Persistent Enhancement of Micronekton Backscatter at the Summits of Seamounts in the Azores
- 1Marine and Environmental Sciences Centre (MARE) and Institute of Marine Research (IMAR), University of the Azores, Horta, Portugal
- 2Department of Oceanography and Fisheries, University of the Azores, Horta, Portugal
- 3Pacific Islands Fisheries Science Center, National Marine Fisheries Service, National Oceanographic and Atmospheric Administration, Honolulu, HI, USA
- 4Hawaii Institute of Marine Biology, University of Hawaii, Kaneohe, HI, USA
- 5Oceanwide Science Institute, Honolulu, HI, USA
- 6Division of Modelling and Management of Fishery Resources, Portuguese Sea and Atmosphere Institute (IPMA), Lisbon, Portugal
- 7Department of Ecology and Animal Biology, Faculty of Marine Science, University of Vigo, Vigo, Spain
- 8Biology Department, Woods Hole Oceanographic Institution, Woods Hole, MA, USA
Knowledge of the dynamics of micronekton at seamounts is critical to understanding the ecological role of these ecosystems. Active acoustic techniques are an effective tool to monitor the distribution and movements of pelagic organisms. We carried out several day- and nighttime active acoustic surveys over a 3-year period (2009–2011) to characterize the spatial and temporal distribution of micronekton backscatter on two seamounts (Condor and Gigante) in the Azores and in the surrounding open-waters. The highest mean volume backscattering strength (MVBS) was consistently found in the water column over the seamount summits, regardless of the season and diel period. MVBS over the summits was 14–26 times higher than over the slopes, and 10 times higher than in open-waters. Diel variations in backscatter intensity were more pronounced in open-waters and in Gigante seamount, with higher values during the day in open-waters, and at night over the summits and slopes of Gigante. Over Condor seamount, diel changes in backscatter intensity were small, but MVBS was generally higher at night than during the day, as in Gigante. Persistence of strong acoustic backscatter over the summits of Condor and Gigante seamounts is a key finding of this study and may be explained by the presence of a seamount-associated micronekton community and by the retention of vertically migrating micronekton. The latter hypothesis is consistent with observed day-night differences in backscatter, suggesting that nocturnal migrants may be passively transported or actively swim above seamount summits and slopes. Possible physical mechanisms leading to the observed patterns in micronekton distribution are discussed. This study contributes to a better understanding of how seamounts may influence the spatial and temporal dynamics of micronekton assemblages.
Introduction
Sound-scattering layers (SLs) are dense aggregations of planktonic and nektonic organisms that reflect sound in water and can be observed acoustically. Scattering layers of micronekton are taxonomically diverse, consisting of small (mostly 2–20 cm long) pelagic crustaceans (adult euphausiids, decapods, and mysids), cephalopods (namely sepiolids, pyroteuthids, and enoploteuthids) and fishes (mainly mesopelagic and juvenile stages of pelagic species) (Barham, 1966; Brodeur and Yamamura, 2005).
Micronekton SLs are abundant worldwide and form a substantial biomass in oceanic waters (Atkinson et al., 2009; Irigoien et al., 2014). The organisms that make these layers are critical components of oceanic food webs: they consume large quantities of phytoplankton and small zooplankton (e.g., copepods) and serve as primary prey for higher trophic levels, including charismatic and endangered marine mammals and seabirds, as well as commercially harvested fishes and squids (Harrison and Seki, 1987; Pauly et al., 1998; Watanabe et al., 2009). Most micronektonic taxa undergo diel vertical migration (DVM), residing in deeper waters during the day and swimming toward the surface to feed at night (Sutton, 2013). Extensive diel horizontal migrations between oceanic and slope waters have also been documented in some areas (Benoit-Bird and Au, 2006). Thus, micronekton SLs also play an important role in the transport of carbon and nutrients between mesopelagic and epipelagic environments (Hudson et al., 2014), and between oceanic and neritic systems (Benoit-Bird and Au, 2004).
Like other topographic features, seamounts can shape the aggregation of pelagic organisms and many seamounts support unusually high biomass of micronekton SLs compared to the surrounding ocean (e.g., Boehlert, 1988; Johnston et al., 2008; Letessier et al., in press). Various non-exclusive mechanisms may explain increased micronekton biomass over seamounts. The abrupt topography of seamounts can interact with background ocean circulation promoting a range of physical processes (e.g., deflection of impinging currents, rectification of internal tides, amplification of internal waves, and formation of Taylor columns) that intensify flow over the summit and upper slopes of the seamount (Genin, 2004). This, in turn, enhances the flux of food particles and plankton over these areas, attracting micronekton foraging on surface layers during the night, and increasing the horizontal advection of migrating and non-migrating micronekton (Koslow, 1997; Porteiro and Sutton, 2007; Morato et al., 2009). Vertically migrating micronekton swept onto seamounts by prevailing currents may also become trapped over the seamount summit and upper slopes (Isaacs and Schwartzlose, 1965). Finally, seamounts may provide calm suitable shelter at the benthic boundary layer for some micronekton (Genin, 2004). Therefore, micronekton may conserve energy by taking advantage of this quiescent habitat during non-feeding intervals, whereas in open-waters they must swim constantly.
The effectiveness and magnitude of each of these mechanisms in increasing or aggregating micronekton biomass will depend on a range of factors, including the topography of the seamount, the extent, and depth of the summit and plateau, the distance to the continental shelf and to other bathymetric features, the hydrographic conditions, and the community composition (Porteiro and Sutton, 2007). For instance, bottom trapping will only be effective for seamounts shallower than the daylight depth of micronekton organisms (Genin, 2004; Martin and Christiansen, 2009; Denda and Christiansen, 2014). Moreover, because different organisms migrate to different daylight depths, the deeper the seamount summit, the fewer the species retained and the lower the biomass (Genin and Dower, 2007). Strong variability in impinging currents leading to instability of Taylor columns can also impact the retention potential over seamounts and affect concentration of micronekton organisms (Diekmann and Piatkowski, 2004). In addition, micronekton may suffer increased predation from benthopelagic predators inhabiting the seamount or they may actively avoid seamount shallow topographies (Pusch et al., 2004).
In fact, not all seamounts appear to hold high SL densities. Denda and Christiansen (2014) found no significant differences in zooplankton biomass at Ampère and Senghor seamounts (summits of 55 and 90 m, respectively) relative to open-waters. Reduced zooplankton biomass was found above the summits of Sedlo (750 m) and Seine (170 m) seamounts compared to the slope and far-field sites (Martin and Christiansen, 2009). Abundance and biomass of mesopelagic organisms was also lower above the summits of Cross seamount (330 m; De Forest and Drazen, 2009; Drazen et al., 2011) and over the summits and slopes of the Great Meteor (330 m) (Pusch et al., 2004).
While most of these studies suggest some kind of effect of seamounts on micronekton communities, they provide only limited information about the spatial and temporal dynamics of SLs around seamounts. Prior research on seamount pelagic communities has largely been based on trawl sampling. Trawl surveys sample only specific depth layers at discrete locations and because they are expensive and time-consuming, usually a small number of samples are obtained from each area. Thus, trawl catches provide a snapshot of the pelagic community living at seamounts. In contrast, active acoustics provide continuous measurements of organisms' abundance and depth distribution and are increasingly used to assess the density and study the movements of fish and zooplankton at various spatial and temporal scales (Benoit-Bird and Au, 2003). Acoustic studies on a wide range of seamounts, especially in different ecological settings, can contribute to our understanding of the dynamics of micronekton communities and provide insights into the underlying forcing mechanisms.
We conducted several day- and night-time active acoustic surveys over a 3-year period (2009–2011) to characterize the spatial and temporal dynamics of micronektonic backscatter at two seamounts in the Azores archipelago. This region has one of the highest densities of seamounts in the Northeast Atlantic (Morato et al., 2013). Several of these seamounts host commercially valuable pelagic and demersal fish species important for local fisheries (Morato et al., 2008b; Menezes et al., 2013) and act as foraging posts for sea turtles, seabirds, marine mammals, and large pelagic fishes (Santos et al., 2007; Morato et al., 2008b; Silva et al., 2013; Afonso et al., 2014b; Tobeña et al., 2016). However, with few exceptions (e.g., Hargreaves, 1975; Martin and Nellen, 2004), previous studies on acoustic backscatter distribution in the Azores have focused only in open ocean areas (e.g., Moore, 1950; McElroy, 1974; Smailes, 1976; Wade and Heywood, 2001). Here we compare the distribution of micronekton backscatter in open ocean waters and at two seamounts with different physical properties to (i) investigate the influence of seamounts in driving distribution patterns of acoustic scatterers, (ii) determine how this effect varies over diel and seasonal scales, and (iii) discuss physical and biological processes controlling dynamics of micronekton in the study areas.
Methods
Study Area
Acoustic surveys were conducted in Condor and Gigante seamounts in the Azores archipelago and in adjacent open-waters (Figure 1). Morato et al. (2008a) classified both seamounts as “large seamount-like features,” i.e., seamounts rising more than 1000 m from the surrounding seafloor.
