Autoimmune Hypothyroidism As a Predictor of Mortality in Chronic Hypersensitivity Pneumonitis
- 1Department of Medicine, Section of Pulmonary and Critical Care Medicine, University of Chicago, Chicago, IL, United States
- 2Department of Medicine, Division of Pulmonary, Critical Care and Sleep Medicine, University of California, Davis, Davis, CA, United States
- 3Department of Pathology, University of Chicago, Chicago, IL, United States
- 4Department of Radiology, University of Chicago, Chicago, IL, United States
Background: Chronic hypersensitivity pneumonitis (CHP) is a fibrotic parenchymal lung disease that occurs when inhalation of environmental antigens leads to immune dysregulation. Autoimmune features have recently been identified as potentially important among patients with CHP. However, the relationship between hypothyroidism (HT) and CHP is unknown. In this study, we investigate the prevalence and impact of HT among patients with CHP.
Methods: We conducted a retrospective, case–control analysis. We identified 121 patients at the University of Chicago Interstitial Lung Disease Center with a multidisciplinary diagnosis of CHP. These patients were matched 3:1 according to age, sex, and race to 363 control subjects with asthma from 2006 to 2015. We analyzed demographics, clinical characteristics, and survival between both groups and assessed the relationship of HT with CHP. Survival analysis was performed using Cox proportional hazards modeling.
Results: Patients with CHP had higher prevalence of HT (25.6%, n = 31) compared to controls (10.7%, n = 39; OR, 2.86; 95% CI, 1.62–4.99; P < 0.0001). Compared to CHP alone, patients with CHP/HT were more likely to be female (80.6 vs 51.1%, P = 0.004), have increased incidence of autoimmune disease (19.4 vs 3.3%, P = 0.009), antinuclear antibody seropositivity (80.6 vs 57.0%, P = 0.019), and higher TSH levels (4.0 vs 1.9 mIU/L, P < 0.0001). HT was a significant independent predictor of mortality among CHP patients with seropositive ANA (HR, 3.39; 95% CI, 1.31–8.80; P = 0.012).
Conclusion: HT is common in patients with CHP and may carry prognostic significance in patients with features of autoimmunity. Further research exploring common pathogenic pathways between autoimmune HT and CHP may illuminate the association of HT with survival.
Summary at a Glance
The prevalence of hypothyroidism and its prognostic impact in chronic hypersensitivity pneumonitis (CHP) are unknown. This study demonstrates a threefold increase in HT compared to the general population and shows that, in patients with CHP and antinuclear antibody (ANA) seropositivity, HT independently predicts mortality, providing insight into its influence on prognosis.
Introduction
Hypersensitivity pneumonitis (HP) is a diffuse interstitial lung disease (ILD) resulting from a dysregulated immune response to inhaled environmental antigens. Due to complex immunologic interactions, up to one-fifth of exposed individuals may develop HP (1–3). When HP results in pulmonary fibrosis, also known as chronic HP (CHP), survival is worse (4). The heterogeneous nature of these immune responses and variation in clinical course suggests, however, that multiple pathways may be involved in disease initiation and progression. Several underlying mechanisms for autoimmune diseases, such as shared genetic pathways, tissue microchimerism, and exposure to environmental antigens, have been implicated in the pathogenesis of CHP (5–8). We recently showed that features of autoimmunity are common among patients with CHP (9) and that HT, an autoimmune process characterized by autoantibody-mediated thyroid inflammation and destruction, is prevalent among patients with idiopathic pulmonary fibrosis (IPF) (10).
Hypothyroidism affects up to 9% of women and 1–2% of men in the general population (11, 12). HT occurs predominantly in developed nations as the sequelae of an underlying autoimmune process, though a minority of cases may be congenital, postpartum, or result of treatment with medications such as glucocorticoids (12–17). Systemic corticosteroid medications, used frequently in the treatment of patients with chronic HP, may also cause HT through suppression of thyroid-stimulating hormone (TSH) (18).
The prevalence and impact of HT in CHP is unknown. We hypothesized that (1) HT is more common in patients with CHP than in matched control subjects and (2) the increased prevalence of HT impacts survival in CHP and is associated with underlying autoimmune processes and not glucocorticoid therapy.
Materials and Methods
Study Population
A retrospective, case–control analysis was performed at the University of Chicago Hospitals, with approval of our Institutional Review Board (IRB16-1235). The University of Chicago ILD registry was screened for patients with CHP followed from 2006 to 2015. CHP cases were matched 3:1 according to age, sex, and race to individuals with a diagnosis of asthma followed at the University of Chicago during the same time period.
The diagnosis of CHP was determined through multidisciplinary review involving pulmonologists, thoracic radiologists, and pathologists according to ATS criteria as previously described (9, 19). Patients with other forms of ILD, including IPF and interstitial pneumonia with autoimmune features (IPAF), were excluded. Eligible controls were all individuals with asthma who had been evaluated at the University of Chicago from 2006 to 2015. Patients with an International Classification of Diseases, Ninth Revision code for asthma were systematically identified by the University of Chicago Center for Research Informatics and included in the matching algorithm. Using a random-number generator, we selected three control individuals per case, frequency matched by age, sex, and race/ethnicity. If race/ethnicity information was missing, the selected control was discarded and the next randomly selected eligible control with complete race/ethnicity information was chosen.
