Eosinophilic Gastrointestinal Disorders Pathology
- 1Division of Pathology and Laboratory Medicine, Department of Pediatrics, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, United States
- 2Department of Pathology and Laboratory Medicine, University of Cincinnati, Cincinnati, OH, United States
- 3Department of Pathology, Children’s Hospital Colorado, Aurora, CO, United States
- 4Department of Pathology, University of Colorado, Denver, CO, United States
- 5Department of Pathology, Feinberg School of Medicine, Northwestern University, Chicago, IL, United States
Eosinophilic gastrointestinal disorders (EGID) are characterized pathologically by excess eosinophils in mucosal biopsies of one or multiple sites in the gastrointestinal (GI) tract, simultaneously or sequentially. Eosinophilic esophagitis (EoE) is the best characterized EGID, and in most patients it is an abnormal immune-mediated response to food antigens. Current recommendations for diagnosis include signs and symptoms of esophageal dysfunction that do not respond to proton-pump inhibitor therapy, and esophageal biopsies that exhibit at least 15 intraepithelial eosinophils in at least one high power field (HPF). Therapy consists of swallowed glucocorticoids or dietary elimination. Eosinophilic gastritis (EG) is the second most common form of EGID, but like all forms of EGID except EoE consensus recommendations for either clinical or pathological diagnosis do not exist. EG may be associated clinically with peripheral blood eosinophilia, hypoalbuminemia, and anemia, and pathologically with marked expansion of lamina propria by dense eosinophilic infiltrates. Eosinophilic enteritis (EE) may be subdivided into eosinophilic duodenitis, eosinophilic jejunitis, and eosinophilic ileitis. Most investigators believe that EE rarely, if ever, exists as a solitary form of EGID and is encountered only in patients who have at least one other affected portion of the GI tract. Eosinophilic colitis (EC) is perhaps the most enigmatic EGID. Distinction of EC from inflammatory bowel disease may be problematic especially in children. Multiple possible etiologies for EGID include hypereosinophilic syndrome, drug reactions, etc. Currently, the only etiology that can be identified histologically is parasitic infestation, if a portion of an invasive parasite is found in mucosal biopsies. This review will provide guidelines for the pathologic diagnosis of the various forms of EGID.
Introduction
In the mid-twentieth century, excess eosinophils in the gastrointestinal (GI) tract were correlated with a multitude of symptoms, based on the examination of resected bowel segments. Increased density of eosinophils in mucosa was found in resected bowels from patients who manifested anemia, hypoproteinemia, and diarrhea, and increased density of eosinophils in the muscularis propria was seen in resected specimens from patients whose major clinical manifestation was bowel obstruction (1). The development of safe and flexible endoscopes resulted in fewer surgical procedures, and therefore resected bowel segments, and greater reliance on mucosal biopsies for diagnosis of and monitoring response to therapy for GI diseases. Currently, eosinophilic gastrointestinal disorders (EGID) are defined pathologically, virtually, and exclusively by endoscopically obtained mucosal biopsies (2, 3) necessitating greater understanding of the role of eosinophils in GI mucosal health and disease (4, 5). Pathologic confirmation of eosinophilic inflammation confined to the muscularis propria can be accomplished currently by laparoscopic mural bowel biopsies that are guided by imaging studies showing bowel wall thickening. There may also be subserosal dense eosinophil infiltrates that are associated with ascites (1, 6), and in those cases large numbers of eosinophils are found in the ascitic fluid.
Eosinophilic gastrointestinal disorders are subclassified according to the affected site(s) as eosinophilic esophagitis (EoE), eosinophilic gastritis (EG), eosinophilic enteritis (EE), and eosinophilic colitis (EC). Eosinophils are normally found in the mucosa of all parts of the GI tract except the esophagus, but there are few studies that quantify eosinophils in normal GI mucosa complicating the ability to recognize pathologic numbers of eosinophils (7). Consensus recommendations for diagnosis of EGID currently exist only for EoE.
Eosinophilic Esophagitis
In the late-twentieth century, pediatric and adult patients who had abundant eosinophils in esophageal mucosal biopsies and who responded clinically and histologically to dietary restrictions were described (8–10). Characteristic esophageal endoscopic findings were reported in such patients (11). Case reports and small series of affected patients appeared increasingly in the literature. Subsequent retrospective studies of archived pathology slides identified esophageal biopsies, displaying numerous intraepithelial eosinophils in files from the 1980s (12–14). Some of those patients were later diagnosed with EoE, and patients who had as few as 5 eosinophils/HPF were statistically more likely to have signs and symptoms of esophageal dysfunction years later compared to patients whose esophageal biopsies had not displayed intraepithelial eosinophils in the remote past (15).
