Diagnosis of Idiopathic Pulmonary Fibrosis “Pragmatic Challenges in Clinical Practice”
- 1First Academic Department of Pneumonology, Hospital for Thoracic Diseases, “Sotiria”, Medical School, National and Kapodistrian University of Athens, Athens, Greece
- 2Division of Immunology, Biomedical Sciences Research Center “Alexander Fleming”, Athens, Greece
- 35th Department of Pneumonology, Hospital for Thoracic Diseases, “Sotiria”, Athens, Greece
- 42nd Pulmonary Medicine Department, Attikon University Hospital, Medical School, National and Kapodistrian University of Athens, Athens, Greece
The past few years have signaled a major breakthrough on the management of idiopathic pulmonary fibrosis (IPF). Finally, we have drugs in our arsenal able to slow down the inexorable disease natural course. On the other hand, the latter evidence has increased the responsibility for a timely and accurate diagnosis. Establishment of IPF diagnosis directly affects the choice of appropriate treatment. The current diagnostic guidelines represent a major step forward providing an evidence-based road map; yet, clinicians are encountering major diagnostic dilemmas that inevitably affect therapeutic decisions. This review article aims to summarize the current state of knowledge on the diagnostic procedure of IPF based on the current guidelines and discuss pragmatic difficulties and challenges encountered by clinicians with regards to their applicability in the everyday clinical practice.
Introduction
Idiopathic pulmonary fibrosis (IPF) represents the most common form of idiopathic interstitial pneumonia (IIPs) and is characterized by the gravest prognosis with a median survival of 3–5 years (1), irrespective of treatment. The past 10 years, large multicenter placebo-controlled clinical trials have significantly shifted the therapeutic dial of IPF (2–4) from harmful agents to Ref. (5) to therapies able to slow down the disease progression (2–4). It is important to note that the efficacy of the antifibrotic agents, pirfenidone, and nintedanib has been tested only in the context of IPF; thus, accurate and timely diagnosis seems to be imperative. It is important to note that disease diagnosis does not represent anymore an academic exercise since it directly influences and guides therapeutic decisions.
Diagnosis of IPF
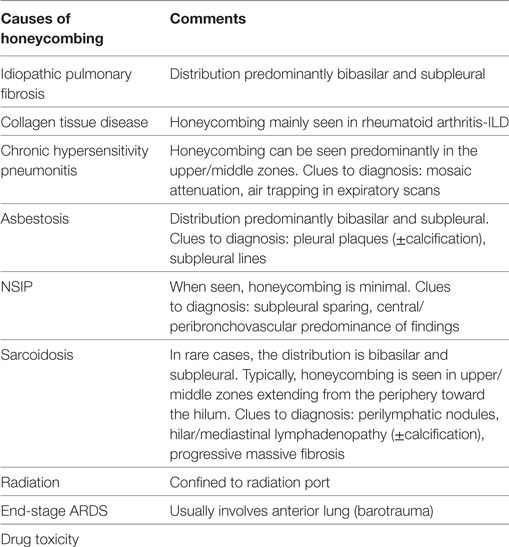
Histologically, IPF is characterized by the pattern of usual interstitial pneumonia (UIP), which is denoted by spatial and temporal heterogeneity (6). It is of utmost importance to highlight the fact that UIP pathology is not exclusive to IPF, as it may characterize other diseases including chronic hypersensitivity pneumonitis (HP), connective tissue disorders-associated ILDs, asbestosis, or drug toxicity. In other words, all IPF have UIP pathology, but not all UIP are IPF. High-resolution computed tomography (HRCT) revolutionized the diagnosis of IPF. The presence of honeycombing in a predominantly peripheral and bibasilar distribution has a sufficient positive-predictive value (PPV) for underlying UIP pathology obviating the need for tissue confirmation (7–9). Even in that case, exclusion of other causes of UIP is mandatory to finally establish IPF diagnosis. Furthermore, IPF diagnosis represents a dynamic process and, therefore, close monitoring of the patient is mandatory. In particular, therapeutic decisions can be altered based on disease natural course and treatment responsiveness on an individual basis. In addition, disease diagnosis could be revisited in light of emerging symptoms compatible with connective tissue disorder or exposure to potentially harmful environmental agents.
The patient with IPF typically presents with progressive dyspnea on exertion of insidious onset and non-productive cough. The most characteristic clinical finding is the presence of Velcro rales that can be proven an extremely useful diagnostic tool for early disease diagnosis (10). Clubbing is almost found in 30–50% of patients; yet, its prevalence is much higher following disease progression and development of pulmonary hypertension. Multisystemic manifestations in the context of IPF are highly uncommon and should alert the physician toward alternative diagnoses. Median time from onset of symptoms to first evaluation in an ILD center quite often exceeds 1 year and the length of delay has been associated with increased mortality (11, 12).
