Species-Specific Coral Calcification Responses to the Extreme Environment of the Southern Persian Gulf
- 1Center for Genomics and Systems Biology, New York University Abu Dhabi, Abu Dhabi, United Arab Emirates
- 2Ecological Marine Services, Millbank, QLD, Australia
- 3Natural History Museum of Denmark, University of Copenhagen, Copenhagen, Denmark
- 4NOAA/NESDIS/STAR Coral Reef Watch, College Park, MD, United States
- 5Marine Geophysical Laboratory, Physics Department, College of Science and Engineering, James Cook University, Townsville, QLD, Australia
- 6ARC Centre of Excellence for Coral Reef Studies, James Cook University, Townsville, QLD, Australia
- 7Experimental Marine Ecology Laboratory, Department of Biological Sciences, National University of Singapore, Singapore, Singapore
Sustained accretion of calcium carbonate (mostly by scleractinian corals) is fundamental for maintaining the structure and function of coral reef ecosystems, but may be greatly constrained by extreme and rapidly changing environmental conditions. Corals in the southern Persian Gulf already experience extreme temperature ranges (<20 to >34°C), chronic hypersalinity (>43 psu) and frequent light limitation (<100 μmol photons m−2 s−1). We compared annual rates of calcification for two of the most common coral species in the region (Platygyra daedalea and Cyphastrea microphthalma) along marked gradients in environmental conditions in the southern Persian Gulf and into the Oman Sea. Overall calcification rates were 32% higher in P. daedalea colonies (x = 1.103 g cm−2 y−1, n = 46) than in C. microphthalma (x = 0.835 g cm−2 y−1, n = 37), probably reflecting inter-specific differences in energy allocation and skeletal density. There was also considerable variation in calcification rates among individual colonies from the same locations that was unrelated to depth or photosymbiont type. However, most interestingly, P. daedalea and C. microphthalma exhibited contrasting trends in mean annual calcification rates across locations. For P. daedalea, calcification rates were lowest at Delma, where the minimum temperatures were lowest and salinity was highest, and increased across the southern Persian Gulf with increases in minimum temperatures and decreases in salinity. These data suggest that calcification rates of P. daedalea are most constrained by minimum temperatures, which is consistent with the strong relationship between annual calcification rates and minimum local temperatures recorded across the Indo-Pacific. Conversely, linear extension and calcification of C. microphthalma in the southern Persian Gulf was lowest at Ras Ghanada, where there was lowest light and highest maximum temperatures. These data reveal striking taxonomic differences in the specific environmental constraints on coral calcification, which will further reinforce changes in the structure of coral assemblages with ongoing global climate change.
Introduction
Scleractinian corals are one of the foremost contributors to carbonate accretion (Vecsei, 2004), which is fundamental for the formation and maintenance of coral reef frameworks (Perry et al., 2012). Moreover, contemporary corals form complex three-dimensional structural habitats that support high biodiversity and productivity of reef-associated organisms (Stella et al., 2010; Graham and Nash, 2013). However, extreme and rapidly changing environmental conditions are expected to increasingly constrain calcification rates of corals (De'ath et al., 2009; Cantin et al., 2010; Pratchett et al., 2015), if not decimate coral populations (e.g., Hughes et al., 2017), and drastically alter the composition of coral assemblages (Loya et al., 2001; Hughes et al., 2012). Most notably, sustained increases in global ocean temperatures, unequivocally linked to anthropogenic climate change, are challenging the thermal limits of most coral species, causing increased incidence and/or severity of mass coral bleaching (Heron et al., 2016b). Even if corals do not actually bleach or die, exposure to supra-optimal temperatures has marked effects on individual fitness of coral colonies, leading to temporary cessation of calcification (Lough and Cantin, 2014) and/or sustained declines in calcification rates (De'ath et al., 2009; Cantin et al., 2010). Calcification rates may be further constrained as corals are exposed to increasing concentrations of dissolved CO2, thereby decreasing seawater pH and aragonite saturation (Schoepf et al., 2013).
Calcification rates of corals, and corresponding rates of linear extension and density, vary in accordance with natural gradients in temperature, seawater chemistry, light and nutrient concentrations (reviewed in Pratchett et al., 2015). Within the range of naturally occurring temperatures, calcification and linear extension rates have mostly shown fairly constant rates of increase with increasing temperature. For example, in Platygyra spp., annual linear extension increased 0.9 mm of every 1°C increase in local summer maximum temperatures across 21 locations in the Indo-Pacific (Weber and White, 1974). However, extreme environmental conditions can pose significant constraints on individual performance (e.g., calcification and reproduction) and abundance of corals, leading to limited reef development in “marginal” reef environments (Kleypas et al., 1999). At high latitudes, for example, annual linear extension of corals may be <30% of extension rates recorded in lower latitudes due to low light, temperature and aragonite saturation (Anderson et al., 2015). Conversely, some corals appear unaffected, or may actually benefit, from prolonged exposure to otherwise adverse environmental conditions (Harriott, 1999; Fabricius et al., 2011; Riegl et al., 2011), implying that specialization via physiological (acclimation) and/or genetic (adaptation) mechanisms may provide a degree of performance compensation. While the specific mechanisms underpinning compensatory responses are often unknown, some coral species and populations can specialize to extreme environments by hosting stress-tolerant symbionts (Symbiodinium; Fabricius et al., 2004; Cooper et al., 2011; Hume et al., 2013), upregulating heterotrophic feeding (Anthony and Fabricius, 2000), altering the expression of stress-response proteins (Brown et al., 2002; Palumbi et al., 2014), or via longer-term genetic adaptation (Dixon et al., 2015; Howells et al., 2016a).
Environmental tolerances of corals, as well as their capacity for acclimatization and adaptation, vary taxonomically (Loya et al., 2001; Hennige et al., 2010; Grottoli et al., 2014). It is expected therefore, that climate change is likely to drive directional shifts in the structure of coral assemblages (Hughes et al., 2012), whereby corals that predominate in locations that are already subject to extreme environmental conditions may be capable of withstanding projected environmental change (Riegl et al., 2011). The hottest environment in the world where corals naturally occur is the southern Persian Gulf (Arabian Gulf). Here, summer temperatures remain above 34°C for several consecutive weeks and reach highs of 36°C, exceeding the survival limits of most corals living in other locations and regions by 2–6°C (Riegl et al., 2011). Due to the shallow and semi-enclosed nature of the Gulf, corals in this region are routinely exposed to not only extremely high temperatures but to a suite of additional harsh conditions (Sheppard, 1993; Riegl and Purkis, 2012). Winter temperatures can reach lows of 18°C, approaching the lower limit for reef development (Kleypas et al., 1999), the seawater is chronically hypersaline (39–46 psu), and frequent sediment resuspension causes periods of light limitation. While several corals (both endemic and pandemic) are known to occur within the southern Persian Gulf (Riegl et al., 2012), there is limited information on population viability and demography of these corals with gradients in environmental conditions (Lough et al., 2003; Howells et al., 2016b; Samiei et al., 2016).
The purpose of this study was to explore spatial variation in calcification rates of two widespread massive coral species, Platygyra daedalea and Cyphastrea microphthalma that co-occur in the southern Persian Gulf. Both P. daedalea and C. microphthalma are abundant in the southern Persian Gulf, especially after the depletion of Acropora spp. in this region from repeated heat stress events (Burt et al., 2011; Coles and Riegl, 2013), but are also widespread throughout the Indo-Pacific (Veron, 2000). To quantify contemporary growth (specifically, the amount of carbonate added in the 12-months prior to collection), individual corals were stained in situ using alizarin red-S to provide a definitive reference for measuring subsequent growth. The depth (linear extension) and density of new skeletal material that was added subsequent to staining was used to calculate the area-specific mass of new skeleton that was added (calcification) during the 12-month study period.
Methods
Species and Site Locations
Coral growth (linear extension, skeletal density, and ultimately calcification) was quantified for replicate colonies of each of two massive scleractinian coral species (P. daedalea and C. microphthalma) across 3 sites within the southern Gulf (Abu Dhabi) separated by 38–246 km (Figure 1). The westernmost site at Delma Island was 47.4 km from the mainland coast, while Saadiyat and Ras Ghanada sites were located 3.6 and 5.1 km from the coast, respectively. Corals were sampled in relatively shallow (6–7 m) areas of emergent hard substrate at all locations. Growth rates from the Gulf were then compared to growth estimates for both P. daedalea and C. microphthalma sampled on shallow (4–6 m) fringing reef in the Oman Sea, as well as published values for Platygyra and Cyphastrea spp. from tropical and subtropical Indo-Pacific reefs (Weber and White, 1974; Romano, 1990; Babcock, 1991; Harriott, 1999; Roberts and Harriott, 2003).
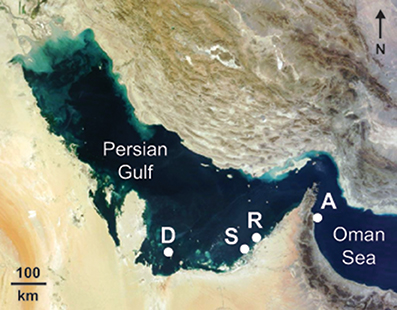
Figure 1. Location of study sites where growth parameters were measured in the corals Platygyra daedalea and Cyphastrea microphthalma. Delma (D: 24.5208°N, 52.2781°E), Saadiyat (S: 24.5986°N, 54.4201°E), Ras Ghanada (R: 24.8482°N, 54.6903°E), and Al Aqah (A: 25.4929°N, 56.3635°E). Background NASA satellite image sourced from https://zoom.earth.
