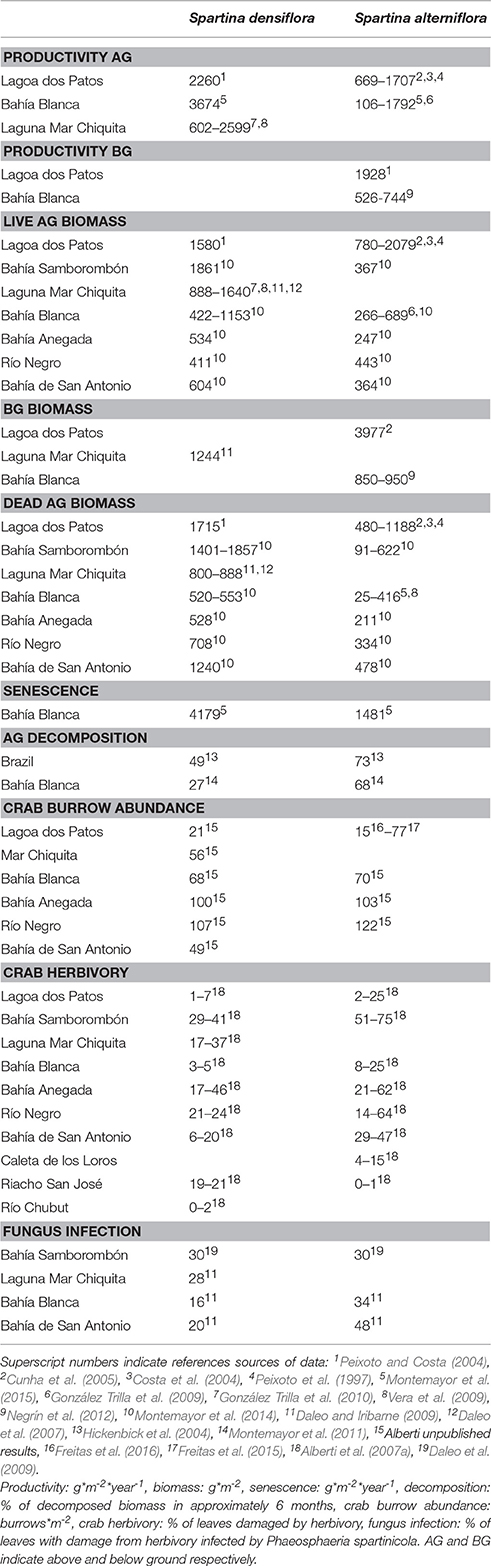
Crab Bioturbation and Herbivory May Account for Variability in Carbon Sequestration and Stocks in South West Atlantic Salt Marshes
- 1Laboratorio de Ecología, Instituto de Investigaciones Marinas y Costeras (Consejo Nacional de Investigaciones Científicas y Técnicas-Universidad Nacional de Mar del Plata), Mar del Plata, Argentina
- 2Laboratório de Biotecnologia de Halófitas, Instituto de Oceanografia, Universidade Federal do Rio Grande, Rio Grande, Brasil
Coastal vegetation plays an important role for climate change mitigation. Compared with terrestrial ecosystems, coastal vegetation shows higher rates of atmospheric CO2 uptake and a more efficient retention of carbon (C) in sediments. Salt marshes present the highest values as C binders, although a global estimation of these values is still pending due to regional gaps in the records predominantly from the southern hemisphere. There are no clear patterns or dominant processes with enough evidence to account for the observed variability, suggesting that context dependent processes are likely greatest influencers on C storage. Salt marshes in the South West Atlantic (SWA) coast are densely populated by the intertidal burrowing and herbivore crab Neohelice (= Chasmagnathus) granulata. Many ecological processes related to C transformation occurring in these salt marshes are influenced by crab activities, either through bioturbation or via herbivory. We hypothesize that N. granulata could have a significant role in the capacity of SWA salt marshes to bind C. Reduction of plant biomass, increased aerobic decomposition in the sediment and facilitation of erosion are some of the multiple effects exerted by N. granulata that can directly and indirectly modify the capacity of salt marshes to bind C. Here, we compiled information available regarding C sequestration and accumulation in SWA coastal salt marshes and propose a hypothetical model including the mechanisms mediated by N. granulata that interfere the transformation paths of C in salt marshes. The data suggest that mechanisms that are top-down regulated, negatively affect C accumulation in the form of aboveground biomass especially in salt marshes dominated by Spartina alterniflora. While, mechanisms mediated by bioturbation can negatively (increasing oxygenation and thus facilitating aerobic degradation) affect as well as positively (increasing retention of macrodetritus) affect the accumulation of C, the latter being of greater magnitude in Spartina densiflora salt marshes.
Introduction
Vegetated coastal ecosystems (salt marshes, mangroves, seagrass meadows) provide many goods and services. For instance they play a fundamental role dissipating wave energy and limiting the effects of sea level rise (Gedan et al., 2011), act as filters, cycling the excess of land-derived nutrients (McGlathery et al., 2007), and provide a habitat for many economically important species (Beck et al., 2001). However, although these benefits are well recognized, degradation and loss of these ecosystems are continuously occurring at accelerating rates (Duarte et al., 2008; Valiela et al., 2009).
Recently, a new service derived from coastal vegetation has been identified concerning the role that these ecosystems play in climate change mitigation, with particular focus on the role of coastal plant communities in sequestering and storing atmospheric CO2 (Nellemann et al., 2009; Duarte et al., 2013). Vegetated coastal ecosystems are efficient carbon binders exceeding the well know capacity of terrestrial forests. Reported average C burial rates (CBR) for coastal vegetation range between 138 and 244 g C m−2 year−1 while temperate, tropical and boreal forests range between 4 and 5 g C m−2 year−1 (McLeod et al., 2011; Ouyang and Lee, 2014). In addition to the efficient CBR, marine vegetated ecosystems can store C for millennia (Mateo et al., 1997; Lo Iacono et al., 2008; Chmura, 2009), while terrestrial vegetation does so for decades or centuries (Chambers et al., 2001). Thus, preservation and restoration of coastal vegetated ecosystems represent a win-win scenario for climate change mitigation implementation: on the one side the preservation and restoration of these ecosystems secure an active and efficient uptake of atmospheric CO2, and in the other, their preservation prevents the emission of greenhouse gases by exposing to degradation the large amount of carbon stored in sediments and live biomass. In this context, the term “blue carbon” has emerged to indicate C that is sequestered and stored in coastal marine environments (Nellemann et al., 2009).
Blue carbon is the most recently acknowledged ecosystem service provided by salt marshes (Chmura, 2013). Historically, the high productivity of salt marshes has been more linked with the export of energy to adjacent systems and the support of a significant fraction of the metabolism that takes place within the water column rather than binding organic matter (OM) in salt marsh sediments (Valiela et al., 2000). There are some characteristics that make these ecosystems particularly good as C binders. For instance, every CO2 molecule stored in salt marsh and mangrove soils has an added value due to the negligible rate of emission of others greenhouse gases such as methane (Chmura, 2009). Marine sediments present large concentrations of sulfate which inhibits the activity of methanogen bacteria (Winfrey and Ward, 1983) limiting, thus, the emission of methane. The average CBR reported for salt marshes is quite similar to that found in mangroves and over 1.75 fold higher than that reported for seagrasses (244.7, 226 and 138 g C m−2 year−1 for salt marshes, mangroves and seagrasses respectively; McLeod et al., 2011; Ouyang and Lee, 2014). Nevertheless, there is a huge variability in CBR for salt marshes around the world, ranging from 18 to 1713 g C m−2 year−1 (Ouyang and Lee, 2014). The maximum CBR reported for salt marshes is almost twice than the maximum reported in mangrove ecosystems (949 g C m−2 year−1) and 9 times higher than the maximum CBR for seagrass meadows (190 g C m−2 year−1; McLeod et al., 2011). This difference among systems highlights the potential of salt marsh ecosystems for binding C as well as the need for further studies on the determinant factors for sequestration and remobilization of C from marsh sediments.