Pulmonary function tests usually exhibit a restrictive pattern with concomitant reduction in diffusing capacity of carbon monoxide (DLco). However, the absence of restriction does not exclude the diagnosis of IPF, especially in the context of combined pulmonary fibrosis and emphysema, which is characterized by relatively preserved lung volumes with disproportionately reduced DLco (13). There are also cases discovered early and presumably with an initial FVC > 100% that do not fulfill the criteria of a restrictive pattern, nevertheless, fibrosis is evident on HRCT.
According to current diagnostic criteria (1), HRCT plays a pivotal role in the diagnostic procedure. There are three diagnostic categories based on HRCT appearance: UIP pattern, possible UIP pattern, and inconsistent with UIP. The definition of each category is based on morphological as well as distribution characteristics. UIP pattern is characterized by the presence of reticular abnormalities and honeycombing (with or without traction bronchiectasis) with a subpleural and basal predominance in the absence of inconsistent features. The above radiological features in the absence of honeycombing constitute the possible UIP pattern. Inconsistent features can be categorized as those involving distribution (upper or mid lung and peribronchovascular predominance) and those involving morphology (extensive ground glass opacities, consolidation, mosaic attenuation, nodules, discrete cysts).
The presence of honeycombing is not synonymous with IPF, as it can be seen in other diseases as chronic HP, collagen tissue-related interstitial lung diseases, asbestosis, drug-induced lung toxicity, sarcoidosis, postradiation pneumonitis, and post ARDS. Minimal honeycombing can also be encountered in cases of fibrotic NSIP, which represents a major component of the differential list.
The distribution of honeycombing can offer significant information. Typically, in IPF, it has a subpleural and basilar distribution. Chronic HP can be a great mimic of IPF. However, sometimes in chronic HP, honeycombing can be more marked in the upper/mid lung zones giving a hint to the actual diagnosis. The same upper lobe predominance of honeycombing can be seen in sarcoidosis as well. Furthermore, in sarcoidosis, the fibrotic process often follows the expected perilymphatic route, thus creating a “swath” of honeycombing extending from the hilum to the periphery of the lung. In patients who develop fibrosis post ARDS, it has a striking anterior distribution (Table 1).
Besides the distribution of honeycombing, there are other findings on HRCT that increase suspicion toward certain diagnoses. Silva et al. (14) studied 66 patients with biopsy proven IPF, HP, and NSIP. The presence of lobular areas with decreased attenuation, centrilobular nodules, and cysts favored the diagnosis of HP. The best predictors of NSIP were the presence of subpleural sparing and the absence of honeycombing.
In the appropriate clinical setting in patients with a UIP pattern, IPF diagnosis can be established without the need for surgical lung biopsy (SLB). In the cases of possible UIP and inconsistent with UIP, a SLB is recommended in order to reach a final diagnosis. When SLB is necessary, close cooperation with the thoracic surgeon is necessary in order to point the optimal sites for biopsy in order to increase the possibility of an accurate diagnosis. Areas of honeycombing should be avoided in lung sampling since they may reveal non-specific end-stage lung damage and absence of spatial and temporal heterogeneity suggestive of UIP. Samples should be obtained from at least two different lobes, because of the possibility of discordant findings (UIP in one lobe and NSIP in another). In such cases, the UIP pattern drives diagnosis and prognosis as well (15, 16).
Regarding histopathology, current guidelines have classified patients into four categories: UIP, probable UIP, possible UIP, and not UIP (1). Specific combinations of HRCT and SLB pattern with the integration of clinical data are evaluated by a multidisciplinary (MDT) team in order to achieve a final diagnosis.
MDT approach is acknowledged as a major advance in IPF diagnosis. It refers to the constructive exchange of views between a respiratory physician, radiologist, and pathologist with expertise in the field of ILDs. The added value of MDT diagnosis is its association with higher levels of diagnostic confidence and better interobserver agreement (17, 18). Walsh et al. (19) were the first to evaluate the agreement between different multidisciplinary teams in diffuse lung diseases after the 2013 ATS/ERS update (20) on the classification of IIPs. Inter-MDT agreement was acceptable for cases of IPF (weighted kappa coefficient, κw = 0.71), while it was moderate for NSIP (κw = 0.42) and rather disappointing for HP (κw = 0.29) reflecting the lack of diagnostic guidelines for the last two clinical entities. This indirectly impacts the accuracy of IPF diagnosis as well, given the fact that NSIP and HP are frequently major components of its differential.
A Pragmatic Application of Guidelines in Every Day Clinical Practice
The 2011 guidelines are a clear step forward considering that they provide clear guidance on an evidence-based approach. The most crucial caveat of these guidelines is that, in a significant percentage of “real-life” patients with IPF, lack clinical practicality regarding diagnosis, prognosis, and therapeutic decisions.
