Fronts at the Surface Ocean Can Shape Distinct Regions of Microbial Activity and Community Assemblages Down to the Bathypelagic Zone: The Azores Front as a Case Study
- 1Department of Marine Science, University of Otago, Dunedin, New Zealand
- 2NIWA/University of Otago Research Centre for Oceanography, Dunedin, New Zealand
- 3Instituto de Oceanografía y Cambio Global, Universidad de Las Palmas de Gran Canaria, Las Palmas, Spain
Oceanic fronts are widespread features which separate distinct water masses. They are well known to control the distribution of microbial communities in surface waters, although there is scarce information on their role in delimiting critical functions that microbes perform, and on whether their effects can be translated down into the dark ocean. Here we carried out the first study on the variability of hydrolysis of organic matter (extracellular enzymatic activity; EEA) across a permanent front (the Azores Front), coupled with changes in microbial assemblage composition, from the surface down to the bathypelagic zone. The front separated the study area (enclosed into the North Atlantic Subtropical Gyral Province) into two distinct latitudinal sub-regions with sharp differences in the abundance of autotrophic and heterotrophic microbial assemblages, as well as in the extracellular enzymes activities of glucosidases, alkaline phosphatase, and leucine aminopeptidase. South of the front there was an abrupt decline in the abundance of picophytoplankton as well as in heterotrophic prokaryotes with high nucleic-acid content, but an increase in the abundance of prokaryotes with high side-scatter, an indication that cells were growing attached to particles. Concomitantly, there was also an increase in the aminopeptidase to glucosidase ratio, a proxy of higher degradation of proteinaceous material relative to carbohydrates. Interestingly, these sharp changes in microbial assemblages and enzymatic activities north and south of the front were translated down to the deep ocean. Our results suggest that permanent fronts, like the Azores Front, can act as ecological boundaries in the ocean (even within a biogeographical province), in terms of microbial community structure and biogeochemical cycling. Oriented studies on oceanic fronts down to the deep ocean will help to understand how the variability of these widely-extended hydrographic futures will impact microbial communities and carbon cycling in a future ocean affected by trends in global warming, de-oxygenation and acidification.
Introduction
Microbes are the engines driving oceanic biogeochemical cycles, regulating the composition of Earth's atmosphere and influencing climate (Falkowski et al., 2008; Kirchman, 2010; Buchan et al., 2014). Thus, understanding the factors controlling the ecological and geographical distribution of marine microbes is crucial if we are to reveal how marine ecosystems will respond to climate change, ocean acidification, and other anthropogenic stressors.
The most recognized exercise performed in the ocean to set geographical boundaries, in terms of biogeochemical features and the interplay of planktonic systems with regional oceanography, corresponds to Longhurst (2006). He divided the ocean in a number of biogeographical provinces, based on regional patterns of phytoplankton ecology, and its response to physical forcing (e.g., depth of the mixed layer, turbulence, depth of the photic zone, etc.) regulating nutrient availability. The boundaries between these provinces were mostly set up using chlorophyll values obtained from remote sensing and a global data set of chlorophyll profiles. Paradoxically, some of these provinces embrace recurrent frontal systems that presumably should be decisive in separating ecological regions in terms of biogeochemical features and microbial dynamics. Indeed, major frontal systems have been related to boundaries of biogeographic zones in the global ocean circulation since the late 80s (Backus, 1986) and are known to play key roles in marine ecosystems (Le Fèvre, 1987; Longhurst, 2006).
Recent studies have endeavored to define oceanic biomes based on microbial data (e.g., Zwirglmaier et al., 2008; Zinger et al., 2011; Gibbons et al., 2013), while other studies have shown that fronts can enhance microbial activity (e.g., Arístegui and Harrison, 2002) or delimit the distribution of the bacterioplankton community composition in surface waters (Baltar et al., 2016). However, there is almost no information on how surface frontal systems influence the distribution of microbial community composition and activity in the dark water column, which contains around 75% of the prokaryotic biomass and 50% of the prokaryotic production of the global ocean (Arístegui et al., 2009). This lack of characterization of ecological regions in the deep ocean is probably based on the presumption that the dark ocean is a stable and homogenous system with rather low microbial activity. Several lines of recent evidence have demonstrated, however, that the dark ocean is more dynamic than anticipated hitherto, revealing a significant spatial and seasonal variability in microbial stocks and activity (Sherry et al., 2002; Tanaka and Rassoulzadegan, 2002, 2004; Gasol et al., 2009; Tamburini et al., 2009; Baltar et al., 2010a,b, 2012).
Here we performed the first study on the variability of organic matter hydrolysis (by looking at EEA), together with the distribution of microbial assemblages, across a permanent front (the Azores Front; AF), from surface to the bathypelagic zone (>1,000 m). EEA are referred as the gatekeepers of the carbon cycle, since, before uptake, microbes need to use EEA to hydrolyze the high molecular dissolved organic matter (DOM) into low molecular weight compounds that can be incorporated (Hoppe, 1991; Hoppe et al., 2002; Arnosti, 2011). Since different enzymes are specific to distinctive substrates, EEA can tell us not only how active heterotrophic microbes are but also how diverse is the organic matter composition (substrates) they are using.
We hypothesized that, even within a biogeographical province, stable fronts can act as delimiting factor of the dynamics of microbial assemblages, and that the surface boundary effect of the front can be translated down to the dark ocean, providing that the front separates two well-defined regions with different hydrographic and biogeochemical properties.
The AF extends over the eastern North Atlantic subtropical gyre basin, delimiting the northern border of the Azores Current (32–35°N), which is a branch of the Gulf Stream, separating the temperate from the subtropical eastern North Atlantic (Gould, 1985). Previous studies have revealed strong differences between the areas north and south of the front in primary production, chlorophyll-a concentration, N2 fixation, deep-ocean particle flux and DOM quality (Fasham et al., 1985; Fründt and Waniek, 2012; Lønborg and Álvarez-Salgado, 2014; Lønborg et al., 2015; Riou et al., 2016). Moreover, the front is a region of instabilities, which are further enhanced due to the passage of the Azores Current across the Mid-Atlantic Ridge, increasing the generation of eddies and vertical mixing (Gould, 1985; Alves et al., 2002; Riou et al., 2016). Despite the evidence of the AF as a potential boundary region or transition zone of different biogeochemical properties, the front does not separate distinct provinces. In fact, the AF zonally crosses the center of the North Atlantic Subtropical Gyre–East, [NAST(E)] province. NAST(E) is bound to the northeast by the bifurcation of the flow between the Azores Current and the North Atlantic Current (about 40–42 N), and to the south by the Subtropical Convergence that lies below the convergence between the trade winds and the westerlies (about 25–30 N) (Longhurst, 2006). Thus, the AF represents an excellent example of a permanent front separating different hydrographic regimes, but located within the same biogeographical province.
Materials and Methods
Study Site and Sampling
Hydrographic casts were performed at 69 stations along a latitudinal section crossing the AF, during the CAIBOX cruise, on board the R/V “Sarmiento de Gamboa” from 25th July to 14th August 2009 (Figure 1). At each station, temperature, salinity, dissolved oxygen, fluorescence, and beam transmission were continuously recorded down to the bottom by means of a SeaBird 9–11+ CTD system implemented with a fluorometer (Sea-Tech 57S) and a transmissometer (WET Labs C-Star), mounted on a General Oceanics rosette sampler equipped with 24 12 l Niskin bottles. Water samples for analyses of salinity, dissolved oxygen and chlorophyll a (chl a) were obtained at discrete depths to calibrate the CTD sensors. Samples for microbial assemblages and microbial enzymatic activities were collected at up to 16 stations from 5 to 7 different depths (Figure 1a).
