Dissolved Organic Matter Influences N2 Fixation in the New Caledonian Lagoon (Western Tropical South Pacific)
- 1Marine Biology Section, Department of Biology, University of Copenhagen, Helsingør, Denmark
- 2Aix-Marseille Université, Université de Toulon, CNRS, IRD, MIO UM 110, Marseille, France
- 3Mina and Everard Goodman Faculty of Life Sciences, Bar-Ilan University, Ramat Gan, Israel
Specialized prokaryotes performing biological dinitrogen (N2) fixation (“diazotrophs”) provide an important source of fixed nitrogen in oligotrophic marine ecosystems such as tropical and subtropical oceans. In these waters, cyanobacterial photosynthetic diazotrophs are well known to be abundant and active, yet the role and contribution of non-cyanobacterial diazotrophs are currently unclear. The latter are not photosynthetic (here called “heterotrophic”) and hence require external sources of organic matter to sustain N2 fixation. Here we added the photosynthesis inhibitor 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) to estimate the N2 fixation potential of heterotrophic diazotrophs as compared to autotrophic ones. Additionally, we explored the influence of dissolved organic matter (DOM) on these diazotrophs along a coast to open ocean gradient in the surface waters of a subtropical coral lagoon (New Caledonia). Total N2 fixation (samples not amended with DCMU) ranged from 0.66 to 1.32 nmol N L−1 d−1. The addition of DCMU reduced N2 fixation by >90%, suggesting that the contribution of heterotrophic diazotrophs to overall N2 fixation activity was minor in this environment. Higher contribution of heterotrophic diazotrophs occurred in stations closer to the shore and coincided with the decreasing lability of DOM, as shown by various colored DOM and fluorescent DOM (CDOM and FDOM) indices. We tested the response of diazotrophs (in terms of nifH gene expression and bulk N2 fixation rates) upon the addition of a mix of carbohydrates (“DOC” treatment), amino acids (“DON” treatment), and phosphonates and phosphomonesters (“DOP” treatment). While nifH expression increased significantly in Trichodesmium exposed to the DOC treatment, bulk N2 fixation rates increased significantly only in the DOP treatment. The lack of nifH expression by gammaproteobacteria, in any of the DOM addition treatments applied, questions the contribution of non-cyanobacterial diazotrophs to fixed nitrogen inputs in the New Caledonian lagoon. While the metabolism and ecology of heterotrophic diazotrophs is currently elusive, a deeper understanding of their ecology and relationship with DOM is needed in the light of increased DOM inputs in coastal zones due to anthropogenic pressure.
Introduction
Biological dinitrogen (N2) fixation provides an important source of fixed nitrogen to fuel primary production in the oceans (Karl et al., 2002), being especially critical in areas devoid of other significant fixed nitrogen sources such as the oligotrophic subtropical gyres. N2 fixation is performed by specialized prokaryotic microbes called “diazotrophs.” These include cyanobacterial and non-cyanobacterial (bacteria and archaea) groups, that are commonly detected and quantified via the presence of the nifH gene, which encodes for a subunit of the nitrogenase enzyme system (Zehr, 2011). Traditionally, marine diazotrophs were hypothesized to be constrained by warm, low inorganic nitrogen waters (Sohm et al., 2011). Yet, several filamentous cyanobacteria are able to obtain energy from dissolved organic matter-DOM- (Rippka et al., 1979), and several reports have shown that the most abundant photoautotrophs in the world's oceans (Prochlorococcus and Synechococcus) harbor the genes necessary to use amino acids, peptides and sugars (Yelton et al., 2016). As evidence accumulates, it seems that the ability of using DOM as an alternative source of nutrients under limiting situations is common in various planktonic groups (Stoecker et al., 2017). Among marine diazotrophs, unicellular cyanobacteria such as Cyanothece have been reported to grow on glycerol (Feng et al., 2010), and filamentous diazotrophs such as Trichodesmium may obtain as much carbon from amino acids assimilation as from CO2 fixation in the field (Benavides et al., 2017). Non-cyanobacterial and non-photosynthetic diazotrophs require external sources of DOM as energy sources (Riemann et al., 2010). The globally widespread non-cyanobacterial diazotroph Gamma A (Langlois et al., 2015) upregulates nifH gene expression upon glucose + mannitol additions (Moisander et al., 2011). Previous work has also shown that N2 fixation activity is stimulated by DOM additions (Bonnet et al., 2013; Rahav et al., 2013, 2015), and correlates with labile DOM compounds in environments where non-cyanobacterial (non-photosynthetic) diazotrophs predominate, such as the aphotic mesopelagic layer (Benavides et al., 2015), or the surface ultraoligotrophic eastern Mediterranean Sea (Rahav et al., 2016).
N2 fixation and diazotroph community studies have mostly focused on populations in the North Atlantic (Benavides and Voss, 2015) and North Pacific Oceans, while other areas such as the Indian and the South Atlantic and Pacific Oceans have been chronically under-sampled (Luo et al., 2012). The western tropical Southwest Pacific (WTSP) Ocean comprises warm, oligotrophic, trace metal rich waters that harbor among the high N2 fixation rates measured at sea (>500 μmol N m−2 d−1; Bonnet et al., 2017). The activity and distribution of diazotrophs in the WTSP has been related to temperature, iron and inorganic nutrient availability thresholds (e.g., Bonnet et al., 2009; Moisander et al., 2010). In austral summer conditions, the relatively warmer and productive waters like the Arafura, Timor and Solomon Seas (~26.5–30°C) are dominated by Trichodesmium and UCYN-B (Messer et al., 2016), while UCYN-A remains confined to the colder and more oligotrophic Coral Sea (~22–26.5°C; Moisander et al., 2010; Bonnet et al., 2015; Messer et al., 2016). Together with the high abundances of diazotroph phylotypes (Moisander et al., 2010; Bonnet et al., 2015), the WTSP has been recently acknowledged as a hotspot of global importance for N2 fixation activity (Bonnet et al., 2017). Thus, understanding how environmental factors (besides temperature and inorganic nutrients) shape the distribution and activity of diazotrophs in the WTSP is of utmost importance to assess their potential to fuel primary production in these oligotrophic waters and their contribution to the oceanic nitrogen budget.
New Caledonia, situated at the eastern edge of the WTSP, is surrounded by a reef enclosing one of the largest coral lagoons in the world. Here, primary production is nitrogen-limited throughout the year (Torréton et al., 2010), prompting diazotrophic activity (Garcia et al., 2007; Biegala and Raimbault, 2008) and the presence of various cyanobacterial diazotroph phylotypes including Trichodesmium (often occurring as large blooms; Rodier and Le Borgne, 2008), diatom-diazotroph symbioses (DDAs) as well the unicellular cyanobacteria UCYN-A1, UCYN-A2, UCYN-B, and UCYN-C (Turk-Kubo et al., 2015). However, non-cyanobacterial diazotrophs have been far less studied, and despite representatives such as the putative gammaproteobacterium γ-24774A11 that has been detected at abundances of 102–103nifH copies L−1 within the lagoon (Turk-Kubo et al., 2015), their contribution to N2 fixation in this environment remains unquantified. Moreover, the importance of DOM as a source of energy and nutrition for both cyanobacterial and non-cyanobacterial diazotrophs has never been explored in this environment. In this study we assess if the inhibition of photosynthesis triggers DOM nutrition in pelagic diazotrophs, and whether the N2 fixation activity and/or nifH expression of these pelagic diazotrophs is enhanced by different model DOM molecules.
