Predicting Success of Range-Expanding Coral Reef Fish in Temperate Habitats Using Temperature-Abundance Relationships
- 1Fish Ecology Lab, Faculty of Science, University of Technology Sydney, Ultimo, NSW, Australia
- 2School of Life and Environmental Sciences, The University of Sydney, Sydney, NSW, Australia
An 18-year database of coral reef fish expatriation poleward in South East Australia was used to estimate persistence of coal reef fish recruits on temperate reefs. Surveys have identified over 150 coral reef fish species recruiting to temperate reefs at latitudes of 34°S (Sydney) and 60 species to 37°S (Merimbula) with 20 and 5 species respectively overwintering in at least 1 year over the study duration. We developed indices of vulnerability of key species to drops in water temperatures, by relating drops in abundances of species to temperature drops. Twenty species were ranked according to their temperature vulnerability. Overall, the families Chaetodontidae (butterflyfishes), Acanthuridae (surgeonfishes), Labridae (wrasses) and Pomacetnridae (damselfishes) had similar cold-water tolerance. However, there was considerable variability within families, for instance in the Pomacentridae, species from the genus Abudefduf appeared to have better cold-temperature tolerance than the other species. Predicted minimum overwintering temperature varied from 15.6°C to 19.8°C, with some species showing lower Tzero at Merimbula, the more poleward location. There was general concordance between a species' tolerance to cold-water and its tendency to occur as an overwinter but also notable exceptions. So while this work demonstrates the potential utility of tolerance to seasonal temperature drops as a means to predict range expansion capacity of vagrant species, the exceptional cases serve to highlight alternative factors. Specifically, tolerance to seasonal cooling of water is not the only important factor when predicting the range expansion capacity of a species. Factors affecting the general abundance of the vagrants, such as habitat suitability and competitor/predator environments will also be critical where overwinter survival becomes a lottery.
Introduction
The future of species globally, both in terrestrial and aquatic environments, is under threat through human-caused climate change (Chen et al., 2011; Poloczanska et al., 2013). Colonization of new, more climatically-suitable, habitat is a key coping strategy used by organisms, and understanding this process is critical to guide conservation strategies (Gilman et al., 2010). Not surprisingly, species' range expansions are difficult to accurately predict (HilleRisLambers et al., 2013; Bates et al., 2014), since they require detailed knowledge of dispersal mechanisms and physiological tolerance of species to the receiving environment. Range expansion is also problematic to measure empirically since it requires longer-term detailed fish observations.
As a result of strengthening western boundary currents under climate change, many coral reef fishes are expected to expand their ranges polewards (Figueira and Booth, 2010; Wernberg et al., 2012; Feary et al., 2014; Vergés et al., 2016). This influx into temperate ecosystems may lead to novel interactions and affect the structure and function of temperate marine communities (Vergés et al., 2014; Luiz et al., 2016). To date, there is still relatively little understanding of factors that constrain or facilitate species' geographic responses to climate change, including those of marine fishes (Li et al., 2011; Yang et al., 2011; Blois et al., 2013; Liancourt et al., 2013). Feary et al. (2014) and Booth et al. (2018a,b) showed that species metrics such as large body size, high swimming ability, large size at settlement and pelagic spawning behavior are more likely to occur in species expatriating into temperate habitats.
Low winter water temperatures are thought to act as a bottleneck for tropical fish survival polewards, with a threshold identified of approximately 18°C, below which few coral reef fish survive (Figueira et al., 2009; Figueira and Booth, 2010). Additionally, temperate waters exhibit short-term drops in temperature (at the scale of days to weeks) which can stress the tropical species. A species that copes better with drops in water temperature may be more able to exploit range-edge habitats, and such species may be more resilient to lower winter temperatures and thus more able to expand their ranges poleward.
The southeast coast of Australia is a known ocean warming hotspot (Ridgway, 2007) and is therefore likely to be particularly susceptible to the process of tropicalization. Every year, tropical fish larvae are expatriated south from the Great Barrier Reef (GBR) via the East Australian Current (EAC), where they arrive and settle in recruitment pulses from January to May (Booth et al., 2007). Our 18-year dataset of coral reef fish abundances across seasons in temperate locations can be used to identify key abundance fluctuations.
