Ice Algae-Produced Carbon Is Critical for Overwintering of Antarctic Krill Euphausia superba
- 1Polar Biological Oceanography, Alfred Wegener Institute Helmholtz Centre for Polar and Marine Research, Bremerhaven, Germany
- 2Centre for Natural History (CeNak), Zoological Museum, University of Hamburg, Hamburg, Germany
- 3Wageningen Marine Research, Den Helder, Netherlands
- 4Ecological Chemistry, Alfred Wegener Institute Helmholtz Centre for Polar and Marine Research, Bremerhaven, Germany
- 5Climate Sciences, Alfred Wegener Institute Helmholtz Centre for Polar and Marine Research, Bremerhaven, Germany
Antarctic krill Euphausia superba (“krill”) constitute a fundamental food source for Antarctic seabirds and mammals, and a globally important fisheries resource. The future resilience of krill to climate change depends critically on the winter survival of young krill. To survive periods of extremely low production by pelagic algae during winter, krill are assumed to rely partly on carbon produced by ice algae. The true dependency on ice algae-produced carbon, however, is so far unquantified. This confounds predictions on the future resilience of krill stocks to sea ice decline. Fatty acid (FA) analysis, bulk stable isotope analysis (BSIA), and compound-specific stable isotope analysis (CSIA) of diatom- and dinoflagellate-associated marker FAs were applied to quantify the dependency of overwintering larval, juvenile, and adult krill on ice algae-produced carbon (αIce) during winter 2013 in the Weddell-Scotia Confluence Zone. Our results demonstrate that the majority of the carbon uptake of the overwintering larval and juvenile krill originated from ice algae (up to 88% of the carbon budget), and that the dependency on ice algal carbon decreased with ontogeny, reaching <56% of the carbon budget in adults. Spatio-temporal variability in the utilization of ice algal carbon was more pronounced in larvae and juvenile krill than in adults. Differences between αIce estimates derived from short- vs. long-term FA-specific isotopic compositions suggested that ice algae-produced carbon gained importance as the winter progressed, and might become critical at the late winter-spring transition, before the phytoplankton bloom commences. Where the sea ice season shortens, reduced availability of ice algae might possibly not be compensated by surplus phytoplankton production during wintertime. Hence, sea ice decline could seriously endanger the winter survival of recruits, and subsequently overall biomass of krill.
Introduction
Antarctic krill Euphausia superba Dana (1850) (hereafter “krill”) is a highly abundant key species in the Southern Ocean, often channeling the majority of dietary carbon from marine microalgae to fishes, seabirds, and marine mammals (Hempel, 1987; Ward et al., 2012). Besides their ecological importance, krill constitute an increasingly harvested fisheries resource. In the period from 2000 to 2015, the annual catch has approximately doubled, accounting for over 200,000 metric tons, which equals a total market value of 300 million US$ in 2015 (Grant et al., 2013; CCAMLR, 2016). While fisheries effort increases, krill populations have suffered from sea ice decline and other climate change-related stressors (Atkinson et al., 2004; Flores et al., 2012a). In the south-west Atlantic sector of the Southern Ocean, decreasing winter sea ice extent was paralleled by a significant decline of krill abundance, indicating a pronounced dependency of krill on winter sea ice (Loeb et al., 1997; Atkinson et al., 2004, 2008; Flores et al., 2012a). Satellite observations show large regional variability with negative trends in sea ice extent (Parkinson and Cavalieri, 2012) and ice season duration (Stammerjohn et al., 2012) in key areas of krill distribution (Atkinson et al., 2008). Although there remains uncertainty in sea ice extent predictions for the Southern Ocean (Turner et al., 2013), the majority of simulations indicate more widespread sea ice decline in the coming decades, particularly during winter and spring (Turner et al., 2014).
Winter survival of krill larvae is considered the most vulnerable component of their life cycle to climate change (Flores et al., 2012a). Krill spawn during austral summer, between December and March. In autumn, the larvae reach their overwintering stage, the furcilia larvae (Fraser, 1937; Hempel et al., 1979). Krill larvae have a lower energy storage capacity than adults (Hagen et al., 2001), and thus a higher need to feed during the winter period to cover their energy demand (Quetin et al., 1994; Meyer et al., 2002; Meyer, 2012). In an experimental study, it has been shown that after 10–15 days of starvation, larvae krill lose their ability to take up food and to recover from nutritional stress (Yoshida et al., 2009). Hence, krill larvae cannot afford long periods of starvation (Quetin et al., 1994; Meyer, 2012). In winter, concentrations of pelagic algae (phytoplankton) are far below critical levels to sustain their food demand (Meyer, 2012). Alternatively, the productivity and/or standing stocks of “ice algae”—microalgae living in sea ice—and other in-ice fauna are assumed to be important in the diet of krill larvae during winter (Daly, 1990). Supporting this theory, primarily young developmental stages of krill were frequently found in high abundances underneath ice floes during winter (Meyer et al., 2009; Flores et al., 2012b; David et al., 2016). In addition, heterotrophic prey, e.g., copepods, can account significantly to the carbon uptake of krill larvae during winter (Meyer et al., 2009), but may in turn also depend on ice algae as a primary carbon source. Altogether, there is ample observational evidence that ice algae-produced carbon is important for the survival of krill larvae during their first winter (Daly, 1990; Frazer et al., 2002; Meyer et al., 2009). In contrast, overwintering adult krill have demonstrated a combination of strategies to survive periods of starvation, e.g., by reducing metabolic rates, mobilizing reserves from body tissues (Ikeda and Dixon, 1982; Meyer et al., 2010), feeding on detritus (Schmidt et al., 2011) or zooplankton (Ju and Harvey, 2004), or even cannibalism (Maihama and Endo, 1986). Temporarily, however, adults may also rely on ice algae-produced carbon (Marschall, 1988; Quetin et al., 1994).
Increasing fishing effort in combination with a presumed high vulnerability of krill to climate change has raised concerns about the future sustainability of krill harvesting and its role in Antarctic ecosystems (Schiermeier, 2010). In spite of extensive efforts to elucidate the winter survival of krill, the true contribution of ice algae-produced carbon to the carbon budget of overwintering krill has remained unresolved. Quantitative knowledge of the dependency of overwintering krill on ice algae-produced carbon, however, is critical for assessing the ability of krill to maintain today's population levels in regions of changing sea ice conditions. In the light of the assumed significant role of sea ice for overwintering krill, such an assessment would be crucial for future resource and conservation management.
In this study, we analyzed the composition of lipid classes, fatty acids (FAs), and stable isotopes in larval, juvenile and adult krill from the Weddell-Scotia Confluence Zone during late winter 2013. Lipid parameters provided information on energy allocation and the FA composition reflected the natural distribution of dietary FAs in krill. Certain FAs biosynthesised by microalgae are not metabolically transformed and can therefore be used as tracers of autotrophic carbon sources in food webs (Dalsgaard et al., 2003 and references therein). Some of these marker FAs are indicative of distinct autotrophic taxa, such as, Bacillariophyceae (“diatoms”), or Dinophyceae (“dinoflagellates”) (Dalsgaard et al., 2003 and references therein). In algal biomolecules, the lighter carbon stable isotope 12C is more reactive during photosynthetic assimilation than the heavier 13C isotope, and is therefore usually enriched. In sea ice, however, metabolic enrichment of 12C is less pronounced in the semi-closed environments of the sea ice brine channels, when CO2 concentrations drop during high productivity with reduced water exchange (Fry and Sherr, 1984; Hecky and Hesslein, 1995). Hence, biomolecules synthesized by ice algae often have a higher relative content of the heavy carbon isotope 13C compared to phytoplankton (Wada et al., 1987; Fischer, 1991; Rau et al., 1991). The isotopic composition of biomolecules from ice algae is transferred through the food web by organisms ingesting ice algae-produced carbon either directly or indirectly by carnivorous or detritivorous feeding. The isotopic fractionation of carbon (δ13C: 13C/12C) has therefore been used in polar organisms to estimate their relative dependency on ice algae-produced carbon (Budge et al., 2008; Wang et al., 2015; Jia et al., 2016; Kohlbach et al., 2016, 2017). In this study, we estimated the relative dependency of krill on carbon produced by ice algae in terms of the proportional contribution of ice algae-produced carbon to the body carbon of krill (αIce), based on bulk stable isotope analysis (BSIA) and compound-specific stable isotope analysis (CSIA) of marker FAs. Quantification of the importance of ice algae-produced carbon for ecological key species is a crucial step to model the overall carbon flux in the Southern Ocean under current and future scenarios. In this way, it ultimately contributes to the development of sustainable approaches in fisheries management and conservation policy.
Materials and Methods
Sample Collection
Sampling was conducted during the RV “Polarstern” expedition PS81 (14 August–16 October 2013) in the Weddell-Scotia Confluence Zone (Figure 1, Table 1). Detailed information on the sampling during PS81 can be found in David et al. (2016).
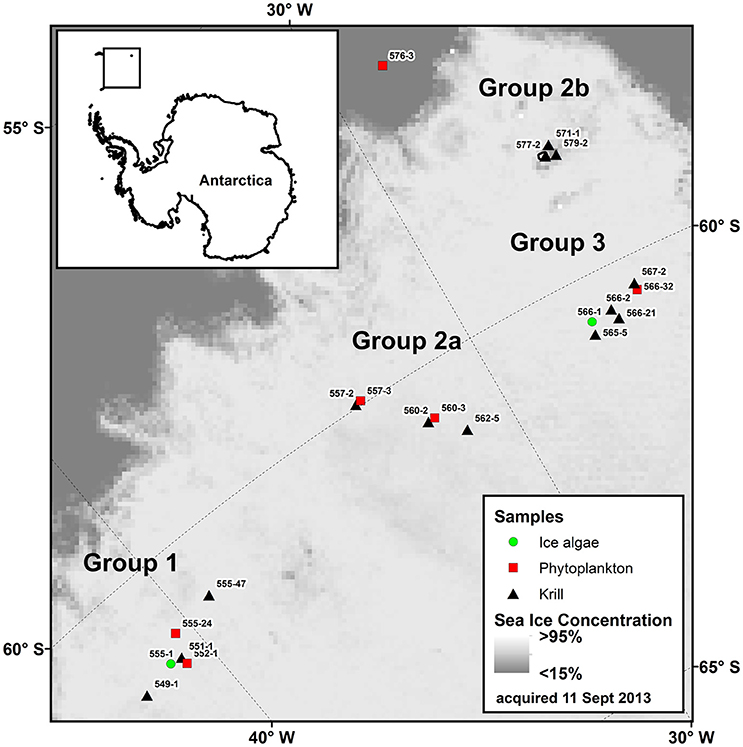
Figure 1. Map of the sampling area during “Polarstern” expedition PS81 in the Weddell-Scotia Confluence Zone (14 August to 16 October 2013) with sea ice concentration (Spreen et al., 2008). Ice camps were performed at stations 555-1 and 566-1. Station information for the individual sampling sites is given in Table 1. Stations were separated into four different groups after Schaafsma et al. (2016).
