Taiwanese Records of Oblong Large-Eye Seabream Gymnocranius oblongus (Teleostei: Lethrinidae) and Other Rare or Undetermined Large-Eye Seabreams
- 1Institute of Oceanography, National Taiwan University, Taipei, Taiwan
- 2Institute of Marine Biology, National Dong Hwa University, Pingtung, Taiwan
- 3National Museum of Marine Biology and Aquarium, Pingtung, Taiwan
- 4Institut de Recherche pour le Développement, UMR 250 “Ecologie Marine Tropicale des Océans Pacifique et Indien”, Denpasar, Indonesia
The oblong large-eye seabream Gymnocranius oblongus Borsa, Béarez and Chen, 2010 has been described based on specimens from New Caledonia, the only locality where this species has been recorded up to now. Here, new records are reported from Taiwan, which is located over 7000 km from the previously known site of occurrence. Nucleotide sequences at the cytochrome b locus of the Taiwanese samples of G. oblongus were provided and compared to those of the conspecific New Caledonian samples. The results showed little intraspecific DNA variation among the sequences (only three segregating sites along the 1140-bp long aligned sequences). Further population genetic analysis revealed a relatively high level of genetic differentiation (ϕst = 0.286) indicating limited gene flow between the two populations. The records thus available suggest a wide and antitropical distribution for this relatively rare and poorly known species. In addition to this finding, we report the first Taiwanese records of Gymnocranius satoi Borsa, Béarez, Paijo and Chen, 2013, a species widespread in the western Pacific, and of undetermined Gymnocranius specimens that potentially represent two new species.
Introduction
Large-eye seabreams (Lethrinidae: Monotaxinae) comprise medium- to large-sized benthic fishes occurring over sandy or rubble substrates at or in the vicinity of coral reefs, at depths ranging from 1 to 180 m (Carpenter and Allen, 1989). Large-eye seabreams regularly occur at local fish markets throughout the tropical Indo-West Pacific (Carpenter and Allen, 1989; authors' personal observations). Despite the importance of several large-eye seabream species for human consumption, our knowledge on their biology, taxonomy, and biogeography is still limited. Currently, 14 species belonging to four genera are recognized in this fish group that is classified as one of two subfamilies of the perciform family Lethrinidae (Carpenter and Allen, 1989; Borsa et al., 2010, 2013; Eschmeyer et al., 2016). Gymnocranius is the most speciose genus, with 10 species described thus far. The oblong large-eye seabream, Gymnocranius oblongus Borsa, Béarez and Chen, 2010 is one of the three species that have been described since the synopsis of Carpenter and Allen (1989); the other two are Gymnocranius satoi Borsa, Béarez, Paijo and Chen, 2013 and Gymnocranius superciliosus Borsa, Béarez, Paijo and Chen, 2013. The specimens used for the description of G. oblongus had all been collected from New Caledonia; its distribution was thought to be limited to the Coral Sea (Borsa et al., 2010). This fish is characterized by an oblong, fusiform body, a slightly rounded snout, an elongate tail with rounded tips and sub-horizontal, wavy blue lines or dashes on snout and cheeks (Borsa et al., 2010). It is distinct from Gymnocranius grandoculis (Valenciennes, 1830), regularly found at fish markets (e.g., in Taiwan), by a more slender body which is also more symmetrical dorso-ventrally and by a more elongated caudal fin. Another remarkable feature of G. oblongus is its prominent forehead, though less conspicuously so than its relative Gymnocranius microdon (Bleeker, 1851).
Two specimens purchased from local fish markets in Taiwan were identified as G. oblongus. The main purpose of the present work is to put the newly discovered Taiwanese specimens of this species on record. Cytochrome b gene sequences obtained from the Taiwanese specimens were compared to those from New Caledonia to evaluate the intraspecific genetic diversity and to estimate the level of genetic differentiation between the two populations. Additional records of rare or undetermined Gymnocranius species from Taiwan were also provided in this paper.