All data were extracted retrospectively from the electronic medical record using the initial clinic visit. These data included demographic information (age, race/ethnicity, sex), patient-reported medical/surgical history [HT, gastroesophageal reflux (GER), diabetes mellitus (DM), coronary artery disease (CAD), tobacco use, hyperthyroidism, thyroid ablation, thyroidectomy], environmental antigen exposure history (avian, mold, other, unknown), patient-reported medications [thyroid replacement, GER and statin therapy, lithium, amiodarone, systemic corticosteroids, radioactive iodine (RAI) history], physical examination findings [body mass index (BMI), clubbing, crackles], laboratory studies (ANA with staining pattern, rheumatoid factors, anticitrullinated protein antibody, myositis-specific antibodies, antineutrophil cytoplasmic antibody, anti-Ro/SSA antibody, anti-La/SSB antibody, anti-Scl-70 antibody, aldolase, TSH, and free thyroxine), diagnostic studies [high-resolution CT (HRCT) scan, surgical lung biopsy (SLB), pulmonary function testing, including FVC, FEV1, and percent predicted diffusion capacity of the lung for carbon monoxide (DLco)] and documented diagnosis of a defined autoimmune disease (Sjogren’s disease, scleroderma, systemic lupus erythematosus, idiopathic inflammatory myopathy, rheumatoid arthritis, and ulcerative colitis). Chronic glucocorticoid therapy was recorded when a patient reported a history of prednisone use equivalent to 5 mg daily or higher for 4 or more weeks (20). HT was recorded when a patient reported a history of HT, was using thyroid replacement therapy, and did not report a previous history of thyroidectomy or RAI ablation. Exploratory analysis was performed among the CHP cases to evaluate any associations between HT, serum TSH, glucocorticoid use, and underlying autoimmune processes. No patients reported the use of lithium, amiodarone, or interferon-gamma, which are known to alter thyroid function. No patients were immediately postpartum or endorsed a history of congenital HT.
Statistical Analysis
Continuous variables are reported as means with SD and are compared using a two-tailed Student’s t-test. Categorical variables are reported as counts and percentages and were compared using the chi-squared test or Fisher exact test, as appropriate. Conditional logistic regression was performed to compare the proportion of HT between cases and control subjects. Survival analysis was performed using univariate and multivariable Cox regression together with the unadjusted log-rank test and was plotted using the Kaplan–Meier survival estimator. Survival time was defined as time from CHP diagnosis to death, transplant, loss-to-follow-up, or end of study period. Survival time was censored on April 30th, 2015 or at the time a patient underwent lung transplant or was lost to follow-up. All statistical analyses were performed using Stata 14 (StataCorp LP).
Results
Of 161 individuals initially identified with a diagnosis of HP based on International Classification of Diseases, Ninth Revision code (Figure S1 in Supplementary Material), 121 were diagnosed with HP after multidisciplinary review, according to the 2013 American Thoracic Society/European Respiratory Society guidelines (19). Of those failing to meet the established guidelines for diagnosis of HP, three were missing relevant clinical information needed to verify the diagnosis: HRCT or SLB; 34 were given a diagnosis of an alternative ILD. Patients who exhibited clinical, radiographic, and pathologic features of HP but had antecedent history of chemotherapy (n = 3) were excluded from the analysis leaving 121 cases for the primary analysis. Of these 121 cases, 75 (62%) had undergone SLB, which demonstrated histopathologic features of HP, whereas the remainder demonstrated positive serum precipitins to specific antigens, lymphocytosis on bronchoalveolar lavage or compatible HRCT abnormalities that were consistent with their exposure history and clinical features of HP. All patients demonstrated HRCT or SLB features of fibrosis.
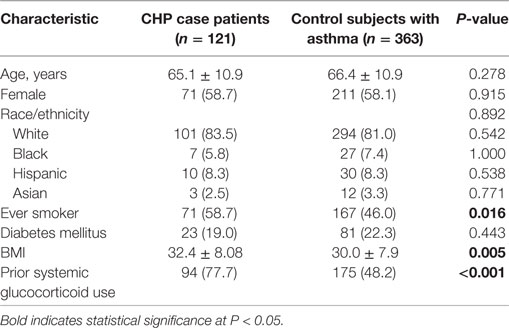
A comparison of baseline characteristics between cases and controls is shown in Table 1. Groups were similar with regard to age (65.1 vs 66.4 years, respectively), female sex (58.7 vs 58.1%), and non-Hispanic Caucasian race/ethnicity (83.5 vs 81.0%), as specified by the study design. Compared with controls, cases had a higher BMI (32.4 vs 30.0, P = 0.005), higher prevalence of ever-smokers (59 vs 46%, P = 0.016), and a higher frequency of documented glucocorticoid use (77.7 vs 48.2%, P < 0.001; respectively). There were no significant differences in the prevalence of DM between case patients and control subjects (19.0 vs 22.3%, respectively).
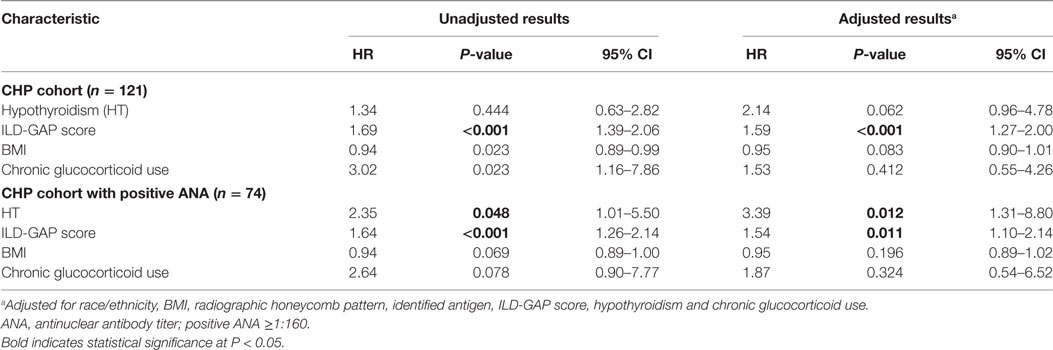
When comparing the proportion of patients with HT between cases and controls (Table 2), HT was identified in 31 (25.6%) cases and 39 (10.7%) controls (OR, 2.86; 95% CI, 1.62–4.99; P < 0.0001). HT was identified in 5% of male cases compared with 2% of male controls and 21% of female cases compared with 9% of female controls. After adjustment for variables previously associated with HP, HT, or both, including BMI (21, 22), smoking history (7, 23), DM (24, 25), and corticosteroid use (16, 17, 26), HT remained significantly associated with CHP (OR, 2.39; 95% CI, 1.36–4.20; P = 0.002).