In spite of the effort to estimate global C storage and sequestration rates in salt marshes, there is a gap in data mostly from the southern hemisphere (Chmura, 2013; Ouyang and Lee, 2014). Given the enormous variability in CBR registered in salt marshes worldwide, global estimations calculated with the current data available is probably biased. There are not yet clear general patterns or a dominant process with enough evidence to account for this variability. Some of the main factors described to influence C sequestration in coastal wetland habitats are: local geomorphology, nutrient availability, hydroperiod, salinity, and suspended sediment supply. For instance, sediment grain size has recently found to be a good predictor of C storage in SE Australian salt marshes (Kelleway et al., 2016). There are also inherent characteristics to plant species that dominate within different salt marshes that are linked to the C burial capacity, such as allocation of plant parts, decomposition rates and primary productivity. These characteristics are, in turn, influenced by physical factors such as temperature, precipitation, tidal range, nutrients, and granulometry; as well as biological (plant competition, bioturbation, trophic cascades; McLeod et al., 2011). A previous study attempting to explain the variability in CBR was based on the type of halophyte dominating the salt marsh. In that analysis, Distichlis was found to have the lowest average CBR, while Spartina had the highest (Ouyang and Lee, 2014). However, when the data is carefully examined, both maximum and minimum CBR reported correspond to Spartina dominated salt marshes. This pattern suggests that there are more site specific characteristics to explain such a large variability related to the ecological functioning of each particular location.
Although, burrowing and herbivorous organisms often inhabit vegetated coastal ecosystems, their effects on C stocks are scarcely known, but evidence shows that they can be relevant to Blue C studies. For instance, the activity of intertidal burrowing crabs (Ucides cordatus and Uca maracoani) enhance the decomposition of OM in the soil of Brazilian mangroves reducing up to 70% the total organic carbon (Araújo et al., 2012). In salt marshes in Cape Cod (Mass, USA), the crab Sesarma reticulatum increases erosion by burrowing near-water sediment and reduces plant biomass by herbivory (Coverdale et al., 2014). These two examples are evidence that the activity of these organisms reduce the capacity of these environments to bind C.
Salt marshes along the Atlantic coast of South America are mostly dominated by two species of Spartina (S. densiflora and S. alterniflora) and Sarcocornia spp. (Figures 1A–C). Most of these salt marshes (except for those located at the southernmost extreme, from 42° 25′ S to 53° 48′ S) are highly bioturbated by the burrowing crab Neohelice (= Chasmagnathus) granulata (Figures 1D–F, Iribarne et al., 1997). This crab is also an herbivore (Iribarne et al., 1997) and exerts a strong top-down control on salt marsh plants (Costa et al., 2003; Alberti et al., 2007a). Many ecological processes related to C transformation occurring in these salt marshes are influenced by crab activities, either through bioturbation or via herbivory (Alberti et al., 2015). Thus, we hypothesize that N. granulata could have a significant role in the capacity of South West Atlantic salt marshes to bind C. Reduction of plant biomass, increment of aerobic decomposition in the sediment and facilitation of erosion are some of the multiple effects exerted by N. granulata that can directly and indirectly modify the C stored in these salt marshes.

Figure 1. Typical South American salt marshes dominated by Spartina densiflora (A), Sarcocornia spp. (B), and S. alterniflora (C). Neohelice granulata burrowing bed in a S. alterniflora salt marsh showing erosion and plant roots exposed through the burrows (D). N. granulata with burrow in S. alterniflora (E) and S. desiflora (F) salt marshes. Photo credits: P. Martinetto (A,C–E), J. Alberti (B), and P. Daleo (F).
In this study we compiled the available information regarding the processes affected by N. granulata linked to the pathways of C in South American salt marshes. Based on this information, we propose a model including the mechanisms that regulate C transformation paths in salt marshes mediated by N. granulata.
The South West Atlantic Salt Marsh Environment
South West Atlantic (SWA) salt marshes span ~ 4300 km of coastline from Rio Mampituba (29° S) in Southern Brazil to Río Grande in the Southern Argentinean Patagonia (53° S, Table 1). The quite large latitudinal range of distribution (~24°) includes geomorphologic as well as climatic variations (Costa and Davy, 1992; Isacch et al., 2006). Dominant plant species are the cordgrasses S. densiflora and S. alterniflora and the glasswort Sarcocornia spp. (formerly named Sarcocornia perennis; Costa and Davy, 1992; Isacch et al., 2006; Bortolus et al., 2009). These plant species dominate ~70% of the SWA salt marshes and their relative abundance is closely related to the input of freshwater in each location. Spartina densiflora is the dominant species in areas with higher freshwater input while S. alterniflora and Sarcocornia spp. dominate more saline sites (Isacch et al., 2006). In those salt marshes where both Spartina species coexist, S. alterniflora occupies low intertidal areas, being daily affected by tides while S. densiflora remains in the upper intertidal zone where the inundation only occurs during high spring tides. The loss of salt marsh area in the SWA coast is almost entirely related to degradation as a result of anthropogenic activities. Eutrophication (Cardoni et al., 2011), land-fill (Costa et al., 2009; Marangoni and Costa, 2009; Pratolongo et al., 2013), fire to improve cattle forage as well as to prevent accidental fires (Bortolus and Iribarne, 1999; Isacch et al., 2004), and farming/ranching (Marangoni and Costa, 2010) are the primary causes of salt marsh loss and conversion.
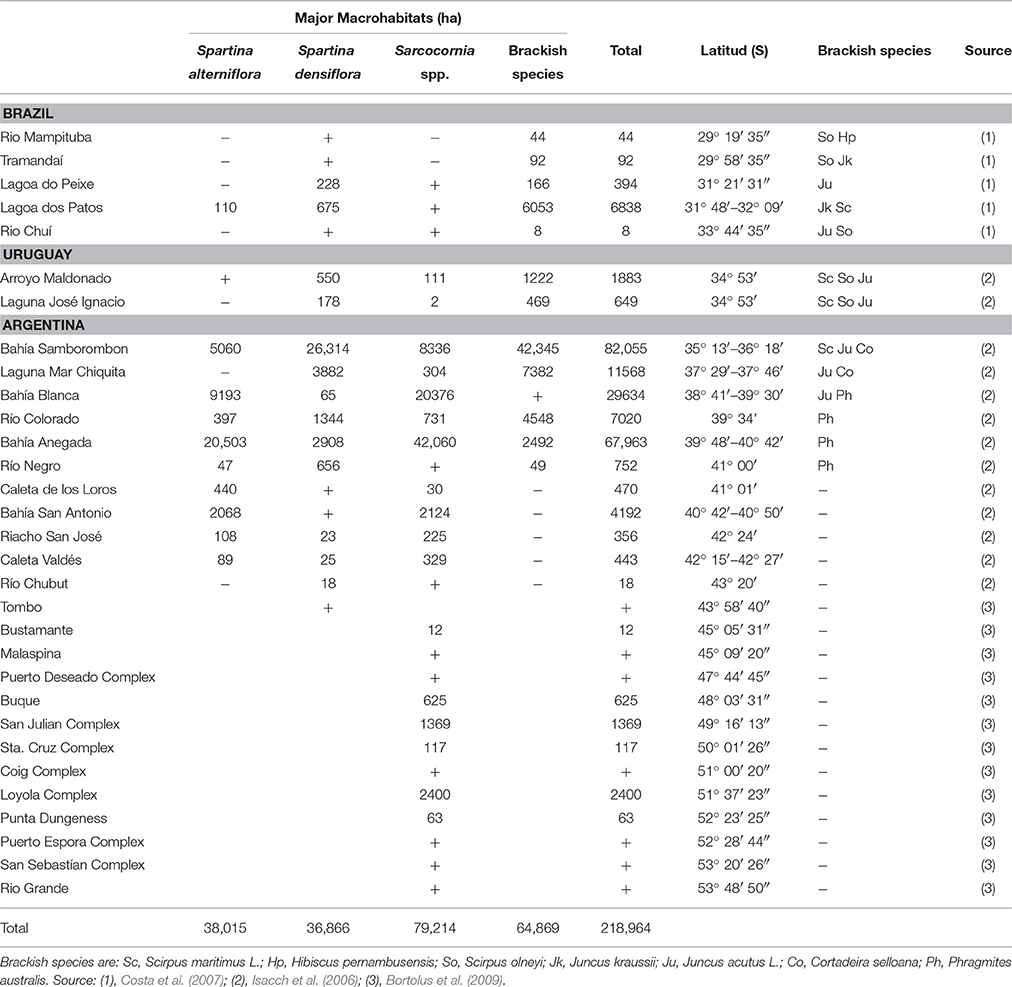
Table 1. Salt marshes in the South West Atlantic coast with dominant plant species, area and location.