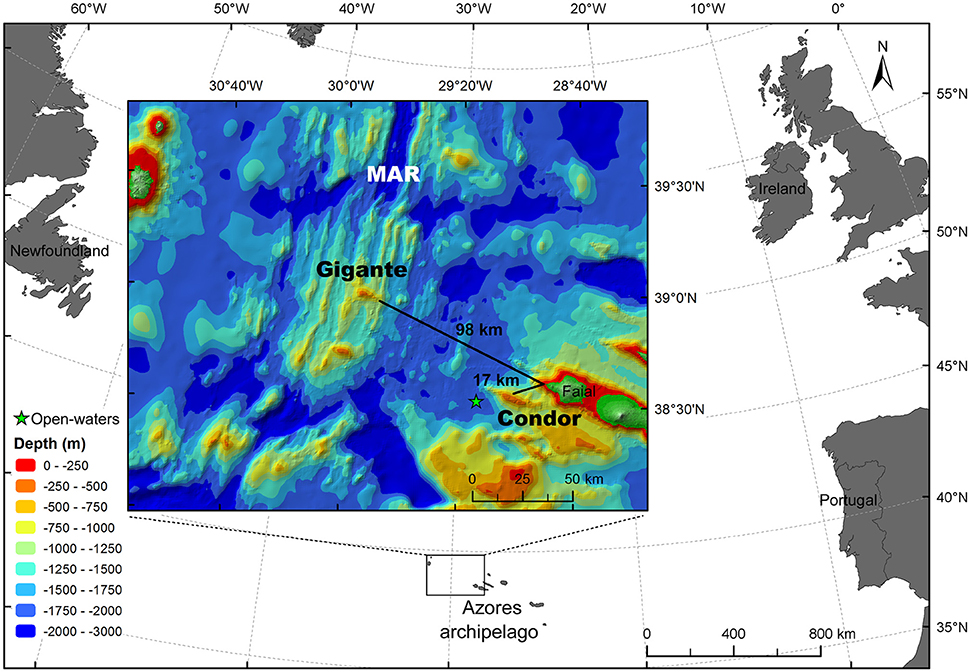
Figure 1. Study area. Map of the study area indicating the locations where acoustic surveys were conducted: Condor seamount, Gigante seamount, and open-water far-field site (green star). MAR: Mid-Atlantic Ridge. Azores bathymetry data credits: (Lourenço et al., 1998).
Condor seamount (38°33′N, 29°02′W) is located approximately 17 km to the WSW of Faial Island and 100 km east of the Mid-Atlantic Ridge (MAR) (Figure 1). Condor is an elongated feature about 26 km long and 7.4 km wide at the 1000-m depth contour. It is a shallow-intermediate seamount with two major peaks: the main summit 182 m depth on the western side, and a secondary peak 214 m deep on the eastern side of the seamount. The summit at the 300-m depth contour is nearly flat, with a total surface area of 11.6 km2 (Figure 2A). The nearest large seamount is at 39 km to the south, while the nearest small seamount (200 m ≤ height < 1000 m) is 23 km to the SSE (Morato et al., 2008a). Tempera et al. (2012) describe in great detail the geomorphological structures on Condor seamount. Condor is characterized by peculiar multi-scale dynamics involving localized upwelling–downwelling patterns, enhanced mixing, and pronounced closed circulation structures over the seamount (Bashmachnikov et al., 2013).
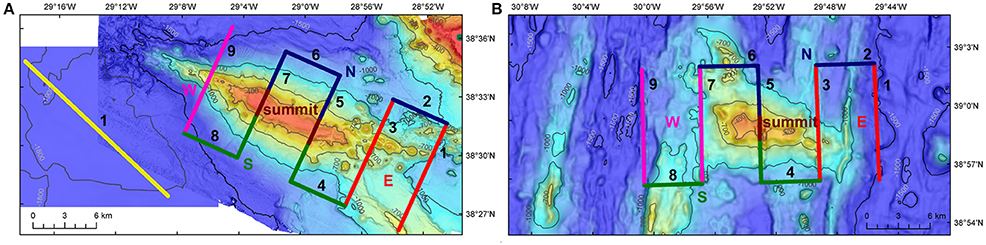
Figure 2. Acoustic surveys. Surveys to collect acoustic data on Condor seamount (A), Gigante seamount (B) and in open-waters (A). Surveys at seamounts were composed of nine transects, while surveys in the open-waters followed a single transect. Condor and Gigante's transects were categorized into five regions: summit, East (E), North (N), South (S), and West (W). Warmer colors indicate shallower depths. On Condor seamount, transect 7 runs across the main summit (182 m deep) and transect 5 crosses the second peak (214 m deep). On Gigante seamount, transect 5 runs across the shallowest peak of 161 m depth. Bathymetry data credits: Open-waters—MeshAtlantic, IMAR-DOP/UAz; Condor—EMEPC, DOP/UAz, Project STRIPAREA/J.Luís/UAlg-CIMA; Gigante—EMEPC, IMAR-DOP/UAz.
Gigante seamount (38°59′N, 29°53′W) is situated about 98 km to the WNW of Faial Island and 6 km east from the MAR (Figure 1). It is approximately 16 km long and 6–13 km wide at the 1000-m depth contour. It is a shallow seamount, reaching 161 m depth, and the summit has a small surface area of 0.7 km2 at the 300-m depth contour (Figure 2B). The nearest large seamount is located 31 km to the south, while the nearest small seamount is 15 km to the SW with a deep summit of 787 m (Morato et al., 2008a). The distance between Condor and Gigante seamounts is about 80 km. No hydrographical data are available for the Gigante area.
In addition to the seamounts, surveys were carried out at an open-water far-field site (38°30′N, 29°13′W), with depths ranging from 1770 to 1900 m (Figure 2A). This station was located 7 km west of Condor seamount (at the 1000-m depth contour; ~10 km from the summit of Condor) and 35 km to the WSW of Faial Island (Figure 1).
Data Collection
Acoustic backscatter data were collected using a split-beam Simrad EK500 scientific echosounder system aboard R/V Arquipélago, operating at 38 and 120 kHz frequencies. Both elliptical transducers had approximately 7° beam widths (38 kHz: 7.2° along and 6.8° athwart; 120 kHz: 7° along and 7.1° athwart). They were set to operate with 1.024 and 0.256 ms pulse durations at 2000 and 1000 W transmit power for the 38 and 120 kHz frequencies, respectively. These settings allowed for 1000 and 300 m sampling depths for the 38 and 120 kHz frequencies, respectively. The transducers were calibrated every year prior to the first survey using a 60-mm-diameter copper sphere for 38 kHz and 23-mm sphere for 120 kHz, according to standard procedures (Demer et al., 2015). To exclude unwanted scatterers, such as plankton and other smaller organisms, the minimum threshold for the mean volume backscattering strength (Sv, hereafter designated as MVBS) was set to −70 dB re 1 m−1. While this threshold level may exclude some scattering from low-density micronekton, −70 dB was selected based on known aggregative behavior of micronekton (Saunders et al., 2013) and to exclude backscatter from larger zooplankton.
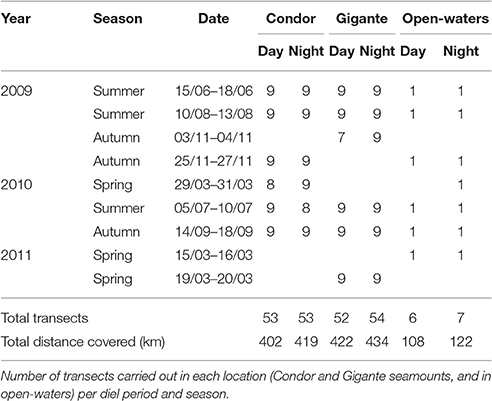
Acoustic surveys on Condor and Gigante seamounts and at the control site were conducted during the day and at night in spring (March), summer (June–August), and autumn (September and November) from 2009 to 2011. Surveys followed a systematic design, and acoustic backscatter data were collected continuously along a pre-defined transect. Acoustic surveys over seamounts covered nine transects: five transects perpendicular to seamounts (each 11.11 km) and four transects along seamounts (each 5.56 km), totaling 77.78 km (Figure 2). Sampling in open-waters followed a single transect 18.52 km long (Figure 2A). Survey speed varied between 7 and 8 knots, depending on sea conditions.
In 2010, trawl surveys were conducted concurrently with acoustic surveys to determine the contribution of different species or taxa to the acoustic backscatter and to estimate the abundance of organisms sampled acoustically. Twenty-five oblique tows were made in open-waters and around Condor using an Isaacs Kid Midwater Trawl (3-m IKMT). Unfortunately, these surveys failed to capture meaningful samples of micronektonic organisms (average of 2.3 fishes captured/tow, no zooplankton or cephalopods were captured), preventing groundtruthing of acoustic data.
Data Analysis
Data Processing
Acoustic backscatter data were recorded using Movies + software (Ifremer) and then processed using Echoview software (Myriax Pty Ltd, Hobart, Tasmania). Data were pre-processed to avoid unreliable backscatter in the analysis. First, the surface layer (0–10 m depth) was excluded to avoid surface bubbles, noise, and near-field effects of the transducers. Then, each echogram was visually inspected to remove bottom echoes and acoustic or electrical noise from the sampled water column.
Only the 38 kHz data were used in this study to characterize the spatial and temporal distribution of acoustic scatterers, since the maximum penetration depth of the 120 kHz echosounder was only 300 m and, therefore, unable to capture the full vertical range of the deep scattering layer (~400–700 m depths). At the 38 kHz frequency, acoustic backscatter is dominated by organisms with gas-filled structures (Kloser et al., 2009; Davison et al., 2015). Many epipelagic and mesopelagic fishes possess a gas-filled swimbladder that present a high density contrast with seawater, making them the most significant contributors to acoustic backscatter at the 38 kHz (Davison et al., 2015). Conversely, large crustaceans, squids and non-gas bearing fishes, are relatively weak scatterers at this frequency (Lavery et al., 2002; Kang et al., 2005) and their importance to total backscatter in this study was likely small. Gelatinous organisms with pneumatophores may also be important sources of scattering at 38 kHz (Warren et al., 2001). Bongo net catches indicate that pteropods, siphonophores, and salpids are not abundant in the surface waters around Condor and at a nearby open-water site (Carmo et al., 2013), and their scattering would have been masked by the much stronger scattering of the dominant swimbladdered fish. Thus, acoustic backscatter patterns reported in this study are likely to be primarily attributed to gas-bearing swimbladder fish.
Since most acoustic scatterers were aggregated in schools or layers, single-target echoes could not be identified. Therefore, acoustic density of micronekton fish was estimated by calculating the MVBS (dB re 1 m−1). We initially calculated MVBS over 10-m deep by 100-m long bins. These data were used to construct variograms to determine the extent and scale of spatial autocorrelation in the acoustic observations (Rivoirard et al., 2000). Analysis of the variograms indicated that data were no longer correlated at distances >1 km. MVBS was recalculated for the entire water column (from 10 m depth to the sea floor) by 1-km long bins.
