Effect of the Silica Content of Diatoms on Protozoan Grazing
- Division of Life Science, The Hong Kong University of Science and Technology, Kowloon, Hong Kong
This study examined the effect that silica content in diatom cells has on the behavior of protists. The diatoms Thalassiosira weissflogii and T. pseudonana were cultured in high or low light conditions to achieve low and high silica contents, respectively. These cells were then fed to a heterotrophic dinoflagellate Noctiluca scintillans and a ciliate Euplotes sp. in single and mixed diet experiments. Our results showed that in general, N. scintillans and Euplotes sp. both preferentially ingested the diatoms with a low silica content rather than those with a high silica content. However, Euplotes sp. seemed to be less influenced by the silica content than was N. scintillans. In the latter case, the clearance and ingestion rate of the low silica diatoms were significantly higher, both in the short (6-h) and long (1-d) duration grazing experiments. Our results also showed that N. scintillans required more time to digest the high silica-containing cells. As the high silica diatoms are harder to digest, this might explain why N. scintillans exhibits a strong preference for the low silica prey. Thus, the presence of high silica diatoms might limit the ability of the dinoflagellate to feed. Our findings suggest that the silica content of diatoms affects their palatability and digestibility and, consequently, the grazing activity and selectivity of protozoan grazers.
Introduction
Diatoms are one of the most prevalent groups of phytoplankton in the world oceans. They are responsible for ~40% of the oceanic primary productivity (Nelson et al., 1995; Smetacek, 1999), which makes them an important component of marine food webs and a major source of carbon export. Diatoms can vary greatly in both size and form, but they all have a characteristic outer cell wall (or frustule) made of biogenic silica. A number of possible functions of the frustule have been suggested, such as offering protection from photoinhibition; providing an increased uptake of nutrients; controlling the sinking rate; and acting as a mechanical defense against grazers (Martin-Jézéquel et al., 2000; Hamm et al., 2003; Raven and Waite, 2004; Finkel and Kotrc, 2010; Spillane, 2016). The silica content of diatoms varies both within and among species, according to size (Paasche, 1973; Durbin, 1977; Waite et al., 1997) and growth phase (Brzezinski et al., 1990; Martin-Jézéquel et al., 2000; Hildebrand, 2006), as well as in response to environmental factors, such as light, temperature, salinity and nutrients (Bienfang et al., 1983; Martin-Jézéquel et al., 2000; Claquin et al., 2002; Vrieling et al., 2007; Shatwell et al., 2013).
Diatoms are an important food source for zooplankton grazers. It has been suggested that large zooplankton such as copepods act as the sole link between oceanic primary production and the higher trophic levels of the food chain (Turner, 2004, 2014). However, other studies have shown that the dominant grazers of marine diatoms are microzooplankton (<200 μm), due to their high abundance, short generation time and various feeding strategies (Lessard, 1991; Strom et al., 2001; Sherr and Sherr, 2002, 2007; Strom, 2002; Landry and Calbet, 2004). Strom et al. (2001) suggested that microzooplankton grazers, which consist largely of protists such as ciliates and heterotrophic dinoflagellates, account for an average of ~65% of the grazing that occurs on bloom-forming diatoms. In contrast, mesozooplankton grazing only contributes to an average of ~22% (or less) of the total predation on diatoms (Calbet, 2001; Strom et al., 2001). These protozoan grazers thus play two important ecological roles in the marine ecosystem: (1) the transfer of organic material up the food chain, when they are themselves consumed by larger zooplankton (Stoecker and Capuzzo, 1990); and (2) the recycling of nutrients, via the release of inorganic and organic matter in both dissolved and particulate forms (Caron, 1990; Nagata and Kirchman, 1991, 1992; Strom et al., 1997). When microzooplankton graze on diatoms, they produce lightweight, easily accessible detritus, which can then be hydrolyzed by bacteria in the microbial loop (Schultes et al., 2010). They also produce detrital bSiO2, which promotes the recycling of Si(OH)4 in the microbial loop (Schultes et al., 2010). For example, when pallium-feeding thecate dinoflagellates feed on diatoms, they discard the empty silica frustules (Jacobson and Anderson, 1986), whereas athecate dinoflagellates, which directly ingest diatoms, produce mini-fecal pellets composed of the frustules with little associated carbon (Buck and Newton, 1995; Strom and Strom, 1996; Saito et al., 2006).
Previous studies investigating the importance of the silicification level of diatoms in diatom-grazer interactions have focused on the changes in diatom silica content in response to grazing pressure (Pondaven et al., 2007; Schultes et al., 2010). For example, Schultes et al. (2010) reported that microzooplankton grazing leads to the enhanced uptake of Si(OH)4 by diatoms, which suggests the potential influence of grazing on the silicification of diatom frustules. Many of the arguments in favor of mechanical defense, however, only considered predation from copepods and there has been relatively little attention paid to that from protists (Liu et al., 2016; Liu and Wu, 2016; Spillane, 2016). Liu et al. (2016) recently showed that the silica content of the diatom Thalassiosira weissflogii could be modulated by changing the intensity of light they were exposed to, whereas other parameters such as the size, cellular content and stoichiometry of other essential elements were not significantly affected. They also demonstrated that copepods can ingest significantly more low silica-containing diatom prey (grown in high light intensity conditions) than those with high silica content (grown in low light intensity conditions). In the present study, we investigated how the cellular silica content of diatoms affect protozoan grazing and digestion. We conducted a series of grazing experiments by feeding T. weissflogii or T. pseudonana, which were cultured under different light intensities (as described by Liu et al., 2016), so that they contained different levels of biogenic silica, to the protists Noctiluca scintillans and Euplotes sp. To further examine the influence of the silica content of diatoms on protist digestion, we determined the food vacuole defecation rate of N. scintillans after it was fed with diatoms cells of different silica content.