Materials and Methods
Hydrography, Oxygen, Inorganic Nutrients, Chl α and DOC Measurements
Sampling took place on 13 January 2016 approximately from 8:00 a.m. to noon at four stations along an anthropogenic pressure gradient in the New Caledonian lagoon onboard the R/V Archamia. Station 1 was close to the rim of the coral reef barrier and Station 4 near the harbor of the city of Nouméa (Figure 1, Table 1). This transect connects the passage of Dumbéa (the main canal for the entrance of ships into the lagoon) with the harbor. Sampling started at Station 1 at high tide and proceeded toward the coast as the tide ebbed.
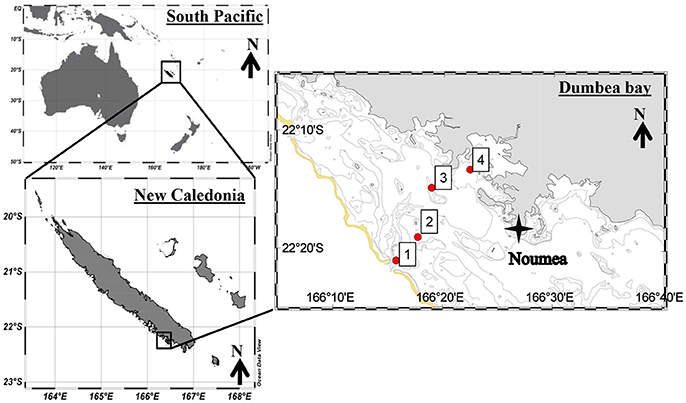
Figure 1. Map of stations sampled in the New Caledonian lagoon. The yellow line represents the coral reef barrier.
At each station, temperature and salinity were measured by casting a SeaBird SB19 plus CTD probe from the surface to the bottom. Water samples for the measurement of oxygen, nutrients, chlorophyll a (Chl a) and DOC concentrations, and colored DOM (CDOM) and fluorescent DOM (FDOM) parameters (see below) were collected from the subsurface (~3 m), using a 5 L Niskin bottle and transported to the lab for analysis in coolers equipped with icepacks within 2 h. Samples for the measurement of dissolved oxygen were taken in triplicate and analyzed ashore using an automated titration system with a photometric endpoint (Langdon, 2010). Samples for the determination of nitrate plus nitrite (NOx) and phosphate () concentrations were taken on acid-washed 20 mL polyethylene tubes, poisoned with 1% HgCl2, and stored at 4°C until analysis. The samples were analyzed on a AA3 Bran+Luebbe autoanalyzer following JGOFS recommendations (JGOFS, 1994). The detection limits were 50 and 10 nM for NOx and , respectively. Chl a concentrations were estimated from seawater samples (500 mL) filtered through 25 mm GF/F filters (Whatman) and stored at −80°C until analysis, extracted in methanol on a AU Turner Designs bench fluorometer, previously calibrated with pure Chl a (Herbland et al., 1985). Samples for DOC were collected on triplicate precombusted (450°C, 6 h) glass ampoules and acidified to pH 2 with 50 μL of 50% phosphoric acid. The samples were analyzed by high temperature catalytic oxidation on a TOC-V analyzer (Sohrin and Sempéré, 2005).
FDOM and CDOM Analyses
Samples for the analysis of FDOM and CDOM were filtered through 0.2 μm polycarbonate filters in a custom-made filtration system pre-cleaned with 1 M HCl and Milli-Q water. Samples for FDOM analyses were stored at 4°C until analysis (within 1 month), while samples for CDOM absorption analyses were directly measured on a liquid waveguide capillary flow cell (WPI) according to the method of Kowalczuk et al. (2013). Excitation-emission matrix (EEM) spectra for analysis of FDOM were determined using a Hitachi F-7000 spectrofluorometer (Tedetti et al., 2011). FDOM components were identified by parallel factor analysis (PARAFAC), a statistical decomposition technique used to extract the most representative fluorescent components within EEMs dataset. To increase the validity of the PARAFAC analysis, data from the present experiment were pooled with 39 other samples obtained from the same waters, 3 months before and after the experiment. Indeed, to avoid systematic biases, remove signals unrelated to fluorescence and to validate the number of FDOM components, a large dataset was needed (Stedmon et al., 2003). For example, in the case where two of the components had very similar fluorescence characteristics and/or were highly correlated in their appearance among samples, the PARAFAC approach would have difficulties to discern between the two. We then determined several spectral indices: the absorption at 442 nm as a proxy of phytoplankton production and the absorption at 350 nm as an indicator of terrestrial inputs in the bay (Passow and Alldredge, 1995), a biological index (BIX) of recent autochthonous FDOM contribution (Huguet et al., 2009), a specific ultraviolet absorbance at 254 nm of DOC (SUVA254), which is an indicator of aromatic DOM (Weishaar et al., 2003), and the ratio of spectral slopes (Sr) at 275–295 nm (S275−295) and 350–400 nm (S350−400) ranges, related to the molecular weight shift of DOM (Helms et al., 2008; Table 2).
N2 and Carbon Fixation Rates
Seawater for the quantification of N2 and carbon fixation rates was sampled using a Teflon pump connected to polyethylene tubing. Bulk N2 and carbon fixation rates were measured at each transect station on triplicate 2.3 L transparent polycarbonate bottles filled to overflow and closed air-free with septum screwcaps. Once in the lab (within ~2 h) the bottles were amended with stable isotopes to assay N2 and carbon fixation simultaneously. Each bottle was amended with NaH13CO3 99 atom% 13C to a final ~10 atom% enrichment, and 2.5 mL 98.9 atom% 15N2 gas (both isotopes acquired from Cambridge Isotope Laboratories) injected through the septum using a gas-tight syringe (Montoya et al., 1996). Commercial 15N2 gas stocks have been recently reported to be contaminated with varying amounts of other nitrogenated forms such as nitrous oxide and nitrite (Dabundo et al., 2014). The 15N2 gas batch used here was tested for contamination levels at J. Granger's lab (University of Connecticut, CT, USA), which were found to be negligible and hence did not affect our N2 fixation rates (Benavides et al., 2015). After isotope additions, the incubation bottles were inverted 20 times, transferred to running seawater incubators covered with blue light screening reproducing the light intensity at the corresponding sampling depth, and incubated for 24 h. To test for non-cyanobacterial (non-photoautotrophic) N2 fixation activity, a second set of incubation bottles was amended with DCMU to a final concentration of 50 μM (Rahav et al., 2015). These bottles were covered with black fabric bags to keep them in the dark at the same conditions as described above.
After incubation, the samples were filtered through precombusted GF/F filters (Whatman), and the filters were stored at −20°C until analysis. Background (time zero δ15N and δ13C) samples were taken at every station to allow an accurate calculation of N2 and carbon fixation rates. The samples were analyzed by continuous-flow isotope ratio mass spectrometry (IRMS) using an Integra2 Analyser (Integra CN), calibrated with International Atomic Energy Agency standards IAEA 310-A for nitrogen (47.2‰) and IAEA 303-B for carbon (466‰). The minimum quantifiable N2 fixation rate was 0.0041 nmol N L−1 d−1, as determined by error propagation analysis (Gradoville et al., 2017). We note that the use of the traditional 15N2 “bubble method” (Montoya et al., 1996) instead of the “dissolved method” (Mohr et al., 2010) may have resulted in an underestimation of the actual rates (Grokopf et al., 2012), although the long incubation time (24 h) likely reduces this possibility.
