Sexually Dimorphic Scale Worms (Annelida: Polynoidae) From Hydrothermal Vents in the Okinawa Trough: Two New Species and Two New Sex Morphs
- 1Department of Biology, Hong Kong Baptist University, Kowloon, Hong Kong
- 2Hong Kong Baptist University Institute of Research and Continuing Education, Shenzhen, China
- 3Department of Subsurface Geobiological Analysis and Research, Japan Agency for Marine-Earth Science and Technology, Yokosuka, Japan
Scale worms in the family Polynoidae are common inhabitants of both shallow-water and deep-sea ecosystems, but their diversity in the deep-sea remains poorly known. In the West Pacific, only 10 polynoid species have been described from deep-sea chemosynthetic ecosystems including hydrothermal vents and methane seeps. Here, we described two new species of polynoids based on specimens collected from hydrothermal vents in the Okinawa Trough. Levensteiniella undomarginata sp. nov. is distinguished from other congeners by having elytra with a wave-shaped edge, and that males possess two pairs of nephridial papillae. Branchinotogluma elytropapillata sp. nov. differs from other congeners by having papillae on the elytral edge, and by having a single pair of nephridial papillae and five pairs of C-shaped lamellae in males. Furthermore, we redescribed Lepidonotopodium okinawae (Sui and Li, 2017) and Branchinotogluma japonicus Miura and Hashimoto, 1991, because the original description of the former species did not cover males and that of the latter did not cover females. Sequencing of the cytochrome oxidase I (COI) gene in these four species confirmed the sexual dimorphism in vent polynoids for the first time, and provided reliable barcoding sequences for identifying these polychaetes.
Introduction
With more than 900 recognized species, Polynoidae is the largest family of Aphroditiformia—a group of scale- or elytra-bearing polychaetes commonly called “scale worms” (Rouse and Pleijel, 2001; Wilson et al., 2003). Polynoids are widely distributed in various shallow-water ecosystems ranging from tropical coral reefs to intertidal shores of the polar regions (Beesley et al., 2000; Barnich and Fiege, 2003). With the increasing exploration of the deep sea over the last four decades, it is now recognized that polynoids are also common inhabitants of deep-sea habitats, including chemosynthetic environments (i.e., hydrothermal vents, methane seeps, and organic falls; Van Dover et al., 1999; Ramirez-Llodra et al., 2007; Levin et al., 2016), cold water coral communities (Henry and Roberts, 2007) and seamounts (Serpetti et al., 2017). To date, 143 species of deep-sea polynoids in 65 genera have been recorded in the World Register of Marine Species database (Read and Fauchald, 2017). Despite the increased intensity of ocean exploration over the last several decades, many ocean regions (such as continental shelf, oceanic basin, seamount, deep ocean trench) remain largely uncharted, with even the relatively well-studied ocean ridge systems being 90% unexplored (Ramirez-Llodra et al., 2007). Much is still to be learnt about the species richness of deep ecosystems, where many new species await discovery, especially with regards to small-sized invertebrates.
Unlike most shallow-water polynoids, deep-sea polynoids often exhibit sexual dimorphism, with female and male individuals exhibiting constant differences in characters such as the number of nephridial papillae, the presence of flattened parapodial lamellae or compressed posterior segments (Van Dover et al., 1999; Glover et al., 2005; Desbruyères et al., 2006). Due to such sexual dimorphism being unrecognized or the authors lacked specimens of both sexes, some species were described based solely on specimens of a single sex (e.g., Branchinotogluma grasslei Pettibone, 1985a and Lepidonotopodium okinawae Sui and Li, 2017), resulting in incomplete morphological descriptions. Indeed, different names were often given to different sex morphs of the same species. For instance, the genus Opisthotrochopodus (Pettibone, 1985a) was erected for O. alvinus (Pettibone, 1985a) to accommodate deep-sea polynoids with a pair of nephridial papillae, five pairs of lamellae and compressed posterior segments, which are in fact characters of the male morph of Branchinotogluma hessleri (Pettibone, 1985a). Desbruyères et al. (2006) therefore synonymized O. alvinus (Pettibone, 1985a) with B. hessleri (Pettibone, 1985a) (although they did not explicitly state the justification). As O. alvinus (Pettibone, 1985a) is the type species of its genus, this resulted in the transfer of all species in Opisthotrochopodus (Pettibone, 1985a) to Branchinotogluma (Pettibone, 1985a) (Read and Fauchald, 2017).
Despite nearly three decades of deep-sea research in the West Pacific, little is known about deep-sea scale-worm diversity in this region (Desbruyères et al., 2006). Among the specialists of hydrothermal vents and cold seeps (i.e., those without lateral antennae), only ten species in four genera of deep-sea polynoids have been described. Among them, most species appear to have limited distribution ranges, as three species (Thermopolynoe branchiata Miura, 1994; Branchinotogluma segonzaci Miura and Desbruyères, 1995; Branchinotogluma trifurcus Miura and Desbruyères, 1995) have been reported only from the North Fiji, Lau, and Manus Back-Arc Basins; four (Branchinotogluma marianus Pettibone, 1989; Branchinotogluma burkensis Pettibone, 1989; Levensteiniella raisae Pettibone, 1989; Lepidonotopodium minutum Pettibone, 1989) from the Mariana Back-Arc Basin and one (Branchinotogluma japonica Miura and Hashimoto, 1991) from the Kaikata Seamount. L. okinawae (Sui and Li, 2017) is currently known only from the Okinawa Trough. Branchipolynoe pettiboneae Miura and Hashimoto, 1991, however, is widely distributed across the West Pacific (Miura and Hashimoto, 1991; Desbruyères et al., 2006; Sui and Li, 2017).
In deep-sea polynoids, mitochondrial molecular markers have been shown to be a promising tool in delimiting species and often led to the discovery of potential new species. For instance, Chevaldonné et al. (1998) applied two mitochondrial gene fragments (COI and 16S rRNA) to examine the genetic diversity among Branchipolynoe collected from three regions. They found a deep sequence divergence in COI (17.2–19.6% of nucleotide difference) between a Pacific species (B. symmytilida Pettibone, 1984) and an Atlantic species (B. seepensis Pettibone, 1986), indicating an ancient split of the two species. In addition, specimens of B. seepensis (Pettibone, 1986) collected from the Gulf of Mexico (the type locality of the species) and those collected from the Mid Atlantic Ridge (MAR) that was also identified as “B. seepensis (Pettibone, 1986)” based on morphology, had a smaller, but still substantial nucleotide difference (5.3%) in COI sequences, indicating that the MAR population belongs to a potential new species. Nevertheless, the sequences used in Chevaldonné et al. (1998) have not been deposited in a public sequence database. So far, only few sequences of such genetic markers are available for deep-sea scale worms.
Samples of polychaetes used in this study were collected during the R/V Natsushima cruise NT15-13 organized by Japan Agency for Marine-Earth Science and Technology (JAMSTEC) in 2015. Examination of the samples revealed two unknown species and previously undescribed sex morphs of two known species of polynoids. The aim of the present study is to describe and characterize them as two new species, as well as provide a full redescription of the two known species to include both sex morphs. To confirm the specific status of the new species and the affinity of the sex morphs, four gene fragments (COI, 16S rRNA, 18S rRNA, 28S rRNA) were amplified from selected specimens and the genetic distances between these sex morphs and species were calculated.
Materials and Methods
Sample Collection and Morphological Observation
Samples of free-living benthic polynoids were collected from four hydrothermal vent sites in the Okinawa Trough using ROV HYPER-DOLPHIN on-board R/V Natsushima during the cruise NT15-13 led by Ken Takai (JAMSTEC) in 2015. The following localities were sampled: Sakai Field (27°31.386′N, 126°59.209′E, 1603 m in depth, dive number 1857 on July 30/27°32.89′N, 126°59.37′E, 1300 m in depth, dive number 1858 on July 31/27°31.01N, 126°58.90′E, 1550 m in depth, dive number 1860 on August 1; Miyazaki et al., 2017); Aki site, Iheya North Field (27°46.00′N, 126°54.25′E, 1083 m in depth, dive number 1859 on July 31); Iheya North Original site, Iheya North Field (27°47.47′N, 126°53.80′E, 986 m in depth, diver number 1861 on August 2; Nakamura et al., 2015). Polynoids, which are often found along with deep-sea squat lobsters, mussels, shrimps, and limpets (Supplementary Figure 1), were sampled using a suction sampler. Table 1 lists the on-board sample IDs of the samples investigated in the present study and their collecting data. Specimens were fixed and preserved in 99% ethanol. All specimens examined in the present study are deposited in the National Museum of Nature and Science, Tsukuba (NSMT), the University Museum, the University of Tokyo (UMUT), or the Natural History Museum and Institute, Chiba (CBM).
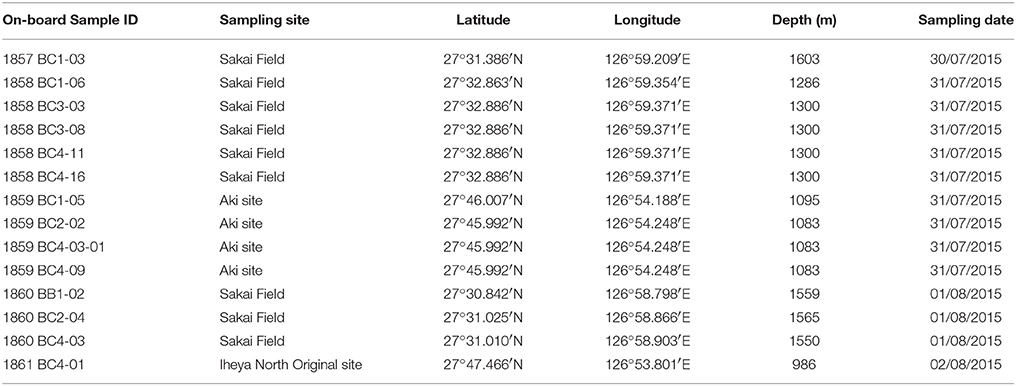
Table 1. On-board sample ID and collecting data for samples used in the present study (often with mixed species).
Polynoid specimens were observed under a dissecting microscope (Olympus SZX9, Tokyo, Japan) and a compound microscope (Motic BA210E Elite, China), both fitted with an ocular micrometer in the eyepiece. Light micrographs were taken using a digital camera (TUCSEN TrueChrome, China) attached to the microscopes. Fine details of the chaetae and some morphological characters were observed using a LEO 1530 FE-SEM scanning electron microscope (Zeiss, Germany). Tissues for SEM were prepared according to a method described in Dunlap and Adaskaveg (1997), which uses Hexamethyldisilazane (HMDS) (Sigma-Aldrich, Missouri, USA) as the drying agent.
Barcoding and Phylogenetic Analyses
An elytron or two parapodia from selected specimens were dissected for use in DNA barcoding and phylogenetic analysis. Genomic DNA was extracted using the CTAB method (Stewart and Via, 1993). A fragment of the cytochrome oxidase I (COI) gene was amplified using the universal primer pairs HCO2198 and LCO1490 (Folmer et al., 1994). PCR reactions were carried out in a Veriti Thermal Cycler (Applied Biosystems, Massachusetts, USA) using a PCR Amplification Kit (Takara, Dalian, China) according to the manufacturer′s protocol. The PCR program was as follows: 5 min at 96°C, 45 s at 94°C, 45 s at 50°C, 60 s at 72°C (30 cycles), and 10 min at 72°C. PCR amplicons were sent to BGI (Hong Kong) for sequencing after purification using a TaKaRa MiniBEST Agarose Gel DNA Extraction Kit. The sequences obtained are deposited in NCBI GenBank.