Growth Measurements
Skeletons of replicate colonies of P. daedalea and C. microphthalma were stained using the approach of Dustan (1975) which has been widely used to measure linear extension in scleractinian corals (Pratchett et al., 2015). At each site, 14–17 colonies of each species were removed from the substrate and aggregated into large (70-L) sealed bags with 10 mg.l−1 of alizarin red-S (AL080, chem-supply) for 4–5 h. Following staining, colonies were tagged, tissue sampled and re-attached to the substrate with rapid-setting mounting resin (FIS V 360 S, Fischer). To eliminate effects of competition on growth, colonies were attached to substrate areas free from contact with other corals. Staining was conducted September 2013 and 76% of colonies (Pd: n = 51; Cm: n = 44) were recovered and collected in September 2014.
All colonies recovered from the field had their tissue removed with a high-pressure water jet, were dried and subsequently cut into 3–4 slices perpendicular to the direction of growth plane using a diamond-tipped rock saw (Figures 2a,c). High-resolution photos were taken at 2–5 non-overlapping regions of each colony slice with fluorescent excitation of the alizarin stain (Figures 2b,d). Five measurements, including the longest and shortest linear extension and three haphazard points across each colony, were taken from the edge of the stain to the periphery of the skeleton to the nearest 0.001 mm using Image J software (https://imagej.nih.gov/ij/). The number of extension measurements taken for each colony was size dependent and ranged from 16 to 65 for P. daedalea colonies and 15 to 75 for C. microphthalma colonies.
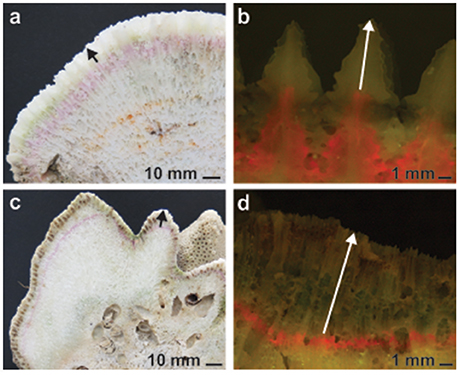
Figure 2. Representative skeletal slices of Platygyra daedalea (a,b) and Cyphastrea microphthalma (c,d) colonies used to measure coral growth in the Persian Gulf and Oman Sea. Annual linear extension was measured across each colony from the edge of the incorporated and alizarin stain (red line, fluorescently excited in b,d) to the periphery of the skeleton (indicated by arrows). Density was measured in skeletal subsamples from each colony, approximating the annual band of extension.
Skeletal density was measured using the water displacement method (e.g., Bucher et al., 1998; Morgan and Kench, 2012). Small ~1–2 cm3 sections of skeleton were cut from the surface of each coral slice (i.e., 3–4 per colony) using a hand-held rotary tool. The depth of section cuts were matched to the alizarin stain to include all growth within the survey year, however, a small amount of growth beneath the alizarin stain line may have been inadvertently included in some samples. Skeletal samples were dried overnight at 60°C and their density measured by dividing the weight of the sample (recorded to the nearest 0.01 g) by the volume of water displaced in a 10 ml graduated cylinder (recorded to the nearest 0.1 ml). When measuring water displacement, samples were gently tapped for 1 min, left still for 1 min, and tapped for one additional minute to remove any air bubbles present. Dry mass and water displacement volumes were recorded twice on average per individual sample (range 1–4). Annual calcification rates were calculated by multiplying density by extension as described in the Data Analysis section.
Environmental Drivers
Symbiont communities, annual variation in environmental conditions, and recent disturbance history were considered as potential drivers of growth variability.
To characterize symbiont communities, DNA samples were taken from each tagged colony at the time of alizarin staining. Symbiodinium clade(s) were identified with PCR amplification of the small-subunit region using ss5Z and ss3Z primers followed by TaqI restriction enzyme digestion of PCR products (Rowan and Powers, 1991). Digests were run alongside size markers on 2% agarose gels and Symbiodinium clades were assigned based on known fragment sizes (e.g., Toller et al., 2001). Sub-cladal Symbiodinium types in P. daedalea and C. microphthalma were assumed consistent with symbiont diversity previously documented for these species and sites (i.e., A1, C3, D1a; Hume et al., 2013; Howells et al., 2016a). In a subset of samples (n = 15 total), these types were confirmed with PCR and sequencing of the internal transcribed spacer 2 region (ITS2: ITSintfor2 and ITS-Reverse primers; LaJeunesse and Trench, 2000).
Sea temperature across the study period was recorded at each site at hourly intervals using in situ loggers attached to the reef substrate (September 2013–2014; HOBO TidbiT v2, Onset). Daily averages were calculated and from these, the warmest and coolest day and month (continuous 28-day period) were used to characterize the thermal environment at each site. Photosynthetically Active Radiation (PAR) at the ocean surface was acquired from the NASA Ocean Biology Processing Group (NASA Goddard Space Flight Center, Ocean Ecology Laboratory Ocean Biology Processing Group, 2014), derived from the MODIS sensor on the Aqua satellite and mapped daily at 4-km resolution. Daily data at each site (September 2013–2014) was averaged across a 3 × 3-pixel box and composited to monthly resolution to provide suitable data density of the product (Frouin et al., 2002). As these satellite values do not account for the effects of depth and turbidity on light attenuation, in situ down-welling PAR was measured at midday on 1 day at each site in August 2016 (LI-192 Underwater Quantum Sensor, LI-COR). Salinity values across the study period were obtained for each day from the 1/12° global HYbrid Coordinate Ocean Model (HYCOM; experiments 91.0, 91.1; Metzger et al., 2010) and compared to ad-hoc in situ measurements (water quality multi-probe, YSI). As model values underestimated in situ measurements by ~8% in the Gulf (Saadiyat and Ras Ghanada) and ~3.5% in the Oman Sea (Al Aqah), summer (May–October), and winter (November–April) correction factors were applied to daily HYCOM values. The difference in deviance between model and in situ values for Saadiyat and Ras Ghanada was negligible and the same correction factors were applied to all three sites in the Gulf (no in-situ data were available for Delma). Summaries from both the uncorrected and corrected HYCOM salinity data are reported. Primary productivity across the study period was evaluated from published monthly values of chlorophyll-a from the VIIRS sensor at 5 km resolution (http://oceanwatch.pifsc.noaa.gov/thredds/ncss/grid/viirs_nasa/monthly/dataset.html).
The primary disturbances impacting Gulf corals are relatively frequent bleaching and disease events (Coles and Riegl, 2013). Only visibly healthy P. daedalea and C. microphthalma colonies were collected for staining and no bleaching or disease was recorded on these colonies at 6 and 12 month monitoring intervals. Consistent with this observation, satellite derived measures of heat stress (i.e., Degree Heating Weeks; https://coralreefwatch.noaa.gov/satellite/; Heron et al., 2016a) indicate that no heat stress was accumulated at any of the sites during the study (i.e., 2013 and 2014).
Data Analysis
Growth data for P. daedalea and C. microphthalma were initially assessed to identify and remove linear extension and density outliers. Significant reductions in linear extension were observed in colonies that experienced injuries or mortality, or were dislodged (Pd: n = 5; Cm: n = 7) and data from these colonies were discarded. The final dataset consisted of 3,700 linear extension measurements and 635 density assays where calcification rates were calculated for 46 colonies of P. daedalea and 37 colonies of C. microphthalma. Summary statistics of raw data are provided in Table S1.
Site differences in linear extension, density and calcification rates were examined using a Bayesian mixed modeling approach employing Markov chain Monte Carlo (MCMC) methods for fitting Generalized Linear Mixed Models (GLMM) (Hadfield, 2010). To account for intrinsic species-specific differences in growth rates (Pratchett et al., 2015), species were modeled separately to accurately estimate random effects to explain variability among sites. Linear extension and density models were defined with fixed site and random colony effects. For density models, skeletal subsamples were also defined as a random effect to account for measurement variation among density assays. Additionally, the random effect estimates for linear extension in each colony (i.e., marginal posterior modes for each colony in linear extension model solutions) were used as a fixed effect covariate in density models to examine how relationships between density and linear extension varied by site. Where nested models with different fixed effect structure were compared, we used Deviance Information Criterion (DIC) values to select the most parsimonious. Parameter estimates, model predictions and model descriptors reported are the posterior means and 95% highest posterior density (HPD) intervals from MCMCglmm model outputs, unless otherwise stated. Within species, parameter estimates were considered statistically significant when HPD intervals did not include 0 and pMCMC values < 0.05. Between species, parameter estimates were considered statistically significant when prediction HPD intervals overlapped <5% of prediction estimates from the other species.
Annual calcification rates (g.cm−2.year−1) were calculated for each colony by multiplying linear extension (cm.year−1) and density (g.cm−3) using the random effect estimates obtained from linear extension and density null-fixed-effect models. A non-parametric bootstrapping approach was used to examine site differences in calcification rate to account for error in modeled colony specific linear extension and density random effects. Here the original dataset was resampled with replacement using the same strata and intensity of the original dataset. The resampled data were then fit to null-fixed-effect models to calculate colony-specific random effects of linear extension and density, which were multiplied to obtain the colony-specific calcification rate. This process was repeated 999 times and this bootstrapped calcification rate data modeled with fixed site and random colony effects as described above. The influence of environmental variables on linear extension and calcification within the Gulf was also evaluated using a mixed modeling approach. For each species, site identity was replaced by information on annual-extreme temperature (maximum and minimum of 28-day averages), salinity (average of corrected model values), and irradiance (mid-point of recorded values) (Table 1). Additionally, colony identity was replaced by the dominant Symbiodinium clade detected within tissues (Table 1). Regional growth comparisons were undertaken by examining local (Gulf and Oman Sea) vs. Indo-Pacific relationships between linear extension and temperature (annual mean, maximum, minimum, and range).
