Bioactivity Screening of Microalgae for Antioxidant, Anti-Inflammatory, Anticancer, Anti-Diabetes, and Antibacterial Activities
- 1Department of Integrative Marine Ecology, Stazione Zoologica Anton Dohrn, Naples, Italy
- 2Marbio, UiT The Arctic University of Norway, Tromsø, Norway
Marine microalgae are considered a potentially new and valuable source of biologically active molecules for applications in the food industry as well as in the pharmaceutical, nutraceutical, and cosmetic sectors. They can be easily cultured, have short generation times and enable an environmentally-friendly approach to drug discovery by overcoming problems associated with the over-utilization of marine resources and the use of destructive collection practices. In this study, 21 diatoms, 7 dinoflagellates, and 4 flagellate species were grown in three different culturing conditions and the corresponding extracts were tested for possible antioxidant, anti-inflammatory, anticancer, anti-diabetes, antibacterial, and anti-biofilm activities. In addition, for three diatoms we also tested two different clones to disclose diversity in clone bioactivity. Six diatom species displayed specific anti-inflammatory, anticancer (blocking human melanoma cell proliferation), and anti-biofilm (against the bacteria Staphylococcus epidermidis) activities whereas, none of the other microalgae were bioactive against the conditions tested for. Furthermore, none of the 6 diatom species tested were toxic on normal human cells. Culturing conditions (i.e., nutrient starvation conditions) greatly influenced bioactivity of the majority of the clones/species tested. This study denotes the potential of diatoms as sources of promising bioactives for the treatment of human pathologies.
Introduction
Cancer, inflammation, and the evolution of antibiotic-resistant pathologies, together with other human diseases, are continuously stimulating the search for new bioactive molecules from natural sources. Unlike drug discovery on land, marine drug discovery is a relatively new field which began in the 1940s with the advent of scuba diving and new sampling technologies that allowed scientists to systematically probe the oceans for useful therapeutics. The number of potential compounds isolated from marine organisms now exceeds 28,000 with hundreds of new compounds being discovered every year (Blunt et al., 2015). However, despite the number of compounds isolated from marine organisms and the biological activities attributed to many of these, those that have either been marketed or are under development are relatively few (Jaspars et al., 2016). To date, the global marine pharmaceutical pipeline consists of seven approved pharmaceuticals in clinical use, four of which are anticancer drugs, and about 26 natural products in Phase I to Phase III clinical trials, 23 as anticancer agents, two for schizophrenia and Alzheimer's, and one for chronic pain (http://marinepharmacology.midwestern.edu/clinPipeline.htm). Most of these natural products have been isolated from Porifera (sponges) and Chordata (including ascidians) but these macroorganisms are often difficult to cultivate and there may be problems to obtain a sustainable supply of the compounds of interest without ecologically impacting natural populations. More recently, there is great interest in exploring the biotechnological potential of microorganisms such as microalgae since they are easier to cultivate, have short generation times and represent a renewable and still poorly explored resource for drug discovery. However, although a range of pharmacological activities have been observed from microalgal extracts, the active principles are often unknown (Mimouni et al., 2012; Guedes et al., 2013; Nigjeh et al., 2013; Samarakoon et al., 2013).
Microalgae are photosynthetic eukaryotes that constitute one of the major components of marine and freshwater phytoplankton; they are primary producers, a food source for other marine organisms, and are also excellent sources/producers of pigments, lipids, carotenoids, ω-3 fatty acids and other fine chemicals (Mimouni et al., 2012). Their long evolutionary and adaptive diversification to a multitude of habitats and extreme conditions (e.g., cold/hot environments, hydrothermal vents) make them good candidates for drug discovery, because they may have evolved compounds for communication, defense and survival that are often unique and may not have any terrestrial counterparts (Landsberg, 2002, Wolfe et al., 2002, Caldwell, 2009). Diatoms are one of the most important groups of microalgae with over 100,000 species that occur in virtually every environment that contains water, including not only oceans and lakes, but also soil. Diatoms have already found important applications as biofuels, health foods, biomolecules, materials relevant to nanotechnology, and as bioremediators of contaminated water (Bozarth et al., 2009). However not much is known on the potential applications of diatoms as pharmaceuticals. Various studies have shown that some diatoms are rich in bioactive compounds such as the sulfated polysaccharide named naviculan isolated from Navicula directa, with antiviral activity (Lee et al., 2006), adenosine from Phaeodactylum tricornutum, an antiarrhythmic agent to treat tachycardia (Prestegard et al., 2009, 2014), and marennine, a blue pigment identified in the marine diatom Haslea ostrearia that has shown allelopathic, antioxidant, antibacterial, antiviral, and growth-inhibiting properties (Gastineau et al., 2014). Other studies have shown that the marine carotenoid fucoxanthin found in brown seaweeds and several diatom species has antioxidant, anti-inflammatory, anticancer, anti-obese, antidiabetic, antiangiogenic, and antimalarial activities (Peng et al., 2011), and that the fatty alcohol ester nonyl 8-acetoxy-6-methyloctanoate (NAMO), isolated from the diatom P. tricornutum, has anticancer effects on three different cancer cell lines including human leukemia (HL-60) and lung carcinoma (A549), and a mouse melanoma (B16F10; Samarakoon et al., 2014). In addition to these, diatom unsaturated aldehydes such as decadienal, octadienal, and heptadienal isolated from Skeletonema marinoi have shown anticancer effects in lung cancer A549 and colon COLO 205 tumor cells, without affecting the viability of normal cells from the same tissue type (Sansone et al., 2014).
In addition to diatoms, other microalgae (e.g., green algae, flagellates, and dinoflagellates) have been screened for possible biotechnological applications (MacKinnon et al., 2006; Kobayashi, 2008; Samarakoon et al., 2013; Blunt et al., 2015). Digalactosyldiacylglycerols and other monogalactosyl analogs isolated from the green algae Nannochloropsis granulata exhibited strong NO inhibitory activity against LPS-induced NO production in RAW264.7 macrophage cells suggesting a strong anti-inflammatory potential (Blunt et al., 2015). Samarakoon et al. (2013) evaluated the anti-inflammatory activity of two species of green algae, Chlorella ovalis and Nannchloropsis oculata, and one species of dinoflagellates, Amphidinium carterae. Even if all the species showed activity, N. oculata hexane and chloroform fractions showed the strongest anti-inflammatory activity. The green alga C. ovalis and the dinoflagellate A. carterae suppressed the growth of HL-60 cells (human promyelocytic leukemia cell line; Samarakoon et al., 2013) also demonstrating anticancer properties.
On dinoflagellates, there are also various studies reporting functional-based metagenomic approaches (as reviewed by Kellmann et al., 2010). Given the fact that polyketides are an important class of bioactive secondary metabolites, major efforts have been focused on isolating polyketide synthase (PKS) genes and new polyketides. PKSs have been found in several microalge, such as Chlamydomonas reinhardtii, Ostreococcus spp., Emiliania huxleyi, Karenia brevis, Ostreopsis cf. ovata, Coolia monotis, Prorocentrum lima, Gymnodinium catenatum, Heterocapsa triqueta, Azadinium spinosum, and Alexandrium ostenfeldii (Eicholz et al., 2012; Van Dolah et al., 2013; Meyer et al., 2015). PKSs very often synthetize potent toxins in microalgae responsible for harmful algal blooms (HABs; Meyer et al., 2015), impacting humans through consumption of contaminated shellfish, finfish, and through water or aerosol exposure. The synthesized polyketides can exert a variety of functions, such as antipredator and allelopathic effects (Kohli et al., 2016), but also have anticancer activity (Kobayashi, 2008) and/or beneficial effects for the treatment of Alzheimer's disease (MacKinnon et al., 2006).
Not much else is known on the biotechnological potential of microalgae as pharmaceutical agents. Few species have been screened for their biological activity and information on how culturing conditions affect bioactivity is almost totally lacking.