The COI sequences obtained were aligned with available COI sequences of other deep-sea polynoid species (Branchipolynoe symmytilida Pettibone, 1984; Branchinotogluma sandersi Pettibone, 1985a; Bathykurila guaymasensis Pettibone, 1989) as well as shallow-water polynoids (Halosydna brevisetosa Kinberg, 1856; Harmothoe oculinarum Storm, 1879 and Lepidonotus squamatus Linnaeus, 1758) downloaded from GenBank. The shallow-water polynoids, which fall into separate clades in the phylogenetic tree of Polynoidae (Norlinder et al., 2012; Gonzalez et al., 2017; Zhang, 2017), served as the outgroup. Specifically, the COI sequences of Branchipolynoe longqiensis (Zhou et al., 2017), B. pettiboneae (Miura and Hashimoto, 1991) (collected from South China Sea), Levensteiniella iris (Hourdez and Desbruyères, 2003), and L. okinawae (Sui and Li, 2017) were extracted from their mitochondrial genomes (Zhang, 2017). The alignment was made using Mesquite, adopting the MUSCLE algorithm (Madison and Madison, 2011). Sequence divergence was calculated using MegAlign (https://www.dnastar.com/t-megalign.aspx). Phylogenetic analysis was conducted using Maximum Likelihood (ML) and Maximum Parsimony (MP) methods. The ML analysis was implemented in RaXML GUI1.3, with the GTR+I+G substitution model being selected as the most suitable evolutionary model using jModelTest (Darriba et al., 2012). The MP analysis was conducted using the heuristic search algorithm based on 100 random addition sequences in PAUP* V4.0 (Swofford, 2003), with Tree Bisection and Reconnection (TBR) applied to reduce the number of topologies searched. Bootstrap analysis was based on heuristic searches of 1,000 random addition replicates.
To provide more phylogenetic information, three additional genes were amplified from the two new species: 16S rRNA using primer pairs 16S arL and 16S brH (Palumbi, 1996), 18S rRNA using F19 (Turbeville et al., 1994) and R1843 (Elwood et al., 1985), 28S rRNA using F63.2 (Passamaneck et al., 2004), and D3AR (Scholin et al., 1994). A phylogenetic tree was constructed based on the concatenated dataset of the four genes using the same method described above.
Results
Systematics
Genus Branchinotogluma Pettibone, 1985
Type species. Branchinotogluma hessleri Pettibone, 1985
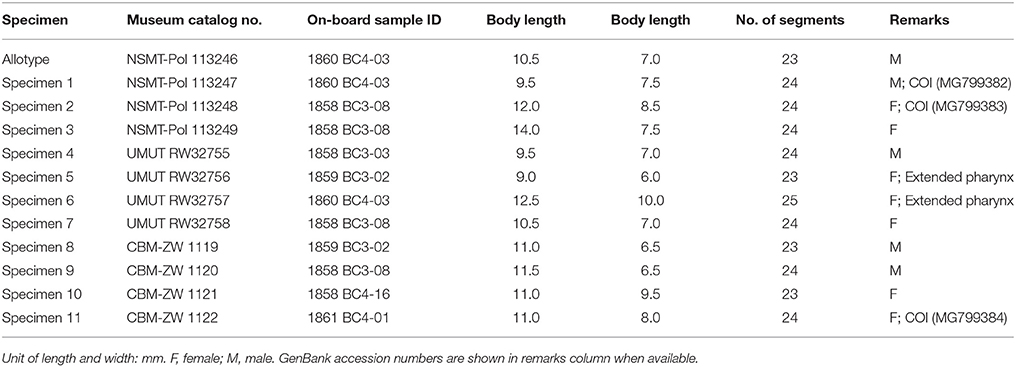
Branchinotogluma elytropapillata sp. nov.
ZooBank registration LSID
urn:lsid:zoobank.org:act:1D4C05F7-4CF2-4820-A5BC-20203FA03C22
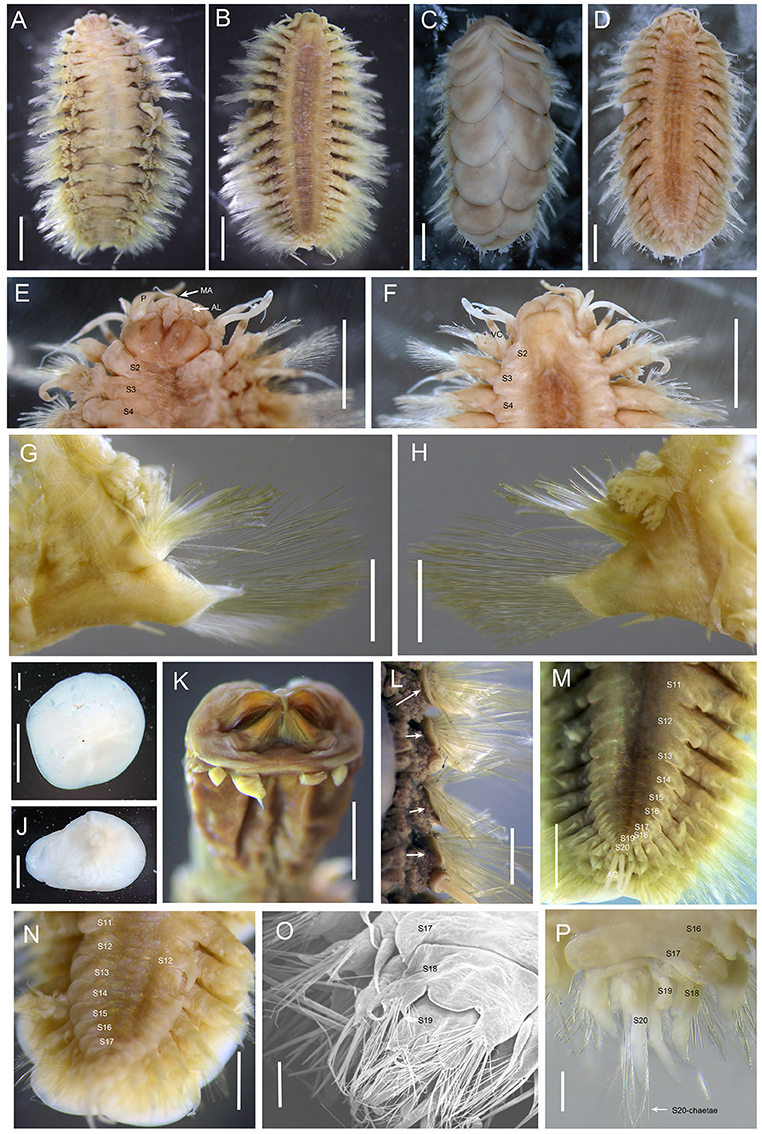
Figure 1. Branchinotogluma elytropapillata sp. nov. (A,B) Paratype 1; (C,D) Holotype. E, F, I to L, Paratype 2; K, Paratype 21; M, Paratype 7; N and O, Paratype 5; P, Paratype 1. (A), dorsal view of female. (B), ventral view of female. (C), dorsal view of male. (D), ventral view of male. (E) dorsal view of anterior part. (F) ventral view of anterior part. (G) anterior view of parapodium (segment 10). (H) posterior view of parapodium (segment 10). (I,J) elytra. (K) ventral view of extended pharynx. (L) dorsal view of neuropodia show neuropodia bract (indicated by arrows). (M) ventral view of female posterior part. (N) ventral view of male posterior part. (O) the detail of posterior end of male (the same as N) from ventral side of view. (P) dorsal view of male posterior part. Scale bar: (A–D) 2 mm; (E–J,L) 1 mm; (K) 0.5 mm; (O,P) 200 μm. MA, medial antenna; AL, anterior lobe; VC, ventral cirri.
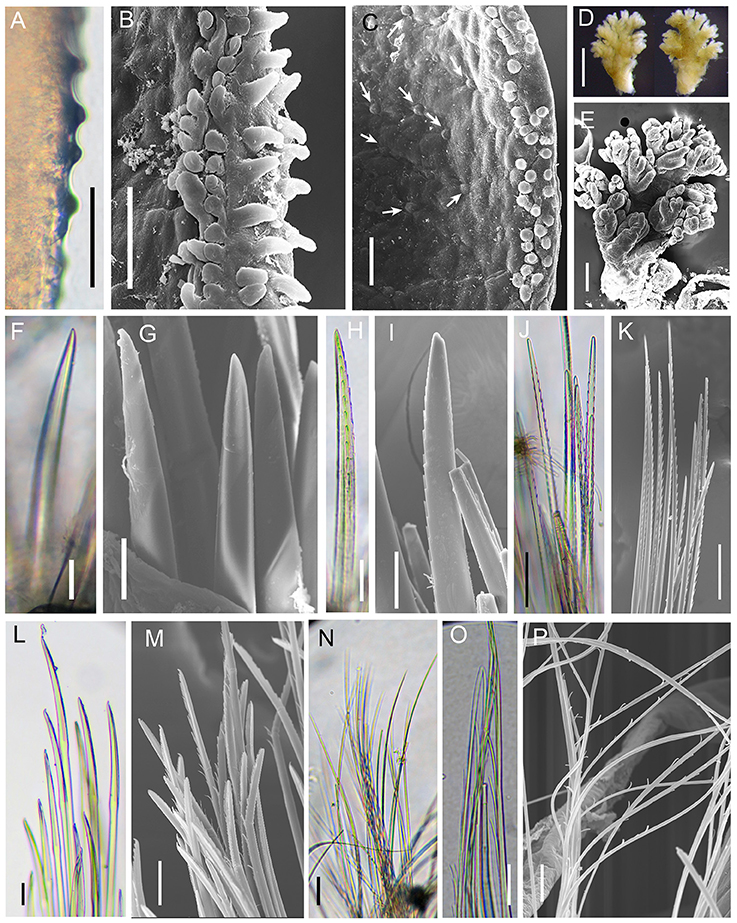
Figure 2. Branchinotogluma elytropapillata sp. nov. Details of scale and chaetae. (A–M) are from Paratype 2. (N–P) are from Paratype 5. (A–C) globular and microtubular papillae on the margin of elytron. Arrows in (C) show the tiny globular papillae. (D–L) are from segment 11. (D,E) shape of branchiae, (F–I) notochaetae. (J,K) upper neurochaetae. (L,M) lower neurochaetae. (N) capillary neurochaetae from posterior end. (O,P) special chaetae from last parapodia. Scale bar: (A,B,C,F,G,L,M,N,O) 40 μm; (D) 500 μm; (H,I) 20 μm; (E,J,K) 100 μm; (H) 60 μm; (P) 10 μm.
Material examined
Twenty-five specimens (Table 2). Holotype: adult, male, 11.0 mm in length, 5.5 mm in width, 20 segments. Paratypes: 24 specimens including 14 females and 10 males, 5.0 to 12.0 mm in length and 3.0 to 7.0 mm in width, 20 segments.
