Protective Ventilation Improves Gas Exchange, Reduces Incidence of Atelectases, and Affects Metabolic Response in Major Pancreatoduodenal Surgery
- 1Department of Anesthesiology and Intensive Care, Northern State Medical University, Arkhangelsk, Russian Federation
- 2Department of Anesthesiology, City Hospital # 1, Arkhangelsk, Russian Federation
- 3Department of Surgery, Northern State Medical University, Arkhangelsk, Russian Federation
Background: Protective perioperative ventilation has been shown to improve outcomes and reduce the incidence of postoperative pulmonary complications. The goal of this study was to assess the effects of ventilation with low tidal volume (VT) either alone or in a combination with moderate permissive hypercapnia in major pancreatoduodenal interventions.
Materials and methods: Sixty adult patients scheduled for elective pancreatoduodenal surgery with duration >2 h were enrolled into a prospective single-center study. All patients were randomized to three groups receiving high VT [10 mL/kg of predicted body weight (PBW), the HVT group, n = 20], low VT (6 mL/kg PBW, the LVT group, n = 20), and low VT combined with a moderate hypercapnia and hypercapnic acidosis (6 mL/kg PBW, PaCO2 45–60 mm Hg, the LVT + HC group, n = 20). Cardiopulmonary parameters and the incidence of complications were registered during surgery and postoperatively.
Results and discussion: The values of VT were 610 (563–712), 370 (321–400), and 340 (312–430) mL/kg for the HVT, the LVT, and the LVT + HC groups, respectively (p < 0.001). Compared to the HVT group, PaO2/FiO2 ratio was increased in the LVT group by 15%: 333 (301–381) vs. 382 (349–423) mm Hg at 24 h postoperatively (p < 0.05). The HVT group had significantly higher incidence of atelectases (n = 6), despite lower incidence of smoking compared with the LVT (n = 1) group (p = 0.017) and demonstrated longer length of hospital stay. The patients of the LVT + HC group had lower arterial lactate and bicarbonate excess values by the end of surgery.
Conclusion: In major pancreatoduodenal interventions, preventively protective VT improves postoperative oxygenation, reduces the incidence of atelectases, and shortens length of hospital stay. The combination of low VT and permissive hypercapnia results in hypercapnic acidosis decreasing the lactate concentration but adding no additional benefits and warrants further investigations.
Introduction
Postoperative pulmonary complications (PPC) can significantly worsen the outcomes of major surgery, thereby increasing the resource utilization and length of hospital stay (1). The benefits of the protective mechanical ventilation with low tidal volume (VT) resulting in improved outcome have been convincingly proved in patients with acute respiratory distress syndrome (ARDS) in large clinical studies and meta-analyses (2, 3). Respiratory support with protective VT of 6–8 mL/kg to limit volumotrauma as well as setting of an adequate positive end-expiratory pressure (PEEP) to prevent atelectotrauma can be considered as key measures for both prevention and management of ARDS (4). Beyond the lower VT, the subgroup of protectively ventilated patients with ARDS with permissive hypercapnia might have certain additional benefits. The precise mechanism of this effect is not completely clear and may involve the suppression of inflammation, mitigation of cell apoptosis, and, finally, counteraction of the biotrauma (5–7).
During the past two decades, we observe a “paradigm shift” of preventive approach from tertiary, targeted on the prevention of the complication and mortality in ARDS, to secondary, aimed for the prevention of the development of PPC and ARDS per se (8). In patients with intact lungs, i.e., those without ARDS, the use of protective perioperative ventilation as “secondary” preventive measure can dramatically improve postoperative outcomes and reduce the risk of PPC (9). The prevention of PPC and its most severe form, postoperative ARDS, is of utmost interest in major abdominal surgery when patients have initially intact lungs but are in a risk group of postoperative respiratory adverse events (10, 11).
The important components of protective perioperative ventilation are low VT and moderate PEEP targeting low plateau and driving pressures to avoid ventilator-associated lung injury (12). However, the independent contributing role of both the parameters as well as their interaction with specific pulmonary characteristics such as lung compliance and non-modifiable risk factors are to be further explored and discussed. In addition, the use of the relatively low VT can be accidentally accompanied by permissive hypercapnia that can interact with systemic inflammatory response, biotrauma, and extrapulmonary organ function (6, 13).
The major pancreatoduodenal interventions include the extensive and complex resection of pancreas and duodenum involving hepatic and biliary structures. This branch of elective surgery may be potentially associated with a high risk of pulmonary and extrapulmonary postoperative complications due to history of smoking, alcohol consumption, high bleeding potential, hypoalbuminemia, and advanced age (14–16).