Here, we explore the idea that an indicator of a species' resilience under climate change scenarios is how they cope with drops in water temperature. Oliver et al. (2015) note the importance of resilience metrics to help assess possible range shifts and future biodiversity loss. Our aim was to see if we can identify species which are more or less sensitive to water temperature changes at extreme temperate latitudes, and use this to predict which species may do better or worse in the future, both in these extreme poleward habitats, and also in their coral reef natal habitats.
Specifically, we aimed to evaluate the utility of short-term abundance change with temperature as a metric for cold-temperature tolerance. We explore how this metric links to overwintering and whether it depends on location, which might indicate local adaptation or selection during transport. We then rank existing vagrant coral reef fish species as to their cold-temperature tolerance, and identify patterns by taxonomic groups (family).
Methods
Vagrant Fish Surveys
Since 2002, seasonal surveys of tropical fish expatriates have been conducted at sites in South East Australia (e.g., Booth et al., 2007). Surveys involve a snorkeler navigating a known area systematically and identifying, counting and sizing all tropical fish observed. Surveys were conducted at four sites around Sydney (34°S) and one in Merimbula (37°S) approximately every 1–3 weeks during the peak recruitment period (December–May) dropping off to approximately every 4–6 weeks for the rest of the year (Table 1). Shelly Beach East and West sites in Sydney as well as the Bar Beach Site in Merimbula have been surveyed since 2002. Two Sydney Harbour sites, Little Manly and Collins Beach, have been surveyed since 2009.
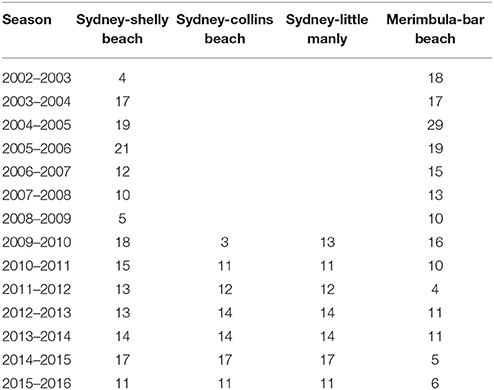
Table 1. Number of surveys conducted at each site between January and June of each summer recruitment season.
Water Temperature
The water temperature at all sites was logged at 30 min intervals with duplicate loggers (HOBO Pendant UA-001-08) placed within each site at 2–3 m depth. Logger data for Shelly Beach was not available for the entire data series so instead we used logger data from nearby Long Reef which is highly correlated (r2 = 0.97). Daily averages for all sites were extracted from the full time series. From this we computed the average temperature over the interval between individual surveys (typically 7–14 days). There are occasional gaps in the temperature series at both sites due to logger failures. With exception of a period of 1 year from July 2012 to July 2013 in Merimbula, most periods are only a few weeks in duration and not during the key period of January-June considered here (see below). Analysis of abundance series (as described below) was not conducted where no temperature data was available.
Identifying Resilient Species
We based our assessment of the resilience of vagrant coral reef fish species on three general characteristics; (1) has the species been observed to overwinter at either location, (2) how common are overwinterers for the species and (3) how sensitive to temperature is the abundance of each species? The status as an overwintering species was based on the observation of at least one overwinter survivor (adult present in surveys after August) in the data series for each location. To evaluate how common overwintering was, we established the relative occurrence of overwinterers for each species as the ratio of the average abundance (per survey for the whole dataset) of overwinter sized individuals (as defined by Figueira and Booth, 2010) at any time of year to the average abundance (per survey for the whole dataset) of recruits (any non-overwinter sized fish) during the main recruitment season (January–May).
Our general approach to characterizing thermal sensitivity for each species was to evaluate the absolute and relative rates of decline in abundance of different species with temperature across all the sites and seasons available within this dataset. Here we define the term “abundance series” as the data series of abundance values for a given species at a given site in a given season. In order to achieve our objective, we first narrowed our list of species to only the top 20 most commonly occurring ones (high density per survey but also represented by at least five abundance series across the whole data set) at these sites based on long-term averages (Table S1). We next normalized each abundance series by the maximum value in the series such that all values varied between zero and one (Figure 1A). When doing this, we excluded, based on size, any fish which would not have recruited during the season in question (and would thus be overwinter survivors). We then identified the “decline period” of each abundance series from its peak (1.0) to its lowest value, typically zero (as numbers reduced through the season). Since these are surveys of rare species in large areas, it is not uncommon for decline periods to show some fluctuations. Where multiple peak values were observed, the start of the decline period was taken to be the latest peak value. We also included up to two zero abundance values in a row (surveys which found no individuals of that species) at the end of the decline period (as opposed to stopping at the first zero) to ensure a true zero had been reached (see Figure 1A).