In 2007, the first set of recommendations for EoE diagnosis and therapy was published (16), and several have subsequently appeared (17–19). All guidelines or recommendations cite a peak eosinophil count of ≥15 eosinophils in at least one high power field (HPF) in an esophageal biopsy from at least one site in the esophagus (distal, mid, or proximal) as the pathologic criterion for diagnosis. All recognize that other pathologic changes are found in EoE biopsies. Recently, an EoE histology scoring system (EoEHSS) was developed that scores eight pathologic features including eosinophil density, but also pathologic features whose definition does not include eosinophils (20). Eosinophil inflammation, basal zone hyperplasia, eosinophil abscess, eosinophil surface layering, dilated intercellular spaces, surface epithelial alteration, dyskeratotic epithelial cells, and lamina propria fibrosis are evaluated in the EoEHSS (Figures 1 and 2; Table 1). The features are scored separately for severity of change (grade) and for the amount of tissue that is affected by each feature (stage). The EoEHSS scores better identified treated compared to untreated subjects’ biopsies than did peak eosinophil count, indicating the importance of evaluating more than eosinophil density in esophageal biopsies obtained to diagnose or monitor EoE, and also indicating the importance of determining the amount of tissue damage in addition to the degree of damage. A systematic method to evaluate the myriad endoscopic features of EoE has also been developed (21).
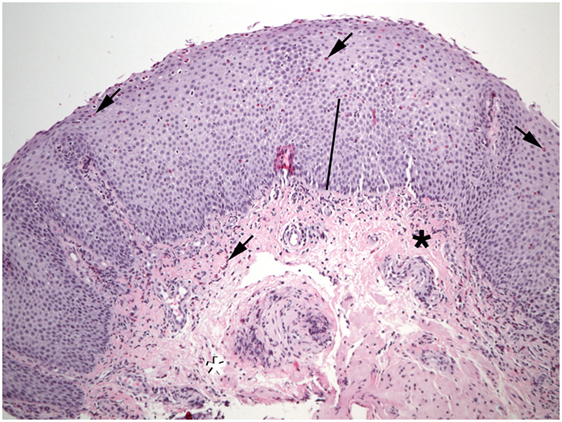
Figure 1. Numerous eosinophils (arrows) are found in the epithelium of this esophageal biopsy. The basal zone is markedly expanded (bar). Lamina propria fibers appear thickened near the epithelium (black asterisk), but not at the deep margin (white asterisk). Eosinophils are also present in the lamina propria (shaded arrows).
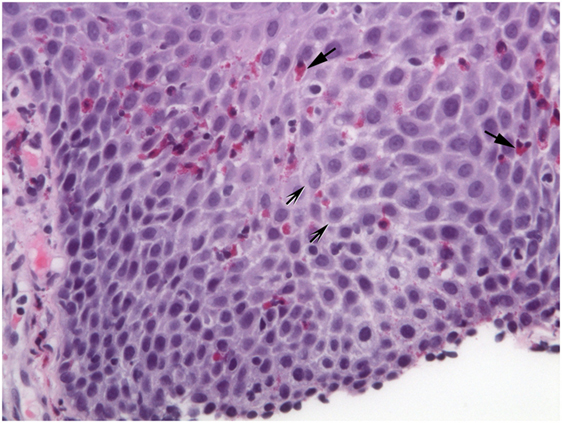
Figure 2. Extracellular eosinophil granules are seen (arrows). Intercellular bridges (shaded arrows) are visible in the dilated intercellular spaces.
A whole-genome messenger RNA esophageal expression analysis identified a unique EoE transcriptome and eotaxin-3 was the most upregulated gene (22). A diagnostic panel derived from the results of the original microarray study and consisting of 96 genes distinguishes EoE from non-EoE biopsies and can be used on paraffin-embedded tissue samples (23).