We then stratified patients with CHP based on HT status (Table 3). The proportion of females in the CHP/HT subgroup was greater than that of the CHP subgroup (80.6 vs 51.1%, P = 0.004). Those with CHP/HT were also found to have significantly greater incidence of autoimmune disease (19.4 vs 3.3%, P = 0.009), ANA seropositivity (80.6 vs 57.0%, P = 0.019); rheumatoid factor/anticitrullinated protein antibody seropositivity (9.7 vs 1.1%, P = 0.021); >1 autoantibody seropositivity (19.4 vs 5.6%, P = 0.027); higher TSH levels (4.0 vs 1.9 mIU/L, P < 0.0001) and radiographic mosaic attenuation (96.8 vs 81.1%, P = 0.035) compared with those with CHP alone. No significant differences were observed between groups with respect to the following: age; race/ethnicity; BMI; crackles; clubbing; smoking history; GER; DM; the use of glucocorticoid therapy; FVC% predicted; DLCO% predicted; requirement for oxygen therapy, 6-min walk distance; radiographic ground glass opacities, traction bronchiectasis or honeycomb pattern; histopathologic presence of poorly formed granulomas, lymphoplasmacytic infiltration or germinal centers, UIP pattern; gender, age, physiology (GAP) stage (27); or lung transplant.
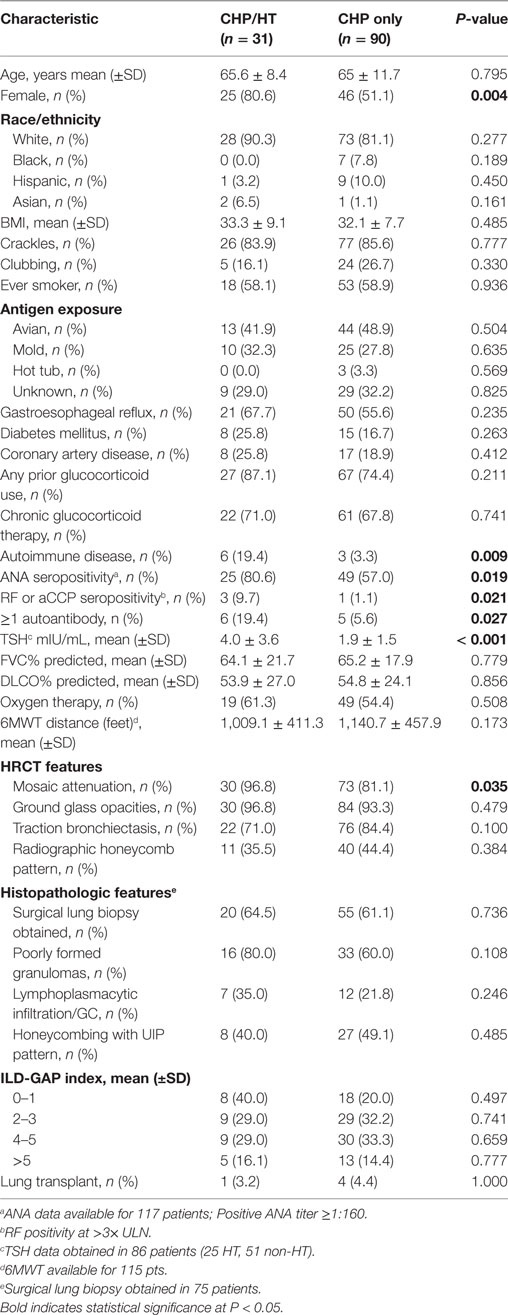
Table 3. Baseline characteristics of hypothyroid cohort among patients with hypersensitivity pneumonitis.
When sub-stratified based on chronic glucocorticoid therapy, the subgroup of CHP patients who had received chronic glucocorticoids had a higher white blood cell count (P = 0.006) (Figure 1A), similar BMI (P = 0.972) (Figure 1B), similar TSH (P = 0.328) (Figure 1C), and higher ILD-GAP score (P = 0.009) (Figure 1D). When comparing the proportion of patients who had received chronic glucocorticoid therapy among CHP patients with HT and CHP patients without HT, there was no significant association between HT and chronic glucocorticoid therapy in univariate analysis (OR, 1.16; 95% CI, 0.44–3.24; P = 0.741) or after multivariate adjustment (OR, 1.12; 95% CI, 0.45–2.79; P = 0.810) (Table S1 in Supplementary Material).
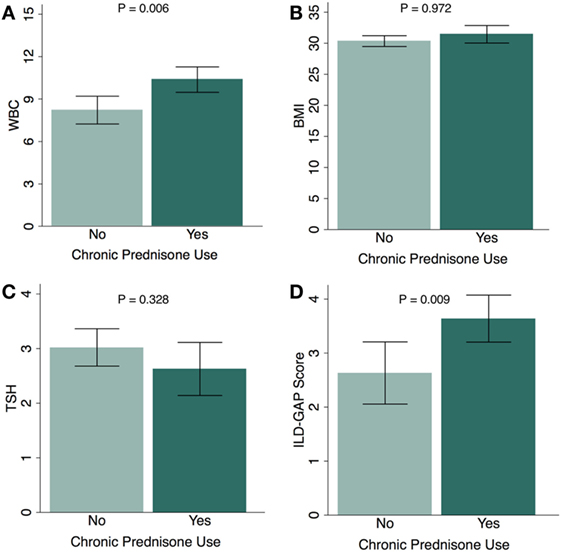
Figure 1. Chronic glucocorticoid use and physiologic parameters in chronic hypersensitivity pneumonitis (CHP) cohort. Relationship between chronic glucocorticoid therapy and (A) white blood cell count (WBC); (B) body mass index (BMI); (C) thyroid-stimulating hormone (TSH); and (D) ILD-GAP Score in patients with CHP. (Patients receiving chronic glucocorticoid therapy, N = 83; patients not receiving chronic glucocorticoid therapy, N = 38.) Exception for number of patients: WBC (n = 111), TSH (n = 86). Results are shown as mean ± SD.
Eighty-six patients had serum TSH levels available for analysis. There was no difference in TSH levels between this subgroup of CHP patients and control subjects (2.52 ± 2.47 vs 3.05 ± 2.78; P = 0.121) (Figure 2). TSH did not correlate with an increase in age (R = −0.036, P = 0.502) (Figure 2A), or BMI (R = 0.086, P = 0.115) (Figure 2B). However, analysis of the CHP cohort revealed a positive correlation of serum TSH levels with ANA titers (R = 0.2997; P = 0.0043) (Figure 2C). Lower TSH levels were associated with traction bronchiectasis on chest imaging (P = 0.04). TSH levels did not differ between patients who had received chronic glucocorticoid therapy and those who had not (P = 0.497) (Figure 2D).
