Polysaccharides As Viscosupplementation Agents: Structural Molecular Characteristics but Not Rheology Appear Crucial to the Therapeutic Response
- 1Rheumatology and Metabolic Bone Diseases Department, Hospital de Santa Maria, CHLN, Lisbon Academic Medical Centre, Lisbon, Portugal
- 2Rheumatology Research Unit, Faculty of Medicine, Instituto de Medicina Molecular, University of Lisbon, Lisbon, Portugal
- 3Faculty of Medicine, Department of Internal Medicine, Federal University of Ceará, Fortaleza, Brazil
Introduction: Most clinical studies and basic research document viscosupplementation (VS) in terms of effectiveness and safety, but only a few highlight its molecular mechanisms of action. Besides, there is generally focus on hyaluronic acid (HA) as being the most relevant polysaccharide to reach the clinical endpoints, attributing its effect mainly to its unique viscoelastic properties, related to a high-molecular weight and gel formulation. Usually, studies do not approach the possible biological pathways where HA may interfere, and there is a lack of reports on other biocompatible polysaccharides that could be of use in VS.
Aim: We briefly review the main proposed mechanisms of action of intra-articular hyaluronic acid (IA-HA) treatment and discuss its effectiveness focusing on the role of rheological and intrinsic structural molecular properties of polysaccharides in providing a therapeutic effect.
Methods: We conducted a literature search using PubMed database to find articles dealing with the mechanisms of action of IA-HA treatment and/or emphasizing how the structural properties of the polysaccharide used influenced the clinical outcomes.
Discussion/conclusion: HA is involved in numerous biochemical interactions that may explain the clinical benefits of VS, most of them resulting from HA–cluster of differentiation 44 receptor interaction. There are other important aspects apart from the molecular size or the colloidal state of the IA-HA involved in VS efficiency that still need to be consolidated. Indeed, it seems that clinical response may be dependent on the intrinsic properties of the polysaccharide, regardless of being HA, rather than to rheology, posing some controversy to previous beliefs.
Introduction
Hyaluronic acid (HA) is a non-sulfated polyanionic polysaccharide that can be highly viscous, formed by synoviocytes, fibroblasts, and chondrocytes inside joints. It is present in the synovial fluid and the extracellular matrix of the cartilage (1) where it naturally occurs as a high-molecular weight (HMW) substance with an average molecular weight (MW) of 6 × 106 Da (2).
In healthy joints, HA is thought to act mainly as a viscoelastic shock absorber during high shear and as a lubricant during slow movement (3), apparently due to its rheological properties. There are claims that reduction of the MW and concentration of HA that occurs in osteoarthritis (OA) would alter the rheological properties of HA resulting in increased friction and reduced protection of the articular cartilage during mechanical stress (2, 4). Viscosupplementation (VS)—the injection of exogenous HA into the synovial joints—has emerged as a therapeutic approach to restore the viscoelasticity of the synovial fluid in the joint (2).
The observation that the clinical relief after a single injection of this compound may last up to 12 months (5, 6) argues against a pure rheological effect to explain the analgesia considering that exogenous HA possibly lasts less than 1 day in the joint (5). The possibility that intra-articular hyaluronic acid (IA-HA) has disease modifying properties does also question a mechanical action as the only explanation for the therapeutic benefit. Several papers have emphasized other clinical benefits arising from VS, such as chondroprotection and induction of proteoglycan and glycosaminoglycan synthesis, as well as anti-inflammatory, subchondral bone sparing, and analgesic actions. Altman et al. (7) summarized the mechanisms of action of VS of the knee described in the literature in a systematic review, with HA–CD44 receptor binding being the most frequently reported source of the effects mentioned.
According to some reports, the therapeutic efficacy of hylans (HA derivatives) is directly associated with their viscoelastic properties, related to their HMW and gel formulation, despite a lack of studies to prove this assumption (5, 8).