DOM Addition Experiments
To test for the enhancement of N2 fixation rates and/or nifH gene expression as a response to DOM enrichments, we sampled seawater in 25 extra 2.3 L polycarbonate bottles at Station 2. Five bottles were amended with a mix of carbohydrates —“DOC treatment”- (sodium pyruvate, sodium acetate and glucose), another five with a mix of amino acids —“DON treatment”- (alanine, leucine, and glutamic acid), and another five were amended with a mix of phosphomonoesters and phosphonates —“DOP treatment”- (methylphosphonic acid, 2-aminoethylphosphonic acid and fructose 1,6-biphosphate). All DOM mixtures were prepared to be equimolar in carbon (all contained 4 μM carbon; Benavides et al., 2015). Five bottles were used as a control (no DOM additions), and another five bottles were used as a “time zero” (i.e., sacrificed at the start of the experiment to know the baseline conditions). All bottles were spiked with 2.5 mL 15N2 and incubated for 24 h as described above. At the end of the incubation period, samples for NOx, and DOC analyses were taken from three bottles of each treatment (i.e., DOC, DON, DOP) as described above. The fourth and fifth bottles from each treatment were kept for RNA analyses, respectively (see below).
DNA and RNA Sampling, Extraction and qPCR Assays
Samples for DNA extractions were obtained at each transect station in 2.3 L polycarbonate bottles. Once ashore, the samples were filtered through 0.2 μm Supor filters (PALL) using a peristaltic pump. Upon filtration the filters were transferred to bead beating tubes including ~50 μL of a mixture of 0.1 mm and 0.5 mm diameter glass beads (BioSpec Products), and stored at −80°C until analysis. DNA was extracted via the phenol-chloroform method (Massana et al., 1997). RNA samples were taken in duplicate from each treatment (see “DOM addition experiments” above), filtered through 0.2 μm Supor filters and stored in sterile bead beating tubes containing 350 μL RLT buffer (Invitrogen) and 3.5 μL ß-mercaptoethanol, and finally stored at −80°C until analysis. The RNA was extracted by TRI Reagent (Invitrogen). 1 mL of Tri reagent was added to each sample that was disrupt and homogenized by pipetting and vortexing in order to break the cells. The cell lysate was incubated at room temperature for 3 h with occasional vortexing. The samples were then centrifuge at 12,000 g for 20 min at 4°C. The supernatant was transferred to a new tube and 10% of Phase Separation Reagent, BCP (Molecular Research Center, Inc., Cincinnati, OH, USA) was added. After additional incubation (30 min) at room temperature and centrifugation (12,000 g, 15 min, 4°C) the supernatant was transferred to a new tube and a cleanup and DNase treatment was performed using the RNA Clean and Concentrator-5 kit (Zymo Research, Irvin, CA, USA). Complementary DNA (cDNA) was created using the qScriptTM cDNA Synthesis Kit (Quantabio, Beverly, MA, USA) using the reverse primers of the nifH (nifH2 and nifH3; Zani et al., 2000). Quantitative PCR (qPCR) assays were performed on a CFX-96 Touch Real-Time PCR Detection System (Bio-Rad) using the TaqMan Fast Advanced Master Mix (Applied Biosystems) and primers for nifH of UCYN-A, UCYN-B, Trichodesmium and γ-24774A11 (here referred to as gammaproteobacteria) (Turk et al., 2011).
Results
Hydrography, Nutrients and in Situ DOM
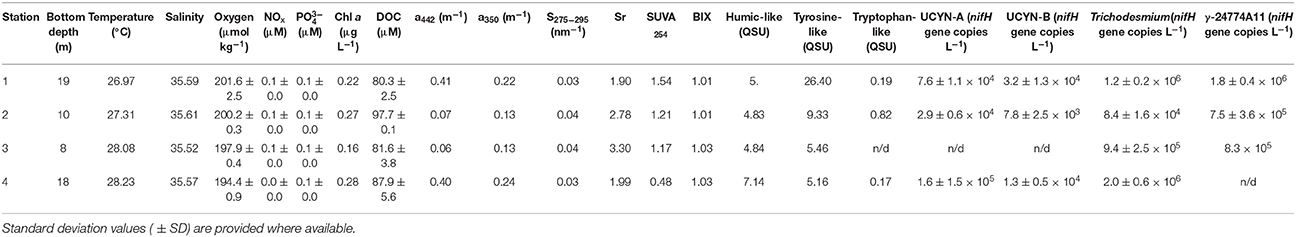
The temperature of surface waters increased from 26.97 to 28.23°C moving onshore from Station 1 toward Station 4, while salinity remained close to 35.6 at all stations (Table 1). The concentration of oxygen decreased onshore from 201 to 194 μmol kg−1, while inorganic nutrient concentrations (NOx and ) did not vary significantly along the transect, and neither did Chl a concentrations, with the exception of Station 3 where Chl a was slightly lower (Table 1). DOC concentrations remained between 80 and 90 μM along the transect, showing a peak of ~98 μM at Station 2 (Table 1).
CDOM absorption at 442 nm and 350 nm (a442 and a350) presented lower values at station 2 and 3 as compared to the values recorded at Stations 1 and 4 (Table 1) which indicated a decrease in CDOM concentrations. Moreover, the spectral slope S275−295 and the spectral ratio Sr were high with values >1 and >0.0.3 nm−1, respectively, which indicated the presence of photodegraded DOM with low molecular weight. In addition, the SUVA254 indices were <4 (this reference value is indicative of DOM with low aromaticity) with an average value of 1.09 ± 0.44. Then CDOM at 442 nm and 350 nm is strongly photodegraded with a low aromaticity. Three fluorescent DOM compounds were identified by the PARAFAC model: terrestrial humic-like (compound 1), tyrosine-like (compound 2) and tryptophan-like (compound 3; Table 3). Humic-like compound signatures were higher closer to the coast (Station 4) and decreased to values around ~5 QSU at the other stations. This pattern is opposite to that of tyrosine-like compounds signals, which increased toward the barrier reef. Tryptophane-like compound concentrations did not follow any coast-open ocean pattern but presented the highest concentrations at Station 2. The BIX indices were homogenous and >1 (this value is indicative of an enrichment in DOM from bacterial origin) along the transect (Table 1). Overall, the optical analyses of DOM suggest that while river-derived refractory DOM was more abundant close to the coast, biological-derived labile DOM compounds were present homogeneously at all stations along the transect.
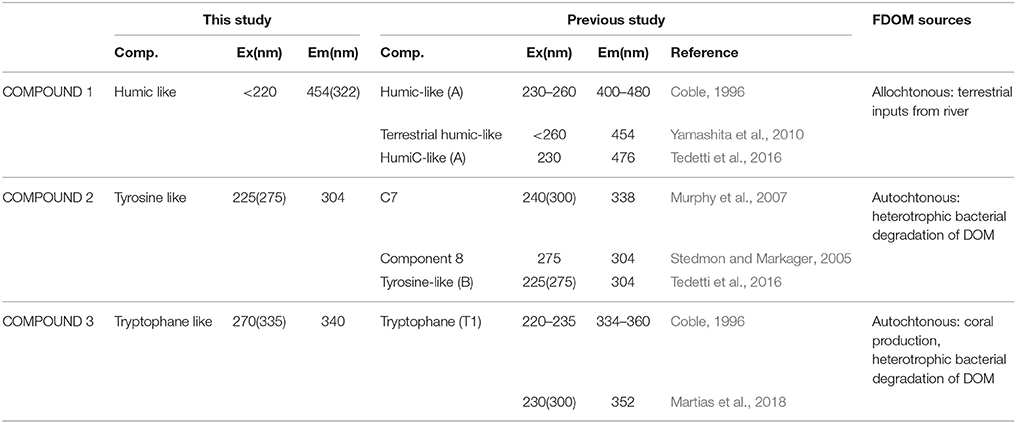
Table 3. Excitation and Emission maxima (Ex/Em) of three CDOM fluorescent components validated by PARAFAC model and identification by comparison with the literature data.
