); coordination amongst these bacteria is likely to be critical in their ability to thrive on the degradation of POC (Vetter et al., 1998; Mislan et al., 2014) and may directly contribute to the acceleration of POC degradation processes. As an indirect consequence of enhanced POC degradation, particle hydrolysis can stimulate the recycling of nutrients (e.g., phosphorus), which can fuel the productivity of free-living microbial populations, especially in nutrient deprived open ocean environments (Azam and Long, 2001; Kiørboe and Jackson, 2001; Stocker et al., 2008).
In this study, in situ quorum sensing activity of particle-associated bacterial communities in various oceanic regions ranging from coastal to open-ocean waters was monitored. Differences in hydrolytic activity rates of bacteria on sinking particles were observed in incubations amended with AHLs; these responses varied by geographic location, post-amendment timescale, and AHL concentration. Results of this study represent another important step toward deciphering the impact of marine bacterial communication systems on the marine biological carbon pump.
Materials and Methods
Field Sampling
Sinking particles were collected from five different locations in the Atlantic and Pacific Ocean (Table 1) using sediment deployments following the techniques of Peterson et al. (2005). Sediment were deployed below the deep chlorophyll maximum (DCM). Individual deployments lasted 8–24 h. After recovery of sediment traps, collected material was sieved through a 500 μm nylon mesh in order to remove contaminating zooplankton.
Incubation Design
The particles recovered from the traps were quantitatively split using a wet sample divider (Lamborg et al., 2008) and transferred into 60 mL polycarbonate incubation bottles. Prior to each experiment, these bottles were thoroughly cleaned with methanol, 10% acid, and milli-Q water. Sets of triplicate bottles were amended with AHLs (described in section AHL amendments), and incubated in the dark at in situ temperature. No-amendment control incubations were also prepared in triplicate, and were dosed with the same solvents used in the AHL solutions. Triplicate 200 μL subsamples were taken from each incubation bottle for each hydrolytic enzyme activity and transferred into sterile 96 multi-well plates (details in section Hydrolytic enzyme activity assays), and further incubated in the dark at in situ temperature. Incubations were terminated after 24 h and sample material from incubation bottles was extracted to collect AHLs (details in section AHL sample collection, extraction, and analysis).
AHL Amendments
Substituted AHLs are delineated by the presence of a 3-oxo (ketone) substituent on the acyl-side chain, whereas unsubstituted AHLs do not have such a ketone group. As a representative substituted AHL we chose N-(3-oxooctanoyl)-L-homoserine lactone (3OC8-HSL). We also constructed a cocktail of unsubstituted AHLs including di-deuterated N-(decanoyl) homoserine lactone (D2-C10-HSL), di-deuterated N-(dodecanoyl) homersine lactone (D2-C12-HSL), and di-deuterated N-(tetradecanoyl) homersine lactone (D2-C14-HSL). The stocks were made to a concentration of 50 μM in dimethylsulfoxide. Incubations were amended with AHLs to a final concentration of 500 nM in all locations. Additional incubation experiments were performed in the western tropical North Atlantic with final AHL concentrations of 100, 250, 750, and 1000 nM. All AHLs were purchased from Sigma-Aldrich.
Hydrolytic Enzyme Activity Assays
The activity of different hydrolytic enzymes (aminopeptidase, lipase, and phosphatase) was measured by monitoring the hydrolysis products of model fluorogenic substrates (Hoppe, 1993). For each enzyme activity, three 200 μL samples from each incubation were analyzed. The model substrates used in these experiments were L-leucine-7-amino-4-methylcoumarin for aminopeptidase activity, 4-methylumbelliferyl-butyrate for lipase activity, and 4-methylumbelliferyl-phosphate for phosphatase activity. All fluorogenic substrates are from Sigma-Aldrich. Fluorescence was determined using a CytoFluor® Multi-Well Plate Reader Series 4000 (PerSeptive Biosystems, MA, USA). Standards of 7-amino-4-methylcoumarin and 4-Methylumbelliferone were prepared in concentrations ranging from 0.002 to 5.6 mM. Chauvenet's Criterion (Dmax = 1.38) was applied in order to identify and remove outliers from the dataset. T-tests were then applied to identify instances were enzyme activity in experimental incubation differed from control incubations (p < 0.1). Type I statistical errors due to multiple comparisons were controlled within each dataset (e.g., Figure 1B) using the false discovery rate criterion described by Benjamini and Hochberg (1995). After these statistical tests, the hydrolytic enzyme activities in incubations amended with AHLs were divided by the average of the control incubation to facilitate location-to-location comparisons.
