Bridging the Gap between Policy and Science in Assessing the Health Status of Marine Ecosystems
- 1AZTI, Marine Research Division, Pasaia, Spain
- 2Institute of Estuarine & Coastal Studies, University of Hull, Hull, UK
- 3Departments of Ocean Sciences and Biology, Memorial University of Newfoundland. St. John's NL, Canada
- 4Plymouth Marine Laboratory, Plymouth, UK
- 5MariLim Aquatic Research GmbH, Schönkirchen, Germany
- 6SALT, Lofoten, Norway
- 7Bioscience, Aarhus University, Roskilde, Denmark
- 8Department of Life and Environmental Sciences, Polytechnic University of Marche, Ancona, and Stazione Zoologica Anton Dohrn, Naples, Italy
- 9Marine Scotland – Science, Marine Laboratory, Aberdeen, UK
- 10Marine Research Centre, Finnish Environment Institute, Helsinki, Finland
- 11Centre for Environment, Fisheries, and Aquaculture Science, Lowestoft, UK
- 12Ecoreach, Ancona, Italy
- 13NILU—IMPEC, Kjeller, Norway
- 14CIMA, University of Algarve, Faro, Portugal
- 15European Commission, Joint Research Centre, Directorate for Sustainable Resources, D.2 Water and Marine Resources Unit, Ispra, Italy
- 16Oceandtm Ltd. Lowestoft, UK
Human activities, both established and emerging, increasingly affect the provision of marine ecosystem services that deliver societal and economic benefits. Monitoring the status of marine ecosystems and determining how human activities change their capacity to sustain benefits for society requires an evidence-based Integrated Ecosystem Assessment approach that incorporates knowledge of ecosystem functioning and services). Although, there are diverse methods to assess the status of individual ecosystem components, none assesses the health of marine ecosystems holistically, integrating information from multiple ecosystem components. Similarly, while acknowledging the availability of several methods to measure single pressures and assess their impacts, evaluation of cumulative effects of multiple pressures remains scarce. Therefore, an integrative assessment requires us to first understand the response of marine ecosystems to human activities and their pressures and then develop innovative, cost-effective monitoring tools that enable collection of data to assess the health status of large marine areas. Conceptually, combining this knowledge of effective monitoring methods with cost-benefit analyses will help identify appropriate management measures to improve environmental status economically and efficiently. The European project DEVOTES (DEVelopment Of innovative Tools for understanding marine biodiversity and assessing good Environmental Status) specifically addressed these topics in order to support policy makers and managers in implementing the European Marine Strategy Framework Directive. Here, we synthesize our main innovative findings, placing these within the context of recent wider research, and identifying gaps and the major future challenges.
Introduction
A recent assessment of marine ecosystem ecology identified eight grand research challenges (Borja, 2014): (i) understanding the role of biodiversity in maintaining ecosystem functionality; (ii) understanding the relationships between human pressures and ecosystems; (iii) understanding the impacts of global change on marine ecosystems; (iv) developing integrative assessment of marine ecosystem health; (v) ensuring delivery of ecosystem services by conserving and protecting the seas; (vi) understanding the way in which ecosystem structure and functioning may recover through restoration; (vii) understanding the need for an ecosystem approach and integrated spatial planning in managing ocean use, and (viii) developing better ecosystem models to support more effective management.
These challenges reflect widespread recognition of clear effects of pressures from established and emerging human activities on marine ecosystems (Halpern et al., 2015) and, consequently, the potential of those pressures to alter the ability of ocean ecosystems to provide services that yield societal and economic benefits (Barbier et al., 2012; Turner and Schaafsma, 2015). Given the multiple pressures society places on marine ecosystems and the broad range of services they provide, a holistic assessment (Borja et al., 2016) of the status of marine ecosystems requires scientific evidence-based Integrated Ecosystem Assessments (IEA; Levin et al., 2009). Indeed, the former European Commissioner for Environment, Janez Potočnik, stated during the closing session of Euromares 2010, on the occasion of the European Maritime Day, that: “We are learning that the [Marine Strategy Framework] Directive has a weakness—and that weakness is the lack of knowledge.” With a lack of knowledge “…these unknown variables pose a real problem for decision-makers. They need to be identified and addressed in a systematic way. And while we need to acknowledge the differences and diversity of our seas, there are some issues which can only be adequately addressed on a European scale.” These statements capture the desire of policy-makers and managers worldwide to fulfill their moral mandate to conserve and protect the seas (Reker et al., 2015) using evidence-based decision-making. Hence, the vision for clean, healthy, biodiverse, and productive oceans and seas with sustainable resource use requires bridging the gap between policy and science in assessing the status of marine ecosystems by increasing scientific knowledge of marine ecosystems and their functioning, including humans and their role as part of the ecosystem (Borja et al., 2013). Indeed, recent European and national policies enshrine the vision of healthy and biologically diverse seas (e.g., DEFRA, 2002; European Marine Board, 2013). More recently, the European Union and United Nations have tried to address problems associated with exploitation of deep fishing resources and associated impacts on biodiversity (St. John et al., 2016).
The development and implementation of policy and legislation globally demonstrate a significant effort to improve the status of the seas, including an ecosystem approach to ocean use management (Browman et al., 2004; Nicholson and Jennings, 2004; Borja et al., 2008, 2016; Curtin and Prellezo, 2010). In the European Union (EU), the Marine Strategy Framework Directive (MSFD; European Commission, 2008) represents the most comprehensive marine environmental legislation. This Directive aims to achieve Good Environmental Status (GES) by 2020 in the four European Regional Seas (Baltic, North Eastern Atlantic, Mediterranean and Black Sea). The MSFD requires that Member States assess ecosystem characteristics, pressures, and impacts with respect to 11 descriptors related to: biological diversity, non-indigenous species, commercial fish and shellfish, food-webs, eutrophication, seafloor integrity, hydrographic conditions, concentration of contaminants in the environment and in fish and other seafood consumed by humans, marine litter, and introduction of energy including underwater noise. Within these 11 descriptors, the European Commission (2010) then defines 29 criteria and 56 indicators necessary in evaluating environmental status.
The assessment of environmental status, while scientifically challenging (Stanley, 1995), simultaneously offers many opportunities for European marine research to support an ecosystem approach to environmental management, which EU Member States have agreed to implement (Borja et al., 2013). The European project DEVOTES (DEVelopment Of innovative Tools for understanding marine biodiversity and assessing GES, www.devotes-project.eu) was started in 2012 to facilitate MSFD implementation. This project considers these complex, inter-related scientific issues and management needs of the MSFD, as well as the challenges shared by the four regional seas identified within the MSFD. Its main objectives were:
- To improve understanding of the cumulative impacts of human activities on marine biodiversity and variation associated with climate, identifying the socio-economic and legislative barriers and bottlenecks that prevent achieving GES;
- To test indicators currently in use (European Commission, 2010) and develop new assessment options, particularly for biodiversity-related descriptors (i.e., D1. Biological diversity, D2. Non-indigenous species, D4. Food-webs, and D6. Seafloor integrity), at several ecological levels (species, habitat, ecosystems), and characterize and classify status of marine waters;
- To develop, test and validate innovative integrative modeling and cost-effective monitoring tools to strengthen understanding of ecosystem function and biodiversity changes in space and time associated with human impacts, including climatic influences.
- To propose and disseminate strategies and measures for adaptive management of ecosystems, including integrative and holistic tools to assess environmental status.
We therefore set an overall goal of better understanding the relationships between pressures from human activities and climate change, and their effects on marine ecosystems, including biological diversity, in order to support ecosystem-based management and attain GES of marine waters. Our harmonized approach to the four European regional seas tested and validated existing indicators, created new indicators when necessary, developed modeling tools for the assessment of biodiversity, tested new monitoring tools and established an integrative approach for assessing environmental status.
This overview describes how this research has contributed to advancing the state-of-the-art since 2012 in bridging the gap between science and policy in marine environmental status assessment. Specifically, this addresses elements such as human pressures, indicator development, model use, innovative monitoring, and integrative assessment tools), in order to achieve healthy and sustainable ocean use. Here we synthesize key responses to major environmental questions and the lessons learnt. This information will support managers and policy-makers in making decisions for improved management of ocean use.
Why Must We Understand Impacts of Human Activities At Sea?
State-of-the-Art
Marine environmental managers primarily aim to protect and maintain natural structure and functioning while simultaneously ensuring that ecosystems provide services, which in turn deliver benefits for society (Atkins et al., 2011; Elliott, 2011). In the management of human activities in the marine environment, it is axiomatic that a regulatory body (i.e., an environmental protection agency, natural conservation body, fisheries body, or marine licensing body), does not have to prove that an activity or its developer (the “user,” “polluter”—those undertaking the activity, such as a dredging company, industrial plan, or wind farm operator) causes an adverse impact (Gray and Elliott, 2009). In contrast, the developer must prove they will not cause an impact, hence creating the scientific and statistical challenge of “proving the negative.” A second key feature, “the precautionary principle” (PP), assumes a deleterious effect resulting from a given activity in the system unless proven otherwise (O'Riordan and Jordan, 1995). However, detractors criticize the vague definition of PP, and balancing scientific uncertainty and appropriate management measures remains a challenge (Steel, 2014).
The third key feature states that any developer wishing to use the marine system must obtain permission from a regulatory body, hence the importance of sufficient administrative bodies (Boyes and Elliott, 2014, 2015; Elliott, 2014); this encompasses the whole of marine governance, defined as the net result of policies, politics, legislation, and administration (Barnard and Elliott, 2015). The fourth feature, the “polluter pays principle,” requires a developer to pay for the costs associated with that use: the licensing of the activity, the monitoring, remediation and mitigation of any damage to the system and, if necessary, compensation. The latter requires integrating natural and economic sciences to enable sustainability within and across generations and it may require developers to compensate affected users, the affected resource (e.g., restocking affected fish), or the affected environment (e.g., by creating new environment; Elliott et al., 2016). However, all of these central features relate to how users use an area of the sea (e.g., dredging, wind farm, fishing, etc.) but superimposing a wider suite of natural and human influences, such as climate change, on all of these activities (Elliott et al., 2015). This complexity demands, as the fifth feature, assessing the anthropogenic change or pressure in question (a “signal”) against a background of inherent variability and natural change or wider influences, i.e., the changes emanating externally to the area being managed (the “noise”; Gray and Elliott, 2009; Elliott, 2011). Finally, a sixth key feature requires quantitative and legally defendable detection of such change with a direct feedback into management.
Progress beyond the State-of-the-Art
These key features require a defendable, holistic, underlying framework, accepted, and communicable to marine managers and wider users. That framework must link causes of potential and actual changes to the marine environment, the types of changes experienced and societal responses to mediating or removing the drivers of change or at least accepting change for the benefits provided. Even in the recent past, stakeholders frequently used the DPSIR (Drivers, Pressures, State change, Impact and Response) interlinking framework (e.g., Atkins et al., 2011; Smith et al., 2014), without clearly defining each element. Hence, the wide use of DPSIR model (Gari et al., 2015; Lewison et al., 2016; Patrício et al., 2016a) not only introduced many variants and perpetuated confusion but also made it not-fit-for-purpose in providing management guidance.
Previous studies document the evolution of the DPSIR approach (Smith et al., 2014, 2016), and here we summarize and focus on the evolution from DPSIR to the most recent derivative DAPSI(W)R(M) (Patrício et al., 2016a; Scharin et al., 2016; Burdon et al., in press). This modified approach adds Activities, and relates the Impact to human Welfare and the Responses to the use of Measures (the term preferred by EU Directives). Drivers describe underlying basic human needs, such as for food, security, space, and well-being, which require Activities (fishing, building wind farms, creating navigation routes). These activities then create Pressures, such as scraping the seabed with bottom trawls or building infrastructure that removes space. Pressures are the mechanisms that change the system, potentially causing concern. Those changes encompass both the natural system, including its structure and functioning (the “State change”; Strong et al., 2015), and the human system [the Impact (on human Welfare)]. The term Welfare is used sensu stricto to include economic welfare and human and societal well-being (Oxford English Dictionary).
Furthermore, all of the activities and external changes could potentially adversely affect that main aim (the protection of the social and ecological systems), and may thus be considered hazards. If these hazards damage parts of the socio-ecological system we value, they may be termed risks, thus providing a hazard and risk typology used in the DEVOTES project (Elliott et al., 2014). Smith et al. (2016) illustrated DPSIR, using fishing activity and the pressure of trawling from abrasion on the seabed and its impacts on particular components as an example. The challenges were addressed in moving from conceptual models to actual assessments including: assessment methodologies (interactive matrices, Bayesian Belief Networks, ecosystem modeling, the Bow Tie approach, assessment tools), data availability, confidence, scaling, cumulative impacts, and multiple simultaneous pressures, which more often occur in multi-use and multi-user areas (Smith et al., 2016).
Society and environmental managers need to know not only the current status of a marine system, but also whether it has been altered, the cause of that alteration, its significance, and what can be done to reverse that change. Therefore, this requirement creates the need to consider how Pressures result in State change, in the natural system, and a societally relevant Impact of sea use (including the assessment of cumulative pressures and impacts, as shown by Korpinen and Andersen, 2016); hence the need to consider not just Welfare (sensu DPSWR in Cooper, 2013) but the Impact (on human Welfare). This need explicitly includes an economic approach and a human health and well-being approach to human-induced changes. Furthermore, while that State change may often relate to the physico-chemical and ecological structure of the marine system, it increasingly requires users to consider the ecological functioning (Strong et al., 2015) especially given that many MSFD descriptors relate to functioning aspects. This “biodiversity-ecosystem functioning debate,” regarding the effect of functioning on biodiversity and vice versa, is an important and developing field (Zeppilli et al., 2016).
The detection or prediction of changes to the natural state and impacts on human welfare require action to minimize, mitigate, compensate, remove, or even accept changes through societal Responses (the R in DPSIR). However, based on terminology used in the EU Directives, environmental managers now refer to those Responses as Measures [hence Responses -using Measures- in DAPSI(W)R(M); Scharin et al., 2016]. During the past decade, management has recognized the need to include all measures which therefore, as referred to as the Programme of Measures in the MSFD, should consider aspects of ecology, technology, economy, legislation, and administration. They should also satisfy societal, cultural and moral imperatives while communicating decisions to stakeholders; hence the so-called “10 tenets” for sustainable and successful marine management (Elliott, 2013; Barnard and Elliott, 2015).
The prevailing governance system provides a central control on adverse effects of human activities. The EU arguably represents the pre-eminent proponent of marine environmental legislation and other aspects of governance (Boyes and Elliott, 2014), but the complexity of the marine system, the need for transboundary action and the joint implementation of different systems have produced anomalies, confusion, and a need for an inter-governmental transboundary approach (Cavallo et al., 2016).
Most of the above framework relates to activities and pressures emanating from within a system such as a sea region, under management, for example the Baltic or North Seas (Andersen et al., 2015; Scharin et al., 2016). These may be termed endogenic managed pressures in which the causes and consequences in the region are managed (Elliott, 2011) and under legislative control (Boyes and Elliott, 2014). Exogenic unmanaged pressures (i.e., those aspects emanating from outside a managed system; for example global climate change Elliott et al., 2015) represent the major current challenge; environmental managers cannot control the causes but must respond to the consequences. Climate change offers a primary example, in which human impacts (e.g., ocean acidification, increase in alien species, sea-level rise, temperature regime change; Danovaro et al., 2013; Katsanevakis et al., 2014, 2016) add to internal pressures in an area. Climate change therefore shifts baselines, complicating evaluation change associated with internal activities in a region, but also potentially nullifying the use of quantitative indicators or at least requiring the target values of those indicators to be continually revised. A Member State not meeting legislative controls, such as directives, may therefore cite climate change as a modifying factor but one outside of its control (Elliott et al., 2015). Targets that cannot be reached due to changes caused by climate change effects are not manageable and need to be revised as a part of the 6 years management cycle.