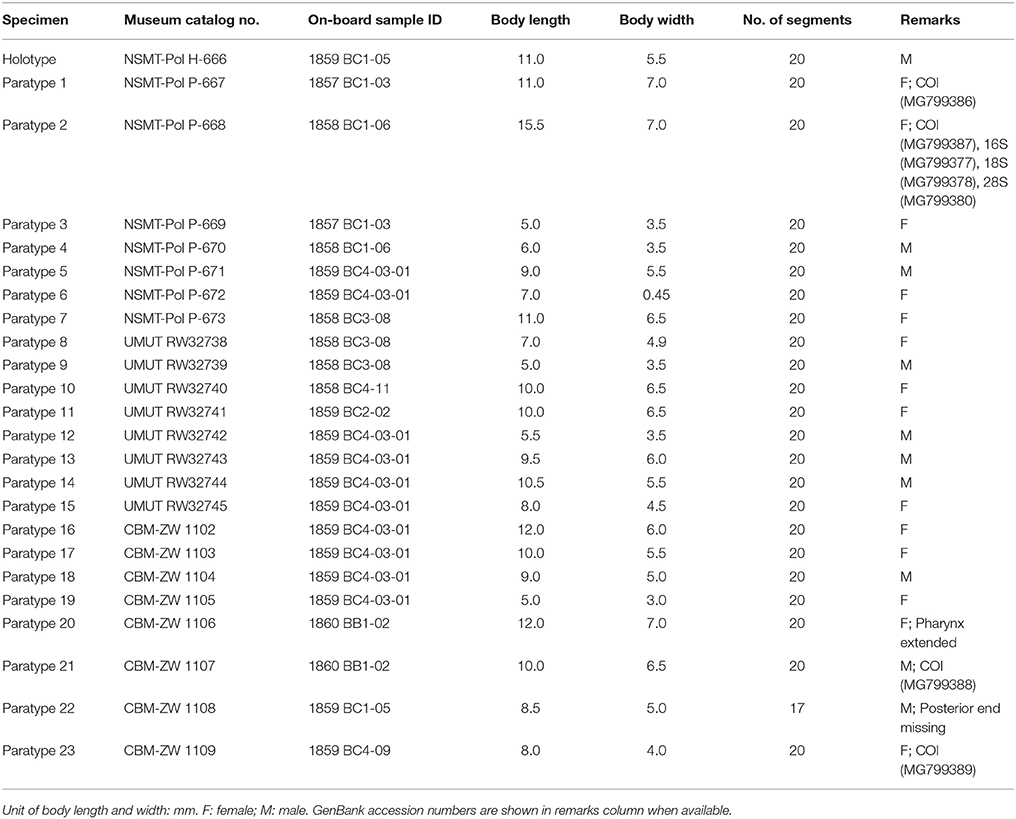
Table 2. General information of specimens of Branchinotogluma elytropapillata sp. nov. used in this study.
Description
Ethanol preserved specimens light brownish. Body short, suboval, dorsoventrally flattened (Figures 1A–D). Dorsum fully covered by 10 pairs of elytra, on segments 2, 4, 5, 7, 9, 11, 13, 15, 17, and 19. Elytra large, opaque, oval, or subreniform (Figures 1I,J) with numerous small papillae along non-overlapping margin (Figures 2A,B). Numerous tiny globular papillae on non-overlapped area of elytral surface (Figure 2C).
Prostomium deeply bilobed (Figure 1E). Anterior lobes subtriangular with short conical end. Median antenna inserted into anterior notch, with short ceratophore and subulate style as long as prostomium. Lateral antennae absent. Palps long, tapered, and smooth, approximately two times as long as prostomium. Eyes absent.
First segment achaetous, fused to prostomium, not distinct dorsally. The dorsal and ventral cirri are enlarged as long as palps (Figure 1F). Segment 2 with biramous parapodia bearing first pair of elytrophores; ventral cirri slightly shorter than those of first segment. Notopodial bract present on segment 2. Mouth located between segments 1 and 2 ventrally. Extended pharynx with four pairs of terminal papillae (two in middle larger than two lateral ones; same pattern on dorsal and ventral sides) and two pairs of arched jaws with ~20 denticles (Figure 1K).
Branchiae present from segment 3 to segment 16, arising from lateral base of elytrophores or dorsal base of notopodia (Figures 1A,G,H). Branchiae separated into 2 clusters, each arborescent with short terminal filaments (Figures 2D,E). Branchiae larger in middle region, smaller anteriorly and posteriorly (Figure 1A).
Parapodia biramous (Figures 1G,H). Dorsal cirri present on parapodia without elytra, as long as neurochaetae, smooth, with tapered end. Notopodia shorter than neuropodia, with bract clearly seen from dorsal view (Figure 1L). Neuropodia with long triangular pre-chaetal acicular lobe (Figure 1G) and shorter triangular lobe (Figure 1H). Ventral cirri thin, short, with tapered end (Figures 1G,H).
Notochaetae and neurochaetae numerous. Notochaetae thicker than neurochaetae, straight, with pairs of short lateral spines (Figures 2F–I). Neurochaetae longer than notochaetae, two kinds: one with pairs of lateral spines and straight tip (Figures 2J,K), another with longer spines and curved tip (Figures 2L,M). Long capillary neurochaetae present on segment 17 (Figure 2N).
In males, parapodia from segment 18 highly compressed and modified. Segment 18 with small notopodial acicular lobe fused to cirrophore of dorsal cirrus, with a few short, stout notochaetae on upper side; neuropodia fused to form a lamina on ventral side with ventral cirrus and small bundle of long capillary neurochaetae (Figures 1O,P). Segment 19 with very small elytrophore; notopodia with only few stout notochaetae; neuropodium fused, similar to segment 18 (Figure 1O).
Notopodial acicular lobe of segment 20 fused to cirrophore of dorsal cirrus, without notochaetae; neuropodial conical acicular lobe with slender capillary neurochaetae bearing widely-spaced long lateral spines (Figures 2O,P); without ventral cirrus. Pygidium small rounded lobe with dorsal anus medial to parapodia of segment 20, and pair of long anal cirri (Figure 1M).
Variation
The number of notochaetae for segment 2 varies from 1 to 6. Branchiae present from segment 3–16 for males, but from segment 3 to 18 for females. Males have one pair of nephridial papillae on ventral side of segments 12 and five pairs of ventral lamellae on segments 13–17 (Figure 1N). Nephridial papillae are subulate, short, as long as one segment only. Ventral lamellae are C-shaped, overlapping. Females do not bear long nephridial papillae or lamellae but have very short papillae from segment 11 to segment 15 (Figure 1M). In posterior segments, the last 3 segments in males are greatly modified and bear capillary chaetae. Posterior segments in females are not modified and they do not bear capillary chaetae.
Etymology
The specific epithet, elytropapillata, is a composite Latinized word derived from “elytra” and “papillae,” referring to the presence of papillae on the edge of elytra.
Distribution
Branchinotogluma elytropapillata sp. nov. is currently only known from the Okinawa Trough, including the Sakai Field and Aki site, Iheya North Field.
Remarks
Branchinotogluma is characterized by having 13–18 pairs of branchiae, 10 pairs of elytra, and in males a greatly modified posterior. There are eight recognized species in this genus. B. elytropapillata sp. nov. can be distinguished from all other congeneric species by the presence of rows of papillae along the edge of the elytra. Sexual dimorphism has been reported in Branchinotogluma. There are usually nephridial papillae and lamellae for males but absent for females. The patterns of nephridial papillae and lamellae for males are different among the species. Specifically, B. sandersi (Pettibone, 1985a) has four or five pairs of short nephridial papillae and three pairs of lamellae; B. hessleri (Pettibone, 1985a) has one pair of long nephridial papillae and seven pairs of lamellae; B. burkensis (Pettibone, 1989) has seven pairs of small nephridial papillae; B. marianus (Pettibone, 1989) has one pair of long nephridial papillae and six pairs of lamellae; B. segonzaci (Miura and Desbruyères, 1995), B. trifurcus (Miura and Desbruyères, 1995) and B. japonicus (Miura and Hashimoto, 1991) have one pair of nephridial papillae and five pairs of lamellae; B. tunnicliffeae (Pettibone, 1988) has four pairs of long nephridial papillae and two pairs of lamellae (Desbruyères et al., 2006). However, C-shaped lamellae present in B. elytropapillata sp. nov. and B. japonicus (Miura and Hashimoto, 1991) only. But these species differ in the shapes of pharyngeal papillae and posterior end. B. elytropapillata sp. nov. is also similar with B. segonzaci (Miura and Desbruyères, 1995), B. trifurcus (Miura and Desbruyères, 1995) in having four pairs of pharynx papillae. However, in B. segonzaci (Miura and Desbruyères, 1995) the pharynx papillae are small, and in B. elytropapillata sp. nov the dorsal cirri do not have rounded tips—a distinct taxonomic character for B. segonzaci (Miura and Desbruyères, 1995). In B. trifurcus (Miura and Desbruyères, 1995), the anterior lobes do not have frontal filaments and there are trifurcate neurochaetae on segment 20 in specimens with modified posterior segments. These two characters are different from those in B. elytropapillata sp. nov.
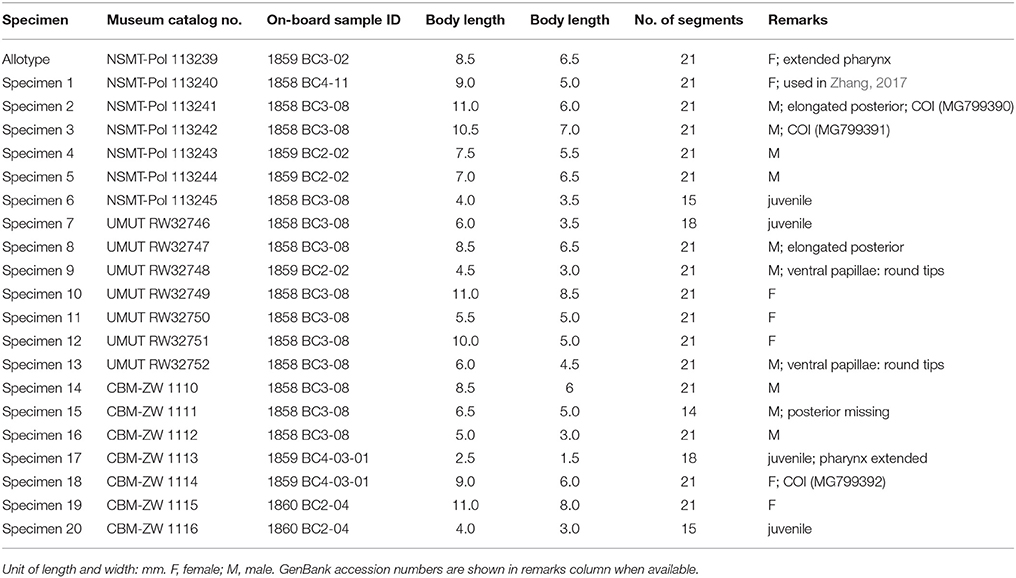
Branchinotogluma japonicus (Miura and Hashimoto, 1991)
(Figures 3, 4; Miura and Hashimoto, 1991: Figures 4–6).