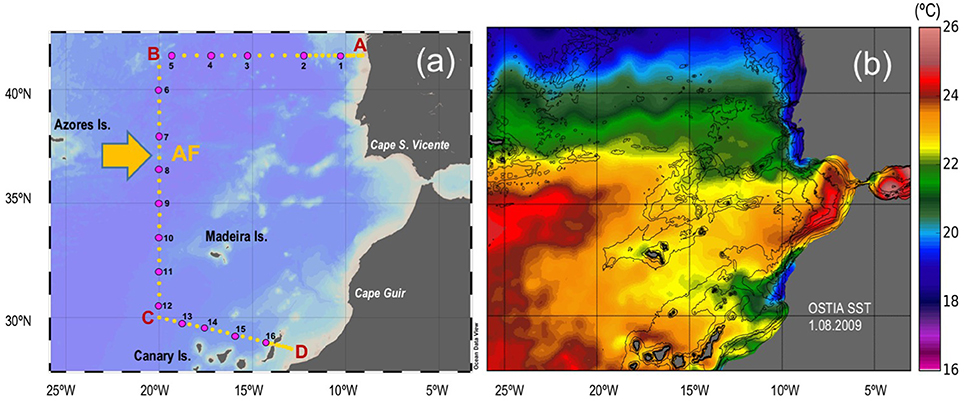
Figure 1. (a) Map of stations (25th July–14th August 2009). Yellow circles: hydrographic stations; Pink circles: biological stations. AF: Azores Front. (b) Map of high resolution analysis of sea-surface temperature (SST) over the study area on 1 August 2009 produced by the Operational Sea Surface Temperature and Sea Ice Analysis (OSTIA) system (Stark et al., 2007).
Salinity, Dissolved Oxygen, and Chl a
Salinity was measured from samples collected at 2–3 deep levels with a Guildline 8410-A Portasal, in order to calibrate the CTD sensor.
Dissolved oxygen was measured at 15 depths by Winkler potentiometric end-point titration using a Titrino 720 analyzer (Metrohm).
Chl a was estimated fluorometrically by means of a Turner Designs bench fluorometer, previously calibrated with pure chl a (Sigma), following the recommendations of Yentsch and Menzel (1963). Seawater samples (500 ml) from 3 depths down to the deep chlorophyll maximum (DCM) were filtered through Whatman GF/F filters. Pigments were extracted in cold acetone for 24 h. For the final determination of chl a, the acetone extracts were acidified allowing chl a and pheopigments to be independently estimated. Extracted chl a samples were used to calibrate the voltage readings of the submersible fluorometer linked to the CTD unit.
Autotrophic and Heterotrophic Picoplankton
Abundances of autotrophic (Prochlorococcus and Synechococcus type cyanobacteria and pigmented picoeukaryotes) and heterotrophic prokaryote assemblages were determined by flow cytometry, from water samples collected at seven depths: surface, intermediate (about 25–50 m), DCM, 100 m, 300 m, oxygen minimum zone (OMZ), and the core of the Mediterranean Water (MW) or 1,100 m, when the MW was not present north of the AF. Samples (1.6 ml) were preserved with paraformaldehyde (2% final concentration), left 15 min at 4°C in the dark to fix, deep frozen in liquid nitrogen and stored at −80°C until analyzed. Once in the lab, fixed samples were thawed, stained in the dark for a few minutes with a DMS-diluted SYTO-13 (Molecular Probes Inc.) stock (10:1) at 2.5 μM final concentration, and run through a BD FACSCalibur cytometer with a laser emitting at 488 nm. High and Low Nucleic Acid content prokaryotes (HNA, LNA; Figure 2A) were identified in bivariate scatter plots of side scatter (SSC-H) vs. green fluorescence (FL1-H). Beside these commonly-described groups, two other groups of LNA and HNA prokaryotes with high SSC (LNA-HSC, and HNA-HSC) were observed south of the AF, probably corresponding to cells attached to particles (Figure 2B; see discussion below). Autotrophic picoplankton were discriminated in plots of orange fluorescence (FL2) vs. red fluorescence (FL3) and picocyanobacteria (Prochlorococcus and Synechococcus) were subtracted from HNA prokaryote counts. Samples were run at low or medium speed until 10.000 events were captured. A suspension of yellow–green 1 μm latex beads (105–106 beads ml−1) was added as an internal standard (Polysciences, Inc.).
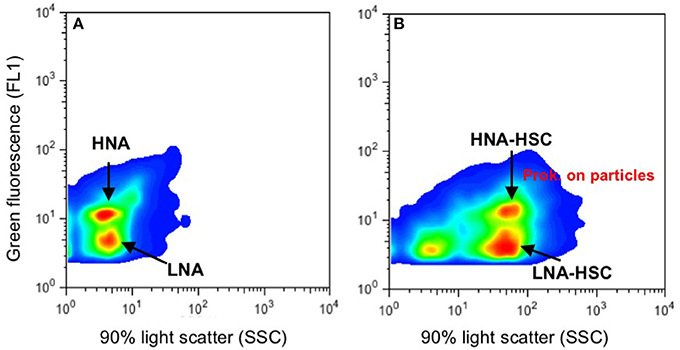
Figure 2. Examples of flow cytometer cytograms (green fluorescence vs. side scatter) from Syto 13-stained heterotrophic prokaryote samples. (A) Typical prokaryotes assemblages with low and high nucleic-acid content (LNA, HNA). (B) LNA and HNA prokaryotes (Prok.) with high side scatter, likely due to cells attached to particles.
Microbial Extracellular Enzymatic Activity (EEA)
The hydrolysis of the fluorogenic substrate analogs L-Leucine-7-amido-4-methylcoumarin, 4-methylumbelliferyl (MUF)- α-D-glucoside, 4-MUF-β-D-glucoside and MUF-phosphate was measured to estimate potential activity rates of leucine aminopeptidase (LAPase), α-glucosidase (AGase), β-glucosidase (BGase), and alkaline phosphatase (APase), respectively (Hoppe, 1983). We followed the same procedure previously described in Baltar et al. (2009). Briefly, EEA was determined after substrate addition and incubation using a spectrofluorometer (Fluorolog-3) with a microwell plate reader (MicroMax 384, Horiba) at excitation and emission wavelengths of 365 and 445 nm, respectively. Samples (300 μl) were incubated in the dark at in situ temperature for 12–24 h. The relative increase in fluorescence with incubation time was checked to be linear on sets of samples incubated for 12–24 h, therefore resulting in the same calculated hydrolytic rates per hour. Subsamples without substrate served as blanks to determine the background fluorescence of the samples (Baltar et al., 2009). Previous experiments showed not significant abiotic hydrolysis of the substrates (Hoppe, 1993; Unanue et al., 1999; Azúa et al., 2003). The fluorescence obtained at the beginning and the end of the incubation was corrected for the corresponding blank. This increase in fluorescence over time was transformed into hydrolysis activity using a standard curve established with different concentrations of the fluorochromes MUF and MCA added to 0.2 μm filtered sample water. A final substrate concentration of 10 μmol l−1 was applied to measure AGase and BGase activities, 100 μmol l−1 for APase and 500 μmol l−1 for LAPase. These concentrations have been previously determined as saturating substrate concentrations in these waters (Baltar et al., 2009, 2013).
Statistical Analyses
A hierarchical clustering analysis was performed to produce a dendrogram represented with even spacing (showing the distance between each node as equal), in order to statistically group the sampled stations into regions. We applied the Ward's minimum variance method, where the distance between two clusters is the ANOVA sum of squares between the two clusters added up over all the variables. To reduce the large number of variables to a few principal components, a principal component analysis (PCA) was also performed. Prior to conducting the hierarchical clustering analyses and the PCA, all targeted variables were standardized by subtracting the mean of all values and dividing by the standard deviation of all values (Dauwe and Middelburg, 1998). The PCA and clustering analyses were carried out with the JMP® Pro 10.0.0 Statistical Software (SAS Institute Inc, Cary, NC).
