Early Development of the Threespine Stickleback in Relation to Water pH
- 1The Bioeconomy Research Team, Novia University of Applied Sciences, Ekenäs, Finland
- 2Tvärminne Zoological Station, University of Helsinki, Hanko, Finland
- 3Department of Environmental Ecology, University of Helsinki, Lahti, Finland
- 4Marine Research Centre, Finnish Environment Institute, Helsinki, Finland
- 5Department of Biosciences, University of Helsinki, Helsinki, Finland
Ocean acidification is a growing environmental problem, and there is a need to investigate how the decreasing pH will affect marine organisms. Here we studied the effects of lowered pH on the growth and development of the threespine stickleback (Gasterosteus aculeatus) eggs. Adult fish, collected from the natural environment, were allowed to mate in aquaria and the newly produced eggs were incubated in an experiment. Eggs and larvae from ambient conditions (produced in the laboratory) were reared at three different pH concentrations (control: pH 7.8; and reduced pH treatments: pH 7.5 and 7.0) for 21 days in the laboratory. Dissolved oxygen concentration (8.1 ± 0.1 mg l−1) and temperature (18.6 ± 0.02°C) were monitored regularly. Then, egg diameter, larval length, weight and survival were measured. There was no relationship between egg diameter and pH or oxygen, but a negative relationship was found with temperature. Survival of larvae was not affected by pH or temperature, whereas dissolved oxygen concentration had a positive effect on number of survivors. The pH did not have a significant effect on the final larval length on day 21, but interacted significantly with dissolved oxygen. Higher temperatures were found to have a positive effect on the final larval length and weight. Larval weight, on the other hand, was not related to pH nor oxygen. Coastal zones are characterized by pH levels that fluctuate due to natural processes, such as upwelling and river runoff. Our results suggest that the threespine stickleback larvae are well adapted to the different pHs tested, and egg development will likely not be affected by decreasing pH, but even slight temperature and oxygen changes can have a great impact on the threespine stickleback development.
Introduction
Since the onset of the industrial revolution, burning of fossil fuels and change in land use have led to the doubling of atmospheric carbon dioxide levels, presently at ~400 ppm (Blunden and Arndt, 2017), and an average warming of nearly 1°C. In seawater, atmospheric carbon dioxide dissolves and causes a decrease in the pH, a phenomenon referred to as ocean acidification. For more than a century, the pH of ocean surface waters has decreased and a doubling in acidity is expected by year 2100 (Feely et al., 2009). The current ocean acidification and change in the equilibrium of the seawater chemistry are so rapid that they will most likely lead to major changes in marine ecosystems and impact marine life (Pelejero et al., 2010). For more than a decade, the biological responses of phytoplankton (Lohbeck et al., 2012), foraminifera (Bijma et al., 2002), pteropods (Bednaršek et al., 2017), bivalves (Jansson et al., 2013), crustaceans (Vehmaa et al., 2013) and fish (reviewed by Clements and Hunt, 2015; Esbaugh, 2017) have been studied under different ocean acidification scenarios. Research suggests that mobile species, such as crustaceans and fish are less sensitive to acidification than sessile species, possibly due to their high metabolic rates and active regulation of internal pH (reviewed by Kroeker et al., 2013). Some fish seem well adapted to low pH (Maneja et al., 2014; Sundin et al., 2017). Other fish appear to experience negative effects on life history, behavior, fitness and performance. For example, fundamental processes can react negatively to low pH, such as changes in auditory or olfactory function (Simpson et al., 2011), growth and survival (Baumann et al., 2012), behavior (Jutfelt et al., 2013; Schmidt et al., 2017), otolith calcification (Checkley et al., 2009), as well as tissue and organ structure (Frommel et al., 2012, 2016).
While ocean acidification will affect ecosystems on a global level, the impact on coastal areas is more complex. Various processes, such as acidic inputs from land and freshwater, eutrophication, and low buffering capacity of brackish waters can influence the pH (Dickinson et al., 2013). Furthermore, CO2 is more soluble in cold water (Fabry et al., 2008) making regions at higher latitudes, including the Baltic Sea, more susceptible to ocean acidification (Havenhand, 2012). In the Baltic Sea, a large seasonal and inter-annual variability in the carbon dioxide system is observed due to primary production, air-sea gas exchange and mixing (Almén et al., 2017 and references therein). The Baltic Sea is currently considered very sensitive to climate change, and is forecasted to be more severely impacted by a changing climate than other seas, due to basin-specific low alkalinity, heavy eutrophication, low biodiversity and low salinity (Jutterström et al., 2014).
The threespine stickleback Gasterosteus aculeatus (L. 1758) is a common model organism in both evolutionary and ecological research (Schluter and McPhail, 1992), and tested protocols for laboratory rearing of this fish are well-established (Des Roches et al., 2013). The threespine stickleback population has increased in the Baltic Sea, which is suggested as a consequence of overfishing of their predators (Bergström et al., 2015). The stickleback is found in a wide range of habitats, showing high tolerance to changes in water chemistry and temperature (Östlund-Nilsson et al., 2006). The species is also euryhaline, and inhabits both freshwater, brackish and marine areas (Defaveri et al., 2012). Although the stickleback thrives in different environments and is tolerant to a wide range of pH, the larval stage may be more sensitive than adults to environmental change, as is the case for a number of other species (Pankhurst and Munday, 2011; Crespel et al., 2017; Lonthair et al., 2017 and references therein).
