Habitat Connectivity of Fish in Temperate Shallow-Water Seascapes
- Department of Ecology, Environment and Plant Sciences, Stockholm University, Stockholm, Sweden
Movements of organisms comprise a fundamental aspect of coastal habitat connectivity. Determining the distribution and co-existence of habitat specialists and generalists in shallow-water seascapes leads to a better understanding of the strength of connectivity-driven community patterns in coastal areas. In this study, unbaited Remote Underwater Video (RUV) systems were used to examine habitat usage and connectivity of fish within six shallow-water coastal seascapes on the Swedish west coast. Within each seascape, video sampling was conducted at three different shallow-water habitats: seagrass meadows, rock-macroalgae and unvegetated areas, in June 2014. Comparative analyses showed that the shallow-water fish community was similar in adjacent habitats within a seascape, though abundances of fish were higher within the structurally complex habitats. All habitats were dominated by juveniles, highlighting the importance of the coastal seascape for early fish life stages. The findings demonstrate that adjacent shallow-water habitats in temperate coastal waters are linked through similar species utilization and that the coastal matrix could be regarded in terms of a seascape nursery for fish. The study highlights the importance of considering shallow-water seascape connectivity in coastal conservation planning and management.
Introduction
Shallow-water habitats are vital for healthy coastal areas and contribute globally to fisheries productivity and maintenance of biodiversity (Stål et al., 2008; Ramos et al., 2015; Nordlund et al., 2017). Fundamental to ecology is an understanding of assemblage composition and interactions. Recent efforts have shifted toward examining connectivity between habitats (and habitat patches) within the seascape mosaic, in an effort to broaden applicability to marine spatial planning and comprehensive management, (e.g., Gillanders et al., 2003; Unsworth et al., 2009; Yeager et al., 2011, 2016; Caldwell and Gergel, 2013).
Fish are capable of utilizing multiple habitats, and are therefore influenced by the complexity and structure of the seascape as a whole (Pittman et al., 2007; Grober-Dunsmore et al., 2009; Staveley et al., 2017). A tremendous effort has been made toward describing and comparing distribution patterns of fish in different shallow-water habitats (e.g., Dean et al., 2000; Mathieson et al., 2000; Able, 2005; Dorenbosch et al., 2007; Reiss et al., 2010), such as the significant role of seagrass meadows as nursery grounds (reviewed by Heck et al., 2003). However, in temperate waters the strength of habitat linkages and the relative importance of multiple habitats from a seascape perspective (considering ecological functions in relation to spatial patterns) has, as yet, been given sparse attention (see Perry et al., 2017; Staveley et al., 2017).
Fish connect habitats by the exchange of biomass and energy via ontogenetic migrations, larval dispersal and daily movement. Many coastal marine fish species use different habitats during different life stages (Gillanders et al., 2003). Indeed, many vulnerable species, such as Gadus morhua, use key habitats (e.g., seagrass meadows) during at least part of their life cycle (Musick et al., 2000; Orth et al., 2006). Studies have shown that fish species richness and abundance are higher for juveniles and subadults in seagrass meadows compared to larger adults (Gullström et al., 2008; Bertelli and Unsworth, 2013). Additionally, research from tropical regions indicates that seagrass meadows close to other habitats, such as mangroves and coral reefs, have a positive influence on nursery species (i.e., fish species that use specific habitats exclusively during juvenile life stages), and are essential in shaping fish assemblage structure (Gullström et al., 2008; Berkström et al., 2013b). Given that mobile species use multiple coastal habitats, it is important to understand patterns and processes at a seascape level.
As human activity fragments, and even destroys, important coastal habitats (Baden et al., 2003, 2012; Pihl et al., 2006; Crook et al., 2015; Nagelkerken et al., 2015), defining critical areas and nursery grounds for fish is imperative. This is of importance given the fact that species possess varying degrees of mobility. Therefore, it can be expected that stationary species suffer more negative consequences than migrating species as a result of habitat fragmentation and destruction (Caldwell and Gergel, 2013). Maintaining the integrity, diversity, and connectivity of shallow-water habitats is important for healthy fish communities. Due to their dependence on these shallow-water biomes during various life-stages, commercially and economically profitable fisheries/fish assemblages will certainly benefit from the preservation of these habitats (Pihl et al., 2006; Stål et al., 2008; Baden et al., 2012; Bertelli and Unsworth, 2013; Ramos et al., 2015).
Research has found that nursery species prefer the clearer water in seagrass meadows compared to the turbid water found in unvegetated sandy areas (Nagelkerken and van der Velde, 2004). The authors argue that this pattern is likely a result of the protection provided by the structural complexity of seagrass meadows rather than the reduced visibility due to turbidity. In addition, the food supply in vegetated habitats is important for food webs within the shallow-water environment (Valentine and Heck, 1999; Baden et al., 2010). Heck et al. (2003) posit that the existence of structure (regardless of type) is critical for shallow-water habitats to function as nursery grounds. Therefore, habitat structure and linkages between areas of complex construction, rather than specific habitats themselves, may drive improved species abundances seen in vegetated areas (vs. unvegetated) (Orth et al., 1984; Hemminga and Duarte, 2000; Wennhage and Pihl, 2002; Pittman et al., 2004; Gullström et al., 2008, 2011).
Nagelkerken et al. (2013) proposed the “seascape nursery” as a conceptual model that defines a mosaic of coastal habitats as an interlinked entity. While some research in tropical regions has evaluated the seascape nursery concept and the strength of connectivity between shallow-water areas (e.g., Nagelkerken et al., 2000; Dorenbosch et al., 2007; Gullström et al., 2012; Berkström et al., 2013a,b), this has seldom been investigated in temperate regions. In temperate shallow coastal waters, fish-habitat associations have been studied in various biomes such as seagrass meadows and rocky bottoms (e.g., Wennhage and Pihl, 2002; Jackson et al., 2006; Pihl et al., 2007; Hutchinson et al., 2013) though the focus on habitat connectivity has often been overlooked.
The primary focus of this study was to examine the nature and strength of habitat usage and connectivity in shallow-water coastal seascapes by comparing fish assemblage composition in three different (but adjacent) key habitats: seagrass meadows, rocky bottoms covered by macroalgae, and unvegetated areas. This was done using field observations at multiple sites on the temperate Swedish Skagerrak coast. Using habitat preferences and the life stage of individuals, we hypothesized high levels of similarity in terms of species composition and distribution patterns of fish between adjoining habitats in shallow-water environments, thereby demonstrating connectivity between neighboring habitats in shallow coastal seascapes.
Materials and Methods
Study Location
The study took place on the Swedish Skagerrak coast (58°00′N−58°5′6′N, 11°00′E−11°67′E) in June 2014 (Figure 1). This area is characterized by low tidal fluctuations with an average of ~30 cm daily, though it can oscillate as much as 2 m depending on the strength of the winds (Johannesson, 1989). The archipelago is a fjord-like system consisting of many rocky islands and islets. The area is a productive transitional zone connecting the oceanic waters of the North Sea with the low saline waters of the Kattegat region in the south, an area influenced by the brackish waters of the Baltic Sea. Within the study area, the surface water salinity varies from 15 to 25 (Baden et al., 2012), though it has been reported to be as high as 33 (Björk and Nordberg, 2003).
