Potential Structuring Forces on a Shelf Edge Upper Mesophotic Coral Ecosystem in the US Virgin Islands
- 1Center for Marine and Environmental Studies, University of the Virgin Islands, Charlotte Amalie, VI, USA
- 2Department of Engineering Science and Materials and Department of Marine Sciences, UPRM Center for Applied Ocean Science and Engineering, University of Puerto Rico at Mayagüez, Mayagüez, PR, USA
Mesophotic coral ecosystems are extensive light-dependent habitats that typically form between 30 and 150 m depth in the tropical oceans. The forces that structure the benthic communities in these ecosystems are poorly understood but this is rapidly changing with technological advances in technical diving and remote observation that allow large-scale scientific investigation. Recent observations of southeastern Puerto Rican Shelf of the US Virgin Islands have shown that this Caribbean mesophotic coral ecosystem has distinct habitats within the same depth ranges and across small horizontal distances (<<1 km), that range from sparse hardbottom to vibrant coral reefs with stony coral cover >25%. High-resolution bathymetric mapping of the shelf edge revealed a topographically distinct semi-continuous 71 km-long relict barrier reef bank system. The purpose of this study was to characterize the pattern of mesophotic habitat development of the shelf edge and use this data to narrow the potential long-term and large-scale structuring forces of this mesophotic coral ecosystem. We hypothesized from limited preliminary observations that the shelf edge coral cover was limited in shallower portions of the bank and on the seaward orientation. Through stratified random surveys we found that increasing depth and decreasing wave driven benthic orbital velocities were positively related to coral abundance on the shelf edge. In addition, low coral cover habitats of the shelf edge contrasted strongly with adjacent on shelf banks surveyed previously in the same depth range, which had relatively high coral cover (>30%). Predictions of benthic orbital velocities during major storms suggested that mechanical disturbance combined with low rates of coral recovery as a possible mechanism structuring the patterns of coral cover, and these factors could be targets of future research.
Introduction
Mesophotic coral ecosystems of the Caribbean are poorly known environments beyond the depth ranges of historical coral reef investigation (30–100 m depth; Ginsburg, 2007; Hinderstein et al., 2010). However, these environments support light dependent stony corals and can be important as reservoirs of biodiversity and, potentially, as refuges or refugia from local and global stressors (Bridge et al., 2013). The scientific focus on mesophotic coral ecosystems has recently turned from descriptive studies of community structure to specific investigations of the factors controlling their geomorphological and community structure. Much of this focus has been on vertical zonation of steep slopes or walls (Grigg, 2006; Bongaerts et al., 2015), with the largest structuring forces suggested as light intensity and quality (Kahng and Kelley, 2007; Lesser et al., 2009), heterotrophic food supply (Leichter and Genovese, 2006) and temperature (Kobluk and Lysenko, 1994; Wolanski et al., 2004; Bak et al., 2005). However, recent observations have shown that bank mesophotic coral ecosystems, areas of hardbottom raised above the seafloor and supporting stony corals, show horizontal variations in coral community structure as well (Bridge et al., 2011, 2012), sometimes over scales <100's of meters (Smith et al., 2010). Thus, unknown forces acting along horizontal gradients are also important in structuring these communities.
A common early assumption was that deeper reef slopes, including at mesophotic depths, are buffered from some disturbances that have historically or recently structured shallow reefs, including storm swell (Goldberg, 1983; Bongaerts et al., 2010). However, studies have documented damage from storms at mesophotic depths on gently sloping mesophotic banks and shelf edges (Robbart et al., 2009; Bongaerts et al., 2013) and in steeply sloping mesophotic shelf edge systems in Jamaica (Woodley et al., 1981), Curaçao (Bak et al., 2005), Okinawa (White et al., 2013), and the US Virgin Islands (Aronson et al., 1994). Much of the damage from tropical storms in shallow water has been attributed to turbulence that declines exponentially with depth (Sheppard, 1982; Done, 1983), whereas in deeper areas (>15 m) damage has been attributed to colony breakage and transport of broken colonies downslope where they impact mesophotic depths (Harmelin-Vivien, 1994). Another mechanism of reef damage is colony breakage from unidirectional or swell generated currents that directly dislodge colonies or impact them with debris. For example, a modeling study using a 100 year climatology of tropical storms impacting the >15 m depth Flower Gardens Bank in the Gulf of Mexico did not directly demonstrate colony breakage during tropical storm disturbance, but did show sediment dispersal and the potential for scour, and did not rule out colony dislodgement (Lugo-Fernández and Gravois, 2010). A study on the East Flower Gardens Bank before and after the passage of Category 3 Hurricane Rita within 83 km in 2005 did show low levels of whole colony dislodgement at depths from 32 to 40 m (Robbart et al., 2008). Thus, mesophotic coral ecosystems do not appear to be invulnerable to acute storm disturbance, raising the possibility that storms can contribute to reef structure even at deep depths.
The extensive bank mesophotic coral ecosystems of the southeastern Puerto Rican Shelf, including the insular shelf of St. Thomas and St. John, US Virgin Islands, have been shown to support very high coral cover (>30%) relative to many shallow Caribbean reefs and are dominated (>80% relative coral cover) by colonies of Orbicella spp. (Smith et al., 2010). These reefs represent part of the upper mesophotic coral zone (30–60 m depth) that is an extension of shallower reef systems (Slattery et al., 2011). The geomorphological structure of these reefs has been shown to consist of a distinct linear shelf edge structure, termed the primary bank, backed by on shelf secondary, and tertiary bank reefs (Figure 1; also see Smith et al., 2010; Weinstein et al., 2014). The edge of the southeastern Puerto Rican shelf has not yet been characterized in situ, but high-resolution bathymetry shows the shelf edge feature as a semi-continuous ridge, with topographically higher areas that generally orient toward the southeast to south. Thus, this geomorphological feature may be a deeper representative of known submerged drowned barrier reefs that have been described from St. Croix, US Virgin Islands (Adey et al., 1977).
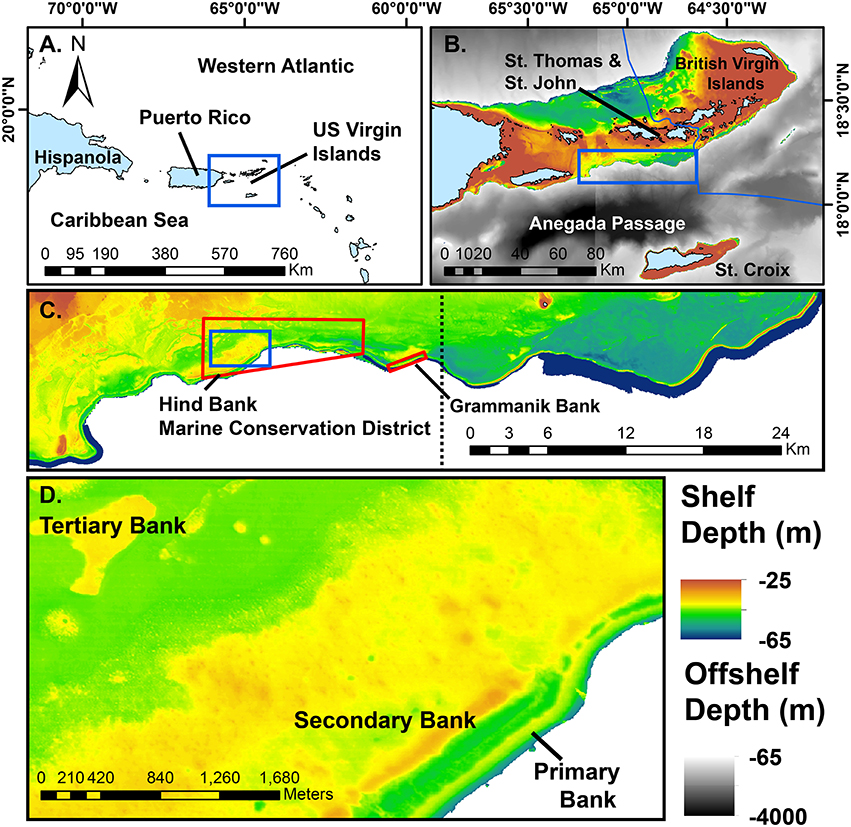
Figure 1. Maps of the study area at successively higher spatial resolution. Blue boxes indicate the subsequent map in the series. (A) The position of the US Virgin Islands in the northeastern region of the Antilles arc. (B) The US Virgin Islands centered on the southeastern Puerto Rican Shelf mesophotic coral ecosystem. (C) The study area of the shelf edge primary bank from Vieques Island (lower left, not shown) to the Border of the British Virgin Islands (upper right). Red polygons indicate marine protected areas mentioned in the text. Dotted line divides the eastern and western study areas as shown in Figure 4. (D) A close-up view of the shelf edge primary bank, a well-defined linear rise adjacent to deep water. Secondary banks of the southeast Puerto Rican Shelf tend to parallel the shelf edge, whereas tertiary banks are much more scattered in distribution. Solid blue areas of bathymetry are multibeam data >65 m depth and drop off rapidly into the Anegada Passage. Multibeam and modeled bathymetry data sets as cited in the text.