Crab beds of Neohelice granulata constitute a conspicuous characteristic of SWA coastal areas from tidal flats to salt marshes. Crab density in the salt marsh is variable both within and among salt marshes ranging from 6 to more than 120 burrows per m2 (Alberti et al., 2007a, Table 2). There is usually one crab and one entrance per burrow (Iribarne et al., 1997). Therefore, burrows density is a good proxy widely used to estimate crab abundance without using destructive sampling methods. N. granulata inhabits SWA salt marshes located above 42° 25′S, and these salt marshes correspond to ~98% of the total area covered by salt marsh in the SWA coast (Table 1). As a bioturbator, this crab can remove up to 2.4 kg of sediment per day per m2 and their burrows can reach up to 1 m depth and range from 2 to 7.5 cm in entrance diameter (Iribarne et al., 1997). The impacts on ecological functions in SWA salt marshes are either via herbivory, consuming large amounts of plant biomass, or via bioturbation through the construction and maintenance of their burrows (Alberti et al., 2015).
Although there are other burrower (e.g., small invertebrates such as Laeonereis culveri in Argentinean salt marshes, Albano et al., 2012 and Nephtys fluviatilis and Kalliapseudes schubartii in Brazil C. Costa per. Obs.) and herbivore organisms (stem-borer moths, Canepuccia et al., 2010 and rodents in the upper marsh Pascual et al., 2015) inhabiting the SWA salt marshes, none have effects comparable to those of N. granulata on salt marsh ecological functioning (Alberti et al., 2015). Rodents can have a large impact via herbivory by reducing plant biomass, but these are restricted to the upper marsh (Costa et al., 2004; Pascual et al., 2015) and they inhabit fully terrestrial vegetation, thus play no role as burrowers in the salt marsh. Cyrtograpsus angulatus is a grapsid crab that builds burrows in salt marshes in Caleta Valdés, at the southern limit of N. granulata distribution (42°15′S, Iribarne et al., 2003). However, when both species coexist in northern coastal areas, C. angulatus is limited to inhabit the low intertidal and it never inhabits salt marsh areas (Martinetto et al., 2007). C. angulatus burrows are similar in shape to those of N. granulata, and it has been proposed that these species play similar roles modifying sediment structure and affecting the infaunal community in soft-bottom intertidal areas (Martinetto et al., 2011); however its effects on salt marsh sediments have never been explored.
Given the large variability in CBR estimated in salt marshes around the world and the difficulties in identifying a variable that can explains this variability (Ouyang and Lee, 2014), local conditions seem to be key, although the principal mechanisms are often unknown. In this context, crabs strongly influence almost every single step from primary production to carbon sequestration in SWA salt marshes and we believe that is a primary driver in modulating their CBR. Below we investigate the multiple ecological functions linked to C sequestration and storage in SWA salt marshes mediated by N. granulata through herbivory and bioturbation.
Effects Mediated by Herbivory
Neohelice granulata consumes large amounts of green Spartina spp. biomass (Bortolus and Iribarne, 1999), in particular at lower intertidal heights (Costa et al., 2003; Alberti et al., 2007a). Herbivory is particularly intense on S. alterniflora likely due to its lower position in the intertidal (up to 75% damaged leaves; Alberti et al., 2007a, Table 2). Reduction of aboveground biomass by herbivory on S. densiflora has been estimated in 20% (Alberti et al., 2010b) while the intensity of herbivory in S. alterniflora can double it (Alberti et al., 2007a; Daleo et al., 2009). In addition to the reduction in biomass by direct consumption, crabs can also reduce plant biomass by facilitating infection by fungi in those leaves damaged by grazing (Daleo et al., 2009; Freitas et al., 2015). This facilitative process amplifies the negative effect that crabs exert reducing plant biomass. Both, grazing and fungal infection suppress S. alterniflora production by more than 50% consequently decreasing the potential of C that can be accumulated in live plant biomass. Thus, herbivory could potentially modulate the amount of C stored in salt marshes dominated by Spartina spp. by reducing aboveground biomass.
Effects Mediated by Bioturbation
Crab burrows are mainly straight, vertical and tubular and long enough to reach the water table (up to ~1 m depth and 2.66 cm average diameter; Iribarne et al., 1997; Bortolus and Iribarne, 1999), and these are maintained permanently open (Escapa et al., 2008). The effects mediated by bioturbation comprise a complex interplay between direct and indirect effects with both positive and negative outcomes in terms of C sequestration and storage.
The presence of crabs and their burrows contributes to the increase of O2 content within the semdiments (Fanjul et al., 2008, 2011), which in turn could increase the aerobic decomposition rates of the OM present in the sediment (Hemminga et al., 1991). In fact, rates of OM degradation and nitrogen remineralization are accelerated in areas bioturbated by N. granulata (Fanjul et al., 2007, 2011). Given that crabs spend most of the time inside their burrows (Méndez Casariego et al., 2011), feces and plant and detritus accumulate there. As a consequence, high quantities of remineralized nutrients are accumulated and concentrated in the water within the burrows (Fanjul et al., 2008); detritus are efficiently remineralized and quickly exported to the water column as CO2 and dissolved organic carbon (Fanjul et al., 2014).
In addition to this negative effect, increasing the sediment O2 content also has a positive effect. Arbuscular mycorrhizal fungi (AMF) are obligate aerobes so their development is often limited in salt marshes. Even when their spores are present in the sediment, the waterlogged and consequently low oxygen conditions characteristic of salt marsh habitats limits extensive development. An increase in O2 in the sediment due to the presence of burrows promotes AMF association with Spartina densiflora roots (Daleo et al., 2008). The occurrence of AMF has strong consequences in the structure of the salt marsh by increasing plant biomass (Daleo et al., 2007) as well as altering salt marsh species zonation (Daleo et al., 2008). When burrows or AMF were experimentally eliminated, the biomass of S. densiflora was reduced by 35% (Daleo et al., 2007). In those salt marshes where both Spartina species coexist, S. alterniflora is limited to more stressful low intertidal areas while S. densiflora dominates more benign high intertidal areas. However, when AMF is inhibited, S. alterniflora can move to higher elevation areas invading S. densiflora zone (Daleo et al., 2008). S. densiflora contributes more to the C accumulation than S. alterniflora due to its higher biomass per m2 and primary production (Montemayor et al., 2014). Thus, a modification in the areas occupied by one or other species will have an impact in the ability of that marsh to accumulate C. These results indicate that the presence of crabs and their burrows can indirectly regulate biomass production by facilitating AMF association in S. densiflora salt marshes or by modulating the area inhabited by each species, which can affect a salt marsh capacity to sequester atmospheric CO2 and to store C in live biomass.
Another indirect effect of crab bioturbation is related to the performance of S. densiflora. By increasing nutrient availability in bioturbated sediments, and promoting nutrient acquisition through AMF associations, N. granulata bioturbation enhances the performance of S. densiflora. Experimental manipulation of crab densities show that Spartina densiflora has lower fiber contents and C/N ratios and produces higher density of seeds at higher crab densities (Canepuccia et al., 2008). This indirect effect could be seen as positive; however, plants with better nutritional conditions are preferred by other herbivores than N. granulata. For instance, stem-borer moths (Haimbachia sp. nov) inhabit and consume a greater proportion of plants that grow in soils with higher burrow densities (Canepuccia et al., 2010). In terms of C stocks, the final outcome of this interplay of effects is variable and depends on the magnitude of the increase in S. densiflora performance, how much the herbivores reduce live biomass and how much of the litter production increased by herbivory is effectively trapped in the sediments or exported to adjacent systems.