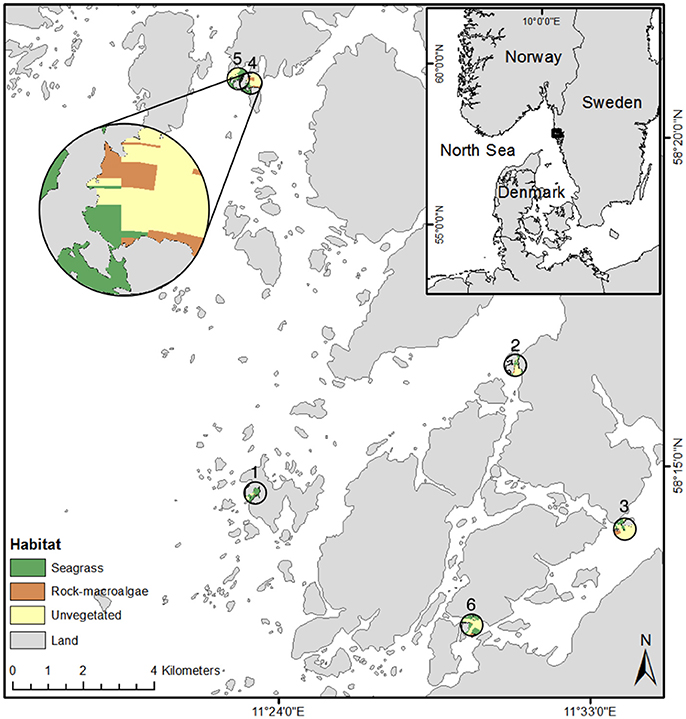
Figure 1. Map of all six seascapes where cameras were placed. The map shows a zoomed image (top left) of one site where habitat locations within the seascape are visible. Site locations are numbered. Coastline: ©Lantmäteriet I2016/00691.
We examined six seascapes each with a diameter of 600 m, all of which had been previously mapped with the use of a drop video system (SeaDrop camera, SeaViewer Cameras, Florida, USA) (Staveley et al., 2017). We use the term “seascape” following the definition by Pittman et al. (2011) stating that seascapes are “wholly or partially submerged marine landscapes.” Each seascape contained the three distinct focal coastal habitats necessary for the current study: (i) seagrass (Zostera marina) meadows, (ii) rocky bottoms covered by macroalgae (rock-macroalgae), and (iii) unvegetated soft bottom areas (unvegetated). The minimum area considered a distinct habitat used for placing the cameras was approximately 850 m2. For each seascape, an unbaited stereo-video system was deployed in each focal habitat.
Camera Surveys and Assessment of Fish Assemblages
Using Remote Underwater Video (RUV) for marine research is a relatively new approach that has grown in popularity because (1) it is a non-extractive method, as opposed to more destructive sample methods employing fishing gear, and (2) it can mitigate problems faced by diver surveys (Underwater Visual Census-UVC), such as disturbance and expense (Langlois et al., 2010). RUV has great potential for studying movements of individuals and connectivity through the use of stereo video cameras. With stereo video, two cameras are mounted on a calibrated frame and then synchronized to record the same object simultaneously (Mallet and Pelletier, 2014). These recordings give the observer highly accurate three-dimensional images, while collecting data on both flora and fauna unobtrusively (Harvey et al., 2003). This ability to observe without disturbance makes this method a valuable tool for studying mobile species such as fish. While the remote underwater camera is a suitable method for studying fish communities, and is well designed for habitats such as rocky areas that are difficult to study with traditional net methods (Cappo et al., 2004; McIlwain et al., 2011), it also has its limitations.
Though RUV methods are able to capture fish behavior undisturbed, they are limited by water visibility; there must be suitable light for recording, and water clarity must be adequate for data collection. Furthermore, it is very difficult to determine if the video is recording a new fish entering the field of view or if it is the same fish previously recorded. For this reason, double counting is an inherent limitation of the method. In order to standardize the methodology, all fish that were out of the field of view for at least 3 s were considered a new fish and therefore counted. Though this is a limitation of the stereo-video method, it would limit all videos equally in the current study and therefore not influence the overall results.
Through calibration and the use of the EventMeasure (www.seagis.com.au) analysis software, data was collected on species identification and abundance information, as well as the length of individuals (Harvey et al., 2001, 2002, 2003). All fish were identified to the lowest taxonomic level possible, counted, and measured. Subsequently, abundances were calculated (m−2) based on fish activity. This was defined as the number of instances fish entered the field of view divided by the recording area, which therefore corrects for the variation in visibility among sites (Hammar et al., 2015; Perry et al., 2017). Field of view is defined by the maximum distance at which a recorded fish is identified for each specific film. To obtain length information fish must be measurable in both camera fields of view with their image overlapping in the two (stereo) images simultaneously. Hence, length measurements were difficult to obtain in highly vegetated habitats where certain parts of the fish could be obstructed. Additionally, cryptic species with little to no movement can be difficult to measure; their ability to camouflage themselves with their surroundings may obscure the edge of the fish in relation to the habitat. However, when fish length data were obtainable, adult and juvenile life stages were determined using their length at maturity (Froese and Pauly, 2015). When specific maturity data were unavailable, a method commonly used to determine life stage was employed, by which individuals 1/3 of the maximum recorded length (according to FishBase) are considered to be juveniles (Dorenbosch et al., 2004, 2007; Unsworth et al., 2009).
The camera system was deployed from a boat and placed in the center of a habitat aided by a snorkeler, angled to record each habitat properly (upward toward the rock-macroalgae area, down toward the unvegetated soft bottom and straight forward/slightly up in the seagrass meadow), ensuring that no vegetation directly obstructed the field of view. Two GoPro® HERO2 cameras mounted on a calibrated frame with 60 cm base separation were used for data collection. Specific calibration (Hammar et al., 2013) and additional methodological details (Perry et al., 2017) can be found in previous investigations. Once positioned, the cameras were left to record for 50 min of continuous filming, with an additional 5 min at the start of all videos to exclude disturbance effects. In total, 900 min of video were analyzed by the same observer. The camera was positioned at depths ranging from approximately 1.5 m to 3 m and the field of view ranged between approximately 0.5 and 3.5 m2 (see Appendix A in Supplementary Material).
All videos were analyzed for fish community information within each of the three habitats. Additionally, species were grouped into guilds based on habitat preference in order to elucidate similarities and differences in fish assemblage structure between various coastal shallow-water habitats, to understand species connectivity between habitats. Guilds were selected based on information from FishBase (Froese and Pauly, 2015) and other sources (Elliott and DeWailly, 1995; Pihl and Wennhage, 2002; Pihl et al., 2006; Perry et al., 2017; Staveley et al., 2017). The guild groupings were as follows: stationary species (SS), shallow-water generalists (SWG), occasional shallow-water visitors (OSV) and juvenile migrants (JM). (1) Stationary species do not actively leave a specific habitat, though they may be found in various habitats and transported via water movement to different areas at early life-stage phases. (2) Shallow-water generalists regularly move between coastal shallow-water habitats but typically not outside the coastal areas. (3) OSV utilize certain shallow-water habitats occasionally as well as other habitats, such as deep-water areas or fresh water rivers. (4) Juvenile migrants use shallow-water habitats in their early life stages before migrating to nearby habitats as adults (or subadults) but may also return occasionally as adults for feeding. Fish within the Pleuronectidae family were not included in the habitat preference guild grouping for this study. We were unable to identify the individuals to species level and different species have different habitat preferences.
Data Analysis
Differences in fish abundance by habitat, number of species and diversity (Shannon-Wiener diversity index) were analyzed by randomized block design analysis of variance (ANOVA) using R (v. 3.2.0). Prior to analyses, data were log10(x+1)-transformed in order to validate statistical assumptions. Pairwise post-hoc Tukey's tests were conducted to determine significant differences between habitats. Additionally, multivariate analyses were conducted on fish assemblage data using the Primer software (v. 6, Plymouth Marine Laboratory). Given the extreme variation in species abundances (because of some highly abundant species), presence/absence data were used for the Sorensen similarity measures. One-way analysis of similarity (ANOSIM) was carried out to test for differences in fish assemblage structure between habitats, and patterns were visualized using non-metric multidimensional scaling (nMDS) ordinations. Moreover, similarity of percentages (SIMPER) analyses were conducted to determine which species were driving the differences in fish assemblage structure among habitats.
