Carbon Bioavailability in a High Arctic Fjord Influenced by Glacial Meltwater, NE Greenland
- 1Department of Biology, University of Bergen, Bergen, Norway
- 2National Institute for Aquatic Resources, Technical University of Denmark, Charlottenlund, Denmark
- 3Marine Biological Section, University of Copenhagen, Helsingør, Denmark
- 4Department of Bioscience, Aarhus University, Roskilde, Denmark
- 5Arctic Research Centre, Aarhus University, Aarhus, Denmark
- 6Climate Research Centre, Greenland Institute of Natural Resources, Nuuk, Greenland
- 7Instituto Andaluz de Ciencias de la Tierra (CSIC-UGR), Armilla, Granada, Spain
- 8Uni Research Environment, Bergen, Norway
The land-to-ocean flux of organic carbon is increasing in glacierized regions in response to increasing temperatures in the Arctic (Hood et al., 2015). In order to understand the response of the coastal ecosystem metabolism to the organic carbon input it is essential to determine the bioavailability of the different carbon sources in the system. We quantified the bacterial turnover of organic carbon in a high Arctic fjord system (Young Sound, NE Greenland) during the ice-free period (July-October 2014) and assessed the quality and quantity of the 3 major organic carbon sources; (1) local phytoplankton production (2) runoff from land-terminating glaciers and a lowland river and (3) inflow from the ocean shelf. We found that despite relatively low concentrations of DOC in the rivers, the bioavailability of the river–DOC was significantly higher than in the fjord, and characterized by high cell-specific bacterial production and low C:N ratios. In contrast, the DOC source entering via inflow of coastal shelf waters had high DOC concentrations with high C:N and low specific bacterial production. The phytoplankton production in the fjord could not sustain the bacterial carbon demand, but was still the major source of organic carbon for bacterial growth. We assessed the bacterial community composition and found that communities were specific for the different water types i.e., the bacterial community of the coastal inflow water could be traced mainly in the subsurface water, while the glacial river community strongly dominated the surface water in the fjord.
Introduction
Carbon consumption and mineralization by pelagic heterotrophic bacteria play a key role in marine ecosystems. Increasing temperatures, with pronounced effects at high latitudes, have raised questions about how reduced ice-cover and increased runoff can affect the ecosystem metabolism i.e., the balance between respiration and primary production. Bacterial carbon turnover was traditionally suggested to be limited by low temperature in high latitude systems, and consequently play only a minor role in turning over primary production (Pomeroy and Deibel, 1986; Pomeroy et al., 1991). Several high latitude studies during the past decade have however reported bacterial production rates similar to those reported in low-latitude non-oligotrophic systems (Børsheim, 2000; Rysgaard and Nielsen, 2006; Sejr et al., 2007). Two seasonal studies found annual ranges in bacterial production of 5–42 mg C m−2 d−1 in Kobbefjord (64°N) (Middelboe et al., 2012) and 90–165 mg C m−2 d−1 in Kongsfjorden, Svalbard (78°N) (Iversen and Seuthe, 2011), with maximum values during spring, coinciding with the spring phytoplankton bloom.
Estimates of bacterial carbon cycling is often based on measurements of net bacterial production, however variability in the factors used to convert radioisotope (e.g., 14C-leucine or 3H-thymidine) incorporation to carbon production affects the growth estimates and potentially complicates comparison of studies. Measurements of the bacterial respiration (BR) are required in order to estimate the bacterial carbon demand (BCD) and growth efficiency (BGE). Such measurements in Arctic systems are few and the BGE reported are highly variable, but all in the low end of those reported in other aquatic systems (del Giorgio and Cole, 1998). It has been hypothesized that low temperatures limit substrate uptake and consequently argued that Arctic bacteria need relatively higher concentrations of carbon to grow at low temperatures (Pomeroy and Wiebe, 2001). Other studies have demonstrated an inverse relationship between BGE and temperature (Rivkin and Legendre, 2001; Apple et al., 2006) leading to speculations that the low efficiency found in the Arctic may instead be a result of poor quality carbon sources (Middelboe et al., 2012). While the concentration of organic matter alone does not reflect the carbon quality (Kirchman et al., 2005), the elemental ratios of the dissolved organic matter (DOM) i.e., the C:N ratio has provided insight on the DOM bioavailability (del Giorgio and Cole, 1998; Pradeep Ram et al., 2003; Kragh and Søndergaard, 2004).
The activity of bacteria is tightly coupled to DOM bioavailability (Amon and Benner, 1996; Kragh and Søndergaard, 2004). Bioavailable dissolved organic carbon (BDOC) has been estimated to constitute on average < 1% of the oceanic DOC pool, however elevated in the surface waters (Hansell, 2013). Studies in the Greenland Sea, Fram Strait, and Kobbefjord have shown that BDOC constitutes 13–36% of total DOC in surface water (Middelboe and Lundsgaard, 2003; Middelboe et al., 2012; Jørgensen et al., 2014). Glacial meltwater from both Alaskan (Hood et al., 2009) and Alpine glaciers (Singer et al., 2012) contained highly bioavailable (>60%) DOC. As the Greenland Ice Sheet is melting at record speed (Nghiem et al., 2012) and the melt is projected to continue increasing (Keegan et al., 2014) it poses the question whether coastal bacterial carbon turnover will increase and drive the fjord systems toward more heterotrophic conditions in the future.
The bioavailability of different DOM types is influenced by a number of factors including composition of substrate, availability of mineral nutrients and the bacterial communities and their enzymatic capabilities (Middelboe and Lundsgaard, 2003; Kritzberg et al., 2010; Traving et al., 2016). Only few studies have tried to directly connect specific bacterial groups to different types of DOM (Kirchman et al., 2007; Baña et al., 2013; Osterholz et al., 2016). Meltwater from glaciers has been found to significantly modify the structure of microbial communities in the connected fjord (Gutiérrez et al., 2015). Consequently, increased runoff associated with warming climate will not only affect the transport of organic matter, it may also change the dynamics of coastal bacterial communities toward a higher influence of riverine bacteria and thus potentially changes in BGE and DOM degradation of the coastal bacterial community (Fortunato et al., 2013). Exploring links between the bacterial community composition and the various DOM sources, are therefore highly relevant in Arctic environments. Young Sound receives most of its runoff from the Greenland Ice Sheet via land terminating glaciers (Citterio et al., 2017), resulting in a clear gradient of allochthonous sources of both organic matter and silt throughout the fjord (Murray et al., 2015). The organic carbon sources in the fjord comprise two allochthonous carbon sources 1) meltwater from land-terminating glaciers and a lowland river and 2) coastal water that contains traces of DOM from the Arctic Ocean (Amon and Budéus, 2003).
Based on previous findings we hypothesize that the glacial runoff in Young Sound contains highly bioavailable DOM compared to the local production and inflowing coastal water. In order to understand the response in ecosystem metabolism we evaluate the importance of each of the 3 carbon sources as substrate for bacterial carbon degradation and examine associations between the bacterial DOM degradation and the genetic diversity.
Materials and Methods
Study Site and Sampling
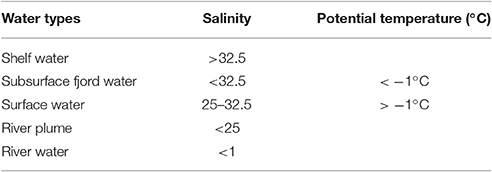
The study was conducted in the high arctic fjord Young Sound, NE Greenland (74.2–74.3 °N, 19.7-21.9 °W). A sill of 45 m depth separates the deeper parts of the fjord from the coastal shelf waters, which are influenced by the East Greenland Current (for more info see Rysgaard et al., 2003). Sampling was conducted at four stations located along a length section from the inner fjord (St. 1) to the shelf waters on the outer side of the sill (St. 4) (Figure 1). The stations 1, 2, 3, and 4 are located according to stations monitored yearly by the Greenland Ecosystem Monitoring (GEM) MarinBasis Zackenberg programme in which they are named Tyro 05, YS 3.18, Standard St. and GH 05, respectively. The fjord stations were each sampled approximately every 10th during the early ice-free period (July 15–August 7) and the late summer period before new ice formation (September 4–October 4) (Figure S1).
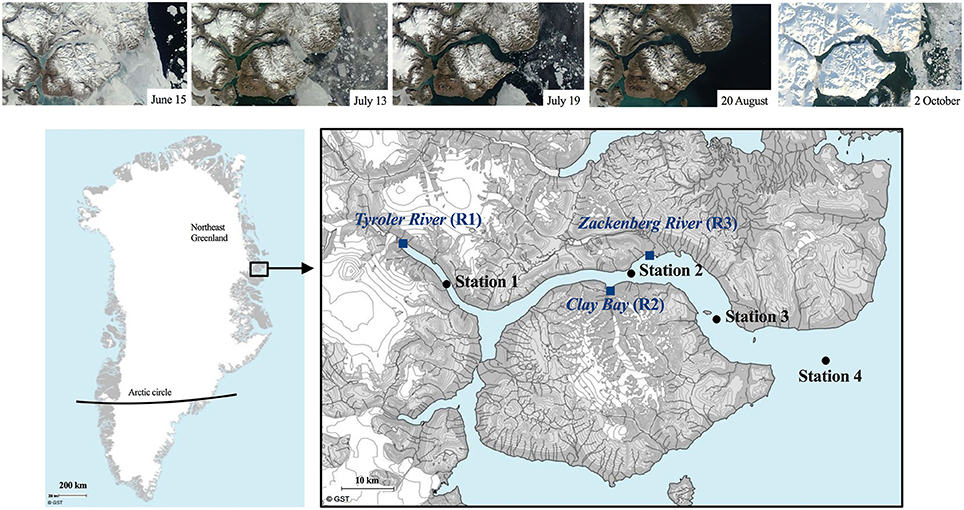
Figure 1. Study area in Northeast Greenland (74 °N, 21 °W) showing the 4 fjord stations (black dots) and the 3 rivers (blue squares). Above are Satellite TERR images from June-August 2014 illustrating the ice and snow conditions as well as the gradient of riverine input (available at http://ocean.dmi.dk/arctic/daneborg.uk.php).
The first sampling was conducted prior to the sea-ice break-up through a hole in the ice at St. 3, when only the central part of the fjord was still ice covered (see satellite photos Figure 1). The ice broke up in the central part on July 15 and the fjord rendered ice-free within 24 h. The remaining sampling was carried out from the research vessel Aage V. Jensen using mini rosette with 12 × 1.7 L Niskin bottles from 6 standard depths (1, 10, 20, 30, 40, and 100 m) and 1–2 additional depths at the deep chlorophyll maximum (DCM) when this did not overlap with one of the standard depths. The DCM was determined prior to every sampling using a Satlantic Free-falling Optical Profiler (Murray et al., 2015). A Seabird SBE 19+ CTD profiler was deployed at every sampling occasion and recorded vertical profiles of temperature (°C), salinity (ratio; no units), chlorophyll fluorescence (fluchl, relative; no units), turbidity (FTU), and photosynthetically active radiation (PAR, μmol m−2 s−1) at every sampling occasion. The light attenuation was estimated from the CTD-profiles using a two-phase Weibull function as described in Murray (2015).
Glaciers cover ca. 33% of the drainage area of the fjord and land-terminating glaciers contribute 50–80% of the annual terrestrial runoff with highest contribution in the inner fjord (Bendtsen et al., 2014; Citterio et al., 2017). Three of the major rivers discharging into the fjord were sampled as long as sufficient water was flowing (last sampling was on September 10). The meltwater in the Tyroler river (R1) and Clay Bay river (R2) flows from glaciers trough rocky sediment basins with close to zero vegetation for a distance of ca. 0.5 and 2 km, respectively, before they reach the fjord. A model study estimate the residence time of river water in the fjord to be about 2 weeks in July and up to a month in August (Bendtsen et al., 2014).