In this study, we screened crude extracts of 32 microalgal species (21 diatoms, 7 dinoflagellates and 4 flagellates) grown in three different culturing conditions (i.e., normal medium, nitrogen-, and phosphate-starvation) for possible antioxidant, anti-inflammation, anticancer, anti-diabetes, antibacterial, and anti-biofilm activities. Oxidative stress is a major cause of inflammatory events implicated in a large number of diseases (e.g., cancer, diabetes, neurodegenerative, and cardio-vascular diseases). The main oxidative stress effectors are reactive intermediates and free radicals such as peroxynitrite, superoxide anion, hydroxyl, and nitric oxide radicals (Lauritano et al., 2012). In low quantities, these are rapidly converted to less reactive forms, but, when present in abnormally high quantities, these free radicals can be very damaging to DNA, RNA, and proteins. We evaluated the antioxidant potential of microalgae using two different assays, the Cellular Lipid Peroxidation Antioxidant Activity (CLPAA) and the Cellular Antioxidant Activity (CAA) Assays. Oxidative stress is thought to be an important contributing factor in the development of inflammation and cancer. In this study, microalgal anti-inflammatory activity was screened by evaluating the capability to inhibit the release of tumor necrosis factor α (TNFα), one of the main effectors of inflammation (Newton and Dixit, 2012), in lipopolysaccharide (LPS)-stimulated monocytic leukemia cells (THP-1). Anticancer properties were evaluated by testing the antiproliferative activity against human melanoma cells (A2058). Melanoma is a neoplastic disorder of melanocytes, occurring in the skin and in the soft tissue of the meninges, mucous membranes, and upper esophagus (Bajetta et al., 2002). The incidence of melanoma is increasing rapidly, especially in the US and Europe, particularly in young adults, mainly due to UV over exposure (Bajetta et al., 2002). In order to discriminate between toxicity and specific anticancer activity, extracts were also tested for toxicity on human fetal lung cells (MRC-5).
We also evaluated the capability of extracts to inhibit the protein tyrosine phosphatase 1B, a negative regulator of insulin receptor signal transduction and a drug target for the treatment of type 2- diabetes (Gum et al., 2003). There is increasing evidence in both experimental and clinical studies suggesting that Type 2-diabetes is a long-term metabolic disorder that is characterized by high blood sugar, insulin resistance, and relative lack of insulin. Common long-term complications from high blood sugar include heart disease, strokes, diabetic retinopathy which can result in blindness, kidney failure, and poor blood flow in the limbs.
Another important and common threat for human health is characterized by bacterial infections. We therefore also performed two different antibacterial assays: minimum inhibitory concentration (MIC) assay, to evaluate if the extracts were able to inhibit the growth of various Gram-negative (Escherichia coli and Pseudomonas aeruginosa) and Gram-positive (Staphylococcus aureus, Enterococcus faecalis, and Streptococcus B) bacteria, and the anti-biofilm assay to check activity against biofilm formation by the bacteria Staphylococcus epidermidis. S. epidermidis is responsible for common hospital-acquired infections (Levinson, 2010) and is of particular concern for people with catheters or other surgical implants (Salyers and Whitt, 2002). The aim of this study was therefore to give a broad overview of microalgal activities, including the testing of different clones and culturing conditions, and to look for promising sources of biologically active molecules for the treatment of different human diseases.
Materials and Methods
Microalgae Culturing and Maintenance
Microalgae (32 species), previously identified by microscopy and 18S sequencing, were selected from the Stazione Zoologica Anton Dohrn culture collection for culturing and activity screening (Table 1). 21 diatoms, 7 dinoflagellates, and 4 flagellates were selected from those that have previously been shown to have anti-grazing and anti-proliferative activities on their predators at sea (Ianora and Miralto, 2010) or species responsible for toxic blooms worldwide (Gorbi et al., 2013, Sampedro et al., 2013). Diatoms and flagellates were grown in Guillard's f/2 medium (Guillard, 1975; For flagellates f/2 without silicates) and dinoflagellates in Keller medium (Keller et al., 1987) in 10 L polycarbonate bottles. Species were grown in normal, nitrogen- and/or phosphate- starved media (90 μM NO3− for N-starved and 0.5 μM PO for P-starved media). Cultures were kept in a climate chamber at 19°C at a 12:12 h light:dark cycle at 100 μmol photons m−2 s−1. Initial cell concentrations were about 50,000 cells/mL for each experiment and at the end of the stationary phase, cultures were centrifuged for 30 min at 4°C at 3900 g and pellets (for the approximate pellet weight and cell concentration used for the chemical extractions of microalgae cultured in control, nitrogen- and phosphate-starvation see Table 1) and kept at −80°C until chemical extraction.
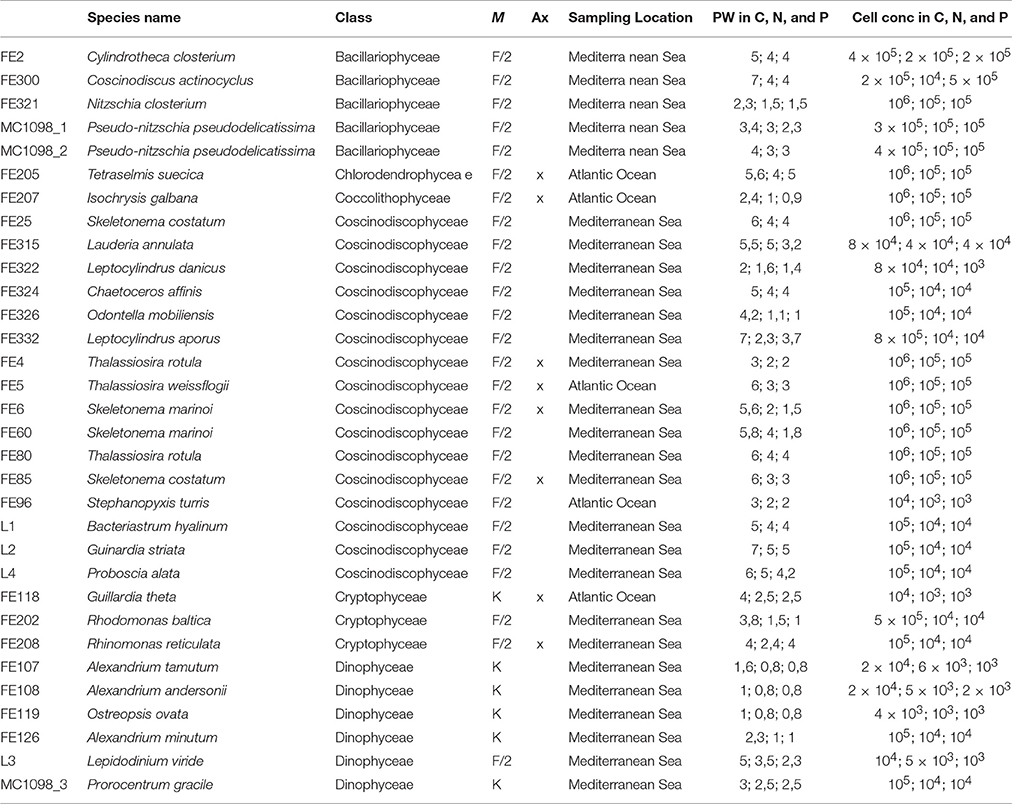
Table 1. The table reports the culture collection code, species name, and class, medium (M) used for culturing, axenicity (Ax) information, and sampling location for each microalgae selected for this study. In addition, it reports the approximate pellet weight (PW) and cell concentration used for the chemical extractions of microalgae cultured in control, nitrogen- and phosphate-starvation (C, N, and P, respectively).
Chemical Extraction and Pre-Fractionation
Fifty millilitre of distilled water was added to the microalgal pellets and samples were sonicated for 1 min at maximum intensity. The same volume of acetone was added and, after 50 min mixing at room temperature, samples were evaporated under nitrogen stream down to half of their volume. In order to start fractionation of the samples we used Amberlite® XAD16N resin that is a macroreticular, styrene-divinylbenzene copolymer, nonionic bead (Sigma-Aldrich). The hydrophobic chemical nature of the resin makes the XAD16N an excellent adsorbent under reversed phase conditions, to bind non-polar solute from water. Only the extracts adsorbed and successively released from the resin were investigated in this study. In detail, about 1 g of Amberlite XAD16N resin (20–60 mesh, Sigma-Aldrich) was added to each sample. After 50 min of mixing at room temperature, samples were centrifuged (15 min at 3500 g at room temperature) and 18 mL of water were added to the resin for a washing step. After 50 min of mixing at room temperature, a centrifugation step (15 min at 3500 g at room temperature) allowed the elimination of water and the resin was incubated with 10 ml acetone for 50 min. Centrifugation (at 3500 g) for 15 min at room temperature allowed the resin to settle and the supernatants, that were the final extracts, were freeze-dried and stored at −20°C until screening. Before performing the assays, extracts were first diluted at 1 mg/mL with MilliQ water and 2.5% DMSO. A triplicate of control plus the same concentration of DMSO found in test wells was used in all assays.