Conclusions
Successful ocean use management relies on adequate and comprehensive monitoring, and identifying appropriate measurements of change. Management response requires a clear understanding of underlying causes and effects of change in the marine environment and their consequences. Hence, the use of conceptual models linking the marine drivers, activities, and pressures can provide that solid foundation to link to state changes, impacts on societal welfare, and the resulting management responses using programmes of measures. Similarly, management relies on the ability to predict and detect future responses of the system to changes with sufficient certainty; prediction requires conceptual, empirical, and deterministic models, whereas detection implies the presence of robust monitoring systems at appropriate spatial and temporal scales. However, the “paradox of environmental assessment” sets the backdrop for this framework whereby increasing national and European legislation (such as the MSFD) requires more understanding and better monitoring but monitoring organizations face reduced budgets (Borja and Elliott, 2013). Therefore, by expanding the concept of DPSIR into DAPSI(W)R(M), understanding the gaps and the Strengths, Weaknesses, Opportunities, and Threats in monitoring, and exploring how climate change could affect GES, DEVOTES has included human welfare in the modified approach, emphasizing the importance for future policy and management measures. Hence, an adequate assessment of marine status can only be achieved through fit-for-purpose monitoring based on sound scientific knowledge.
Why do We Need Better Indicators to Assess the Status?
State-of-the-Art
The multifaceted concept of biodiversity encompasses everything from the genetic composition of species to the organization of habitats and ecosystems (CBD, 1992). Despite the widely recognized need to maintain biodiversity, its many interpretations make difficult any comprehensive evaluation and therefore it is necessary to use indicators, or simplified measures, that reflect or synthesize the status of important aspects of ecosystem structure or function. Marine assessments depend upon indicators to detect and evaluate changes in environmental status driven by either natural or human pressures, often in the context of implementing management targets for environmental objectives and measures. Therefore, scientists and managers worldwide seek accurate and reliable indicators that represent all relevant aspects of marine biodiversity either as individual aspects or as surrogates (proxies) for series of changes (for example the use of the breeding health of piscivorous seabirds as a proxy for the whole marine trophic system).
Although, many nations worldwide recognize the need for an ecosystem approach to ocean management, the EU has led in developing specific metrics toward that objective. The European Commission (2010) Decision specifies criteria and methodological standards to evaluate environmental status of marine waters, based upon a set of 56 MSFD indicators. Some indicators used in the assessment of coastal ecosystems under the Water Framework Directive (WFD; European Commission, 2000; Birk et al., 2012) also apply to the MSFD assessment beyond the narrow coastal strip where MSFD and WFD overlap (Borja et al., 2010; Boyes et al., 2016). In practice, during the first phase of the MSFD implementation, EU Member States used different methodological approaches to determine and assess ecosystem status (European Commission, 2014; Palialexis et al., 2014). Data availability, regional specificities, and potentially different interpretations of the EU Commission Decision led to discrepancies within methodologies reported by Member States, increasing the potential for non-harmonized approaches to status determination. Managers require further guidance on criteria for “good” indicators, and assessment of status (Patrício et al., 2014), and such a plan is currently being developed by the EU and its Member States, ICES (International Council for the Exploration of the Sea), EEA (European Environment Agency), and RSCs (The Regional Sea Conventions). Concurrently, the RSCs are developing indicators for holistic marine assessments (e.g., HELCOM, 2013; OSPAR, 2015; UNEP, 2016).
Progress Beyond the State-of-the-Art
Overview of Existing Indicators and Gaps in Relation to MSFD Requirements
To support the MSFD process, we completed a comprehensive overview of existing MSFD biodiversity-related indicators (MSFD descriptors: D1—biological diversity, D2—non-indigenous species, D4—food-webs, and D6—seafloor integrity), identified gaps, and developed/tested new indicators to assess the status in the marine environment (Patrício et al., 2014).
We created an inventory of current MSFD biodiversity indicators, which includes over 600 entries, and developed complementary software (DEVOTool; www.devotes-project.eu/devotool) to help users navigate the metadata. The DEVOTool includes instructions for its use as well as a description of the database contents. Developing the inventory demonstrated that, despite many available marine biodiversity indicators, obvious gaps remain regarding some biotic components and criteria required for the MSFD implementation (Teixeira et al., 2014). Furthermore, information regarding the quality and confidence of the indicators is currently insufficient. Most available operational indicators target coastal and shelf ecosystems and cover WFD biological quality elements, such as macroinvertebrates, fish, phytoplankton, macroalgae, and seagrasses. Major current gaps include ecosystem level and genetic population level indicators, as well as indicators for microbes, pelagic and planktonic invertebrates, reptiles, ice-associated species, and communities, and deep-sea habitats. Most indicators lack regional targets or GES threshold values, and few measure confidence levels or demonstrably link to pressures. Thus, although current indicators may be regarded as operational in the way that they have been used in marine assessments, their applicability to fulfill the criteria of MSFD indicators and to comply with indicator quality criteria (Queirós et al., 2016) has not been assessed.
Development of New Indicators
We developed 16 new indicators and refined another 13 indicators (Berg et al., 2016; Table 1) to address gaps in MSFD implementation (Teixeira et al., 2014). These indicators mainly relate to the biodiversity-related Descriptors (D1, D2, D4, and D6), and cover the full range of biological components (i.e., from microbes to seabirds and marine mammals). In addition, we developed indicator quality criteria, which were used to evaluate these indicators (Queirós et al., 2016). For example, we developed four new indicators for microbes (bacteria and cyanobacteria), but their poor score on pressure responsiveness and the potential to set targets indicated a need for further development and validation (Berg et al., 2016). Some phytoplankton biomass indicators, such as chlorophyll-a concentration from satellite measurements, provide valuable assessments of pressures leading to eutrophication, but linking changes in diverse and rapidly fluctuating phytoplankton composition with impacts of nutrient loading has proved challenging (Camp et al., 2015; Carstensen et al., 2015).
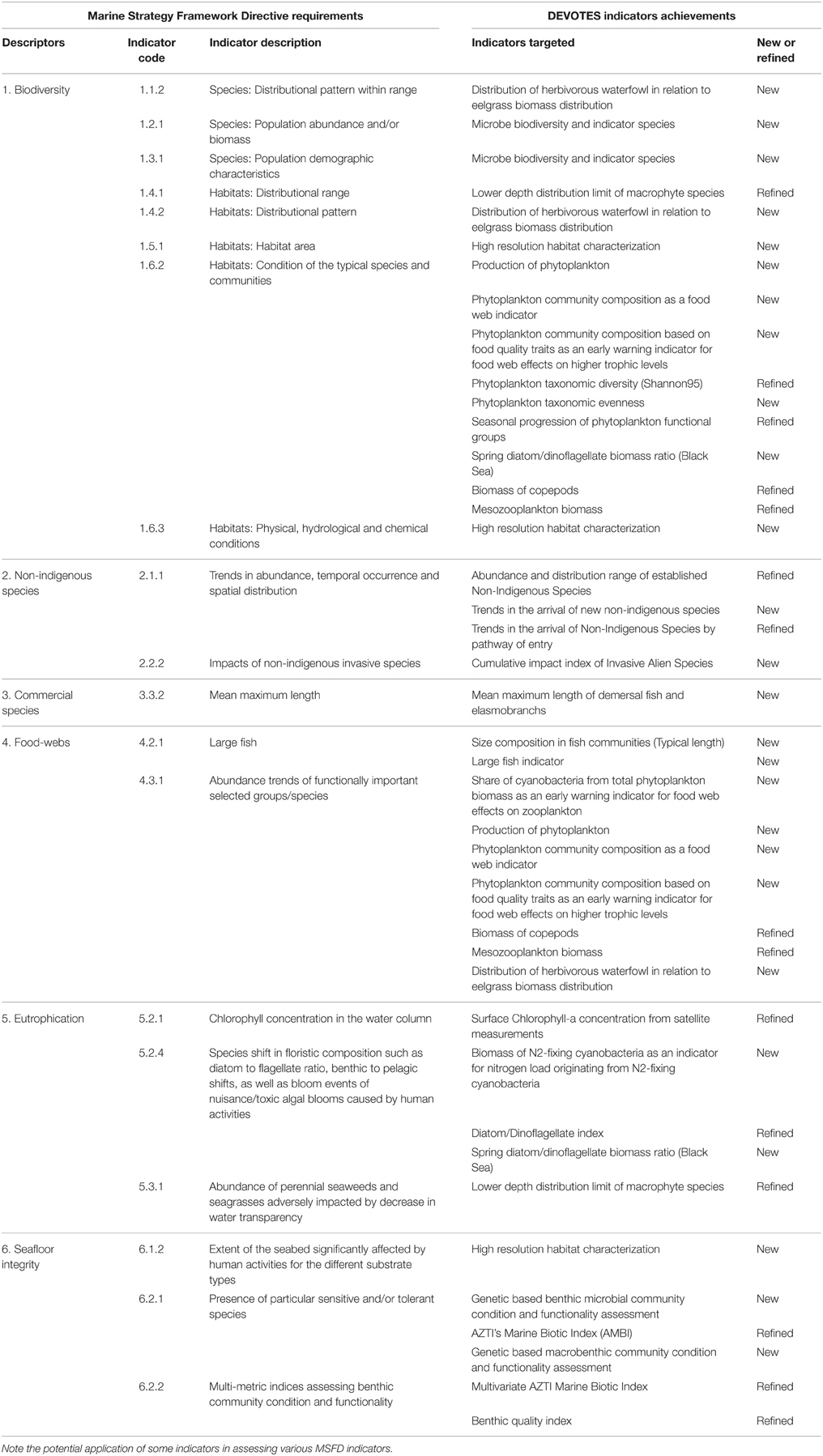
Table 1. Indicators developed or refined within DEVOTES project, in relation to some of the indicators proposed within the Marine Strategy Framework Directive (MSFD).
Also indicators were developed to address the environmental impacts of invasive non-indigenous species in European regional seas (Minchin and Zaiko, 2013; Zaiko et al., 2014; Katsanevakis et al., 2016). Moreover, the project developed new food-web indicators focusing on primary and secondary producers, both for phytoplankton and fish. Of those, the novel food-web indicator “Phytoplankton community composition as a food-web indicator” was a highly-evaluated indicator and hence it is currently a candidate HELCOM core indicator for holistic ecosystem assessment. An indicator for systematic high-resolution habitat mapping and characterization scored high in the indicator-evaluation as it may be a proxy for many of the 56 MSFD indicators. We also recently developed and tested numerous promising indicators that capture effects of fishing on marine biodiversity, e.g., on the positive effects of fishing effort reduction on the increase of large fish indicator (Engelhard et al., 2015) and on the need of using biodiversity and conservation-based indicators complementarily to ecological indicators of fishing pressure to evaluate the overall impact of fishing on exploited marine ecosystems (Fu et al., 2015; Coll et al., 2016). Furthermore, a newly developed indicator based on DNA metabarcoding assesses genetic diversity of macroinvertebrates and microorganisms (Aylagas et al., 2014, 2016; Carugati et al., 2015; Dell'Anno et al., 2015).
We also applied Signal Detection Theory (SDT) to assess the accuracy, sensitivity and specificity of refined benthic indicators (such as the Benthic Quality Index—BQI) and their response to eutrophication. In general, we found SDT to be a robust and scientifically sound strategy for setting threshold values for indicators (Chuševė et al., 2016). Finally, we introduced a new approach to set indicator targets in relation to ecosystem resilience (i.e., the ability to recover rapidly and predictably from pressures) and to select indicators and their target ranges (Rossberg et al., 2017). This approach is a specific, quantitative interpretation of the concepts of GES and sustainable use in terms of indicators and associated targets. Importantly, it distinguishes between current and future uses to satisfy societal needs and preferences.
Conclusions
Increasing legal challenges of marine and coastal management, both to the EU Member State implementation of Directives and industry compliance with national laws, which hinge upon detecting and demonstrating marine environmental change (Elliott et al., 2015), increases the need for scientifically defensible indicators. Those indicators must be comprehensive, either in covering all relevant aspects of the marine system or as conceptually defensible surrogates that represent a well-defined and well-accepted causal link (e.g., the health of breeding populations of top seabird and fish predators being dependent on the health of seabed populations).
We tested and refined 13 available biodiversity indicators, developed 16 new options for assessment, particularly for biological descriptors (considering species, habitat and ecosystem levels), identified gaps for future research, developed indicator performance criteria, and provided a user-friendly tool to select and rank indicators (Table 1). These publicly-available contributions (Berg et al., 2016), support the second phase of the MSFD implementation and assist marine management in Europe and elsewhere.
Why Models are Necessary in Marine Studies and Assessment?
State-of-the-Art
Understanding how changes in biodiversity link to food-web functioning, anthropogenic pressures, and climate changes requires novel, integrative modeling tools. Similarly, scaling determining change from small to large areas and from the present to future, also requires such modeling approaches. Once validated, modeling tools can elucidate expected risks and rewards for a range of management options, aimed at achieving or maintaining GES. The evidence base from such scenario testing thus provides a suitable platform to enable informed decision-making. Prior to 2012, the proposals for using models in the MSFD implementation were very limited (Cardoso et al., 2010) but now, in the context of using models in assessments, Pinnegar et al. (2014) for example have demonstrated the value of food-web models in assessing potential responses of ecosystems to invasions.
Progress beyond State-of-the-Art
We assessed the capabilities of state-of-art models to provide information about current and candidate indicators outlined in the MSFD, particularly on biological diversity, food-webs, non-indigenous species, and seafloor integrity descriptors (Piroddi et al., 2015; Tedesco et al., 2016). We demonstrated that models could explain food-webs and biological diversity, but poorly-addressed non-indigenous (alien) species, habitats and seafloor integrity (Lynam et al., 2016).
Habitats and Non-Indigenous (Alien) Species
In order to address the key gap related to non-indigenous (alien) species, we developed a method to model the vulnerability of areas to invasions, using the Mediterranean Sea as a case study (Katsanevakis et al., 2016). This conservative additive model accounts for the Cumulative IMPacts of invasive ALien species (CIMPAL index) on marine ecosystems. It estimates cumulative impact scores based on distributions of invasive species and ecosystems, considering both the reported magnitude of ecological impacts and the strength of such evidence.
Theory and New Approaches to Model Ecosystem Function
The theory supporting advanced modeling of food-webs and biodiversity was extended (Rossberg, 2013; James et al., 2015). Through different projects, including DEVOTES, Fung et al. (2015) used this theory to explore links between Biodiversity-Ecosystem Functioning (BEF) in marine ecosystems to fill in a key knowledge gap. Strong et al. (2015), furthermore, showed the importance and potential of such functional indicators. The BEF relationship can change (Mora et al., 2014; Fung et al., 2015), but the protection of fish from predation provided the mechanism in this case, and BEF relationships depended upon species richness and fishing impacts. Previous studies by Danovaro et al. (2008) revealed that the BEF relationships can be exponential and thus extremely sensitive to changes in environmental conditions determining a biodiversity loss. Nagelkerke and Rossberg (2014) also developed a theoretical understanding whereby resource and consumer traits predict trophic space, such that empirical data can be used to determine trophic traits related to food-web functioning (James et al., 2015).
New modeling approaches using mass-balanced models were also developed to identify ecosystem structure, function (including Ecological Network Analyses) and reaction to disturbance (Lassalle et al., 2013, 2014a,b; Niquil et al., 2014; Chaalali et al., 2015; Guesnet et al., 2015).