Challenge 1: Interpretation of HRCT
High-resolution computed tomography plays a pivotal role in disease diagnosis and determines the need of SLB to establish a definite diagnosis. However, accurate identification of honeycombing is not straightforward even amongst thoracic radiologists. Interobserver agreement has been proven poor, and this has been validated in recent studies (21–24). This problem is further accentuated on a community level.
Possible UIP Pattern
An increasing number of studies has shown that in patients with a high suspicion of IPF, a possible UIP pattern retains sufficient PPV for underlying UIP pathology in order to obviate the need for tissue based diagnosis. Raghu et al. (25) studied 315 patients with IPF study that had both HRCT and SLB samples. As expected, UIP pattern had a high PPV for UIP pathology (97.3%). This high PPV was retained for patients with a possible UIP pattern (94%). Though a selection bias is quite obvious given that this evidence refers to patients screened for recruitment into clinical trials and thus should not be generalized; yet, this study highlights the importance of pretest clinical probability. Chung et al. (26) studied 201 patients with pulmonary fibrosis that were subjected to lung biopsy within 1 year of chest CT. Patients with possible UIP on CT scan were more likely to have histologic probable/definite UIP comparing to patients with indeterminate UIP on CT scan (82.4 vs 54.2%, p = 0.01). Finally, in the INPULSIS trials, a significant proportion of patients (31.9%, n = 338) were enrolled based on possible UIP pattern (with traction bronchiectasis) and no confirmation by SLB. This group of patients exhibited the same progression of disease based on the annual decline of FVC compared to patients with honeycombing on HRCT and/or confirmation by SLB as well as similar treatment response to nintedanib (27). This observation adds further to the notion that in the appropriate clinical setting, possible UIP pattern carries sufficient PPV for UIP pathology.
Recently, a study by Brownell et al. offered valuable new information on this topic (28). Avoiding selection bias, they elegantly showed that the PPV of possible UIP for predicting UIP pathology directly depends on the pretest probability of IPF and the prevalence of IPF in the examined population. In the derivation cohort, possible UIP had a specificity of 91.2% and a PPV of 62.5%. By using two key clinical predictors (male sex, increasing age) and a radiographic predictor (total traction bronchiectasis score), the PPV increased above the acceptable threshold of 90%.
Inconsistent with UIP Pattern
According to guidelines, even if histology is that of a typical UIP pattern when the HRCT appearance is inconsistent with UIP, the diagnosis of IPF is deemed only to be possible. However, the term inconsistent seems to be a misnomer. IPF is actually a great mimic. UIP pathology can exhibit a wide variety of radiological expressions ranging from the typical UIP pattern with peripheral, bibasilar honeycombing, to a pattern resembling HP with areas of mosaic attenuation or to a pattern characterized by extensive ground glass opacities (29, 30). Interestingly, NSIP pathology seems to be much more consistent regarding its radiological expression (31). The key point is that an inconsistent with UIP radiological pattern does not rule out the diagnosis of IPF, but mandates histological confirmation regardless the pretest probability of the patient. In the study by Brownell et al., the maximum PPV achieved for the inconsistent UIP pattern regarding IPF diagnosis was just 38% (28). Figure 1 summarizes a proposed algorithm for IPF diagnosis based on recent findings as described previously.
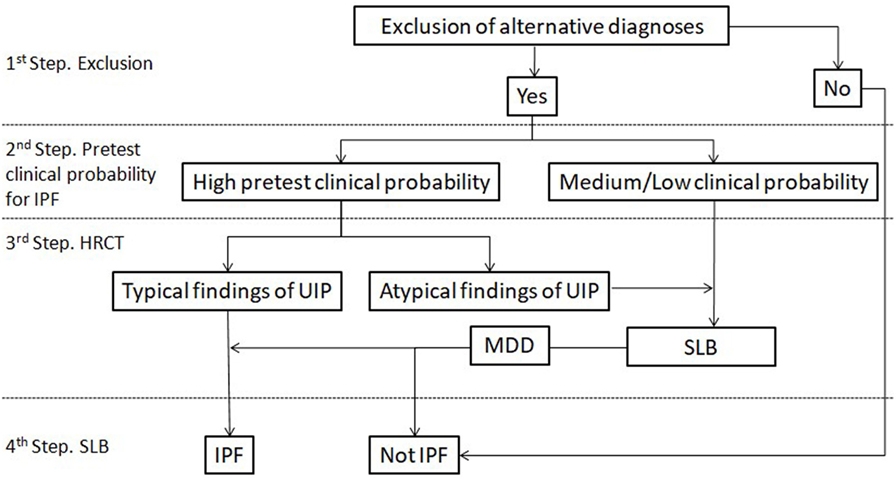
Figure 1. Proposed diagnostic algorithm for idiopathic pulmonary fibrosis (IPF). Typical findings of UIP are radiological signs of lung fibrosis (honeycombing, traction bronchiolectasis, and/or irregular reticular pattern) with a predominantly peripheral/subpleural and bibasilar distribution. Atypical findings of UIP are according to the current definition of inconsistent with UIP pattern. HRCT, high-resolution computed tomography; SLB, surgical lung biopsy.