N2 and Carbon Fixation Activity and Diazotroph Abundance
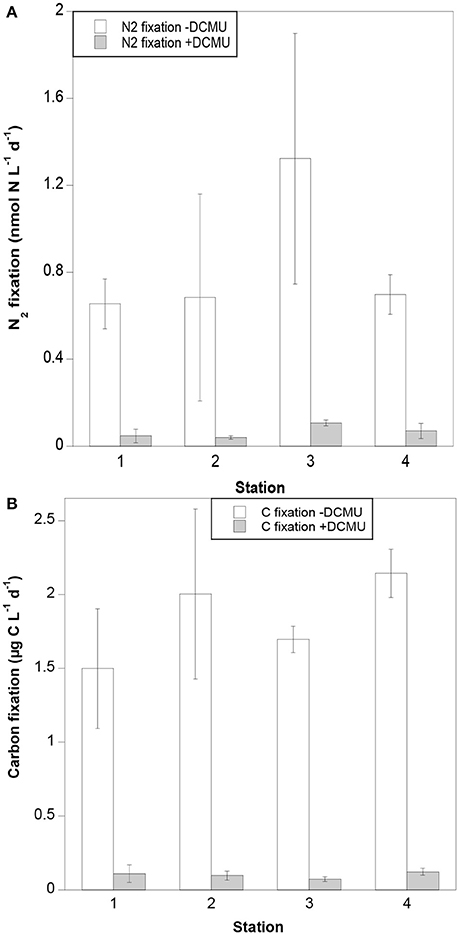
No clear spatial trends were observed in either N2 or carbon fixation rates along the transect. N2 fixation rates in samples not amended with DCMU ranged from 0.66 to 1.32 nmol N L−1 d−1, with the highest rates measured at Station 3 (Figure 2A). Carbon fixation rates in samples without DCMU ranged from 1.50 to 2.14 μg C L−1 d−1 (Figure 2B). Both N2 and carbon fixation were effectively inhibited in DCMU-amended samples (>90%; Figures 2A,B).
Among the diazotroph phylotypes quantified by qPCR, Trichodesmium and gammaproteobacteria showed the highest abundance, ranging between 8.4 × 104 and 2 × 106, and 7.5 × 105 and 1.8 × 106 nifH copies L−1, respectively (Table 1). UCYN-A and UCYN-B phylotypes were one to two orders of magnitude lower, and undetectable at station 3 (Table 1).
N2 Fixation, Diazotroph Abundance and Optical Response to Model DOM Compound Additions
At Station 2 we tested whether the addition of DOC, DON or DOP upregulated nifH expression and/or enhanced N2 fixation rates. N2 fixation rates were similar in the control, DOC and DON treatments (~0.8 nmol N L−1 d−1), yet they doubled to ~2 nmol N L−1 d−1 when DOP was added (Figure 3). However, due to the variability among replicates, the enhancement of either N2 fixation or expression of nifH genes was not statistically significant in any treatment (Kruskal-Wallis, p > 0.05).
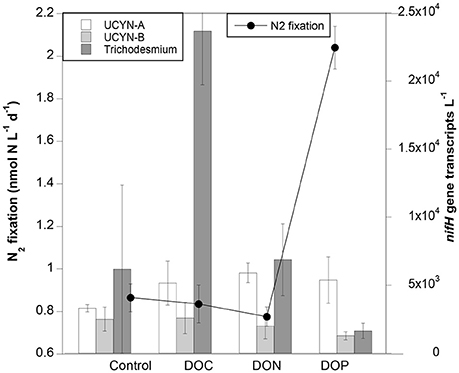
Figure 3. N2 fixation rates (line) and nifH gene expression (nifH transcripts L−1; bars) in control, DOC, DON, and DOP treatments performed at Station 2. Error bars indicate the standard deviation of the mean.
The DOC, DON, and DOP treatments yielded slightly higher abundances of UCYN-A nifH transcripts compared to transcripts measured in the controls, yet similar transcript numbers were with obtained in each of the three treatments (~5–6 × 103 nifH transcripts L−1; Figure 3). UCYN-B did not show differential expression among treatments, while Trichodesmium increased by one order of magnitude when exposed to the DOC treatment (Figure 3). Despite being the most abundant ambient group as determined by DNA qPCR counts, gammaproteobacteria did not show detectable nifH gene expression in our DOM enrichment experiments (including control bottles). The prolonged incubation (24 h) in polycarbonate bottles could have affected nifH expression in this group (Moisander et al., 2014).
Regarding optical parameters, the DOC treatment resulted in a peak of tyrosine-like compounds concentrations (+51%) whereas no changes were recorded in humic-like and tryptophan-like compounds concentrations (Table 4). The effect of DON and DOP treatments on concentrations of fluorescent compounds are not shown as FDOM results were considered outliers in the validation of PARAFAC model.
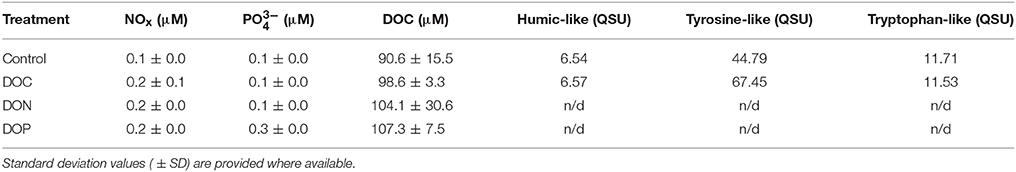
Table 4. NOx, , DOC concentrations and values of FDOM-related ratios for the different DOM additions (DOC, DON, and DOP treatments) performed at Station 2.
Discussion
Spatial Variability of N2 Fixation Rates and Diazotrophic Phylotypes
Gammaproteobacteria and Trichodesmium were the most abundant diazotrophs among the four phylotypes quantified (Table 1). The nifH copies of these two phylotypes were higher than in a previous study performed in the lagoon during the austral summer. The abundances, reported by Turk-Kubo et al. in nifH copies, ranged from 2.8 × 103 to 6.4 × 104 (UCYN-A), 3.5 × 102 to 2.7 × 103 (UCYN-B), 3.4 × 102 and 1.4 × 105 (Trichodesmium), and 4 × 102 to 1.9 × 103 (gammaproteobacteria) nifH copies L−1. Typically, the nifH copy numbers reported here are similar to published numbers from Berthelot et al. (2017) who reported abundances up to 1.3 × 103 (UCYN-A), 7.5 × 104 (UCYN-B), 1.6 × 105 (Trichodesmium), and 2.7 × 104 (gammaproteobacteria) nifH copies L−1 from the Solomon Sea.