Results and Discussion
Oceanographic and Biogeochemical Settings Across the Azores Front
A detailed characterization of the different water masses observed during the CAIBOX cruise is described in Lønborg and Álvarez-Salgado (2014) and Lønborg et al. (2015). Briefly, in the upper 200 m salinity and temperature increase west- and southwards, with marked gradients at about 36.5°N, coinciding with the presence of the AF (Figure 1). The location of the AF is confirmed by the abrupt deepening of the 16°C isotherm from 50 m down to >200 m depth (Gould, 1985). Sharp differences north and south of the AF are also observed in salinity, dissolved oxygen, chlorophyll a (chl a) and beam transmission in the upper 200 m (Figure 3). In particular, chla forms a DCM around 50–75 m depth reaching concentrations up to 1.3 mg m−3 north of the AF. South of the front, the DCM deepens down to 100 m and decreases to concentrations <0.3 mg m−3 (Figure 3b), while beam transmission decreases below the DCM, indicating a higher concentration of suspended particles south of the AF (Figure 3d).
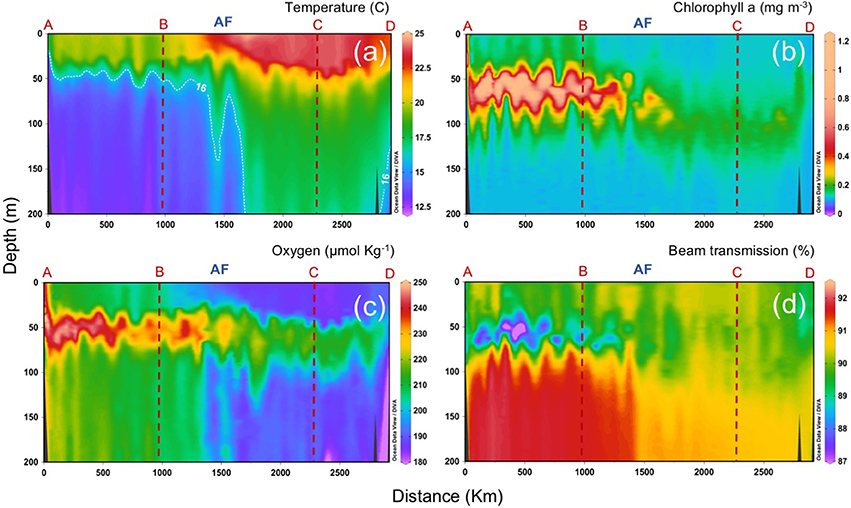
Figure 3. Vertical distributions (0 to 200 m) of (a) temperature, (b) chlorophyll a, (c) dissolved oxygen, and (d) beam transmission, along the sampling section (a–d; refer to Figure 1). AF: Azores Front.
The AF delimits also, at about 200–500 m depth, the confluence of Eastern North Atlantic Central Water (ENACW) from the north with Madeira Mode Water (MMW) from the south. Below the central waters, overflow Mediterranean Water (MW) spreads into the Atlantic, mostly north of the AF, giving rise to a salinity maximum between 600 and 1,400 m depth, while Antarctic Intermediate Water (AAIW), characterized by lower salinity and dissolved oxygen, replaces the MW south of the AF (Figure 4). The presence of AAIW can be tracked by the signature of high apparent oxygen utilization (AOU) and high inorganic nutrients, as corresponds to an old water mass long time exposed to mineralization during his life history (Lønborg et al., 2015). At deeper layers, North East Atlantic Deep Water (NEADW), influenced by the deep waters of the Labrador Sea Water (LSW) from the north and Antarctic Bottom Water (AABW) from the south, extends down to the bottom. The large AOU variability reflects strong differences in mineralization processes within the CAIBOX section, supported by a significant relation between inorganic nutrients concentrations, and AOU (Lønborg and Álvarez-Salgado, 2014).
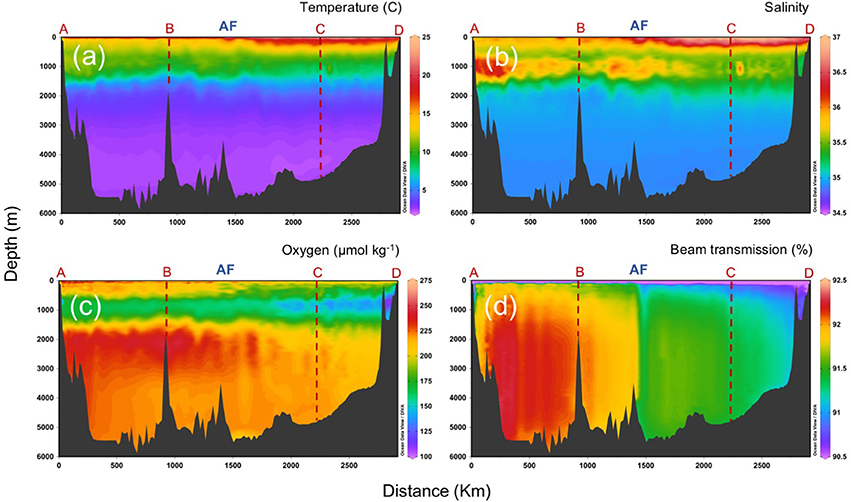
Figure 4. Vertical distributions (surface to bottom) of (a) temperature, (b) salinity, (c) dissolved oxygen, and (d) beam transmission, along the sampling section (a–d; refer to Figure 1). AF: Azores Front.
One of the most interesting patterns of distribution north and south of the front is on beam transmission, which yields a proxy of small suspended particles in the water column. Figure 4d illustrates beam transmission after removing outliers from the plot, which correspond to sparse peaks of low transmission, probably due to large sinking particles that intercepted the light path of the transmissometer. The figure shows the lowest values (more particles) at surface and close to the coastal region, with a clear gradient from lower to higher values in the deep ocean along section D-C. The AF delimits a sharp front in transmission that, surprisingly, extends straight down to the ocean bottom.
Recent and past studies support the view that the eastern boundary Canary Current, south of the AF, is a region affected by intense coastal—offshore transport of suspended particles (see Lovecchio et al., 2017, for a review and references therein). The combined effect of strong upwelling filaments, like those stretching from Cape Sao Vicente (Relvas and Barton, 2002) and Cape Guir (Sangrà et al., 2015), and mesoscale eddies (Sangrà et al., 2009) may extend the transport hundreds of miles to the open ocean. Particularly, the Cape Guir filament is known to export a large fraction of the coastal primary production to the open ocean (García-Muñoz et al., 2004; Pelegrí et al., 2005; Santana-Falcón et al., 2016). Lovecchio et al. (2017), used a Regional Ocean Modeling System (ROMS) coupled to a Nutrient, Phytoplankton, Zooplankton and Detritus (NPZD) ecosystem model to quantify and assess the lateral export of particulate organic carbon (POC) from the Canary Upwelling System to the open waters of the subtropical Northeast Atlantic. They estimated that the offshore transport of coastal production may extend beyond 1,500 km, contributing as nearly 60% of the local net community production in the open ocean. Alonso-González et al. (2009) inferred, using a box-model approach southwest of the Canary Islands, that the lateral fluxes of POC from the Canary Upwelling System to the subtropical open ocean could account for up to 59% of the total mesopelagic (200–1,000 m) respiration. Figure 2A of their study shows a strong westward decreasing gradient in POC, with high values down to 3,000 m, at the Canary Islands latitude. In another study around two seamounts—Seine (close to Madeira Island; south of the AF) and Sedlo (close to the Azores Islands; north of the AF), (Vilas et al., 2009; see their Figure 4) found that POC concentrations were more than doubled in Seine with respect to Sedlo down to at least 1,000 m, and that peaks in POC in the water column at Seine corresponded to discontinuities in density gradients, suggesting a lateral transport of neutrally-buoyant particles. Conversely, particulate organic nitrogen (PON) was higher in Sedlo, with a sharp decline in the upper 200 m, suggesting a more labile nature of the particles, with a surface water origin.
