Repeated Thermal Stress, Shading, and Directional Selection in the Florida Reef Tract
- Department of Biological Sciences, Florida Institute of Technology, Melbourne, FL, United States
Over the last three decades reef corals have been subjected to an unprecedented frequency and intensity of thermal-stress events, which have led to extensive coral bleaching, disease, and mortality. Over the next century, the climate is predicted to drive sea-surface temperatures to even higher levels, consequently increasing the risk of mass bleaching and disease outbreaks. Yet, there is considerable temporal and spatial variation in coral bleaching and in disease prevalence. Using data collected from 2,398 sites along the Florida reef tract from 2005 to 2015, this study examined the temporal and spatial patterns of coral bleaching and disease in relation to coral-colony size, depth, temperature, and chlorophyll-a concentrations. The results show that coral bleaching was most prevalent during the warmest years in 2014 and 2015, and disease was also most prevalent in 2010, 2014, and 2015. Although the majority of the corals surveyed were found in habitats with low chlorophyll-a concentrations, and high irradiance, these same habitats showed the highest prevalence of coral bleaching and disease outbreaks during thermal-stress events. These results suggest that directional selection in a warming ocean may favor corals able to tolerate inshore, shaded environments with high turbidity and productivity.
Introduction
Predicting and responding to the effects of climate change is critical if we wish to preserve marine life in the oceans. The global effects of climate change are becoming glaringly apparent on coral reefs, where repeated thermal anomalies are forcing temperatures beyond the physiological tolerance range of many coral species. These stress events are causing widespread coral bleaching and mortality (Loya et al., 2001; Baker et al., 2008). Yet, predicting where corals are less likely to bleach, and more likely to survive thermal-stress, is an important scientific endeavor that might redirect ocean conservation efforts at both local and regional scales (Cacciapaglia and van Woesik, 2015). Here we use an 11-year dataset (from 2005 to 2015) to examine the spatial relationships between coral bleaching, coral disease, and environmental conditions at 2,398 study sites along the Florida reef tract.
Coral bleaching is a combination of irradiance and temperature stress (Takahashi et al., 2004). High seasonal irradiance causes photoinhibition of the coral symbionts, from which they can recover, unless water temperatures are high. When irradiance and water temperatures are both high, the symbiont's photosystems are compromised, and nighttime recovery from daytime photoinhibition is minimal (Warner et al., 1999; Takahashi et al., 2004). Weeks of high-water temperatures leads to chronic photoinhibition, or bleaching, that can lead to the expulsion of coral symbionts. This bleaching can be temporary and non-fatal for some coral species (Goreau, 1964; Loya et al., 2001), or fatal for other coral species that rely heavily on their symbionts as a food source (Grottoli et al., 2006).
Coral bleaching can also lower the tolerance of corals to pathogens, and bleaching can lead to disease (Muller et al., 2008; Brandt and McManus, 2009; Randall et al., 2014). The causative agents of most coral diseases are still unknown, but laboratory and field studies have shown an influence from a variety of stressors in addition to water temperatures, including population density (Bruno et al., 2007), nutrient enrichment (Bruno et al., 2003; Voss and Richardson, 2006), and irradiance (Kuta and Richardson, 2002). It is also likely that physiological and environmental parameters interact to influence the virulence of the pathogens, and the susceptibility of coral hosts (Randall and van Woesik, 2015).
By contrast, reducing irradiance during temperature-stress events relieves stress on the symbiont's photosystem, buffers corals from temperature stress, and reduces the likelihood of coral bleaching (Warner et al., 1999; Takahashi et al., 2004). Such reductions in irradiance are common on deep reef slopes (Smith et al., 2014), or nearshore, where turbidity and chlorophyll-a concentrations are high (Wagner et al., 2010; van Woesik et al., 2012). Yet, reef corals generally prefer low-nutrient waters (Tomascik and Sander, 1987), and nearshore environments support high nutrient concentrations, which in combination with high temperatures are detrimental to reef corals (Wooldridge and Done, 2009; Wagner et al., 2010; Wiedenmann et al., 2013). Still, if shading in nearshore, turbid environments provides protection to corals under thermal-stress events, then the corals that can tolerate those nearshore conditions may be selected for when thermal-stress events become frequent. We hypothesize that although shading by high turbidity and high organics are less than optimal for reef-building corals, these conditions may be physiologically beneficial for corals under thermal stress. Indeed, the benefits of shading under extreme thermal stress may override the costs of living in these less suitable nearshore environments, with high thermal variation, low irradiance, and high nutrients. We question whether global warming is progressively driving reef corals away from clear, oligotrophic waters, to more nearshore, turbid habitats.
The present study uses an extensive dataset collected between 2005 and 2015 (http://www.frrp.org) at 2,398 sites along the Florida reef tract to examine the spatial distribution of scleractinian corals and the environmental variables that cause and relieve stress. Specifically, the objectives of this study were to: (i) quantify the water-quality characteristics of the study sites, particularly temperature and chlorophyll-a concentrations, (ii) determine the spatial extent of bleaching and coral disease, and (iii) examine interactions between coral bleaching, coral disease, and water quality.