Figure 3. Branchinotogluma japonicus. (A,B,M,O), Specimen 3; (C,D,H,I) allotype; (E–G), Specimen 1; (L) Specimen 13; (J,K,N,P) Specimen 2. (A) dorsal view of male. (B) ventral view of male. (C) dorsal view of female. (D) ventral view of female. (E) dorsal view of anterior part. (F) ventral view of anterior part. (G) elytra. (H) ventral view of extended pharynx. (I) five papillae on the lateral side of pharynx (one side). (J) anterior view of parapodium (segment 10). (K) posterior view of parapodium (segment 10). (L–N), ventral view of posterior part of males. (O) dorsal view of (M). (P) dorsal view of N. Scale bar: (A–D) 1 mm; (E,F,G,L,M,N) 1 mm; (H–K), (O,P) 0.5 mm.

Figure 4. Branchinotogluma japonicus. Details of scale and chaetae. (A–L) are from Specimen 1; (M,N) is from Specimen 5. (A,B) elytral surface. (C,D) branchiae on segment 11. (E–G) notochaetae and the details of tips. H and I, upper neurochaetae and the details of the tips. J and K, lower neurochaetae. (L,M) special chaetae from last parapodia. (C–K) are all from right segment 11 parapodium. Scale bar: (A,B,H,J,K,L,M) 20 μm; (C) 500 μm; (D) 100 μm; (E,F,I) 30 μm; (G) 10 μm.
Material examined
21 specimens (including 4 juveniles; Table 3). Allotype (designated in this study): female, 8.5 mm in length, 6.5 mm in width, 21 segments. Non-type specimens: 10 males and 6 females, 4.5 to 11.0 mm in length and 3.0 to 8.5 mm in width, 15 to 21 segments. Smaller individuals (2.5 to 6.0 mm in length, 1.5 to 3.5 mm in width), considered juveniles, have fewer segments (15 to 18).
Description
Ethanol preserved specimens light brownish. Body short, oval, dorsoventrally flattened (Figures 3A–D). Dorsum covered by 10 pairs of elytra, on segments 2, 4, 5, 7, 9, 11, 13, 15, 17, 19 (Figures 3A,C). Elytra very large, soft, and overlapping, oval to subreniform with branched veins (Figure 3G). At higher magnification, irregular squares and pentagons present on surface of non-overlapping region (Figures 4A,B).
Prostomium bilobed (Figure 3E). Anterior lobes subtriangular with frontal filament. Median antenna inserted into anterior notch with short cylindrical ceratophore, and subulate style longer than prostomium. Lateral antennae absent. Palps thick, tapered and smooth, approximately three times as long as prostomium. Eyes absent.
First segment achaetous, fused to prostomium, not distinct dorsally (Figure 3F). The dorsal and ventral cirri are enlarged as long as palps. Mouth located between segments 1 and 2 ventrally. Extended pharynx with two pairs of dorsal and ventral arched soft jaws without teeth (Figure 3H), and five pairs of lateral tapered papillae (Figure 3I).
Parapodia from segment 2 to segment 17 biramous (Figures 3J,K). Dorsal cirri present on parapodia without elytra, as long as neurochaetae, smooth, with tapered end.
Notopodium shorter than neuropodia. Neuropodium triangular with longer conical pre-chaetal acicular lobe (Figure 3J) and shorter post-chaetal lobe with rounded tip (Figure 3K). Ventral cirri thin, as long as neuropodial post-chaetal lobe, with tapered end (Figures 3J,K).
Branchiae present from segment 3 to segment 16, arising from lateral base of elytrophores or dorsal base of notopodia (Figures 4C,D). Branchiae separated into 2 clusters, each arborescent with short terminal filaments. Branchiae larger in middle region, smaller anteriorly and posteriorly. Branchiae reduced to two or three terminal globular papillae on segment 18 and disappeared on segment 19 (Figure 3P).
Notochaetae and neurochaetae numerous. Notochaetae thicker than neurochaetae, with rows of fine spines arranged perpendicularly to growth axis on one side (Figures 4E–G). Neurochaetae on average thinner and longer than notochaetae, two kinds: one with pairs of long lateral spines and pointed tip (Figures 4H,I); another with pasta server-like inflated end (Figures 4J,K). Capillary neurochaetae present from segment 17.
In males, parapodia from segment 18 slightly compressed and much smaller (Figures 3L,P). Segment 18 with small notopodial acicular lobe with a few short stout notochaetae on upper side; neuropodium with some long capillary neurochaetae and ventral cirrus. Segment 19 biramous; elytrophore and ventral cirri small; notopodia with few stout notochaetae; neuropodium with spinous neurochaetae and capillary chaetae. Segment 20 sub-biramous with notopodial acicular lobe fused to cirrophore of dorsal cirrus, without notochaetae; neuropodial conical acicular lobe with few short capillary chaetae; ventral cirri tapered, similar length to dorsal cirri. Segment 21 subbiramous similar to segment 20; neuropodium small, with short conical acicular lobe with few long modified chaetae (Figures 4L,M). Pygidium small, with pair of tapered anal cirri (Figure 3M).
Variation
Males have a pair of prominent nephridial papillae on ventral side of segment 12 and five pairs of ventral lamellae on segments 13–17 (Figures 3L–N). Nephridial papillae are ampullar, constricted subterminally, or digitiform; approximately as long as two segments (Figures 3L–N). Ventral lamellae are wedge-shaped. Female specimens do not have ventral papillae or lamellae. While in most male specimens, posterior parapodia are compressed (Figure 3M). In two of the males, posterior segments are elongated (Figure 3N). In smaller male specimens, ventral papillae are thinner and ventral lamellae are smaller (Figure 3L), not as distinct as in adults. Branchiae are present from segment 3 to segment 18 for females, but missing from segment 17 for males. There are capillary chaetae on several posterior segments (from segment 17). For juveniles, there are fewer elytra (seven pairs).
Distribution
Branchinotogluma japonicus has been reported from the Okinawa Trough and Kaikata seamount in the West Pacific.
Remarks
In the original description of B. japonicus based on two male specimens, Miura and Hashimoto (1991) considered its large elytra, one pair of elongate nephridial papillae on segment 12 and 5 or 6 pairs of short lamellae on the following segments, and compressed posterior segments to be distinguishing characters for this species. Here, we provide supplementary information on the morphology of this species. First, the females do not bear nephridial papillae, which is a common trait shared by several species of Branchinotogluma (Desbruyères et al., 2006). Second, instead of having compressed posterior segments, some male specimens have elongated posterior segments and the last parapodia (i.e., segment 21) do not bear modified chaetae. Third, there is variation in the shape of nephridial papillae and lamellae for males: in most of them (8 out of 10), nephridial papillae have a tapered end and the lamellae are thin, with a rounded edge; in other males, ventral papillae are ampulla shaped with constriction subterminally, and the papillae are fleshy with a straight edge. Fourth, males and females differ in segments bearing branchiae: males do not bear branchiae from segment 17, whereas females bear branchiae until segment 18. Fifth, the extended pharynx of this species has two pairs of arched soft jaws without teeth and five pairs of tapered papillae. Overall, B. japonicus is characterized by its large elytra, prominent ventral papillae on segment 12 and lamellae on several following segments in males, and a special pharynx with small lateral papillae instead of terminal ones.
Genus Levensteiniella Pettibone, 1985
Type species. Levensteiniella kincaidi Pettibone, 1985
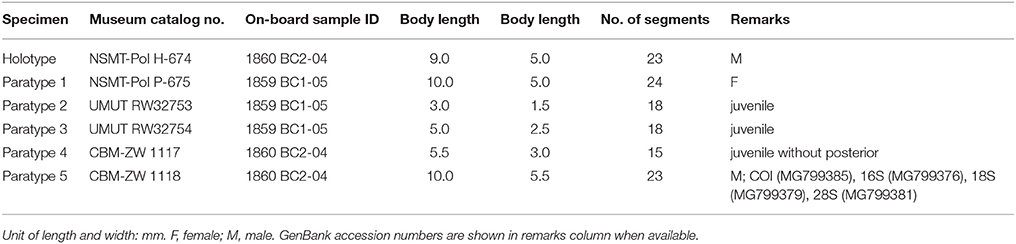
Levensteiniella undomarginata sp. nov.
ZooBank registration LSID.
urn:lsid:zoobank.org:act:21702740-DFB1-496D-BF6B-BBA018B24AE4
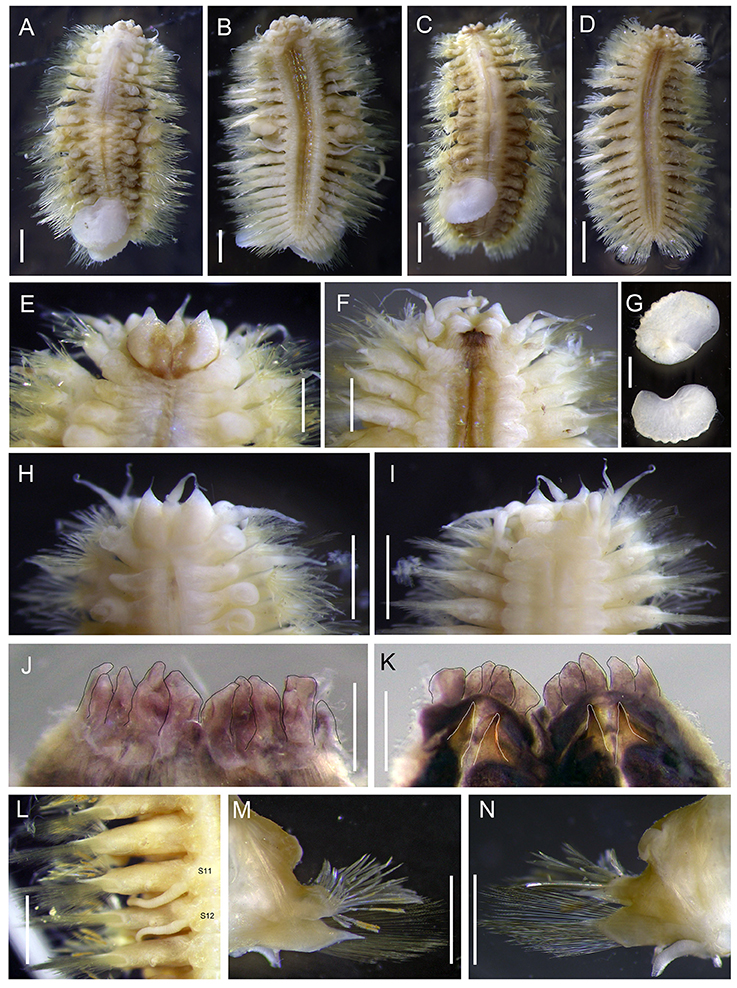
Figure 5. Levensteiniella undomarginata sp. nov. (A,B) specimen: Holotype; (C,D) Paratype 1; (E–G,J–N) Paratype 5; (H,I) Paratype 2. (A) dorsal view of male. (B) ventral view of male. (C) dorsal view of female. (D) ventral view of female. (E) dorsal view of anterior part. (F) ventral view of anterior part. (G) elytra. (H) dorsal view of anterior part. (I) ventral view of anterior part. (J) ventral view of pharynx (cut open) showing some of the terminal papillae (outlined with a black line). (K) dorsal view of pharynx (cut open) showing two pairs of jaws (outlined with a white line). (L) ventral view of segment 11, 12 (male). (M) anterior view of parapodium (segment 11). (N) posterior view of parapodium (segment 11). Scale bar: (A,B,G–I,L–N) 1 mm; (C,D) 2 mm; (E,F,J,K) 0.5 mm.
