Short-Term Thermal Acclimation Modifies the Metabolic Condition of the Coral Holobiont
- 1Laboratory for Biological Geochemistry, School of Architecture, Civil and Environmental Engineering, École Polytechnique Fédérale de Lausanne, Lausanne, Switzerland
- 2Hawaii Institute of Marine Biology, University of Hawaii, Kaneohe, HI, United States
- 3Department of Biological Sciences, University of Rhode Island, Kingston, RI, United States
- 4327A Leidy Laboratories, Department of Biology, University of Pennsylvania, Philadelphia, PA, United States
- 5Center for Advanced Surface Analysis, Institute of Earth Sciences, University of Lausanne, Lausanne, Switzerland
The nutritional symbiosis between coral hosts and photosynthetic dinoflagellates is fundamental to the functioning of coral reefs. Rising seawater temperatures destabilize this relationship, resulting in drastic declines in world-wide coral cover. Thermal history is thought to play an important role in shaping a coral's response to subsequent thermal stress. Here, we exposed Pocillopora damicornis to two thermal acclimation regimes (ambient vs. warm) and compared the effect that acclimation had on the coral holobiont's response to a subsequent seven day heat stress event. We conducted daily physiological measurements at the holobiont level (gross photosynthesis, respiration, host protein content, symbiont density and chlorophyll content) throughout the heating event, as well as cellular-level imaging of 13C-bicarbonate and 15N-nitrate assimilation (using NanoSIMS) at the end of the heat stress event. Thermal acclimation history had a negligible effect on the measurements conducted at the holobiont level during the heat stress event. No differences were observed in the O2-budget between ambient and warm-acclimated corals and only small fluctuations in host protein, symbiont density and chlorophyll content were detected. In contrast, this lack of differential response, was not mirrored at the cellular level. Warm-acclimated corals had substantially higher 13C enrichment in the host gastrodermis and lipid bodies, but significantly lower 15N-nitrate assimilation in the symbionts and the host tissue layers, relative to the ambient-acclimated corals. We discuss potential reasons for the disconnect that occurred between symbiont bicarbonate and nitrate assimilation (in the absence of photosynthetic breakdown) in the warm-acclimated corals. We suggest this represents either a shift in nitrogen utilization, or supply limitation by the host. Our findings raise several interesting hypotheses regarding the role that nitrogen metabolism plays in thermal stress, which will warrant further investigation if we are to understand the acclimatization capacity of the coral holobiont.
Introduction
The mutualistic relationship that exists between reef-building corals, photosynthetic microalgae (genus: Symbiodinium) and a consortium of microbial partners (the “coral holobiont”; Rosenberg et al., 2007) is critical for the health and persistence of reefs. The symbiotic partners exist in a closely-regulated, yet highly dynamic metabolic landscape, which is fuelled by the reciprocal exchange of nutrients (Wang and Douglas, 1999). The coral host affords Symbiodinium access to inorganic nutrients (CO2, NH3, , ) present in the seawater, as well as metabolic waste products (Muscatine and Porter, 1977; Muller-Parker and D'Elia, 1997), which the Symbiodinium cells recycle to synthesize organic compounds such as sugars, alcohols, amino acids, fatty acids and lipids (Burriesci et al., 2012). Most of the photosynthates produced are then translocated back to the coral or lost via respiration. The metabolic condition of the holobiont is strongly influenced by light (Falkowski et al., 1990), which imposes limitations on the rate of photosynthetic carbon fixation (Wangpraseurt et al., 2016) and nitrogen availability (Rädecker et al., 2015), which plays a central role in controlling the population density of the symbionts (Falkowski et al., 1993). In ambient conditions, Symbiodinium can meet up to 90–100% of the coral's energetic demands (Falkowski et al., 1984; Muscatine, 1990), but in more extreme conditions (such as elevated temperatures and/or high solar irradiance), the symbiont contribution is greatly reduced (Grottoli et al., 2004). Sustained exposures to such conditions lasting weeks to months initiates a predicable cascade of cellular responses (Weis, 2008), which triggers the progressive loss of photosynthetic pigments and/or the expulsion of symbiont cells (Brown, 1997); a process known as coral bleaching. If a coral is deprived of its principle energy source for prolonged periods it will eventually starve and die. The devastating loss of reefs that followed the mass bleaching events of 1998, 2010, and 2016 (Hughes et al., 2017), are testament to the severe consequences that can arise from this symbiotic breakdown.
With ocean temperatures continuing to rise over the coming century (IPCC, 2014), an increase in the frequency and severity of global mass bleaching events is expected (Hoegh-Guldberg et al., 2007). Reef recovery following such events depends on the capacity of coral holobionts' for acclimation and/or adaptation (Weis, 2010; Logan et al., 2014; Van Oppen et al., 2015). Reefs that have been subjected to frequent thermal anomalies and/or highly variable thermal regimes over the past century tend to exhibit higher bleaching thresholds than those exposed to stable conditions suggesting a certain degree of plasticity exists in the coral holobionts' thermal resistance (Carilli et al., 2012). Indeed, some coral species exhibit increased energy reserves (Rodrigues and Grottoli, 2007; Grottoli et al., 2014) and/or changes in the dominant Symbiodinium type after bleaching events (Howells et al., 2012; Boulotte et al., 2016), while others modify their microbial community (Ziegler et al., 2017). A recent analysis of temperature fluctuations recorded over 27 years on the Great Barrier Reef, Australia, found that 75% of all thermal stress events were characterized by temperatures greater than the maximum monthly mean temperature (MMM) but less than the bleaching threshold (MMM+1–2°C). These events were followed by a recovery period lasting 10 days on average, during which temperatures returned to levels below the MMM. Such fluctuations have been termed “protective” because they change the expression of key thermal stress-related genes and thus confer thermal resilience to the coral (Ainsworth et al., 2016). This could be an additional reason why increased resilience has been observed after consecutive bleaching events on some reefs (Maynard et al., 2008; Guest et al., 2012; Penin et al., 2013).
There is some experimental support for the hypotheses outlined above. For example, Acropora aspera acclimated in elevated but sub-lethal temperatures (31°C, 2 days) for 1 or 2 weeks before a simulated bleaching event (34°C, 6 days), retained more of their symbionts and exhibited enhanced photosynthetic efficiency than those exposed to ambient (27°C) conditions prior to the simulated bleaching event (Middlebrook et al., 2008). Similarly, A. millepora that were exposed to elevated temperatures (28°C, 10 days) performed better than corals kept in control conditions when subsequently subjected to a simulated bleaching experience event (31°C, 8 days) (Bellantuono et al., 2011). However, any benefits derived from short-term thermal acclimation (e.g., 33°C, 2 days) were lost if A. millepora was transplanted back into the environment before the bleaching event (Middlebrook et al., 2012), showing that the mechanism(s) involved in thermal acclimation function over much shorter time scales than initially expected. A recent transcriptomic study monitored A. nana in three acclimation temperatures (29°C, 31°C, and variable: 29–33°C), across four time-points (0, 2, 7, and 11 days) and found that 11 days was sufficient to observe significant decreases in the transcription of genes related to rRNA processing, carbohydrate metabolism and the production of heat shock proteins: a finding that suggests that short-term acclimation reduces heat stress-related protein damage (Bay and Palumbi, 2015).