Bioturbation also affects the retention and exportation of detritus, and again, it may have positive or negative effects on C balance. In salt marshes located in the Bahía Blanca estuary (Table 1) Spartina species produced the same amount of detritus (Montemayor et al., 2011). However, even though S. alterniflora is more frequently inundated, which could increase the export of detritus, it had a greater proportion of trapped detritus in the sediment than S. densiflora. This could be related to the larger number of crab burrows in that zone (Montemayor et al., 2011). In fact crab burrows act as passive traps increasing detrita entrapment in the sediment (Botto et al., 2006). Moreover, the C content trapped inside the burrows as a result of tidal sediment deposition is greater than the C content in the sediment excavated by crabs and exposed in the surface resulting in a net decrease in the amount of C that can be exported from the marsh by tidal processes (Gutiérrez et al., 2006). In this context, crab bioturbation seems to reduce the export of particulate OM to estuarine and coastal waters.
The presence of vegetation in the SWA coast ameliorates harsh physical conditions in the sediment facilitating the establishment of N. granulata and their burrows (Bortolus et al., 2002). Particularly in SWA salt marshes dominated by Sarcocornia spp., these crabs and their burrows can then promote erosion (Escapa et al., 2008). The ability of Sarcocornia spp. to trap sediments is very low contributing very little to sedimentation (Townend et al., 2011). In addition, during the construction and maintenance of burrows, crabs remove large amounts of sediment depositing it in the surface as mounds in the burrow entrances (Iribarne et al., 1997). These mounds are easily eroded in areas subjected to high speed currents, especially at the head of tidal creeks, basins, and banks where a net loss of sediment occurs (Escapa et al., 2008). At a landscape scale, this process increases the inland growth rate of tidal creeks (Escapa et al., 2007). Furthermore, the experimental exclusion of crabs at the head of creeks and basins demonstrate a direct link between crab bioturbation and erosion by decreasing the inland growth rate of tidal creeks (Escapa et al., 2007). In areas less affected by currents, such as mudflat plains and inside the salt marsh matrix, the sedimentary balance is positive showing a net increase in sediment deposited into burrows of 380 and 1200 g sediment per m2 per day in salt marshes and mudflat plains respectively (Escapa et al., 2008). These studies demonstrate that the impact of bioturbation on Sarcocornia spp salt marshes is context dependent: it promotes sediment (and C) losses at the head of tidal creeks, basins and banks, while promotes sediment (and C) deposition in the marsh matrix.
Overall, bioturbation can both positively and negatively affect C sequestration and storage through direct and indirect mechanisms. This complex interplay of effects leads to large (between) and small (within salt marshes) context dependent variations in C sequestration and storage.
Carbon Stocks Gains and Losses
With all the previously summarized information we constructed a conceptual model showing C sequestration and storage pathways that involves: (1) CO2 uptake by photosynthesis, (2) the transfer of C through the food web by herbivores and detritivores, (3) senescence of above and below ground plant biomass, (4) decomposition of dead plant biomass, (5) import and export of dead plant biomass from/to adjacent systems and, (6) burial of dead plant biomass and C accumulation in sediments. The crab Neohelice granulata affects all these processes through both herbivory and bioturbation (Figure 2). Summarizing, crabs eat Spartina leaves and their detritus and the damaged leaves suffer from more fungal infection and consequently less production and increased senescence. Crab burrows increase soil oxygenation promoting aerobic decomposition of the OM in the sediment and affecting also the architecture and the production of roots (Daleo and Iribarne, 2009). The increment of O2 in the sediment promotes AMF association which in turn increases the productivity of the salt marsh. Plants growing in soils with a greater number of burrows have better quality and are preferred by herbivores. Burrowing activity positively affects detrita burial by increasing the trapping of detritus inside burrows as well as by covering fallen detritus with sediment. However, burrowing also negatively affects C burial by removing, mixing and disrupting the structure of the sediment, and exposing the buried C to degradation and erosion.
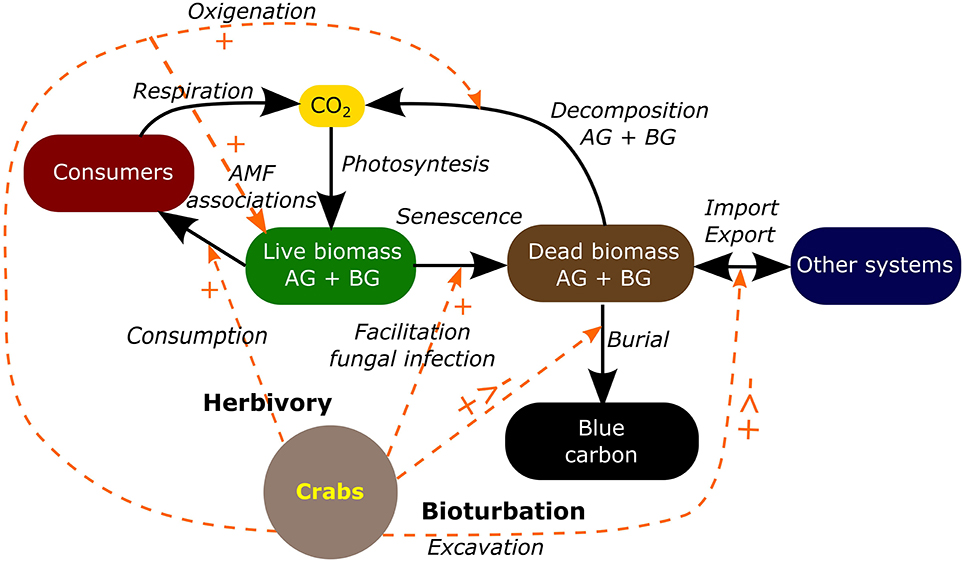
Figure 2. Carbon transformation pathways in a salt marsh (solid line arrows) and the multiple effects of Neohelice granulata in each carbon transformation pathway (dashed line arrows). AB, above ground; BG, below ground; AMF, arbuscular mycorrhizal fungi.
Most of these crab effects, however, are context dependent, with clear differences in the key pathways between the two dominant Spartina species. While S. alterniflora is more sensitive to herbivory (Figure 3A, Table 2), indirect effects related to bioturbation are more significant in S. densiflora salt marshes (Figure 3B, Table 2). In particular, the facilitation of AMF associations allows S. densiflora to increase its biomass by 35% and delineates the zonation when both species coexist. These differences may result in different C stock gains and losses; thus, the resulting scenarios for both types of salt marshes may be different.