The aim of this work was to assess the development of eggs and larvae of the threespine stickleback at different pH levels. Those pH levels were selected based on the ambient pH (7.8) and the intensive emission scenario of the RCP 8.5 scenario for the year 2100 (ΔpH ~−0.3, IPCC, 2013) and an extreme scenario (ΔpH ~−0.7). We hypothesized that eggs and larvae are not affected by lowered pH levels. Eggs and larvae were reared across a range of three different pH conditions. The effects of low pH on egg diameter, larval length and survival of G. aculeatus were investigated during a 3-week experiment.
Materials and Methods
Fish Collection
Adult threespine sticklebacks were collected with seine nets during the breeding season in May 2014 from Tvärminne archipelago (Brännskär, Långskär, Vindskär Islands) at the entrance to the Gulf of Finland, Baltic Sea. They were stored in 40 l coolers with ambient water for transport to the laboratory, where they were separated by sex into holding aquaria (50 × 60 × 32 cm; 96 l) with oxygenation combined with continuous seawater exchange, attached to heaters, set at 18°C. The fish were kept no longer than 4 weeks, and were fed with frozen chironomids twice daily, and occasionally with mysid shrimps collected in local waters. The average surface temperature (0–3 m) was 9.88 ± 0.4°C (mean ± S.E.), average surface salinity (0–3 m) was 5.87 ± 0.02 (mean ± S.E.), and average pH (0 and 5 m) was 8.11 (min and max: 8.01, 8.28) of the study area in May and June 2014 (monitoring data of Tvärminne Zoological Station). The study (i.e., 0-class experiment) was performed in accordance with the current Finnish legislation of animal welfare, and meets the terms of the Animal Care Committee at the County Board.
Mating Procedure
The mating of the fish (sex ratio 1:1) was performed according to the protocol in Candolin et al. (2008). Mating aquaria (33 × 21 × 15 cm; 10 l) were connected to a seawater flow-through system (ambient daylight: 17 h light: 07 h dark; salinity: 5.1; water temperature: 18°C, pH 7.8), and assembled with clean sand, a plastic plant as shelter, and green algae Cladophora glomerata for nest building. Once the males exhibited readiness for breeding by changing colors, they were individually placed in a mating aquarium for 1 week to build their nest. A mature female was haphazardly chosen, introduced to the aquarium and allowed to spawn with the male. If the female did not spawn within the two first hours, a new female was presented to the male. The mating procedure was repeated with 20 couples. The fertilized egg masses were allowed to harden for 2 h, removed from the nest and transferred to the laboratory. The clutch sizes and the total weights of egg clutches (Table 1) were monitored using a cold light microscope and a Mettler Ae100 Scale (precision 0.01 g). The parents were weighed (wet weight) on a Mettler Toledo Scale (precision 0.001 g) and their length measured with graph paper (Table 1).
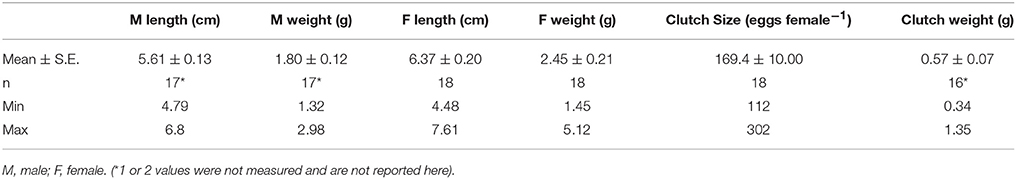
Table 1. Background information on parents' sticklebacks Gasterosteus aculeatus and their clutches used in this study.
Seawater Manipulation
Seawater was filtered through a 10 μm filter, and subsequently added to three tanks of 126 l (70 × 45 × 40 cm), one for each pH level. The ambient pH (7.8) was used as control, and two treatments of reduced pH were used: pH 7.5 and 7.0. The controls and treatments were oxygenated continuously using an air pump. In addition, the two reduced pH levels were achieved by adding CO2 (gas bottle, 99.7%) through non-silicone tubing and diffusers to the bottom of the tanks. This provided rapid CO2 mixing and efficient water circulation. The amount of CO2 added to the tanks was regulated by magnetic valves using a feedback mechanism, which was connected to a Sera Precision Controller (see below). The pH levels were monitored with a Sera Precision Controller (NBS scale, precision 0.1, calibrated daily using buffer solutions 4 and 7). All calibration buffers used met the criteria of NIST (US National Institute of Standards and Technology; also referred to as NBS, National Bureau of Standards).
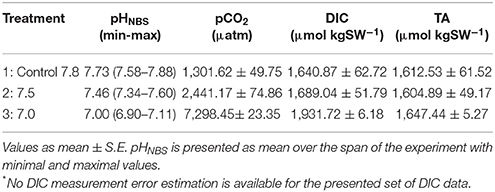
The controls and pH manipulated waters were added to 3.6 l plastic aquaria (no flow-through, of volume changed daily), while keeping pH, water temperature, and dissolved oxygen (DO) concentration as constant as possible (Table 2). However, there was slight variation in pH, temperature and DO, due to variation in room temperature and DO consumption (larval respiration), and these variables were recorded in each aquarium twice per day. The pH was measured with a hand-held laboratory glass pH meter (VWR pH10, accuracy 0.1, resolution 0.01), calibrated daily with pH 4 and 7 buffer solutions. DO and temperature were measured using YSI ProDSS Sensor (Oxygen resolution 0.1, accuracy 0.1 mg l−1; temperature resolution 0.1°C, accuracy 0.2°C). Samples for total dissolved inorganic carbon (DIC) were collected in triplicate from the header tanks(treatments and control) on day 10 and at the end (day 21) of the experiment. Samples were stored dark in glass vials without airspace on ice in +3°C, until measured within 12 h, using the acidification/gas stripping/infrared detection method (Salonen, 1981). The carbonate chemistry parameters including pCO2 and Total alkalinity (TA) were calculated in CO2SYS (V2.1, Pierrot et al., 2006) based on measured DIC, pHNBS, temperature and salinity using the dissociation constants K1 and K2 from Roy et al. (1993), Dickson (1990) for KHSO4 and Lee et al. (2010) for the total Boron. The NBS scale was applied as the pH scale. pH values reported in the manuscript may contain a systematic error in the absolute pH values (cf., Dickson, 2010), as no additional calibration was performed.