The studies detailed above provide valuable information about the responses of the host and the symbiont to short-term acclimation at sub-lethal temperatures, but no studies to date have addressed how the metabolic condition of the holobiont (in terms of the nutritional interactions between the two partners) is affected. Nanoscale secondary ion mass spectrometry (NanoSIMS) imaging enables the visualization and quantification of the flux of nutrients between Symbiodinium and corals and their utilization in different host compartments at high-spatial resolution (Pernice et al., 2012, 2015; Kopp et al., 2013, 2015). This approach has recently been used to determine how long-term exposure to sub-lethal/sub-bleaching temperatures (29°C, 6 weeks) impacts carbon and nitrate utilization in Northern Red Sea coral Stylophora pistillata (Krueger et al., 2017). These authors found subtle decreases in the amount of carbon (−17%) and nitrate (−6%) that was assimilated by the symbionts and greater decreases in the amount of carbon and nitrate translocated to the host gastrodermis (−31 and −35%, respectively) without visible signs of bleaching, suggesting that this species is able to mount some form of acclimation response. Here we investigated whether these types of sub-cellular changes (which occur during short-term exposure to sub-lethal temperatures) are able to modulate the metabolic condition of the holobiont (specifically the acquisition and allocation of autotrophic carbon and nitrogen) during a subsequent heat stress event. We chose to use Pocillopora damicornis in Kaneohe Bay, Hawaii for this study; a coral living closer to its upper thermal limit (Jokiel and Coles, 1977; Jokiel and Brown, 2004; Bahr et al., 2015) than S. pistillata in the Red Sea, as the response of Northern Red Sea corals to thermal stress is not likely to be representative of most corals because of the strong evolutionary selection they have undergone in the past for heat tolerance and their current location in the cooler parts of the Red Sea (Fine et al., 2013; Krueger et al., 2017; Osman et al., 2017).
Materials and Methods
Coral Collection, Recovery, Acclimation, and Heat Stress Conditions
On the 25th of May 2016, four Pocillopora damicornis colonies were collected from a shallow fringing reef in Kaneohe Bay, Hawaii. Each colony was divided into 40 identical fragments (3 × 2 cm), which were mounted atop plastic plugs using hot glue and placed in a ~1,300 L flow-through common garden tank outdoors with natural solar irradiation. Fragments were left to recover in these tanks for 19 days (from 25th May to 14th of June) in flowing seawater pumped directly from Kaneohe Bay. High flow rates (~6 L min−1) were generated within the tank using recirculating pumps (700 gph Magnetic Drive, Danner Manufacturing Inc. Islandia, NY, USA). Temperature and light conditions were recorded every 15 min using underwater temperature (Hobo Water Temp Pro v2, Onset Computer Corporation, Bourne, MA, USA) and light loggers (Odyssey PAR loggers, Dataflow Systems Ltd, Christchurch, NZ). Temperatures and light fluctuated on natural diurnal cycles. Daily temperatures averaged 26.5 ± 0.4°C but ranged from 25.9 to 27.2°C. Daytime light (between the hours of 06:00 to 18:00) averaged 135 μmol photons m−2 s−1 but ranged from 10 to 564 μmol photons m−2 s−1 (Figure 1C).
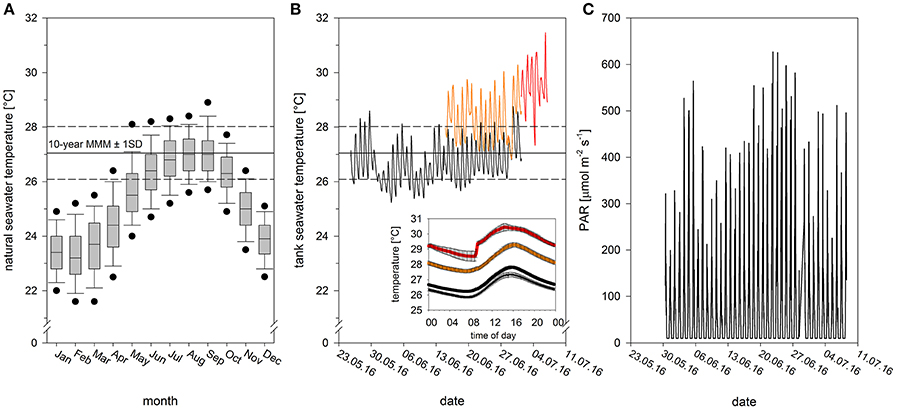
Figure 1. Abiotic conditions during recovery, acclimation and heat stress. (A) Monthly seawater temperature range for a representative shallow coral reef location in Kaneohe Bay between 2006 and 2015 (data derived from automatic weather station of PacIOOS at HIMB, Point Lab, from ~1 m water depth). The maximum monthly mean is reached in September with the current 10-year MMM at 27.05 ± 0.97°C (mean ± SD, 2006–2015, N = 7,200). Black dots in boxplots show the 5th and 95th percentiles. (B) Experimental thermal profile with initial tank acclimation (black thin line), temperature acclimation to ambient (black thick line) and elevated temperature (orange), and heat phase (red). Values for tank and temperature acclimation phase are mean values of two tanks. Graph inset shows daily temperature variation (mean ± SE) during the respective experimental phases (cf. Figure S1 for daily natural variation). (C) Photosynthetically active radiation (PAR) over the course of the experiment, monitored using underwater Odyssey light loggers.
At the end of this recovery period, fragments were evenly distributed between four acclimation tanks (n = 10 fragments per tank, per colony), where they were kept for an additional 2 weeks (from 15th of June to 1st of July). Two tanks were maintained at ambient temperatures (daily average: 26.8 ± 0.6°C, range: 26.4 to 27.5°C, hereafter referred to as “ambient-acclimated corals”), while two were raised to elevated but sub-lethal conditions (daily average: 28.3 ± 0.3°C, range: 27.9 to 28.7°C, hereafter referred to as “warm-acclimated corals”). Desired temperatures were achieved by mixing ambient seawater with seawater pre-heated to 36°C. Manipulating the proportion of ambient to hot water enabled us to adjust temperature without modifying flow rates in the tanks. Daytime light levels during this time averaged 180 μmol photons m−2 s−1, but ranged from 10 to 627 μmol photons m−2 s−1 (Figure 1C).
All fragments were then transferred to a common garden flow-through tank, where they were exposed to a heat stress event for 7 days (from 1st of July to 7th of July). Temperatures during this experimental period averaged ~2.5°C above the current 10-year maximum monthly mean (Figure 1; daily average: 29.6 ± 0.1°C, range: 29.4–28.7°C). By the end of the heat stress event, warm-acclimated corals had experienced cumulative heat stress equivalent to 4.5 degree heating weeks (DHW; Liu et al., 2014), and ambient-acclimated corals had experienced 2.5 DHW (calculations based on daily mean temperatures). The diurnal fluctuation range (ca. 1.5°C in all phases of the experiment), matched the diurnal natural seawater temperature fluctuation (Figure 1B inset; Figure S1). Daytime light levels during the heat stress averaged 128 μmol photons m−2 s−1 but ranged from 10 to 511 μmol photons m−2 s−1 (Figure 1C).
Holobiont Scale Physiological Measurements
One coral fragment from each colony and acclimation treatment was sampled daily and used to measure five physiological parameters: gross photosynthesis (Pgross), respiration (R), host protein content, symbiont density and chlorophyll content. Pgross and R were calculated from the evolution and consumption of oxygen in light and dark conditions, respectively. The measurements were conducted in 250 mL Plexiglas chambers, fitted with specially designed rubber O-ring sealed lids into which temperature and oxygen probes were placed. Each chamber contained identical stands, which held the coral fragment above a stir bar. The chambers were placed in a water bath, maintained at 29.5 ± 1°C by aquarium heaters (WON pro-heat IC heaters, Fredericksburg, Virginia, USA) and the bath was placed atop a magnetic stir plate to prevent seawater stratification in the chamber. Measurements were conducted at the same time each day. Fragments were kept in darkness for 20 min before the lights were turned on and oxygen measurements were started to ensure the fragments had time to recover from any stress incurred from handling. Oxygen evolution was measured under 350 μmol photons m−2 s−1 of light, provided by an actinic white LED system (AI Sol, 72 W 100 to 240 VAC/50–60 Hz, C2 Development Inc., Ames, Iowa). Oxygen measurements were recorded every second using a ten channel fiber-optic optode oxygen meter (Presens, Regensburg, Germany), pre-calibrated with O2-saturated (air-bubbled) and zero O2 (10 mg ml−1 sodium sulfite) seawater. Oxygen evolution was recorded until it was linear, a period that lasted a minimum of 20 min. The lights were then switched off, the chambers covered with aluminum foil and oxygen was recorded until a steady-state of respiration (light enhanced dark respiration, LEDR) was observed. Rates of oxygen flux were adjusted to account for the volume of each fragment (via buoyant weighing using Archimedes' principle) before fragments were snap-frozen in liquid nitrogen and stored at −80°C. Rates of net photosynthesis (Pnet) and R were derived from the number of moles of O2 that were produced or consumed per hour using linear regression controlling for any oxygen flux in the water using rates from blank chambers. R was then subtracted from Pnet to generate Pgross.