N. scintillans is a cosmopolitan red tide-forming heterotrophic dinoflagellate (of ~200–2,000 μm in diameter), which is widely distributed throughout temperate-to-subtropical coastal waters (Elbrächter and Qi, 1998; Harrison et al., 2011). Many studies have shown that N. scintillans feeds mainly on phytoplankton, particularly diatoms (Schaumann et al., 1988; Umani et al., 2004; Harrison et al., 2011; Zhang et al., 2016). N. scintillans blooms are also known to co-occur with diatom blooms (Kiørboe et al., 1998; Nakamura, 1998; Tiselius and Kiørboe, 1998; Dela-Cruz et al., 2002). Euplotes sp., which are in the ciliate order Hypotrichida, are common in both freshwater and marine habitats. They are known for their ability to “walk” on a substrate, and they are filter feeders of suspended prey (Fenchel, 1986), feeding planktonically when sufficient suspended prey are available (Dolan, 1991). The results of our study suggest that these different grazers have different levels of tolerance for the silica content of their diatom prey. Overall, however, the silica content of the diatoms does affect the grazing and digestive behavior of both the protozoan grazers tested. These findings might have potential implications for the dynamics of bloom formation, food web structure, and biogeochemical cycles of carbon and nutrients in the coastal oceans.
Materials and Methods
Experimental Organisms and Culture Conditions
The diatoms Thalassiosira. weissflogii and T. pseudonana were maintained in exponential growth in f/2+Si medium (Guillard and Ryther, 1962). For most experiments, both species were kept at 22 ± 1°C in a 12:12 h light:dark cycle, under light intensities of 15 μmol photons m−2 s−1 and 200 μmol photons m−2 s−1, to generate cells with high and low cellular silica content, respectively. In the first grazing experiment, however, T. pseudonana was cultured at 23 ± 1°C in a 14:10 h light:dark cycle with light intensities of 5 μmol photons m−2 s−1 and 88 μmol photons m−2 s−1 to generate cells with high and low cellular silica content, respectively. The diatom cultures were transferred every 4 and 8 days for the high- and low-light batches, respectively.
The heterotrophic dinoflagellate N. scintillans and the ciliate Euplotes sp. were used as grazers in the present study. N. scintillans was fed with T. weissflogii at a concentration of ~1 mg C L−1, and this algal food was replenished every 3 d. As early experiments showed that continuous shaking and rotation caused deleterious effects on N. scintillans growth, these cultures were only gently agitated manually 1–2 times a day to keep the prey items homogeneously distributed. N. scintillans cells were collected by reverse filtration via a 100 μm mesh, and transferred to freshly filtered and autoclaved seawater every 2 wk. Euplotes sp. was kept in autoclaved filtered seawater containing 0.005% yeast extract, and transferred to fresh medium every 2 wk. These predator cultures were maintained in low light levels under the same conditions as their diatom prey.
Cultures were kept under the respective light conditions for at least 3 transfers before each experiment. The amount of biogenic silica (bSiO2) in the diatom cells was measured following the procedures described by Grasshoff et al. (1999). Cellular C, N and P of the diatoms in the second grazing experiment were also measured by filtering 10–15 ml of culture onto a pre-combusted GF/F filter, and storing the filters at −80°C until analysis. Cellular C and N were analyzed with a CHN elemental analyzer (Perkin-Elmer) and cellular P was analyzed as ortho-phosphate after acidic oxidative hydrolysis with 1% HCl (Grasshoff et al., 1999). The cell size of the diatom prey was determined using a Coulter Counter (Beckman Coulter, Z2 Coulter Particle Count and Size Analyzer).
Grazing Experiments
Two sets of grazing experiments were conducted. In the first series of experiments, the heterotrophic dinoflagellate N. scintillans and the ciliate Euplotes sp. were used as grazers, and they were fed separately with T. pseudonana that contained high or low levels of silica (Table 1), achieved via culture at different intensities of light as described above. N. scintillans and Euplotes sp. were adapted to T. pseudonana 1 week before the experiment. Stocks of these two predators were concentrated using reverse filtration to remove the prey from the medium. They were then resuspended in 0.2 μm filtered autoclaved seawater, and starved for 1–2 days to void their food vacuoles prior to the start of the experiment. Microscopic examination was carried out to verify that the predators were still active after starvation. Starved N. scintillans (final conc. 4 cells ml−1) and Euplotes sp. (final conc. 75 cells ml−1) were then placed into each food suspension (~ 1mg C L−1) in 125 ml polycarbonate bottles with five replicates. Two bottles containing prey items without grazers were used as controls. All the bottles were kept in low-light conditions (i.e., 5 μmol photons m−2 s−1), under the same conditions as described above (23 ± 1°C in a 14:10 h light:dark cycle), for 1 day. The cultures were gently agitated manually 2–3 times a day to avoid cell aggregation and settlement.
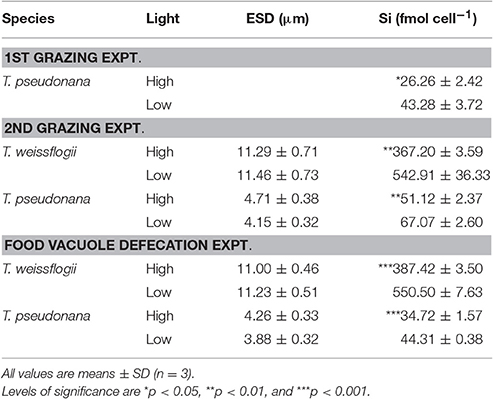
Table 1. Cell size (ESD, μm) and silica content (fmol cell−1) of the diatom prey used in each experiment.
As mentioned previously, N. scintillans blooms are usually associated with diatom blooms (Elbrächter and Qi, 1998; Harrison et al., 2011). Thus, in the second series of grazing experiments N. scintillans alone was used as the predator, in order to investigate further if the diatom silica content might affect the feeding preference of this bloom-forming dinoflagellate. Because it is hard to distinguish between the diatom cells that contain high or low levels of silica based on morphology alone, in this experiment, N. scintillans were fed a mixed diet by pairing a diatom containing a high or low level of silica, with a reference algal prey. Thus T. weissflogii was paired with Platymonas helgolandica and T. pseudonana was paired with Dunaliella salina. N. scintillans was also fed single prey, as described in the first grazing experiment, but in this experiment the feeding duration only lasted for 6 h. The two reference algae have previously been reported to be good prey for N. scintillans, and they are within a similar size range as the paired diatom species (Zhang et al., 2016). The ratio of two prey items in the mixed diet was ~1:1 based on the carbon biomass measured previously (Zhang et al., 2016), and the total prey concentration for all the food treatments was adjusted to ~1 mg C L−1.