Indeed, the clinical benefit resulting from HA preparations injected into OA joints was also obtained with preparations of lower MW (in the order of 1 × 106 Da or less) (9). Further, the benefit of using a viscous or saline-soluble compound has not been brought to scrutiny. In keeping with this statement, it was shown (8) that the administration of a polysaccharide derived from guar gum (GG) injected into the knee of rats subjected to anterior cruciate ligament transection (ACLT), used as an OA model, provided significant analgesia that was similar to Hylan G-F 20™, as a comparator, regardless of using a saline-soluble or a reticulated, highly viscous, polysaccharide preparation, this way suggesting that polysaccharide compounds used in VS provide analgesia independent of the gel state (8).
The controversy on whether HA preparations are worth in treating OA persists. Whereas a recent systematic review and meta-analysis considered IA-HA effective for pain relief in knee OA (10), this therapy was ruled out by the American Academy of Orthopedic Surgeons OA guidelines (11). We do not intend to discuss the controversy on the clinical efficacy or relevance of using “viscosupplements” in OA treatment. Rather, we briefly review some of the proposed mechanisms of action of VS while strictly addressing the issue that the intrinsic polysaccharide structure, rather than its rheological properties, probably account for the pharmacologic effect linked to the analgesia provided by this therapy.
Mechanisms of Action
The most relevant clinical results of VS described are chondroprotection, induction of proteoglycan and glycosaminoglycan synthesis, mechanical, anti-inflammatory, subchondral, and analgesic actions (7). Mechanisms of action refer to the specific biochemical interaction through which HA as a molecule produces the listed pharmacological effects. Most of them result from the interaction between HA and various cellular receptors such as the CD44 receptor, the intercellular adhesion molecule (ICAM)-1, and the receptor for hyaluronan-mediated motility (RHAMM).
The Role of CD44 Receptor
CD44 is a cell-surface glycoprotein receptor expressed in a large number of mammalian cell types. HA binding to CD44 elicits several biomolecular processes, namely inhibition of interleukin (IL)-1β expression, reduction of a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTs) expression, decrease of prostaglandin E2 (PGE2) synthesis, overexpression of the anti-apoptotic heat shock protein 70 (Hsp70), and stimulation of the intrinsic production of proteoglycans inside the joint.
Inhibition of IL-1β expression, a dominant catabolic cytokine present in the inflamed articular joints (12), results in an overall anti-inflammatory effect (7, 13). This inhibition is carried out through induction of mitogen-activated protein kinase phosphatase (MKP)-1, a negative regulator of IL-1β (7, 14). Likewise, IL-1β suppression leads to a decline in the activation of matrix metalloproteinases (MMPs) -1, 2, 3, 9, and 13 (7, 15, 16), thereby decreasing catabolic enzyme activity within the joint cartilage, as well as chondrocyte apoptosis (7, 17–19). Anti-inflammatory effects are further accomplished by a decreased release of other pro-inflammatory mediators, such as IL-6 (7, 20), as well as HA binding to ICAM-1 and subsequent downregulation of the nuclear factor kappa-light-chain enhancer of activated B cells (12). Given that MMPs and IL-6 are linked to inflammatory bone resorption, this HA effect could be bone sparing (7, 20). Most studies on HA mechanisms were done in vitro, but reduced levels of peripheral CD4+ T lymphocytes, particularly those of the T helper-17 subtype, were shown following intra-articular injection of an HA preparation, suggesting in vivo modulation of the inflammatory response by HA with a systemic relevance (21).
Coupling of HA to CD44, via reduction of ADAMTs expression (7), a family of peptidases that are involved in the cleavage of important synovial components (including aggrecan, versican, and brevican) (7, 22, 23), enhances chondroprotection and decreases inflammation also through inhibiting PGE2 synthesis and increasing the anti-apoptotic Hsp70 overexpression (7).
Hyaluronic acid–CD44 interaction, in addition with HA binding to ICAM-1, appears to stimulate the intrinsic production of proteoglycans inside the joint (7, 24), apparently depending, at least partially, on the stimulation of insulin-like growth factor-1 pathway (7, 24), thus representing an anabolic activity of HA that may protect the osteoarthritic joint.