The coral communities on the southeastern Puerto Rican Shelf edge may be consistently structured by depth and orientation to the open ocean. Research over a 1 km section of the Grammanik Bank marine protected area (Figure 1) has indicated that there is far less development of coral on the shelf edge than on adjacent secondary and tertiary bank reefs at the same depths (30–40 m; Smith et al., 2010; Nemeth and Kadison, 2013). Furthermore, preliminary unpublished observations on the Grammanik Bank suggest that the leeward sides of the primary bank have higher coral development than the seaward sides, These observations pose the question, does this pattern hold across the 71 km long primary bank edge and, if so, why does this reef complex seem relatively depauperate relative to reefs within 100 m of horizontal distance at the same depths?
Tropical cyclones frequently pass near the southeastern Puerto Rican shelf edge and are dominant structuring force in very shallow reefs in the US Virgin Islands (Hubbard et al., 1991). Notable examples of hurricanes affecting the US Virgin Islands in recent years include Hurricane Marilyn that passed directly over the study area as a category 2–3 storm in 1995 and Hurricane Hugo that passed over the west of the study area as a category 4 storm in 1989. On the south coast of St. John within 15 km of the closest shelf edge mesophotic reef site in this study, a shallow water (11–13 m) location showed a 40% decline of Orbicella annularis cover attributed to Hurricane Hugo (Rogers et al., 1991). The impact of Hurricane Hugo on mesophotic coral ecosystems was documented at the Salt River Canyon, St. Croix, US Virgin Islands by Aronson et al. (1994). The impact of the storm on this steeply sloping habitat increased from shallow to mesophotic depths, and at 27 and 34 m the relative cover of Agaricia lamarcki declined by 69.9 and 57.6%, respectively. This was attributed to water movement, although Hubbard (1992) also found considerable sediment scour and transport during the storm, which may have also impacted mesophotic corals. Aronson et al. (1994) also suggested that, given the published growth rates for agariciid corals, recovery could take as long as 100 years.
The objectives of this study were two-fold. First, we assessed the benthic community structure of the southeastern Puerto Rican shelf edge mesophotic coral ecosystem to understand if distinct small-scale patterns noted at the Grammanik Bank applied more broadly to the shelf edge. Specifically, we hypothesized that patterns of coral development are consistent across the shelf edge, with lower coral development in shallow areas and areas oriented toward the open ocean. We also suspected that shelf edge coral development was limited, in general, relative to proximal on shelf coral reefs banks described in earlier studies (Armstrong et al., 2006; Menza et al., 2008; Smith et al., 2008, 2010). Our second objective was to understand if the patterns of coral development on the southeastern Puerto Rican shelf mesophotic coral ecosystem were consistent with chronic processes and stressors, such as sedimentation, benthic turbulence, disease, or competition, or where potentially patterned with respect to acute disturbance, such as tropical storms. We hypothesized that the relatively low coral cover upper mesophotic shelf edge might indicate acute disturbances from tropical storms or tsunamis as responsible for patterns of benthic communities. Given that direct observations of infrequent disturbance events and their impact on large-scale community structuring is difficult, we used an oceanographic model to show the potential role of storm-generated swell in structuring coral abundance. We also explore the potential role of common chronic stressors in generating the revealed patterns of coral development. This work suggests the potential for large-scale mesophotic coral ecosystem benthic habitat structuring by mechanical disturbance generated from surface swell, although alternative explanations are possible and could be explored in future research.
Materials and Methods
Study Area and Sampling Design
High-resolution benthic imagery shows that the shelf edge of the southeastern Puerto Rican shelf from St. John, US Virgin Islands to Vieques, Puerto Rico is a well-defined ridge, particularly in areas facing the southeast (Figure 1). This shelf edge ridge was sampled by in situ observation (divers or remote camera) using unbiased sample allocation. Benthic sample points were allocated with a stratified random design within defined areas of the southeastern Puerto Rican primary bank shelf edge (Figure 1). All allocations were completed using Environmental Systems Research Institute, Inc. ArcGIS 10.1 and associated Arc Toolbox tools. Different bathymetric data layers ranging from 1 to 5 m resolution were obtained for the southeastern Puerto Rican shelf from the National Oceanic and Atmospheric Administration's (NOAA) National Geophysical Data Center (www.ngdc.noaa.gov), the US Geological Survey (Chaytor and ten Brink, 2015), and Rivera et al. (2006). A polygon was drawn to encompass the shelf edge and Arc Toolbox spatial analyst raster extraction was performed to delineate the bank shelf edge from 27 to 43 m depth, corresponding to the depth limit of orbicellid dominated reefs in this area (Smith et al., 2010).
The shelf edge habitat was stratified into leeward and seaward portions using the Arc Hydro Toolset. The ridgeline of the shelf edge was defined by producing a map of virtual flow that allowed delineation of the break in flow to either side of the ridge (seaward and leeward of ridge). This also highlighted flat areas at the top of the shelf edge ridgeline. A 5 m buffer was placed around the defined ridgeline and its associated flat areas to keep sampling areas in a well-defined leeward or seaward shelf edge slope area where benthic contrasts should be highest. Thus, results of the study characterize the slope areas of the shelf edge, but the narrow high point of the shelf edge was not typically characterized. The leeward and seaward shelf edge areas outside of the excluded ridgeline to a depth of 43 m were clipped to produce the final sampling areas. Arc Toolbox Data management was used to create 200 random sampling points in each polygon. A total of 142 of these stratified random coordinates were sampled in random order for drop camera surveys (n = 100) and diver surveys (n = 42).
Benthic Surveys and Data Processing
Benthic cover was estimated at sample points via remote drop camera and diver-based transect surveys. Drop camera surveys were used to rapidly sample broad areas for benthic cover. Diver surveys were used for more comprehensive assessments of fish communities (not presented in this paper), benthic cover (high-definition video), and coral community health (in situ assessment). Diver surveys of benthic video transects and coral health were based upon the methods described in Smith et al. (2010) and detailed below. Diver transects are closer to the substrate and can reveal more taxonomic detail than drop camera. Comparison among the random sampling sites (t-test or Wilcoxon test) showed that drop camera methods estimated more epilithic algae and less sponge, macroalgae, Lobophora variegata, Agaricia spp., and filamentous cyanobacteria cover than diver methods (Supplementary Table 1). However, because surveys site and sampling method were randomly allocated in shelf edge surveys this bias did not affect habitat comparisons. On shelf data collected from a previous study (see below) was only from diver video, and comparisons between shelf edge (drop camera and diver video) and on shelf habitats for Agaricia spp. and epilithic algae may be biased to toward lower and higher cover, respectively, in the shelf edge habitats.
Drop Camera Surveys
Drop camera surveys were designed to capture one wide-view image of the seafloor that could be processed for benthic cover (Figure 2). Random sample points allocated in GIS were loaded onto the shipboard global positioning system and the research vessel was piloted to within 5 m of the sample point. At this point, the weighted drop camera or surface buoy (diver surveys) was released and allowed to freely fall to the bottom. Rapid descent limited drift from the point of release. A downward facing GoPro Hero 3 camera was set to photograph 12 megapixel images every 5 s. This camera was attached to a plastic fin set into a grooved foam buoy to dampen swing and vibration. The camera buoy was attached to the drop line 3 m from the weighted bottom during sampling and deployed at each site for 1 to 2 min prior to retrieval, producing ~15–20 photographs of the benthos. The clearest photograph from each set was selected for processing (see below). Known spherical aberration of this camera system was likely to have lowered resolution at the picture margins and lessened the ability of observers to define all benthic sampling points to the lowest taxonomic resolution. As outlined above, because sample points were randomly allocated this bias should not affect habitat comparisons.
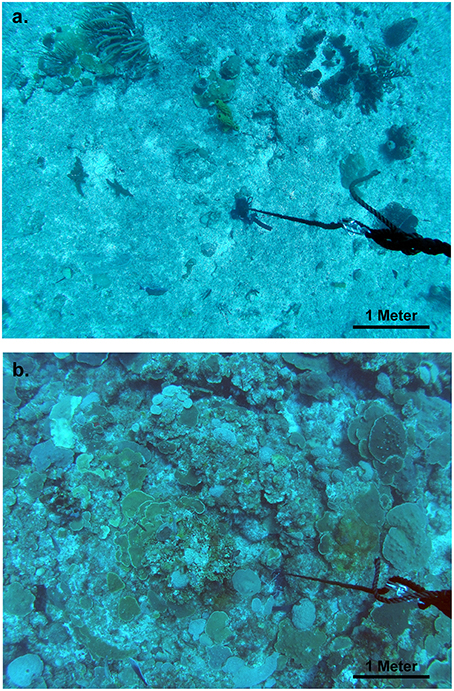
Figure 2. Example image captures from drop camera surveys used in analysis of benthic cover. (A) A seaward-shallow site at 18.203794, −64.70561, and a depth of 31.1 m. (B) A leeward-deep site at 18.191797, −64.948269, and a depth of 38.7 m.