Cytotoxicity Assay
Cytotoxicity was evaluated after 24 h exposure in human hepatocellular liver carcinoma (HepG2, ATCC HB-8065™) and 72 h exposure in normal human lung fibroblast (MRC-5, ATCC CCL-171™) cells. For the 24 h study, 20,000 HepG2 cells were seeded per well. For the 72 h study, 6000 MRC-5 cells were used. HepG2 and MRC5 were grown overnight, and then incubated with 50 μg/mL test extract diluted in MEM Earle's supplemented with gentamycin (10 μg/mL), non-essential amino acids (1%), sodium pyruvate (1 mM), L-alanyl-L-glutamine (2 mM), but without FBS (total volume was 100 μl). Ten μL of CellTiter 96® AQueous One Solution Reagent (Promega, Madison, WI, USA) was added and plates were then further incubated for 1 h. Absorbance was measured at 485 nm in a DTX 880 Multimode Detector. Results were calculated as % survival compared to negative (assay media) and positive (Triton X-100; Sigma-Aldrich) controls. The screening was performed using 2–3 biological replicates and 6–9 technical replicates (i.e., duplicate/triplicate chemical extractions from each microalgae).
Cellular Lipid Peroxidation Antioxidant Activity (CLPAA) Assay
Approximately 90,000 HepG2 cells per well (100 μl final volume) were seeded in black 96 well-plates with clear bottoms (Corning, NY, USA) with MEM Earle's medium (F0325) and incubated at 37°C with 5% CO2 overnight (Pap et al., 1999; Lind et al., 2013). Briefly, cells were labeled with 5 μM C11-BODIPY (#D3861, Invitrogen, Eugene, OR, USA) for 30 min and incubated for 1 h with 50 μg/mL test extracts. Cumene hydroperoxide (CumOOH, Sigma-Aldrich; final concentration 50 μM) was added to initiate lipid peroxidation and the plate was immediately installed in a Victor3 Plate Reader. Both red (590/632 nm) and green (485/520 nm) fluorescence were recorded. Cells in Hanks buffer were used as negative control and cells treated with CumOOH as positive control. The screening was performed using at least two biological replicates.
Antioxidant Assay for Cellular Antioxidant Activity (CAA)
The CAA assay was performed as in Lind et al. (2013). Briefly, HepG2 80,000 cells/well were seeded in black 96-well-plates (#3603, Corning, NY, USA) and incubated with 25 μM DCFH-DA (2′7′-dichlorofluorescin diacetate; Fluka, 35847) and the test extracts (50 μg/mL) in duplicate for 1 h. Luteolin (final concentration of 50 μg/mL) was used as antioxidant control. After incubation, Hank's saline solution without phenol red (Biochrom, BCHRL2035) supplemented with 2,2-azobis (2-amidinopropane) dihydrochloride AAPH (600 μM) was added to all the wells, except for the negative control (without AAPH). Fluorescence was recorded in a Victor3 Plate Reader (485 nm). The screening was performed using at least two biological replicates.
Anti-Inflammatory Assay
The assay was performed as in Lind et al. (2013). Briefly, ~106 human monocyte (acute monocytic leukemia) THP-1 cells/mL (ATCC TIB-202) supplemented with 50 ng/mL phorbol 12-myristate 13-acetate (PMA, Sigma Aldrich) were seeded in 96 well plates and incubated at 37°C, 5% CO2 for 48 h in RPMI-1640 medium (Biochrom; 10% FBS). After 72 h, 80 μL fresh RPMI medium and 10 μL/well (tested concentration 100 μg/mL) of test extract were added. The test was performed in triplicate. After incubation for 1 h, all samples were incubated with 1 ng/mL lipopolysaccharide (LPS; final concentration) for another 6 h at 37°C. The reactions were stopped by freezing the plates at −80°C immediately after incubation. Enzyme-linked immunosorbent Assay (ELISA) was used to test TNFα secretion. One day prior to ELISA testing TNFα secretion, MaxiSorp 96F-well plates (Nunc) were coated with 2 μg/mL capture antibody (eBioscience, San Diego, CA, USA) and placed in the refrigerator overnight. Between each step, plates were washed with Tris-buffered saline (TBS; at pH 7.4, with 0.05% Tween-20). All incubations were at room temperature with shaking. A volume of 200 μL blocking buffer (TBS w/2% BSA) was added to the plates and incubated for 1 h. Standard concentration of TNFα were added to each plate before incubation for 2 h. Biotin coupled anti-human antibody (eBioscience) was diluted in assay diluent (TBS with 1% BSA) to 3 μg/mL and added to each well and incubated for 1 h. Diluted ExtrAvidin®-Alkaline Phosphatase (Sigma-Aldrich) was added and plates incubated for 30 min. 100 μL pNPP substrate (Sigma-Aldrich, 1 mg/mL in 1 M diethanolamin buffer pH 9.8) was added to each well, incubated for 45 min and results read at 405 nm. The dose-dependent response of the active extracts (10, 25, 50, and 100 μg/mL) was further investigated. Experiments were performed in triplicate. The pre-screening was performed using at least two biological replicates. For the active species, biological, and technical triplicates have been used for serial dilution testing.
Anticancer Assay
Anticancer activity was tested on the human melanoma A2058 cancer cell line [American Type Culture Collection (ATCC) CRL-11147TM, Manassas, VA, USA]. Cell lines were seeded in 96-well-microtitre plates (Nunc, Thermo Fisher Scientific, United States) at 2000 cells/well in RPMI medium with 10% fetal bovine serum (FBS) and 10 μg/mL gentamicin. Cells were incubated for 24 h before microalgal extracts (50 μg/mL) were added and thereafter incubated for 72 h. Cell viability was determined by a colorimetric 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay. At the end of the exposure time, 10 μl Cell Titer 96® Aqueous One Solution Reagent (Promega, Madison, WI, USA) was added to each well, as for the cytotoxicity test, and results measured after 1 h at 485 nm. The dose-dependent response of the active extracts (10 ng/mL, 100 ng/mL, 1, 2.5, 5, 10, 12.5, 25, 50, and 100 μg/mL) was further investigated in triplicate. The pre-screening was performed using at least two biological replicates. For the active species, biological, and technical triplicates have been used for serial dilution testing.
Anti-Diabetes Protein Tyrosine Phosphatase 1B (PTP1B) Inhibition Assay
The assay was carried out in triplicate using the fluorogenic substrate 6,8-difluoro-4-methylumbelliferyl phosphate (DiFMUP; VWR, Leuven, Belgium) and recombinant human PTP1B (Merck-Calbiochem, Darmstadt, Germany) as in Ingebrigtsen et al. (2016). Microalgal extracts (50 μg/mL) were incubated with PTP1B (1.56 ng enzyme/well) in 96-well plates. After 30-min incubation in the dark, 25 μl of a 10 μM DiFMUP solution were added and the fluorescent signal measured after 10 min at 360 nm. The positive control was a 0.16 mM solution of PTP inhibitor IV (Merck-Calbiochem). Assay buffer was used as the negative control. The pre-screening was performed using at least two biological replicates.
Antibacterial Assay
The Gram-negative bacteria E. coli (ATCC 25922) and P. aeruginosa (ATCC 27853) and the Gram-positive bacteria S. aureus (ATCC 9144), E. faecalis (ATCC 29212), and Streptococcus B (ATCC 12386) were used as test organisms and antibacterial tests were performed as in Ingebrigtsen et al. (2016). The tests were performed in 96-well-flat-bottomed plates (Nunc, Thermo Fisher Scientific, United States), in which 50 μl of test extracts (final concentration of 50 μg/mL, three biological replicates) were incubated overnight at 37°C with 50 μl of a suspension of an actively growing (log phase) culture of bacteria. In wells where the extracts did not have an effect on the bacteria, the bacteria grew, and the growth media appeared opaque. In wells, where the extracts inhibited the growth or killed the bacteria, the growth medium was clear. The absorbance was measured after about 20 h at OD600 in a Victor3 Plate Reader. Bacteria plus MilliQ water were used as positive control, while growth media plus MilliQ water was used as negative control. Fractions were considered active when the optical density was under 0.05.
Anti-Biofilm Assay
The biofilm-forming bacteria S. epidermidis (1% glucose was added in the culture media to induce the biofilm formation) was incubated with extracts at 50 μg/mL overnight at 37°C in 96 well-plates with clear bottoms (Corning, NY, USA). For controls, no test agents were added to the wells. Wells were carefully washed with 100 μl PBS to eliminate free-floating bacteria. Formation of the bottom biofilm was fixed by incubating the plates at 65°C for 1 h. Plates were then stained with 100 μl 0.1% crystal violet for 10 min. Excess stain was thoroughly rinsed off with distilled water and plates were left to dry at 65°C for at least 1 h. OD600 of stained biofilm was measured with a Victor3 Plate Reader. These optical density values were considered as a measure of bacteria adhering to the surface and forming biofilms and OD values under 0.25 were considered active. S. epidermidis plus MilliQ water were used as positive control, the control bacteria Staphylococcus haemolyticus plus MilliQ water as negative control. Experiments were performed in triplicate. The screening was performed using three biological replicates and nine technical replicates.