Samples were thawed and tissue was brushed from the skeleton in 10 mL of buffer (50 mM KH2PO4/K2HPO4, 0.1 mM EDTA, 10% [v/v] glycerol, pH 7.0, 4°C) using a waterpik. The resulting slurry was homogenized (electric hand-held homogenizer) and centrifuged (5 min, 2,000 × g, 4°C) to form an algae pellet. The host-tissue containing supernatant was decanted and re-centrifuged (10 min, 16,000 × g, 4°C). A 1 mL aliquot of this was snap-frozen and stored at −20°C for subsequent analysis of the host protein content, using the improved Bradford assay (590/450 nm) with bovine serum albumin as the standard (Zor and Selinger, 1996). The algal pellet was re-suspended in 1.1 mL of the aforementioned buffer, 100 μL of which was fixed in 4% formaldehyde for determination of symbiont density, 1 mL of which was re-pelleted (5 min, 4,000 × g, 4°C) and stored at −20°C for subsequent chlorophyll extraction. Chlorophyll content was quantified after extraction in 3 mL of 90% acetone (48 h at 4°C). The absorbance of triplicate aliquots of 200 μL was determined at 663, 630, and 750 nm (to account for seawater turbidity) in a 96-well plate (UV Star, Greiner), using a spectrophotometer (Molecular Devices, Sunnyvale, California, USA). Chlorophyll a and c2 content was calculated using modified equations, specific to dinoflagellates (Jeffrey and Humphrey, 1975), which was corrected for the different path length (0.555 cm; Equations 1 and 2).
Finally, symbiont density was calculated from six cell counts, which were performed using an Improved Neubauer haemocytometer (Boeco, Hamburg, Germany). All physiological measurements are normalized to the surface area of the coral, calculated using the paraffin wax dipping method (Johannes and Wiebe, 1970).
Microscale Physiological Measurements
On the last day of the heat stress event (i.e., day 7), one coral fragment from each colony and acclimation treatment was incubated with stable isotopes of carbon and nitrogen to assess their assimilation efficiency. The stable isotope experiment was conducted in 500 mL glass beakers containing small stir bars, which were placed in the same water bath (and therefore exposed to the same light and temperature conditions) that was used for the photosynthesis and respiration measurements. Isotopically-labeled seawater was prepared immediately before use. Final concentrations of 2 mM NaH13CO3 and 3 μM K15NO3 (both 98% atom, Sigma-Aldrich, Germany) were achieved by acidifying the seawater with a defined amount of 1 M HCl (pH < 3), shifting the carbon equilibrium to >99% dissolved CO2, before bubbling with air for 2 h (DIC stripping), and subsequently spiking the water with 2 mM NaH13CO3 before returning the pH to normal values (pH 8) using a defined amount of 1 M NaOH. Fragments were incubated in the isotopically labeled seawater for 6 h. After, samples were preserved in fixative 2.5% [v/v] glutaraldehyde, 0.5% [v/v] formaldehyde in 0.22 μm filtered Sörensen-sucrose phosphate buffer (0.1 M phosphate at pH 7.5, 0.6 M sucrose) for 2 h at room temperature followed by 22 h at 4°C. Fragments were decalcified (0.5 M EDTA) for 3 days at room temperature before being returned to Sörensen phosphate buffer for storage. Samples were later transferred to the Laboratory for Biological Geochemistry (EPFL, Switzerland) under CITES permit 16US98467B, where they were micro-dissected into segments of connective coenosarc tissue using a binocular microscope. Post-fixation lasted for 1 h in 1% osmium tetroxide in distilled water before samples were dehydrated and embedded in Spurr's resin (Kopp et al., 2015). Semi-thin sections (500 nm) were cut using an Ultracut S microtome (Leica Microsystems, Germany), equipped with a 45° diamond knife (Diatome, Hatfield, PA, USA). Sections were then gold-coated and imaged using a NanoSIMS 50 L ion microprobe. NanoSIMS images (40 × 40 μm, 256 × 256 pixels, 5 ms/pixel dwell time, 5 layers) were obtained with a 16 keV Cs+ primary ion beam, which was focused to a spot size of ca. 150 nm. Secondary ions (12, 13C12C−, 12C14N−, and 12C15N− were counted in individual electron-multiplier detectors at a mass resolution power of about 9000 (Cameca definition), sufficient to resolve all potential interferences. A minimum of 30 symbiont cells and their surrounding tissue were imaged from each sample (10–20 images per sample). In most cases, this required multiple sections to be imaged. When this arose, sections were taken a minimum of 20 μm apart in the original tissue to ensure imaged symbiont cells were independent replicates. Analysis was conducted using the NanoSIMS software, L'IMAGE (Dr. Larry Nittler, Carnegie Institution of Washington). Drift-corrected maps of 13C- and 15N-enrichment were obtained from the ratios of 13C12C− to 12 and 15N12C− to 14N12C−, respectively. Regions of interest (ROI) were then hand-drawn around Symbiodinium cells, host gastrodermis (excluding symbionts), host epidermis, and host lipid bodies in each image.
Statistical Analysis
Holobiont scale physiological measurements (Pgross, R, host protein content, symbiont density and total chlorophyll content per cell) were analyzed by a restricted maximum-likelihood (REML) linear mixed model with acclimation temperature (ambient or warm) and time (days 1, 2, 4, 5, 6, or 7) as fixed factors. Colony was included as a random factor to assess how much variance in the data was explained by the source of each fragment (variance components analysis). A minimal model approach was employed where the interaction term between acclimation and time was removed from the model when it was non-significant. When terms were found to be significant, least square means contrasts were performed to identify where the differences lay.
The microscale physiological measurements were analyzed using a repeated measures analysis of variance (rmANOVA), using acclimation temperature (ambient or warm) and colony (matched pairs) as factors. Carbon and nitrogen enrichment were tested separately for each region of interest in the oral layer of the coenosarc coral tissue (symbiont cells, gastrodermis, host lipid bodies and epidermis). This approach enabled multiple ROI data points to be contained within each replicate, rather than being treated as independent data points.
All dependent variables were tested for normal distribution using the Shapiro–Wilks test and were transformed to achieve normality if required and all statistical analyses were performed using the software JMP (SAS Institute, Cary, NC, USA).
Results
All colonies from both treatments survived the heat stress event. Acclimation temperature did not significantly affect either oxygen production or consumption and no significant changes were observed throughout the heat stress event (Table S1, Figure 2A). Acclimation temperature did have a significant effect on host protein content; warm-acclimated corals contained 22% more protein on average than the ambient-acclimated corals (Figure 2B). Host protein also varied significantly across the heat stress event, being highest on days 5 and 6. Symbiont density significantly differed between the two acclimation temperatures; warm-acclimated corals contained 3% fewer symbionts than ambient-acclimated corals. Symbiont density also differed across the heat stress event (Table S1), although the only significant difference occurred on days 1 and 2; corals on day 2 containing 4% less symbionts on average, than corals on day 1 (Figure 2C). In contrast, total chlorophyll content per symbiont was lowest on day 1 of the heat stress event and showed no relationship to the acclimation temperature (Table S1; Figure 2D).