Diver Surveys
Diver pairs using technical NITROX descended on a buoyed drop line and proceeded to lay and survey a transect in a random direction from the buoy line base. Each diver had unique responsibilities: diver one deployed the transect tape while conducting a fish survey (presented elsewhere) and diver two conducted a coral health survey. At the end of the 30 m transect length, diver one returned along the transect recording the substrate with a video camera, pointed downward with a spacing of 40 cm above the bottom maintained by a guide dropper weight (Canon Vixia HF G10 high definition video system, Canon Corp., Tokyo; Bluefin housing, 2 Sola 1200 lights, and Fathom wide angle lens, Light and Motion, California, USA). Diver two conducted a coral health survey along the same transect (see Supplemental Data Section 2).
Benthic Image Processing
Images from video transects were used to capture a mean of 42.4 ± 14.5 S.D. non-overlapping images in Sony Vegas Studio video processing software (Sony Corp., Tokyo). Resultant images from transects and a single clear image from the drop camera survey were analyzed for percent cover using Coral Point Count with Excel Extensions (CPCe; Kohler and Gill, 2006). The selection of the number of points per image to analyze in transect and drop camera methodologies was based on a leveling of the running mean of coral and macroalgal cover in four test images for each methodology. Each transect image was overlaid with 15 points under which the uppermost biotic (e.g., gorgonian frond) or abiotic feature was identified to the lowest possible taxonomic level or abiotic category. Each single drop camera image was overlaid with 50 points and analyzed similarly. A list of the benthic categories analyzed is presented in Supplementary Data Section 1.
Modeling Benthic Orbital Velocities
We modeled benthic orbital velocities (mean and maximum) using the CariCOOS Nearshore Wave Model (CNWM), which is an operational model based on the Simulating Waves Nearshore (SWAN) spectral wave model (Booij et al., 1999). Details of the setup and validation of the CariCOOS Nearshore Wave Model may be found in Anselmi et al. (2010) and Canals et al. (2012).
The SWAN model for the outer Puerto Rico shelf has a resolution of 1.1 km. While this model resolution is not sufficient to resolve small scale features of the study area, given the depths of the sampling sites (>27 m), large cross-shelf differences in wave height due to depth-induced shoaling or bottom friction are not expected over the distances between sampling points. The factors controlling the orbital velocities at the sites are then the local water depth and exposure due to geographic location.
The model was used to predict significant wave height and peak wave period at 3-h intervals in each grid cell from the period 2012 to 2014. This wave data was then used to determine the orbital velocities using the actual depth of each sampling site using linear wave theory, as follows:
Where d is the local water depth at the study site, H is the significant wave height, Tp is the peak wave period, and L is the local wavelength corresponding to the peak wave period and the local water depth. The local wavelength L is obtained by solving the linear dispersion relation given by
where ω = 2π/Tp is the wave angular frequency and k = 2 π/L is the wavenumber. The mean and maximum values were calculated for the two-year time series.
There were no major storms near the study area in the modeled period of 2012–2014. Therefore, to assess potential effects of storm generated swells, we computed orbital velocities for a major storm passing near the study area. Orbital velocities were computed analytically for a typical hurricane with a significant wave height of 7 m and a peak period of 13 s, assuming a spatially homogeneous wave climate (same wave parameters at all sampling points). This assumption of spatial homogeneity makes the water depth the sole controlling factor in the orbital velocities experienced during the hypothetical storm at each of the sampling points. Given the limited fetch near the southeast PR shelf, these values of significant wave height and peak period are assumed to be representative of a Category 2 or 3 Hurricane making a direct pass over the study area.
Analyses
Benthic cover categories identified as important in multidimensional tests (Supplemental Data Section 1) were compared with multiple linear regression using the strata distance from ridgeline and the mean orbital velocity (umean). Distance from the ridgeline was calculated as the distance from the sampling point to the nearest portion of the ridgeline polygon defined in GIS. Distance to the ridgeline values provided a continuous variable for multiple linear regression that indicated whether the sampling point was leeward or seaward of the shelf edge ridge. However, actual distance to the ridgeline was not expected to influence benthic cover on the shelf edge. Negative values indicated seaward sampling points and positive values are leeward sampling points. All comparisons were made in R using the “stats package.” Benthic cover categories were arcsine square root transformed prior to analysis.
Mean benthic orbital velocity at shelf edge sampling points was related to depth and longitude, decreasing with depth and at more western sites (Figure 3; linear regression for depth vs. umean, D.F.num.∕den. = 1/138, F = 585.4, p < 0.0001, R2 = −0.8079). Statistical results were the same using depth or umean, with the exception of filamentous cyanobacteria, which showed a significant interaction between depth and seaward orientation, whereas no comparison with umean was significant. Because depth and umean were similar for most benthic categories tested, single variable benthic components are shown compared only against mean benthic orbital velocity in figures and in statistical comparisons.
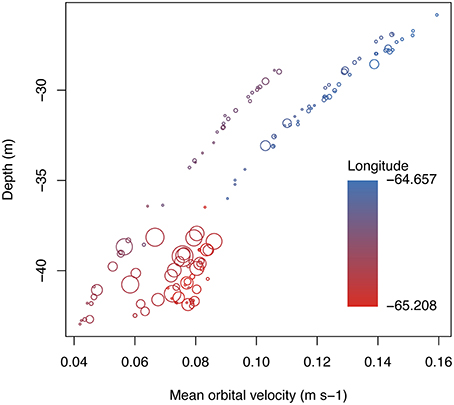
Figure 3. Relationship between depth, mean orbital velocity, and longitude at each of the shelf edge sampling sites along the southeastern Puerto Rican shelf. Bubble sizes represent coral cover and are scaled to 40% (largest cover value recorded). Points equal to 0%.
Benthic cover comparisons were also conducted between shelf edge and on shelf habitats within the Hind Bank Marine Conservation District (“Hind Bank”; Figures 1C,D), in order to test the hypothesis that benthic structure varies from shelf edge to on shelf habitats. Data was from Hind Bank shelf edge habitats collected in this study (n = 28 transects) and data collected in a previous study in the Hind Bank from on shelf secondary and tertiary coral banks (n = 20; Smith et al., 2010). Mean depths of sampling points were not different between studies (Mean Depthon shelf = 40.3m ± 3.2 S.D., Mean Depthshelf edge = 40.7 ± 1.5 S.D; Wilcoxon Rank Sums test, χ2 = 0.25, p = 0.6155) and mean benthic orbital velocities were not different between studies (u-meanon shelf = 0.058 ± 0.02 S.D., u-meanshelf edge = 0.062 ± 0.01; t ratio = 1.12, p = 0.265). Therefore, only shelf position was considered in the comparisons, although depth and umean are plotted in figures for reference. In addition, distance to the ridgeline is used in the resultant figure to show the relative position cross shelf, but not used as a continuous variable in comparisons. All statistical comparisons were one-way ANOVA with the levels on shelf, leeward shelf edge, and seaward shelf edge. The categories sponges and epilithic algae were log transformed to meet parametric assumptions.
Results
Shelf Edge Benthic Structure
The southeastern Puerto Rican shelf edge mesophotic coral ecosystem has benthic communities that showed distinct spatial patterning of coral, gorgonian, sponge, and algal development (Table 1, Supplementary Data Section 1). Across the shelf edge study area overall stony coral cover was low (7.1% ± 0.8 SE), but, spatially, coral cover increased with depth and with decreasing longitude (westward) (Figure 4). Stony coral and Orbicella spp. cover had significant interactions between modeled mean benthic orbital velocity (umean) and distance to the ridgeline of the shelf edge, with cover increasing with decreasing umean, most strongly in the lee of the shelf edge (Figure 5). A similar pattern was seen for Agaricia spp.; however, treatment level comparisons were not significant, perhaps because Agaricia spp. cover was very low in general in this upper mesophotic habitat. Epilithic algae, as a measure carbonate substrate not colonized by sessile macrophytes, was the most abundant benthic category (cover>60%; Supplementary Table 4) and was unrelated to distance to the ridgeline or orbital velocity. Overall macroalgal cover was also not related to umean or distance to the shelf edge ridgeline, whereas Lobophora variegata cover was negatively related to the umean. Filamentous cyanobacteria had a trend toward increasing abundance with increasing umean, but this was not significant. However, depth comparisons (not shown) showed significantly higher cover in the shallow leeward areas, as is apparent in Figure 5. Increased umean was associated with significantly higher gorgonian cover, whereas epibenthic sponges were not significantly related to umean or distance to the ridgeline.
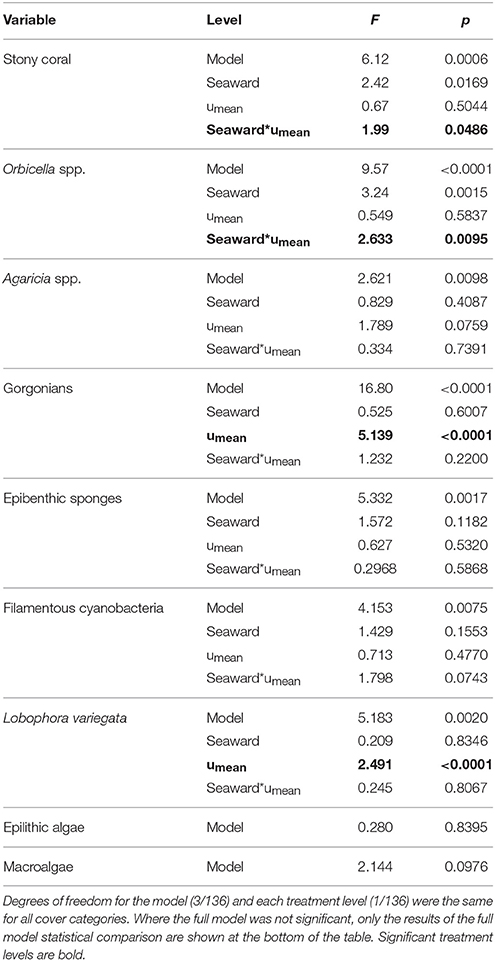
Table 1. Multiple linear regression of selected benthic cover categories for the treatments shelf edge position (seaward) and benthic orbital velocity (umean).