Community Assemblages and Organic Matter Hydrolysis Shifts along the Azores Front
The picophytoplankton community (Procholococcus, Synechococcus, and picoeukaryotes) in the upper 100 m (Figure 5) presents the highest concentrations north of the AF, at the DCM and about 25–50 m above it (“intermediate” depth). There is a progressive decreasing gradient in abundances from north to south, with a sharp drop (up to five-fold) at the AF, particularly in Synechococcus and picoeukaryotes, coinciding with the decline in chlorophyll concentration at the DCM (Figures 3b, 5). South of the AF, picophytoplankton concentrations remain rather low and stable, with no significant differences in abundances at each group among sampling depths. North of the AF the stronger DCM is caused both by Synechococcus and picoeukaryotes, while south of the front the weaker and deeper DCM is due to Procholococcus and picoeukaryotes.
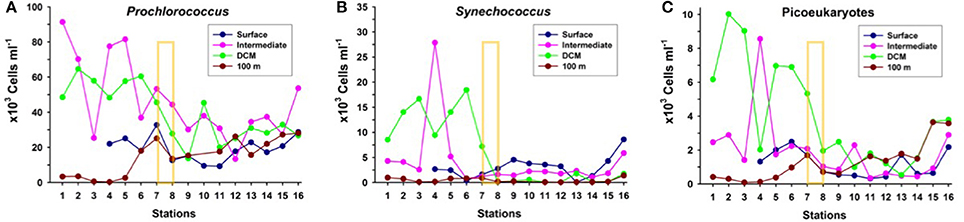
Figure 5. Distribution of the abundances of (A) Prochlorococcus, (B) Synechococcus, and (C) Picoeukaryotes at the biological stations, at four depth layers: surface, intermediate (25 to 50 m), deep chlorophyll maximum (DCM) and 100 m depth. The yellow rectangle indicates the approximate location of the Azores Front.
Like with picophytoplankton, there are marked changes in heterotrophic prokaryote abundances north and south of the AF in the upper 100 m, more evident in the depth layer from surface down to the DCM (Figure 6). Particularly, HNA prokaryotes decline two- to four- fold from north to south at the AF. Nevertheless, this drop in total prokaryotic abundances could be more apparent than real, since just south of the AF appear LNA and HNA cells with HSC (high side scatter), which summed up to the common HNA and LNA groups yield comparable abundances north and south of the front. Although, we did not collect samples for microscopic observations, we believe that the high side scatter in these HNA and LNA prokaryotes are due to their association with particles. Similar cytograms of prokaryotic assemblages with HSC were recently observed associated with the discharge of vent material in stations close to a shallow submarine volcano in the Canary Islands (Ferrera et al., 2015).
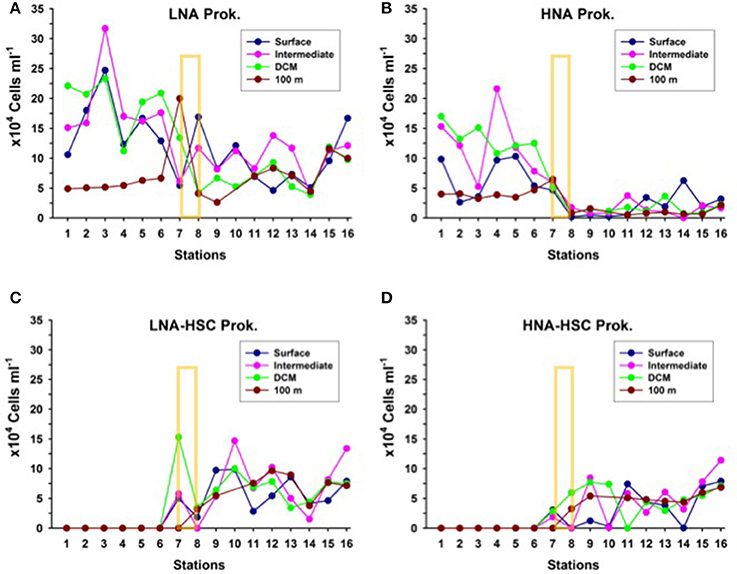
Figure 6. Distribution of the abundances of (A) Low nucleic-acid heterotrophic prokaryotes (LNA) (B) High nucleic-acid heterotrophic prokaryotes (HNA), (C) Low nucleic-acid heterotrophic prokaryotes with high side scatter (LNA-HSC), and (D) High nucleic-acid heterotrophic prokaryotes with high side scatter (HNA-HSC), at the biological stations, at four depth layers: surface, intermediate (25 to 50 m), deep chlorophyll maximum (DCM) and 100 m depth. The yellow rectangle indicates the approximate location of the Azores Front.
Prokaryotic abundances decrease exponentially with depth to values an order of magnitude lower at 1,000 m than in the upper 100 m, with no significant differences in LNA and HNA assemblages north and south of the AF (Figures 7A,B). However, like in shallower depths, LNA-HSC and HNA-HSC appear south of the AF with abundances comparable to free-living prokaryotes at the same depths (Figures 7C,D).
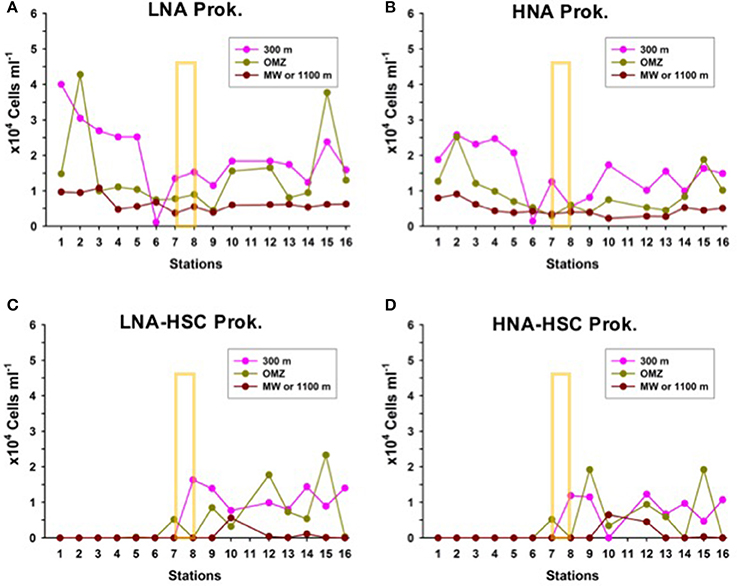
Figure 7. Distribution of the abundances of (A) Low nucleic-acid heterotrophic prokaryotes (LNA) (B) High nucleic-acid heterotrophic prokaryotes (HNA), (C) Low nucleic-acid heterotrophic prokaryotes with high side scatter (LNA-HSC), and (D) High nucleic-acid heterotrophic prokaryotes with high side scatter (HNA-HSC), at the biological stations, at three depth layers: 300 m depth, oxygen minimum zone (OMZ) and the core of the Mediterranean Water (MW) or 1,100 m depth. The yellow rectangle indicates the approximate location of the Azores Front.
The distribution of these HSC prokaryotes, presumably associated with particles, coincides with the region of lower beam transmission south of the AF, indicative of a greater presence of small suspended particles in the water column. This observation adds support to previous studies in the subtropical Northeast Atlantic that ascribe a predominant particle-attached way of life to deep-ocean prokaryotes thriving on suspended particles (Baltar et al., 2009, 2010a,b).
Although, there are some reports suggesting similar taxonomic composition of HNA and LNA populations (Servais et al., 2003; Longnecker et al., 2005), most of the recent research indicates that these populations are generally different (e.g., (Bernard et al., 2000; Zubkov et al., 2001; Mary et al., 2006; Schattenhofer et al., 2011; Vila-Costa et al., 2012; García et al., 2015). For example, there seems to be a widespread prevalence of SAR11 in the LNA fraction (Maruyama and Heslinga, 1997; Schattenhofer et al., 2011; García et al., 2015). In fact, a flow-cytometric based differentiation of microbial populations/communities was recently shown to be in good agreement with molecular analysis (16S rRNA sequencing; García et al., 2015), suggesting that cytometric diversity represents a useful complementary tool in the macroecology of aquatic microbes. Thus, some of the differences we observe in the LNA and HNA patterns could be linked to taxonomic changes of the microbial populations.