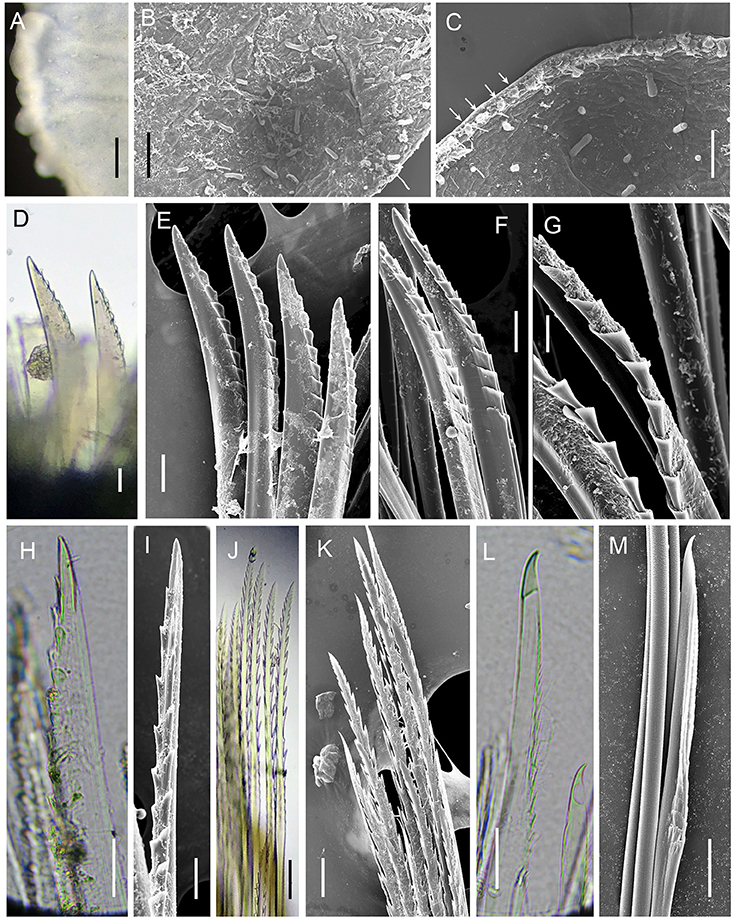
Figure 6. Levensteiniella undomarginata sp. nov. Details of scale and chaetae. All the materials are from Paratype 5. (A–C) micropapillae on the elytral surface. Arrows in (C) show micropapillae along the edge. (D,E), shorter notochaetae on the dorsal side. (F,G) longer notochaetae in the middle. (H,I) notochaetae from the lower ventral side. (J,K) upper neurochaetae. (L,M) lower neurochaetae. D to M are all from right segment 11 parapodium. Scale bar: (A) 250 μm; (B) 80 μm; (C) 60 μm; (D,E,J,K) 30 μm; (F,H,I) 20 μm; (G,L,M) 10 μm.
Material examined
Six specimens (Table 4). Holotype: male, 9.0 mm in length, 5.0 mm in width, 23 segments. Paratypes: one male, 9.0 mm in length and 5 mm in width including chaetae, 23 segments; one female 10.0 mm in length and 5.0 to 5.5 mm in width including chaetae, 24 segments. Three juveniles 3.0 to 5.5 mm long for 18 segments with fewer elytra (8 or 9 pairs), 1.5 to 3.0 mm wide including chaetae.
Description
Ethanol preserved specimens light brownish. Body short, oval, dorsoventrally flattened (Figures 5A–D). Adults with 11 pairs elytra, located on segments 2, 4, 5, 7, 9, 11, 13, 15, 17, 19, and 21 (Figures 5A,C). Elytra opaque, oval to subreniform, with thicker and wave-shaped posterior edge (Figure 5G); irregularly-spaced macrotubercles present on border of non-overlapping area (Figures 5G, 6A). At higher magnification, globular micropapillae and rod-shaped microtubercles present on elytral surface (Figures 6A–C) and micropapillae located on margin of elytron (Figure 6C).
Prostomium bilobed (Figures 5E,H). Anterior lobes prominent, triangular with frontal terminal filament. Median antenna inserted into anterior notch, with short ceratophore, and subulate style approximately half length of prostomium (Figures 5E,H). Lateral antennae absent. Palps thick, slightly subulate, more than twice as long as prostomium (Figure 5F). Eyes absent.
First segment achaetous, fused to prostomium, not distinct dorsally. The dorsal and ventral cirri are enlarged, as long as palps (Figure 5F).
Mouth ventral, located between segments 1 and 2. Pharynx dark purplish, located between segments 2 and 9 when not extended. Extended pharynx with seven pairs of terminal papillae forming circle, middle one smaller than side ones (Figures 5J,K). Two pairs of hooked jaws without teeth, located dorsally, and ventrally inside pharynx (Figure 5K).
Parapodia biramous (Figures 5M,N). On parapodia without elytra, dorsal cirri present, shorter than neurochaetae, smooth, with tapered end. Notopodia shorter than and located anterodorsally of neuropodia, with post-chaetal triangular acicular lobe hidden by numerous notochaetae from frontal view. Neuropodia with long triangular pre-chaetal acicular lobe, and shorter triangular post-chaetal lobe. Ventral cirri present on base of neuropodia, very thin, short, with tapered end (Figures 5B,D,M,N).
Notochaetae and neurochaetae numerous (Figures 5M,N). Notochaetae arranged in two fan-shaped bundles, one bundle with shorter chaetae bending frontally and another with longer chaetae pointing to the side. Notochaetae two kinds: one short and thick, bent with blunt end, with pocket-like serration along one edge (Figures 6D–G); another long and thin, with two long rows of paired spines near tip (Figures 6H,I). Neurochaetae thinner and longer than notochaetae, arranged in fan-shaped bundle. Neurochaetae in upper position with straight tip, and paired spines along sides (Figures 6J,K); those in lower position with slightly hooked tip and minute serration on one side (Figures 6L,M).
Pygidium small, with anus located dorsally, and pair of long anal cirri as long as the last parapodia.
Variation
Males have two pairs of ventral nephridial papillae on segments 11 and 12, subulate, approximately as long as three segments (Figures 6B,L). Females do not have nephridial papillae. Most of elytra have been detached from the body in the ethanol preserved specimens. Some specimens with dark brownish pigment on prostomium and ventral side, as well as dorsal and ventral cirri. Other specimens without distinct pigmentation. Anal cirri are usually missing.
Etymology
The specific epithet, undomarginata, is a composite word derived from the Latin words undo, meaning wave-formed, and margino, meaning edge.
Distribution
Levensteiniella undomarginata sp. nov. is currently known from Aki site, Iheya North Field and Sakai Field in the Okinawa Trough only.
Remarks
The genus Levensteiniella is characterized by having neither branchiae nor notopodial bract, and having 11 pairs of elytra. Of the five recognized species in Levensteiniella, three [L. intermedia (Pettibone, 1990), L. kincaidi (Pettibone, 1985b), L. plicata (Hourdez and Desbruyères, 2000)] are from the East Pacific, one from the Mariana Trough (L. raisae Pettibone, 1989) and one from the Mid-Atlantic Ridge (L. iris Hourdez and Desbruyères, 2003). Although L. undomarginata sp. nov., L. intermedia (Pettibone, 1990) and L. iris (Hourdez and Desbruyères, 2003) are similar in having wave-shaped elytra, their males differ in the number of nephridial papillae. Both L. intermedia and L. iris (Hourdez and Desbruyères, 2003) have one pair of nephridial papillae only, but L. undomarginata sp. nov. has two pairs. Among the congeners whose chaetae have been examined using SEM (i.e., Paratype 5), L. undomarginata sp. nov. is unique in having a special type of notochaetae with pocket-like serration along the edge. In addition, the elytra of L. undomarginata sp. nov. have micro- and macro tubercles and bulbous papillae on the surface and posterior border and they are much larger and more distinct than those in L. raisae (Pettibone, 1989) and L. plicata (Hourdez and Desbruyères, 2000). Moreover, L. undomarginata sp. nov. has fewer segments (23–24) than L. raisae (Pettibone, 1989) (27). The lengths of ventral papillae are different: they reach the middle of neurochaetae in L. undomarginata sp. nov., but they are longer than neurochaetae in L. kincaidi (Pettibone, 1985b), and shorter than neuropodia in L. raisae (Pettibone, 1989).
Genus Lepidonotopodium (Pettibone, 1983)
Type species. Lepidonotopodium fimbriatum (Pettibone, 1983)
Lepidonotopodium okinawae (Sui and Li, 2017)
(Figures 7, 8; Sui and Li, 2017: Figures 4–6).
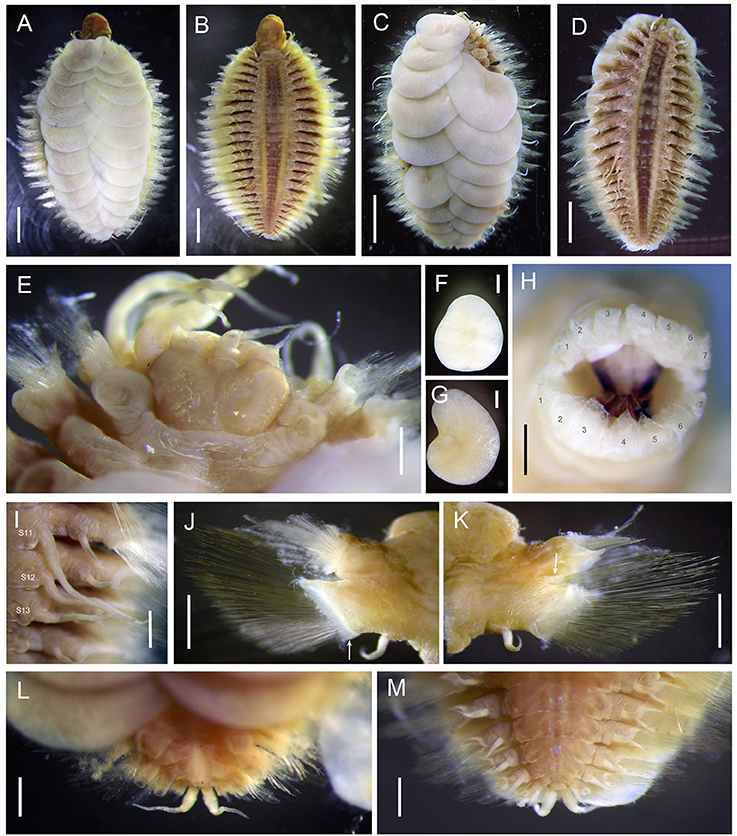
Figure 7. Lepidonotopodium okinawae. (A,B,E,F,G) Specimen 5; (C,D) Allotype; (H) Specimen 6; (J,K) Specimen 10; (I,L,M) Specimen 1. (A) dorsal view of female. (B) ventral view of female. (C) dorsal view of male. (D) ventral view of male. (E) dorsal view of anterior part. (F) first elytra. (G) second elytra. (H) top view of extended pharynx. (I) ventral view of segments 11–13 (male). (J) anterior view of parapodium (segment 9). The arrow indicates the membrane-like edge. (K) posterior view of parapodium (segment 9). The arrow indicates the tubular attachment on the dorsal base of neuropodia. (L) dorsal view of posterior part. (M) ventral view of posterior part. Scale bar: (A–D) 2 mm; (E) 1 mm; (F,G,I,J,K,L,M) 0.5 mm; (H) 0.25 mm.