Day- and night-time acoustic data were examined separately, excluding crepuscular data from the analysis (1 h before and after sunrise and sunset) to avoid migratory periods of the sound scattering organisms. The sunrise and sunset times for the Azores were acquired from the U.S. Naval Observatory Astronomical Applications Department database. Echograms were visually inspected to ensure no migratory periods were included.
Statistical Analysis
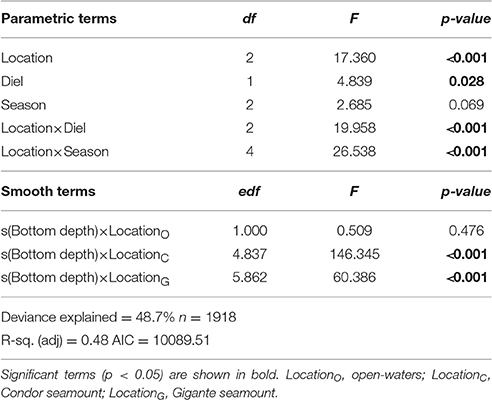
Generalized Linear Models (GLMs) and Generalized Additive Models (GAMs) were used to investigate spatial and temporal differences in MVBS in the study areas, and to assess the influence of physiographic variables. Latitude and longitude were strongly collinear (0.96) and were therefore not included in the analysis. We also tried to model the spatial distribution of backscattering strength using transect as a covariate, but these models provided a poor fit to the data. Instead, MVBS was compared between locations (open-waters, Condor, Gigante) and between seamount regions (summit, East, North, South, West) identified based on their orientation relative to the summits (Figure 2). Bottom depth at sampling locations was derived from in situ measurements of the echosounder, and slope values were extracted from a Digital Terrain Model created from depth data using ArcGIS™ Spatial Analyst tools. Possible effects of physical and biological variables other than physiography, such as currents, temperature, salinity, or chlorophyll-a, were not investigated because spatial and temporal resolution of those variables was too coarse to compare with backscatter data.
Prior to analysis, normality of the data was verified by plotting histograms and normal Q-Q plots of all variables, and boxplots were used to detect extreme values and outliers in the data. Boxplots, coplots and lattice graphs were used to inspect the relationship between covariates and the response variable, and pairplots were used to assess collinearity among covariates.
The GLM approach was inappropriate for our dataset since the relationship between MVBS and bottom depth was non-linear. Thus, backscattering data were modeled using GAMs with a Gaussian distribution and an identity link function, using the “mgcv” R package. Two different models were fitted to investigate the effect of bottom depth, slope, diel period, and season in MVBS in seamounts (summits and slopes) and open-waters (Model 1), and to compare MVBS and its diel pattern between the slopes of each seamount (Model 2):
where MVBS was the response variable, α the intercept, f the smoothing function for predictor variables, and εi the residuals. The model contained three categorical explanatory variables: location (open-waters—LocationO, Condor—LocationC, and Gigante—LocationG), diel (day and night) and season (spring, summer, and autumn). Bottom depth and slope were fitted as smoothing functions, and the model allowed for a different smoother at each location.
where region had four levels corresponding to seamount sides (East, North, South, and West), and diel and bottom depth were fitted as in the previous model. Bottom depth was included as a covariate to account for differences in topography between seamount regions. A separate model was built for Condor and Gigante.
A backward stepwise selection procedure was used to identify the best fitting model based on the Akaike Information Criterion (AIC) value and analysis of deviance. The adequacy of the best fitting model was inspected using normal Q-Q plots, histograms of the residuals, and plots of residuals and observed values versus fitted values (Supplementary Figures 1, 2).
Maps of predicted MVBS were produced to visualize the distribution of acoustic scatterers in the study areas. As differences in MVBS between seamount slopes were small and not significant (see Results below), maps were generated using the best fitting Model 1. A 1 × 1 km spatial grid was created for the study area and values for the variable bottom depth were extracted at the mid-point of each grid cell. MVBS and respective standard errors were predicted for each grid cell, diel period, and season. Standard errors of predicted MVBS were also mapped to visualize regions of model uncertainty (Supplementary Figures 3, 4).
Results
Over 3 years, nine acoustic surveys were completed in 29 days, totaling 1907 km surveyed (Table 1). Similar sampling effort was achieved at Condor (821 km) and Gigante (856 km) seamounts, whereas in open-waters 230 km of acoustic surveys were carried out. The day-night sampling was equally distributed at each study location, but there were considerable differences in sampling between seasons. Most of the survey effort was conducted in summer due to favorable weather conditions, followed by autumn and spring. Sampled bottom depths around Condor seamount ranged between 182 and 1604 m, at Gigante between 161 and 1609 m, and in open-waters between 1774 and 1904 m. Seamounts had steeper slopes (Condor: 0.05°–73.08°; Gigante: 0.16°–51.30°) than open-waters (0.02°–3.94°).
Distribution of Acoustic Scatterers: Open-Waters vs. Seamounts
Acoustic scatterers were observed throughout the study area in all seasons and diel periods. Overall, the highest MVBS was found in the open-water site (−73.50 ± 2.47 dB), followed by Condor (−75.20 ± 5.19 dB) and Gigante (−75.74 ± 4.40 dB). MVBS was significantly related to seamounts bottom depth (Table 2). In Condor and Gigante seamounts, the highest MVBS was found over the shallowest areas. Backscattering strength then decreased rapidly with increasing seafloor depths with the lowest values occurring in areas 800–1100 m deep (Figures 3A,B). Conversely, in open-waters, MVBS decreased linearly but only moderately with depth (Figure 3C). Average backscattering strength above the summits of Condor (−63.28 ± 4.71 dB) and Gigante (−63.59 ± 6.76 dB) was 18 times higher the MVBS measured over the slopes (Condor: −75.90 ± 4.29 dB; Gigante: −76.04 ± 3.88 dB) and about 10 times higher MVBS in open-waters (Figure 4).
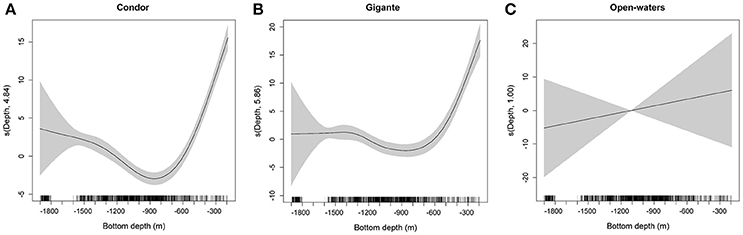
Figure 3. Response curves for the GAM of MVBS relative to bottom depth for seamounts and open-waters. Fitted smoothing function (solid line) for bottom depth for Condor seamount (A), Gigante seamount (B), and open-waters (C) obtained by the best GAM. Estimated degrees of freedom (edf) are displayed on the y-axes. Tick marks on the x-axis show sample values. Shaded areas denote the approximate 95% confidence bands.
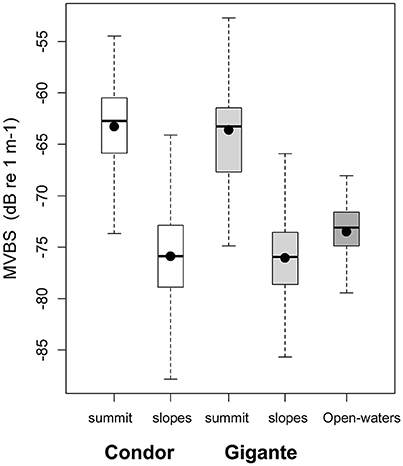
Figure 4. Backscatter in seamount summits and slopes, and in open-waters. Box plots of MVBS for the summits and slopes of Condor seamount (white), Gigante seamount (light gray) and in open-waters (dark gray). Black dots represent the mean; black bars the median; boxes the 25 and 75% quartiles; whiskers extend 1.5 times the interquartile range (spread) from the box edges or indicate the most extreme values of the spread.
There was also a significant interaction between location and diel period, and between location and season (Table 2). In open-waters, the highest backscattering occurred during the day, while the reverse pattern was observed in Gigante. Diel differences in acoustic backscatter were small in Condor (Figure 5A, Supplementary Table 1). In Condor, MVBS peaked in spring, decreasing substantially in summer (ΔMVBS = 1.52 dB) and autumn (ΔMVBS = 5.27 dB) (Figure 5B, Supplementary Table 1). In contrast, mean backscatter was slightly higher in summer than in the other seasons in Gigante and open-waters.
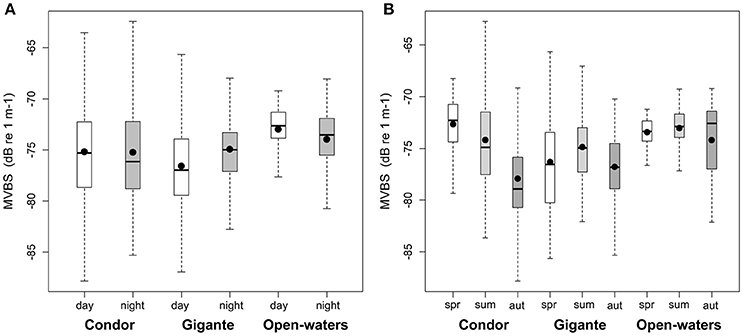
Figure 5. Diel and seasonal patterns in backscatter in seamounts and open-waters. Box plots of MVBS for: (A) day (white boxes) and night (gray boxes) and (B) seasons—spring (white boxes), summer (light gray boxes) and autumn (dark gray boxes). MVBS for seamount summits and slopes were pooled into an overall value. Black dots represent the mean; black bars the median; boxes the 25 and 75% quartiles; whiskers extend 1.5 times the interquartile range (spread) from the box edges or indicate the most extreme values of the spread.