Subsamples to determine the predator and prey abundances were collected at the start and end of the incubation period, and preserved in acidic Lugol's solution (final concentration 2%). Ingestion and clearance rates, and the prey consumption index of N. scintillans for each of the prey were determined. Ingestion rates, clearance rates and average prey and predator concentrations were calculated, as described by Frost (1972). The prey consumption index (values from 0 to 1; Zhang et al., 2016), an indicator of food preference, was calculated by comparing the frequency distribution of specific prey in the ambient environment and in the diet, as described by Chesson (1978).
Food Vacuole Evacuation Experiment
A food vacuole evacuation experiment was conducted to investigate the influence of the diatom silica content on the digestion of N. scintillans. In this experiment, N. scintillans cells in stock cultures were starved for 1 day before the start of the experiment. Then ~1,500 vacuole-free N. scintillans cells were separately inoculated into 300 ml food suspensions containing equal cell concentrations (~1 mg C L−1) of T. weissflogii or T. pseudonana with high or low silica contents. After incubation for 24 h, ~10 active N. scintillans cells containing food vacuoles were separated from the prey items, and transferred to 6-well plates that contained 10 ml of 0.2 μm-filtered autoclaved seawater (4–6 replicates). Cells were inspected under a dissecting microscope every hour over a period of 10 h for those previously reared on T. weissflogii, and every 3–7 h over a period of ~30 h for those previously reared on T. pseudonana. Digestion was considered to be complete only when there was no trace of food in the cell. The food vacuole defecation rate of N. scintillans on different types of diatom prey were calculated as the constant of the exponential decay plot of the percentage of the cells with food vacuoles vs. starvation time (Zhang et al., 2016). The volume of the food vacuoles in N. scintillans cells (n = 30) after 1 d incubation was calculated from their areas, which were measured using the SPOT image program (Version 3.5.0) and ImageJ (Version 2.0.0), and by assuming that they are spherical in shape.
Statistical Analysis
Student's t-tests (2-tailed) were performed using SigmaPlot 12.5 (Systat Software), and with significance levels of p < 0.05.
Results
Diatom Silica Content
The diatom prey T. weissflogii and T. pseudonana grown in low light (i.e., at 15 μmol photons m−2 s−1) consistently contained significantly higher levels of biogenic silica than those grown in the high light (i.e., at 200 μmol photons m−2 s−1) throughout our study (Table 1). A further comparison of the diatoms grown at high and low light intensity levels showed no significant difference in their equivalent spherical diameter (Table 1), but the cells grown under low light conditions (i.e., with high silica content, HSi) yielded higher contents of cellular carbon (C), nitrogen (N) and phosphorus (P) (Table 2). However, the significance of the difference of these elemental contents between the high and low silica diatoms varied between T. weissflogii and T. pseudonana. For example, the P content of T. pseudonana with high vs. low silica content was significantly different, whereas that of T. weissflogii was not (Table 2; Student's t-test, n = 6, p < 0.05). In addition, variations in the elemental stoichiometry of the diatom cells containing high and low silica were almost opposite for T. weissflogii and T. pseudonana (Table 2). Moreover, the elemental ratios of T. weissflogii with low and high silica content were all significantly different, apart from the Si:N ratio; whereas those of T. pseudonana showed no significant difference, except the C:P ratio.
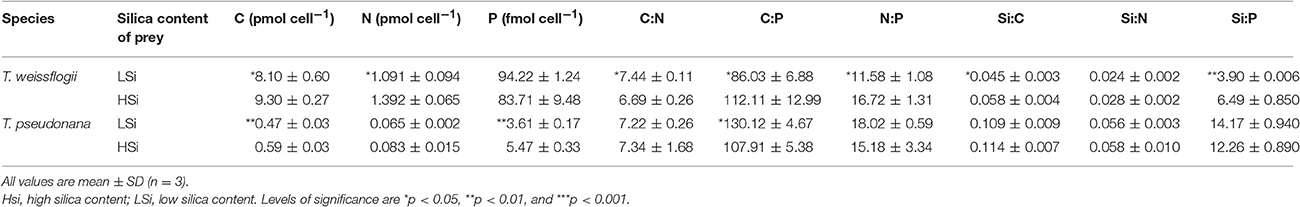
Table 2. Summary of the elemental contents and ratios of the diatom prey in the 2nd grazing experiment.
Grazing Response of Protists to the Diatoms with Different Levels of Silica Content
In the first series of grazing experiments (i.e., 1 d), both N. scintillans and Euplotes sp. showed higher clearance and ingestion rates for T. pseudonana with low silica content as compared to those with high silica content (Figure 1), although the difference was not significant for Euplotes sp. (Student's t-test, n = 6, p < 0.05).
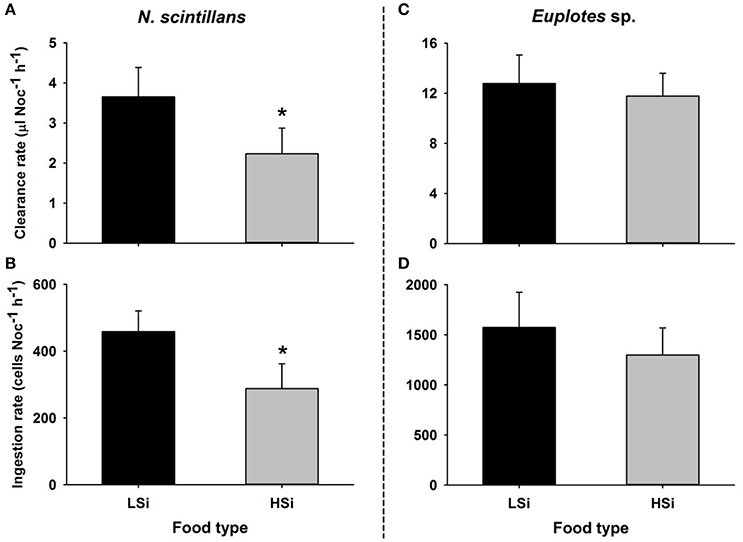
Figure 1. Clearance and ingestion rates of Noctiluca scintillans (A,B) and Euplotes sp. (C,D) on the diatom Thalassiosira pseudonana with different silica contents. LSi and HSi are low and high silica diatom prey, respectively. The error bars show 1 SD (n = 3). *indicates statistically significant differences at p < 0.05 (Student's t-test, 2-tailed).