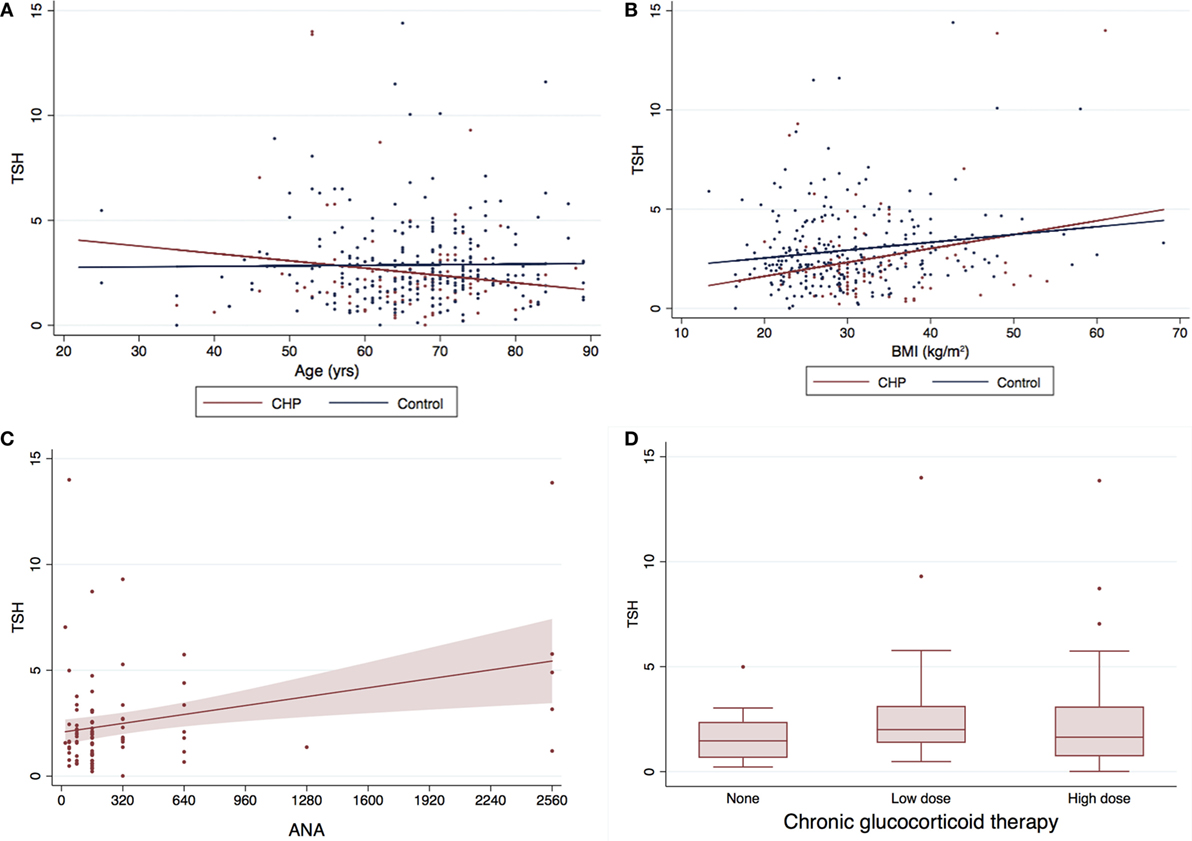
Figure 2. Serum TSH levels and baseline characteristics of CHP cohort*. The mean TSH for the CHP cohort was marginally lower (0.53 mIU/L) than the control population (2.52 ± 2.47 vs 3.05 ± 2.78; P = 0.121). There was no correlation between (A) TSH and age (R = −0.036, P = 0.502) or (B) TSH and body mass index (BMI) (R = 0.086, P = 0.115). Serum TSH levels (C) correlated positively with antinuclear antibody (ANA) titers (R = 0.2997; P = 0.0043), and (D) did not differ with/without glucocorticoid therapy (P = 0.497). Panel (C) only includes patients with CHP and no controls. *Two data points with TSH >20 included in the analysis were not depicted in the graph above for the purpose for clarity. TSH, thyroid-stimulating hormone; CHP, chronic hypersensitivity pneumonitis.
Although the presence of HT did not independently predict survival in the entire CHP cohort (HR, 2.14; 95% CI, 0.96–4.78; P = 0.062), survival analysis of the CHP cohort demonstrated significant interaction between HT and positive ANA (interaction term P-value; P = 0.024). When unadjusted survival analysis of CHP patients who had a positive ANA was performed, those with HT demonstrated significantly shorter survival compared with those without HT (log-rank test P = 0.04) (Figure 3). To identify predictors of mortality in the cohort of CHP patients who had a positive ANA, we performed univariate and multivariable Cox regression analysis (Table 4). Univariate analysis revealed HT to be a significant predictor of mortality [hazard ratio (HR), 2.35; 95% CI, 1.01–5.50; P = 0.048]. Each increase in GAP stage was also demonstrated to predict mortality (HR, 1.64; 95% CI, 1.26–2.14; P = 0.001). While the predictive value of BMI and chronic glucocorticoid therapy trended towards statistical significance (HR, 0.94; 95% CI, 0.89–1.00; P = 0.069 and HR, 2.64; 95% CI, 0.90–7.77; P = 0.078, respectively), other variables including radiographic honeycomb pattern of fibrosis (28, 29) and antigen identification (7) were not predictive of survival in univariate analysis. Inclusion of these variables in a multivariable model, along with BMI and race/ethnicity, demonstrated that HT remained significantly predictive of mortality (HR, 3.39; 95% CI, 1.31–8.80; P = 0.012), as did incremental change in the GAP stage (HR, 1.54; 95% CI, 1.10–2.14; P = 0.011).