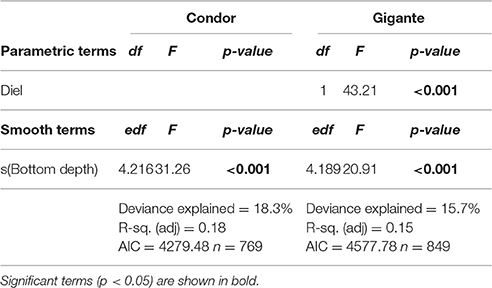
Distribution of Acoustic Scatterers between Seamount Slopes
Model 2 was built to investigate differences in MVBS and its diel pattern between the slopes of Condor and Gigante seamounts. The best fitting models for each seamount explained 15.7–18.3% of the deviance (Table 3). The interaction between region and diel period, and the main term region had no significant effect on MVBS in any of the seamounts. Little variation was observed in MVBS between seamount flanks and the differences were not consistent between seamounts (Figure 6). Slightly lower values of MVBS were found on the eastern (−77.50 ± 3.87 dB) and northern (−75.58 ± 4.38 dB) slopes of Condor, and on the southern (−76.53 ± 4.10 dB) and northern (−76.27 ± 4.15 dB) slopes of Gigante.
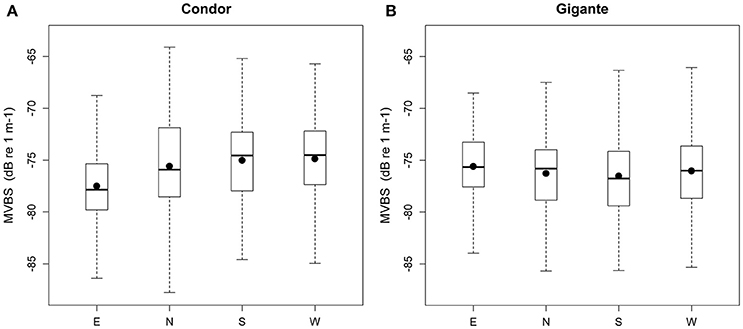
Figure 6. Backscatter in seamount slopes. Box plots of MVBS for the slopes in Condor seamount (A) and Gigante seamount (B). Black dots represent the mean; black bars the median; boxes the 25 and 75% quartiles; whiskers extend 1.5 times the interquartile range (spread) from the box edges or indicate the most extreme values of the spread.
Predicted Spatial Density of Acoustic Scatterers
The predictions of MVBS for Condor and Gigante seamounts and for the open-water site for each diel and season from the best fitting model (48.7% of deviance explained; Table 2) are shown in Figures 7, 8. The maps highlight the spatial heterogeneity in backscattering strength around seamounts related to the depth gradient. The model predicted the highest MVBS over the summits and upper slopes of Condor and Gigante seamounts, irrespective of the season and diel period. Acoustic density decreased drastically along the seamount slopes, reaching the minimum values at the base of both seamounts. A slight enhancement in backscatter was predicted toward deeper waters away from Condor and Gigante seamounts and in the open-water site. Average MVBS predicted for the open-water site was 3% higher (ΔMVBS = ~2.4 dB) than MVBS in seamount slope areas but 15% lower (ΔMVBS = ~10 dB) than MVBS predicted for Condor and Gigante summits.
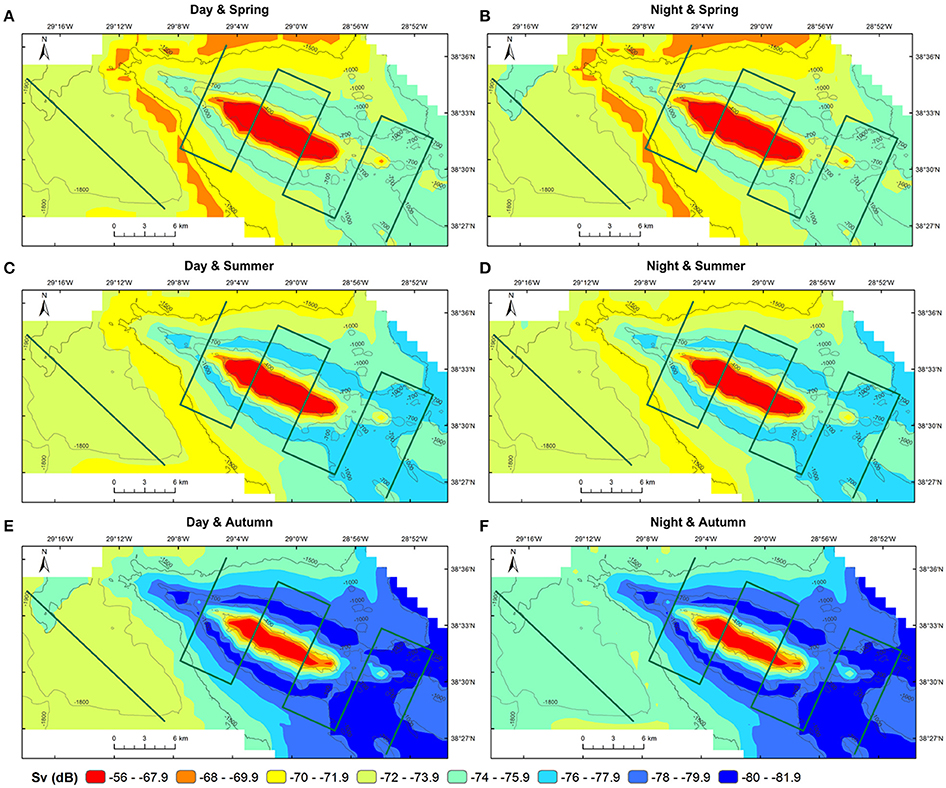
Figure 7. Predicted backscatter in Condor seamount and in open-waters. Predicted MVBS obtained from the best GAM (Model 1) for each diel period (day—left; night—right) and season (spring—top; summer—middle; autumn—bottom). Warmer colors indicate higher Sv. Green lines illustrate transect surveys.
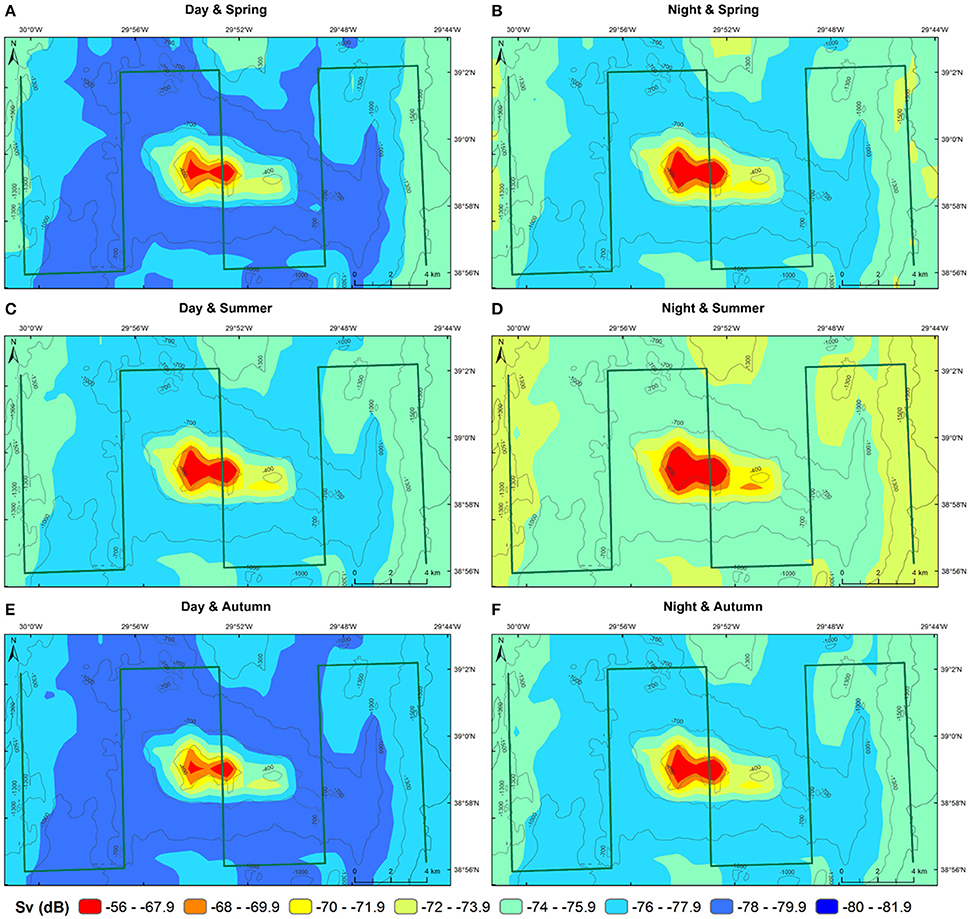
Figure 8. Predicted backscatter in Gigante seamount. Predicted MVBS obtained from the best GAM (Model 1) for each diel period (day—left; night—right) and season (spring—top; summer—middle; autumn—bottom). Warmer colors indicate higher Sv. Green lines illustrate transect surveys.
Whilst the acoustic scatterer's hotspot over the Condor summit was a consistent feature in all seasons it decreased in size from spring to autumn (Figures 5B, 7). Still, it was in autumn that the spatial gradient in acoustic density was more evident because of lower backscatter over the slopes and in areas farther away from the seamount. Compared to Condor and to the open-water site, diel differences in MVBS were much more pronounced in Gigante (Figures 5B, 8) with consistently higher nighttime MVBS over the summit and slopes.
Discussion
Our results clearly show that Condor and Gigante seamounts significantly affected the spatial and temporal distribution of micronekton, and this effect could be detected up to ~7 km from the seamount summit. Even though the two seamounts differ in a number of physical characteristics (e.g., size, shape, flatness of the summit, and distance to other topographic features), patterns and estimates of acoustic density were similar between them. Strong sound-scattering aggregations were a permanent feature above the summits of Condor and Gigante in all seasons, both during the day and at night. This contrasted with the reduced scattering in the water column above the seamount flanks, which was even lower than that measured in open ocean waters just a few kilometers away. Also, differences in scattering strength between seamount slopes were generally small, providing no evidence for micronekton concentrations at the upstream or downstream side of the seamounts. Finally, on seamount summits and slopes, acoustic backscatter increased during the night, while the opposite diel pattern was observed in open-waters.