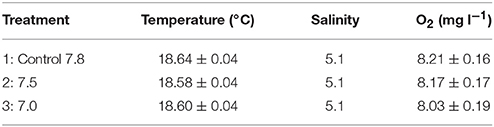
Table 2. Mean (± S.E.) temperature, salinity and oxygen over the course of experiments with stickleback (G. aculeatus) eggs and larvae.
Experimental Set-Up
Eggs were carefully separated using forceps and disposable plastic pipettes. This procedure has no significant effect on egg survival (Kraak et al., 1997; Candolin, 2000).
Eggs produced during the mating procedure by one mother in ambient conditions (~3 h since laying) were put into three treatments (20 eggs aquarium−1); the remaining eggs were discarded (Figure 1). The same procedure was repeated with 20 different couples. The eggs were checked twice daily during the manipulations; any dead eggs were recorded and removed instantly. Once the eggs hatched and the larval yolk sacs diminished, larvae were fed with liquid food (JBL Nobil Fluid Artemia). When larvae were ~7 days old, we switched to powdered food (JBL Novo Tom Artemia). Food was added twice a day and always ad libitum. To prevent egg mortality, aquarium fungicide (Tetra Medica, FungiStop) was added each time new water was prepared (twice daily) at a concentration of 0.1 ml l−1 seawater (Socha et al., 2012).
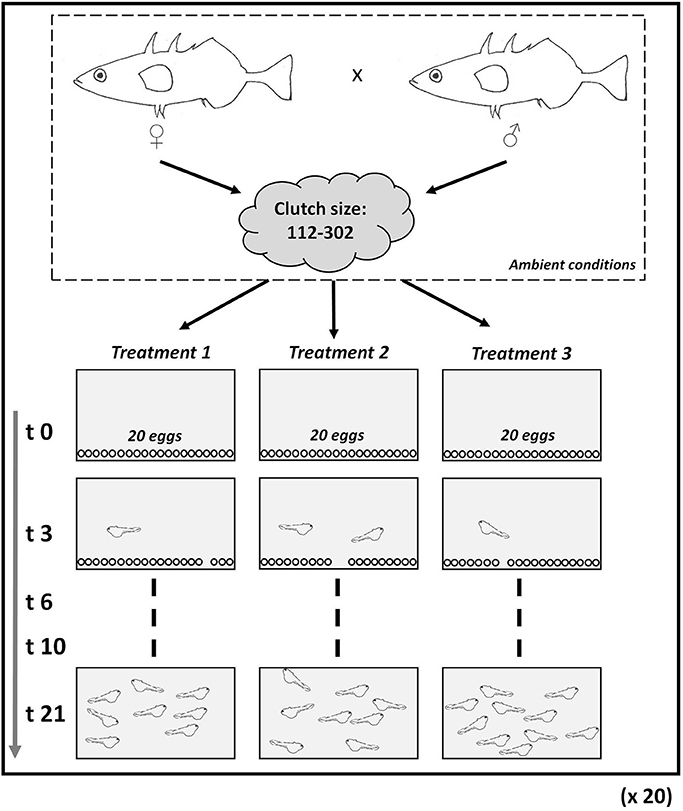
Figure 1. Experimental set-up. After spawning, eggs of Gasterosteus aculeatus were distributed into three different aquaria and exposed to ambient pH (Treatment 1, i.e., control), intermediate pH (Treatment 2), and low pH (Treatment 3). The experiment started when eggs were placed gently into the aquarium (t0) and were terminated after 3 weeks (t21). The procedure was repeated 20 times with eggs from 20 couples (18 were analyzed; see Methods).
The lids for the aquaria were kept loosely on to minimize outgassing between the water and the atmosphere. The eggs and larvae were photographed using a microscope camera (Nikon DS-L3 Leica MZ 12 with a cold light microscope). Egg diameter and the total length of larvae were measured. A random subset of 5 individuals (eggs before hatching, and larvae after hatching) from each aquarium were measured in petri dishes on days 0, 3, 6, and 10, and afterwards returned to their respective aquarium. Day 0 refers to the day when eggs were put to the aquarium to hatch. On day 21, the length of each remaining larva was measured and their total wet weight was measured for each aquarium. The experiment was set up using 20 replicates per treatment, but two families (including control and treatments) were removed due to fungal infection (N = 18) (see Table 1 for details).