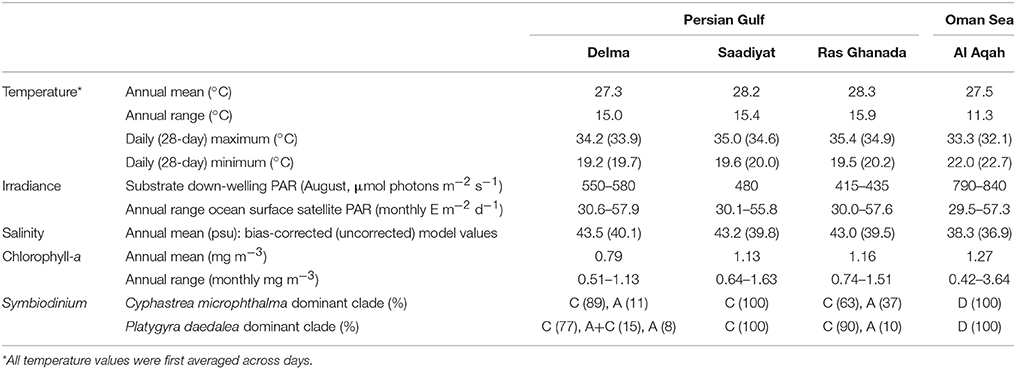
Table 1. Environmental parameters at each site where coral growth was measured in the Persian Gulf and Oman Sea.
All analyses were performed in R (R Core Team, 2016). The MCMCglmm package was used for Bayesian mixed modeling (Hadfield, 2010). Diffuse, weakly-informative inverse gamma priors and default priors were used for variance components and fixed effects, respectively [G = list (G1 = list (V = 1, nu = 0.002)), R = list(V = 1,nu = 0.002)]. Model fits were evaluated by visual inspection of trace plots and by ensuring autocorrelation between successive values was low (Hadfield, 2015). Model comparisons were made using the MuMIn package (Barton, 2016). Bootstrapping for analysis of calcification rates was performed using a custom script applying functions from the Sampling package (Tillé and Matei, 2009) and dplyr package (Wickham and Francois, 2015). Reported model results are posterior means and their 95% HPD intervals unless otherwise stated. Sampling effort, summary statistics, raw data, and R code for model analyses are provided in the Supplementary Materials.
Results
Growth Variation within and among Coral Colonies and Species
Growth rates varied considerably within and among colonies of C. microphthalma and P. daedalea. Across all Gulf and Oman Sea sites, average rates of linear extension in both species were highly similar (Cm: 4.51 mm.y−1; Pd: 4.66 mm.y−1). Individual colonies exhibited extension rates that ranged from 1.77 to 7.81 mm.y−1 for C. microphthalma and 1.94 to 9.63 mm.y−1 for P. daedalea (Figure S1; Table S1) and this variation was unrelated to colony size (Table S2). Additionally, intra-colony variation in extension rates was often equivalent to or more variable than extension rates among different colonies (Figure S1). In particular, there were generally faster extension rates on colony nodules and slower extension between nodules for C. microphthalma (Figures 2c,d), whereas the more even surface of P. daedalea colonies tended to result in more uniform intra-colony extension and thus less intra-colony variation in extension measurements (Figures 2a,b; Table S1). In contrast, skeletal density differed markedly between species. On average, density was 26% higher in P. daedalea (2.36 g.cm−3) than C. microphthalma (1.87 g.cm−3) with minimal variation among and within colonies of different sizes (Tables S1, S2). Incorporating both of these parameters, calcification rates in C. microphthalma ranged from 0.79 to 0.97 g.cm−2.y−1 (mean: 0.84) and in P. daedalea from 0.89 to 1.35 g.cm−2.y−1 (mean: 1.10; Figure S1; Table S1).
Growth Comparisons among Southern Persian Gulf Sites
Spatial variation in linear extension was greatest for C. microphthalma, where the highest rates were recorded on Delma Island, the westernmost and furthest offshore site (5.4 mm.y−1, 4.3–6.4; mean, 95% HPD intervals; Figure 3A). Linear extension declined eastward across southern Gulf sites by 20% at Saadiyat (4.3 mm.y−1, 3.3–5.5) and by 37% at Ras Ghanada (3.4 mm.y−1, 2.4–4.3; Table 2A). Conversely, for P. daedalea, linear extension was highest at Ras Ghanada (4.9 mm.y−1, 4.1–5.7) and declined westward (Figure 3A), 10% lower at Saadiyat (4.4 mm.y−1, 3.7–5.3) and 20% lower at Delma (3.9 mm.y−1, 3.2–4.6; Table 2B). Skeletal density exhibited minimal variation within the southern Gulf for both species (Table S1), such that calcification rates reflected the patterns of linear extension (Figure 3B). For C. microphthalma, calcification was 19 and 35% higher at Delma (0.97 g.cm−2.y−1, 0.78–1.15) than at Saadiyat (0.79 g.cm−2.y−1, 0.59–0.98) and Ras Ghanada (0.63 g.cm−2.y−1, 0.47–0.81), respectively (Table 2A). For P. daedalea, calcification was 5 and 21% higher at Ras Ghanada (1.12 g.cm−2.y−1, 0.93–1.31) than at Saadiyat (1.06 g.cm−2.y−1, 0.88–1.25) and Delma (0.89 g.cm−2.y−1, 0.71–1.05), respectively, although not statistically significant (Table 2B). As a result of the opposite spatial patterns in growth, relative differences between species were not consistent among sites. For instance, at Ras Ghanada, P. daedalea had 44% higher linear extension and 78% higher calcification than C. microphthalma. However, at Delma, while linear extension was 28% lower in P. daedalea, calcification rates were similar between the two species.
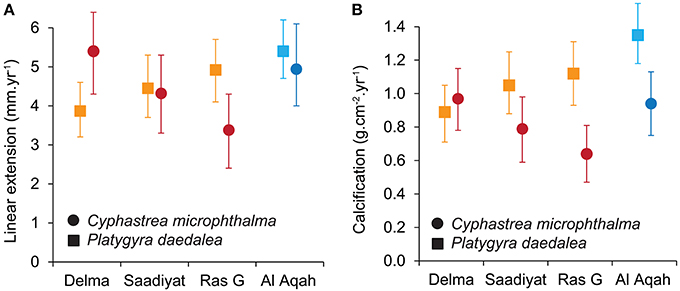
Figure 3. Site variation in linear extension (A) and calcification (B) for the corals Cyphastrea microphthalma and Platygyra daedalea in the Persian Gulf (red/orange) and Oman Sea (dark blue/light blue). Values are means and 95% highest posterior density (HPD) intervals from model outputs.
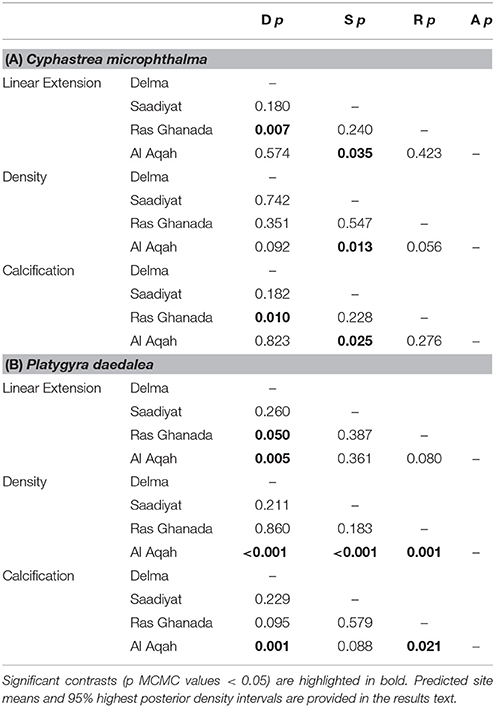
Table 2. Summary of site contrasts in linear extension, density, and calcification Generalized Linear Mixed Models for (A) Cyphastrea microphthalma and (B) Platygyra daedalea in the Persian Gulf and Oman Sea.
Coral growth patterns were associated with east-west gradients in temperature, irradiance, salinity and primary productivity in the Gulf (Table 1). Annual average temperatures among sites were similar, however Ras Ghanada in the east had the hottest summer (28 day maximum: 34.9°C) and Delma in the west had the coldest winter (28 day minimum: 19.7°C). Similarly, Ras Ghanada and Saadiyat had the lowest irradiance (midday PAR: <500 μmol. photons m−2.s−1) and highest productivity (annual mean chlorophyll-a: ~1.2 mg.m−3), while Delma was the most hypersaline (42–43 psu). Correspondingly, in C. microphthalma, linear extension and calcification was negatively associated with maximum temperatures and primary productivity, and positively associated with irradiance (Table 3). Conversely, P. daedalea growth was positively associated with minimum temperature and primary productivity and negatively associated with hypersalinity. In terms of disturbance, no bleaching or diseases were recorded on colonies at any sites during winter or summer surveys. Finally, Symbiodinium identity had no discernible influence on the growth rates of either C. microphthalma or P. daedalea within the Gulf. Symbiodinium was identified in 96% of colonies and the majority (86%) had dominant associations with clade C (Table 1). The remaining samples had either dominant associations with clade A (10%) or a mix of both clades (4%). Clade A was more prevalent at Delma and Ras Ghanada than at Saadiyat. However, for both coral species, there was no effect of Symbiodinium clade on colony rates of linear extension (Table 3). Sequencing of the Symbiodinium ITS2 region in a subset of colonies across species and sites confirmed these symbionts as types C3 (n = 12) and A1 (n = 1) in line with earlier studies (e.g., Hume et al., 2013; Howells et al., 2016b).
