Light Thresholds to Prevent Dredging Impacts on the Great Barrier Reef Seagrass, Zostera muelleri ssp. capricorni
- 1Centre for Tropical Water and Aquatic Ecosystem Research, James Cook University, Cairns, QLD, Australia
- 2Plant Functional Biology and Climate Change Cluster, University of Technology Sydney, Sydney, NSW, Australia
Coastal seagrass habitats are at risk from a range of anthropogenic activities that modify the natural light environment, including dredging activities associated with coastal and port developments. On Australia's east coast, the tropical seagrass Zostera muelleri ssp. capricorni dominates intertidal mudbanks in sheltered embayments which are also preferred locations for harbors and port facilities. Dredging to establish and maintain shipping channels in these areas can degrade water quality and diminish light conditions that are required for seagrass growth. Based on this potential conflict, we simulated in-situ light attenuation events to measure effects on Z. muelleri ssp. capricorni condition. Semi-annual in situ shading studies conducted over 3 years were used to quantify the impact of prolonged light reduction on seagrass morphometrics (biomass, percent cover, and shoot density). Experimental manipulations were complimented with an assessment of 46 months of light history and concurrent natural seagrass change at the study site in Gladstone Harbour. There was a clear light-dependent effect on seagrass morphometrics during seagrass growing seasons, but no effect during senescent periods. Significant seagrass declines occurred between 4 and 8 weeks after shading during the growing seasons with light maintained in the range of 4–5 mol photons m−2 d−1. Sensitivity to shading declined when applied in 2-week intervals (fortnightly) rather than continuous over the same period. Field observations were correlated to manipulative experiments to derive an applied threshold of 6 mol photons m−2 d−1 which formed the basis of a reactive light-based management strategy which has been successfully implemented to ensure positive ecological outcomes for seagrass during a large-scale dredging program.
Introduction
Seagrasses cover 38,079 km2 of habitat on Australia's east coast within the boundary of the Great Barrier Reef World Heritage Area (GBRWHA; Coles et al., 2015). Coastal seagrasses are an integral part of the health and ecosystem function of the GBRWHA and provide key habitat linkages, feeding grounds for globally threatened turtles and dugong, habitat for commercially important fisheries, sediment trapping and stabilization, effective nutrient filtering from coastal inputs, and carbon sequestration (Hemminga and Duarte, 2000; Jackson et al., 2001; Orth et al., 2006; Romero et al., 2006; Heck et al., 2008; Duarte et al., 2010). Despite being highly valued globally for their contribution to ecosystem services, seagrass habitats are threatened by a range of anthropogenic activities including coastal development and declining water quality from poor catchment management activities (Waycott et al., 2009; Grech et al., 2012; Costanza et al., 2014). Anthropogenic pressures on seagrasses are often compounded by natural events such as severe storms and flooding that may cumulatively lead to widespread seagrass decline. This has occurred on the tropical and sub-tropical east coast of Australia where severe tropical storms have contributed to widespread seagrass declines in recent years (Devlin et al., 2012; Rasheed et al., 2014).
A major cause of seagrass losses globally relates to human induced changes to the inshore environment that reduce available light, the primary driver of seagrass growth and distribution (Dennison, 1987; Duarte, 1991; Ralph et al., 2007). The risk of these types of impacts along the Great Barrier Reef (GBR) coast tends to be highest in areas where urban development and port infrastructure have a strong foothold (Grech et al., 2011). In the GBRWHA, extensive seagrass meadows commonly occur in proximity to large port facilities (Grech and Coles, 2010). Recent, well-publicized port expansions (BREE, 2012; Grech et al., 2013) place adjacent seagrass meadows under increased pressure. The capital works required for port developments can include large-scale dredging programs, which can have negative impacts on seagrass through direct burial and/or physical removal, and indirectly from turbidity plumes and the associated reduction in available light (Erftemeijer and Robin Lewis, 2006). In the GBRWHA, recent studies have shown that these plumes can have a substantial impact on seagrass (York et al., 2015). While physical damage to seagrass is relatively easy to quantify or directly avoid, it is the potential for large and persistent sediment plumes which are much harder to effectively forecast the scale of impact or to mitigate against seagrass loss.
The impact of dredge plumes are typically managed using measures not directly related to the ecological requirements of marine plants, such as reference to a background level of turbidity (Sofonia and Unsworth, 2010). Using the plant's light requirements to ensure minimal impacts is seldom attempted, largely due to a lack of understanding on what the in situ light requirements are for most seagrass species (Ralph et al., 2007). Turbidity can provide a measure of added pressure from dredging activity to the ecosystem, but does not necessarily have any direct biological relevance or account for the in-built resilience of an organism or whole system over short timescales (Sofonia and Unsworth, 2010). Adopting a direct measure of available light as a threshold for seagrass management is directly related to the plant's growth requirements making it far more preferable to turbidity.
Determining an appropriate light threshold for seagrasses involves several challenges: the light environment can be naturally highly variable over multiple timescales; plants can have dramatically different light requirements depending on time of year (Staehr and Borum, 2011); seagrasses can tolerate periods of time below their minimum light requirement without long-term impacts; and a range of other environmental parameters including water temperature and sediment chemistry can further influence in situ light requirements (Koch, 2001; Lee et al., 2007). The plant response to fluctuating light begins with explicit gene regulation driving changes in photosystems and pigment composition before growth rates and eventual plant morphology or meadow scale reductions become apparent (Abal et al., 1994; Collier C. J. et al., 2012). While laboratory experiments have helped to resolve the fundamental timeline of many of these responses (Abal et al., 1994; Collier C. J. et al., 2012; McMahon et al., 2013), the actual timeline of in situ seagrass growth dynamics is likely to be quite different due to additional extrinsic factors that cannot easily be replicated in laboratory or mesocosm trials such as nutrient availability, water temperature, hydrodynamics, epiphyte loads, water column oxygen fluxes and sediment chemistry (Carruthers et al., 2002; Waycott et al., 2005; Raun and Borum, 2013). In situ shading studies provide an empirical approach to measuring impacts of prolonged incident light attenuation and identify potential warning signs of decline in meadow-scale seagrass health as related to dredging or other anthropogenic-induced light reduction under realistic field conditions (Longstaff and Dennison, 1999; Collier C. et al., 2012).
Identifying the relevant timeframe to elicit a negative response by local seagrasses is a key component of developing a regionally-specific light threshold. Most seagrasses can tolerate periods of time below their minimum light requirement without long-term impacts (Alcoverro et al., 1999; Collier C. J. et al., 2012). Short-term re-allocation of carbon from storage tissues and adjustments to photosynthetic machinery can help bide time until conditions improve (Alcoverro et al., 2001; Cayabyab and Enríquez, 2007). A light threshold must establish the juncture at which compensatory physiological mechanisms are superseded by plant-scale declines (Collier C. J. et al., 2012). An applied light management strategy must consider the light quantity, quality and duration of light that is required to sustain local seagrass populations.
Many coastal seagrass species are well-adapted to the variable conditions that occur in a near-shore environment, including naturally turbid waters related to runoff, large tidal fluxes, complex hydrodynamics and oscillating temperatures creating constantly shifting optical and metabolic challenges (de los Santos et al., 2010; Collier et al., 2011; Petrou et al., 2013). Strategies to tolerate temporary light reduction are broadly the same for all species: adjusting light harvesting capacity and the efficiency of light use (Abal et al., 1994; Enriquez, 2005); adjustments to rates of growth and plant turnover (Collier et al., 2009; Collier C. J. et al., 2012; and drawing upon carbohydrate reserves to maintain a positive carbon balance (Burke et al., 1996; Touchette and Burkholder, 2000). While seagrasses adapted to marginal environments may be tolerant of wide fluctuations in light, they can also be acutely sensitive to reductions in light beyond the natural range of conditions (Ralph et al., 2007). When light drops below a critical level, seagrass productivity is compromised and significant physiological, biochemical and structural changes begin to take place eventually manifesting into broader meadow-scale losses with consequences for ecosystem function (Lee and Dunton, 1997; Ralph et al., 2007; Hughes et al., 2008).
Zostera muelleri ssp. capricorni is a key coastal seagrass species found along the tropical east coast of Australia (Waycott et al., 2004) and occurs in the muddy, inshore estuarine environments few other seagrass species inhabit (Lee Long et al., 1993; Carruthers et al., 2002). In port areas of the GBRWHA it is often the dominant species present, including in the Gladstone region, where it is found in monospecific intertidal meadows covering up to 40 km2 within the port limits (Thomas et al., 2010; Supplementary Figure 1). With no known functional replacement, a large-scale dieback due to a stress event such as dredging could have wider implications for the ecological success of the inshore marine community.
The goal of this study was to develop a species-specific, light threshold for the effective management of Zostera muelleri ssp. capricorni in Gladstone, Australia. Recent expansion of port infrastructure and shipping channels around Gladstone has involved large-scale dredging and the removal of ~26 million m3 of sediment over 3 years. In situ shading studies were used to elicit a response in a local seagrass population to determine a light threshold at which seagrasses will decline and over what time scale a decline is detectable in plant abundance. The approach used does not attempt to simulate a given dredging scenario but rather to apply information on how locally-adapted seagrasses withstand constant light attenuation or how regular short-term reprieves from light attenuation events affect the overall seagrass condition and its' recovery in order to better manage threats from dredging related turbidity plumes. This information was used to apply a management-based light threshold to protect seagrasses from light stress during dredging. Long-term monitoring of the seagrass meadow at an adjacent site also provided information on the status and trend of local seagrass in relation to seasonality, light history, and water temperature. The adjacent site also provides a testing ground to assess the suitability of our light threshold against seagrass condition over the long term.
Our study focused on the development of locally-relevant light thresholds that can be applied for effective management of coastal and port development activities in a way that maintains seagrass health. The term threshold, as used here, is defined as the point at which a change in external conditions causes a significant negative change in seagrass physical condition, i.e., above-ground biomass, cover, or shoot density. It is important to note that this is different to defining a minimum light requirement (MLR) for effective seagrass photosynthesis. Rather, the goal is focused around developing a biologically relevant management tool, which incorporates other local environmental drivers such as tidal cycles, seasonality and sediment chemistry dynamics that influence seagrass condition together with light in vivo.