The goal of our study was to assess the effects of protective ventilation on hemodynamics, gas exchange, incidence of PPC and extrapulmonary complications, and clinical outcome. We hypothesized that the protective ventilation with low VT results in the similar postoperative outcome and the incidence of PPC on Day 28 in the relatively homogenous population of the patients subjected to major pancreatoduodenal surgery. The secondary hypothesis was that protective ventilation combined with permissive hypercapnia does not improve organ functions compared with low VT alone.
Materials and Methods
The study protocol and informed consent were approved by the Ethical Committee of the Northern State Medical University, Arkhangelsk, Russian Federation. During the period of 2014–2016, 60 patients [28 females/32 males, age 54 (45–60) years] scheduled for major pancreatoduodenal surgery (mostly, extended pancreatic resection of pancreatic cancer or chronic calcific pancreatitis) with expected duration of the intervention exceeding 2 h were included into a prospective randomized study. All the patients were visited 12 h before the intervention in the surgical ward and signed an informed consent.
Perioperative Ventilation
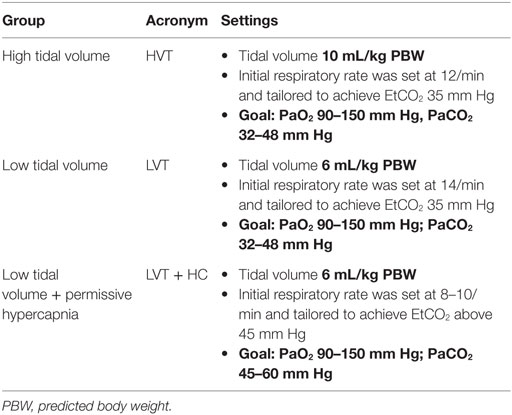
Before anesthesia and start of mechanical ventilation, the patients were randomized using the envelope method to three groups receiving either high VT [10 mL/kg of predicted body weight (PBW); the HVT group, n = 20] or low VT (6 mL/kg PBW; the LVT group, n = 20). An additional group combined low VT with moderate permissive hypercapnia (VT 6 mL/kg PBW and PaCO2 45–60 mm Hg: the LVT + HC group, n = 20). In all the groups, PEEP of 4 cm H2O was set (Table 1).
The standard preoxygenation lasting for at least 3 min was performed in all the patients using 80% oxygen (Datex Ohmeda Avance, GE, Madison, WI, USA). Initial FiO2 was set at 30% to achieve SpO2 at least 95%. In case of SpO2 below 95%, FiO2 was increased with increment of 5% to achieve the target SpO2 value. In all the patients, the respiratory support was discontinued using standard criteria and spontaneous breathing trial. The tracheal extubation was performed in the ICU by an independent ICU physician. The criteria for discontinuation of respiratory support were as follows: the ability to tolerate 30 min of spontaneous breathing trial via the pressure support ventilation with pressure support level of 6–8 cm H2O, PaO2/FiO2 >200 mm Hg, spontaneous minute volume <10 L/min, and respiratory rate <30/min (f/VT < 65 1/L and VT > 6 mL/kg PBW) as well as normal body temperature, no obvious bleeding or anemia, hemodynamic stability, and adequate analgesia.
Anesthesia
Before the interventions, all patients received premedication with sedative (phenazepam 1.0 mg) and antacid (omeprazol 20 mg). After transferring to the operating room, the catheterization of peripheral vein was performed and sedation with diazepam 5–10 mg intravenously was provided. The radial arterial line and thoracic epidural catheterization (Th7–Th9) were set in all patients. Epidural anesthesia (ropivacain 30–50 mg bolus with continuous infusion, fentanyl 100 μg bolus) was induced prior the start of surgery. General anesthesia was induced using propofol (1.5–2.0 mg/kg) and fentanyl (100 μg). Muscular blockade for the tracheal intubation was achieved with atracurium besilate (0.6 mg/kg). Thereafter, the anesthesia was maintained with sevoflurane 1.5–2.5 vol.% with fresh gas flow of 1 L/min and continuous infusion of fentanyl (100 μg/h) and atracurium (25 mg/h). Gastric tube and urinary catheter were set after the induction and intubation of the patients. Mean arterial pressure was maintained >55 mm Hg, if necessary using the titrated infusion of norepinephrine. Continuous infusion of a balanced crystalloid solution (4–5 mL/kg/h) was performed intraoperatively.
Perioperative Measurements and Monitoring
Hemodynamics, gas exchange, and laboratory parameters were registered at the beginning of surgery, at the end of the intervention, and every 6–12 h during 72 h of the postoperative period. The invasive arterial blood pressure (radial artery), central venous pressure, and SpO2 were monitored continuously (B40 Patient monitor, GE Medical Systems, Freiburg, Germany). Inspiratory and end-expiratory sevoflurane concentration, FiO2 and FeO2, and EtCO2 were monitored using integrated monitor of anesthesia machine and Capnostream™ 20 monitor (Covidien, USA). Intra- and postoperatively, arterial and venous blood gases, lactate concentration, bicarbonate excess (BE), and hemoglobin concentration were registered.