The Role of RHAMM
Hyaluronic acid binding to RHAMM (a cytosolic and surface protein) promotes wound repair, activates pro-migration and invasion functions, regulates cellular responses to growth factors, and plays a role in fibroblast migration and motility (7). This HA activity does also aid in chondroprotection in addition to CD44 binding (7).
Other Molecular Pathways
Hyaluronic acid has been demonstrated to suppress numerous inflammatory mediators, not only by interacting with CD44 and ICAM-1 but also through toll-like receptors (TLRs) 2 and 4 binding, this way suppressing tumor necrosis factor-α, IL-1-β, IL-17, MMP-13, and inducible nitrogen oxide synthase expression (7, 25).
Hyaluronic acid analgesic effects have been shown to occur at mechano-sensitive stretch-activated ion channels inside the joint, where channel activity is significantly decreased upon HA binding (7).
Hyaluronic acid may suppress fibrinolytic activity mediated by the urokinase-type plasminogen activator and its receptor, this way downregulating the activation of proteinases, including gelatinases and other MMPs (12).
Structural Properties of the Polysaccharides Used in VS
As shown above, HA dynamic interactions with other molecules modulate cell proliferation and gene expression. It has been suggested that these physicochemical and biologic properties of HA may be due to characteristics such as its MW and the gel formulation of the compound. Most reports seem to agree that HMW HA is superior to low-molecular weight (LMW) HA formulations in achieving the pharmacological effects mentioned. Besides, the gel formulation, as opposed to a saline-soluble preparation, apparently mimics the structural characteristics, particularly the intrinsic viscosity, of the synovial fluid. Allegedly, exogenous HA preparations should be viscous mimicking the “shock absorbing” effect provided by endogenous HA. However, we believe that there are no studies to prove these assumptions (8).
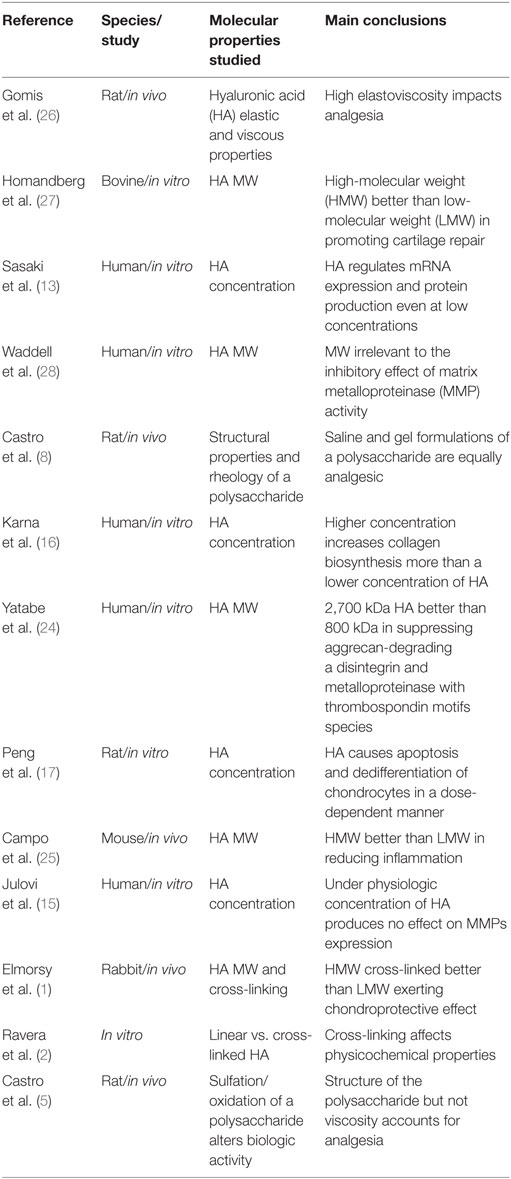
Table 1 summarizes the reviewed studies addressing molecular properties and “viscosupplements” proposed effect.