R1 receives water directly from the Greenland Ice Sheet and has the largest catchment area (Bendtsen et al., 2014), while R2 receives meltwater from smaller local glaciers (Figure 1). The largest river, R3 (Zackenberg river) has the second largest catchment area and is connected to 2 lakes. It flows through lowland permafrost soils covered with vegetation types like dwarf shrub heath (Salix arctica) and grasses (e.g., Arctagrostis latifolia), however the riverbed is rocky and without vegetation (Elberling et al., 2008). River water was collected just below surface in 5 L plastic bottles. A 10-year time series of temperature and the organic and inorganic particulate biomass and the dissolved organic carbon (DOC) recorded from the Zackenberg river by the GeoBasis programme by Greenland Ecosystem Monitoring are included in Figure S3.
A total of 25 profiles were sampled at the fjord stations and the rivers were sampled each 3 times. Based on the salinity and temperature (Figure 2) five water types were defined (Table 1). Bacterial abundance, production and chemical parameters (nutrients, DOC, DON, and chl a) were measured in total 174 times each. A total of 42 samples were collected in the rivers and at 1 m and DCM for “extra” analysis of carbon bioavailability, particle associated bacterial production and community composition analysis. These “focus samples” are marked with large symbols in Figure 2. Environmental data associated with these 42 focus samples are given in Table S1. Note no focus samples were collected from the water mass defined as Shelf water.
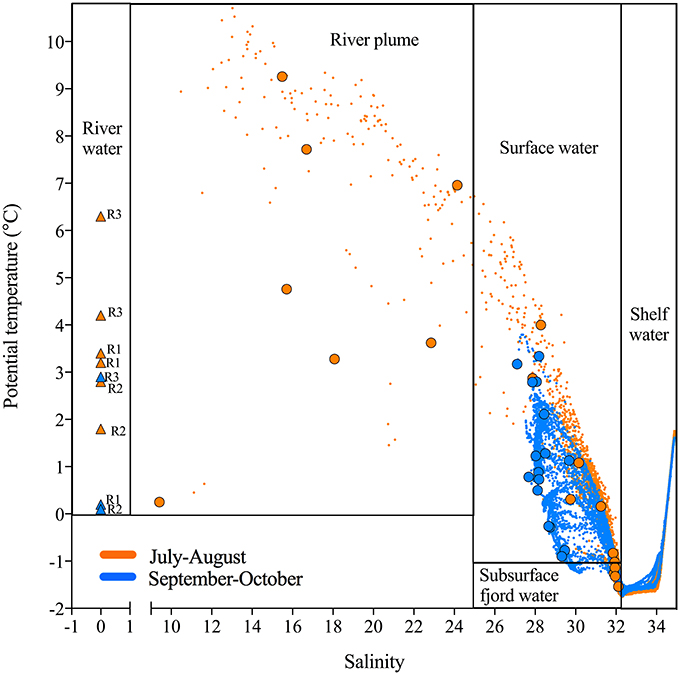
Figure 2. Potential temperature (θ) and salinity plot for the open-water season in Young Sound 2014 with water types divided into five categories following the criteria presented in Table 1 and selected based on salinity and temperature profiles (Figure S1): River water, River plume, Surface water, Subsurface fjord water, and Shelf water. The season is split into two periods: July-August (orange) and September-October (blue). All data points from CTD casts throughout the fjord is shown as small triangles, while larger symbols (circles and triangles) represent the “focus depths” which include measurements of bioavailability, particle associated production and bacterial community composition. The river samples (triangles) are labeled according to origin; Tyroler river (R1), Clay Bay river (R2), and Zackenberg river (R3) and their salinity is set to 0.
Chlorophyll a and Primary Production
Concentrations of chlorophyll a (chl a) were determined according to Jespersen and Christoffersen (1987). Triplicates of 250 mL water was filtered onto GF/F, 2 and 10 μm polycarbonate filters and chl a was extracted in 5 mL 96% ethanol for 12–24 h and analyzed on a Turner Design Fluorometer calibrated against a chl a standard. The measurements were done in triplicates. Primary production (PP) was measured as 14C-uptake (Nielsen, 1952) according to Markager et al. (1999) and measured for 3 size fractions; dissolved (< 0.7 μm), small phytoplankton (0.7–10 μm) and lager phytoplankton (>10 μm). Samples were collected at 1 m depth and at one or two additional depth with a notable DCM (26 samples in total). The areal primary production was calculated according to Lyngsgaard et al. (2014). The daily area production was estimated by integrating over 24 h and with depth. The light intensity at each depth was calculated from the light attenuation and the surface light measured at the nearby Zackenberg research station as part of the GEM Programme.
Flow Cytometry
The abundance of bacteria was determined on an Attune® Acoustic Focusing Flow Cytometer (Applied Biosystems by Life technologies) with a syringe-based fluidic system and a 20 mW 488 nm (blue) laser. Samples were fixed with glutaraldehyde (0.5% final conc.) and kept dark at 4°C until analysis within 12 h. Samples were stained with SYBR Green I (Molecular Probes, Eugene, Oregon, USA) for min 0.5 h at low flow rate of 25 μL min−1 following the protocol of Marie et al. (1999). It should be noted that the bacterial abundance in the rivers was not easily counted by flow cytometry as the inorganic particle signal was high and obscured the counts of free-living bacteria. To reduce the problem the samples were diluted x10 with TE-buffer.
Nutrients
Unfiltered seawater was filled directly from the Niskin bottles into 30 mL acid washed HDPE bottles and stored at −20°C. Nitrite and nitrate (), phosphate () and silicic acid (H4SiO4) were measured on a Smartchem200 (by AMS Alliance) autoanalyser following procedures as outlined in Wood et al. (1967) for , Murphy and Riley (1962) for and Koroleff (1983) for the determination of H4SiO4. Concentration of was determined directly in fresh samples using ortho-phthaladehyde according to Holmes et al. (1999).
Organic Matter Concentration
DOC and DON samples for determining the initial concentration of DOC were collected in 60 mL acid washed HDPE (high-density polyethylene) bottles and stored frozen (−20°C) until analysis. DOC is here considered to equal total organic carbon (TOC) according to (Anderson, 2002). DOC concentrations were determined by high temperature combustion (720°C) using a Shimadzu TOC-V CPH-TN carbon and nitrogen analyser calibrated using a standard series of acetoanilide and the accuracy of the instrument was evaluated using seawater reference material provided by the Hansell CRM (consensus reference material) program. DON was calculated by subtraction of inorganic nitrogen. For particulate organic carbon and nitrogen (POC and PON) a total of 85 samples were collected. 15 L samples from the rivers and 30 L samples from the fjord (collected at 1 m, DCM and 100 m) were filtered onto 47 mm pre-combusted GFF filters using a peristaltic pump. The filters were placed in a desiccator containing concentrated 37% HCl for 12–14 h to remove inorganic carbon. POC and PON was measured using a Carlo Elba NC1500 (Milan, Italy) CHN elemental analyser following the method of Hedges and Stern (1984).
Bacterial Production
Bacterial production was estimated from incorporation of 3H-thymidine (Riemann et al., 1982). From each water sample, four replicates of 10 mL unfiltered seawater samples were transferred to 20 mL plastic vials. One replicate was immediately amended with 500 μL of 100% trichloroacetic acid (TCA) and served as control. Samples were incubated with 10 nM 3H-thymidine (final concentration) for 3–5 h at in situ temperature and stopped by addition of 500 μL 100% TCA. Samples were filtered onto 0.2 μm cellulose-nitrate filters, which were subsequently washed 10 times with ice-cold 5% TCA. Filters were transferred to 6-mL plastic vials and stored at −20°C until analysis. In the laboratory 5 mL of scintillation liquid was added and the radioactivity was counted on a Perkin Elmer Liquid Scintillation Analyzer Tri-Carb 2800TR. The measured thymidine incorporation was converted to cell production assuming 2.0 x1018 cells produced per mole 3H thymidine incorporated (Fuhrman and Azam, 1980). Bacterial population growth rate (GR) was calculated as cell production (cells mL−1 d−1) divided by the cell abundance (cells mL−1). Cell production was converted to bacterial carbon production (BP) assuming 2.0 × 10−14 g C cell−1 (Lee and Fuhrman, 1987). For the calculation of area-integrated bacterial carbon consumption, the bacterial production was depth-integrated across the upper 100 m, and divided by the BGE measured in July (see below). Additionally, at the “focus depths” and in the rivers the particulate bacterial production was measured as the fraction associated with particles larger than 3 μm and calculated as the total BP subtracted the < 3 μm fraction.
Bacterial Respiration and Growth Efficiency (BGE)
Bacterial respiration (BR) was measured as oxygen consumption for ~48 h at constant temperature in water from 1 m and DCM in triplicate 12 mL gas tight Exetainers equipped with an optical sensor. Water was pre-filtered through a 3 μm- polycarbonate filter to reduce bias from eukaryotic cell respiration and grazing. A sample with 20 μL HgCl served as control. Oxygen was measured every 5 min for >24 h using a 4-channel Fiber-Optic Oxygen Meter (FireSting, Pyroscience) using the program Pyro Oxygen Logger Software version 2.37 (PyroScience). Respiration rates were calculated as the decrease in oxygen concentration (μM) over the incubation time after subtracting control values. Conversion from oxygen consumption to carbon respiration was done assuming a respiratory quotient (RQ) of 0.82 (Søndergaard and Middelboe, 1995). BGE was calculated from measurements of net bacterial production (NBP) and bacterial respiration (BR) in the 3 μm-filtered samples as: BGE (%) = BP/ (BP+BR) × 100. BGE was measured at all four stations at 1 m depth and DCM, and from the three rivers, however only BR measurements that fulfilled the three following criteria were used: (1) constant incubation temperature (± 0.1°C) for > 24 h, (2) low abundance (< 5,000 cells mL−1) of small phytoplankton (< 3 μm) (as we saw indications of possible respiration contamination from these), and finally (3) the measured respiration rate should not exceed the total respiration rate of the system (measured by T. Dalsgaard unpublished).
Long Term BDOC Experiments
The quantity of DOC available for bacterial degradation (BDOC) was measured in long term (126–148 d) oxygen consumption experiments. A total of 36 incubations were established from the focus depths and rivers (Table S1). 2 L were 0.22 μm filtered (Millipore® Sterivex) into acid washed HDPE bottles (the filters were later used for extraction of nucleic acids). Note we thus did not measure the bioavailability of the particulate organic matter. The bacterial inoculum was prepared by GF/F filtering 100 mL into 2 × 50 mL falcon tubes. The 0.22 μm-filtered water and bacterial inoculum was stored cold (2°C) until experimental set-up. The oxygen consumption experiment was set up with five replicate 65 mL Winkler glass bottles equipped with an optical oxygen sensor for each sample and incubated in dark at 8°C for 148 days (samples collected in July and August) or 126 days (samples collected September and October). Prior to incubation and (final conc. 5 μM and 1 μM, respectively) were added along with the bacterial inoculum (10% vol.) to ensure that N or P was not limiting C-degradation during incubation. Consequently, these measurements do not reflect in situ conditions neither the bacterial communities at the time of sampling, but are rather quantitative measure of the bioavailable DOC pool. All Winkler bottles contained a magnet to ensure mixing, and were incubated in a water bath in order to minimize oxygen contamination. In addition, parallel bottles containing 100 and 0% air saturated seawater were measured to correct oxygen measurements for deviations in the 100% control. As control incubations, triplicates of sample water were incubated without bacterial inoculum as well as a sample with 20 μL HgCl. The change in % air saturation over time was monitored every 7–14 days using a Fibox 3 fiber optic patch oxygen sensor (Presense) calibrated with 100% and 0% air saturation using the program OxyView–PST3-V6.02. As for the BGE measurements the change in oxygen concentration over time was converted to carbon consumption assuming an RQ of 0.82 (Søndergaard and Middelboe, 1995). DOC concentration was measured initially and by the end of the incubation.