DCMU blocks photosynthesis and thus the energy source required by autotrophic cyanobacterial diazotrophs to fix N2. Hence, measured N2fixation rates from DCMU-amended samples should be representative of non-cyanobacterial N2 fixation. The lack of nifH expression in gammaproteobacteria in our DOM addition experiments (see below) questions whether this specific group was active. Nevertheless, other non-cyanobacterial diazotrophs such as Bradyrhizobium and Mesorhizobium, previously detected in the lagoon (Pfreundt et al., 2016), could be responsible for the measured N2 fixation rates in DCMU-amended bottles. N2 fixation rates in DCMU-amended incubations represented 4–7% of N2 fixation measured without amendments. The New Caledonian lagoon is dominated by autotrophic cyanobacterial diazotrophs (Rodier and Le Borgne, 2008; Turk-Kubo et al., 2015), where non-cyanobacterial phylotypes have been reported to represent <2% of the diazotrophic community (Turk-Kubo et al., 2015; Pfreundt et al., 2016). Hence, such a low contribution to total N2 fixation rates may not be surprising, but deserves further study in other parts of the lagoon and other times of the year. Still, N2 fixation rates in DCMU-amended bottles ranged between 0.04 and 0.11 nmol N L−1 d−1, which is in the range of N2 fixation rates measured in environments where non-cyanobacterial diazotrophs prevail (Moisander et al., 2017).
It has to be noted that putative non-cyanobacterial N2 fixation was higher at stations closer to the coast (Stations 3 and 4), which may be linked to the Dumbéa river inputs importing bioavailable CDOM at 350 nm and terrestrial humic-like DOM compounds into the bay. Spectral indices (Sr and S275−295) revealed low molecular weight and photodegraded terrestrial CDOM/FDOM at Station 4 (the most impacted by the river), which has been seen to enhance its availability to heterotrophic bacteria in oligotrophic waters (Su et al., 2017). At Station 1, DOM sources come from autochthonous phytoplankton production), which is also available for bacterial growth (based on a442 values >0.1 as compared to Stations 2 and 3; Steinberg et al., 2004). The observed relationship between DOM lability proxies and non-cyanobacterial N2 fixation activity in the present study support the findings of previous studies in the WTSP and the Mediterranean Sea (Benavides et al., 2015, 2016; Rahav et al., 2016). Nevertheless, a mechanistic understanding of how non-cyanobacterial diazotrophs benefit from different DOM molecules (e.g., metabolic pathways, affinity for different substrates) is still lacking (Bombar et al., 2016).
Diazotrophic Responses to DOM Additions
The influence of the three different DOM treatments applied (DOC, DON, and DOP) differentially influenced nifH gene expression of different phylotypes detected, with transcript abundance increasing similarly in UCYN-A for all amendments as compared to the controls (from ~3 to 5–6 × 103 nifH transcripts L−1), while no differences were observed between controls and amendments for UCYN-B transcripts (Figure 3). UCYN-B fixes carbon during the day and N2 at night to avoid nitrogenase enzyme inhibition by photosynthetically derived oxygen. Thus, nifH expression in UCYN-B is typically higher at night (Church et al., 2005; Zehr et al., 2007). Hence, the absence of nifH expression in UCYN-B during the day (when our RNA samples were taken) is not surprising, even in the presence of external DOM sources. Similarly, Moisander et al. (2011) observed that nifH expression was not upregulated in UCYN-B when incubated with a mixture of glucose and mannitol. UCYN-A do not have a tricarboxylic acid pathway (Tripp et al., 2010), and thus require reduced organic carbon sources provided by their hosts (Thompson et al., 2012). It is therefore plausible that DOM additions in our experiments enhanced the diazotrophic activity of UCYN-A, similar to the experiments of Moisander et al. (2011), who demonstrated upregulated nifH gene expression in UCYN-A upon the addition of a glucose and mannitol mixture. In our experiments, however, a preference for DOC, DON or DOP was not observed and the nifH expression response was similar across treatments (Figure 3).
Active assimilation of both DOC and DON was measured in natural Trichodesmium colonies from the WTSP open ocean waters in similar experiments (Benavides et al., 2017). However, in that previous study only the addition of DON resulted in an increase of filament-specific N2 fixation rates (Benavides et al., 2017). The bulk water N2 fixation measurements presented here do not allow us to discern the contribution of each diazotroph phylotype. Nevertheless, while bulk N2 fixation rates were highest in the DOP treatment, nifH gene expression by Trichodesmium was highest in the DOC treatment (Figure 3). The peak of tyrosine-like compounds coinciding with the highest nifH expression of Trichodesmium observed in the DOC treatment may be related to the production of CDOM by this cyanobacterium (Steinberg et al., 2004). Surprisingly, DOM additions did not result in any nifH expression in the group of non-cyanobacterial diazotrophs tested in this study. In marine waters, maximum nifH expression of gammaproteobacteria typically reaches × 104 nifH transcripts L−1 (Luo et al., 2012; Langlois et al., 2015). For example, Church et al. (2005) reported an average of 4 × 102 nifH transcripts L−1 for gammaproteobacteria in a diel cycle study at station ALOHA in the North Pacific Ocean, which is in the same order of magnitude as in a study in the WTSP by Moisander et al. (2014). Nevertheless, a study including samples from a wide range of oceanographic cruises across the North and South Atlantic and Pacific Oceans reported up to ×105 gammaproteobacterial nifH transcripts per liter in sunlit surface waters (Langlois et al., 2015). In offshore waters of the WTSP, the addition of glucose and mannitol upregulated nifH expression in gammaproteobacteria (Moisander et al., 2011).
The lack of significant responses to DOM additions in this study could reflect sufficient DOM availability (based on Sr,S275−295 and SUVA254 indexes) potentially provided by corals in the lagoon which are known for producing tryptophane-like compounds (available DOM sources for bacteria; Weishaar et al., 2003). However, the absence of detected gammaproteobacterial nifH transcripts, also in the control bottles, suggests that bottle confinement effects may have inhibited this group (Moisander et al., 2014). In addition to the diel changes in nifH gene expression between phylotypes (Church et al., 2005; Turk et al., 2011), it is possible that the nifH expression measured in our samples does not reflect the immediate response of diazotrophs to DOM additions. It must be noted that while RNA samples represent a snapshot of nifH expression at the time of sampling, N2 fixation rates integrate the nitrogenase activity that has taken place over a period of 24 h. Such differences probably also account for the uncoupling observed between N2 fixation rates and nifH gene expression.
N2 Fixation Rates Enhanced by DOP Additions
Diazotrophs differ in how they access DOP. For example, Crocosphaera watsonii (here represented by the UCYN-B phylotype) obtains inorganic phosphorus using high affinity phosphate binding systems, and organic phosphorus sources such as phosphomonoesters utilizing alkaline phosphatases (Dyhrman and Haley, 2006). In addition to these phosphorus sources, Trichodesmium is also able to grow on phosphonates and use phosphite (Dyhrman et al., 2006; Polyviou et al., 2015). It is intriguing why the nifH gene expression of Trichodesmium-or of any of the other diazotroph phylotypes tested- was not enhanced when DOP was enriched, and deserves further study. However, an enhancement of N2 fixationactivity was observed in the DOP treatment (Figure 3), and could be due to inorganic phosphorus limitation alleviation. Unfortunately, we do not have alkaline phosphatase activity data to confirm whether the DOP added to our incubations was ultimately transformed to inorganic phosphorus compounds, but concentrations nearly doubled in DOP-amended samples with respect to the control (data not shown), suggesting that active alkaline phosphatases were functioning. The fact that N2 fixation rates enhanced by DOP additions was not reflected in a significant enhancement of nifH expression of any of the diazotroph phylotypes tested suggests that the N2 fixation activity measured may be provided by a diazotroph not accounted for in our nifH gene q-RT-PCR assays. Alternatively, transcription may be interrupted when the number of transcripts is enough to activate the nitrogenase protein, resulting in an uncoupling between expression and nitrogenase activity, as previously reported in Trichodesmium cultures (Levitan et al., 2010).