Challenge 2: Interpretation of SLB
The interpretation of histological findings is subject to the same limitations as HRCT. The pathologic distinction between UIP and fibrotic NSIP can be especially difficult and is the main reason (>50%) for interobserver variation in the evaluation of diffuse parenchymal lung disease (32). In the same study, the median prevalence for diagnoses with low confidence (<70% likelihood) was 18% while diagnoses made with 100% confidence were reached in only 39% of cases. Thus, it is not surprising that the pathological diagnosis can be reconsidered in almost 20% of cases following integration of clinical and HRCT data (17). The variability in interpretation is accentuated between thoracic and general pathologists with a poor level of agreement (κ: 0.21), which was shown to have direct clinical implications (33). Also, it is known that biopsies from different sites can produce discordant results, specifically, NSIP vs UIP histology (15, 16). Consequently, sampling error is a possibility and in cases with a definite UIP radiology pattern and NSIP on histology, the radiologic diagnosis actually prevails over histology (34). Finally, histology patterns in interstitial lung diseases are not exclusive to certain diseases. In fact, the same histology can correspond to different diseases and furthermore to the same disease but with strikingly different progression and natural course. SLB is not a gold standard and the risk vs benefit ratio should be carefully examined in each patient.
Challenge 3: Safety of SLB
Surgical lung biopsy in patients with ILDs as it can trigger an acute exacerbation (35, 36), regardless disease severity (37). An alarming observation is that the same parameters that increase the likelihood of IPF (increased age and male sex) (28, 38, 39) represent risk factors that increase mortality following SLB in patients with ILDs (40, 41). Actually, a provisional diagnosis of IPF was identified as a risk factor for increased mortality. Other risk factors are the presence of comorbidities, hypoxemic respiratory failure, severe physiological impairment, pulmonary hypertension, rapidly progressive disease (42).
Two large series (40, 41) reported postoperative hospital mortality rates (1.7%) similar to those reported in patients with lung cancer undergoing lobectomy. The actual postoperative mortality may vary depending on the nature of the procedure (elective vs non-elective) and the risk factors for the individual patient. Thus, the decision to proceed to SLB [via video-assisted thoracoscopic surgery (VATS)] should be carefully considered on an individual basis, weighing risks vs diagnostic benefit.
Challenge 4: Current Clinical Practice
By strictly adhering to current guidelines, a large number of patients (almost 50% with a suspicion of IPF) will need to be subjected to SLB. Clinical practice seems to have endorsed the facts that SLB carries a small but significant risk and that the possible UIP pattern in a patient with a high pretest probability of IPF retains sufficient PPV for UIP pathology. It is common practice that in patients with a high clinical probability of IPF (28, 38, 39) (male sex, increased age, and/or extent of fibrosis), we establish a working diagnosis of IPF (43, 44) without resorting to surgical biopsy. Biopsies are reserved for patients in whom establishing a tissue-based diagnosis is clinically meaningful and are fit enough to undergo such a procedure. This is vividly depicted in the study by Hutchinson et al. (40). In a UK study held between 1997 and 2008, only 4.5% of new cases with a provisional diagnosis J84.1 were subjected to SLB (40). Given the fact that the ICD-10 of J84.1 does not accurately describe the IPF population (45, 46), this percentage is likely to be even smaller for actual IPF cases. We eagerly wait to see how the above will be translated in the upcoming guidelines for the diagnosis of IPF.
Future Directions
Bronchoscopic Lung Cryobiopsy (BLC)
Bronchoscopic lung cryobiopsy is dynamically emerging during the past few years as an alternative diagnostic tool to SLB, claiming the same diagnostic efficacy and reduced mortality (47). Cryobiopsies are considerably larger and have minimal crash artifacts as opposed to forceps biopsies when performed by experienced bronchoscopists in appropriate organized centers. Therefore, they allow confident recognition of histological patterns. The vital question that should be addressed is their safety and diagnostic yield against lung biopsies obtained via VATS. A recent meta-analysis including 16 studies with BLC (642 patients) and 14 studies with VATS (1,594 patients) (48) reported comparable diagnostic yields for BLC (83.7%) and VATS (92.7%). With regards to safety profile, BLC was associated with severe bleeding in 4.9% of cases and pneumothorax in 9.5% of cases while short-term mortality was similar between BLC (0.7%) and VATS (1.8%). Similar findings have been demonstrated by earlier studies (49, 50). In order to generalize the use of BLC beyond expert centers, it is important to standardize the procedure (e.g., size of cryoprobe, number and site of biopsies, degree of sedation), offer proper training, since it is an operator-dependent procedure (51) and prospectively evaluate safety and diagnostic profile of BLC as opposed to VATS.