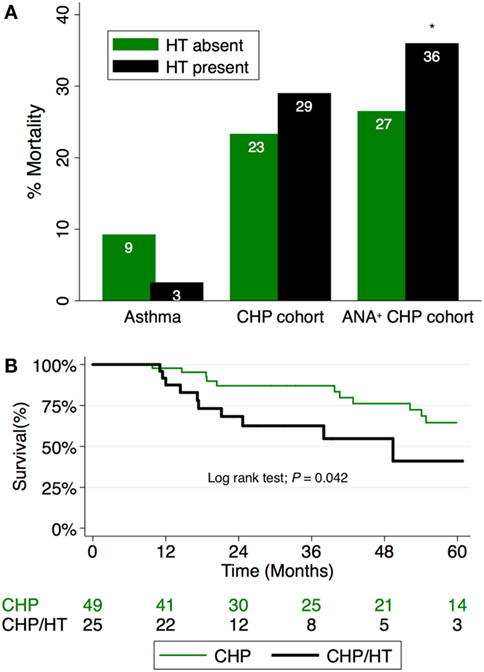
Figure 3. (A) Percentage mortality in CHP cohort compared to controls. Mortality associated with hypothyroidism (HT) in CHP is worsened in patients with positive antinuclear antibody (ANA) titers (interaction term P-value; P = 0.024). (B) Survival among patients with CHP and positive ANA stratified by HT status. Patients with combined HT and CHP demonstrate significantly reduced survival time compared with those with CHP alone (log-rank test; P = 0.042). CHP, chronic hypersensitivity pneumonitis; HT, hypothyroidism.
Discussion
We report, for the first time, an association between HT and CHP. Compared to the general population in which HT affects up to 9% of women and 2% of men, 21% of the women, and 5% of the men in our CHP cohort had HT. Our study showed that the proportion of CHP/HT cases also exceeded that of matched control subjects with asthma. We found that the presence of HT was associated with autoimmune serologies. We also show that in those subjects with CHP and a positive ANA, HT was an independent predictor of mortality.
Although specific reasons for HT in our CHP cohort cannot be determined in this retrospective analysis, several factors previously associated with autoimmune HT are prevalent in our CHP cohort. Exposure to environmentally inhaled antigens, a characteristic feature of CHP, has been associated with development of autoimmune HT (8, 30). The majority of our CHP cohort were smokers or had identifiable exposure to inhaled environmental antigens. In addition, X-chromosome inactivation has been described as an important contributor to the increased female risk for autoimmune HT and carries prognostic value in affected patients (30–32). In our study, an overwhelming majority of HT subjects were female. The increased prevalence of environmental exposure in our cohort supports postulations by other investigators that link differences in individual environmental exposure to gender differences in prevalence of CHP (28, 33, 34). In our study, the greater female predominance and a high rate of exposure to identifiable environmentally inhaled antigens could have contributed to the increased prevalence of HT in our CHP cohort.
Further, HT has been associated with several autoimmune diseases such as Sjogren syndrome, systemic lupus erythematosus, and rheumatoid arthritis (35–37). This is consistent with data from our cohort which demonstrates an increased prevalence of autoimmune diseases in the CHP subset with HT. This suggests that shared biological pathways may contribute to development of HT in CHP patients with autoimmune diseases. The female predominance in our CHP/HT subpopulation may also reflect the increased prevalence of autoimmune HT in women. As expected, those with CHP/HT demonstrated a significant increase in the incidence of autoimmune disease and serologies concurrent with an increase in their TSH levels.
In our study, the proportion of control subjects with HT was notably higher than that of the general population. Thyroid hormones are thought to influence the inflammatory component of asthma possibly through enhancing IgE production (38). A large population study by Goldacre et al. suggested a positive association of asthma with HT (39). Harrison et al. measured serum thyroxine values and specific airway conductance as an index of beta-adrenergic responsiveness in hypothyroid patients (40). They demonstrated an inverse relationship between airway beta-adrenergic responsiveness and the level of thyroid function. Hemminki et al. also demonstrated an increased risk for HT in obese individuals (21), which constituted a significant proportion of subjects with asthma in our control population. We explored this association in our control population but found that the proportion of HT did not differ among obese and non-obese subjects with asthma (data not shown).
Our findings add to an increasing body of recent evidence that links HT with greater susceptibility to lung injury through mechanisms that involve epithelial cell apoptosis and TGF-B signaling. We have previously shown that HT is common in patients with IPF, and independently predicted mortality (10). Alonso-Merino et al. demonstrated the ability of the thyroid hormone triiodothyronine to antagonize fibrotic processes in vivo through inhibition of TGF-β/SMAD-dependent transcriptional activation (41). In their study, they showed the potential therapeutic (anti-inflammatory and anti-fibrotic) effects of triiodothyronine in experimental models of ventilator-induced lung injury, skin, and hepatic fibrosis. Their results suggest that binding of triiodothyronine to its nuclear receptors could be beneficial in blocking progression of pulmonary fibrosis. Similarly, Barca-Mayo et al. showed that increase in lung deiodinase type-2, a critical mediator of thyroid hormone metabolism, protects against ventilator-induced lung injury in mouse models of functional HT (42). They found that treatment with triiodothyronine reversed the increased chemokine and cytokine inflammatory profiles within the lungs. Taken together, these findings might represent a plausible mechanistic explanation for the increased mortality observed in CHP/HT patients, and adequate repletion of the deficient thyroid hormones holds potential therapeutic appeal in decreasing progression of pulmonary fibrosis and mortality.
Thyroid transcription factor-1 (TTF-1) controls the expression of select genes in thyroid and lung tissue, and optimal levels are essential to maintain thyroid and lung function (43). Mutations in the gene encoding TTF-1, NKX2-1, have been associated with development of ILD and pulmonary fibrosis (44). Genetic factors resulting in immune dysregulation have also been associated with autoimmune HT, including polymorphisms in the genes for human leukocyte antigen (HLA) and cytotoxic T-lymphocyte antigen 4 (CTLA-4). HLA haplotypes, such as HLA-DRB1/3, HLA-DQA1, HLA-DQB1, and HLA-DPB1, are present in persons with autoimmune HT (45, 46). Recent studies demonstrate an increased prevalence of these same HLA gene polymorphisms in subjects with CHP from different genetic backgrounds (47, 48). Likewise, CTLA-4 has been implicated in the susceptibility to autoimmune HT, and may reduce inflammatory lung disease in murine models of CHP (49–52). Our findings support these studies, which implicate common genetic pathways in the pathogenesis of CHP and autoimmune HT.