Methods
Water-Quality Data
Water-quality data were obtained from the Southeast Environmental Research Center, Florida International University (http://serc.fiu.edu/wqmnetwork/). These data were collected at 215 sites along the Florida Keys sampled quarterly from January 2005 to December 2015. We were particularly interested in both the benthic water temperature that the corals experienced (Figure 1), and the chlorophyll-a concentrations, used as a proxy for primary productivity in the water column. To geographically align the water-quality data with the coral data, we spatially cropped the coral data to the same extent as the water-quality data, and then spatially interpolated the water-quality data using ordinary kriging with the R package “gstat” (Pebesma, 2004). The interpolated data were combined to reflect patterns (i) over the entire study period, (ii) from 2005 to 2009, and (iii) from 2010 to 2015, before and after the major coral bleaching event in 2010.
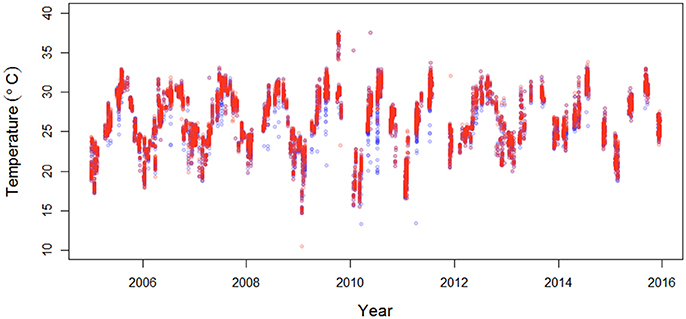
Figure 1. Sea surface (red) and benthic (blue) temperatures (°C) along the Florida Keys (data from Southeast Environmental Research Center, http://serc.fiu.edu/wqmnetwork/) (n = 6,849).
Coral Sampling
The study used data that stemmed from the Florida Reef Resilience Program (FRRP) (http://www.frrp.org), which was a two-stage stratified-random survey design to assess the condition of scleractinian corals along the Florida reef tract every summer from 2005 to 2015 (Wagner et al., 2010; Smith et al., 2011; Burman et al., 2012). The region was stratified into geographic sub-regions and habitats. To date the FRRP has surveyed 2,398 sites, using replicated 10 m by 1 m belt transects. Along each transect each coral colony was identified to species, measured for diameter, and examined for bleaching and disease. The database is available from The Nature Conservancy upon request.
Data Analysis
We used semivariograms to estimate the extent of autocorrelation of the coral and water-quality data, and plotted the semivariance of each variable expressed as a function of distance across the spatial field. The semivariogram value γ(d), or estimated semivariance, for lag distance d was defined as:
where N(d) is the number of pairs of points separated by d, z(xi) are the data values for points xi, and z(xi+d) are the data at cells separated from xi by the lag distance d in the chosen direction. The semivariogram estimates assumed that: (i) the process that generated the data was random, (ii) the variance of the process was constant and independent across space, and (iii) the process was only dependent on the separation distance between points. We examined the semivariogram estimates for inherent changes through time (i.e., stationarity), and for directionality (i.e., isotrophy).
We used generalized linear and non-linear models to examine relationships between coral bleaching and the water-quality parameters of interest, in particular water temperature and chlorophyll-a concentration. All linear and non-linear models showed spurious results, with significant negative slopes, suggesting that high-water temperature, for example, predicted low coral bleaching. We have long known that the opposite is true, because high-water temperatures cause coral bleaching. Therefore, the linear and non-linear models provided misleading predictions. We suspect that the negative slope is, in part, related to the high density of data, particularly around the 29.5°C, reducing the central tendency of the relationship. We instead considered using point pattern processes, which gain strength from high density data, because they utilize the intensity of spatially explicit geographic data as predictors. We therefore examined the coral responses as spatial point patterns and determined the dependence of those point patterns on environmental covariates (Baddeley et al., 2012), using the following:
where λ(u) is an intensity function of a finite set of spatial data points of the coral localities (u), X(u) is a spatial covariate (i.e., benthic temperature and chlorophyll-a in this study) at every locality. The data were modeled as a spatial point Poisson process, and ρ was determined using a nonparametric estimator with the R package “spatstat” (Baddeley et al., 2015). Although we analyzed the relationship between all stony corals present on the reefs and chlorophyll-a concentrations and temperature, we were also particularly interested in the corals Acropora cervicornis, which is a threatened species, and Orbicella annularis, which was common in the Florida reef tract in the past. We therefore further examined the relationship between these two species and chlorophyll-a concentrations and temperature. We also wanted to know whether the size of the coral colonies influenced the relationships with the environmental covariates, and therefore ran similar spatial point pattern analyzes after categorizing the coral colony diameters as either small (4–50 cm), medium (51–100 cm), or large (>100 cm). All analyses were run in R (R Core Team, 2016).
Results
Water-Quality Data
Over the 11-year study period, within the study region, the benthic water temperature ranged from 13.4°C to 37.6°C, and chlorophyll-a concentrations ranged from 0.002 μg l−1 to 12.29 μg l−1. Sea surface temperatures (SSTs) were more variable than benthic temperatures (Figure 1), ranging from 10.5°C to 37.6°C. The highest temperatures were recorded in 2010, and the lowest temperatures were recorded in 2009 (Figure 1). The extent of homogeneous patches of benthic temperatures, evident from the range in the semivariograms, averaged approximately 15 km (SD ± 9.9 km) for the time period from 2005 to 2009, and approximately 17 km (SD ± 12 km) for the time period from 2010 to 2015. The semivariogram range of chlorophyll-a concentrations averaged approximately 23 km (SD ± 16 km) for the time period from 2005 to 2009, and approximately 33 km (SD ± 18 km) for the time period from 2010 to 2015 (see Supplementary document).