Materials and Methods
We examined 81 specimens of large-eye seabream species collected between 2011 and 2015 at several local fish markets in the eastern and southern parts of Taiwan and on Little Liuqiu and Penghu Islands in the Taiwan Strait (see Table S1). The two specimens of G. oblongus, which had been caught by line, were purchased from fish sellers, one by HCH in Hengchun town, southern Taiwan on 05 August 2011, and the other one by WJC in Magong, Penghu Islands (Taiwan Strait) on 03 May 2015. The specimens were photographed shortly after collection. Standard length (SL), body depth at origin of first dorsal fin (BD), head length, snout length, eye diameter, inter-orbital width, and other parameters were measured to the nearest millimeter. Species identification followed Borsa et al. (2010). Other monotaxine specimens collected by us in Taiwanese waters were identified using diagnostic morphological traits, as provided in the relevant literature (Carpenter and Allen, 1989; Borsa et al., 2013; Nakabo, 2013) and/or assessed against the genetic library that we are currently building for the Lethrinidae.
Small pieces of muscle-tissue were excised from the specimens, preserved in 95% ethanol, and stored at −20°C in the Marine Biodiversity and Phylogenomics laboratory at Institute of Oceanography, National Taiwan University (NTU), Taipei with tissue identification numbers LET439 and LET1209, respectively. The Hengchun specimen was lost afterwards. The Penghu specimen was preserved in formaldehyde and deposited at the ichthyological collection of the NTU Museums, Taipei under registration no. NTUM 10819. In total, we accessed tissue samples from seven G. oblongus specimens including the two specimens from Taiwan and five specimens previously collected from New Caledonia (including the holotype and all three paratypes of the species), one G. satoi specimen, and two unidentified Gymnocranius specimens from Taiwan (Table 1). Genomic DNA was extracted using an automated DNA-extractor (LabTurbo 48 Compact System with LGD 480–220 kits: Taigene Bioscience Corporation, Taipei) following the manufacturer's protocol. Protocols for cytochrome b gene amplification and sequencing have been outlined in Borsa et al. (2013). The sequences were deposited in GenBank (http://www.ncbi.nlm.nih.gov/) as a genetic reference for future DNA-identification and research on large-eye seabreams.
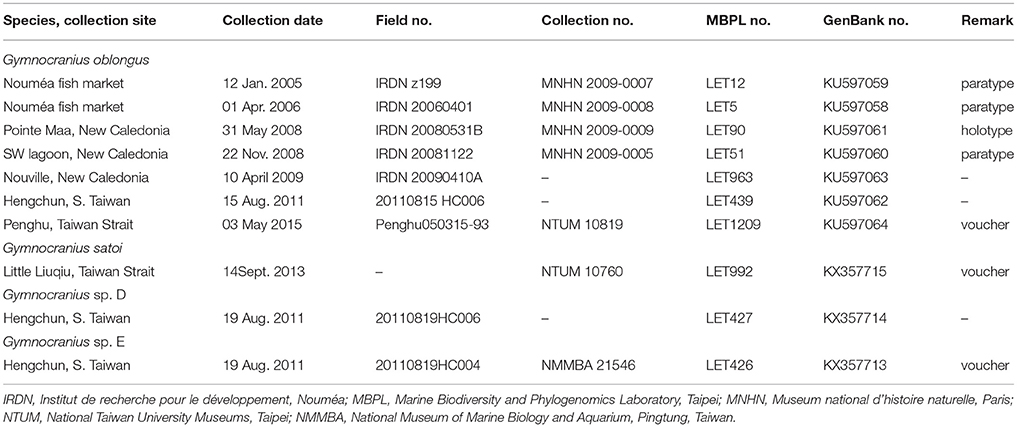
Table 1. List of Gymnocranius specimens referred to in present study, with sampling details, collection nos. and GenBank accession nos.
The sequences were aligned with homologous sequences from Gymnocranius elongatus Senta, 1973, G. grandoculis, and Gymnocranius griseus (Temminck and Schlegel, 1843) published by Lo Galbo et al. (2002) and retrieved from GenBank (accession nos. AF381260, AF381275, and AF381259, respectively), and those of G. superciliosus (from New Caledonia) and G. satoi (from West Papua) that have been produced previously (Borsa et al., 2013).