Statistical Analyses
A one-way analysis of variance (ANOVA) following a post-hoc Tukey test were used to compare the three treatments consisting of a low (7.0), an intermediate (7.5) and ambient (7.8) pH level (the control). The same analysis was used for comparing oxygen and temperature between treatments. Although we tried to keep temperature and DO as constant as possible, there was some variation and we included these variables (in addition to pH) in a general linear model (GLM) to analyze egg diameter (day 3), larval length and larval weight on day 21. The assumptions of normality and homogeneity of variances were checked before analysis. Environmental factors (temperature, oxygen) were considered as co-variates and the treatment (pH) as a fixed variable. A general linear mixed model (GLMM) with binomial distribution was used for survival data (1 = survived, 0 = dead) in relation to the environment (average pH, O2, temperature). The pH concentrations were transformed to hydrogen ions before calculating means. All data were analyzed using SPSS 21.0.
Results
Experimental Conditions
The pH levels measured (7.00, 7.46, and 7.73) varied significantly between treatments [F(2, 1, 077) = 9,327.3, p < 0.001] and a post-hoc Tukey test showed that the three treatments differed significantly from each other (p < 0.001). Differences were also found for O2 between treatments 1 and 3, and treatments 2 and 3 [F(2, 1, 077) = 13.941, p < 0.001]. The temperatures measured varied significantly between treatments [F(2, 1, 077) = 9.538, p < 0.001] and these differences were observed between treatment 1 and 2 (p < 0.001), and 1 and 3 (p = 0.02).
The carbonate chemistry parameters including pCO2 and TA, calculated from measured pH and DIC are summarized in Table 3.
Egg Diameter
The diameter of the stickleback eggs ranged between 1.33 and 2.16 mm (1.75 ± 0.01 mm, mean ± Standard Error, S.E.). Egg diameter was analyzed on day 3 to study the effects of environmental variables. No significant relationships were observed between egg diameter and the pH level, nor the dissolved oxygen concentration, whereas the water temperature had a significant negative effect on egg diameter [GLM, F(1, 417) = 4.140, t = −2.035, p = 0.043].
Egg and Larval Survival
Survival (from 20 eggs added to each aquarium at start) was high throughout the experiment and varied between 17.1 ± 0.6 (ambient, mean ± S.E.), 16.6 ± 0.5 (intermediate pH) and 17.1 ± 0.7 (low pH) surviving individuals during the final day of the experiment (Figure 2). Egg and larval binomial survival were analyzed in relation to environmental conditions in the aquaria; mean pH and mean temperature did not have a significant effect on survivorship of the fish in the different treatments on the final day of the experiment [Binomial GLMM, F(1, 1, 076) = 0.19, p = 0.665, pH; F(1, 1, 076) = 2.76, p = 0.097, temperature], whereas average dissolved oxygen concentration affected survival significantly positively [F(1, 1, 076) = 5.76, p = 0.017].
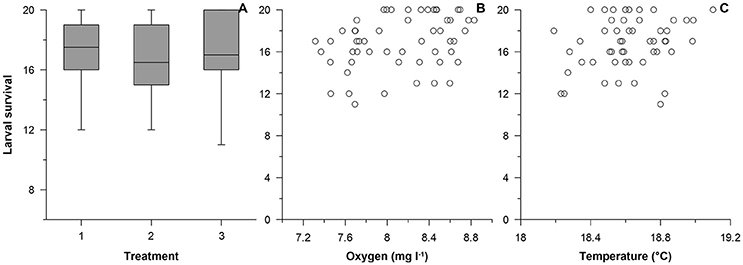
Figure 2. Relation between the larval survival (at day 21) of Gasterosteus aculeatus and treatment (A; 1: ambient, 2: intermediate pH and 3: low pH), oxygen concentration (B) or the temperature (C).
Larval Length and Weight
The final larval length on day 21 was 9.03 ± 0.04 mm, 9.05 ± 0.04 mm, 9.08 ± 0.05 mm (mean ± S.E.), respectively in treatment 1, 2 and 3 (Figure 3, Supplementary Material). Mean dissolved oxygen concentration showed an interaction with treatment, i.e., pH [F(2, 904) = 3.454, p = 0.032] on larval length and with temperature [F(1, 904) = 47.612, p < 0.001]. The treatment pH [F(2, 41) = 0.542, p = 0.585] and oxygen [F(1, 41) = 0.668, p = 0.418] were not significant predictors of the final total wet weight of larvae on day 21 whereas the temperature [F(1, 41) = 5.989, p = 0.018] had an effect.
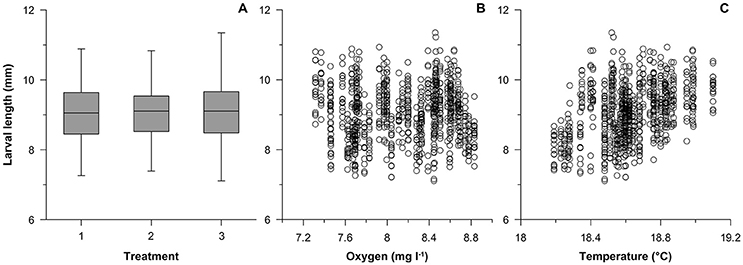
Figure 3. Relation between the final larval length (at day 21) of Gasterosteus aculeatus and treatment (A; 1: ambient, 2: intermediate pH and 3: low pH), oxygen concentration (B) or temperature (C).
Discussion
Although juvenile and adult fish are considered tolerant to future changes in carbonate chemistry (Fabry et al., 2008; Ishimatsu et al., 2008; Maneja et al., 2014; Sundin et al., 2017), the early stages can be more vulnerable to elevated pCO2, because their acid-base competency is still developing (Murray et al., 2016; Crespel et al., 2017 and references therein). Recently, Esbaugh (2017) reviewed the observed effects of elevated CO2 on early life stage survival in a variety of marine teleost species. The responses ranged from high (Baumann et al., 2012) to low mortality (Lonthair et al., 2017), or even no observed changes in high CO2 conditions (Frommel et al., 2013).