Materials and Methods
Shading Study Experimental Design
This study was conducted at Pelican Banks, Gladstone Harbour (151° 18′ 30″E, 23° 45′ 58″S), Australia (see Supplementary Figure 1) from 2010 to 2013. At Pelican Banks the tropical sub-species Z. muelleri ssp. capricorni forms a predominantly mono-specific intertidal seagrass meadow on intertidal mud banks. Studies were carried out during two growing seasons for local seagrasses (ca. July to December) and two senescent seasons (ca. January to June) when seagrasses naturally decline with the onset of the tropical monsoon and subsequent cooler months in the austral winter (Mellors et al., 1993; McKenzie, 1994). Studies are described accordingly: growing seasons 1 and 2 (G1 and G2) and senescent seasons 1 and 2 (S1 and S2). The study location was chosen for its accessibility, semi-firm sediment composition for repeated measurements during emergence at low tide without compromising site integrity, and year-round seagrass cover to assess seasonal effects. A semi-diurnal tide cycle with a maximum range of 5 m meant seagrasses were exposed at least fortnightly, depending on the time of year.
The study site was ~30 × 20 m with experimental plots randomly assigned to each of three shade treatments or as controls (n = 4). Vertical isolation borders (sever root connection between shaded and non-shaded areas) were inserted for the shade experiments by hammering 0.25 m2 quadrats with a 0.25 m depth into the sediment until flush with the sediment surface to isolate plots where seagrass would be measured. This ensured seagrass outside of the experimental plot could not translocate nutrients/carbohydrates to seagrass within treatment plots. Plots were also “gardened” around the isolation border perimeter prior to each sampling event to prevent seagrass growing over the border and into experimental plots. Aluminium frames were secured into the sediment and covered with 1 m2 neutral density polyethelene shade cloth of varying intensities fixed 0.15 m above the sediment surface. Shade treatments were used to assess three levels of reduced light on seagrass health; high, medium and low shade, equivalent to ~15, 30, and 45% of incident benthic light, respectively. Control plots were established using quadrats with steel frames and isolation borders but without shade screens. No control was used for the effect of rhizome severing based on the work of Rasheed (1999) which found no border effect using an identical experimental design and field materials to measure shading effects on the same species. Controlling for the additional effect of shade screens on water movement was not possible without creating additional shading or fouling over control plots (see Fitzpatrick and Kirkman, 1995). Shade screens were changed and cleaned fortnightly to reduce the effects of fouling on shade treatments. Light intensities under shade treatments fluctuated with natural insolation but maintained consistent patterns among treatments and relative differences to naturally occurring benthic light, indicating that fouling of the shade screens was minimal. Shade screens were removed at the end of each experiment to track potential recovery from treatment conditions.
Experimental plots were randomly assigned to varying durations of continuous shading (between 1 and 3 months) during each seasonal study (Table 1). This variation in shading study duration and tracking of recovery was necessary to align the program with expected timeframes for managing impacts to seagrass health during dredging operations as required by managers and regulators. Therefore, comparison among seasonal studies was limited to shading durations comparable between studies. In addition, fortnightly cyclic shading was carried out during G1 to assess the impact of periodic turbidity plumes (i.e., shorter periods of reduced light and subsequent respites) on seagrass condition.
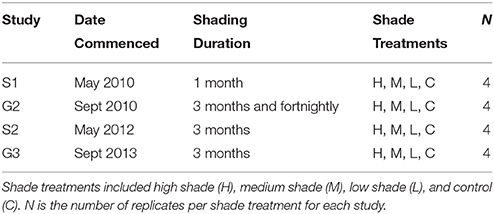
Table 1. Shading study design during senescent seasons 1 and 2 (S1, S2) and growing seasons 1 and 2 (G1, G2).
Light Climate
Light (photosynthetically active radiation, PAR) was measured within the seagrass canopy and under shade treatments using 2π cosine-corrected irradiance loggers (Submersible Odyssey Photosynthetic Irradiance Recording System, Dataflow Systems Pty. Ltd., New Zealand) calibrated using a cosine corrected Li-Cor underwater quantum sensor (LI-190SA; Li-Cor Inc., Lincoln, Nebraska USA) and corrected for immersion using a factor of 1.33 (Kirk, 1994). Loggers were deployed on site for the duration of shading and maintained using automated wiper units. Readings were made at 15 min intervals and used to measure total daily light (mol photons m−2 day−1) reaching seagrasses under each shading treatment.
Substantial tidal flux in Gladstone Harbour leads to dramatic shifts in daily light intensities on the intertidal banks due to fortnightly intertidal exposure cycles and this has the potential to control light availability to the plant (Koch and Beer, 1996). To evaluate light over a practical timeframe for measuring impacts, light data was integrated as a rolling 14 day mean of the total daily benthic light under each shading treatment, controls, as well as the long-term monitoring site (detailed below). Current understanding of seagrass response indicates under low light stress conditions, physiological adjustments first occur over a matter of days, whereas plant-scale changes take place after a number of weeks and are a reflection of the integrated light history over that period rather than short term daily fluxes (McMahon et al., 2013). This 2 week rolling average incorporated spring and neap tide conditions, variation in tide height, and the associated degree of exposure that affects the light conditions reaching the seagrass. An assessment of integrated light over a 2-week period is therefore in line with both tidally-driven fluxes in light, as well as a period of time preceding apparent morphological changes to seagrass.
Seagrass Morphometrics
Seagrass above-ground biomass, percent cover and shoot density were measured at fortnightly or monthly intervals in each treatment plot during S1 and G1 studies, while only biomass and percent cover were recorded during S2 and G2 studies. Above-ground biomass was measured using a “visual estimates of biomass” technique (Kirkman, 1978; Mellors, 1991; Rasheed, 1999). Biomass was estimated for each plot by an experienced observer recording a rank of seagrass biomass from photographs of each plot taken during sampling. Biomass ranks were assigned in reference to a series of photographs of similar seagrass habitats for which above-ground biomass has previously been measured. The same observer was used for the duration of each study to remove any inter-observer variability. At the completion of recording ranks, the observer ranked a series of additional photographs that had been previously harvested, dried, and weighed and which represented the range of seagrass biomass in the survey. A regression of ranks and biomass from these calibration quadrats was generated for each observer (r2 = 0.97; see Supplementary Figure 2) and applied to the measuring plot ranks to determine above-ground biomass estimates. Biomass ranks were then converted into above-ground biomass estimates in grams dry weight per square meter (g DW m−2). Shoot density was estimated by counting all shoots within a mini-quadrat (0.01 m2) randomly placed three times in each measuring plot except where total-plot shoot density was less than 30 shoots and all shoots were counted within the 0.25 m2 plot. Seagrass percent cover estimates were made for each plot by an observer using a standardized photo guide sheet.
Light History, Environmental Conditions and Seagrass Trend in the Meadow
A monitoring site was established in the Z. muelleri ssp. capricorni meadow adjacent to the shading study site to assess incident light and temperature at the seagrass canopy and its potential influence on seagrass meadow condition over longer time scales under natural harbor conditions. Light was recorded continuously between November 2009 and September 2013. Light loggers were deployed and operated in the same manner as in the shading studies through June 2012. From July 2012, irradiance loggers were replaced with LiCor underwater sensors with inbuilt wiper units and customized telemeted systems (Vision Environment QLD., 2013) to ensure continuous data collection and immediate availability of data during dredging operations. Water temperature was measured in the seagrass canopy (Thermodata Pty Ltd, Melbourne, Australia), daily rainfall (Bureau of Meteorology Australia1) and total hours of daytime tidal air exposure of the meadow (Maritime Safety Queensland, Department of Transport and Main Roads) were also collected.
Seagrass condition was assessed at three 50 m transects nested in two 50 x 50 m sites. Sites were selected within a relatively homogenous section of the Z. muelleri ssp. capricorni meadow. Seagrass above-ground biomass was estimated within a 0.25 m2 sampling quadrat placed at 0 m and then every 5 m along each transect (eleven sampling points per transect) using the same technique described above (observer regression of ranks, r2 = 0.95). Mean biomass was calculated for each sampling event (n = 66 quadrats) with change in biomass calculated from consecutive sampling events.
Data Analysis
All values displayed are means ± standard error (SE). Differences in morphological responses of seagrass among shading treatments and over time were assessed using repeated measures analysis of variance (rmANOVA). Data were checked for homogeneity of variance by assessing residual plots. Significant deviations from normal variance were found in G1 biomass data which were log-transformed prior to analysis. If data still did not meet the criteria, the p-value was set to 0.01 to minimize the risk of a Type I error (Underwood, 1997). For repeated measures ANOVAs, matrices were tested for sphericity using Mauchly's test. If the assumption of sphericity was not met (p < 0.05) the Greenhouse-Geisser (G-G) epsilon adjustment was applied to the numerator and denominator degrees of freedom. Differences among treatment effects at a given sampling time were compared using Tukey's post-hoc analysis. For data collected during the “recovery phase,” a one-way ANOVA was performed when a single recovery time point was measured with shading intensity as a fixed effect and tests for homogeneity of variance and transformation applied as previously described. Statistical analyses were performed using Statistica 7.0. When multiple recovery period measurements were taken, rmANOVA methods as described for the shading period were applied.
Results
Seagrass Morphometrics
Shading treatments did not have a significant effect on Z. muelleri ssp. capricorni morphology during either senescent season study (S1 and S2). However, after 1 month of shading there was a significant increase in shoot density during S1 (p < 0.05), but no significant changes in biomass or percent cover (p > 0.05, Table 2; Figures 1–3). Above-ground biomass and percent cover declined significantly over the 12 weeks of shading among all treatments during S2 (both p < 0.001); significantly lower above-ground biomass and percent cover in treatments compared to control plots; this was apparent from the start of the study (both p < 0.05, Table 2; Figures 1–2).