Plain chest X-ray was performed as a standard procedure at 24 h of the postoperative period in the semi-recumbent position; the films were interpreted by an independent specialist. In cases when PPC (e.g., atelectasis, pleuritis, nosocomial pneumonia, etc.) were suspected, chest X-ray or computed tomography was performed within the period of observation up to Day 28 on request either in the ICU or in the radiology department.
The incidence of the postoperative complications including atelectases, postoperative ileus, nosocomial pneumonia, bleeding, and anastomosis leakage, as well as lengths of the ICU and hospital stay, and mortality were registered up to Day 28 after surgery.
Statistics
The data distribution was assessed using Shapiro–Wilk test. The data are presented as median (25th–75th percentiles). For data analysis, we used SPSS Statistics software (IBM, USA). Intergroup comparisons were performed using Kruskal–Wallis H-test followed by pair-wise post hoc Mann–Whitney U-test. The nominal data were compared using Pearson χ2-test followed with Exact Fisher’s test when appropriate. The intragroup differences were explored using Wilcoxon test. p values below 0.05 were regarded as statistically significant.
Results
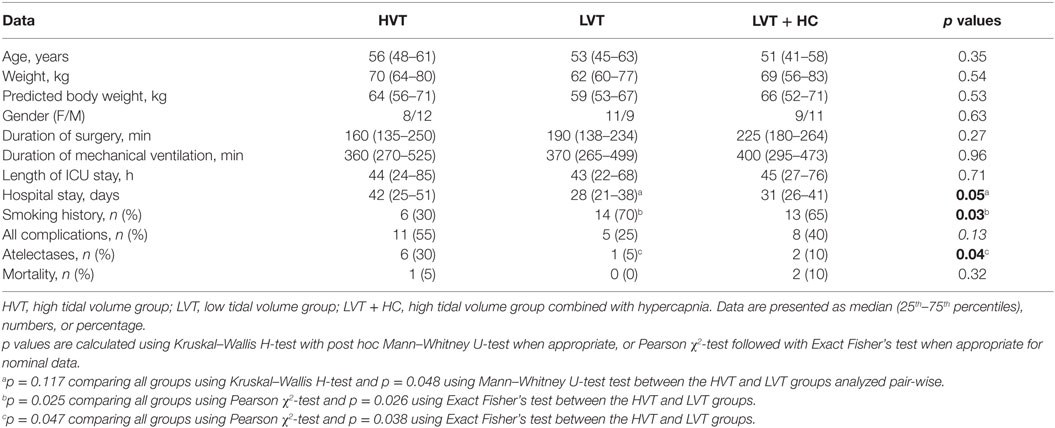
We did not find any significant baseline differences between the groups except for the history of smoking that was significantly lower in the HVT group (Table 2). The duration of both intra- and postoperative respiratory support as well as the overall duration of surgery were not different between all the three groups.
The values of VT and PaCO2 at the start and completion of the surgery for the HVT, the LVT, and the LVT + HC groups are depicted in Figure 1. Compared with the HVT group, PaO2/FiO2 ratio at 24 h postoperatively was higher in the LVT group – 333 (301–381) vs. 382 (349–423) mm Hg (p = 0.027) but not in the LVT + HC group (Figure 2). Notably, the transient improvement of the postoperative oxygenation was achieved, regardless of the significantly higher incidence of smokers in the LVT group compared with the HVT group (p = 0.025; Table 2).
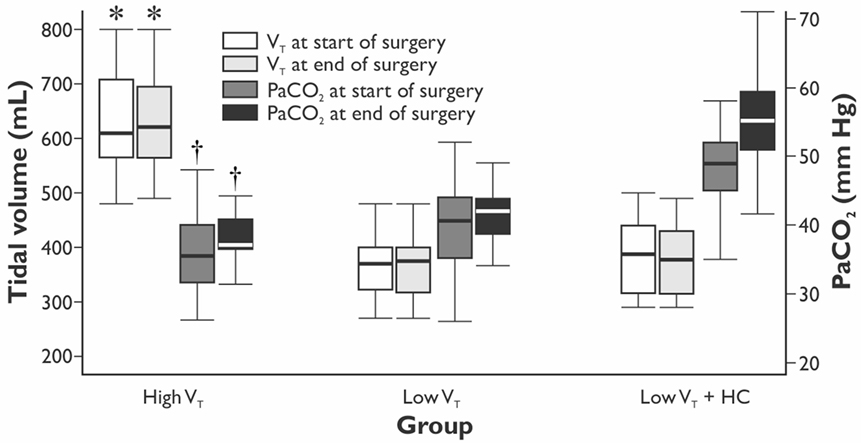
Figure 1. The values of tidal volume and arterial partial pressure of CO2 in the groups at the beginning and end of the surgery. Data are presented as median (25th–75th percentiles). p values are calculated using Kruskal–Wallis H-test followed by post hoc Mann–Whitney U-test. *p < 0.001 between the LVT, LVT + HC, and the HVT groups for the tidal volume set at the start and end of surgery. † p < 0.001 between the LVT group and the LVT + HC group for PaCO2 at the start and end of surgery.