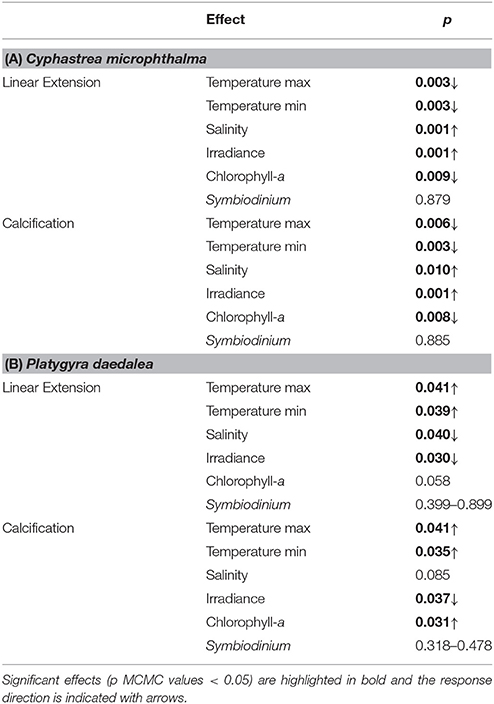
Table 3. Summaries of environmental effects on linear extension and calcification for (A) Cyphastrea microphthalma and (B) Platygyra daedalea in the Persian Gulf.
Growth Comparisons between the Southern Persian Gulf and Oman Sea
In the Oman Sea, linear extension in C. microphthalma was variable among colonies, but on average (5.0 mm.y−1, 4.0–6.1; mean, 95% HPD intervals), comparable to Delma (5.4 mm.y−1) in the western Gulf and 16–59% higher than the remaining two Gulf sites (Figure 3A; Table 2A). In contrast, extension in P. daedalea in the Oman Sea (5.4 mm.y−1, 4.7–6.2) was up to 38% higher than at all Gulf sites (but this comparison was only statistically significant for the Delma site; Table 2B). For both species, colonies from the Oman Sea had marginally higher skeletal density than those within the Gulf (Figure 4). For C. microphthalma, site variation in density was only apparent when modeled with linear extension, and was 6-8% higher in the Oman Sea (2.00 g.cm−3, 1.88–2.11 HPD intervals) for any given colony extension estimate than at Gulf sites, but only significantly so in relation to Ras Ghanada (1.79 g.cm−3, 1.68–1.90; Figure 4; Table 2A). For P. daedalea, there was no covariation of density with extension, and colonies had significantly higher density (by 9–13%) in the Oman Sea (2.58 g.cm−3, 2.49–2.66) than at all Gulf sites (2.26–2.35 g.cm−3; Table 2B). Despite the higher density of colony skeletons in the Oman Sea, regional calcification differences were primarily driven by linear extension. Correspondingly, for C. microphthalma, calcification in the Oman Sea (0.94 g.cm−2.y−1, 0.75–1.13) was similar to Delma (0.97 g.cm−2.y−1), 49% higher than Ras Ghanada and 19% higher than Saadiyat (non-significant) (Figure 3B; Table 2A). For P. daedalea, calcification in the Oman Sea (1.36 g.cm−2.y−1, 1.18–1.54) was higher than all Gulf sites by 53% at Delma, 28% at Saadiyat, and 21% at Ras Ghanada (non-significant) (Table 2B).
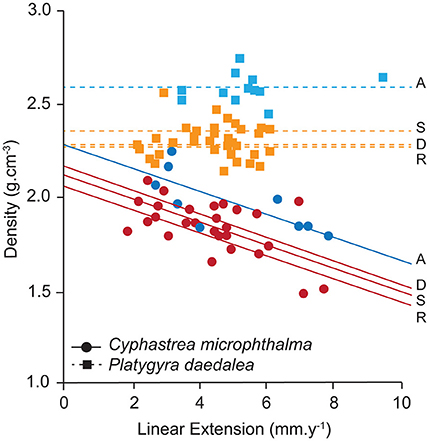
Figure 4. Site variation in density and its relationship to linear extension for the corals Cyphastrea microphthalma and Platygyra daedalea in the Persian Gulf (red/orange) and Oman Sea (dark blue/light blue). Values are means of colonies (symbols) and sites (lines) from model outputs and raw data summaries provided in Table S1. D, Delma; S, Saadiyat; R, Ras Ghanada; A, Al Aqah.
Marked variation in environmental conditions was apparent among the three study locations in the southern Persian Gulf, and relative to the Oman Sea. In the Oman Sea, temperature extremes were considerably milder than the Gulf by up to 3°C in summer (28-day maximum: 32.1°C) and winter (28-day minimum: 22.7°C; Table 1). Additionally, benthic light availability was ~40–90% higher in the Oman Sea than in the Gulf (midday PAR: 790–840 μmol photons.m−2.s−1) and salinity approached oceanic values (37–37.5 psu). A further factor differentiating the Oman Sea from the Gulf was apparent in the symbiont communities in colonies of both species. All C. microphthalma or P. daedalea colonies hosted Symbiodinium clade D as a dominant symbiont (confirmed as type D1a; Table 1), although several colonies (27%) also harbored clade C at detectable background levels.
Growth Comparisons among Conspecifics in the Indo-Pacific
For C. microphthalma, Gulf and Oman Sea growth rates were relatively comparable to the few available records for Cyphastrea (Figure 5A). The range of annual linear extension for C. microphthalma in this study was similar to values reported from C. serailia at Lord Howe Island (3.0 mm.yr−1, Harriott, 1999) and C. ocellina in Hawaii (3.9 mm.yr−1, Romano, 1990), but about half that of C. serailia at Moreton Bay (7.2–10.4 mm.yr−1, Roberts and Harriott, 2003). Among these published records, there was no clear relationship between annual linear extension and local temperature parameters.
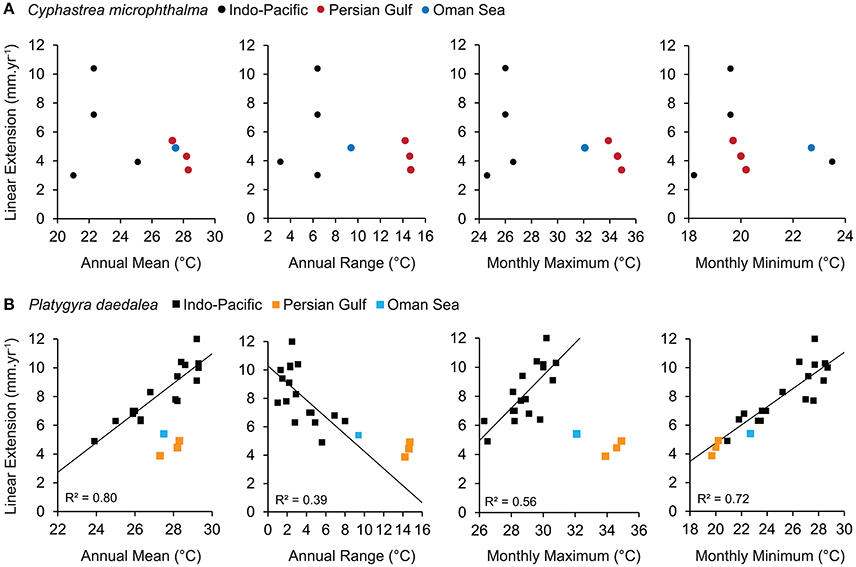
Figure 5. Relationship between linear extension and sea temperature for the corals (A) Cyphastrea spp. and (B) Platygyra spp. Data from the Persian Gulf and Oman Sea in this study are compared to relationships from available records for the wider Indo-Pacific. Relationships between linear extension and sea temperature were only apparent for Platygyra records and are indicated with trend lines and R2 values.
In contrast, growth rates of P. daedalea in this study were among the lowest recorded for this genus (Figure 5B). In particular, annual linear extension of P. daedalea from all Gulf sites was equivalent or below the previous lowest record from the Southern Great Barrier Reef (4.9 mm.yr−1, Weber and White, 1974) and 14–82% lower than other Indo-Pacific localities (Knutson et al., 1972; Weber and White, 1974; Simpson, 1988; Babcock, 1991). Linear extension of Platygyra from previous studies shows a strong positive relationships with annual temperature means (R2 = 0.80) and maxima (R2 = 0.56), however both Gulf and Oman Sea extension rates of P. daedalea fell well below expected values based on these relationships. Conversely, a strong positive effect of temperature minima (R2 = 0.72) is also a good predictor of P. daedalea extension in the Gulf and Oman Sea indicating universal constraint of cold temperatures on Platygyra growth. Additionally, P. daedalea extension somewhat exceeded expectations when the range of seasonal temperatures was considered (R2 = 0.39), suggesting a degree of local adaptation to extreme environments.
Discussion
This study shows that annual rates of calcification and linear extension for P. daedalea and C. microphthalma in the extreme southern Persian Gulf are highly constrained relative to growth rates recorded for conspecifics in the wider Indo-Pacific. This adds to a very limited number of regional growth estimates for the wider Gulf region (Lough et al., 2003; Samiei et al., 2016), which are potentially important in understanding the environmental tolerances of corals subject to increasing environmental change. Despite considering two different coral species (P. daedalea and C. microphthalma) with shared taxonomic affinities (Family Merulinidae) and similarities in their growth form and life-histories (Veron, 2000; Darling et al., 2012; Huang et al., 2014), this study revealed marked taxonomic differences in both the investment in calcification, as well as specific environmental constraints on annual rates of calcification and linear extension. Substantial variation in the performance of individual colonies was also detected within each reef despite uniform depth and the majority of colonies hosting identical symbiont types. Such inter- and intra-species heterogeneity in coral growth has implications for accurately detecting and predicting the effects of environmental change on the productivity of species assemblages.