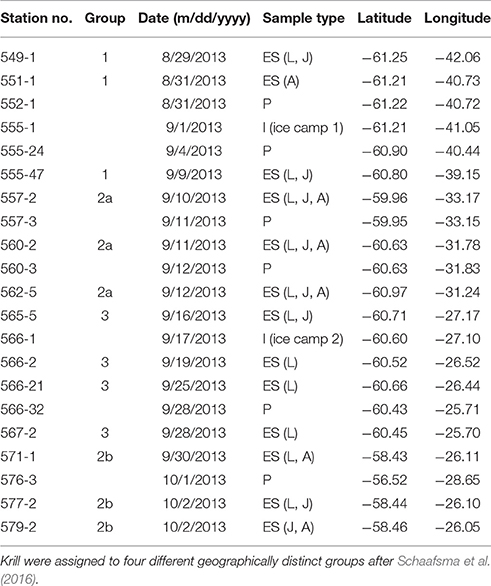
Table 1. Sample information for ice algae (I), phytoplankton (P), and larval (L), juvenile (J) and adult (A) Euphausia superba (ES) collected during RV “Polarstern” expedition PS81 in the Weddell-Scotia Confluence Zone.
The ice algae community was sampled by taking ice cores with a 9 cm interior diameter ice corer (Kovacs Enterprises) at two ice camps (n = 28) (Figure 1, Table 1). The bottom 10 cm of the ice cores were melted on board. Particulate Organic Matter (POM) from sea ice was concentrated by filtering the water from the melted ice cores via a vacuum pump through pre-combusted GF/F filters (1.0–1.5 L). The phytoplankton community (n = 5) was sampled at the chlorophyll a maximum depth (between 20 and 50 m) by a carousel water sampler connected to a CTD probe (Seabird SBE9+) with attached fluorometer (Table 1). Depending on the biomass concentration, POM from the water column was obtained by filtering between 3 and 21 L of water through pre-combusted GF/F filters. All filters were stored at –80°C until further processing.
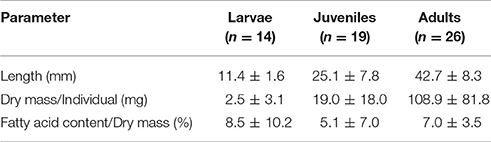
Krill was caught in the uppermost 2 m from directly underneath the sea ice by a Surface and Under-Ice Trawl (SUIT) (van Franeker et al., 2009). Larval krill were staged based on the number of terminal spines on the telson (Kirkwood, 1982). All analyzed larvae were staged as furcilia VI krill. Krill which already lost one pair of post-lateral spines from their telson (Fraser, 1937), but had not yet developed external sexual characteristics, were classified as juveniles (Makorov and Denys, 1981). Krill bearing external sexual characteristics were categorized into females and males (Makorov and Denys, 1981), but grouped together as “adults” since no statistical differences in biomarker parameters were apparent. After determination of the body length and developmental stage (Table 2), samples were immediately frozen at –80°C in pre-combusted and pre-weighed sample vials (Wheaton, 6 h, 500°C). Analytical work took place at the Alfred Wegener Institute, Bremerhaven, Germany.
To determine source areas and pathways of sampled sea ice, the individual sample positions were tracked backward in time using a combination of low resolution ice drift and concentration products from satellites. Sea ice concentration data used in this study were obtained from the National Snow and Ice Data Center (NSIDC). The applied sea ice motion information was obtained from NSIDC (prior 2013) and from OSISAF (Ocean and Sea Ice Satellite Application Facility, Norway, from 2013). Sea ice motion information is available on a daily basis and provided on a 25 km (NSIDC, Fowler et al., 2013) and 62.5 km grid (OSISAF), respectively. The tracking algorithm works as follows: Using motion and concentration data, a specific ice area is tracked backwards until: (a) the ice reaches a position next to a coastline, or (b) the ice concentration at a specific location reaches a threshold value of <40% when ice parcels are considered lost. For a detailed description of the applied algorithm, we refer to Krumpen et al. (2016).
Lipid Class and Fatty Acid Analyses
Prior to analysis, POM filters and whole krill individuals were freeze-dried for 24 h. Dry masses of krill were determined gravimetrically (Table 2). After homogenization, lipids were extracted with dichloromethane/methanol (2:1, v/v) (Folch et al., 1957). The lipid class composition of krill was investigated by High Performance Liquid Chromatography (HPLC) (Graeve and Janssen, 2009) in order to determine ontogenetic differences in the proportions of storage and membrane lipids. Extracted lipids were converted into fatty acid methyl esters (FAMEs) and free fatty alcohols by transesterification with methanol, containing 3% concentrated sulfuric acid. The proportional mass contributions of the diatom-associated marker FAs 16:1n-7 and 20:5n-3 (Graeve et al., 1994, 1997; Falk-Petersen et al., 1998; Volkman et al., 1998; Scott et al., 1999), the dinoflagellate-associated marker FAs 18:4n-3 and 22:6n-3 (Viso and Marty, 1993; Graeve et al., 1994; Phleger et al., 1998), and the copepod-associated marker FA 20:1n-9 and 22:1n-11 (Dahl et al., 2000; Virtue et al., 2016) were analyzed via gas chromatography. The percentage of each individual fatty acid of the total fatty acid mass was determined by an internal standard (23:0) added prior to lipid extraction. The total fatty acid content was determined gravimetrically by the internal standard (Table 2). 16:1n-7 and 18:4n-3 are mainly incorporated in storage lipids, and 20:5n-3 and 22:6n-3 mainly serve as membrane FAs. Storage FAs are assumed to reflect the more recent carbon source composition, whereas membrane FAs are more conserved (Stübing et al., 2003). To distinguish the short- and long-term importance of diatom- vs. dinoflagellate-derived carbon in the krill diet, the fatty acid ratios 16:1n-7/18:4n-3 and 20:5n-3/22:6n-3 were investigated. For details on sample preparation and measurements as well as analytical equipment see Kohlbach et al. (2016).
Bulk and Compound-Specific Stable Isotope Analyses
Length, dry weight, lipid content, and relative composition of storage and membrane lipids of krill used in BSIA and CSIA measurements were in the same range as those for lipid class and fatty acid composition (Table 2). Bulk nitrogen and carbon isotopic compositions of POM and krill were determined from freeze-dried bulk sample material (BSIA). Lipids were not removed prior to BSIA in order to avoid inducing changes in the isotopic composition of the krill samples (Mintenbeck et al., 2008). The FA-specific stable isotope composition of carbon was measured in FAME derivatives of the diatom-associated FAs 16:1n-7 and 20:5n-3, and the dinoflagellate-associated FAs 18:4n-3 and 22:6n-3 in POM and krill (CSIA; Kohlbach et al., 2016). During transesterification, a methyl group is added to the FA, which can result in a difference in δ13C values between the free FA and the corresponding FAME (e.g., Budge et al., 2011). In a preparatory experiment for this study, however, we did not find significant differences between the δ13C values of the free FA and the FAME (e.g., 16:0 FA: −28.56 ± 0.12‰, 16:0 FAME: −28.57 ± 0.16‰; C. Albers unpubl.). Therefore, we did not correct for these potential differences.
All isotopic ratios were expressed in the δ notation as parts per thousand (‰) differences from the primary (calibration) standards Vienna Pee Dee Belemnite (VPDB) for carbon measurements (δ13C), and atmospheric nitrogen for nitrogen measurements (δ15N).
Verification of accuracy and precision of BSIA measurements was done by measuring the secondary reference material USGS41 (δ15N = 47.6 ± 0.2‰, δ13C = 37.6 ± 0.1‰, measured: δ15N = 46.8‰, δ13C = 36.8‰), provided by the International Atomic Energy Agency (IAEA, Vienna). Measurement errors were indicated as ±0.8‰ for nitrogen and ±0.5‰ for stable carbon measurements, respectively (representing ±1 standard deviation of 17 analyses). Furthermore, the laboratory standards isoleucine (δ15N = −11.9‰, δ13C = −3.1‰) and peptone (δ15N = 8.0‰, δ13C = −15.7‰) were analyzed every five samples (Sigma Aldrich). Measurement errors were ±0.3‰ for nitrogen and ±0.6‰ for carbon isotope ratios of isoleucine (representing ±1 standard deviation of 16 analyses) and ±0.3‰ for both peptone measurements (representing ±1 standard deviation of 8 analyses). For CSIA measurements, quality assurance and analytical precision of the carbon stable isotope ratios were established by analyzing the certified standard FAME 14:0 (certified: δ13C = −30.0‰, measured: δ13C = 29.8‰), 16:0 (certified: δ13C = −30.7‰, measured: δ13C = −30.2‰), and 18:0 (certified: δ13C = −23.2‰, measured: δ13C = −23.8‰), supplied by Indiana University, every 5 samples. Analytical error was ±0.2‰ for 14:0 and ±0.3‰ for both 16:0 for 18:0 (representing ±1 standard deviation of 8 analyses). The samples were analyzed in duplicates, and true δ-values were obtained after two-point linear normalization (Paul et al., 2007). For details on sample preparation, measurements and analytical equipment for BSIA and CSIA see Kohlbach et al. (2016).
Quantification of Ice Algal Carbon
We estimated the proportional contribution of ice algae-produced carbon αIce to the body carbon of krill from the bulk and FA-specific carbon stable isotope compositions of POM from sea ice (simplified to ice algae) and the water column (simplified to phytoplankton), and krill by applying Bayesian multi-source stable isotope mixing models (MixSIAR) (Parnell et al., 2013; Stock and Semmens, 2015). The mixing models can account for different isotopic turnover rates in the consumers. However, krill-specific trophic enrichment of 13C is unknown, and was thus assumed to be zero for both BSIA and CSIA models (Budge et al., 2011; Graham et al., 2014; Wang et al., 2015). For the BSIA models, trophic enrichment of 15N was assumed to be 3.4‰ per trophic level (Minagawa and Wada, 1984). For the FA-specific modeling, αIce was calculated separately for (1) FAs associated with storage lipids (16:1n-7 + 18:4n-3) and for (2) FAs associated with biomembranes (20:5n-3 + 22:6n-3). Additionally, αIce was calculated by weighting the average mass proportions of storage and membrane lipids in each developmental stage, to account for differences in the contribution of the two lipid fractions to the total lipid content.
Data Analysis
Sea ice drift data suggested a different origin of the ice sampled at ice camp 1 and ice sampled at ice camp 2 (Figure 2). To assess the potential influence of spatio-temporal variability of ice algae samples on the results, we investigated algal FA profiles and isotopic values separately for the two ice camps. Conversely, we assessed the potential effect of the spatial variability of the krill population structure by comparing FA profiles and isotopic values between geographically separated cohort groups identified by Schaafsma et al. (2016) for larval and juvenile krill (Figure 1, Table 1). Cohort 1 comprised predominantly juvenile krill from station 555-47. Cohort 2a was dominated by larval krill from stations 557-2 to 562-5. Krill from cohort 2b had similar length distribution and developmental stages to krill from cohort 2a, but were sampled considerably later in the season and farther north (stations 571-1 to 579-2). Cohort 3 krill (stations 565-5 to 567-2) differed considerably in the composition of krill developmental stages to the other cohorts (Schaafsma et al., 2016). Because the overall krill population was dominated by larval and juvenile krill in the sampling area, the cohort grouping was the basis of grouping the krill population for this study into four groups (Groups 1, 2a, 2b, and 3). Krill from stations 549-1 and 551-1 were classified as group 1 krill due to the close spatio-temporal proximity to station 555-47.