Habitats and Function
To further understand the role of habitat in regulating function in marine food-webs, and thus link to other descriptors, such as seafloor integrity and biological diversity, we studied marine habitats at local [i.e., Basque coast, Galparsoro et al. (2015); Eastern Aegean Sea, Lynam et al. (2015b); Western Adriatic deep-sea, Zeppilli et al. (2016)], sub-regional (i.e., North Sea, Stephens and Diesing, 2015; van Leeuwen et al., 2015), and regional (i.e., Mediterranean, Katsanevakis et al., 2016) scales. For example, we developed a process-driven characterization of sedimentary habitats for the Basque continental shelf and demonstrated that species richness decreases rapidly with increased sediment resuspension (Galparsoro et al., 2013). Habitat modeling of elasmobranchs in the southern North Sea demonstrated the extirpation of some species such as common skate over time (Sguotti et al., 2016). Modeling spatial distribution of three common seabird species in the southern North Sea demonstrated the importance of habitat type and availability fish prey to seabird distributions (see Lynam et al., 2015a). Additionally, we demonstrated in Stephens and Diesing (2015) the feasibility of predicting substratum composition spatially across a large swath of seabed (North Sea) using legacy grain-size data and environmental predictors. We also demonstrated the suitability of such a quantitative prediction for further analyses of habitat suitability compared to traditional grid cell categorization (Stephens and Diesing, 2015).
We applied Benthic Traits Analysis (Alves et al., 2014; van der Linden et al., 2016a; Van der Linden et al., 2016b) specifically to understand benthic community function in relation to habitat. This analysis identified typological groups of benthic macroinvertebrates in the North Sea, based on response and effect traits, as potential ecological indicators for MSFD Descriptors 1 (Biological Diversity) and 6 (Seafloor integrity; Veríssimo et al., 2015). The creation and analysis of large data set on population genetics in species groups with different dispersal abilities linked genetic variation to constraints in movement within benthic habitats in macroinvertebrates. This finding appears consistent with a “neutral theory” explanation for marine biodiversity spatial patterns (Chust et al., 2013, 2016).
A major challenge in marine management and assessment relates to the ability to link the physico-chemical and ecological systems. For example, for pelagic habitats, we identified distinct physical regimes in the North Sea based on density stratification characteristics, and modeling identified five hydrodynamic regimes (van Leeuwen et al., 2015). These findings are valuable to support assessment at a sub-divisional scale within MSFD subregions. Effective marine management must consider these regimes and their likely biological interactions. These zones form the basis for the OSPAR biodiversity (pelagic habitat) assessment based on lifeforms, together with considering oxygen and eutrophication when assessing primary production for food webs.
Scenario Testing to Inform Management Decisions
Our research demonstrated that fisheries management may enhance biological diversity (such as the size-structure of the fish and elasmobranch community) but potentially produce unintended consequences for other ecosystem components (Lynam and Mackinson, 2015). For example, decreases in bentho-piscivores component. However, the system may nonetheless sustain economic yields with minimal risk of stock collapse (Lynam et al., 2015b) if managed through an ecosystem approach. In the long term, climate change may shift baselines for indicators (Lynam et al., 2015b) and so assessments of GES should recognize these effects (Elliott et al., 2015).
Conclusions
Marine research and assessment require modeling studies that can support the use of indicators in fully encompass the functional linkages between ecosystem components and overwhelming pressures on the marine environment, such as climate change and ocean acidification. Such modeling provides the evidence for setting realistic targets and thus supporting better long-term marine planning. We used case studies to illustrate that modeling can assist in MSFD implementation, contributing to each step of the assessment and management cycle. Modeling can help to develop and refine novel indicators to support indicator-based assessment of GES. Modeling can incorporate indicator trends and responses, incorporating prevailing climatic conditions and anthropogenic pressures and, in this way, support the review of objectives, targets and indicators. Moreover, modeling can both inform adaptive monitoring programmes and be used in scenario testing to inform management decisions.
Monitoring Networks in European Regional Seas: Is Traditional Monitoring Sufficient to Assess the Status of Marine Ecosystems?
State-of-the-Art
Methods traditionally used in marine monitoring to investigate spatial and temporal variation in abiotic and biotic variables are time-consuming, costly and often limited in resolution (de Jonge et al., 2006; Borja and Elliott, 2013; Carstensen, 2014; Fraschetti et al., 2016). These constraints can severely limit our capacity to detect spatial and temporal changes in marine environmental health. In addition, most countries lack the tools to expand marine monitoring to the deep sea (Ramirez-Llodra et al., 2011), severely constraining the expected implementation of the MSFD in the open ocean and deep sea (Zeppilli et al., 2016). Moreover, marine monitoring methods currently limit analyses of some descriptors. For example, detecting cryptic and/or alien species (including those causing harmful algal blooms) will benefit from molecular approaches (Bourlat et al., 2013).
Activities that smoother, abrade or permanently-remove seabed habitat represent the greatest threats to seafloor integrity (Rice et al., 2012). Previous studies used benthic faunal analysis to indicate general seafloor integrity (Pearson and Rosenberg, 1978), drawing on an extensive catalog of methods and approaches for such a fundamental change (Gray and Elliott, 2009), but increasingly together with various visual assessment tools (Solan et al., 2003). Specific benthic faunal indicators exist for trawl abrasion (Jorgensen et al., 2016) but deriving these indicators is time-consuming and expensive to implement. Video inspection of seafloor smothering using Remotely Operated Vehicles (ROV), such as from seabed drilling activities, can visually map the environmental footprint (Gates and Jones, 2012), but we lack data to validate the uncertainty of the method compared to conventional biological sample collection.
The implementation of the assessments of marine environmental status required by the MSFD thus requires development and/or testing of innovative monitoring systems. Despite creating recent methodologies/technologies in DEVOTES, these are not yet used in routine monitoring of the MSFD descriptors. We encourage this through our summary analysis encompassing a catalog of monitoring networks and a wide array of potential tools, including: (i) molecular approaches (e.g., barcoding and metagenomic tools), (ii) remote sensing/acoustic methods, and (iii) in situ monitoring techniques.
Progress beyond the State-of-the-Art
We have produced a catalog with the biodiversity monitoring networks, currently available in European Seas, with the aim to: (i) present a critical overview of the monitoring activities in Europe (i.e., the amount and reason for ongoing monitoring, whether it fulfills its objectives and to what pressures it is links), (ii) identify areas where no monitoring occurs, and (iii) recommend the further development and improvements for optimizing marine biodiversity monitoring in the context of the MSFD. Since the publication of the catalog (Patrício et al., 2014), new material has been added so that it currently identifies 865 monitoring activities corresponding to 298 monitoring programmes. A gap and SWOT (Strengths, Weaknesses, Opportunities, and Threat) analysis of the catalog (Patrício et al., 2016b), highlights uneven distributions of monitoring across regional seas (i.e., more monitoring activities in the North Eastern Atlantic and Mediterranean). Specifically, we note uneven monitoring effort between descriptors (e.g., more monitoring for Descriptor 1 on Biological diversity and Descriptor 4 on Food webs), between biological components (e.g., monitoring emphasis on fish and phytoplankton) and between pressures (e.g., high level of monitoring of organic matter enrichment across all regional sea). In addition, we consider whether monitoring networks are fit-for-purpose or sufficient for adequate implementation of the MSFD within the context of the need for better coordination, harmonization of methodologies, and cost-effectiveness considerations (Patrício et al., 2016b). This allowed us to explore different innovative monitoring approaches. Below we discuss these new approaches in terms of their potential applications to some of the 11 descriptors of the MSFD investigated by DEVOTES, in order to evaluate their broader applicability to future marine environmental monitoring.
Descriptors 1 (Biological Diversity) and 2 (Non-indigenous Species)
Future monitoring is increasingly likely to use molecular tools to complement classical taxonomic techniques in providing timely and inexpensive results (Bourlat et al., 2013). Classical biodiversity assessment is time-consuming and requires diverse taxonomic expertise. Metabarcoding could expedite biodiversity assessment, especially for microscopic organisms (either algae or animals) for which morphological identification is difficult (Carugati et al., 2015). For example, Dell'Anno et al. (2015) provided the first comparison of different DNA extraction procedures and their suitability for sequencing analyses of 18S rDNA of marine nematodes. They subsequently analyzed intra-genomic variation in 18S rRNA gene repeats and reported that morphological identification of deep-sea nematodes matches the results obtained by metabarcoding analysis only at the order-family level. These results illustrate the importance of metabarcoding for exploring the diversity of benthic metazoans, but currently available databases have a limited coverage in quantifying the species encountered. Metabarcoding studies should therefore carefully consider these limitations in quantitative ecological research and monitoring programmes of marine biodiversity (Aylagas et al., 2016).
The routine use of microarrays for rapid detection of specific phytoplankton taxa, and particularly the presence of harmful algal blooms, requires further development to increase reliability and reduce associated time and expense. Nonetheless, monitoring strategies should include different molecular approaches [e.g., quantitative, in situ Polymerase Chain Reaction (PCR)] as these approaches offer far greater sensitivity to detect the presence, for example, of pathogenic bacteria compared to traditional approaches.
In addition to the above molecular tools, comparing biodiversity across different habitats and seas represents a critically important aspect of marine biodiversity monitoring, which metabarcoding can address. For example, in order to use metabarcoding to investigate the benthic biodiversity colonizing identical structures in different habitats, we deployed and later recovered Autonomous Reef Monitoring Structures (ARMS), initially developed by NOAA for coral reefs, after 12 months on hard bottoms at shallow depths at three sites (triplicates) within different regional seas (Baltic Sea, English Channel in the NE Atlantic, Adriatic Sea, Black Sea, and Red Sea). This highly reproducible approach allows a standardized comparison of colonizing biodiversity in different systems.
In parallel, molecular tools allowed us to identify aspects of biodiversity that classical tools could not, such as identifying microbial assemblages as indicators of biodiversity (Caruso et al., 2016), monitoring picoplankton (Ferrera et al., 2016), an early detection of invasive species (Ardura et al., 2015; Zaiko et al., 2015a,b), a census of meiofauna (Carugati et al., 2015), identifying functional gene diversity and plankton phylogeny (Reñé et al., 2013, 2015; Ferrera et al., 2015), revealing benthic eukaryotic diversity (Pearman et al., 2016a,b), or assessing the status of benthic macroinvertebrates (Aylagas et al., 2014).
The MSFD recognizes spatial changes in species and population distributions as key indicators. Numerous DEVOTES studies demonstrated the value of combining seabed geological information with biological variables (e.g., Galparsoro et al., 2013, 2014). However, whilst multiple needs drive the collection of such geological data (e.g., safety of navigation, renewable energy infrastructure, planning), mapping the entire marine area will require considerable time (although perhaps less than a decade with existing capabilities). Despite this potential, even after a comprehensive baseline survey, further monitoring for change will always be necessary. Existing monitoring programmes have enabled collection of high-resolution multibeam sonar data over a large area and extrapolation of these properties across 100,000 km2 in the western English Channel. Only by addressing and interrogating environmental variables at scales and a resolution relevant to the biota will we understand the context of local ecosystem change and status.
Descriptor 3 (Commercial Fish Species and Shellfish)
At present, other than acoustic surveys that lack taxonomic resolution and exceed the science capability of developing nations, we lack novel approaches to replace traditional surveys and stock recruitment assessment in fish population studies. However, emerging molecular tools can identify connectivity among fish populations and help elucidate the role of connectivity in maintenance of fish stocks.
Descriptor 4 (Food-Webs)
Researchers can now cost-effectively monitor the functioning at the base of the food-web (i.e., primary and secondary production) using ferrybox systems [such as the Continuous Automated Litter and Plankton Sampler -CALPS-, developed on the RV Endeavor CONISMA, 2013] on research vessels and ships of opportunity. The zooplankton data collected by CALPS identifies broad geographic patterns in abundance and diversity and can be integrated within existing multidisciplinary surveys at minimal extra cost. As another example, semi-automated classification of zooplankton samples usefully provided data for a range of food web related indicators even in the northern Baltic Sea, where the generally small-bodied zooplankton is difficult to be classified using semi-automated methods (Uusitalo et al., 2016a). The OSPAR-led EU project “Applying an ecosystem approach to (sub) regional habitat assessments” (EcapRHA, www.ospar.org/work-areas/bdc/ecaprha) has further investigated this approach. Monitoring of phytoplankton community composition (i.e., ratio between diatoms and flagellates) by a combination of remote sensing, microscopy, and bio-optical methods can clarify food-web effects on higher trophic levels (Goela et al., 2015).
Descriptor 5 (Eutrophication)
Current instruments that can analyze chlorophyll-a from in situ sampling can ground-truth satellite image analysis for monitoring of phyto-pigments concentrations in surface waters (Cristina et al., 2014, 2016) or assess aquaculture impacts (Mirto et al., 2010, 2014; Luna et al., 2013; Bengil and Bizsel, 2014). In addition, pigment color analysis (particularly in situ flow cytometry) can provide insights on phytoplankton biodiversity (Goela et al., 2015), estimate and calculate time series of annual gross primary production, and support MSFD implementation (Cristina et al., 2015). We also investigated the influence of benthic trophic state on meiofaunal biodiversity and found that the benthic trophic status based on organic matter variables is not sufficient to provide a sound assessment of the environmental quality in marine coastal ecosystems. However, the integration of the meiofaunal variable allows providing robust assessments of the marine environmental status (Bianchelli et al., 2016).
Descriptor 8 (Contaminants)
Andrade et al. (accepted) developed a high frequency non-invasive (HFNI) bio-sensor as a potential tool for marine monitoring which uses the biorhythmic gaping behavior of clams (such as the Icelandic scallop Chlamys islandica and the Pacific oyster Crassostrea gigas) in response to environmental cues such as day length. These innovative microsensors measure the distance between the valves of bivalves held in underwater baskets at strategic locations, and can operate unattended for several years. Measurements every 1.6 s are telemetered from the field to the laboratory and further transferred to a “big-data” storage system for analysis. Minimal operational costs and online, real-time data availability offer major advantages of the system once installed.
Beyond biorhythm research (including growth and spawning behavior) in relation to climatic factors, the method has potential for monitoring marine contamination. Exposure to stressors such as sudden changes in water quality, temperature increases (e.g., around power plants), toxic algal blooms, or a plume of water-borne contaminants, interrupts otherwise regular gaping behavior. The automated, real-time detection could provide an early-warning system, with potential applications including monitoring of water quality at swimming beaches, harbors, petroleum installations (produced water and unintentional spillages), and aquaculture sites. This “talking clam” method can improve cost-efficiency by alerting users to periods of potential risk, narrowing the need for more labor-intensive physical sampling, as long as it is assumed that normal gaping behavior reflects good water quality status.
Conclusions
As indicated above, we currently face a “paradox of environmental assessment”—with increasing monitoring requirements set against a backdrop of decreasing budgets. This paradox ensures the need for more cost-efficient and effective monitoring, and may eventually produce cheaper traditional monitoring, especially where monitoring requirements span large areas, as in the MSFD. The paradox requires wide-scale and rapid surveillance techniques, including innovative tools such as genomic approaches, remote sensing and acoustic sensors.
Why do We Need an Integrative Assessment of Status?
State-of-the-Art
The European Commission (2010) identified 56 indicators to consider when evaluating environmental status, but at least an order of magnitude more indicators already exist (Berg et al., 2015). Despite this, many of these indicators are variants on similar themes and hence measure related attributes, and are often geographical derivations, for example the health of seabed communities. The relevance and availability of indicators vary substantially among regional seas and their subdivisions; however, the MSFD provides no guidance on integration principles, despite multiple approaches to aggregating indicators whose selection may produce highly diverging results (Borja et al., 2014). These choices challenge the scientific community to develop harmonized approaches for integrating these indicators to compare across different assessment areas.