Until recently, little was known about the threespine stickleback responses to low pH. Schade et al. (2014) investigated transgenerational effects on the life history of juvenile sticklebacks by acclimating parents and offspring to different CO2 levels (~400 and ~1,000 μatm). They detected positive effects of low pH on both larval growth and survival. Moreover, breeding pairs of this species held at elevated levels of CO2 (1,000 μatm) produced more eggs than control fish. The authors demonstrated that parental acclimation to elevated CO2 concentrations can modify these effects without improving offspring fitness. Sundin et al. (2017) looked in to the reproductive behavior of the stickleback under ambient conditions (400 μatm) and future levels of CO2 (1,000 μatm). They found no effect of elevated carbon dioxide on the reproductive behavior of this species and the numbers of offspring produced remained unchanged.
In the present study, we did not find differences in total survival of eggs and larvae incubated under different pH environments (from ambient 7.8 to low pH 7.0). In addition, the final larval length was not affected by pH conditions, whereas a temperature increase of 1°C had a significant positive effect. The larvae grew normally and their body sizes correspond to reported values (Lehtiniemi, 2005). The increase of larval growth with one-degree rise in temperature is to be expected, as the optimal growth temperature for the species is 21.7°C (Lefébure et al., 2011). The threespine stickleback is, however, flexible concerning temperature, and is able to grow between 3.6 and 30.7°C (Lefébure et al., 2011), and future warming might not necessarily cause any serious problems for this species.
The dissolved oxygen concentration (cf. Table 2), interacting with treatment pH, showed a significant effect on final larval length; O2 probably fluctuated due to food addition and larval respiration. This result was expected as dissolved oxygen and pH correlate strongly in nature (Jansson et al., 2015). Some studies suggest that species residing in shallow waters are more resilient to CO2 changes, as the coastal environment is subject to large variability (Waldbusser and Salisbury, 2014). As an example, Lonthair et al. (2017) were interested in the tolerance of early life stages of the red drum (Sciaenops ocellatus) to elevated pCO2 (1,000 and 3,000 μatm) as this estuarine species experiences large natural fluctuations of pCO2 on a variety of time scales. They observed tolerance to elevated pCO2 on a number of different levels (i.e., survival, growth, yolk consumption, heart rate and scototaxis).
Ishimatsu et al. (2008) stated that littoral fish, in general, can compensate their acid-base balance, especially when exposed to mild hypercapnia. Munday et al. (2011) demonstrated that juveniles of the damselfish Acanthochromis polyacanthus (Bleeker 1855), a commonly occurring coral reef species, have no clear response to reduction in pH, possibly due to habitat use. The juveniles reside in caves with high night-time fluctuations in CO2; the young experience natural fluctuation in pH as photosynthesis is increasing during the day and respiration during the night in the reef matrix. Melzner et al. (2011) showed that hypercapnia increases during food limitation, suggesting that some responses measured in laboratory experiments during CO2 stress can be masked if fish are fed ad libitum (Esbaugh, 2017), as is the case in the present work.
The threespine stickleback in the current study area resides in a fluctuating habitat consisting of small lagoons with green algal mats, where pH is high during the day and lower during night. Ahlnäs (2015) measured pH at different depths in the Tvärminne archipelago, showing large variation (7.17–8.59) between sites, months and times of day, at sites all being close to the shore and potential stickleback habitats. In Jansson et al. (2015), pH variability in the field was even larger in the same area, fluctuating between 6.91 and 8.23. The on-going eutrophication in the Baltic Sea is estimated to increase seasonal variability of coastal pH even further in the future: increasing CO2 concentrations decrease pH in particular during low productive winter months, and increasing primary production (caused by eutrophication) converts CO2 to organic carbon, and causes elevation of the pH during the summer months (cf. Jutterström et al., 2014; Waldbusser and Salisbury, 2014). McNeil and Sasse (2016) pointed out that coastal marine environments experience large CO2 fluctuations, and many organisms living in these areas have naturally evolved resilience to CO2 changes. Even though we did not study the effect of changes in pH that sticklebacks can experience on a daily base, our results show that the threespine stickleback seems tolerant to shifts up to −0.7 units in average pH. Future studies should investigate combined effects of ocean acidification and warming on the thermal tolerance of the threespine stickleback, as the optimal thermal window may decrease in lowered pH (Pörtner, 2008). It is also important to study behavioral disturbances (Nilsson et al., 2012), as the potential to acclimate to CO2 is minimal in some fish (Welch et al., 2014).
Author Contributions
All authors listed, have made substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Tvärminne Zoological Station is thanked for board and accommodation. Special thanks to B. Grönlund for precious assistance and hard work in the laboratory, to A. Vehmaa for help in lab, and to M. öst for statistical advice. J. Norkko is acknowledged for help with practical things. Academy of Finland (project nr. 276947), Norden Havgrupp and Walter and Andrée de Nottbeck Foundation are greatly acknowledged for funding the work. Great thanks to Jon Havenhand and one reviewer for constructive comments.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2017.00427/full#supplementary-material
References
Ahlnäs, A. (2015). Kusthabitats Naturliga dygns- och Säsongsvariationer i pH. MSc thesis, Abo Akademi University, Finland.