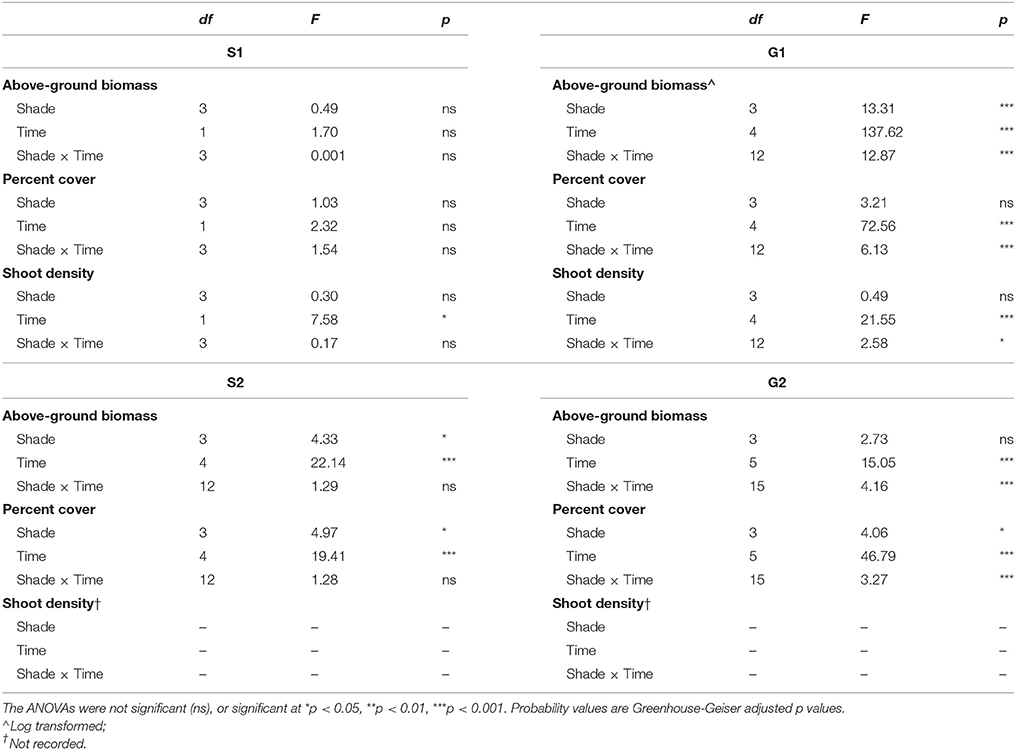
Table 2. Repeated measures ANOVA of the effects of shading treatment (among groups effect) and time (within groups effect) for biomass, percent cover and shoot density during senescent seasons 1 and 2 (S1, S2) and growing seasons 1 and 2 (G1, G2).
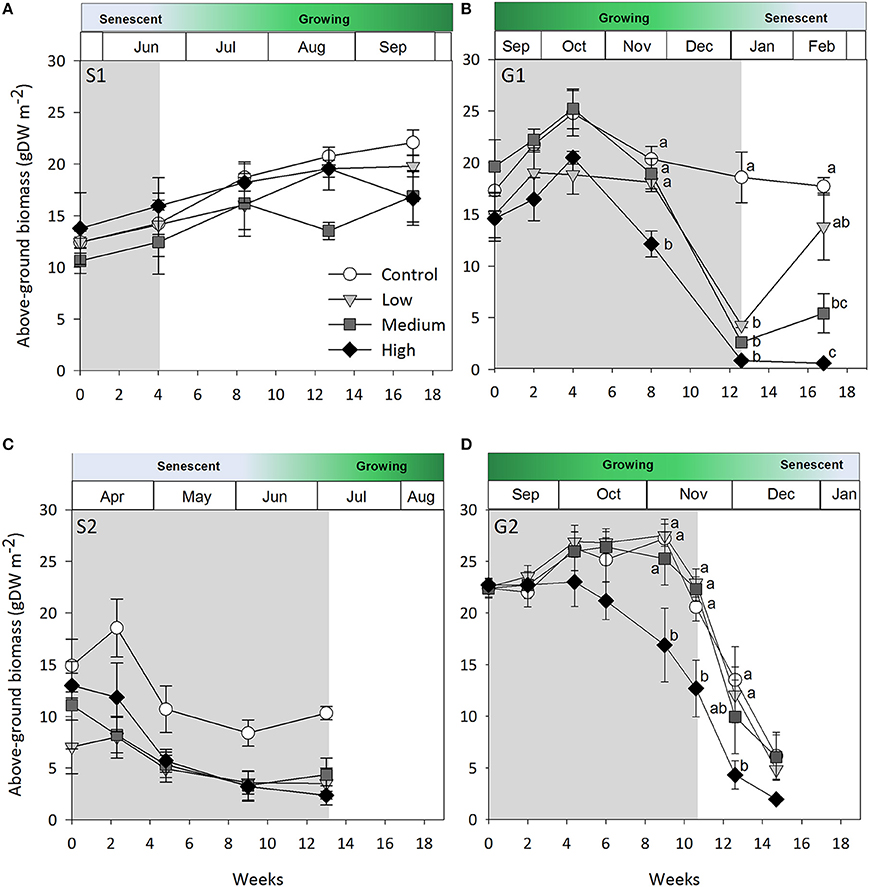
Figure 1. Seagrass above-ground biomass over time. (A) Senescent season 1 (S1); (B) growing season 1 (G1); (C) senescent season 2 (S2); (D) growing season 2 (G2). Grayed area represents shading periods and white area represents monitored recovery periods where data was recorded. Data represent mean ± SEM (n = 4). Superscripted identical letters indicate no significant difference among shading treatment (control, low, medium, high) at p < 0.05 (Tukey's post-hoc test).
Shading had a detrimental effect on Z. muelleri ssp. capricorni above-ground biomass during the growing seasons (G1 and G2, shade × time interaction p < 0.001, Table 2; Figure 1). During both growing season studies, biomass was significantly lower by the 8 week sampling under high shade treatments compared to controls and other treatments (Figure 1). This occurred between 4 and 8 weeks in G1 and 6 and 8 weeks in G2. There was significant loss of above-ground biomass under all treatments compared to control plots by 12 weeks during G1, including near total loss of above-ground biomass under high shade plots (Figure 1B). Within 4 weeks of shade removal, above-ground biomass under low shade treatments recovered to control levels, whereas biomass under medium and high shade treatments remained significantly lower than control plots (p < 0.001; Figure 1B). Control plots did decline somewhat from a peak at 4–16 week measurements, likely due to the onset of characteristic seasonal senescence which occurred toward the end of the study (Jan–Feb 2011). Similarly, above-ground biomass under high shade was significantly lower than under control, low and medium shade treatments by 8 weeks of shading during G2. Declines in above-ground biomass and percent cover from mid-November in G1 and G2 across controls and all treatment plots are consistent with seasonal declines with the onset of the senescent season (Figures 1B,D, 2B,D).
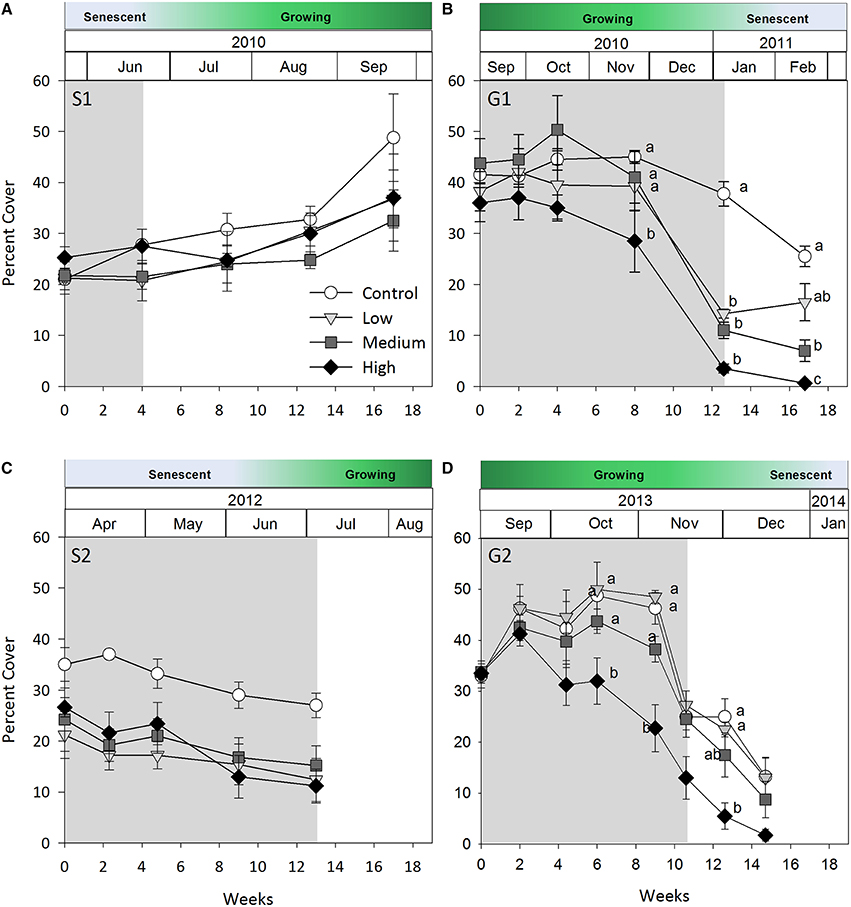
Figure 2. Seagrass percent cover over time. (A) Senescent season 1 (S1); (B) growing season 1 (G1); (C) senescent season 2 (S2); (D) growing season 2 (G2). Grayed area represents shading periods and white area represents monitored recovery periods where data was recorded. Data represent mean ± SEM (n = 4).
Negative effects of shading on percent cover during both growing seasons were similar to those recorded for above-ground biomass (both p-values for shade × time interaction < 0.001, Table 2; Figure 2). Percent cover was significantly lower under high shade treatments compared with control, low and medium shade treatments for G1and G2 within 8 and 6 weeks, respectively, (Figures 2B,D). Within 12 weeks percent cover under all shade treatments was significantly lower than control plots during G1 (Figure 2B). Recovery of seagrass during G1to a percent cover similar to control plots occurred within 4 weeks of shades being removed for the low shade treatment, but there were no similar signs of recovery for treatments that had been under medium or high shade treatment (Figure 2B; Table 3). Percent cover of seagrass under high shade similarly demonstrated no sign of recovery 2 weeks following shade removal during G2 (Figure 2D; Table 3). High shade plots were nearly devoid of seagrass cover 4 weeks after shade removal for G1 and G2 (Figures 2B,D).