The sharp transition observed for picophytoplankton and heterotrophic prokaryotes across the front is also evident in microbial EEA (Figure 8). Leucine aminopeptidase (LAPase) and alkaline phosphatase (APase) activities are generally higher in shallower waters and south of the AF (with the exception of APase in the bathypelagic waters near the African upwelling). In contrast, α-glucosidase (AGase) and β-glucosidase (BGase) activities are generally lower at surface and south of the front. The leucine aminopeptidase to glucosidases ratios (LAPase:AGase and LAPase:BGase), which are suggested to be indicative of the relative degradation of polysaccharides relative to proteinaceous material (Middelboe et al., 1995), decrease with depth, with a marked shift coinciding with the location of the AF. The reduction with depth of the these ratios is in agreement with a previous study in the subtropical North Atlantic (Baltar et al., 2009) that reports a sharp decline in the LAPase:BGase ratio from the epipelagic (0–200 m) down to the bathypelagic (>1,000 m) zone. It is also consistent with the general rise in the molar C:N:P ratios of the oceanic DOM pool (Hopkinson and Vallino, 2005) and the decrease in the amino acid:carbohydrate ratio of sinking particulate organic matter (POM) with depth (Haake et al., 1993). Previous experiments have shown that bacteria degrade proteins faster than polysaccharides during decomposition of phytoplankton-derived detritus (Skopintsev, 1981; Middelboe et al., 1995), suggesting that proteinaceous components of sinking POM are more rapidly degraded than the polysaccharide fraction (Smith et al., 1992; Skoog and Benner, 1997). Thus, the relative concentration of polysaccharides in sinking POM would increase with depth, resulting in an intensive supply of polysaccharide-rich material to deeper waters and a decrease in the LAPase:BGase ratio with depth. This would explain the low ratios in the mesopelagic and bathypelagic waters north of the AF, with a predominant vertical flux of particles. South of the AF, DOM is more recalcitrant than north of the AF (Lønborg et al., 2015). Thus, the higher ratios south of AF should be linked to the influence of the observed higher concentration of suspended particles south of AF. This would produce higher LAPase (and APase) activities of those microbial organisms attached to small particles with low sedimentation rates (and relatively more labile than the bulk of the DOM).
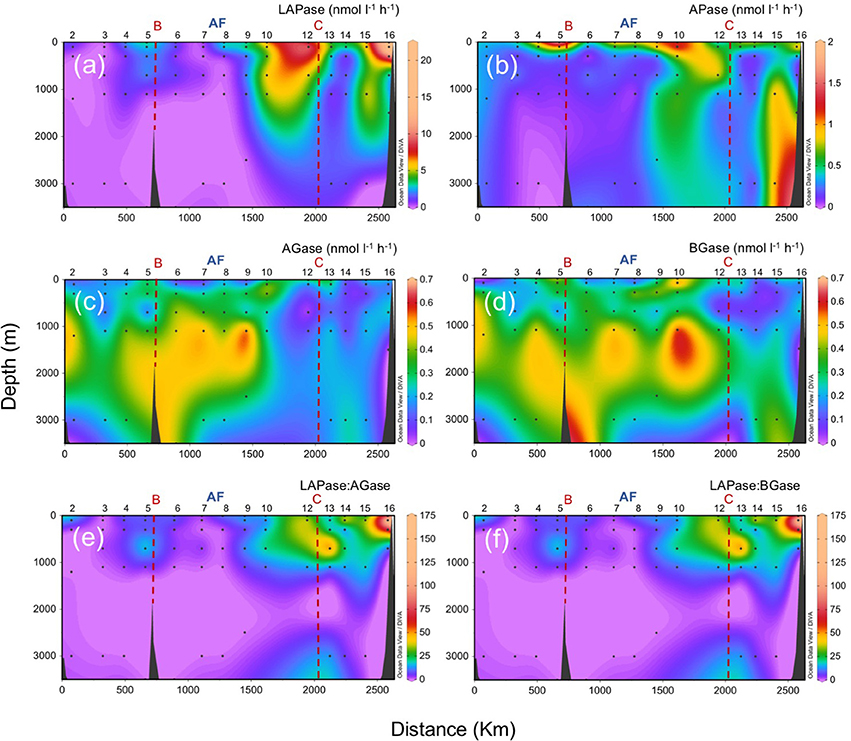
Figure 8. Vertical distributions (surface to 3,000 m) of microbial extracellular enzymatic activities at the biological stations along the sampling section (refer to Figure 1). (a) leucine aminopeptidase (LAPase), (b) alkaline phosphatase (APase), (c) α-glucosidase (AGase), (d) β-glucosidase (BGase), (e) the ratio of LAPase:AGase, (f) the ratio of LAPase:BGase. AF: Azores Front.
Compiling all the biological data together (i.e., the abundance of microbial assemblages and the EEA) along the transect, a clear clustering arises separating the stations north and south of the AF into two regions (Figure 9). The distance (9.2) between the two main clusters separating the waters at both sides of the AF was almost twice as high as the distance between any of the other clusters. The clustering is based on the almost absence of LNA-HSC and HNA-HSC, the lower activities of APase, LAPase, and the lower ratios of LAPase:AGase and LAPase:BGase to the north of the AF. A PCA, which explains around 64% of the variance, illustrates the direct relation between the abundance of HSC prokaryotes and LAPase, which is inverse with AGase and BGase (Figure 10). The statistical analysis also shows that APase is halfway between the HSC prokaryotes and picophytoplankton vectors, in agreement with the notion that APase can be expressed not only by heterotrophs but also by autotrophs (Hoppe, 2003).
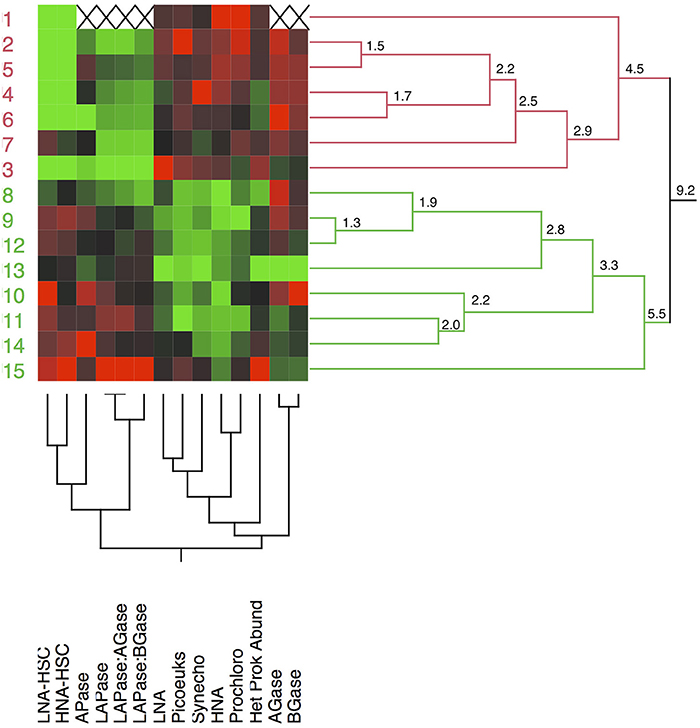
Figure 9. Dendrogram showing the two-way hierarchical clustering of the stations based on the biological data collected (abundance of microbial assemblages and extracellular enzymatic activities). LAPase, leucine aminopeptidase; APase, alkaline phosphatase; AGase, alpha- glucosidase; BGase, beta-glucosidase; Het Prok Abund, total heterotrophic prokaryote abundance; Synecho, Synechococcus, Prochloro, Prochlorococcus; Picoeuk, picoeukaryotes; LNA and HNA, low and high acid-nucleic content heterotrophic prokaryotes; LNA-HSC and HNA-HSC, low and high acid-nucleic content heterotrophic prokaryotes with high side scatter.