Nucleic Acids Extraction, Amplification, and Amplicon Sequencing
All environmental samples for molecular analysis were collected by filtering water onto 0.22 μm pore size Millipore® Sterivex filters (the filtrate was used for bioavailability measurements described above). Note that samples were not prefiltered i.e., also the particle associated bacteria are included in this analysis. The filters were immediately frozen and stored at –80°C until nucleic acid extraction. DNA and RNA were extracted simultaneously using the AllPrep DNA/RNA Mini Kit (Qiagen, Hilden, Germany) according to manufacturer's instructions with modifications for extraction from Sterivex filters as in Paulsen et al. (2016). RNA was subsequently treated with the DNA-free DNA Removal kit (Invitrogen, CA, USA) and reverse transcribed using the SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen). Amplification of cDNA and DNA was performed using a two-step nested PCR approach with primers 519F (CAGCMGCCGCGGTAA; Øvreås et al. (1997) and 806R (GGACTACHVGGGTWTCTAAT; Caporaso et al. (2011) targeting the bacterial (and Archaeal) 16S rRNA gene V4 hypervariable region. For the first PCR step triplicate samples were amplified in reaction volumes of 20 μL including 10 ng DNA or cDNA, 10 μL HotStarTaq Master Mix (Qiagen), 500 nM of each primer and nuclease free water. PCR cycles consisted of an initial denaturation of 15 min at 95°C, followed by 25 cycles of 95°C for 20 s, 55°C for 30 s and 72°C for 30 s and a final extension step of 72°C for 7 min. Triplicate PCR products were pooled, purified using the DNA Clean & Concentrator-5 kit (Zymo Research Corporation, CA, USA) and quantified using the Qubit 3.0 Fluorometer. For the second PCR step, 10 ng of pooled PCR product was used in a reaction mixture containing 25 μL HotStarTaq Master Mix, 500 nM of each nested primer with an unique eight-nucleotide barcode (total of 96 combinations) and nuclease-free water to bring the mixture to the total volume of 50 μL. Thermal cycles had an initial denaturation for 15 min at 95°C, followed by 15 cycles at 95°C for 20 s, 62°C for 30 s, 72°C for 30 s, and a final extension step of 72°C for 7 min. PCR products were purified using Agencourt AMPure XP Beads (Beckman Coulter Inc., CA, USA) and prepared for sequencing by pooling the amplicons in equimolar amounts. The quality and concentration of the amplicon pool were assessed by agarose gel electrophoresis and by using a Qubit 3.0 Fluorometer, before sending to the Norwegian Sequencing Centre (Oslo, Norway) for High-Throughput Sequencing on a MiSeq platform (Illumina, CA, USA) using the MiSeq Reagent Kit v2 (Illumina). Sequencing data is available at “The European Bioinformatics Institute” under study accession number PRJEB16067 (http://www.ebi.ac.uk).
16S rRNA Gene Sequence Analysis
Paired-end sequences were processed using different bioinformatic tools incorporated on a qiime-processing platform (Caporaso et al., 2010). FASTQ files were quality end-trimmed at a phred quality score ≥24 using Trimmomatic (Bolger et al., 2014) and merged using PANDAseq (Masella et al., 2012), while all reads < 200 bp were removed. Prokaryotic OTUs were selected at a sequence similarity threshold of 97% using a de novo uclust (Edgar, 2010) OTU clustering method with default parameters and taxonomy assigned using the Silva 111 reference database (Quast et al., 2013). OTUs with a taxonomic identification were assembled to an OTU table providing abundances for each sample excluding singletons and rarefied to the number of sequences of the smallest samples (5,000 sequences). A total of 4,096,371 sequences were retrieved from the Illumina sequencing of the 16S rRNA gene V4 hypervariable region from total RNA across 52 samples. After removal of singletons, unassigned OTUs and chloroplast reads, sequences were rarefied to 5,000 reads per sample, with a total of 15,922 unique OTUs at 97% sequence identity. Multivariate statistical analysis was performed on basis of the rarefied OTU matrix to explain variations in the data and test for multivariate environmental correlation with the prokaryotic community structure. Bray–Curtis resemblance, ANOSIM, principal component analysis and redundancy analysis were calculated using primer-e version 6 (Plymouth, UK) and Canoco 5 (Ter Braak and Šmilauer, 2012).
Source Tracker Analysis
To illustrate the spread of bacterial communities in the fjord the SourceTracker 0.9.5 software (Knights et al., 2011) was applied in QIIME. It is designed to track the relative contribution of predefined microbial sources in sink samples using a Bayesian approach, as done in Storesund et al., in review. The relative abundance of 16S rRNA genes of all focus samples was used as input data. The OTU table, comprising all OTUs with a taxonomic identification (excluding singletons and chloroplast reads) was rarefied to 1,000 sequences and filtered to only include OTUs that were abundant in more than 3 samples. The rivers (R1, R2 and R3) and as a proxy for inflowing coastal water St. 4 DCM samples were used as “source populations.” All remaining stations during the sampling period from July until October were defined as the “sink samples.” The result is given as % likely origin from the 4 defined sources. The remaining are categorized as “unknown source”. The result were visualized as fjord transects with weighted-average extrapolation between points using Ocean Data View (Schlitzer, 2016).
Results
Hydrography
During the first period (July-August) the fjord was stratified by a strong halocline at 5–6 m depth with low salinity (< 20) in the surface and more saline bottom water (>30). The stratification was strongest in the inner fjord and the salinity of the surface water increased eastwards toward the shelf (Figure S1). The later period (September-October) was in general colder (surface water < 2°C) and frequent storms mixed the upper layer, which deepened to 30 m at the two inner stations and down to 50 and 80 m at St. 3 and 4, respectively (Figure S1). Five water types were defined for this study based on the thermohaline properties (Table 1 and Figure 2). This was done to facilitate the interpretation of the results as the dominant carbon sources can be expected to differ across these water types. The “Shelf water” represented the mixing of waters from the East Greenland Current (T~1.5°C, S < 34) with warmer and more saline Atlantic water (S > 34.4). “Surface water” is influenced by runoff and the seasonal surface heating, while “Subsurface fjord” water represents the shelf water that has entered the fjord passing over the sill and gradually being mixed with the “Surface water.” We defined the “River plume waters” to be the fjord surface waters that were under direct influence of the river discharge. The change between the two periods is apparent in the T-S plot (Figure 2). The runoff was strongest in July and during the last river sampling on September 10 the flow from the glacial rivers (R1 and R2) had almost terminated, while Zackenberg river (R3) was still flowing until end-September. The inner stations were strongly affected by the river silt, with a high turbidity of surface water and the photic zone was therefore initially shallow at St. 1 and 2 (< 5 and 10 m, respectively), however later in September it deepened to 25–35 m as turbidity decreased. The opposite trend was observed at the outermost station where the photic depth decreased from 35 to 10 m from July to October, due to the decreasing irradiation.
Bacterial Abundance and Chlorophyll a
While the central fjord was still ice-covered (Figure 1), the inner part was ice-free and a reduction in nutrients measured below the ice at St. 3; , , SiO4, in the surface (0.01, 0.33, 1 μM) compared to 100 m values (3.2, 0.7, 6.4 μM) indicated that a phytoplankton spring bloom had already initiated in the inner fjord prior to current sampling program. Chl a was highest (up to 3.1 μg L−1) in the outer region of the fjord in July and August with a deep maximum at 20–40 m (Figure 3D), while in the late period chl a was highest in the inner fjord (Figure 3A). The average bacterial abundance (BA) in the surface water was 3.2 ± 1.0 × 105 mL−1 and generally peaked at 0–20 m in the first period, with a maximum abundance in the outer fjord. BA was significantly higher in the upper 20 m for 19 out of 25 profiles, thus the BA maxima were decoupled from the chl a, especially in the first period where the chl a max was deep (20–40 m) (Figure 3). Overall only a weak significant linear correlation was found between BA and chl a (r2 = 0.03, p = 0.03, n = 164). At the innermost station BA did not correlate with chl a at any time, but there was a positive correlation between BA and turbidity (r2 = 0.757, p < 0.01, n = 31). At the outermost station chl a correlated with BA during the study (r2 = 0.44, p < 0.01). At the two mid-fjord stations (2 and 3), correlation between BA and chl a was found only after the runoff had ceased (St. 2: r2 = 0.83, p < 0.01 and St. 3: r2 = 0.64, p < 0.01). BA in the rivers was significantly lower than in the fjord surface waters (Table 3). Abundances ranged from a minimum of 1.3 × 105 in the glacial rivers (R1 and R2) to 6.4 × 105 cells mL−1 in R3 (Table 2). Note that only the free-living bacteria were enumerated.
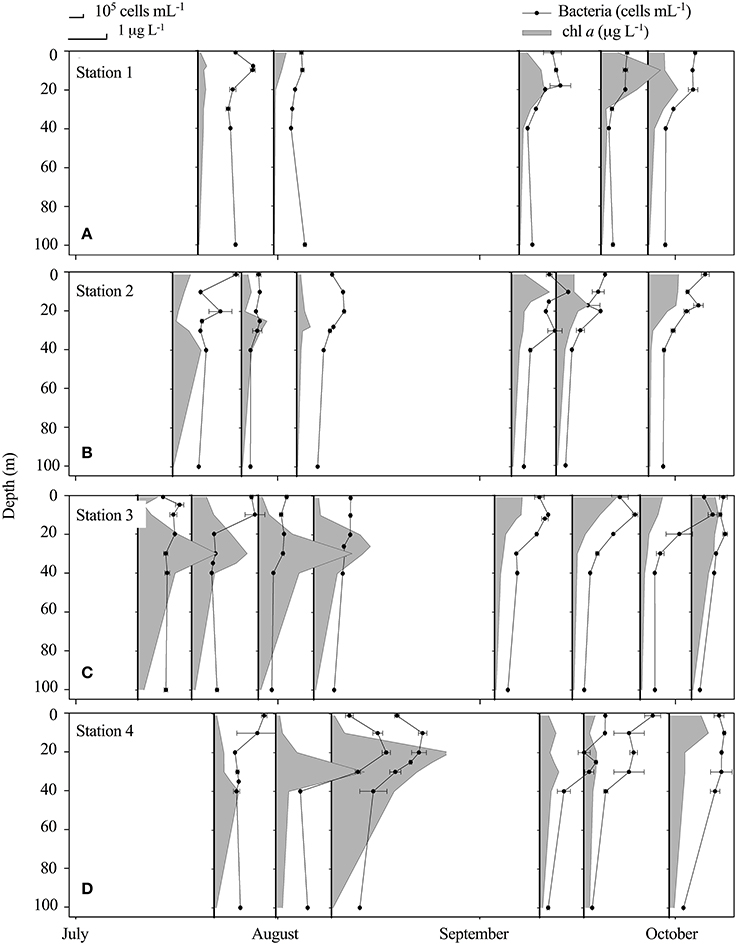
Figure 3. Vertical distribution (upper 100 m) at Station 1 (A), Station 2 (B), Station 3 (C) and Station 4 (D) of bacteria (105 cells mL−1 ± SD) (black dots) and chl a (gray area) (μg chl a L−1 ± SD) at the fjord stations from July to October.
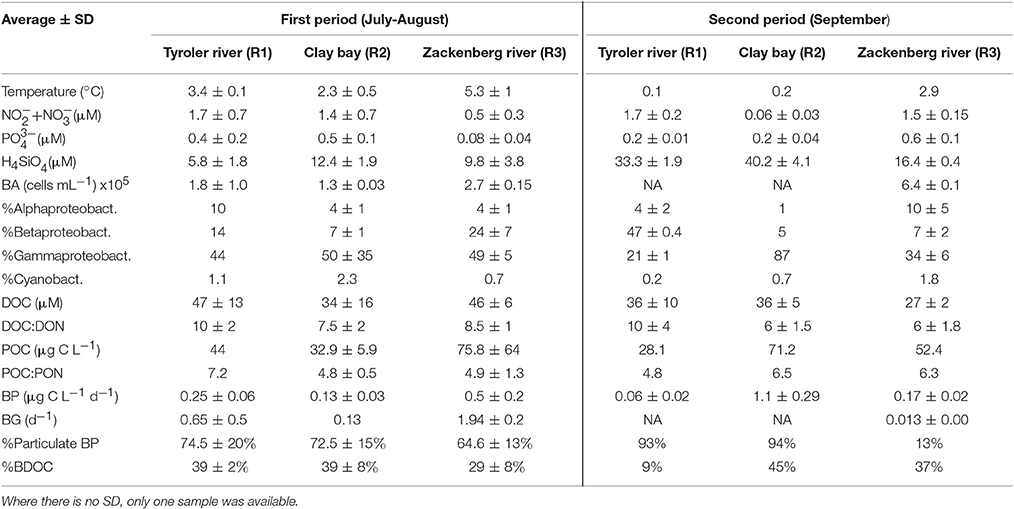
Table 2. River properties presented as average ± SD (n varies from 3 to 6) for each of the two time periods.