Coral Bleaching and Diseases
Coral bleaching was most prevalent in 2014 and 2015 (Figure 2), with average bleaching around 45 and 33% respectively. Coral bleaching was recorded at depths between 2 m and 28 m, although bleaching was most prevalent at depths between 6 and 8 m (Figure 3). The size of homogenous patches of bleached corals averaged at approximately 46 km (SD ± 47 km), although during extremely warm years the patch sizes were larger than in other years (see online Supplementary document). There was clear directionality (i.e., anisotropy) in the coral bleaching data, with most time periods showing east-west alignment along the geographic axis of the Florida Keys (see online Supplementary document).
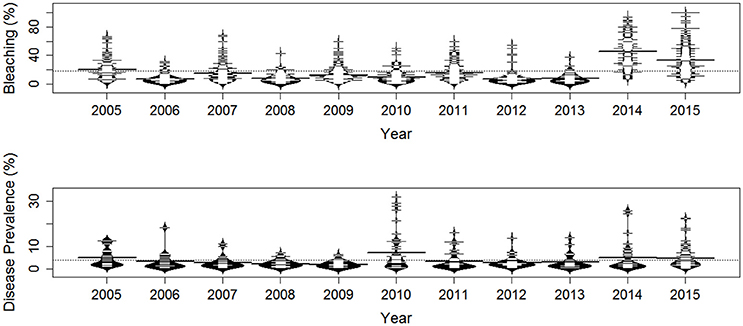
Figure 2. Time series of the percentage of coral bleaching (upper panel), and the prevalence of coral disease (lower panel) at 2,398 sites along the Florida reef tract from 2005 to 2015. The longest horizontal line for each time period represents the yearly mean, and the dotted horizontal line displays the overall mean.
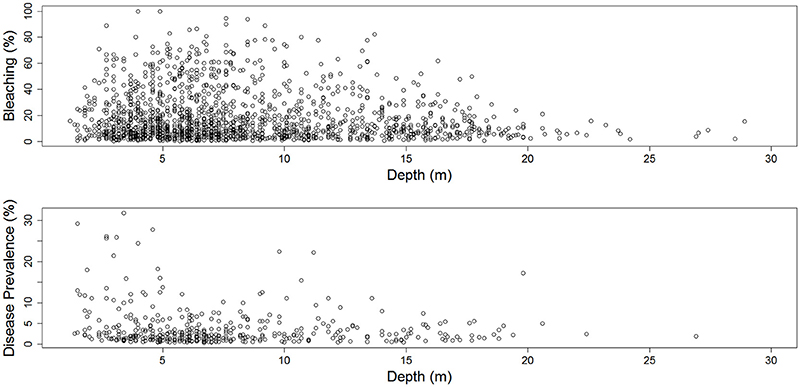
Figure 3. The percentage of coral bleaching (upper panel), and the prevalence of disease (lower panel) across depth (m) at 2,398 sites along the Florida reef tract from 2005 to 2015.
Coral diseases were most prevalent in 2010, 2014, and 2015 (Figure 2), with average disease prevalence at 7, 5, and 4%, respectively. Diseases were most common at depths between 2 and 7 m (Figure 3). The average range of homogenous coral disease patches was typically 5 km or less, except in 2005 and 2014, when the sea surface and benthic temperatures were high, and when the range of homogeneous coral-disease patches averaged 14 km (SD ± 19 km). As occurred with the coral bleaching data, there was clear directionality (i.e., anisotropy) in the coral disease data, with most time periods showing east-west alignment along the geographic axis of the Florida Keys (see online Supplementary document).
Environmental Relationships
Most reef-building corals along the Florida reef tract were geographically located in habitats where the water temperatures were above 24°C during the winter season, and below 30°C in the summer season (Figure 4). Similarly, most corals favored habitats with low chlorophyll-a concentrations, yet these same oligotrophic conditions increased the prevalence of coral bleaching and disease (Figure 5). There was a considerable decline in coral bleaching and coral disease where chlorophyll-a concentrations were >0.3 μg l−1. The two main reef-building corals, Acropora cervicornis and Orbicella annularis, showed similar responses to chlorophyll-a concentrations and bleaching. Both coral species were most common in oligotrophic waters, with low chlorophyll-a concentrations, yet these same clear-water habitats were conducive to coral bleaching (Figure 6). When we categorized the sizes of the coral colonies, and analyzed each size-class separately, both species showed a similar response, with most colonies occurring in waters with low chlorophyll-a concentrations independent of size class (Figure 7). By contrast, Acropora cervicornis colonies in localities with chlorophyll-a concentrations >0.3 μg l−1 were less susceptible to bleaching, and Orbicella annularis in localities with chlorophyll-a concentrations > 0.4 μg l−1 were less susceptible to bleaching. Similarly, when summer temperatures and chlorophyll-a concentrations were considered together, as spatial covariates of the spatial-point-process-intensity estimates, the corals were most common at low chlorophyll-a concentrations and at 27.5°C. Bleached corals were most prevalent at low chlorophyll-a concentrations and at temperatures >29°C (Figure 8).