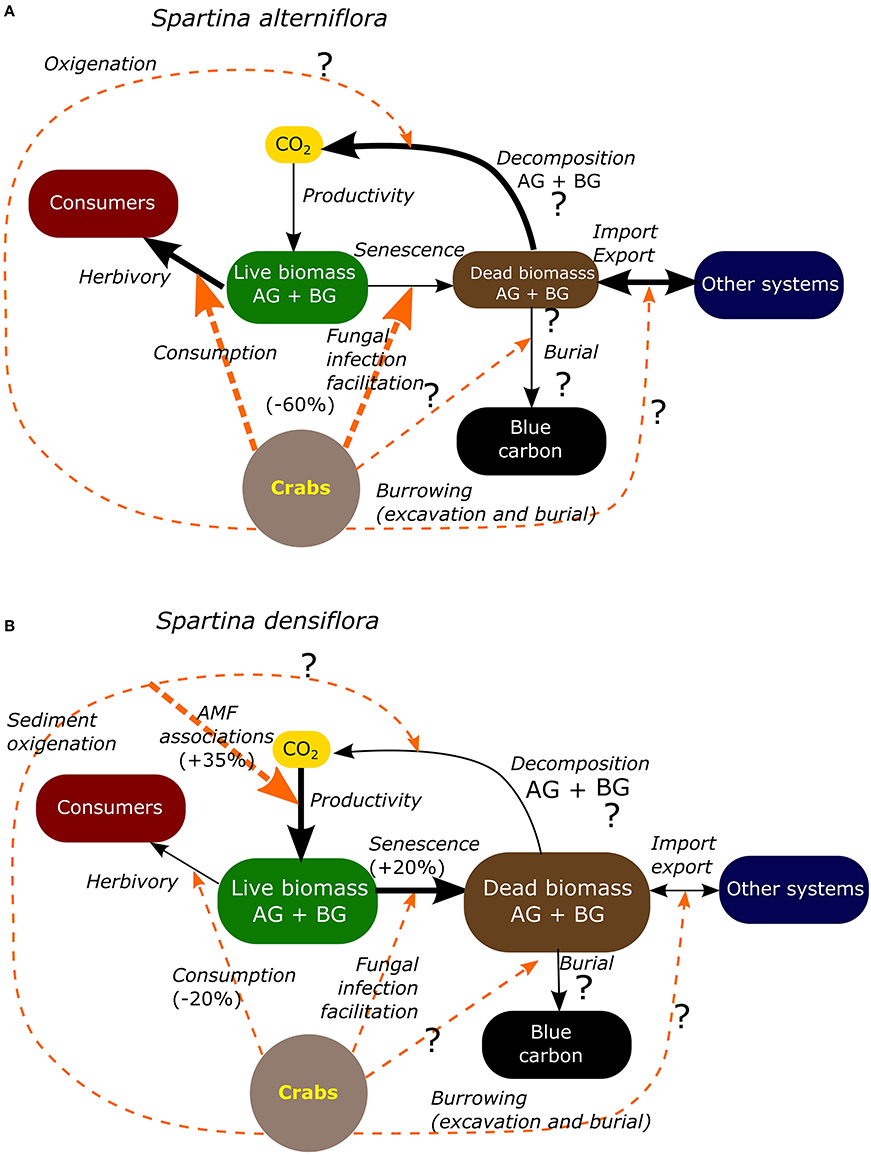
Figure 3. Carbon transformation pathways in South American salt marsh dominated by Spartina alterniflora (A) and by Spartina densiflora (B). The effects of Neohelice granulata are indicated by dashed line arrows. The relative magnitude of the effects is indicated by arrow width. Percentages indicate reported changes in biomass driven by crabs in S. alterniflora (herbivory and fungus infection: Daleo et al., 2009) and S. densiflora (senescence, Alberti et al., 2011; consumption, Alberti et al., 2010a; AMF, Daleo et al., 2007) salt marshes. AB, above ground; BG, below ground; AMF, arbuscular mycorrhizal fungi.
The present review is focused on the effects of N. granulata on C stocks and sequestration but of course no single factor can explain the variability in salt marsh C sequestration. There are, for instance, large scale external factors that can modify crab effects (Alberti et al., 2007b). Marangoni and Costa (2012) found a similar inhibition of S. densiflora growth and invasion and displacement by S. alterniflora tillers by increasing the flooding frequency of the intertidal area, which occurs under moderate-strong El Niño (ENSO) events, as a result of excessive rainfall and subsequent high fluvial discharge in the microtidal estuaries located along the southern Brazilian and NE Argentinean coasts. This response may or may not be related to AMF sensitivity to flood-induced anaerobic conditions. Previously, other studies had pointed out important effects of ENSO events on the productivity and C accumulation of microtidal SWA marshes. Cunha et al. (2005) observed significantly higher biomass production (aerial and belowground biomass) during a strong ENSO (1992-93) than a non ENSO period (1993–1994), and they associate this result to a 2.8°C higher average air temperature during ENSO. Significantly increased night time temperatures occur during strong ENSO events in southern Brazil (Marengo, 2007), and this may explain why biomass production by the C4 S. alterniflora was more affected by temperature, than solar radiation or salinity in a study by Cunha et al. (2005). On the other hand, low salinity due to excessive rainfall and high discharge of rivers in SWA estuaries during ENSO events can stimulate the spreading and herbivory pressure by rodents, such as Myocastor coypus (Costa et al., 2004), Cavia aperea (Canepuccia et al., 2010), and Akodon azarae (Pascual et al., 2015) into estuaries, which strongly affect the standing biomass of Spartina species.
Conclusion
South American salt marshes are highly productive systems year round (Montemayor et al., 2015) resulting in large stocks of C stored in above and below ground biomass. Herbivory by crabs has a greater incidence on S. alterniflora than on S. densiflora. Thus, top-down mediated processes will probably have a stronger impact in the C transformation path in S. alterniflora salt marshes. There is a gap in information related to C content in salt marsh sediments. However, several mechanisms mediated by bioturbation suggest that crabs may increase degradation as well as increase retention of detritus, especially in S. densiflora salt marshes where detritus production and dead biomass is high. Although, we are presenting here a hypothetical model that requires evaluation, it is based on strong scientific evidence. Overall, these results show a potential context dependency of C accumulation in salt marshes, reinforcing the need to be very careful if extrapolations from other systems are going to be used in order to accurately estimate the value of ecosystem services.
In this sense, context dependency should be seen as a scientific challenge rather than an obstacle redirecting the effort to increase the geographic scale. A recent review on the ecological functioning of South American salt marshes highlights the difficulties to establish global generalization when paradigms are built on partial information (Alberti et al., 2015). For South American salt marshes there are no CBRs reported yet; however, the extensive research on the ecological functioning of these ecosystems that has been undertaken over the last two decades (Alberti et al., 2015) provides the basis to generate hypotheses on the functioning of salt marshes in terms of C sequestration and storage. Testing these hypotheses will improve our knowledge on the factors that drive salt marsh C sequestration variability.
The notion that one single species can be responsible for the major functioning of an ecosystem has been stressed in ecological studies in the second half of the XX century. Even concepts as “key-stone species” (Paine, 1969) and “ecosystem engineers” (Jones et al., 1994) have been developed to account for the paramount role of certain species within an ecosystem. By the end of the XX century the focus changed to consider the importance of species in terms of biodiversity and its relationship with ecosystem stability maintenance (Kareiva and Levin, 2003). Thus, ecological studies have moved from detailed study of plants, animals and their interactions to more qualitative and experimental approaches (helped by methodological advances) where, in many cases, energy flows are the focus. Beside the framework in which ecologist have settled their studies, it is undeniable that in some cases a single species can control the functioning of an ecosystem (e.g., dominant plant species, bivalve beds, beavers). To maintain a historical perspective will improve our ability to reconsider the importance of older approaches in order to foresee productive new research directions.
Author Contributions
All authors listed, have made substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study has been benefitted from discussions during the Blue C Initiative workshop in Brazil (October 2014). Sources of funds: PIP (CONICET), PICT (Agencia Nacional de Promoción de Científica y Tecnológica, Argentina) y UNMdP to O. Iribarne. We thank Dr. Raymond Ward from Brighton University for comments and suggestions that improved the manuscript.
References
Albano, M. J., Obenat, S., and Luppi, T. (2012). “Community composition, assemblages and latitudinal pattern of benthic intertidal invertebrates at marshes in the southwestern Atlantic, Argentina. Conferencia,” in CERF's Inaugural International Conference of the Americas (Mar del Plata), 48–49.