Bacterial Production and Particle Association
Bacterial production (BP) was highest in the beginning of the sampling period (17–21 July) with a maximum of 2 μg C L−1 d−1 in the river plume water (St.1 + 2, 1 m) (Table 2, Figures 4A,B). The third highest measure of BP (1.7 μg C L−1 d−1) was found in the fresh water layer just below the ice sampled on July 11. BP was lowest within the Shelf water at St. 4 where it remained below 0.09 μg C L−1 d−1 throughout the entire study period, despite BA being relatively high. BP in the rivers was on average 0.39 ± 0.07 μg C L−1 d−1 and 0.32 ± 0.06 μg C L−1 d−1 in the first and the second period, respectively, and thus generally higher than those measured in the fjord despite low BA (Table 3). We found a larger fraction of BP in the R3 to be free-living (Table 2), which is possibly due to the presence of lakes acting as sedimentation basins. The contribution of particle-associated (>3 μm) bacterial production was considerable in both the fjord and rivers. In 17 out of 25 cases particle association was higher in the DCM sample than in the associated 1 m sample, however there was no significant difference between the %particle associations at the two depths (Figure 4).
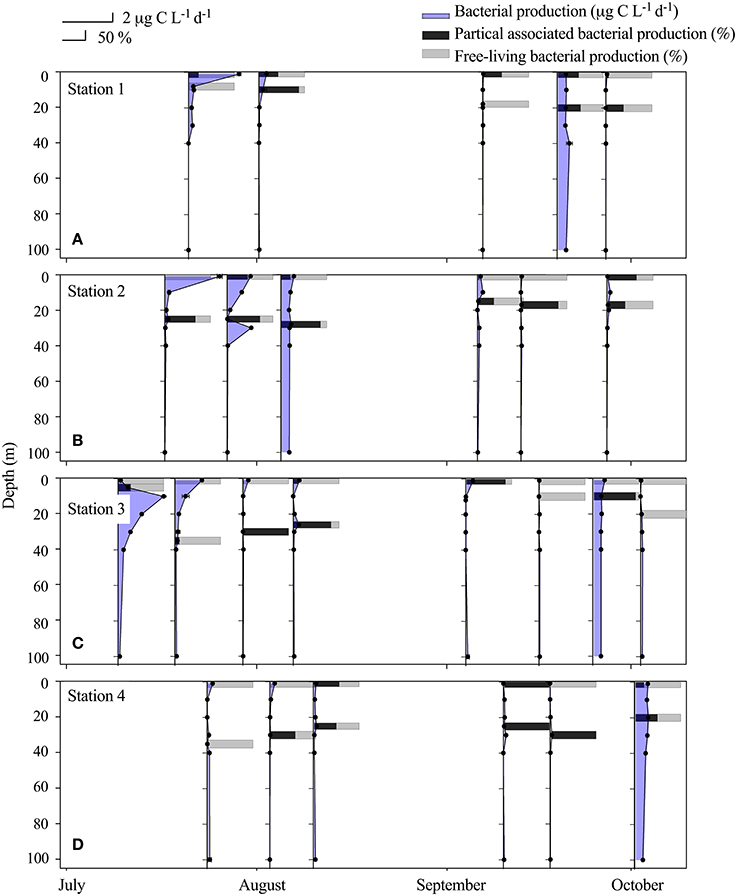
Figure 4. Vertical distribution (upper 100 m) at Station 1 (A), Station 2 (B), Station 3 (C) and Station 4 (D) of bacterial production ± SD, n = 3 (μg C L−1 d−1). The horizontal bars indicate the relative distribution between particle-associated bacterial production and free-living production (%) at 1 m and at the deep chlorophyll max (DCM) from July to October at the 4 stations.
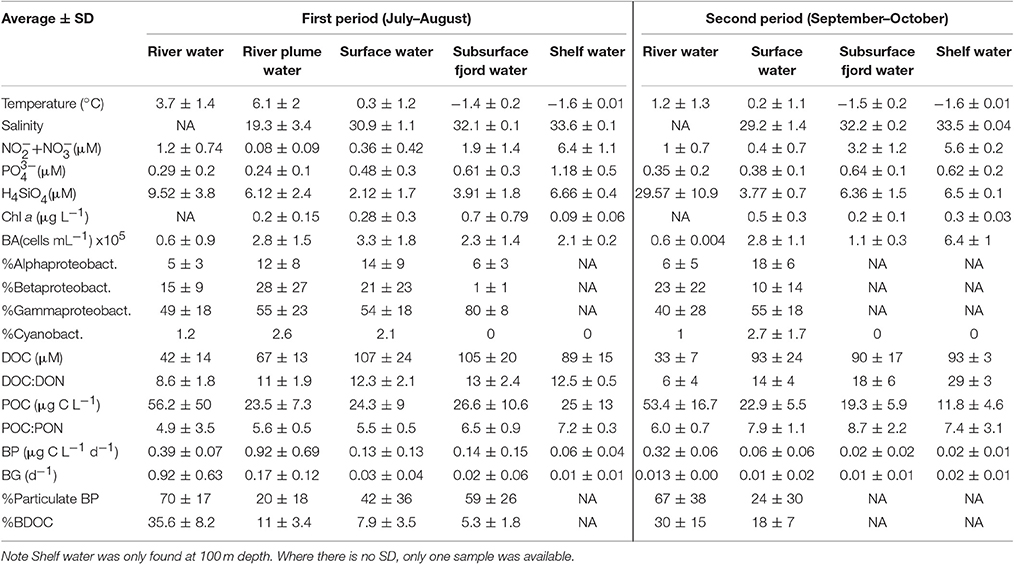
Table 3. Water type characteristics and properties shown as average ± SD of the 5 water types for each of the two periods.
Bacterial Carbon Demand and Primary Production
Only two of the BR measurements fulfilled all criteria for solid measurements of oxygen consumption throughout the incubation. These gave growth efficiency values of 7.3 ± 1.0 and 6.4 ± 2.0%, during incubation at the in situ temperature of −1.1°C (St. 3, July) and at 3°C (St. 4), respectively. The average BGE of 6.9% was applied for determination of bacterial carbon demand (BCD) to allow comparison with the total amount of carbon fixed by planktonic primary production (PP). When integrated over the photic zone the estimated BCD:PP was on average 1.7 ± 1.2 across the sampling period, suggesting that bacterial carbon demand could not be sustained by the local phytoplankton production. There were no clear spatial or temporal trends in the BCD:PP ratio (Figure 5), and estimates were similar when integrated to 100 m. PP was highest initially and could support a high BCD at St. 1 and 3, while bacteria were not sustained by fresh PP initially at St. 2 and 4 in this period. Toward the end of the open water period different patterns developed as bacteria in the inner fjord could be sustained by fresh PP, while BCD was decoupled (up to 5 times higher) from PP at the outermost station (Figure 5). The dissolved fraction (< 0.7 μm) of PP was in general high in the fjord and contributed 39–52% in the first period and less, 27-36%, in the late period. The largest fraction contributed most at the outer station (max 32%) and in the last period it was never higher than 13%, whereas the fraction 0.7–10 μm became dominant in the late period (Figure 5).
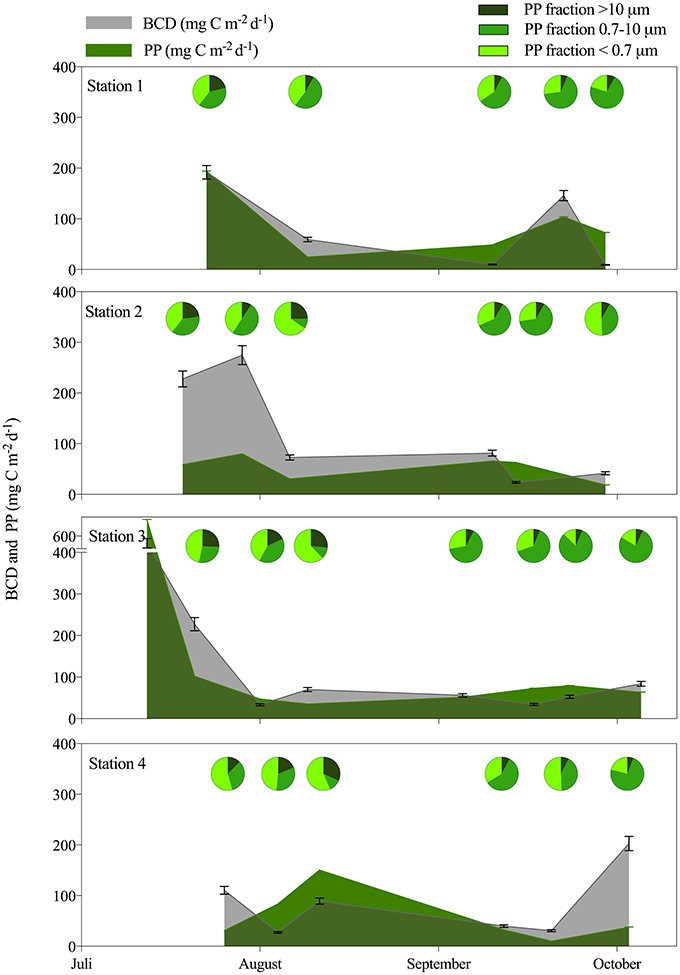
Figure 5. Colored areas show the bacterial carbon demand (BCD) in gray and primary production (PP) in green both integrated over the photic zone (mg C m−2 d−1) for each of the 4 stations from July to October. The pie diagrams illustrate the contribution of different size fractions of PP averaged for the 1 m and DCM sample.
Organic Matter Concentrations and C:N Ratios
DOC concentrations showed little systematic vertical variability except for the surface samples at 1 m, which had significantly lower concentrations in 19 out of 25 profiles (Figure S2). On average, the DOC concentration decreased from the outer part to the inner fjord from 130 ± 16 μM at St. 4 to 106 ± 30 μM at St. 1 (Figure 6). From the first to the second sampling period where the mixed layer deepened, the DOC concentration at 1 m increased, while at the DCM there was a slight decrease. The DOC concentration was highest in the deep water types characterized as Subsurface fjord water (101 ± 20 μM, n = 49) and slightly lower in the Surface water (97 ± 27 μM, n = 97). The River water had significantly lower DOC (40 ± 13 μM, n = 24), and thus the River plume water was diluted to an averaged concentration of 67 ± 13 μM, n = 7. DOC:DON was also lowest in the River water (8 ± 2) and the River plume water (11 ± 2), while the Shelf water had a significantly higher C:N ratio (21 ± 8) (Table 3).
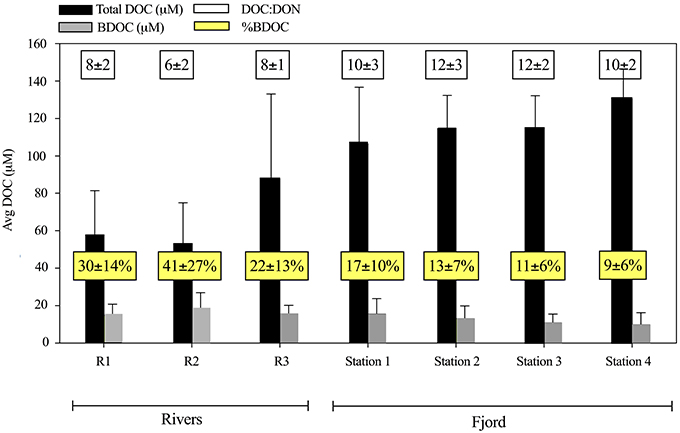
Figure 6. The concentration of dissolved organic matter (DOC) (black) and the bioavailable dissolved organic matter (BDOC) (gray) in μM ± SD for the three rivers (n = 3) and the 4 fjord stations average including both 1 m and DCM (n = 8–10). White boxes present the averaged DOC:DON ratio of the organic matter. Yellow boxes present the BDOC percentage of the total DOC (%BDOC).