The Ocean Health Index (OHI; Halpern et al., 2012) was developed to assess the consequences of human impacts as well as societal benefits by calculating a weighted average of scores for pressure, status and resilience goals in different areas globally. Borja et al. (2011) were the first to address specifically the challenges of the MSFD, using weighting averaging principles for integrating indicator information. The MARMONI (Innovative approaches for MARine biodiversity MONItoring and assessment of conservation status of nature values in the Baltic Sea) assessment tool (Martin et al., 2015) then used an aggregation principle based on the hierarchical structure laid out by the European Commission (2010), rather than using aggregation approaches based on the structures of marine ecosystems. Nevertheless, all assessment methods standardize indicators to a common scale prior to aggregation (Borja et al., 2016). This standardization relies upon defining of targets or reference states, which MSFD describes as targets for GES (Borja et al., 2013). The OHI uses the relative deviation from a reference state, whereas the MARMONI tool uses a binary scoring system to determine whether GES has been achieved (score of 100) or not (score of 0). However, these standardization approaches do not always achieve translating indicator values to a common scale. A relative deviation from a reference state of 50% could indicate a minor human disturbance for one indicator but a major human disturbance for another. Similarly, a binary standardization approach does not differentiate between whether minimal attainment or high status level of GES was achieved.
Progress beyond the State-of-the-Art
We developed and released software for NEAT (Nested Environmental status Assessment Tool; freely available at: www.devotes-project.eu/neat), to overcome some of the deficiencies of current integrated assessment tools (e.g., aggregation of multiple indicators at multiple temporal and spatial scales; absence of uncertainty determination, etc.) NEAT is loosely based on previous tools (Andersen et al., 2014, 2016) and translates indicator values to a common scale ranging from 0 (worst possible status) to 1 (best possible status), with 0.6 defining GES or the good-moderate boundary according to the WFD. Similarly, NEAT also allows users to set boundaries representing high-good status (value of 0.8), moderate-poor status (value of 0.4), and poor-bad status (value of 0.2). It also employs stepwise linear interpolation between these fixed points to produce transformations with a high degree of flexibility spanning the entire scale (0–1) and in which 0.6 always represent GES. In comparison with the OHI and MARMONI tool, this transformation produces a more comparable scale for integrating standardized indicator values. NEAT also employs weighted averaging of standardized indicators, but bases averaging on ecosystem features to represent the whole ecosystem. The approach primarily divides the entire ecosystem into multiple Spatial Assessment Units (SAU) that are nested to define a hierarchy of SAUs. Habitat information and relevant indicators according to organism groups are used to describe the environmental status which the given habitat may enter at different levels of the hierarchy, depending on the spatial representativity of the indicator and organism. First, averaging aggregate indicators at the organism level to produce a more even representation of relevant organism groups, i.e., to avoid an assessment biased by many indicators for the same organism group, before aggregating across habitats and SAU (Clark et al., 2011). Spatial information of the different SAUs, if provided, is used for weighting and habitats can be prioritized to weight complex habitats such as vegetated sea bottoms more heavily than deep, muddy sediments. In addition, NEAT indicators are associated with the different MSFD descriptors, supporting assessments based on various descriptor combinations (essentially from one to all).
Application of NEAT to 10 case studies across European marine waters with very different challenges, environmental conditions, and scales (Uusitalo et al., 2016b) highlights its flexibility adapting to these very different cases. This also highlighted the need for careful evaluation of the indicator set, their GES boundaries, and the selection of the SAUs, all of which can increase the accuracy of the GES assessment.
Finally, NEAT includes an uncertainty assessment at all levels of integration based on the propagation of errors (uncertainties) associated with the provided indicator information (Uusitalo et al., 2015). Therefore, assessing the confidence in the integrated assessment requires including an indicator value with an estimate of the standard error of that indicator value. Noting that few studies report or even determine the standard error of an indicator value, Carstensen and Lindegarth (2016) provide a framework for quantifying indicator uncertainty to enable such calculations. Knowing the distributions of the indicator estimates enables the calculation of the distribution of the standardized indicators as well as their aggregated values.
Conclusions
A true ecosystem approach for ocean use management requires an integrative assessment of marine water status. In this way, NEAT provides a second-generation, integrated assessment tool that builds on the hierarchical structure of marine ecosystems and the organisms inhabiting different compartments within this structure, thereby improving upon previous tools. Such a hierarchical approach allows users to interrogate the results to understand the reasons for the failure or success at achieving GES. However, the integrated assessment is only as good as indicator information allows, and missing or omitting information on specific groups (e.g., biological components or descriptors relevant to the assessed area) can bias the assessment results. Therefore, managers should produce guidelines stipulating indicator minimum requirements [e.g., type, coverage (ecosystem components, area, etc.), number] and the integrated assessment tool should clearly indicate if there is non-compliance with such guidelines. Moreover, because NEAT includes a comprehensive uncertainty assessment, researchers should incorporate this information as part of their interpretation of outcomes and decision support, thus needing guidelines for confidence levels of decisions.
In conclusion, environmental managers must assess the status of marine waters, not only to comply with current legislation (i.e., MSFD, WFD), but also to determine how far from targets marine ecosystems may be. Such information will allow managers to make informed decisions on sustainable resource use and the adequate restoration of degraded systems.
What Economic and Social Dimensions Affect Marine Management?
State-of-the-Art
Inevitably, new legislative framework directives bring about unforeseen challenges to the different stakeholders who need to be involved in their implementation, particularly when first applied. Managers already apply the MSFD, which is itself complex, to complex, heterogeneous, and dynamic environments. Furthermore, initiation of the MSFD coincided with a period of a growing, global economic crisis. The numerous objectives can potentially conflict with one another from the perspective of different government departments within the Member States and also between Member States sharing a regional sea. The MSFD legal status and implementation deadlines demand that scientists and decision makers ensure a collaborative and multidisciplinary approach to deliver multi-sectoral objectives that test the abilities of existing institutions. The rapid identification of the issues, and the problems that they can create, can help those responsible for MSFD implementation to consider best how to address such issues and ensure that the MSFD can provide the intended sustainable environmental benefits.
The introduction of complex and integrative environmental legislation such as the MSFD also inevitably incurs additional costs, such as establishing new monitoring and improving existing monitoring of multiple indicators across European seas. This demand can be economically challenging. Policy makers and regulators in all EU countries are obliged to manage their resources carefully and hence they will seek to comply with the MSFD in the most cost-effective way. Yet they have many choices on which types of monitoring to apply as they select the approaches that best comply with the legislative needs within the limits of their budgets (Veidemane and Pakalniete, 2015). Although the MSFD does not require consideration of the socio-economic aspects of monitoring, Borja and Elliott (2013) noted that limited financial resources represent the most significant threat to ensuring adequate monitoring.
Furthermore, the law requires that EU countries determine whether they need new management measures and monitoring schemes to enable them to achieve GES and, if so, to implement them. Here, the socio-economic analysis of the use of marine waters, the cost of present-date degradation of the marine environment, and the cost-benefit analysis of implementing monitoring and new management measures required under the MSFD could motivate Member States to achieve GES. However, whilst a dominant tool of all governments, economic analysis approaches to achieve such analyses specifically for the MSFD in relation to the marine environment and its management were not developed at the start of the MSFD process.
Progress Beyond the State-of-the-Art
Barriers to Achieving Good Environmental Status
A comprehensive review of the documented barriers to achieving GES indicated that Member States have encountered and reported legislative, governance, and socio-economic barriers during this first phase of implementing the MSFD (Boyes et al., 2015, 2016). Barriers include ambiguity in the text of the Directive resulting in different interpretations by Member States, creating uncertainty, and different levels of conformity and governance complications. For example, GES [Article 3(5)] is neither well defined nor quantitatively described (Boyes et al., 2016), not easily understandable, and requires specific guidance to achieve common understanding and to enable coherent practices between the Member States and across regional seas. The next revision of the European Commission (2010) Decision regarding MSFD implementation will provide more guidance on GES definition (for example the operational definition proposed by DEVOTES; Borja et al., 2013), and thus the input from different stakeholders, including the scientific community, will be extremely important. The effectiveness with which MSFD can achieve GES partially relates to the success of other EU legislation [e.g., the WFD, the reformed Common Fisheries Policy (CFP), Maritime Spatial Planning Directive (MSP), Integrated Maritime Policy (IMP)], acknowledging the ambiguity of the role and contribution of each individual piece of legislation. Despite limited reference to specific policies in the MSFD, it provides a framework that can incorporate earlier and future legislation to ensure that legislation provides spatially and temporally complete coverage for the protection of marine environment. The MSFD article 6 is quite clear on the purpose and role of RSC: “…Member States shall, where practical and appropriate, use existing regional institutional cooperation structures, including those under Regional Sea Conventions…” and “…Member States shall, as far as possible, build upon relevant existing programmes and activities developed in the framework of structures stemming from international agreements such as Regional Sea Conventions….” However, in the absence of clear guidance on how this objective should be implemented or the actual competence of the RSCs, Member States have not adopted the regional coordination and integration to achieve MSFD objectives. Boyes et al. (2015, 2016) offer recommendations to address these legislative and governance barriers, such as “continued clarification and harmonization of the definitions and methodologies within and between Member States and the different RSCs.” The aims of other directives should be consistently included in considerations for GES together with clear reference to MSFD and other existing, forthcoming and amended directives. Systematic use of standards that already used within other EU legislation must be applied as minimum requirements. Implementation of the MSP Directive particularly provides measures that will support delivery of the goals of MSFD by facilitating a balance with blue growth objectives (Boyes and Elliott, 2014; Boyes et al., 2016). The RSC must have a mandate supported by their contracting parties in order to ensure that the measures implemented in EU countries are supported and complemented by respective measures also in non-EU countries. Achieving RSC aims requires continuous cooperation in regional seas between EU-Member and non-Member States in the context of RSCs (Cavallo et al., 2016).
Socio-economic barriers include a lack of appropriate biological, environmental, and socio-economic data, a limited application of the ecosystem-based approach and of economic impact analyses by Member States. Effective use of the findings of EU funded projects and pilot projects (involving both non-EU countries and Member States) can both boost the evidence and knowledge required. It can also provide a vehicle to improve and support regional coordination and encourage the coherent implementation of the MSFD in regional sea areas, and ensure engaging non-EU countries in programmes that enable measures to achieve true regional GES.
Discussions with stakeholders showed that often public and stakeholder consultations on the programmes of measures were only open for limited periods of 1–2 months, and stakeholders in most Member States, particularly NGOs, felt that they were not sufficiently involved in the MSFD process, with only limited integration of their feedback (Boyes et al., 2015). In recognizing the complexity of marine ecosystems, the existence of multiple stakeholders with imperfect and impartial knowledge, as well as resource constraints, we developed a workshop approach “to engage and share different perspectives, and develop models of the system under consideration that are seen to be valid and useful aids to decision making” (Boyes et al., 2015). This multi-stakeholder workshop based modeling approach, which focused on Causal Loop Diagrams (CLD) to describe and understand the case site, was developed and then trialed in a case study site in England (Boyes et al., 2015). Managers should consider this approach, which effectively engaged stakeholders in understanding the complex environment associated with GES and the barriers and opportunities for its achievement, is exemplary for moving forward in MSFD implementation.
Cost-Effectiveness of Monitoring
Building on the ecological criteria for monitoring developed in Queirós et al. (2016), we developed an approach that uses multi-criteria-decision-analysis (MCDA) for cost-effectiveness analysis incorporating both ecological and economic criteria as attributes of monitoring systems. This approach encompassed a standardized scoring system for each of the different attributes, readily adaptable to the analysis undertaken with the attributes and the scores used as input to the MCDA. The cost-effectiveness of a given monitoring approach can be determined using the Rapfish software (www.rapfish.org), a non-parametric multivariate analysis tool, developed and tested in different contextual case studies of MSFD monitoring in Finland, Spain, and the UK. We also developed flow charts to help users identify the different elements of operational costs during monitoring. The tool can be applied to examine both the cost-effectiveness of the different monitoring elements and whether the monitoring programmes satisfy the requirements of the MSFD monitoring objectives. The tool has demonstrated, for example, a mixed ability of current monitoring programmes in Bay of Biscay to comply with the need to monitor changes in quality and quantity of different MSFD Descriptors. In addition, monitoring open sea areas in the Gulf of Finland becomes more cost-efficient when combining monitoring with research cruises on scientific vessels, which make up the largest single monitoring cost.
Cost-Benefit Analysis of New Management Measures to Achieve GES
By 2015, EU Member States had to define the Programme of Measures, including new measures if any, required to achieve GES. Oinonen et al. (2016a) stated that “the specific application of methods and uptake of resulting information are currently still evolving in the ecosystem-based and adaptive management framework that the Directive stipulates.” They further recommend the use of environmental economics delivered through interdisciplinary research to support the needs of MSFD.
Three different case-studies showed interdisciplinary approaches to the cost-benefit analysis of management measures to achieve GES. In Finland, a quantitative cost-effectiveness analysis of implementing different management measures, based on opinion of interdisciplinary experts, identified the costs, and most cost-effective measures. Researchers estimated economic benefits of the management measures based on existing valuation studies (i.e., willingness to pay) on the benefits of improving the state of the Baltic Sea; these analyses connected the benefit estimates directly to the change in the status of the GES descriptors (Oinonen et al., 2016b). Extending from this analysis into a full cost-benefit analysis, the net value of achieving GES for indicators of biodiversity, food webs, and eutrophication alone in 2020 is placed at ~2 bn € (although the planned management measures will not achieve GES of these Descriptors by 2020).
Alternative approaches to cost-benefit analysis of management measures were developed and applied in the Bay of Biscay and the East Coast of England Marine Plan Areas (ECE). These approaches built on research to determine changes in ecosystem services and the benefits that identified, mapped and modeled ecosystem services, and considered valuation of their benefits (e.g., Hattam et al., 2014, 2015; Galparsoro et al., 2014; Borja et al., 2015; Kleisner et al., 2015; Laurila-Pant et al., 2015).
The Bay of Biscay approach examined the links between ecosystem services and their benefits and management measures to control the development of maritime activities creating those benefits. We used the Fishrent bioeconomic model (Salz et al., 2011) to quantitatively assess the impacts, in terms of percentage changes in net present value, of implementing some of the measures under the European Common Fisheries Policy (CFP) expected to support attainment of GES.
The ECE approach used structured analysis of changes in ecosystem services and benefits arising from potential new management measures (ballast water treatment, underwater noise reduction) to identify the benefits of achieving GES alongside the costs of implementing the measures. Insufficient availability of valuation data, needed to quantify ecosystem benefit impacts in monetary terms, precluded the possibility of extending the analysis into a quantitative cost-benefit analysis.
Conclusions
Ensuring that Member States implement sufficient and non-overlapping measures to achieve GES will require the continuous review of legislation and policy, and the assessment of its implementation (Boyes et al., 2016). Furthermore, effective stakeholder engagement is likely to facilitate the acceptance of the measures and associated costs. The effective application of the MSFD requires knowledge and databases but these currently are limited by economics (Borja and Elliott, 2013). It remains to be seen how the different Member States identify the additional measures needed to improve the marine environment toward GES and close the gap between current status and GES in 2020. However, Member States must use existing budgets carefully to avoid further economic hardship resulting from financial penalties due to legal infraction proceedings in the European Court. For example, reduced funding for monitoring, if not guided toward more effective monitoring tools (see Section Monitoring Networks in European Regional Seas: Is Traditional Monitoring Sufficient to Assess the Status of Marine Ecosystems?), can reduce the quality of monitoring (e.g., by reducing spatial and/or temporal coverage). Such reduction can ultimately entail a greater cost than investment in monitoring as inaccurate evaluation could increase the risk of decision-making errors, potentially resulting in reduced ecosystem services and a devaluation of ecosystem benefits. Political decision makers may consider other aspects of monitoring as societally important, such as maintaining a bank of knowledge, technological development, professional skill and experience development and enhancing public engagement. Tools for determining the cost-effectiveness of monitoring and of management measures, as well as use of the ecosystem service approach to determine ecosystem benefits in cost-benefit analysis can support decisions on activities undertaken to comply with the MSFD. However, many member states implementing the MSFD lack both the data required to underpin rigorous economic analysis of costs of monitoring and the valuation data for assessing changes in ecosystem benefits from improvements in ecosystem services. Furthermore, the relationship between MSFD indicators and ecosystem services still requires better understanding and the implementation of the MSFD still urgently requires such data and information.