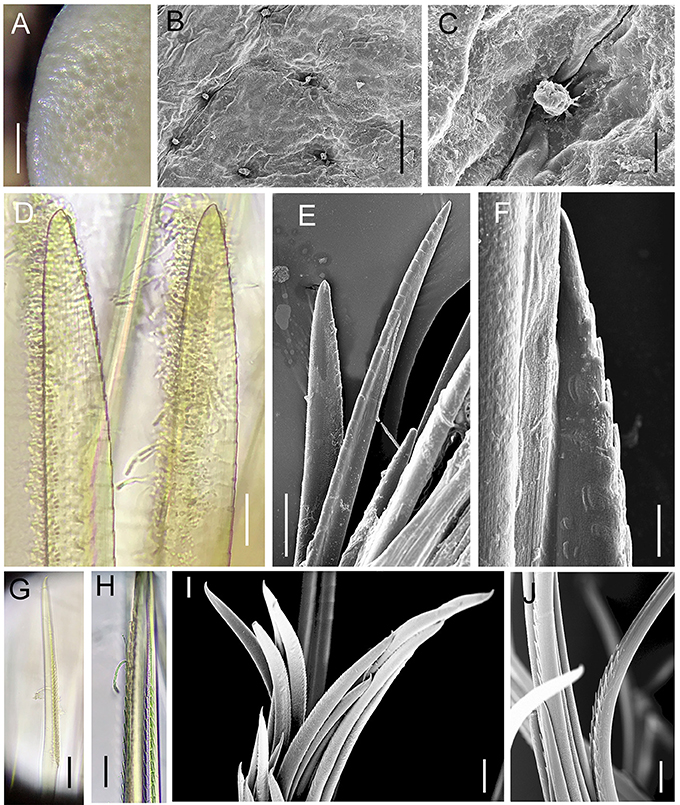
Figure 8. Lepidonotopodium okinawae. Details of scale (A–C, from Specimen 3) and chaetae (D–H, from Specimen 10). (A–C) foveolae and micropapillae on the elytral surface. (D–E) shorter notochaetae from the dorsal side. (F) lower longer notochaetae. (G–J) neurochaetae. (D–J) are all from 10th right parapodium. Scale bar: (A) 0.5 mm; (B) 80 μm; (E) 40 μm; (F,H) 10 μm; (D,E,G,I,J) 20 μm.
Material examined
Twelve specimens (Table 5). Allotype (designated in this study): male, 10.5 mm in length, 7.0 mm in width including chaetae, 23 segments. Non-type specimens: 4 males and 7 females, 9.0 to 14.0 mm in length and 6.0 to 10.0 mm in width, 23 to 25 segments.
Description
Ethanol preserved specimens light brownish. Body short, oval, dorsoventrally flattened (Figures 7A–D). Dorsum fully covered by 11 pairs of elytra, on segments 2, 4, 5, 7, 9, 11, 13, 15, 17, 19, and 21. Elytra large, whitish, opaque, oval or subreniform (Figures 7F,G), with numerous small pits in non-overlapping area (Figure 8A). At higher magnifications, a globular micropapilla present in each pit (Figures 8B,C).
Prostomium bilobed (Figure 7E). Anterior lobes subtriangular, each with short frontal filament. Median antenna inserted into anterior notch, with short ceratophore and subulate style slightly longer than prostomium. Lateral antennae absent. Palps thick, slightly subulate, more than twice as long as prostomium. Eyes absent.
Mouth located between segments 1 and 2 ventrally. Extended pharynx with seven pairs of terminal papillae (Figure 7H). Median papillae larger than lateral papillae (Figure 7H). Two pairs of hooked jaws without teeth, located dorsally and ventrally inside pharynx (Figure 7H).
First segment achaetous, fused to prostomium, not distinct dorsally. The dorsal and ventral cirri are enlarged, as long as palps (Figure 7E). Mouth located between segments 1 and 2 ventrally. Extended pharynx with seven pairs of terminal soft papillae, and two pairs of jaws (Figure 7H).
Parapodia biramous (Figures 7J,K). Dorsal cirri present on parapodia without elytra; as long as neurochaetae, smooth, with tapered end. Notopodia shorter than and located anterodorsally of neuropodia. Notopodia with projecting post-chaetal acicular lobe (Figures 7K) hidden anteriorly by numerous notochaetae and enclosed anteriorly and dorsally by flaring bract (Figure 7J). Neuropodia dorsally separated into two lobes by neurochaetae, pre-chaetal lobe acicular lobe long, triangular, post-chaetal lobe with short digit projecting outward from notch between notopodia and neuropodia, and wider lower ligule with membrane-like edge (Figure 7K); cleft present ventrally (Figures 7K). Ventral cirri short, tapered, attached near base of neuropodia.
Notochaetae and neurochaetae numerous. Notochaetae stout, forming radiating bundle, each with fine spinous rows on one side (Figures 8D–F). Neurochaetae thinner and longer than notochaetae, forming fan-shaped bundle, with two rows of paired spines distributed along one edge sub-terminally, tip slightly curved (Figures 8G–J).
Pygidium small, with anus located dorsally (Figure 7L), and pair of ventral cirri approximately as long as three segments (Figure 7M).
Variation
Male specimens are consistent in having three pairs of nephridial papillae on segments 11–13. They are subulate, each papilla approximately as long as three segments (Figures 7D,I). Females do not have ventral papillae.
Distribution
L. okinawae is known only from the Okinawa Trough.
Remarks
Lepidonotopodium is morphologically similar with Levensteiniella in having 11 pairs of elytra but it has notopodial bract. The original description of L. okinawae (Sui and Li, 2017) was based on six specimens collected on 20 May 2014 from the Okinawa Trough (27°33.06928′N, 126°58.13082′E, 1361 m), only 3.5 km from Sakai Field—one of our sampling sites. As their specimens were all females, no information about the ventral papillae was included in the original species description. Our comparison of this new species with its congeners indicates that having three pairs of nephridial papillae is a distinguishing character for the males of L. okinawae (Sui and Li, 2017): L. fimbriatum (Pettibone, 1983) and L. riftense (Pettibone, 1984) have two pairs, L. williamsae (Pettibone, 1984) and L. atalantae (Desbruyères and Hourdez, 2000a) have four pairs, and L. piscesae (Pettibone, 1988) and L. jouinae (Desbruyères and Hourdez, 2000b) have five pairs. Of the other two characters considered to be distinct in L. okinawae by Sui and Li (2017), the number of segments vary depending on the size of the adult, thus may not be a reliable character for use in species delimitation, as our specimens have slightly more segments (23–25) than those used in the originally description (23–24). The special feature on the surface of elytra (i.e., small pits on elytral surface with a papilla inside each pit) is indeed unique among all described species in this genus.
Key to Deep-Sea Polynoids Without Lateral Antennae From the West Pacific
1. With branchiae……………………………………………2
Without branchiae………………………….…………….3
2. With 11 pairs of elytra….……….Thermopolynoe branchiata
With 10 pairs of elytra……….……….………………….4
3. With notopodial bract…………….………………………7
Without notopodial bract.……….……………….……….8
4. Anterior lobes without frontal filaments……….………….5
Anterior lobes with frontal filaments……….…………….6
5. Anterior lobes rounded………Branchipolynoe pettiboneae
Anterior lobes prominent………Branchinotogluma segonzaci
6. Dorsal cirri with rounded tips…….Branchinotogluma trifurcus
Dorsal cirri with tapered tips….….………….…………….9
7. Elytra with numerous pits…………….Lepidonotopodium okinawae
Elytra with scattered micropapillae……Lepidonotopodium minutum
8. 23 to 24 segments ……………….………. Levensteiniella undomarginata sp. nov
27 segments …….……………………Levensteiniella raisae
9. Elytral edge with rows of papillae……. Branchinotogluma elytropapillata sp. nov.
Elytra edge smooth …………………….……………….10
10. Pharynx with terminal papillae………….……………….11
Pharynx without terminal papillae but with five pairs of lateral papillae………………….…………….…Branchinotogluma japonicus
11. Jaws with numerous denticles (about 50)……………. Branchinotogluma burkensis
Jaws without denticles……………………Branchinotogluma marianus.
Molecular Analysis
A fragment of the mitochondrial cytochrome c oxidase I (COI) gene, ~680 bp long, was successfully amplified and sequenced from at least one male and one female of L. okinawae, B. japonicus, B. elytropapillata sp. nov. and one male of L. undomarginata sp. nov. After removing the low-quality sites at the two ends, sequences around 620 bp long were used for the phylogeny and comparison of sequence divergence. For those species with both male and female sequences, the individuals were clustered by species, rather than by the presence or absence of nephridial papillae or ventral lamellae in both ML and MP trees (Figure 9). For those species with at least two sequences, the intra-specific divergence is very low (0–4.2%), when compared with inter-specific divergence that exhibits a clear barcoding gap within the same genus (14.8% for Branchinotogluma, 20.3% for Lepidonotopodium). These results thus indicate that the COI gene is a suitable barcoding gene for deep-sea polynoids as it can be used to confirm the sexual dimorphism, as well as to distinguish different species. In addition, for species with variation in morphological details within the same sex (i.e., males of B. japonicus with either compressed or elongate posterior end), COI can be used to confirm the species identity (sequence divergence from 0 to 3.3%). The phylogenetic analysis using the concatenated genes (COI, 16S, 18S, 28S) showed that the 13 deep-sea species in eight genera form a well-supported clade (Supplementary Figure 2). The six species of branchiae-bearing deep-sea polynoids, including B. elytropapillata sp. nov. and B. japonicus, formed a well-supported clade. L. undomarginata sp. nov. is sister species to L. iris.