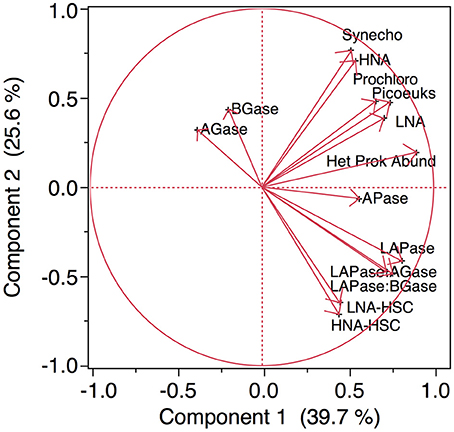
Figure 10. Principal Component Analysis of all the biological data collected in all the stations. Labels like in Figure 9.
There are several potential reasons to explain the shifts in microbial abundances and activities observed at both sides of the front. First, primary production, chlorophyll a and zooplankton biomass have been reported to be higher in the colder waters north of the AF (Riou et al., 2016). Although, our study was carried out during summer, when the seasonal planktonic cycle presents its lowest productivity, and phytoplankton is concentrated at the thermocline forming a DCM, there are still conspicuous differences in the magnitude and depth of the DCM at both sides of the front. The higher productivity north of the AF would also yield more excreted labile DOM in the epipelagic zone, as reported by Lønborg et al. (2015), which could be responsible of the presence of more free-living HNA bacteria (Figure 6A; Fasham et al., 1985).
On the other hand, summer is the period of more intense trade winds and consequently higher upwelling activity in the NW African coast, favoring the coastal-ocean transport of organic matter by enhancing both Ekman transport and the development of upwelling filaments (Figure 1b). During the time of our study and the previous months, the region south of the AF would have been affected by strong advection of organic matter from the continental margins, explaining the lower beam transmission values, the association of heterotrophic prokaryotes to particles, and the increases in the LAPase:BGase and LAPase:AGase ratios, indicative of more labile material. Alonso-González et al. (2010), using a sediment trap that separate particles as function of their settling velocity, found over a 1.5-year period deployment in the Canary Current region that more than 60% of total organic carbon is contained in small slowly settling particles (0.7–11 m d−1), at least during half of the year (from June to December). The origin of these particles could be both from the surface waters or advected from the more productive continental shelf. Analyses of organic biomarkers revealed that these particles have the same degradation state or are even fresher than rapidly sinking particles (Alonso-González et al., 2010). The large eddy activity in the region south of the AF could be responsible of the re-distribution of these slowly sinking particles in the deep ocean. Indeed, oceanic eddies near the AF have been detected as deep as 2,000 m (Gould, 1985), and the high interannual variability found in the deep ocean particle flux in the region has been explained by the propagation of the AF and by strong variations in the catchment areas of sediment traps due to the associated eddy activity in the frontal region (Fründt and Waniek, 2012).
Conclusions
The results of this study strongly suggest a critical role of the Azores Front (AF) as a border separating two biogeochemically distinct regions. This role is particularly relevant since the AF is a permanent structure (Gould, 1985), and because these two regions are located within the same biogeographical province (NAST-E; (Longhurst, 2006). Moreover, evidences of this bordering effect are not restricted to the surface waters (geostrophic circulation), but are found down to the bathypelagic zone, strengthening the potential importance of permanent fronts, like the AF, as oceanographic features defining biogeochemical regions. We show that fronts can, not only delimit the distribution of different planktonic assemblages, but also the crucial activities and services they perform (e.g., hydrolysis and degradation of organic matter). Our results are in agreement with recent studies attempting to define oceanic biomes based on microbial data (e.g., Zwirglmaier et al., 2008; Zinger et al., 2011; Gibbons et al., 2013), and calls for studying other permanent fronts in the ocean that could delimit biogeographical regions down to the deep ocean.
Author Contributions
Both authors listed have equally contributed to the different aspects of this work including the design, sampling, data analysis, writing and proofing of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the captain and crew of the R/V Sarmiento de Gamboa for their support at sea, Gabriel Rosón for the CTD data, C Lønborg and XA Alvarez-Salgado for the chlorophyll data and GJ Herndl for the spectrofluorometer. Thanks also to Josep Coca for providing Figure 1b, and Blair Thomson for proofing the manuscript. This research was carried out in the frame of the IMBER-endorsed Spanish project CAIBEX (CTM2007-66408-C02-02) “Plan Nacional de I+D” (MEC), coordinated by JA. Partial support was obtained by a Research Grant of the University of Otago and the Rutherford Discovery Fellowship from Government funding, administered by the Royal Society of New Zealand to FB, and by funding from the FLUXES project (CTM2015-69392-C3-1-R) “Plan Nacional de I+D” (MEC) to JA.
References
Alonso-González, I. J., Arístegui, J., Lee, C., and Calafat, A. (2010). Regional and temporal variability of sinking organic matter in the subtropical Northeast Atlantic Ocean: a biomarker diagnosis. Biogeoscience 7, 2101–2115. doi: 10.5194/bg-7-2101-2010
Alonso-González, I. J., Arístegui, J., Vilas, J. C., and Hernández-Guerra, A. (2009). Lateral POC transport and consumption in surface and deep waters of the Canary Current region: a box model study. Global Biogeochem. Cycles 23:GB2007. doi: 10.1029/2008GB003185
Alves, M., Gaillard, F., Sparrow, M., Knoll, M., and Giraud, S. (2002). Circulation patterns and transport of the Azores Front-Current system. Deep Sea Res. Part II 49, 3983–4002. doi: 10.1016/S0967-0645(02)00138-8
Arístegui, J., and Harrison, W. G. (2002). Decoupling of primary production and community respiration in the ocean: implications for regional carbon studies. Aquatic Microb. Ecol. 29, 199–209. doi: 10.3354/ame029199
Arístegui, J., Gasol, J. M., Duarte, C. M., and Herndl, G. J. (2009). Microbial Oceanography of the dark ocean's pelagic realm. Limnol. Oceanogr. 54, 1501–1529. doi: 10.4319/lo.2009.54.5.1501
Arnosti, C. (2011). Microbial extracellular enzymes and the marine carbon cycle. Ann. Rev. Mar. Sci. 3, 401–425. doi: 10.1146/annurev-marine-120709-142731
Azúa, I., Uanue, M., Ayo, B., Arrtolozaga, I., Arrieta, J. M., and Iriberri, J. (2003). Influence of organic matter quality in the cleavage of polymers by marine bacterial communities. J. Plankton Res. 25, 1451–1460. doi: 10.1093/plankt/fbg105
Backus, R. (1986). “Biogeographic boundaries in the open ocean,” in Pelagic Biogeography (UNESCO Tech. Pap. Mar. Sc.), 9–13.