Filling in the Gap between Science and Policy
Some of the challenges in marine ecosystems ecology identified by Borja (2014) relate to socio-ecological topics, especially given the recognition of humans (and the activities they perform and pressures they pose in the oceans) as an integral part of the marine ecosystem in recent decades. The human dimension of marine systems remains poorly documented, and discussions on ecosystem-based management of seas often minimize the importance of social sciences (Fréon et al., 2009), despite the explicit role of humans in implementing the Ecosystem Approach since its adoption in the Convention on Biological Diversity (CBD, 1992). Despite progress in recent years in connecting natural and social sciences, the gap between science (social and natural) and policy remains large in marine research (Nicholson et al., 2012). Europe has made efforts to close the research project-policy circuit in relation to the WFD (Oliver et al., 2005; Quevauviller et al., 2005; Hering et al., 2010), although until recently, few attempts have been made to close such a circuit for the MSFD (Borja et al., 2010).
Many challenges remain in bridging the gap between science and policy to support improved policy decisions in marine management (von Winterfeldt, 2013; Choi et al., 2015). As noted by Rodwell et al. (2014), improvement would require identifying: (i) the gap or perceived gap between marine science and policy; (ii) the obstacles that prevent us from bridging the gap, and (iii) the possible solutions.
More than 30 years ago, Sebek (1983) identified some of the reasons for marine public policy failure in incorporating scientific knowledge, but researchers since have removed some of the impediments (Table 2). Specific examples include:
(i) Lack of international regulation encompasses both EU and non-EU countries adjacent to the regional seas, which the different RSCs and, especially, the WFD and MSFD has overseen in recent years. DEVOTES brought together different pieces of legislation, identified gaps and overlaps and provided advice on future needs for the satisfactory implementation of the MSFD for the new Commission Decision expected in the coming months (Patrício et al., 2014);
(ii) Although monitoring increased after enacting the WFD and should continue to improve during the MSFD implementation (Patrício et al., 2014; Patrício et al., 2016b), it remains insufficient. DEVOTES contributed by identifying, testing and validating multiple innovative tools for monitoring and modeling large areas;
(iii) Independent scientific input into international conferences provided a means to organize multiple stakeholder meetings and offer training courses and sessions at international conferences aimed at disseminating and making project findings operational;
(iv) Incorporating scientific advice into regulations requires political compromise and we contributed by developing tools to assess environmental status (NEAT) that we hope will be incorporated into the implementation of the environmental quality directives (e.g., MSFD and WFD). Furthermore, a series of workshops and webinars with key stakeholders and end-users (e.g., policy-makes, relevant Member State representatives, RSC, etc.) has accompanied this development for all to understand the basis, capacities and functioning on this tool;
(v) Insufficient use of economic analysis to assess and support implementation of MSFD exacerbated a lack of appropriate monitoring costs, impacts and valuation data relevant to the assessment of marine ecosystems, their services and benefits. We developed new approaches to undertake cost effectiveness and cost- benefit analysis of monitoring and management measures, respectively, in the context of MSFD. Furthermore, we explored linkages between ecosystems and provision of ecosystem services as well as between management measures and ecosystem services.
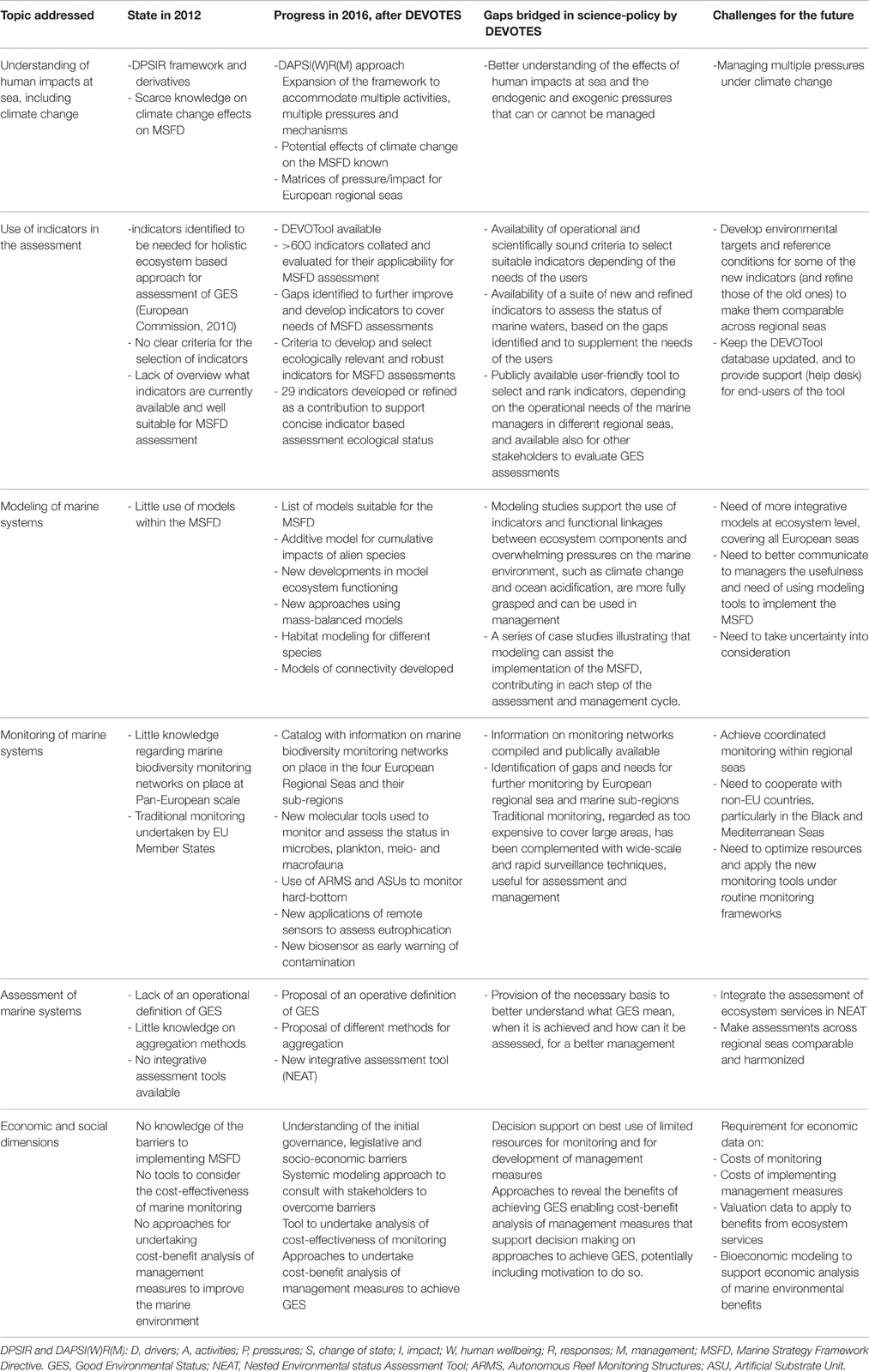
Table 2. Progress beyond the state-of-the-art achieved by DEVOTES, within the different topics addressed by the project, and gaps bridged in science-policy.
DEVOTES was conceived as an integrative project that aimed to expand and merge natural and social sciences, enable the natural scientists to understand the economic and legal requirements and economic and governance specialists to understand the limitations of natural science. Figure 1 encapsulates the work done and the integration of pieces to assess the status and respond to multiple stakeholders and end-users (i.e., scientists, policy-makers, managers, industry, conservation organizations, and society). Hence, our outputs not only increased knowledge of marine assessment and assisted marine managers, but also communicated these findings to increase stakeholder uptake.
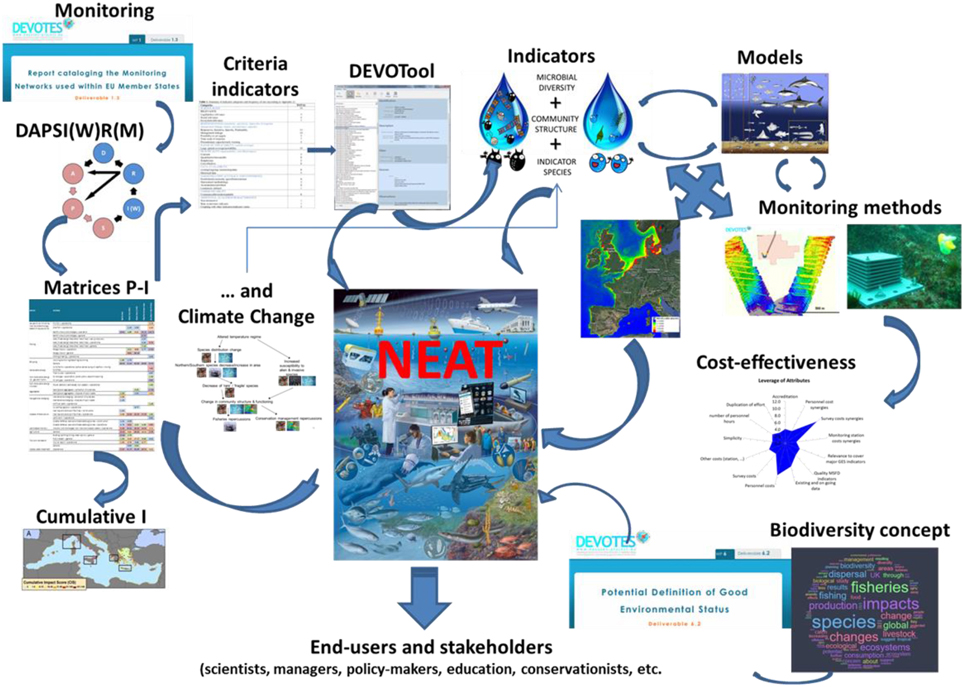
Figure 1. Synthesis of the knowledge generated within the DEVOTES project, to assess the status of marine waters in an integrative manner. D, drivers; A, activities; P, pressures; S, change of state; I, impact; W, human wellbeing; R, responses; M, measures. NEAT, Nested Environmental status Assessment Tool. The central plate, about NEAT, was drawn by Alberto Gennari.
In connection with the development of the MSFD programme of measures, Oinonen et al. (2016b) developed a pragmatic approach to holistic cost-effectiveness analysis. This allows users to select a cost-effective set of candidate measures in order to reach the multidimensional environmental objectives of the MSFD. They concluded that the major challenge in applying cost-effectiveness analysis was in assessing the current state of the environment and the multiple effects of different measures in evaluating marine ecosystem components rather than in the concepts of economic analysis. Despite this, economics helped to determine socially optimal level of marine protection.
Many challenges remain despite the body of work undertaken over the last 4 years (Table 2). These challenges include, among others: (i) understanding how multiple pressures act in marine ecosystems, and managing those pressures in the context of climate change; (ii) identifying key indicators and setting targets and reference conditions for those indicators so they can be used in assessments, noting the need for comparability across regional seas, and recognizing scenarios of climate change that shift baselines; (iii) the need to develop models capable of operating at an ecosystem level, with powerful computational capacities able to handle big data; (iv) getting EU Members States to consider adopting new monitoring tools routinely, and coordinating monitoring activities within regional sea research activities; (v) the need for intercomparable and harmonized assessments across regional seas that include ecosystem services in the assessment, and (vi) the urgent need for a harmonized framework under which indicator development follows specific rules and aligns with specific criteria to readily use in an integrative assessment tool.
General Conclusions
Defining, attaining and maintaining the GES of the seas spans from the technical details of monitoring and indicator implementation to major social and economic issues of how to optimize the long-term delivery of ecosystem goods and services, and how to govern society fairly in relation to use of the sea. It requires integrating natural and social sciences, horizontal integration across stakeholders, and vertical integration through governance, and feedback between monitoring, measures, and management. The MSFD aspires to bring all these aspects under the same umbrella, an ambitious and highly relevant objective.
DEVOTES advanced the state-of-the-art and identified major gaps within various aspects of MSFD implementation, contributed to filling these gaps, and identified additional scientific and development needs. The further development and validation of marine biodiversity indicators requires improved data with better spatial and temporal coverage, based on novel and cost-efficient monitoring methods. Better ecological relevance and indicator responsiveness to pressures will require experimental research on different levels of biological organization from the cell to the ecosystem. Such research will also enable incorporation of indicators into models in order to extrapolate marine assessment results to larger spatial and temporal scales. Each of these aspects requires comprehensive and integrated natural and social sciences which cross international boundaries and regional seas.
Author Contributions
AB conceived the paper and wrote the first draft; ME, AB contributed to the pressures section; TB, SC, MM, AH, JP, LU contributed to the indicators section; CL, CW contributed to the modeling section; RD, AB, AN, JP, SC, PS, MU contributed to the monitoring section; JC, AB, TB, SC, MU contributed to the integration section; MA, MU contributed to the socio-economic area; all authors contributed equally to the discussion and conclusions, AB, ME, SG, PS, MU made a revision of the whole text.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This manuscript is a result of DEVOTES (DEVelopment Of innovative Tools for understanding marine biodiversity and assessing GES) project, funded by the European Union under the 7th Framework Programme, “The Ocean of Tomorrow” Theme (grant agreement no. 308392), www.devotes-project.eu. MU is partially funded through the Spanish programme for Talent and Employability in R+D+I “Torres Quevedo”. This is publication number 777 from AZTI's Marine Research Division.
References
Alves, A. S., Veríssimo, H., Costa, M. J., and Marques, J. C. (2014). Taxonomic resolution and Biological Traits Analysis (BTA) approaches in estuarine free-living nematodes. Estuar. Coast. Shelf Sci. 138, 69–78. doi: 10.1016/j.ecss.2013.12.014
Andersen, J. H., Dahl, K., Göke, C., Hartvig, M., Murray, C., Rindorf, A., et al. (2014). Integrated assessment of marine biodiversity status using a prototype indicator-based assessment tool. Front. Mar. Sci. 1:55. doi: 10.3389/fmars.2014.00055
Andersen, J. H., Halpern, B. S., Korpinen, S., Murray, C., and Reker, J. (2015). Baltic Sea biodiversity status vs. cumulative human impacts. Estuar. Coast. Shelf Sci. 161, 88–92. doi: 10.1016/j.ecss.2015.05.002
Andersen, J. H., Murray, C., Larsen, M. M., Green, N., Høgåsen, T., Korpinen, S., et al. (2016). Development and testing of a prototype tool for integrated assessment of chemical status in marine environments. Environ. Monit. Assess. 188, 1–13. doi: 10.1007/s10661-016-5121-x
Andrade, H., Massabuau, J. C., Cochrane, S., Tran, D., Ciret, P., and Sow, M. (accepted). High frequency non-invasive (HFNI) bio-sensors as a potential tool for marine monitoring assessments. Front. Mar. Sci. 3.