Conclusions
Although cyanobacterial diazotrophs predominate in the New Caledonian lagoon, here we demonstrate that non-cyanobacterial diazotrophs contribute ~10% of total N2 fixation rates. Comparable to previous findings (see Moisander et al., 2017) gammaproteobacteria were the most abundant based on DNA-derived qPCR nifH gene counts, yet we did not detect any nifH gene expression for this group throughout the experiments. This lack of detectable expression together with the low N2 fixation rates measured in DCMU-treated samples questions the contribution of non-cyanobacterial diazotrophs to biologically fixed nitrogen inputs in the lagoon. Yet, the response of the cyanobacterial diazotroph phylotypes to the amended DOM treatments suggests that mixotrophic nutrition may be important in these organisms and should be investigated further.
Author Contributions
Conceived and designed the experiments: MB, CM, SB; Performed the experiments: MB, CM; Analyzed the data: MB, CM, HE; Contributed reagents, materials, analysis tools: CM, SB, HE, CD, IB-F; Wrote the paper: MB, CM, CD, HE, IB-F, SB.
Funding
MB was funded by the People Programme (Marie Skłodowska-Curie Actions) of the European Union's Seventh Framework Programme (FP7/2007-2013) under REA grant agreement number 625185. Funding was obtained for IB-F through a collaborative grant from Ministry of Science and Technology (MOST) Israel and the High Council for Science and Technology (HCST)-France (2013). CM and SB were funded by the Institut de Recherche pour le Développement (IRD, France).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer AF-C and handling Editor declared their shared affiliation.
Acknowledgments
We thank the captain of R/V Archamia for his invaluable help at sea. We would like to thank O. Grosso for IRMS analyses. We are also grateful to P. Gérard and L. Jamet from the Laboratoire des Moyens Analytiques (LAMA) at IRD in Nouméa (New Caledonia) for oxygen, nutrient and DOC analyses.
References
Benavides, M., Berthelot, H., Duhamel, S., Raimbault, P., and Bonnet, S. (2017). Dissolved organic matter uptake by Trichodesmium in the Southwest Pacific. Sci. Rep. 7, 1–6. doi: 10.1038/srep41315
Benavides, M., Bonnet, S., Hernández, N., Martínez-Pérez, A. M., Nieto-Cid, M., Álvarez-Salgado, X. A., et al. (2016). Basin-wide N2 fixation in the deep waters of the Mediterranean Sea. Glob. Biogeochem. Cycles 30, 1–19. doi: 10.1002/2015GB005326
Benavides, M. H., Moisander, P., Berthelot, H., Dittmar, T., Grosso, O., and Bonnet, S. (2015). Mesopelagic N2 fixation related to organic matter composition in the Solomon and Bismarck Seas (Southwest Pacific). PLoS ONE 10:e0143775. doi: 10.1371/journal.pone.0143775
Benavides, M., and Voss, M. (2015). Five decades of N2 fixation research in the North Atlantic Ocean. Front. Mar. Sci. 2, 1–20. doi: 10.3389/fmars.2015.00040
Berthelot, H., Benavides, M., Moisander, P. H., Grosso, O., and Bonnet, S. (2017). High-nitrogen fixation rates in the particulate and dissolved pools in the Western Tropical Pacific (Solomon and Bismarck Seas). Geophys. Res. Lett. 44, 8414–8423. doi: 10.1002/2017GL073856
Biegala, I. C., and Raimbault, P. (2008). High abundance of diazotrophic picocyanobacteria (<3 μm) in a Southwest Pacific coral lagoon. Aquat. Microb. Ecol. 51, 45–53. doi: 10.3354/ame01185
Bombar, D., Paerl, R. W., and Riemann, L. (2016). Marine non-cyanobacterial diazotrophs: moving beyond molecular detection. Trends Microbiol. 24, 916–927. doi: 10.1016/j.tim.2016.07.002
Bonnet, S., Biegala, I. C., Dutrieux, P., Slemons, L. O., and Capone, D. G. (2009). Nitrogen fixation in the western equatorial Pacific: rates, diazotrophic cyanobacterial size class distribution, and biogeochemical significance. Global Biogeochem. Cycles 23, 1–13. doi: 10.1029/2008GB003439
Bonnet, S., Caffin, M., Berthelot, H., and Moutin, T. (2017). Hot spot of N2 fixation in the western tropical South Pacific pleads for a spatial decoupling between N2 fixation and denitrification. Proc. Natl. Acad. Sci. U.S.A. 114, E2800–E2801. doi: 10.1073/pnas.1619514114
Bonnet, S., Dekaezemacker, J., Turk-Kubo, K. A., Moutin, T., Hamersley, R. M., Grosso, O., et al. (2013). Aphotic N2 fixation in the eastern tropical South Pacific Ocean. PLoS ONE 8:e81265. doi: 10.1371/journal.pone.0081265
Bonnet, S., Rodier, M., Turk-Kubo, K. A., Germineaud, C., Menkes, C., Ganachaud, A., et al. (2015). Contrasted geographical distribution of N2 fixation rates and nifH phylotypes in the Coral and Solomon Seas (southwestern Pacific) during austral winter conditions. Global Biogeochem. Cycles 29, 1874–1892. doi: 10.1002/2015GB005117
Church, M. J., Short, C. M., Jenkins, B. D., Karl, D. M., and Zehr, J. P. (2005). Temporal patterns of nitrogenase gene (nifH) expression in the oligotrophic North Pacific Ocean. Appl. Environ. Microbiol. 71, 5362–5370. doi: 10.1128/AEM.71.9.5362-5370.2005
Coble, P. G. (1996). Characterization of marine and terrestrial DOM in seawater using excitation-emission matrix spectroscopy. Mar. Chem. 51, 325–346.
Dabundo, R., Lehmann, M. F., Treibergs, L., Tobias, C. R., Altabet, M. A., Moisander, P. H., et al. (2014). The contamination of commercial 15N2 gas stocks with 15N-labeled nitrate and ammonium and consequences for nitrogen fixation measurements. PLoS ONE 9:e110335. doi: 10.1371/journal.pone.0110335
Dyhrman, S. T., Chappell, P. D., Haley, S. T., Moffett, J. W., Orchard, E. D., Waterbury, J. B., et al. (2006). Phosphonate utilization by the globally important marine diazotroph Trichodesmium. Nature 439, 68–71. doi: 10.1038/nature04203
Dyhrman, S. T., and Haley, S. T. (2006). Phosphorus scavenging in the unicellular marine diazotroph Crocosphaera watsonii. Appl. Environ. Microbiol. 72, 1452–1458. doi: 10.1128/AEM.72.2.1452-1458.2006
Feng, X., Bandyopadhyay, A., Berla, B., Page, L., Wu, B., Pakrasi, H. B., et al. (2010). Mixotrophic and photoheterotrophic metabolism in Cyanothece sp. ATCC 51142 under continuous light. Microbiology 156, 2566–2574. doi: 10.1099/mic.0.038232-0
Garcia, N., Raimbault, P., and Sandroni, V. (2007). Seasonal nitrogen fixation and primary production in the Southwest Pacific: nanoplankton diazotrophy and transfer of nitrogen to picoplankton organisms. Mar. Ecol. Prog. Ser. 343, 25–33. doi: 10.3354/meps06882
Gradoville, M. R., Bombar, D., Crump, B. C., Letelier, R. M., Zehr, J. P., and White, A. E. (2017). Diversity and activity of nitrogen-fixing communities across ocean basins. Limnol. Oceanogr. 62, 1895–1909. doi: 10.1002/lno.10542
Grokopf, T., Mohr, W., Baustian, T., Schunck, H., Gill, D., Kuypers, M. M. M., et al. (2012). Doubling of marine dinitrogen-fixation rates based on direct measurements. Nature 488, 361–364. doi: 10.1038/nature11338
Helms, J. R., Stubbins, A., Ritchie, J. D., Minor, E. C., Kieber, D. J., and Mopper, K. (2008). Absorption spectral slopes and slope rations as indicators of molecular weight, source, and photoleaching of chromophoric dissolved organic matter. Limnol. Oceanogr. 53, 955–969. doi: 10.2307/40058211
Herbland, A., Le Bouteiller, A., and Raimbault, P. (1985). Size structure of phytoplankton biomass in the equatorial Atlantic Ocean. Deep. Res. 32, 819–836. doi: 10.1016/0198-0149(85)90118-9
Huguet, A., Vacher, L., Relexans, S., Saubusse, S., Froidefond, J. M., and Parlanti, E. (2009). Properties of fluorescent dissolved organic matter in the Gironde estuary. Org. Geochem. 40, 706–719. doi: 10.1016/j.orggeochem.2009.03.002
JGOFS (1994). Protocols For The Joint Global Ocean Flux Study (JGOFS) core measurements, IOC Manuals Guid. 29, 180.