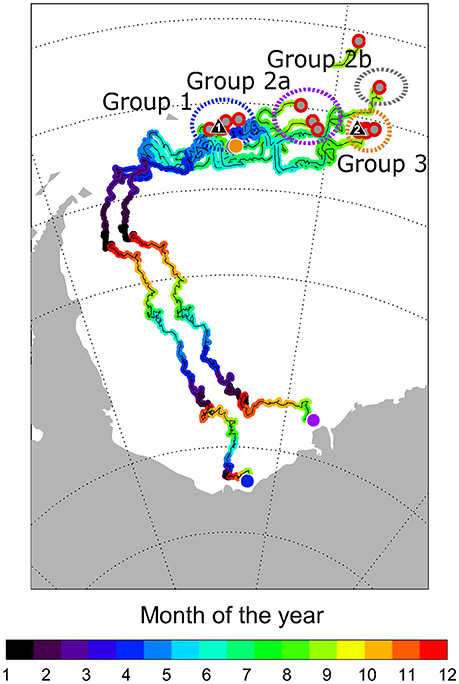
Figure 2. Backward-projected drift trajectories of sea ice areas in this study. Specific ice area is tracked backwards until the ice reaches a position next to a coastline, or the ice concentration at a specific location reaches a threshold value of <40% when ice parcels are considered lost (Krumpen et al., 2016). Stations with a distance <60 km to each other were represented as one dot. Triangular symbols mark the approximate position of the two ice camps. Dashed circles grouping stations by krill cohort group are distinguished by different colors. Dots in corresponding colors mark the back-tracked origin of sea ice drift trajectories from these station groups. Color code of drift trajectories represents the monthly sea ice position.
Based on the proportional αIce quantified for young krill (larvae + juveniles) derived from the CSIA model incorporating the stable isotope values of the storage FAs, ingestion rates (Pakhomov et al., 2004) and species abundances in the upper 2 m of the water column and the first 500 m of the water column during the sampling period (Schaafsma et al., 2016), we estimated the ice algae-produced carbon demand of young krill.
Variations in lipid class, FA and stable isotope compositions were tested using 1-way ANOVA followed by Tukey HSD post-hoc tests. For testing between ice algae and phytoplankton and to assess variability between the two ice camps, Student's t-tests were applied. A 2-way ANOVA was applied to determine if there was an interaction between the two independent variables (factors), carbon source (ice algae vs. phytoplankton), and sampling area (ice camp 1 vs. ice camp 2), on the dependent variable (stable isotope values). A Principal Component Analysis (PCA) was applied to visualize differences in krill FA proportions between the different groups. A distance-based analysis of similarities (ANOSIM) was applied to test for significant differences in FA proportions between krill from group 3 compared to all other groups, using a Euclidean distance matrix (Clarke and Ainsworth, 1993). FA data were transformed applying an arcsine square root function in order to achieve the near-normal distribution assumed by these parametric tests. In all statistical tests, we considered results with a statistical threshold value of α = 0.05 as significant. All performed data analyses were conducted using Software R, version 3.2.3 (R Core Team, 2015).
Results
Trophic Baseline
In the ice algae samples, the diatom-associated marker FAs 16:1n-7 and 20:5n-3 were the most abundant marker FAs, altogether contributing over 30% to the total fatty acid mass. Conversely, the dinoflagellate-associated marker FAs 18:4n-3 and 22:6n-3 were only present in small amounts (mean < 5%). In the phytoplankton samples, the mean proportional contributions of both diatom- and dinoflagellate-associated marker FAs ranged between 5 and 7%.
In the ice algae samples, the mean δ13C values of the bulk algal material and the diatom-associated marker FAs (16:1n-7, 20:5n-3) were on average significantly higher by 5–6‰ compared to the phytoplankton samples (Table 3). In the two dinoflagellate-associated marker FAs, significant differences in the mean δ13C values ranged between 9‰ (22:6n-3) and 14‰ (18:4n-3; Table 3). Only for 16:1n-7 δ13C values (dependent variable), the 2-way ANOVA indicated an interaction between the two carbon sources (ice algae and phytoplankton) and the ice camps (p < 0.05).
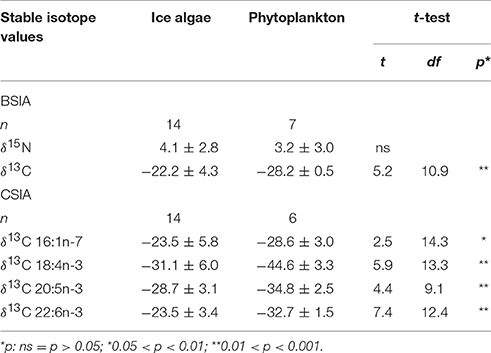
Table 3. Bulk nitrogen (δ15N) and carbon stable isotope compositions (δ13C) and δ13C compositions of marker fatty acids in ice algae and phytoplankton (mean ± 1 standard deviation ‰).
Between the two ice camps, the bulk δ13C values were significantly higher in the ice algal samples from ice camp 1 vs. ice camp 2. Contrastingly, the δ13C values in the FAs 16:1n-7 and 22:6n-3 were significantly higher in the ice algae samples from ice camp 2 vs. ice camp 1 (Supplementary A). In phytoplankton, there was no significant spatial variability in the δ13C values.
Krill
Lipid Class and Fatty Acid Compositions
The mean proportions of the membrane-associated phosphatidylcholines (PCs) were very similar among the different developmental stages (41–42% of the total lipid content; Table 4). Adult individuals, however, displayed significantly higher mean proportions of the storage-associated triacylglycerols (TAGs) (39%) relative to larval (12%) and juvenile krill (20%). Conversely, the mean proportions of the membrane-associated phosphatidylethanolamine (PE) were significantly lower in adult krill (10%) than in larvae (20%) and juveniles (18%; Table 4). Adding TAGs, PCs, and other typical storage lipids, such as, sterols and free fatty acids, the lipid pool was clearly dominated by storage lipids in all three developmental stages. Adults, however, had a significantly higher mass proportion of storage lipids (87.2 ± 6.2%) than larval (72.5 ± 5.4%) and juvenile krill [75.2 ± 7.6%; ANOVA F(2, 20) = 4.7, p < 0.05, Tukey HSD p < 0.05]. The variability in lipid class composition between the different groups in each developmental stage was insignificant, but larvae and juvenile krill from group 3 indicated lower proportions of TAGs (5.5 ± 8.4 and 2.9%, respectively) compared to the other groups (larvae: 17.3 ± 8.7%, juveniles: 25.3 ± 9.7%).
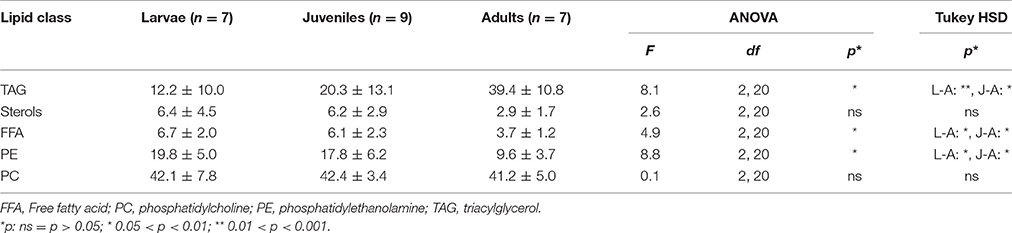
Table 4. Proportions of most abundant lipid classes in larval (L), juvenile (J), and adult (A) krill (mean ± 1 standard deviation mass % of total lipid content).
In all three developmental stages, the most abundant marker FAs were 20:5n-3 and 22:6n-3 (Table 5). Both polyunsaturated FAs were found in significantly higher concentrations in larval and juvenile krill vs. adult individuals. Contrastingly, the FA profiles of the adult individuals were characterized by significantly higher amounts of 16:1n-7 compared to the younger developmental stages. The sum of the storage-associated FA proportions of 16:1n-7 and 18:4n-3 was significantly higher [ANOVA F(2, 55) = 8.2, p < 0.001] in adults (8.5 ± 1.8%) vs. larvae (6.2 ± 2.0%, Tukey HSD p < 0.001) and juveniles (7.0 ± 1.7%, Tukey HSD p < 0.05). The proportional sum of the membrane-associated FAs 20:5n-3 and 22:6n-3 was significantly higher [ANOVA F(2, 55) = 83.7, p < 0.001] in larvae (49.4 ± 3.8%, Tukey HSD p < 0.001) and juvenile krill (44.8 ± 6.7%, Tukey HSD p < 0.001) than in adult krill (27.3 ± 6.0%). The levels of 20:1n-9 and 22:1n-11 were very low in all krill samples (mean < 1%; Table 5). Accordingly, the PCA showed a clear ontogenetic pattern in the marker fatty acid composition along Principal Component axis 1. Furthermore, larval and juvenile krill from group 3 clustered separately from all other groups along Principal Component axis 2, whereas groups 1–2b did not cluster apart from each other (Figure 3). A distinct marker fatty acid profile of group 3 compared to all other groups was confirmed with ANOSIM (R = 0.6853, p = 0.001). Krill from groups 1, 2a, and 2b indicated the same trend in fatty acid compositions as found in ice algae from ice camp 1, with higher proportions of the diatom-associated FA 20:5n-3 and lower proportions of the dinoflagellate-associated FA 22:6n-3 compared to krill from group 3 and ice algae from ice camp 2.
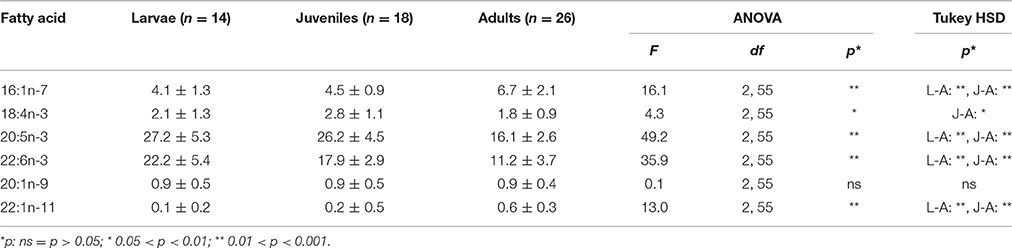
Table 5. Proportions of marker fatty acids in larval (L), juvenile (J), and adult (A) krill (mean ± 1 standard deviation mass % of total fatty acid content).
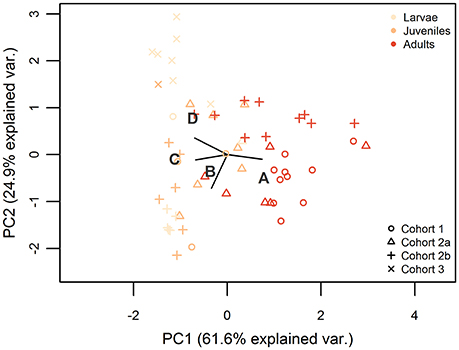
Figure 3. PCA biplot of marker fatty acids (FAs) in krill. 16:1n-7 (A) and 20:5n-3 (C) represent diatom-associated marker FAs, 18:4n-3 (B) and 22:6n-3 (D) represent dinoflagellate-associated marker FAs.
In all three developmental stages and both lipid fractions of krill, the mean ratio of the diatom-associated vs. dinoflagellate-associated marker FA (16:1n-7/18:4n-3 in storage lipids and 20:5n-3/22:6n-3 in membrane lipids) was on average > 1, indicating a dominance of diatom-associated carbon sources (Figure 4). Furthermore, this mean ratio was higher in storage lipids (2.3–5.5) than in membrane lipids (1.3–1.5) in all three developmental stages. The mean ratio in membrane lipids was lower in larvae (0.9 ± 0.3) and juveniles (1.0) from group 3 compared to the other three groups (range larvae 1.5–1.7, range juveniles 1.4–1.6).