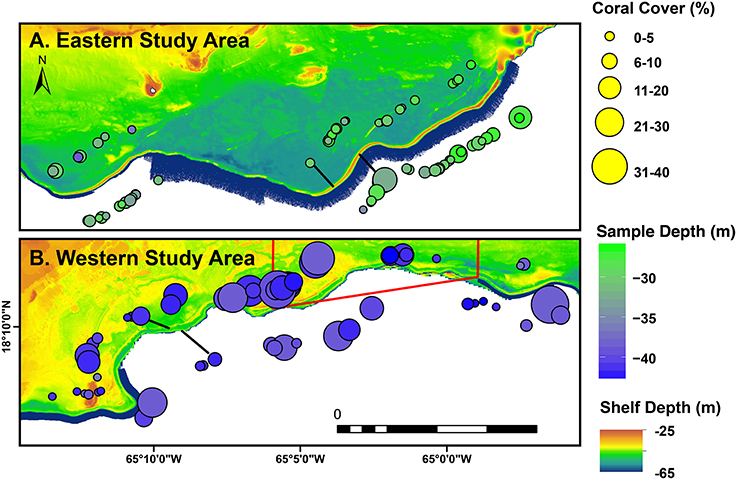
Figure 4. The eastern (A) and western (B) study areas of the southeastern Puerto Rican shelf edge as defined in Figure 1. Bubbles are sized by observed coral cover (%), colored according to mean depth, and are divided above (leeward) or below (seaward) of the shelf edge. Black joining lines indicate the direction of offset for leeward and seaward bubbles in each picture, respectively.
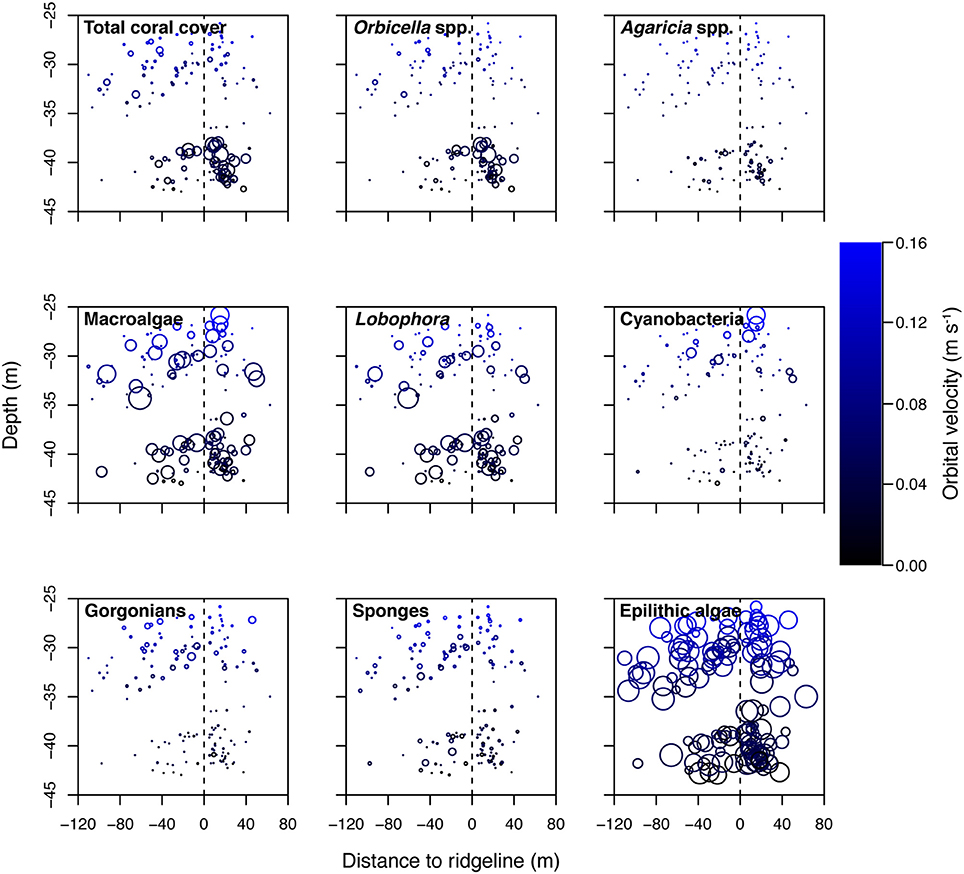
Figure 5. Select benthic cover categories shown against depth, distance to ridgeline (negative values seaward of the ridgeline and positive values leeward), and modeled benthic orbital velocity (umean). Bubble sizes in the epilithic algae plot scale to 100% (the highest value), whereas all other plots scale to 57% cover (based on the highest value of macroalgae). Points equal to 0%.
Shelf Edge to On Shelf Differences in Benthic Structure
Within the Hind Bank Marine Conservation District of the western research area, coral community structure on the shelf edge was depauperate and open space more abundant compared with coral communities located immediately behind the shelf edge at the same overall depths and mean benthic orbital velocities (Figure 6, Table 2). Overall coral cover and Orbicella spp. cover on the seaward shelf and leeward shelf edge were only about a fifth to a half of the coral cover of dense reefs of the on shelf banks (Table 2). In the opposite pattern, the cover of epilithic algae increased significantly on the shelf edge bank compared to on shelf banks. However, this estimate may be biased by differing methodologies used in field sampling. Substrate uncolonized by sessile animals increased significantly across each habitat successively from on shelf to the leeward shelf edge to the seaward shelf edge.
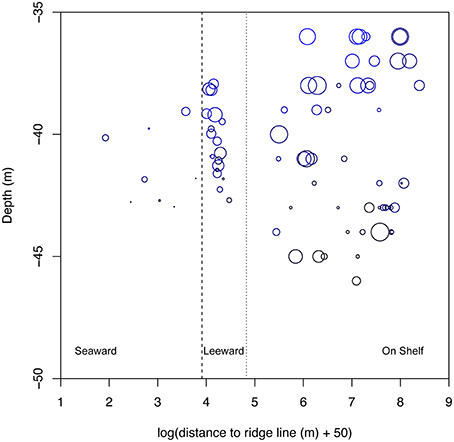
Figure 6. Comparison of coral cover across randomly sampled sites in the Hind Bank Marine Conservation District, St. Thomas from shelf edge (this study) and on shelf (Smith et al., 2010) habitats. Sites are plotted with depth and distance to ridgeline as in Figure 5. Log of distance to ridgeline is plotted, with a correction for negative values, to condense the on shelf distances. Solid dash indicates separation between seaward (left on plot) and leeward habitats, and dotted line indicates transition between shelf edge and on shelf habitats. Bubbles scale to the maximum coral cover value of 50% and points indicate 0% cover. Mean benthic orbital velocity scale in color of bubbles the same as in Figure 5.
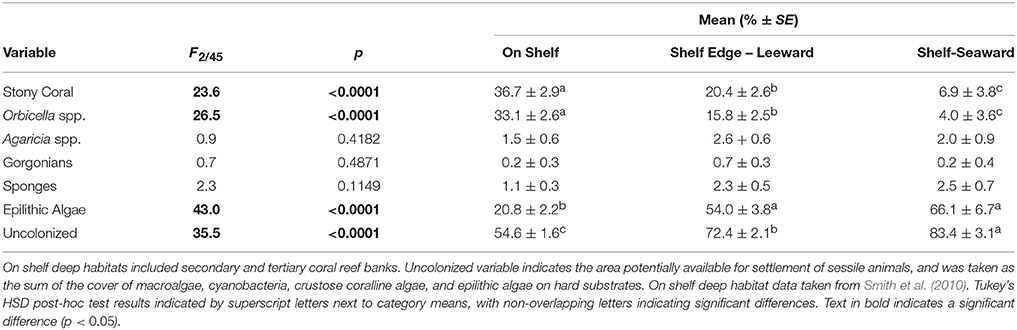
Table 2. Comparison of benthic cover categories within the Hind Bank Marine Conservation District between on shelf (n = 20), leeward shelf edge (n = 19), and seaward shelf edge (n = 9) habitats by one-way ANOVA.
Benthic Orbital Velocity Characteristics
Modeled mean benthic orbital velocities over the period 2012–2014 showed a correspondence with depth whereas maximum orbital velocity did not (Figure 7). Maximum orbital velocity was a record of transient swell events over the sampling sites during a period when there were no tropical storms passing near the region. Major storm benthic orbital velocities (Figure 7), a function of depth only, showed an apparent correspondence with coral cover. There appeared to be a break point of coral cover at an orbital velocity of 1.3 m s−1 and a depth of 35 m.