It should be noted that Atlantic herring, Clupea harengus, was removed from the analysis when abundance data were utilized, given that this species was found in extremely high numbers compared to all other species identified (or shown with/without C.harengus for comparative purposes). When only presence/absence data were considered C. harengus was included. The reason for removal was to avoid the extremely high abundance muting all other fish abundance results, however it was included in presence/absence calculations because we felt it important to show that it is occasionally a part of the shallow-water habitats in the area. Atlantic herring are a coastal pelagic fish exhibiting schooling behavior and are therefore expected in all types of coastal habitats, as well as in the open ocean, and when seen are found in very high densities (Reid et al., 1999).
Results
Fish Assemblage Compositions
A total of 11,744 fish were recorded, including 15 taxa (13 to species level) within 9 families. Clupeidae was the most abundant family followed by Gobiidae and Labridae, while Gobiidae exhibited the highest species richness (Table 1). At species level, C. harengus was by far the most abundant species throughout all habitats, followed by Gobiusculus flavescens (Table 1). Total fish abundance was significantly higher in seagrass meadows and rock-macroalgae habitats, compared to unvegetated soft bottom areas (S-U p < 0.001; R-U p = 0.002), while seagrass meadows and rock-macroalgae habitat abundances did not differ from each other [(S-R p = 0.217) Block ANOVA; Habitat: F(2, 10) = 24.482, p < 0.001; Site: F(5, 10) = 2.644, p = 0.090] (Figure 2). Interestingly, this is the case despite the fact that the total area of the unvegetated habitat, on average over the six seascapes, was the largest, followed by the area of seagrass, while the smallest coverage area was that of the rock-macroalgae habitat (Figure 2). Although the abundance differed significantly between some of the habitats, neither the Shannon-Wiener diversity index (excluding the abundance of C. harengus) [Block ANOVA; Habitat: F(2, 10) = 0.602, p = 0.567; Site: F(5, 10) = 0.453, p = 0.802] nor the number of species [Habitat: F(2, 10) = 0.278, p = 0.763; Site: F(5, 10) = 0.056, p = 0.997] showed any differences among habitats or across sites (Table 2).
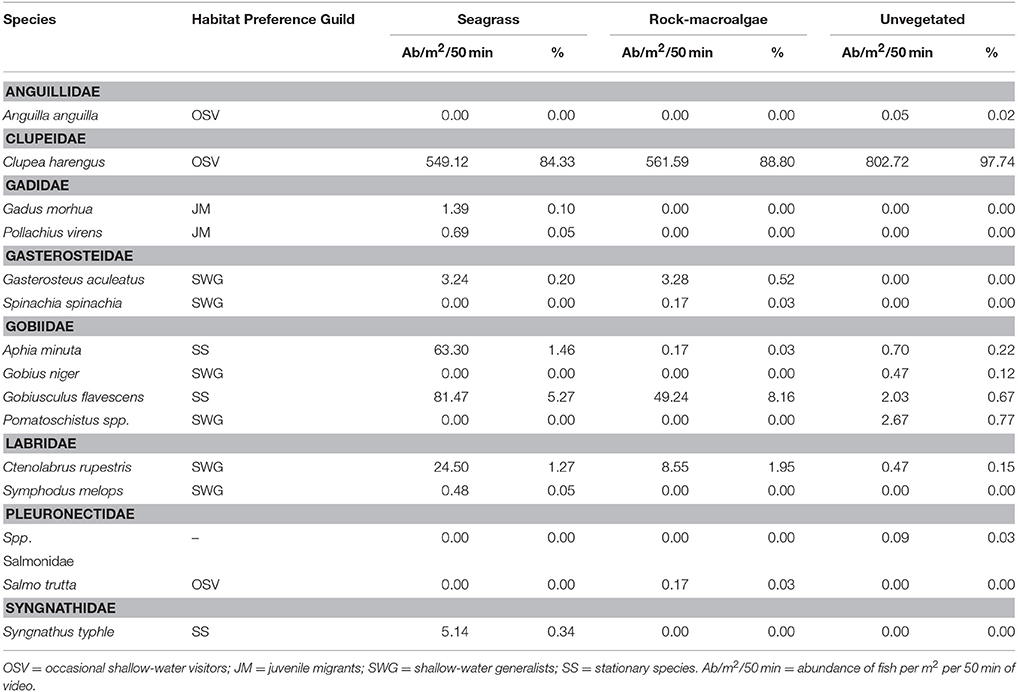
Table 1. List of fish taxa identified from camera surveys in each of the three studied habitats (seagrass meadows, rocky bottoms covered by macroalgae and unvegetated soft bottom areas), their habitat preference guilds and the mean activity per m2 (proxy for density) per 50 min sampling period for each fish taxon as well as the percent of each species compared to the total abundance of fish within each habitat.
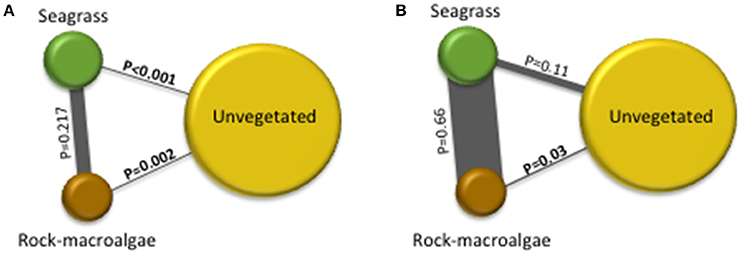
Figure 2. The illustration is based on pairwise post-hoc (Tukey's test) results from randomized block-designed ANOVAs comparing fish abundance per m2 in the three studied habitats. The size of the circle is proportional to the average size of the habitat area for all six seascapes. The lines between the habitats are proportionally related to the strength of the similarity between habitats; the thicker the gray line is the more similar the fish abundance is between those habitats. Significant differences between fish abundances in different habitats are indicated by bolded p-values (where p < 0.05). No differences were found between sites with p = 0.09 for illustration (A) and p = 0.179 for (B). *Clupea harengus abundance was removed from the analysis in the image on the left (A) and included in the image on the right (B).
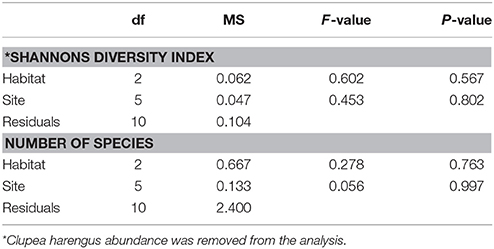
Table 2. Randomized block analysis of variance of log-transformed number of species per habitat and Shannon-Wiener diversity index.
The assemblage structure of fish (Figure 3) varied significantly among the three habitats (ANOSIM; Global R = 0.435, p < 0.001), with seagrass- and rock-macroalgae-habitats differing from the unvegetated areas (Seagrass vs. Unvegetated: R = 0.594, p < 0.01; Rock-macroalgae vs. Unvegetated: R = 0.635, p < 0.01), while the two vegetated habitats did not differ from each other (Seagrass vs. Rock-macroalgae: R = 0.026, p = 0.37). When evaluating the assemblage structure based on presence/absence data in order to control for the disproportionately large abundance of C. harengus, the SIMPER analysis showed that the taxon contributing most to the dissimilarities between the rock-macroalgae- and unvegetated habitats was Pomatoschistus spp., which was only present in unvegetated areas, whereas G. flavescens was found more often in the rock-macroalgae habitat (Table 2). Gobius niger, on the other hand, was only found in unvegetated soft bottom habitats (Table 2). Similarly, these were also the same species driving statistical differences between seagrass meadows and unvegetated areas (Table 3). Species such as C. harengus, A. minuta, G. flavescens, and C. rupestris were, however, observed in all three habitats.