Ardura, A., Zaiko, A., Martinez, J. L., Samulioviene, A., Semenova, A., and Garcia-Vazquez, E. (2015). eDNA and specific primers for early detection of invasive species – A case study on the bivalve Rangia cuneata, currently spreading in Europe. Mar. Environ. Res. 112(Pt B), 48–55. doi: 10.1016/j.marenvres.2015.09.013
Atkins, J. P., Burdon, D., Elliott, M., and Gregory, A. J. (2011). Management of the marine environment: integrating ecosystem services and societal benefits with the DPSIR framework in a systems approach. Mar. Pollut. Bull. 62, 215–226. doi: 10.1016/j.marpolbul.2010.12.012
Aylagas, E., Borja, Á., Irigoien, X., and Rodríguez-Ezpeleta, N. (2016). Benchmarking DNA metabarcoding for biodiversity-based monitoring and assessment. Front. Mar. Sci. 3:96. doi: 10.3389/fmars.2016.00096
Aylagas, E., Borja, Á., and Rodríguez-Ezpeleta, N. (2014). Environmental status assessment using DNA metabarcoding: towards a genetics based Marine Biotic Index (gAMBI). PLoS ONE 9:e90529. doi: 10.1371/journal.pone.0090529
Barbier, E. B., Hacker, S. D., Koch, E. W., Stier, A., and Silliman, B. (2012). “Chapter 6: Estuarine and coastal ecosystems and their services,” in Ecological Economics of Estuaries and Coasts, Vol. 12, eds M. van den Belt and R. Costanza (Waltham, MA: Academic Press), 109–127.
Barnard, S., and Elliott, M. (2015). The 10-tenets of adaptive management and sustainability - applying an holistic framework for understanding and managing the socio-ecological system. Environ. Sci. Policy 51, 181–191. doi: 10.1016/j.envsci.2015.04.008
Bengil, F., and Bizsel, K. C. (2014). Assessing the impact of aquaculture farms using remote sensing: an empirical neural network algorithm for Ildırı Bay. Turkey Aquac. Environ. Interact. 6, 67–79. doi: 10.3354/aei00115
Berg, T., Aylagas, E. T., Balsby, J. S., Borja, A., Bučas, M., Zenetos, A., et al. (2016). Report on the New Indicators and Methods for Setting Reference and Target Values. Deliverable D3-3, 269. DEVOTES Project. Available online at: http://www.devotes-project.eu/deliverables-and-milestones/
Berg, T., Fürhaupter, K., Teixeira, H., Uusitalo, L., and Zampoukas, N. (2015). The Marine Strategy Framework Directive and the ecosystem-based approach – pitfalls and solutions. Mar. Pollut. Bull. 96, 18–28. doi: 10.1016/j.marpolbul.2015.04.050
Bianchelli, S., Pusceddu, A., Buschi, E., and Danovaro, R. (2016). Trophic status and meiofauna biodiversity in the Northern Adriatic Sea: insights for the assessment of good environmental status. Mar. Environ. Res. 113, 18–30. doi: 10.1016/j.marenvres.2015.10.010
Birk, S., Bonne, W., Borja, A., Brucet, S., Courrat, A., Poikane, S., et al. (2012). Three hundred ways to assess Europe's surface waters: an almost complete overview of biological methods to implement the Water Framework Directive. Ecol. Indic. 18, 31–41. doi: 10.1016/j.ecolind.2011.10.009
Borja, A. (2014). Grand challenges in marine ecosystems ecology. Front. Mar. Sci. 1:1. doi: 10.3389/fmars.2014.00001
Borja, A., Bricker, S. B., Dauer, D. M., Demetriades, N. T., Ferreira, J. G., Forbes, A. T., et al. (2008). Overview of integrative tools and methods in assessing ecological integrity in estuarine and coastal systems worldwide. Mar. Pollut. Bull. 56, 1519–1537. doi: 10.1016/j.marpolbul.2008.07.005
Borja, A., and Elliott, M. (2013). Marine monitoring during an economic crisis: the cure is worse than the disease. Mar. Pollut. Bull. 68, 1–3. doi: 10.1016/j.marpolbul.2013.01.041
Borja, A., Elliott, M., Andersen, J. H., Berg, T., Carstensen, J., Rodriguez-Ezpeleta, N., et al. (2016). Overview of integrative assessment of marine systems: the Ecosystem Approach in practice. Front. Mar. Sci. 3:20. doi: 10.3389/fmars.2016.00020
Borja, A., Elliott, M., Andersen, J. H., Cardoso, A. C., Carstensen, J., Joγo, G., et al. (2013). Good Environmental Status of marine ecosystems: what is it and how do we know when we have attained it? Mar. Pollut. Bull. 76, 16–27. doi: 10.1016/j.marpolbul.2013.08.042
Borja, Á., Elliott, M., Carstensen, J., Heiskanen, A.-S., and van de Bund, W. (2010). Marine management – Towards an integrated implementation of the European Marine Strategy Framework and the Water Framework Directives. Mar. Pollut. Bull. 60, 2175–2186. doi: 10.1016/j.marpolbul.2010.09.026
Borja, Á., Galparsoro, I., Irigoien, X., Iriondo, A., Menchaca, I., Muxika, I., et al. (2011). Implementation of the European Marine Strategy Framework Directive: a methodological approach for the assessment of environmental status, from the Basque Country (Bay of Biscay). Mar. Pollut. Bull. 62, 889–904. doi: 10.1016/j.marpolbul.2011.03.031
Borja, A., Murillas, A., Pascual, M., and Uyarra, M. C. (2015). “Marine and coastal ecosystems: delivery of goods and services through conservation,” in Ecosystem Services and River Basin Ecohydrology, eds L. Chícharo, F. Muller, and N. Fohrer (London: Springer), 83–105.
Borja, A., Prins, T., Simboura, N., Andersen, J. H., Berg, T., Uusitalo, L., et al. (2014). Tales from a thousand and one ways to integrate marine ecosystem components when assessing the environmental status. Front. Mar. Sci. 1:72. doi: 10.3389/fmars.2014.00072
Bourlat, S., Borja, A., Gilbert, J., Taylor, M. I., Davies, N., Weisberg, S. B., et al. (2013). Genomics in marine monitoring: new opportunities for assessing marine health status. Mar. Pollut. Bull. 74, 19–31. doi: 10.1016/j.marpolbul.2013.05.042
Boyes, S. J., and Elliott, M. (2014). Marine legislation – The ultimate ‘horrendogram’: international law, European directives & national implementation. Mar. Pollut. Bull. 86, 39–47. doi: 10.1016/j.marpolbul.2014.06.055
Boyes, S. J., and Elliott, M. (2015). The excessive complexity of national marine governance systems – Has this decreased in England since the introduction of the Marine and Coastal Access Act 2009. Mar. Policy 51, 57–65. doi: 10.1016/j.marpol.2014.07.019
Boyes, S. J., Elliott, M., Murillas-Maza, A., Papadopoulou, N., and Uyarra, M. C. (2016). Is existing legislation fit-for-purpose to achieve Good Environmental Status in European seas? Mar. Pollut. Bull. doi: 10.1016/j.marpolbul.2016.06.079. [Epub ahead of print].
Boyes, S., Murillas-Maza, A., Uyarra, M. C., Eronat, H., Bizsel, K. C., Oinonen, S., et al. (2015). Key Barriers of Achieving Good Environmental Status (GES). Deliverable 2.2, 100. Available online at: DEVOTES Project http://www.devotes-project.eu/wp-content/uploads/2015/05/DEVOTES-Deliverable -2_2.pdf
Browman, H. I., Stergiou, K. I., Cury, P. M., Hilborn, R., Jennings, S., Zeller, D., et al. (2004). Perspectives on ecosystem-based approaches to the management of marine resources. Mar. Ecol. Prog. Ser. 274, 269–303. doi: 10.3354/meps274269
Burdon, D., Boyes, S. J., Elliott, M., Smyth, K., Atkins, J. P., and Barnes, R. A. (in press). Integrating natural social marine science to sustainably manage vectors of change: dogger Bank transnational case study. Estuar. Coast. Shelf Sci. doi: 10.1016/j.ecss.2015.09.012.
Camp, J., Flo, E., Vila, M., Arin, L., Reñé, A., Sampedro, N., et al. (2015). “Pros and cons of biological quality element phytoplankton as a water-quality indicator in the NW Mediterranean Sea,” in Experiences from Ground, Coastal and Transitional Water Quality Monitoring. The Handbook of Environmental Chemistry, eds A. Munné, A. Ginebreda, and N. Prat (London: Springer), 135–160.
Cardoso, A. C., Cochrane, S., Doemer, H., Ferreira, J. G., Galgani, F., Hagebro, C., et al. (2010). Scientific Support to the European Commission on the Marine Strategy Framework Directive. Management Group Report. EUR 24336 EN – Joint Research Centre, Luxembourg: Office for Official Publications of the European Communities, 57.
Carstensen, J. (2014). Need for monitoring and maintaining sustainable marine ecosystem services. Front. Mar. Sci. 1:33. doi: 10.3389/fmars.2014.00033
Carstensen, J., Cloern, J. E., and Klais, R. (2015). Phytoplankton blooms in estuarine and coastal waters: seasonal patterns and key species. Estuar. Coast. Shelf Sci. 162, 98–109. doi: 10.1016/j.ecss.2015.05.005
Carstensen, J., and Lindegarth, M. (2016). Confidence in ecological indicators: a framework for quantifying uncertainty components from monitoring data. Ecol. Indic. 67, 306–317. doi: 10.1016/j.ecolind.2016.03.002
Carugati, L., Corinaldesi, C., Dell'Anno, A., and Danovaro, R. (2015). Metagenetic tools for the census of marine meiofaunal biodiversity: an overview. Mar. Genomics 24, 11–20. doi: 10.1016/j.margen.2015.04.010
Caruso, G., La Ferla, R., Azzaro, M., Zoppini, A., Marino, G., Petochi, T., et al. (2016). Microbial assemblages for environmental quality assessment: knowledge, gaps and usefulness in the European Marine Strategy Framework Directive. Crit. Rev. Microbiol. 42, 883–904. doi: 10.3109/1040841X.2015.1087380
Cavallo, M., Elliott, M., Touza, J., and Quintino, V. (2016). The ability of regional coordination and policy integration to produce coherent marine management: implementing the Marine Strategy Framework Directive in the North-East Atlantic. Mar. Policy 68, 108–116. doi: 10.1016/j.marpol.2016.02.013
CBD (1992). Convention on Biological Diversity. Available online at: https://www.cbd.int/doc/legal/cbd-en.pdf
Chaalali, A., Saint-béat, B., Lassalle, G., Le Loc, H. F., Tecchio, S., Safi, G., et al. (2015). A new modeling approach to define marine ecosystems food-web status with uncertainty assessment. Prog. Oceanogr. 135, 37–47. doi: 10.1016/j.pocean.2015.03.012
Choi, B. C. K., Li, L., Lu, Y., Zhang, L. R., Zhu, Y., Little, J., et al. (2015). Bridging the gap between science and policy: an international survey of scientists and policy makers in China and Canada. Implement. Sci. 11, 16. doi: 10.1186/s13012-016-0377-7
Chuševė, Nygård, R., Nygård, H., Vaičiūtė, D, Daunys, D., and Zaiko, A. (2016). Application of signal detection theory approach for setting thresholds in benthic quality assessments. Ecol. Indic. 60, 420–427. doi: 10.1016/j.ecolind.2015.07.018
Chust, G., Albaina, A., Aranburu, A., Borja, Á., Diekmann, O. E., Estonba, A., et al. (2013). Connectivity, neutral theories and the assessment of species vulnerability to global change in temperate estuaries. Estuar. Coast. Shelf Sci. 131, 52–63. doi: 10.1016/j.ecss.2013.08.005
Chust, G., Villarino, E., Chenuil, A., Irigoien, X., Bizsel, N., Bode, A., et al. (2016). Dispersal similarly shapes both population genetics and community patterns in the marine realm. Sci. Rep. 6:28730. doi: 10.1038/srep28730
Clark, J. S., Bell, D. M., Hersh, M. H., Kwit, M. C., Moran, E., Salk, C., et al. (2011). Individual-scale variation, species-scale differences: inference needed to understand diversity. Ecol. Lett. 14, 1273–1287. doi: 10.1111/j.1461-0248.2011.01685.x
Coll, M., Shannon, L. J., Kleisner, K. M., Juan-Jordá, M., J, Bundy, A., Shin, Y. J., et al. (2016). Ecological indicators to capture the effects of fishing on biodiversity and conservation status of marine ecosystems. Ecol. Indic. 60, 947–962. doi: 10.1016/j.ecolind.2015.08.048
CONISMA (2013). Report on the Set Up of the Field and Experimental Activities. Deliverable 5.1. DEVOTES Project.
Cooper, P. (2013). Socio-ecological accounting: DPSWR, a modified DPSIR framework, and its application to marine ecosystems. Ecol. Econ. 94, 106–115. doi: 10.1016/j.ecolecon.2013.07.010
Cristina, S., D'Alimonte, D., Costa Goela, P., Kajiyama, T., Icely, J., Moore, G., et al. (2016). Standard and regional bio-optical algorithms for chlorophyll a estimates in the atlantic off the Southwestern Iberian Peninsula. IEEE Geosci. Remote Sens. Lett. 13, 757–761. doi: 10.1109/LGRS.2016.2529182
Cristina, S., Icely, J., Goela, P. C., DelValls, T. A., and Newton, A. (2015). Using remote sensing as a support to the implementation of the European Marine Strategy Framework Directive in SW Portugal. Cont. Shelf Res. 108, 169–177. doi: 10.1016/j.csr.2015.03.011
Cristina, S. C. V., Moore, G. F., Goela, P. R. F. C., Icely, J. D., and Newton, A. (2014). In situ validation of MERIS marine reflectance off the southwest Iberian Peninsula: assessment of vicarious adjustment and corrections for near-land adjacency. Int. J. Remote Sens. 35, 2347–2377. doi: 10.1080/01431161.2014.894657
Curtin, R., and Prellezo, R. (2010). Understanding marine ecosystem based management: a literature review. Mar. Policy 34, 821–830. doi: 10.1016/j.marpol.2010.01.003
Danovaro, R., Cerrano, C., Cardini, U., Bianchelli, S., Corinaldesi, C., and Pusceddu, A. (2013). Red coral extinction risk enhanced by ocean acidification. Sci. Rep. 3, 1–7. doi: 10.1038/srep01457
Danovaro, R., Gambi, C., Dell'Anno, A., Corinaldesi, C., Fraschetti, S., Vanreusel, A., et al. (2008). Exponential decline of deep-sea ecosystem functioning linked to benthic biodiversity loss. Curr. Biol. 18, 1–8. doi: 10.1016/j.cub.2007.11.056
DEFRA (2002). Safeguarding Our Seas. A Strategy for the Conservation and Sustainable Development of Our Marine Environment. Department for Environment, Food & Rural Affairs, DEFRA Publications, London, 80. Available online at: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/69321/pb6187-marine-stewardship-020425.pdf
de Jonge, V. N., Elliott, M., and Brauer, V. S. (2006). Marine monitoring: its shortcomings and mismatch with the EU Water Framework Directive's objectives. Mar. Pollut. Bull. 53, 5–19. doi: 10.1016/j.marpolbul.2005.11.026
Dell'Anno, A., Carugati, L., Corinaldesi, C., Riccioni, G., and Danovaro, R. (2015). Unveiling the biodiversity of deep-sea nematodes through metabarcoding: are we ready to bypass the classical taxonomy? PLoS ONE 10:e0144928. doi: 10.1371/journal.pone.0144928
Elliott, M. (2011). Marine science and management means tackling exogenic unmanaged pressures and endogenic managed pressures – A numbered guide. Mar. Pollut. Bull. 62, 651–655. doi: 10.1016/j.marpolbul.2010.11.033
Elliott, M. (2013). The 10-tenets for integrated, successful and sustainable marine management. Mar. Pollut. Bull. 74, 1–5. doi: 10.1016/j.marpolbul.2013.08.001
Elliott, M. (2014). Integrated marine science and management: wading through the morass. Mar. Pollut. Bull. 86, 1–4. doi: 10.1016/j.marpolbul.2014.07.026
Elliott, M., Borja, Á., McQuatters-Gollop, A., Mazik, K., Birchenough, S., Andersen, J. H., et al. (2015). Force majeure: will climate change affect our ability to attain Good Environmental Status for marine biodiversity? Mar. Pollut. Bull. 95, 7–27. doi: 10.1016/j.marpolbul.2015.03.015
Elliott, M., Cutts, N. D., and Trono, A. (2014). A typology of marine and estuarine hazards and risks as vectors of change: a review for vulnerable coasts and their management. Ocean Coast. Manag. 93, 88–99. doi: 10.1016/j.ocecoaman.2014.03.014
Elliott, M., Mander, L., Mazik, K., Simenstad, C., Valesini, F., Whitfield, A., et al. (2016). Ecoengineering with ecohydrology: successes and failures in estuarine restoration. Estuar. Coast. Shelf Sci. 176, 12–35. doi: 10.1016/j.ecss.2016.04.003
Engelhard, G. H., Lynam, C. P., Garcia-Carreras, B., Dolder, P. J., and Mackinson, S. (2015). Effort reduction and the large fish indicator: spatial trends reveal positive impacts of recent European fleet reduction schemes. Environ. Conserv. 42, 227–236. doi: 10.1017/S0376892915000077
European Commission (2000). Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for community action in the field of water policy. Off. J. Eur. Union L327, 1–72.