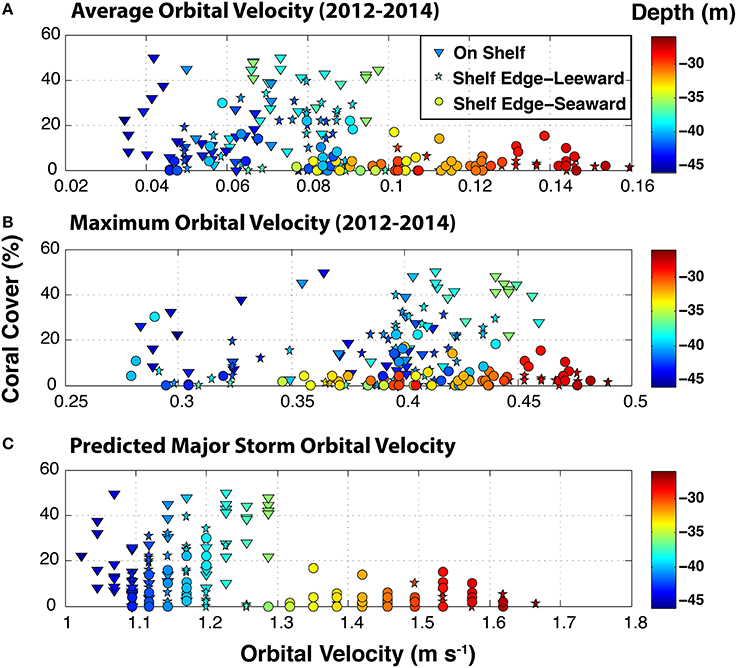
Figure 7. Benthic orbital velocities (m s−1) predicted from the CariCOOS Simulating Waves Nearshore (SWAN) spectral wave model at the surveyed sites on the southeastern Puerto Rican Shelf. Average (A) and maximum (B) velocities are based on the significant wave height, peak period, and water depth at each site and were simulated for the period 2012–2014. Surface wave conditions from SWAN were validated with in situ observations at oceanographic buoy VIA03 (www.caricoos.org; Linear comparison of modeled and empirical wave height: r2 = 0.865, RSME = 0.14 m). Major predicted storm benthic orbital velocities (C) were calculated for surface conditions of 7 m wave heights and 13 s wave periods.
Discussion
The mesophotic coral ecosystem of the southeastern Puerto Rican Shelf is strongly structured by depth, orientation to the open ocean, and position at the shelf edge or on shelf. Two major patterns were apparent. First, there was increasing shelf edge coral cover with increasing depth and decreasing mean orbital velocity, particularly in leeward portions of the shelf edge at deeper western study sites. Second, on shelf banks with a minimum depth of 36 m near the shelf edge had higher coral cover for the same depths and mean benthic orbital velocities. Coral cover was also higher on shelf at shallower depths of ~30 m, more similar to the habitat depths of the eastern shelf edge study region. For example, in the Hind Bank Marine Conservation District a 6.5 km long secondary bank feature about 250 m from the shelf edge and with a top at 28–31 m (Figure 1D) had consistent coral cover greater than 30% (“College Shoal” in Smith et al., 2013; authors, unpub. obs). These two general patterns were largely controlled by the distribution of orbicellid corals, which composed 50, 70, and 90% of total coral cover in seaward, leeward, and on shelf habitats, respectively. In addition, the benthic patterns are likely long-term and have not been caused by recent anthropogenic activities, since the low coral cover pavements appear to have little “standing dead” colonies that would represent the loss to recent disturbance (Figure 2).
Chronic Processes as a Control on Mesophotic Habitat Structure
Explanations for the pattern of coral development could be numerous, since chronic processes/stress, acute disturbances, and failure to recover can limit coral cover. The pattern suggests that the structuring force(s) that limit coral development increase(s) with decreasing depth, except on shelf, and is stronger on the seaward side of the shelf edge. Chollett and Mumby (2012) used daily wind speed on the Belize barrier reef to accurately predict the occurrence of shallow water orbicellid-dominated communities to more sheltered habitats. They suggested chronic inhibition of larval establishment by sand scour as a controlling mechanism. However, on the southeastern Puerto Rican shelf MCE the statistically significant relationship between mean benthic orbital velocity at a sampling site and coral cover may not reflect chronic inhibition due swell. On the edge of the southeastern Puerto Rican Shelf non-storm wave conditions are mild (MeanSignificant Wave Height = 1.04 m ± 0.36 S.D., MeanDominant Wave Period = 6.7 s ± 2.0, MeanWave Direction = 104.3° ± 14.5, N = 33048 hourly observations from Apr. 2011 to Feb. 2015, CariCOOS Oceanographic Buoy VI1, 18.25150° –64.76700°), average benthic orbital speeds are low even in the shallowest areas (<0.2 m s−1) and mean unidirectional currents are also low (<0.15 m s−1; Smith et al., 2010). Thus, the relationship between coral cover and mean orbital velocities may be more indicative of a general relationship with depth, and some process or processes that are correlated with depth. However, for gorgonian species that are dependent on orbital current for feeding or sediment removal (Yoshioka and Yoshioka, 1989) the positive relationship with benthic orbital velocity may indicate a direct relationship.
Other chronic stresses that recur on time scales of less than a year and are known to cause coral mortality do not occur in a spatial pattern that fits the pattern of coral development. Physical factors that might cause intra-annual stress include low and high temperatures and high rates of sedimentation. In general, low temperature stress increases with depth (Bak et al., 2005; Bongaerts et al., 2015) and were not harmful in the study area [i.e., lowest recorded daily mean temperature from 2005 to 2013 at the shelf edge Grammanik Bank Tiger mesophotic reef site (Figure 1C) was 25.1°C at 38 m depth, Smith et al., 2013]. High temperature stress causing coral bleaching has been shown to impact the seaward edge of shallow (<5 m) fringing reefs more severely in the Red Sea, possibly because of lowered thermal tolerance in the moderated mean thermal climate, relative to inshore reefs, caused by the nearby connection to cooler oceanic water (Pineda et al., 2013). The southeastern Puerto Rican shelf within a few kilometers of the shelf edge is sufficiently deep to allow relatively free exchange with oceanic water; during the annual thermal maximum (September to November) mean daily temperatures decrease only slightly (~0.2°C) going inshore at similar depths. This shows that there is no strong horizontal temperature gradient that could drive coral stress and limit coral development (Smith et al., 2016). Chronic sedimentation can also have strong controlling effects on mesophotic coral community structure (Sherman et al., 2010), but the shelf edge is a topographic high which would limit bed transport effects on coral, and total sedimentation recorded by benthic traps was very low on the southeastern Puerto Rican shelf mesophotic reef system (<<1 mg cm−2 day−1, Smith et al., 2008) and had no terrestrial component (Weinstein, 2014).
Biological factors could also affect patterns of shelf edge coral community development. Some disease outbreaks or suspected disease outbreaks can increase with depth, but in the southeastern Puerto Rican shelf mesophotic coral ecosystem are patchily distributed and do not clearly increase or decrease with depth (Menza et al., 2007; Calnan et al., 2008; Smith et al., 2008). One exception was a disease outbreak that was confined to specific mesophotic basin habitat between 42 and 45 m depth where a common environmental driver was suspected (Smith et al., 2010). It is not clear what common environmental driver would operate so specifically on the shelf edge and not affect adjacent on shelf habitats at the same depths and within a few hundred horizontal meters. Described algal blooms affecting mesophotic depths and attributed to nutrient enrichment were localized to anthropogenic waste discharges (Lapointe, 1997) or localized to reef passes and associated with upwelling (Wolanski et al., 1988), and would not explain widespread inhibition of reef development increasing in shallower mesophotic depths. Furthermore, another algal bloom with negative impacts to mesophotic coral and attributed to the invasive Indo-Pacific lionfish (Lesser and Slattery, 2011; Slattery and Lesser, 2014) was not depth-specific and is relatively recent (lionfish were first observed in the mesophotic habitats of the southeastern Puerto Rican Shelf in 2011; Smith et al., 2013).
In addition, if different shelf edge habitats were impacted by different levels of chronic stress then coral health should follow this same pattern, but this was not the case (Supplementary Data Section 2). While coral-cyanobacteria interactions and coral-sediment interactions were increased in the more turbulent, shallow shelf edge habitat, this was not mirrored in patterns of coral partial mortality, disease, or bleaching. This suggests the effects of higher coral interactions or any unknown chronic stressor is not being recorded by coral health characteristics known to respond to external impacts in shallow reefs (Nugues and Roberts, 2003) or MCE (Smith et al., 2008).