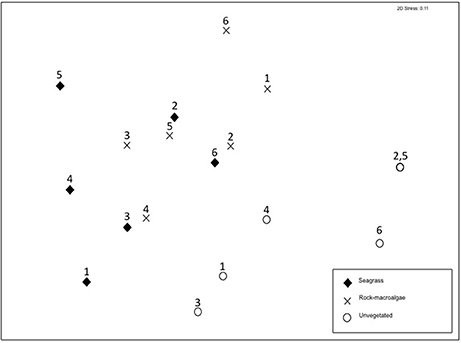
Figure 3. Non-metric multidimensional scaling (nMDS) based on Bray-Curtis similarity coordinates for presence/absence of fish species separated into the three studied shallow-water habitats. Site numbers are labeled (compare with Figure 1); note that two unvegetated habitat sites overlap.
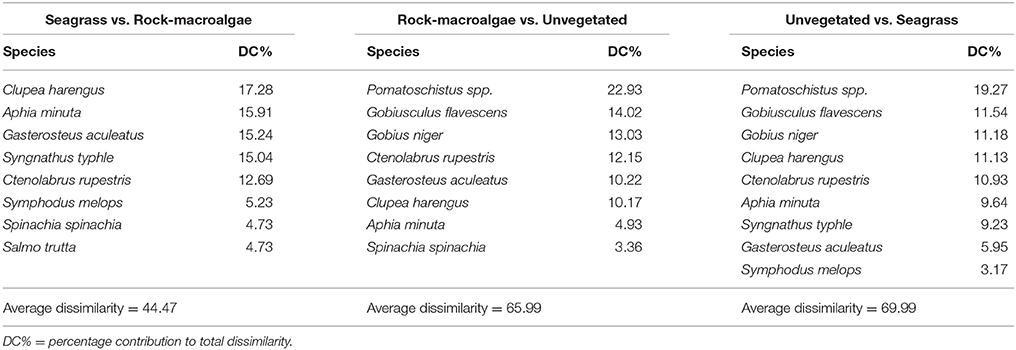
Table 3. SIMPER analysis results for fish species (based on presence/absence data) contributing most to dissimilarities among habitats (cumulative limit of 90%).
Habitat Preference Guilds
When reviewing all camera data the habitat preference guild with the highest abundance was the OSV with approximately 90–98% of the total abundance in all three habitats when C. harengus was included. Excluding C. harengus, the OSV yielded an approximate abundance less than half a percent in both the unvegetated- and rock-macroalgae habitats. When omitting C. harengus, the seagrass- and rock-macroalgae habitats were dominated by the SS guild, while the SWG was only about 20% of the total abundance. However, the opposite was found in the unvegetated habitat as the SWG was most abundant followed by the SS. Taxa from the JM guild were only seen within the seagrass habitat (Figure 4).
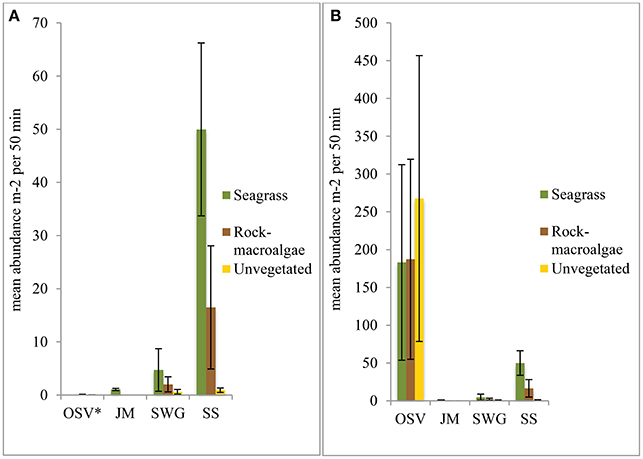
Figure 4. Mean fish abundance per m2 over the 50 min sampling period for each habitat preference guild (OSV—occasional shallow-water visitors, JM—juvenile migrants, SWG—shallow-water generalists, SS—stationary species) per habitat. The figure on the left (A) excludes C. harengus in the OSV guild (indicated by *), while the figure on the right (B) includes C. harengus in the OSV guild. Noteworthy is the large difference between the two y-axes.
Length and Life Stage of Fish
The average length of fish did not differ significantly among habitats [ANOVA; F(2, 5) = 2.065, p = 0.178], though the fish within the unvegetated areas were, on average, slightly larger than those in the seagrass, followed by the rock-macroalgae habitats (5.7 cm ± 0.9 SE, 5.3 cm ± 0.3 SE, 4.9 cm ± 0.2 SE, respectively). It must be noted that the number of measurable fish was not equal to the number of identified fish as not all fish are seen within both cameras' field of view (refer to Methods section for the discussion of methodological limitations). For the unvegetated soft bottom habitat, 2% (n = 108/6008) of the observed fish were measurable, 4% (n = 78/2048) within the seagrass meadow and 16% (n = 582/3688) from the rock-macroalgae habitat. While length, and therefore life stage information, was estimated on an individual basis, the limited number of measurable fish made analysis of averages necessary. From these measurable fish an evaluation of the proportion of juveniles was performed using ANOVA and it was shown that there was no significant difference between habitats [F(2, 5) = 0.634, p = 0.551], with approximately 75% juveniles comprising all three habitats (Figure 5).
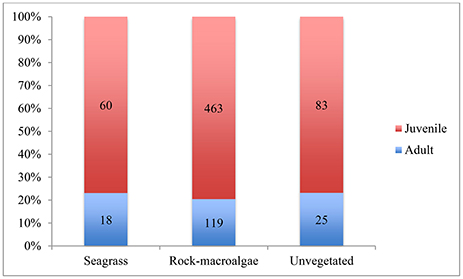
Figure 5. Proportion of juvenile and adult fish for each habitat. Numbers shown in the bars represent number of fish specimens that could be measured for length and therefore analyzed for life stage. The figure includes C. harengus.
Discussion
The concept that the complexity and interconnectedness of habitats (rather than evaluating each specific habitat individually), is of importance for those organisms dependent thereon, has been gaining momentum (Heck et al., 2003). In this study, we tried to understand in more detail how fish assemblages within three adjacent shallow-water habitats overlap. We did this by assessing variation between habitat types, as well as explicitly focusing on habitat variation within different coastal seascapes, to see whether differences exist between habitats and/or seascapes. In support of our hypothesis, we found that there was high similarity in species composition between adjoining habitats. Specifically, we show that neither the number of species nor species diversity differed among the three shallow-water habitats, with many specimens of the same species found in all three habitat types implying a degree of connectivity between habitats. This indicates that the fish community is similar in adjoining habitats of temperate shallow-water seascapes, though structurally complex habitats (i.e., seagrass meadows and macroalgae beds) had significantly higher abundances of fish compared to unvegetated areas. Also, interestingly, the proportion of juveniles found within each habitat did not differ between the three habitats.
The mean fish abundance was highest within the seagrass habitat, a result initially appearing to contrast with those found previously on the Swedish west coast by Stål et al. (2007), where rocky areas contained higher abundances than soft bottom areas. However, the soft bottom areas examined in the study by Stål et al. (2007) included depths (6–10 m) not evaluated in the current study and where seagrass does not grow, and thus is the most probable cause for the contradictory findings. While seagrass beds had the highest fish abundance in our study, they did not differ significantly from the rock-macroalgae habitat. These findings emphasize the need to consider the shallow-water seascape as an integrally-linked coastal area important for species assemblages, rather than an area of isolated homogenous habitats important for particular species. This is consistent with recent results from the tropics showing that both seagrass beds and macroalgae habitats are important for juvenile fish (Tano et al., 2017).