Aggregations of micronekton are taxonomically diverse and include fish, cephalopods, and crustaceans but acoustic observations at the 38 kHz frequency used in this study are dominated by air-filled swimbladder fish (Kloser et al., 2009; Davison et al., 2015). Therefore, we assume that variations in backscattering strength mostly reflect changes in the relative density of micronekton fish, although occasional contribution of large nekton fishes cannot be completely ruled out. Still, the sound scatter produced by organisms depends not only on their numerical density but also on their morphological, physiological and behavioral characteristics (Godø et al., 2009; Davison, 2011; Davison et al., 2015). While it is impossible to identify species from acoustic scattering alone, the acoustic properties of micronekton fish, together with information on micronekton composition from studies in Condor and at other seamounts in the Atlantic, may provide clues about the identity of sound scatterers and help explain the patterns documented here.
Midwater trawls conducted over the slopes of Condor and at a far-field station (located very close to our open-water site) captured 46 taxa but only a few species/families were numerically important (Porteiro et al., 2011). Cyclothone spp. represented 66% of the total catches in number, followed by myctophids (18%), the stomiids Stomias boa ferox and Chauliodus sloani (8%), and the sternoptychid Argyropelecus hemigymnus (2%). The majority of fishes ranged from 20 to 80 mm, well within the size range of micronekton.
Slope samples were dominated by species that also occurred in open-waters (Porteiro et al., 2011). Mid- and deep-water (400–800 m) catches were largely dominated by Cyclothone spp., a group of non-migratory mesopelagic fish very abundant worldwide (Nelson, 2006). Most Cyclothone species are strong scatterers at the 38 kHz frequency (Peña et al., 2014; Ariza et al., 2016) and this group likely was the major source of the day and nighttime backscatter over seamount slopes and in open-waters. Another important contributor to the backscatter outside the summits may be A. hemigymnus, also known to reside at depth all day long (Peña et al., 2014). Myctophids (especially Lobianchia dofleini, Diaphus rafinesquei, and Lampanyctus pusillus) were the second most abundant group overall but the first in shallow waters at night (Porteiro et al., 2011), consistent with their well-known DVM (Sutton, 2013). Catches were dominated by species with functional swimbladders as adults (Davison, 2011) which are strong scatterers. However, swimbladder resonance of myctophids and other mesopelagic fishes seems to increase with depth depending on swimbladder size, potentially biasing estimates of backscatter in deeper waters (Kloser et al., 2002; Godø et al., 2009; Yasuma et al., 2010). The effects of this bias in our estimates will be discussed below. In contrast to the former taxa, the two stomiids possibly contributed little to acoustic scattering because they lack air-filled swimbladders.
Unfortunately, a single nighttime trawl was conducted over the summit of Condor, capturing 3 fish specimens (myctophids D. rafinesquei and S. boa ferox; Porteiro et al., 2011). Video images from a baited lander (Fontes and Menezes, 2011) and from remotely operated vehicles (ROV) (Porteiro et al., 2013) showed that, during the day, the pelagic fish fauna on Condor summit was dominated by dense swarms of small sized zooplanktivorous fishes, including the seaperches (Anthias anthias and Callanthias ruber), the snipefish (Macrorhamphosus scolopax) and the blue jack mackerel (Trachurus picturatus), with occasional records of unidentified myctophids (Porteiro et al., 2013). Care must be taken, however, as ROV observations may be biased due to the known avoidance behavior of some micronekton fishes, including myctophids (Porteiro et al., 2013). Most zooplanktivorous fish were absent from the slopes of Condor and the few species present below 300 m depth had densities 20 times lower than those observed at the summit (Porteiro et al., 2013).
These and other zooplanktivorous fish may well be part of the permanent micronekton community inhabiting the summits and upper slopes of Condor, and possibly Gigante, as reported in other shallow and intermediate seamounts in the subtropical Northeast Atlantic (see Morato and Clarke, 2007). Most of these fishes are good sound reflectors and possibly accounted for a significant part of the enhanced backscatter above the summits of Condor and Gigante in all seasons and diel periods. Seamount-associated micronekton fishes live a benthopelagic lifestyle, feeding in the water column above the seamount when conditions are right and resting at the benthic boundary layer (Boehlert, 1988; Genin, 2004). Presence of a resident micronekton community implies the existence of an abundant and predictable supply of prey. While there is no information on the oceanographic conditions or biological communities in Gigante, physical processes at Condor may be responsible for concentrating prey for resident micronekton fish. Condor is characterized by a quasi-persistent anticyclonic cap located 50–60 m above the summit, possibly generated by tidal forcing and/or a steady impinging flow (Bashmachnikov et al., 2013). Taylor caps penetrating into the euphotic zone can locally enhance primary production by bringing nutrient-rich waters to the surface (Genin, 2004). In the case of Condor, however, trapping induced by the Taylor cap occurs mostly below 170 m depth (i.e., below the seasonal pycnocline) (Bashmachnikov et al., 2013) and is unlikely to boost primary production. In addition, changes in water circulation and mixing result in periodic shedding of the Taylor cap lasting from weeks to months (Bashmachnikov et al., 2013). Thus, presence of the Taylor cap will hardly cause a persistent enrichment in primary productivity over Condor. Consistent with these findings, there is no evidence of increased phytoplankton concentrations in the top 100 m above the summit and slopes of Condor (Santos et al., 2013). Rather, the Taylor cap may retain sufficient concentrations of autochthonous or allochtonous prey above the seamount to enable self-sustainability of the resident micronekton (Genin, 2004; Genin and Dower, 2007). Alternatively, or in combination with this mechanism, the seamount micronekton community might rely on vertically migrating zooplankton that becomes trapped above the seamount summits.
Similar DVM behavior by mesopelagic fishes would add to the backscatter from the seamount resident community, amplifying the contrast between summits and slopes and causing diel changes in backscatter above the summits. Mesopelagic fish ascending from deeper waters in the vicinity and above the seamount flanks could be attracted to the summits to feed on concentrations of zooplankton created by physical forcing or topographic blockage. Despite being strong swimmers, micronekton may also be passively advected onto the summits by currents (Kaartvedt et al., 2009). The seamounts' shallow topography aggregates these fishes in near-surface waters at night and possibly retains part of this community during the day (Genin, 2004). Obviously, this would result in fewer fishes returning to bottom waters at the seamount flanks. The effect of topographic blockage should be more pronounced in Condor than in Gigante, because the smaller plateau of Gigante likely is less efficient at blocking the fish descent. We believe this is one of the reasons for the much clear day-night differences in backscattering intensity in Gigante, as opposed to Condor where diel changes were small. Although video imagery and net sampling provided only limited evidence of the presence of vertically migrant fishes on the Condor plateau (Porteiro et al., 2011, 2013), topographic blockage has been suggested as one of the mechanisms responsible for the increased nighttime micronekton abundance at other shallow/intermediate seamounts (Boehlert, 1988; Johnston et al., 2008).
As a result of the interaction of the local flow field with the seamount topography, distribution of micronekton fish often exhibits small-scale spatial variability, with enhanced biomass or densities on the downstream flanks of seamounts. At Southeast Hancock Seamount, micronekton fish moving into waters above the seamount on a diel basis were usually displaced by strong currents to the downstream side of the summit by the end of the night (Wilson and Boehlert, 2004). Porteiro et al. (2011) reported lower abundance and biomass of midwater fishes on the northern slope (upstream) of Condor when compared to the southern slope (downstream), consistent with the northern-southerly direction of the upper ocean current (Bashmachnikov et al., 2013). Since Gigante is under the influence of the same regional current, we assumed that acoustic density would be higher on the southern slope of the two seamounts. Against our expectation, total backscatter and its diel patterns were similar upstream and downstream the seamounts. In the case of Condor, it is possible that the effects of the anticyclonic vortex prevail over the background oceanic flow (Bashmachnikov et al., 2013), trapping micronekton fish and their prey and preventing them from being advected downstream the summit. Nonetheless, the presence of a Taylor cap has not been documented in Gigante and the potential retention of such mechanism in Condor is unknown. Concurrent acoustic and oceanographic observations will help understanding how different physical processes shape micronekton distribution at the scale of the seamounts.
Another interesting finding of this study is the reduced acoustic density above the seamount slopes in comparison to open-waters. As already mentioned, samples from midwater trawls conducted over the slopes and off the seamount were similar (Porteiro et al., 2013), suggesting that differences in species composition are unlikely to be responsible for backscattering variations between those regions. Assuming identical composition of scatterers, then a difference in mean backscattering strength of 2.4 dB indicates that density of micronekton above the slopes was nearly half of that found in the open ocean (Simmonds and MacLennan, 2005). Such difference may result from: (i) the retention at summit of part of the population of migrant fishes that never return to their daytime depths above the slopes, and (ii) the upward migration in open-waters of some mesopelagic species from depths beyond the seamount base (~1000–1200 m depth).
Contrasting with the diel pattern at seamounts (more evident in Gigante), backscatter measured in open-waters was consistently higher during the day. Different behavioral processes and physical mechanisms may explain this pattern. On one hand, the upward migration of taxa from depths beyond >1000 m; i.e., outside the range of the 38 kHz (Domokos, 2009) should contribute to greater nighttime backscattering. This could be offset by the effects of the horizontal movements of micronekton and of swimbladder resonance. During the day, myctophids occur in discrete, dense patches, but at night they tend to disperse horizontally and vertically in the upper water column (Benoit-Bird and Au, 2006). As the horizontal range of myctophids increases, estimates of volume backscattering strength decrease. Swimbladder resonance increases acoustic backscatter from fishes and such effect is more pronounced at greater depths (>300 m) (Godø et al., 2009; Yasuma et al., 2010). Consequently, bias from swimbladder resonance mainly affects daytime acoustic observations, when mesopelagic fish occur deeper in the water column. At present, it is impossible to know to what extent resonance may have overestimated daytime backscatter measurements. Future studies examining the vertical distribution of micronekton may help elucidating the role played by each of these factors.