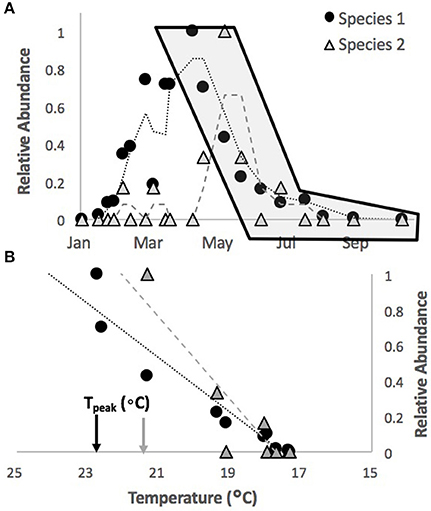
Figure 1. Illustration on methodology for extracting abundance-temperature relationships. From normalized abundance series over time (A, illustrated for two random species here) data are extracted for the region of decline, starting with normalized relative abundance value of one (the point in the gray shaded area of A). For each survey in this period the relative abundance on that survey is plotted against the temperature between that survey and the previous (B). The best fit line to this data (dashed line in B) is used to estimate the slope and temperature at which relative abundance was 1.0 (Tpeak, identified on the X-axis for each species).
We next regressed the proportional abundance for a survey against the average temperature over the interval between that survey and the previous one for all decline periods (see Figure 1B). We only included decline periods where the peak abundance had at least 3 individuals observed and consisted of at least 4 survey data points. As indicated above, periods where no temperature data was available were not considered in this analysis. We then excluded relationships where large fluctuations in numbers from one survey to the next led to slopes that deviated wildly from the positive relationship we observed for most of the decline periods. The abundance series which had these issues were typically those which only barely met the criteria for inclusion. In total this lead to exclusion of 19 of the 380 initial abundance series (5%, see Table S1 for summary of number of series by species).
From the best-fit line to each of these 361 decline periods, we extracted two metrics which describe the sensitivity of abundance to temperature for given species at each site and season:
(a) Tpeak: The temperature at which the species had its peak abundance for the series (proportional abundance of 1.0). This is the temperature after which it started to decline in abundance. Lower values would suggest a more cold-tolerant species.
(b) Slope: This is the slope of the relationship between temperature and proportional abundance. This relationship is positive and thus a lower value suggests a more cold-tolerant species whose abundance drops off more slowly with reductions in temperature.
Results
Over the whole period of this dataset there was a relatively consistent pattern of incursion of tropical fish recruits in January/February, stable populations through March/April and then a drop off in May or June which occurred more rapidly in Merimbula than in Sydney (Figure 2). Tropical fish were very rarely observed after June in Merimbula but were found more commonly through August in Sydney.
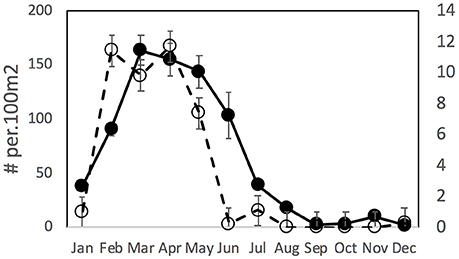
Figure 2. Mean (±SE) density of tropical vagrant fishes overall in Sydney (solid line, solid symbol curve, left Y axis) and Merimbula (dashed line, open symbol, right Y axis) over 12 months, n = 14 years.
Overwinter survival was generally much more common in Sydney than in Merimbula (Figure 3A) with the highest ratio observed for the territorial damselfish Stegastes gascoynei, although overwintering was much more limited for the other Pomacentridae aside from Chromis nitida. Naso unicornis was relatively abundant as an overwinterer amongst the Acanthuridae and overwintering was very uncommon for the Chaetodontiade. Both labrid species had high overwintering ratios in Sydney, but not in Merimbula.