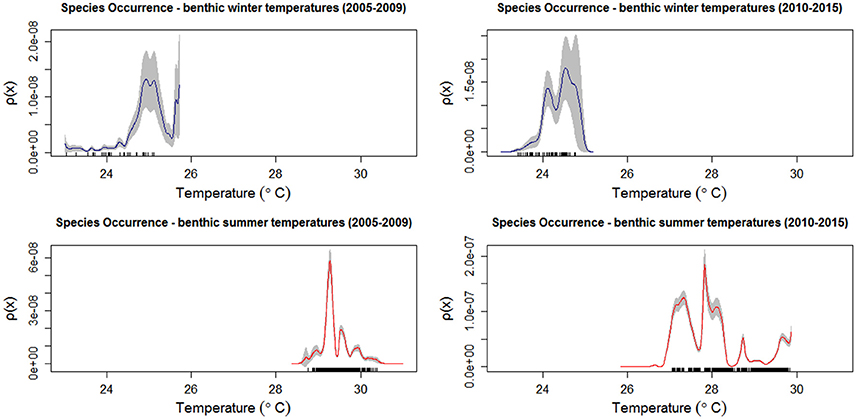
Figure 4. Occurrence of all recorded coral species relative to winter and summer benthic temperatures (°C) in the Florida reef tract at 2,398 sites from 2005 to 2015. Kernel estimates of ρ (equation 2) (solid lines), and two-standard deviation confidence limits (gray shading) for winter (blue) and summer (red) temperatures. The rug plot indicates the number of sites that supported corals.
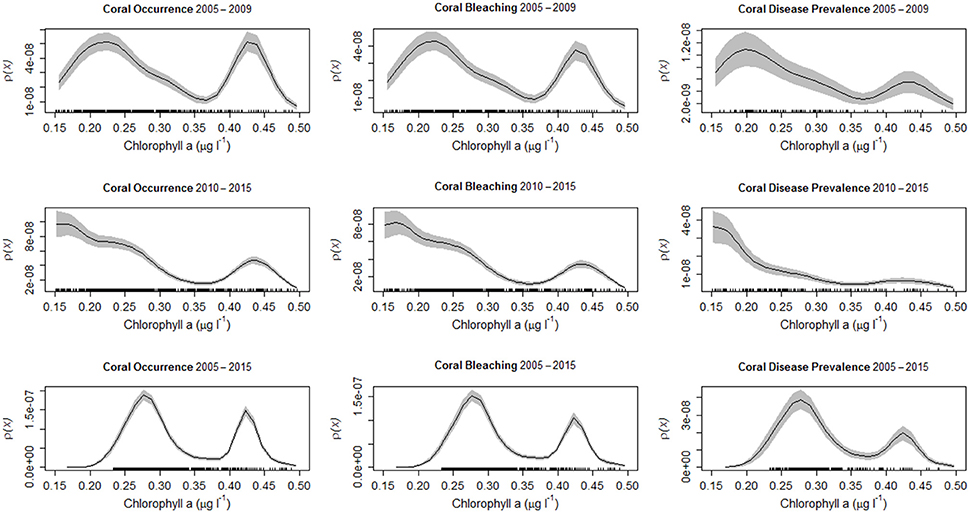
Figure 5. All coral colonies in the Florida reef tract from 2005 to 2009 (upper row), from 2010 to 2015 (middle row), and throughout the entire study period (2005–2015) (lower row). Kernel estimates of ρ (equation 2) (solid black line), with two-standard deviation confidence limits (gray shading) for: (left column) the occurrence of all coral colonies as a function of chlorophyll-a concentrations (μg l−1) in the water column, (central column) the prevalence of coral bleaching as a function of chlorophyll-a concentrations (μg l−1), and (right column) the prevalence of coral disease as a function of chlorophyll-a concentrations (μg l−1). The rug plots indicate the number of sites surveyed.
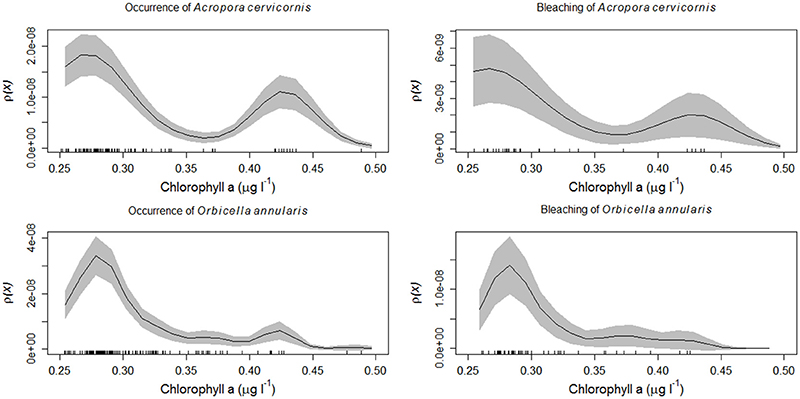
Figure 6. Acropora cervicornis and Orbicella annularis from the Florida reef tract from 2005 to 2015. Kernel estimates of ρ (equation 2) (solid black line), with two-standard deviation confidence limits (gray shading) for: (top left panel) the occurrence of Acropora cervicornis as a function of chlorophyll-a concentrations (μg l−1), (top right panel) the prevalence of coral bleaching of Acropora cervicornis as a function of chlorophyll-a concentrations (μg l−1), (lower left panel) the occurrence of Orbicella annularis as a function of chlorophyll-a concentrations (μg l−1), (lower right panel) the prevalence of coral bleaching of Orbicella annularis as a function of chlorophyll-a concentrations (μg l−1). The rug plot indicates the number of sites surveyed that supported the coral species.