We found a well-defined seasonal pattern in acoustic density at Condor seamount, with an evident peak in spring, lower backscatter in summer and the lowest values in autumn. In comparison, seasonal variations were not so clear in Gigante and in open-waters. In Condor, maximum concentrations of chlorophyll-a occurred in spring, associated with the well-mixed water column and lower surface temperature, whereas minimum values were recorded in summer with stratified, warmer waters (Santos et al., 2013). Zooplankton develops rapidly following the onset of the phytoplankton bloom, with the highest abundances recorded from spring to early summer (Carmo et al., 2013). More importantly, increased prey availability likely attracts greater numbers of micronekton fish to the seamount. As zooplankton becomes scarcer in upper layers in late summer and autumn, due to the decline in primary production and to predation, but also because some species descend to mid-waters to overwinter, several mesopelagic fishes, including myctophids, restrain from migrating to the surface at night (Dypvik et al., 2012 and references therein). Density of migrant micronekton fish above the seamount summits will therefore decrease, following the decrease in zooplankton biomass after the spring-early summer peak. In addition to prey distribution, life history patterns of seamount fishes may also contribute to seasonal peaks in backscatter. While several species are year-round residents at seamounts, others aggregate at seamounts periodically for spawning (e.g., M. scolopax, Capros aper) (Morato and Clarke, 2007). Arrival of high-density spawning aggregations at seamounts (Jorgensen et al., 2016) could potentially explain seasonal variations in acoustic density, although we have no way of confirming this hypothesis.
Acoustic sampling is a powerful method to estimate the distribution and biomass of micronekton fish but it has its own limitations, uncertainties and potential bias, which ultimately constrained interpretation of some of the findings of this study. First, our study area is certainly inhabited by many micronekton species not visible in the echograms, either because they are non-resonant at the 38 kHz frequency or because their weak signal is masked by dominant sound reflectors. Second, acoustics does not allow direct taxonomic identification of sound scatterers nor does it provide certain quantitative data (e.g., size and weight of organisms) necessary to estimate absolute density or biomass. In this study we did not attempt to convert MVBS into biomass; instead, MVBS was interpreted as a proxy for the relative density of micronekton. We also didn't assume equal composition of scattering layers within the study area or across the water column but tried to support our findings on available (albeit limited) knowledge about local pelagic communities. As an example, we avoided comparisons between Gigante and Condor because we lacked information on possible scattering layer constituents in Gigante. Future studies should consider using multi-frequency acoustics to better discriminate sound scatterers, providing insights into the relative composition and density of micronekton layers. Another constraint of this study is that acoustic data were not integrated with oceanographic observations to determine how bio-physical parameters influenced the distribution of sound-scattering micronekton fish. The only data available on sea surface temperature or chlorophyll-a were weekly averages at 4-km resolution which was judged too coarse considering the size of the seamounts, the duration of the surveys and the spatial variability of the SL. Therefore, we chose to use static seabed features (bottom depth and slope), as well as spatial (location and region) and temporal (diel and season) variables. Future work will be necessary to refine these analyses by including more biological-relevant environmental variables. Despite these shortcomings, this work fills an important knowledge gap of micronekton, the “missing link” between lower and higher trophic levels, and provides some clues to the mechanisms responsible for the variation in micronekton distribution across and within seamounts, contributing to our growing understanding of the functioning of seamount ecosystems.
Summary
Through a series of acoustic surveys conducted over a 3-year period, this study provides the first comprehensive view of the distribution and temporal dynamics of micronekton at seamounts in the Azores. Our work showed that presence of seamounts affected the horizontal distribution of acoustic scatterers, contributing to the formation of persistent higher MVBS in near-surface waters over the summits, which contrasted with lower acoustic density in the water column above the slopes and in the open ocean. The dynamics of micronekton around seamounts likely result from a combination of behavioral and physical mechanisms acting over different spatial and temporal scales. The DVM brings huge concentrations of mesopelagic fishes to surface waters to feed under the cover of darkness. Fishes may actively seek seamounts to take advantage of prey aggregated around the top by the Taylor cap (Bashmachnikov et al., 2013). Micronekton fishes may also be advected from upstream the summit and become entrained in the circular flow generated by the Taylor cap (Pitcher and Bulman, 2007). Fishes present in the water column immediately above the plateau could then be trapped at the seamount when trying to descend the next dawn. In addition to vertically migrant fishes, we suggest these seamounts support an abundant community of year-round or seasonal residents.
The dynamics of micronekton likely has profound effects on the structure and function of seamount ecosystems, and on their linkages to other pelagic or coastal systems. Stable isotopes analysis showed that the food chain on Condor seamount is composed of five trophic levels, and that mesopelagic organisms play a crucial role in the trophic web, linking the epipelagic environment to benthic, and benthopelagic organisms (Colaço et al., 2013). Increased micronekton abundance at seamounts likely supports important biomasses of benthic fishes and attracts numerous pelagic visitors (Morato et al., 2008b). Studies conducted in the Azores have shown that several benthic and benthopelagic fish species rely on SL constituents for food (Gomes et al., 1998; Morato et al., 2001; Colaço et al., 2013) and that the vertical distribution of these fishes at seamounts is driven by the dynamics of the SL (Afonso et al., 2014a). Large pelagic predators, such as tuna, seabirds, and marine mammals, feed on various micronektonic organisms (Choy et al., 2016). The effect of seamounts at aggregating micronekton may play an important role in the feeding success of these top predators, especially in oligotrophic environments, where food is often scarce and too patchy to be efficiently exploited. Indeed, several of these predators are known to intensively use and forage at seamounts (Awkerman et al., 2005; Garrigue et al., 2015; Jorgensen et al., 2016). In the Azores, several dolphin species preferentially distribute in areas with high density of seamounts (Tobeña et al., 2016) and baleen whales instrumented with satellite tags spent days to weeks apparently foraging around seamounts (Silva et al., 2013). Preliminary analyses of long-term acoustic recordings from hydrophones deployed at Condor and Gigante showed that dolphins use these seamounts nearly every day (Silva and Cascão, 2011). By concentrating micronekton prey in near-surface waters, these seamounts likely provide increased foraging opportunities for shallow divers.
Author Contributions
Conceived and designed the acoustic surveys: IC, ML, MS. Executed data collection: IC. Performed data analyses: IC, RéD, MS. Wrote the paper: IC, MS. Reviewed the manuscript and approved the final version: IC, ML, RéD, VM, RuD, RS, MS.
Funding
This research was supported by FEDER funds, through the Competitiveness Factors Operational Programme - COMPETE, QREN European Social Fund, by national funds, through FCT - Foundation for Science and Technology, under project TRACE (PTDC/MAR/74071/2006), EEA Grants Financial Mechanism—Iceland, Liechtenstein, and Norway, through research CONDOR (PT0040). We acknowledge funds provided by FCT to MARE, through the strategic project UID/MAR/04292/2013. MS was supported by POPH, QREN European Social Fund and the Portuguese Ministry for Science and Education, through an FCT Investigator grant (IF/00943/2013). IC was supported by an FCT doctoral grant (SFRH/BD/41192/2007). The FCT Exploratory project (IF/00943/2013/CP1199/CT0001) paid the fees for this open-access publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are grateful to the crew of the R/V Arquipélago, João Medeiros, Yves Cuenot, and colleagues from the Oceanography group, especially Alexandre Medeiros and Sergio Gomes, for their support during data collection. Special thanks to Alexandre Morais for providing technical assistance on the EK500 echosounder and Yorgos Stratoudakis for hosting IC at IPMA for technical support on Movies+ software. We thank the reviewers for the fruitful discussions that significantly improved the original manuscript. This study is an output of research project TRACE (PTDC/MAR/74071/2006)—Cetacean-habitat associations in oceanic ecosystems: an integrated approach.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/article/10.3389/fmars.2017.00025/full#supplementary-material
References
Afonso, P., McGinty, N., Graça, G., Fontes, J., Inácio, M., Totland, A., et al. (2014a). Vertical migrations of a deep-sea fish and its prey. PLoS ONE 9:e97884. doi: 10.1371/journal.pone.0097884
Afonso, P., McGinty, N., and Machete, M. (2014b). Dynamics of whale shark occurrence at their fringe oceanic habitat. PLoS ONE 9:e102060. doi: 10.1371/journal.pone.0102060
Ariza, A., Landeira, J. M., Escánez, A., Wienerroither, R., Aguilar de Soto, N., Røstad, A., et al. (2016). Vertical distribution, composition and migratory patterns of acoustic scattering layers in the Canary Islands. J. Mar. Syst. 157, 82–91. doi: 10.1016/j.jmarsys.2016.01.004
Atkinson, A., Siegel, V., Pakhomov, E. A., Jessopp, M. J., and Loeb, V. (2009). A re-appraisal of the total biomass and annual production of Antarctic krill. Deep Sea Res. I 56, 727–740. doi: 10.1016/j.dsr.2008.12.007
Awkerman, J. A., Fukuda, A., Higuchi, H., and Anderson, D. J. (2005). Foraging activity and submesoscale habitat use of waved albatrosses Phoebastria irrorata during chick-brooding period. Mar. Ecol. Prog. Ser. 291, 289–300. doi: 10.3354/meps291289
Barham, E. G. (1966). Deep scattering layer migration and composition: observations from a diving saucer. Science 151, 1399–1403. doi: 10.1126/science.151.3716.1399
Bashmachnikov, I., Loureiro, C. M., and Martins, A. (2013). Topographically induced circulation patterns and mixing over Condor seamount. Deep Sea Res. II 98, 38–51. doi: 10.1016/j.dsr2.2013.09.014
Benoit-Bird, K. J., and Au, W. W. L. (2003). Spatial dynamics of a nearshore, micronekton sound-scattering layer. ICES J. Mar. Sci. 60, 899–913. doi: 10.1016/S1054-3139(03)00092-4
Benoit-Bird, K. J., and Au, W. W. L. (2004). Diel migration dynamics of an island-associated sound-scattering layer. Deep Sea Res. I 51, 707–719. doi: 10.1016/j.dsr.2004.01.004
Benoit-Bird, K. J., and Au, W. W. L. (2006). Extreme diel horizontal migrations by a tropical nearshore resident micronekton community. Mar. Ecol. Prog. Ser. 319, 1–14. doi: 10.3354/meps319001
Boehlert, G. W. (1988). Current-topography interactions at mid-ocean seamounts and the impact on pelagic ecosystems. Geojournal 16, 45–52. doi: 10.1007/BF02626371
Carmo, V., Santos, M., Menezes, G. M., Loureiro, C., Lambardi, P., and Martins, A. (2013). Variability of zooplankton communities at Condor seamount and surrounding areas, Azores (NE Atlantic). Deep Sea Res. II 98, 63–74. doi: 10.1016/j.dsr2.2013.08.007
Choy, C. A., Wabnitz, C. C. C., Weijerman, M., Woodworth-Jefcoats, P. A., and Polovina, J. J. (2016). Finding the way to the top: how the composition of oceanic mid-trophic micronekton groups determines apex predator biomass in the central North Pacific. Mar. Ecol. Prog. Ser. 549, 9–25. doi: 10.3354/meps11680
Colaço, A., Giacomello, E., Porteiro, F., and Menezes, G. M. (2013). Trophodynamic studies on the Condor seamount (Azores, Portugal, North Atlantic). Deep Sea Res. II 98, 178–189. doi: 10.1016/j.dsr2.2013.01.010
Davison, P. (2011). The specific gravity of mesopelagic fish from the northeastern Pacific Ocean and its implications for acoustic backscatter. ICES J. Mar. Sci. 68, 2064–2074. doi: 10.1093/icesjms/fsr140
Davison, P. C., Koslow, J. A., and Kloser, R. J. (2015). Acoustic biomass estimation of mesopelagic fish: backscattering from individuals, populations, and communities. ICES J. Mar. Sci. 72, 1413–1424. doi: 10.1093/icesjms/fsv023
De Forest, L., and Drazen, J. (2009). The influence of a Hawaiian seamount on mesopelagic micronekton. Deep Sea Res. I 56, 232–250. doi: 10.1016/j.dsr.2008.09.007
Demer, D. A., Berger, L., Bernasconi, M., Bethke, E., Boswell, K., Chu, D., et al. (2015). Calibration of Acoustic Instruments. ICES Cooperative Research Report.