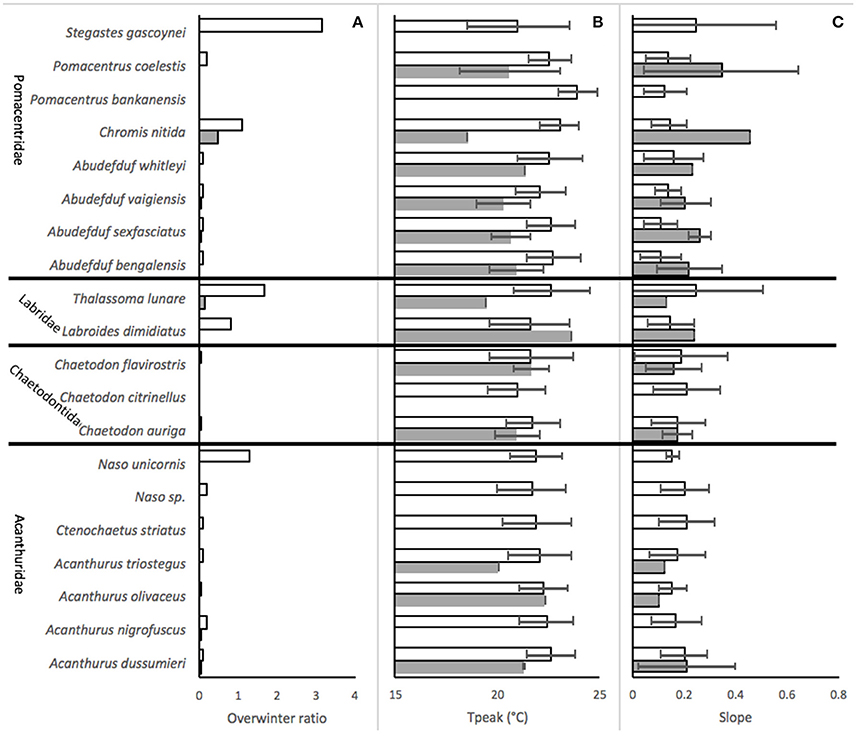
Figure 3. Performance of 20 species of expatiating coral reef fish in SE Australia, (mean ± SD of values derived from all abundance series in the 2002–2017 dataset), at Sydney (empty bars) and Merimbula (filled bars), for (A) the ratio of average over winterers to recruits, (B) temperature after which abundance started to fall (Tpeak), and (C) slope of relationship between temperature and proportional abundance.
Temperatures at the peak of abundance (Tpeak) differed between 18 and 24°C across the dataset and were generally lower at Merimbula than Sydney for the same species (Figure 3B) although there was a large overlap in error bars suggesting that this is not a significant effect. There was considerable variation across the Pomacentridae with members of the genus Abudefduf showing relatively high cold-water tolerance (low Tpeak). S. gascoynei and C. nitida had moderate Tpeak values within this family while Pomacentrus bankanensis had the highest value of in the whole dataset. The chaetodontids had generally lower Tpeak values than the than any of the other families as a whole, but only by a half a degree or so. Acanthurids tended to have Tpeak values which were intermediate to those of Pomacentrids and Chaetodontids, while the Labrids, represented by only two species had quite variable values (Figure 3B). Cold water tolerance expressed by the slope of the temperature-abundance relationships generally matched that suggested by Tpeak values (Figures 3C, 4).
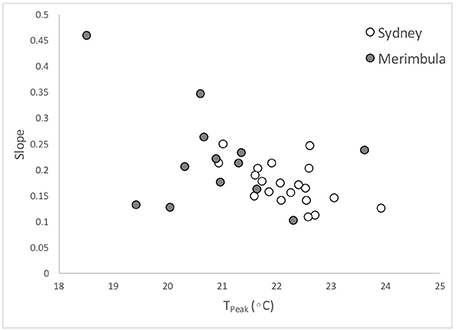
Figure 4. Relationship between predicted Tpeak and slope of temperature-drop, fish abundance drop curves, for the 20 species. Each point is average of all values available for each species. Not all species occurred in Merimbula.