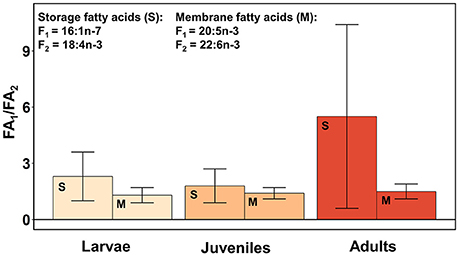
Figure 4. Ratios of diatom—vs. dinoflagellate-associated marker fatty acids (FAs) in krill (mean ± 1 standard deviation). The diatom-associated marker FA 16:1n-7 (FA1) and the dinoflagellate-associated marker FA 18:4n-3 (FA2) were considered storage FAs (S). The diatom-associated marker FA 20:5n-3 (FA1) and the dinoflagellate-associated marker FA 22:6n-3 (FA2) were considered membrane FAs (M).
Stable Isotope Compositions
In the krill samples, the δ13C values varied significantly between the developmental stages. The δ13C values of the bulk measurements and all marker fatty acids were lower in adults than in the younger stages (Table 6). The bulk δ15N values increased with ontogeny from larvae to adults, indicating a higher degree of heterotrophy in the diet of adult vs. young krill (Table 6).
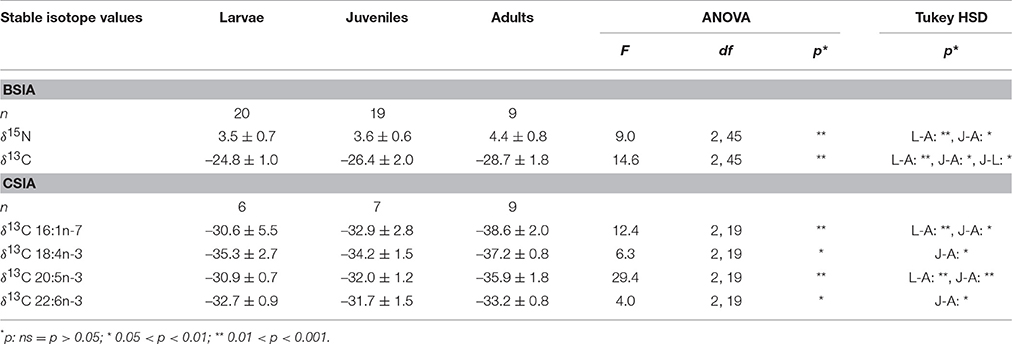
Table 6. Bulk nitrogen (δ15N) and carbon stable isotope compositions (δ13C) and δ13C compositions of marker fatty acids in larval (L), juvenile (J), and adult (A) krill (mean ± 1 standard deviation ‰).
Larvae from group 3 showed the lowest bulk δ13C values (−26.8 ± 0.5‰) among all four groups (range −24.3 to −25.7‰), which were significantly lower compared to larvae from groups 2a and 2b [ANOVA F(3, 16) = 29.6, p < 0.001, Tukey HSD p < 0.001]. Larval krill from group 3 also had significantly lower δ15N values (2.4 ± 0.5‰) than larvae from all other groups [range 3.3 to 4.1‰; ANOVA F(3, 16) = 21.4, p < 0.001, Tukey HSD p < 0.001]. Variability in FA-specific δ13C values between groups was insignificant in all three developmental stages.
Proportional Contribution of Ice Algal Carbon
The different drift history of the two ice camps in combination with differences in the isotopic composition of ice algae between the two ice camps indicated that the variability of ice algae parameters needed to be accounted for in the computation of the proportional contribution of ice algae-produced carbon αIce of the analyzed krill individuals. Therefore, we calculated αIce using ice algae stable isotope values from ice camp 1 for krill from groups 1 and 2a due to the close spatio-temporal proximity between the sampling, and the similarities in FA compositions. Krill from cohort 2b were also included in this group due to their similarity in fatty acid and stable isotope parameters with groups 1 and 2a. The trophic dependency on ice algal carbon in krill from group 3 was calculated with the ice algae stable isotope values from ice camp 2, based on the close spatio-temporal proximity between the sampling, the similarity in isotopic values between krill from group 3 and ice algae from ice camp 2, and the different drift trajectory of the sea ice region where group 3 krill were sampled compared to groups 1 and 2 (Figure 2).
Both BSIA and CSIA indicated higher mean αIce estimates in larval and juvenile krill compared to adult krill. BSIA-based mean proportions of ice algae-produced carbon αIce, considering the mean estimates of the different groups in each developmental stage, were 44% in larvae, 29% in juveniles, and 20% in adults (Figure 5A). CSIA-based estimates of αIce were calculated separately for storage lipids (FAs 16:1n-7 and 18:4n-3) and membrane lipids (FAs 20:5n-3 and 22:6n-3) in each individual krill. This procedure ensured integrating the trophic signal of both diatom- and dinoflagellate-associated marker FAs, respectively in each of the two lipid pools. In the CSIA-based mixing model using storage-associated FAs, mean αIce-values were between 69% in larvae and 65% in juveniles, and 53% in adults (Figure 5B). Mean αIce-values in membrane-associated FAs were considerably lower, with values between 26 and 33% in larvae and juveniles, and 17% in adults (Figure 5C). To account for the differences between storage lipids and membrane lipids in their relative contribution to the lipid mass, we estimated the overall CSIA-based αIce, weighted by the proportional mass of the two lipid fractions in each developmental stage. As a result, the mean CSIA-based relative dependency on ice algae-produced carbon was 39% in larvae, 46% in juveniles, and 33% in adults (Figure 5D).
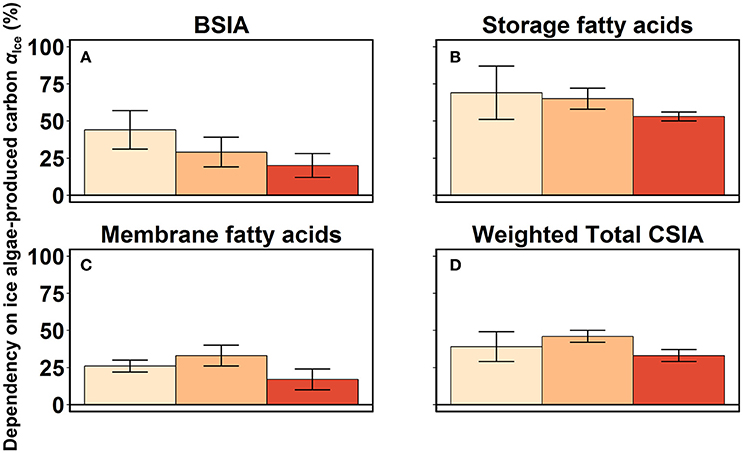
Figure 5. Proportional contribution of ice algae-produced carbon (αIce) to the body carbon of krill (mean ± 1 standard deviation %). Estimates of αIce based on the bulk stable isotope compositions are shown in (A). The estimates in (B,C) were based on the stable isotope compositions of the storage fatty acids 16:1n-7 and 18:4n-3 (B), and the membrane fatty acids 20:5n-3 and 22:6n-3 (C) from CSIA measurements. The Weighted Total CSIA estimates of αIce (D) were weighted by the proportional mass of storage and membrane lipids in each life stage.
The variability in αIce estimates between the groups was higher in larval and juvenile krill than in adults (Table 7). Variability in young krill was particularly evident in the αIce estimates based on the storage-associated FAs 16:1n-7 and 18:4n-3. Mean αIce estimates using these two storage-associated FAs indicated the highest trophic dependency on ice algae-produced carbon in larvae from group 2a (88%) and the lowest contribution of ice algal carbon to larval krill from group 3 (52%). In juveniles, mean αIce estimates were somewhat higher in krill from group 2b vs. groups 1 and 2a, based on the storage-associated FAs. Furthermore, BSIA results indicated a higher contribution of ice algae-produced carbon to juveniles from groups 2a and 2b vs. group 1. The weighted total CSIA estimates were similar among the groups in each developmental stage. Only the estimate for larvae from group 2a was considerably higher compared to the estimates from groups 2b and 3 (Table 7).
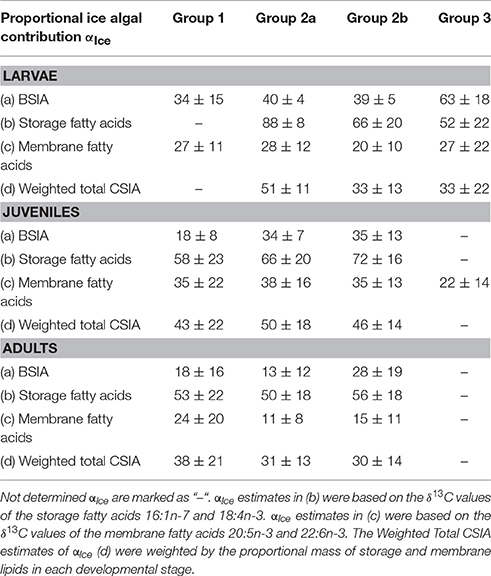
Table 7. Proportional contribution of ice algae-produced carbon αIce to the body carbon of krill from the four different groups (mean ± 1 standard deviation %).
Discussion
Lipid and Fatty Acid Parameters
Lipids provide two essential functions in living organisms: (1) they constitute structural components in membranes, and (2) they are a component for cellular energy storage (Lee et al., 2006). In most Antarctic euphausiids, energy is mainly stored in wax esters, providing long-term energy compared to triacylglycerols (TAGs), which are faster metabolized (Fricke et al., 1984; Stübing et al., 2003; Ko et al., 2015). Krill, however, mainly rely on TAGs and phosphatidylcholines (PCs) as energy stores for the survival of food-limited periods (Hagen et al., 1996). The observed ontogenetic differences in lipid class compositions among the different developmental stages were consistent with the known higher energy storage capacity of adults compared to younger stages (Meyer, 2012).
The distribution of marker FAs in the ice algae samples confirmed the well-known dominance of diatoms in the ice algal community (Garrison and Close, 1993; Lizotte, 2001). The evenly distributed proportions of diatom- and dinoflagellate-associated FAs in the phytoplankton samples indicated a co-dominance of both algal taxa (Buck and Garrison, 1983; Garrison et al., 1991). The high proportions of the long-chain FAs 20:5n-3 and 22:6n-3 in all three krill developmental stages were in agreement with previous studies (Falk-Petersen et al., 2000; Virtue et al., 2016). Hagen et al. (2001) also found higher levels of 20:5n-3 and 22:6n-3 accompanied by lower levels of 16:1n-7 in krill furcilia larvae compared to adults, suggesting that the fatty acid signatures of larvae might provide a more evident dietary input of primary synthesized FAs compared to the FA content of the adult krill. The diatom-associated marker FA 16:1n-7 and the dinoflagellate-associated marker FA 18:4n-3 are mainly incorporated into storage lipids, whereas the diatom-associated marker FA 20:5n-3 and the dinoflagellate-associated marker FA 22:6n-3 are predominantly incorporated in membrane lipids (Stübing et al., 2003). Hence, in this study we considered the two pairs of FAs separately in their two corresponding lipid fractions. The two storage-associated marker FAs are metabolically more dynamic, and hence more likely to reflect dietary patterns during the sampling periods than the highly conserved membrane-associated marker FAs, which possibly still reflected in parts the trophic signal from autumn grazing (Stübing et al., 2003). The higher ratio of diatom-associated vs. dinoflagellate-associated FAs in storage vs. membrane lipids in all three developmental stages indicated that diatoms were a more important carbon source for krill during the weeks of our sampling than integrated over the months before.