Our study was limited by several factors. First, our findings represent an association but do not infer causality due to the retrospective design of our investigation. Second, it was not possible to biochemically confirm autoimmune HT using anti-TPO or anti-thyroglobulin in the entire cohort because the diagnosis had been made several years before referral to our institution. Thus, we used a previously published algorithmic approach to the diagnosis of HT in ILD patients (10). Additionally, as the high prevalence of ANA seropositivity in autoimmune HT is well described (53–56), we elected to use ANA seropositivity as a marker of autoimmune HT in our CHP/HT cohort. Third, we used data from electronic medical records containing patient-reported medical history and medications. Data were collected as a part of clinical care, and not specifically for this study.
Conclusion
We demonstrate that in patients with CHP, HT is a common finding and may have prognostic value in the subset of patients with a positive ANA. In a well-characterized cohort of subjects with CHP, HT was not associated with glucocorticoid use. As the role of the immune system is increasingly studied in the pathogenesis and progression of CHP, further research identifying common pathogenic pathways between autoimmune HT and CHP may elucidate the association of HT with survival.
Ethics Statement
Patient consent was obtained for enrollment and participation in the University of Chicago ILD registry and the study was approved by the University of Chicago Institutional Review Board (IRB16-1235).
Author Contributions
Conception and design—AA, JO, RV, IN, and MS; data acquisition—AA, JO, LC, SH, AH, SM, JC, RV, IN, and MS; data analysis and interpretation—AA, JO, RV, and MS; drafting of manuscript for important intellectual content—AA, JO, LC, SH, AH, SM, JC, RV, IN, and MS. All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to express our appreciation to the support staff of the University of Chicago Respiratory Clinical Research and the Interstitial Lung Disease Clinic, in particular Nancy Trojan, Catherine Brown, and Spring Holland. We also extend our gratitude to the patients with HP who made this research possible.
Funding
AA is supported by a National Institute of Health grant (T32 HL 07605). MS has received institutional funding for idiopathic pulmonary fibrosis research from Genentech, Gilead and MedImmune and she serves on a data monitoring committee for Boehringer Ingelheim.
Supplementary Material
The Supplementary Material for this article can be found online at http://www.frontiersin.org/article/10.3389/fmed.2017.00170/full#supplementary-material.
Abbreviations
BMI, body mass index; CAD, coronary artery disease; CHP, chronic HP; CTLA-4, cytotoxic T-lymphocyte antigen 4; DLco, diffusion capacity of the lung for carbon monoxide; DM, diabetes mellitus; GAP, gender age physiology; GER, gastroesophageal reflux; HRCT, high-resolution CT; HLA, human leukocyte antigen; HP, hypersensitivity pneumonitis; HT, hypothyroidism; ILD, interstitial lung disease; IPF, idiopathic pulmonary fibrosis; RAI, radioactive iodine; SLB, surgical lung biopsy; TSH, thyroid-stimulating hormone.
References
1. Rodriguez de Castro F, Carrillo T, Castillo R, Blanco C, Diaz F, Cuevas M. Relationships between characteristics of exposure to pigeon antigens. Clinical manifestations and humoral immune response. Chest (1993) 103:1059–63. doi:10.1378/chest.103.4.1059
2. Christensen LT, Schmidt CD, Robbins L. Pigeon breeders’ disease – a prevalence study and review. Clin Allergy (1975) 5:417–30. doi:10.1111/j.1365-2222.1975.tb01881.x
3. Hendrick DJ, Faux JA, Marshall R. Budgerigar-fancier’s lung: the commonest variety of allergic alveolitis in Britain. Br Med J (1978) 2:81–4. doi:10.1136/bmj.2.6130.81
4. Mooney JJ, Elicker BM, Urbania TH, Agarwal MR, Ryerson CJ, Nguyen ML, et al. Radiographic fibrosis score predicts survival in hypersensitivity pneumonitis. Chest (2013) 144:586–92. doi:10.1378/chest.12-2623
5. Bustos ML, Frias S, Ramos S, Estrada A, Arreola JL, Mendoza F, et al. Local and circulating microchimerism is associated with hypersensitivity pneumonitis. Am J Respir Crit Care Med (2007) 176:90–5. doi:10.1164/rccm.200608-1129OC
6. Newton CA, Batra K, Torrealba J, Kozlitina J, Glazer CS, Aravena C, et al. Telomere-related lung fibrosis is diagnostically heterogeneous but uniformly progressive. Eur Respir J (2016) 48:1710–20. doi:10.1183/13993003.00308-2016
7. Fernandez Perez ER, Swigris JJ, Forssen AV, Tourin O, Solomon JJ, Huie TJ, et al. Identifying an inciting antigen is associated with improved survival in patients with chronic hypersensitivity pneumonitis. Chest (2013) 144:1644–51. doi:10.1378/chest.12-2685
8. Brent GA. Environmental exposures and autoimmune thyroid disease. Thyroid (2010) 20:755–61. doi:10.1089/thy.2010.1636
9. Adegunsoye A, Oldham JM, Demchuk C, Montner S, Vij R, Strek ME. Predictors of survival in coexistent hypersensitivity pneumonitis with autoimmune features. Respir Med (2016) 114:53–60. doi:10.1016/j.rmed.2016.03.012
10. Oldham JM, Kumar D, Lee C, Patel SB, Takahashi-Manns S, Demchuk C, et al. Thyroid disease is prevalent and predicts survival in patients with idiopathic pulmonary fibrosis. Chest (2015) 148:692–700. doi:10.1378/chest.14-2714
11. Asvold BO, Vatten LJ, Bjoro T. Changes in the prevalence of hypothyroidism: the HUNT study in Norway. Eur J Endocrinol (2013) 169:613–20. doi:10.1530/EJE-13-0459
12. Vanderpump MP, Tunbridge WM, French JM, Appleton D, Bates D, Clark F, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham survey. Clin Endocrinol (1995) 43:55–68. doi:10.1111/j.1365-2265.1995.tb01894.x
13. Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, Mechanick JI, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract (2012) 18:988–1028. doi:10.4158/EP12280.GL
14. Sawin CT, Castelli WP, Hershman JM, McNamara P, Bacharach P. The aging thyroid. Thyroid deficiency in the Framingham study. Arch Intern Med (1985) 145:1386–8. doi:10.1001/archinte.1985.00360080056006
15. Haugen BR. Drugs that suppress TSH or cause central hypothyroidism. Best Pract Res Clin Endocrinol Metab (2009) 23:793–800. doi:10.1016/j.beem.2009.08.003
16. Jensen J, Nolan G, Jubiz W. The effect of prednisone on serum thyrotropin, thyroxine and triiodothyronine concentrations in hypothyroid patients. J Endocrinol Invest (1978) 1:171–3. doi:10.1007/BF03350367
17. Fatourechi V, Aniszewski JP, Fatourechi GZ, Atkinson EJ, Jacobsen SJ. Clinical features and outcome of subacute thyroiditis in an incidence cohort: Olmsted County, Minnesota, study. J Clin Endocrinol Metab (2003) 88:2100–5. doi:10.1210/jc.2002-021799
18. Brabant A, Brabant G, Schuermeyer T, Ranft U, Schmidt FW, Hesch RD, et al. The role of glucocorticoids in the regulation of thyrotropin. Acta Endocrinol (Copenh) (1989) 121:95–100.