Comparable fish communities within shallow-water habitats on the Swedish west coast have been demonstrated by Pihl and Wennhage (2002). However, the perspective of a continuous and interconnected shallow-water seascape has not previously been highlighted as in the current research. Here, we evaluated all habitats within the same seascape together (600 m diameter), whereas previous studies utilized random habitat sampling methods. In our design and analyses all three habitats were adjacent and therefore part of the same contiguous shallow-water seascape. Many of the species from the current study were found in at least two of the three shallow-water habitats examined, with a few species found in all three habitats. Clearly, the species within this study utilize multiple habitats within the shallow-water seascape, indicating that species may be moving between the adjacent habitats. However, some species, such as those from the stationary species guild, may be habitat-specific at the individual level as opposed to the species level. Similar findings of a substantial overlap of species within multiple coastal habitats have been found in different areas of the world (Nagelkerken and van der Velde, 2004; Franco et al., 2006; Unsworth et al., 2009; Berkström et al., 2013b; Lilley and Unsworth, 2014), including temperate Swedish waters (Pihl and Wennhage, 2002; Wennhage and Pihl, 2002).
Habitat Connectivity
Our results strengthen the postulation that habitat connectivity is strong in temperate shallow-water seascapes. Different species, such as C. harengus, G. flavescens and C. rupestris, were observed within all three focal habitats, which made up three of the four habitat preference guilds assessed (OSV, SS, and SWG, respectively). These data, when combined with life-stage history information from the literature, show highly connected seascapes where all shallow-water habitats evaluated are important for these species as well as the juvenile migrant (JM) G. morhua (Figure 6). Clupea harengus is a species known for its schooling behavior utilizing coastal pelagic waters as juveniles as well as the open ocean during all life stages (Reid et al., 1999). As adults, specimens of C. harengus are found to make vertical migrations between deep and shallow waters The stationary species G. flavescens is ecologically associated with both macroalgae- and seagrass habitats as adults, while different parts of the water column are used at different life stages in response to predator avoidance (Folkestad, 2005; Froese and Pauly, 2015). Juveniles have been found in seagrass meadows (Staveley et al., 2017) as the eggs are demersal; after hatching the larvae are typically seen in the sublittoral zone (Folkestad, 2005). While G. morhua was only seen in the seagrass habitat in the current study, previous studies have shown that juveniles may be associated with rocky- and unvegetated areas as well (Wennhage and Pihl, 2002). It is possible that our results are due to a limitation of the camera method, which could potentially be limited by species behavior; G. morhua can respond strongly to changes in light, and therefore are more active at dawn and dusk (Staveley unpublished data). Adults are known to perform vertical migrations from deep- to shallow-water areas and the eggs and larval stage have been seen in the pelagic zone (Petitgas et al., 2013). For C. rupestris, while seen in all habitats within the shallow-water seascape, literature suggests that juveniles and adults are typically associated with seagrass, and with rocky areas (Pihl and Wennhage, 2002; Staveley et al., 2017). Clearly, the species in Figure 6, among others in the current study, are associated with many habitats, both coastal and offshore, showing strong connectivity patterns between various habitats.
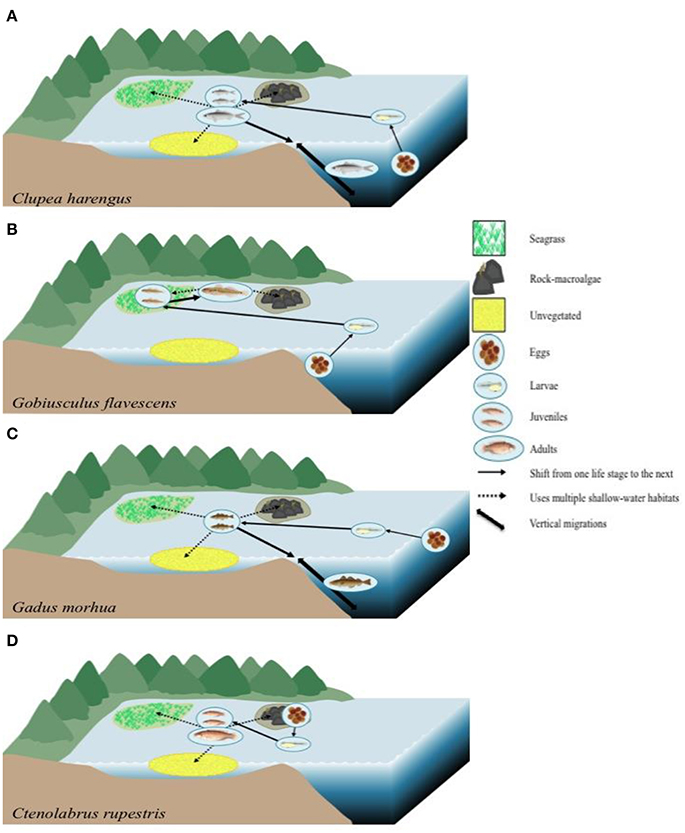
Figure 6. Theoretical diagrams illustrating the habitat connectivity based on ontogenetic migrations of four species found within our study representing the four habitat preference guilds, based on literature as described in the text. (A) Clupea harengus, an occasional shallow-water visitor (OSV) habitat usage. Eggs found in deep water with larvae located in the pelagic zone (Reid et al., 1999; Petitgas et al., 2013). Juveniles and adults in large schools utilizing coastal waters and the open ocean (Reid et al., 1999). (B) Stationary species (SS) Gobiusculus flavescens; adults linked to seagrass and rock-macroalgae areas (Folkestad, 2005; Froese and Pauly, 2015), juveniles in seagrass habitats (Staveley et al., 2017), eggs demersal and larvae in the sublittoral zone (Folkestad, 2005). (C) Juvenile migrant (JM) Gadus morhua; adults perform vertical migrations, juveniles utilize multiple shallow-water habitats (Staveley et al., unpublished data; Wennhage and Pihl, 2002), and larvae and eggs are in the pelagic zone (Petitgas et al., 2013). (D) Shallow-water generalist (SWG) Ctenolabrus rupestris. Juveniles and adults are associated with seagrass and rock-macroalgae (Pihl and Wennhage, 2002; Staveley et al., 2017), eggs are laid in the rock-macroalgae habitats (Darwall et al., 1992) and larvae in the pelagic zone (Kullander et al., 2012). Fish illustrations of G. flavescens and C. rupestris by Maj Persson. Other symbols courtesy of the Integration and Application Network (IAN).
The species observed in multiple habitats represented the habitat preference guilds occasional shallow-water visitors (OSV), stationary species (SS), and shallow-water generalists (SWG), while juvenile migrants (JM) were identified only in seagrass meadows. These findings are similar to a study in tropical waters by Kendall et al. (2011), who found that when evaluating the seascape influence on fish mobility guilds, all guilds (residents, mobile fish and transient fish) were influenced by the surrounding seascape, including seagrass meadows, sand/soft bottom and hard bottom areas. In this study, the vegetated habitats consisted of more than 75% of specimens belonging to the SS guild, while half of the specimens in unvegetated areas represented the SWG guild. This may be attributable to the fact that stationary species are reliant on specific habitat to provide them with food and protection, and therefore structurally complex areas offer more opportunity to hide (e.g., while foraging) (Heck et al., 2003; Nagelkerken and van der Velde, 2004). In contrast, unvegetated areas may largely be used for temporary passage between nearby habitats or by cryptic, sand-colored species such as Pomatoschistus spp. and some bottom-dwelling flatfish (Froese and Pauly, 2015). It is also possible that the stationary species are found in the unvegetated area as a result of passive transport via water current movement.