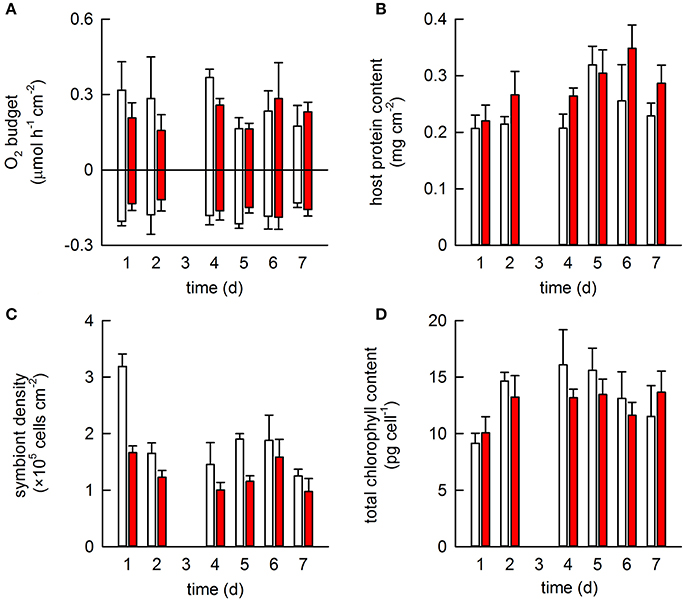
Figure 2. The effect of acclimation temperature on the physiological response of Pocillopora damicornis exposed to heat stress. Four coral colonies were exposed to two different acclimation temperatures for 2 weeks: ambient (26.8°C; white) and warm (28.2°C; red) in a paired design. All were then exposed to heat stress (29.5°C) for 1 week. One fragment from each colony and each acclimation treatment was sampled daily (except for day 3, when data was lost) and used to monitor a suite of physiological measurements: (A) gross photosynthesis and respiration; (B) host protein content; (C) symbiont density and (D) chlorophyll content per symbiont cell. Bars represent mean ± SE (N = 4).
The final measurements at the end of the heat stress event showed there was no statistical effect of acclimation temperature on the density, total chlorophyll content, or oxygen productivity of the symbiont population in the tested colonies (Figure 3A). However, three of the four warm-acclimated colonies showed a consistent trend, characterized by a reduction in symbiont density, but increased chlorophyll pigmentation per cell and enhanced O2-production (Figure 3A). Notably, the between colony variance in the specific Chl a:Chl c2 ratio was much smaller in warm-acclimated corals.
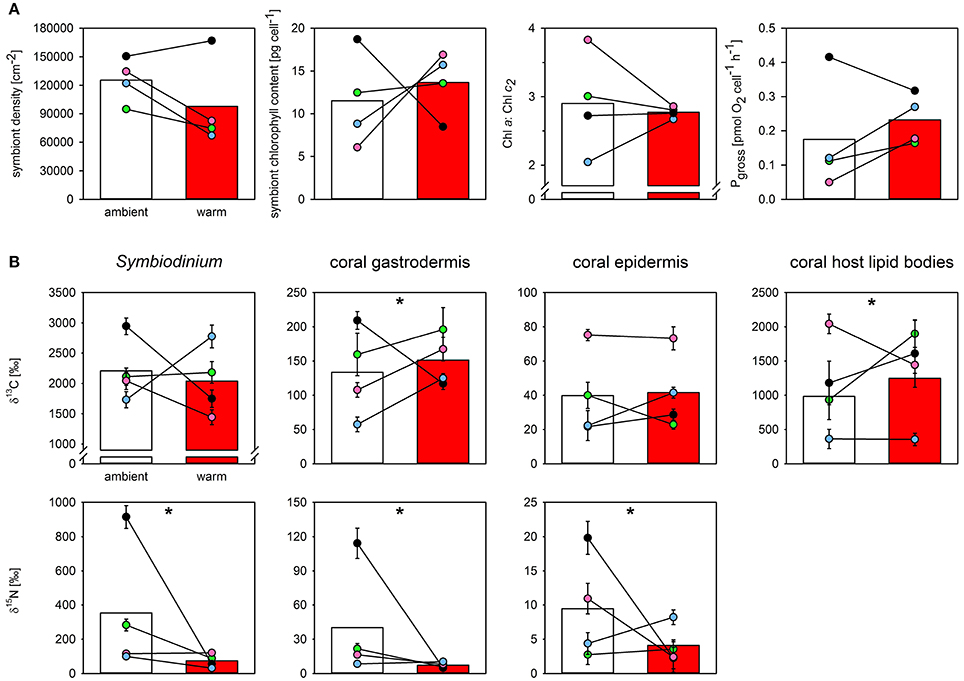
Figure 3. The effect of acclimation temperature on the physiological function and the carbon and nitrogen assimilation of Pocillopora damicornis at the end of 7-days of heat stress. Shown are the effect of acclimation temperature on (A) final symbiont density, symbiont chlorophyll content, Chl a:Chl c2 ratio, and symbiont oxygen production (from left to right), and (B) carbon and nitrate assimilation (top and bottom row, respectively) in different compartments of the oral coenosarc tissue, as assessed by NanoSIMS. Bars represent mean response of ambient- (white) and warm-acclimated (red) corals with the individual colony performance indicated as colored dots (paired design; blue, green, magenta, black for colonies 1–4, respectively). NanoSIMS data represent means ± SE of imaged symbionts (N = 28–38 per sample), surrounding gastrodermal and epidermal area (N = 11–17), and host lipid bodies contained within the imaged gastrodermal area (N = 5–45). Nitrogen enrichment in lipid bodies was below detection limit in both treatments. Significant effects of thermal acclimation on mean response are indicated by asterisks. Note that thermal acclimation effects were significantly different between individual colonies (cf. Table 2).
At the cellular scale, acclimation temperature had no consistent effect on the carbon assimilation in P. damicornis, which was highly variable between colonies (Table 1, Figure 3B). The level of 13C-carbon enrichment in the symbionts significantly varied between colonies; ranging from 1,700 to 3,000. Significant effects of acclimation temperature were only observed for 13C-enrichment in the gastrodermis and host lipid bodies, but all regions of interest had significant interactions between acclimation temperature and colony (Table 1). In three out of the four colonies, warm-acclimation resulted in greater enrichment of 13C in the gastrodermis (+22–117%) and similar between-colony variation was evident in the amount of 13C assimilated into host lipids (Table S2).
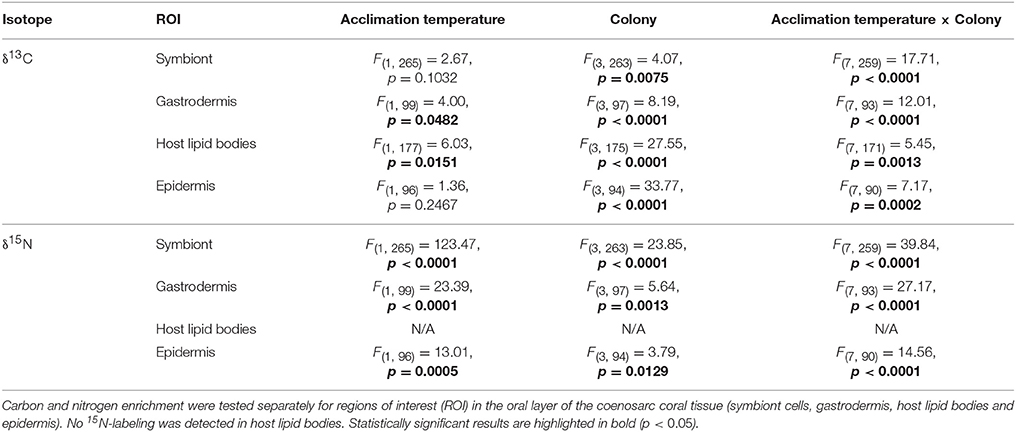
Table 1. The effect of acclimation temperature on carbon and nitrogen assimilation rates in Pocillopora damicornis at the end of a 7-day heat stress event. Statistical output of rmANOVA (n = 4), using acclimation temperature (ambient or warm) and colony (matched pairs) as factors.