Denda, A., and Christiansen, B. (2014). Zooplankton distribution patterns at two seamounts in the subtropical and tropical NE Atlantic. Mar. Ecol. 35, 159–179. doi: 10.1111/maec.12065
Diekmann, R., and Piatkowski, U. (2004). Species composition and distribution patterns of early life stages of cephalopods at Great Meteor Seamount (subtropical North-east Atlantic). Arch. Fish. Mar. Res. 51, 115–131. Available online at: https://www.researchgate.net/publication/234118856_Species_composition_and_distribution_patterns_of_early_life_stages_of_cephalopods_at_Great_Meteor_Seamount_subtropical_North-east_Atlantic
Domokos, R. (2009). Environmental effects on forage and longline fishery performance for albacore (Thunnus alalunga) in the American Samoa exclusive economic zone. Fish. Oceanogr. 18, 419–438. doi: 10.1111/j.1365-2419.2009.00521.x
Drazen, J. C., De Forest, L. G., and Domokos, R. (2011). Micronekton abundance and biomass in Hawaiian waters as influenced by seamounts, eddies, and the moon. Deep Sea Res. I 58, 557–566. doi: 10.1016/j.dsr.2011.03.002
Dypvik, E., Røstad, A., and Kaartvedt, S. (2012). Seasonal variations in vertical migration of glacier lanternfish, Benthosema glaciale. Mar. Biol. 159, 1673–1683. doi: 10.1007/s00227-012-1953-2
Fontes, J., and Menezes, G. (2011). “Baited image lander for deep water fish counts and biodiversity studies - a tool for MPA science and fisheries management,” in CONDOR Observatory for Long-Term Study and Monitoring of Azorean Seamount Ecosystems – Final Project Report, eds E. Giacomello and G. Menezes (Horta: Arquivos do DOP, Série Estudos 1/2012), 17–23.
Garrigue, C., Clapham, P. J., Geyer, Y., Kennedy, A. S., and Zerbini, A. N. (2015). Satellite tracking reveals novel migratory patterns and the importance of seamounts for endangered South Pacific humpback whales. R. Soc. Open Sci. 2:150489. doi: 10.1098/rsos.150489
Genin, A. (2004). Bio-physical coupling in the formation of zooplankton and fish aggregations over abrupt topographies. J. Mar. Syst. 50, 3–20. doi: 10.1016/j.jmarsys.2003.10.008
Genin, A., and Dower, J. F. (2007). “Seamount plankton dynamics,” in Seamounts: Ecology, Fisheries and Conservation, eds T. J. Pitcher, T. Morato, P. J. B Hart, M. R. Clark, N. Haggan, and R. S. Santos (Oxford: Blackwell Publishing), 85–100.
Godø, O. R., Patel, R., and Pedersen, G. (2009). Diel migration and swimbladder resonance of small fish: some implications for analyses of multifrequency echo data. ICES J. Mar. Sci. 66, 1143–1148. doi: 10.1093/icesjms/fsp098
Gomes, M. T., Sola, E., Grós, M. P., Menezes, G., and Pinho, M. R (1998). Trophic Relationships and Feeding Habits of Demersal Fishes from the Azores: Importance to Multispecies Assessment. ICES CM 1998/O:7. Available online at: https://www.researchgate.net/publication/239280707_TROPHIC_RELATIONSHIPS_AND_FEEDING_HABITS_OF_DEMERSAL_FISHES_FROM_THE_AZORES_IMPORTANCE_TO_MULTISPECIES_ASSESSMENT
Hargreaves, P. M. (1975). Some observations on the relative abundance of biological sound scatterers in the North-eastern Atlantic Ocean, with particular reference to apparent fish shoals. Mar. Biol. 29, 71–87. doi: 10.1007/BF00395529
Harrison, C. S., and Seki, M. P. (1987). “Trophic relationships among tropical seabirds at the Hawaiian Islands,” in Seabirds: Feeding Ecology and Role in Marine Ecosystems, ed J. P. Croxall (Cambridge: Cambridge University Press), 305–326.
Hudson, J. M., Steinberg, D. K., Sutton, T. T., Graves, J. E., and Latour, R. J. (2014). Myctophid feeding ecology and carbon transport along the northern Mid-Atlantic Ridge. Deep Sea Res. I 93, 104–116. doi: 10.1016/j.dsr.2014.07.002
Irigoien, X., Klevjer, T. A., Røstad, A., Martinez, U., Boyra, G., Acuña, J. L., et al. (2014). Large mesopelagic fishes biomass and trophic efficiency in the open ocean. Nat. Commun. 5, 1–10. doi: 10.1038/ncomms4271
Isaacs, J. D., and Schwartzlose, R. A. (1965). Migrant sound scatterers: interaction with the sea floor. Science 150, 1810–1813. doi: 10.1126/science.150.3705.1810
Johnston, D. W., McDonald, M., Polovina, J., Domokos, R., Wiggins, S., and Hildebrand, J. (2008). Temporal patterns in the acoustic signals of beaked whales at Cross Seamount. Biol. Lett. 4, 208–211. doi: 10.1098/rsbl.2007.0614
Jorgensen, S. J., Klimley, A. P., Muhlia-Melo, A., and Morgan, S. G. (2016). Seasonal changes in fish assemblage structure at a shallow seamount in the Gulf of California. PeerJ 4:e2357. doi: 10.7717/peerj.2357
Kaartvedt, S., Røstad, A., Klevjer, T., and Staby, A. (2009). Use of bottom-mounted echo sounders in exploring behavior of mesopelagic fishes. Mar. Ecol. Prog. Ser. 395, 109–118. doi: 10.3354/meps08174
Kang, D., Mukai, T., Iida, K., Hwang, D., and Myoung, J. G. (2005). The influence of tilt angle on the acoustic target strength of the Japanese common squid (Todarodes pacificus). ICES J. Mar. Sci. 62, 779–789. doi: 10.1016/j.icesjms.2005.02.002
Kloser, R. J., Ryan, T. E., Young, J. W., and Lewis, M. E. (2009). Acoustic observations of micronekton fish on the scale of an ocean basin: potential and challenges. ICES J. Mar. Sci. 66, 998–1006. doi: 10.1093/icesjms/fsp077
Kloser, R. J., Ryan, T., Sakov, P., Williams, A., and Koslow, J. A. (2002). Species identification in deep water using multiple acoustic frequencies. Can. J. Fish. Aquat. Sci. 59, 1065–1077. doi: 10.1139/f02-076
Koslow, J. A. (1997). Seamounts and the ecology of deep-sea fisheries: the firm-bodied fishes that feed around seamounts are biologically distinct from their deepwater neighbors and may be especially vulnerable to overfishing. Am. Sci. 85, 168–176.