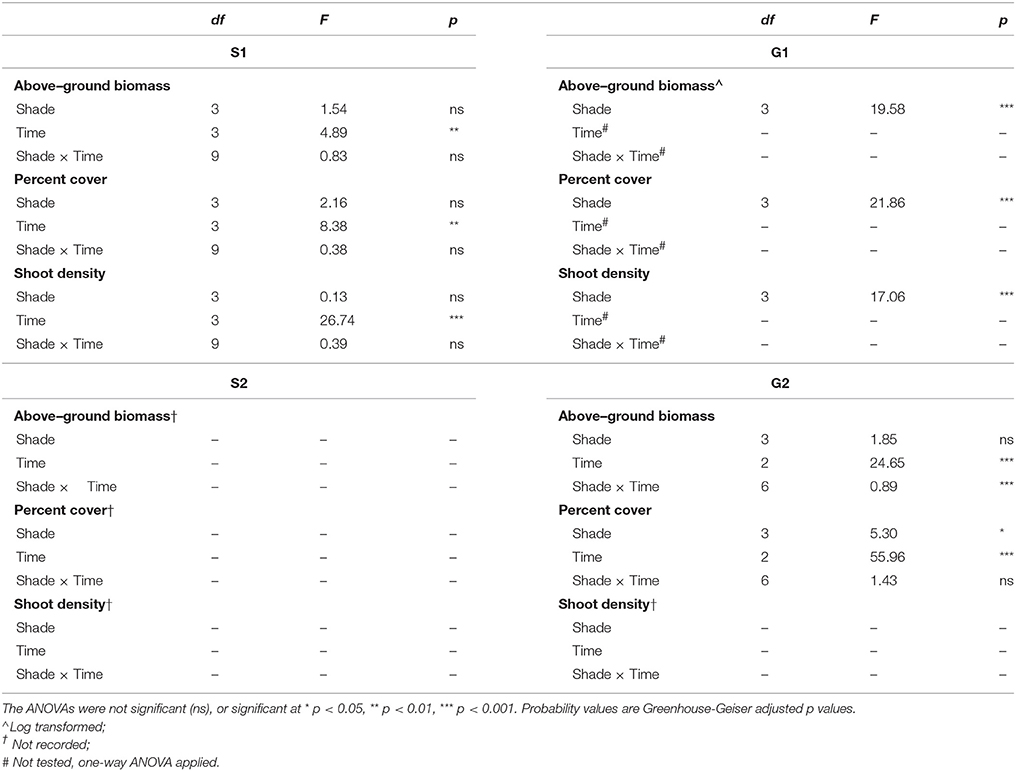
Table 3. Repeated measures and one-way ANOVA of recovery from shading treatments (among groups effect) and time (within groups effect) for biomass, percent cover and shoot density during senescent seasons 1 and 2 (S1, S2) and growing seasons 2 (G2).
Shoot density was less sensitive to shading than percent cover and above-ground biomass. Seagrass shoot density decreased significantly by 12 weeks under the high shade treatment compared with control and low shade treatment plots during the growing season (G1 study, shading x time interaction p < 0.05, Table 2; Figure 3B). There were no signs of recovery to control levels 4 weeks after shades were removed (Figure 3B). Shading had no significant effect on temporal fluctuations in shoot density during the senescent season (S1 study, p > 0.05, Table 2; Figure 3A).
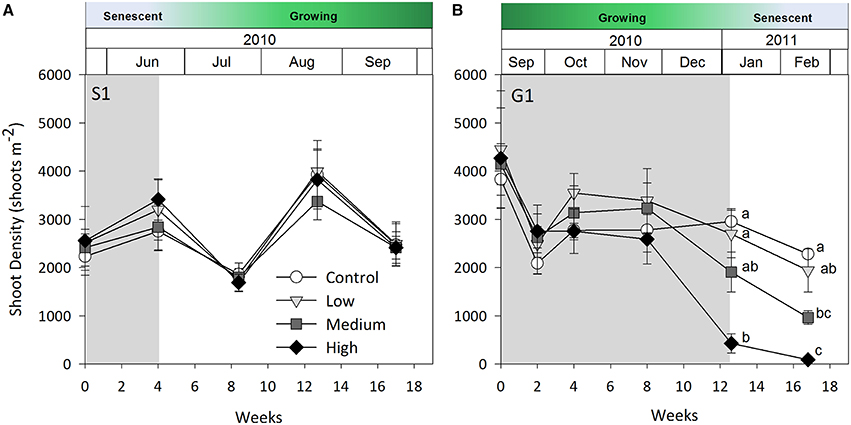
Figure 3. Seagrass shoot density over time. (A) Senescent season 1 (S1); (B) growing season 1 (G1); note shoot density not recorded during S2 or G2 (see Results). Grayed area represents shading periods and white area represents monitored recovery periods where data was recorded. Data represent mean ± SEM (n = 4).
Seagrass was less sensitive to fortnightly cyclic shading than to continuous shading when tested during G1. Above-ground biomass data is only presented, but shoot density and percent cover results were analogous. Above-ground biomass under all shade treatments was similar to control plots for the first 8 weeks of the study; however, by week 12 biomass under all shade treatments was equally and significantly lower than under control plots (two-way rmANOVA, shade x time interaction, p < 0.01, Figure 4). After 4 additional weeks without shading (weeks 12–16), no biomass recovery occurred under high shade treatments relative to controls (p < 0.05). While seagrass loss was delayed under cyclic shading, the magnitude of impact of these treatments was similar to those found under continuous shading after 12 weeks.
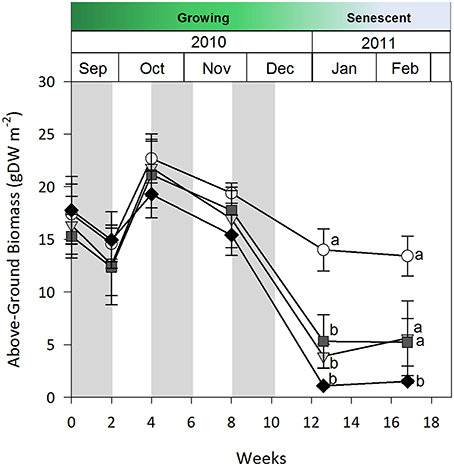
Figure 4. Seagrass above-ground biomass over time during the 2-week cyclic shading experiment in growing season 1 (G1). Grayed area represents shading periods and white area represents monitored recovery periods where data was recorded. Data represent mean ± SEM (n = 4).
Above-ground biomass and percent cover in control plots throughout all studies was similar to that measured at the nearby long-term monitoring site (see Figure 6) indicating no effect of the physical presence of frames holding shade screens otherwise on the experiment.
Light Climate in Relation to Morphometric Results
During both senescent season studies (S1 and S2), light levels were strongly attenuated under all shade treatments compared to controls, while no measured loss of seagrass biomass, percent cover or shoot density was recorded after 4 and 13 weeks, respectively, when shades were in place (Figures 5A,C). Light intensities measured under S1 and S2 shades were generally between 2 and 6 mol photons m−2 d−1, a similar range recorded during the G1 study under the same shading treatments.
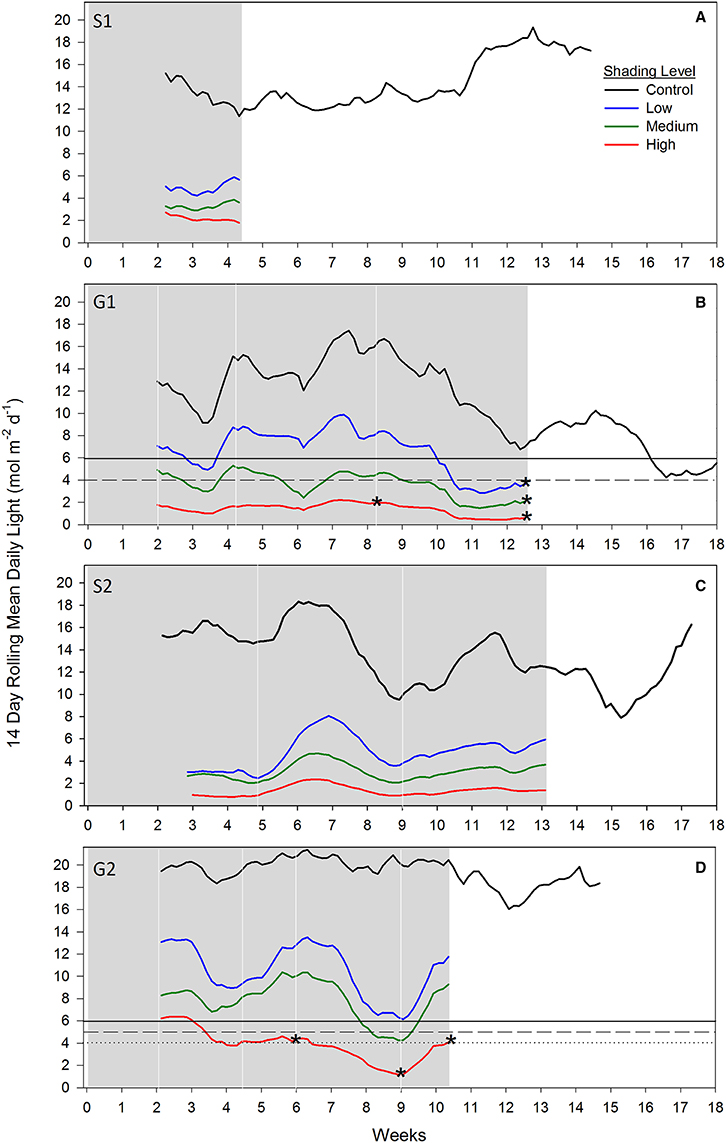
Figure 5. Fourteen day rolling mean benthic light recorded under shade treatments across four shading studies. (A) Senescent season 1 (S1); (B) growing season 1 (G1); (C) senescent season 2 (S2); (D) growing season 2 (G2). Grayed area represents when shades were over experimental plots and white area when shades were removed. White vertical lines indicate sampling days; asterisks overlaying shade treatment light data indicates a significant reduction in seagrass above-ground biomass and percent cover relative to control for that sampling event (percent cover only for week 6 in G2); dashed lines indicate a biologically significant light threshold based on shading study results; solid black lines denote the derived management light threshold.
During the first growing season (G1), light intensities under the high shade treatment measured consistently below 2 mol photons m−2 d−1 leading to significant declines in above-ground biomass and percent cover recorded by 8 weeks (Figure 5B). Light remained at or below 2 mol photons m−2 d−1 for the remaining 4 weeks of shading over which time seagrass was completely lost from high shaded plots. Light under medium shade treatments was higher and more variable over the course of G1, but generally stayed above 4 mol photons m−2 d−1 for the initial 10 weeks of the study, while light under low shades remained above 6 mol photons m−2 d−1 during the same period. Light declined between weeks 10 and 12 of the experiment across controls and all treatments during a period of high rainfall in November and December 2010 (Australian Bureau of Meteorology2). Light levels were consistently below 4 mol photons m−2 d−1 under all shade treatments in the fortnight leading up to the 12 week sampling event, when biomass and percent cover were significantly lower for all treatments compared with control plots (Figure 5B). Four subsequent weeks with shades removed (recovery; weeks 12–16) were insufficient reprieve for biomass, percent cover or shoot density to recover under medium and high shade treatments while low shade treatments recovered when returned to ambient light conditions (Figures 1B, 2B, 3B).