Biomarkers
According to the Bayesian diagnostic approach of IPF, it would be very useful to have biomarkers that would increase the pretest probability of IPF. Ideally, these biomarkers would not only have diagnostic value but would also offer clinically relevant prognostic information regarding not only the natural course of the disease but also response to therapy. While for pulmonary embolism, d-dimers are an established diagnostic indicator in a complex disease as IPF, it is unlikely that just one “diagnosticator” will suffice. Considering that IPF diagnosis represent the least critical question for clinicians (given the major improvements of HRCT), in the real-world clinical practice, an ideal biomarker would be the one who could fulfill the unmet need for timely prediction of disease progressiveness and treatment responsiveness. In line with this, most of the studied biomarkers were mostly used as disease prognosticators rather than disease-specific diagnostic tools. Matrix metalloproteinase-(MMP)-7 represents the most extensively studied molecular biomarker that showed promising prognostic value in several independent cohorts of patients with IPF. Despite the fact that elevated MMP-7 levels clearly discriminated patients with IPF from those with HP (52); yet, they showed lack of discriminatory ability between IPF and RA-ILD (53). Further studies using highly standardized sample collection procedures and collection matrices are needed to produce reproducible and reliable diagnostic and prognostic cutoff thresholds (54). The latter observation represents an amenable need for precision medicine approaches (55, 56).
Conclusion
The ILD community has made significant progress in understanding IPF. With the development of antifibrotic agents, accurate diagnosis is crucial. Guidance is needed to focus on practical implementation of current guidelines in a real-world clinical setting. An integral first step of the diagnostic process is the exclusion of alternative diagnoses. Equally important is the definition of the pretest probability for every patient with suspected IPF. The diagnostic significance of possible or even definite UIP pattern is completely different when facing a 45-year-old female or a 70-year-old male. Possible UIP pattern seems to carry sufficient PPV in patients with a high pretest probability of IPF. SLB should be considered in patients with inconsistent with UIP pattern after evaluating the individualized benefit risk ratio (Table 2). BLC seems an attractive, safer alternative to SLB; yet, standardization and prospective evaluation of the process is required in order to “escape” from expert centers and be embraced by common practice. Finally, biomarkers are sorely needed to fulfill the unmet need of current clinical practice: early prediction of disease progressiveness and treatment responsiveness that will timely guide therapeutic decisions.
Author Contributions
VT, AT, and SC wrote the manuscript. SP and DB revised the manuscript for important intellectual content.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med (2011) 183:788–824. doi:10.1164/rccm.2009-040GL
2. Noble PW, Albera C, Bradford WZ. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomized trials. Lancet (2011) 377:1760–9. doi:10.1016/S0140-6736(11)60405-4
3. King TE Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med (2014) 370:2083–92. doi:10.1056/NEJMoa1402582
4. Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med (2014) 370:2071–82. doi:10.1056/NEJMoa1402584
5. Idiopathic Pulmonary Fibrosis Clinical Research Network, Raghu G, Anstrom KJ, King TE Jr, Lasky JA, Martinez FJ. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med (2012) 366(21):1968–77. doi:10.1056/NEJMoa1113354
6. Smith M, Dalurzo M, Panse P, Parish J, Leslie K. Usual interstitial pneumonia-pattern fibrosis in surgical lung biopsies. Clinical, radiological and histopathological clues to aetiology. J Clin Pathol (2013) 66(10):896–903. doi:10.1136/jclinpath-2013-201442