Almén, A. K., Glippa, O., Pettersson, H., Alenius, P., and EngströmÖst, J. (2017). Changes in wintertime pH and hydrography of the Gulf of Finland (Baltic Sea) with focus on depth layers. Environ. Monit. Assess. 189, 147. doi: 10.1007/s10661-017-5840-7
Baumann, H., Talmage, S. C., and Gobler, C. J. (2012). Reduced early life growth and survival in a fish in direct response to increased carbon dioxide. Nat. Clim. Chang. 2, 38–41. doi: 10.1038/nclimate1291
Bednaršek, N., Feely, R. A., Tolimieri, N., Hermann, A. J., Siedlecki, S. A., Walbusser, G. G., et al. (2017). Exposure history determines pteropod vulnerability to ocean acidification along the US West Coast. Sci. Rep. 7, 4526. doi: 10.1038/s41598-017-03934-z
Bergström, U., Olsson, J., Casini, M., Eriksson, B. K., Fredriksson, R., Wennhage, H., et al. (2015). Stickleback increase in the Baltic Sea–a thorny issue for coastal predatory fish. Estuar. Coast. Shelf Sci. 163, 134–142. doi: 10.1016/j.ecss.2015.06.017
Bijma, J., Hönisch, B., and Zeebe, R. E. (2002). Impact of the ocean carbonate chemistry on living foraminiferal shell weight: comment on ‘carbonate ion concentration in glacial-age deep waters of the Caribbean Sea’ by WS Broecker and E. Clark. Geochem. Geophys. Geosyst. 3, 1–7. doi: 10.1029/2002GC000388
Blunden, J., and Arndt, D. S. (2017). A look at 2016: takeaway points from the state of the climate supplement. Bull. Am. Meteor. Soc. 98, 1563–1572. doi: 10.1175/BAMS-D-17-0148.1
Candolin, U. (2000). Increased signalling effort when survival prospects decrease: male–male competition ensures honesty. Anim. Behav. 60, 417–422. doi: 10.1006/anbe.2000.1481
Candolin, U., Engström-Öst, J., and Salesto, T. (2008). Human-induced eutrophication enhances reproductive success through effects on parenting ability in sticklebacks. Oikos 117, 459–465. doi: 10.1111/j.2007.0030-1299.16302.x
Checkley, D. M., Dickson, A. G., Takahashi, M., Radich, J. A., Eisenkolb, N., and Asch, R. (2009). Elevated CO2 enhances otolith growth in young fish. Science 324, 1683. doi: 10.1126/science.1169806
Clements, J. C., and Hunt, H. L. (2015). Marine animal behaviour in a high CO2 ocean. Mar. Ecol. Prog. Ser. 536, 259–279. doi: 10.3354/meps11426
Crespel, A., Zambonino-Infante, J.-L., Mazurais, D., Koumoundouros, G., Fragkoulis, S., Quazuguel, D., et al. (2017). The development of contemporary European sea bass larvae (Dicentrarchus labrax) is not affected by projected ocean acidification scenarios. Mar. Biol. 164:155. doi: 10.1007/s00227-017-3178-x
Defaveri, J., Sjikano, T., Ghani, N. I. A., and Merilä, J. (2012). Contrasting population structures in two sympatric fishes in the Baltic Sea basin. Mar. Biol. 159, 1659–1672. doi: 10.1007/s00227-012-1951-4
Des Roches, S., Shurin, J. B., Schluter, D., and Harmon, L. J. (2013). Ecological and evolutionary effects of stickleback on community structure. PLoS ONE 8:e59644. doi: 10.1371/journal.pone.0059644
Dickinson, G. H., Matoo, O. B., Tourek, R. T., Sokolova, I. M., and Beniash, E. (2013). Environmental salinity modulates the effects of elevated CO2 levels on juvenile hard-shell clams, Mercenaria mercenaria. J. Exp. Biol. 216, 2607–2618. doi: 10.1242/jeb.082909
Dickson, A. G. (1990). Standard potential of the reaction: AgCl(s) + 12H2(g) = Ag(s) + HCl(aq), and the standard acidity constant of the ion HSO4− in synthetic sea water from 273.15 to 318.15 K. J. Chem. Thermodyn. 22, 113–127. doi: 10.1016/0021-9614(90)90074-Z
Dickson, A. G. (2010). “The carbon dioxide system in seawater: equilibrium chemistry and measurements,” in Guide to Best Practices for Ocean Acidification Research and Data Reporting, eds U. Riebesell, V. J. Fabry, L. Hansson, and J.-P. Gattuso (Luxembourg: Publications Office of the European Union), 17–40.
Esbaugh, A. J. (2017). Physiological implications of ocean acidification for marine fish: emerging patterns and new insights. J. Comp. Physiol. B. doi: 10.1007/s00360-017-1105-6. [Epub ahead of print].
Fabry, V. J., Seibel, B. A., Feely, R. A., and Orr, J. C. (2008). Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J. Mar. Sci. 65, 414–432. doi: 10.1093/icesjms/fsn048
Feely, R. A., Doney, S. C., and Cooley, S. R. (2009). Ocean acidification: present conditions and future changes in a high-CO2 world. Oceanography 22, 36–47. doi: 10.5670/oceanog.2009.95
Frommel, A. Y., Maneja, R., Lowe, D., Malzahn, A. M., Geffen, A. J., Folkvord, A., et al. (2012). Severe tissue damage in Atlantic cod larvae under increasing ocean acidification. Nat. Clim. Chang. 2, 42–46. doi: 10.1038/nclimate1324
Frommel, A. Y., Margulies, D., Wexler, J. B., Stein, M. S., Scholey, V. P., Williamson, J. E., et al. (2016). Ocean acidification has lethal and sub-lethal effects on larval development of yellowfin tuna, Thunnus albacares. J. Exp. Mar. Biol. Ecol. 482, 18–24. doi: 10.1016/j.jembe.2016.04.008
Frommel, A. Y., Schubert, A., Piatkowski, U., and Clemmesen, C. (2013). Egg and early larval stages of Baltic cod, Gadus morhua, are robust to high levels of ocean acidification. Mar. Biol. 160, 1825–1834. doi: 10.1007/s00227-011-1876-3
Havenhand, N. (2012). How will ocean acidification affect Baltic sea ecosystems? An assessment of plausible impacts on key functional groups. Ambio 41, 637–644. doi: 10.1007/s13280-012-0326-x
IPCC (2013). “Near-term climate change: projections and predictability,” in Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (Cambridge; New York, NY: Cambridge University Press), 33.