Fish Assemblage
The SIMPER analysis indicated that similar species contributed to the variation in seagrass meadows and rock-macroalgae habitats compared to unvegetated areas. The differences in fish assemblage structure between the structurally complex habitats and the unvegetated sites were mainly driven by the shallow-water generalists Pomatoschistus spp. and Gobius niger, and the stationary species G. flavescens, with G. flavescens found in the more structurally complex habitats while Pomatoschistus spp. and G. niger were only found in the unvegetated areas. Interestingly, G. niger has been reported to have very strong habitat selection shifts based on the presence of predators, avoiding seagrass meadows completely when certain predator species are present and only inhabiting unvegetated soft bottom areas (Kruschel and Schultz, 2011), illustrating the complexity of trophic interactions.
While the abundance of different species differed among certain shallow-water habitats, the average size of fish within each habitat did not differ significantly. All habitats consisted of approximately 75% juveniles, indicating an important nursery function. These results are very strong evidence in support of the holistic seascape nursery concept initially posited by Nagelkerken et al. (2013). Although RUV is limited with regard to the number of fish that can be measured, its results are consistent across all seascapes and habitats. The number of measurable fish obtained is likely sufficient to draw relevant conclusions from, such as similarities in the life stage proportions among habitats.
Conclusions
Shallow-water habitats are integrally linked through nutrient flow and the movements of mobile organisms such as fish, and should therefore be studied and analyzed with this holistic perspective in mind (Ng et al., 2013). Here, we found support for the idea that coastal habitats, while differing in the abundance of fish contained within each habitat, all support juvenile fish assemblages. Our results, together with life history information of specific species, show that habitats within the shallow-water seascape are connected via species movements and ontogenetic shifts. Type of habitat was important for the abundance of fish but not for the diversity or number of species. Therefore, we suggest that the different habitats of shallow-water seascapes are of similar importance for fish assemblages and as a seascape nursery. Thus, management should mimic the ecological system and focus on a holistic-, heterogeneous- and interconnected-seascape approach, rather than take a single-habitat or single-species perspective.
Author Contributions
DP, TS, and MG all contributed to the design of the study, analysis and interpretation of the data. DP and TS were responsible for data acquisition. DP drafted the work and DP, TS, and MG participated in all manuscript revisions. DP, TS, and MG all gave final approval of the submitted manuscript and all agree to be accountable for every aspect of the work.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We wish to thank Said Mgeleka and Mathew Silas for their help in assisting with all the field work as well as Linus Hammar for assistance during a portion of the field work. We are also grateful to Maria Asplund for her generosity in loaning equipment as well as the Sven Lovén Centre for Marine Sciences (Kristineberg) facility. Thank you Earl and Alex Perry for reviewing and editing the language of the manuscript. We gratefully acknowledge the support from the Swedish Research Council Formas (grant number 2011-1640). Additional support was graciously provided by a scholarship from the Royal Swedish Academy of Sciences.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2017.00440/full#supplementary-material
References
Able, K. W. (2005). A re-examination of fish estuarine dependence: evidence for connectivity between estuarine and ocean habitats. Estuar. Coast. Shelf Sci. 64, 5–17. doi: 10.1016/j.ecss.2005.02.002
Baden, S., Boström, C., Tobiasson, S., Arponen, H., and Moksnes, P. O. (2010). Relative importance of trophic interactions and nutrient enrichment in seagrass ecosystems: A broad-scale field experiment in the Baltic-Skagerrak area. Limnol. Oceanogr. 55, 1435–1448. doi: 10.4319/lo.2010.55.3.1435
Baden, S., Emanuelsson, A., Pihl, L., Svensson, C.-J., and Åberg, P. (2012). Shift in seagrass food web structure over decades is linked to overfishing. Mar. Ecol. Prog. Ser. 451, 61–73. doi: 10.3354/meps09585
Baden, S., Gullström, M., Lundén, B., Pihl, L., and Rosenberg, R. (2003). Vanishing Seagrass (Zostera Swedish Coastal Waters L.) in Swedish Coastal Waters. Ambio 32, 374–377. doi: 10.1579/0044-7447-32.5.374
Berkström, C., Jörgensen, T. L., and Hellström, M. (2013a). Ecological connectivity and niche differentiation between two closely related fish species in the mangrove – seagrass – coral reef continuum. Mar. Ecol. Prog. Ser. 477, 201–215. doi: 10.3354/meps10171
Berkström, C., Lindborg, R., Thyresson, M., and Gullström, M. (2013b). Assessing connectivity in a tropical embayment: fish migrations and seascape ecology. Biol. Conserv. 166, 43–53. doi: 10.1016/j.biocon.2013.06.013
Bertelli, C. M., and Unsworth, R. K. (2013). Protecting the hand that feeds us: Seagrass (Zostera marina) serves as commercial juvenile fish habitat. Mar. Pollut. Bull. 83, 425–429. doi: 10.1016/j.marpolbul.2013.08.011
Björk, G., and Nordberg, K. (2003). Upwelling along the Swedish west coast during the 20th century. Cont. Shelf Res. 23, 1143–1159. doi: 10.1016/S0278-4343(03)00081-5
Caldwell, I. R., and Gergel, S. E. (2013). Thresholds in seascape connectivity: influence of mobility, habitat distribution, and current strength on fish movement. Landsc. Ecol. 28, 1937–1948. doi: 10.1007/s10980-013-9930-9
Cappo, M., Speare, P., and De'Ath, G. (2004). Comparison of baited remote underwater video stations (BRUVS) and prawn (shrimp) trawls for assessments of fish biodiversity in inter-reefal areas of the Great Barrier Reef Marine Park. J. Exp. Mar. Biol. Ecol. 302, 123–152. doi: 10.1016/j.jembe.2003.10.006
Crook, D. A., Lowe, W. H., Allendorf, F. W., Eros, T., Finn, D. S., Gillanders, B. M., et al. (2015). Human effects on ecological connectivity in aquatic ecosystems: Integrating scientific approaches to support management and mitigation. Sci. Total Environ. 534, 52–64. doi: 10.1016/j.scitotenv.2015.04.034
Darwall, W. R. T., Costello, M. J., Donnelly, R., and Lysaght, S. (1992). Implications of life-history strategies for a new wrasse fishery. J. Fish. Biol. 41, 111–123. doi: 10.1111/j.1095-8649.1992.tb03873.x
Dean, T. A., Haldorson, L., Laur, D. R., Jewett, S. C., and Blanchard, A. (2000). The distribution of nearshore fishes in kelp and eelgrass communities in Prince William Sound, Alaska: associations with vegetation and physical habitat characteristics. Environ. Biol. Fishes 57, 271–287. doi: 10.1023/A:1007652730085
Dorenbosch, M., Van Riel, M. C., Nagelkerken, I., and Van Der Velde, G. (2004). The relationship of reef fish densities to the proximity of mangrove and seagrass nurseries. Estuar. Coast. Shelf Sci. 60, 37–48. doi: 10.1016/j.ecss.2003.11.018
Dorenbosch, M., Verberk, W., Nagelkerken, I., and van der Velde, G. (2007). Influence of habitat configuration on connectivity between fish assemblages of Caribbean seagrass beds, mangroves and coral reefs. Mar. Ecol. Prog. Ser. 334, 103–116. doi: 10.3354/meps334103
Elliott, M., and DeWailly, F. (1995). The structure and composition of european estuarine fish assemblages. Neth. J. Aquat. Ecol. 29, 397–417. doi: 10.1007/BF02084239
Folkestad, H. (2005). Stage dependent habitat use under conflicting predation pressure: An experimental test with larval and juvenile two-spotted gobies, Gobiusculus flavescens Fabricius. J. Exp. Mar. Biol. Ecol. 323, 160–171. doi: 10.1016/j.jembe.2005.04.005
Franco, A., Franzoi, P., Malavasi, S., Riccato, F., and Torricelli, P. (2006). Fish assemblages in different shallow water habitats of the Venice Lagoon. Hydrobiologia 555, 159–174. doi: 10.1007/s10750-005-1113-5
Froese, R., and Pauly, D. (eds.). (2015). FishBase. version (10/2015). World Wide Web Electronic Publication. Available online at: www.fishbase.org
Gillanders, B., Able, K., Brown, J., Eggleston, D. B., and Sheridan, P. F. (2003). Evidence of connectivity between juvenile and adult habitats for mobile marine fauna: an important component of nurseries. Mar. Ecol. Prog. Ser. 247, 281–295. doi: 10.3354/meps247281
Grober-Dunsmore, R., Pittman, S. J., Caldow, C., Kendall, M. S., and Frazer, T. K. (2009). Ecological Connectivity among Tropical Coastal Ecosystems. Dordrecht: Springer.