The softwares PopART (University of Otago, available from http://popart.otago.ac.nz) and PAUP* (Swofford, 2002) were used to compute number of haplotypes, minimum spanning network, pairwise p distances, nucleotide diversity, and segregating (polymorphic) sites to assess the intra- and inter-specific genetic diversity of the cytochrome b sequences. The level of genetic differentiation between populations was estimated by ϕST, Excoffier et al.'s (1992) estimator of Wright's (1951) FST, the standardized among-population variance in allele frequency using ARLEQUIN v. 3.5 (Excoffier and Lischer, 2010). The nucleotide-substitution model chosen was TN93, the best model according to the Bayesian information criterion (MEGA6; Tamura et al., 2013) among the suite of models implemented in ARLEQUIN v. 3.5.
Estimates of genetic differentiation in G. oblongus were compared to those between populations of shore fishes from the Coral Sea and the northern South China Sea or adjacent locations. In blacktip grouper Epinephelus fasciatus and largescale mullet Planiliza sp. H (one of 10 cryptic species in the genus Planiliza; Durand and Borsa, 2015), genetic differences were estimated between populations from the Coral Sea or from nearby Fiji and the northern South China Sea and Philippines or nearby Ryukyu Islands, Japan, based on the nucleotide sequence data compiled or produced by Borsa et al. (2016) and Durand et al. (2012), respectively. For all other species examined, FST values or equivalents were taken directly from the relevant literature.
Results and Discussion
The photographs of the two oblong large-eye seabream specimens from Taiwan are shown in Figure 1. The measurements on the single specimen kept as voucher (NTUM 10819) were the following: SL 241.4 mm, BD 94.5 mm, head length 67.5 mm, snout length 28.0 mm, eye diameter 21.0 mm, and inter-orbital width 28.5 mm; the count of pored scales on lateral line was 48. The ratio of SL to BD was 2.6, which was at the lower end of the range reported for six specimens from New Caledonia (2.6–2.8; Borsa et al., 2010). The SL/BD ratio in the Hungchen specimen (Figure 1A) was around 2.5. The combination of three features diagnose adult specimens of G. oblongus relative to all other species described to date in the genus Gymnocranius, namely, G. audleyi Ogilby, 1916, G. elongatus, G. euanus (Günther, 1879), G. frenatus Bleeker, 1873, G. grandoculis, G. griseus, G. microdon, G. satoi and G. superciliosus (Carpenter and Allen, 1989; Borsa et al., 2010, 2013). These are: (1) “sub-horizontal, wavy blue lines or dashes on snout and cheeks”; (2) “fusiform body”; and (3) “elongate tail” (Borsa et al., 2010, p. 245). The sub-horizontal wavy blue lines on snout were observed in the larger of our two Taiwanese specimens (Figure 1B), but not in the smaller specimen (Figure 1A). Both specimens had a fusiform body with remarkable dorso-ventral symmetry (Figure 1). Both possessed an elongate tail (Figure 1).

Figure 1. Gymnocranius oblongus from Taiwanese waters. (A) Specimen from Hengchun, southern Taiwan, collected on 15 August 2011; standard length ~150 mm (photographed by HCH); arrow indicates oblique dark bar; (B) Specimen no. NTUM 10819 from Penghu Islands, Taiwan Strait, collected on 03 May 2015; standard length 244 mm (photographed by WJC).
Juvenile and pre-adult Gymnocranius species usually present several transversal dark bars on body sides (Carpenter and Allen, 1989). One of these dark bars runs from around the base of the second soft ray of the dorsal fin to the base of the anal fin spines (Carpenter and Allen, 1989). The equivalent transversal dark bar in the smaller G. oblongus specimen from Taiwan (indicated by an arrow on Figure 1A) ran forward and crossed with another transversal dark bar running from the base of 5th–6th dorsal spin to ventral part of body at approximately the place of the tip of the pectoral fin tip; a faint dark blotch was visible at the crossing position (Figure 1A). These pigmentation patterns also characterize an early juvenile specimen from New Caledonia (Borsa et al., 2010: Figure 3).