The same trend was also observed in the second series of grazing experiments (i.e., 6 h), in that N. scintillans consumed significantly more T. weissflogii and T. pseudonana containing low silica content than they did those with high silica content (Figure 2). This trend was observed in both the single and mixed diet treatments. For example, in the mixed diet treatments, N. scintillans cleared T. weissflogii and T. pseudonana with low silica content at rates of 2.98 ± 0.22 and 2.78 ± 0.44 μl Noc−1 h−1, respectively, whereas they cleared T. weissflogii and T. pseudonana with high silica content at 1.86 ± 0.12 and 0.65 ± 0.08 μl Noc−1 h−1, respectively (Figures 2A,C). Accordingly, in the mixed diet treatments, the prey consumption index of N. scintillans for diatoms with low silica content was significantly higher than for those with high silica content (Student's t-test, n = 6, p < 0.05; Figure 3). These results indicate that the silica content of diatoms can affect the grazing activity and feeding preference of protozoan grazers.
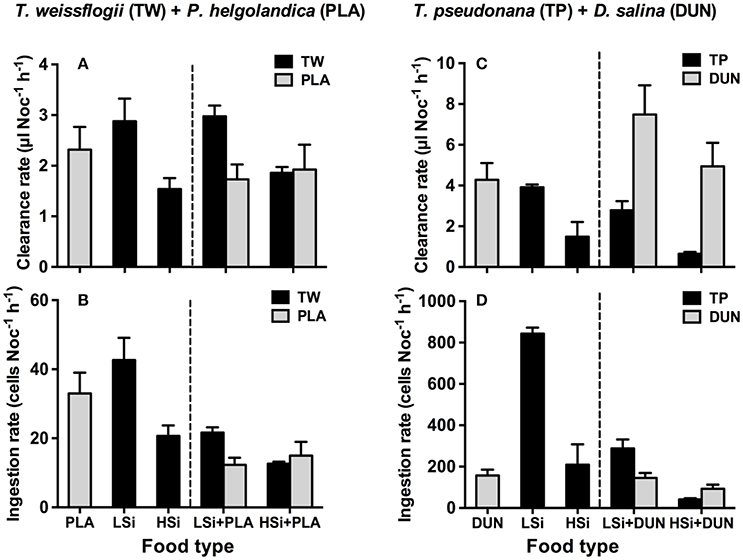
Figure 2. Clearance and ingestion rates of Noctiluca scintillans on Thalassiosira weissflogii (A,B) and T. pseudonana (C,D) that contain different levels of biogenic silica in the single and mixed diet treatments (i.e., T. weissflogii + Platymonas helgolandica, T. pseudonana + Dunaliella salina). LSi and HSi are low and high silica diatom prey, respectively. Error bars are 1 SD (n = 3).
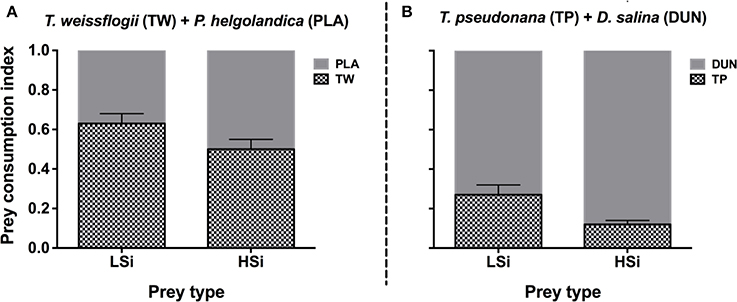
Figure 3. Prey consumption index of Noctiluca scintillans on either T. weissflogii + P. helgolandica (A), or T. pseudonana + D. salina (B). LSi and HSi are low and high silica-containing diatom prey, respectively. Error bars indicate 1 SD (n = 3).
Digestion of Diatoms with Different Levels of Silica Content
After a 1-d incubation with T. weissflogii or T. pseudonana, the N. scintillans that had been fed on low-silica diatoms contained more food vacuoles than those fed on high-silica diatoms (Figure 4). When N. scintillans consumed T. pseudonana with low or high silica content, the resulting food vacuoles occupied on average ~11.34 and ~9.1% of the cell volume, respectively. Similarly, consumption of T. weissflogii with low or high silica content resulted in food vacuoles comprising 5.36 and 5.15% of the cell volume, respectively (Figure 4). Moreover, food vacuoles containing the high silica forms of both T. weissflogii and T. pseudonana yielded a much slower decay plot than those containing low silica diatoms (Figure 5), suggesting that diatom cells with a high silica content are digested more slowly. It was also noted that the time it took for N. scintillans to digest T. weissflogii and T. pseudonana was different. In the case of T. weissflogii, it took ~10 h for >80% of the food vacuoles to be evacuated, whereas for T. pseudonana, it took >30 h for a similar amount of food vacuoles to be evacuated.
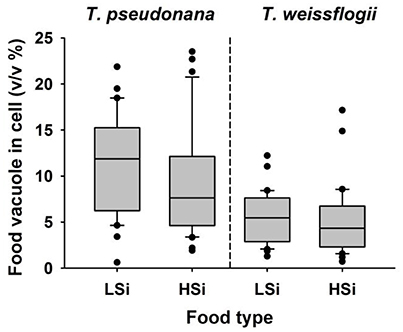
Figure 4. Percentage (v/v %) of Noctiluca scintillans cells that contain food vacuoles after 1 day feeding on T. weissflogii or T. pseudonana that contain different levels of biogenic silica (n = 30). LSi and HSi are low and high silica diatom prey, respectively.
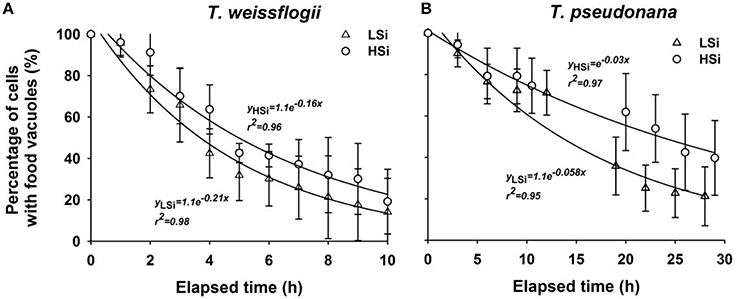
Figure 5. Change in percentage of food vacuoles over time in Noctiluca scintillans cells that have ingested T. weissflogii (A) or T. pseudonana (B) with different silica contents. LSi and HSi are low and high silica diatom prey, respectively. Error bars indicate 1 SD (n = 4–6).