Gullström, M., Berkström, C., Öhman, M. C., Bodin, M., and Dahlberg, M. (2011). Scale-dependent patterns of variability of a grazing parrotfish (Leptoscarus vaigiensis) in a tropical seagrass-dominated seascape. Mar. Biol. 158, 1483–1495. doi: 10.1007/s00227-011-1665-z
Gullström, M., Bodin, M., Nilsson, P. G., and Öhman, M. C. (2008). Seagrass structural complexity and landscape configuration as determinants of tropical fish assemblage composition. Mar. Ecol. Prog. Ser. 363, 241–255. doi: 10.3354/meps07427
Gullström, M., Dorenbosch, M., Lugendo, B. R., Mwandya, A. W., Mgaya, Y. D., and Berkström, C. (2012). “Connectivity and nursery function of shallow-water habitats in Chwaka Bay,” in People, Nature and Research: Past, Present and Future of Chwaka Bay, Zanzibar, eds M. de la Torre-Castro and T. J. Lyimo (Zanzibar: WIOMSA), 175–192.
Hammar, L., Andersson, S., Eggertsen, L., Haglund, J., Gullström, M., Ehnberg, J., et al. (2013). Hydrokinetic turbine effects on fish swimming behaviour. PLoS ONE 8:e84141. doi: 10.1371/journal.pone.0084141
Hammar, L., Eggertsen, L., Andersson, S., Ehnberg, J., Arvidsson, R., Gullström, M., et al. (2015). A probabilistic model for hydrokinetic turbine collision risks: exploring impacts on fish. PLoS ONE 10:e0117756. doi: 10.1371/journal.pone.0117756
Harvey, E., Cappo, M., Shortis, M., Robson, S., Buchanan, J., and Speare, P. (2003). The accuracy and precision of underwater measurements of length and maximum body depth of southern bluefin tuna (Thunnus maccoyii) with a stereo–video camera system. Fish. Res. 63, 315–326. doi: 10.1016/S0165-7836(03)00080-8
Harvey, E., Fletcher, D., and Shortis, M. (2001). A comparison of the precision and accuracy of estimates of reef-fish lengths determined visually by divers with estimates produced by a stereo-video system. Fish. Bull. 99, 63–71.
Harvey, E., Fletcher, D., and Shortis, M. (2002). Estimation of reef fish length by divers and by stereo-video: a first comparison of the accuracy and precision in the field on living fish under operational conditions. Fish. Res. 57, 255–265. doi: 10.1016/S0165-7836(01)00356-3
Heck, K. L., Hays, G., and Orth, R. J. (2003). Critical evaluation of the nursery role hypothesis for seagrass meadows. Mar. Ecol. Prog. Ser. 253, 123–136. doi: 10.3354/meps253123
Hemminga, M. A., and Duarte, C. (2000). Seagrass ecology. Am. Soc. Limnol. Oceanogr. 47, 611. doi: 10.1017/CBO9780511525551
Hutchinson, N., Jenkins, G. P., Brown, A., and Smith, T. M. (2013). Variation with depth in temperate seagrass-associated fish assemblages in Southern Victoria, Australia. Estuar. Coasts 37, 801–814. doi: 10.1007/s12237-013-9742-9
Jackson, E. L., Attrill, M. J., and Jones, M. B. (2006). Habitat characteristics and spatial arrangement affecting the diversity of fish and decapod assemblages of seagrass (Zostera marina) beds around the coast of Jersey (English Channel). Estuar. Coast. Shelf Sci. 68, 421–432. doi: 10.1016/j.ecss.2006.01.024
Johannesson, K. (1989). The bare zone of Swedish rocky shores : why is it there? Oikos 54, 77–86. doi: 10.2307/3565899
Kendall, M., Miller, T., and Pittman, S. (2011). Patterns of scale-dependency and the influence of map resolution on the seascape ecology of reef fish. Mar. Ecol. Prog. Ser. 427, 259–274. doi: 10.3354/meps08945
Kruschel, C., and Schultz, S. T. (2011). Juvenile Gobius niger avoids seagrass in the presence and uncertain absence of seagrass-inhabiting predators. J. Exp. Mar. Biol. Ecol. 409, 240–246. doi: 10.1016/j.jembe.2011.09.001
Kullander, S. O., Nyman, L., Jilg, K., and Delling, B. (2012). Nationalnyckeln: Strålfeniga fiskar Actinopterygii ArtDatabanken. Uppsala: SLU.
Langlois, T., Harvey, E., Fitzpatrick, B., Meeuwig, J. J., Shedrawi, G., and Watson, D. L. (2010). Cost-efficient sampling of fish assemblages: comparison of baited video stations and diver video transects. Aquat. Biol. 9, 155–168. doi: 10.3354/ab00235
Lilley, R. J., and Unsworth, R. K. F. (2014). Atlantic Cod (Gadus morhua) benefits from the availability of seagrass (Zostera marina) nursery habitat. Glob. Ecol. Conserv. 2, 367–377. doi: 10.1016/j.gecco.2014.10.002
Mallet, D., and Pelletier, D. (2014). Underwater video techniques for observing coastal marine biodiversity: a review of sixty years of publications (1952–2012). Fish. Res. 154, 44–62. doi: 10.1016/j.fishres.2014.01.019
Mathieson, S., Cattrijsse, A., Costa, M. J., Drake, P., Elliott, M., Gardner, J., et al. (2000). Fish assemblages of European tidal marshes: A comparison based on species, families and functional guilds. Mar. Ecol. Prog. Ser. 204, 225–242. doi: 10.3354/meps204225
McIlwain, J. L., Harvey, E. S., Grove, S., Shiell, G., Al Oufi, H., and Al Jardani, N. (2011). Seasonal changes in a deep-water fish assemblage in response to monsoon-generated upwelling events. Fish Oceanogr. 20, 497–516. doi: 10.1111/j.1365-2419.2011.00598.x
Musick, J., Harbin, M., Berkeley, S., Burgess, G. H., Eklund, A. M., Findley, L. T., et al. (2000). Marine, estuarine, and diadromous fish stocks at risk of extinction in North America (Exclusive of Pacific Salmonids). Fisheries 37–41. doi: 10.1577/1548-8446(2000)025<0006:MEADFS>2.0.CO;2
Nagelkerken, I., Dorenbosch, M., Verberk, W. C. E. P., de la Morinière, E. C., and van der Velde, G. (2000). Importance of shallow-water biotopes of a Caribbean bay for juvenile coral reef fishes: patterns in biotope association, community structure and spatial distribution. Mar. Ecol. Prog. Ser. 202, 175–192. doi: 10.3354/meps202175
Nagelkerken, I., Russell, B. D., Gillanders, B. M., and Connell, S. D. (2015). Ocean acidification alters fish populations indirectly through habitat modification. Nat. Clim. Change 6, 89–93. doi: 10.1038/nclimate2757
Nagelkerken, I., Sheaves, M., Baker, R., and Connolly, R. M. (2013). The seascape nursery: a novel spatial approach to identify and manage nurseries for coastal marine fauna. Fish Fish. 16, 362–371. doi: 10.1111/faf.12057
Nagelkerken, I., and van der Velde, G. (2004). A comparison of fish communities of subtidal seagrass beds and sandy seabeds in 13 marine embayments of a Caribbean island, based on species, families, size distribution and functional groups. J. Sea. Res. 52, 127–147. doi: 10.1016/j.seares.2003.11.002
Ng, C. N., Xie, Y. J., and Yu, X. J. (2013). Integrating landscape connectivity into the evaluation of ecosystem services for biodiversity conservation and its implications for landscape planning. Appl. Geogr. 42, 1–12. doi: 10.1016/j.apgeog.2013.04.015
Nordlund, L. M., Unsworth, R., Gullström, M., and Cullen-Unsworth, L. (2017). Global significance of seagrass fishery activity. Fish Fisher. doi: 10.1111/faf.12259. [Epub ahead of print].