During the second growing season (G2), light under high shaded plots was less than 5 mol photons m−2 d−1 in the fortnight leading up to detection of a significant decline in seagrass percent cover at 6 weeks (Figure 5D). Light declined further to <4 mol photons m−2 d−1 for the fortnight leading up to sampling at 9 weeks, when significant declines in percent cover and above-ground biomass were detected. Light under low and medium shade treatments mostly stayed above 5 mol photons m−2 d−1 for the duration of the G2 shading study; one exception was when light dropped below 5 mol photons m−2 d−1 under medium shade for ~1 week at week 9; although with no detectable change in seagrass biomass or percent cover recorded. In contrast, significant declines in seagrass biomass and/or percent cover were recorded following more prolonged periods of light < 5 mol photons m−2 d−1 under high shade treatments at weeks 6, 9, and 10.
Climate History and Seagrass Trend
From September 2009 to September 2013, seagrass above-ground biomass at the monitoring site followed a typical oscillating seasonal pattern. Z. muelleri ssp. capricorni reached maximum biomass between October and December each year which coincided with higher water temperatures and ambient light (Figure 6). Light levels in the meadow were relatively high during the growing season which paralleled net positive growth. Light intensities remained above 8 mol photons m−2 d−1; well above the levels at which significant impacts were measured under shade treatments. Annual seagrass senescence began at approximately the start of the year when temperatures consistently reached >30°C in the meadow and the onset of rain and flooding events led to reductions in light (Figure 6). The relationship between seagrass above-ground biomass and mean maximum daily water temperature for the month prior to sampling in the growing period likewise indicated water temperature correlated with seagrass biomass (p < 0.01, r2 = 0.55) until water temperature exceeded 30°C and seagrass declined, despite high light intensities over the same period. Seagrass abundance typically reached a minimum by April/May after which a return to growth and increased seagrass biomass was observed around July each year.
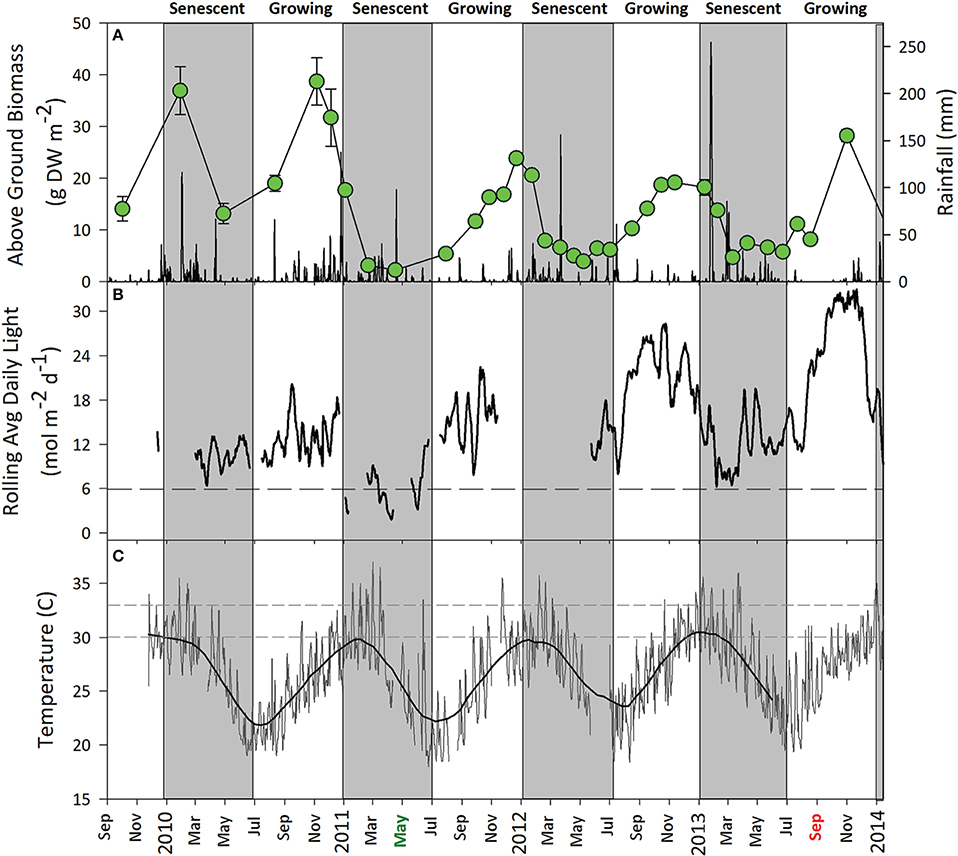
Figure 6. Environmental conditions in the Zostera muelleri ssp. capricorni meadow, September 2009 to July 2013. (A) Mean ± SE seagrass percent cover (open circles) and daily rainfall. (B) Rolling mean total daily benthic light with trialed species- and region-specific light threshold for Z. muelleri ssp. capricorni (dashed line). (C) Daily maximum water temperature with critical temperature threshold for Z. muelleri ssp. capricorni (Collier et al., 2011) (dashed line). Grayed areas represent indicative senescent periods (~Jan-July) for local seagrasses; however, the onset of senescence and return to growing periods is environmentally driven rather than a fixed date. Dredging activity in Gladstone Harbour is indicated on the x-axis starting in May 2011 (green) and finishing in September 2013 (red).
Discussion
Z. muelleri ssp. capricorni condition (biomass, shoot density and percent cover) was measurably driven by light reductions tested during the growing seasons but was unaffected by a reduction in light applied during either senescent season. Similar field shading experiments have demonstrated time-of-year is a critical factor in defining the magnitude of the plant's response to reduced light conditions, linked to seasonal light and water temperatures (Lavery et al., 2009). We found that Z. muelleri ssp. capricorni declined in the growing season when light was ≤ 5 mol quanta m−2 d−1 for periods of time exceeding 4 weeks. This was successfully used to develop a conservative management threshold to protect seagrasses during dredging operations by maintaining light levels above 6 mol quanta m−2 d−1.
The significant and consistent decline in Z. muelleri ssp. capricorni during the growing season shading studies highlights the sensitivity of this species during its period of peak productivity and expansion. Z. muelleri ssp. capricorni carbon fixation and above-ground biomass have been shown to significantly decline when grown under saturating or limiting light levels in conjunction with extreme temperatures (>33°C; Collier et al., 2011) and for temperate Z. muelleri when grown under 30°C conditions (York et al., 2013). Similar results have been found for the congeneric northern hemisphere species, Zostera marina, with summertime declines coinciding with low light and high temperatures (Zimmerman et al., 1989; Olesen and Sand-Jensen, 1993).
The high metabolic demand that comes with warmer conditions was typically supported by higher light (approximately July to December) at our study site (Figure 6). This likely allowed an increase in photosynthetic processes to keep up with rising seasonal temperatures up until a point, after which respiration would continue to increase without a concomitant increase in photosynthesis (Bulthuis, 1987; Lee et al., 2007). When such an imbalance occurs this can lead to die-off, whether seasonal or driven by episodic reductions in light. It was likely that Z. muelleri ssp. capricorni was not meeting its metabolic requirements during these warmer months when subjected to reduced light levels, leading to a dieback under our shading treatments. Similar trends were seen at our permanent monitoring location adjacent to the study site where seasonal cycles of seagrass growth and decline paralleled temperature and light regimes (Figure 6).
Seasonal seagrass growth rates are closely linked to light and temperature patterns (Lee et al., 2007). Intertidal Z. muelleri ssp. capricorni meadows along the Queensland coast follow typical seasonal fluctuations in condition linked to light, temperature and tidal exposure (Mellors et al., 1993; McKenzie, 1994; Carruthers et al., 2002; Petrou et al., 2013). From August to December, clearer waters and warmer temperatures spur rapid growth and expansion of seagrass meadows in the Gladstone region before typical dieback in late austral summer with the onset of high temperatures and wet season conditions.
The lack of a low light response in the senescent season could be due to a decrease in extrinsic energy requirements due to the lower seagrass standing crop and preferential use of carbohydrate reserves to support seagrass metabolic requirements (Burke et al., 1996; Touchette and Burkholder, 2000). Lavery et al. (2009) also found shading imposed over winter did not produce morphological changes; in contrast to their late summer results. They associated the effect of temperature on gross photosynthetic requirements of the plant to explain the disparity in seasonal effects. The saturating irradiance for photosynthesis (Ik) and respiration typically increase with temperature (Masini and Manning, 1997; Lee et al., 2007) equating to higher overall light requirements during summer growing periods compared to cooler months.
When light levels are sufficient, carbohydrate reserves are enhanced which help offset periods of high light attenuation by supporting short-term energy demands of the plant. In the first growing season study, medium shaded plots were not measurably affected until the 12 week sampling event and did not recover from losses within 4 weeks. While light under medium shaded plots during the first 10 weeks (4–5 mol photons m−2 d−1) sustained Z. muelleri ssp. capricorni in vivo, it was likely near its' light requirement limit and may have exhausted energy reserves, making recovery unachievable in the short-term once shades were removed. Alternatively, light during G1 under low shaded plots, which received by and large > 6 mol photons m−2 d−1 during the study, likely enabled excess energy to be stored in the plant and used to support recovery when shades were removed. These differences in treatment response illustrate that conditions leading up to an acute stress event are important in determining recovery success. Ensuring light is maintained at a level that not only sustains seagrass cover, but also provides energy reserves to be maintained or increased when conditions are good is likely important to ensure short-term stress events do not push the plant past a point of no return.
The quality of the light environment reaching seagrasses may be as important as the quantity of light received. Dredging, for example, typically increases particulate matter in the water column which affects spectral quality (Kirk, 1994). The size and type of particles re-suspended by dredging activity alter PAR transmission in a non-linear manner, with some wavelengths being more attenuated than others, resulting in a reduced light environment with a shift toward yellow wavelengths (Kirk, 1994; Gallegos et al., 2009). Therefore, a light threshold value used for monitoring seagrass health during a dredging campaign, as determined according to the full PAR spectrum available, may overestimate the actual light available for photosynthesis as PAR measurements do not distinguish spectral shifts (Van Duin et al., 2001; Zimmerman, 2003). Light quality in Gladstone waters has explicit spatial variability, with broader spectral transmission in the outer harbor compared to the inner harbor, yet dredging had no effect on these spectral signatures when measured during the dredging campaign that occurred during this study (Chartrand et al., 2012). The region is naturally highly turbid and therefore already exhibits a yellow-enhanced light signature due to the particle load in the water column and was not further skewed with additional sediment re-suspension from the dredge operation. While a more accurate threshold applying photosynthetic usable radiation (PUR) in place of PAR could resolve any effects of wavelength-specific water column absorption we did not need to alter light threshold values to incorporate spectral shifts from dredging in this instance.