Baltar, F., Arísstegui, J., Gasol, J. M., and Herndl, G. J. (2012). Microbial functioning and community structure variability in the Mesopelagic and Epipelagic waters of the Subtropical Northeast Atlantic Ocean. Appl. Environ. Microbiol. 78, 3309–3316. doi: 10.1128/AEM.07962-11
Baltar, F., Arístegui, J., Gasol, J. M., and Herndl, G. J. (2010a). Prokaryotic carbon utilization in the dark ocean: growth efficiency, leucine-to-carbon conversion factors, and their relation. Aquatic Microb. Ecol. 60, 227–232. doi: 10.3354/ame01422
Baltar, F., Arístegui, J., Gasol, J. M., Lekunberri, I., and Herndl, G. J. (2010b). Mesoscale eddies: hotspots of prokaryotic activity and differential community structure in the ocean. ISME J. 4, 975–988. doi: 10.1038/ismej.2010.33
Baltar, F., Arístegui, J., Gasol, J. M., Yokokawa, T., and Herndl, G. J. (2013). Bacterial versus archaeal origin of extracellular enzymatic activity in the Northeast Atlantic Deep Waters. Microb. Ecol. 65, 277–288. doi: 10.1007/s00248-012-0126-7
Baltar, F., Currie, K., Stuck, E., Roosa, S., and Morales, S. E. (2016). Oceanic fronts: transition zones for bacterioplankton community composition. Environ. Microbiol. Rep. 8, 132–138. doi: 10.1111/1758-2229.12362
Baltar, F., Sintes, E., Van Aken, H. M., Gasol, J. M., Arístegui, J., and Herndl, G. J. (2009). Prokaryotic extracellular enzymatic activity in relation to biomass production and respiration in the meso- and bathypelagic waters of the (sub)tropical Atlantic. Environ. Microbiol. 11, 1998–2014. doi: 10.1111/j.1462-2920.2009.01922.x
Bernard, L., Courties, C., Servais, P., Troussellier, M., Petit, M., and Lebaron, P. (2000). Relationships among bacterial cell size, productivity, and genetic diversity in aquatic environments using cell sorting and flow cytometry. Microb. Ecol. 40, 148–158. doi: 10.1007/s002480000046
Buchan, A., Lecleir, G. R., Gulvik, C. A., and González, J. M. (2014). Master recyclers: features and functions of bacteria associated with phytoplankton blooms. Nat. Rev. Microbiol. 12, 686–698. doi: 10.1038/nrmicro3326
Dauwe, B., and Middelburg, J. J. (1998). Amino acids and hexosamines as indicators of organic matter degradation state in North Sea sediments. Limnol. Oceanogr. 43, 782–798. doi: 10.4319/lo.1998.43.5.0782
Falkowski, P. G., Fenchel, T., and Delong, E. F. (2008). The microbial engines that drive Earth's biogeochemical cycles. Science 320, 1034–1039. doi: 10.1126/science.1153213
Fasham, M., Platt, T., Irwin, B., and Jones, K. (1985). Factors affecting the spatial pattern of the deep chlorophyll maximum in the region of the Azores front. Prog. Oceanogr. 14, 129–165. doi: 10.1016/0079-6611(85)90009-6
Ferrera, I., Arístegui, J., González, J. M., Montero, M. F., Fraile-Nuez, E., and Gasol, J. M. (2015). Transient changes in bacterioplankton communities induced by the submarine volcanic eruption of El Hierro (Canary Islands). PLoS ONE 10:e0118136. doi: 10.1371/journal.pone.0118136
Fründt, B., and Waniek, J. J. (2012). Impact of the azores front propagation on deep ocean particle flux. Central Eur. J. Geosci. 4, 531–544. doi: 10.2478/s13533-012-0102-2
García, F. C., Alonso-Sáez, L., Morán, X. A. G., and López-Urrutia, Á. (2015). Seasonality in molecular and cytometric diversity of marine bacterioplankton: the re-shuffling of bacterial taxa by vertical mixing. Environ. Microbiol. 17, 4133–4142. doi: 10.1111/1462-2920.12984
García-Muñoz, M., Arístegui, J., Montero, M. F., and Barton, E. D. (2004). Distribution and transport of organic matter along a filament-eddy system in the Canaries–NW Africa coastal transition zone region. Prog. Oceanogr. 62, 115–129. doi: 10.1016/j.pocean.2004.07.005
Gasol, J. M., Alonso-Sáez, L., Vaqué, D., Baltar, F., Calleja, M. L., Duarte, C. M., et al. (2009). Mesopelagic prokaryotic bulk and single-cell heterotrophic and community composition in the NW Africa-Canary Islands coastal-transition zone. Prog. Oceanogr. 83, 189–196. doi: 10.1016/j.pocean.2009.07.014
Gibbons, S. M., Caporaso, J. G., Pirrung, M., Field, D., Knight, R., and Gilbert, J. A. (2013). Evidence for a persistent microbial seed bank throughout the global ocean. Proc. Natl. Acad. Sci. U.S.A. 110, 4651–4655. doi: 10.1073/pnas.1217767110
Gould, W. (1985). Physical oceanography of the Azores Front. Prog. Oceanogr. 14, 167–190. doi: 10.1016/0079-6611(85)90010-2
Haake, B., Ittekot, V., Honjo, S., and Manganini, S. (1993). Amino acid, hexosamine and carbohydrate fluxes to the deep Subarctic Pacific (Station P). Deep Sea Res. 40, 547–560. doi: 10.1016/0967-0637(93)90145-S
Hopkinson, C. S., and Vallino, J. J. (2005). Efficient export of carbon to the deep ocean through dissolved organic matter. Nature 433, 142–145. doi: 10.1038/nature03191
Hoppe, H. G. (1993). “Use of fluorogenic model substrates for extracellular enzyme activity (EEA) measurement of bacteria,” in Handbook of Methods in Aquatic Microbial Ecology, eds P. F. Kemp, B. F. Sherr, E. B. Sherr, and J. J. Cole (Boca Raton, FL: Lewis Publishers), 423–431.
Hoppe, H. G. (2003). Phosphatase activity in the sea. Hydrobiologia 493, 187–200. doi: 10.1023/A:1025453918247
Hoppe, H.-G. (1983). Significance of exoenzymatic activities in the ecology of brackish water: measurements by means of methylumbelliferyl-substrates. Mar. Ecol. Prog. Ser. 11, 299–308. doi: 10.3354/meps011299
Hoppe, H.-G. (1991). “Microbial extracellular enzyme activity: a new key parameter in aquatic ecology,” in Microbial Enzymes in Aquatic Environments (New York, NY: Springer Verlag), 60–83.
Hoppe, H.-G., Arnosti, C., and Herndl, G. J. (2002). “Ecological significance of bacterial enzymes in the marine environment,” in Enzymes in the Environment: Activity, Ecology, and Applications, eds R. G. Burns and R. P. Dick (New York, NY: Marcel Dekker, Inc.), 73–108.
Le Fèvre, J. (1987). Aspects of the biology of frontal systems. Adv. Mar. Biol. 23, 163–299. doi: 10.1016/S0065-2881(08)60109-1
Lønborg, C., and Álvarez-Salgado, X. A. (2014). Tracing dissolved organic matter cycling in the eastern boundary of the temperate North Atlantic using absorption and fluorescence spectroscopy. Deep Sea Res. Part I 85, 35–46. doi: 10.1016/j.dsr.2013.11.002
Lønborg, C., Yokokawa, T., Herndl, G. J., and Álvarez-Salgado, X. A. (2015). Production and degradation of fluorescent dissolved organic matter in surface waters of the eastern north Atlantic ocean. Deep Sea Res. Part I 96, 28–37. doi: 10.1016/j.dsr.2014.11.001
Longnecker, K., Sherr, B. F., and Sherr, E. B. (2005). Activity and phylogenetic diversity of bacterial cells with high and low nucleic acid content and electron transport system activity in an upwelling ecosystem. Appl. Environ. Microbiol. 71, 7737–7749. doi: 10.1128/AEM.71.12.7737-7749.2005
Lovecchio, E., Gruber, N., Münnich, M., and Lachkar, Z. (2017). On the long-range offshore transport of organic carbon from the Canary Upwelling System to the open North Atlantic. Biogeosciences 14, 3337–3369. doi: 10.5194/bg-14-3337-2017
Maruyama, T., and Heslinga, G. A. (1997). Fecal discharge of zooxanthellae in the giant clam Tridacna derasa, with reference to their in situ rate. Mar. Biol. 127, 473–477. doi: 10.1007/s002270050035
Mary, I., Heywood, J. L., Fuchs, B. M., Amann, R., Tarran, G. A., Burkill, P. H., et al. (2006). SAR11 dominance among metabolically active low nucleic acidd bacterioplankton in surface waters among an Atlantic meridional transect. Aquat. Microb. Ecol. 45, 107–113. doi: 10.3354/ame045107