European Commission (2008). Directive 2008/56/EC of the European Parliament and of the Council establishing a framework for community action in the field of marine environmental policy (Marine Strategy Framework Directive). Off. J. Eur. Union L164, 19–40.
European Commission (2010). Commission Decision of 1 September 2010 on criteria and methodological standards on good environmental status of marine waters (notified under document C(2010) 5956)(2010/477/EU). Off. J. Eur. Union L232, 12–24.
European Commission (2014). Annex accompanying the document Commission Report to the Council and the European Parliament: The first phase of implementation of the Marine Strategy Framework Directive (2008/56/EC) - The European Commission's assessment and guidance. Available online at: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52014DC0097
European Marine Board (2013). Linking Oceans and Human Health: A Strategic Research Priority for Europe. Position paper 19 of the European Marine Board, Ostend, 116. Available onlie at: http://www.marineboard.eu/publications-full-list
Ferrera, I., Giner, C., Reñé, A., Camp, J., Massana, R., Gasol, J., et al. (2016). Evaluation of alternative high-throughput sequencing methodologies for the monitoring of marine picoplanktonic biodiversity based on rRNA gene amplicons. Front. Mar. Sci. 3:147. doi: 10.3389/fmars.2016.00147
Ferrera, I., Sebastian, M., Acinas, S. G., and Gasol, J. M. (2015). Prokaryotic functional gene diversity in the sunlit ocean: stumbling in the dark. Curr. Opin. Microbiol. 25, 33–39. doi: 10.1016/j.mib.2015.03.007
Fraschetti, S., Guarnieri, G., Gambi, C., Bevilacqua, S., Terlizzi, A., and Danovaro, R. (2016). Impact of offshore gas platforms on the structural and functional biodiversity of nematodes. Mar. Environ. Res. 115, 56–64. doi: 10.1016/j.marenvres.2016.02.001
Fréon, P., Barange, M., and Arístegui, J. (2009). Eastern boundary upwelling ecosystems: integrative and comparative approaches. Prog. Oceanogr. 83, 1–14. doi: 10.1016/j.pocean.2009.08.001
Fu, C., Large, S., Knight, B., Richardson, A. J., Bundy, A., Shin, J., et al. (2015). Relationships among fisheries exploitation, environmental conditions, and ecological indicators across a series of marine ecosystems. J. Mar. Syst. 148, 101–111. doi: 10.1016/j.jmarsys.2015.01.004
Fung, T., Farnsworth, K. D., Reid, D. G., and Rossberg, A. G. (2015). Impact of biodiversity loss on production in complex marine food webs mitigated by prey-release. Nat. Commun. 6:6657. doi: 10.1038/ncomms7657
Galparsoro, I., Borja, Á., Kostylev, V. E., Rodríguez, J. G., Pascual, M., and Muxika, I. (2013). A process-driven sedimentary habitat modelling approach, explaining seafloor integrity and biodiversity assessment within the European Marine Strategy Framework Directive. Estuar. Coast. Shelf Sci. 131, 194–205. doi: 10.1016/j.ecss.2013.07.007
Galparsoro, I., Borja, A., and Uyarra, M. C. (2014). Mapping ecosystem services provided by benthic habitats in the European North Atlantic Ocean. Front. Mar. Sci. 1:23. doi: 10.3389/fmars.2014.00023
Galparsoro, I., Rodríguez, J. G., Menchaca, I., Quincoces, I., Garmendia, J. M., and Borja, A. (2015). Benthic habitat mapping on the Basque continental shelf (SE Bay of Biscay) and its application to the European Marine Strategy Framework Directive. J. Sea Res. 100, 70–76. doi: 10.1016/j.seares.2014.09.013
Gari, S. R., Newton, A., and Icely, J. D. (2015). A review of the application and evolution of the DPSIR framework with an emphasis on coastal social-ecological systems. Ocean Coast. Manag. 103, 63–77. doi: 10.1016/j.ocecoaman.2014.11.013
Gates, A. R., and Jones, D. O. B. (2012). Recovery of benthic megafauna from anthropogenic disturbance at a hydrocarbon drilling well (380 m depth in the Norwegian Sea). PLoS ONE 7:e0044114. doi: 10.1371/journal.pone.0044114
Goela, P. C., Icely, J., Cristina, S., Danchenko, S. Angel DelValls, T., and Newton, A. (2015). Using bio-optical parameters as a tool for detecting changes in the phytoplankton community (SW Portugal). Estuar. Coast. Shelf Sci. 167(Pt A), 125–137. doi: 10.1016/j.ecss.2015.07.037
Gray, J. S., and Elliott, M. (2009). Ecology of Marine Sediments. From Science to Managemen, 2nd Edn. New York, NY: Oxford University Press.
Guesnet, V., Lassalle, G., Chaalali, A., Kearney, K., Saint-Béat, B., Karimi, B., et al. (2015). Incorporating food-web parameter uncertainty into Ecopath-derived ecological network indicators. Ecol. Modell. 313, 29–40. doi: 10.1016/j.ecolmodel.2015.05.036
Halpern, B. S., Frazier, M., Potapenko, J., Casey, K. S., Koenig, K., Longo, C., et al. (2015). Spatial and temporal changes in cumulative human impacts on the world's ocean. Nat. Commun. 6:7615. doi: 10.1038/ncomms8615
Halpern, B. S., Longo, C., Hardy, D., McLeod, K. L., Samhouri, J. F., Katona, S. K., et al. (2012). An index to assess the health and benefits of the global ocean. Nature 488, 615–620. doi: 10.1038/nature11397
Hattam, C., Atkins, J. P., Beaumont, N. Börger, T., Böhnke-Henrichs, A., Burdon, D., et al. (2014). Marine ecosystem services: linking indicators to their classification. Ecol. Indic. 49, 61–75. doi: 10.1016/j.ecolind.2014.09.026
Hattam, C., Böhnke-Henrichs, A., Börger, T., Burdon, D., Hadjimichael, M., Delaney, A., et al. (2015). Integrating methods for ecosystem service assessment and valuation: mixed methods or mixed messages? Ecol. Econ. 120, 126–138. doi: 10.1016/j.ecolecon.2015.10.011
HELCOM (2013). “HELCOM core indicators: Final report of the HELCOM CORESET project,” in Baltic Sea Environment Proceedings No. 136 (Helsinki).
Hering, D., Borja, A., Carstensen, J., Carvalho, L., Elliott, M., Feld, C., et al. (2010). The European Water Framework Directive at the age of 10: a critical review of the achievements with recommendations for the future. Sci. Tot. Environ. 408, 4007–4019. doi: 10.1016/j.scitotenv.2010.05.031
James, A., Plank, M. J., Rossberg, A. G., Beecham, J., Emmerson, M., and Pitchford, J. W. (2015). Constructing random matrices to represent real ecosystems. Am. Nat. 185, 680–692. doi: 10.1086/680496
Jorgensen, L. L., Planque, B., Thangstad, T. H., and Certain, G. (2016). Vulnerability of megabenthic species to trawling in the Barents Sea. ICES J. Mar. Sci. 73, 84–97. doi: 10.1093/icesjms/fsv107
Katsanevakis, S., Tempera, F., and Teixeira, H. (2016). Mapping the impact of alien species on marine ecosystems: the Mediterranean Sea case study. Divers. Distrib. 22, 694–707. doi: 10.1111/ddi.12429
Katsanevakis, S., Wallentinus, I., Zenetos, A., Leppäkoski, E., Çinar, M. E., Oztürk, B., et al. (2014). Impacts of invasive alien marine species on ecosystem services and biodiversity: a pan-European review. Aquat. Invas. 9, 391–423. doi: 10.3391/ai.2014.9.4.01
Kleisner, K. M., Coll, M., Lynam, C. P., Bundy, A., Shannon, L., Shin, Y. J., et al. (2015). Evaluating changes in marine communities that provide ecosystem services through comparative assessments of community indicators. Ecosyst. Serv. 16, 413–429. doi: 10.1016/j.ecoser.2015.02.002
Korpinen, S., and Andersen, J. (2016). A global review of cumulative pressure and impact assessments in marine environment. Front. Mar. Sci. 3:153. doi: 10.3389/fmars.2016.00153
Lassalle, G., Bourdaud, P., Saint-Béat, B., Rochette, S., and Niquil, N. (2014a). A toolbox to evaluate data reliability for whole-ecosystem models: application on the Bay of Biscay continental shelf food-web model. Ecol. Modell. 285, 13–21. doi: 10.1016/j.ecolmodel.2014.04.002
Lassalle, G., Chouvelon, T., Bustamante, P., and Niquil, N. (2014b). An assessment of the trophic structure of the Bay of Biscay continental shelf food web: comparing estimates derived from an ecosystem model and isotopic data. Prog. Oceanogr. 120, 205–215. doi: 10.1016/j.pocean.2013.09.002
Lassalle, G., Nelva Pasqual, J.-S., Boët, P. H, Rochet, M.-J., and Trenkel, V. (2013). Combining quantitative and qualitative models to identify key interactions in the Bay of Biscay continental shelf exploited food web. ICES J. Mar. Sci. 71, 105–117. doi: 10.1093/icesjms/fst107
Laurila-Pant, M., Lehikoinen, A., Uusitalo, L., and Venesjärvi, R. (2015). How to value biodiversity in environmental management? Ecol. Indic. 55, 1–11. doi: 10.1016/j.ecolind.2015.02.034
Levin, P. S., Fogarty, M. J., Murawski, S. A., and Fluharty, D. (2009). Integrated ecosystem assessments: developing the scientific basis for ecosystem-based management of the ocean. PLoS Biol. 7:e1000014. doi: 10.1371/journal.pbio.1000014
Lewison, R. L., Rudd, M. A., Al-Hayek, W., Baldwin, C., Beger, M., Lieske, S. N., et al. (2016). How the DPSIR framework can be used for structuring problems and facilitating empirical research in coastal systems. Environ. Sci. Policy 56, 110–119. doi: 10.1016/j.envsci.2015.11.001
Luna, G. M., Corinaldesi, C., Dell'Anno, A., Pusceddu, A., and Danovaro, R. (2013). Impact of aquaculture on benthic virus–prokaryote interactions in the Mediterranean Sea. Water Res. 47, 1156–1168. doi: 10.1016/j.watres.2012.11.036
Lynam, C., Niquil, N., Andonegi, E., Queirós, A., Tecchio, S., Fernandes, J. A., et al. (2015b). Report on the Analysis of Reference Levels for Indicators of Biodiversity for Selected pilot areas. Modelling Studies Integrating Uncertainty, Trophic Flows and Prevailing Climatic Conditions. Deliverable 4.3, 66 + Annexes. DEVOTES Project, Lowestoft.
Lynam, C., Tempera, F., Teixeira, H., Diesing, M., Stephens, D., van Leeuwen, S., et al. (2015a). Report Describing Spatial Ecosystem Models. Linking Habitats to Functional Biodiversity and Modelling Connectivity between Regional Seas. Deliverable 4.2, 186 + Annexes. DEVOTES Project, Lowestoft.
Lynam, C. P., and Mackinson, S. (2015). How will fisheries management measures contribute towards the attainment of good environmental status for the North Sea ecosystem? Glob. Ecol. Conserv. 4, 160–175. doi: 10.1016/j.gecco.2015.06.005
Lynam, C. P., Uusitalo, L., Patrício, J., Piroddi, C., Queirós, A. M., Teixeira, H., et al. (2016). Uses of innovative modelling tools within the implementation of the marine strategy framework directive. Front. Mar. Sci. 3:182. doi: 10.3389/fmars.2016.00182
Martin, G., Fammler, H., Veidemane, K., Wijkmark, N., Auniņvs, A., and Hällfors, H., (eds.). et al. (2015). The MARMONI Approach to Marine Biodiversity Indicators - Volume I: Development of Indicators for Assessing the State of Marine Biodiversity in the Baltic Sea within the LIFE MARMONI Project. Estonian Marine Institute Report Series, No. 16.
Minchin, D., and Zaiko, A. (2013). “Variability of the zebra mussel (Dreissena polymorpha) impacts in the Shannon River system,” in Quagga and Zebra Mussels: Biology, Impacts and Control, 2nd Edn, eds T. Nalepa and D. Schlosser (Boca Ratón, FL: CRC Press), 587–597.
Mirto, S., Arigò, C., Genovese, L., Pusceddu, A., Gambi, C., and Danovaro, R. (2014). Nematode assemblage response to fish-farm impact in vegetated (Posidonia oceanica) and non-vegetated habitats. Aquac. Environ. Interact. 5, 17–28. doi: 10.3354/aei00091
Mirto, S., Bianchelli, S., Gambi, C., Krzelj, M., Pusceddu, A., Scopa, M., et al. (2010). Fish-farm impact on metazoan meiofauna in the Mediterranean Sea: analysis of regional vs. habitat effects. Mar. Environ. Res. 69, 38–47. doi: 10.1016/j.marenvres.2009.07.005
Mora, C., Danovaro, R., and Loreau, M. (2014). Alternative hypotheses to explain why biodiversity-ecosystem functioning relationships are concave-up in some natural ecosystems but concave-down in manipulative experiments. Sci. Rep. 4:5427. doi: 10.1038/srep05427
Nagelkerke, L. A. J., and Rossberg, A. G. (2014). Trophic niche-space imaging, using resource and consumer traits. Theor. Ecol. 7, 423–434. doi: 10.1007/s12080-014-0229-5
Nicholson, E., Collen, B., Barausse, A., Blanchard, J. L., Costelloe, B. T., Milner-Gulland, E. J., et al. (2012). Making robust policy decisions using global biodiversity indicators. PLoS ONE 7:e41128. doi: 10.1371/journal.pone.0041128
Nicholson, M. D., and Jennings, S. (2004). Testing candidate indicators to support ecosystem-based management: the power of monitoring surveys to detect temporal trends in fish community metrics. ICES J. Mar. Sci. 61, 35–42. doi: 10.1016/j.icesjms.2003.09.004
Niquil, N., Baeta, A., Marques, J. C., Chaalali, A., Lobry, J., and Patrício, J. (2014). Reaction of an estuarine food web to disturbance: lindeman's perspective. Mar. Ecol. Prog. Ser. 512, 141–115. doi: 10.3354/meps10885
Oinonen, S., Börger, T., Hynes, S., Buchs, A. K., Heiskanen, A.-S., Hyytiäinen, K., et al. (2016a). The role of economics in ecosystem based management: the case of the EU Marine Strategy Framework Directive; first lessons learnt and way forward. J. Ocean Coast. Econ. 2:3. doi: 10.15351/2373-8456.1038
Oinonen, S., Hyytiäinen, K., Ahlvik, L., Laamanen, M., Lehtoranta, V., Salojärvi, J., et al. (2016b). Cost-effective marine protection - a pragmatic approach. PLoS ONE 11:e0147085. doi: 10.1371/journal.pone.0147085
Oliver, M., Sorkin, L., and Kaschl, A. (2005). Science and policy interface: the LIFE programme and its links to the EU Water Framework Directive. Environ. Sci. Policy 8, 253–257. doi: 10.1016/j.envsci.2005.03.004
O'Riordan, T., and Jordan, A. (1995). The precautionary principle in the contemporary environmental policy. Environ. Values 4, 191–212. doi: 10.3197/096327195776679475
OSPAR (2015). “Programme of work for the Biodiversity Committee - 2015/2016,” in Meeting of the OSPAR Commission (OSPAR) (Ostend).