Discussion
Many environmental conditions such as nutrient concentration, salinity, temperature and light, can affect the degree of silicification in diatoms (Bienfang et al., 1983; Martin-Jézéquel et al., 2000; Claquin et al., 2002; Vrieling et al., 2007; Shatwell et al., 2013). In non-silica limiting conditions, light intensity has been shown to affect the amount of silica per cell mainly due to a reduction in the growth rate (Paasche, 1980; Martin-Jézéquel et al., 2000; Liu et al., 2016; Liu and Wu, 2016). Our new results support those from previous studies (Liu et al., 2016; Liu and Wu, 2016), and show that when T. weissflogii and T. pseudonana are grown under low light intensity, they consistently contain a higher amount of biogenic silica, and when they are grown under high light intensity they contain a lower amount of biogenic silica (Table 1). In contrast, changes in cell volume and the content of other essential elements are negligible (Tables 1, 2).
Previous studies have shown that copepods preferentially feed on diatom species with more weakly silicified frustules and a higher growth rate, and their fecal pellets are produced and degraded rapidly. In contrast, they feed less on diatoms possessing more complex frustules with a high silica content and the fecal pellets produced exhibit greater stability (Friedrichs et al., 2013; Liu et al., 2016; Liu and Wu, 2016). Our results confirm that the protists N. scintillans and Euplotes sp. prefer to ingest low silica-containing diatoms, which suggests that their grazing activity might be (similar to copepods) affected by the silica content of the available food. However, it was also noted that the silica content of the diatoms did not significantly affect the grazing behavior of Euplotes sp. We hypothesize that the difference between the two protozoan grazers tested, might be due to their different feeding behaviors. N. scintillans has negligible swimming ability and usually exhibits positive buoyancy; it relies on its high ascent velocity for encountering prey (Kiørboe and Titelman, 1998). In contrast, Euplotes sp. are interstitial ciliates, which are normally associated with surfaces. Thus, although Euplotes sp. can swim freely, they are reported to prefer creeping along a substratum (Ricci et al., 1987). One might think that increasing the silica content of prey would result in an increase in cell density, which would lead to a higher sinking rate. Therefore, it is reasonable to assume that the positively-buoyant N. scintillans might have a problem ingesting diatoms with high silica content, whereas the substratum-associated Euplotes sp. are less likely to be affected. However, it has previously been reported that the extent of silicification is not directly correlated with the sinking rate (Bienfang et al., 1983; Culver and Smith, 1989; Waite et al., 1997). For example, Waite et al. (1997) demonstrated that T. weissflogii and T. pseudonana exhibit a contrasting hyperbolic relationship between the cell sinking rate and light intensity. T. pseudonana had higher sinking rate when grown under low light conditions (10 μmol m−2 s−1) than it did when grown in high light conditions (and the sinking rate was almost constant at irradiance >50 μmol m−2 s−1), whereas T. weissflogii exhibited the opposite sinking rate pattern. The same two diatom species were used as prey in our study; however, N. scintillans constantly consumed more, and preferentially fed on the low silica-containing cells of both species in both the short- (6 h) and long- (1 d) duration grazing experiments, as well as in the single and mixed diet treatment experiments (Figures 1, 2). The experimental bottles were gently agitated manually 2–3 times a day to avoid cell aggregation and settlement. Therefore, differences in the sinking rate of the high and low silica diatoms are not relevant for explaining the higher ingestion rate of (and preference for) the low silica-containing diatoms by the protists.
It has been previously suggested that thicker frustules reduce the digestibility of diatom cells (Raven and Waite, 2004). In our present study, the food vacuole evacuation experiment showed that N. scintillans find it harder to digest diatoms with a higher silica content. Therefore, the lower ingestion rate, and reduced preference of N. scintillans for high silica-containing diatoms, might be because the latter are harder to digest. The diatoms therefore remain in the food vacuoles longer, and thus limit the ability of N. scintillans to feed. This general phenomenon has been widely observed in zooplankton (Donk et al., 1997; Smetacek, 1999; Hamm et al., 2003; Spillane, 2016). Most recently, Spillane (2016) observed a significantly lower digestion rate of N. scintillans when feeding on thick-frustuled diatoms, which lead to lower carbon-based ingestion rates and hence growth rates. In addition, Liu and Wu (2016) showed that when Calanus sinicus were fed on a high concentration of highly silicified diatom prey (induced by changing the level of light irradiance), they exhibited a lower grazing and fecal pellet production rate when compared with those fed on a similar amount of low silica content diatom prey. Therefore, diatoms with the higher silica content might be less palatable and harder to digest, which could explain the lower ingestion and clearance rates observed both in protists (the present study) and in copepods (Liu et al., 2016; Liu and Wu, 2016).
In addition to prey digestibility, the nutritional quality of the available food source is also considered to be an important factor governing the feeding preference of grazers (Sterner, 1989; Landry et al., 1991; Verity, 1991; DeMott, 1998). Zhang et al. (2015) reported that the ΣALA (α-linolenic acid) +EPA (eicosapentaneoic acid) and P content of diets represent good indicators of food quality for N. scintillans. In this study, we observed some differences in the elemental composition of the diatom cells grown in the different light intensities (Table 2), although the differences were not always consistent or significant (Liu et al., 2016). Species-specific differences were also observed with regards to the effect of light intensity. For example, for T. pseudonana, a significantly higher P content was observed when cells were grown in low light (i.e., with a high silica content), but for T. weissflogii the P content was not significantly different when comparing the low- and high-light intensity-exposed cells (Table 2). Although we did not measure the composition of fatty acids in this study, it has previously been shown that in T. pseudonana the lipid quota and percentage of EPA (the major polyunsaturated fatty acid in diatoms) are similar (or even lower) when they are grown in high or low light intensity conditions (i.e., 6 μmol m−2 s−1 and 25 μmol m−2 s−1, respectively; Harrison et al., 1990). Therefore, the variance in the nutritional quality of the diatom prey (after being subjected to different light intensities) does not appear to be the cause of the higher preference of N. scintillans for low silica diatoms. Rather, the silica content of the diatoms per se might play a more prominent role in determining their palatability and digestibility as a food source for N. scintillans. Obviously, the level of such influence varies depending on the types of prey and predator examined.