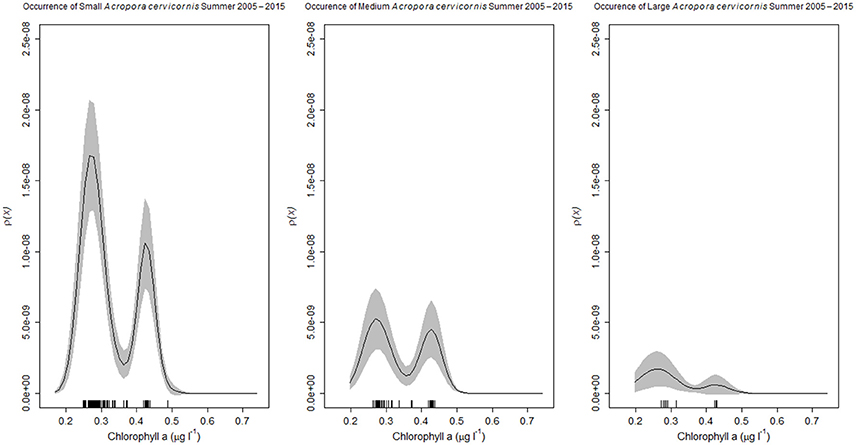
Figure 7. Acropora cervicornis colonies of three size classes, small (4–50 cm), medium (51–100 cm), or large (> 100 cm), in the Florida reef tract from 2005 to 2015. Kernel estimates of ρ (equation 2) (solid black line), with two-standard deviation confidence limits (gray shading) for the occurrence of Acropora cervicornis as a function of chlorophyll-a concentrations (μg l−1). The rug plot indicates the number of sites surveyed that supported the coral species of the specific size class.
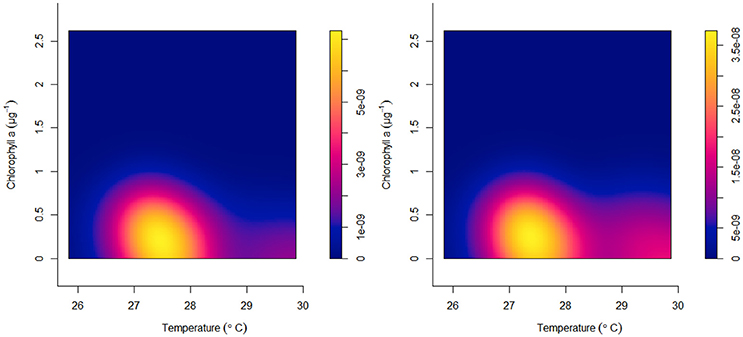
Figure 8. Acropora cervicornis from 633 sites in the Florida reef tract from 2005 to 2015. Kernel intensity estimates of ρ (equation 2) for all colony occurrences (left panel) and all bleached corals (right panel) against chlorophyll-a concentrations (μg l−1) and benthic temperatures (°C).
Discussion
The analysis of this extensive dataset from the Florida reef tract suggests that thermal-stress events are responsible for coral bleaching and associated coral disease, but water clarity also plays an important role. We found that throughout the decade of observation, the clearer the water the more likely it was that corals bleached. Corals in general, and the two major reef-building corals, Acropora cervicornis and Orbicella annularis, in particular, occurred in greater numbers in habitats with low chlorophyll-a concentrations. Yet coral colonies in these low chlorophyll-a habitats showed more extensive bleaching during thermal stress events than coral colonies in high chlorophyll-a habitats. It is most likely that the high concentrations of chlorophyll-a acted as a thermal refuge for coral colonies because of the shading they provided to the corals.
Physiological studies have confirmed the benefits of reducing irradiance during temperature-stress events. Shading reduces stress on the symbiont's photosystem, which in turn reduces the likelihood of coral bleaching (Warner et al., 1999; Takahashi et al., 2004). Notwithstanding the obviously adverse effects that poor-water quality, with high levels of pollutants and high nutrient concentrations, has on corals (Wooldridge and Done, 2009; Wagner et al., 2010; Wiedenmann et al., 2013), some shading provided by high-primary productivity and high turbidity can benefit corals during thermal-stress events (Cacciapaglia and van Woesik, 2016). Indeed, since coral bleaching is essentially extreme photoinhibition, and high temperatures make that photoinhibition worse (Warner et al., 1999; Takahashi et al., 2004), high productivity and high turbidity during high temperature events should effectively reduce the probability of photoinhibition and coral bleaching.