Statistical Analysis
Statistical differences between treated and control cells for all the assays performed in this study were determined by Student's t-test using GraphPad Prim statistic software, V4.00 (GraphPad Software, San Diego, California, USA). Data were considered significant when at least p was <0.05 (* for p < 0.05, ** for p < 0.01, and *** for p < 0.001).
Results
Cytotoxicity Assay
Toxicity tests were performed to avoid confusion between cytotoxicity and possible bioactivity. Considering that HepG2 cells were used for <24 h in the CAA and CLPAA assays, these cells were also used for 24 h toxicity tests. Results showed that only Ostreopsis ovata extracts (CTRL, N- and P-starved extracts; named FE119/1, FE119/2, and FE119/3, respectively) were toxic and had antiproliferative activity on these liver cells (Student's t-test, p < 0.001), while all the other extracts did not alter cell survival (Figure 1A, Student's t-test, p > 0.05). Hepatocytes are good models for studying toxicity since the liver is the primary site for drug metabolism and biotransformation (Gómez-Lechón et al., 2007; Nakamura et al., 2011). In order to compare normal and cancer cells (A2058) used for the anticancer assay, we also included a toxicity test on normal lung fibroblast (MRC-5) cells. MRC-5 cytotoxicity test was assessed after 72 h of extract incubations. O. ovata extracts (CTRL, N- and P-starved extracts; FE119/1, FE119/2, and FE119/3, respectively) were also toxic on MRC-5 cells (Student's t-test, p < 0.001). In addition, Alexandrium tamutum (CTRL and P-starved extracts; FE107/1 and FE107/3, respectively), Alexandrium minutum (CTRL and N-starved extracts; FE126 and FE126/2, respectively), Alexandrium andersoni (FE108/1, CTRL extract) and Coscinodiscus actinocyclus (FE300/2, N-starved extract) also showed cytotoxic activities on MRC-5 cells, by considerably reducing cell survival (Figure 1B, Student's t-test, p < 0.001 for all, except p < 0.01 for FE108/1).
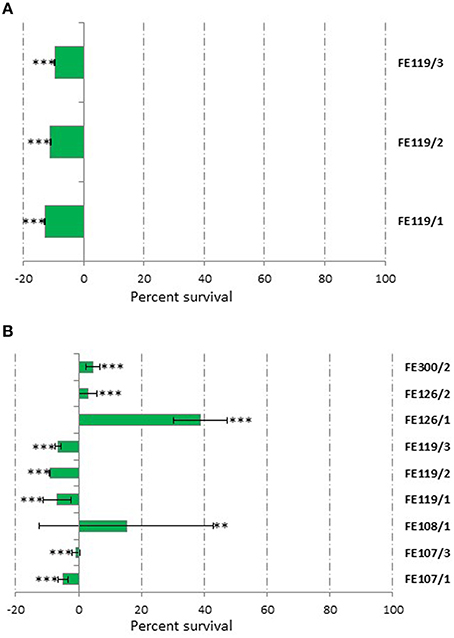
Figure 1. Cytotoxicity Assay. Results from cytotoxic extracts using HepG2 cells for 24 h (A) and MCF-7 cells for 72 h (B) compared to controls. Results are expressed as percent survival after 24 and 72 h exposure respectively (n = 9, data represent means for three biological and nine technical triplicates). FE119 corresponds to Ostreopsis ovata, FE107 to Alexandrium tamutum, FE126 to Alexandrium minutum, FE108 to Alexandrium andersoni, and FE300 to Coscinodiscus actinocyclus (/1, /2, and /3 correspond to Normal, N-starved or P-starved extracts, respectively; ** for p < 0.01 and *** for p < 0.001, Student's t-test).
Antioxidant Activity (CLPAA and CAA)
Even if antioxidant biochemical assays are fast and cost-effective, cellular assays can give more biological relevant information since they take into account the bioavailability and metabolism of the tested compounds. We used two antioxidant cellular-based assays, CAA and CLPAA, in order to assess both general antioxidant capacity and the ability of the extracts to reduce lipid peroxidation (CAA and CLPAA, respectively). In both assays, O. ovata showed antioxidant properties when grown in CTRL, N- and P-starved media (FE119/1, FE119/2, and FE119/3, respectively). The FE119/1 showed 66% of oxidative degeneration inhibition in the CAA assay and 74% in the CLPAA assay, FE119/2 had 70% inhibition for the CAA and 61% for the CLPAA, while FE119/3 showed 69% inhibition for both CAA and CLPAA assays. In addition, also A. minutum (FE126/1) had 100% antioxidant activity in the CLPAA assay. However, since both A. minutum and O. ovata showed toxicity in the cytotoxicity assay we did not proceed with dose-response analysis.
Anti-Inflammatory Assay
Microalgal anti-inflammatory potential was screened using the human acute monocytic leukemia cell line (THP-1) and ELISA, by monitoring the release of tumor necrosis factor α (TNFα). Species that showed TNFα inhibition were Cylindrotheca closterium (FE2/1), Odontella mobiliensis (FE326/1), Pseudonitzschia pseudodelicatissima (FE1098_1/1), C. actinocyclus (FE300/2) and A. minutum (FE126/1). We found a dose-dependent inhibition of TNFα production with increasing concentration of C. closterium FE2/1 (Student's t-test, p < 0.01 when tested at 10 and 25 μg/mL and p < 0.001 when tested at 50 and 100 μg/mL), O. mobiliensis FE326/1 (Student's t-test, p < 0.01 when tested at 25 μg/mL and p < 0.001 when tested at 10, 50, and 100 μg/mL) and P. pseudodelicatissima FE1098/1 (Student's t-test, p < 0.01 when tested at 25 μg/mL and p < 0.001 when tested at 50 and 100 μg/mL, Figure 2). The other active strains, C. actinocyclus (FE300/2) and A. minutum (FE126/1), were significantly active only when tested at 100 μg/mL (Student's t-test, p < 0.001), did not show a dose-response pattern and were positive in the cytotoxicity assay. In addition, it was interesting to note that all three diatoms, FE2/1, FE326/1, and FE1098_1/1, were active only when grown in normal medium, and inactive when grown in nutrient starvation conditions. This suggests that in stressful conditions, such as nutrient starvation, these species will not produce the compound/compounds responsible for this activity (or they produce very low amounts). Of these three species, C. closterium was previously shown to have antioxidant (evaluated only by enzymatic assay, without using cells) and anti-inflammatory properties (Affan et al., 2009). On the contrary, no information is available regarding the bioactivities of O. mobiliensis and P. pseudodelicatissima.
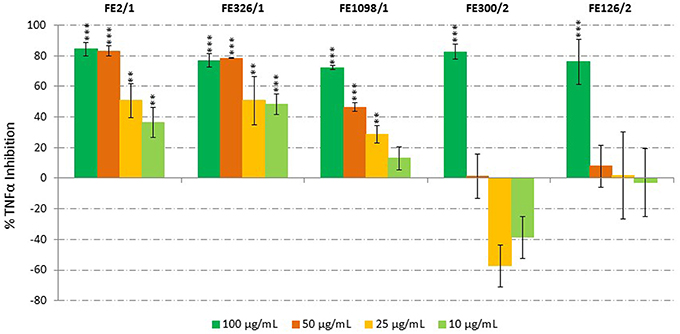
Figure 2. Anti-Inflammatory Assay. Inhibition of TNFα secretion from LPS-stimulated THP-1 cells treated with extracts of Cylindrotheca closterium (FE2/1), Odontella mobiliensis (FE326/1), Pseudonitzschia pseudodelicatissima (FE1098_1/1), Coscinodiscus actinocyclus (FE300/2), and Alexandrium minutum (FE126/1) (n = 9, data represent means for 3 biological and 9 technical triplicates; ** for p < 0.01 and *** for p < 0.001, Student's t-test) compared to control.