Furthermore, in addition to top-down processes (predation, disturbance) described above, bottom-up processes (resource availability) can also influence the distribution of organisms in MCE (Leichter and Genovese, 2006; Lesser and Slattery, 2013). Two recent studies of coral skeletal growth on the northern and southern Puerto Rican shelf and partly within the study area showed that orbicellid linear extension was very low and not significantly different in depths >28 m, regardless of shelf position (Weinstein, 2014; Groves, 2016). This shows that bottom up processes may not be influencing growth patterns of coral cover development. Light decreases with depth and does not appear to be limiting to Orbicella spp. growth except perhaps at the maximum depth of Orbicella spp. occurrence (~45 m). Furthermore, the similarity in mean temperatures and temperature variability between the shelf edge and on shelf habitats (Smith et al., 2016) suggests that there is not a strong difference in upwelling conditions experienced by shelf edge and nearby on shelf banks.
Mechanical Disturbance as a Structuring Force
We suggest that a factor that could account for the upper mesophotic shelf edge coral development pattern is large-scale mechanical disturbance with force originating from the open ocean and declining with depth. Since we did not directly observe this type of disturbance event we cannot estimate the relative importance of storm orbital velocities compared with other factors and, thus, our discussion is hypothetical and will need further testing. However the pattern of the data and the lack of fit to other possible structuring forces points to a role for mechanical disturbance from storm-generated swell and tsunamis. The large total energy of tsunamis could cause damage in mesophotic depths given their behavior as shallow water waves in very deep water and the potential of very strong depth-independent orbital velocities (Holthuijsen, 2007). The last large tsunami to affect the south coast of St. Thomas was 1867 (Watlington, 2006) and, if shelf edge MCE were impacted, there should have been sufficient time in 146 years to increase coral cover above 4%. However, the structuring force of a large tsunami might be preserved for over a century if combined with the more frequent disturbance from tropical storms and large long period swells.
On the southeastern Puerto Rican Shelf edge major storm wave impacts can reach mesophotic depths and may have sufficient strength to directly damage corals. Instrument observations on the northern Puerto Rican shelf at a leeward shelf edge bank 39.5 m depth showed that long-period swell of greater than 13 s and wave heights >4 m caused notable benthic turbulence (Bright et al., 2016). Both Hurricane Hugo and Hurricane Marilyn that passed within 25 years prior to the study were sufficiently strong to generate sea states predicted for a major storm in our calculations. In addition, both hurricanes passed with a northwestward motion with all or much of the study area on the stronger rightward side of the storm, suggesting even stronger translational winds from the south-southeast, an offshelf to onshelf direction. On the southeastern Puerto Rican Shelf predicted benthic orbital velocities for a major storm are strong, ~1.0–1.7 m s−1 between 27 and 43 m. Orbital velocities >1 m s−1 are associated with breakage or dislodgement of branching coral colonies in shallow water reef flats (Baldock et al., 2014). The process of dislodgement is complex and may rely as much on the strength of the underlying substrate than the strength of individual colonies (Madin, 2005). Many coral colonies at mesophotic depths in this study system assume a plating morphology, often stacked on pillars that rise above the substrate 50–100 cm (Smith et al., 2010). This may give these structures a high hydrodynamic colony shape factor (Madin and Connolly, 2006) and increase the risk of dislodgement. Further, in the southeastern Puerto Rican Shelf study system internal bioerosion increases with depth and external bioerosion is the same as shallow water corals where there are abundant parrotfish, such as shallow parts of the banks (Weinstein et al., 2014). This could lead to weakened colony or pillar bases that would increase the possibility of dislodgement.
The long-term consequence of storm damage on shelf edge mesophotic habitats may be limitation of coral development. In shallow water, recovery from high intensity and frequent storm disturbance has been predicted to take decades (Dollar and Tribble, 1993). If the recovery times of mesophotic reefs are even slower than shallow reefs than this could increase the potential of disturbance to shape the community structure in MCE. Corals in the genus Orbicella are the dominant components of coral cover in these upper mesophotic banks (Armstrong, 2007; Smith et al., 2010) and their ability to recover after disturbance should be critical in determining their long-term persistence in certain habitats. Studies, including in the Virgin Islands, have shown that the growth rates (skeletal linear extension) of Orbicella spp. decline precipitously with depth, from ~12 mm yr−1 shallower than 10 m, to as low as 2 mm yr−1 at 30 m, and 0.8 mm yr−1 at 30–40 m (Baker and Weber, 1975; Grauss and Macintyre, 1982; Bosscher, 1993; Weinstein, 2014; Groves, 2016). In addition, four long-term mesophotic study sites on the southeastern Puerto Rican Shelf have not recovered coral cover lost in the 2005 bleaching event (Smith et al., 2016). This would support the potential for slowed recovery in mesophotic depths and the potential for infrequent but high impact disturbances to have lasting impacts on coral community development. The same mechanisms might also apply if other mesophotic coral demographic rates, such as recruitment, are slower than in shallow reefs. Faster recovery in shallow reefs might explain why shallow orbicellids have been able to maintain high coral cover on many shelf edge ecosystems that are exposed to storm disturbance (Foster et al., 2007). Demographic parameters of corals in mesophotic depths are not well-established and deserve increased attention.
Differences in the impacts of storm waves do not explain the change in mean coral cover from shelf edge to on shelf habitats, which was one of the more dramatic patterns seen in the western study area. Unlike shallow water coral reefs where storm waves can break and rapidly dissipate energy (Baldock et al., 2014), mesophotic reefs are sufficiently deep that wave forces from the open ocean are not likely to be strongly attenuated due to bottom friction or depth-induced breaking at the shelf edge. This is especially true given the relatively small cross-shelf scales of the study area, which is on the order of several hundred meters to a few kilometers. Thus, the seaward shelf edge may not be acting as a strong baffle for oceanic storm waves, and benthic orbital velocities and the potential for damage may be similar at a given depth for seaward, leeward and on shelf habitats.
A hypothetical alternate mechanism is that the antecedent geomorphology of the banks allows retention of colonies dislodged by water motion in some areas but not others. The steep slopes of the shelf edge means that colonies dislodged at the top of the bank (27–34 m depth) would be transported seaward and off shelf or into the leeward zone. In the leeward zone they may end up too deep to establish, which might tend to occur in the eastern portion of the study area below St. John where the lee shelf is very steep and levels out in water >50 m. On the other hand, where the leeward deep habitat levels out in depths appropriate for orbicellids (35–43 m depth) the colonies may persist and eventually reattach, such as in the western study area where deep leeward coral cover was higher than anywhere else on the shelf edge. Flat bank tops located on shelf, with dense networks of coral pillars, may further enhance retention of dislodged colonies, allowing maintenance of high coral cover even when the reefs are damaged. Quantification of the response to a major storm at long-term, permanent monitoring sites will help to reveal if these processes are indeed structuring this large mesophotic coral ecosystem.
While the impact of tropical storms at mesophotic depths is not a new observation, the possibility that storms structure mesophotic communities over large horizontal scales is novel. Previous studies of storm and swell impacts to mesophotic habitats presented an episodic disturbance with localized impacts. Here we are suggesting acute but infrequent swell impacts may have structured an entire mesophotic habitat. If true, then in the wider Caribbean we might expect that many shelf margins that would otherwise support mesophotic orbicellid-dominated coral reefs might be limited by swell impacts. It would be valuable for researchers to attempt broad-scale sampling of mesophotic coral ecosystems before and after disturbance events to add to our understanding of storm or tsunami impacts with depth and their potential to structure reef habitats.
Author Contributions
TS, VB, MC, MB, DH, JM, and EK designed the research. DH, TS, VB, and MC analyzed and interpreted the data. All authors conducted data acquisition, reviewed and edited the manuscript, and approved the final version.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank A. Sabine, L.M. Henderson, and R. Sjoken for assistance in the field; I. Byrne and S. Prosterman for critical logistical support; J. Jossart and J. Gyory for analysis of temperature data, and R.S. Nemeth to helpful discussions. This work was funded by the Black Coral Penalty Fund, the Black Coral Community Service fund, The Lana Vento Charitable Trust, and the VI Experimental Program to Stimulate Competitive Research (NSF#0814417), and the NOAA Coral Reef Conservation Program (#NA11NOS4820001). The funders had no input on the design or interpretations of the study. This is contribution #129 from the Center for Marine and Environmental Studies.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/article/10.3389/fmars.2016.00115
References
Adey, W. H., Macintyre, I. G., and Stuckenrath, R. (1977). “Relict barrier reef system off St. Croix: its implication with respect to late Cenozoic coral reef development in the Western Atlantic,” in Proceedings of Third International Coral Reef Symposium, Vol. 2 (Miami, FL: Rosenstiel School of Marine and Atmospheric Science, University of Miami), 15–21.
Anselmi, C., Canals, M., Morell, J., Gonzalez, J., Capella, J., and Mercado, A. (2010). Development of an operational nearshore wave forecast system for Puerto Rico and the U.S. Virgin Islands. J. Coast. Res. 28, 1049–1056. doi: 10.2112/JCOASTRES-D-11-00132.1
Armstrong, R. A. (2007). Deep zooxanthellate coral reefs of the Puerto Rico: U.S. Virgin Islands insular platform. Coral Reefs 26:945. doi: 10.1007/s00338-007-0286-y
Armstrong, R. A., Singh, H., Torres, J., Nemeth, R. S., Can, A., Roman, C., et al. (2006). Characterizing the deep insular shelf coral reef habitat of the Hind Bank marine conservation district (U.S. Virgin Islands) using the Seabed autonomous underwater vehicle. Cont. Shelf Res. 26, 194–205. doi: 10.1016/j.csr.2005.10.004
Aronson, R. B., Sebens, K. P., and Ebersole, J. P. (1994). “Hurricane Hugo's impact on Salt River submarine canyon, St. Croix, U.S. Virgin Islands,” in Proceedings of the Colloquim on Global Aspects of Coral Reefs: Health, Hazards, and History (Miami, FL: University of Miami).