19. Travis WD, Costabel U, Hansell DM, King TE Jr, Lynch DA, Nicholson AG, et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med (2013) 188:733–48. doi:10.1164/rccm.201308-1483ST
20. Recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis: 2001 update. American College of Rheumatology Ad Hoc Committee on Glucocorticoid-Induced Osteoporosis. Arthritis Rheum (2001) 44:1496–503. doi:10.1002/1529-0131(200107)44:7<1496::AID-ART271>3.0.CO;2-5
21. Hemminki K, Li X, Sundquist J, Sundquist K. Risk of asthma and autoimmune diseases and related conditions in patients hospitalized for obesity. Ann Med (2012) 44:289–95. doi:10.3109/07853890.2010.547515
22. Iacobellis G, Ribaudo MC, Zappaterreno A, Iannucci CV, Leonetti F. Relationship of thyroid function with body mass index, leptin, insulin sensitivity and adiponectin in euthyroid obese women. Clin Endocrinol (2005) 62:487–91. doi:10.1111/j.1365-2265.2005.02247.x
23. Nystrom E, Bengtsson C, Lapidus L, Petersen K, Lindstedt G. Smoking – a risk factor for hypothyroidism. J Endocrinol Invest (1993) 16:129–31. doi:10.1007/BF03347665
24. Benvenga S, Pintaudi B, Vita R, Di Vieste G, Di Benedetto A. Serum thyroid hormone autoantibodies in type 1 diabetes mellitus. J Clin Endocrinol Metab (2015) 100:1870–8. doi:10.1210/jc.2014-3950
25. Distiller LA, Polakow ES, Joffe BI. Type 2 diabetes mellitus and hypothyroidism: the possible influence of metformin therapy. Diabet Med (2014) 31:172–5. doi:10.1111/dme.12342
26. Wilber JF, Utiger RD. The effect of glucocorticoids on thyrotropin secretion. J Clin Invest (1969) 48:2096–103. doi:10.1172/JCI106176
27. Ryerson CJ, Vittinghoff E, Ley B, Lee JS, Mooney JJ, Jones KD, et al. Predicting survival across chronic interstitial lung disease: the ILD-GAP model. Chest (2014) 145:723–8. doi:10.1378/chest.13-1474
28. Gaxiola M, Buendia-Roldan I, Mejia M, Carrillo G, Estrada A, Navarro MC, et al. Morphologic diversity of chronic pigeon breeder’s disease: clinical features and survival. Respir Med (2011) 105:608–14. doi:10.1016/j.rmed.2010.11.026
29. Churg A, Sin DD, Everett D, Brown K, Cool C. Pathologic patterns and survival in chronic hypersensitivity pneumonitis. Am J Surg Pathol (2009) 33:1765–70. doi:10.1097/PAS.0b013e3181bb2538
30. Effraimidis G, Wiersinga WM. Mechanisms in endocrinology: autoimmune thyroid disease: old and new players. Eur J Endocrinol (2014) 170:R241–52. doi:10.1530/EJE-14-0047
31. Yin X, Latif R, Tomer Y, Davies TF. Thyroid epigenetics: X chromosome inactivation in patients with autoimmune thyroid disease. Ann N Y Acad Sci (2007) 1110:193–200. doi:10.1196/annals.1423.021
32. Ishido N, Inoue N, Watanabe M, Hidaka Y, Iwatani Y. The relationship between skewed X chromosome inactivation and the prognosis of Graves’ and Hashimoto’s diseases. Thyroid (2015) 25:256–61. doi:10.1089/thy.2014.0318
33. Kanerva L, Jolanki R, Toikkanen J. Frequencies of occupational allergic diseases and gender differences in Finland. Int Arch Occup Environ Health (1994) 66:111–6. doi:10.1007/BF00383366
34. Bourke SJ, Dalphin JC, Boyd G, McSharry C, Baldwin CI, Calvert JE. Hypersensitivity pneumonitis: current concepts. Eur Respir J Suppl (2001) 32:81s–92s.