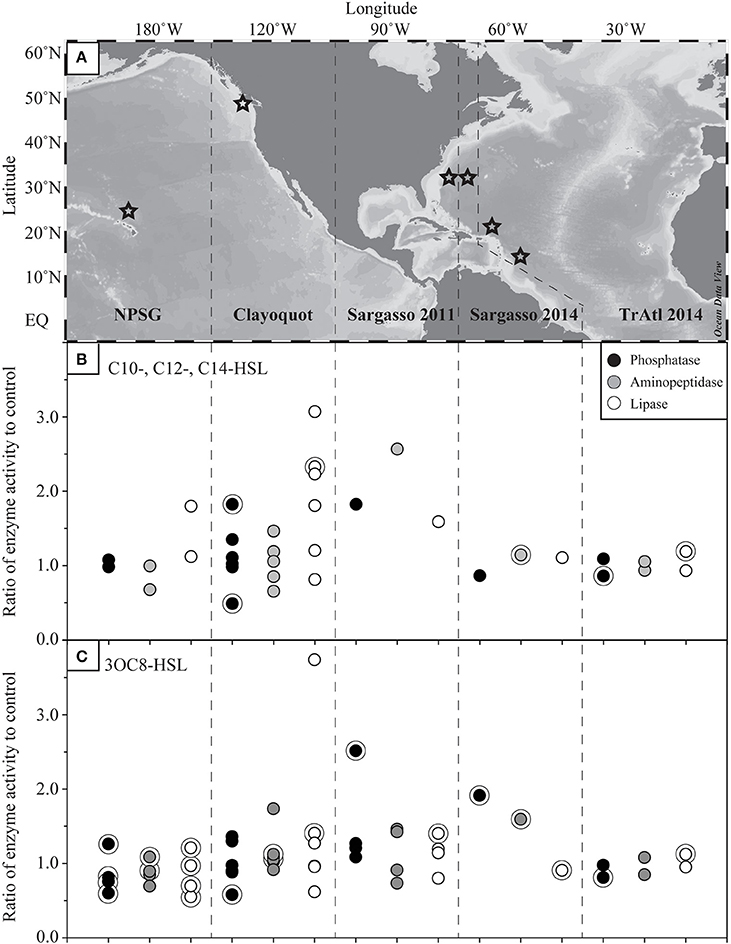
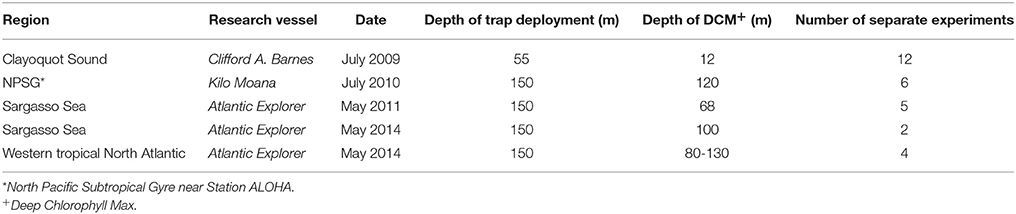
Figure 1. Response in hydrolytic enzyme activity to 500 nM AHL amendments by particle-associated bacteria after 24 h of incubation. (A) study sites (B) experimental response to an amendment of mixture of unsubstituted AHLs and (C) response to 3OC8-HSL. Each data point represents a single experiment and is the average of results of triplicate incubations, which were in turn determined by triplicate assays. Circled data point indicated significant responses to AHLs (p < 0.1) controlled for the false discovery rate in each panel. Investigated regions are North Pacific Subtropical Gyre (NPSG), Clayoquot Sound off Vancouver Island, B.C. (Clayoquot), Sargasso Sea (Sargasso) and the western tropical North Atlantic (TropAtl). Samples from Clayoquot Sound were incubated with C8-HSL only instead of the AHL-cocktail and data points were previously published by Hmelo et al. (2011).
AHL Sample Collection, Extraction, and Analysis
Triplicate incubation samples were pooled after 24 h (~90 mL total), gently filtered through BondEluent solid phase extraction cartridges (Mega BE-C18, 1 g; Agilent Technologies, MA, USA) using a vacuum filtration manifold, and stored at −80°C until further processing. AHLs that were retained by the SPE cartridges were extracted following Li et al. (2006) with minor modifications. SPE cartridges were eluted into 2.0 mL HPLC vials using 1.5 mL solvent mixture of hexane and isopropanol (30/70 v/v) (ThermoFisher, MA, USA). Eluent was blown dry using a Vacuufuge (Eppendorf, HH, Germany). These two steps were repeated 3 times and subsequently residual material was dissolved in 1.5 mL solvent of Milli-Q water and acetonitrile (50/50 v/v). Hereafter, HPLC vials containing AHL extracts were analyzed via HPLC/MS/MS (Hmelo and Van Mooy, 2009) in order to detect and determine AHLs present in our experiments. An HPLC 1200 Series (Agilent Technologies, MA, USA) connected to a TSQ Vantage triple quadrupole mass spectrometer (TQMS; Thermo Scientific, HB, Germany) was used as described by Van Mooy et al. (2012). Data were processed with Excalibur software (Thermo Scientific, HB, Germany).
Results
We examined geographic variability in the effects of two classes of AHLs on three bacterial enzyme activities from samples of sinking particles collected at five locations across the Pacific Ocean and the Atlantic Ocean (Figure 1). Each experiment consisted of three incubations amended with either unsubstituted AHLs or three incubations amended with 3OC8-HSL, and three corresponding control incubations amended only with the solvent used to make the amendment stocks. A total of 29 experiments were conducted for this geographical assessment. All of these incubations were conducted for 24 h and amended with AHLs to final concentrations of 500 nM.
Across the different sampling sites, average hydrolytic enzyme activity rates in experiments amended with AHLs varied by factors of 0.4–3.7 compared to control incubations (Figures 1B,C). Of the 87 separate comparisons of average enzyme activities in incubations amended with AHLs, 26 showed rates that were statistically different than the no-amendment control incubations, indicating a response of particle-associated bacteria to AHLs. These responses were nearly equally likely to be stimulatory (15 of 26 comparisons) as inhibitory (11 comparisons).