Families were overall similar in their cold water tolerance ranking. Acanthuridae (surgeonfishes), Labridae (wrasses) and Pomacentridae (damselfishes) were similarly represented across the rankings, each having around 50% of member species considered here in the top 50% of rankings. There is some evidence that the Chaetodontidae (butterflyfishes) may be less cold-tolerant since all three species were in the bottom 50% of rankings, although two of them were at positions 11 and 12, so the generalization is weak.
However, there was again considerable variability within the Pomacentrids with the Abudefdufs appearing to have better cold-temperature tolerance than the other species. Of note here is the very low slope value for P. bankanensis which is not consistent with its very high Tpeak value, though this was not a very common species, with only 5 abundance series upon which to base the analyses.
Based on the average of ranked values for each of the metrics, Naso unicornis is indicated as the most cold-tolerant while Pomacentrus bankensis the least (Table 2). While there is a very general concordance with these rankings and the categorization of a species as an overwinterer based on observations, there are certainly notable exceptions. We expected to see the species which have been observed to overwinter at both sites ranked consistent near the top. While this pattern holds for A. vaigiensis, which is the most commonly observed tropical vagrant fish in the dataset, the other two species in this category, C. nitida and Thalasomma lunare are ranked 11 and 16 out of 20, respectively. However, the species that are not known to overwinter at either location are ranked near the bottom of the list, as expected, including P. bankanensis, which is ranked lowest.
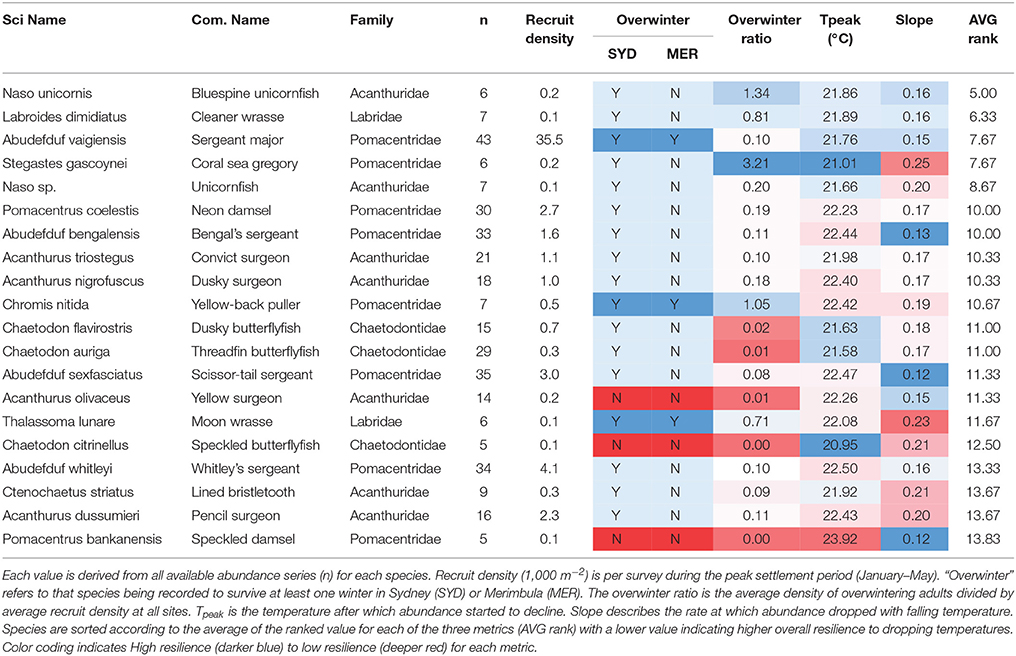
Table 2. Key temperature resilience metrics for 20 species of expatriating coral reef fish in SE Australia.
Discussion
How coral reef fishes respond to key stressors such as water temperature will determine their ability to cope with the projected effects of human-caused climate change. For the tropical vagrant species considered here there were strong and consistent drops in abundance as water temperature changed through the season. The nature of these changes, as characterized by the Tpeak and slope values, allows us to characterize the relative cold-temperature tolerance of the more common tropical vagrant species. Importantly, the tolerance suggested by these metrics is broadly supported by the characterization of a species as an “overwinterer” but provide a much greater resolution amongst species. The species that occur as vagrants quite commonly and in high abundance, such as A. vaigiensis and P. coelestis did appear near the top of the list of cold-tolerance. And of course many of the rare species, such as P. bankanensis and T. lunare appeared near the bottom of the list.