35. Jara LJ, Navarro C, Brito-Zeron Mdel P, Garcia-Carrasco M, Escarcega RO, Ramos-Casals M. Thyroid disease in Sjogren’s syndrome. Clin Rheumatol (2007) 26:1601–6. doi:10.1007/s10067-007-0638-6
36. Markenson JA. Rheumatic manifestations of endocrine diseases. Curr Opin Rheumatol (2010) 22:64–71. doi:10.1097/BOR.0b013e328333dd38
37. Shiroky JB, Cohen M, Ballachey ML, Neville C. Thyroid dysfunction in rheumatoid arthritis: a controlled prospective survey. Ann Rheum Dis (1993) 52:454–6. doi:10.1136/ard.52.6.454
38. Jafarzadeh A, Poorgholami M, Izadi N, Nemati M, Rezayati M. Immunological and hematological changes in patients with hyperthyroidism or hypothyroidism. Clin Invest Med (2010) 33:E271–9. doi:10.25011/cim.v33i5.14352
39. Goldacre M, Kurina L, Yeates D, Seagroatt V, Gill L. Use of large medical databases to study associations between diseases. QJM (2000) 93:669–75. doi:10.1093/qjmed/93.10.669
40. Harrison RN, Tattersfield AE. Airway response to inhaled salbutamol in hyperthyroid and hypothyroid patients before and after treatment. Thorax (1984) 39:34–9. doi:10.1136/thx.39.1.34
41. Alonso-Merino E, Martin Orozco R, Ruiz-Llorente L, Martinez-Iglesias OA, Velasco-Martin JP, Montero-Pedrazuela A, et al. Thyroid hormones inhibit TGF-beta signaling and attenuate fibrotic responses. Proc Natl Acad Sci U S A (2016) 113:E3451–60. doi:10.1073/pnas.1506113113
42. Barca-Mayo O, Liao XH, DiCosmo C, Dumitrescu A, Moreno-Vinasco L, Wade MS, et al. Role of type 2 deiodinase in response to acute lung injury (ALI) in mice. Proc Natl Acad Sci U S A (2011) 108:E1321–9. doi:10.1073/pnas.1109926108
43. Boggaram V. Thyroid transcription factor-1 (TTF-1/Nkx2.1/TITF1) gene regulation in the lung. Clin Sci (Lond) (2009) 116:27–35. doi:10.1042/CS20080068
44. Hamvas A, Deterding RR, Wert SE, White FV, Dishop MK, Alfano DN, et al. Heterogeneous pulmonary phenotypes associated with mutations in the thyroid transcription factor gene NKX2-1. Chest (2013) 144:794–804. doi:10.1378/chest.12-2502
45. Hunt PJ, Marshall SE, Weetman AP, Bunce M, Bell JI, Wass JA, et al. Histocompatibility leucocyte antigens and closely linked immunomodulatory genes in autoimmune thyroid disease. Clin Endocrinol (2001) 55:491–9. doi:10.1046/j.1365-2265.2001.01356.x
46. Zeitlin AA, Heward JM, Newby PR, Carr-Smith JD, Franklyn JA, Gough SC, et al. Analysis of HLA class II genes in Hashimoto’s thyroiditis reveals differences compared to Graves’ disease. Genes Immun (2008) 9:358–63. doi:10.1038/gene.2008.26
47. Falfan-Valencia R, Camarena A, Pineda CL, Montano M, Juarez A, Buendia-Roldan I, et al. Genetic susceptibility to multicase hypersensitivity pneumonitis is associated with the TNF-238 GG genotype of the promoter region and HLA-DRB1*04 bearing HLA haplotypes. Respir Med (2014) 108:211–7. doi:10.1016/j.rmed.2013.11.004
48. Camarena A, Juarez A, Mejia M, Estrada A, Carrillo G, Falfan R, et al. Major histocompatibility complex and tumor necrosis factor-alpha polymorphisms in pigeon breeder’s disease. Am J Respir Crit Care Med (2001) 163:1528–33. doi:10.1164/ajrccm.163.7.2004023
49. Yanagawa T, Hidaka Y, Guimaraes V, Soliman M, DeGroot LJ. CTLA-4 gene polymorphism associated with Graves’ disease in a Caucasian population. J Clin Endocrinol Metab (1995) 80:41–5. doi:10.1210/jcem.80.1.7829637
50. Pastuszak-Lewandoska D, Sewerynek E, Domanska D, Gladys A, Skrzypczak R, Brzezianska E. CTLA-4 gene polymorphisms and their influence on predisposition to autoimmune thyroid diseases (Graves’ disease and Hashimoto’s thyroiditis). Arch Med Sci (2012) 8:415–21. doi:10.5114/aoms.2012.28593
51. Jimenez-Alvarez L, Arreola JL, Ramirez-Martinez G, Ortiz-Quintero B, Gaxiola M, Reynoso-Robles R, et al. The effect of CTLA-4Ig, a CD28/B7 antagonist, on the lung inflammation and T cell subset profile during murine hypersensitivity pneumonitis. Exp Mol Pathol (2011) 91:718–22. doi:10.1016/j.yexmp.2011.09.010
52. Israel-Assayag E, Fournier M, Cormier Y. Blockade of T cell costimulation by CTLA4-Ig inhibits lung inflammation in murine hypersensitivity pneumonitis. J Immunol (1999) 163:6794–9.
53. Segni M, Pucarelli I, Truglia S, Turriziani I, Serafinelli C, Conti F. High prevalence of antinuclear antibodies in children with thyroid autoimmunity. J Immunol Res (2014) 2014:150239. doi:10.1155/2014/150239
54. Tektonidou MG, Anapliotou M, Vlachoyiannopoulos P, Moutsopoulos HM. Presence of systemic autoimmune disorders in patients with autoimmune thyroid diseases. Ann Rheum Dis (2004) 63:1159–61. doi:10.1136/ard.2004.022624
55. Torok KS, Arkachaisri T. Autoimmune thyroiditis in antinuclear antibody positive children without rheumatologic disease. Pediatr Rheumatol Online J (2010) 8:15. doi:10.1186/1546-0096-8-15
Keywords: autoimmunity, hypothyroidism, hypersensitivity pneumonitis, extrinsic allergic alveolitis, pulmonary fibrosis
Citation: Adegunsoye A, Oldham JM, Husain AN, Chen L, Hsu S, Montner S, Chung JH, Vij R, Noth I and Strek ME (2017) Autoimmune Hypothyroidism As a Predictor of Mortality in Chronic Hypersensitivity Pneumonitis. Front. Med. 4:170. doi: 10.3389/fmed.2017.00170
Received: 03 August 2017; Accepted: 26 September 2017;
Published: 16 October 2017
Edited by:
Argyrios Tzouvelekis, Alexander Fleming Biomedical Sciences Research Center, GreeceReviewed by:
Gabriela Leuschner, Ludwig-Maximilians-Universität München, GermanyEffrosyni D. Manali, National and Kapodistrian University of Athens, Greece
Copyright: © 2017 Adegunsoye, Oldham, Husain, Chen, Hsu, Montner, Chung, Vij, Noth and Strek. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ayodeji Adegunsoye, deji@uchicago.edu
 Ayodeji Adegunsoye
Ayodeji Adegunsoye Justin M. Oldham
Justin M. Oldham Aliya N. Husain3
Aliya N. Husain3
 Imre Noth
Imre Noth