Seven new cytochrome b gene sequences of G. oblongus (GenBank nos. KU597058-KU597064) produced in this study resulted in three haplotypes. The nucleotide diversity was 0.18%. Individual LET439 from Taiwan possessed the same haplotype as 4/5 individuals from New Caledonia. The haplotype represented by the Penghu specimen (LET1209) diverged by two mutation steps from it. Three segregating sites were observed along the 1140-bp long aligned sequences of G. oblongus. A total of 323 variable nucleotide sites were observed among all included sequences of Gymnocranius species. The average interspecific nucleotide divergence (estimated from pairwise uncorrected p-distances) was 11.02% whereas the average intraspecific nucleotide divergence was 0.08% in G. oblongus and 0.4% in G. satoi (see below).
The estimate of genetic differentiation (ϕST) between the New Caledonian and Taiwanese populations of G. oblongus was 0.286 (P = 0.057; permutation test; ARLEQUIN), suggesting limited gene flow. This was the fourth highest-ranking value in Table 2. The three species with higher FST were E. fasciatus, the coral trout Plectropomus leopardus and the narrow-barred Spanish mackerel Scomberomorus commerson. The high value in E. fasciatus reflected the occurrence of a divergent mitochondrial lineage within a sample (Borsa et al., 2016). The high value in P. leopardus was caused by the occurrence of two mitochondrial clades with allopatric distribution (van Herwerden et al., 2009). The high values reported for S. commerson stemmed from differences between multiple divergent mitochondrial clades (Fauvelot and Borsa, 2011). The phylogeographic structure uncovered in all three cases suggests the occurrence of cryptic species. Homologous estimates of genetic differentiation in all other species listed in Table 2 were <0.200. The high ϕST value observed here suggests geographically isolated populations, hence an antitropical distribution.
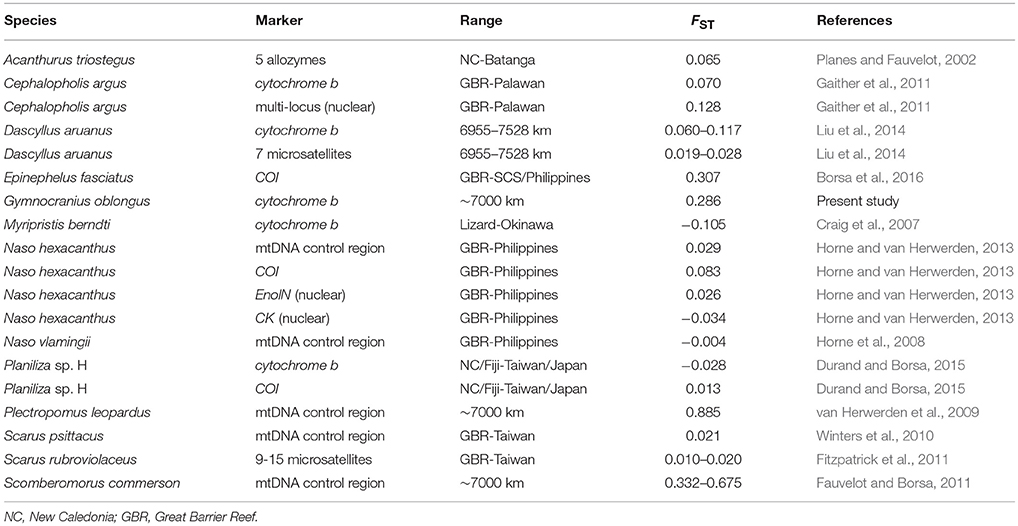
Table 2. Estimates of genetic differentiation (FST or equivalent) between populations of shore fishes from the Coral Sea and the northern South China Sea (SCS) (or adjacent location).