7. Raghu G, Mageto YN, Lockhart D, Schmidt RA, Wood DE, Godwin JD. The accuracy of the clinical diagnosis of new-onset idiopathic pulmonary fibrosis and other interstitial lung disease: a prospective study. Chest (1999) 116:1168–74. doi:10.1378/chest.116.5.1168
8. Hunninghake GW, Zimmerman MB, Schwartz DA, King TE Jr, Lynch J, Hegele R, et al. Utility of a lung biopsy for the diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med (2001) 164:193–6. doi:10.1164/ajrccm.164.2.2101090
9. Flaherty KR, Thwaite EL, Kazerooni EA, Gross BH, Toews GB, Colby TV, et al. Radiological versus histological diagnosis in UIP and NSIP: survival implications. Thorax (2003) 58:143–8. doi:10.1136/thorax.58.2.143
10. Cottin V, Cordier JF. Velcro crackles: the key for early diagnosis of idiopathic pulmonary fibrosis? Eur Respir J (2012) 40(3):519–21. doi:10.1183/09031936.00001612
11. Purokivi M, Hodgson U, Myllärniemi M, Salomaa ER, Kaarteenaho R. Are physicians in primary health care able to recognize pulmonary fibrosis? Eur Clin Respir J (2017) 4(1):1290339. doi:10.1080/20018525.2017.1290339
12. Lamas DJ, Kawut SM, Bagiella E, Philip N, Arcasoy SM, Lederer DJ. Delayed access and survival in idiopathic pulmonary fibrosis: a cohort study. Am J Respir Crit Care Med (2011) 184(7):842–7. doi:10.1164/rccm.201104-0668OC
13. Cottin V, Nunes H, Brillet PY, Delaval P, Devouassoux G, Tillie-Leblond I, et al. Combined pulmonary fibrosis and emphysema: a distinct underrecognised entity. Eur Respir J (2005) 26:586–93. doi:10.1183/09031936.05.00021005
14. Silva CI, Müller NL, Lynch DA, Curran-Everett D, Brown KK, Lee KS, et al. Chronic hypersensitivity pneumonitis: differentiation from idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia by using thin-section CT. Radiology (2008) 246(1):288–97. doi:10.1148/radiol.2453061881
15. Flaherty KR, Travis WD, Colby TV, Toews GB, Kazerooni EA, Gross BH, et al. Histopathologic variability in usual and nonspecific interstitial pneumonias. Am J Respir Crit Care Med (2001) 164:1722–7. doi:10.1164/ajrccm.164.9.2103074
16. Monaghan H, Wells AU, Colby TV, du Bois RM, Hansell DM, Nicholson AG. Prognostic implications of histologic patterns in multiple surgical lung biopsies from patients with idiopathic interstitial pneumonias. Chest (2004) 125:522–6. doi:10.1378/chest.125.2.522
17. Flaherty KR, King TE Jr, Raghu G, Lynch JP 3rd, Colby TV, Travis WD, et al. Idiopathic interstitial pneumonia: what is the effect of a multidisciplinary approach to diagnosis? Am J Respir Crit Care Med (2004) 170:904–10. doi:10.1164/rccm.200402-147OC
18. Thomeer M, Demedts M, Behr J, Buhl R, Costabel U, Flower CD, et al. Multidisciplinary interobserver agreement in the diagnosis of idiopathic pulmonary fibrosis. Eur Respir J (2008) 31:585–91. doi:10.1183/09031936.00063706
19. Walsh SL, Wells AU, Desai SR, Poletti V, Piciucchi S, Dubini A, et al. Multicentre evaluation of multidisciplinary team meeting agreement on diagnosis in diffuse parenchymal lung disease: a case-cohort study. Lancet Respir Med (2016) 4(7):557–65. doi:10.1016/S2213-2600(16)30033-9
20. Travis WD, Costabel U, Hansell DM, King TE Jr, Lynch DA, Nicholson AG, et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med (2013) 188(6):733–48. doi:10.1164/rccm.201308-1483ST
21. Lynch DA, Godwin JD, Safrin S, Starko KM, Hormel P, Brown KK, et al. Idiopathic Pulmonary Fibrosis Study Group. High-resolution computed tomography in idiopathic pulmonary fibrosis: diagnosis and prognosis. Am J Respir Crit Care Med (2005) 17:488–93. doi:10.1164/rccm.200412-1756OC
22. Watadani T, Sakai F, Johkoh T, Noma S, Akira M, Fujimoto K, et al. Interobserver variability in the CT assessment of honeycombing in the lungs. Radiology (2013) 26:936–44. doi:10.1148/radiol.12112516
23. Sundaram B, Gross BH, Martinez FJ, Oh E, Müller NL, Schipper M, et al. Accuracy of high-resolution CT in the diagnosis of diffuse lung disease: effect of predominance and distribution of findings. AJR Am J Roentgenol (2008) 191(4):1032–9. doi:10.2214/AJR.07.3177
24. Walsh SL, Calandriello L, Sverzellati N, Wells AU, Hansell DM; UIP Observer Consort. Interobserver agreement for the ATS/ERS/JRS/ALAT criteria for a UIP pattern on CT. Thorax (2016) 7:45–51. doi:10.1136/thoraxjnl-2015-207252