HMW vs. LMW HA
One reason for HMW HA preparations being superior could be a more effective binding to the CD44 receptor (7). Additionally, HMW HA would produce superior friction coefficients, thus reducing attrition between structures, as compared to LMW HA (29). However, one must consider that in vitro evaluations of friction coefficients and intrinsic viscosity may not reflect in vivo conditions.
Hyaluronic acid with HMW are increasingly being used in clinical practice such as Hylan G-F 20™—a mixture of a HMW HA with a cross-linked HA preparation, the combination having an MW range of 6 × 106 to 23 × 106 Da (9). However, other HA preparations currently used to treat OA have MW in the order of 1 × 106 Da or less, and most are reported to be effective as well (9). A recent network meta-analysis showed that HA preparations had the highest effect size for analgesia in knee OAs without specifically addressing the MW issue (10).
Meanwhile, studies using large animal models of OA have shown that HA with MW within the range of 0.5 × 106 to 1.0 × 106 Da were generally more effective in reducing indices of synovial inflammation and restoring the rheological properties of synovial fluid than HA with MW > 2.3 × 106 Da (9). It is possible but as yet unproven, that HA preparations within a specific size range could provoke a pattern of CD44 clustering and cross-linking on binding, which then triggers an intracellular signal, whereas the larger HA molecules may occupy these multiple CD44 linking sites but preventing receptor cross-link and a cellular reaction (9). On the other hand, LMW HA preparations may have a greater penetration through the extracellular matrix of the synovium, thereby maximizing its concentration and facilitating interaction with target synovial cells (9). Nevertheless, the interpretation of these studies that favor the use of LMW HA formulations is clouded by the number of protocol variations used (9).
Hyaluronic acid preparations, at least those present in clinical practice, probably do not last more than 3 days inside a joint. Realistically, considering the high synovial blood flow, the effect of movement increasing clearance of intra-articular fluid substances and the presence of proteases that may digest exogenous HA, particularly in inflamed joints, relevant levels of “viscosupplements” probably do not last more than 24 h inside an osteoarthritic knee (29). In keeping with this statement, there are scant data to support any difference in the clinical results obtained with preparations of variable MW (10). Using human synovial explants, Waddell et al. (28) showed that inhibition of MMP activity was similar whether incubating with 1.2 × 106Da HA (Supartz®) or 12.8 × 106Da HA (Synvisc®) preparations, thus suggesting that mechanisms other than the MW of the VS agents are relevant to their effect. Also, Ronchetti et al. (30) showed HMW hylans (around 106Da) had similar effectiveness as compared to other compounds of LMW (5–7.5 × 105 Da) in providing pain relief in OA (8).
Ghosh and Guidolin (9) have suggested that the ideal MW of HA is still to be determined but it is probably not a fixed size range, varying with characteristics as the extent of inflammation that would affect synovial tissue permeability and cell populations entering the joint, synovial fluid volume, and its rate of clearance.
Colloidal State
The gel formulation is also claimed to be fundamental for VS effectiveness, mostly considering its analgesic actions. In fact, one of the proposed mechanisms for the antinociceptive activity of hylans is a decrease of the activation of mechano-sensitive receptors located in periarticular structures, as well as in the synovium, by embedding these nerve endings in a highly viscous medium reducing their firing (8, 26). However, this mechanism has been put into scrutiny, as aforementioned (8). As a matter of fact, by using an experimental OA model in rats, it was demonstrated (8) that the intra-articular injection of a protein-free GG derivative—a galactomannan polysaccharide derived from the seed endosperm of the plant Cyamopsis tetragonolobus—provided analgesia similar to that of Hylan G-F 20™. Moreover, that GG-derived galactomannan analgesia occurred both with a highly viscous and a saline-soluble preparation, suggesting that the effect was independent of the gel state. Recently, the same group showed (5) that modifications of the chemical structure of that galactomannan, namely oxidation or sulfation, abrogated the analgesia provided by the original compound, using the ACLT rat OA model. Additionally, weekly administrations, starting 14 days until 70 days post-ACLT prevented structural joint damage to the knee, as compared to controls. As a whole, these data suggested that the analgesia provided by a GG-derived galactomannan depends on the chemical structure of the compound, which was also shown to be chondroprotective, questioning that the rheological properties of polysaccharides are responsible for the therapeutic benefit of “viscosupplements” (5).