Among the other Monotaxinae collected from Taiwan, four specimens were identified as G. satoi and 15 others could only be identified to genus Gymnocranius based on the available morphological keys (Table S1). These undetermined Gymnocranius specimens could be classified into two different morphological types based on their transversal dark bar patterns on body sides and fin ray colors (reddish or yellowish; Figures 2, 3). They also showed substantial genetic divergence (p-distance >10.7%) with all other known species in the genus for which cytochrome b sequence data were available, indicating potential new species. These two species have been mentioned for the first time by Borsa et al. (2013) as Gymnocranius sp. D (Figure 2) and Gymnocranius sp. E (Figure 3). G. satoi is the red-finned “Gymnocranius sp.” depicted previously (Sato, 1986; Carpenter and Allen, 1989; Nakabo, 2013) and formally described in Borsa et al. (2013). Its distribution includes New Caledonia, Japan and Raja Ampat in West Papua, and possibly the Great Barrier Reef, the Solomon Sea, the Bismarck Sea, the Pacific coast of New Guinea and the Lesser Sunda Islands (Borsa et al., 2013, and references therein). The present record from Taiwan is new.

Figure 2. Gymnocranius sp. D from Taiwanese waters. Specimen from Hengchun, southern Taiwan, collected on 19 August 2011; standard length 148 mm (photographed by HCH).

Figure 3. Gymnocranius sp. E from Taiwanese waters. Specimen no. NMMBA 21547 from Hengchun, southern Taiwan, collected on 19 August 2011; standard length 183 mm (photographed by HCH).
Concluding Remarks
The oblong large-eye seabream likely has an antitropical distribution in the western Pacific. Thus, most monotaxine species, now including G. oblongus, have a relatively wide distribution in the Indo-West Pacific (Carpenter and Allen, 1989; Borsa et al., 2013; this study). The new records and species reported in this study make up to 10 known and two undescribed monotaxine species in Taiwanese waters (Shao, 2016; this study).
Author Contributions
WJC and PB contributed to the conception and design of the work, analyzed, and interpreted the data, and wrote the paper; WJC and HCH collected and managed the samples; HCH provided precious photographies taken from the fresh fish specimens. WJC collected the molecular data and led the project including the search of funding support for completing this study.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank WJC's lab members, especially Pei-Chun Lo, for assistance in the laboratory, and Hsiu-Chen Lin and Jhen-Nien Chen, for management. This work was supported by research grants from the Ministry of Science and Technology, Taiwan (MOST 101-2611-M-002-016-MY3 and MOST 104-2611-M-002-002-MY3 to WC).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/article/10.3389/fmars.2016.00107
References
Bleeker, P. (1851). Nieuwe bijdrage tot de kennis der ichthyologische fauna van Celebes. Nat. Tijdschr. Ned.-Indië 2, 209–224.
Bleeker, P. (1873). Mededeelingen omtrent eene herziening der Indisch-archipelagische soorten van Epinephelus, Lutjanus, Dentex en verwante geslachten. Verslag. Mededeel. Koninkl. Akad. Wetenschappen Afdeel. Natuurk. 7, 40–46.
Borsa, P., Béarez, P., and Chen, W.-J. (2010). Gymnocranius oblongus (Teleostei: Lethrinidae), a new large-eye bream species from New Caledonia. C. R. Biol. 333, 241–247. doi: 10.1016/j.crvi.2009.12.015
Borsa, P., Béarez, P., Paijo, S., and Chen, W.-J. (2013). Gymnocranius superciliosus and Gymnocranius satoi, two new large-eye breams (Sparoidea: Lethrinidae) from the Coral Sea and adjacent regions. C. R. Biol. 336, 233–240. doi: 10.1016/j.crvi.2013.06.003
Borsa, P., Durand, J.-D., Chen, W.-J., Hubert, N., Muths, D., Mou-Tham, G., et al. (2016). Comparative phylogeography of the western Indian Ocean reef fauna. Acta Oecol. 72, 72–86. doi: 10.1016/j.actao.2015.10.009
Carpenter, K. E., and Allen, G. R. (1989). FAO species catalogue, vol. 9, Emperor fishes and large-eye breams of the world (family Lethrinidae), an annotated and illustrated catalogue of lethrinid species known to date. FAO Species Synopsis 125, 1–118.