Maximum POC concentration was found in the rivers with up to 14 μM, while in the fjord a maximum of 4 μM POC was found at the DCM. In the fjord the particulate fraction (%) of the total organic matter was in general minor (avg. 2.3 ± 1, max = 5%), while in the rivers the contribution of POC was significant (avg. 13 ± 8, max = 35%) (Tables 2, 3). This gave a significant negative relationship between salinity and POC (r2 = 0.3, p < 0.001, n = 86). POC did not correlate with chl a, turbidity or to particulate bacterial production. The C:N ratio of the particulate matter was significantly lower in the rivers than in the fjord (p = 0.047, F-test). In the fjord, the C:N ratio increased from the first period to the end of the open water season (p = 0.0023, F-test), whereas no trend was observed in the rivers.
Inorganic nitrogen () was the limiting inorganic nutrient for primary production and was reduced to 0.4 μM in the surface water during the entire period, while the background level in the Shelf water was ca. 6 μM (Table 3, Figure S2). Ammonium () was only measured at St. 3 and ranged between 0.05 and 0.4 μM, with a maximum at 40 m (below DCM).
Bioavailability of DOM
Despite the increase in average DOC concentration from St. 1 to 4, there was a slight decrease in the concentration of BDOC from 19 ± 10 to 11 ± 8 μM from St. 1 and 4, respectively (when values were averaged for 1 m and DCM over the entire period). The fraction of BDOC relative to total DOC (%BDOC) thus decreased from the inner to the outer part of the fjord (Figure 6). BDOC concentrations in the rivers were relatively high (avg. 18 ± 8 μM), resulting in significantly higher %BDOC in the rivers than in the fjord (p < 0.005, t-test) (Figure 6, Table 3). Averaging %BDOC within each water type revealed a decrease as river water was mixed with the fjord water, i.e., River water 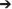 River plume
River plume 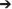 Surface water
Surface water 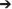 Subsurface water (Table 3). There was a significant negative correlation between the relative abundance of C to N (DOC:DON) and %BDOC (r2 = 0.17, p = 0.08, n = 42). C:N ratios were generally higher in the last period and the highest C:N of 29 ± 3 was found in the Shelf water.
Subsurface water (Table 3). There was a significant negative correlation between the relative abundance of C to N (DOC:DON) and %BDOC (r2 = 0.17, p = 0.08, n = 42). C:N ratios were generally higher in the last period and the highest C:N of 29 ± 3 was found in the Shelf water.
Bacterial Community Composition
In general, Proteobacteria were the most abundant bacteria phylum in both fjord and river samples (~86% of the bacterial phylum). Differences in community composition were observed at class level, with fjord DCM samples containing more Alphaproteobacteria than the rivers and the Subsurface water being strongly dominated by Gammaproteopbacteria (80%), opposed to surface samples and rivers samples with a higher share of Betaproteobacteria (Table 3). Cyanobacteria were present in all rivers and constituted up to 2.7 ± 1.7% of the microbial community in the innermost stations. Alpha diversity (described by the Shannon index) ranged from 4.9 to 9 (Table S2) indicating a greater diversity in the rivers and lowest in fjord samples from July and August (Figure 7). A redundancy analysis (including spatial and temporal parameters) was performed to identify factors that significantly affect bacterial community composition. This explained 47.2% of the total variation in the OTU data, with “water type” (19.5%; river (13%), p = 0.002) and time of sampling expressed as “month” (10.8%; September (8.5%), p = 0.002) being the most significant variables. As “station” generally explained < 5% this variable was not included. Samples from all three rivers with overall higher species richness clustered together. The majority of the September fjord samples clustered tightly together, independent of their respective water types and differences between surface and DCM were minimal. Samples from July and August however clustered according to sample depth (i.e., surface or DCM) (Figure 7). Explanatory variables (water type, month and station) explain in total less than 50% of the variation in diversity for the entire data set.
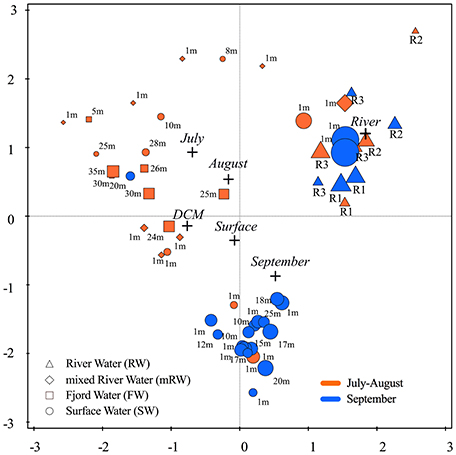
Figure 7. Redundancy analysis biplot summarizing the variation of bacterial diversity based on sequenced 16S rRNA gene fragments at RNA level plotted in relation to nonnumeric explanatory variables (black cross symbol): (1) the month of sampling (July, August, September) and (2) sample origin [River, Surface, and deep chlorophyll max (DCM)]. The size of the symbols relates to the sample richness at family level. The water type of each sample is given by symbols and the depth of sample collection is added as caption and the period of sampling is indicated by color; the early (orange) and the later (blue) open water period.
To elucidate changes in community composition throughout the fjord the complexity of the dataset was reduced by constraining a phylogenetic analysis to only include the most abundant bacterial taxa (relative abundance >1%). Further the temporal factor was reduced by restricting the analysis to cover a 10-day period in early August (Figure 8). The heat map shows that all three rivers were very different from the fjord community, and that each river had unique taxa as the most abundant (blue = high, red = low relative abundance). While bacteria found in R1 (with the closest connection to the Greenland Ice Sheet) all could be found in the two other rivers, the R3 (that runs through vegetation-covered catchment area) included some unique families e.g., Granulosicoccaceae, Alcaligenaceae, and an unknown family from the order vadinha64 (the latter presented as “uncultured_bacterium” from the class Opitutae in Figure 8). In early August the majority of the most abundant river taxa were absent in the fjord samples, with the exception of R1 that shared a great number of its most abundant taxa with the nearby Station 1 surface sample which had more unique taxa than any other station (Figures 1, 8). Stations 2, 3, and 4 showed a higher number of shared taxa indicating a gradient from the inner to the outer fjord. The opposite was observed for the deeper DCM samples, which showed a gradual decrease of certain taxa from the outer St. 4 toward the innermost St. 1.
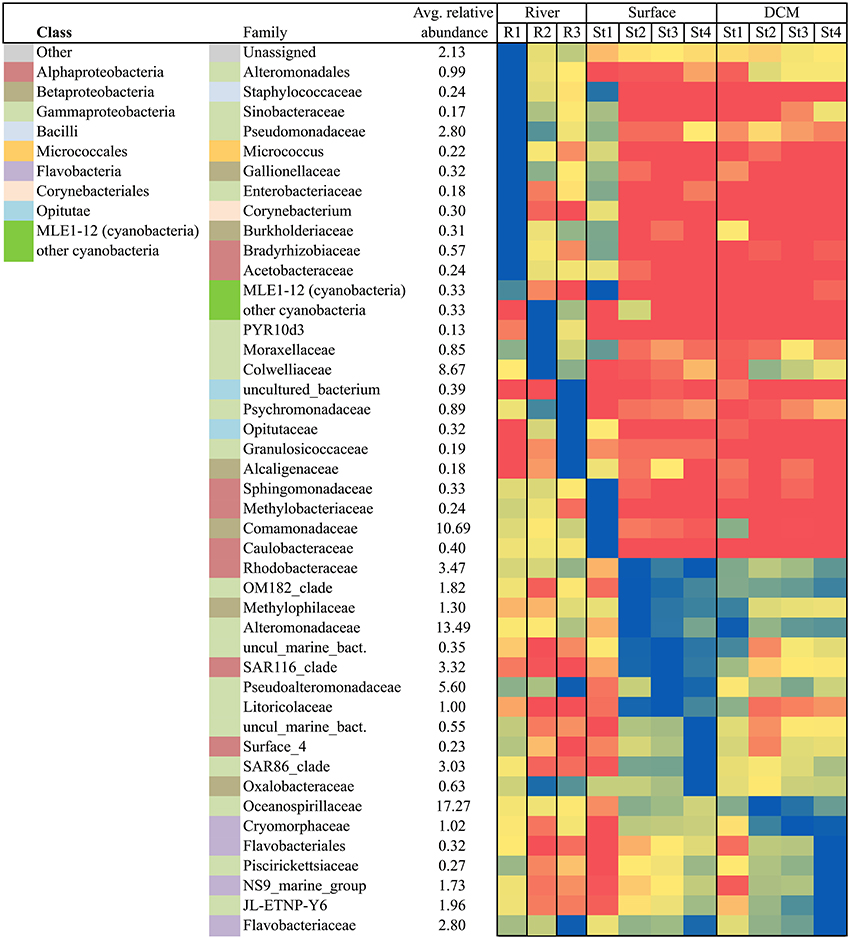
Figure 8. Heat map displaying the highly represented (relative abundance>1%) bacterial taxa at family level (the class level is indicated from the left hand color-code) across each of the 3 rivers and the 4 fjord stations (1 m and DCM) all sampled from August 1 to 10. The highest abundance of each of the 45 bacterial families (including cyanobacteria) was used as respective maximum and the remaining samples are given as a percentage relative to the maximum. The darkest blue illustrates maximum, yellow medium, and red lowest abundances.
The SourceTracker analysis (including the full dataset at OTU level) showed clear contribution of especially the R1 community to the surface fjord stations with maxima of 75, 63, and 66% similarity at St. 1, 3, and 4, respectively in July-August and ca. 40% in September (Figure 9). The coastal community (source set as St. 4 DCM) entering the outer part of the fjord dominated strongly in the deeper fjord samples throughout the entire study (Figure 9 and Table S1). However, the coastal communities also dominated at the surface waters of St. 2 in the first period, where R1 only contributed 15%. As the R1 community was also present in R2 and R3, overall river contribution is incorporated in the R1 plot (Figures 9A,B). The community unique to R2 and R3 only contributed to minor degree (7–15%) to the fjord surface community.
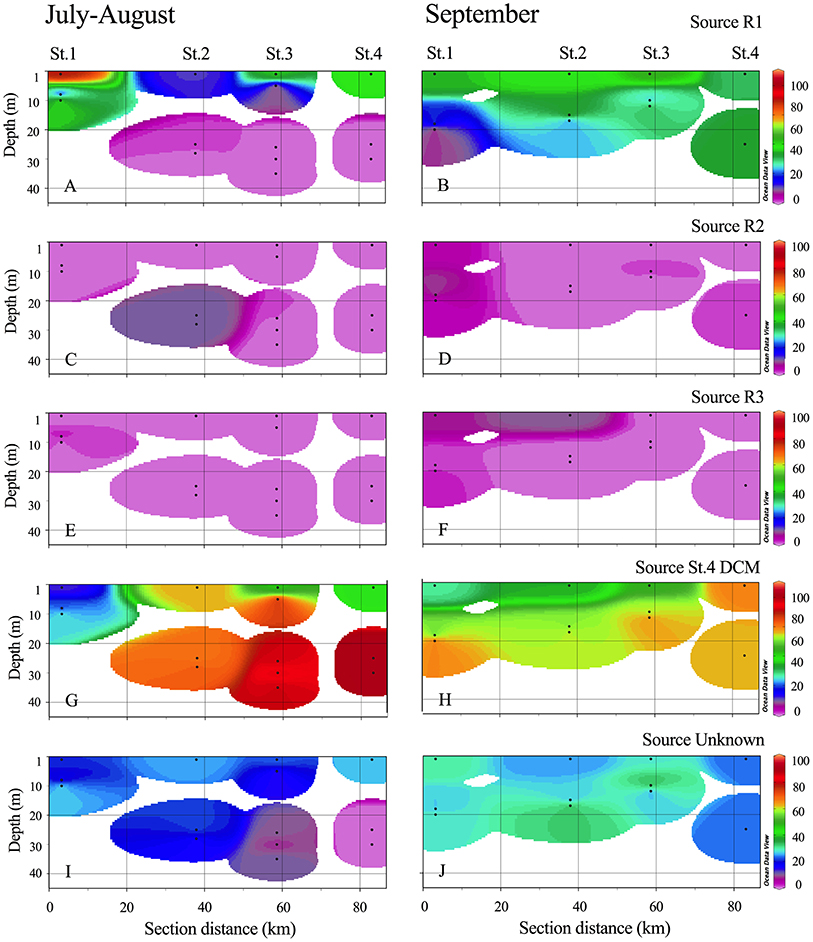
Figure 9. The likely contribution (%) of each defined source bacterial populations from R1 (A–B), R2 (C–D), R3 (E–F), St.4 DCM (G–H) and when a source could not be identified (I–J) in the fjord (upper 45 m) is plotted for the two periods using SourceTracker analysis. Transects are plotted using weighted-average extrapolation between points using Ocean Data View (Schlitzer, 2016).