Palialexis, A., Tornero, V., Barbone, E., Gonzalez, D., Hanke, G., Cardoso, A., et al. (2014). In-Depth Assessment of the EU Member States' Submissions for the Marine Strategy Framework Directive under Articles 8, 9 and 10. JRC 88072. European Union.
Patrício, J., Elliott, M., Mazik, K., Papadopoulou, K.-N., and Smith, C. J. (2016a). DPSIR - Two decades of trying to develop a unifying framework for marine environmental management. Front. Mar. Sci. 3:177. doi: 10.3389/fmars.2016.00177
Patrício, J., Little, S., Mazik, K., Papadopoulou, K.-N., Smith, C. J., Teixeira, H., et al. (2016b). European biodiversity monitoring networks: strengths, weaknesses, opportunities and threats. Front. Mar. Sci. 3:161. doi: 10.3389/fmars.2016.00161
Patrício, J., Teixeira, H., Borja, A., Elliott, M., Berg, T., Papadopoulou, N., et al. (2014). DEVOTES Recommendations for the Implementation of the Marine Strategy Framework Directive. Deliverable 1.5, 71. DEVOTES project. JRC92131. Available online at: http://www.devotes-project.eu/wp-content/uploads/2014/10/DEVOTES_Deliverable-1-5.pdf
Pearman, J. K., Anlauf, H., Irigoien, X., and Carvalho, S. (2016b). Please mind the gap – Visual census and cryptic biodiversity assessment at central Red Sea coral reefs. Mar. Environ. Res. 118, 20–30. doi: 10.1016/j.marenvres.2016.04.011
Pearman, J. K., Irigoien, X., and Carvalho, S. (2016a). Extracellular DNA amplicon sequencing reveals high levels of benthic eukaryotic diversity in the central Red Sea. Mar. Genomics 26, 29–39. doi: 10.1016/j.margen.2015.10.008
Pearson, T., and Rosenberg, R. (1978). Macrobenthic succession in relation to organic enrichment and pollution of the marine environment. Oceanogr. Mar. Biol. 16, 229–311.
Pinnegar, J. K., Tomczak, M. T., and Link, J. S. (2014). How to determine the likely indirect food-web consequences of a newly introduced non-native species: a worked example. Ecol. Modell. 272, 379–387. doi: 10.1016/j.ecolmodel.2013.09.027
Piroddi, C., Teixeira, H., Lynam, C. P., Smith, C., Alvarez, M. C., Uyarra, M. C., et al. (2015). Using ecological models to assess ecosystem status in support of the European Marine Strategy Framework Directive. Ecol. Indic. 58, 175–191. doi: 10.1016/j.ecolind.2015.05.037
Queirós, A. M., Strong, J. A., Mazik, K., Carstensen, J., Bruun, J., Krause-Jensen, D., et al. (2016). An objective framework to test the quality of candidate indicators of good environmental status. Front. Mar. Sci. 3:73. doi: 10.3389/fmars.2016.00073
Quevauviller, P., Balabanis, P., Fragakis, C., Weydert, M., Oliver, M., Kaschl, A., et al. (2005). Science-policy integration needs in support of the implementation of the EU Water Framework Directive. Environ. Sci. Pol. 8, 203–211. doi: 10.1016/j.envsci.2005.02.003
Ramirez-Llodra, E., Tyler, P. A., Baker, M. C., Bergstad, O. A., Clark, M. R., Escobar, E., et al. (2011). Man and the last great wilderness: human impact on the Deep Sea. PLoS ONE 6:e22588. doi: 10.1371/journal.pone.0022588
Reñé, A., Camp, J., and Garcés, E. (2015). Diversity and phylogeny of Gymnodiniales (Dinophyceae) from the NW Mediterranean Sea revealed by a morphological and molecular approach. Protist 166, 234–263. doi: 10.1016/j.protis.2015.03.001
Reñé, A., de Salas, M., Camp, J., Balagué, V., and Garcés, E. (2013). A new clade, based on partial LSU rDNA sequences, of unarmoured dinoflagellates. Protist 164, 673–685. doi: 10.1016/j.protis.2013.07.002
Reker, J. C., de Carvalho Belchior and Royo Gelabert, E. (2015). State of Europe's Seas. EEA Report, 2, 220. Copenhagen: European Environment Agency.
Rice, J., Arvanitidis, C., Borja, A., Frid, C., Hiddink, J. G., Krause, J., et al. (2012). Indicators for Sea-floor Integrity under the European Marine Strategy Framework Directive. Ecol. Indic. 12, 174–184. doi: 10.1016/j.ecolind.2011.03.021
Rodwell, L. D., Fletcher, S., Glegg, G. A., Campbell, M., Rees, S. E., Ashley, M., et al. (2014). Marine and coastal policy in the UK: challenges and opportunities in a new era. Mar. Policy 45, 251–258. doi: 10.1016/j.marpol.2013.09.014
Rossberg, A. G. (2013). Food Webs and Biodiversity: Foundations, Models, Data. Chichester: John Wiley & Sons.
Rossberg, A. G., Uusitalo, L., Berg, T., Zaiko, A., Chenuil, A., Uyarra, M., et al. (2017). Quantitative criteria for choosing targets and indicators for sustainable use of ecosystems. Ecol. Indic. 72, 215–224. doi: 10.1016/j.ecolind.2016.08.005
Salz, P., Buisman, E., Soma, K. K., Hans, F., Acadia, P., and Prellezo, R. (2011). FISHRENT, Bioeconomic Simulation and Optimisation Model for Fisheries. LEI Report 2011-024, May 2011. The Hague: LEI, part of Wageningen UR.
Scharin, H., Ericsdotter, S., Elliott, M., Turner, R. K., Niiranen, S., Rockström, J., et al. (2016). Processes for the sustainable stewardship of marine environments. Ecol. Econ. 128, 55–67. doi: 10.1016/j.ecolecon.2016.04.010
Sebek, V. (1983). Bridging the gap between environmental science and policy-making: why public policy often fails to reflect current scientific knowledge. Ambio 12, 118–120.
Sguotti, C., Lynam, C. P., García-Carreras, B., Ellis, J. R., and Engelhard, G. H. (2016). Distribution of skates sharks in the North Sea: 112 years of change. Glob. Change Biol. 22, 2729–2743. doi: 10.1111/gcb.13316
Smith, C., Papadopoulou, K.- N., Barnard, S., Mazik, K., Elliott, M., Patrício, J., et al. (2016). Managing the marine environment, conceptual models and assessment considerations for the european marine strategy framework directive. Front. Mar. Sci. 3:144. doi: 10.3389/fmars.2016.00144
Smith, C., Papadopoulou, N., Barnard, S., Mazik, K., Patrício, J., Elliott, M., et al. (2014). Conceptual Models for the Effects of Marine Pressures on Biodiversity. Deliverable 1.1, 82. DEVOTES Project. Available onlie at: http://www.devotes-project.eu/wp-content/uploads/2014/06/DEVOTES-D1-1-ConceptualModels.pdf
Solan, M., Germano, J. D., Rhoads, D. C., Smith, C., Michaud, E., Parry, D., et al. (2003). Towards a greater understanding of pattern, scale and process in marine benthic systems: a picture is worth a thousand worms. J. Exp. Mar. Biol. Ecol. 285–286, 313–338. doi: 10.1016/S0022-0981(02)00535-X
Stanley, T. R. (1995). Ecosystem management and the arrogance of humanism. Conserv. Biol. 9, 255–262. doi: 10.1046/j.1523-1739.1995.9020255.x
Steel, D. (2014). Philosophy and the Precautionary Principle. Science, Evidence, and Environmental Policy. Cambridge: Cambridge University Press.
Stephens, D., and Diesing, M. (2015). Towards quantitative spatial models of seabed sediment composition. PLoS ONE 10:e0142502. doi: 10.1371/journal.pone.0142502
St. John, M. A., Borja, A., Chust, G., Heath, M., Grigorov, I., Mariani, P., et al. (2016). A dark hole in our understanding of marine ecosystems and their services: perspectives from the mesopelagic community. Front. Mar. Sci. 3:31. doi: 10.3389/fmars.2016.00031
Strong, J. A., Andonegi, E., Bizsel, K. C., Danovaro, R., Elliott, M., Franco, A., et al. (2015). Marine biodiversity and ecosystem function relationships: the potential for practical monitoring applications. Estuar. Coast. Shelf Sci. 161, 46–64. doi: 10.1016/j.ecss.2015.04.008
Tedesco, L., Piroddi, C., Kämäri, M., and Lynam, C. P. (2016). Capabilities of Baltic Sea models to assess Good Environmental Status for marine biodiversity. Mar. Policy 70, 1–12. doi: 10.1016/j.marpol.2016.04.021
Teixeira, H., Berg, T., Fürhaupter, K., Uusitalo, L., Papadopoulou, N., Can Bizsel, K., et al. (2014). Existing Biodiversity, Non-Indigenous Species, Food-Web and Seafloor Integrity GEnS Indicators. Deliverable D3-1. within the DEVOTES Project, 198. Available online at: http://www.devotes-project.eu/deliverables-and-milestones/
Turner, R. K., and Schaafsma, M. (eds.). (2015). “Coastal zones ecosystem services: from science to values and decision making,” in Springer Ecological Economic Series (London: Springer International Publisher).
UNEP (2016). Draft Decision: Integrated Monitoring and Assessment Programme of the Mediterranean Sea and Coast and Related Assessment Criteria. UNEP(DEPI)/MED IG.22/10, London.
Uusitalo, L., Blanchet, H., Andersen, J. H., Beauchard, O., Berg, T., Bianchelli, S., et al. (2016b). Indicator-based assessment of marine biological diversity – lessons from 10 case studies across the European Seas. Front. Mar. Sci. 3:159. 10.3389/fmars.2016.00159
Uusitalo, L., Fernandes, J. A., Bachiller, E., Tasala, S., and Lehtiniemi, M. (2016a). Semi-automated classification method addressing marine strategy framework directive (MSFD) zooplankton indicators. Ecol. Indic. 71, 398–405. doi: 10.1016/j.ecolind.2016.05.036
Uusitalo, L., Lehikoinen, A., Helle, I., and Myrberg, K. (2015). An overview of methods to evaluate uncertainty of deterministic models in decision support. Environ. Modell. Softw. 63, 24–31. doi: 10.1016/j.envsoft.2014.09.017
Van der Linden, P., Borja, A., Rodriguez, J. G., Muxika, I., Galparsoro, I., Patrício, J., et al. (2016b). Spatial and temporal response of multiple trait-based indices to natural- and anthropogenic seafloor disturbance (effluents). Ecol. Indic. 69, 617–628. doi: 10.1016/j.ecolind.2016.05.020
van der Linden, P., Marchini, A., Dolbeth, M., Patrício, J., Veríssimo, H., and Marques, J. C. (2016a). The performance of trait-based indices in an estuarine environment. Ecol. Indic. 61, 378–389. doi: 10.1016/j.ecolind.2015.09.039
van Leeuwen, S., Tett, P., Mills, D., and van der Molen, J. (2015). Stratified and nonstratified areas in the North Sea: long-term variability and biological and policy implications. J. Geophys. Res. Oceans 120, 4670–4686. doi: 10.1002/2014jc010485
Veidemane, K., and Pakalniete, K. (eds.). (2015). Socio-Economic Assessment of Indicator-Based Marine Biodiversity Monitoring Programmes and Methods. MARMONI Report. Baltic Environmental Forum, Riga.
Veríssimo, H., Beauchard, O., Lynam, C., Somerfield, P., Borja, A., Galparsoro, I., et al. (2015). Fuzzy Analysis Complete. Biological Traits and Ecological Function. Milestone 17, 64. DEVOTES project, Coimbra.
von Winterfeldt, D. (2013). Bridging the gap between science and decision making. Proc. Natl. Acad. Sci. U.S.A. 110, 14055–14061. doi: 10.1073/pnas.1213532110
Zaiko, A., Martinez, J. L., Schmidt-Petersen, J., Ribicic, D., Samuloviene, A., and Garcia-Vazquez, E. (2015a). Metabarcoding approach for the ballast water surveillance - an advantageous solution or an awkward challenge? Mar. Pollut. Bull. 92, 25–34. doi: 10.1016/j.marpolbul.2015.01.008
Zaiko, A., Minchin, D., and Olenin, S. (2014). “The day after tomorrow”: anatomy of an ‘r’ strategist aquatic invasion. Aquat. Invas. 9, 145–155. doi: 10.3391/ai.2014.9.2.03
Zaiko, A., Samulioviene, A., Ardura, A., and Garcia-Vazquez, E. (2015b). Metabarcoding approach for non-indigenous species surveillance in marine coastal waters. Mar. Pollut. Bull. 100, 53–59. doi: 10.1016/j.marpolbul.2015.09.030
Keywords: environmental status, marine health, status assessment, management, ecosystem approach, socio-ecology
Citation: Borja A, Elliott M, Snelgrove PVR, Austen MC, Berg T, Cochrane S, Carstensen J, Danovaro R, Greenstreet S, Heiskanen A-S, Lynam CP, Mea M, Newton A, Patrício J, Uusitalo L, Uyarra MC and Wilson C (2016) Bridging the Gap between Policy and Science in Assessing the Health Status of Marine Ecosystems. Front. Mar. Sci. 3:175. doi: 10.3389/fmars.2016.00175
Received: 15 June 2016; Accepted: 31 August 2016;
Published: 12 September 2016.
Edited by:
Christos Dimitrios Arvanitidis, Hellenic Centre for Marine Research, GreeceReviewed by:
Matt Terence Frost, Marine Biological Association of the United Kingdom, UKMarco Uttieri, Parthenope University of Naples, Italy
Copyright © 2016 Borja, Elliott, Snelgrove, Austen, Berg, Cochrane, Carstensen, Danovaro, Greenstreet, Heiskanen, Lynam, Mea, Newton, Patrício, Uusitalo, Uyarra and Wilson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Angel Borja, aborja@azti.es
 Angel Borja
Angel Borja Michael Elliott
Michael Elliott Paul V. R. Snelgrove3
Paul V. R. Snelgrove3  Melanie C. Austen
Melanie C. Austen Torsten Berg
Torsten Berg Sabine Cochrane
Sabine Cochrane Jacob Carstensen
Jacob Carstensen Roberto Danovaro
Roberto Danovaro Anna-Stiina Heiskanen
Anna-Stiina Heiskanen Christopher P. Lynam
Christopher P. Lynam Marianna Mea
Marianna Mea Laura Uusitalo
Laura Uusitalo María C. Uyarra
María C. Uyarra Christian Wilson
Christian Wilson