Short term repeated shading and respite (fortnightly) in the present study was carried out to mimic repeated acute attenuation events from turbidity plumes followed by subsequent “relief” intervals. In providing a 14 day period of respite after shading was applied, Z. muelleri ssp. capricorni appeared to cope for 12 weeks with even the highest shade treatment, which had significantly impacted treatment plots shaded continuously after only 6–8 weeks. A study by Biber et al. (2009) also explored extreme attenuation events interspersed with recovery periods of varying length. They found that recovery intervals at least equal to the period of light deprivation were essential for long term survival.
Other investigations into in situ light requirements on Zostera spp. agree with the measured light effects and management threshold derived in this study (Dennison and Alberte, 1985; Moore et al., 1997; Thom et al., 2008; Collier C. J. et al., 2012). Collier C. J. et al. (2012) tested reduced light conditions during laboratory shading experiments on Z. muelleri ssp. capricorni also collected from Gladstone Harbour and found shoot density declined after 8.7 weeks under 4.4 mol photons m−2 d−1 and 10.6 weeks under 9.5 mol photons m−2 d−1. For the congeneric Z. marina, Dennison and Alberte (1985) found a significant reduction in Z. marina production rates with average daily scalar light levels of ~3.7 mol photons m−2 d−1 under shades compared to unshaded controls (8 mol photons m−2 d−1) during critical summer growing conditions. Moore et al. (1997) found similar results where sites with high light attenuation (2.7 mol photons m−2 d−1) over 30 days was lethal to Z. marina transplants compared to those with higher water clarity (13.4 mol photons m−2 d−1). More recent work on Z. marina found light requirements for long-term survival is 3 mol photons m−2 d−1 and at least 7 mol photons m−2 d−1 for non-light-limiting growth conditions during critical growing months (Thom et al., 2008).
Deriving a Light Threshold for Management
Developing effective management tools and appropriate mitigation strategies to protect seagrasses from a large-scale dredging campaign requires information on the distribution, light requirements and tolerances of local seagrass communities. Shading studies and the 4-year seagrass and light monitoring program provided the means to develop an effective and ecologically-derived management threshold. A 14 day integrated daily light value was used to establish a light threshold, which if maintained, would allow sufficient light to maintain local Z. muelleri ssp. capricorni seagrass condition in Gladstone Harbour during dredging.
With no significant effects of shading on seagrass growth during either of the senescent seasons, a seagrass light management threshold was only defined for the growing season when Z. muelleri ssp. capricorni was sensitive to shading treatments. Both growing season studies clearly indicated light below 4 mol photons m−2 d− is insufficient to maintain seagrass growth and or survival. In the second growing season study, light levels 2 weeks prior to a decline in seagrass measured between 4 and 5 mol photons m−2 d−1, indicating morphological changes in Z. muelleri ssp. capricorni can take place in Gladstone at light intensities of ≤ 5 mol photons m−2 d−1.
While the time to measurable loss in the first growing season was between 4 and 8 weeks, more frequent sampling during the second growing season documented appreciable declines in seagrass cover as early as 6 weeks under light limiting conditions. A study by Adams et al. (2015) found the timeframe over which light history and Z. muelleri above-ground biomass best correlated was from 8 to 35 weeks, however, they recognized management actions also should be triggered well before these measured reductions in biomass occur.
A range of bioindicators have been reviewed for use in seagrass monitoring programs to measure environmental pressures such as dredging (McMahon et al., 2013). While some metrics may be more sensitive on shorter time scales (e.g., rhizome sugars or ETRmax) to changes in the light climate (reviewed in McMahon et al., 2013), the ability to measure changes rapidly in relation to anthropogenic pressures (i.e., dredge operations) is important to apply an appropriate and timely management response. In the current study, above-ground abundance (either biomass or percent cover) reacted to light conditions within a timeframe that would allow a management response to be applied that could abate seagrass loss (i.e., move dredge to a new location), whereas shoot density was less sensitive to attenuated light. Other studies have also found shoot density to be a less sensitive metric; Z. muelleri ssp. capricorni alters leaf morphology before shoot loss under reduced light treatments, making above-ground biomass or cover a more sensitive indicator of change than shoot density as a consequence of environmental conditions (Rasheed, 1999; Collier C. J. et al., 2012).
As a conservative approach to protecting seagrass, a management light threshold needed to provide >5 mol photons m−2 d−1 with some degree of buffer from potential impact to the plants and to ensure the plants not only maintained physical presence, but could generate energy stores. The threshold needed to ensure protection of seagrasses from deteriorating light conditions, while also having a credible fit with natural background light variability within the local meadow. If the threshold value was set too high and therefore routinely breached without measureable impacts to seagrass condition, it would be ineffective as a management tool. Conversely, a value too low that was never measured in situ in spite of concurrent declines in seagrass cover would likewise be inappropriate. A light threshold of 6 mol photons m−2 d−1 was therefore used in a compliance framework by government regulators and management authorities to prevent measurable loss of seagrass from dredge related light attenuation in required management zones during dredging activity in Gladstone Harbour. This light threshold was considered in parallel with turbidity monitoring to ensure effects of turbidity related to the dredge vs. background conditions could be resolved (GPCL, 2012b). During the dredging campaign light was maintained above the management threshold for the growing season at all of the prescribed seagrass management zones (GPCL, 2012a). This coincided with the presence of the largest seagrass meadows in the greater region during and post-dredging (Carter et al., 2015) and provides confidence that the approach used could be applied elsewhere for managing seagrasses.
While much research is focused on quantifying seagrass light requirements (Dennison, 1987; Staehr and Borum, 2011; Collier et al., 2016), this work has focused on the application of seagrass light requirements for use in a management setting of a large-scale dredging program. The absolute threshold value detailed here is not as critical as the approach used to derive a light-based model for seagrasses. The successful approach developed could readily be applied in other settings with sufficient knowledge of local seagrass dynamics and light conditions.
A range of additional measures would further improve the use of light thresholds to effectively manage seagrasses during dredging and other anthropogenic activities impacting on the light environment:
1. Combine threshold assessments with effective sub-lethal bio-indicators of light stress-A bioindicator that responds over days rather than weeks, and prior to actual physical declines in the plant, would dramatically improve the reaction time for management decisions to adjust dredging activities before declines occur. McMahon et al. (2013) identified a range of indicators that may be useful to measure sub-lethal changes, however, most still require substantial processing time. An indicator would ideally be measured and processed within 24–48 h for effective reactive management of dredging operations. Progress toward developing molecular indicators of sub-lethal seagrass light stress provides the most promising approach (Macreadie et al., 2014).
2. Further investigations of the effect of water temperature-Temperature is a known driver of temperate seagrass meadow dynamics and plant metabolism (Zimmerman et al., 1989; Olesen and Sand-Jensen, 1994; Staehr and Borum, 2011). However, the role of seasonally-driven temperature fluctuations on tropical seagrasses is inadequately described (McKenzie, 1994; Rasheed and Unsworth, 2011) despite work showing temperature governs the light intensity needed for a net carbon balance (Lee et al., 2007; Collier et al., 2011). Such effects need to be studied in other species and in greater detail to understand how temperature may act as a secondary driver of seagrass light thresholds for management.
3. Research on the impacts of whole plant dynamics on light requirements-Recent work has implicated cascade effects of reduced light on degradation of below-ground structures and the surrounding micro-environment (Terrados et al., 1999; Borum et al., 2006; Koren et al., 2015). Compromising below-ground root/rhizome integrity has negative implications for meadow resilience and the ability to resist short-term stresses (Vonk et al., 2015). Understanding whole plant dynamics and how light reduction affects oxygen transport and below ground viability is vital to understand whether thresholds are in line with whole plant coping strategies.
4. Modification of light requirements under cumulative long-term impacts-Poor water quality prior to a major development may exacerbate efforts to manage additional impacts on already chronically stressed seagrass. Prolonged physiological strain from cumulative pressure over time may alter the plant's capacity to cope with further reduced light and may influence the light levels required for recovery.
Conclusion
This study characterized the tolerance of Z. muelleri ssp. capricorni to light attenuation on an intra- and inter-annual cycle using in situ shading studies and light history monitored over a 4-year period. This information was used to develop a locally-relevant management plan to protect seagrasses from dredging-related impacts to the light environment. A light threshold of 6 mol photons m−2 d−1 was successfully trialed as part of a compliance program for mitigating dredging impacts. This minimized the risk that Z. muelleri ssp. capricorni, the dominant local species, was affected by dredge turbidity plumes within prescribed management zones. When implementing a light management strategy it is critical that local conditions, species and context are considered.
Author Contributions
KC, MR, CB, and PR together designed the research project. KC led the study and drafted the manuscript with the assistance of MR and AC. CB and AC provided major assistance in field execution and data analysis. All co-authors commented on and approved the final manuscript draft.
Conflict of Interest Statement
Funding for this research came from two industry bodies as detailed in the Funding Statement, creating a perceived conflict of interest. However, all data, results, analysis and conclusions were delivered through an independent government-mandated Dredge Technical Review Panel with a suite of scientific experts and engineers appointed to establish potential impacts of dredging on local seagrasses and to implement (as mandated under permit approvals) a light-based approach to dredge management.
Acknowledgments
We would like to thank Leonie Andersen and Vision Environment Pty Ltd for light data and assistance in maintaining field equipment. We would also like to acknowledge Brett Kettle from Babel-Sbf Pty Ltd and Queensland Gas Corporation who initially supported and commissioned this research. Further funding and support was provided by Gladstone Ports Corporation Pty Ltd and Australian Research Council Grant LP110200454. We thank James Cook University TropWATER staff for field support and data collection and K Petrou and I Jimenez for their invaluable input in the field and larger research program.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/article/10.3389/fmars.2016.00106
Footnotes
References
Abal, E. G., Loneragan, N., Bowen, P., Perry, C. J., Udy, J. W., and Dennison, W. C. (1994). Physiological and morphological responses of the seagrass Zostera capricorni Aschers, to light intensity. J. Exp. Mar. Biol. Ecol. 178, 113–129. doi: 10.1016/0022-0981(94)90228-3
Adams, M., Ferguson, A., Collier, C., Baird, M., Gruber, R., and O'Brien, K. (2015). “Assessment of light history indicators for predicting seagrass biomass,” in 21st International Congress on Modelling and Simulation (Gold Coast, QLD), 1303–1309.