In order to investigate the influence of environmental parameters exerted on the bacterial community structure, we performed canonical correspondence analysis (CCA) between numerical factors, such as bacterial production, chl a fluorescence, temperature, salinity, DOC, and %BDOC, (Figure S2). Due to the complexity of the dataset no strong correlations were found with this analysis. However, when single taxa were correlated to specific environmental parameters we found that especially genera from the class Gammaproteobacteria showed correlations with specific DOM characteristics e.g., a strong positive correlation was found between the genus Glaciecola (order: Alteromonadales) and bacterial production (r2 = 0.5283; p < 0.0001) with a maximum relative abundance of 25% when BP was highest. Another unknown genus from the order Alteromonadales showed a positive correlation with %BDOC (r2 = 0.2720; p < 0.0075).
Discussion
Bioavailability of Allochthonous DOM Sources
River input in marine systems are often a source of high DOM concentrations and therefore DOM often correlate negatively to salinity (Cauwet, 2002). The Young Sound system however deviates from this trend due to two factors. Firstly the freshwater input in fjord has low DOC concentrations due to the dominance of glacial meltwater and limited catchment vegetation. Secondly, we hypothesize that the coastal shelf waters entering the fjord are characterized by high levels of terrestrial organic matter that originates predominantly from Siberian rivers (we did however not sample sufficiently deep at St. 4 to capture the pure Polar water). These rivers discharge into the Arctic Ocean (Amon and Budéus, 2003) and the terrestrial DOC is retained in the surface waters exiting the Arctic Ocean via the Fram Strait as part of the East Greenland Current (Granskog et al., 2012). A large fraction of the terrestrially derived DOM transported by the major rivers Ob and Yenisei is found to be refractory (Meon and Amon, 2004), which explain the low bacterial activity and low DOC bioavailability we found in the Shelf water despite high concentrations. These conditions therefore result in a positive correlation between DOC and salinity (r = 0.51, p < 0.001, df = 175) in Young Sound.
In addition to the quantitative difference, the qualitative C:N ratio of the two allochthonous DOM sources differ. The ratio between bioavailable DOC and inorganic nitrogen exceeded the Redfield ratio (C:N:P = 106:16:1) by thousand fold, whereas the BDOC: ratio was 45 ± 30. This emphasizes the importance of DON as the main source of nitrogen for bacteria. The low C:N ratios in the river water resemble those reported from Alaskan glacial rivers, where the relatively high source of DON is explained by microbial production of protein-rich DOM in the subglacial environment (Hood and Scott, 2008). The concentration of bioavailable DOM in the rivers entering Young Sound was slightly lower than values obtained in Alaskan glacier outflow (Hood et al., 2009), but very similar to other studies from the Greenland ice sheet meltwater (Lawson et al., 2014). In contrast to the river DOC, the allochthonous DOC entering the fjord from the open ocean had high C:N ratios, similar to Arctic surface water (Benner et al., 2005). Together, our results demonstrate that open ocean DOC is less labile than the DOC produced in the fjord and that supplied from the rivers.
As expected the load of particulate organic carbon was relatively high in the rivers (Hood et al., 2015). However a high POC-signal was not traceable in the surface water of the inner fjord stations as was the case e.g., for the silt particles (measured as turbidity) and silicate. This indicates that the POC has been lost from water column either by sedimentation, dissolution or bacterial degradation. Bioavailability of POC was not determined, and BDOC values thus potentially represent an underestimation of labile organic carbon concentration. However, since POC on average accounted for 2.3 ± 1.0% and 13 ± 8% of total DOC in the fjord and rivers, respectively, the contribution of bioavailable POC to total bioavailable carbon is probably relatively small. The spatial gradient in %BDOC along the fjord transect suggests a gradual consumption of the labile DOC entering the fjord via the rivers. Consumption of riverine DOC in the fjord is supported by the high BP in the river and river plume water (Table 3) and the negative correlation of BP to salinity. The differences in DOM concentration and composition has been suggested as a driver for diversification of bacterial communities (Crump and Hobbie, 2005; Blanchet et al., 2016; Roiha et al., 2016). Our study suggests that the labile character of the river-DOM may have a role stimulating and shaping the activity and structure of bacterial communities in the fjord, by favoring fast growing bacteria. In samples where the community structure was analyzed, high BP was negatively correlated to salinity (r2 = 0.2743; p < 0.0103). Further, high bacterial production was associated with growth of specific taxa, such as a positive correlation between BP and the relative abundance of the genus Glaciecola.
Bacterial Community Composition
In general, our results suggest that the bacterial community is largely structured by the different water sources to the fjord and changes along the salinity gradient, as also found in other coastal environments (del Giorgio and Bouvier, 2002; Crump and Hobbie, 2005; Gutiérrez et al., 2015). Especially in the early period large differences were observed between 1 m and DCM bacterial communities, which were explained by the strong stratification at 5–7 m in this period (Figure S1). This resulted in lower species richness during stratification in July and August (Figure 8), possibly because bacterial communities exhibit environmental niche partitioning when the water masses remain separated in this period (Morris et al., 2005; Delong et al., 2006; Chow et al., 2013; Salter et al., 2014). As river runoff decreased and mixing events increased (September) the resemblance between communities at 1 m and DCM naturally increased. One specific strong storm that lasted for several days (21–26 September) may explain the high similarity of 1 m surface samples from St. 1 and 2 to the river samples (Figure 8), as samples were collected immediately after the storm where the disturbance apparently caused transport of terrestrial bacteria to the fjord (Crump and Hobbie, 2005).
In temperate and Polar Regions Flavobacteriia often dominate during phytoplankton bloom (Wilson et al., 2017). We also found this group to dominate when chl a concentrations were high (i.e., DCM samples at St. 4), while they contributed only marginally (< 0.1%) to the total bacterial community in the remaining fjord and particularly little in the rivers (Figure 8). Cyanobacteria were relatively abundant in the glacial runoff and can be considered as freshwater tracer, since marine cyanobacteria are usually not found in these Arctic regions (Paulsen et al., 2016). The bacterial communities in the rivers were highly specific to each of them (Figure 8), which we suggest is due to their difference in origin and catchment area; close connection (0.5 km) to the Greenland Ice Sheet (R1), longer distance (2 km) from smaller local glacier (R2), and the lowland vegetation rich river with lake connection (R3). This is in agreement with a recent study from West Greenland that similarly show distinct communities in rivers and a proglacial lake over a 2 km distance (Hauptmann et al., 2016), and find less terrestrial species in river samples with a more direct connection to glaciers.
In the present study R1 has the closest connection to the glacier and all the bacterial families found in R1 were also found in the two other rivers. The SourceTracker analysis (Figure 9) revealed that the R1 community had the far most influence on the surface water, despite not being the largest river. The species unique to R3 hardly contributed to the fjord community, despite being the largest river in terms of volume. This suggests that there is a higher potential for the glacial bacterial community to persist in the fjord than terrestrial communities. Gutiérrez et al. (2015) also found persistence of specific bacterial communities in the surface water of a glacier influenced fjord in Patagonia, and suggested that the glacial meltwater community in the surface was maintained by the competitive advantage of tolerating the cold fresh conditions. Given the averaged doubling time of bacteria in the surface water of Young Sound of 11 ± 7 days (fastest doubling time of 0.9 days) and a transport time of 14–30 days from innermost to the mouth of the fjord, the persistence of the specific communities may be a result of fast transport within the surface water and limited mixing. The differentiation between surface and subsurface communities was lost when the thermal stratification broke down in present study and in Gutiérrez et al. (2015).
Annual Estimations of Carbon Production and Turnover in Young Sound
Due to the strong stratification, high turbidity and input of allochthonous carbon sources, relatively heterotrophic conditions were expected to prevail in the fjord system as concluded in Nielsen et al. (2007). The seasonal and spatial resolution of bacterial carbon demand and primary production allowed a rough, but more robust than previous estimates of the annual carbon budget in Young Sound (Rysgaard and Nielsen, 2006; Nielsen et al., 2007), as previous studies cover a shorter period of the productive season and use a literature BGE of 33%. However, the present study did not cover the early productive season during ice cover in June. As an attempt to account for that we extrapolate the carbon uptake and primary production during the ice-covered productive period using the values obtained on July 11 at St. 3 during ice-cover and by assuming a productive period of 125 days. Based on these assumptions, the annual bacterial production amounted to 1.3 g C m−2 year−1, corresponding to a carbon demand of 18 g C m−2 year−1 in the upper 100 m. This BP value is ~3 times lower than a previous estimate of net annual bacterial production in Young Sound of 4.2 g C m−2 year−1, based on measurements conducted during ice-cover at St. 3 (Rysgaard and Nielsen, 2006), emphasizing that the conditions at St. 3 in June are not representative for the late open water period (August-October) or for the entire Fjord system.
The assessment of bacterial carbon demand rely greatly on estimates of BGE, which is known to depend on a number of factors including DOC composition and lability, bacterial community and temperature, thus it is important to emphasize that BGE is likely to vary across time and space in Young Sound. The BGE we found (6.9%) is low, but still in line with other Arctic studies ranging from 2.2 ± 2.1% in the Chucki Sea (Cota et al., 1996), 6.3 ± 3% in the Fram Strait (Kritzberg et al., 2010), 19.1 ± 9.5% in the rivers Ob, Yenisei and the adjacent Kara Sea (Meon and Amon, 2004) and 9.5 ± 8.7% in Kobbefjord, Greenland (Middelboe et al., 2012).
The bacterial carbon demand on average exceeded primary production during the study period (Figure 5) and indicated that only about 30% of the PP is dissolved and thus potentially available for bacterial uptake. Further, protist and copepod grazing of phytoplankton consumed a substantial fraction of the particulate PP (Middelbo et al. submitted; Arendt et al., 2016). Even though some of the particulate primary production are eventually degraded by pelagic bacteria as detrital matter, our results strongly indicated that primary production could not sustain bacterial carbon demand in the fjord within the productive 125 days and much less on an annual basis. Consequently, the allochthonous DOM sources contributes significantly to the bacterial carbon turnover in Young Sound.
The current study presents an overview of the organic carbon sources and their turnover in the high Arctic Young Sound. Further it highlights that the meltwater associated bacterial community from the glacial rivers persists and is actively transforming the river-DOM within the freshwater lens. The calculations of annual carbon production and turnover are obviously associated with large uncertainties regarding extrapolations across time and space and use of factors for converting thymidine incorporation to carbon production and net bacterial production to carbon demand. However, by applying a high temporal and spatial resolution of sampling and on-site estimation of BGE, this study provides a relatively solid estimation of the annual carbon budget in a high Arctic Fjord compared to previous studies. The fjord is net heterotrophic and in future scenarios with increasing temperatures the relative contribution of riverine DOC is expected to increase, driving the system toward more heterotrophic conditions. However, more measurements of bacterial growth efficiency and factors controlling this are required to provide more solid budget estimates of bacterial carbon consumption on an ecosystem scale.
Author Contributions
MP and SN led the collection and analysis of data and the writing of the paper with equal contribution. MM led the planning of the study, and contributed with sample collection, data analysis and contributing greatly to the writing of the paper. OM led the collection, laboratory work and analysis of the molecular data. SM led the planning and calculations of primary production. AD led the collection and measurements of POC and PON. All other authors contributed to writing the paper. In addition EM, AL, TJ, and MS helped collecting data and CS helped analysing the organic matter samples.