Anticancer Assay
Antiproliferative activity on human melanoma A2058 cells was assessed for all microalgal extracts. Results showed that S. marinoi (FE60/2), A. minutum (FE126/1), A. tamutum (FE107/1 and FE107/3) and A. andersoni (FE108/1) induced a significant reduction in cell survival at 100 μg/mL. Successively, a dilution series was performed and the active extracts were tested at 100, 50, 25, 12.5, 10, 2.5, and 10 ng/mL. Figure 3 shows that S. marinoi FE60/2 inhibits cell survival in a dose-dependent manner while for the other species, the pattern was not clear. However, A. minutum, A. tamutum, and A. andersoni also showed toxicity in the cytotoxicity assay. We also tested two different S. marinoi clones, FE6 isolated in 1997 and FE60 isolated in 2005 in the Adriatic Sea (Mediterranean Sea). Both strains are known to produce secondary metabolites such as short-chain polyunsaturated aldehydes (PUAs) and other oxygenated fatty acid degradation products such as hydroxides, oxoacids, epoxy alcohols, and hydroperoxides that induce reproductive failure in zooplankton grazers (Miralto et al., 1999; Ianora et al., 2004; Fontana et al., 2007; Romano et al., 2010; Gerecht et al., 2011). PUAs isolated from FE6 have been shown to have antiproliferative activity on colon carcinoma cells (Miralto et al., 1999), while FE60 has never been tested before for anticancer properties. Interestingly, extracts of FE6 did not show activity for human melanoma cells (A2058), suggesting that the anticancer activity was specific for a particular cancer cell line. On the other hand, FE60 was active against A2058 cells only when grown in nitrogen-starved medium (FE60/2) at 25, 50, and 100 μg/mL (Student's t-test, p < 0.05 for 25 μg/mL and p < 0.001 for 50 and 100 μg/mL), while CTRL and phosphate-starved conditions (FE60/1 and FE60/3, respectively) were not active. These results confirm once again that environmental conditions, such as nutrient availability, may alter cellular metabolism, enhancing or reducing the synthesis of the metabolites of interest.
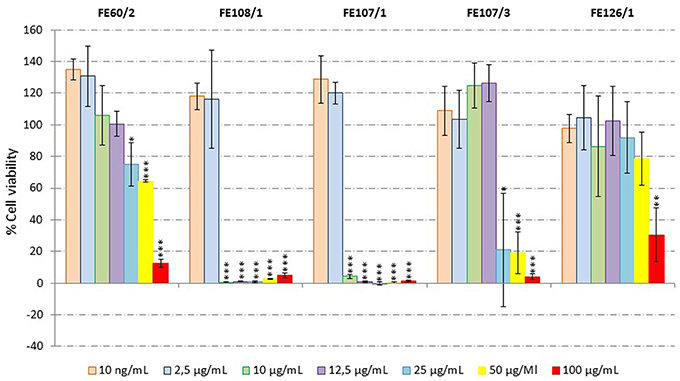
Figure 3. Anticancer Assay. Percentage viability of human melanoma cells (A2058) after incubation for 72 h with microalgal extracts (Skeletonema marinoi FE60/2, Alexandrium andersoni FE108/1, Alexandrium tamutum FE107/1 and FE107/3, and Alexandrium minutum FE126/1) at 100, 50, 25, 12.5, 10, and 2.5 μg/mL and 10 ng/mL (n = 9, data represent means for three biological and nine technical triplicates; * for p < 0.05, ** for p < 0.01 and *** for p < 0.001, Student's t-test) compared to control.
Protein Tyrosine Phosphatase 1B (PTP1B) Inhibition Assay
Protein thyrosine phosphatase 1B (PTP1B) is an enzyme that is associated with the development of type 2 diabetes. PTP1B dephosphorylates the insulin receptor and its substrates, thereby inhibiting the effect of insulin. The potential of microalgae to block PTP1B activity was screened, but no apparent bioactivity was observed (data not shown).
Antibacterial Assays
Microalgal extracts were also screened against a set of bacterial strains composed of Gram-negative bacteria E. coli and P. aeruginosa and the Gram-positive bacteria S. aureus, E. faecalis, and Streptococcus B. The concentration tested (50 μg/mL) was the same as for the biofilm assay. No apparent bioactivity against any of the tested strains was observed (data not shown), except for FE60/2 and FE6/3 that inhibited S. aureus growth by 97 and 96%, respectively (Student's t-test, p < 0.001 for both). Extracts were then tested for possible inhibitory activity on biofilm formation by the bacteria S. epidermidis. Interestingly, two species that did not show cytotoxicity in the previous antibacterial tests showed strong anti-biofilm formation. These two species belong to the same microalgal genus and were Leptocylindrus danicus (FE322) and Leptocylindrus aporus (FE332). In particular, FE322 was able to inhibit biofilm formation only when the alga was grown in N-starved medium (90% Inhibition), while FE332 was active in all three culturing conditions: CTRL (64%), N-starved (90%) and P-starved (85%) (Student's t-test, p < 0.001 for all conditions). For both species, the nitrogen-starvation condition was the most active.
Discussion
Our study is one of the few to simultaneously test a wide range of marine microalgal species using the same protocols for culturing, extraction, and testing on human cancer cell lines and other bioactivity screenings (antioxidant, anti-inflammatory, anti-diabetes, antibacterial, and anti-biofilm). Of the 32 species tested (21 diatom, 7 dinoflagellate and 4 flagellate species; for three diatoms we also tested two different clones), only diatoms displayed bioactivity denoting the potential of this microalgal group to produce bioactive compounds for the treatment of human diseases. To our knowledge this is the first report on the wide screening of marine microalgae in three different nutrient culturing conditions for the treatment of human pathologies. Guedes et al. (2013) tested the antioxidant capacity of 23 microalgae (P. tricornutum was the only diatom) cultured in only one growth condition, Samarakoon et al. (2013) tested the anti-inflammatory (nitric oxide production inhibition) and anticancer (i.e., on human promyelocytic leukemia cell line HL60, mouse melanoma cell line B16F10 and human lung carcinoma cell line A549) activity for one diatom and one dinoflagellate cultured in only one growth condition, Shah et al. (2014) tested the antioxidant (DPPH radical scavenging assay), anti-inflammatory (nitric oxide production inhibition) and anticancer (human promyelocytic leukemia cell line HL-60) activities of 11 benthic dinoflagellates collected in Jeju Island (Korea), cultured in two different culture media (IMK and f/2 medium). Finally, Ingebrigtsen et al. (2016) tested the bioactivity (for antioxidant, antibacterial, anti-inflammatory, anti-diabetes, and anticancer) of five North-Atlantic diatoms grown in four different light/temperature conditions. In addition to these broad screenings, there are a few other studies on the activity of single species, such as the anticancer activity of the diatom Chaetoceros calcitrans (strain UPMAAHU10) on human breast cell line MCF-7 (Nigjeh et al., 2013), the anticancer effect of Dunaliella salina (green microalgae, strain from Iran) on skin carcinoma cell line A431 (Emtyazjoo et al., 2012), the anticancer activity of amphidinolides H and N, isolated from the dinoflagellate A. carterae, on human tumor cell lines (Kobayashi, 2008) and beneficial effects in a transgenic mouse model of Alzheimer's disease (Alonso et al., 2013) inferred by 13-desmethyl spirolide C (polyketide-derived), synthetized by the dinoflagellate A. ostenfeldii (MacKinnon et al., 2006).
In the current study, of the 32 species tested three showed anti-inflammatory properties (C. closterium FE2/1, O. mobiliensis FE326/1, and P. pseudodelicatissima FE1098_1/1), one species had anticancer activity (S. marinoi FE60/2), two species showed antibacterial properties (S. marinoi FE60/2 and S. marinoi FE6/3) and another two blocked bacteria biofilm formation (L. danicus FE322/2, L. aporus FE332/1, FE332/2, and FE332/3) (see Summary in Figure 4). Inflammation is a protective response that involves immune cells, blood vessels, and different molecular mediators (e.g., TNFα, IL1, nitric oxide, and prostaglandins). Anti-inflammatory properties were previously found for other microalgae, such as the green algae Dunaliella bardawil (Lee et al., 2013), the diatoms Porosira glacialis, Attleya longicornis (Ingebrigtsen et al., 2016), and P. tricornutum (Samarakoon et al., 2013), and the dinoflagellate A. carterae (Samarakoon et al., 2013). Here we evaluated the anti-inflammatory potential of 32 species analyzing the capacity of the microalgal extracts to inhibit TNFα release in LPS-treated acute monocytic leukemia THP-1 cells and we found activity for three diatoms C. closterium FE2/1, O. mobiliensis FE326/1, and P. pseudodelicatissima FE1098_1/1. Ingebrigtsen et al. (2016) used the same protocol and found activity for the North-Atlantic diatoms P. glacialis and A. longicornis. Interestingly, C. closterium FE2/1, O. mobiliensis FE326/1, and P. pseudodelicatissima FE1098_1/1 were active only when grown in normal medium, and inactive when grown in nutrient starvation conditions (i.e., nitrogen- and phosphate-starved media) suggesting that in stressful conditions, such as nutrient starvation, these species will not produce the compound/compounds responsible for this activity (or they produce very low amounts). This is in contrast to other secondary metabolites produced by diatoms such as PUAs and domoic acid (DA), that are produced in higher quantities under stress conditions, i.e., aging and nutrient starvation conditions (Maldonado et al., 2002; Fehling et al., 2004 Ribalet et al., 2007; Martin-Jézéquel et al., 2015). Similarly, Fehling et al. (2004) found an increased production of DA when Pseudo-nitzschia seriata (Cleve) was cultured under silicate-limitation, while Martin-Jézéquel et al. (2015) found higher toxin content in nitrogen-starvation conditions for Pseudo-nitzschia multiseries CCL70 and Pseudo-nitzschia australis PNC1. In addition, Maldonado et al. (2002) observed an increased DA production for both P. multiseries and P. australis in Fe-deficient and Cu-stressed conditions.
