Liver Abscess Caused by Pannonibacter phragmitetus: Case Report and Literature Review
- 1Yun Leung Laboratory for Molecular Diagnostics, School of Biomedical Sciences, Huaqiao University, Xiamen, Fujian, China
- 2Department of Medical Laboratory, Institute of Nanomedicine Technology, Weifang Medical University, Weifang, Shandong, China
- 3Department of Clinical Laboratory, Quanzhou First Hospital Affiliated to Fujian Medical University, Fujian, China
- 4Department of Microbiology and Molecular Genetics, Michigan State University, East Lansing, MI, USA
Background: Bacterial hepatic abscess is a common occurrence in developing countries, which is mostly caused by Klebsiella pneumoniae and Escherichia coli. Pannonibacter phragmitetus is a Gram-negative alkali-tolerant bacillus that exists in the natural environment. Human infection by this bacterium is rare, with only four cases reported.
Method: We presented one of these cases with a bacterial liver abscess by a polymicrobial infection involving P. phragmitetus and Streptococcus oralis, with P. phragmitetus being the predominate isolate.
Result and discussion: Our strain of P. phragmitetus was resistant to more antibiotics than the other reported two strains. This case further verified the infectivity of P. phragmitetus.
Background
Pannonibacter phragmitetus (P. phragmitetus) is a Gram-negative, motile rod that is frequently found to be star-shaped aggregates under microscopy (1). It is a facultative anaerobe and chemo-organotrophic (1). Before 2006, it was misidentified as Achromobacter groups B and E. Actually, P. phragmitetus and Achromobacter groups B and E are a single taxon (2). P. phragmitetus can survive in a high alkaline environment, such as Hungarian soda lake (1), and geothermal habitat (3, 4). It has the capacities of reducing hexavalent chromium (5–12). Isolation of this bacterium from a human sample was first reported in 1975 (13). So far, only four cases of infection by P. phragmitetus were reported: one case of replacement valve endocarditis (14), two cases of septicemia (15), and one case of recurrent septicemia (16). Here, we presented one case of liver abscess infected by P. phragmitetus, suspiciously combined with Streptococcus oralis (S. oralis).
Case Report
A 44-year-old male consumed more than 150 g of alcohol and smoked 20 cigarettes per day for 20 years. He was admitted to our hospital due to pain in the right upper abdomen for 14 days with normal body temperature. On a physical examination, he displayed right lateral upper abdominal tenderness. The liver edge was palpated 5 and 10 cm under the right costal margin and the xiphoid process, respectively, with surface protuberance and tenderness. Laboratory tests showed an elevated inflammatory reaction [white blood cell count (WBC) 1.7 × 1010/L, neutrophil 81.6%, C-reactive protein (CRP) level 325 mg/L] and anemia (RBC 3.28 × 1012/L, HGB 112 g/L). Abnormal liver function parameters were also noted [aspartate aminotransferase 47 IU/L, gamma-glutamyl transpeptidase 139 IU/L, and alkaline phosphatase 133 IU/L]. Ultrasonography detected a large abscess (82 mm × 78 mm) within the right liver lobe (Figure 1).
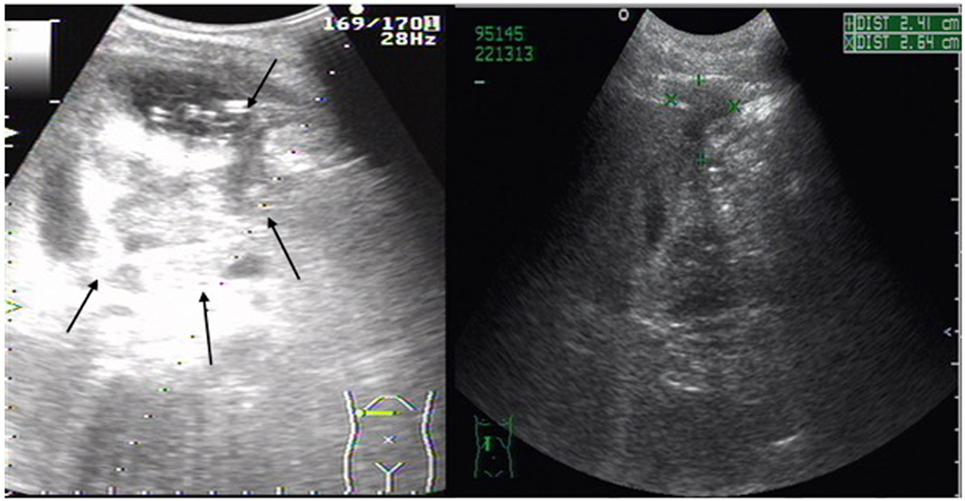
Figure 1. Abdominal ultrasonography scan of liver abscess in right lobe of the liver. Large (82 mm × 78 mm) multiple low-signal areas (shown by arrows) were detected before percutaneous abscess drainage. Its size (+, ×) was greatly reduced after percutaneous liver abscess drainage.