In contrast to carbon metabolism, acclimation temperature had a strong impact on 15N-assimilation in symbiont cells and the enrichment in gastrodermis and epidermis (Table 1; Figure 3B). In general, warm-acclimation decreased 15N-assimilation, although again, the magnitude of the reduction was colony-specific (Table 1; Figure 3B). Symbionts in three out of the four warm-acclimated colonies assimilated less 15N (−69 to −94%) than ambient-acclimated corals, yet those in one colony were unaffected. Similar intra-colony variability was observed in assimilation in the coral gastrodermis: three of the warm-acclimated colonies assimilated less 15N (−51 to −96%), while one, incorporated more (+23 %). Finally, two warm-acclimated colonies exhibited enhanced 15N-enrichment in their epidermis (+86 and 33%), relative to ambient-acclimated corals, while two incorporated less (−78 and −89%). 15N-assimilation into host lipid bodies was not detectable for either acclimation temperature.
Discussion
Short-term temperature anomalies have been shown in some instances to modify a coral's response to subsequent heat events (Middlebrook et al., 2008; Bellantuono et al., 2011). The coral species that we used in this study, Pocillopora damicornis, lives close to its thermal maximum in Kaneohe Bay, Hawaii and is usually among the most bleaching-susceptible species in the region (Jokiel and Brown, 2004; Bahr et al., 2015). Comparison of our values with those reported in previous studies, revealed that our photosynthesis and respiration rates and the symbiont densities were substantially less for Pgross and R; (Lesser et al., 1994) and symbiont density (Stimson and Kinzie, 1991; Wall et al., 2017); than usual for P. damicornis, yet we observed no visible signs of bleaching. Acclimation temperature had little effect on final holobiont level measurements: with no detectable differences between the O2-budget (both Pgross and R) of ambient and warm-acclimated corals, and negligible differences in symbiont density and total chlorophyll content per symbiont cell. In fact, the only holobiont level variable that differed was host protein, which was 22% higher in warm-acclimated corals (which could represent a change in either tissue thickness and/or protein content per cell). The lack of observed differences is likely a reflection of the heat and light conditions that the corals had experienced. By the end of the 7-day heat stress event, ambient corals had experienced 2.5 DHW and warm-acclimated corals had experienced 4.5 DHW (conditions classified as “likely” to cause bleaching by NOAA), yet the light levels during the experiment were relatively low (arising from an abnormal period of cloudy weather; Figure 1C). Since temperature and light are synergistic stressors of coral bleaching, it is possible that the low light conditions that prevailed during the experiment alleviated the impact of the 4.5 DHW treatment to some extent (Skirving et al., 2018). While unexpected, such conditions emphasized the impact of heat stress on coral holobiont metabolism, without the interference of excessive light.
Temperature acclimation did not affect carbon incorporation in the symbionts, but it did increase carbon assimilation in the host gastrodermis (+15%) and the host lipid bodies (+13%), of warm-acclimated corals. There are two explanations for this finding: (i) symbionts in warm-acclimated corals are more efficient at fixing carbon, but retain the same amount of carbon for themselves as the symbionts in ambient-acclimated corals, allowing excess carbon to be translocated to their coral host and invested in tissue production or energy storage in lipid bodies; or (ii) the same amount of carbon is translocated to the host, but warm-acclimated corals allocate more to storage and/or have a higher general turnover of carbon in their host tissue reserves (note that soluble compounds are lost in conventional NanoSIMS sample preparation). Since host protein content increased in warm-acclimated corals in the absence of changes in respiration rates we favor the first hypothesis, although this would require a higher efficiency of carbon capture or enhanced electron cycling because the activity of photosystem II (Pgross) was unchanged.
The most striking finding of this study is the effect that acclimation had on nitrogen metabolism. Warm-acclimation reduced nitrogen incorporation from external seawater nitrate on average by 79% in the symbionts, 81% in the gastrodermis, and 55% in the epidermis. Significant reductions in nitrate uptake at elevated temperatures have been observed in Stylophora pistillata (Godinot et al., 2011) and P. damicornis (Ezzat et al., 2016) but, in both cases the decreases were linked to a decline in photosynthesis. Here, the relative reduction in nitrate uptake in warm-acclimated corals observed at the end of the heat stress event is independent of the photosynthetic performance of the symbionts (i.e., O2-production), suggesting a general impact on nitrate supply to the symbiont and/or specific thermal impact on the enzymes involved in nitrate reduction.
Dissolved inorganic nitrogen (DIN) can be acquired in many forms by the coral holobiont. Measurements for S. pistillata show the majority is acquired as ammonia (: 42%), but that nitrate (: 34%), dissolved free amino acid (DFAA: 21%) and urea (3%) can all be sources of nitrogen for corals (Grover et al., 2008). It is possible that decreases in nitrate assimilation and translocation represent a switch to ammonia rather than a reduction in the overall amount of nitrogen fixed. Symbiodinium is known to favor ammonia over nitrate if both are present. The concentration of ambient ammonia also influences nitrate uptake (D'Elia et al., 1983; Domotor and D'elia, 1984; Wilkerson and Trench, 1985; Taguchi and Kinzie, 2001). In the intact symbiosis, 3–4 μM of ammonia is sufficient to completely inhibit nitrate uptake in S. pistillata (Grover et al., 2003). Ammonia concentrations in Kaneohe Bay typically range between 0.6 and 0.8 μM (Drupp et al., 2011; Silbiger et al., 2014), so it seems unlikely that ambient ammonia concentrations induce a substantial shift toward ammonia assimilation. Temperature-dependent nitrate incorporation has been observed in Acropora tenuis, but these differences were caused by the genetic identity of the symbiont clade that they hosted (Baker et al., 2013). We did not genotype the symbionts in our study since P. damicornis in Hawaii consistently and almost exclusively hosts Symbiodinium ITS2 type C1 (Lajeunesse et al., 2004; Magalon et al., 2007). The endodermal location of the symbionts means that DIN acquired from seawater present in the coelenteron must cross a minimum of two host-derived membranes (the plasma membrane and the symbiosome) before it reaches the symbionts, and so it stands to reason that the symbionts' capacity to fix nitrate depends on the uptake capabilities of both the host and the symbiont (Grover et al., 2003). It is possible that the reductions in 15 assimilation and translocation that we observed in the warm-acclimated corals simply reflect changes in the ability of the host to supply nitrate to the symbionts. Nothing is known about how the host regulates the supply of nitrate, nor its response to thermal stress. Previous studies that monitored the gene expression of nitrate-transporter 2 (nrt2) in Symbiodinium C under different thermal regimes and in different host species found that temperature had little impact on nrt2 expression (Mayfield et al., 2013a,b, 2014), but no studies have examined the activity of nitrate reductase of in hospite Symbiodinium under thermal stress.
The disconnect that we observed between carbon and nitrate assimilation in the warm-acclimated corals (in the absence of holobiont level changes) can be viewed from two perspectives: either this is a mechanistic response to delay bleaching by the coral host, or the warm-acclimated corals are simply one step closer to their bleaching threshold than their ambient-acclimated counterparts. The first hypothesis relies on the reduction in acquisition being caused by limited host supply and not being a direct negative effect on the ability of the symbiont to fix nitrate. If a limited host supply is indeed the case, then the stable symbiont O2-production, reduced nitrate acquisition and enhanced carbon delivery to the host could feasibly represent a host response to limit nitrogen supply to the symbiont population. A number of studies have shown that nitrogen deprivation can enhance lipid formation and modify the amount of carbon outflow from Symbiodinium (Muller-Parker et al., 1996; Zhu et al., 2011; Weng et al., 2014; Pasaribu et al., 2016; Suescún-Bolívar et al., 2016). Such a mechanism could potentially boost the hosts long-term energy reserves (Grottoli et al., 2004, 2014; Rodrigues and Grottoli, 2007), while reducing symbiont population growth. If on the other hand, warm-acclimated corals are simply closer to their bleaching threshold than ambient-acclimated corals, then it is possible that the reduced nitrate assimilation we observed in warm-acclimated corals represents a heightened state of nitrogen-limitation in the holobiont. Changes in nitrogen assimilation are thought to be critical in determining the transition to a carbon-limited state and thus determining the onset of bleaching (Cunning et al., 2017). Since our corals did not progress into the bleaching phase, we cannot conclude which hypothesis is more likely. However, the consistent response of three of the four colonies showing a reduction in symbiont density but an increase in Pgross and gastrodermal 13C-enrichment offers support for a host mechanism, although further investigation is warranted.