Discussion/Conclusion
By reviewing the current literature, it seems that there are multiple mechanisms of action that may explain VS clinical benefits. The dynamic interaction between HA and various cellular receptors appears to be crucial to that therapy, perhaps with a major relevance to CD44 receptor coupling, resulting in several biologic effects such as inhibition of pro-inflammatory mediators and suppression of MMPs activity. Other possible pathways include modulation by ICAM-1, RHAMM, and coupling to TLR-2 and 4, as well as interference with specific ion channels relevant to pain mechanisms inside the joint.
According to some reports, the therapeutic effectiveness of hylans have been claimed to be directly associated with their viscoelastic properties, mostly HMW and gel formulation. However, there may be more than rheology to explain the yet to be proven efficacy of “viscosupplements.” In fact, HA that naturally occurs in the joints has an average MW of 6 × 106 Da, but experiments showed that HA of LMW may present the same or greater effectiveness depending on the specific clinical endpoint and animal species studied. On the other hand, even taking into account the lack of a human study, a plant-derived polysaccharide, obtained from a commonly used compound in the cosmetic, food, and pharmaceutical industries, provided analgesia and chondroprotection regardless of the gel state, this way implying that the biochemical structure and pharmacologic mechanisms rather than rheological properties account to explain the clinical outcome.
Given that intra-articular injection of a protein-free, saline-soluble, easily obtained polysaccharide is probably less complex as compared to that of a highly viscous preparation, it would be interesting to see the experimental data obtained with the GG polysaccharide reproduced in patients with OA.
Author Contributions
All authors wrote the main manuscript text and approved the final version.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This work was supported by grant 459334/2014-0 from CNPq (Conselho Nacional de Desenvolvimento e Tecnológico, Brasil).
Abbreviations
ACLT, anterior cruciate ligament transection; ADAMTs, a disintegrin and metalloproteinase with thrombospondin motifs; CD, cluster of differentiation; GG, guar gum; HA, hyaluronic acid; HMW, high-molecular weight; Hsp70, heat shock protein 70; IA-HA, intra-articular hyaluronic acid; ICAM, intercellular adhesion molecule; IL, interleukin; LMW, low-molecular weight; MMP, matrix metalloproteinase; MW, molecular weight; OA, osteoarthritis; PGE2, prostaglandin E2; RHAMM, receptor for hyaluronan-mediated motility; TLRs, toll-like receptors; VS, viscosupplementation.
References
1. Elmorsy S, Funakoshi T, Sasazawa F, Todoh M, Tadano S, Iwasaki N. Chondroprotective effects of high-molecular-weight cross-linked hyaluronic acid in a rabbit knee osteoarthritis model. Osteoarthritis Cartilage (2014) 22(1):121–7. doi: 10.1016/j.joca.2013.10.005
2. Ravera E, Fragai M, Parigi G, Luchinat C. Differences in dynamics between crosslinked and non-crosslinked hyaluronates measured by using fast field-cycling relaxometry. Chemphyschem (2015) 16(13):2803–9. doi:10.1002/cphc.201500446
3. Johal H, Devji T, Schemitsch EH, Bhandari M. Viscosupplementation in knee osteoarthritis: evidence revisited. JBJS Rev (2016) 4(4):e1. doi:10.2106/JBJS.RVW.15.00098