The two different AHL amendments rarely elicited the same response; for example, in the NPSG amendments of 3OC8-HSL impacted the rates of all three hydrolytic enzyme activities in most experiments, while the unsubstituted AHLs had no effect. In total, 3OC8-HSL much more frequently elicited a response (20 comparisons) than the unsubstituted AHLs (6 comparisons). In addition, the effects of either amendment on any individual hydrolytic enzyme activity were remarkably inconsistent at any single location. In general, while AHLs often impacted rates of hydrolytic enzyme activity by bacteria associated with sinking particles, there were no obvious geographical patterns to these responses.
In order to assess the timescales of the response of the particle-associated bacteria on sinking particles to amendments of AHLs, multiple time-series incubation experiments were performed in the Sargasso Sea and western tropical Atlantic Ocean (Figures 2A–D). Generally, there was significant variability in the separate assays for each experiment, as can be seen by the spread in the data points for any one AHL concentration. However, many significant responses were observed, and these tended to fluctuate throughout the 24 h time course. In particular, 3OC8-HSL amendments elicited a response in enzyme activity after 6 h in 5 of 6 comparisons (Figures 2B,D); this effect is particularly noticeable in the Sargasso Sea. Furthermore, rates of lipase activity in the Sargasso Sea and phosphatase in the western tropical Atlantic showed a marked decay in rates after the 6-h time point. Interestingly, after 6 h the addition of unsubstituted AHLs affected only two responses in hydrolytic activity: the inhibition of lipase and aminopeptidase activity in the western tropical North Atlantic (Figure 2C). The most consistent effects, on 5 of 6 comparisons, occurred after only 3 h. In general, the addition of substituted 3OC8-HSL more pronounced stimulatory effects on hydrolytic activity throughout the incubation time of 24 h compared to unsubstituted AHLs, especially in the Sargasso Sea (Figures 2A–D).
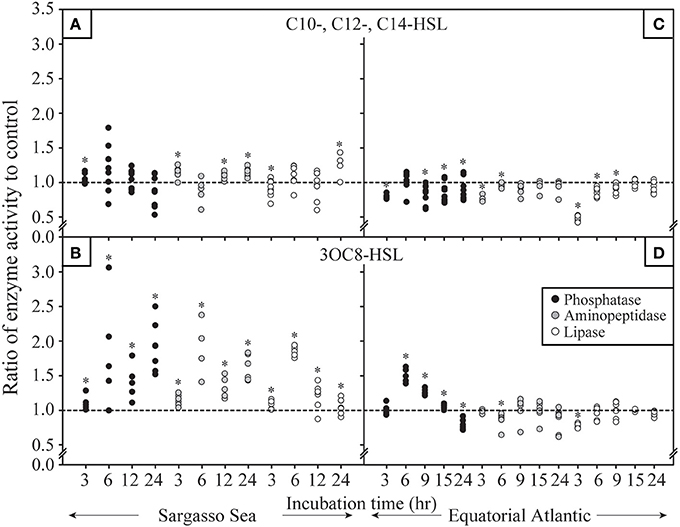
Figure 2. Response in hydrolytic enzyme activity to AHLs amendments by particle-associated bacteria over a 24 h time-course. Results from samples collected in 2014 representing (A,B) the Sargasso Sea and (C,D) the western tropical North Atlantic (TropAtl). Response in hydrolytic enzyme activity is shown for a (A,C) mixture of unsubstituted long chain acylated homoserine lactones (AHLs) and (B,D) substituted 3OC8-HSL. Each data point represents the result from a single hydrolytic enzyme activity assay. Asterisks denote statistical significance (p < 0.1) controlled for the false discovery rate in each panel.
We further aimed to understand the impact of the concentrations of AHLs on the response of particle-associated bacteria. Therefore, we conducted incubations with AHL concentrations ranging from 100 to 1000 nM in the western tropical North Atlantic for both unsubstituted AHLs and substituted AHLs (Figures 3A,B, Supplementary Figures 1A–C, 2A–C). After an incubation period of 24 h, activity rates for phosphatase, aminopeptidase and lipase showed a number of significant increases and decreases under all AHL concentrations (Figures 3A,B). While there appeared to be discernable concentration-dependent trends in lipase activity in response to unsubstituted AHLs and in phosphatase activity to 3OC8-HSL, there was no general trend in the response to different AHL concentrations. In addition, few concentration-dependent effects were observed after other time periods of incubation (Supplementary Figures 1, 2).
Figure 3. Response in hydrolytic enzyme activity by particle-associated bacteria after 24 h of incubationto different concentrations of (A) unsubstituted long chain acylated homoserine lactones (AHLs) mixture and (B) substituted 3OC8-HSL. Enzymatic response in phosphatase, aminopeptidase and lipase are shown for samples collected in 2014 in the western tropical North Atlantic. Hydrolytic activity is shown under varying concentrations of AHLs ranging from 100 to 1000 nM. Each data point represents the result from a single hydrolytic enzyme activity assay. Asterisks denote statistical significance (p < 0.1) controlled for the false discovery rate in each panel.
At the end of the incubation experiments in the Sargasso Sea and western tropical North Atlantic, we used solid phase extraction to collect dissolved AHLs for analysis by HPLC/MS/MS. In our control incubations, we found no evidence for endogenous dissolved AHLs in our extracts. In the incubations amended with AHLs we did find evidence for both the exogenous 3OC8-HSL and the exogenous di-deuterated unsubstituted AHLs used as amendments (Figures 4A–D). The quantities detected were substantially less than the initial concentrations of the amendments; using a first-order degradation equation as applied by Hmelo and Van Mooy (2009) we determined that residence time of these dissolved AHLs was approximately 7 h.