Bak, R., Nieuwland, G., and Meesters, E. (2005). Coral reef crisis in deep and shallow reefs: 30 years of constancy and change in reefs of Curacao and Bonaire. Coral Reefs 24, 475–479. doi: 10.1007/s00338-005-0009-1
Baker, P. A., and Weber, J. N. (1975). Coral growth rate: variation with depth. Phys. Earth Planet Inter. 10, 135–139. doi: 10.1016/0031-9201(75)90031-X
Baldock, T. E., Karampour, H., Sleep, R., Vyltla, A., Albermani, F., Golshani, A., et al. (2014). Resilience of branching and massive corals to wave loading under sea level rise – A coupled computational fluid dynamics-structural analysis. Mar. Pollut. Bull. 86, 91–101. doi: 10.1016/j.marpolbul.2014.07.038
Bongaerts, P., Frade, P. R., Hay, K. B., Englebert, N., Latijnhouwers, K. R. W., Bak, R. P. M., et al. (2015). Deep down on a Caribbean reef: lower mesophotic depths harbor a specialized coral-endosymbiont community. Sci. Rep. 5:7652. doi: 10.1038/srep07652
Bongaerts, P., Muir, P., Englebert, N., Bridge, T. C. L., and Hoegh-Guldberg, O. (2013). Cyclone damage at mesophotic depths on Myrmidon Reef (GBR). Coral Reefs 32, 935–935. doi: 10.1007/s00338-013-1052-y
Bongaerts, P., Ridgway, T., Sampayo, E., and Hoegh-Guldberg, O. (2010). Assessing the ‘deep reef refugia’ hypothesis: focus on Caribbean reefs. Coral Reefs 29, 309–327. doi: 10.1007/s00338-009-0581-x
Booij, N., Ris, R. C., and Holthuijsen, L. (1999). A third generation wave model for coastal regions. Part 1: model description and validation. J. Geophys. Res. C4, 7649–7666. doi: 10.1029/98JC02622
Bosscher, H. (1993). Computerized tomography and skeletal density of coral skeletons. Coral Reefs 12, 97–103. doi: 10.1007/BF00302109
Bridge, T., Beaman, R., Done, T., and Webster, J. (2012). Predicting the location and spatial extent of submerged coral reef habitat in the Great Barrier Reef World Heritage Area, Australia. PLoS ONE 7:e48203. doi: 10.1371/journal.pone.0048203
Bridge, T. C. L., Hughes, T. P., Guinotte, J. M., and Bongaerts, P. (2013). Call to protect all coral reefs. Nat. Clim. Chang. 3, 528–530. doi: 10.1038/nclimate1879
Bridge, T., Done, T., Beaman, R., Friedman, A., Williams, S., Pizarro, O., et al. (2011). Topography, substratum and benthic macrofaunal relationships on a tropical mesophotic shelf margin, central Great Barrier Reef, Australia. Coral Reefs 30, 143–153. doi: 10.1007/s00338-010-0677-3
Bright, A. J., Rogers, C., Brandt, M., Muller, E., and Smith, T. B. (2016). Disease prevalence and snail predation associated with swell-generated damage on the threatened coral, Acropora palmata (Lamarck). Front. Marine Sci. 3:77. doi: 10.3389/fmars.2016.00077
Calnan, J., Smith, T. B., Nemeth, R., Kadison, E., and Blondeau, J. (2008). Coral disease prevalence and host susceptibility on mid-depth and deep reefs in the U.S. Virgin Islands. Revista Biol. Trop. 56, 223–224. doi: 10.15517/rbt.v56i0.5589
Canals, M., Morell, J., Corredor, J. E., and Leonardi, S. (2012). “Expanding the Caribbean Coastal Ocean Observing System into the nearshore region,” in Oceans, 2012 (Hampton Roads, VA: IEEE). doi: 10.1109/OCEANS.2012.6404936
Chaytor, J. D., and ten Brink, U. S. (2015). Event sedimentation in low-latitude deep-water carbonate basins, Anegada passage, northeast Caribbean. Basin Res. 27, 310–335. doi: 10.1111/bre.12076
Chollett, I., and Mumby, P. (2012). Predicting the distribution of Montastraea reefs using wave exposure. Coral Reefs 31, 493–503. doi: 10.1007/s00338-011-0867-7
Dollar, S. J., and Tribble, G. W. (1993). Recurrent storm disturbance and recovery: a long-term study of coral communities in Hawaii. Coral Reefs 12, 223–233. doi: 10.1007/BF00334481
Done, T. (1983). “Coral zonation: its nature and significance,” in Perspectives on Coral Reefs, eds D. J. Barnes and B. Clouston (Townsville, QLD: Australian Institute of Marine Science), 107–147.
Foster, N. L., Baums, I. B., and Mumby, P. J. (2007). Sexual vs. asexual reproduction in an ecosystem engineer: the massive coral Montastraea annularis. J. Anim. Ecol. 76, 384–391. doi: 10.1111/j.1365-2656.2006.01207.x
Ginsburg, R. N. (2007). “Mesophotic coral reefs (30-100m) are the frontier of reef exploration and research,” in 33rd Scientific Meeting of the Association of the Marine Labs of the Caribbean (St. Thomas, U.S. Virgin Islands).
Goldberg, W. M. (1983). Cay Sal Bank, Bahamas: a biologically impoverished physically controlled environment. Atoll Res. Bull. 271, 1–17. doi: 10.5479/si.00775630.271.1
Grauss, R. R., and Macintyre, I. G. (1982). Variation in growth forms of the reef coral Montastrea annularis (Ellis and Solander): a quantitative evaluation of growth response to light distribution using computer simulation. Smithson. Contrib. Mar. Sci. 12, 441–464.
Grigg, R. (2006). Depth limit for reef building corals in the Au'au Channel, S.E. Hawaii. Coral Reefs 25, 77–84. doi: 10.1007/s00338-005-0073-6
Groves, S. (2016). Physical Drivers of Community Structure and Growth among Mesophotic Coral Ecosystems Surrounding. Master's Thesis, University of the Virgin Islands, St. Thomas, U.S. Virgin Islands.
Harmelin-Vivien, M. L. (1994). The effects of storms and cyclones on coral reefs: a review. J. Coast. Res. 12, 211–231. Available online at: http://www.jstor.org/stable/25735600
Hinderstein, L., Marr, J., Martinez, F., Dowgiallo, M., Puglise, K., Pyle, R., et al. (2010). Theme section on mesophotic coral ecosystems: characterization, ecology, and management. Coral Reefs 29, 247–251. doi: 10.1007/s00338-010-0614-5
Holthuijsen, L. H. (2007). Waves in Oceanic and Coastal Waters. Cambridge: Cambridge University Press.
Hubbard, D. K. (1992). Hurricane-induced sediment transport in open-shelf tropical systems - an example from St. Croix, U.S. Virgin Islands. J. Sediment. Petrol. 62, 946–960.
Hubbard, D. K., Parsons, K. M., Bythell, J. C., and Walker, N. D. (1991). The effects of Hurricane Hugo on the reefs and associated environments of St. Croix, U.S. Virgin Islands—a preliminary assessment. J. Coast. Res. 8, 33–48.
Kahng, S., and Kelley, C. (2007). Vertical zonation of megabenthic taxa on a deep photosynthetic reef (50–140 m) in the Au'au Channel, Hawaii. Coral Reefs 26, 679–687. doi: 10.1007/s00338-007-0253-7
Kobluk, D. R., and Lysenko, M. A. (1994). Ring bleaching in southern Caribbean Agaricia agaricites during rapid water cooling. Bull. Mar. Sci. 54, 142–150.