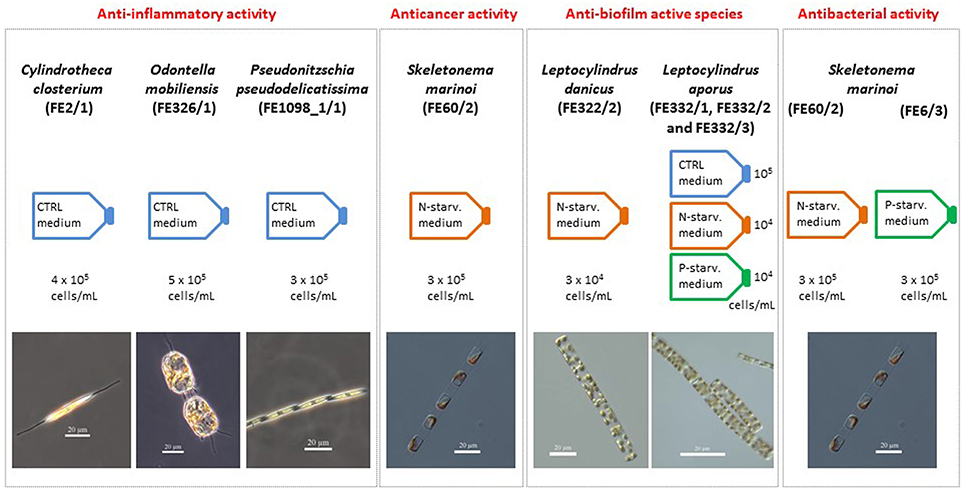
Figure 4. Summary chart. Summary of the species and culturing conditions (CTRL, nitrogen- and phosphate-starved media, indicated with N- and P-starv. medium, respectively) that showed anti-inflammatory (Cylindrotheca closterium FE2/1, Odontella mobiliensis FE326/1, Pseudonitzschia pseudodelicatissima FE1098_1/1), anticancer (Skeletonema marinoi FE60/2), and anti-biofilm properties (Leptocylindrus danicus FE322/2 and Leptocylindrus aporus FE332/1, FE332/2, and FE332/3). In addition, approximate cell concentration used for the preparation of the chemical extracts tested and species photos are reported.
The diatom S. marinoi showed very interesting results in the current study. One clone of this species S. marinoi (FE60) showed anticancer activity on human melanoma A2058 cells, but only when cultured in nitrogen-starvation conditions. The other clone FE6 was not active against cancer cells. In addition, both clones (S. marinoi FE60 and FE6) showed antibacterial properties by inhibiting the survival of S. aureus. However, FE60 had antibacterial properties only when cultured in nitrogen-starvation conditions, while FE6 only in phosphate-starvation condition. These data indicated clone-specific differences in biological activity, but both were active when cultured under nutrient-starvation.
To our knowledge, this is the first report of so many diatoms (and microalgae in general) tested on human melanoma A2058 cells. Ingebrigtsen et al. (2016) were the only authors to test a clone of S. marinoi against this extremely malignant cancer cell line. They tested a North Atlantic clone and found that this clone had antiproliferative activities on A2058 cells only when cultured at low temperature-high light, while it was not active in other culturing conditions. In addition, their clone was not active against bacteria. S. marinoi, as other diatoms, are known to produce a series of secondary metabolites (PUAs and other oxylipins) with anticancer properties (Miralto et al., 1999; Sansone et al., 2014). Considering that all S. marinoi clones produce oxylipins, even if in different amounts (Gerecht et al., 2011), we suggest that the compounds responsible for the observed anticancer and antibacterial activities are not oxylipins because only one clone has anticancer activity. Taking into account that in this study the resin Amberlite (which binds non-polar solutes in the microalgal extract, Sigma-Aldrich) was used for the pre-fractionation, we suggest that other non-polar compounds may be responsible for the observed bioactivities.
This is the first time that anti-biofilm activity is reported for microalgae (activity against the biofilm-forming bacteria S. epidermidis). Of the 32 species tested, the two species L. danicus (FE322) and L. aporus (FE332), belonging to the same microalgal genus, showed strong anti-biofilm activity. In particular, FE322 was able to inhibit biofilm formation only when the alga was grown in N-starved medium (90% Inhibition), while FE332 was active in all three culturing conditions: CTRL (64%), N-starved (90%), and P-starved (85%). For both species, the nitrogen-starvation condition was the most active, indicating that this stress condition increased the production of the compound/compounds responsible for this activity. S. epidermidis is a permanent and ubiquitous colonizer of human skin. Treatment is complicated by specific antibiotic resistance genes and the formation of biofilms, multicellular agglomerations that have intrinsic resistance to antibiotics (Otto, 2009). S. epidermidis infections have gained increasing attention because they represent the most common source of hospital-acquired infections (Levinson, 2010) and are of particular concern for people with catheters or other surgical indwelling medical devices (Salyers and Whitt, 2002). The discovery that microalgae possess anti-biofilm activity against this bacterium opens new possibilities for the treatment of S. epidermidis infections. Future chemical investigations may allow the identification of the compounds responsible of the reported activities.
In conclusion, the results reported in this study show the absence of correlations between bioactivity and a specific microalgal class or a specific culturing condition. When the culturing parameters are modified, the same organism may show different bioactivities and produce diverse metabolites (Bode et al., 2002). This strategy, termed OSMAC (One strain–many compounds), has successfully been applied for biodiscovery in bacteria (Bode et al., 2002) and could potentially also work in microalgae. The production of primary and secondary metabolites in microalgae can vary depending on e.g., growth phases (Vidoudez and Pohnert, 2012), clones (Gerecht et al., 2011), light (Depauw et al., 2012), temperature (Huseby et al., 2013), culturing media (Alkhamis and Qin, 2015), grazing pressure (Pohnert, 2002), extraction method (Jüttner, 2001), and probably many other factors (Chen et al., 2011). This metabolic plasticity positively influences drug discovery by triggering bioactivities and hopefully leading to the discovery of novel bioactive compounds for the treatment of human diseases.
Author Contributions
CL, JA, EH, GR, AI conceived and designed the experiments; CL, EH, MA, LE, FE, KH, KH performed the experiments; CL, EH, MA, KH analyzed the data; all authors co-wrote the paper.
Funding
The research leading to these results has received funding from European Union 7th Framework Program PHARMASEA (312184).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Massimo Perna and Mariano Amoroso (Stazione Zoologica Anton Dohrn SZN, Napoli, Italy) and Evaldas Kasiulis (Marbank, Institute of Marine Research, Tromsø, Norway) for technical assistance. The authors also thank Flora Palumbo from SZN for graphics and two anonymous reviewers for their helpful suggestions.