All considered, our NanoSIMS data provides a pre-bleaching snap shot of the differences in metabolic condition of differentially-acclimated coral holobionts, exposed to different degrees of sub-lethal thermal stress. We show that sub-lethal temperatures can have a substantial impact on nutrient metabolism and therefore modify the energetic basis of the symbiotic association. It is possible that the modifications play an important role in delaying the onset of coral bleaching. It is equally possible that the changes we observed reflect the transition phase that occurs just before the onset of bleaching. Either way, our study opens many interesting avenues for future research. One particular question requiring attention is the role of nitrogen metabolism under thermal stress.
Authors Contributions
EG, TK, and HP designed the experiment. EG, TK, HP, and KB conducted the experiment. EG, TK, and JB analyzed the samples. EG and TK performed data analysis. EG produced the first draft of the manuscript, and all authors contributed to the final version.
Funding
This work was funded by a Swiss National Science Foundation (grant no. CR2312-141048) and the Weizmann-EPFL Collaboration Program (grant no. 721236), both awarded to AM. EG was supported by an EPFL-Marie Skłodowska-Curie Post-doctoral Fellowship (grant no. 665667) and HP was supported by funding from a National Science Foundation Ocean Sciences Post-doctoral Fellowship (grant no. 1323822).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Jen Davidson and Elizabeth Lenz from the Hawai'i Institute of Marine Biology (HIMB) for help with coral maintenance and assistance with the experimental setup. The Electron Microscopy Facility (EMF) at the University of Lausanne is acknowledged for access and help with sample preparation for NanoSIMS. This work was supported by a Swiss National Science Foundation grant no. CR2312-141048 to AM.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2018.00010/full#supplementary-material
Figure S1. Daily temperature variation (mean ± 95%CI) over the annual cycle for a representative shallow reef location in Kaneohe Bay between 2006 and 2015 (data derived from automatic weather station of PacIOOS at HIMB, Point Lab, from ~1 m water depth). Solid line indicates 10-year MMM ± 1SD.
Table S1. The effect of acclimation on the global scale physiological response of Pocillopora damicornis to a simulated bleaching event. Dependent variables were analyzed by a restricted maximum-likelihood (REML) linear mixed model with acclimation and time as fixed factors. Colony was included as a random factor to assess how much of the variance in the data was explained by the source colony that each fragment originated from (variance components analysis). A minimal model approach was employed whereby acclimation and time were initially crossed to assess their combined effect, but removed if their interaction was non-significant (–).Degrees of freedom (d.f.), F-ratios (F) and probability levels (P) are provided and significant effects (P < 0.05) are in bold.
Table S2. Summary of enrichment values for carbon and nitrogen from NanoSIMS image analysis. Regions of interest (ROI) were based on coenosarc cross sections and refer to the oral tissue layer. Gastrodermis ROIs refer to gastrodermis excluding symbionts, but including host lipids.
References
Ainsworth, T. D., Heron, S. F., Ortiz, J. C., Mumby, P. J., Grech, A., Ogawa, D., et al. (2016). Climate change disables coral bleaching protection on the Great Barrier Reef. Science 352, 338–342. doi: 10.1126/science.aac7125
Bahr, K. D., Jokiel, P. L., and Rodgers, K. S. (2015). The 2014 coral bleaching and freshwater flood events in Kānéohe Bay, Hawaíi. PeerJ 3:e1136. doi: 10.7717/peerj.1136
Baker, D. M., Andras, J. P., Jordán-Garza, A. G., and Fogel, M. L. (2013). Nitrate competition in a coral symbiosis varies with temperature among Symbiodinium clades. ISME J. 7, 1248–1251. doi: 10.1038/ismej.2013.12
Bay, R. A., and Palumbi, S. R. (2015). Rapid acclimation ability mediated by transcriptome changes in reef-building corals. Genome Biol. Evol. 7, 1602–1612. doi: 10.1093/gbe/evv085
Bellantuono, A. J., Hoegh-Guldberg, O., and Rodriguez-Lanetty, M. (2011). Resistance to thermal stress in corals without changes in symbiont composition. Proc. Biol. Sci. 279, 1100–1107. doi: 10.1098/rspb.2011.1780
Boulotte, N. M., Dalton, S. J., Carroll, A. G., Harrison, P. L., Putnam, H. M., Peplow, L. M., et al. (2016). Exploring the Symbiodinium rare biosphere provides evidence for symbiont switching in reef-building corals. ISME J. 10, 2693–2701. doi: 10.1038/ismej.2016.54
Brown, B. E. (1997). Coral bleaching: causes and consequences. Coral Reefs 16, S129–S138. doi: 10.1007/s003380050249
Burriesci, M. S., Raab, T. K., and Pringle, J. R. (2012). Evidence that glucose is the major transferred metabolite in dinoflagellate–cnidarian symbiosis. J. Exp. Biol. 215, 3467–3477. doi: 10.1242/jeb.070946
Carilli, J., Donner, S. D., and Hartmann, A. C. (2012). Historical temperature variability affects coral response to heat stress. PLoS ONE 7:e34418. doi: 10.1371/journal.pone.0034418
Cunning, R., Muller, E. B., Gates, R. D., and Nisbet, R. M. (2017). A dynamic bioenergetic model for coral-symbiodinium symbioses and coral bleaching as an alternate stable state. J. Theor. Biol. 431, 49–62. doi: 10.1016/j.jtbi.2017.08.003
D'Elia, C., Domotor, S., and Webb, K. (1983). Nutrient uptake kinetics of freshly isolated zooxanthellae. Mar. Biol. 75, 157–167. doi: 10.1007/BF00405998
Domotor, S., and D'elia, C. (1984). Nutrient uptake kinetics and growth of zooxanthellae maintained in laboratory culture. Mar. Biol. 80, 93–101. doi: 10.1007/BF00393132
Drupp, P., De Carlo, E. H., Mackenzie, F. T., Bienfang, P., and Sabine, C. L. (2011). Nutrient inputs, phytoplankton response, and CO2 variations in a semi-enclosed subtropical embayment, Kaneohe Bay, Hawaii. Aquat. Geochem. 17, 473–498. doi: 10.1007/s10498-010-9115-y
Ezzat, L., Maguer, J.-F., Grover, R., and Ferrier-Pagès, C. (2016). Limited phosphorus availability is the Achilles heel of tropical reef corals in a warming ocean. Sci. Rep. 6:31768. doi: 10.1038/srep31768
Falkowski, P. G., Dubinsky, Z., Muscatine, L., and Mccloskey, L. (1993). Population control in symbiotic corals. Bioscience 43, 606–611. doi: 10.2307/1312147
Falkowski, P. G., Dubinsky, Z., Muscatine, L., and Porter, J. W. (1984). Light and the bioenergetics of a symbiotic coral. Bioscience 34, 705–709. doi: 10.2307/1309663
Falkowski, P. G., Jokiel, P. L., and Kinzie, R. (1990). Irradiance and corals. Ecosyst. world 25, 89–107.