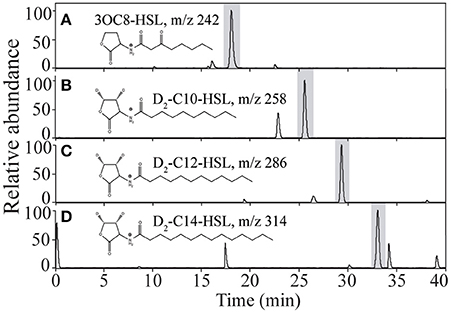
Figure 4. HPLC/TQMS chromatogram of selective reactions diagnostic of the different AHLs used to amend incubations in this study. Samples collected in (A) the western tropical North Atlantic, and (B–D) in the Sargasso Sea.
Discussion
Sinking particles represent so-called hot spots for microbial activity, where particulate organic carbon (POC) is hydrolyzed and remineralized into dissolved form (Stocker and Seymour, 2012). Assays of hydrolytic enzyme activity show cell-specific rates that are markedly higher than cells in surrounding waters (Smith et al., 1992). This observation suggests a cell-density dependence of organic matter degradation that could require coordinated activity amongst particle-associated cells (Vetter et al., 1998; Mislan et al., 2014). Such a coordination, if mediated by biochemically inexpensive infochemical signaling pathways (e.g., quorum sensing systems based on AHLs), could theoretically the relative physiological cost of enhanced hydrolytic activity by particle-associated bacteria (Vetter et al., 1998) and fuel the intensification of sinking particle degradation and concomitant particle flux attenuation (Mislan et al., 2014). Many characteristic behaviors of particle-associated bacteria, such as enhanced enzyme activity, motility, and biofilm formation, have been observed to be regulated by quorum sensing systems in cultured bacteria (Miller and Bassler, 2001). To date, only a few studies have investigated the potential impact of infochemicals on the activity of particle-associated bacterial assemblages, but they have thus far affirmed that bacterial signaling systems are involved in, and may either enhance or inhibit the rate of the degradation of sinking POC (Hmelo et al., 2011; Van Mooy et al., 2012; Edwards et al., 2015).
Across different sampling sites, 30% of AHL amendment experiments elicited a significant response in enzyme activity experiments although the sign of the response (inhibitory or stimulatory) was inconsistent (Figures 1B,C). We observed considerable variability in replicates within individual experiments, but all AHL amendments elicited a significant response in enzyme activity during at least one time point over a 24 h time course experiment (Figures 2A–D). Variability within replicates likely reflects microspatial heterogeneity inherent in the microbial populations contained within the particle slurries (Long and Azam, 2001; Seymour et al., 2004) and such heterogeneity may also contribute to the between-experiment variability observed across sampling sites (Figures 1A–C).
Despite some variability in the type of response each AHL amendment elicited, we assert that the aggregated results of the AHL amendments are clear evidence of a response to quorum sensing signals by bacteria associated with sinking particles. Although, it is possible that AHL amendments were simply an external carbon and/or nitrogen food source, as outlined by Hmelo et al. (2011) we exclude this possibility for three reasons. First, the addition of greater concentrations of AHLs did not necessarily lead to enhanced hydrolytic activity, suggesting a threshold response characteristic for quorum sensing. Second, in most instances responses were detected in incubations treated with either unsubstituted or substituted AHLs, but not both; this is indicative of specific regulatory functions of these AHL classes, whereas if AHLs were a nutrient source, then similar responses would be expected for amendments of both types of AHLs. Third, the amount of added carbon in the form of AHLs (0.0003–0.00035 mM) is negligible compared to DOC concentrations in the interstitial water of typical marine aggregates (~1–5 mM) (Alldredge, 2000). Based on these three arguments we posit that responses to AHL amendments do indeed reflect activation of AHL-regulated pathways in a manner characteristic of quorum sensing.
The results of this study affirm that exogenous AHLs activate quorum sensing response that impact hydrolytic enzyme activity by bacteria associated with sinking POC (Figures 3A,B). Furthermore, the results corroborate the general observations of Hmelo et al. (2011) and extend them to the world's oceans. In many locations across the North Pacific and North Atlantic Oceans, hydrolytic responses after 24 h changed significantly in response to AHL amendments compared to control conditions (Figures 1B,C). Such changes could be critical in affecting the rates of POC degradation, particle flux attenuation, and the depths at which carbon is remineralized and sequestered in the deep sea (Armstrong et al., 2001; Kwon et al., 2009). Furthermore, the relative balance of two of the major processes that contribute to particle flux attenuation, microbial respiration and enzyme hydrolysis (Collins et al., 2015), may also be affected. Thus, quorum sensing mechanisms certainly have the potential to affect the biological carbon pump on a global scale. Yet, the responses to AHLs were so inconsistent (Figure 1) that it is impossible to conclude whether the net effect of quorum sensing is to increase or decrease particle flux attenuation.
The employed experimental design investigated both substituted and unsubstituted AHLs because these two broad classes of AHLs are produced and used in quorum sensing processes by a wide range of cultured heterotrophic bacterial representatives that are likely to inhabit marine particles (Gram et al., 2002; Wagner-Döbler et al., 2005; Hmelo et al., 2011). Furthermore, these two classes of AHLs have been directly observed in sinking particles (Hmelo et al., 2011; Jatt et al., 2015). Similar to the observations of Hmelo et al. (2011), the two different AHL amendments tended to have inconsistent effects on enzyme hydrolysis activity rates. This suggests that there may be multiple parallel quorum sensing systems at play amongst bacteria associated with sinking particles.