Differential Investment in Growth within and among Species
For both P. daedalea and C. microphthalma, large variation in annual rates of linear extension and calcification were apparent both within and among individual colonies, which typically exceeded differences among sites (Figure S1; Table S1). This may be attributable to fine-scale differences in environmental conditions (micro-climates), specific genotypic differences or individual variation in symbiont types. Coral transplant, breeding, and symbiont inoculation studies indicate that growth rates are at least partially determined by genetic factors (e.g., Little et al., 2004; Howells et al., 2013; Kenkel et al., 2015; Drury et al., 2017). For example, colony genotype accounted for 6% of growth variation in Acropora cervicornis fragments transplanted to eight Florida reefs (Drury et al., 2017), and parental effects (genetic + epigenetic + maternal) were responsible for 10–30% of the early growth variation in Porites astreoides juveniles (Kenkel et al., 2015). Additionally, in juvenile corals (Acropora millepora), Symbiodinium clade identity has been shown to be a stronger determinant of growth rate than both parental population and grow out environment (Mieog et al., 2009). Furthermore, reduced access to water flow and/or irradiance in sheltered and shaded reef areas can suppress growth rates relative to colonies in adjacent exposed areas (Sebens et al., 2003). In this study, there was no clear contribution of Symbiodinium type to growth rates. In the Persian Gulf, the minority of P. daedalea and C. microphthalma colonies hosting predominantly Symbiodinium type A1 did not consistently grow faster or slower than the majority of colonies hosting type C3 (although there was limited power to detect a difference due to the small number of colonies hosting A1). As Symbiodinium type D1a associations were unique to the Oman Sea, any effect of this Symbiodinium type on individual growth responses are confounded by site-specific factors (discussed below). This suggests that host genotypes and/or micro-climates were potentially more important determinants of individual growth responses. Regardless of the ultimate causes of variation, our results highlight the need for large sample sizes to generate representative estimates of skeletal extension and density in target populations.
Overall, mean linear extension rates were highly similar between the two species (4.67 vs. 4.51 mm.y−1), however P. daedalea had higher rates of calcification to achieve similar rates of linear extension, owing to its denser skeleton (2.36 vs. 1.88 g.cm−3). Colonies of Platygyra are generally more vulnerable to environmental stress relative to sympatric Cyphastrea. Meta-analyses of thermal stress events show Platygyra is moderately susceptible to bleaching whereas Cyphastrea is highly resistant (McClanahan et al., 2004; Hoey et al., 2016). Additionally, during anomalously cold temperatures in the western Persian Gulf, widespread mortality has been observed in Platygyra but not Cyphastrea colonies (Coles and Fadlallah, 1991). Increased investment in stress tolerance in C. microphthalma could limit the allocation of resources to growth, thereby accounting for the observed trade-off between linear extension and density among individual colonies (Figure 4). Similar negative relationships between extension and density have been documented in stress-tolerant massive Porites (Scoffin et al., 1992; Lough and Barnes, 2000). Inter-specific differences in growth strategies could also be explained by variation in energy acquisition. While the clade and type identity of Symbiodinium communities of P. daedalea and C. microphthalma was highly similar at each site (Table 1), symbiosis nutrition is influenced by several potential factors that were not accounted for in this study. These include functional differences among fine-scale symbiont genotypes (Howells et al., 2012; Parkinson et al., 2016), host species effects on the internal light environment (Wangpraseurt et al., 2012; Enríquez et al., 2017) and species differences in heterotrophic feeding capacity (Goreau et al., 1971; Anthony and Fabricius, 2000).
Regional Effects of Environmental Drivers on Growth Rates
In our study, opposing spatial gradients in linear extension and calcification between P. daedalea and C. microphthalma demonstrate that effects of environmental drivers on coral growth are likely to be species-specific (Figure 3). Declines in growth rates for P. daedalea from the Oman Sea across the southern Gulf corresponded with decreasing minimum monthly temperatures and primary productivity, and increasing hypersalinity. Annual minimum temperatures are a strong predictor of Indo-Pacific wide linear extension rates in Platygyra (R2 = 0.72, Figure 5B) and calcification rates in massive Porites (Lough and Barnes, 2000). Thus, we infer that the duration of cold exposure is the most plausible constraint on P. daedalea growth rates from east to west in our study, where sites experienced temperatures below 20°C for 0, 8, 16, and 33 days, respectively (Table S3). Furthermore, the reduced growth of P. daedalea in colder waters was consistent with their higher sensitivity to cold stress (bleaching and mortality), relative to C. microphthalma (Coles and Fadlallah, 1991). However, negative impacts of hypersalinity (e.g., Lirman and Manzello, 2009) and limited sources of heterotrophic food (potentially indicated by low chlorophyll-a levels; Anthony and Fabricius, 2000; Houlbrèque et al., 2003) warrant further investigation as potential additional constratints on the growth rates of P. daedalea.
The opposite gradient of decline in C. microphthalma extension and calcification from west to east across the southern Gulf was associated with hot temperatures and light limitation. Throughout the year, the three Gulf sites experienced 0, 27, and 34 days, respectively, above the regional bleaching threshold of 34.5°C (Shuail et al., 2016; Table S2). Additionally, the sites with lowest growth in C. microphthalma (Saadiyat and Ras Ghanada) were also those where coral populations experienced bleaching 1 year prior to the study (in September 2012). While this information points to a primary constraint of hot temperatures on the growth of C. microphthalma, we do not interpret that this is the case due to the known high heat tolerance of Cyphastrea (McClanahan et al., 2004) and the lack of an effect of bleaching on reproductive energy reserves at the study sites (Howells et al., 2016b). Instead we infer that light availability is a more likely driver of growth rates in C. microphthalma. Despite the shallow depth of all sites (<7 m), the high sediment resuspension in the Gulf potentially reduces PAR to sub-saturating levels at the inshore Saadiyat and Ras Ghanada. In-situ midday values of <500 μmol photons m−2 s−1 were recorded on calm summer days (Table 1) but these drop to less than 100 μmol photons m−2 s−1 following moderate to strong winds. The constraint of light limitation on the growth of massive corals has been shown in a meta-analysis of >200 growth records, where linear extension typically declined with increasing depth (Pratchett et al., 2015). However, when individual records are examined, it is evident that the importance of light availability to growth varies among massive species (Highsmith, 1979; Huston, 1985). For example, at a Central Pacific atoll, Highsmith (1979) found that the extension rates of Favia pallida only decreased slightly from 2 to 30 m, whereas declines in extension with depth were comparatively steep (~3 times higher) in both Porites lutea and Goniastrea retiformis.
Given the collinearity of environmental variables among sites, controlled experimental studies are required to validate the importance of specific factors in constraining or enhancing growth rates in both C. microphthalma and P. daedalea. Furthermore, understanding how environmental factors influence performance would benefit from measuring multi-trait responses in the same individuals.
Coral Growth under Extreme Conditions Is Slow but Exceeds Expectations
At broad spatial scales, annual mean sea temperature has been shown to be a reliable predictor of growth rates in several massive scleractinian genera including Platygyra (R2 = 0.80, Figure 5B; Weber and White, 1974), Orbicella (Weber and White, 1977), and Porites (Lough, 2008). However, mean temperatures are a poor representation of the thermal environment of the Persian Gulf (and the Oman Sea). While the Gulf sites in our study experience annual means similar to many low latitude reefs (27–28°C), summers (≥34°C) and winters (≤21°C) are considerably more extreme; for example, 4–6°C hotter and up to 10°C colder than sites within comparable growth records (Figure 5B). Consequently, it is not surprising that linear extension rates in P. daedalea in this study were only half (Gulf, 0.47–0.52) to two thirds (Oman Sea, 0.35) of the expected values based on mean sea temperatures. When looking at the ends of the temperature spectrum, it could be interpreted that while the effect of cold winters is entirely consistent with an Indo-Pacific wide constraint of cold temperatures on growth, the hot summers of Gulf (and the Oman Sea) far exceed the optimal conditions for coral growth. However, confirming these interpretations requires seasonal based growth measurements that track growth rates during the hottest and coldest months of the year. For the continuous annual data in our study, the range in temperatures between seasons provides the best representation of regional thermal environments for global comparisons. Relative to the Indo-Pacific decline in extension with increasing temperature range (R2 = 0.39), P. daedalea extension at Gulf sites was 1.3–2.5 times higher than expected.
For Cyphastrea, there was no obvious Pacific-wide relationship between linear extension and any temperature parameters (Figure 5A). While there are relatively few records for Cyphastrea, comparison with these did not indicate linear extension is strongly constrained by environmental conditions in the Gulf. Similarly, records from other coral genera also suggest that coral growth rates in the Persian Gulf somewhat exceed environmental expectations. Annual linear extension and calcification in massive Porites colonies in the central-western Persian Gulf did not deviate from Indo-Pacific relationships with annual temperature means (Lough, 2008) despite experiencing seasonal extremes in temperature of similar magnitude to the southern Gulf. Additionally, estimated annual calcification rates of Acropora downingi from the north-eastern Persian Gulf were comparable to values from several Acropora records from the Indo-Pacific and exceeded values from similar thermal environments in the central Red Sea (Roik et al., 2016; Samiei et al., 2016).
Can Locally Adapted Growth Performance Be Maintained under Climate Warming?
This study, along with previous estimates of coral growth from the Persian Gulf (Lough, 2008; Samiei et al., 2016) provide evidence that at least some corals have adapted (or acclimatized) to the marginal and extreme environmental conditions in this region. The capacity of these corals to withstand extreme temperatures and chronic hypersalinity, whether due to coral host attributes or Symbiodinium partners (D'Angelo et al., 2015; Howells et al., 2016a), indicates that at least some corals (species or genotypes) could potentially withstand more moderate predictions of projected global climate change. However, in the Persian Gulf, annual rates of calcification and linear extension for corals are very low, especially for P. daedalea, and are likely to become increasingly constrained with ongoing changes in environmental conditions within this region. The Arabian Peninsula is one of the most rapidly warming regions, with recent decadal increases in minimum and maximum air temperatures of 0.49 and 0.74°C, respectively (Saudi Arabia, 1979–2009, Almazroui et al., 2012). As the Persian Gulf is a very shallow semi-enclosed sea, regional air temperatures are the predominant driver of local sea surface temperatures (Sheppard and Loughland, 2002), and these are expected to rise a further 5–7°C by 2100 (RCP 8.5, Collins et al., 2013).