Intravenous injections of cefodizime sodium (1.5 g, b.i.d) and metronidazole (0.5 g, b.i.d) were administered daily from the first day after his admission to the end of the hospitalization (20 days). The patient underwent percutaneous abscess drainage using an 8.5-Fr catheter guided by ultrasonography on the fifth day of his hospitalization, which was left in place for 7 days. The patient fully recovered with the combination of antibiotic therapy and percutaneous abscess drainage. This was demonstrated by normal blood WBC count, CRP, negative blood, and abscess culture.
Using BD BACTEC 9240 auto blood culture system, P. phragmitetus strain 31801 was isolated from a blood sample collected within 24 h after his admission. The identity of P. phragmitetus strain 31801 was verified by 16S rRNA gene sequencing (GenBank number FJ882624.1) and whole genome sequencing strategy (accession number CP013068). The antimicrobial susceptibility test (AST) was conducted with the Kirby–Bauer disk diffusion test (OXOID, England) and BD Phoenix™ 100 Automated Microbiology System using NMIC/ID-109 identification/antibiotic susceptibility cards (Becton, Dickinson and Company). P. phragmitetus 31801 was sensitive to amikacin, imipenem, ceftazidime, cefepime, amoxicillin, piperacillin/tazobactam, gatifloxacin, and levofloxacin, while it was immediately sensitive to cefotaxime and ceftriaxone. It was resistant against gentamicin, tobramycin, piperacillin, trimethoprim/sulfamethoxazole, furazolidone, and tetracycline. Further studies showed that this strain was positive for extra-extended-spectrum β-lactamase as shown by the Kirby–Bauer test. It was shown to carry the ampicillin-inducible β-lactamase with some clones in the zone of CAZ + CA disk and ant (3″)-I that were verified by polymerase chain reaction. Using a blood agar plate, S. oralis was cultured from the liver abscess and collected on the fifth day after the patient’s admission when the abscess drainage was performed. It was unable to be recovered from the pus and blood sample collected later.
Discussion
In China, the most common bacterial hepatic abscesses have been consistently caused by Escherichia coli and Klebsiella sp. (accounts for 83%) (17). However, other bacteria were also reported to be isolated from the liver abscess. A recent survey showed that Gram-negative bacteria Enterobacter spp. (9%), Proteus spp. (6%), and Pseudomonas spp. (5%) were factors that caused this disease, next to E. coli and Klebsiella sp. (17). Frequently, many cases were caused by Gram-positive bacteria, mainly including Staphylococcus spp. (13%), Streptococcus spp. (8%), and Enterococcus spp. (7%) (17). It is uncommon for polymicrobial infections in hepatic abscesses due to most of the cases being monomicrobial (18). In our patient, S. oralis, a Gram-positive bacterium, was isolated from the pus. It should be noted that liver abscess due to its infection is quite rare. It was only isolated from the pus samples from two liver abscess patients (19, 20). However, no report was found on the application of cefodizime for treating S. oralis infections, and it was known that S. oralis was not sensitive to metronidazole (19). Our patient was cured by metronidazole, cefodizime, and catheter drainage. Although we could not rule out the possibility that S. oralis was acquired from environmental contamination, we speculated that its contribution to this liver abscess was not significant.
Instead, P. phragmitetus 31801 may be the real pathogen of our patient seeing as it was isolated from the blood sample collected within 24 h after his admission to the hospital, and the liver abscess in our patient could be diagnosed by abdominal ultrasonography along with an elevated inflammatory reaction on the first day that he was admitted. The chance of contamination during collection of the blood is low. It was initially identified as Achromobacter species, with the use of BD Phoenix™ 100 Automated Microbiology System and verified by 16S rRNA gene sequencing. Although it was not isolated from abscess fluid, we believed that P. phragmitetus strain 31801 was the actual pathogen. We had misidentified P. phragmitetus strain 31801 as Sphingomonas paucimobilis during our interpretation of the result of BD Phoenix™ 100 Automated Microbiology System before it was verified by 16S rRNA gene sequencing. The infectivity of P. phragmitetus had been demonstrated even though only four cases of infection have been reported (13, 15, 16). The pathogenic mechanisms of P. phragmitetus were unknown; in fact, we found many virulence factors in P. phragmitetus when we analyzed the sequenced genome (unpublished). In an antimicrobial susceptibility test, this bacterium was resistant against several classifications of antibiotics mentioned above. Furthermore, it has extra-extended-spectrum β-lactamase, which was inducible and ant (3″)-I (21).