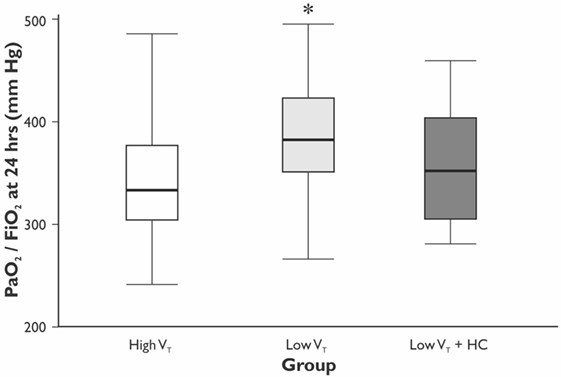
Figure 2. The ratio of PaO2/FiO2 at 24 h after surgery. Data are presented as median (25th–75th percentiles). p values are calculated using Mann–Whitney U-test between the HVT and the LVT groups; *p = 0.027 compared with the HVT group.
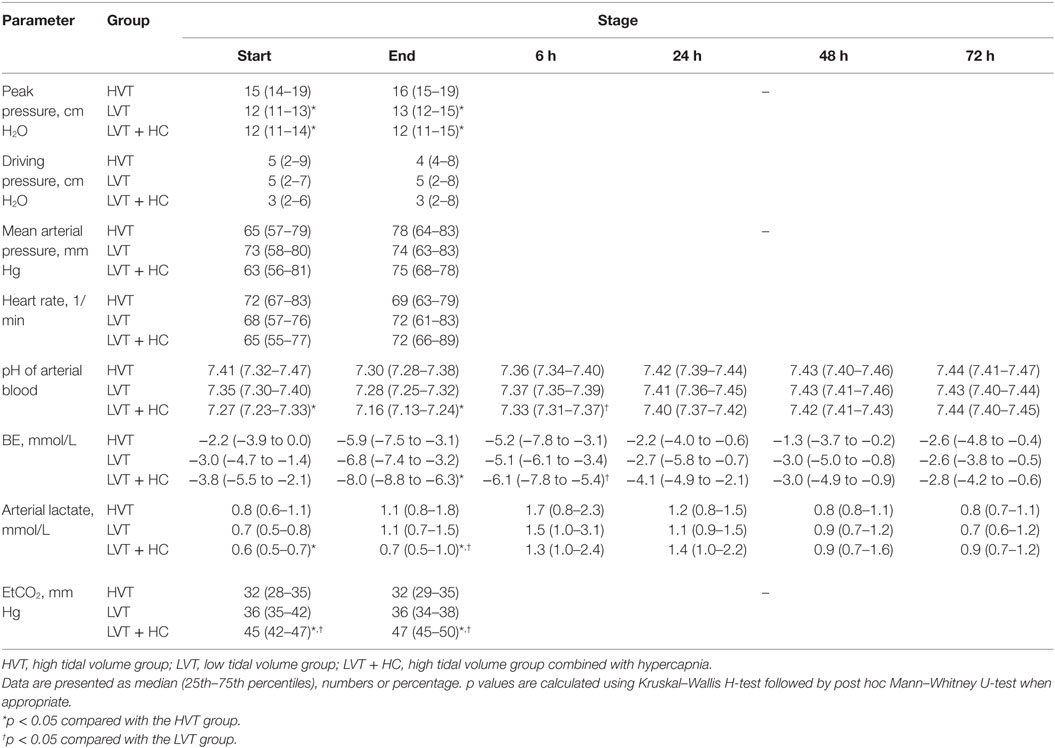
During surgery, we observed significantly increased VE and peak pressures in the HVT group (Figure 1; Table 3). The LVT + HC group had significantly higher PaCO2 and EtCO2 compared with the other groups. In parallel with development of hypercapnia, arterial pH, BE, and lactate concentration reduced significantly (p < 0.03 and p < 0.02 compared with the HVT and the LVT groups, respectively, Table 3).
The length of hospital, but not the length of ICU stay, was significantly longer in the HVT group compared with the LVT group when compared without LVT + HC group (Figure 3). The overall mortality at Day 28 was 5% (n = 1 in the HVT group and n = 2 in the LVT + HC group), and the overall incidence of postoperative complications was 40% (n = 24). We registered a tendency for higher incidence of the postoperative complications and significantly higher rate of the atelectases in the HVT group compared with the LVT group (Figure 4). We found no differences in the overall incidence of complications and atelectases between the HVT and the LVT + HC groups.