More extreme summer temperatures in the Gulf would likely increase the seasonal duration at which optimal growth temperatures are exceeded, especially as corals in this region are living within a small margin (<1°C) of their upper thermal limits of survival (Howells et al., 2016a). Recent declines in the linear extension and calcification rates of corals have been observed in several species and regions and have been linked to climate warming (Cooper et al., 2008; De'ath et al., 2009, 2013; Cantin et al., 2010; Carricart-Ganivet et al., 2012; Tanzil et al., 2013). Such negative effects of warming on coral growth in the Gulf would likely be exacerbated by any increase in coral bleaching episodes which already occur at relatively high frequency in the southern Gulf (Coles and Riegl, 2013) as the energetic costs of bleaching survival often include a period of stunted growth (Goreau and Macfarlane, 1990; Carilli et al., 2010). An alternative outcome of sea temperature rise for Gulf corals is that an increase in winter minima alleviates cold constraints on coral growth. For example, on subtropical reefs, some corals have achieved gains in extension and calcification over periods of warming (Cooper et al., 2012; Anderson et al., 2015).
At present, we are unable to speculate on the responses of Gulf corals to recent warming or predict their responses to future warming. Multi-year skeletal extension and density data are needed to determine how summer, winter, and annual growth rates have changed in the Gulf in response to rising baseline temperatures as well as a high frequency of acute heat stress events (i.e., ~7 in the past 20 years in Abu Dhabi; Coles and Riegl, 2013; Howells et al., 2016b). Future investigations should aim to incorporate several coral species as it is clear from our study that sympatric corals can exhibit divergent responses to the extreme conditions in the Gulf.
Author Contributions
Designed the study: AB, MP, EH. Performed the fieldwork: EH, AB, GD, DM. Processed and measured samples: EH, AB, GD, DM, GV. Provided environmental data: JB, GV, SH, EH. Analyzed the data: GD. Wrote the manuscript: EH with assistance from MP. Revised and approved the manuscript: all authors.
Funding
The project was funded by New York University Abu Dhabi (JB, EH) and the AXA Research Fund (AB, Postdoctoral Fellowship 154-000-649-507). Additional support was provided from the NOAA Coral Reef Conservation Program and the NOAA/NESDIS Center for Satellite Applications and Research (SH).
Conflict of Interest Statement
GD was employed by company Ecological Marine Services throughout the duration of this study. Ecological Marine Services received no payment in relation to this work and GD declares no competing interest in relation to his employment with a private company.
The other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling Editor declared a past co-authorship with one of the authors SH.
Acknowledgments
This research was permitted by the Environment Agency Abu Dhabi, Fujairah Municipality, and Dibba Municipality in the UAE. We thank New York University Abu Dhabi, the Palms Dive Center, Al Mahara Dive Center and Ben Hume for their assistance with fieldwork. We also thank Al Hashem Marble in Abu Dhabi for sectioning our coral specimens, the Environment Agency Abu Dhabi for providing temperature logger data for the Delma site, and the NASA Ocean Biology Processing Group for providing satellite PAR data. The scientific results and conclusions, as well as any views or opinions expressed herein, are those of the author(s) and do not necessarily reflect the views of NOAA or the Department of Commerce.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2018.00056/full#supplementary-material
References
Almazroui, M., Nazrul Islam, M., Athar, H., Jones, P., and Rahman, M. A. (2012). Recent climate change in the Arabian Peninsula: annual rainfall and temperature analysis of Saudi Arabia for 1978–2009. Int. J. Climatol. 32, 953–966. doi: 10.1002/joc.3446
Anderson, K. D., Heron, S. F., and Pratchett, M. S. (2015). Species-specific declines in the linear extension of branching corals at a subtropical reef, Lord Howe Island. Coral Reefs 34, 479–490. doi: 10.1007/s00338-014-1251-1
Anthony, K. R., and Fabricius, K. E. (2000). Shifting roles of heterotrophy and autotrophy in coral energetics under varying turbidity. J. Exp. Mar. Biol. Ecol. 252, 221–253. doi: 10.1016/S0022-0981(00)00237-9
Babcock, R. C. (1991). Comparative demography of three species of scleractinian corals using age-and size-dependent classifications. Ecol. Monogr. 61, 225–244. doi: 10.2307/2937107
Brown, B. E., Downs, C. A., Dunne, R. P., and Gibb, S. W. (2002). Exploring the basis of thermotolerance in the reef coral Goniastrea aspera. Mar. Ecol. Progr. Ser. 242, 119–129. doi: 10.3354/meps242119
Bucher, D. J., Harriott, V. J., and Roberts, L. G. (1998). Skeletal micro-density, porosity and bulk density of acroporid corals. J. Exp. Mar. Biol. Ecol. 228, 117–136. doi: 10.1016/S0022-0981(98)00020-3
Burt, J., Al-Harthi, S., and Al-Cibahy, A. (2011). Long-term impacts of coral bleaching events on the world's warmest reefs. Mar. Environ. Res. 72, 225–229. doi: 10.1016/j.marenvres.2011.08.005
Cantin, N. E., Cohen, A. L., Karnauskas, K. B., Tarrant, A. M., and McCorkle, D. C. (2010). Ocean warming slows coral growth in the central Red Sea. Science 329, 322–325. doi: 10.1126/science.1190182
Carilli, J. E., Norris, R. D., Black, B., Walsh, S. M., and McFieldm, M. (2010). Century-scale records of coral growth rates indicate that local stressors reduce coral thermal tolerance threshold. Glob. Chang. Biol. 16, 1247–1257. doi: 10.1111/j.1365-2486.2009.02043.x
Carricart-Ganivet, J. P., Cabanillas-Teran, N., Cruz-Ortega, I., and Blanchon, P. (2012). Sensitivity of calcification to thermal stress varies among genera of massive reef-building corals. PLoS ONE 7:e32859. doi: 10.1371/journal.pone.0032859
Coles, S. L., and Fadlallah, Y. H. (1991). Reef coral survival and mortality at low temperatures in the Arabian Gulf: new species species-specific lower temperature limits. Coral Reefs 9, 231–237. doi: 10.1007/BF00290427
Coles, S. L., and Riegl, B. M. (2013). Thermal tolerances of reef corals in the Gulf: a review of the potential for increasing coral survival and adaptation to climate change through assisted translocation. Mar. Pollut. Bull. 72, 323–332. doi: 10.1016/j.marpolbul.2012.09.006
Collins, M., Knutti, R., Arblaster, J., Dufresne, J.-L., Fichefet, T., Friedlingstein, P., et al. (2013). “Long-term climate change: projections, commitments and irreversibility,” in Climate Chage 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernment Panel on Climate Change, eds T. Stocker, D. Qin, G. K. Plattner, M. Tignor, S. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex, and P. Midgley (Cambridge: Cambridge University Press), 1029–1136.
Cooper, T. F., Berkelmans, R., Ulstrup, K. E., Weeks, S., Radford, B., Jones, A. M., et al. (2011). Environmental factors controlling the distribution of Symbiodinium harboured by the coral Acropora millepora on the Great Barrier Reef. PLoS ONE 6:e25536. doi: 10.1371/journal.pone.0025536
Cooper, T. F., De'Ath, G., Fabricius, K. E., and Lough, J. M. (2008). Declining coral calcification in massive Porites in two nearshore regions of the northern Great Barrier Reef. Glob. Chang. Biol. 14, 529–538. doi: 10.1111/j.1365-2486.2007.01520.x
Cooper, T. F., O'Leary, R. A., and Lough, J. M. (2012). Growth of Western Australian corals in the Anthropocene. Science 335, 593–596. doi: 10.1126/science.1214570
D'Angelo, C., Hume, B. C., Burt, J., Smith, E. G., Achterberg, E. P., and Wiedenmann, J. (2015). Local adaptation constrains the distribution potential of heat-tolerant Symbiodinium from the Persian/Arabian Gulf. ISME J. 9, 2551–2560. doi: 10.1038/ismej.2015.80
Darling, E. S., Alvarez-Filip, L., Oliver, T. A., McClanahan, T. R., and Côté, I. M. (2012). Evaluating life-history strategies of reef corals from species traits. Ecol. Lett. 15, 1378–1386. doi: 10.1111/j.1461-0248.2012.01861.x
De'ath, G., Fabricius, K., and Lough, J. (2013). Yes—Coral calcification rates have decreased in the last twenty-five years! Mar. Geol. 346, 400–402. doi: 10.1016/j.margeo.2013.09.008
De'ath, G., Lough, J. M., and Fabricius, K. E. (2009). Declining coral calcification on the Great Barrier Reef. Science 323, 116–119. doi: 10.1126/science.1165283
Dixon, G. B., Davies, S. W., Aglyamova, G. A., Meyer, E., Bay, L. K., and Matz, M. V. (2015). Genomic determinants of coral heat tolerance across latitudes. Science 348, 1460–1462. doi: 10.1126/science.1261224
Drury, C., Manzello, D., and Lirman, D. (2017). Genotype and local environment dynamically influence growth, disturbance response and survivorship in the threatened coral, Acropora cervicornis. PLoS ONE 12:e0174000. doi: 10.1371/journal.pone.0174000
Dustan, P. (1975). Growth and form in the reef-building coral Montastrea annularis. Mar. Biol. 33, 101–107. doi: 10.1007/BF00390714
Enríquez, S., Méndez, E. R., Hoegh-Guldberg, O., and Iglesias-Prieto, R. (2017). Key functional role of the optical properties of coral skeletons in coral ecology and evolution. Proc. Biol. Sci. 284:20161667. doi: 10.1098/rspb.2016.1667
Fabricius, K. E., Langdon, C., Uthicke, S., Humphrey, C., Noonan, S., De'ath, G., et al. (2011). Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nat. Clim. Chang. 1, 165–169. doi: 10.1038/nclimate1122
Fabricius, K. E., Mieog, J. C., Colin, P. L., Idip, D., and Van Oppen, M. J. H. (2004). Identity and diversity of coral endosymbionts (zooxanthellae) from three Palauan reefs with contrasting bleaching, temperature and shading histories. Mol. Ecol. 13, 2445–2458. doi: 10.1111/j.1365-294X.2004.02230.x
Frouin, R., Franz, B. A., and Werdell, P. J. (2002). “The SeaWiFS PAR product,” in Algorithm Updates for the Fourth SeaWiFS Data Reprocessing, NASA Tech. Memo. 2003–206892, Vol. 22, eds S. B. Hooker and E. R. Firestone (Greenbelt: NASA Goddard Space Flight Center), 46–50.