Prior to treatment, children who have EoE commonly experience vomiting and poor weight gain or weight loss, but adolescents and adults commonly experience dysphagia that may include food impaction (24). Therapy commonly consists of swallowed glucocorticoids (25–30) and elimination diet (31, 32). Response to dietary antigen-removal suggests that Th2 immunity is important in EoE pathogenesis; in fact, IL-13 and IL-5 levels are increased in EoE biopsies (33), and monoclonal antibodies to each of those cytokines reduce esophageal inflammation (34–38). Proton pump inhibitors (PPI) were used to distinguish EoE patients from those who had gastroesophageal reflux disease (GERD): clinical response to a PPI was considered not consistent with EoE and patients were believed to have GERD (16). However, a group of patients who respond initially to PPI, but subsequently may become refractory to PPI emerged and is believed to be a phenotype of EoE (39). Their pre-PPI biopsies are indistinguishable from patients who do not respond to PPI therapy and the genotype identified in their biopsies is very similar to nonresponders (40).
Eosinophilic esophagitis has increased in both prevalence and incidence (41–43) and is currently an important cause for food bolus impaction (44) and esophagitis (41, 45). Care of afflicted patients in the U.S. is estimated to consume approximately one billion dollars annually (46).
Eosinophilic Gastritis
In contrast to the esophagus, eosinophils are normally present in gastric mucosa, but in lower concentrations than in the small and large bowel (47–49). The criteria for diagnosing EG histologically include ≥30/HPF in ≥5 HPF and ≥70/HPF in ≥3 HPF (49, 50). Common features of EG biopsies are eosinophil sheets in expanded lamina propria, excess intraepithelial eosinophils, eosinophil cryptitis/abscess, and eosinophils in the muscularis mucosa and submucosa (Figure 3). Mast cells and FOXP3-positive lymphocytes are more abundant in EG biopsies compared to controls (51).
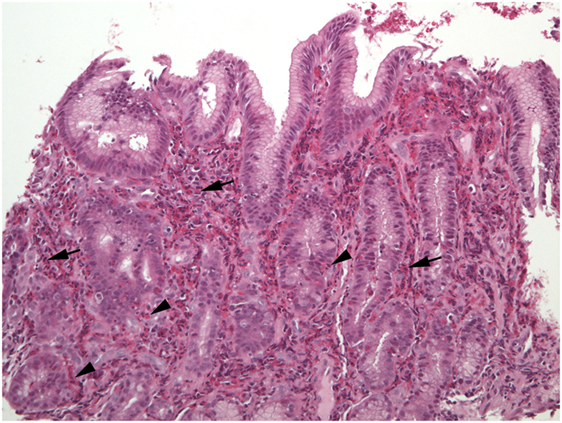
Figure 3. The lamina propria of this section of gastric mucosa is almost entirely occupied by sheets of eosinophils (arrows). Numerous intraepithelial eosinophils are found in gland epithelium (arrowheads).
An EG transcriptome overlaps minimally with the EoE transcriptome (52). However, cadherin 26 (CDH26) is the most upregulated gene in EG and is also among the most upregulated genes in EoE. CDH26 is expressed by esophageal and gastric epithelial cells in EoE and EG respectively, binds to α4 and αE integrins, and regulates leukocyte adhesion and activation. Importantly, CDH26 inhibits CD4+ T-cells in vitro, suggesting a role as a downregulator of inflammation in EGID (52).
Clinical features of EG include epigastric pain, peripheral blood eosinophilia, anemia, and hypoalbuminemia (49–51). Endoscopic abnormalities include nodular mucosa, erythema, and ulcers/erosions, but the mucosa may appear normal (49–51). EG may occur in isolation, or may be associated with excess eosinophil infiltrates in the rest of the GI tract, especially the esophagus (50, 51), either simultaneously or sequentially. Antigen restriction successfully reduces symptoms and tissue eosinophilia in some pediatric EG patients (50).
Eosinophilic Enteritis
Excess eosinophils in the small intestine could be considered a multiple of the maximum count found in normal biopsies, such as 2 × 26/HPF or 52/HPF in duodenal mucosa, and 2 × 28/HPF or 56/HPF in ileum (7). Excess eosinophils restricted to the small intestine appear to be exceedingly rare, i.e., small bowel mucosal eosinophilia appears to be commonly, perhaps exclusively, associated with excess eosinophil density in the mucosa of other parts of the GI tract. Patients who report dyspepsia and do not have ulcers have increased numbers of duodenal mucosal eosinophils compared to patients who do not report dyspepsia (53). Increased numbers of eosinophils in the lamina propria, increased intraepithelial eosinophils compared to normal counts (48), blunt villi (54), and eosinophils in the muscularis mucosa and submucosa may be found in EE (Figures 4–6). Parts of invasive helminths may be found in small bowel mucosal biopsies permitting identification of a specific cause for excess mucosal eosinophils (55).