It should be mentioned that the concentration of prey used in our experiments was typically higher than that found in the natural environment. Feeding selectively requires the expenditure of energy, so there should be a tradeoff between the cost of selective feeding and the benefits it provides. This is why selectivity usually eases off when the concentration of prey is low and the differences in the type of prey are minor. Nevertheless, the concentration of prey we used in this study is comparable to those observed during the diatom/Noctiluca blooms that occur in the eastern waters of Hong Kong every winter (Zhang et al., in press). It is therefore likely that during the early stages of a diatom bloom, the silica content of cells is low due to sufficient levels of light and nutrients, and the zooplankton feeding rate is concomitantly relatively high, which leads to a rapid increase of N. scintillans. As the bloom proceeds, the silica content of diatom cells increases due to the depletion of silicate and the growth rate decreases because of nutrient exhaustion and/or shading due to the increase in algal density. This results in a lower ingestion rate for N. scintillans and other zooplankton grazers. This might explain why diatom blooms persist longer than other competing algae (Gobler and Sunda, 2012).
In conclusion, the results of our study suggest that the silica content of diatom cells affects their ingestion and digestion by protists. In addition, the reduction in the feeding preference and ingestion rate, and the increase in the digestion time of protists, such as N. scintillans, when consuming high silica-containing diatom cells, might explain the long-lasting nature of diatom blooms.
Author Contributions
SZ and HL conceived the study. HL obtained funding. SZ, YK, and BL conducted the experiments, and SZ and HL wrote the paper. All the authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the Research Grant Council of Hong Kong via GRF awards: 661610, 661911, and 661912; and the TUYF Charitable Trust (TUYF10SC08). Additional support from the State Key Laboratory in Marine Pollution (SKLMP) Seed Collaborative Research Fund (SKLMP/ SCRF/0006) is also acknowledged.
References
Bienfang, P., Szyper, J., and Laws, E. (1983). Sinking rate and pigment responses to light-limitation of a marine diatom-implications to dynamics of chlorophyll maximum layers. Oceanol. Acta 6, 55–62.
Brzezinski, M., Olson, R., and Chisholm, S. (1990). Silicon availability and cell-cycle progression in marine diatoms. Mar. Ecol. Prog. Ser. 67, 83–96. doi: 10.3354/meps067083
Buck, K. R., and Newton, J. (1995). Fecal pellet flux in Dabob Bay during a diatom bloom - contribution of microzooplankton. Limnol. Oceanogr. 40, 306–315. doi: 10.4319/lo.1995.40.2.0306
Calbet, A. (2001). Mesozooplankton grazing effect on primary production: a global comparative analysis in marine ecosystems. Limnol. Oceanogr. 46, 1824–1830. doi: 10.4319/lo.2001.46.7.1824
Caron, D. A. (1990). “Protozoan nutrient regeneration,” in Ecology of Marine Protozoa, ed G. M. Capriulo (New York, NY: Oxford University Press), 283–306.
Chesson, J. (1978). Measuring preference in selective predation. Ecology 59, 211–215. doi: 10.2307/1936364
Claquin, P., Martin-Jézéquel, V., Kromkamp, J. C., Veldhuis, M. J. W., and Kraay, G. W. (2002). Uncoupling of silicon compared with carbon and nitrogen metabolisms and the role of the cell cycle in continuous cultures to Thalassiosira pseudonana (Bacillariophyceae) under light, nitrogen, and phosphorus control. J. Phycol. 38, 922–930. doi: 10.1046/j.1529-8817.2002.t01-1-01220.x
Culver, M. E., and Smith, W. O. (1989). Effects of environmental variation on sinking rates of marine phytoplankton. J. Phycol. 25, 262–270. doi: 10.1111/j.1529-8817.1989.tb00122.x
Dela-Cruz, J., Ajani, P., Lee, R., Pritchard, T., and Suthers, I. (2002). Temporal abundance patterns of the red tide dinoflagellate Noctiluca scintillans along the southeast coast of Australia. Mar. Ecol. Prog. Ser. 236, 75–88. doi: 10.3354/meps236075
DeMott, W. R. (1998). Utilization of a cyanobacterium and a phosphorus-deficient green alga as complementary resources by Daphnids. Ecology 79, 2463–2481. doi: 10.1890/0012-9658(1998)079[2463:UOACAA]2.0.CO;2
Dolan, J. R. (1991). Guilds of ciliate microzooplankton in the Chesapeake Bay. Estuar. Coast. Shelf Sci. 33, 137–152. doi: 10.1016/0272-7714(91)90003-T
Donk, V. E., Lürling, M., Hessen, D., and Lokhorst, G. (1997). Altered cell wall morphology in nutrient-deficient phytoplankton and its impact on grazers. Limnol. Oceanogr. 42, 357–364. doi: 10.4319/lo.1997.42.2.0357
Durbin, E. G. (1977). Studies on the autecology of the marine diatom Thalassiosira nordenskioeldii. The influence of cell size on growth rate, and carbon, nitrogen, chlorophyll a and silica content. J. Phycol. 13, 150–155.
Elbrächter, M., and Qi, Y. (1998). “Aspects of Noctiluca (Dinophyceae) population dynamics,” in Ecology of Harmful Algal Blooms, ed M. D. Anderson (Berlin: Springer-Verlag), 315−335.
Fenchel, T. (1986). “Protozoan filter feeding,” in Progress in Protistology, eds J. O. Corliss and D. J. Patterson (Bristol: Biopress Ltd.), 65–113.