Understanding the temporal scale on which quorum sensing affects POC degradation is particularly important because of the potential to impact remineralization depth. The timescales at which AHL amendments impacted enzyme hydrolysis rates showed marked inconsistencies between amendments, locations, and enzyme activity (Figures 2A–D). Such observations underscore the dynamic role that quorum sensing is likely to play on enzymatic activity within the particle-associated bacterial community and bely a highly complex relationship between quorum sensing and particle flux attenuation. The short-term responses in hydrolytic activity to AHL amendments are explained, at least in part, by induced physiological changes by particle-associated bacteria as opposed to a shift in community composition. Generation times for marine bacteria attached to sinking particles can be 0.4–2.0 days (Jacobsen and Azam, 1984; Ploug and Grossart, 2000). As such, the 3- and 6-h time-periods probably are not long enough to observe a substantial shift in the composition of the bacterial community on sinking particles. Additionally, studies on marine microorganisms have shown that the molecular machinery regulating the expression and production of specific enzymes can respond to changing ambient conditions within a few hours (Fuqua and Greenberg, 2002; Arnosti, 2004). Our results support such findings and suggest that quorum sensing amongst particle-associated bacteria influence bacterial physiology on short timescales. It is possible that the responses we observed over longer timescales (i.e., 12- and 24-h) were influenced by community shifts, although we have no data that speaks to this directly. Edwards et al. (2015) recently showed that additions of diatom-derived polyunsaturated aldehydes led to similar changes in enzyme activity after 24 h of incubation, but did not affect major shifts in community structure except at very high concentrations.
Quorum sensing is a cell-density dependent communication mechanism, and therefore, the concentration of infochemicals is an important parameter controlling community driven bacterial activities. Hmelo et al. (2011) found that amendments of 500 nM were sufficient to affect hydrolytic responses in marine bacteria. Our measurements show that different AHL concentrations elicited changes in hydrolytic enzyme activity that varied as a function of the type of AHL, but are independent of geographic location. This observation is in marked contrast to the response of particle-associated bacteria to different concentrations of polyunsaturated aldehydes, which varied systematically across a broad range of marine environments (Edwards et al., 2015).
AHLs and other quorum sensing molecules have been observed directly in a wide range of marine environments (Decho et al., 2009; Van Mooy et al., 2012; Zimmer et al., 2014) including sinking particles (Hmelo et al., 2011) and endogenous AHLs are often interpreted to be direct evidence of quorum sensing. We argue that AHLs must truly be dissolved in order to be actively involved in quorum sensing, and therefore sought to identify AHLs present solely in the porewaters of sinking particles by using a solid phase extraction method. Hmelo et al. (2011) used direct liquid-liquid extraction of samples, which recovered AHLs from both porewaters and the particulate matter itself. We report here that endogenous dissolved AHLs were not observed in any of our samples. Yet, the partial recovery of AHLs from most of our amended incubation experiments confirms that these molecules were dissolved and therefore had the potential to elicit changes in microbial pathways regulated by quorum sensing (Figures 4A–D). We did not detect added AHLs in every sample at the end of each set of incubation, likely because these molecules have the tendency to degrade quickly in seawater, especially substituted short chain AHLs (Hmelo and Van Mooy, 2009). Estimated residence times of added AHLs in our experiments average ~7 h, explaining their absence at the end of the incubation period in some experiments. Hmelo and Van Mooy (2009) observed that AHLs were exceptionally short-lived in natural marine waters, and estimated that only 3–7% of the AHL amendments they made remained after a 24-h incubation period. These results underscore the inherent abiotic chemical instability of AHLs at seawater pH, but also suggest ubiquitous enzymatic degradation (i.e., quorum quenching) in marine environments, which makes capturing natively produced AHLs a challenge (Hmelo and Van Mooy, 2009; Tait et al., 2010; Van Mooy et al., 2012).
The vertical flux of POC can vary greatly in different marine regions (Lutz et al., 2002; Buesseler et al., 2007; Buesseler and Boyd, 2009) and our results suggest that quorum sensing could contribute to this variability through its role in coordinating the stimulation or repression of hydrolytic enzyme activity in marine particle-associated bacterial communities. Our study showed variability in the response of AHLs amendments as a function of geographic location, AHL class, response time, and AHL concentration. Therefore, the AHL signaling systems employed by particle-associated bacteria appear to be extraordinarily nuanced, and the links between AHL-based quorum sensing and particle export and flux attenuation may presently be too complex to model (Mislan et al., 2014). Future work should begin to address the synergistic or inhibitory interactions between the different pathways affected by AHLs. Such investigations will strengthen our abilities to translate the various languages of marine bacteria by developing a Rosetta Stone that will help to better understand how cell-cell signaling systems are affecting the efficiency of POC degradation.