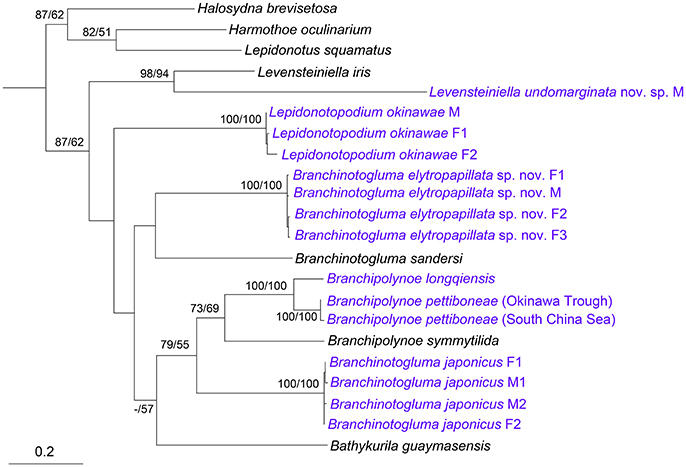
Figure 9. Phylogenetic tree constructed using COI sequences. Numbers above branches represent ML/MP bootstrap values with 100 as the highest value. M, male; F, female. Species we sequenced were marked as blue. The subfamilies are shown on the right. Accession numbers: Harmothoe oculinarum, AY894314; Halosydna brevisetosa, AY894313; Lepidonotus squamatus, AY894316; Bathykurila guaymasensis, DQ074766; Branchinotogluma sandersi, JN852923; Branchipolynoe symmytilida, AY646021; Levensteiniella iris, KY753827; Branchipolynoe longqiensis, KY753826; Branchipolynoe pettiboneae, KY753825 (South China Sea), MG799393 (Okinawa Trough); Branchinotogluma elytropapillata sp. nov., MG799386 (F1, Paraytpe 1), MG799387 (F2, Paratype 2), MG799388 (M, Paratype 21), MG799389 (F3, Paratype 23); Branchinotogluma japonicus, KY753824 (F1, Specimen 1), MG799390 (M1, Specimen 2), MG799391 (M2, Specimen 3), MG799392 (F2, Specimen 18); Levensteiniella undomarginata sp. nov., MG799385 (M, Paratype 5); Lepidonotopodium okinawae, MG799382 (M, Specimen 1), MG799383 (F1, Specimen 2), MG799384 (F2, Specimen 11).
Discussion
The deep-sea polynoid fauna in the chemosynthesis-based ecosystems of the West Pacific is poorly known. Only 10 species that belong to a monophyletic clade containing five exclusively deep-sea genera have been reported from this region. Our study has increased the recognized species in this clade of polynoids from 10 to 12, and provided complete redescriptions of two species with only one previously known sex morph.
Using molecular tools, we confirmed for the first time that sexual dimorphism is common in polynoids inhabiting hydrothermal vents. Sexual dimorphism has been recognized in several vent dwelling polynoids (Van Dover et al., 1999; Jollivet et al., 2000; Glover et al., 2005): females of Branchipolynoe seepensis (Pettibone, 1986) have two pairs of nephridial papillae but males have one pair only; males of Branchinotogluma spp. have nephridial papillae, ventral lamellae and modified posterior end, and females lack papillae; males of B. guaymasensis (Pettibone, 1989) have one pair of ventral papillae but females have no nephridial papillae; males of Levensteiniella spp. have one or two pairs of long nephridial papillae; Lepidonotopodium spp. and T. branchiata have one pair to five pairs of nephridial papillae and females lack papillae. Nevertheless, although it is now generally accepted that many deep-sea polynoids from hydrothermal vents and cold seeps exhibit sexual dimorphism (Desbruyères et al., 2006), Branchipolynoe pettiboneae, (Miura and Hashimoto, 1991) does not seem to exhibit sexual dimorphism, indicating empirical observation from more species is needed before we can generalize about the pattern of sexual dimorphism in this clade of polynoids.
Although several previous studies have pointed out sexual dimorphism in deep-sea polynoids, no assessment of the nucleotide sequence divergence between the presumably sex morphs of the same species has been conducted. Our study showed that COI can be used to confirm sexual dimorphism in this group of polynoids, and to infer their phylogenetic relationship. Nevertheless, due to the limited information of partial COI (only ~620 bp), the bootstrap values in several nodes of the phylogenetic trees are low. The bootstrap values are much higher for the phylogenetic tree based on concatenated dataset, indicating the multi-gene approach, like those used in Norlinder et al. (2012), Gonzalez et al. (2017), and Zhang (2017), should be employed in the future to better resolve the phylogenetic relationships of deep-sea polynoids when better taxon sampling becomes available.
It should be noted that our study was conducted based on a limited sampling scheme (i.e., four hydrothermal sites within a radius of 50 km in the Okinawa Trough) during a single research cruise. More intensive and systematic sampling should provide specimens for a better understanding of the true diversity of polynoids in the study area. The combined morphology and molecular approach used in our study can be applied to understand the diversity of this group of deep-sea polychaetes that are often common members of the benthic fauna of hydrothermal vents (Tunnicliffe, 1992; Van Dover et al., 1999; Levin et al., 2016) in other unexplored areas of the deep sea.
Author Contributions
YZ: examined the specimens, identified the species, and conducted the molecular experiments and data analyses; YZ and J-WQ: prepared the first draft of the manuscript; CC: collected the samples, provided site photos and edited several versions of the manuscript. All co-authors contributed to the final version.
Funding
This work was supported by General Research Fund of Hong Kong (Project No. 12302917), Shenzhen Science and Technology Innovation Committee of Shenzhen (Project No. JCYJ20170307161326613), and HKBU Strategic Development Fund (SDF 15-1012-P04). YZ received a Ph.D. studentship from Hong Kong Baptist University. During the time of writing CC is supported by a JAMSTEC International Postdoctoral Fellowship.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank the operation team of ROV HYPER-DOLPHIN as well as the Captain and crew of R/V Natsushima for their tireless support of the scientific activity during the research cruise NT15-13, the principal scientist, Ken Takai (JAMSTEC), for his leadership during the NT15-13 cruise, and Hiromi K. Watanabe (JAMSTEC) for facilitating collaboration among the authors and for valuable discussions.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2018.00112/full#supplementary-material
Supplementary Figure 1. In situ photographs of polynoid individuals (marked by red circles) and their associated fauna in the Sakai Field of the Okinawa Trough. (A) With the mussel Bathymodiolus platifrons Hashimoto and Okutani, 1994, the snail Provanna subglabra Sasaki et al., 2016, limpet-form gastropods Lepetodrilus nux (Okutani et al., 1993) and Bathyacmaea sp., shrimps Alvinocaris longirostris Kikuchi and Ohta, 1995 and Lebbeus shinkaiae Komai et al., 2012, the squat lobster Shinkaia crosnieri Baba and Williams, 1998, the barnacle Neoverruca intermedia Sha and Ren, 2015, and the tubeworm Alaysia sp. (27°32.89′N, 126°59.37′E, 1300 m in depth); B: With L. nux limpets, A. longirostris shrimps, S. crosnieri squat lobsters, and N. intermedia barnacles (27°31.01N, 126°58.90′E, 1550 m in depth).
Supplementary Figure 2. Phylogenetic tree constructed using the concatenated sequences of four genes (COI, 16S, 18S, and 28S) by the ML and MP methods. Numbers above branches represent ML/MP bootstrap values with 100 as the highest value. The sequences of the two new species were generated in this study. The sequences of Branchinotogluma japonicus and Lepidonotopodium okinawae are from Zhang (2017). The sequences of the other species are from Gonzalez et al. (2017).
References
Baba, K., and Williams, A. B. (1998). New Galatheoidea (Crustacea, Decapoda, Anomura) from hydrothermal systems in the West Pacific Ocean: Bismarck Archipelago and Okinawa Trough. Zoosystema 20, 143–156.
Barnich, R., and Fiege, D. (2003). The Aphroditoidea (Annelida: Polychaeta) of the-Mediterranean Sea. Abhandlungen der Senckenbergischen Naturforschenden Gesellschaft, Band 559. E. Schweizerbart'sche Verlagsbuchh.
Beesley, P. L., Ross, G. J., and Glasby, C. J. (2000). Polychaetes & Allies: the Southern Synthesis, Vol. 4. Australia: CSIRO Publishing.
Chevaldonné, P., Jollivet, D., Feldman, R. A., Desbruyères, D., Lutz, R. A., and Vrijenhoek, R. C. (1998). Commensal scale-worms of the genus Branchipolynoe (Polychaeta: Polynoidae) at deep-sea hydrothermal vents and cold seeps. Cah. Biol. Mar. 39, 347–350.
Darriba, D., Taboada, G. L., Doallo, R., and Posada, D. (2012). jModelTest 2: more models, new heuristics and parallel computing. Nat. Methods 9, 772. doi: 10.1038/nmeth.2109
Desbruyères, D., and Hourdez, S. (2000a). A new species of scale-worm (Polychaeta: Polynoidae), Lepidonotopodium atalantae sp nov., from the East Pacific Rise at 13°N and 9 °50'N. Cah. Biol. Mar. 41, 47–54. doi: 10.21411/CBM.A.53816BF9
Desbruyères, D., and Hourdez, S. (2000b). A new species of scale-worm (Polychaeta: Polynoidae), Lepidonotopodium jouinae sp nov., from the Azores Triple Junction on the Mid-Atlantic Ridge. Cah. Biol. Mar. 41, 399–405. doi: 10.21411/CBM.A.3111279A
Desbruyères, D., Segonzac, M., and Bright, M. (2006). Handbook of Deep-Sea Hydrothermal vent Fauna, 2nd Edn. Austria: Biologiezentrum Linz.
Dunlap, M., and Adaskaveg, J. E. (1997). Introduction to the Scanning Electron Microscope: Theory, Practice, and Procedures. Davis: CA, Facility for Advance Instrumentation.
Elwood, H. J., Olsen, G. J., and Sogin, M. L. (1985). The small-subunit ribosomal RNA gene sequences from the hypotrichous ciliates Oxytricha nova and Stylonychia pustulata. Mol. Biol. Evol. 2, 399–410.
Folmer, O., Black, M., Hoeh, W., Lutz, R., and Vrijenhoek, R. (1994). DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 3, 294–299.
Glover, A. G., Goetze, E., Dahlgren, T. G., and Smith, C. R. (2005). Morphology, reproductive biology and genetic structure of the whale-fall and hydrothermal vent specialist, Bathykurila guaymasensis (Annelida: Polynoidae). Mar. Ecol. 26, 223–234. doi: 10.1111/j.1439-0485.2005.00060.x
Gonzalez, B. C., Martínez, A., Borda, E., Iliffe, T. M., Eibye-Jacobsen, D., and Worsaae, K. (2017). Phylogeny and systematics of Aphroditiformia. Cladistics. doi: 10.1111/cla.12202. [Epub ahead of print].
Hashimoto, J., and Okutani, T. (1994). Four new mytilid mussels associated with deep-sea chemosynthetic communities around Japan. Venus 53, 61–83.