Alberti, J., Cebrian, J., M9ndez Casariego, A., Canepuccia, A., Escapa, M., and Iribarne, O. (2011). Effects of nutrient enrichment and crab herbivory on a SW Atlantic salt marsh productivity. J. Exp. Mar. Biol. Ecol. 405, 99–104. doi: 10.1016/j.jembe.2011.05.023
Alberti, J., Daleo, P., Fanjul, E., Escapa, M., Botto, F., and Iribarne, O. (2015). Can a single species challenge paradigms of salt marsh functioning? Estuar. Coasts 38, 1178–1188. doi: 10.1007/s12237-014-9836-z
Alberti, J., Daleo, P., Iribarne, O., Silliman, B. R., and Bertness, M. (2007a). Local and geographic variation in grazing intensity by herbivorous crabs in SW Atlantic salt marshes. Mar. Ecol. Prog. Ser. 349, 235–243. doi: 10.3354/meps07089
Alberti, J., Escapa, M., Daleo, P., Méndez Casariego, A., and Iribarne, O. (2010a). Crab bioturbation and herbivory reduce pre- and postgermination success of Sarcocornia perennis in bare patches of SW Atlantic salt marshes. Mar. Ecol. Prog. Ser. 400, 55–61. doi: 10.3354/meps08440
Alberti, J., Méndez Casariego, A., Daleo, P., Fanjul, E., Silliman, B., Bertness, M., et al. (2010b). Abiotic stress mediates top-down and bottom-up control in a Southwestern Atlantic salt marsh. Oecologia 163, 181–191. doi: 10.1007/s00442-009-1504-9
Alberti, J., Montemayor, D., Álvarez, F., Méndez Casariego, A., Luppi, T., Canepuccia, A., et al. (2007b). Changes in rainfall pattern affect crab herbivory rates in a SW Atlantic salt marsh. J. Exp. Mar. Biol. Ecol. 353, 126–133. doi: 10.1016/j.jembe.2007.09.007
Araújo, J. M. C. Jr., Otero, X. L., Marques, A. G. B., Nóbrega, G. N., Silva, J. R. F., and Ferreira, T. O. (2012). Selective geochemistry of iron in mangrove soils in a semiarid tropical climate: effects of the burrowing activity of the crabs Ucides cordatus and Uca maracoani. Geo-Mar. Lett. 32, 289–300. doi: 10.1007/s00367-011-0268-5
Beck, M. W., Heck, K. L. Jr., Able, K. W., Childers, D. L., Eggleston, D. B., Gillanders, B. M., et al. (2001). The identification, conservation and management of estuarine and marine nurseries for fish and invertebrates. Bioscience 51, 633–641. doi: 10.1641/0006-3568(2001)051[0633:TICAMO]2.0.CO;2
Bortolus, A., and Iribarne, O. O. (1999). The effect of the southwestern Atlantic burrowing crab Chasmagnathus granulata on a Spartina salt-marsh. Mar. Ecol. Prog. Ser. 178, 79–88. doi: 10.3354/meps178079
Bortolus, A., Schwindt, E., Bouza, P. J., and Idaszkin, Y. L. (2009). A characterization of Patagonian salt marshes. Wetlands 29, 772–780. doi: 10.1672/07-195.1
Bortolus, A., Schwindt, E., and Iribarne, O. (2002). Positive plant-animal interactions in the high marsh of an Argentinean coastal lagoon. Ecology 83, 733–742. doi: 10.1890/0012-9658(2002)083[0733:PPAIIT]2.0.CO;2
Botto, F., Iribarne, O., Gutierrez, J., Bava, J., Gagliardini, A., and Valiela, I. (2006). Ecological importance of passive deposition of organic matter into burrows of the SW Atlantic crab Chasmagnathus granulatus. Mar. Ecol. Prog. Ser. 312, 201–210. doi: 10.3354/meps312201
Canepuccia, A. D., Alberti, J., Pascual, J., Alvarez, G., Cebrian, J., and Iribarne, O. O. (2010). ENSO episodes modify plant/terrestrial–herbivore interactions in a southwestern Atlantic salt marsh, J. Exp. Mar. Biol. Ecol. 396, 42–47. doi: 10.1016/j.jembe.2010.09.013
Canepuccia, A. D., Farias, A. A., Escalante, A. H., Iribarne, O., Novaro, A., and Isacch, J. P. (2008). Differential responses of marsh predators to rainfall-induced habitat loss and subsequent variations in prey availability. Can. J. Zool. 86, 407–418. doi: 10.1139/Z08-007
Cardoni, D. A., Isacch, J. P., Fanjul, M. E., Escapa, M., and Iribarne, O. O. (2011). Relationship between anthropogenic sewage discharge, marsh structure and bird assemblages in a SW Atlantic saltmarsh. Mar. Environ. Res. 71, 122–130. doi: 10.1016/j.marenvres.2010.12.003
Chambers, J. Q., Higuchi, N., Tribuzy, E. S., and Trumbore, S. E. (2001). Carbon sink for a century. Nature 410:429. doi: 10.1038/35068624
Chmura, G. L. (2009). “Tidal salt marshes,” in The Management of Natural Coastal Carbon Sinks, eds D. d'A. Laffoley and G. Grimsditch (Gland: IUCN), 5–11.
Chmura, G. L. (2013). What do we need to assess the sustainability of the tidal salt marsh carbon sink? Ocean Coast. Manag. 83, 25–31. doi: 10.1016/j.ocecoaman.2011.09.006
Costa, C. S. B., and Davy, A. J. (1992). “Coastal saltmarsh communities of Latin America,” in Coastal plant communities of Latin America, ed U. Seeliger (New York, NY: Academic Press), 179–199.
Costa, C. S. B., Gianuca, D., and Tormena, T. (2004). Ação de Herbívoros Sobre a Produtividade Das Marismas do sul do Brasil: Experimento Piloto de Exclusão de Roedores e Caranguejos Grapsidae. Anais do VI Simpósio de Ecossistemas Brasileiros, Vol. 2. Academia de Ciências do Estado de São Paulo – ACIESP, São Paulo.
Costa, C. S. B., Iribarne, O. O., and Farina, J. M. (2009). “Human impacts and threats to the conservation of South American salt marshes,” in Salt Marshes under Global Siege, eds B. R. Silliman, T. Grosholtz, and M. D. Bertness (Berkeley: University of California Press), 337–359.
Costa, C. S. B., Marangoni, J. C., and Azevedo, A. M. G. (2003). Plant zonation in irregularly flooded salt marshes: relative importance of stress tolerance and biological interactions. J. Ecol. 91, 951–965. doi: 10.1046/j.1365-2745.2003.00821.x
Costa, C. S. B., Seeliger, U., and Bemvenuti, C. E. (2007). Diagnóstico de Alterações Hidrológicas Devido ao Impacto das Mudanças Climáticas Sobre o Ecossistema Costeiro Temperado Brasileiro Através da Vegetação e do Macrozoobentos. Brasília: Ministério do Meio Ambiente/PROBIO.