Similarly, the results showed that corals in habitats with high water-column productivity had a lower prevalence of disease than elsewhere. Previously, Lesser et al. (2007) suggested that reducing chronic photoinhibition and bleaching reduces the likelihood of coral disease. Subsequent field studies have validated these observations. For example, Muller et al. (2008) showed that bleached Acropora palmata colonies were more likely to suffer disease than coral colonies that did not bleach.
Still, reducing irradiance can be detrimental to the calcification process, causing reductions in coral growth rates (Tomascik and Sander, 1987). Moreover, high sedimentation environments can deplete coral resources and increase their susceptibility to disease (Sheridan et al., 2014). Therefore, reducing irradiance, through primary productivity in the water column and high turbidity, can have beneficial effects up to a point, beyond which the costs may override the benefits for reef corals. Indeed, high and persistent productivity and extremely high turbidity can reduce the capacity of corals to build reefs entirely, because the photic zone becomes so narrow that there is insufficient irradiance for photosynthesis and calcification (Tomascik et al., 1993; Kleypas, 1996; Toth et al., 2012). Previously, Kleypas et al. (1999) showed that reef building is unlikely in habitats with an average irradiance of <250 μE m−2 s−1 (at a depth of 3 m). Therefore, locating optimal niches, where corals can survive through thermal-stress events, should be a research priority, to not only detect natural refuges, but also for the rapidly burgeoning coral restoration endeavor that is expanding throughout Florida and the Caribbean.
Ocean temperatures are clearly increasing globally (Hansen et al., 2010; IPCC, 2013), and as the oceans continue to warm, thermal anomalies will most likely continue to cause coral bleaching and subsequent diseases (Hoegh-Guldberg, 1999; Harvell et al., 2002; Donner et al., 2005; Hoegh-Guldberg et al., 2007; Muller and van Woesik, 2012). Some researchers question the capacity of corals to adapt to rapid climate change (Hoegh-Guldberg, 2006; Frieler et al., 2013), whereas other researchers suggest that adaptive radiations and directional selection of thermally tolerant genotypes are likely (Thompson, 1998; Hoffmann and Sgro, 2011; Guest et al., 2012; Poloczanska et al., 2016). The present study suggests an additional, more nuanced, effect of ocean warming—causing geographical shifts of reef corals toward more turbid environments.
Adaptation, however, will occur only under persistent selective pressure. In the past, there may have been little selective pressure for corals to live in habitats with consistently high turbidity and high chlorophyll-a-concentrations, since reef-building corals grow best under moderate irradiance (Done, 1982). Most recently however that selective pressure may have increased. The almost annual reoccurrence of thermal-stress events and coral bleaching in the Florida reef tract, shown by this study, may be selecting for coral genotypes that can live in naturally shaded environments, with high primary productivity and turbidity. Corals in these shaded habitats are less likely to suffer thermal stress than elsewhere. Therefore, in a rapidly warming ocean, there is likely to be a fitness advantage for corals that can live in habitats with less than optimal irradiance, because those same environments shield corals from short-term temperature-stress events. This study suggests that directional selection in a warming ocean may favor corals that are able to tolerate inshore environments with high turbidity and productivity. Because of reduced thermal stress in those shaded environments, selection for colonies in these habitats may provide the genetic pool of corals needed to survive through climate change.
Author Contributions
RvW designed the work, wrote the R code to conduct analysis, and wrote the manuscript. KM conducted the kriging analysis, wrote some of the R code, and edited the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Our thanks extend to the Florida Reef Resilience Program (FRRP) project and all the volunteers for collecting the field data, and Sandra J. van Woesik for editorial comments on the manuscript. We would also like to thank the Florida Fish and Wildlife Commission for award 16008 to RvW that partially supported this research.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/article/10.3389/fmars.2017.00182/full#supplementary-material
References
Baddeley, A., Chang, Y.-M., Song, Y., and Turner, R. (2012). Nonparametric estimation of the dependence of a spatial point process on spatial covariates. Stat. Interface 5, 221–236. doi: 10.4310/SII.2012.v5.n2.a7
Baddeley, A., Rubak, E., and Turner, R. (2015). Spatial Point Patterns: Methodology and Applications with R. London: Chapman and Hall/CRC Press.
Baker, A. C., Glynn, P. W., and Riegl, B. (2008). Climate change and coral reef bleaching: an ecological assessment of long-term impacts, recovery trends and future outlook. Estuar. Coast. Shelf Sci. 80, 435–471. doi: 10.1016/j.ecss.2008.09.003
Brandt, M. E., and McManus, J. W. (2009). Disease incidence is related to bleaching extent in reef-building corals. Ecology 90, 2859–2867.