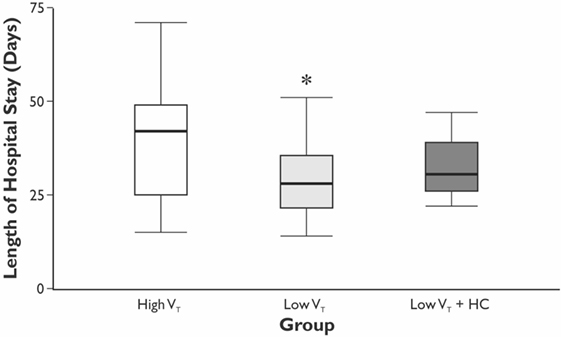
Figure 3. The length of the hospital stay. Data are presented as median (25th–75th percentiles). p values are calculated using Kruskal–Wallis H-test followed by post hoc Mann–Whitney U-test between the HVT and the LVT groups only; *p = 0.048 using Mann–Whitney U-test test between the HVT and LVT groups analyzed pair-wise.
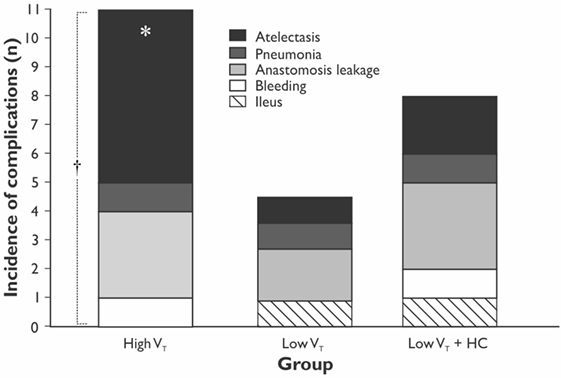
Figure 4. The incidence of the postoperative complications in the study groups. Data are presented as stacked numbers of the complications. p values are calculated using χ2-test between the HVT and the LVT groups; *p = 0.047 comparing all groups using Pearson χ2-test and p = 0.038 using Exact Fisher’s test between the HVT and LVT groups. † p = 0.13 for incidence of all the complications between the HVT and the LVT groups.
Discussion
Our study demonstrated transiently improved postoperative oxygenation, reduced incidence of postoperative pulmonary atelectases, and shortened length of hospital stay in the patients ventilated with protective VT of 6 mL/kg PBW during major pancreatoduodenal surgery. The combination of protective VT with moderate hypercapnia and hypercapnic acidosis did not affect pulmonary function but could potentially interplay with perioperative acid–base balance.
The improvement of oxygenation (PaO2/FiO2) was relatively minor (15%) and transient as registered only at 24 h of the postoperative period in the LVT but not LVT + HC group. Moreover, we showed the decreased incidence of atelectases, mostly registered at 24 h postoperatively in parallel with changing PaO2/FiO2 ratio, and tendency to reduced overall incidence of the postoperative complications in the LVT group that was associated with increased length of the hospital stay in the group ventilated with high VT. In compliance with our results, Severgnini et al. have shown that open abdominal surgery lasting more than 2 h ventilation with relatively high VT of 9 mL/kg and zero PEEP resulted in compromised pulmonary function, worsened oxygenation, and increased incidence of PPC in comparison with protective ventilation (VT of 7 mL/kg of ideal body weight, PEEP of 10 cm H2O, and recruitment maneuvers) (10). In contrast, Treschan et al. demonstrated that the application of low VT of 6 mL/kg PBW in major abdominal surgery did not improve postoperative lung function as compared with high VT values of 12 mL/kg PBW with the similar PEEP level (5 cm H2O) (17). In the large randomized controlled trial, Futier et al. have demonstrated a reduction in the incidence of the major pulmonary and extrapulmonary complications within 7 days following major abdominal surgery by 17% in the protective ventilation (VT 6–8 mL/kg PBW and PEEP 6–8 cm H2O) compared with the conventional ventilation group (VT 10–12 mL/kg PBW and 0 PEEP) (11). In consistency with our results, this study convincingly proved that protective ventilation was associated with shorter length of hospital stay. The protective ventilation can prevent both volumotrauma, that triggers the PPC, and ARDS, as well as pulmonary cytokine release, i.e., biotrauma that can result in the propagation of systemic inflammatory response and distant organ injury. The involvement of the whole-body response to the perioperative pulmonary stress can explain the increased rate of extrapulmonary complications and, finally, the adverse clinical outcomes (11, 18). The potential overdistension of lungs in the HVT group could increase the risk of atelectases due to alveolar stretch and shear stress, thereby resulting in inflammation and repeated closure and opening of dependent areas (8, 18).