Several Antarctic copepod species, including Oithona spp., Metridia spp., Calanus propinquus, and Calanoides acutus, produce the long-chain FA isomers 20:1 and 22:1 de novo in large amounts, which can be used as carnivory marker FAs in omnivorous or carnivorous predators (Kattner et al., 1994; Cripps and Hill, 1998; Ju and Harvey, 2004). The low levels of these FAs in all krill samples suggested that copepods did not serve as major food source during our sampling period. It has to be considered, however, that the FAs 20:1n-9 and 22:1n-11 are largely egested with the krill's fecal pellets (Stübing et al., 2003; Stübing, 2004). Therefore, the estimation of the absolute copepod-derived carbon uptake based on relative proportions of these FAs in the consumers can be difficult when they are metabolized quickly. Results from stomach analysis indicated that other copepod genera (such as, Stephos longipes) were an important diet component at least during the hours and days of our sampling (Schaafsma et al., 2017).
Quantification of the Dependency on Ice Algal Carbon
Estimates of the proportional contribution of ice algal carbon αIce based on BSIA can be influenced by the varying metabolic fractionations of δ13C in the different organic compounds, i.e. lipids, proteins, and carbohydrates, of the bulk material (DeNiro and Epstein, 1978; Kohlbach et al., 2016, 2017). In contrast, δ13C values of trophic marker FAs are assumed to be unchanged by metabolic processes, and independent from the chemical composition of organisms. CSIA allows estimating δ13C in individual marker FAs to accurately trace and quantify sea ice-produced carbon in food webs (Wang et al., 2015; Kohlbach et al., 2016). Here, we applied for the first time both stable isotope methods to quantify the relative dependency of overwintering krill on ice algae-produced carbon. Both BSIA- and weighted CSIA-based estimates of αIce consistently showed a high dependency on ice algae-produced carbon by larval krill, and a lower dependency in adults (Figure 5).
The isotopic turnover rate in an organism depends on its feeding rate and the incorporation of new dietary carbon when switching to a different food source, e.g., from pelagic algae to ice algae. Particularly in winter, krill larvae have higher feeding rates compared to adult krill, and a large proportion of the ingested carbon is utilized for growth (Atkinson et al., 2002; Meyer et al., 2002). Contrastingly, in adults the signal of more recent ice algae-derived carbon uptake may have been buffered by their older and more modified lipid pool (Stübing et al., 2003). Because of the varying carbon turnover rates between the different developmental stages, the lower αIce estimates from both the short-term and the long-term isotopic compositions in adults may have been negatively biased compared to larvae and juveniles. Overall, CSIA-based estimates of αIce in membrane FAs probably retained in parts the trophic signal of pre-winter feeding, because these FAs are highly conserved and accumulated over long time periods (Stübing et al., 2003). Conversely, CSIA-based estimates of αIce in storage-associated FAs rather represented carbon sources during our sampling period, indicating that about two-thirds of the carbon demand in larval and juvenile krill originated from ice algae in late winter. Our weighted CSIA-based estimate of αIce should therefore be considered as a conservative estimate.
Spatio-Temporal Variability in the Stable Isotope Compositions and the Utilization of Ice Algal Carbon
δ13C values in POM can vary interanually, seasonally and regionally, ranging from −16 to −28‰ in ice-associated POM and from −25 to −31‰ in pelagic POM from the Weddell Sea (Rau et al., 1991). The large range of ice algae δ13C values in our study suggests that the sampled ice algae were representative for the range of values that krill have encountered throughout the research area. Thus, we are confident that our mixing models of the ice algae-derived carbon uptake in krill were not biased by the specific temporal and spatial framework of our sampling. In the areas around both ice camps, δ13C from bulk material and marker FAs could be used to confidently discriminate between ice algae—and pelagic algae-associated carbon sources. However, the FA and stable isotope compositions varied considerably between ice algae from the two ice camps (Supplementary A). Such differences can occur due to variations in the isotopic baseline during ice formation, variations in ice thickness, snow cover, and light intensity, subsequently affecting growth conditions of the ice algae. Our backward-projected sea ice drift data indicated that such variations could have been related to differences in the origin of ice formation, ice age, and drift trajectory of the sea ice in the areas of the two ice camps (Figure 2). The sea ice tracking indicated that much of the sea ice near ice camp 1 fostered the development of microbial in-ice communities over a longer period than the sea ice near ice camp 2, where the drift possibly only allowed for the development of microbial in-ice communities with lower food quality, coinciding with differences in the FA composition and a reduced physiological condition of krill larvae of group 3 (Schaafsma et al., 2016; Figure 2). Passive-microwave retrieved ice drift products are provided by different institutions and have been widely used in sea ice studies and for model assimilation (e.g., Spreen et al., 2011; Sumata et al., 2014; Krumpen et al., 2016). According to Sumata et al. (2015), the uncertainty of satellite-based drift products in the Arctic range from 0.9 to 2.0 cm s−1, depending on the type of product, drift speed, ice concentration and season. Uncertainty estimates are based on a comparison of buoy derived sea ice motion with satellite derived drift. Unfortunately, the uncertainty of motion products in the Antarctic has not been studied in detail. Therefore, we assume the uncertainty to be within the range of estimates carried out in the Arctic.
Accounting for the spatio-temporal variability in ice algal δ13C values between the two ice camps, we determined the utilization of ice algal carbon in krill grouped into four different groups (Schaafsma et al., 2016). Accordingly, ice algae-produced carbon contributed up to 88% to the carbon budget of larval krill at the earlier sampled locations near ice camp 1 in the closed pack-ice (group 2a), whereas the dependency on ice algal carbon was approximately 20% less in individuals sampled near the ice edge (group 2b), based on the combined isotopic information of the short-term FAs 16:1n-7 and 18:4n-3 and the weighted total CSIA results (Table 7). Supporting these results, the impact of diatom-vs. dinoflagellate-associated FAs in the storage lipid fraction was higher in young krill from group 2a vs. group 2b, possibly reflecting the higher impact of ice algae—vs. pelagic algae-produced FAs in the weeks before the sampling. The juvenile krill from group 1 mainly comprised individuals in their second year, whereas the other groups consisted predominantly of juveniles in their first year (Schaafsma et al., 2016). Thus, a decrease in the utilization of ice algae-produced carbon with ontogeny might have been reflected by the considerably lower αIce values in juveniles from group 1 compared to groups 2a and 2b based on the bulk stable isotope values and δ13C values in storage-associated FAs.
A good body condition of young krill from group 2a was indicated by high proportions of stored lipids in form of TAG, which further supports the assumption of the constant feeding on ice-associated biota. The higher δ15N values in all three krill developmental stages from group 2b in comparison to the other groups indicated an elevated indirect uptake of ice algae-produced carbon via predatory feeding. These results suggest a somewhat lower trophic association to sea ice when other food sources than ice algal carbon become more abundant in the water column, triggered by enhanced melting and pelagic productivity at the onset of spring.
Krill from group 3 differed considerably in lipid, fatty acid and stable isotope composition compared to the other three cohort groups. The high proportions of the dinoflagellate-associated FA 22:6n-3 were in agreement with the considerably lower αIce estimates for larval krill from group 3 vs. groups 2a and 2b (Table 7), and might have reflected the higher proportions of 22:6n-3 in ice algae from ice camp 2 vs. ice camp 1. The low δ13C and δ15N values in larval krill from group 3 suggest that the sea ice conditions and availability of sea ice resources were less favorable for krill in this region compared to the other regions. However, the trophic dependency on ice algal carbon by young krill was overall significant throughout the entire sampling area and sampling period, and still above 60% in krill near the ice edge (group 2b), suggesting a generally high importance of ice algae-produced carbon during winter.
Importance of Ice Algal Carbon for Overwintering Krill
With a good agreement between both isotopic approaches, this study demonstrates for the first time that up to two-thirds of the carbon demand of young krill during their first winter is covered from ice algae production. It has been suggested that high abundances of young developmental stages of krill underneath ice floes indicate that feeding on sea ice biota is important for their winter survival, but this behavior may also be related to other factors, e.g., predator avoidance (Hamner et al., 1989; Frazer et al., 2002; Meyer et al., 2009; Flores et al., 2012b; David et al., 2016). A high importance of ice algae in the diet was supported by analyses of stomach contents and FA compositions in previous studies, finding that diatoms were an important carbon source of young krill (Daly, 1990; Schmidt et al., 2014). Stomach content and FA analyses alone, however, cannot accurately discriminate between the signal of ice algae and phytoplankton, because these two communities overlap significantly in species composition (Garrison and Buck, 1985; Garrison et al., 1987; Melnikov, 1998). Heterotrophic prey species sometimes also account for a significant part of the diet of krill (Perissinotto et al., 2000), but their link to ice algae as a carbon source is not always clear, because the trophic signal is diluted from one trophic level to another (Meyer et al., 2009; Schmidt et al., 2014). Overcoming the uncertainty of these well-established methods to reliably trace the trophic signal of ice algae, our isotopic biomarker approach provides quantitative evidence of a crucial dependency of particularly larval and juvenile krill on ice algae-produced carbon, integrated over several trophic levels.
In a recent study by Jia et al. (2016), bulk stable isotope data of krill collected from East Antarctica during two winter-spring transitions are compared. As found in our study, adult specimens had lower δ13C values than larval and juvenile krill, confirming the common notion that our observed ontogenetic trend of a higher utilization of ice algal carbon by younger krill is a rather general pattern in different regions of the Southern Ocean (Meyer, 2012). A lower dependency of adult krill on ice algae-produced carbon (Figure 5) in combination with a higher proportion of storage lipids confirms the paradigm that adult krill survive the winter mainly by reducing metabolism and mobilizing lipid reserves accumulated during summer and autumn (Meyer, 2012). This assumption was supported by previous studies on the feeding behavior of postlarval krill, reporting very low feeding rates (Morris and Priddle, 1984; Quetin and Ross, 1991) and no significant growth during austral winter (Kawaguchi et al., 1986; Siegel, 1987). In our study, mean lipid contents in adults of 7% dry mass were at the low end of the typical range of 5–30% for winter and spring (Hagen et al., 1996; Ju and Harvey, 2004), indicating that energy reserves were almost depleted. For the comparison of the lipid levels with other studies, it has to be considered that instead of the total lipid content, we determined the mass percentage of the total fatty acid content. The total lipid content, containing molecule backbones besides the single FAs, can be assumed to be somewhat higher. The low lipid levels in our study in combination with the high αIce estimates based on the storage fatty acids, representing the more recent carbon source, suggest that ice algae-produced carbon could become critical for adult krill to survive the late winter-spring period, as long as phytoplankton production remained low (Meyer, 2012). Winter-feeding on sea ice biota by post-larval krill is corroborated by reported concentrations of juvenile and adult krill at the ice underside during winter and early spring (Marschall, 1988; Flores et al., 2012b). Both in larvae and adults, however, our knowledge about the seasonal variability of their dependency on ice algae-produced carbon is still limited. Therefore, trans-seasonal studies on the role of ice algae in the carbon budget of krill are needed to pinpoint critical time periods for krill survival.