Alcoverro, T., Manzanera, M., and Romero, J. (2001). Annual metabolic carbon balance of the seagrass Posidonia oceanica: the importance of carbohydrate reserves. Mar. Ecol. Prog. Ser. 211, 105–116. doi: 10.3354/meps211105
Alcoverro, T., Zimmerman, R. C., Kohrs, D. G., and Alberte, R. S. (1999). Resource allocation and sucrose mobilization in light-limited eelgrass Zostera marina. Mar. Ecol. Prog. Ser. 187, 121–131. doi: 10.3354/meps187121
Biber, P. D., Kenworthy, W. J., and Paerl, H. W. (2009). Experimental analysis of the response and recovery of Zostera marina (L.) and Halodule wrightii (Ascher) to repeated light-limitation stress. J. Exp. Mar. Biol. Ecol. 369, 110–117. doi: 10.1016/j.jembe.2008.10.031
Borum, J., Larkum, A. W. D., Orth, R. J., Duarte, C. M., Sand-Jensen, K., Binzer, T., et al. (2006). “Oxygen Movement in Seagrasses,” in Seagrasses: Biology, Ecology and Conservation (Springer Netherlands), 255–270.
BREE (2012). Australian Bulk Commodity Export and Infrastructure: Outlook to 2025. Canberra, CN: Australian Department of Resources; Energy and Tourism Vision Environment.
Bulthuis, D. A. (1987). Effects of temperature on photosynthesis and growth of seagrasses. Aquat. Bot. 27, 27–40. doi: 10.1016/0304-3770(87)90084-2
Burke, M., Dennison, W., and Moore, K. (1996). Non-structural carbohydrate reserves of eelgrass Zostera marina. Mar. Ecol. Prog. Ser. 137, 195–201. doi: 10.3354/meps137195
Carruthers, T. J. B., Dennison, W. C., Longstaff, B. J., Waycott, M., Abal, E. G., McKenzie, L. J., et al. (2002). Seagrass habitats of Northeast Australia: models of key processes and controls. Bull. Mar. Sci. 71, 1153–1169.
Carter, A., Jarvis, J. C., Bryant, C. V., and Rasheed, M. A. (2015). Development of Seagrass Indicators for the Gladstone Healthy Harbour Partnership Report Card, ISP011: Seagrass. Publication 15/29 ed (Cairns: Centre for Tropical Water & Aquatic Ecosystem Research).
Cayabyab, N. M., and Enríquez, S. (2007). Leaf photoacclimatory responses of the tropical seagrass Thalassia testudinum under mesocosm conditions: a mechanistic scaling−up study. New Phytol. 176, 108–123. doi: 10.1111/j.1469-8137.2007.02147.x
Chartrand, K. M., Ralph, P. J., Petrou, K., and Rasheed, M. A. (2012). Development of a Light-Based Seagrass Management Approach for the Gladstone Western Basin Dredging Program. Cairns, QLD: Fisheries Queensland.
Coles, R. G., Rasheed, M. A., McKenzie, L. J., Grech, A., York, P. H., Sheaves, M., et al. (2015). The great barrier reef world heritage area seagrasses: managing this iconic Australian ecosystem resource for the future. Estuar. Coast. Shelf Sci. 153, A1–A12. doi: 10.1016/j.ecss.2014.07.020
Collier, C., Adams, M., Langlois, L., Waycott, M., O'Brien, K., Maxwell, P., et al. (2016). Thresholds for morphological response to light reduction for four tropical seagrass species. Ecol. Indic. 67, 358–366. doi: 10.1016/j.ecolind.2016.02.050
Collier, C. J., Lavery, P. S., Ralph, P. J., and Masini, R. J. (2009). Shade-induced response and recovery of the seagrass Posidonia sinuosa. J. Exp. Mar. Biol. Ecol. 370, 89–103. doi: 10.1016/j.jembe.2008.12.003
Collier, C. J., Uthicke, S., and Waycott, M. (2011). Thermal tolerance of two seagrass species at contrasting light levels: implications for future distribution in the Great Barrier Reef. Limnol. Oceanogr. 56, 2200–2210. doi: 10.4319/lo.2011.56.6.2200
Collier, C. J., Waycott, M., and Ospina, A. G. (2012). Responses of four Indo-West Pacific seagrass species to shading. Mar. Pollut. Bull. 65, 342–354. doi: 10.1016/j.marpolbul.2011.06.017
Collier, C., Waycott, M., and McKenzie, L. (2012). Light thresholds derived from seagrass loss in the coastal zone of the northern Great Barrier Reef, Australia. Ecol. Indic. 23, 211–219. doi: 10.1016/j.ecolind.2012.04.005
Costanza, R., de Groot, R., Sutton, P., van der Ploeg, S., Anderson, S. J., Kubiszewski, I., et al. (2014). Changes in the global value of ecosystem services. Glob. Environ. Change 26, 152–158. doi: 10.1016/j.gloenvcha.2014.04.002
de los Santos, C., Brun, F., Bouma, T., Vergara, J., and Perez-Llorens, J. (2010). Acclimation of seagrass Zostera noltii to co-occurring hydrodynamic and light stresses. Mar. Ecol. Prog. Ser. 398, 127–135. doi: 10.3354/meps08343
Dennison, W. C. (1987). Effects of light on seagrass photosynthesis, growth and depth distribution. Aquat. Bot. 27, 15–26. doi: 10.1016/0304-3770(87)90083-0
Dennison, W. C., and Alberte, R. S. (1985). Role of daily light periodin the depth distribution of Zostera marina (eelgrass). Mar. Ecol. Prog. Ser. 25, 51–61. doi: 10.3354/meps025051
Devlin, M., Brodie, J., Wenger, A., da Silva, E., Alvarez-Romero, J., Waterhouse, J., et al. (2012). “Chronic and acute influences on the Great Barrier Reef: Impacts of extreme weather conditions,” in Proceedings of the 12th International Coral Reef Symposium (Cairns, QLD), 9–13.
Duarte, C. M. (1991). Seagrass depth limits. Aquat. Bot. 40, 363–377. doi: 10.1016/0304-3770(91)90081-F
Duarte, C. M., Marbà, N., Gacia, E., Fourqurean, J. W., Beggins, J., Barrón, C., et al. (2010). Seagrass community metabolism: assessing the carbon sink capacity of seagrass meadows. Glob. Biogeochem. Cycles 24, GB4032. doi: 10.1029/2010GB003793
Enriquez, S. (2005). Light absorption efficiency and the package effect in the leaves of the seagrass Thalassia testudinum. Mar. Ecol. Prog. Ser. 289, 141–150. doi: 10.3354/meps289141
Erftemeijer, P. L., and Robin Lewis, R. R. (2006). Environmental impacts of dredging on seagrasses: a review. Mar. Pollut. Bull. 52, 1553–1572. doi: 10.1016/j.marpolbul.2006.09.006
Fitzpatrick, J., and Kirkman, H. (1995). Effects of prolonged shading stress on growth and survival of seagrass Posidonia australis in Jervis Bay, New South Wales, Australia. Mar. Ecol. Prog. Ser. 127, 279–289. doi: 10.3354/meps127279
Gallegos, C. L., Kenworthy, W. J., Biber, P. D., and Wolfe, B. S. (2009). Underwater spectral energy distribution and seagrass depth limits along an optical water quality gradient. Smithson. Contrib. Mar. Sci. 38, 359–368.
GPCL (2012a). Gladstone Ports Corporation Limited [Online]. Available online at: http://www.westernbasinportdevelopment.com.au/
GPCL (2012b). Seagrass Light Based Management: Western Basin Dredging and Disposal Project. Gladstone, MO: GPCL.
Grech, A., Bos, M., Brodie, J., Coles, R., Dale, A., Gilbert, R., et al. (2013). Guiding principles for the improved governance of port and shipping impacts in the Great Barrier Reef. Mar. Pollut. Bull. 75, 8–20. doi: 10.1016/j.marpolbul.2013.07.013
Grech, A., Chartrand-Miller, K., Erftemeijer, P., Fonseca, M., McKenzie, L., Rasheed, M., et al. (2012). A comparison of threats, vulnerabilities and management approaches in global seagrass bioregions. Environ. Res. Lett. 7:024006. doi: 10.1088/1748-9326/7/2/024006
Grech, A., and Coles, R. (2010). An ecosystem−scale predictive model of coastal seagrass distribution. Aquat. Conserv. Mar. Freshwater Ecosyst. 20, 437–444. doi: 10.1002/aqc.1107
Grech, A., Coles, R., and Marsh, H. (2011). A broad-scale assessment of the risk to coastal seagrasses from cumulative threats. Mar. Policy 35, 560–567. doi: 10.1016/j.marpol.2011.03.003
Heck, K. L. J., Carruthers, T. J., Duarte, C. M., Hughes, A. R., Kendrick, G., Orth, R. J., et al. (2008). Trophic transfers from seagrass meadows subsidize diverse marine and terrestrial consumers. Ecosystems 11, 1198–1210. doi: 10.1007/s10021-008-9155-y
Hughes, A. R., Williams, S. L., Duarte, C. M., Heck, K. L Jr, and Waycott, M. (2008). Associations of concern: declining seagrasses and threatened dependent species. Front. Ecol. Environ. 7, 242–246. doi: 10.1890/080041
Jackson, E. L., Rowden, A. A., Attrill, M. J., Bossey, S., and Jones, M. (2001). The importance of seagrass beds as a habitat for fishery species. Oceanogr. Mar. Biol. 39, 269–304.
Kirk, J. T. O. (1994). Light and Photosynthesis in Aquatic Ecosystems. Cambridge: Cambridge University Press.
Kirkman, H. (1978). Decline of seagrass in northern areas of Moreton Bay, Queensland. Aquat. Bot. 5, 63–76. doi: 10.1016/0304-3770(78)90047-5
Koch, E., and Beer, S. (1996). Tides, light and the distribution of Zostera marina in Long Island Sound, USA. Aquat. Bot. 53, 97–107. doi: 10.1016/0304-3770(95)01015-7
Koch, E. W. (2001). Beyond light: physical, geological, and geochemical parameters as possible submersed aquatic vegetation habitat requirements. Estuaries 24, 1–17. doi: 10.2307/1352808
Koren, K., Brodersen, K. E., Jakobsen, S. L., and Kühl, M. (2015). Optical sensor nanoparticles in artificial sediments–a new tool to visualize O2 dynamics around the rhizome and roots of seagrasses. Environ. Sci. Technol. 49, 2286–2292. doi: 10.1021/es505734b
Lavery, P. S., McMahon, K., Mulligan, M., and Tennyson, A. (2009). Interactive effects of timing, intensity and duration of experimental shading on Amphibolis griffithii. Mar. Ecol. Prog. Ser. 394, 21–33. doi: 10.3354/meps08242
Lee, K.-S., and Dunton, K. H. (1997). Effect of in situ light reduction on the maintenance, growth and partitioning of carbon resources in Thalassia testudinum banks ex König. J. Exp. Mar. Biol. Ecol. 210, 53–73. doi: 10.1016/S0022-0981(96)02720-7
Lee, K.-S., Park, S. R., and Kim, Y. K. (2007). Effects of irradiance, temperature, and nutrients on growth dynamics of seagrasses: a review. J. Exp. Mar. Biol. Ecol. 350, 144–175. doi: 10.1016/j.jembe.2007.06.016
Lee Long, W., Mellors, J., and Coles, R. (1993). Seagrasses between Cape York and Hervey Bay, Queensland, Australia. Mar. Freshwater Res. 44, 19–31.