Middelboe, M., Søndergaard, M., Letarte, Y., and Borch, N. H. (1995). Attached and free-living bacteria: production and polymer hydrolysis during a diatom bloom. Microb. Ecol. 29, 231–248. doi: 10.1007/BF00164887
Pelegrí, J., Arístegui, J., Cana, L., González-Dávila, M., Hernández-Guerra, A., Hernández-León, S., et al. (2005). Coupling between the open ocean and the coastal upwelling region off northwest Africa: water recirculation and offshore pumping of organic matter. J. Mar. Syst. 54, 3–37. doi: 10.1016/j.jmarsys.2004.07.003
Relvas, P., and Barton, E. D. (2002). Mesoscale patterns in the Cape Sao Vicente (Iberian peninsula) upwelling region. J. Geophys. Res. 107, 28-1–28-23. doi: 10.1029/2000JC000456
Riou, V., Fonseca-Batista, D., Roukaerts, A., Biegala, I. C., Loureiro, C. M., Santos, M., et al. (2016). Importance of N 2-Fixation on the Productivity at the North-Western Azores Current/Front System, and the Abundance of Diazotrophic Unicellular Cyanobacteria. PLoS ONE 11:e0150827. doi: 10.1371/journal.pone.0150827
Sangrà, P., Pascual, A., Rodríguez-Santana, Á., Machín, F., Mason, E., McWilliams, J. C., et al. (2009). The Canary Eddy Corridor: a major pathway for long-lived eddies in the subtropical North Atlantic. Deep Sea Res. Part I 56, 2100–2114. doi: 10.1016/j.dsr.2009.08.008
Sangrà, P., Troupin, C., Barreiro-González, B., Desmond Barton, E., Orbi, A., and Arístegui, J. (2015). The Cape Ghir filament system in August 2009 (NW Africa). J. Geophys. Res. 120, 4516–4533. doi: 10.1002/2014JC010514
Santana-Falcón, Y., Benavides, M., Sangrà, P., Mason, E., Barton, E. D., Orbi, A., et al. (2016). Coastal–offshore exchange of organic matter across the Cape Ghir filament (NW Africa) during moderate upwelling. J. Mar. Syst. 154, 233–242. doi: 10.1016/j.jmarsys.2015.10.008
Schattenhofer, M., Wulf, J., Kostadinov, I., Glöckner, F. O., Zubkov, M. V., and Fuchs, B. M. (2011). Phylogenetic characterisation of picoplanktonic populations with high and low nucleic acid content in the North Atlantic Ocean. Syst. Appl. Microbiol. 34, 470–475. doi: 10.1016/j.syapm.2011.01.008
Servais, P., Casamayor, E. O., Courties, C., Catala, P., Parthuisot, N., and Lebaron, P. (2003). Activity and diversity of bacterial cells with high and low nucleic acid content. Aquatic Microb. Ecol. 33, 41–51. doi: 10.3354/ame033041
Sherry, N. D., Imanian, B., Sugimoto, K., Boyd, P. W., and Harrison, P. J. (2002). Seasonal and interannual trends in heterotrophic bacterial processes between 1995 and 1999 in the subarctic NE Pacific. Deep-Sea Res. II 49, 5775–5791. doi: 10.1016/S0967-0645(02)00214-X
Skoog, A., and Benner, R. (1997). Aldoses in various size fractions of marine organic matter: implications for carbon cycling. Limnol. Oceanogr. 42, 1803–1813. doi: 10.4319/lo.1997.42.8.1803
Skopintsev, B. A. (1981). “Decomposition of organic matter of plankton, humification and hydrolysis,” in Marine Organic Chemistry; Evolution, Composition, Interactions, and Chemistry of Organic Matter in Seawater, eds K. Duursma and R. Dawson. (Elsevier), 125–177. doi: 10.1016/S0422-9894(08)70328-3
Smith, D. C., Simon, M., Alldredge, A. L., and Azam, F. (1992). Intense hydrolytic enzyme activity on marine aggregates and implications for rapid particle dissolution. Nature 359, 139–142. doi: 10.1038/359139a0
Stark, J. D., Donlon, C. J., Martin, M. J., and Mcculloch, M. E. (2007). “OSTIA: an operational, high resolution, real time, global sea surface temperature analysis system,” in Oceans 2007-Europe (Aberdeen: IEEE), 1–4. doi: 10.1109/OCEANSE.2007.4302251
Tamburini, C., Garel, M., Ali, B. A., Mérigot, B., Kriwy, P., Charrière, B., et al. (2009). Distribution and activity of Bacteria and Archaea in the different water masses of the Tyrrhenian Sea. Deep-Sea Res. Part II 56, 700–712. doi: 10.1016/j.dsr2.2008.07.021
Tanaka, T., and Rassoulzadegan, F. (2002). Full-depth profile (0-2000 m) of bacteria, heterotrophic nanoflagellates and ciliates in the NW Mediterranean Sea: vertical partitioning of microbial trophic structures. Deep Sea Res. II 49, 2093–2107. doi: 10.1016/S0967-0645(02)00029-2
Tanaka, T., and Rassoulzadegan, F. (2004). Vertical and seasonal variations of bacterial abundance and production in the mesopelagic layer of the NW Mediterranean Sea: bottom-up and top-down controls. Deep Sea Res. I 51, 531–544. doi: 10.1016/j.dsr.2003.12.001
Unanue, M., Ayo, B., Agis, M., Slezak, D., Herndl, G. J., and Iriberri, J. (1999). Ectoenzymatic activity and uptake of monomers in marine bacterioplankton described by a biphasic kinetic model. Microb. Ecol. 37, 36–48. doi: 10.1007/s002489900128
Vila-Costa, M., Gasol, J. M., Sharma, S., and Moran, M. A. (2012). Community analysis of high-and low-nucleic acid-containing bacteria in NW Mediterranean coastal waters using 16S rDNA pyrosequencing. Environ. Microbiol. 14, 1390–1402. doi: 10.1111/j.1462-2920.2012.02720.x
Vilas, J., Arístegui, J., Kiriakoulakis, K., Wolff, G., Espino, M., Polo, I., et al. (2009). Seamounts and organic matter—Is there an effect? The case of Sedlo and Seine Seamounts: Part 1. Distributions of dissolved and particulate organic matter. Deep Sea Res. Part II 56, 2618–2630. doi: 10.1016/j.dsr2.2008.12.023
Yentsch, C. S., and Menzel, D. W. (1963). A method for the determination of phytoplankton chlorophyll and phaeophytin by fluorescence. Deep Sea Res. Oceanogr. Abst. 10, 221–231. doi: 10.1016/0011-7471(63)90358-9
Zinger, L., Amaral-Zettler, L. A., Fuhrman, J. A., Horner-Devine, M. C., Huse, S. M., Welch, D. B. M., et al. (2011). Global patterns of bacterial beta-diversity in seafloor and seawater ecosystems. PLoS ONE 6:e24570. doi: 10.1371/journal.pone.0024570
Zubkov, M. V., Fuchs, B. M., Archer, S. D., Kiene, R. P., Amann, R., and Burkill, P. H. (2001). Linking the composition of bacterioplankton to rapid turnover of dissolved dimethylsulphoniopropionate in an algal bloom in the North Sea. Environ. Microbiol. 3, 304–311. doi: 10.1046/j.1462-2920.2001.00196.x
Zwirglmaier, K., Jardillier, L., Ostrowski, M., Mazard, S., Garczarek, L., Vaulot, D., et al. (2008). Global phylogeography of marine Synechococcus and Prochlorococcus reveals a distinct partitioning of lineages among oceanic biomes. Environ. Microbiol. 10, 147–161. doi: 10.1111/j.1462-2920.2007.01440.x
Keywords: oceanic fronts, extracellular enzymatic activity, microbial community assemblage, suspended particulate organic matter, biogeographical regions, Azores Front
Citation: Baltar F and Arístegui J (2017) Fronts at the Surface Ocean Can Shape Distinct Regions of Microbial Activity and Community Assemblages Down to the Bathypelagic Zone: The Azores Front as a Case Study. Front. Mar. Sci. 4:252. doi: 10.3389/fmars.2017.00252
Received: 08 June 2017; Accepted: 21 July 2017;
Published: 03 August 2017.
Edited by:
Toshi Nagata, University of Tokyo, JapanReviewed by:
Mauro Celussi, National Institute of Oceanography and Experimental Geophysics, ItalyGordon T. Taylor, Stony Brook University, United States
Copyright © 2017 Baltar and Arístegui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Federico Baltar, federico.baltar@otago.ac.nz
Javier Arístegui, javier.aristegui@ulpgc.es
 Federico Baltar
Federico Baltar Javier Arístegui
Javier Arístegui