Craig, M. T., Eble, J. A., Bowen, B. W., and Robertson, D. R. (2007). High genetic connectivity across the Indian and Pacific Oceans in the reef fish Myripristis berndti (Holocentridae). Mar. Ecol. Prog. Ser. 334, 245–254. doi: 10.3354/meps334245
Durand, J.-D., and Borsa, P. (2015). Mitochondrial phylogeny of grey mullets (Acanthopterygii: Mugilidae) suggests high proportion of cryptic species. C. R. Biol. 338, 266–277. doi: 10.1016/j.crvi.2015.01.007
Durand, J.-D., Shen, K.-N., Chen, W.-J., Jamandre, B.-W., Blel, H., Diop, K., et al. (2012). Systematics of the grey mullets (Teleostei: Mugiliformes: Mugilidae): molecular phylogenetic evidence challenges two centuries of morphology-based taxonomy. Mol. Phylogenet. Evol. 64, 73–92. doi: 10.1016/j.ympev.2012.03.006
Eschmeyer, W. N., Fricke, R., and van der Laan, R. (2016). Catalog of Fishes: Genera, Species, References. Available online at: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp Electronic version (Accessed May 25, 2016).
Excoffier, L., and Lischer, H. E. (2010). Arlequin suite ver. 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 10, 564–567. doi: 10.1111/j.1755-0998.2010.02847.x
Excoffier, L., Smouse, P. E., and Quattro, J. M. (1992). Analysis of molecular variance inferred from metric distances among DNA haplotypes - application to human mitochondrial-DNA restriction data. Genetics 131, 479–491.
Fauvelot, C., and Borsa, P. (2011). Patterns of genetic isolation in a widely distributed pelagic fish, the narrow-barred Spanish mackerel (Scomberomorus commerson). Biol. J. Linn. Soc. 104, 886–902. doi: 10.1111/j.1095-8312.2011.01754.x
Fitzpatrick, J. M., Carlon, D. B., Lippe, C., and Robertson, D. R. (2011). The West Pacific diversity hotspot as a source or sink for new species? Population genetic insights from the Indo-Pacific parrotfish Scarus rubroviolaceus. Mol. Ecol. 20, 219–234. doi: 10.1111/j.1365-294X.2010.04942.x
Gaither, M. R., Bowen, B. W., Bordenave, T. R., Rocha, L. A., Newman, S. J., Gomez, J. A., et al. (2011). Phylogeography of the reef fish Cephalopholis argus (Epinephelidae) indicates Pleistocene isolation across the Indo-Pacific Barrier with contemporary overlap in the Coral Triangle. BMC Evol. Biol. 11:189. doi: 10.1186/1471-2148-11-189
Günther, A. (1879). Notice of two new species of fishes from the South seas. Ann. Mag. Nat. Hist. 4, 136–137.
Horne, J. B., and van Herwerden, L. (2013). Long-term panmixia in a cosmopolitan Indo-Pacific coral reef fish and a nebulous genetic boundary with its broadly sympatric sister species. J. Evol. Biol. 26, 783–799. doi: 10.1111/jeb.12092
Horne, J. B., van Herwerden, L., Choat, J. H., and Robertson, D. R. (2008). High population connectivity across the Indo-Pacific: congruent lack of phylogeographic structure in three reef fish congeners. Mol. Phylogenet. Evol. 49, 629–638. doi: 10.1016/j.ympev.2008.08.023
Liu, S.-Y. V., Chang, F.-T., Borsa, P., Chen, W.-J., and Dai, C.-F. (2014). Phylogeography of the humbug damselfish, Dascyllus aruanus (Linnaeus, 1758): evidence of Indo-Pacific vicariance and genetic differentiation of peripheral populations. Biol. J. Linn. Soc. 113, 931–942. doi: 10.1111/bij.12378
Lo Galbo, A., Carpenter, K. E., and Reed, D. L. (2002). Evolution of trophic types in emperor snappers (Lethrinus, Lethrinidae, Percoidei) based on cytochrome b gene sequence variation. J. Mol. Evol. 54, 754–762. doi: 10.1007/s0023901-0076-z
Nakabo, T. (2013). Fishes of Japan with Pictorial Keys to the Species, 3rd Edn. Tokyo: Tokai University Press.