Pannonibacter phragmitetus 31801 was resistant to more antibiotics than the strain isolated by Jenks and Shaw, which was sensitive to gentamicin, trimethoprim/sulfamethoxazole, and tetracycline (16), and the strain isolated by McKinley et al., which was sensitive to trimethoprim/sulfamethoxazole and tetracycline (14), while P. phragmitetus 31801 was resistant against these three antibiotics: tobramycin, piperacillin, and furazolidone.
In conclusion, this case report raises awareness to the possibility of infectivity and multiple-drug resistance of P. phragmitetus.
Ethics Statement
The written informed consent for the publication of this case report was obtained from the patient. This work was already approved by the Ethics Committee of Quanzhou First Hospital.
Author Contributions
Conception and design of the work: MW, XZ, and YZ. Data collection: MW, XZ, TJ, SH, ZY, YZ, and DM. Data analysis and interpretation, manuscript writing, and critical revision of the article: MW, XZ, YZ, and SC. Approval of the final version of the article: MW, XZ, TJ, SH, ZY, YZ, DM, and SC.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This work was supported by Huaqiao University Graduate Student Scientific Research Innovation Ability Cultivation Plan Projects, the Fong Shu Fook Tong and Fong Yun Wah Foundations (14X30127), the Xiamen Southern Oceanographic Center (14GYY008NF08), the Technology Planning Projects of Quanzhou Social Development Fields (2014Z24), and the Major Support Research Project of National Key Colleges Construction of Quanzhou Medical College (2013A13).
References
1. Borsodi AK, Micsinai A, Kovács G, Tóth E, Schumann P, Kovács AL, et al. Pannonibacter phragmitetus gen. nov., sp. nov., a novel alkalitolerant bacterium isolated from decomposing reed rhizomes in a Hungarian soda lake. Int J Syst Evol Microbiol (2003) 53(Pt 2):555–61. doi:10.1099/ijs.0.02356-0
2. Holmes B, Segers P, Coenye T, Vancanneyt M, Vandamme P. Pannonibacter phragmitetus, described from a Hungarian soda lake in 2003, had been recognized several decades earlier from human blood cultures as Achromobacter groups B and E. Int J Syst Evol Microbiol (2006) 56(Pt 12):2945–8. doi:10.1099/ijs.0.64563-0
3. Coman C, Drugă B, Hegedus A, Sicora C, Dragoş N. Archaeal and bacterial diversity in two hot spring microbial mats from a geothermal region in Romania. Extremophiles (2013) 17(3):523–34. doi:10.1007/s00792-013-0537-5
4. Bandyopadhyay S, Schumann P, Das SK. Pannonibacter indica sp. nov., a highly arsenate-tolerant bacterium isolated from a hot spring in India. Arch Microbiol (2013) 195(1):1–8. doi:10.1007/s00203-012-0840-z
5. Xu L, Luo M, Jiang C, Wei X, Kong P, Liang X, et al. In vitro reduction of hexavalent chromium by cytoplasmic fractions of Pannonibacter phragmitetus LSSE-09 under aerobic and anaerobic conditions. Appl Biochem Biotechnol (2012) 166(4):933–41. doi:10.1007/s12010-011-9481-y
6. Wang Y, Yang Z, Peng B, Chai L, Wu B, Wu R. Biotreatment of chromite ore processing residue by Pannonibacter phragmitetus BB. Environ Sci Pollut Res Int (2013) 20(8):5593–602. doi:10.1007/s11356-013-1526-z
7. Xu L, Luo M, Yang L, Wei X, Lin X, Liu H. Encapsulation of Pannonibacter phragmitetus LSSE-09 in alginate-carboxymethyl cellulose capsules for reduction of hexavalent chromium under alkaline conditions. J Ind Microbiol Biotechnol (2011) 38(10):1709–18. doi:10.1007/s10295-011-0960-5