This work also highlights the interplay between two important factors which help to determine the probability of overwinter survival, thermal tolerance and abundance. There are species such as Chaetodon auriga, A. sexfasciatus, A. whitleyi, and A. dussumieri which are all relatively common vagrants at these sites (occurring most years and in moderate numbers) but who would appear to have relatively low capacity to deal with seasonal temperature drops. For these species, in absence of adaptation/acclimation, range expansion may depend strongly on high recruitment numbers. Conversely species such as L. dimidiatus and Naso sp. may do well into the future not because of their high numbers (they tend to be rare) but because of their high tolerance to cold-temperatures.
While there was not strong evidence of a of a location effect (Sydney 34°S vs. Merimbula 37°S) which would support local adaptation or selection during transport, it appears that Tpeak was lower overall in Merimbula (e.g., Figure 4). This may indicate either local adaptation to colder waters, or selection of more cold-resistant individuals (this may occur during advection from tropical waters) at this higher latitude location.
How might these metrics indicate the resilience of these species and their ability to range shift poleward? That is, can a short term performance metric predict long term changes? While this is unclear at present, such metrics have been established in other systems (e.g., McLeod, 2009; Fogarty et al., 2017). There seems to be more variability in the Tpeak than Slope value. However, a suite of factors including likelihood of advection of larvae to a location (e.g., Booth and Parkinson, 2011; Feary et al., 2014; Fogarty et al., 2017) and local habitat effects (e.g., Beck et al., 2017) must also be considered in prediction a species' persistence.
Annual settlement of surgeonfishes is rapidly increasing, and has been suggested to indicate a “tropicalization” phenomenon similar to that occurring in the Mediterranean (Vergés et al., 2014). Our results suggest that several of these may be among the most cold-tolerant species (lower Tpeak, lower Slope) which has implications for their invasiveness in future.
A recent review of range shifts of young-of-the-year of many of the species considered here (Fowler et al., 2017) shows that even less resilient species had shifted. Of our “High Ranked” species, 2 of 5 species showed recruitment further poleward (recruitment, not overwintering) while 3 of 5 species of “Low Ranked” species had shifted. However, this does not indicate establishment or even temperature tolerance, rather offshore advection of larvae and early survival. How species respond to temperature drops at the temperate habitat (as reported here) would be more indicative of range shift potential (i.e. establishment). For instance, in Austral summer 2016, EAC activity and warmer waters lead to unprecedented arrivals of acanthurids and Abudefduf whitleyi at the southern location (Merimbula), however these species quickly disappeared once temperatures dropped (Booth unpub. data).
Future extensions of this study would investigate responses of key tropical species in their natal habitats to ambient temperature drops, and linking more- and less-resilient species to overwinter success. As noted by Shoo et al. (2006), investigation of fine grained responses to climate, such as how expatriating reef fishes respond to short-term temperate changes, may increase our ability to predict range shifts.
Ethics Statement
Animal ethics approval for this project was granted by the University of Technology, Sydney (UTS) Animal Care and Ethics Committee (ACEC) under the ethics permit of DB (Permit ETH17 – 1117). Collections were under NSW DPI F94/696.
Author Contributions
DB contributed in leading the study, gathering data, analysing data and writing the manuscript. WF contributed in gathering data, analysing data and writing the manuscript. GB contributed in gathering data and writing the manuscript. LB contributed in gathering data and writing the manuscript.