Lavery, A. C., Stanton, T. K., McGehee, D. E., and Chu, D. (2002). Three-dimensional modeling of acoustic backscattering from fluid-like zooplankton. J. Acoust. Soc. Am. 111, 1197–1210. doi: 10.1121/1.1433813
Letessier, T. B., De Grave, S., Boersch-Supan, P. H., Kemp, K. M., Brierley, A. S., and Rogers, A. D. (in press). Seamount influences on mid-water shrimps (Decapoda) gnathophausiids (Lophogastridea) of the South-West Indian Ridge. Deep Sea Res. II. doi: 10.1016/j.dsr2.2015.05.009
Lourenço, N., Miranda, J. M., Luis, J. F., Ribeiro, A., Mendes Victor, L. A., Madeira, J., et al. (1998). Morpho-tectonic analysis of the Azores Volcanic Plateau from a new bathymetric compilation of the area. Mar. Geophys. Res. 20, 141–156. doi: 10.1023/A:1004505401547
Martin, B., and Christiansen, B. (2009). Distribution of zooplankton biomass at three seamounts in the NE Atlantic. Deep Sea Res. II 56, 2671–2682. doi: 10.1016/j.dsr2.2008.12.026
Martin, B., and Nellen, W. (2004). Composition and distribution of zooplankton at the Great Meteor Seamount, subtropical North-east Atlantic. Arch. Fish. Mar. Res. 51, 89–100. Available online at: https://www.researchgate.net/publication/292316515_Composition_and_distribution_of_zooplankton_at_the_Great_Meteor_Seamount_subtropical_North-East_Atlantic
McElroy, P. T. (1974). Geographic patterns in volume-reverberation spectra in the North Atlantic between 33°N and 63°N. J. Acoust. Soc. Am. 56, 394–407. doi: 10.1121/1.1903272
Menezes, G. M., Diogo, H., and Giacomello, E. (2013). Reconstruction of demersal fisheries history on the Condor seamount, Azores archipelago (Northeast Atlantic). Deep Sea Res. II 98, 190–203. doi: 10.1016/j.dsr2.2013.02.031
Moore, H. B. (1950). The relation between the scattering layer and the Euphausiacea. Biol. Bull. 99, 181–212. doi: 10.2307/1538738
Morato, T., Bulman, C., and Pitcher, T. J. (2009). Modelled effects of primary and secondary production enhancement by seamounts on local fish stocks. Deep Sea Res. II 56, 2713–2719. doi: 10.1016/j.dsr2.2008.12.029
Morato, T., Kvile, K. Ø., Taranto, G. H., Tempera, F., Narayanaswamy, B. E., Hebbeln, D., et al. (2013). Seamount physiography and biology in the north-east Atlantic and Mediterranean Sea. Biogeosciences 10, 3039–3054. doi: 10.5194/bg-10-3039-2013
Morato, T., Machete, M., Kitchingman, A., Tempera, F., Lai, S., Menezes, G., et al. (2008a). Abundance and distribution of seamounts in the Azores. Mar. Ecol. Prog. Ser. 357, 17–21. doi: 10.3354/meps07268
Morato, T. M., and Clarke, M. R. (2007). “Seamount fishes: ecology and life histories,” in Seamounts: Ecology, Fisheries and Conservation, eds T. J. Pitcher, T. Morato, P. J. B Hart, M. R. Clark, N. Haggan, and R.S. Santos (Oxford: Blackwell Publishing), 170–188.
Morato, T., Solà, E., Grós, M. P., and Menezes, G. (2001). Feeding habits of two congener species of seabreams, Pagellus bogaraveo and Pagellus acarne, off the Azores (northeastern Atlantic) during spring of 1996 and 1997. Bull. Mar. Sci. 69, 1073–1087. Available online at: http://www.ingentaconnect.com/contentone/umrsmas/bullmar/2001/00000069/00000003/art00002
Morato, T., Varkey, D. A., Damaso, C., Machete, M., Santos, M., Prieto, R., et al. (2008b). Evidence of a seamount effect on aggregating visitors. Mar. Ecol. Prog. Ser. 257, 23–32. doi: 10.3354/meps07269
Pauly, D., Trites, A. W., Capuli, E., and Christensen, V. (1998). Diet composition and trophic levels of marine mammals. ICES J. Mar. Sci. 55, 467–481. doi: 10.1006/jmsc.1997.0280
Peña, M., Olivar, M. P., Balbín, R., López-Jurado, J. L., Iglesias, M., and Miquel, J. (2014). Acoustic detection of mesopelagic fishes in scattering layers of the Balearic Sea (Western Mediterranean). Can. J. Fish. Aquat. Sci. 71, 1186–1197. doi: 10.1139/cjfas-2013-0331
Pitcher, T. J., and Bulman, C. (2007). “Raiding the larder: a quantitative evaluation framework and trophic signature for seamount food webs (Chapter 14),” in Seamounts: Ecology, Fisheries and Conservation, eds T. J. Pitcher, T. Morato, P. J. B Hart, M. R. Clark, N. Haggan, and R.S. Santos (Oxford: Blackwell Publishing), 282–295.
Porteiro, F. M., Giacomello, E., Carmo, V., Graça, G., Cascão, I., Morais, A., et al. (2011). “Mesopelagic fishes around the Condor seamount,” in CONDOR Observatory for Long-Term Study and Monitoring of Azorean Seamount Ecosystems – Final Project Report, eds E. Giacomello and G. Menezes (Horta: Arquivos do DOP, Série Estudos 1/2012), 81–91.
Porteiro, F. M., Gomes-Pereira, J. N., Pham, C. K., Tempera, F., and Santos, R. S. (2013). Distribution and habitat association of benthic fish on the Condor seamount (NE Atlantic, Azores) from in situ observations. Deep Sea Res. II 98, 114–128. doi: 10.1016/j.dsr2.2013.09.015
Porteiro, F. M., and Sutton, T. (2007). “Midwater fish assemblages and seamounts (Chapter 6),” in Seamounts: Ecology, Fisheries and Conservation, eds T. J. Pitcher, T. Morato, P. J. B Hart, M. R. Clark, N. Haggan, and R. S. Santos (Oxford: Blackwell Publishing), 101–116.
Pusch, C., Beckman, A., Porteiro, F. M., and Westernhagen, H. V. (2004). The influence of seamounts on mesopelagic fish communities. Arch. Fish. Mar. Res. 51, 165–186. Available online at: http://epic.awi.de/9197/
Rivoirard, J., Simmonds, J., Foote, K. G., Fernandes, P., and Bez, N. (2000). Geostatistics for Estimating Fish Abundance. Oxford: Blackwell Science.
Santos, M. A., Bolten, A. B., Martins, H. R., Riewald, B., and Bjorndal, K. A. (2007). “Air-breathing visitors to seamounts: Sea turtles (Chapter 12B),” in Seamounts: Ecology, Fisheries and Conservation, eds T. J. Pitcher, T. Morato, P. J. B Hart, M. R. Clark, N. Haggan, and R. S. Santos (Oxford: Blackwell Publishing), 239–244.
Santos, M., Moita, M. T., Bashmachnikov, I., Menezes, G. M., Carmo, V., Loureiro, C. M., et al. (2013). Phytoplankton variability and oceanographic conditions at Condor Seamount, Azores (NE Atlantic). Deep Sea Res. II 98, 52–62. doi: 10.1016/j.dsr2.2013.05.037
Saunders, R. A., Fielding, S., Thorpe, S. E., and Tarling, G. A. (2013). School characteristics of mesopelagic fish at South Georgia. Deep Sea Res. I 81, 62–77. doi: 10.1016/j.dsr.2013.07.007
Silva, M. A., and Cascão, I. (2011). “Investigating the occurrence of cetaceans at Condor seamount using information from visual observations and passive acoustic monitoring,” in CONDOR Observatory for Long-Term Study and Monitoring of Azorean Seamount Ecosystems – Final Project Report, eds E. Giacomello and G. Menezes (Horta: Arquivos do DOP, Série Estudos 1/2012), 183–190.
Silva, M. A., Prieto, R., Jonsen, I., Baumgartner, M. F., and Santos, R. S. (2013). North Atlantic blue and fin whales suspend their spring migration to forage in middle latitudes: building up energy reserves for the journey? PLoS ONE 8:e76507. doi: 10.1371/journal.pone.0076507
Simmonds, J., and MacLennan, D. (2005). Fisheries Acoustics: Theory and Practice, 2nd Edn. Oxford: Blackwell Publishing.
Smailes, I. C. (1976). Observations of volume scattering strength in the Northeast Atlantic. J. Acoust. Soc. Am. 60, 1056–1060. doi: 10.1121/1.381196
Sutton, T. T. (2013). Vertical ecology of the pelagic ocean: classical patterns and new perspectives. J. Fish Biol. 83, 1508–1527. doi: 10.1111/jfb.12263
Tempera, F., Giacomello, E., Mitchell, N. C., Campos, A. S., Braga-Henriques, A., Bashmachnikov, I., et al. (2012). “Mapping Condor seamount seafloor environment and associated biological assemblages (Azores, NE Atlantic),” in Seafloor Geomorphology as Benthic Habitat: Geohab Atlas of Seafloor Geomorphic Features and Benthic Habitats, eds E. Baker and P. Harris (London: Elsevier), 807–818.
Tobeña, M., Prieto, R., Machete, M., and Silva, M. A. (2016). Modeling the potential distribution and richness of cetaceans in the Azores from fisheries observer program data. Front. Mar. Sci. 3:202. doi: 10.3389/fmars.2016.00202
Wade, I. P., and Heywood, K. J. (2001). Acoustic backscatter observations of zooplankton abundance and behaviour and the influence of oceanic fronts in the northeast Atlantic. Deep Sea Res. II 48, 899–924. doi: 10.1016/S0967-0645(00)00113-2
Warren, J. D., Stanton, T. K., Benfield, M. C., Wiebe, P. H., Chu, D., and Sutor, M. (2001). In situ measurements of acoustic target strengths of gas-bearing siphonophores. ICES J. Mar. Sci. 58, 740–749. doi: 10.1006/jmsc.2001.1047
Watanabe, H., Kubodera, T., and Yokawa, K. (2009). Feeding ecology of the swordfish Xiphias gladius in the subtropical region and transition zone of the western North Pacific. Mar. Ecol. Prog. Ser. 396, 111–122. doi: 10.3354/meps08330
Wilson, C. D., and Boehlert, G. W. (2004). Interaction of ocean currents and resident micronekton at a seamount in the Central North Pacific. J. Mar. Syst. 50, 39–60. doi: 10.1016/j.jmarsys.2003.09.013
Keywords: micronekton, acoustic scatterers, seamount, spatial and temporal dynamics, Azores
Citation: Cascão I, Domokos R, Lammers MO, Marques V, Domínguez R, Santos RS and Silva MA (2017) Persistent Enhancement of Micronekton Backscatter at the Summits of Seamounts in the Azores. Front. Mar. Sci. 4:25. doi: 10.3389/fmars.2017.00025
Received: 31 March 2016; Accepted: 23 January 2017;
Published: 07 February 2017.
Edited by:
Ellen Hines, San Francisco State University, USAReviewed by:
Brett W. Molony, Department of Fisheries, AustraliaThor Aleksander Klevjer, Institute of Marine Research, Norway
Copyright © 2017 Cascão, Domokos, Lammers, Marques, Domínguez, Santos and Silva. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Irma Cascão, irma.cascao@gmail.com
 Irma Cascão
Irma Cascão Réka Domokos
Réka Domokos Marc O. Lammers4,5
Marc O. Lammers4,5  Vítor Marques
Vítor Marques Rula Domínguez
Rula Domínguez Ricardo S. Santos
Ricardo S. Santos Mónica A. Silva
Mónica A. Silva