Ishimatsu, A., Hayashi, M., and Kikkawa, T. (2008). Fishes in high-CO2, acidified oceans. Mar. Ecol. Prog. Ser. 373, 295–302. doi: 10.3354/meps07823
Jansson, A., Norkko, J., and Norkko, A. (2013). Effects of reduced pH on Macoma balthica larvae from a system with naturally fluctuating pH-dynamics. PLoS ONE 8:e68198. doi: 10.1371/journal.pone.0068198
Jansson, A., Norkko, J., Dupont, S., and Norkko, A. (2015). Growth and survival in a changing environment: combined effects of moderate hypoxia and low pH on juvenile bivalve Macoma balthica. J. Sea Res. 102, 41–47. doi: 10.1016/j.seares.2015.04.006
Jutfelt, F., Bresolin de Souza, K., Vuylsteke, A., and Sturve, J. (2013). Behavioural disturbances in a temperate fish exposed to sustained high-CO2 levels. PLoS ONE 8:e65825. doi: 10.1371/journal.pone.0065825
Jutterström, S., Andersson, H. C., Omstedt, A., and Malmaeus, J. M. (2014). Multiple stressors threatening the future of the Baltic Sea–Kattegat marine ecosystem: implications for policy and management actions. Mar. Pollut. Bull. 86, 468–480. doi: 10.1016/j.marpolbul.2014.06.027
Kraak, S. B. M., Bakker, T. C. M., and Mundwiler, B. (1997). How to quantify embryo survival in nest-building fishes, exemplified with three-spined sticklebacks. J. Fish Biol. 51, 1262–1264. doi: 10.1111/j.1095-8649.1997.tb01141.x
Kroeker, K. J., Kordas, R. L., Crim, R., Hendriks, I. E., Ramajo, L., Singh, G. S., et al. (2013). Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Glob. Chang. Biol. 19, 1884–1896. doi: 10.1111/gcb.12179
Lee, K., Kim, T. W., Byrne, R. H., Millero, F. J., Feely, R. A., and Liu, Y. M. (2010). The universal ratio of boron to chlorinity for the North Pacific and North Atlantic oceans. Geochim. Cosmochim. Ac. 74, 1801–1181. doi: 10.1016/j.gca.2009.12.027
Lefébure, R., Larsson, S., and Byström, P. (2011). A temperature-dependent growth model for the three-spined stickleback Gasterosteus aculeatus. J. Fish Biol. 79, 1815–1827. doi: 10.1111/j.1095-8649.2011.03121.x
Lehtiniemi, M. (2005). Swim or hide: predator cues cause species specific reactions in young fish larvae. J. Fish Biol. 66, 1285–1299. doi: 10.1111/j.0022-1112.2005.00681.x
Lohbeck, K. T., Riebesell, U., and Reusch, T. B. (2012). Adaptive evolution of a key phytoplankton species to ocean acidification. Nat. Geosci. 5, 346–351. doi: 10.1038/ngeo1441
Lonthair, J., Ern, R., and Esbaugh, A. J. (2017). The early life stages of an estuarine fish, the red drum (Sciaenops ocellatus), are tolerant to high pCO2. ICES J. Mar. Sci. 74, 1042–1050. doi: 10.1093/icesjms/fsw225
Maneja, R. H., Dineshram, R., Thiyagarajan, V., Skiftesvik, A. B., Frommel, A. Y., Clemmesen, C., et al. (2014). The proteome of Atlantic herring (Clupea harengus L.) larvae is resistant to elevated pCO2. Mar. Pollut. Bull. 86, 154–160. doi: 10.1016/j.marpolbul.2014.07.030
McNeil, B. I., and Sasse, T. P. (2016). Future ocean hypercapnia driven by anthropogenic amplification of the natural CO2 cycle. Nature 529, 383–386. doi: 10.1038/nature16156
Melzner, F., Stange, P., Trübenbach, K., Thomsen, J., Casties, I., Panknin, U., et al. (2011). Food supply and seawater pCO2 impact calcification and internal shell dissolution in the blue mussel Mytilus edulis. PLoS ONE 6:e24223. doi: 10.1371/journal.pone.0024223
Munday, P. L., Gagliano, M., Donelson, J. M., Dixson, D. L., and Thorrold, S. R. (2011). Ocean acidification does not affect the early life history development of a tropical marine fish. Mar. Ecol. Prog. Ser. 423, 211–221. doi: 10.3354/meps08990
Murray, C. S., Fuiman, L. A., and Baumann, H. (2016). Consequences of elevated CO2 exposure across multiple life stages in coastal forage fish. ICES J. Mar. Sci. 74, 1051–1061. doi: 10.1093/icesjms/fsw179
Nilsson, G. E., Dixson, D. L., Domenici, P., McCormick, M. I., Sørensen, C., Watson, S.-A., et al. (2012). Near-future carbon dioxide levels alter fish behaviour by interfering with neurotransmitter function. Nat. Clim. Chang. 2, 201–204. doi: 10.1038/nclimate1352
Östlund-Nilsson, S., Mayer, I., and Huntingford, F. A. (2006). Biology of the Three-Spined Stickleback. Boca Raton, FL: CRC Press.