The ability of preventive low VT to counteract the potential injurious and pro-inflammatory effects of inadvertent lung overdistension related to conventional ventilation is still a matter of debates (19, 20). Thus, Cai et al. showed by means of computed tomography that ventilation with protective VT of 6 mL/kg alone without PEEP was not associated with any difference both in the incidence of atelectases and in oxygenation compared with the VT of 10 mL/kg (21). In routine practice, low VT is associated with the rather unjustified fair of atelectases, which probably could be counteracted by an adequate PEEP. Since our study included the patients with body mass index within the relatively normal range of 23.2 (21.3–28.4) kg/m2, the empiric and relatively low PEEP of 4 cm H2O could be considered adequate to reduce the risk of atelectases. As a result, low VT was accompanied by a significant reduction of the atelectases incidence compared with the HVT group that makes this approach attractive for a wider use in clinical practice.
As noted, the PPC have been considered to be strongly associated with prolonged hospital stay (22), which was also confirmed by the presented results. Despite our study confirms the conclusions of several similar investigations showing that protective ventilation can improve gas exchange and lung mechanics and attenuate the risk of PPC and extrapulmonary adverse events (10, 11, 23), its results could contribute to the pool of the evidences favoring protective ventilation in major pancreatoduodenal surgery due to relatively homogenous patient population and insights into effects of permissive hypercapnia.
In our study, we induced a moderate degree of hypercapnia in the LVT + HC group aiming to prevent significant hemodynamic effects, risk of organ dysfunction, and increased consumption of anesthetic drugs. The patients assigned to this group did not show any additional improvement in oxygenation or reduced incidence of PPC and, namely, atelectases compared with both the LVT and the HVT groups. We found minor and transient metabolic effects in the LVT + HC group, namely, reduced arterial lactate concentration combined with respiratory acidosis and lower BE values. Hypercapnia and acidosis could interact with inflammation, modulate biotrauma, and attenuate ARDS that mostly explored so far in isolated lungs and in vivo experimental studies (7, 24, 25). In addition, the exact values of hypercapnic acidosis and hypercapnia are not settled ranging from 6.90 to 7.40 and 40 to 100 mm Hg, respectively, and the distinct mechanism of the protection remains unrevealed (26, 27). However, beyond the experimental attenuation of the cytokine release, hypercapnia can exert several deleterious effects via overproduction of nitric oxide, impaired plasma membrane repair, immunosuppression, and possible promotion of the bacterial growth (6). These effects combined with influence of hypercapnia on cardiovascular and central nervous systems can prevent physician to avoid this maneuver in patients without ARDS (28–30). The reduction in lactate concentration observed in our study can be explained by the metabolic acid–base effect of hypercapnic acidosis rather than any modification of organ perfusion. Indeed, it is suggested that the decreased lactate concentration during hypercapnia might actually result from the inhibition of phosphofructokinase activity, suppressed transport of lactic acid from muscles, and augmented rate of lactate oxidation (31–33). Therefore, the effects of hypercapnia associated with hypercapnic acidosis and low VT on PPC incidence might worth further investigations to clarify the value and safety of this approach in the routine clinical practice.
Limitations
The limitations of our study include the relatively small number of observations. Applying low VT, we did not consider the specific targets for pulmonary compliance, peak, plateau, and driving pressures that can also limit the applicability of the findings. The population of patients is relatively homogeneous in respect of the type of surgery but is heterogeneous for underlying pathology (both cancer- and not cancer-related interventions were included). Unfortunately, we could not explain clearly why the combination of low VT and hypercapnia resulted in the potentially worse oxygenation, hospital length of stay, and the incidence of complications. We can hypothesize only that even moderate hypercapnia might be potentially detrimental for the patients without ARDS and multiple organ failure, and this risk forced us to limit the enrollment into this study after the intrinsic analysis.
Conclusion
In major elective pancreatoduodenal surgery, preventive reduction of VT to protective values results in transiently improved postoperative oxygenation, reduced incidence of atelectases, and shortened length of the hospital stay. The combination of low VT and permissive hypercapnia leads to transient decrease in lactate concentration but does not add any substantial benefits to the outcome and organ function and warrants further investigations.
Author Contributions
VK planned the design, performed data collection and analysis, and drafted the manuscript. LR planned the design, enrolled the patients, performed data collection and analysis, and drafted the manuscript. YI enrolled the patients, performed data collection, and drafted the manuscript. MS participated in the data collection and randomization of the patients. AU enrolled the patients, performed data collection, and drafted the manuscript. EF planned the design, performed analysis, and drafted the manuscript. BD participated in the data analysis and drafted the manuscript. MK planned the design of the study and drafted the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors appreciate the assistance of Andrey A. Papko, MD, and Maria A. Feoktistova, MD.
Funding
The study was supported, in part, by the Grant of the President of Russian Federation (grant number MD-4984.2015.7).