Karl, D., Michaels, A., Bergman, B., Capone, D., Carpenter, E., Letelier, R., et al. (2002). Dinitrogen fixation in the world's oceans. Biogeochemistry 57/58, 47–98. doi: 10.1023/A:1015798105851
Kowalczuk, P., Tilstone, G. H., Zabłocka, M., Röttgers, R., and Thomas, R. (2013). Composition of dissolved organic matter along an Atlantic meridional transect from fluorescence spectroscopy and parallel factor analysis. Mar. Chem. 157, 170–184. doi: 10.1016/j.marchem.2013.10.004
Langdon, C. (2010). “Determination of dissolved oxygen in seawater by Winkler titration using the amperometric technique,” in Go-sh. Repeat Hydrogr. Man. A Collect. Expert Reports Guidel, 18.
Langlois, R., Großkopf, T., Mills, M., Takeda, S., and LaRoche, J. (2015). Widespread distribution and expression of Gamma A (UMB), an uncultured, diazotrophic, γ-proteobacterial nifH phylotype. PLoS ONE 10:e0128912. doi: 10.1371/journal.pone.0128912
Levitan, O., Brown, C. M., Sudhaus, S., Campbell, D., LaRoche, J., and Berman-Frank, I. (2010). Regulation of nitrogen metabolism in the marine diazotroph Trichodesmium IMS101 under varying temperatures and atmospheric CO2concentrations. Environ. Microbiol. 12, 1899–1912. doi: 10.1111/j.1462-2920.2010.02195.x
Luo, Y.-W., Doney, S. C., Anderson, L. A., Benavides, M., Berman-Frank, I., Bode, A., et al. (2012). Database of diazotrophs in global ocean: abundance, biomass and nitrogen fixation rates. Earth Syst. Sci. Data 4, 47–73. doi: 10.5194/essd-4-47-2012
Martias, C., Tedetti, M., Lantoine, F., Jamet, L., and Dupouy, C. (2018). Characterization and sources of colored dissolved organic matter in a coral reef ecosystem subject to ultramafic erosion pressure (New Caledonia, Southwest Pacific). Sci. Total Environ. 616–617, 438–452. doi: 10.1016/j.scitotenv.2017.10.261
Massana, R., Murray, A. E., Preston, C. M., and DeLong, E. F. (1997). Vertical distribution and phylogenetic characterization of marine planktonic Archaea in the Santa Barbara channel. Appl. Environ. Microbiol. 63, 50–56.
Messer, L. F., Mahaffey, C., Robinson, C. M., Jeffries, T. C., Baker, K. G., Isaksson, J. B., et al. (2016). High levels of heterogeneity in diazotroph diversity and activity within a putative hotspot for marine nitrogen fixation. ISME J. 10, 1499–1513. doi: 10.1038/ismej.2015.205
Mohr, W., Großkopf, T., Wallace, D. W. R., and LaRoche, J. (2010). Methodological underestimation of oceanic nitrogen fixation rates. PLoS ONE 5:e0012583. doi: 10.1371/journal.pone.0012583
Moisander, P. H., Beinart, R. A., Hewson, I., White, A. E., Johnson, K. S., Carlson, C. A., et al. (2010). Unicellular cyanobacterial distributions broaden the oceanic N2 fixation domain. Science 327, 1512–1514. doi: 10.1126/science.1185468
Moisander, P. H., Benavides, M., Bonnet, S., Berman-Frank, I., White, A. E., and Riemann, L. (2017). Chasing after non-cyanobacterial nitrogen fixation in marine pelagic environments. Front. Microbiol. 8:1736. doi: 10.3389/fmicb.2017.01736
Moisander, P. H., Serros, T., Paerl, R. W., Beinart, R. A., and Zehr, J. P. (2014). Gammaproteobacterial diazotrophs and nifH gene expression in surface waters of the South Pacific Ocean. ISME J. 8, 1962–1973. doi: 10.1038/ismej.2014.49
Moisander, P. H., Zhang, R., Boyle, E. A., Hewson, I., Montoya, J. P., and Zehr, J. P. (2011). Analogous nutrient limitations in unicellular diazotrophs and Prochlorococcus in the South Pacific Ocean. ISME J. 6, 733–744. doi: 10.1038/ismej.2011.152
Montoya, J. P., Voss, M., Kahler, P., and Capone, D. G. (1996). A simple, high-precision, high-sensitivity tracer assay for N2 fixation. Appl. Environ. Microbiol. 62, 986–993.