Coverdale, T. C., Brisson, C. P., Young, E. W., Yin, S. F., Donnelly, J. P., and Bertness, M. D. (2014). Indirect human impacts reverse centuries of carbon sequestration and salt marsh accretion. PLoS ONE 9:e93296. doi: 10.1371/journal.pone.0093296
Cunha, S. R., Asmus, M., and Costa, C. S. B. (2005). Production dynamics of Spartina alterniflora salt marshes in the estuary of Patos Lagoon (RS, Brazil): a Simulation model approach. Brazil. J. Aquat. Sci. Technol. Itajaí 9, 75–85. doi: 10.14210/bjast.v9n2.p75-85
Daleo, P., Alberti, J., Canepuccia, A., Escapa, M., Fanjul, E., Silliman, B. R., et al. (2008). Mycorrhizal fungi determine salt-marsh plant zonation depending on nutrient supply. J. Ecol. 96, 431–437. doi: 10.1111/j.1365-2745.2007.01349.x
Daleo, P., Fanjul, E., Mendez Casariego, A., Silliman, B. R., Bertness, M. D., and Iribarne, O. (2007). Ecosystem engineers activate mycorrhizal mutualism in salt marshes. Ecol. Lett. 10, 902–908. doi: 10.1111/j.1461-0248.2007.01082.x
Daleo, P., and Iribarne, O. (2009). The burrowing crab Neohelice granulata affects the root strategies of the cordgrass Spartina densiflora in SW Atlantic salt marshes. J. Exp. Mar. Biol. Ecol. 373, 66–71. doi: 10.1016/j.jembe.2009.03.005
Daleo, P., Silliman, B., Alberti, J., Escapa, M., Canepuccia, A., Peña, N., et al. (2009). Grazer facilitation of fungal infectionand the control of plant growth in SW Atlantic salt marshes. J. Ecol. 97, 781–787. doi: 10.1111/j.1365-2745.2009.01508.x
Duarte, C. M., Dennison, W. C., Orth, R. J. W., and Carruthers, T. J. B. (2008). The charisma of coastal ecosystems: addressing the imbalance. Estuar. Coast. 31, 233–238. doi: 10.1007/s12237-008-9038-7
Duarte, C. M., Losada, I. J., Hendriks, I. E., Mazarrasa, I., and Marbà, N. (2013). The role of coastal plant communities for climate change mitigation and adaptation. Nat. Clim. Change 3, 961–968. doi: 10.1038/nclimate1970
Escapa, M., Minkoff, D. R., Perillo, G. M. E., and Iribarne, O. (2007). Direct and indirect effects of burrowing crab Chasmagnathus granulatus activities on erosion of southwest Atlantic Sarcocornia-dominated marshes. Limnol. Oceanogr. 52, 2340–2349. doi: 10.4319/lo.2007.52.6.2340
Escapa, M., Perillo, G. M. E., and Iribarne, O. (2008). Sediment dynamics modulated by burrowing crab activities in contrasting SW Atlantic intertidal habitats. Estuar. Coast. Shelf Sci. 80, 365–373. doi: 10.1016/j.ecss.2008.08.020
Fanjul, E., Bazterrica, M. C., Escapa, M., Grela, M. A., and Iribarne, O. (2011). Impact of crab bioturbation on benthic flux and nitrogen dynamics of Southwest Atlantic intertidal marshes and mudflats. Estuar. Coast. Shelf Sci. 92, 629–638. doi: 10.1016/j.ecss.2011.03.002
Fanjul, E., Escapa, M., Montemayor, D., Addino, M., Alvarez, M. F., Grela, M. A., et al. (2014). Effect of crab bioturbation on organic matter processing in South West Atlantic intertidal sediments. J. Sea Res. 94, 194–212. doi: 10.1016/j.seares.2014.05.005
Fanjul, E., Grela, M. A., Canepuccia, A., and Iribarne, O. (2008). The Southwest Atlantic intertidal burrowing crab Neohelice granulata modifies nutrient loads of phreatic waters entering coastal area. Estuar. Coast. Shelf Sci. 79, 300–306. doi: 10.1016/j.ecss.2008.04.005
Fanjul, E., Grela, M. A., and Iribarne, O. (2007). Effects of the dominant SW Atlantic intertidal burrowing crab Chasmagnathus granulatus on sediment chemistry and nutrient distribution. Mar. Ecol. Prog. Ser. 341, 177–190. doi: 10.3354/meps341177
Freitas, R. F., Schrack, E. C., He, Q., Silliman, B. R., Furlong, E. B., Telles, A. C., et al. (2016). Consumer control of the establishment of marsh foundation plants in intertidal mudflats. Mar. Ecol. Prog. Ser. 547, 79–89. doi: 10.3354/meps11624
Freitas, R. F., Schrack, E. C., Sieg, R. D., Silliman, B. R., and Costa, C. S. B. (2015). Grazing scar characteristics impact degree of fungal facilitation in Spartina alterniflora leaves in a South American salt marsh. Braz. Arch. Biol. Technol. 58, 103–108. doi: 10.1590/S1516-8913201400030
Gedan, K. B., Kirwan, M. L., Wolanski, E., Barbier, E. B., and Silliman, B. R. (2011). The present and future role of coastal wetland vegetation in protecting shorelines: answering recent challenges to the paradigm. Clim. Change 106, 7–29. doi: 10.1007/s10584-010-0003-7
González Trilla, G., De Marco, S., Marcovecchio, J., Vicari, R., and Kandus, P. (2010). Net primary productivity of Spartina densiflora Brong in an SW Atlantic coastal salt marsh. Estuar. Coast. 33, 953–962. doi: 10.1007/s12237-010-9288-z
González Trilla, G., Kandus, P., Negrin, V., and Marcovecchio, J. (2009). Tiller dynamic andproduction on a SW Atlantic Spartina alterniflora marsh. Estuar. Coast. Shelf Sci. 85, 126–133. doi: 10.1016/j.ecss.2009.07.034
Gutiérrez, J. L., Jones, C. G., Groffman, P. M., Findlay, S. E. G., Iribarne, O. O., Ribeiro, P. D., et al. (2006). The contribution of crab burrow excavation to carbon availability in surficial salt-marsh sediments. Ecosystems 9, 647–658. doi: 10.1007/s10021-006-0135-9
Hemminga, M. A., de Leeuw, J., de Mune, W., and Koutstaal, B. P. (1991). Decomposition in estuarine salt marshes: the effect of soil salinity and soil water content. Vegetatio 94, 25–33.
Hickenbick, G. R., Ferro, A. L., and Abreu, P. C. (2004). Produção de detrito de macrófitas emergentes em uma marisma do estuário da lagoa dos Patos: Taxas de decomposição e dinâmica microbiana. Atlânt. Rio Grande 26, 61–75. doi: 10.5088/atl2ntica.v26i1.2233. Available online at: http://www.seer.furg.br/atlantica/article/view/2233
Iribarne, O., Bortolus, A., and Botto, F. (1997). Between-habitat differences in burrow characteristics and trophic modes in the southwestern Atlantic burrowing crab Chasmagnathus granulata. Mar. Ecol. Prog. Ser. 155, 137–145. doi: 10.3354/meps155137
Iribarne, O., Martinetto, P., Schwindt, E., Botto, F., Bortolus, A., and García Borboroglu, P. (2003). Evidence of habitat displacement between two common soft-bottom SW Atlantic intertidal crabs. J. Exp. Mar. Biol. Ecol. 296, 167–182. doi: 10.1016/S0022-0981(03)00318-6
Isacch, J. P., Costa, C. S. B., Rodríguez-Gallego, L., Conde, D., Escapa, M., Gagliardini, D. A., et al. (2006). Distribution of saltmarsh plant communities associated with environmental factors along a latitudinal gradient on the south-west Atlantic coast. J. Biogeography 33, 888–900. doi: 10.1111/j.1365-2699.2006.01461.x
Isacch, J. P., Holz, S., Ricci, L., and Martínez, M. M. (2004). Post-fire vegetation change and bird use of a salt marsh in coastal Argentina. Wetlands 24, 235–243. doi: 10.1672/0277-5212(2004)024[0235:PVCABU]2.0.CO;2
Jones, C. G., Lawton, J. H., and Shacha, M. (1994). Organisms as ecosystem engineers. Oikos 69, 373–386.
Kareiva, P., and Levin, S. E. (2003). The Importance of Species: Perspectives on Expendability and Triage. Princeton, NJ: Princeton University Press.
Kelleway, J. J., Saintilan, N., Macreadie, P. I., and Ralph, P. J. (2016). Sedimentary factors are 532 key predictors of carbon storage in SE Australian saltmarshes. Ecosystems 1–16. doi: 10.1007/s10021-016-9972-3
Lo Iacono, C., Mateo, M. A., Gràcia, E., Guasch, L., Carbonell, R., Serrano, L., et al. (2008). Very high-resolution seismo-acoustic imaging of seagrass meadows (Mediterranean sea): implications for carbón sink estimates. Geophysic. Res. Lett. 35:L18601. doi: 10.1029/2008gl034773
Marangoni, J. C., and Costa, C. S. B. (2009). Natural and anthropogenic effects on salt marsh over five decades in the Patos Lagoon (Southern Brazil). Braz. J. Oceanog. 57, 345–350. doi: 10.1590/S1679-87592009000400009
Marangoni, J. C., and Costa, C. S. B. (2010). Caracterização das atividades económicas tradicionais no entorno das marismas no estuário da Lagoa dos Patos (RS). Desenvol. Meio. Amb. 21, 129–142. doi: 10.5380/dma.v21i1.12702
Marangoni, J. C., and Costa, C. S. B. (2012). Short- and long-term vegetative propagation of two Spartina species on a salt marsh in Southern Brazil. Estuar. Coast. 35, 763–773 doi: 10.1007/s12237-011-9474-7
Marengo, J. A. (2007). Mudanças Climáticas Globais e Seus Efeitos Sobre a Biodiversidade: Caracterização do Clima Atual e Definição Das Alterações Climáticas Para o Território Brasileiro ao Longo do Século XXI. 2nd Edn. Brasília: Ministério do Meio Ambiente.