References
1. Canet J, Gallart L. Postoperative respiratory failure: pathogenesis, prediction, and prevention. Curr Opin Crit Care (2014) 20:56–62. doi: 10.1097/MCC.0000000000000045
2. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. Acute Respiratory Distress Syndrome Network. N Engl J Med (2000) 342:1301–8. doi:10.1056/NEJM200005043421801
3. Putensen C, Theuerkauf N, Zinserling J, Wrigge H, Pelosi P. Meta-analysis: ventilation strategies and outcomes of the acute respiratory distress syndrome and acute lung injury. Ann Intern Med (2009) 151:566–76. doi:10.7326/0003-4819-151-8-200910200-00011
4. Gonga MN, Thompson BT. Acute respiratory distress syndrome: shifting the emphasis from treatment to prevention. Curr Opin Crit Care (2016) 22:21–37. doi:10.1097/MCC.0000000000000275
5. Laffey JG, O’Croinin D, McLoughlin P, Kavanagh BP. Permissive hypercapnia – role in protective lung ventilator strategies. Intensive Care Med (2004) 30:347–56. doi:10.1007/s00134-003-2051-1
6. Ismaiel NM, Henzler D. Effects of hypercapnia and hypercapnic acidosis on attenuation of ventilator-associated lung injury. Minerva Anestesiol (2011) 77:723–33.
7. Sinclair SE, Kregnow DA, Lamm WJE, Starr IR, Chi EY, Hlastala MP. Hypercapnic acidosis is protective in an in vivo model of ventilator-induced lung injury. Am J Respir Crit Care Med (2002) 166:403–8. doi:10.1164/rccm.200112-117OC
8. Neto AS, Nagtzaam L, Schultz MJ. Ventilation with lower tidal volumes for critically ill patients without the acute respiratory distress syndrome: a systematic translational review and meta-analysis. Curr Opin Crit Care (2014) 20:25–32. doi:10.1097/MCC.0000000000000044
9. Neto AS, Hemmes SN, Barbas CS, Beiderlinden M, Biehl M, Binnekade JM, et al. Protective versus conventional ventilation for surgery: a systematic review and individual patient data meta-analysis. Anesthesiology (2015) 123:66–78. doi:10.1097/ALN.0000000000000706
10. Severgnini P, Selmo G, Lanza C, Chiesa A, Frigerio A, Bacuzzi A, et al. Protective mechanical ventilation during general anesthesia for open abdominal surgery improves postoperative pulmonary function. Anesthesiology (2013) 118:1307–21. doi:10.1097/ALN.0b013e31829102de
11. Futier E, Constantin JM, Jaber S. Protective lung ventilation in operating room: a systematic review. Minerva Anestesiol (2014) 80:726–35.
12. Neto AS, Hemmes SN, Barbas CS, Beiderlinden M, Fernandez-Bustamante A, Futier E, et al. Association between driving pressure and development of postoperative pulmonary complications in patients undergoing mechanical ventilation for general anaesthesia: a meta-analysis of individual patient data. Lancet Respir Med (2016) 4:272–80. doi:10.1016/S2213-2600(16)00057-6
13. Masterson C, Otulakowski G, Kavanagh BP. Hypercapnia: clinical relevance and mechanisms of action. Curr Opin Crit Care (2015) 21:7–12. doi:10.1097/MCC.0000000000000164
14. Arozullah AM, Daley J, Henderson WG, Khuri SF. Multifactorial risk index for predicting postoperative respiratory failure in men after major noncardiac surgery. The National Veterans Administration Surgical Quality Improvement Program. Ann Surg (2000) 232:242–53. doi:10.1097/00000658-200008000-00015
15. Metreveli RE, Sahm K, Abdel-Misih R, Petrelli NJ. Major pancreatic resections for suspected cancer in a community-based teaching hospital: lessons learned. J Surg Oncol (2007) 95:201–6. doi:10.1002/jso.20662
16. Canet J, Gallart L, Gomar C, Paluzie G, Valles J, Castillo J, et al. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology (2010) 113:1338–50. doi:10.1097/ALN.0b013e3181fc6e0a
17. Treschan TA, Kaisers W, Schaefer MS, Bastin B, Schmalz U, Wania V, et al. Ventilation with low tidal volumes during upper abdominal surgery does not improve postoperative lung function. Br J Anaesth (2012) 109:263–71. doi:10.1093/bja/aes140
18. Lionetti V, Recchia FA, Ranieri VM. Overview of ventilator-induced lung injury mechanisms. Curr Opin Crit Care (2005) 11:82–6. doi:10.1097/00075198-200502000-00013