Ecological Implications
Krill are distributed throughout the Southern Ocean, extending over about 27 degrees of latitude, from coastal waters to deep-sea and from year-round ice-covered to virtually ice-free regions, and are adapted to survive a wide range of environmental conditions (Flores et al., 2012a). The dependency of overwintering krill on ice algae-produced carbon is therefore likely to vary regionally, particularly driven by the duration of the sea ice season (Jia et al., 2016). A large part of the circum-Antarctic krill population (Atkinson et al., 2008) as well as major recruitment areas (Meyer, 2012) and fishing grounds (Flores et al., 2012a) are found in the Weddell-Scotia Confluence Zone and adjacent Scotia Sea. Stretching over a distance of approximately 900 km from west to east, our study area represented a major part of this key region of krill distribution and recruitment (Figure 1).
Based on an ingestion rate of 23 μg C ind. d−1 (Pakhomov et al., 2004), and αIce values for larval and juvenile krill derived from storage FAs (69 and 65%, respectively), first-year krill took up about 0.016 mg ice algal carbon and 0.007 mg phytoplankton carbon ind. d−1 during our sampling period. The northern Weddell Sea/Scotia Sea has experienced a shortening of the sea ice season of about 10 days per decade between 1979 and 2006, and it may be delayed by up to 40 days by the end of this century (Flores et al., 2012a; Piñones and Fedorov, 2016). Without sea ice, carbon availability in the water column would need to at least triple in order to support the 2012 standing stock of overwintering first-year krill in our study region. In the Weddell-Scotia Confluence Zone, chlorophyll a and pigment concentrations in the water column rarely exceed 0.1 mg m−3 between April and August even in ice-free waters (Comiso et al., 1993; David et al., 2016). In contrast, typical Antarctic bottom ice algal chlorophyll a concentrations during these months are in the order of 2–4 mg m−2 (Meiners et al., 2012). This suggests that the gap created in the krill energy budget by a loss of ice algae is highly unlikely to be filled by phytoplankton in an ice-free winter. More importantly, sea ice preserves carbon in the form of ice algae and heterotrophic sea ice biota throughout the dark period, when even ice algae production is nearly zero (Arrigo and Thomas, 2004). This carbon storage capacity will be reduced when delayed sea ice formation shortens the period for ice algal production before winter darkness (Arrigo and Thomas, 2004).
There is some evidence, however, that in the Southern Ocean climate change-related decline of sea ice and, consequently, ice algae production, may be balanced by an increase of overall phytoplankton productivity due to a longer ice-free growth season, higher temperatures and better iron supply (Arrigo and Thomas, 2004; Bopp et al., 2013; Vancoppenolle et al., 2013). This effect, however, may be counter-acted by deeper mixing due to increasing wind speeds in the future Southern Ocean (Lovenduski and Gruber, 2005; Cai, 2006). Furthermore, climate change could come at the cost of changes in phytoplankton composition toward taxa less supportive of krill, e.g., from diatoms to cryptophytes (Moline et al., 2004). With a comprehensive study comparing multiple models of the distribution of krill spawning habitats at the end of the twenty first century under various scenarios of environmental change, Piñones and Fedorov (2016) concluded that in most scenarios the available spawning habitat in the Southern Ocean declines, even when the models accounted for increased phytoplankton productivity and hence better energy reserves of krill at the onset of winter. Altogether, a prolonged growth season and/or increased primary production of Antarctic phytoplankton seem unlikely to significantly counter-act the detrimental effect of sea ice decline on the winter survival of first-year krill.
Conclusions
This study substantiates concerns with quantitative data that loss of ice algae habitat constitutes a serious threat to particularly young overwintering krill. In regions of on-going or future sea ice decline, this will lead to declining recruitment success, caused by reduced winter survival of larval krill, impacting significantly on the overall krill biomass and the sustainability of the krill fishery. Due to their position as a key secondary producer in the Southern Ocean, changing krill populations could incur significant ramifications on the structure and biogeochemical cycling of Antarctic ecosystems.
Author Contributions
DK was the main author of this paper and accomplished the laboratory analyses and data evaluation. HF designed and supervised this study and contributed substantially to the drafting of the manuscript. MG and HF provided laboratory materials, methodological expertise, and laboratory space. DK, HF, and BL contributed significantly to statistical analyses and data interpretation. JvF provided sampling logistics. CD, FS, and JvF accomplished the sampling. MV assisted the performance of the laboratory analyses. TK re-constructed drift trajectories of the sea ice. All authors contributed significantly to the drafting of this research article.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the captain Stefan Schwarze and the crew of the RV “Polarstern” expedition PS81 for their support with work at sea. We thank Michiel van Dorssen and André Meijboom for technical support with work at sea. SUIT was developed by Wageningen Marine Research with support from the Netherlands Ministry of EZ (project WOT-04-009-036) and the Netherlands Polar Program (project ALW-NWO 866.13.009). We thank Dieter Janssen and Theresa Geißler for their help with the laboratory analyses. We thank Bettina Meyer for providing ice algae samples. Sea ice concentration data were acquired from www.meereisportal.de. This study is part of the Helmholtz Association Young Investigators Group Iceflux: Ice-ecosystem carbon flux in polar oceans (VH-NG-800). We thank the editor Michael Arthur St. John, and the reviewers Shiway Wang and Eugene J. Murphy for their helpful suggestions and comments during the review process.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/article/10.3389/fmars.2017.00310/full#supplementary-material
References
Arrigo, K. R., and Thomas, D. N. (2004). Large scale importance of sea ice biology in the Southern Ocean. Antarct. Sci. 16, 471–486. doi: 10.1017/S0954102004002263
Atkinson, A., Siegel, V., Pakhomov, E. A., Rothery, P., Loeb, V., Ross, R. M., et al. (2008). Oceanic circumpolar habitats of Antarctic krill. Mar. Ecol. Prog. Ser. 362, 1–23. doi: 10.3354/meps07498
Atkinson, A., Meyer, B., Bathmann, U., Stübing, D., Hagen, W., and Schmidt, K. (2002). Feeding and energy budget of Antarctic krill Euphausia superba at the onset of winter-I Juveniles, I., and adults. Limnol. Oceanogr. 47, 953–996. doi: 10.4319/lo.2002.47.4.0953
Atkinson, A., Siegel, V., Pakhomov, E., and Rothery, P. (2004). Long-term decline in krill stock and increase in salps within the Southern Ocean. Nature 432, 100–103. doi: 10.1038/nature02996
Bopp, L., Resplandy, L., Orr, J. C., Doney, S. C., Dunne, J. P., Gehlen, M., et al. (2013). Multiple stressors of ocean ecosystems in the 21st century: projections with CMIP5 models. Biogeosciences 10, 6225–6245. doi: 10.5194/bg-10-6225-2013
Buck, K. R., and Garrison, D. L. (1983). Protists from the ice-edge region of the Weddell Sea. Deep Sea Res. (A Oceanogr. Res. Pap.) 30, 1261–1277. doi: 10.1016/0198-0149(83)90084-5
Budge, S. M., Wang, S. W., Hollmén, T. E., and Wooller, M. J. (2011). Carbon isotopic fractionation in eider adipose tissue varies with fatty acid structure: implications for trophic studies. J. Exp. Biol. 214, 3790–3800. doi: 10.1242/jeb.057596
Budge, S. M., Wooller, M. J., Springer, A. M., Iverson, S. J., Mcroy, C. P., and Divoky, G. J. (2008). Tracing carbon flow in an arctic marine food web using fatty acid-stable isotope analysis. Oecologia 157, 117–129. doi: 10.1007/s00442-008-1053-7
Cai, W. (2006). Antarctic ozone depletion causes an intensification of the Southern Ocean super-gyre circulation. Geophys. Res. Lett. 33, L03712. doi: 10.1029/2005GL024911
CCAMLR (2016). Krill Fisheries. Available online at: www.ccamlr.org/en/fisheries/krill-fisheries
Clarke, K. R., and Ainsworth, M. (1993). A method of linking multivariate community. Mar. Ecol. Prog. Ser. 92, 205–219. doi: 10.3354/meps092205
Comiso, J. C., McClain, C. R., Sullivan, C. W., Ryan, J. P., and Leonard, C. L. (1993). Coastal zone color scanner pigment concentrations in the Southern Ocean and relationships to geophysical surface features. J. Geophys. Res. 98, 2419–2451. doi: 10.1029/92JC02505
Cripps, G., and Hill, H. (1998). Changes in lipid composition of copepods and Euphausia superba associated with diet and environmental conditions in the marginal ice zone, Bellingshausen Sea, Antarctica. Deep Sea Res. 45, 1357–1381. doi: 10.1016/S0967-0637(98)00022-3
Dahl, T. M., Lydersen, C., Kovacs, K. M., Falk-Petersen, S., Sargent, J., Gjertz, I., et al. (2000). Fatty acid composition of the blubber in white whales (Delphinapterus leucas). Polar Biol. 23, 401–409. doi: 10.1007/s003000050461
Dalsgaard, J., St. John, M., Kattner, G., Müller-Navarra, D., and Hagen, W. (2003). Fatty acid trophic markers in the pelagic marine environment. Adv. Mar. Biol. 46, 225–340. doi: 10.1016/S0065-2881(03)46005-7
Daly, K. L. (1990). Overwintering development, growth, and feeding of larval Euphausia superba in the antarctic marginal ice zone. Limnol. Oceanogr. 35, 1564–1576. doi: 10.4319/lo.1990.35.7.1564
David, C., Schaafsma, F. L., van Franeker, J. A., Lange, B., Brandt, A., and Flores, H. (2016). Community structure of under-ice fauna in relation to winter sea-ice habitat properties from the Weddell Sea. Polar Biol. 40, 247–261. doi: 10.1007/s00300-016-1948-4
DeNiro, M. J., and Epstein, S. (1978). Influence of diet on the distribution of carbon isotopes in animals. Geochim. Cosmochim. Acta 42, 495–506. doi: 10.1016/0016-7037(78)90199-0
Falk-Petersen, S., Hagen, W., Kattner, G., Clarke, A., and Sargent, J. (2000). Lipids, trophic relationships, and biodiversity in Arctic and Antarctic krill. Can. J. Fish. Aquat. Sci. 57, 178–191. doi: 10.1139/f00-194
Falk-Petersen, S., Sargent, J. R., Henderson, J., Hegseth, E. N., Hop, H., and Okolodkov, Y. B. (1998). Lipids and fatty acids in ice algae and phytoplankton from the Marginal Ice Zone in the Barents Sea. Polar Biol. 20, 41–47. doi: 10.1007/s003000050274
Fischer, G. (1991). Stable carbon isotope ratios of plankton carbon and sinking organic matter from the Atlantic sector of the Southern Ocean. Mar. Chem. 35, 581–596. doi: 10.1016/S0304-4203(09)90044-5
Flores, H., Atkinson, A., Kawaguchi, S., Krafft, B. A., Milinevsky, G., Nicol, S., et al. (2012a). Impact of climate change on Antarctic krill. Mar. Ecol. Prog. Ser. 458, 1–19. doi: 10.3354/meps09831
Flores, H., van Franeker, J. A., Siegel, V., Haraldsson, M., Strass, V., Meesters, E. H., et al. (2012b). The association of Antarctic krill Euphausia superba with the under-ice habitat. PLoS ONE 7:e31775. doi: 10.1371/journal.pone.0031775
Folch, J., Lees, M., and Sloane-Stanley, G. H. (1957). A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226, 497–509.