Martinetto, P., Palomo, G., Bruschetti, M., and Iribarne, O. (2011). Similar effects on sediment structure aand infaunal community of two competitive intertidal soft-bottom burrowing crab species. J. Mar. Biol. Ass. UK 91, 1385–1393. doi: 10.1017/S0025315411000075
Martinetto, P., Valiñas, M., Palomo, G., and Iribarne, O. (2007). Negative interactions between two SW Atlantic intertidal crabs in soft bottom habitats. Mar. Biol. 151, 1479–1490. doi: 10.1007/s00227-006-0585-9
Mateo, M. A., Romero, J., Pérez, M., Littler, M. M., and Littler, D. S. (1997). Dynamics of millenary organic deposits resulting from the growth of the Mediterranean seagrass Posidonia oceanica. Estuar. Coast. Shelf Sci. 44, 103–110. doi: 10.1006/ecss.1996.0116
McGlathery, K. J., Sundbäck, K., and Anderson, I. C. (2007). Eutrophication in coastal bays and lagoons: the role of plants in the coastal filter. Mar. Ecol. Prog. Ser. 348, 1–18. doi: 10.3354/meps07132
McLeod, E., Chmura, G. L., Bouillon, S., Salm, R., Bjök, M., Duarte, C. M., et al. (2011). A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 9, 552–560. doi: 10.1890/110004
Méndez Casariego, A., Alberti, J., Luppi, T., Daleo, P., and Iribarne, O. (2011). Habitat shifts and spatial distribution of the intertidal crab Neohelice (Chasmagnathus) granulata Dana. J. Sea Res. 66, 87–94. doi: 10.1016/j.seares.2011.05.001
Montemayor, D. I., Addino, M., Valiñas, M., Fanjul, E., Alvarez, M. F., and Iribarne, O. (2015). Biomass dynamics of the two dominant SW Atlantic Spartina species and its implications on the saltmarsh organic matter accumulation/exportation. Aquat. Bot. 120, 201–204. doi: 10.1016/j.aquabot.2014.05.017
Montemayor, D. I., Canepuccia, A. D., Pascual, J., and Iribarne, O. O. (2014). Aboveground biomass patterns of dominant Spartina Species and their relationship with selected abiotic nariables in Argentinean SW Atlantic marshes. Estuar. Coast. 37, 411–420. doi: 10.1007/s12237-013-9688-y
Montemayor, D. I. Addino, M., Fanjul, E., Escapa, M., Alvarez, M. F., Botto, F., et al. (2011). Effect of dominant Spartina species on salt marsh detritus production in SWAtlantic estuaries. J. Sea Res. 66, 104–110. doi: 10.1016/j.seares.2011.05.003
Negrín, V. L., de Villalobos, A. E., González Trilla, G., Botté, S. E., and Marcovecchio, J. E. (2012). Above- and belowground biomass and nutrient pools of Spartina alterniflora (smooth cordgrass) in a South American salt marsh. Chem. Ecol. 28, 391–404. doi: 10.1080/02757540.2012.666529
Nellemann, C., Corcoran, E., Duarte, C. M., Valdés, L., DeYoung, C., Fonseca, L., et al. (2009). Blue Carbon. A Rapid Response Assessment. United Nations Environment Programme. Birkelant; GRID-Arendal. Available online at: http://www.grida.no/
Ouyang, X., and Lee, S. Y. (2014). Update estimates of carbon accumulation rates in coastal marsh sediments. Biogeosciences 11, 5057–5071. doi: 10.5194/bg-11-5057-2014
Paine, R. T. (1969). A note on trophic complexity and community stability. Am. Natur. 103, 91–93. doi: 10.1086/282586
Pascual, J., Canepuccia, A. D., Alberti, J., Daleo, P., and Iribarne, O. (2015). Rainfall intensity modulates the interaction between the marsh cordgrass Spartina densiflora and the mouse Akodon azarae. Mar. Ecol. Prog. Ser. 523, 71–80. doi: 10.3354/meps11119
Peixoto, A. E., and Costa, C. S. B. (2004). Produção primária líquida aérea de Spartina densiflora Brong. (Poaceae) no estuário da laguna dos Patos, Rio Grande do Sul, Brasil. Iheringia Sér. Bot., Porto Alegre 59, 27–34. Available online at: https://isb.emnuvens.com.br/iheringia/article/view/225/231; http://www.fzb.rs.gov.br/upload/20140328141133ih59_027_034.pdf
Peixoto, R. P., Gaona, C. A. P., and Costa, C. S. B. (1997). “Produção primária líquida aérea de cinco comunidades vegetais de uma marisma no estuário da laguna dos Patos, RG, Brasil,” in VII Congreso Latino-Americano Sobre Ciências do Mar, Vol. II., ed Associação Latino-americana de Pesquisadores em Ciências do Mar – ALICMAR (São Paulo: Resumos Expandidos), 274–276.
Pratolongo, P., Mazzon, C., Zapperi, G., Piovan, M. J., and Brinson, M. M. (2013). Land cover changes in tidal salt marshes of the Bahía Blanca estuary (Argentina) during the past 40 years. Estuar. Coast. Shelf. Sci. 133, 23–31. doi: 10.1016/j.ecss.2013.07.016
Townend, I., Fletcher, C., Knappen, M., and Rossington, K. (2011). A review of salt marsh dynamics. Water Environ. J. 25, 477–488. doi: 10.1111/j.1747-6593.2010.00243.x
Valiela, I., Cole, M. L., McClelland, J., Hauxwell, J., Cebrian, J., and Joye, S. B. (2000). “Role of salt marshes as part of coastal landscapes,” in Concepts and Controversies in Tidal Marsh Ecology, eds M. P. Weinstein and D. A. Kreeger (Dordrecht: Kluwer Academic Publisher), 23–36.
Valiela, I., Kinney, E., Culbertson, J., Peacock, E., and Smith, S. (2009). “Global losses of mangroves and salt marshes,” in Global Loss of Coastal Habitats: Rates, Causes and Consequences, ed C. M. Duarte (Bilbao: Fundación BBVA), 107–138.
Vera, F., Gutiérrez, J. L., and Ribeiro, P. D. (2009). Aerial and detritus production of the cordgrass Spartina densiflora in a southwestern Atlantic salt marsh. Botany 87, 482–491. doi: 10.1139/B09-017
Keywords: Blue C, salt marshes, herbivory, bioturbation, coastal vegetation
Citation: Martinetto P, Montemayor DI, Alberti J, Costa CSB and Iribarne O (2016) Crab Bioturbation and Herbivory May Account for Variability in Carbon Sequestration and Stocks in South West Atlantic Salt Marshes. Front. Mar. Sci. 3:122. doi: 10.3389/fmars.2016.00122
Received: 08 April 2016; Accepted: 23 June 2016;
Published: 07 July 2016.
Edited by:
Iris Eline Hendriks, University of the Balearic Islands, SpainReviewed by:
Jonne Kotta, University of Tartu, EstoniaAndrew‘ Stanley Wozniak, Old Dominion University, USA
Copyright © 2016 Martinetto, Montemayor, Alberti, Costa and Iribarne. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paulina Martinetto, pmartin@mdp.edu.ar
 Paulina Martinetto
Paulina Martinetto Diana I. Montemayor
Diana I. Montemayor Juan Alberti
Juan Alberti César S. B. Costa2
César S. B. Costa2  Oscar Iribarne
Oscar Iribarne