8. Xu L, Yang L, Luo M, Liang X, Wei X, Zhao J, et al. Reduction of hexavalent chromium by Pannonibacter phragmitetus LSSE-09 coated with polyethylenimine-functionalized magnetic nanoparticles under alkaline conditions. J Hazard Mater (2011) 189(3):787–93. doi:10.1016/j.jhazmat.2011.03.009
9. Xu L, Luo M, Li W, Wei X, Xie K, Liu L, et al. Reduction of hexavalent chromium by Pannonibacter phragmitetus LSSE-09 stimulated with external electron donors under alkaline conditions. J Hazard Mater (2011) 185(2–3):1169–76. doi:10.1016/j.jhazmat.2010.10.028
10. Shi Y, Chai L, Yang Z, Jing Q, Chen R, Chen Y. Identification and hexavalent chromium reduction characteristics of Pannonibacter phragmitetus. Bioprocess Biosyst Eng (2012) 35(5):843–50. doi:10.1007/s00449-011-0668-y
11. Chai LY, Huang SH, Yang ZH, Peng B, Huang Y, Chen YH. Hexavalent chromium reduction by Pannonibacter phragmitetus BB isolated from soil under chromium-containing slag heap. J Environ Sci Health A Tox Hazard Subst Environ Eng (2009) 44(6):615–22. doi:10.1080/10934520902784690
12. Chai L, Huang S, Yang Z, Peng B, Huang Y, Chen Y. Cr (VI) remediation by indigenous bacteria in soils contaminated by chromium-containing slag. J Hazard Mater (2009) 167(1–3):516–22. doi:10.1016/j.jhazmat.2009.01.030
13. Holmes B, Lapage SP, Malnick H. Strains of Pseudomonas putrefaciens from clinical material. J Clin Pathol (1975) 28(2):149–55. doi:10.1136/jcp.28.2.149
14. McKinley KP, Laundy TJ, Masterton RG. Achromobacter group B replacement valve endocarditis. J Infect (1990) 20(3):262–3. doi:10.1016/0163-4453(90)91294-N
15. Holmes B, Lewis R, Trevett A. Septicaemia due to Achromobacter group B: a report of two cases. Med Microbiol Lett (1992) 1:177–84.
16. Jenks PJ, Shaw EJ. Recurrent septicaemia due to Achromobacter group B. J Infect (1997) 34:143–5. doi:10.1016/S0163-4453(97)92490-7
17. Luo M, Yang X, Tan B, Zhou P, Xia HM, Xue J, et al. Distribution of common pathogens in patients with pyogenic liver abscess in China: a meta-analysis. Eur J Clin Microbiol Infect Dis (2016) 44(6):615–22. doi:10.1007/s10096-016-2712-y
18. Brogden KA, Guthmiller JM, Taylor CE. Human polymicrobial infections. Lancet (2005) 365(9455):253–5. doi:10.1016/S0140-6736(05)17745-9
19. Tabarelli W, Bonatti H, Cejna M, Hartmann G, Stelzmueller I, Wenzl E. Clostridium perfringens liver abscess after pancreatic resection. Surg Infect (2009) 10(2):159–62. doi:10.1089/sur.2008.014
20. Hu BS, Liu CY, Wang FD. Clinical efficacy of cefmetazole in intraabdominal infection. Zhonghua Yi Xue Za Zhi (Taipei) (1993) 51(6):436–9.
Keywords: bacterial hepatic abscess, Klebsiella pneumoniae, Pannonibacter phragmitetus, polymicrobial infection, Streptococcus oralis
Citation: Wang M, Zhang X, Jiang T, Hu S, Yi Z, Zhou Y, Ming D and Chen S (2017) Liver Abscess Caused by Pannonibacter phragmitetus: Case Report and Literature Review. Front. Med. 4:48. doi: 10.3389/fmed.2017.00048
Received: 30 October 2016; Accepted: 10 April 2017;
Published: 25 April 2017
Edited by:
Dongsheng Zhou, Beijing Institute of Microbiology and Epidemiology, ChinaReviewed by:
T. G. Nagaraja, Kansas State University, USAJose M. Ramia, SESCAM Regional Public Health System of Castilla La Mancha, Spain
Copyright: © 2017 Wang, Zhang, Jiang, Hu, Yi, Zhou, Ming and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yajun Zhou, 1400216018@hqu.edu.cn;
Desong Ming, mds6430@126.com;
Shicheng Chen, shicheng@msu.edu
†These authors have contributed equally to this work.
 Mingxi Wang1,2†
Mingxi Wang1,2†
 Shaohua Hu
Shaohua Hu Yajun Zhou
Yajun Zhou Shicheng Chen
Shicheng Chen