Author Contributions
AK, BV designed research. AK, BV, LH, and JO performed research. AK, BV, and TM analyzed data. AK, BV wrote the paper.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the captain and crew of the R/V Atlantic Explorer, R/V Clifford A. Barnes, and the R/V Kilo Moana. We thank Laura E. Sofen for her assistance in the North Pacific and Sargasso Sea, and Bethanie R. Edwards for her assistance in the Sargasso Sea. Shawn R. Campagna and Amanda L. May made an invaluable contribution of deuterated AHLs, and Helen F. Fredricks assisted with AHL analyses. Finally, we thank Sara J. Bender for manuscript feedback. This study was funded by collaborative grants from the National Science Foundation (NSF) between BV and TM (OCE-0825407), and BV and Sonya T. Dyhrman (OCE-1332898).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/article/10.3389/fmars.2016.00055
Supplementary Figure 1. Response in hydrolytic enzyme activity from particle-attached bacteria over the course of 24 h of incubation to different concentrations of unsubstituted acylated homoserine lactones (AHLs; Extension to Figure 3A). Hydrolytic enzyme activity of (A) phosphatase, (B) aminopeptidase, and (C) lipase are shown for samples collected in 2014 during the AE1409 cruise in the western tropical North Atlantic (TropAtl) at station 8 and 10. Hydrolytic activity is shown under five different concentrations of AHLs ranging from 100 to 1000 nM. Asterisks denote statistical significance (p < 0.01).
Supplementary Figure 2. Response in hydrolytic enzyme activity from particle-attached bacteria over the course of 24 h of incubation to different concentrations of substituted N-Oxooctanoyl-homoserine lactones (3OC8-HSL; Extension to Figure 3B). Hydrolytic enzyme activity of (A) phosphatase, (B) aminopeptidase, and (C) lipase across 24 h of incubation in samples collected in 2014 during the AE1409 cruise in the western tropical North Atlantic (TropAtl). from station 8 and 10. Hydrolytic activity is shown under five different concentrations of 3OC8-HSL ranging from 100 to 1000 nM. Asterisks denote statistical significance (p < 0.01).
References
Alldredge, A. L. (2000). Interstitial dissolved organic carbon (DOC) concentrations within sinking marine aggregates and their potential contribution to carbon flux. Limnol. Oceanogr. 45, 1245–1253. doi: 10.4319/lo.2000.45.6.1245
Armstrong, R. A., Lee, C., Hedges, J. I., Honjo, S., and Wakeham, S. G. (2001). A new, mechanistic model for organic carbon fluxes in the ocean based on the quantitative association of POC with ballast minerals. Deep Sea Res. Pt II 49, 219–236. doi: 10.1016/S0967-0645(01)00101-1
Arnosti, C. (2004). Speed bumps and barricades in the carbon cycle: substrate structural effects on carbon cycling. Mar. Chem. 92, 263–273. doi: 10.1016/j.marchem.2004.06.030
Azam, F., and Long, R. A. (2001). Oceanography: Sea snow microcosms. Nature 414, 495–498. doi: 10.1038/35107174
Benjamini, Y., and Hochberg, Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Statis. Soc. B (Methodol.) 57, 289–300.
Buesseler, K. O., and Boyd, P. W. (2009). Shedding light on processes that control particle export and flux attenuation in the twilight zone of the open ocean. Limnol. Oceanogr. 54, 1210–1232. doi: 10.4319/lo.2009.54.4.1210
Buesseler, K. O., Lamborg, C. H., Boyd, P. W., Lam, P. J., Trull, T. W., Bidigare, R. R., et al. (2007). Revisiting carbon flux through the ocean's twilight zone. Science 316, 567–570. doi: 10.1126/science.1137959
Caron, D. A., Davis, P. G., Madin, L. P., and Sieburth, J. M. (1986). Enrichment of microbial populations in macroaggregates (marine snow) from surface waters of the North Atlantic. J Mar Res 44, 543–565. doi: 10.1357/002224086788403042
Certner, R. H., and Vollmer, S. V. (2015). Evidence for autoinduction and quorum sensing in white band disease-causing microbes on Acropora cervicornis. Sci. Rep. 5:11134. doi: 10.1038/srep11134
Collins, J. R., Edwards, B. R., Thamatrakoln, K., Ossolinski, J. E., DiTullio, G. R., Bidle, K. D., et al. (2015). The multiple fates of sinking particles in the North Atlantic Ocean. Glob. Biogeochem. Cycles 29, 1471–1494. doi: 10.1002/2014GB005037
Decho, A. W., Visscher, P. T., Ferry, J., Kawaguchi, T., He, L., Przekop, K. M., et al. (2009). Autoinducers extracted from microbial mats reveal a surprising diversity of N-acylhomoserine lactones (AHLs) and abundance changes that may relate to diel pH. Environ. Microbiol. 11, 409–420. doi: 10.1111/j.1462-2920.2008.01780.x
DeLong, E. F., Franks, D. G., and Alldredge, A. L. (1993). Phylogenetic diversity of aggregate–attached vs. free–living marine bacterial assemblages. Limnol. Oceanogr. 38, 924–934. doi: 10.4319/lo.1993.38.5.0924