Funding
This study was funded by research grants from the Danish Ministry of the Environment (DANCEA), the project MicroPolar (RCN 225956) funded by the Norwegian Research Council, Carlsberg Foundation and the Arctic Research Centre at Aarhus University. CS was funded by the Danish Research Council for Independent Research (DFF 1323–00336). The Arctic Science Partnership and the Greenland Ecosystem Monitoring program facilitated this work.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Big thanks to Egon Frandsen, Kunuk Lennert, and Ivali Lennert for organization, expertise in the area and technical assistance in the field and to Johan the polar fox for entertainment. We are grateful to be able to include data from the Greenland Ecosystem Monitoring Programme were provided by the Department of Bioscience, Aarhus University, Denmark in collaboration with Department of Geosciences and Natural Resource Management, Copenhagen University, Denmark.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/article/10.3389/fmars.2017.00176/full#supplementary-material
Figure S1. Profiles of salinity, temperature (°C), DOC (μM), DOC:DON and the concentration of nutrients and Si (μM) at the 4 stations in the periods from July-August (red colors) and September-October (blue colors).
Figure S2. Canonical correspondence analysis (CCA) for sequencing data of 16S rRNA gene fragments at RNA level (most abundant OTUs >1%). Arrows indicate selected environmental variables: bacterial production, dissolved organic carbon (DOC), percent bioavailable dissolved organic carbon (%BDOC), fluorescence, temperature and salinity. The OTUs selected for further analysis are labeled with their genus classification.
Figure S3. Time-series measurements from Zackenberg River of temperature (°C), Sediment (mg L−1), LOI (mg L−1) (LOI is organic matter determined by Loss On Ignition when heated at 105°C ≈ particulate organic matter), DOC (μM). Data is collected by Greenland Ecosystem Monitoring, the GEOBASIS Programme.
References
Amon, R. M. W., and Benner, R. (1996). Bacterial utilization of different size classes of dissolved organic matter. Limnol. Oceanogr. 41, 41–51. doi: 10.4319/lo.1996.41.1.0041
Amon, R. M. W., and Budéus, G. (2003). Dissolved organic carbon distribution and origin in the Nordic Seas : exchanges with the Arctic Ocean and the North Atlantic. J. Geophys. Res. 108, 1–17. doi: 10.1029/2002JC001594
Anderson, L. G. (2002). “DOC in the Arctic Ocean,” in Biogeochemistry of Marine Dissolved Organic Matter, eds D. A. Hansell and C. A. Carlson (Academic Press Inc.), 665–683.
Apple, J. K., del Giorgio, P. A., and Kemp, W. M. (2006). Temperature regulation of bacterial production, respiration, and growth efficiency in a temperate salt-marsh estuary. Aquat. Microb. Ecol. 43, 243–254. doi: 10.3354/ame043243
Arendt, K. E., Agersted, M. D., Sejr, M. K., and Juul-pedersen, T. (2016). Glacial meltwater influences on plankton community structure and the importance of top-down control (of primary production) in a NE Greenlan fjord. Estuar. Coast. Shelf Sci. 183, 123–135. doi: 10.1016/j.ecss.2016.08.026
Baña, Z., Ayo, B., Marrasé, C., Gasol, J. M., and Iriberri, J. (2013). Changes in bacterial metabolism as a response to DOM modification during protozoan grazing in coastal Cantabrian and Mediterranean waters. Environ. Microbiol. 16, 498–511. doi: 10.1111/1462-2920.12274
Bendtsen, J., Mortensen, J., and Rysgaard, S. (2014). Seasonal surface layer dynamics and sensitivity to runoff in a high Arctic fjord (Young Sound/Tyrolerfjord, 74°N). J. Geophys. Res. Ocean. 119, 6461–6478. doi: 10.1002/2014JC010077
Benner, R., Louchouarn, P., and Amon, R. M. W. (2005). Terrigenous dissolved organic matter in the Arctic Ocean and its transport to surface and deep waters of the North Atlantic. Global Biogeochem. Cycles 19, 1–11. doi: 10.1029/2004GB002398
Blanchet, M., Pringault, O., Panagiotopoulos, C., Lefe, D., Fernandez, C., Aparicio, F. L., et al. (2016). When riverine dissolved organic matter (DOM) meets labile DOM in coastal waters : changes in bacterial community activity and composition. Aquat. Sci. 79, 27–43. doi: 10.1007/s00027-016-0477-0
Bolger, A. M., Lohse, M., and Usadel, B. (2014). Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120. doi: 10.1093/bioinformatics/btu170
Børsheim, K. Y. (2000). Bacterial production rates and concentrations of organic carbon at the end of the growing season in the Greenland Sea. Aquat. Microb. Ecol. 21, 115–123. doi: 10.3354/ame021115
Caporaso, J. G., Kuczynski, J., Stombaugh, J., Bittinger, K., Bushman, F. D., Costello, E. K., et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336. doi: 10.1038/nmeth.f.303
Caporaso, J. G., Lauber, C., Walters, W. A., Berg-Lyons, D., Lozupone, C. A., Turnbaugh, P. J., et al. (2011). Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. U.S.A. 108, 4516–4522. doi: 10.1073/pnas.1000080107
Cauwet, G. (2002). “DOM in the coastal zone,” in Biogeochemistry of Marine Dissolved Organic Matter, eds D. A. Hansell and C. A. Carlson (San Diego, CA: Academica Press), 579–609.
Chow, C. T., Sachdeva, R., Cram, J. A., Steele, J. A., Needham, D. M., Patel, A., et al. (2013). Temporal variability and coherence of euphotic zone bacterial communities over a decade in the Southern California Bight. ISME J. 7, 2259–2273. doi: 10.1038/ismej.2013.122
Citterio, M., Sejr, M. K., Langen, P. L., Mottram, R. H., Abermann, J., Larsen, S. H., et al. (2017). Towards quantifying the glacial runoff signal in the freshwater input to Tyrolerfjord – Young Sound, NE Greenland. Ambio 46, 146–159. doi: 10.1007/s13280-016-0876-4
Cota, G. F., Pomeroy, L. R., Harrison, W. G., Jones, E. P., Peters, F., Sheldon Jr, W. M., et al. (1996). Nutrients, primary production and microbial heterotrophy in the southeastern Chukchi Sea: Arctic summer nutrient depletion and heterotrophy. Mar. Ecol. Prog. Ser. 135, 247–258. doi: 10.3354/meps135247
Crump, B. C., and Hobbie, J. E. (2005). Synchrony and seasonality in bacterioplankton communities of two temperate rivers. Limnol. Oceanogr. 50, 1718–1729. doi: 10.4319/lo.2005.50.6.1718
del Giorgio, P. A., and Bouvier, T. C. (2002). Linking the physiologic and phylogenetic successions in free-living bacterial communities along an estuarine salinity gradient. Limnol. Oceanogr. 47, 471–486. doi: 10.4319/lo.2002.47.2.0471
del Giorgio, P. A., and Cole, J. J. (1998). Bacterial growth efficiency in natural aquatic systems. Annu. Rev. Ecol. Syst. 29, 503–541. doi: 10.1146/annurev.ecolsys.29.1.503
Delong, E. F., Preston, C. M., Mincer, T., Rich, V., Hallam, S. J., Frigaard, N., et al. (2006). Community genomics among stratified microbial assemblages in the ocean's interior. Science 311, 496503. doi: 10.1126/science.1120250
Edgar, R. C. (2010). Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 26, 2460–2461. doi: 10.1093/bioinformatics/btq461
Elberling, B., Tamstorf, M. P., Michelsen, A., Arndal, M. F., Sigsgaard, C., Illeris, L., et al. (2008). Soil and plant community-characteristics and dynamics at Zackenberg. Adv. Ecol. Res. 40, 223–248. doi: 10.1016/S0065-2504(07)00010-4
Fortunato, C. S., Eiler, A., Herfort, L., Needoba, J. A., Peterson, T. D., and Crump, B. C. (2013). Determining indicator taxa across spatial and seasonal gradients in the Columbia River coastal margin. ISME J. 7, 1899–1911. doi: 10.1038/ismej.2013.79
Fuhrman, J. A., and Azam, F. (1980). Bacterioplankton secondary production estimates for coastal waters of British Columbia, Antarctica, and California. Appl. Environ. Microbiol. 39, 1085–1095.
Granskog, M. A., Stedmon, C. A., Dodd, P. A., Amon, R. M. W., Pavlov, A. K., De Steur, L., et al. (2012). Characteristics of colored dissolved organic matter (CDOM) in the Arctic outflow in the Fram strait : assessing the changes and fate of terrigenous CDOM in the Arctic Ocean. J. Geophys. Res. 117, 1–13. doi: 10.1029/2012JC008075
Gutiérrez, M. H., Galand, P. E., Moffat, C., and Pantoja, S. (2015). Melting glacier impacts community structure of Bacteria, Archaea and fungi in a Chilean Patagonia fjord. Environ. Microbiol. 17, 3882–3897. doi: 10.1111/1462-2920.12872
Hansell, D. A. (2013). Recalcitrant dissolved organic carbon fractions. Ann. Rev. Mar. Sci. 5, 421–445. doi: 10.1146/annurev-marine-120710-100757
Hauptmann, A. L., Markussen, T. N., Stibal, M., and Olsen, N. S. (2016). Upstream freshwater and terrestrial sources are differentially reflected in the bacterial community structure along a small arctic river and its estuary. Front. Microbiol. 7:1474. doi: 10.3389/fmicb.2016.01474
Hedges, J. I., and Stern, J. H. (1984). Carbon and nitrogen determinations of carbonate-containing solids. Limnol. Oceanogr. 29, 657–663. doi: 10.4319/lo.1984.29.3.0657
Holmes, R. M., Aminot, A., Kérouel, R., Hooker, B. A., and Peterson, B. J. (1999). A simple and precise method for measuring ammonium in marine and freshwater ecosystems. Can. J. Fish. Aquat. Sci. 56, 1801–1808. doi: 10.1139/f99-128
Hood, E., Battin, T. J., Fellman, J., Neel, S. O., and Spencer, R. G. M. (2015). Storage and release of organic carbon from glaciers and ice sheets. Nat. Geosci. 8, 91–96. doi: 10.1038/ngeo2331
Hood, E., Fellman, J., Spencer, R. G. M., Hernes, P. J., Edwards, R., Amore, D. D., et al. (2009). Glaciers as a source of ancient and labile organic matter to the marine environment. Nature 462, 1044–1047. doi: 10.1038/nature08580
Hood, E., and Scott, D. (2008). Riverine organic matter and nutrients in southeast Alaska affected by glacial coverage. Nat. Geosci. 583–587. doi: 10.1038/ngeo280
Iversen, K. R., and Seuthe, L. (2011). Seasonal microbial processes in a high-latitude fjord (Kongsfjorden, Svalbard): I. Heterotrophic bacteria, picoplankton and nanoflagellates. Polar Biol. 34, 731–749. doi: 10.1007/s00300-010-0929-2
Jespersen, A. M., and Christoffersen, K. (1987). Measurement of chlorophyll-a from phytoplankton using ethanol as extraction solvent. Arch. Hydrobiol. 109, 445–454.
Jørgensen, L., Stedmon, C. A., Granskog, M. A., and Middelboe, M. (2014). Tracing the long-term microbial production of recalcitrant fluorescent dissolved organic matter. Geophys. Res. Lett. 41, 2481–2488. doi: 10.1002/2014GL059428
Keegan, K. M., Albert, M. R., McConnell, J. R., and Baker, I. (2014). Climate change and forest fires synergistically drive widespread melt events of the Greenland ice sheet. Proc. Natl. Acad. Sci. U.S.A. 111, 7964–7967. doi: 10.1073/pnas.1405397111
Kirchman, D. L., Elifantz, H., Dittel, A. I., Malmstrom, R. R., and Cottrell, M. T. (2007). Standing stocks and activity of Archaea and Bacteria in the western Arctic Ocean. Limnol. Oceanogr. 52, 495–507. doi: 10.4319/lo.2007.52.2.0495
Kirchman, D. L., Malmstrom, R. R., and Cottrell, M. T. (2005). Control of bacterial growth by temperature and organic matter in the Western Arctic. Deep Sea Res. Part II Top. Stud. Oceanogr. 52, 3386–3395. doi: 10.1016/j.dsr2.2005.09.005
Knights, D., Kuczynski, J., Charlson, E. S., Zaneveld, J., Mozer, M. C., Collman, R. G., et al. (2011). Bayesian community-wide culture-independent microbial source tracking. Nat. Methods 8, 761–763. doi: 10.1038/nmeth.1650
Koroleff, F. (1983). “Determination of nutrients,” in Methods of Seawater Analysis, eds K. Grasshoff, M. Erhardt and K. Kremling (Weinheim: Verlag Chemie), 125–187.