Fine, M., Gildor, H., and Genin, A. (2013). A coral reef refuge in the Red Sea. Glob. Chang. Biol. 19, 3640–3647. doi: 10.1111/gcb.12356
Godinot, C., Houlbrèque, F., Grover, R., and Ferrier-Pagès, C. (2011). Coral uptake of inorganic phosphorus and nitrogen negatively affected by simultaneous changes in temperature and pH. PLoS ONE 6:e25024. doi: 10.1371/journal.pone.0025024
Grottoli, A. G., Warner, M. E., Levas, S. J., Aschaffenburg, M. D., Schoepf, V., Mcginley, M., et al. (2014). The cumulative impact of annual coral bleaching can turn some coral species winners into losers. Glob. Chang. Biol. 20, 3823–3833. doi: 10.1111/gcb.12658
Grottoli, A., Rodrigues, L., and Juarez, C. (2004). Lipids and stable carbon isotopes in two species of Hawaiian corals, Porites compressa and Montipora verrucosa, following a bleaching event. Mar. Biol. 145, 621–631. doi: 10.1007/s00227-004-1337-3
Grover, R., Maguer, J.-F., Allemand, D., and Ferrier-Pages, C. (2003). Nitrate uptake in the scleractinian coral Stylophora pistillata. Limnol. Oceanogr. 48, 2266–2274. doi: 10.4319/lo.2003.48.6.2266
Grover, R., Maguer, J.-F., Allemand, D., and Ferrier-Pagès, C. (2008). Uptake of dissolved free amino acids by the scleractinian coral Stylophora pistillata. J. Exp. Biol. 211, 860–865. doi: 10.1242/jeb.012807
Guest, J. R., Baird, A. H., Maynard, J. A., Muttaqin, E., Edwards, A. J., Campbell, S. J., et al. (2012). Contrasting patterns of coral bleaching susceptibility in 2010 suggest an adaptive response to thermal stress. PLoS ONE 7:e33353. doi: 10.1371/journal.pone.0033353
Hoegh-Guldberg, O., Mumby, P. J., Hooten, A. J., Steneck, R. S., Greenfield, P., Gomez, E., et al. (2007). Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742. doi: 10.1126/science.1152509
Howells, E., Beltran, V., Larsen, N., Bay, L., Willis, B., and Van Oppen, M. (2012). Coral thermal tolerance shaped by local adaptation of photosymbionts. Nat. Clim. Chang. 2, 116–120. doi: 10.1038/nclimate1330
Hughes, T. P., Kerry, J. T., Álvarez-Noriega, M., Álvarez-Romero, J. G., Anderson, K. D., Baird, A. H., et al. (2017). Global warming and recurrent mass bleaching of corals. Nature 543, 373–377. doi: 10.1038/nature21707
IPCC (2014). Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Geneva: IPCC, 151.
Jeffrey, S. T., and Humphrey, G. (1975). New spectrophotometric equations for determining chlorophylls a, b, c 1 and c 2 in higher plants, algae and natural phytoplankton. Biochem. Physiol. der Pflanzen 167, 191–194. doi: 10.1016/S0015-3796(17)30778-3
Johannes, R. E., and Wiebe, W. J. (1970). Method for determination of coral tissue biomass and composition. Limnol. Oceanogr. 15, 822–824. doi: 10.4319/lo.1970.15.5.0822
Jokiel, P., and Coles, S. (1977). Effects of temperature on the mortality and growth of Hawaiian reef corals. Mar. Biol. 43, 201–208. doi: 10.1007/BF00402312
Jokiel, P. L., and Brown, E. K. (2004). Global warming, regional trends and inshore environmental conditions influence coral bleaching in Hawaii. Glob. Chang. Biol. 10, 1627–1641. doi: 10.1111/j.1365-2486.2004.00836.x
Kopp, C., Domart-Coulon, I., Escrig, S., Humbel, B. M., Hignette, M., and Meibom, A. (2015). Subcellular investigation of photosynthesis-driven carbon assimilation in the symbiotic reef coral Pocillopora damicornis. MBio 6, e02299–e02214. doi: 10.1128/mBio.02299-14
Kopp, C., Pernice, M., Domart-Coulon, I., Djediat, C., Spangenberg, J., Alexander, D., et al. (2013). Highly dynamic cellular-level response of symbiotic coral to a sudden increase in environmental nitrogen. MBio 4, e00052–e00013. doi: 10.1128/mBio.00052-13
Krueger, T., Horwitz, N., Bodin, J., Giovani, M.-E., Escrig, S., Meibom, A., et al. (2017). Common reef-building coral in the Northern Red Sea resistant to elevated temperature and acidification. R. Soc. Open Sci. 4:170038. doi: 10.1098/rsos.170038
Lajeunesse, T. C., Thornhill, D. J., Cox, E. F., Stanton, F. G., Fitt, W. K., and Schmidt, G. W. (2004). High diversity and host specificity observed among symbiotic dinoflagellates in reef coral communities from Hawaii. Coral Reefs 23, 596–603. doi: 10.1007/s00338-004-0428-4
Lesser, M. P., Weis, V. M., Patterson, M. R., and Jokiel, P. L. (1994). Effects of morphology and water motion on carbon delivery and productivity in the reef coral, Pocillopora damicornis (Linnaeus): diffusion barriers, inorganic carbon limitation, and biochemical plasticity. J. Exp. Mar. Biol. Ecol. 178, 153–179. doi: 10.1016/0022-0981(94)90034-5
Liu, G., Heron, S. F., Eakin, C. M., Muller-Karger, F. E., Vega-Rodriguez, M., Guild, L. S., et al. (2014). Reef-scale thermal stress monitoring of coral ecosystems: new 5-km global products from NOAA Coral Reef Watch. Remote Sens. 6, 11579–11606. doi: 10.3390/rs61111579
Logan, C. A., Dunne, J. P., Eakin, C. M., and Donner, S. D. (2014). Incorporating adaptive responses into future projections of coral bleaching. Glob. Chang. Biol. 20, 125–139. doi: 10.1111/gcb.12390
Magalon, H., Flot, J.-F., and Baudry, E. (2007). Molecular identification of symbiotic dinoflagellates in Pacific corals in the genus Pocillopora. Coral Reefs 26, 551–558. doi: 10.1007/s00338-007-0215-0
Mayfield, A. B., Chen, M.-N., Meng, P.-J., Lin, H.-J., Chen, C.-S., and Liu, P.-J. (2013a). The physiological response of the reef coral Pocillopora damicornis to elevated temperature: results from coral reef mesocosm experiments in Southern Taiwan. Mar. Environ. Res. 86, 1–11. doi: 10.1016/j.marenvres.2013.01.004
Mayfield, A. B., Fan, T.-Y., and Chen, C.-S. (2013b). Physiological acclimation to elevated temperature in a reef-building coral from an upwelling environment. Coral Reefs 32, 909–921. doi: 10.1007/s00338-013-1067-4
Mayfield, A. B., Wang, Y. B., Chen, C. S., Lin, C. Y., and Chen, S. H. (2014). Compartment-specific transcriptomics in a reef-building coral exposed to elevated temperatures. Mol. Ecol. 23, 5816–5830. doi: 10.1111/mec.12982
Maynard, J., Anthony, K., Marshall, P., and Masiri, I. (2008). Major bleaching events can lead to increased thermal tolerance in corals. Mar. Biol. 155, 173–182. doi: 10.1007/s00227-008-1015-y
Middlebrook, R., Anthony, K. R., Hoegh-Guldberg, O., and Dove, S. (2012). Thermal priming affects symbiont photosynthesis but does not alter bleaching susceptibility in Acropora millepora. J. Exp. Mar. Biol. Ecol. 432, 64–72. doi: 10.1016/j.jembe.2012.07.005
Middlebrook, R., Hoegh-Guldberg, O., and Leggat, W. (2008). The effect of thermal history on the susceptibility of reef-building corals to thermal stress. J. Exp. Biol. 211, 1050–1056. doi: 10.1242/jeb.013284
Muller-Parker, G., Lee, K. W., and Cook, C. B. (1996). Changes in the ultrastructure of symbiotic zooxanthellae (Symbiodinium sp., Dinophyceae) in fed and starved sea anemones maintained under high and low light. J. Phycol. 32, 987–994. doi: 10.1111/j.0022-3646.1996.00987.x
Muller-Parker, G., and D'Elia, C. (1997). Interactions between Corals and Their Symbiotic Algae. 96-113. Life and Death of Coral Reefs. Nueva York, NY: Chapman and Hall.