Henry, L. A., and Roberts, J. M. (2007). Biodiversity and ecological composition of macrobenthos on cold-water coral mounds and adjacent off-mound habitat in the bathyal Porcupine Seabight, NE Atlantic. Deep Sea Res. I. 54, 654–672. doi: 10.1016/j.dsr.2007.01.005
Hourdez, S., and Desbruyères, D. (2000). A new species of scale-worm (Polychaeta: Polynoidae), Levensteiniella plicata sp. nov., from the East Pacific Rise. Cah. Biol. Mar. 41, 97–102. doi: 10.21411/CBM.A.F5EE852D
Hourdez, S., and Desbruyères, D. (2003). A new species of scale-worm (Polychaeta: Polynoidae), Levensteiniella iris sp nov., from the Rainbow and Lucky Strike vent fields (Mid-Atlantic Ridge). Cah. Biol. Mar. 44, 13–21. doi: 10.21411/CBM.A.97F52D3
Jollivet, D., Empis, A., Baker, M. C., Hourdez, S., Comtet, T., Jouin-Toulmond, C., et al. (2000). Reproductive biology, sexual dimorphism, and population structure of the deep sea hydrothermal vent scale-worm, Branchipolynoe seepensis (Polychaeta: Polynoidae). J. Mar. Biol. Assoc. U.K. 80, 55–68. Available online at: http://archimer.ifremer.fr/doc/00000/615/
Kikuchi, T., and Ohta, S. (1995). Two caridean shrimps of the families Bresiliidae and Hippolytidae from a hydrothermal field on the Iheya Ridge, off the Ryukyu Islands, Japan. J. Crustacean Biol. 15, 771–785. doi: 10.1163/193724095X00172
Kinberg, J. G. H. (1856). Nya slägter och arter af Annelider. 1. Aphroditea Savigny. Öfversigt af Kongl. Vetensk-Akad Förhandl Stockh 12, 381–388.
Komai, T., Tsuchida, S., and Segonzac, M. (2012). Records of species of the hippolytid genus Lebbeus White, 1847 (Crustacea: Decapoda: Caridea) from hydrothermal vents in the Pacific Ocean, with descriptions of three new species. Zootaxa 3241, 35–63. doi: 10.5281/zenodo.280458
Levin, L. A., Baco, A. R., Bowden, D. A., Colaco, A., Cordes, E. E., Cunha, M. R., et al. (2016). Hydrothermal vents and methane seeps: rethinking the sphere of influence. Front. Mar. Sci. 3:72. doi: 10.3389/fmars.2016.00072
Madison, W. P., and Madison, D. R. (2011). Mesquite: A Modular System for Evolutionary Analysis. Version 2.75. Available online at: http://mesquiteproject.org
Miura, T. (1994). Two new scale-worms (Polynoidae: polychaeta) from the Lau back-arc and North Fiji basins, South Pacific Ocean. Proc. Biol. Soc. Wash. 107, 532–543.
Miura, T., and Desbruyères, D. (1995). Two new species of Opisthotrachopodus (Polychaeta: Polynoidae: Branchinotogluminae) from the Lau and the North Fiji Back-arc Basins, southwestern Pacific Ocean. Proc. Biol. Soc. Wash. 108, 583–595.
Miura, T., and Hashimoto, J. (1991). Two new branchiate scale-worms (Polynoidae: Polychaeta) from the hydrothermal vent of the Okinawa through and the volcanic seamount off Chichijima Island. Proc. Biol. Soc. Wash. 104, 166–174.
Miyazaki, J., Makabe, A., Matsui, Y., Ebina, N., Tsutsumi, S., Ishibashi, J.-I., et al. (2017). WHATS-3: an improved flow-through multi-bottle fluid sampler for deep-sea geofluid research. Front. Earth Sci. 5:45. doi: 10.3389/feart.2017.00045
Nakamura, K., Kawagucci, S., Kitada, K., Kumagai, H., Takai, K., and Okino, K. (2015). Water column imaging with multibeam echo-sounding in the mid-Okinawa Trough: implications for distribution of deep-sea hydrothermal vent sites and the cause of acoustic water column anomaly. Geochem. J. 49, 579–596. doi: 10.2343/geochemj.2.0387
Norlinder, E., Nygren, A., Wiklund, H., and Pleijel, F. (2012). Phylogeny of scale-worms (Aphroditiformia, Annelida), assessed from 18SrRNA, 28SrRNA, 16SrRNA, mitochondrial cytochrome c oxidase subunit I (COI), and morphology. Mol. Phylogenet. Evol. 65, 490–500. doi: 10.1016/j.ympev.2012.07.002
Okutani, T., Fujikura, K., and Sasaki, T. (1993). New taxa and new distribution records of deepsea gastropods collected from or near the chemosynthetic communities in the Japanese waters. Bull. Natn. Sci. Mus. 19, 123–143.
Palumbi, S. R. (1996). “Nucleic acid II: the polymerase chain reaction,” in Molecular Systems, eds D. M. Hillis and B. K. Mable (Sunderland, MA: Sinauer Associates), 205–247.
Passamaneck, Y. J., Schander, C., and Halanych, K. M. (2004). Investigation of molluscan phylogeny using large-subunit and small-subunit nuclear rRNA sequences. Mol. Phylogenet. Evol. 32, 25–38. doi: 10.1016/j.ympev.2003.12.016
Pettibone, M. H. (1983). A new scale worm (Polychaeta: Polynoidae) from the hydrothermal rift-area off western Mexico at 21°N. Proc. Biol. Soc. Wash. 96, 392–399.
Pettibone, M. H. (1984). A new scale-worm commensal with deep-sea mussels on the Galapagos hydrothermal vent (Polychaeta: Polynoidae). Proc. Biol. Soc. Wash. 97, 226–239.
Pettibone, M. H. (1985a). Additional branchiate scale-worms (Polychaeta: Polynoidae) from Galapagos hydrothermal vent and rift-area off Western Mexico at 21°N. Proc. Biol. Soc. Wash. 98, 447–469.
Pettibone, M. H. (1985b). New genera and species of deep-sea Macellicephalinae and Harmothoinae (Polychaeta: Polynoidae) from the hydrothermal rift areas off the Galapagos and Western Mexico at 21°N and from the Santa Catalina Channel. Proc. Biol. Soc. Wash. 98, 740–757.
Pettibone, M. H. (1986). A new scale-worm commensal with deep-sea mussels in the seep-sites at the Florida Escarpment in the eastern Gulf of Mexico (Polychaeta: Polynoidae: Branchipolynoinae). Proc. Biol. Soc. Wash. 99, 444–451.
Pettibone, M. H. (1988). New species and new records of scaled polychaetes (Polychaeta: Polynoidae) from hydrothermal vents of the Northeast Pacific Explorer and Juan de Fuca Ridges. Proc. Biol. Soc. Wash. 101, 192–208.
Pettibone, M. H. (1989). New species of scale-worms (Polychaeta: Polynoidae) from the hydrothermal rift-area of the Mariana Back-arc Basin in the Western Central Pacific. Proc. Biol. Soc. Wash. 102, 137–153.
Pettibone, M. H. (1990). New species and new records of scaled polychaetes (Polychaeta: Polynoidae) from the axial seamount caldera of the Juan de Fuca Ridge in the Northeast Pacific and the East Pacific Ocean off Northern California. Proc. Biol. Soc. Wash. 103, 825–838.
Ramirez-Llodra, E., Shank, T. M., and German, C. R. (2007). Biodiversity and biogeography of hydrothermal vent species: thirty years of discovery and investigations. Oceanography 20, 30–41. doi: 10.5670/oceanog.2007.78
Read, G., and Fauchald, K. (2017). World Polychaeta Database. Avaliable online at: http://www.marinespecies.org/aphia.php?p=taxdetails&id=324929 (Accessed April 17, 2017).
Sasaki, T., Ogura, T., Watanabe, H. K., and Fujikura, K. (2016). Four new species of Provanna (Gastropoda: Provannidae) from vents and a seep off Nansei-shoto area, southwestern Japan. Venus 74, 1–17. doi: 10.18941/venus.74.1-2_1
Scholin, C. A., Herzog, M., Sogin, M., and Anderson, D. M. (1994). Identification of group-and strain-specific genetic markers for globally distributed Alexandrium (Dinophyceae). II. Sequence analysis of a fragment of the LSU rRNA gene. J. Phycol. 30, 999–1011.
Serpetti, N., Taylor, M. L., Brennan, D., Green, D. H., Rogers, A. D., Paterson, G. L. J., et al. (2017). Ecological adaptations and commensal evolution of the Polynoidae (Polychaeta) in the Southwest Indian Ocean Ridge: A phylogenetic approach. Deep-Sea Res. (2 Top. Stud. Oceanogr.) 137, 273–281. doi: 10.1016/j.dsr2.2016.06.004
Sha, Z. L., and Ren, X. Q. (2015). A new species of the genus Neoverruca (Cirripedia, Thoracica, Verrucidea, Neoverrucidae) from a hydrothermal vent area in the Okinawa Trough. Crustaceana 88, 991–1001. doi: 10.1163/15685403-00003462
Stewart, C. N. Jr., and Via, L. E. (1993). A rapid CTAB DNA isolation technique useful for RAPD fingerprinting and other PCR applications. BioTechniques 14, 748–750.
Storm, V. (1879). Bidrag til Kundskab om Throndhjemsfjordens Fauna. Det Kongelige Norske Videnskabers Selskabs Skrifter, 9–36.
Sui, J., and Li, X. (2017). A new species and new record of deep-sea scale-worms (Polynoidae: Polychaeta) from the Okinawa Trough and the South China Sea. Zootaxa 4238, 562–570. doi: 10.11646/zootaxa.4238.4.4
Swofford, D. L. (2003). PAUP*: Phylogenetic Analysis Using Parsimony, version 4.0 b10. Available online at: http://paup.sc.fsu.edu/
Tunnicliffe, V. (1992). The nature and origin of the modern hydrothermal vent fauna. Palaios 338–350.
Turbeville, J. M., Schulz, J. R., and Raff, R. A. (1994). Deuterostome phylogeny and the sister group of the chordates: evidence from molecules and morphology. Mol. Biol. Evol. 11, 648–655.
Van Dover, C. L., Trask, J., Gross, J., and Knowlton, A. (1999). Reproductive biology of free-living and commensal polynoid polychaetes at the Lucky Strike hydrothermal vent field (Mid-Atlantic Ridge). Mar. Ecol. Prog. Ser. 201–214.
Wilson, R. S., Hutchings, P. A., and Glasby, C. J. (2003). Polychaetes: An Identification Guide. CSIRO Publishing.
Zhang, Y. (2017). Systematics and Evolution of Scale Worms (Aphroditiformia, Polychaeta), with a Focus on Deep-sea Species. Ph.D. thesis, Hong Kong Baptist University.
Keywords: hydrothermal vent, polychaete, Branchinotogluma, Branchipolynoe, Lepidonotopodium, Levensteiniella
ZooBank registration publication LSID: urn:lsid:zoobank.org:pub:A15584E4-E33D-44C3-9D9D-059D75074439
Citation: Zhang Y, Chen C and Qiu J-W (2018) Sexually Dimorphic Scale Worms (Annelida: Polynoidae) From Hydrothermal Vents in the Okinawa Trough: Two New Species and Two New Sex Morphs. Front. Mar. Sci. 5:112. doi: 10.3389/fmars.2018.00112
Received: 29 November 2017; Accepted: 15 March 2018;
Published: 03 April 2018.
Edited by:
Wei-Jen Chen, National Taiwan University, TaiwanReviewed by:
Stephane Hourdez, Centre National de la Recherche Scientifique (CNRS), FranceGreg W. Rouse, University of California, San Diego, United States
Copyright © 2018 Zhang, Chen and Qiu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian-Wen Qiu, qiujw@hkbu.edu.hk
 Yanjie Zhang
Yanjie Zhang Chong Chen
Chong Chen Jian-Wen Qiu
Jian-Wen Qiu