Longstaff, B. J., and Dennison, W. C. (1999). Seagrass survival during pulsed turbidity events: the effects of light deprivation on the seagrasses Halodule pinifolia and Halophila ovalis. Aquat. Bot. 65, 105–121. doi: 10.1016/S0304-3770(99)00035-2
Macreadie, P., Schliep, M., Rasheed, M., Chartrand, K., and Ralph, P. (2014). Molecular indicators of chronic seagrass stress: a new era in the management of seagrass ecosystems? Ecol. Indic. 38, 279–281. doi: 10.1016/j.ecolind.2013.11.017
Masini, R. J., and Manning, C. R. (1997). The photosynthetic responses to irradiance and temperature of four meadow-forming seagrasses. Aquat. Bot. 58, 21–36. doi: 10.1016/S0304-3770(97)00008-9
McKenzie, L. (1994). Seasonal changes in biomass and shoot characteristics of a Zostera capricorni Aschers. dominant meadow in cairns harbour, northern queensland. Mar. Freshwater Res. 45, 1337–1352. doi: 10.1071/MF9941337
McMahon, K., Collier, C., and Lavery, P. S. (2013). Identifying robust bioindicators of light stress in seagrasses: a meta-analysis. Ecol. Indic. 30, 7–15. doi: 10.1016/j.ecolind.2013.01.030
Mellors, J. E. (1991). An evaluation of a rapid visual technique for estimating seagrass biomass. Aquat. Bot. 42, 67–73. doi: 10.1016/0304-3770(91)90106-F
Mellors, J., Marsh, H., and Coles, R. (1993). Intra-annual changes in seagrass standing crop, Green Island, Northern Queensland. Mar. Freshwater Res. 44, 33–41.
Moore, K. A., Wetzel, R. L., and Orth, R. J. (1997). Seasonal pulses of turbidity and their relations to eelgrass (Zostera marina L) survival in an estuary. J. Exp. Mar. Biol. Ecol. 215, 115–134. doi: 10.1016/S0022-0981(96)02774-8
Olesen, B., and Sand-Jensen, K. (1993). Seasonal acclimatization of eelgrass Zostera marina growth to light. Mar. Ecol. Prog. Ser. 94, 91–99. doi: 10.3354/meps094091
Olesen, B., and Sand-Jensen, K. (1994). Demography of shallow eelgrass (Zostera marina) populations–shoot dynamics and biomass development. J. Ecol. 82, 379–390. doi: 10.2307/2261305
Orth, R. J., Carruthers, T. J., Dennison, W. C., Duarte, C. M., Fourqurean, J. W., Heck, K. L. Jr, et al. (2006). A global crisis for seagrass ecosystems. Bioscience 56, 987–996. doi: 10.1641/0006-3568(2006)56[987:AGCFSE]2.0.CO;2
Petrou, K., Jimenez-Denness, I., Chartrand, K., McCormack, C., Rasheed, M., and Ralph, P. (2013). Seasonal heterogeneity in the photophysiological response to air exposure in two tropical intertidal seagrass species. Mar. Ecol. Prog. Ser. 482, 93–106. doi: 10.3354/meps10229
Ralph, P. J., Durako, M. J., Enríquez, S., Collier, C. J., and Doblin, M. A. (2007). Impact of light limitation on seagrasses. J. Exp. Mar. Biol. Ecol. 350, 176–193. doi: 10.1016/j.jembe.2007.06.017
Rasheed, M. A. (1999). Recovery of experimentally created gaps within a tropical Zostera capricorni (Aschers) seagrass meadow, Queensland Australia. J. Exp. Mar. Biol. Ecol. 235, 183–200. doi: 10.1016/S0022-0981(98)00158-0
Rasheed, M. A., McKenna, S. A., Carter, A. B., and Coles, R. G. (2014). Contrasting recovery of shallow and deep water seagrass communities following climate associated losses in tropical north Queensland, Australia. Mar. Pollut. Bull. 83, 491–499. doi: 10.1016/j.marpolbul.2014.02.013
Rasheed, M. A., and Unsworth, R. K. (2011). Long-term climate-associated dynamics of a tropical seagrass meadow: implications for the future. Mar. Ecol. Prog. Ser. 422, 93–103. doi: 10.3354/meps08925
Raun, A. L., and Borum, J. (2013). Combined impact of water column oxygen and temperature on internal oxygen status and growth of Zostera marina seedlings and adult shoots. J. Exp. Mar. Biol. Ecol. 441, 16–22. doi: 10.1016/j.jembe.2013.01.014
Romero, J., Lee, K.-S., Pérez, M., Mateo, M. A., and Alcoverro, T. (2006). “Nutrient dynamics in seagrass ecosystems,” in Seagrasses: Biology, Ecology and Conservation, eds A. W. D. Larkum, R. J. Orth, and C. M. Duarte (Dordrecht: Springer), 227–254.
Sofonia, J. J., and Unsworth, R. K. (2010). Development of water quality thresholds during dredging for the protection of benthic primary producer habitats. J. Environ. Monit. 12, 159–163. doi: 10.1039/B904986J
Staehr, P. A., and Borum, J. (2011). Seasonal acclimation in metabolism reduces light requirements of eelgrass (Zostera marina). J. Exp. Mar. Biol. Ecol. 407, 139–146. doi: 10.1016/j.jembe.2011.05.031
Terrados, J., Duarte, C. M., Kamp-Nielsen, L., Agawin, N. S. R., Gacia, E., Lacap, D., et al. (1999). Are seagrass growth and survival constrained by the reducing conditions of the sediment? Aquat. Bot. 65, 175–197. doi: 10.1016/S0304-3770(99)00039-X
Thom, R. M., Southard, S. L., Borde, A. B., and Stoltz, P. (2008). Light requirements for growth and survival of eelgrass (Zostera marina L) in Pacific Northwest (USA) estuaries. Estuaries Coasts 31, 969–980. doi: 10.1007/s12237-008-9082-3
Thomas, R., Unsworth, R. K. F., and Rasheed, M. (2010). Seagrasses of Port Curtis and Rodds Bay and Long Term Seagrass Monitoring, November 2009. (Cairns, QLD: DEEDI).
Touchette, B. W., and Burkholder, J. M. (2000). Overview of the physiological ecology of carbon metabolism in seagrasses. J. Exp. Mar. Biol. Ecol. 250, 169–205. doi: 10.1016/S0022-0981(00)00196-9
Underwood, A. J. (1997). Experiments in Ecology: their Logical Design and Interpretation Using Analysis of Variance. Cambridge: Cambridge University Press.
Van Duin, E. S., Blom, G., Los, F. J., Maffione, R., Zimmerman, R., Cerco, C., et al. (2001). Modeling underwater light climate in relation to sedimentation, resuspension, water quality and autotrophic growth. Hydrobiologia 444, 25–42. doi: 10.1023/A:1017512614680
Vision Environment QLD (2013). Western Basin Dredging Disposal Project Water Quality Monitoring - Methodology Overview. (Gladstone, MO).
Vonk, J. A., Christianen, M. J., Stapel, J., and O'Brien, K. R. (2015). What lies beneath: why knowledge of belowground biomass dynamics is crucial to effective seagrass management. Ecol. Indic. 57, 259–267. doi: 10.1016/j.ecolind.2015.05.008
Waycott, M., Duarte, C. M., Carruthers, T. J., Orth, R. J., Dennison, W. C., Olyarnik, S., et al. (2009). Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc. Natl Acad Sci. U.S.A 106, 12377–12381. doi: 10.1073/pnas.0905620106
Waycott, M., Longstaff, B. J., and Mellors, J. (2005). Seagrass population dynamics and water quality in the Great Barrier Reef region: a review and future research directions. Mar. Pollut. Bull. 51, 343–350. doi: 10.1016/j.marpolbul.2005.01.017
Waycott, M., McMahon, K. M., Mellors, J., Calladine, A., and Kleine, D. (2004). A Guide to Tropical Seagrasses in the Indo-West Pacific. Townsville, QLD: James Cook University.
York, P. H., Carter, A. B., Chartrand, K., Sankey, T., Wells, L., and Rasheed, M. A. (2015). Dynamics of a deep-water seagrass population on the Great Barrier Reef: annual occurrence and response to a major dredging program. Sci. Rep. 5:13167. doi: 10.1038/srep13167
York, P. H., Gruber, R. K., Hill, R., Ralph, P. J., Booth, D. J., and Macreadie, P. I. (2013). Physiological and morphological responses of the temperate seagrass Zostera muelleri to multiple stressors: investigating the interactive effects of light and temperature. PLoS ONE 8:e76377. doi: 10.1371/journal.pone.0076377
Zimmerman, R. C. (2003). A biooptical model of irradiance distribution and photosynthesis in seagrass canopies. Limnol. Oceanogr. 48, 568–585. doi: 10.4319/lo.2003.48.1_part_2.0568
Keywords: seagrass, shading, light attenuation, thresholds, dredging management, Zostera muelleri, indicators
Citation: Chartrand KM, Bryant CV, Carter AB, Ralph PJ and Rasheed MA (2016) Light Thresholds to Prevent Dredging Impacts on the Great Barrier Reef Seagrass, Zostera muelleri ssp. capricorni. Front. Mar. Sci. 3:106. doi: 10.3389/fmars.2016.00106
Received: 14 April 2016; Accepted: 08 June 2016;
Published: 08 July 2016.
Edited by:
Jacob Carstensen, Aarhus University, DenmarkReviewed by:
Nomiki Simboura, Hellenic Centre for Marine Research, GreeceNuria Marba, Consejo Superior de Investigaciones Cientificas, Spain
Peter Anton Staehr, Aarhus University, Denmark
Copyright © 2016 Chartrand, Bryant, Carter, Ralph and Rasheed. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kathryn M. Chartrand, Katie.Chartrand@jcu.edu.au
 Kathryn M. Chartrand
Kathryn M. Chartrand Catherine V. Bryant
Catherine V. Bryant Alex B. Carter1
Alex B. Carter1  Michael A. Rasheed
Michael A. Rasheed