25. Raghu G, Lynch D, Godwin JD, Webb R, Colby TV, Leslie KO, et al. Diagnosis of idiopathic pulmonary fibrosis with high-resolution CT in patients with little or no radiological evidence of honeycombing: secondary analysis of a randomised, controlled trial. Lancet Respir Med (2014) 2:277–84. doi:10.1016/S2213-2600(14)70011-6
26. Chung JH, Chawla A, Peljto AL, Cool CD, Groshong SD, Talbert JL, et al. CT scan findings of probable usual interstitial pneumonitis have a high predictive value for histologic usual interstitial pneumonitis. Chest (2015) 147:450–9. doi:10.1378/chest.14-0976
27. Raghu G, Wells AU, Nicholson AG, Richeldi L, Flaherty KR, Le Maulf F, et al. Effect of nintedanib in subgroups of idiopathic pulmonary fibrosis by diagnostic criteria. Am J Respir Crit Care Med (2017) 195(1):78–85. doi:10.1164/rccm.201602-0402OC
28. Brownell R, Moua T, Henry TS, Elicker BM, White D, Vittinghoff E, et al. The use of pretest probability increases the value of high-resolution CT in diagnosing usual interstitial pneumonia. Thorax (2017) 72(5):424–9. doi:10.1136/thoraxjnl-2016-209671
29. Sverzellati N, Wells AU, Tomassetti S, Desai SR, Copley SJ, Aziz ZA, et al. Biopsy-proved idiopathic pulmonary fibrosis: spectrum of nondiagnostic thin-section CT diagnoses. Radiology (2010) 254(3):957–64. doi:10.1148/radiol.0990898
30. Yagihashi K, Huckleberry J, Colby TV, Tazelaar HD, Zach J, Sundaram B, et al. Radiologic-pathologic discordance in biopsy-proven usual interstitial pneumonia. Eur Respir J (2016) 47(4):1189–97. doi:10.1183/13993003.01680-2015
31. Sumikawa H, Johkoh T, Fujimoto K, Arakawa H, Colby TV, Fukuoka J, et al. Pathologically proved nonspecific interstitial pneumonia: CT pattern analysis as compared with usual interstitial pneumonia CT pattern. Radiology (2014) 272(2):549–56. doi:10.1148/radiol.14130853
32. Nicholson AG, Addis BJ, Bharucha H, Clelland CA, Corrin B, Gibbs AR, et al. Interobserver variation between pathologists in diffuse parenchymal lung disease. Thorax (2004) 59:500–5. doi:10.1136/thx.2003.011734
33. Lettieri CJ, Veerappan GR, Parker JM, Franks TJ, Hayden D, Travis WD, et al. Discordance between general and pulmonary pathologists in the diagnosis of interstitial lung disease. Respir Med (2005) 99(11):1425–30. doi:10.1016/j.rmed.2005.03.008
34. Zander DS. Idiopathic interstitial pneumonias and the concept of the trump card. Chest (2004) 125(2):359–60. doi:10.1378/chest.125.2.359
35. Utz JP, Ryu JH, Douglas WW, Hartman TE, Tazelaar HD, Myers JL, et al. High short-term mortality following lung biopsy for usual interstitial pneumonia. Eur Respir J (2001) 17:175–9. doi:10.1183/09031936.01.17201750
36. Kreider ME, Hansen-Flaschen J, Ahmad NN, Rossman MD, Kaiser LR, Kucharczuk JC, et al. Complications of video-assisted thoracoscopic lung biopsy in patients with interstitial lung disease. Ann Thorac Surg (2007) 83:1140–4. doi:10.1016/j.athoracsur.2006.10.002
37. Chida M, Ono S, Hoshikawa Y, Kondo T. Subclinical idiopathic pulmonary fibrosis is also a risk factor of postoperative acute respiratory distress syndrome following thoracic surgery. Eur J Cardiothorac Surg (2008) 34:878–81. doi:10.1016/j.ejcts.2008.07.028
38. Fell CD, Martinez FJ, Liu LX, Murray S, Han MK, Kazerooni EA, et al. Clinical predictors of a diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med (2010) 181(8):832–7. doi:10.1164/rccm.200906-0959OC
39. Salisbury ML, Xia M, Murray S, Bartholmai BJ, Kazerooni EA, Meldrum CA, et al. Predictors of idiopathic pulmonary fibrosis in absence of radiologic honeycombing: a cross sectional analysis in ILD patients undergoing lung tissue sampling. Respir Med (2016) 118:88–95. doi:10.1016/j.rmed.2016.07.016
40. Hutchinson JP, McKeever TM, Fogarty AW, Navaratnam V, Hubbard RB. Surgical lung biopsy for the diagnosis of interstitial lung disease in England: 1997-2008. Eur Respir J (2016) 48(5):1453–61. doi:10.1183/13993003.00378-2016
41. Hutchinson JP, Fogarty AW, McKeever TM, Hubbard RB. In-hospital mortality after surgical lung biopsy for interstitial lung disease in the United States 2000 to 2011. Am J Respir Crit Care Med (2016) 193:1161–7. doi:10.1164/rccm.201508-1632OC