Bruno, J. F., Petes, L. E., Harvell, C. D., and Hettinger, A. (2003). Nutrient enrichment can increase the severity of coral diseases. Ecol. Lett. 6, 1056–1061. doi: 10.1046/j.1461-0248.2003.00544.x
Bruno, J. F., Selig, E. R., Casey, K. S., Page, C. A., Willis, B. L., Harvell, C. D., et al. (2007). Thermal stress and coral cover as drivers of coral disease outbreaks. PLoS Biol. 5:e124. doi: 10.1371/journal.pbio.0050124
Burman, S., Aronson, R., and van Woesik, R. (2012). Homogenization of coral assemblages along the Florida reef tract. Mar. Ecol. Prog. Ser. 467, 89–96. doi: 10.3354/meps09950
Cacciapaglia, C., and van Woesik, R. (2015). Reef-coral refugia in a rapidly changing ocean. Glob. Change Biol. 21, 2272–2282. doi: 10.1111/gcb.12851
Cacciapaglia, C., and van Woesik, R. (2016). Climate-change refugia: shading reef corals by turbidity. Glob. Change Biol. 22, 1145–1154. doi: 10.1111/gcb.13166
Done, T. J. (1982). Patterns in the distribution of coral communities across the central Great Barrier Reef. Coral Reefs 1, 95–107. doi: 10.1007/BF00301691
Donner, S. D., Skirving, W. J., Little, C. M., Oppenheimer, M., and Hoegh-Guldberg, O. (2005). Global assessment of coral bleaching and required rates of adaptation under climate change. Glob. Change Biol. 11, 2251–2265. doi: 10.1111/j.1365-2486.2005.01073.x
Frieler, K., Meinshausen, M., Golly, A., Mengel, M., Lebek, K., Donner, S. D., et al. (2013). Limiting global warming to 2°C is unlikely to save most coral reefs. Nat. Clim. Change 3, 165–170. doi: 10.1038/nclimate1674
Goreau, T. F. (1964). Mass expulsion of zooxanthellae from Jamaican reef communities after Hurricane Flora. Science 145, 383–386. doi: 10.1126/science.145.3630.383
Grottoli, A. G., Rodrigues, L. J., and Palardy, J. E. (2006). Heterotrophy plasticity and resilience in bleached corals. Nature 440, 1186–1189. doi: 10.1038/nature04565
Guest, J. R., Baird, A. H., Maynard, J. A., Muttaqin, E., Edwards, A. J., Campbell, S. J., et al. (2012). Contrasting patterns of coral bleaching susceptibility in 2010 suggest and adaptive response to thermal stress. PLoS ONE 7:e33353. doi: 10.1371/journal.pone.0033353
Hansen, J., Ruedy, R., Sato, M., and Lo, K. (2010). Global surface temperature change. Rev. Geophys. 48, 1–29. doi: 10.1029/2010RG000345
Harvell, C. D., Mitchell, C. E., Ward, J. R., Altizer, S., Dobson, A. P., Ostfeld, R. S., et al. (2002). Climate warming and disease risks for terrestrial and marine biota. Science 296, 2158–2162. doi: 10.1126/science.1063699
Hoegh-Guldberg, O. (1999). Climate change, coral bleaching and the future of the world's coral reefs. Mar. Freshw. Res. 50, 839–866. doi: 10.1071/MF99078
Hoegh-Guldberg, O. (2006). Thermal biology of coral reefs: will coral reefs survive global warming? Comp. Biochem. Physiol. A Mol. 143:S131.
Hoegh-Guldberg, O., Mumby, P. J., Hooten, A. J., Steneck, R. S., Greenfield, P., Gomez, E., et al. (2007). Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742. doi: 10.1126/science.1152509
Hoffmann, A. A., and Sgro, C. M. (2011). Climate change and evolutionary adaptation. Nature 470, 479–485. doi: 10.1038/nature09670
IPCC, A. (2013). “Climate change 2013: the physical science basis,” in Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, eds T. F. Stocker, D. Qin, G.-K. Plattner, M. Tignor, S. K. Allen, J. Boschung, et al. (Cambridge; New York, NY: Cambridge University Press), 1535.
Kleypas, J. A. (1996). Coral reef development under naturally turbid conditions: fringing reefs near Broad Sound, Australia. Coral Reefs 15, 153–167. doi: 10.1007/BF01145886
Kleypas, J. A., McManus, J. W., and Meñez, L. A. (1999). Environmental limits to coral reef development: where do we draw the line? Am. Zool. 39, 146–159.