Kohler, K., and Gill, S. M. (2006). Coral Point Count with Excel extensions (CPCe): a visual basic program for the determination of coral and substrate coverage using random point count methodology. Comput. Geosci. 32, 1259–1269. doi: 10.1016/j.cageo.2005.11.009
Lapointe, B. (1997). Nutrient thresholds for bottom-up control of macroalgal blooms on coral reefs in Jamaica and southeast Florida. Limnol. Oceanogr. 42, 1119–1131. doi: 10.4319/lo.1997.42.5_part_2.1119
Leichter, J. J., and Genovese, S. J. (2006). Intermittent upwelling and subsidized growth of the scleractinian coral Madracis mirabilis on the deep fore-reef of Discovery Bay, Jamaica. Mar. Ecol. Prog. Ser. 316, 95–103. doi: 10.3354/meps316095
Lesser, M. P., and Slattery, M. (2011). Phase shift to algal dominated communities at mesophotic depths associated with lionfish (Pterois volitans) invasion on a Bahamian coral reef. Biol. Invasions 13, 1855–1868. doi: 10.1007/s10530-011-0005-z
Lesser, M. P., and Slattery, M. (2013). Ecology of Caribbean sponges: are top-down or bottom-up processes more important? PLoS ONE 8:e79799. doi: 10.1371/journal.pone.0079799
Lesser, M. P., Slattery, M., and Leichter, J. J. (2009). Ecology of mesophotic coral reefs. J. Exp. Mar. Biol. Ecol. 375, 1–8. doi: 10.1016/j.jembe.2009.05.009
Lugo-Fernández, A., and Gravois, M. (2010). Understanding impacts of tropical storms and hurricanes on submerged bank reefs and coral communities in the northwestern Gulf of Mexico. Cont. Shelf Res. 30, 1226–1240. doi: 10.1016/j.csr.2010.03.014
Madin, J. (2005). Mechanical limitations of reef corals during hydrodynamic disturbances. Coral Reefs 24, 630–635. doi: 10.1007/s00338-005-0042-0
Madin, J. S., and Connolly, S. R. (2006). Ecological consequences of major hydrodynamic disturbances on coral reefs. Nature 444, 477–480. doi: 10.1038/nature05328
Menza, C., Kendall, M., and Hile, S. (2008). The deeper we go the less we know. Revista Biol. Trop. 56, 11–24.
Menza, C., Kendall, M., Rogers, C., and Miller, J. (2007). A deep reef in deep trouble. Cont. Shelf Res. 27, 2224–2230. doi: 10.1016/j.csr.2007.05.017
Nemeth, R., and Kadison, E. (2013). Temporal patterns and behavioral characteristics of aggregation formation and spawning in the Bermuda chub (Kyphosus sectatrix). Coral Reefs 32, 1067–1076. doi: 10.1007/s00338-013-1050-0
Nugues, M. M., and Roberts, C. M. (2003). Partial mortality in massive reef corals as an indicator of sediment stress on coral reefs. Mar. Pollut. Bull. 46, 314–323. doi: 10.1016/S0025-326X(02)00402-2
Pineda, J., Starczak, V. R., Tarrant, A., Blythe, J., Davis, K., Farrar, J., et al. (2013). Two spatial scales in a bleaching event: corals from the mildest and the most extreme thermal environments escape mortality. Limnol. Oceanogr. 58, 1531–1545. doi: 10.4319/lo.2013.58.5.1531
Rivera, J., Prada, M., Arsenault, J.-L., Moody, G., and Benoit, N. (2006). Detecting Fish Aggregations from Reef Habitats Mapped with High Resolution Side Scan Sonar Imagery. Washington, DC: NOAA Professional Paper NMFS.
Robbart, M. L., Aronson, R. B., Deslarzes, K. J. P., Precht, W. F., Duncan, L., Zimmer, B., et al. (2009). Post-Hurricane Assessment of Sensitive Habitats of the Flower Garden Banks Vicinity (New Orleans, LA: U.S. Department of Interior; Minerals Managament Service; Gulf of Mexico, OCS Region).
Robbart, M. L., Deslarzes, K. J. P., Precht, W. F., Aronson, R. B., Zimmer, B., Duncan, L., et al. (2008). “Post-hurricane assessment (Hurricane Rita, September 2005) and recovery at the East Flower Garden Bank, Northwestern Gulf of Mexico,” in Proceedings of the 11th International Coral Reef Symposium (Fort Lauderdale, FL: Nova Southeastern University), 795–799.
Rogers, C. S., Mclain, L. N., and Tobias, C. R. (1991). Effects of Hurricane Hugo (1989) on a coral reef in St. John USVI. Mar. Ecol. Prog. Ser. 78, 189–199. doi: 10.3354/meps078189
Sheppard, C. R. C. (1982). Coral populations on reef slopes and their major controls. Mar. Ecol. Prog. Ser. 7, 83–115. doi: 10.3354/meps007083
Sherman, C., Nemeth, M., Ruíz, H., Bejarano, I., Appeldoorn, R., Pagán, F., et al. (2010). Geomorphology and benthic cover of mesophotic coral ecosystems of the upper insular slope of southwest Puerto Rico. Coral Reefs 29, 347–360. doi: 10.1007/s00338-010-0607-4
Slattery, M., and Lesser, M. P. (2014). Allelopathy in the tropical alga Lobophora variegata (Phaeophyceae): mechanistic basis for a phase shift on mesophotic coral reefs? J. Phycol. 50, 493–505. doi: 10.1111/jpy.12160
Slattery, M., Lesser, M. P., Brazeau, D., Stokes, M. D., and Leichter, J. J. (2011). Connectivity and stability of mesophotic coral reefs. J. Exp. Mar. Biol. Ecol. 408, 32–41. doi: 10.1016/j.jembe.2011.07.024
Smith, T. B., Blondeau, J., Nemeth, R. S., Pittman, S. J., Calnan, J. M., Kadison, E., et al. (2010). Benthic structure and cryptic mortality in a Caribbean mesophotic coral reef bank system, the Hind Bank Marine Conservation District, U.S. Virgin Islands. Coral Reefs 29, 289–308. doi: 10.1007/s00338-009-0575-8
Smith, T. B., Gyory, J., Brandt, M. E., Miller, W. J., Jossart, J., and Nemeth, R. S. (2016). Caribbean mesophotic coral ecosystems are unlikely climate change refugia. Glob. Chang. Biol. doi: 10.1111/gcb.13175. [Epub ahead of print].
Smith, T. B., Kadison, E., Henderson, L., Gyory, J., Brandt, M. E., Wright, V., et al. (2013). The United States Virgin Islands Territorial Coral Reef Monitoring Program. 2013 Annual Report (U.S. Virgin Islands: University of the Virgin Islands).
Smith, T. B., Nemeth, R. S., Blondeau, J., Calnan, J. M., Kadison, E., and Herzlieb, S. (2008). Assessing coral reef health across onshore to offshore stress gradients in the U.S. Virgin Islands. Mar. Pollut. Bull. 56, 1983–1991. doi: 10.1016/j.marpolbul.2008.08.015
Watlington, R. (2006). “An 1867-class tsunami: potential devastation in the U.S. Virgin Islands,” in Caribbean Tsunami Hazard, eds A. Mercado-Irizarry and P. L. Liu (Hackensack, NJ: World Scientific Publishing Co., Inc.), 341.
Weinstein, D. K. (2014). Deep Reef Bioerosion and Deposition: Sedimentology of Mesophotic Coral Reefs in the U.S. Virgin Islands. Ph.D. Thesis, University of Miami, Miami, FL.
Weinstein, D. K., Smith, T. B., and Klaus, J. S. (2014). Mesophotic bioerosion: variability and structural impact on U.S. Virgin Island deep reefs. Geomorphology 222, 14–24. doi: 10.1016/j.geomorph.2014.03.005
White, K. N., Ohara, T., Fujii, T., Kawamura, I., Mizuyama, M., Montenegro, J., et al. (2013). Typhoon damage on a shallow mesophotic reef in Okinawa, Japan. Peer J. 1:e151. doi: 10.7717/peerj.151
Wolanski, E., Colin, P., Naithani, J., Deleersnijder, E., and Golbuu, Y. (2004). Large amplitude, leaky, island-generated, internal waves around Palau, Micronesia. Estuar. Coast. Shelf Sci. 60, 705–716. doi: 10.1016/j.ecss.2004.03.009
Wolanski, E., Drew, E., Abel, K., and O'brien, J. (1988). Tidal jets, nutrient upwelling and their influence on the productivity of the alga Halimeda in the Ribbon Reefs, Great Barrier Reef. Estuar. Coast. Shelf Sci. 26, 169–201. doi: 10.1016/0272-7714(88)90049-2
Woodley, J. D., Chornesky, E. A., Clifford, P. A., Jackson, J. B. C., Kaufman, L. S., Knowlton, N., et al. (1981). Hurricane Allen's impact on Jamaican coral reefs. Science 214, 749–755. doi: 10.1126/science.214.4522.749
Keywords: shelf edge reefs, Orbicella spp., physical disturbance, chronic stress, swell, wave modeling, orbital velocities, remote sensing
Citation: Smith TB, Brandtneris VW, Canals M, Brandt ME, Martens J, Brewer RS, Kadison E, Kammann M, Keller J and Holstein DM (2016) Potential Structuring Forces on a Shelf Edge Upper Mesophotic Coral Ecosystem in the US Virgin Islands. Front. Mar. Sci. 3:115. doi: 10.3389/fmars.2016.00115
Received: 09 December 2015; Accepted: 16 June 2016;
Published: 29 June 2016.
Edited by:
Andrew Stanley Mount, Clemson University, USAReviewed by:
Peter Edmunds, California State University, Northridge, USAMarc Slattery, University of Mississippi, USA
Copyright © 2016 Smith, Brandtneris, Canals, Brandt, Martens, Brewer, Kadison, Kammann, Keller and Holstein. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tyler B. Smith, tsmith@uvi.edu
 Tyler B. Smith
Tyler B. Smith Viktor W. Brandtneris
Viktor W. Brandtneris Miguel Canals2
Miguel Canals2  Marilyn E. Brandt
Marilyn E. Brandt Justin Martens
Justin Martens Jessica Keller
Jessica Keller