Muscatine, L. (1990). “The role of symbiotic algae in carbon and energy flux in coral reefs,” in Ecosystems of the World 25. Coral Reefs, ed Z. Dubinsky (Amsterdam: Elsevier Science Publishing Company, Inc.), 75–87.
Muscatine, L., and Porter, J. W. (1977). Reef corals: mutualistic symbioses adapted to nutrient-poor environments. Bioscience 27, 454–460. doi: 10.2307/1297526
Osman, E. O., Smith, D. J., Ziegler, M., Kürten, B., Conrad, C., El-Haddad, K. M., et al. (2017). Thermal refugia against coral bleaching throughout the northern Red Sea. Glob. Chang. Biol. doi: 10.1111/gcb.13895. [Epub ahead of print].
Pasaribu, B., Li, Y.-S., Kuo, P.-C., Lin, I.-P., Tew, K. S., Tzen, J. T., et al. (2016). The effect of temperature and nitrogen deprivation on cell morphology and physiology of Symbiodinium. Oceanologia 58, 272–278. doi: 10.1016/j.oceano.2016.04.006
Penin, L., Vidal-Dupiol, J., and Adjeroud, M. (2013). Response of coral assemblages to thermal stress: are bleaching intensity and spatial patterns consistent between events? Environ. Monit. Assess. 185, 5031–5042. doi: 10.1007/s10661-012-2923-3
Pernice, M., Dunn, S. R., Tonk, L., Dove, S., Domart-Coulon, I., Hoppe, P., et al. (2015). A nanoscale secondary ion mass spectrometry study of dinoflagellate functional diversity in reef-building corals. Environ. Microbiol. 17, 3570–3580. doi: 10.1111/1462-2920.12518
Pernice, M., Meibom, A., Van Den Heuvel, A., Kopp, C., Domart-Coulon, I., Hoegh-Guldberg, O., et al. (2012). A single-cell view of ammonium assimilation in coral–dinoflagellate symbiosis. ISME J. 6, 1314–1324. doi: 10.1038/ismej.2011.196
Rädecker, N., Pogoreutz, C., Voolstra, C. R., Wiedenmann, J., and Wild, C. (2015). Nitrogen cycling in corals: the key to understanding holobiont functioning? Trends Microbiol. 23, 490–497. doi: 10.1016/j.tim.2015.03.008
Rodrigues, L. J., and Grottoli, A. G. (2007). Energy reserves and metabolism as indicators of coral recovery from bleaching. Limnol. Oceanogr. 52, 1874–1882. doi: 10.4319/lo.2007.52.5.1874
Rosenberg, E., Koren, O., Reshef, L., Efrony, R., and Zilber-Rosenberg, I. (2007). The role of microorganisms in coral health, disease and evolution. Nat. Rev. Microbiol. 5, 355–362. doi: 10.1038/nrmicro1635
Silbiger, N. J., Guadayol, Ò., Thomas, F. I., and Donahue, M. J. (2014). Reefs shift from net accretion to net erosion along a natural environmental gradient. Mar. Ecol. Prog. Ser. 515, 33–44. doi: 10.3354/meps10999
Skirving, W., Enríquez, S., Hedley, J. D., Dove, S., Eakin, C. M., Mason, R. A., et al. (2018). Remote sensing of coral bleaching using temperature and light: progress towards an operational algorithm. Remote Sens. 10:18. doi: 10.3390/rs10010018
Stimson, J., and Kinzie, R. A. (1991). The temporal pattern and rate of release of zooxanthellae from the reef coral Pocillopora damicornis (Linnaeus) under nitrogen-enrichment and control conditions. J. Exp. Mar. Biol. Ecol. 153, 63–74. doi: 10.1016/S0022-0981(05)80006-1
Suescún-Bolívar, L. P., Traverse, G. M., and Thom,é, P. E. (2016). Glycerol outflow in Symbiodinium under osmotic and nitrogen stress. Mar. Biol. 163:128. doi: 10.1007/s00227-016-2899-6
Taguchi, S., and Kinzie, R. (2001). Growth of zooxanthellae in culture with two nitrogen sources. Mar. Biol. 138, 149–155. doi: 10.1007/s002270000435
Van Oppen, M. J., Oliver, J. K., Putnam, H. M., and Gates, R. D. (2015). Building coral reef resilience through assisted evolution. Proc. Natl. Acad. Sci. U.S.A. 112, 2307–2313. doi: 10.1073/pnas.1422301112
Wall, C., Mason, R., Ellis, W., Cunning, R., and Gates, R. (2017). Elevated pCO2 affects tissue biomass composition, but not calcification, in a reef coral under two light regimes. R. Soc. Open Sci. 4:170683. doi: 10.1098/rsos.170683
Wang, J., and Douglas, A. (1999). Essential amino acid synthesis and nitrogen recycling in an alga–invertebrate symbiosis. Mar. Biol. 135, 219–222. doi: 10.1007/s002270050619
Wangpraseurt, D., Pernice, M., Guagliardo, P., Kilburn, M. R., Clode, P. L., Polerecky, L., et al. (2016). Light microenvironment and single-cell gradients of carbon fixation in tissues of symbiont-bearing corals. ISME J. 10, 788–792. doi: 10.1038/ismej.2015.133
Weis, V. M. (2008). Cellular mechanisms of cnidarian bleaching: stress causes the collapse of symbiosis. J. Exp. Biol. 211, 3059–3066. doi: 10.1242/jeb.009597
Weis, V. M. (2010). The susceptibility and resilience of corals to thermal stress: adaptation, acclimatization or both? Mol. Ecol. 19, 1515–1517. doi: 10.1111/j.1365-294X.2010.04575.x
Weng, L.-C., Pasaribu, B., Lin, I.-P., Tsai, C.-H., Chen, C.-S., and Jiang, P.-L. (2014). Nitrogen deprivation induces lipid droplet accumulation and alters fatty acid metabolism in symbiotic dinoflagellates isolated from Aiptasia pulchella. Sci. Rep. 4:5777. doi: 10.1038/srep05777
Wilkerson, F., and Trench, R. (1985). Nitrate assimilation by zooxanthellae maintained in laboratory culture. Mar. Chem. 16, 385–393. doi: 10.1016/0304-4203(85)90059-3
Zhu, B., Pan, K., and Wang, G. (2011). Effects of host starvation on the symbiotic dinoflagellates from the sea anemone Stichodactyla mertensii. Marine Ecol. 32, 15–23. doi: 10.1111/j.1439-0485.2010.00405.x
Ziegler, M., Seneca, F. O., Yum, L. K., Palumbi, S. R., and Voolstra, C. R. (2017). Bacterial community dynamics are linked to patterns of coral heat tolerance. Nat. Commun. 8:14213. doi: 10.1038/ncomms14213
Keywords: scleractinian corals, Symbiodinium, NanoSIMS, global climate change, nitrogen, acclimation
Citation: Gibbin EM, Krueger T, Putnam HM, Barott KL, Bodin J, Gates RD and Meibom A (2018) Short-Term Thermal Acclimation Modifies the Metabolic Condition of the Coral Holobiont. Front. Mar. Sci. 5:10. doi: 10.3389/fmars.2018.00010
Received: 20 October 2017; Accepted: 12 January 2018;
Published: 13 February 2018.
Edited by:
Sönke Hohn, Leibniz Centre for Tropical Marine Research (LG), GermanyReviewed by:
Christian Robert Voolstra, King Abdullah University of Science and Technology, Saudi ArabiaSusana Enríquez, Universidad Nacional Autónoma de México, Mexico
Copyright © 2018 Gibbin, Krueger, Putnam, Barott, Bodin, Gates and Meibom. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Emma M. Gibbin, emma.gibbin@epfl.ch
Anders Meibom, anders.meibom@epfl.ch
 Emma M. Gibbin
Emma M. Gibbin Thomas Krueger
Thomas Krueger Hollie M. Putnam
Hollie M. Putnam Katie L. Barott2,4
Katie L. Barott2,4  Julia Bodin
Julia Bodin Anders Meibom
Anders Meibom