Pankhurst, N. W., and Munday, P. L. (2011). Effects of climate change on fish reproduction and early life history stages. Mar. Freshw. Res. 62, 1015–1026. doi: 10.1071/MF10269
Pelejero, C., Calvo, E., and Hoegh-Guldberg, O. (2010). Paleo-perspectives on ocean acidification. Trends Ecol. Evol. 25, 332–344. doi: 10.1016/j.tree.2010.02.002
Pierrot, D. E., Lewis, E., and Wallace, D. W. R. (2006). MS Excel Program Developed for CO2 System Calculations. ORNL/CDIAC-105a. Oak Ridge: Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, U.S. Department of Energy.
Pörtner, H.-O. (2008). Ecosystem effects of ocean acidification in times of ocean warming: a physiologist's view. Mar. Ecol. Prog. Ser. 373, 203–217. doi: 10.3354/meps07768
Roy, R. N., Roy, L. N., Vogel, K. M., Porter-Moore, C., Pearson, T., Good, C. E., et al. (1993). The dissociation constants of carbonic acid in seawater at salinities 5 to 45 and temperatures 0 to 45°C. Mar. Chem. 44, 249–267. doi: 10.1016/0304-4203(93)90207-5
Salonen, K. (1981). Rapid and precise determination of total inorganic carbon and some gases in aqueous solutions. Water Res. 15, 403–406. doi: 10.1016/0043-1354(81)90049-X
Schade, F., Clemmesen, C., and Wegner, M. K. (2014). Within- and transgenerational effects of ocean acidification on life history of marine three-spined stickleback (Gasterosteus aculeatus). Mar. Biol. 161, 1667–1676. doi: 10.1007/s00227-014-2450-6
Schluter, D., and McPhail, J. D. (1992). Ecological character displacement and speciation in sticklebacks. Am. Nat. 140, 85–108. doi: 10.1086/285404
Schmidt, M., Gerlach, G., Leo, E., Kunz, K. L., Swoboda, S., Pörtner, H.-O., et al. (2017). Impact of ocean warming and acidification on the behavior of two co-occuring gadid species, Boreogadus saida and Gadus morhua, from Svalbard. Mar. Ecol. Prog. Ser. 571, 183–191. doi: 10.3354/meps12130
Simpson, S. D., Munday, P. L., Wittenrich, M. L., Manassa, R., Dixson, D. L., Gagliano, M., et al. (2011). Ocean acidification erodes crucial auditory behaviour in a marine fish. Biol. Lett. 7, 917–920. doi: 10.1098/rsbl.2011.0293
Socha, M., Sokołowska-Mikołajczyk, M., Szczerbik, P., Chyb, J., Mikołajczyk, T., and Epler, P. (2012). The effect of polychlorinated biphenyls mixture (Aroclor 1254) on the embryonic development and hatching of Prussian carp, Carassius gibelio, and common carp, Cyprinus carpio (Actinopterygii: Cypriniformes: Cyprinidae). Acta Ichthyol. Piscat. 42, 31–35. doi: 10.3750/AIP2011.42.1.04
Sundin, J., Vossem, L. E., Nilsson-Sköld, H., and Jutfelt, F. (2017). No effect of elevated carbon dioxide on reproductive behaviors in the three-spined stickleback. Behav. Ecol. 28, 1482–1491. doi: 10.1093/beheco/arx112
Vehmaa, A., Hogfors, H., Gorokhova, E., Brutemark, A., Holmborn, T., and Engström-Öst, J. (2013). Projected marine climate change: effects on copepod oxidative status and reproduction. Ecol. Evol. 3, 4548–4557. doi: 10.1002/ece3.839
Waldbusser, G. G., and Salisbury, J. E. (2014). Ocean acidification in the coastal zone from an organism's perspective: multiple system parameters, frequency domains, and habitats. Ann. Rev. Mar. Sci. 6, 221–247. doi: 10.1146/annurev-marine-121211-172238
Keywords: climate change, coastal area, Gasterosteus aculeatus, ocean acidification, survival, young stages
Citation: Glippa O, Brutemark A, Johnson J, Spilling K, Candolin U and Engström-Öst J (2017) Early Development of the Threespine Stickleback in Relation to Water pH. Front. Mar. Sci. 4:427. doi: 10.3389/fmars.2017.00427
Received: 16 October 2017; Accepted: 14 December 2017;
Published: 22 December 2017.
Edited by:
Iris Eline Hendriks, University of the Balearic Islands, SpainReviewed by:
Jon Havenhand, University of Gothenburg, SwedenJose Henrique Muelbert, Fundação Universidade Federal do Rio Grande, Brazil
Copyright © 2017 Glippa, Brutemark, Johnson, Spilling, Candolin and Engström-Öst. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Olivier Glippa, olivg62@gmail.com
†Present Address: Andreas Brutemark, Calluna AB, Nacka, Sweden
 Olivier Glippa
Olivier Glippa Andreas Brutemark1†
Andreas Brutemark1†  Kristian Spilling
Kristian Spilling Jonna Engström-Öst
Jonna Engström-Öst