Doberva, M., Sanchez-Ferandin, S., Toulza, E., Lebaron, P., and Lami, R. (2015). Diversity of quorum sensing autoinducer synthases in the Global Ocean Sampling metagenomic database. Aquat. Microb. Ecol. 74, 107–119. doi: 10.3354/ame01734
Eberhard, A., Burlingame, A. L., Eberhard, C., Kenyon, G. L., Nealson, K. H., and Oppenheimer, N. J. (1981). Structural identification of autoinducer of Photobacterium fischeri luciferase. Biochemistry 20, 2444–2449. doi: 10.1021/bi00512a013
Edwards, B. R., Bidle, K. D., and Van Mooy, B. A. (2015). Dose-dependent regulation of microbial activity on sinking particles by polyunsaturated aldehydes: implications for the carbon cycle. Proc. Natl. Acad. Sci. U.S.A. 112, 5909–5914. doi: 10.1073/pnas.1422664112
Fuqua, C., and Greenberg, E. P. (2002). Listening in on bacteria: acyl-homoserine lactone signalling. Nat. Rev. Mol. Cell Biol. 3, 685–695. doi: 10.1038/nrm907
Fuqua, C., Parsek, M. R., and Greenberg, E. P. (2001). Regulation of gene expression by cell–to–cell communication: acyl-homoserine lactone quorum sensing. Annu. Rev. Genet. 35, 439–468. doi: 10.1146/annurev.genet.35.102401.090913
Gram, L., Grossart, H.-P., Schlingloff, A., and Kiørboe, T. (2002). Possible quorum sensing in marine snow bacteria: production of acylated homoserine lactones by Roseobacter strains isolated from marine snow. Appl. Environ. Microbiol. 68, 4111–4116. doi: 10.1128/AEM.68.8.4111-4116.2002
Hmelo, L. R., Mincer, T. J., and Van Mooy, B. A. S. (2011). Possible influence of bacterial quorum sensing on the hydrolysis of sinking particulate organic carbon in marine environments. Environ. Microbiol. Rep. 3, 682–688. doi: 10.1111/j.1758-2229.2011.00281.x
Hmelo, L. R., and Van Mooy, B. A. S. (2009). Kinetic constraints on acylated homoserine lactone–based quorum sensing in marine environments. Aquat. Microb. Ecol. 54, 127–133. doi: 10.3354/ame01261
Hoppe, H.-G. (1993). Use of fluorogenic model substrates for extracellular enzyme activity (EEA) measurement of bacteria. Handb. Methods Aquat. Microbial. Ecol. 1, 423–431.
Jacobsen, T. R., and Azam, F. (1984). Role of bacteria in copepod fecal pellet decomposition: colonization, growth rates and mineralization. Bull. Mar. Sci. 35, 495–502.
Jatt, A. N., Tang, K., Liu, J., Zhang, Z., and Zhang, X.-H. (2015). Quorum sensing in marine snow and its possible influence on production of extracellular hydrolytic enzymes in marine snow bacterium Pantoea ananatis B9. FEMS Microbiol. Ecol. 91, 1–13. doi: 10.1093/femsec/fiu030
Kiørboe, T., and Jackson, G. A. (2001). Marine snow, organic solute plumes, and optimal chemosensory behavior of bacteria. Limnol. Oceanogr. 46, 1309–1318. doi: 10.4319/lo.2001.46.6.1309
Kwon, E. Y., Primeau, F., and Sarmiento, J. L. (2009). The impact of remineralization depth on the air–sea carbon balance. Nat. Geosci. 2, 630–635. doi: 10.1038/ngeo612
Lamborg, C., Buesseler, K., Valdes, J., Bertrand, C., Bidigare, R., Manganini, S., et al. (2008). The flux of bio-and lithogenic material associated with sinking particles in the mesopelagic “twilight zone” of the northwest and North Central Pacific Ocean. Deep Sea Res. Pt II 55, 1540–1563. doi: 10.1016/j.dsr2.2008.04.011
Li, X., Fekete, A., Englmann, M., Götz, C., Rothballer, M., Frommberger, M., et al. (2006). Development and application of a method for the analysis of N-acylhomoserine lactones by solid-phase extraction and ultra high pressure liquid chromatography. J. Chrom. 1134, 186–193. doi: 10.1016/j.chroma.2006.09.047
Long, R. A., and Azam, F. (2001). Microscale patchiness of bacterioplankton assemblage richness in seawater. Aquat. Microb. Ecol. 26:103. doi: 10.3354/ame026103
Lutz, M., Dunbar, R., and Caldeira, K. (2002). Regional variability in the vertical flux of particulate organic carbon in the ocean interior. Glob. Biogeochem. Cycles 16, 1–18. doi: 10.1029/2000GB001383
Miller, M. B., and Bassler, B. L. (2001). Quorum sensing in bacteria. Annu. Rev. Microbiol. 55, 165–199. doi: 10.1146/annurev.micro.55.1.165
Mislan, K., Stock, C. A., Dunne, J. P., and Sarmiento, J. L. (2014). Group behavior among model bacteria influences particulate carbon remineralization depths. J. Mar. Res. 72, 183–218. doi: 10.1357/002224014814901985
Moeseneder, M. M., Winter, C., and Herndl, G. J. (2001). Horizontal and vertical complexity of attached and free-living bacteria of the eastern Mediterranean Sea, determined by 16S rDNA and 16S rRNA fingerprints. Limnol. Oceanogr. 46, 95–107. doi: 10.4319/lo.2001.46.1.0095
Ng, W.-L., and Bassler, B. L. (2009). Bacterial quorum-sensing network architectures. Annu. Rev. Genet. 43, 197–222. doi: 10.1146/annurev-genet-102108-134304