Finkel, Z. V., and Kotrc, B. (2010). Silica use through time: macroevolutionary change in the morphology of the diatom fustule. Geomicrobiol. J. 27, 596–608. doi: 10.1080/01490451003702941
Friedrichs, L., Hörnig, M., Schulze, L., Bertram, A., Jansen, S., and Hamm, C. (2013). Size and biomechanic properties of diatom frustules influence food uptake by copepods. Mar. Ecol. Prog. Ser. 481, 41–51. doi: 10.3354/meps10227
Frost, B. (1972). Effects of size and concentration of food particles on the feeding behavior of the marine planktonic copepod Calanus pacificus. Limnol. Oceanogr. 17, 805−815. doi: 10.4319/lo.1972.17.6.0805
Gobler, C. J., and Sunda, W. G. (2012). Ecosystem disruptive algal blooms of the brown tide species, Aureococcus anophagefferens and Aureoumbra lagunensis. Harmful Algae 14, 36–45. doi: 10.1016/j.hal.2011.10.013
Grasshoff, K., Kremling, K., and Ehrhardt, M. (1999). Methods of seawater analysis. Weinheim: Wiley-Vch. doi: 10.1002/9783527613984
Guillard, R. R. L., and Ryther, J. H. (1962). Studies of marine plankton diatoms: I. Cyclotella nana Hustedt, and Detonula confervacea (Cleve) Gran. Can. J. Microbiol. 8, 229–239. doi: 10.1139/m62-029
Hamm, C. E., Merkel, R., Springer, O., Jurkojc, P., Maier, C., Prechtel, K., et al. (2003). Architecture and material properties of diatom shells provide effective mechanical protection. Nature 421, 841–843. doi: 10.1038/nature01416
Harrison, P., Furuya, K., Glibert, P., Xu, J., Liu, H., Yin, K., et al. (2011). Geographical distribution of red and green Noctiluca scintillans. Chin. J. Oceanol. Limnol. 29, 807–831. doi: 10.1007/s00343-011-0510-z
Harrison, P., Thompson, P., and Calderwood, G. (1990). Effects of nutrient and light limitation on the biochemical composition of phytoplankton. J. Appl. Phycol. 2, 45–56. doi: 10.1007/BF02179768
Hildebrand, M. (2006). “Silicic acid transport and its control during cell wall silicification in diatoms,” in Biomineralization: From Biology to Biotechnology and Medical Application, ed E. Baeuerlein (Weinheim; New York: Wiley-VCH), 171–188.
Jacobson, D. M., and Anderson, D. M. (1986). Thecate heterotrophic dinoflagellates: feeding behavior and mechanisms. J. Phycol. 22, 249–258. doi: 10.1111/j.1529-8817.1986.tb00021.x
Kiørboe, T., Tiselius, P., Mitchell-Innes, B., Hansen, J. L., Visser, A. W., and Mari, X. (1998). Intensive aggregate formation with low vertical flux during an upwelling-induced diatom bloom. Limnol. Oceanogr. 43, 104–116. doi: 10.4319/lo.1998.43.1.0104
Kiørboe, T., and Titelman, J. (1998). Feeding, prey selection and prey encounter mechanisms in the heterotrophic dinoflagellate Noctiluca scintillans. J. Plankton Res. 20, 1615–1636. doi: 10.1093/plankt/20.8.1615
Landry, M., Lehner-Fournier, J., Sundstrom, J., Fagerness, V., and Selph, K. (1991). Discrimination between living and heat-killed prey by a marine zooflagellate, Paraphysomonas vestita (Stokes). J. Exp. Mar. Ecol. Biol. 146, 139–151. doi: 10.1016/0022-0981(91)90021-N
Landry, M. R., and Calbet, A. (2004). Microzooplankton production in the oceans. ICES J. Mar. Sci. 61, 501–507. doi: 10.1016/j.icesjms.2004.03.011
Lessard, E. (1991). The trophic role of heterotrophic dinoflagellates in diverse marine environments. Mar. Microb. Food Webs 5, 49–58.
Liu, H., Chen, M., Zhu, F., and Harrison, P. J. (2016). Effect of diatom silica content on copepod grazing, growth and reproduction. Front. Mar. Sci. 3:89. doi: 10.3389/fmars.2016.00089
Liu, H., and Wu, C.-J. (2016). Effect of the silica content of diatom prey on the production, decomposition and sinking of fecal pellets of the copepod Calanus sinicus. Biogeosciences 13, 4767–4775. doi: 10.5194/bg-13-4767-2016
Martin-Jézéquel, V., Hildebrand, M., and Brzezinski, M. A. (2000). Silicon metabolism in diatoms: implications for growth. J. Phycol. 36, 821–840. doi: 10.1046/j.1529-8817.2000.00019.x
Nagata, T., and Kirchman, D. L. (1991). Release of dissolved free and combined amino acids by bacterivorous marine flagellates. Limnol. Oceanogr. 36, 433–443. doi: 10.4319/lo.1991.36.3.0433
Nagata, T., and Kirchman, D. L. (1992). Release of macromolecular organic complexes by heterotrophic marine flagellates. Mar. Ecol. Prog. Ser. 83, 233–240. doi: 10.3354/meps083233
Nakamura, Y. (1998). Biomass, feeding and production of Noctiluca scintillans in the Seto Inland Sea, Japan. J. Plankton Res. 20, 2213–2222. doi: 10.1093/plankt/20.11.2213
Nelson, D. M., Tréguer, P., Brzezinski, M. A., Leynaert, A., and Quéguiner, B. (1995). Production and dissolution of biogenic silica in the ocean: Revised global estimates, comparison with regional data and relationship to biogenic sedimentation. Global Biogeochem. Cycl. 9, 359–372. doi: 10.1029/95GB01070
Paasche, E. (1973). The influence of cell size on growth rate, silica content, and some other properties of four marine diatom species. Norw. J. Bot. 20, 197–204.
Paasche, E. (1980). Silicon content of five marine plankton diatom species measured with a rapid filter method. Limnol. Oceanogr. 25, 474–480. doi: 10.4319/lo.1980.25.3.0474
Pondaven, P., Gallinari, M., Chollet, S., Bucciarelli, E., Sarthou, G., Schultes, S., et al. (2007). Grazing-induced changes in cell wall silicification in a marine diatom. Protist 158, 21–28. doi: 10.1016/j.protis.2006.09.002
Raven, J., and Waite, A. (2004). The evolution of silicification in diatoms: inescapable sinking and sinking as escape? New Phytol. 162, 45–61. doi: 10.1111/j.1469-8137.2004.01022.x
Ricci, N., Giannetti, R., and Micelli, C. (1987). The ethogram of Euplotes crassus (Ciliata, Hypotrichida). I. The wild type. Eur. J. Protistol. 23, 129–140. doi: 10.1016/S0932-4739(88)80056-7
Saito, H., Ota, T., Suzuki, K., Nishioka, J., and Tsuda, A. (2006). Role of heterotrophic dinoflagellate Gyrodinium sp. in the fate of an iron induced diatom bloom. Geophysical. Res. Let. 33:L09602. doi: 10.1029/2005gl025366
Schaumann, K., Gerdes, D., and Hesse, K. (1988). Hydrographic and biological characteristics of a Noctiluca scintillans red tide in the German Bight, 1984. Meeresforschung 32, 77–91.