Murphy, K. R., Stedmon, C. A., Waite, T. D., and Ruiz, G. M. (2007). Distinguishing between terrestrial and autochthonous organic matter sources in marine environments using fluorescence spectroscopy. Mar. Chem. 108, 40–58. doi: 10.1016/j.marchem.2007.10.003
Passow, U., and Alldredge, A. L. (1995). A dye-binding assay for the spectrophotometric measurement of transparent exopolymer particles (TEP) in the ocean. Limnol. Oceanogr. 40, 1326–1335. doi: 10.4319/lo.1995.40.7.1326
Pfreundt, U., Van Wambeke, F., Caffin, M., Bonnet, S., and Hess, W. R. (2016). Succession within the prokaryotic communities during the VAHINE mesocosms experiment in the New Caledonia lagoon. Biogeosciences 13, 2319–2337. doi: 10.5194/bg-13-2319-2016
Polyviou, D., Hitchcock, A., Baylay, A. J., Moore, C. M., and Bibby, T. S. (2015). Phosphite utilization by the globally important marine diazotroph Trichodesmium. Environ. Microbiol. Rep. 7, 824–830. doi: 10.1111/1758-2229.12308
Rahav, E., Bar-Zeev, E., Ohayon, S., Elifantz, H., Belkin, N., Herut, B., et al. (2013). Dinitrogen fixation in aphotic oxygenated marine environments. Front. Microbiol. 4:227. doi: 10.3389/fmicb.2013.00227
Rahav, E., Giannetto, M. J., and Bar-Zeev, E. (2016). Contribution of mono and polysaccharides to heterotrophic N2 fixation at the eastern Mediterranean coastline. Sci. Rep. 6:27858. doi: 10.1038/srep27858
Rahav, E., Herut, B., Mulholland, M. R., Belkin, N., Elifantz, H., and Berman-Frank, I. (2015). Heterotrophic and autotrophic contribution to dinitrogen fixation in the Gulf of Aqaba. Mar. Ecol. Prog. Ser. 522, 67–77. doi: 10.3354/meps11143
Riemann, L., Farnelid, H., and Steward, G. F. (2010). Nitrogenase genes in non-cyanobacterial plankton: prevalence, diversity and regulation in marine waters. Aquat. Microb. Ecol. 61, 235–247. doi: 10.3354/ame01431
Rippka, R., Deruelles, J., Waterbury, J. B., Herdman, M., and Stanier, R. Y. (1979). Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology 111, 1–61. doi: 10.1099/00221287-111-1-1
Rodier, M., and Le Borgne, R. (2008). Population dynamics and environmental conditions affecting Trichodesmium spp. (filamentous cyanobacteria) blooms in the south-west lagoon of New Caledonia. J. Exp. Mar. Bio. Ecol. 358, 20–32. doi: 10.1016/j.jembe.2008.01.016
Sohm, J. A., Webb, E. A., and Capone, D. G. (2011). Emerging patterns of marine nitrogen fixation. Nat. Rev. Microbiol. 9, 499–508. doi: 10.1038/nrmicro2594
Sohrin, R., and Sempéré, R. (2005). Seasonal variation in total organic carbon in the northeast Atlantic in 2000–2001. J. Geophys. Res. 110:C10S90. doi: 10.1029/2004JC002731
Stedmon, C. A., and Markager, S. (2005). Resolving the variability of dissolved organic matter fluorescence in a temperate estuary and its catchment using PARAFAC analysis. Limnol. Oceanogr. 50, 686–697. doi: 10.4319/lo.2005.50.2.0686
Stedmon, C. A., Markager, S., and Bro, R. (2003). Tracing dissolved organic matter in aquatic environments using a new approach to fluorescence spectroscopy. Mar. Chem. 82, 239–254. doi: 10.1016/S0304-4203(03)00072-0
Steinberg, D. K., Nelson, N. B., Carlson, C. A., and Prusak, A. C. (2004). Production of chromophoric dissolved organic matter (CDOM) in the open ocean by zooplankton and the colonial cyanobacterium Trichodesmium spp. Mar. Ecol. Prog. Ser. 267, 45–56. doi: 10.3354/meps267045
Stoecker, D. K., Hansen, P. J., Caron, D. A., and Mitra, A. (2017). Mixotrophy in the marine plankton. Ann. Rev. Mar. Sci. 9, 311–335. doi: 10.1146/annurev-marine-010816-060617
Su, Y., Hu, E., Feng, M., Zhang, Y., Chen, F., and Liu, Z. (2017). Comparison of bacterial growth in response to photodegraded terrestrial chromophoric dissolved organic matter in two lakes. Sci. Total Environ. 579, 1203–1214. doi: 10.1016/j.scitotenv.2016.11.104
Tedetti, M., Cuet, P., Guigue, C., and Goutx, M. (2011). Characterization of dissolved organic matter in a coral reef ecosystem subjected to anthropogenic pressures (La Réunion Island, Indian Ocean) using multi-dimensional fluorescence spectroscopy. Sci. Total Environ. 409, 2198–2210. doi: 10.1016/j.scitotenv.2011.01.058
Tedetti, M., Marie, L., Röttgers, R., Rodier, M., Van Wambeke, F., Helias, S., et al. (2016). Evolution of dissolved and particulate chromophoric materials during the VAHINE mesocosm experiment in the New Caledonian coral lagoon (south-west Pacific). Biogeosciences 13, 3283–3303. doi: 10.5194/bg-13-3283-2016
Thompson, A. W., Foster, R. A., Krupke, A., Carter, B. J., Musat, N., Vaulot, D., et al. (2012). Unicellular cyanobacterium symbiotic with a single-celled eukaryotic alga. Science 337, 1546–1550. doi: 10.1126/science.1222700
Torréton, J. P., Rochelle-Newall, E., Pringault, O., Jacquet, S., Faure, V., and Briand, E. (2010). Variability of primary and bacterial production in a coral reef lagoon (New Caledonia). Mar. Pollut. Bull. 61, 335–348. doi: 10.1016/j.marpolbul.2010.06.019
Tripp, H. J., Bench, S. R., Turk, K. A., Foster, R. A., Desany, B. A., Niazi, F., et al. (2010). Metabolic streamlining in an open-ocean nitrogen-fixing cyanobacterium. Nature 464, 90–94. doi: 10.1038/nature08786
Turk, K. A., Rees, A. P., Zehr, J. P., Pereira, N., Swift, P., Shelley, R., et al. (2011). Nitrogen fixation and nitrogenase (nifH) expression in tropical waters of the eastern North Atlantic. ISME J. 5, 1201–1212. doi: 10.1038/ismej.2010.205
Turk-Kubo, K. A., Frank, I. E., Hogan, M. E., Desnues, A., Bonnet, S., and Zehr, J. P. (2015). Diazotroph community succession during the VAHINE mesocosm experiment (New Caledonia lagoon). Biogeosciences 12, 7435–7452. doi: 10.5194/bg-12-7435-2015
Weishaar, J. L., Aiken, G. R., Bergamaschi, B. A., Fram, M. S., Fujii, R., and Mopper, K. (2003). Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ. Sci. Technol. 37, 4702–4708. doi: 10.1021/es030360x
Yamashita, Y., Maie, N., Briceño, H., and Jaffé, R. (2010). Optical characterization of dissolved organic matter in tropical rivers of the Guayana Shield, Venezuela. J. Geophys. Res. 115:G00F10. doi: 10.1029/2009JG000987
Yelton, A. P., Acinas, S. G., Sunagawa, S., Bork, P., Pedrós-Alió, C., and Chisholm, S. W. (2016). Global genetic capacity for mixotrophy in marine picocyanobacteria. ISME J. 10, 2946–2957. doi: 10.1038/ismej.2016.64
Zani, S., Mellon, M. T., Collier, J. L., and Zehr, J. P. (2000). Expression of nifH genes in natural microbial assemblages in lake george, New York, detected by reverse transcriptase PCR. Appl. Environ. Microbiol. 66, 3119–3124.
Zehr, J. P. (2011). Nitrogen fixation by marine cyanobacteria. Trends Microbiol. 19, 162–173. doi: 10.1016/j.tim.2010.12.004
Keywords: non-cyanobacterial diazotrophs, nifH, DCMU, DOM, CDOM, FDOM
Citation: Benavides M, Martias C, Elifantz H, Berman-Frank I, Dupouy C and Bonnet S (2018) Dissolved Organic Matter Influences N2 Fixation in the New Caledonian Lagoon (Western Tropical South Pacific). Front. Mar. Sci. 5:89. doi: 10.3389/fmars.2018.00089
Received: 30 November 2017; Accepted: 05 March 2018;
Published: 22 March 2018.
Edited by:
Beatriz Mouriño-Carballido, University of Vigo, SpainReviewed by:
Ana Fernandez-Carrera, University of Vigo, SpainArvind Singh, Physical Research Laboratory, India
Copyright © 2018 Benavides, Martias, Elifantz, Berman-Frank, Dupouy and Bonnet. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mar Benavides, mar.benavides@mio.osupytheas.fr
 Mar Benavides
Mar Benavides Chloé Martias
Chloé Martias Hila Elifantz
Hila Elifantz Ilana Berman-Frank
Ilana Berman-Frank Cécile Dupouy2
Cécile Dupouy2  Sophie Bonnet
Sophie Bonnet