Fowler, C., Emery, W., and Tschudi, M. (2013). Polar Pathfinder Daily 25 km EASE-Grid Sea Ice Motion Vectors, Version 2 (Daily and Mean Gridded Field). Boulder, CO: NASA DAAC at the Natl. Snow and Ice Data Cent.
Fraser, F. C. (1937). On the Development and Distribution of the Young Stages of Krill (Euphausia superba), 1st Edn. Cambridge: Cambridge University Press.
Frazer, T. K., Quetin, L. B., and Ross, R. M. (2002). Abundance, sizes and developmental stages of larval krill, Euphausia superba, during winter in ice-covered seas west of the Antarctic Peninsula. J. Plankton Res. 24, 1067–1077. doi: 10.1093/plankt/24.10.1067
Fricke, H., Gercken, G., Schreiber, W., and Oehlenschläger, J. (1984). Lipid, sterol and fatty acid composition of Antarctic krill (Euphausia superba Dana). Lipids 19, 821–827. doi: 10.1007/BF02534510
Fry, B., and Sherr, E. B. (1984). δ13C measurements as indicators of carbon flow in marine and freshwater ecosystems. Contrib. Mar. Sci. 27, 13–47.
Garrison, D. L., and Buck, K. R. (1985). “Sea-ice algal communities in the Weddell Sea: species composition in ice and plankton assemblages,” in Marine Biology of Polar Regions and Effects of Stress on Marine Organisms, eds J. S. Gray, and M. E. Christiansen (New York, NY: Wiley), 103–122.
Garrison, D. L., and Close, A. R. (1993). Winter ecology of the sea ice biota in Weddell sea pack ice. Mar. Ecol. Prog. Ser. 96, 17–31. doi: 10.3354/meps096017
Garrison, D. L., Buck, K. R., and Fryxell, G. A. (1987). Algal assemblages in Antarctic pack-ice and in ice-edge plankton. J. Phycol. 23, 564–572. doi: 10.1111/j.1529-8817.1987.tb04206.x
Garrison, D. L., Buck, K. R., and Gowing, M. M. (1991). Plankton assemblages in the ice edge zone of the Weddell Sea during the austral winter. J. Mar. Syst. 2, 123–130. doi: 10.1016/0924-7963(91)90018-P
Graeve, M., and Janssen, D. (2009). Improved separation and quantification of neutral and polar lipid classes by HPLC-ELSD using a monolithic silica phase: application to exceptional marine lipids. J. Chromatogr. B 877, 1815–1819. doi: 10.1016/j.jchromb.2009.05.004
Graeve, M., Kattner, G., and Hagen, W. (1994). Diet-induced changes in the fatty acid composition of Arctic herbivorous copepods: experimental evidence of trophic markers. J. Exp. Mar. Biol. Ecol. 182, 97–110. doi: 10.1016/0022-0981(94)90213-5
Graeve, M., Kattner, G., and Piepenburg, D. (1997). Lipids in Arctic benthos: does the fatty acid and alcohol composition reflect feeding and trophic interactions? Polar Biol. 18, 53–61. doi: 10.1007/s003000050158
Graham, C., Oxtoby, L., Wang, S. W., Budge, S. M., and Wooller, M. J. (2014). Sourcing fatty acids to juvenile polar cod (Boreogadus saida) in the Beaufort Sea using compound-specific stable carbon isotope analyses. Polar Biol. 37, 697–705. doi: 10.1007/s00300-014-1470-5
Grant, S. M., Hill, S. L., Trathan, P. N., and Murphy, E. J. (2013). Ecosystem services of the Southern Ocean: trade-offs in decision-making. Antarct. Sci. 25, 603–617. doi: 10.1017/S0954102013000308
Hagen, W., Kattner, G., Terbrüggen, A., and van Vleet, E. (2001). Lipid metabolism of the Antarctic krill Euphausia superba and its ecological implications. Mar. Biol. 139, 95–104. doi: 10.1007/s002270000527
Hagen, W., van Vleet, E., and Kattner, G. (1996). Seasonal lipid storage as overwintering strategy of Antarctic krill. Mar. Ecol. Progr. Ser. 134, 85–89. doi: 10.3354/meps134085
Hamner, W. M., Hamner, P. P., Obst, B. S., and Carleton, J. H. (1989). Field observations on the ontogeny of schooling of Euphausia superba furciliae and its relationship to ice in Antarctic waters. Limnol. Oceanogr. 34, 451–456. doi: 10.4319/lo.1989.34.2.0451
Hecky, R. E., and Hesslein, R. H. (1995). Contributions of benthic algae to lake food webs as revealed by stable isotope analysis. J. N. Am. Benthol. Soc. 14, 631–653. doi: 10.2307/1467546
Hempel, G. (1987). The krill-dominated pelagic system of the southern Ocean. Environ. Int. 13, 33–36. doi: 10.1016/0160-4120(87)90041-9
Hempel, I., Hempel, G., and Baker, A. D. C. (1979). Early life history stages of krill (Euphausia superba) in Bransfield Strait and Weddell Sea. Rep. Mar. Res. 27, 267–281.
Ikeda, T., and Dixon, P. (1982). Body shrinkage as a possible overwintering mechanism of the Antarctic krill, Euphausia superba Dana. J. Exp. Mar. Biol. Ecol. 62, 143–151. doi: 10.1016/0022-0981(82)90088-0
Jia, Z., Swadling, K. M., Meiners, K. M., Kawaguchi, S., and Virtue, P. (2016). The zooplankton food web under East Antarctic pack ice–A stable isotope study. Deep Sea Res. 131, 189–202. doi: 10.1016/j.dsr2.2015.10.010
Ju, S.-J., and Harvey, H. R. (2004). Lipids as markers of nutritional condition and diet in the Antarctic krill Euphausia superba and Euphausia crystallorophias during austral winter. Deep Sea Res. 51, 2199–2214. doi: 10.1016/j.dsr2.2004.08.004
Kattner, G., Graeve, M., and Hagen, W. (1994). Ontogenetic and seasonal changes in lipid and fatty acid/alcohol compositions of the dominant Antarctic copepods Calanus propinquus, Calanoides acutus, and Rhincalanus gigas. Mar. Biol. 118, 637–644. doi: 10.1007/BF00347511
Kawaguchi, K., Ishikawa, S., and Matsuda, O. (1986). The overwintering strategy of Antarctic krill (Euphausia superba Dana) under the coastal fast ice off the Ongul Islands in Lutzow-Holm Bay, Antarctica. Mem. Natl. Inst. Polar Res. 44, 67–85.
Kirkwood, J. M. (1982). A Guide to the Euphausiacea of the Southern Ocean, Vol. 1, ANARE Res. Notes. Information Services Section, Antarctic Division, Dept. of Science and Technology.
Ko, A.-R., Yang, E. J., Kim, M-S., and Ju, S-J. (2015). Trophodynamics of Euphausiids in the Amundsen Sea during the Austral Summer by Fatty Acid and Stable Isotopic Signatures. Deep Sea Res. 123, 78–85. doi: 10.1016/j.dsr2.2015.04.023
Kohlbach, D., Schaafsma, F. L., Graeve, M., Lebreton, B., Lange, B. A., David, C., et al. (2017). Strong linkage of polar cod (Boreogadus saida) to sea ice algae-produced carbon: evidence from stomach content, fatty acid and stable isotope analyses. Prog. Oceanogr. 152, 62–74. doi: 10.1016/j.pocean.2017.02.003
Kohlbach, D., Graeve, M., Lange, B. A., David, C., Peeken, I., and Flores, H. (2016). The importance of ice algae-produced carbon in the central Arctic Ocean ecosystem: food web relationships revealed by lipid and stable isotope analyses. Limnol. Oceanogr. 61, 2027–2044. doi: 10.1002/lno.10351
Krumpen, T., Gerdes, R., Haas, C., Hendricks, S., Herber, A., Selyuzhenok, V., et al. (2016). Recent summer sea ice thickness surveys in Fram Strait and associated ice volume fluxes. Cryosphere 10:523. doi: 10.5194/tc-10-523-2016
Lee, R. F., Hagen, W., and Kattner, G. (2006). Lipid storage in marine zooplankton. Mar. Ecol. Prog. Ser. 307, 273–306. doi: 10.3354/meps307273
Lizotte, M. P. (2001). The contributions of sea ice algae to Antarctic marine primary production. Am. Zool. 41, 57–73. doi: 10.1093/icb/41.1.57
Loeb, V., Siegel, V., Holm-Hansen, O., Hewitt, R., Fraser, W., Trivelpiece, W., et al. (1997). Effects of sea-ice extent and krill or salp dominance on the Antarctic food web. Nature 387, 897–900. doi: 10.1038/43174
Lovenduski, N. S., and Gruber, N. (2005). Impact of the Southern Annular Mode on Southern Ocean circulation and biology. Geophys. Res. Lett. 32, L11603. doi: 10.1029/2005GL022727
Maihama, Y., and Endo, Y. (1986). Laboratory observations on molting and growth of Antarctic krill, Euphausia superba Dana. Mem. Natl. Inst. Pol. Res. 44, 125–127.
Makorov, R. R., and Denys, C. J. I. (1981). Stages of Sexual Maturity of Euphausia Superba, Vol. 11. Cambridge: SCAR and SCOR Polar Res. Inst. 1–13.
Marschall, H.-P. (1988). The overwintering strategy of Antarctic krill under the pack-ice of the Weddell Sea. Polar Biol. 9, 129–135. doi: 10.1007/BF00442041
Meiners, K. M., Vancoppenolle, M., Thanassekos, S., Dieckmann, G. S., Thomas, D. N., Tison, J.-L., et al. (2012). Chlorophyll a in Antarctic sea ice from historical ice core data. Geophys. Res. Lett. 39:L21602. doi: 10.1029/2012GL053478
Melnikov, I. A. (1998). Winter production of sea ice algae in the western Weddell Sea. J. Mar. Syst. 17, 195–205. doi: 10.1016/S0924-7963(98)00038-4
Meyer, B. (2012). The overwintering of Antarctic krill, Euphausia superba, from an ecophysiological perspective. Polar Biol. 35, 15–37. doi: 10.1007/s00300-011-1120-0
Meyer, B., Atkinson, A., Stübing, D., Oettl, B., Hagen, W., and Bathmann, U. (2002). Feeding and energy budget of Antarctic krill Euphausia superba at the onset of winter-I. Furcilia III larvae. Limnol. Oceanogr. 47, 943–952. doi: 10.4319/lo.2002.47.4.0943
Meyer, B., Auerswald, L., Siegel, V., Spahic, S., Pape, C., Fach, B. A., et al. (2010). Seasonal variation in body composition, metabolic activity, feeding, and growth of adult krill Euphausia superba in the Lazarev Sea. Mar. Ecol. Prog. Ser. 398, 1–18. doi: 10.3354/meps08371
Meyer, B., Fuentes, V., Guerra, C., Schmidt, K., Atkinson, A., Spahic, S., et al. (2009). Physiology, growth, and development of larval krill Euphausia superba in autumn and winter in the Lazarev Sea, Antarctica. Limnol. Oceanogr. 54, 1595–1614. doi: 10.4319/lo.2009.54.5.1595
Minagawa, M., and Wada, E. (1984). Stepwise enrichment of d15N along food chains: further evidence and the relation between d15N and animal age. Geochim. Cosmochim. Acta 48, 1135–1140. doi: 10.1016/0016-7037(84)90204-7
Mintenbeck, K., Brey, T., Jacob, U., Knust, R., and Struck, U. (2008). How to account for the lipid effect on carbon stable-isotope ratio (δ13C): sample treatment effects and model bias. J. Fish Biol. 72, 815–830. doi: 10.1111/j.1095-8649.2007.01754.x
Moline, M. A., Claustre, H., Frazer, T. K., Schofield, O., and Vernet, M. (2004). Alteration of the food web along the Antarctic Peninsula in response to a regional warming trend. Global Change Biol. 10, 1973–1980. doi: 10.1111/j.1365-2486.2004.00825.x
Morris, D. J., and Priddle, J. (1984). Observations on the feeding and moulting of the Antarctic krill, Euphausia superba Dana, in winter. Bull. Brit. Antarct. Surv. 65, 57–63.