Goreau, T., and Macfarlane, A. (1990). Reduced growth rate of Montastrea annularis following the 1987–1988 coral-bleaching event. Coral Reefs 8, 211–215. doi: 10.1007/BF00265013
Goreau, T. F., Goreau, N. I., and Yonge, C. (1971). Reef corals: autotrophs or heterotrophs? Biol. Bull. 141, 247–260. doi: 10.2307/1540115
Graham, N. A. J., and Nash, K. L. (2013). The importance of structural complexity in coral reef ecosystems. Coral Reefs 32, 315–326. doi: 10.1007/s00338-012-0984-y
Grottoli, A. G., Warner, M. E., Levas, S. J., Aschaffenburg, M. D., Schoepf, V., McGinley, M., et al. (2014). The cumulative impact of annual coral bleaching can turn some coral species winners into losers. Glob. Chang. Biol. 20, 3823–3833. doi: 10.1111/gcb.12658
Hadfield, J. (2015). MCMCglmm Course Notes. Available online at: https://cran.r-project.org/web/packages/MCMCglmm/vignettes
Hadfield, J. D. (2010). MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33, 1–22. doi: 10.18637/jss.v033.i02
Harriott, V. (1999). Coral growth in subtropical eastern Australia. Coral Reefs 18, 281–291. doi: 10.1007/s003380050195
Hennige, S. J., Smith, D. J., Walsh, S.-J., McGinley, M. P., Warner, M. E., and Suggett, D. J. (2010). Acclimation and adaptation of scleractinian coral communities along environmental gradients within an Indonesian reef system. J. Exp. Mar. Biol. Ecol. 391, 143–152. doi: 10.1016/j.jembe.2010.06.019
Heron, S. F., Johnston, L., Liu, G., Geiger, E. F., Maynard, J. A., De La Cour, J. L., et al. (2016a). Validation of reef-scale thermal stress satellite products for coral bleaching monitoring. Remote Sens. 8:59 doi: 10.3390/rs8010059
Heron, S. F., Maynard, J. A., and Ruben van Hooidonk, C. (2016b). Warming trends and bleaching stress of the world's coral reefs 1985–2012. Sci. Rep. 6:38402. doi: 10.1038/srep38402
Highsmith, R. C. (1979). Coral growth rates and environmental control of density banding. J. Exp. Mar. Biol. Ecol. 37, 105–125. doi: 10.1016/0022-0981(79)90089-3
Hoey, A. S., Howells, E., Johansen, J. L., Hobbs, J.-P. A., Messmer, V., McCowan, D. M., et al. (2016). Recent advances in understanding the effects of climate change on coral reefs. Diversity 8:12. doi: 10.3390/d8020012
Houlbrèque, F., Tambutté, E., and Ferrier-Pagès, C. (2003). Effect of zooplankton availability on the rates of photosynthesis, and tissue and skeletal growth in the scleractinian coral Stylophora pistillata. J. Exp. Mar. Biol. Ecol. 296, 145–166. doi: 10.1016/S0022-0981(03)00259-4
Howells, E. J., Abrego, D., Meyer, E., Kirk, N. L., and Burt, J. A. (2016a). Host adaptation and unexpected symbiont partners enable reef-building corals to tolerate extreme temperatures. Glob. Chang. Biol. 22, 2702–2714 doi: 10.1111/gcb.13250
Howells, E. J., Beltran, V. H., Larsen, N. W., Bay, L. K., Willis, B. L., and van Oppen, M. J. H. (2012). Coral thermal tolerance shaped by local adaptation of photosymbionts. Nat. Clim. Chang. 2, 116–120. doi: 10.1038/nclimate1330
Howells, E. J., Berkelmans, R., van Oppen, M. J., Willis, B. L., and Bay, L. K. (2013). Historical thermal regimes define limits to coral acclimatization. Ecology 94, 1078–1088. doi: 10.1890/12-1257.1
Howells, E. J., Ketchum, R. N., Bauman, A. G., Mustafa, Y., Watkins, K. D., and Burt, J. A. (2016b). Species-specific trends in the reproductive output of corals across environmental gradients and bleaching histories. Mar. Pollut. Bull. 105, 532–539. doi: 10.1016/j.marpolbul.2015.11.034
Huang, D., Benzoni, F., Fukami, H., Knowlton, N., Smith, N. D., and Budd, A. F. (2014). Taxonomic classification of the reef coral families Merulinidae, Montastraeidae, and Diploastraeidae (Cnidaria: Anthozoa: Scleractinia). Zool. J. Linn. Soc. 171, 277–355. doi: 10.1111/zoj.12140
Hughes, T. P., Baird, A. H., Dinsdale, E. A., Moltschaniwskyj, N. A., Pratchett, M. S., Tanner, J. E., et al. (2012). Assembly rules of reef corals are flexible along a steep climatic gradient. Curr. Biol. 22, 736–741. doi: 10.1016/j.cub.2012.02.068
Hughes, T. P., Kerry, J. T., Álvarez-Noriega, M., Álvarez-Romero, J. G., Anderson, K. D., Baird, A. H., et al. (2017). Global warming and recurrent mass bleaching of corals. Nature 543, 373–377. doi: 10.1038/nature21707
Hume, B., D'Angelo, C., Burt, J., Baker, A., Riegl, B., and Wiedenmann, J. (2013). Corals from the Persian/Arabian Gulf as models for thermotolerant reef-builders: prevalence of clade C3 Symbiodinium, host fluorescence and ex situ temperature tolerance. Mar. Pollut. Bull. 72, 313–322. doi: 10.1016/j.marpolbul.2012.11.032
Huston, M. (1985). Variation in coral growth rates with depth at discovery Bay, Jamaica. Coral Reefs 4, 19–25. doi: 10.1007/BF00302200
Kenkel, C., Setta, S., and Matz, M. (2015). Heritable differences in fitness-related traits among populations of the mustard hill coral, Porites astreoides. Heredity 115, 509–516 doi: 10.1038/hdy.2015.52
Kleypas, J. A., McManus, J. W., and Meñez, L. A. (1999). Environmental limits to coral reef development: where do we draw the line? Am. Zool. 39, 146–159.
Knutson, D. W., Buddemeier, R. W., and Smith, S. V. (1972). Coral chronometers: seasonal growth bands in reef corals. Science 177, 270–272. doi: 10.1126/science.177.4045.270
LaJeunesse, T. C., and Trench, R. K. (2000). Biogeography of two species of Symbiodinium (Freudenthal) inhabiting the intertidal sea anemone Anthopleura elegantissima (Brandt). Biol. Bull. 199, 126–134. doi: 10.2307/1542872
Lirman, D., and Manzello, D. (2009). Patterns of resistance and resilience of the stress-tolerant coral Siderastrea radians (Pallas) to sub-optimal salinity and sediment burial. J. Exp. Mar. Biol. Ecol. 369, 72–77. doi: 10.1016/j.jembe.2008.10.024
Little, A. F., van Oppen, M. J. H., and Willis, B. L. (2004). Flexibility in algal endosymbioses shapes growth in reef corals. Science 304, 1492–1494. doi: 10.1126/science.1095733
Lough, J., and Barnes, D. (2000). Environmental controls on growth of the massive coral Porites. J. Exp. Mar. Biol. Ecol. 245, 225–243. doi: 10.1016/S0022-0981(99)00168-9
Lough, J. J., Devereux, M. M., and Barnes, D. D. (2003). Porites Coral Growth Records from the Arabian Gulf. Townsville, QLD: Australian Institute of Marine Science.