References
Affan, A., Heo, S. J., Jeon, Y. J., and Lee, J. B. (2009). Optimal growth conditions and antioxidative activities of Cylindrotheca closterium (Bacillariophyceae). J. Phycol. 45, 1405–1415. doi: 10.1111/j.1529-8817.2009.00763.x
Alkhamis, Y., and Qin, J. G. (2015). Comparison of pigment and proximate compositions of Tisochrysis lutea in phototrophic and mixotrophic cultures. J. Appl. Phycol. 28, 35–42. doi: 10.1007/s10811-015-0599-0
Alonso, E., Otero, P., Vale, C., Alfonso, A., Antelo, A., Giménez-Llort, L., et al. (2013). Benefit of 13-desmethyl spirolide C treatment in triple transgenic mouse model of Alzheimer disease: beta-amyloid and neuronal markers improvement. Curr. Alzheimer Res. 10, 279–289. doi: 10.2174/1567205011310030007
Bajetta, E., Del Vecchio, M., Bernard-Marty, C., Vitali, M., Buzzoni, R., Rixe, O., et al. (2002). Metastatic melanoma: chemotherapy. Semin. Oncol. 29, 427–445. doi: 10.1053/sonc.2002.35238
Blunt, J. W., Copp, B. R., Keyzers, R. A., Munro, M. H. G., and Prinsep, M. R. (2015). Marine natural products. Nat. Prod. Rep. 32, 116–211. doi: 10.1039/C4NP00144C
Bode, H. B., Bethe, B., Höfs, R., and Zeeck, A. (2002). Big effects from small changes: possible ways to explore nature's chemical diversity. Chembiochem. 3, 619–627. doi: 10.1002/1439-7633(20020703)3:7<619::AID-CBIC619>3.0.CO;2-9
Bozarth, A., Maier, U. G., and Zauner, S. (2009). Diatoms in biotechnology: modern tools and applications. Appl. Microbiol. Biotechnol. 82, 195–201. doi: 10.1007/s00253-008-1804-8
Caldwell, G. S. (2009). The influence of bioactive oxylipins from marine diatoms on invertebrate reproduction and development. Mar. Drugs 7, 367–400. doi: 10.3390/md7030367
Chen, C. Y., Yeh, K. L., Aisyah, R., Lee, D. J., and Chang, J. S. (2011). Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: a critical review. Bioresour. Technol. 102, 71–81. doi: 10.1016/j.biortech.2010.06.159
Depauw, F. A., Rogato, A., d'Alcalá, M. R., and Falciatore, A. (2012). Exploring the molecular basis of responses to light in marine diatoms. J. Exp. Bot. 63, 1575–1591. doi: 10.1093/jxb/ers005
Eicholz, K., Beszteri, B., and John, U. (2012). Putative monofunctional type I polyketide synthase units:a dinoflagellate-specific feature? PLoS One 7:e48624. doi: 10.1371/journal.pone.0048624
Emtyazjoo, M., Moghadasi, Z., Rabbani, M., Emtyazjoo, M., Samadi, S., and Mossaffa, N. (2012). Anticancer effect of Dunaliella salina under stress and normal conditions against skin carcinoma cell line A431 in vitro. Iran. J. Fish. Sci. 11, 283–293.
Fehling, J., Green, D. H., Davidson, K., Bolch, C. J., and Bates, S. S. (2004). Domoic acid production by Pseudo-nitzschia seriata (bacillariophyceae) in scottish waters. J. Phycol. 40, 622–630. doi: 10.1111/j.1529-8817.2004.03200.x
Fontana, A., d'Ippolito, G., Cutignano, A., Miralto, A., Ianora, A., Romano, G., et al. (2007). Chemistry of oxylipin pathways in marine diatoms. Pure Appl. Chem. 79, 481–490. doi: 10.1351/pac200779040481
Gastineau, R., Turcotte, F., Pouvreau, J. B., Morançais, M., Fleurence, J., Windarto, E., et al. (2014). Marennine, promising blue pigments from a widespread haslea diatom species complex. Mar. Drugs 12, 3161–3189. doi: 10.3390/md12063161
Gerecht, A., Romano, G., Ianora, A., d'Ippolito, G., Cutignano, A., and Fontana, A. (2011). Plasticity of oxilipin metabolism among clones of the marine diatom skeletonema marinoi (Bacillariophyceae). J. Phycol. 47, 1050–1056. doi: 10.1111/j.1529-8817.2011.01030.x
Gómez-Lechón, M. J., Castell, J. V., and Donato, M. T. (2007). Hepatocytes-the choice to investigate drug metabolism and toxicity in man: in vitro variability as a reflection of in vivo. Chem. Biol. Interact. 168, 30–50. doi: 10.1016/j.cbi.2006.10.013
Gorbi, S., Avio, G. C., Benedetti, M., Totti, C., Accoroni, S., Pichierri, S., et al. (2013). Effects of harmful dinoflagellate ostreopsis cf. ovata exposure on immunological, histological and oxidative responses of mussels Mytilus galloprovincialis. Fish Shellfish Immunol. 35, 941–950. doi: 10.1016/j.fsi.2013.07.003
Guedes, A. C., Gião, M. S., Seabra, R., Ferreira, A. C. S., Tamagnini, P., Moradas-Ferreira, P., et al. (2013). Evaluation of the antioxidant activity of cell extracts from microalgae. Mar. Drugs 11, 1256–1270. doi: 10.3390/md11041256
Guillard, R. R. L. (1975). “Culture of phytoplankton for feeding marine invertebrates,” in Culture of Marine Invertebrate Animals, eds W. L. Smitha and M. H. Chanley (New York, NY: USA: Plenum Press), 26–60.
Gum, R. J., Gaede, L. L., Koterski, S. L., Heindel, M., Clampit, J. E., Zinker, B. A., et al. (2003). Reduction of protein tyrosine phosphatase 1B increases insulin-dependent signaling in ob/ob mice. Diabetes 52, 21–28. doi: 10.2337/diabetes.52.1.21
Huseby, S., Degerlund, M., Eriksen, G. K., Ingebrigtsen, R. A., Eilertsen, H. C., and Hansen, E. (2013). Chemical diversity as a function of temperature in six northern diatom species. Mar. Drugs 11, 4232–4245. doi: 10.3390/md11114232
Ianora, A., and Miralto, A. (2010). Toxigenic effects of diatoms on grazers, phytoplankton and other microbes: a review. Ecotoxicology 19, 493–511. doi: 10.1007/s10646-009-0434-y
Ianora, A., Miralto, A., Poulet, S. A., Carotenuto, Y., Buttino, I., Romano, G., et al. (2004). Aldehyde suppression of copepod recruitment in blooms of a ubiquitous planktonic diatom. Nature 429, 403–407. doi: 10.1038/nature02526
Ingebrigtsen, R. A., Hansen, E., Andersen, J. H., and Eilertsen, H. C. (2016). Light and temperature effects on bioactivity in diatoms. J. Appl. Phycol. 28, 939–950. doi: 10.1007/s10811-015-0631-4
Jaspars, M., De Pascale, D., Andersen, J. H., Reyes, F., Crawford, A. D., and Ianora, A. (2016). The marine biodiscovery pipeline and ocean medicines of tomorrow. J. Mar. Biol. Assoc. U.K. 96, 151–158. doi: 10.1017/S0025315415002106
Jüttner, F. (2001). Liberation of 5, 8, 11, 14, 17-eicosapentaenoic acid and other polyunsaturated fatty acids from lipids as a grazer defense reaction in epilithic diatom biofilms. J. Phycol. 37, 744–755. doi: 10.1046/j.1529-8817.2001.00130.x
Keller, M. D., Selvin, R. C., Claus, W., and Guillard, R. R. L. (1987). Media for the culture of oceanic ultraphytoplankton. J. Phycol. 23, 633–638. doi: 10.1111/j.1529-8817.1987.tb04217.x
Kellmann, R., Stüken, A., Orr, R. J. S., Svendsen, H. M., and Jakobsen, K. S. (2010). Biosynthesis and molecular genetics of polyketides in marine dinoflagellates. Mar. Drugs 8, 1011–1048. doi: 10.3390/md8041011
Kobayashi, J. (2008). Amphidinolides and its related macrolides from marine dinoflagellates. J Antibiot 61, 271–284. doi: 10.1038/ja.2008.39
Kohli, G. S., John, U., Van Dolah, F. M., and Murray, S. A. (2016). Evolutionary distinctiveness of fatty acid and polyketide synthesis in eukaryotes. ISME J. doi: 10.1038/ismej.2015.263. [Epub ahead of print].
Landsberg, J. H. (2002). The effects of harmful algal blooms on aquatic organisms. Rev. Fish. Sci. 10, 113–390. doi: 10.1080/20026491051695
Lauritano, C., Procaccini, G., and Ianora, A. (2012). Gene expression patterns and stress response in marine copepods. Mar. Environ. Res. 76, 22–31. doi: 10.1016/j.marenvres.2011.09.015
Lee, J. B., Hayashi, K., Hirata, M., Kuroda, E., Suzuki, E., Kubo, Y., et al. (2006). Antiviral sulfated polysaccharide from Navicula directa, a diatom collected from deep-sea water in Toyama Bay. Biol. Pharm. Bull. 29, 2135–2139. doi: 10.1248/bpb.29.2135
Lee, J. C., Hou, M. F., Huang, H. W., Chang, F. R., Yeh, C. C., Tang, J. Y., et al. (2013). Marine algal natural products with anti-oxidative, anti-inflammatory, and anti-cancer properties. Cancer Cell Int. 13:55. doi: 10.1186/1475-2867-13-55
Levinson, W. (2010). Review of Medical Microbiology and Immunology, 11th Edn. New York, NY: McGraw-Hill Press.