42. Raj R, Raparia K, Lynch DA, Brown KK. Surgical lung biopsy for interstitial lung diseases. Chest (2017) 151(5):1131–40. doi:10.1016/j.chest.2016.06.019
43. Wells AU. Any fool can make a rule and any fool will mind it. BMC Med (2016) 14:23. doi:10.1186/s12916-016-0562-1
44. Tzilas V, Bouros D. Usual interstitial pneumonia pattern in the diagnosis of idiopathic pulmonary fibrosis? Lancet Respir Med (2016) 4(10):770–2. doi:10.1016/S2213-2600(16)30231-4
45. Tzilas V, Bouros D. Inherent weaknesses of the current ICD coding system regarding idiopathic pulmonary fibrosis. Eur Respir J (2015) 45(4):1194–6. doi:10.1183/09031936.00205914
46. Kaunisto J, Kelloniemi K, Sutinen E, Hodgson U, Piilonen A, Kaarteenaho R, et al. Re-evaluation of diagnostic parameters is crucial for obtaining accurate data on idiopathic pulmonary fibrosis. BMC Pulm Med (2015) 15:92. doi:10.1186/s12890-015-0074-3
47. Colby TV, Tomassetti S, Cavazza A, Dubini A, Poletti V. Transbronchial cryobiopsy in diffuse lung disease: update for the pathologist. Arch Pathol Lab Med (2017) 141(7):891–900. doi:10.5858/arpa.2016-0233-RA
48. Iftikhar IH, Alghothani L, Sardi A, Berkowitz D, Musani AI. Transbronchial lung cryobiopsy and video-assisted thoracoscopic lung biopsy in the diagnosis of diffuse parenchymal lung disease: a meta-analysis of diagnostic test accuracy. Ann Am Thorac Soc (2017) 14(7):1197–211. doi:10.1513/AnnalsATS.201701-086SR
49. Ravaglia C, Bonifazi M, Wells AU, Tomassetti S, Gurioli C, Piciucchi S, et al. Safety and diagnostic yield of transbronchial lung cryobiopsy in diffuse parenchymal lung diseases: a comparative study versus video-assisted thoracoscopic lung biopsy and a systematic review of the literature. Respiration (2016) 91(3):215–27. doi:10.1159/000444089
50. Johannson KA, Marcoux VS, Ronksley PE, Ryerson CJ. Diagnostic yield and complications of transbronchial lung cryobiopsy for interstitial lung disease. A systematic review and metaanalysis. Ann Am Thorac Soc (2016) 13:1828–38. doi:10.1513/AnnalsATS.201606-461SR
51. Poletti V, Hetzel J. Transbronchial cryobiopsy in diffuse parenchymal lung disease: need for procedural standardization. Respiration (2015) 90(4):275–8. doi:10.1159/000439313
52. Rosas IO, Richards TJ, Konishi K, Zhang Y, Gibson K, Lokshin AE, et al. MMP1 and MMP7 as potential peripheral blood biomarkers in idiopathic pulmonary fibrosis. PLoS Med (2008) 5(4):e93. doi:10.1371/journal.pmed.0050093
53. White ES, Xia M, Murray S, Dyal R, Flaherty CM, Flaherty KR, et al. Plasma surfactant protein-D, matrix metalloproteinase-7, and osteopontin index distinguishes idiopathic pulmonary fibrosis from other idiopathic interstitial pneumonias. Am J Respir Crit Care Med (2016) 194(10):1242–51. doi:10.1164/rccm.201505-0862OC
54. Tzouvelekis A, Herazo-Maya JD, Slade M, Chu JH, Deiuliis G, Ryu C, et al. Validation of the prognostic value of MMP-7 in idiopathic pulmonary fibrosis. Respirology (2017) 22(3):486–93. doi:10.1111/resp.12920
55. Tzouvelekis A, Herazo-Maya J, Sakamoto K, Bouros D. Biomarkers in the evaluation and management of idiopathic pulmonary fibrosis. Curr Top Med Chem (2016) 16(14):1587–98. doi:10.2174/1568026616666150930120959
Keywords: idiopathic pulmonary fibrosis, diagnosis, challenges, pragmatic, clinical practice
Citation: Tzilas V, Tzouvelekis A, Chrysikos S, Papiris S and Bouros D (2017) Diagnosis of Idiopathic Pulmonary Fibrosis “Pragmatic Challenges in Clinical Practice”. Front. Med. 4:151. doi: 10.3389/fmed.2017.00151
Received: 11 July 2017; Accepted: 04 September 2017;
Published: 20 September 2017
Edited by:
Bethany B. Moore, University of Michigan, United StatesReviewed by:
Venerino Poletti, Aarhus University Hospital, DenmarkYe Cui, Brigham and Women’s Hospital, United States
Copyright: © 2017 Tzilas, Tzouvelekis, Chrysikos, Papiris and Bouros. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Demosthenes Bouros, debouros@med.uoa.gr, debouros@gmail.com
 Vasilios Tzilas
Vasilios Tzilas Argyris Tzouvelekis
Argyris Tzouvelekis Serafim Chrysikos3
Serafim Chrysikos3
 Demosthenes Bouros
Demosthenes Bouros