Kragh, T., and Søndergaard, M. (2004). Production and bioavailability of autochthonous dissolved organic carbon: effects of mesozooplankton. Aquat. Microb. Ecol. 36, 61–72. doi: 10.3354/ame036061
Kritzberg, E. S., Duarte, C. M., and Wassmann, P. (2010). Changes in Arctic marine bacterial carbon metabolism in response to increasing temperature. Polar Biol. 33, 1673–1682. doi: 10.1007/s00300-010-0799-7
Lawson, E. C., Wadham, J. L., Tranter, M., Stibal, M., Lis, G. P., Butler, C. E. H., et al. (2014). Greenland ice sheet exports labile organic carbon to the Arctic oceans. Biogeosciences 11, 4015–4028. doi: 10.5194/bg-11-4015-2014
Lee, S., and Fuhrman, J. A. (1987). Relationships between biovolume and biomass of naturally derived marine bacterioplankton. Appl. Environ. Microbiol. 53, 1298–1303.
Øvreås, L., Forney, L., and Daae, F. L. (1997). Distribution of bacterioplankton in meromictic lake sælenvannet, as determined by denaturing gradient gel electrophoresis of PCR-amplified gene fragments coding for 16S rRNA. Appl. Environ. Microbiol. 63, 3367–3373.
Lyngsgaard, M. M., Markager, S., and Richardson, K. (2014). Changes in the vertical distribution of primary production in response to land-based nitrogen loading. Limnol. Oceanogr. 59, 1679–1690. doi: 10.4319/lo.2014.59.5.1679
Marie, D., Brussaard, C. P. D., Thyrhaug, R., Bratbak, G., and Vaulot, D. (1999). Enumeration of marine viruses in culture and natural samples by flow cytometry. Appl. Environ. Microbiol. 65, 45–52.
Markager, S., Vincent, W. F., and Tang, E. P. Y. (1999). Carbon fixation by phytoplankton in high Arctic lakes: Implications of low temperature for photosynthesis. Limnol. Oceanogr. 44, 597–607. doi: 10.4319/lo.1999.44.3.0597
Masella, A. P., Bartram, A. K., Truszkowski, J. M., Brown, D. G., and Neufeld, J. D. (2012). PANDAseq : PAired-eND assembler for illumina sequences. BMC Bioinformatics 13:1. doi: 10.1186/1471-2105-13-31
Meon, B., and Amon, R. M. W. (2004). Heterotrophic bacterial activity and fluxes of dissolved free amino acids and glucose in the Arctic rivers Ob, Yenisei and the adjacent Kara Sea. Aquat. Microb. Ecol. 37, 121–135. doi: 10.3354/ame037121
Middelboe, M., Glud, R. N., and Sejr, M. K. (2012). Bacterial carbon cycling in a subarctic fjord: a seasonal study on microbial activity, growth efficiency, and virus-induced mortality in Kobbefjord, Greenland. Limnol. Oceanogr. 57, 1732–1742. doi: 10.4319/lo.2012.57.6.1732
Middelboe, M., and Lundsgaard, C. (2003). Microbial activity in the Greenland sea: role of DOC lability, mineral nutrients and temperature. Aquat. Microb. Ecol. 32, 151–163. doi: 10.3354/ame032151
Morris, R. M., Vergin, K. L., Rappe, M. S., Carlson, C. A., and Giovannoni, S. J. (2005). Temporal and spatial response of bacterioplankton lineages to annual convective overturn at the Bermuda Atlantic Time-series Study site. Limnol. Oceanogr. 50, 1687–1696. doi: 10.4319/lo.2005.50.5.1687
Murphy, J., and Riley, J. P. (1962). A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta. 26, 31–36. doi: 10.1016/S0003-2670(00)88444-5
Murray, C. (2015). Light Attenuation in Natural Waters. Ph. D. thesis. Department of Earth Sciences, Aarhus University, Risø National Laboratory.
Murray, C., Markager, S., Stedmon, C. A., Juul-Pedersen, T., Sejr, M. K., and Bruhn, A. (2015). The influence of glacial melt water on bio-optical properties in two contrasting Greenlandic fjords. Estuar. Coast. Shelf Sci. 163, 72–83. doi: 10.1016/j.ecss.2015.05.041
Nghiem, S. V., Hall, D. K., Mote, T. L., Tedesco, M., Albert, M. R., Keegan, K., et al. (2012). The extreme melt across the Greenland ice sheet in 2012. Geophys. Res. Lett. 39, 6–11. doi: 10.1029/2012GL053611
Nielsen, S. E. (1952). The use of radio-active carbon (14C) for measuring organic production in the sea. ICES J. Mar. Sci. 18, 117–140. doi: 10.1093/icesjms/18.2.117
Nielsen, T. G., Ottosen, L. D., and Hansen, B. W. (2007). Structure and function of the pelagic ecosystem in young sound, NE Greenland. Carbon Cycl. Arct. Mar. Ecosyst. Case Study Young Sound 58, 88–107. doi: 10.3354/meps179013
Osterholz, H., Singer, G., Wemheuer, B., Daniel, R., Simon, M., Niggemann, J., et al. (2016). Deciphering associations between dissolved organic molecules and bacterial communities in a pelagic marine system. 10, 1–14. doi: 10.1038/ismej.2015.231
Paulsen, M. L., Dor,é, H., Garczarek, L., Seuthe, L., Müller, O., Sandaa, R., et al. (2016). Synechococcus in the Atlantic Gateway to the Arctic Ocean Synechococcus in the Atlantic Gateway to the Arctic Ocean. Front. Mar. Sci. 3:191. doi: 10.3389/fmars.2016.00191
Pomeroy, L. R., and Deibel, D. (1986). Temperature regulation of bacterial activity during the spring bloom in newfoundland coastal waters. Science 233, 359–361. doi: 10.1126/science.233.4761.359
Pomeroy, L. R., and Wiebe, W. J. (2001). Temperature and substrates as interactive limiting factors for marine heterotrophic bacteria. Aquat. Microb. Ecol. 23, 187–204. doi: 10.3354/ame023187
Pomeroy, L. R., Wiebe, W. J., Deibel, D., Thompson, R. J., Rowe, G. T., and Pakulski, J. D. (1991). Bacterial responses to temperature and substrate concentration during the newfoundland spring bloom. Mar. Ecol. Prog. Ser. 75, 143–159. doi: 10.3354/meps075143
Pradeep Ram, A. S., Nair, S., and Chandramohan, D. (2003). Bacterial growth efficiency in the tropical estuarine and coastal waters of Goa, Southwest Coast of India. Microb. Ecol. 45, 88–96. doi: 10.1007/s00248-002-3005-9
Quast, C., Pruesse, E., Yilmaz, P., Gerken, J., Schweer, T., Glo, F. O., et al. (2013). The SILVA ribosomal RNA gene database project : improved data processing and web-based tools. Nucleic Acid Res. 41, 590–596. doi: 10.1093/nar/gks1219
Riemann, B., Fuhrman, J., and Azam, F. (1982). Bacterial secondary production in freshwater measured by super(3)H-thymidine incorportion method. Microb. Ecol. 8, 101–114. doi: 10.1007/BF02010444
Rivkin, R. B., and Legendre, L. (2001). Biogenic carbon cycling in the upper ocean: effects of microbial respiration. Science 291, 2398–2400. doi: 10.1126/science.291.5512.2398
Roiha, T., Peura, S., Cusson, M., and Rautio, M. (2016). Allochthonous carbon is a major regulator to bacterial growth and community composition in subarctic freshwaters. Sci. Rep. 6:34456. doi: 10.1038/srep34456
Rysgaard, S., and Nielsen, T. G. (2006). Carbon cycling in a high-arctic marine ecosystem – young Sound, NE Greenland. Prog. Oceanogr. 71, 426–445. doi: 10.1016/j.pocean.2006.09.004
Rysgaard, S., Vang, T., Stjernholm, M., Rasmussen, B., Windelin, A., and Kiilsholm, S. (2003). Physical conditions, carbon transport, and climate change impacts in a Northeast Greenland Fjord. Arct. Antarct. Alp. Res. 35, 301–312. doi: 10.1657/1523-0430(2003)035[0301:PCCTAC]2.0.CO;2
Salter, I., Galand, P. E., Fagervold, S. K., Lebaron, P., Obernosterer, I., Oliver, M. J., et al. (2014). Seasonal dynamics of active SAR11 ecotypes in the oligotrophic Northwest Mediterranean Sea. ISME J. 9, 347–360. doi: 10.1038/ismej.2014.129
Schlitzer, R. (2016). Ocean Data View. Available online at: http://odv.awi.de
Sejr, M. K., Nielsen, T. G., Rysgaard, S., Risgaard-Petersen, N., Sturluson, M., and Blicher, M. E. (2007). Fate of pelagic organic carbon and importance of pelagic-benthic coupling in a shallow cove in Disko Bay, West Greenland. Mar. Ecol. Prog. Ser. 341, 75–88. doi: 10.3354/meps341075
Singer, G. A., Fasching, C., Wilhelm, L., Niggemann, J., Steier, P., Dittmar, T., et al. (2012). Biogeochemically diverse organic matter in Alpine glaciers and its downstream fate. Nat. Geosci. 5, 710–714. doi: 10.1038/ngeo1581
Søndergaard, M., and Middelboe, M. (1995). A cross-system analysis of labile dissolved organic carbon. Mar. Ecol. Prog. Ser. 118, 283–294. doi: 10.3354/meps118283
Ter Braak, C. J. F., and Šmilauer, P. (2012). Canoco Reference Manual and User's Guide: Software for Ordination, Version 5.0 (Ithaca, NY: Microcomputer Power), 496.
Traving, S. J., Bentzon-tilia, M., Knudsen-leerbeck, H., and Mantikci, M. (2016). Coupling bacterioplankton populations and environment to community function in coastal temperate waters coupling bacterioplankton populations and environment to community function in coastal temperate waters. Front. Microbiol. 7:1533. doi: 10.3389/fmicb.2016.01533
Wilson, B., Müller, O., Nordmann, E.-L., Seuthe, L., Bratbak, G., and Øvreås, L. (2017). Changes in marine prokaryote composition with season and depth over an arctic polar year. Front. Mar. Sci. 4:95. doi: 10.3389/fmars.2017.00095
Keywords: bacterial carbon demand, bacterial diversity, dissolved organic matter, runoff, glacial meltwater, high arctic ecosystems, young sound
Citation: Paulsen ML, Nielsen SEB, Müller O, Møller EF, Stedmon CA, Juul-Pedersen T, Markager S, Sejr MK, Delgado Huertas A, Larsen A and Middelboe M (2017) Carbon Bioavailability in a High Arctic Fjord Influenced by Glacial Meltwater, NE Greenland. Front. Mar. Sci. 4:176. doi: 10.3389/fmars.2017.00176
Received: 18 October 2016; Accepted: 19 May 2017;
Published: 08 June 2017.
Edited by:
Ingrid Obernosterer, FR3724 Observatoire Océanologique de Banyuls sur Mer (OOB), FranceReviewed by:
Emma Jane Rochelle-Newall, Institut de Recherche Pour le Développement, FranceXosé Anxelu G. Morán, King Abdullah University of Science and Technology, Saudi Arabia
Copyright © 2017 Paulsen, Nielsen, Müller, Møller, Stedmon, Juul-Pedersen, Markager, Sejr, Delgado Huertas, Larsen and Middelboe. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria L. Paulsen, maria.l.paulsen@uib.no
 Maria L. Paulsen
Maria L. Paulsen Sophia E. B. Nielsen
Sophia E. B. Nielsen Oliver Müller
Oliver Müller Eva F. Møller
Eva F. Møller Colin A. Stedmon
Colin A. Stedmon Thomas Juul-Pedersen
Thomas Juul-Pedersen Stiig Markager
Stiig Markager Mikael K. Sejr
Mikael K. Sejr Antonio Delgado Huertas
Antonio Delgado Huertas Aud Larsen
Aud Larsen Mathias Middelboe
Mathias Middelboe