Orth, R., Heck, K., and Montfrans, J. (1984). Faunal communities in seagrass beds: a review of the influence of plant structure and prey characteristics on predator-prey relationships. Estuaries 7, 339–350. doi: 10.2307/1351618
Orth, R. J., Carruthers, T. J. B., Dennison, W. C., Duarte, C. M., Fourqurean, J. W., Kenneth, L. H. Jr., et al. (2006). A global crisis for seagrass ecosystems. Am. Inst. Biol. Sci. 56, 987–996. doi: 10.1641/0006-3568(2006)56[987:AGCFSE]2.0.CO;2
Perry, D., Staveley, T. A. B., Hammar, L., Meyers, A., Lindborg, R., and Gullström, M. (2017). Temperate fish community variation over seasons in relation to large-scale geographic seascape variables. Can. J. Fish. Aquat. Sci. doi: 10.1139/cjfas-2017-0032
Petitgas, P., Rijnsdorp, A. D., Dickey-Collas, M., Engelhard, G. H., Peck, M. A., Pinnegar, J. K., et al. (2013). Impacts of climate change on the complex life cycles of fish. Fish Oceanogr. 22, 121–139. doi: 10.1111/fog.12010
Pihl, L., Baden, S., Kautsky, N., Rönnbäck, P., Söderqvistc, T., Troell, M., et al. (2006). Shift in fish assemblage structure due to loss of seagrass Zostera marina habitats in Sweden. Estuar. Coast. Shelf Sci. 67, 123–132. doi: 10.1016/j.ecss.2005.10.016
Pihl, L., Sta, J., Stål, J., and Pihl, L. (2007). Quantitative assessment of the area of shallow habitat for fish on the Swedish west coast. ICES J. Mar. Sci. 64, 446–452. doi: 10.1093/icesjms/fsm018
Pihl, L., and Wennhage, H. (2002). Structure and diversity of fish assemblages on rocky and soft bottom shores on the Swedish west coast. J. Fish Biol. 61, 148–166. doi: 10.1111/j.1095-8649.2002.tb01768.x
Pittman, S., Caldow, C., Hile, S., and Monaco, M. (2007). Using seascape types to explain the spatial patterns of fish in the mangroves of SW Puerto Rico. Mar. Ecol. Prog. Ser. 348, 273–284. doi: 10.3354/meps07052
Pittman, S. J., Kneib, R., Simenstad, C., and Nagelkerken, I. (2011). Seascape ecology: application of landscape ecology to the marine environment. Mar. Ecol. Prog. Ser. 427, 187–190. doi: 10.3354/meps09139
Pittman, S., McAlpine, C., and Pittman, K. (2004). Linking fish and prawns to their environment: a hierarchical landscape approach. Mar. Ecol. Prog. Ser. 283, 233–254. doi: 10.3354/meps283233
Ramos, D. A. E., Aragones, L. V., and Rollon, R. N. (2015). Linking integrity of coastal habitats and fisheries yield in the Mantalip reef system. Ocean Coast. Manag. 111, 62–71. doi: 10.1016/j.ocecoaman.2015.04.009
Reid, R. N., Cargnelli, L. M., Griesbach, S. J., Packer, D. B., Johnson D. L.„ Zetlin, C. A., et al. (1999). Atlantic herring, clupea harengus, life history and habitat characteristics. NOAA Tech. Memo. NMFS-TM-12, 1–48.
Reiss, H., Degraer, S., Duineveld, G. C. A., Kröncke, I., Aldridge, J., Craeymeersch, J. A., et al. (2010). Spatial patterns of infauna, epifauna, and demersal fish communities in the North Sea. ICES J. Mar. Sci. 67, 278–293. doi: 10.1093/icesjms/fsp253
Stål, J., Paulsen, S., Pihl, L., Rönnbäck, P., Söderqvist, T., and Wennhage, H. (2008). Coastal habitat support to fish and fisheries in Sweden: Integrating ecosystem functions into fisheries management. Ocean Coast. Manag. 51, 594–600. doi: 10.1016/j.ocecoaman.2008.06.006
Stål, J., Pihl, L., and Wennhage, H. (2007). Food utilisation by coastal fish assemblages in rocky and soft bottoms on the Swedish west coast: Inference for identification of essential fish habitats. Estuar. Coast. Shelf Sci. 71, 593–607. doi: 10.1016/j.ecss.2006.09.008
Staveley, T. A. B., Perry, D., Lindborg, R., and Gullström, M. (2017). Seascape structure and complexity influence temperate seagrass fish assemblage composition. Ecography 40, 936–946. doi: 10.1111/ecog.02745
Tano, S. A., Eggertsen, M., Wikström, S. A., Berkström, C., Buriyo, A. S., and Halling, C. (2017). Tropical seaweed beds as important habitats for juvenile fish. Mar. Freshw. Res. 68, 1921–1934. doi: 10.1071/MF16153
Unsworth, R. K. F., Garrard, S. L., De León, P. S., Cullen-Unsworth, L. C., Smith, D. J., Sloman, K. A., et al. (2009). Structuring of Indo-Pacific fish assemblages along the mangrove-seagrass continuum. Aquat. Biol. 5, 85–95. doi: 10.3354/ab00139
Valentine, J. F., and Heck, K. L. (1999). Seagrass herbivory: evidence for the continued grazing of marine grasses. Mar. Ecol. Prog. Ser. 176, 291–302. doi: 10.3354/meps176291
Wennhage, H., and Pihl, L. (2002). Fish feeding guilds in shallow rocky and soft bottom areas on the Swedish west coast. J. Fish Biol. 61, 207–228. doi: 10.1111/j.1095-8649.2002.tb01772.x
Yeager, L. A., Layman, C. A., and Allgeier, J. E. (2011). Effects of habitat heterogeneity at multiple spatial scales on fish commutity assembly. Oecologia 167, 157–168. doi: 10.1007/s00442-011-1959-3
Keywords: fish assemblages, RUV, seascape nursery, habitat connectivity, marine coastal ecosystem
Citation: Perry D, Staveley TAB and Gullström M (2018) Habitat Connectivity of Fish in Temperate Shallow-Water Seascapes. Front. Mar. Sci. 4:440. doi: 10.3389/fmars.2017.00440
Received: 01 September 2017; Accepted: 22 December 2017;
Published: 18 January 2018.
Edited by:
Alberto Basset, University of Salento, ItalyReviewed by:
Austin T. Humphries, University of Rhode Island, United StatesMario Barletta, Departamento de Oceanografia da Universidade Federal de Pernambuco (UFPE), Brazil
Copyright © 2018 Perry, Staveley and Gullström. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Diana Perry, diana.perry@su.se
 Diana Perry
Diana Perry Thomas A. B. Staveley
Thomas A. B. Staveley Martin Gullström
Martin Gullström