Schultes, S., Lambert, C., Pondaven, P., Corvaisier, R., Jansen, S., and Ragueneau, O. (2010). Recycling and uptake of Si(OH)4 when protozoan grazers feed on diatoms. Protist 161, 288–303. doi: 10.1016/j.protis.2009.10.006
Shatwell, T., KÃűhler, J., and Nicklisch, A. (2013). Temperature and photoperiod interactions with silicon-limited growth and competition of two diatoms. J. Plankton Res. 35, 957–971. doi: 10.1093/plankt/fbt058
Sherr, E. B., and Sherr, B. F. (2002). Significance of predation by protists in aquatic microbial food webs. Anton. Leeuw. Int. J. G. 81, 293–308. doi: 10.1023/A:1020591307260
Sherr, E. B., and Sherr, B. F. (2007). Heterotrophic dinoflagellates: a significant component of microzooplankton biomass and major grazers of diatoms in the sea. Mar. Ecol. Prog. Ser. 352, 187–197. doi: 10.3354/meps07161
Smetacek, V. (1999). Diatoms and the ocean carbon cycle. Protist 150, 25–32. doi: 10.1016/S1434-4610(99)70006-4
Spillane, T. (2016). Diatom Frustules as a Mechanical Defense Against Predation by Heterotrophic Dinoflagellates [dissertation/master's thesis]. Western Washington University.
Sterner, R. W. (1989). “The role of grazers in phytoplankton succession,” in Plankton Ecology – Succession in Plankton Communities, ed U. Sommer (Berlin: Springer-Verlag), 107–170.
Stoecker, D. K., and Capuzzo, J. M. (1990). Predation on protozoa: its importance to zooplankton. J. Plankton Res. 12, 891–908. doi: 10.1093/plankt/12.5.891
Strom, S. (2002). Novel interactions between phytoplankton and microzooplankton: their influence on the coupling between growth and grazing rates in the sea. Hydrobiologia 480, 41–54. doi: 10.1023/A:1021224832646
Strom, S., Brainard, M., Holmes, J., and Olson, M. (2001). Phytoplankton blooms are strongly impacted by microzooplankton grazing in coastal North Pacific waters. Mar. Biol. 138, 355–368. doi: 10.1007/s002270000461
Strom, S. L., Benner, R., Ziegler, S., and Dagg, M. J. (1997). Planktonic grazers are a potentially important source of marine dissolved organic carbon. Limnol. Oceanogr. 42, 1364–1374. doi: 10.4319/lo.1997.42.6.1364
Strom, S. L., and Strom, M. W. (1996). Microplankton growth, grazing, and community structure in the northern Gulf of Mexico. Mar. Ecol. Prog. Ser. 130, 229–240. doi: 10.3354/meps130229
Tiselius, P., and Kiørboe, T. (1998). Colonization of diatom aggregates by the dinoflagellate Noctiluca scintillans. Limnol. Oceanogr. 43, 154–159. doi: 10.4319/lo.1998.43.1.0154
Turner, J. T. (2004). The importance of small planktonic copepods and their roles in pelagic marine food webs. Zool. Stud. 43, 255–266.
Turner, J. T. (2014). Planktonic marine copepods and harmful algae. Harmful Algae 32, 81–93. doi: 10.1016/j.hal.2013.12.001
Umani, S. F., Beran, A., Parlato, S., Virgilio, D., Zollet, T., De Olazabal, A., et al. (2004). Noctiluca scintillans Macartney in the Northern Adriatic Sea: long-term dynamics, relationships with temperature and eutrophication, and role in the food web. J. Plankton Res. 26, 545–561. doi: 10.1093/plankt/fbh045
Verity, P. G. (1991). Measurement and simulation of prey uptake by marine planktonic ciliates fed plastidic and aplastidic nanoplankton. Limnol. Oceanogr. 36, 729–750. doi: 10.4319/lo.1991.36.4.0729
Vrieling, E. G., Sun, Q., Tian, M., Kooyman, P. J., Gieskes, W. W., van Santen, R. A., et al. (2007). Salinity-dependent diatom biosilicification implies an important role of external ionic strength. Proc. Natl. Acad. Sci. U.S.A. 104, 10441–10446. doi: 10.1073/pnas.0608980104
Waite, A., Fisher, A., Thompson, P. A., and Harrison, P. J. (1997). Sinking rate versus cell volume relationships illuminate sinking rate control mechanisms in marine diatoms. Mar. Ecol. Prog. Ser. 157, 97–108. doi: 10.3354/meps157097
Zhang, S., Liu, H., Chen, B., and Wu, C.-J. (2015). Effects of diet nutritional quality on the growth and grazing of Noctiluca scintillans. Mar. Ecol. Prog. Ser. 527, 73–85. doi: 10.3354/meps11219
Zhang, S., Liu, H., Guo, C., and Harrison, P. J. (2016). Differential feeding and growth of Noctiluca scintillans on monospecific and mixed diets. Mar. Ecol. Prog. Ser. 549, 27–40. doi: 10.3354/meps11702
Keywords: diatom silica content, protozoa, grazing, food preference, digestion
Citation: Zhang S, Liu H, Ke Y and Li B (2017) Effect of the Silica Content of Diatoms on Protozoan Grazing. Front. Mar. Sci. 4:202. doi: 10.3389/fmars.2017.00202
Received: 20 January 2017; Accepted: 12 June 2017;
Published: 23 June 2017.
Edited by:
Agostino Merico, Leibniz Centre for Tropical Marine Research (LG), GermanyReviewed by:
Francesc Peters, Institut de Ciències del Mar (CSIC), SpainHans Uwe Dahms, Kaohsiung Medical University, Taiwan
Copyright © 2017 Zhang, Liu, Ke and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongbin Liu, liuhb@ust.hk
 Shuwen Zhang
Shuwen Zhang Hongbin Liu
Hongbin Liu Ying Ke
Ying Ke Beatrice Li
Beatrice Li