Kuta, K., and Richardson, L. (2002). Ecological aspects of black band disease of corals: relationships between disease incidence and environmental factors. Coral Reefs 21, 393–398. doi: 10.1007/s00338-002-0261-6
Lesser, M. P., Bythell, J. C., Gates, R. D., Johnstone, R. W., and Hoegh-Guldberg, O. (2007). Are infectious diseases really killing corals? Alternative interpretations of the experimental and ecological data. J. Exp. Mar. Biol. Ecol. 346, 36–44. doi: 10.1016/j.jembe.2007.02.015
Loya, Y., Sakai, K., Yamazato, K., Nakano, Y., Sambali, H., and van Woesik, R. (2001). Coral bleaching: the winners and the losers. Ecol. Lett. 4, 122–131. doi: 10.1046/j.1461-0248.2001.00203.x
Muller, E. M., Rogers, C. S., Spitzack, A. S., and van Woesik, R. (2008). Bleaching increases likelihood of disease on Acropora palmata (Lamarck) in Hawksnest Bay, St John, US Virgin Islands. Coral Reefs 27, 191–195. doi: 10.1007/s00338-007-0310-2
Muller, E. M., and van Woesik, R. (2012). Caribbean coral diseases: primary transmission or secondary infection? Glob. Change Biol. 18, 3529–3535. doi: 10.1111/gcb.12019
Pebesma, E. J. (2004). Multivariable geostatistics in S: the gstat package. Comput. Geosci. 30, 683–691. doi: 10.1016/j.cageo.2004.03.012
Poloczanska, E. S., Burrows, M. T., Brown, C. J., Molinos, J. G., Halpern, B. S., et al. (2016). Responses of marine organisms to climate change across oceans. Front. Mar. Sci. 3:62. doi: 10.3389/fmars.2016.00062
R Core Team (2016). R: A language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Randall, C. J., Jordan-Garza, A. G., Muller, E. M., and van Woesik, R. (2014). Relationships between the history of thermal stress and the relative risk of diseases of Caribbean corals. Ecology 95, 1981–1994. doi: 10.1890/13-0774.1
Randall, C. J., and van Woesik, R. (2015). Contemporary white-band disease in Caribbean corals driven by climate change. Nat. Clim. Change 5, 375–379. doi: 10.1038/nclimate2530
Sheridan, C., Grosjean, P. h., Leblud, J., Palmer, C. V., Kushmaro, A., and Eeckhaut, I. (2014). Sedimentation rapidly induces an immune response and depletes energy stores in a hard coral. Coral Reefs 33, 1067–1076. doi: 10.1007/s00338-014-1202-x
Smith, S. G., Swanson, D. W., Chiappone, M., Miller, S. L., and Ault, J. S. (2011). Probability sampling of stony coral populations in the Florida Keys. Environ. Monit. Assess. 183, 121–138. doi: 10.1007/s10661-011-1912-2
Smith, T. B., Glynn, P. W., Maté, J. L., Toth, L. T., and Gyory, J. (2014). A depth refugium from catastrophic coral bleaching prevents regional extinction. Ecology 95, 1663–1673.
Takahashi, S., Nakamura, T., Sakamizu, M., van Woesik, R., and Yamasaki, H. (2004). Repair machinery of symbiotic photosynthesis as the primary target of heat stress for reef-building corals. Plant Cell Physiol. 45, 251–255. doi: 10.1093/pcp/pch028
Thompson, J. N. (1998). Rapid evolution as an ecological process. Trends Ecol. Evol. 13, 329–332. doi: 10.1016/S0169-5347(98)01378-0
Tomascik, T., and Sander, F. (1987). Barbados, West Indies. Mar. Biol. 94, 53–75. doi: 10.1007/BF00392900
Tomascik, T., and Suharsono Mah, A. J. (1993). “Case histories: a historical perspective of the natural and anthropogenic impacts in the Indonesian Archipelago with a focus on the Kepulauan Seribu, Java Sea,” in Proceedings of the Colloquium on Global Aspects of Coral Reefs: Health, Hazards and History 1993, ed R. N. Ginsburg (Florida, FL: University of Miami), 304–310.
Toth, L. T., Aronson, R. B., Vollmer, S. V., Hobbs, J. W., Urrego, D. H., Cheng, H., et al. (2012). ENSO drove 2500-year collapse of eastern Pacific coral reefs. Science 337, 81–84. doi: 10.1126/science.1221168
van Woesik, R., Erik, C., Franklin, E. C., O'Leary, J., McClanahan, T. R., Klaus, J. S., et al. (2012). Hosts of the Plio-Pleistocene past reflect modern-day coral vulnerability. Proc. R. Soc. B 279, 2448–2456. doi: 10.1098/rspb.2011.2621
Voss, J. D., and Richardson, L. L. (2006). Nutrient enrichment enhances black band disease progression in corals. Coral Reefs 25, 569–576. doi: 10.1007/s00338-006-0131-8
Wagner, D., Kramer, P., and van Woesik, R. (2010). Species composition, habitat, and water quality influence coral bleaching in southern Florida. Mar. Ecol. Prog. Ser. 408, 65–78. doi: 10.3354/meps08584
Warner, M. E., Fitt, W. K., and Schmidt, G. W. (1999). Damage to photosystem II in symbiotic dinoflagellates: a determinant of coral bleaching. Proc. Natl. Acad. Sci. U.S.A. 96, 8007–8012. doi: 10.1073/pnas.96.14.8007
Wiedenmann, J., D'Angelo, C., Smith, E. G., Hunt, A. N., Legiret, F. E., Postle, A. D., et al. (2013). Nutrient enrichment can increase the susceptibility of reef corals to bleaching. Nat. Clim. Change 3, 160–164. doi: 10.1038/nclimate1661
Keywords: corals, bleaching, disease outbreaks, climate change, directional selection
Citation: van Woesik R and McCaffrey KR (2017) Repeated Thermal Stress, Shading, and Directional Selection in the Florida Reef Tract. Front. Mar. Sci. 4:182. doi: 10.3389/fmars.2017.00182
Received: 12 January 2017; Accepted: 24 May 2017;
Published: 08 June 2017.
Edited by:
David Suggett, University of Technology Sydney, AustraliaReviewed by:
Charles Alan Jacoby, St. Johns River Water Management District, United StatesAldo Cróquer, Simón Bolívar University, Venezuela
Copyright © 2017 van Woesik and McCaffrey. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Robert van Woesik, rvw@fit.edu
 Robert van Woesik
Robert van Woesik Kelly R. McCaffrey
Kelly R. McCaffrey