Pakhomov, E. A., Atkinson, A., Meyer, B., Oettl, B., and Bathmann, U. (2004). Daily rations and growth of larval krill Euphausia superba in the Eastern Bellingshausen Sea during austral autumn. Deep Sea Res. 51, 2185–2198. doi: 10.1016/j.dsr2.2004.08.003
Parkinson, C. L., and Cavalieri, D. J. (2012). Antarctic sea ice variability and trends, 1979–2010. Cryosphere 6, 871–880. doi: 10.5194/tc-6-871-2012
Parnell, A. C., Phillips, D. L., Bearhop, S., Semmens, B. X., Ward, E. J., Moore, J. W., et al. (2013). Bayesian stable isotope mixing models. Environmetrics 24, 387–399. doi: 10.1002/env.2221
Paul, D., Skrzypek, G., and Forizs, I. (2007). Normalization of measured stable isotopic compositions to isotope reference scales-a review. Rapid Commun. Mass Spectrom. 21, 3006–3014. doi: 10.1002/rcm.3185
Perissinotto, R., Gurney, L., and Pakhomov, E. (2000). Contribution of heterotrophic material to diet and energy budget of Antarctic krill, Euphausia superba. Mar. Biol. 136, 129–135. doi: 10.1007/s002270050015
Phleger, C. F., Nichols, P. D., and Virtue, P. (1998). Lipids and trophodynamics of Antarctic zooplankton. Comp. Biochem. Physiol. B 120, 311–323. doi: 10.1016/S0305-0491(98)10020-2
Piñones, A., and Fedorov, A. V. (2016). Projected changes of Antarctic krill habitat by the end of the 21st century. Geophys. Res. Lett. 43, 8580–8589. doi: 10.1002/2016GL069656
Quetin, L. B., and Ross, R. M. (1991). Behavioral and physiological characteristics of the Antarctic krill, Euphausia superba. Am. Zool. 31, 49–63. doi: 10.1093/icb/31.1.49
Quetin, L. B., Ross, R. M., and Clarke, A. (1994). “Krill energetics: seasonal and environmental aspects of the physiology of Euphausia superba,” in Southern Ocean Ecology: the BIOMASS Perspective, ed S. El-Sayed (Cambridge: Cambridge University Press), 165–184.
Rau, G. H., Sullivan, C. W., and Gordon, L. I. (1991). δ13C and δ15N variations in Weddell Sea particulate organic matter. Mar. Chem. 35, 355–369. doi: 10.1016/S0304-4203(09)90028-7
R Core Team (2015). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: www.R-project.org
Schaafsma, F. L., David, C., Pakhomov, E. A., Hunt, B. P. V., Lange, B. A., Flores, H., et al. (2016). Size and stage composition of age class 0 Antarctic krill (Euphausia superba) in the ice–water interface layer during winter/early spring. Polar Biol. 39, 1515–1526. doi: 10.1007/s00300-015-1877-7
Schaafsma, F. L., Kohlbach, D., David, C., Lange, B. A., Graeve, M., Flores, H., et al. (2017). Spatio-temporal variability in the winter diet of larval and juvenile Antarctic krill Euphausia superba in ice-covered waters. Mar. Progr. Ser. doi: 10.3354/meps12309
Schiermeier, Q. (2010). Ecologists fear Antarctic krill crisis. Nature 467, 15–15. doi: 10.1038/467015a
Schmidt, K., Atkinson, A., Pond, D. W., and Ireland, L. C. (2014). Feeding and overwintering of Antarctic krill across its major habitats: the role of sea ice cover, water depth, and phytoplankton abundance. Limnol. Oceanogr. 59, 17–36. doi: 10.4319/lo.2014.59.1.0017
Schmidt, K., Atkinson, A., Steigenberger, S., Fielding, S., Lindsay, M. C. M., Pond, D. W., et al. (2011). Seabed foraging by Antarctic krill: implications for stock assessment, bentho-pelagic coupling, and the vertical transfer of iron. Limnol. Oceanogr. 56, 1411–1428. doi: 10.4319/lo.2011.56.4.1411
Scott, C. L., Falk-Petersen, S., Sargent, J. R., Hop, H., Lønne, O. J., and Poltermann, M. (1999). Lipids and trophic interactions of ice fauna and pelagic zooplankton in the marginal ice zone of the Barents Sea. Polar Biol. 21, 65–70. doi: 10.1007/s003000050335
Siegel, V. (1987). Age and growth of Antarctic Euphausiacea (Crustacea) under natural conditions. Mar. Biol. 96, 483–495. doi: 10.1007/BF00397966
Spreen, G., Kaleschke, L., and Heygster, G. (2008). Sea ice remote sensing using AMSR-E 89-GHz channels. J. Geophys. Res. 113, C02S03. doi: 10.1029/2005JC003384
Spreen, G., Kwok, R., and Menemenlis, D. (2011). Trends in Arctic sea ice drift and role of wind forcing: 1992–2009. Geophys. Res. Lett. 38:L19501. doi: 10.1029/2011GL048970
Stammerjohn, S., Massom, R., Rind, D., and Martinson, D. (2012). Regions of rapid sea ice change: an inter-hemispheric seasonal comparison. Geophys. Res. Lett. 39:L06501. doi: 10.1029/2012GL050874
Stock, B. C., and Semmens, B. X. (2015). MixSIAR User Manual, Version 3.0. Available online at: https://github.com/brianstock/MixSIAR/
Stübing, D. (2004). Lipid Biochemistry of Antarctic Euphausiids-Energetic Adaptations and a Critical Appraisal of Trophic Biomarkers. Ph.D. thesis, University of Bremen.
Stübing, D., Hagen, W., and Schmidt, K. (2003). On the use of lipid biomarkers in marine food web analyses: an experimental case study on the Antarctic krill, Euphausia superba. Limnol. Oceanogr. 48, 1685–1700. doi: 10.4319/lo.2003.48.4.1685
Sumata, H., Lavergne, T., Girard-Ardhuin, F., Kimura, N., Tschudi, M. A., Kauker, F., et al. (2014). An intercomparison of Arctic ice drift products to deduce uncertainty estimates. J. Geophys. Res. 119, 4887–4921. doi: 10.1002/2013JC009724
Sumata, H., Kwok, R., Gerdes, R., Kauker, F., and Karcher, M. (2015). Uncertainty of Arctic summer ice drift assessed by high-resolution SAR data. J. Geophys. Res. 120, 5285–5301. doi: 10.1002/2015JC010810
Turner, J., Bracegirdle, T. J., Phillips, T., Marshall, G. J., and Hosking, J. S. (2013). An initial assessment of Antarctic sea ice extent in the CMIP5 models. J. Clim. 26, 1473–1484. doi: 10.1175/JCLI-D-12-00068.1
Turner, J., Barrand, N. E., Bracegirdle, T. J., Convey, P., Hodgson, D. A., Jarvis, M., et al. (2014). Antarctic climate change and the environment: an update. Polar Rec. 50, 237–259. doi: 10.1017/S0032247413000296
van Franeker, J. A., Flores, H., and van Dorssen, M. (2009). “The Surface and Under Ice Trawl (SUIT),” in Frozen Desert Alive-The Role of Sea Ice for Pelagic Macrofauna and its Predators ed H. Flores (Ph.D thesis. University of Groningen, Groningen), 181–188.
Vancoppenolle, M., Meiners, K. M., Michel, C., Bopp, L., Brabant, F., Carnat, G., et al. (2013). Role of sea ice in global biogeochemical cycles: emerging views and challenges. Q. Sci. Rev. 79, 207–230. doi: 10.1016/j.quascirev.2013.04.011
Virtue, P., Meyer, B., Freier, U., Nichols, P. D., Jia, Z., King, R., et al. (2016). Condition of larval (furcilia VI) and one year old juvenile Euphausia superba during the winter–spring transition in East Antarctica. Deep Sea Res. 131, 182–188. doi: 10.1016/j.dsr2.2016.02.001
Viso, A.-C., and Marty, J.-C. (1993). Fatty acids from 28 marine microalgae. Phytochemistry 34, 1521–1533. doi: 10.1016/S0031-9422(00)90839-2
Volkman, J. K., Barrett, S. M., Blackburn, S. I., Mansour, M. P., Sikes, E. L., and Gelin, F. (1998). Microalgal biomarkers: a review of recent research developments. Org. Geochem. 29, 1163–1179. doi: 10.1016/S0146-6380(98)00062-X
Wada, E., Terazaki, M., Kabaya, Y., and Nemoto, T. (1987). 15N and 13C abundances in the Antartic Ocean with emphasis on the biogeochemical structure of the food web. Deep Sea Res. 34, 829–841. doi: 10.1016/0198-0149(87)90039-2
Wang, S. W., Budge, S. M., Iken, K., Gradinger, R. R., Springer, A. M., and Wooller, M. J. (2015). Importance of sympagic production to Bering Sea zooplankton as revealed from fatty acid-carbon stable isotope analyses. Mar. Ecol. Prog. Ser. 518, 31–50. doi: 10.3354/meps11076
Ward, P., Atkinson, A., Venables, H. J., Tarling, G. A., Whitehouse, M. J., Fielding, S., et al. (2012). Food web structure and bioregions in the Scotia Sea: a seasonal synthesis. Deep Sea Res. 59, 253–266. doi: 10.1016/j.dsr2.2011.08.005
Keywords: Antarctic krill, Euphausia superba, fisheries resources, carbon sources, ice algae, marker fatty acids, compound-specific stable isotope analysis, winter survival
Citation: Kohlbach D, Lange BA, Schaafsma FL, David C, Vortkamp M, Graeve M, van Franeker JA, Krumpen T and Flores H (2017) Ice Algae-Produced Carbon Is Critical for Overwintering of Antarctic Krill Euphausia superba. Front. Mar. Sci. 4:310. doi: 10.3389/fmars.2017.00310
Received: 16 May 2017; Accepted: 12 September 2017;
Published: 26 September 2017.
Edited by:
Michael Arthur St. John, Danish Technical University, Institute for Aquatic Resources, DenmarkReviewed by:
Shiway Wang, Independent Researcher, Anchorage, United StatesEugene J. Murphy, British Antarctic Survey (BAS), United Kingdom
Copyright © 2017 Kohlbach, Lange, Schaafsma, David, Vortkamp, Graeve, van Franeker, Krumpen and Flores. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Doreen Kohlbach, doreen.kohlbach@awi.de
 Doreen Kohlbach
Doreen Kohlbach Benjamin A. Lange
Benjamin A. Lange Fokje L. Schaafsma
Fokje L. Schaafsma Carmen David1,2
Carmen David1,2  Martin Graeve
Martin Graeve Jan A. van Franeker
Jan A. van Franeker Hauke Flores
Hauke Flores