Funding
Funding for this long-term study has come from Australian Research Council, NSW Environmental Trust, Sydney Aquarium Conservation Fund.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Thanks to the fish surveyors over the years, inclining James van den Broek, Hayden Beck, Ash Fowler, and Georgia Poyner. Appreciation to David Suggett for inviting our submission to Frontiers, and to two reviewers for helpful comments.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2018.00031/full#supplementary-material
References
Bates, A. E., Pecl, G. T., Frusher, S., Hobday, A. J., Wernberg, T., Smale, D. A., et al. (2014). Defining and observing stages of climate-mediated range shifts in marine systems. Glob. Environ. Change 26, 27–38. doi: 10.1016/j.gloenvcha.2014.03.009
Beck, H. J., Feary, D. A., Nakamura, Y., and Booth, D. J. (2017). Temperate macroalgae impacts tropical fish recruitment at forefronts of range expansion. Coral Reefs 36, 639–651. doi: 10.1007/s00338-017-1553-1
Blois, J. L., Zarnetske, P. L., Fitzpatrick, M. C., and Finnegan, S. (2013). Climate change and the past, present, and future of biotic interactions. Science 341, 499–504. doi: 10.1126/science.1237184
Booth, D. J., Feary, D., Kobayashi, D., Luiz, O., and Nakamura, Y. (2018a). “Tropical marine fishes and fisheries and climate change,” in Climate Change Impacts on Fisheries and Aquaculture: A Global Analysis, 1st Edn., Vol. 1, eds B. F. Phillips and M. Pérez-Ramírez (West Sussex, UK: John Wiley & Sons Ltd), 875–896.
Booth, D. J., Figueira, W. F., Gregson, M. A., Brown, L., and Beretta, G. (2007). Occurrence of tropical fishes in temperate southeastern Australia: role of the east Australian current. Estuar. Coast. Mar. Sci. 72, 102–114. doi: 10.1016/j.ecss.2006.10.003
Booth, D. J., Poloczanska, E., Donelson, J. M., Molinos, J. G., and Burrows, M. (2018b). “Biodiversity and climate change in the oceans,” in Climate Change Impacts on Fisheries and Aquaculture: A Global Analysis, 1st Edn., Vol 1, eds B. F. Phillips and M. Pérez-Ramírez (West Sussex, UK: John Wiley & Sons Ltd), 63–89.
Booth, D. J., and Parkinson, K. (2011). Pelagic larval duration is similar across 23 degrees of lattitude for two species of butterflyfish (Chaetodontidae) in eastern Australia. Coral Reefs 30, 1071–1075. doi: 10.1007/s00338-011-0815-6
Chen, I. C., Hill, J. K., Ohlemüller, R., Roy, D. B., and Thomas, C. D. (2011). Rapid range shifts of species associated with high levels of climate warming. Science 333, 1024–1026. doi: 10.1126/science.1206432
Feary, D. A., Pratchett, M. S., Emslie, M. J., Fowler, A. M., Figueira, W. F., Luiz, O. J., et al. (2014). Latitudinal shifts in coral reef fishes: why some species do and others do not shift. Fish Fish. 15, 593–615. doi: 10.1111/faf.12036
Figueira, W. F., Biro, P., Booth, D. J., and Valenzuela Davie, V. C. (2009). Performance of tropical fish recruiting to temperate habitats: role of ambient temperature and implications of climate change. Mar. Ecol. Prog. Ser. 384, 231–239. doi: 10.3354/meps08057
Figueira, W. F., and Booth, D. J. (2010). Increasing ocean temperatures allow tropical fishes to survive over winter in temperate waters. Glob. Chang. Biol. 16, 506–516. doi: 10.1111/j.1365-2486.2009.01934.x
Fogarty, H. E., Burrows, M. T., Pecl, G. T., Robinson, L. M., and Poloczanska, E. S. (2017). Are fish outside their usual ranges early indicators of climate-driven range shifts? Glob. Chang. Biol. 23, 2047–2057. doi: 10.1111/gcb.13635
Fowler, A. M., Parkinson, K., and Booth, D. J. (2017). New poleward observations of 30 tropical reef fishes in temperate southeastern Australia. Mar. Bio. 47, 1–6. doi: 10.1007/s12526-017-0748-6
Gilman, S. E., Urban, M. C., Tewksbury, J., Gilchrist, G. W., and Holt, R. D. (2010). A framework for community interactions under climate change. Trends Ecol. Evol. 25, 325–331. doi: 10.1016/j.tree.2010.03.002