Ogilby, J. D. (1916). Edible fishes of Queensland. Parts IV through IX. Mem. Queensland Mus. 5, 127–177.
Planes, S., and Fauvelot, C. (2002). Isolation by distance and vicariance drive genetic structure of a coral reef fish in the Pacific Ocean. Evolution 56, 378–399. doi: 10.1554/0014-3820(2002)056[0378:IBDAVD]2.0.CO;2
Sato, T. (1986). “A systematic review of the sparoid fishes of the subfamily Monotaxinae,” in Indo-Pacific Fish Biology: Proceedings of the Second International Conference on Indo-Pacific Fishes, eds T. Uyeno, R. Arai, T. Taniuchi, and K. Matsuura (Tokyo: Ichthyol. Soc. Japan), 602–612.
Senta, T. (1973). A new sparoid fish, Gymnocranius elongatus from the southern South China Sea. Jpn. J. Ichthyol. 20, 135–144.
Shao, K. T. (2016). Taiwan Fish Database.WWW Web electronic publication. Available online at: http://fishdb.sinica.edu.tw (Accessed January 30, 2016).
Swofford, D. L. (2002). PAUP*. Phylogenetic Analysis using Parsimony (*and other methods). Version 4. Sunderland, MA: Sinauer Associates.
Tamura, K., Stecher, G., Peterson, D., Filipski, A., and Kumar, S. (2013). MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729. doi: 10.1093/molbev/mst197
Temminck, C. J., and Schlegel, H. (1843). “Pisces,” in Fauna Japonica: Sive, Descriptio Animalium Quae in Itinere Per Japoniam, Jussu Et Auspiciis Superiorum, Qui Summum in India Batava Imperium Tenent, Suscepto, Annis 1823-30 Collegit, Notis Observationibus Et Adumbrationibus, Parts 2–4, eds P. F. von. Siebold, C. J. Temminck, and W. de Haan (Batavorum: Lugduni), 21–72.
Valenciennes, A. (1830). “Livre sixième, partie I, Des Sparoïdes,” in Histoire Naturelle Des Poissons, Tome Sixième, eds G. Cuvier and A. Valenciennes (Paris: F. G. Levrault), 141–163.
van Herwerden, L., Choat, J. H., Newman, S. J., Leray, M., and Hillersøy, G. (2009). Complex patterns of population structure and recruitment of Plectropomus leopardus (Pisces: Epinephelidae) in the Indo-West Pacific: implications for fisheries management. Mar. Biol. 156, 1595–1607. doi: 10.1007/s00227-009-1195-0
Winters, K. L., van Herwerden, L., Choat, J. H., and Robertson, D. R. (2010). Phylogeography of the Indo-Pacific parrotfish Scarus psittacus: isolation generates distinctive peripheral populations in two oceans. Mar. Biol. 157, 1679–1691. doi: 10.1007/s00227-010-1442-4
Keywords: fishes, new record, new species, distribution, Taiwan, New Caledonia, diversity
Citation: Chen W-J, Ho H-C and Borsa P (2016) Taiwanese Records of Oblong Large-Eye Seabream Gymnocranius oblongus (Teleostei: Lethrinidae) and Other Rare or Undetermined Large-Eye Seabreams. Front. Mar. Sci. 3:107. doi: 10.3389/fmars.2016.00107
Received: 26 May 2016; Accepted: 08 June 2016;
Published: 24 June 2016.
Edited by:
Tito Monteiro Da Cruz Lotufo, University of São Paulo, BrazilReviewed by:
Barry Russell, Museum and Art Gallery of the Northern Territory, AustraliaRonald Fricke, Stuttgart State Museum of Natural History (SMNS), Germany
Copyright © 2016 Chen, Ho and Borsa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei-Jen Chen, wjchen.actinops@gmail.com
 Wei-Jen Chen
Wei-Jen Chen Hsuan-Ching Ho
Hsuan-Ching Ho Philippe Borsa
Philippe Borsa