Parsek, M. R., and Greenberg, E. P. (2000). Acyl–homoserine lactone quorum sensing in gram–negative bacteria: a signaling mechanism involved in associations with higher organisms. Proc. Natl. Acad. Sci. U.S.A. 97, 8789–8793. doi: 10.1073/pnas.97.16.8789
Pearson, J. P., Gray, K. M., Passador, L., Tucker, K. D., Eberhard, A., Iglewski, B. H., et al. (1994). Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc. Natl. Acad. Sci. U.S.A. 91, 197–201. doi: 10.1073/pnas.91.1.197
Peterson, M. L., Wakeham, S. G., Lee, C., Askea, M. A., and Miquel, J. C. (2005). Novel techniques for collection of sinking particles in the ocean and determining their settling rates. Limnol. Oceanogr. Methods 3, 520–532. doi: 10.4319/lom.2005.3.520
Ploug, H., and Grossart, H. P. (2000). Bacterial growth and grazing on diatom aggregates: respiratory carbon turnover as a function of aggregate size and sinking velocity. Limnol. Oceanogr. 45, 1467–1475. doi: 10.4319/lo.2000.45.7.1467
Ransome, E., Munn, C. B., Halliday, N., Cámara, M., and Tait, K. (2014). Diverse profiles of N-acyl-homoserine lactone molecules found in cnidarians. FEMS Microbiol. Ecol. 87, 315–329. doi: 10.1111/1574-6941.12226
Rath, J. (1998). High phylogenetic diversity in a marine–snow–associated bacterial assemblage. Aquat. Microb. Ecol. 14, 261–269. doi: 10.3354/ame014261
Seymour, J. R., Mitchell, J. G., and Seuront, L. (2004). Microscale heterogeneity in the activity of coastal bacterioplankton communities. Aquat. Microb. Ecol. 35, 1–16. doi: 10.3354/ame035001
Simon, M., Grossart, H.–P., Schweitzer, B., and Ploug, H. (2002). Microbial ecology of organic aggregates in aquatic ecosystems. Aquat. Microb. Ecol. 28, 175–211. doi: 10.3354/ame028175
Smith, D. C., Simon, M., Alldredge, A. L., and Azam, F. (1992). Intense hydrolytic enzyme activity on marine aggregates and implications for rapid particle dissolution. Nature 359, 139–142. doi: 10.1038/359139a0
Stocker, R., and Seymour, J. R. (2012). Ecology and physics of bacterial chemotaxis in the ocean. Microbiol. Mol. Biol. Rev. 76, 792–812. doi: 10.1128/MMBR.00029-12
Stocker, R., Seymour, J. R., Samadani, A., Hunt, D. E., and Polz, M. F. (2008). Rapid chemotactic response enables marine bacteria to exploit ephemeral microscale nutrient patches. Proc. Natl. Acad. Sci. U.S.A. 105, 4209–4214. doi: 10.1073/pnas.0709765105
Tait, K., Hutchison, Z., Thompson, F. L., and Munn, C. B. (2010). Quorum sensing signal production and inhibition by coral-associated vibrios. Environ. Microbiol. Rep. 2, 145–150. doi: 10.1111/j.1758-2229.2009.00122.x
Turley, C. M., and Mackie, P. J. (1994). Biogeochemical significance of attached and free-living bacteria and the flux of particles in the NE Atlantic Ocean. Mar. Ecol. Prog. Ser. 115, 191–191. doi: 10.3354/meps115191
Van Mooy, B. A. S., Hmelo, L. R., Sofen, L. E., Campagna, S. R., May, A. L., Dyhrman, S. T., et al. (2012). Quorum sensing control of phosphorus acquisition in Trichodesmium consortia. ISME J. 6, 422–429. doi: 10.1038/ismej.2011.115
Vetter, Y., Deming, J., Jumars, P., and Krieger-Brockett, B. (1998). A predictive model of bacterial foraging by means of freely released extracellular enzymes. Microb. Ecol. 36, 75–92. doi: 10.1007/s002489900095
Wagner-Döbler, I., Thiel, V., Eberl, L., Allgaier, M., Bodor, A., Meyer, S., et al. (2005). Discovery of complex mixtures of novel long-chain quorum sensing signals in free-living and host-associated marine alphaproteobacteria. Chembiochem 6, 2195–2206. doi: 10.1002/cbic.200500189
Whiteley, M., Lee, K. M., and Greenberg, E. P. (1999). Identification of genes controlled by quorum sensing in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U.S.A. 96, 13904–13909. doi: 10.1073/pnas.96.24.13904
Keywords: acylated homoserine lactones, infochemical signaling, sinking particles, particle-associated bacteria, marine carbon cycle
Citation: Krupke A, Hmelo LR, Ossolinski JE, Mincer TJ and Van Mooy BAS (2016) Quorum Sensing Plays a Complex Role in Regulating the Enzyme Hydrolysis Activity of Microbes Associated with Sinking Particles in the Ocean. Front. Mar. Sci. 3:55. doi: 10.3389/fmars.2016.00055
Received: 21 November 2015; Accepted: 11 April 2016;
Published: 06 May 2016.
Edited by:
Michael William Lomas, Bigelow Laboratory for Ocean Sciences, USAReviewed by:
Arvind Singh, GEOMAR Helmholtz Centre for Ocean Research Kiel, GermanyNazli Olgun, Istanbul Technical University, Turkey
Copyright © 2016 Krupke, Hmelo, Ossolinski, Mincer and Van Mooy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andreas Krupke, andreas.krupke@gmail.com
 Andreas Krupke
Andreas Krupke Laura R. Hmelo
Laura R. Hmelo Justin E. Ossolinski1
Justin E. Ossolinski1  Tracy J. Mincer
Tracy J. Mincer Benjamin A. S. Van Mooy
Benjamin A. S. Van Mooy