19. Wrigge H, Uhlig U, Zinserling J, Behrends-Callsen E, Ottersbach G, Fischer M, et al. The effects of different ventilatory settings on pulmonary and systemic inflammatory responses during major surgery. Anesth Analg (2004) 98:775–81. doi:10.1213/01.ANE.0000100663.11852.BF
20. Determann RM, Wolthuis EK, Choi G, Bresser P, Bernard A, Lutter R, et al. Lung epithelial injury markers are not influenced by use of lower tidal volumes during elective surgery in patients without preexisting lung injury. Am J Physiol Lung Cell Mol Physiol (2008) 294:L344–50. doi:10.1152/ajplung.00268.2007
21. Cai H, Gong H, Zhang L, Wang Y, Tian Y. Effect of low tidal volume ventilation on atelectasis in patients during general anesthesia: a computed tomographic scan. J Clin Anesth (2007) 19:125–9. doi:10.1016/j.jclinane.2006.08.008
22. Pearse RM, Moreno RP, Bauer P, Pelosi P, Metnitz P, Spies C, et al. Mortality after surgery in Europe: a 7 day cohort study. Lancet (2012) 380:1059–65. doi:10.1016/S0140-6736(12)61148-9
23. Weingarten TN, Whalen FX, Warner DO, Gajic O, Schears GJ, Snyder MR, et al. Comparison of two ventilatory strategies in elderly patients undergoing major abdominal surgery. Br J Anaesth (2010) 104:16–22. doi:10.1093/bja/aep319
24. Takeshita K, Suzuki Y, Nishio K, Takeuchi O, Toda K, Kudo H, et al. Hypercapnic acidosis attenuates endotoxin-induced nuclear factor-kappaB activation. Am J Respir Cell Mol Biol (2003) 29:124–32. doi:10.1165/rcmb.2002-0126OC
25. Costello J, Higgins B, Contreras M, Chonghaile MN, Hassett P, O’Toole D, et al. Hypercapnic acidosis attenuates shock and lung injury in early and prolonged systemic sepsis. Crit Care Med (2009) 37:2412–20. doi:10.1097/CCM.0b013e3181a385d3
26. Hickling KG, Walsh J, Henderson S, Jackson R. Low mortality rate in adult respiratory distress syndrome using low-volume, pressure-limited ventilation with permissive hypercapnia: a prospective study. Crit Care Med (1994) 22:1568–78. doi:10.1097/00003246-199410000-00011
27. Broccard AF, Hotchkiss JR, Vannay C, Markert M, Sauty A, Feihl F, et al. Protective effects of hypercapnic acidosis on ventilator-induced lung injury. Am J Resp Crit Care Med (2001) 164:802–6. doi:10.1164/ajrccm.164.5.2007060
28. Marhong J, Fan E. Carbon dioxide in the critically ill: too much or too little of a good thing? Respir Care (2014) 59:1597–605. doi:10.4187/respcare.03405
29. Curley G, Contreras MM, Nichol AD, Higgins BD, Laffey JG. Hypercapnia and acidosis in sepsis: a double-edged sword? Anesthesiology (2010) 112:462–72. doi:10.1097/ALN.0b013e3181ca361f
30. Cullen DJ, Eger EI II. Cardiovascular effects of carbon dioxide in man. Anesthesiology (1974) 41:345–9. doi:10.1097/00000542-197410000-00006
31. Graham TE, Wilson BA, Sample M, Dijk JV, Bonen A. The effects of hypercapnia on the metabolic response to progressive exhaustive work. Med Sci Sports Exerc (1980) 14:278–84.
32. Graham TE, Barclay JK, Wilson BA. Skeletal muscle lactate release and glycolitic intermediates during hypercapnia. J Appl Physiol (1986)60:568–75.
Keywords: protective ventilation, postoperative pulmonary complications, atelectasis, permissive hypercapnia, pancreatoduodenal surgery
Citation: Kuzkov VV, Rodionova LN, Ilyina YY, Ushakov AA, Sokolova MM, Fot EV, Duberman BL and Kirov MY (2016) Protective Ventilation Improves Gas Exchange, Reduces Incidence of Atelectases, and Affects Metabolic Response in Major Pancreatoduodenal Surgery. Front. Med. 3:66. doi: 10.3389/fmed.2016.00066
Received: 08 October 2016; Accepted: 21 November 2016;
Published: 06 December 2016
Edited by:
Andrey V. Kozlov, L. Boltzmann Institute for Traumatology, AustriaReviewed by:
Enrico Calzia, University of Ulm, GermanyInge Bauer, University Hospital Duesseldorf, Germany
Copyright: © 2016 Kuzkov, Rodionova, Ilyina, Ushakov, Sokolova, Fot, Duberman and Kirov. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vsevolod V. Kuzkov, v_kuzkov@mail.ru
†These authors have an equal contribution.
 Vsevolod V. Kuzkov
Vsevolod V. Kuzkov Ludmila N. Rodionova1,2†
Ludmila N. Rodionova1,2†
 Maria M. Sokolova
Maria M. Sokolova Eugenia V. Fot
Eugenia V. Fot Mikhail Y. Kirov
Mikhail Y. Kirov