Lind, K. F., Hansen, E., Østerud, B., Eilertsen, K. E., Bayer, A., Engqvist, M., et al. (2013). Antioxidant and anti-inflammatory activities of barettin. Mar. Drugs 11, 2655–2666. doi: 10.3390/md11072655
MacKinnon, S. L., Cembella, A. D., Burton, I. W., Lewisn, N., LeBlanc, P., and Walter, J. A. (2006). Biosynthesis of 13-desmethyl spirolide C by the dinoflagellate Alexandrium ostenfeldii. J. Org. Chem. 71, 8724–8731. doi: 10.1021/jo0608873
Maldonado, M. T., Hughes, M. P., Rue, E. L., and Wells, M. L. (2002). The effect of Fe and Cu on growth and domoic acid production by Pseudo-nitzschia multiseries and Pseudo-nitzschia australis. Limnol. Oceanogr. 47, 515–526. doi: 10.4319/lo.2002.47.2.0515
Martin-Jézéquel, V., Calu, G., Candela, L., Amzil, Z., Jauffrais, T., Séchet, V., et al. (2015). Effects of organic and inorganic nitrogen on the growth and production of domoic acid by Pseudo-nitzschia multiseries and P. australis (Bacillariophyceae) in culture. Mar. Drugs 13, 7067–7086. doi: 10.3390/md13127055
Meyer, J. M., Rödelsperger, C., Eichholz, K., Tillmann, U., Cembella, A., McGaughran, A., et al. (2015). Transcriptomic characterisation and genomic glimps into the toxigenic dinoflagellate Azadinium spinosum, with emphasis on polykeitde synthase genes. BMC Genomics 16:27. doi: 10.1186/s12864-014-1205-6
Mimouni, V., Ulmann, L., Pasquet, V., Mathieu, M., Picot, L., Bougaran, G., et al. (2012). The potential of microalgae for the production of bioactive molecules of pharmaceutical interest. Curr. Pharm. Biotechnol. 13, 2733–2750. doi: 10.2174/138920112804724828
Miralto, A., Barone, G., Romano, G., Poulet, S. A., Ianora, A., Russo, G. L., et al. (1999). The insidious effect of diatoms on copepod reproduction. Nature 402, 173–176.
Nakamura, K., Mizutani, R., Sanbe, A., Enosawa, S., Kasahara, M., Nakagawa, A., et al. (2011). Evaluation of drug toxicity with hepatocytes cultured in a micro-space cell culture system. J. Biosci. Bioeng. 111, 78–84. doi: 10.1016/j.jbiosc.2010.08.008
Newton, K., and Dixit, V. M. (2012). Signaling in innate immunity and inflammation. Cold Spring Harb. Perspect. Biol. 4:a006049. doi: 10.1101/cshperspect.a006049
Nigjeh, E. S., Yusoff, F. M., Alitheen, M. N. B., Rasoli, M., Keong, Y. S., and Omar, A. R. (2013). Cytotoxic effect of ethanol extract of microalga, Chaetoceros calcitrans, and its mechanisms in inducing apoptosis in human breast cancer cell line. Biomed. Res. Int. 2013:783690. doi: 10.1155/2013/783690
Otto, M. (2009). Staphylococcus epidermidis – the “accidental” pathogen. Nat. Rev. Microbiol. 7, 555–567. doi: 10.1038/nrmicro2182
Pap, E. H. W., Drummen, G. P., Winter, V. J., Kooij, T. W., Rijken, P., Wirtz, K. W., et al. (1999). Ratio-fluorescence microscopy of lipid oxidation in living cells using C11-BODIPY581/591. FEBS Lett. 453, 278–282. doi: 10.1016/S0014-5793(99)00696-1
Peng, J., Yuan, J. P., Wu, C. F., and Wang, J. H. (2011). Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: metabolism and bioactivities relevant to human health. Mar. Drugs 9, 1806–1828. doi: 10.3390/md9101806
Pohnert, G. (2002). Phospholipase A2 activity triggers the wound-activated chemical defense in the diatom Thalassiosira rotula. Plant Physiol. 129, 103–111. doi: 10.1104/pp.010974
Prestegard, S. K., Oftedal, L., Coyne, R. T., Nygaard, G., Skjaerven, K. H., Knutsen, G., Døskeland, S. O., et al. (2009). Marine benthic diatoms contain compounds able to induce leukemia cell death and modulate blood platelet activity. Mar. Drugs 7, 605–623. doi: 10.3390/md7040605
Prestegard, S. K., Knutsen, G., and Herfindal, L. (2014). Adenosine content and growth in the diatom Phaeodactylum tricornutum (Bacillariophyceae): effect of salinity, light, temperature and nitrate. Diatom Res. 29, 361–369. doi: 10.1080/0269249X.2014.889040
Ribalet, F., Wichard, T., Pohnert, G., Ianora, A., Miralto, A., and Casotti, R. (2007). Age and nutrient limitation enhance polyunsaturated aldehyde production in marine diatoms. Phytochemistry 68, 2059–2067. doi: 10.1016/j.phytochem.2007.05.012
Romano, G., Miralto, A., and Ianora, A. (2010). Teratogenic effects of diatom metabolites on sea urchin Paracentrotus lividus embryos. Mar. Drugs 8, 950–967. doi: 10.3390/md8040950
Salyers, A. A., and Whitt, D. D. (2002). Bacterial Pathogenesis: A Molecular Approach, 2nd Edn. Washington, DC: ASM Press.
Samarakoon, K. W., Ko, J. Y., Lee, J. H., Kwon, O. N., Kim, S. W., and Jeon, Y. J. (2014). Apoptotic anticancer activity of a novel fatty alcohol ester isolated from cultured marine diatom, Phaeodactylum tricornutum. J. Funct. Foods 6, 231–240. doi: 10.1016/j.jff.2013.10.011
Samarakoon, K. W., Ko, J. Y., Shah, Md. M. R., Lee, J. H., Kang, M. C., O-Nam, K., et al. (2013). In vitro studies of anti-inflammatory and anticancer activities of organic solvent extracts from cultured marine microalgae. Algae 28, 111–119. doi: 10.4490/algae.2013.28.1.111
Sampedro, N., Franco, J. M., Zapata, M., Riobò, P., Garcés, E., Penna, A., et al. (2013). The toxicity and intraspecific variability of Alexandrium andersonii Balech. Harmful Algae 25, 26–38. doi: 10.1016/j.hal.2013.02.003
Sansone, C., Braca, A., Ercolesi, E., Romano, G., Palumbo, A., Casotti, R., et al. (2014). Diatom-derived polyunsaturated aldehydes activate cell death in human cancer cell lines but not normal cells. PLoS One 9:e101220. doi: 10.1371/journal.pone.0101220
Shah, Md. M. R., Samarakoon, K. W., Ko, J. Y., Chaminda, Lakmal, H. H., Lee, J. H., An, S. J., et al. (2014). Potentiality of benthic dinoflagellate cultures and screening of their bioactivities in Jeju Island, Korea. Afr. J. Biotechnol. 13, 792–805. doi: 10.5897/AJB2013.13250
Van Dolah, F. M., Zippay, M. L., Pezzolesi, L., Rein, K. S., Johnson, J. G., Morey, J. S., et al. (2013). Subcellular localization of dinoflagellate polyketide synthases and fatty acid synthase activity. J. Phycol. 49, 118–1127. doi: 10.1111/jpy.12120
Vidoudez, C., and Pohnert, G. (2012). Comparative metabolomics of the diatom Skeletonema marinoi in different growth phases. Metabolomics 8, 654–669. doi: 10.1007/s11306-011-0356-6
Keywords: drug discovery, marine biotechnology, nutrient starvation, clones, diatoms, anti-inflammatory, anticancer, anti-biofilm
Citation: Lauritano C, Andersen JH, Hansen E, Albrigtsen M, Escalera L, Esposito F, Helland K, Hanssen KØ, Romano G and Ianora A (2016) Bioactivity Screening of Microalgae for Antioxidant, Anti-Inflammatory, Anticancer, Anti-Diabetes, and Antibacterial Activities. Front. Mar. Sci. 3:68. doi: 10.3389/fmars.2016.00068
Received: 11 March 2016; Accepted: 21 April 2016;
Published: 10 May 2016.
Edited by:
Antonio Trincone, Consiglio Nazionale delle Ricerche, ItalyReviewed by:
Carole Anne Llewellyn, Swansea University, UKDr. G. Venkata Subhash, Reliance Industries Limited, India
Copyright © 2016 Lauritano, Andersen, Hansen, Albrigtsen, Escalera, Esposito, Helland, Hanssen, Romano and Ianora. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chiara Lauritano, chiara.lauritano@szn.it
 Chiara Lauritano
Chiara Lauritano Jeanette H. Andersen2
Jeanette H. Andersen2  Espen Hansen
Espen Hansen Laura Escalera
Laura Escalera Francesco Esposito
Francesco Esposito Kine Ø. Hanssen
Kine Ø. Hanssen Giovanna Romano
Giovanna Romano Adrianna Ianora
Adrianna Ianora