Lough, J. M. (2008). Coral calcification from skeletal records revisited. Mar. Ecol. Prog. Ser. 373, 257–264. doi: 10.3354/meps07398
Lough, J. M., and Cantin, N. E. (2014). Perspectives on massive coral growth rates in a changing ocean. Biol. Bull. 226, 187–202. doi: 10.1086/BBLv226n3p187
Loya, Y., Sakai, K., Yamazato, K., Nakano, Y., Sambali, H., and van Woesik, R. (2001). Coral bleaching: the winners and the losers. Ecol. Lett. 4, 122–131. doi: 10.1046/j.1461-0248.2001.00203.x
McClanahan, T. R., Baird, A. H., Marshall, P. A., and Toscano, M. A. (2004). Comparing bleaching and mortality responses of hard corals between southern Kenya and the Great Barrier Reef, Australia. Mar. Pollut. Bull. 48, 327–335. doi: 10.1016/j.marpolbul.2003.08.024
Metzger, E., Hurlburt, H., Xu, X., Shriver, J. F., Gordon, A., Sprintall, J., et al. (2010). Simulated and observed circulation in the Indonesian Seas: 1/12 global HYCOM and the INSTANT observations. Dyn. Atmos. Oceans 50, 275–300. doi: 10.1016/j.dynatmoce.2010.04.002
Mieog, J. C., Olsen, J. L., Berkelmans, R., Bleuler-Martinez, S. A., Willis, B. L., and van Oppen, M. J. H. (2009). The roles and interactions of symbiont, host and environment in defining coral fitness. PLoS ONE 4:e6364. doi: 10.1371/journal.pone.0006364
Morgan, K. M., and Kench, P. S. (2012). Skeletal extension and calcification of reef-building corals in the central Indian Ocean. Mar. Environ. Res. 81 78–82. doi: 10.1016/j.marenvres.2012.08.001
NASA Goddard Space Flight Center, Ocean Ecology Laboratory Ocean Biology Processing Group (2014). MODIS-Aqua Mapped Daily 4 km Photosynthetically Available Radiation(PAR). Available online at: https://oceandata.sci.gsfc.nasa.gov/ (Accessed March 2015).
Palumbi, S. R., Barshis, D. J., Traylor-Knowles, N., and Bay, R. A. (2014). Mechanisms of reef coral resistance to future climate change. Science 344, 895–898. doi: 10.1126/science.1251336
Parkinson, J. E., Baumgarten, S., Michell, C. T., Baums, I. B., LaJeunesse, T. C., and Voolstra, C. R. (2016). Gene expression variation resolves species and individual strains among coral-associated dinoflagellates within the genus Symbiodinium. Genome Biol. Evol. 8, 665–680. doi: 10.1093/gbe/evw019
Perry, C., Edinger, E., Kench, P., Murphy, G., Smithers, S., Steneck, R., et al. (2012). Estimating rates of biologically driven coral reef framework production and erosion: a new census-based carbonate budget methodology and applications to the reefs of Bonaire. Coral Reefs 31, 853–868. doi: 10.1007/s00338-012-0901-4
Pratchett, M. S., Anderson, K. D., Hoogenboom, M. O., Widman, E., Baird, A. H., Pandolfi, J. M., et al. (2015). Spatial, temporal and taxonomic variation in coral growth—implications for the structure and function of coral reef ecosystems. Oceanogr. Mar. Biol. Annu. Rev. 53, 215–296. doi: 10.1201/b18733-7
R Core Team (2016). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: https://www.R-project.org/
Riegl, B. M., Benzoni, F., Samimi-Namin, K., and Sheppard, C. (2012). “The hermatypic scleractinian (hard) coral fauna of the Gulf,” in Coral Reefs of the Gulf, eds B. Riegl and S. J. Purkis (Dordrecht: Springer), 187–224.
Riegl, B. M., and Purkis, S. J. (eds.). (2012). “Environmental constraints for reef building in the Gulf,” in Coral Reefs of the Gulf (Dordrecht: Springer), 5–32.
Riegl, B. M., Purkis, S. J., Al-Cibahy, A. S., Abdel-Moati, M. A., and Hoegh-Guldberg, O. (2011). Present limits to heat-adaptability in corals and population-level responses to climate extremes. PLoS ONE 6:e24802. doi: 10.1371/journal.pone.0024802
Roberts, L., and Harriott, V. (2003). Can environmental records be extracted from coral skeletons from Moreton Bay, Australia, a subtropical, turbid environment? Coral Reefs 22, 517–522. doi: 10.1007/s00338-003-0337-y
Roik, A., Roder, C., Röthig, T., and Voolstra, C. R. (2016). Spatial and seasonal reef calcification in corals and calcareous crusts in the central Red Sea. Coral Reefs 35, 681–693. doi: 10.1007/s00338-015-1383-y
Romano, S. L. (1990). Long-term effects of interspecific aggression on growth of the reef-building corals Cyphastrea ocellina (Dana) and Pocillopora damicomis (Linnaeus). J. Exp. Mar. Biol. Ecol. 140, 135–146. doi: 10.1016/0022-0981(90)90087-S
Rowan, R., and Powers, D. A. (1991). Molecular genetic identification of symbiotic dinoflagellates (zooxanthellae). Mar. Ecol. Prog. Ser. 71, 65–73. doi: 10.3354/meps071065
Samiei, J. V., Saleh, A., Shirvani, A., Fumani, N. S., Hashtroudi, M., and Pratchett, M. S. (2016). Variation in calcification rate of Acropora downingi relative to seasonal changes in environmental conditions in the northeastern Persian Gulf. Coral Reefs 35, 1371–1382. doi: 10.1007/s00338-016-1464-6
Schoepf, V., Grottoli, A. G., Warner, M. E., Cai, W.-J., Melman, T. F., Hoadley, K. D., et al. (2013). Coral energy reserves and calcification in a high-CO2 world at two temperatures. PLoS ONE 8:e75049. doi: 10.1371/journal.pone.0075049
Scoffin, T., Tudhope, A., Brown, B., Chansang, H., and Cheeney, R. (1992). Patterns and possible environmental controls of skeletogenesis of Porites lutea, South Thailand. Coral Reefs 11, 1–11. doi: 10.1007/BF00291929
Sebens, K., Helmuth, B., Carrington, E., and Agius, B. (2003). Effects of water flow on growth and energetics of the scleractinian coral Agaricia tenuifolia in Belize. Coral Reefs 22, 35–47. doi: 10.1007/s00338-003-0277-6
Sheppard, C., and Loughland, R. (2002). Coral mortality and recovery in response to increasing temperature in the southern Arabian Gulf. Aquat. Ecosyst. Health Manag. 5, 395–402. doi: 10.1080/14634980290002020
Sheppard, C. R. (1993). Physical environment of the Gulf relevant to marine pollution: an overview. Mar. Pollut. Bull. 27, 3–8. doi: 10.1016/0025-326X(93)90003-3
Shuail, D., Wiedenmann, J., D'angelo, C., Baird, A. H., Pratchett, M. S., Riegl, B., et al. (2016). Local bleaching thresholds established by remote sensing techniques vary among reefs with deviating bleaching patterns during the 2012 event in the Arabian/Persian Gulf. Mar. Pollut. Bull. 105, 654–659. doi: 10.1016/j.marpolbul.2016.03.001
Simpson, C. J. (1988). Ecology of Schleractinian Corals in the Dampier Archipelago, Western Australia. Melbourne, VIC: Environmental Protection Authority.
Stella, J. S., Jones, G. P., and Pratchett, M. S. (2010). Variation in the structure of epifaunal invertebrate assemblages among coral hosts. Coral Reefs 29, 957–973. doi: 10.1007/s00338-010-0648-8
Tanzil, J. T., Brown, B. E., Dunne, R. P., Lee, J. N., Kaandorp, J. A., and Todd, P. A. (2013). Regional decline in growth rates of massive Porites corals in Southeast Asia. Glob. Chang. Biol. 19, 3011–3023. doi: 10.1111/gcb.12279
Toller, W. W., Rowan, R., and Knowlton, N. (2001). Repopulation of zooxanthellae in the Caribbean corals Montastraea annularis and M. faveolata following experimental and disease-associated bleaching. Biol. Bull. 201, 360–373. doi: 10.2307/1543614
Vecsei, A. (2004). A new estimate of global reefal carbonate production including the fore-reefs. Glob. Planet. Change 43, 1–18. doi: 10.1016/j.gloplacha.2003.12.002
Veron, J. E. (2000). Corals of the World Vol. 1–3. Townsville, QLD: Australian Institute of Marine Science.
Wangpraseurt, D., Larkum, A. W., Ralph, P. J., and Kühl, M. (2012). Light gradients and optical microniches in coral tissues. Front. Microbiol. 3:316. doi: 10.3389/fmicb.2012.00316
Weber, J., and White, E. (1974). Activation energy for skeletal aragonite deposited by the hermatypic coral Platygyra spp. Mar. Biol. 26, 353–359. doi: 10.1007/BF00391518
Weber, J. N., and White, E. W. (1977). “Caribbean reef corals Montastrea annularis and Montastrea cavernosa: long-term growth data as determined by skeletal X-radiography,” in Reefs and Related Carbonates: Ecology and Sedimentology, eds S. H. Frost, M. P. Weiss, and J. B. Saunders (Tulsa, OK: American Association of Petroleum Geologists), 171–179.
Keywords: coral growth, coral calcification, extreme environments, Persian Gulf, Arabian Gulf, Gulf of Oman, Platygyra, Cyphastrea
Citation: Howells EJ, Dunshea G, McParland D, Vaughan GO, Heron SF, Pratchett MS, Burt JA and Bauman AG (2018) Species-Specific Coral Calcification Responses to the Extreme Environment of the Southern Persian Gulf. Front. Mar. Sci. 5:56. doi: 10.3389/fmars.2018.00056
Received: 30 October 2017; Accepted: 06 February 2018;
Published: 27 February 2018.
Edited by:
Jessica Carilli, University of Massachusetts Boston, United StatesReviewed by:
Colleen Brynn Bove, University of North Carolina at Chapel Hill, United StatesAndrew A. Shantz, Pennsylvania State University, United States
Copyright © 2018 Howells, Dunshea, McParland, Vaughan, Heron, Pratchett, Burt and Bauman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Emily J. Howells, em.howells@gmail.com
 Emily J. Howells
Emily J. Howells Glenn Dunshea
Glenn Dunshea Scott F. Heron
Scott F. Heron Morgan S. Pratchett
Morgan S. Pratchett John A. Burt
John A. Burt Andrew G. Bauman
Andrew G. Bauman