4. Fraser JRE, Laurent TC, Laurent UBG. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med (1997) 242(1):27–33. doi:10.1046/j.1365-2796.1997.00170.x
5. Castro RR, Silva CM, Nunes RM, Cunha PL, de Paula RC, Feitosa JP, et al. Structural characteristics are crucial to the benefits of guar gum in experimental osteoarthritis. Carbohydr Polym (2016) 150:392–9. doi:10.1016/j.carbpol.2016.05.031
6. Kirwan J. Is there a place for intra-articular hyaluronate in osteoarthritis of the knee? Knee (2001) 8(2):93–101. doi:10.1016/S0968-0160(01)00075-8
7. Altman RD, Manjoo A, Fierlinger A, Niazi F, Nicholls M. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: a systematic review. BMC Musculoskelet Disord (2015) 16(1):321. doi:10.1186/s12891-015-0775-z
8. Castro RR, Feitosa JPA, da Cunha PLR, da Rocha FAC. Analgesic activity of a polysaccharide in experimental osteoarthritis in rats. Clin Rheumatol (2007) 26(8):1312–9. doi:10.1007/s10067-007-0570-9
9. Ghosh P, Guidolin D. Potential mechanism of action of intra-articular hyaluronan therapy in osteoarthritis: are the effects molecular weight dependent? Semin Arthritis Rheum (2002) 32(1):10–37. doi:10.1053/sarh.2002.33720
10. Bannuru RR, Schmid CH, Kent DM, Vaysbrot EE, Wong JB, McAlindon TE. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis. A systematic review and network meta-analysis. Ann Intern Med (2015) 162(1):46–54. doi:10.7326/M14-1231
11. Brown GA. AAOS clinical practice guideline: treatment of osteoarthritis of the knee: evidence-based guideline. J Am Acad Orthop Surg (2013) 21(9):577–9. doi:10.5435/JAAOS-21-09-577
12. Masuko K, Murata M, Yudoh K, Kato T, Nakamura H. Anti-inflammatory effects of hyaluronan in arthritis therapy: not just for viscosity. Int J Gen Med (2009) 2:77–81. doi:10.2147/IJGM.S5495
13. Sasaki A, Sasaki K, Konttinen YT, Santavirta S, Takahara M, Takei H, et al. Hyaluronate inhibits the interleukin-1β-induced expression of matrix metalloproteinase (MMP)-1 and MMP-3 in human synovial cells. Tohoku J Exp Med (2004) 204(2):99–107. doi:10.1620/tjem.204.99
14. Mihara M, Hashizume M. The effect of high molecular hyaluronic acid on the induction of matrix degradation enzymes By IL-6, IL-1β and TNF-α. Osteoarthritis Cartilage (2012) 20:S134–5. doi:10.1016/j.joca.2012.02.181
15. Julovi SM, Yasuda T, Shimizu M, Hiramitsu T, Nakamura T. Inhibition of interleukin-1β-stimulated production of matrix metalloproteinases by hyaluronan via CD44 in human articular cartilage. Arthritis Rheum (2004) 50(2):516–25. doi:10.1002/art.20004
16. Karna E, Miltyk W, Surażyński A, Pałka JA. Protective effect of hyaluronic acid on interleukin-1-induced deregulation of β1-integrin and insulin-like growth factor-I receptor signaling and collagen biosynthesis in cultured human chondrocytes. Mol Cell Biochem (2008) 308(1–2):57–64. doi:10.1007/s11010-007-9612-5
17. Peng H, Zhou JL, Liu SQ, Hu QJ, Ming JH, Qiu B. Hyaluronic acid inhibits nitric oxide-induced apoptosis and dedifferentiation of articular chondrocytes in vitro. Inflamm Res (2010) 59(7):519–30. doi:10.1007/s00011-010-0156-x
18. Takahashi K, Hashimoto S, Kubo T, Hirasawa Y, Lotz M, Amiel D. Hyaluronan suppressed nitric oxide production in the meniscus and synovium of rabbit osteoarthritis model. J Orthop Res (2001) 19(3):500–3. doi:10.1016/S0736-0266(00)90024-X
19. Kalacı A, Yılmaz HR, Aslan B, Söğüt S, Yanat AN, Uz E. Effects of hyaluronan on nitric oxide levels and superoxide dismutase activities in synovial fluid in knee osteoarthritis. Clin Rheumatol (2007) 26(8):1306–11. doi:10.1007/s10067-006-0504-y