HilleRisLambers, J., Harsch, M. A., Ettinger, A. K., Ford, K. R., and Theobald, E. J. (2013). How will biotic interactions influence climate change–induced range shifts? Ann. N. Y. Acad. Sci. 1297, 112–125. doi: 10.1111/nyas.12182
Li, G., Liu, Y., Frelich, L. E., and Sun, S. (2011). Experimental warming induces degradation of a Tibetan alpine meadow through trophic interactions. J. Appl. Ecol. 48, 659–667. doi: 10.1111/j.1365-2664.2011.01965.x
Liancourt, P., Spence, L. A., Song, D. S., Lkhagva, A., Sharkhuu, A., Boldgiv, B., et al. (2013). Plant response to climate change varies with topography, interactions with neighbours, and ecotype. Ecology 94, 444–453. doi: 10.1890/12-0780.1
Luiz, O. J., Allen, A. P., Robertson, D. R., Floeter, S. R., Kulbicki, M., Vigliola, L., et al. (2016). Adult and larval traits as determinants of geographic range size among tropical reef fishes. Proc. Natl. Acad. Sci. U.S.A. 110, 16498–16502. doi: 10.1073/pnas.1304074110
McLeod, C. D. (2009). “Endangered Species Research” in Global climate change, range changes and potential implications for the conservation of marine cetaceans: a review and synthesis. 7, 125–136. doi: 10.3354/esr00197
Oliver, T. H., Isacc, N. J., August, T. A., Woodcock, B. A., Roy, D. B., and Bullock, J. M. (2015). Declining resilience of ecosystem functions under biodiversity loss. Nat. Commun. 6:10122. doi: 10.1038/ncomms10122
Poloczanska, E. S., Brown, C. J., Sydeman, W. J., Kiessling, W., Schoeman, D. S., Moore, P. J., et al. (2013). Global imprint of climate change on marinelife. Nat. Clim. Change 3, 919–925. doi: 10.1038/nclimate1958
Ridgway, K. R. (2007). Long-term trend and decadal variability of the southward penetration of the East Australian current. Geophys. Res. Lett. 34, L13613 doi: 10.1029/2007GL030393
Shoo, L. P., Williams, S. E., and Hero, J. (2006). Detecting climate change induced range shifts: where and how should we be looking? Austral. Ecol. 31, 22–29. doi: 10.1111/j.1442-9993.2006.01539.x
Vergés, A., Doropoulos, C., Malcolm, H. A., Skye, M., Garcia-Pizá M., Marzinelli, E. M., et al. (2016). Long-term empirical evidence of ocean warming leading to tropicalization of fish communities, increased herbivory, and loss of kelp. Proc. Natl. Acad. Sci. U.S.A. 113, 13791–13796. doi: 10.1073/pnas.1610725113
Vergés, A., Steinberg, P. D., Hay, M. E., Poore, A. G., Campbell, A. H., Ballesteros, E., et al. (2014). The tropicalisation of temperate marine ecosystems: climate-mediated changes in herbivory and community phase shifts. Proc. R. Soc. B 281:20140846. doi: 10.1098/rspb.2014.0846
Wernberg, T., Smale, D. A., Tuya, F., Thomsen, M. S., Langlois, T. J., de Bettignies, T., et al. (2012). An extreme climatic event alters marine ecosystem structure in a global biodiversity hotspot. Nat. Clim. Change 3, 78–82. doi: 10.1038/nclimate1627
Keywords: Acanthurid surgeonfishes, Chaetodontid butterflyfish, overwintering, Pomacentrid damselfish, water temperature drop, vagrant coral reef fishes
Citation: Booth DJ, Beretta GA, Brown L and Figueira WF (2018) Predicting Success of Range-Expanding Coral Reef Fish in Temperate Habitats Using Temperature-Abundance Relationships. Front. Mar. Sci. 5:31. doi: 10.3389/fmars.2018.00031
Received: 28 September 2017; Accepted: 24 January 2018;
Published: 13 February 2018.
Edited by:
Verena Schoepf, University of Western Australia, AustraliaReviewed by:
Jesús Ernesto Arias González, Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional (CINVESTAV-IPN), MexicoPaul Carl Sikkel, Arkansas State University, United States
Copyright © 2018 Booth, Beretta, Brown and Figueira. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David J. Booth, david.booth@uts.edu.au
 David J. Booth
David J. Booth Giglia A. Beretta
Giglia A. Beretta Luke Brown1
Luke Brown1