20. Hiraoka N, Takahashi KA, Arai Y, Honjo K, Nakagawa S, Tsuchida S, et al. Hyaluronan and intermittent hydrostatic pressure synergistically suppressed MMP-13 and IL-6 expressions in osteoblasts from OA subchondral bone. Osteoarthritis Cartilage (2009) 17:S97. doi:10.1016/S1063-4584(09)60186-2
21. Lùrati A, Laria A, Mazzocchi D, Re KA, Marrazza M, Scarpellini M. Effects of hyaluronic acid (HA) viscosupplementation on peripheral Th cells in knee and hip osteoarthritis. Osteoarthritis Cartilage (2015) 23(1):88–93. doi:10.1016/j.joca.2014.09.010
22. Hui AY, McCarty WJ, Masuda K, Firestein GS, Sah RL. A systems biology approach to synovial joint lubrication in health, injury, and disease. Wiley Interdiscip Rev Syst Biol Med (2012) 4(1):15–37. doi:10.1002/wsbm.157
23. Lin EA, Liu CJ. The role of ADAMTSs in arthritis. Protein Cell (2010) 1(1):33–47. doi:10.1007/s13238-010-0002-5
24. Yatabe T, Mochizuki S, Takizawa M, Chijiiwa M, Okada A, Kimura T, et al. Hyaluronan inhibits expression of ADAMTS4 (aggrecanase-1) in human osteoarthritic chondrocytes. Ann Rheum Dis (2009) 68(6):1051–8. doi:10.1136/ard.2007.086884
25. Campo GM, Avenoso A, Nastasi G, Micali A, Prestipino V, Vaccaro M, et al. Hyaluronan reduces inflammation in experimental arthritis by modulating TLR-2 and TLR-4 cartilage expression. Biochim Biophys Acta (2011) 1812(9):1170–81. doi:10.1016/j.bbadis.2011.06.006
26. Gomis A, Pawlak M, Balazs EA, Schmidt RF, Belmonte C. Effects of different molecular weight elastoviscous hyaluronan solutions on articular nociceptive afferents. Arthritis Rheum (2004) 50(1):314–26. doi:10.1002/art.11421
27. Homandberg GA, Ummadi V, Kang H. The role of insulin-like growth factor-I in hyaluronan mediated repair of cultured cartilage explants. Inflamm Res (2004) 53(8):396–404. doi:10.1007/s00011-004-1276-y
28. Waddell DD, Kolomytkin OV, Dunn S, Marino AA. Hyaluronan suppresses IL-1 [beta]-induced metalloproteinase activity from synovial tissue. Clin Orthop Relat Res (2007) 465:241–8. doi:10.1097/BLO.0b013e31815873f9
29. Hunter DJ. Viscosupplementation for osteoarthritis of the knee. N Engl J Med (2015) 372(11):1040–7. doi:10.1056/NEJMct1215534
30. Ronchetti IP, Guerra D, Taparelli F, Boraldi F, Bergamini G, Mori G, et al. Morphological analysis of knee synovial membrane biopsies from a randomized controlled clinical study comparing the effects of sodium hyaluronate (Hyalgan®) and methylprednisolone acetate (Depomedrol®) in osteoarthritis. Rheumatology (2001) 40(2):158–69. doi:10.1093/rheumatology/40.2.158
Keywords: viscosupplementation, hyaluronic acid, polysaccharide, osteoarthritis, pain, cartilage, guar gum
Citation: Machado RC, Capela S and Rocha FAC (2017) Polysaccharides As Viscosupplementation Agents: Structural Molecular Characteristics but Not Rheology Appear Crucial to the Therapeutic Response. Front. Med. 4:82. doi: 10.3389/fmed.2017.00082
Received: 31 March 2017; Accepted: 06 June 2017;
Published: 19 June 2017
Edited by:
Ying Ying Leung, Duke-NUS Medical School, SingaporeReviewed by:
Hamid Rahmatullah Bin Abd Razak, Singapore General Hospital, SingaporePedro Weingrill, Univille, Brazil
Copyright: © 2017 Machado, Capela and Rocha. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francisco A. C. Rocha, arocha@ufc.br
 Rita C. Machado
Rita C. Machado Susana Capela1,2
Susana Capela1,2  Francisco A. C. Rocha
Francisco A. C. Rocha