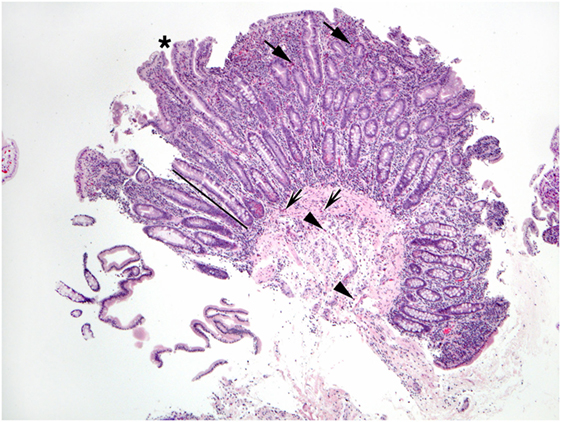
Figure 4. This duodenal biopsy shows few preserved short villi (asterisk), elongated crypts (bar), and numerous eosinophils in the lamina propria (arrows), muscularis mucosa (shaded arrows), and submucosa (arrowheads).
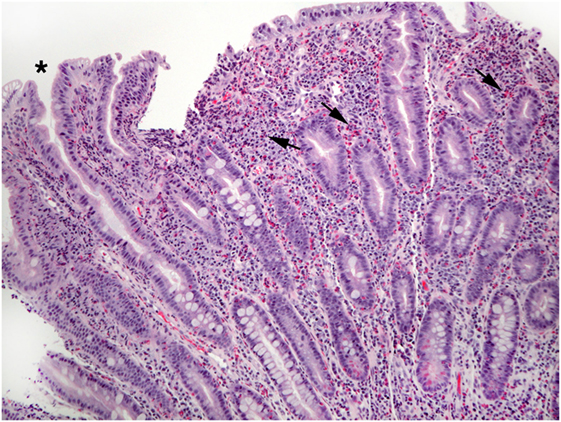
Figure 5. This view of Figure 4 illustrates blunt villi (asterisk) and confirms numerous lamina propria eosinophils (arrows).
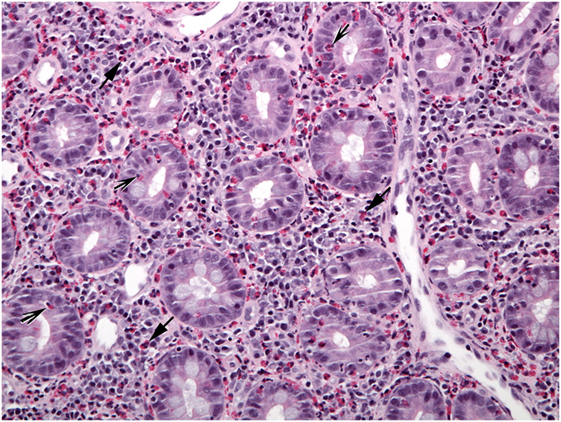
Figure 6. A different biopsy shows numerous eosinophils in duodenal lamina propria (arrows) and crypt epithelium (shaded arrows).
Eosinophilic Colitis
Eosinophils are normally present in colon mucosa and are most abundant in the right colon of both children (47, 48, 56) and adults (57). Therefore, using a single threshold value to identify increased eosinophil density is less accurate and potentially misleading compared to applying threshold values appropriate for each colon site (right, transverse, left, rectosigmoid) to properly labeled and separately submitted colon mucosal biopsies. Excess eosinophils could be considered a multiple of the peak count/HPF in normal biopsies, including 2 × 50/HPF or 100/HPF in cecum and ascending colon, 2 × 42/HPF or 84/HPF in transverse and descending colon, and 2 × 32/HPF or 64/HPF in rectosigmoid mucosa (7). Histologic features in colon biopsies showing increased eosinophil density include eosinophil cryptitis/crypt abscesses, crypt architectural abnormalities, increased intraepithelial eosinophils, and eosinophils in muscularis mucosa and submucosa (Figures 7 and 8) (57–63).
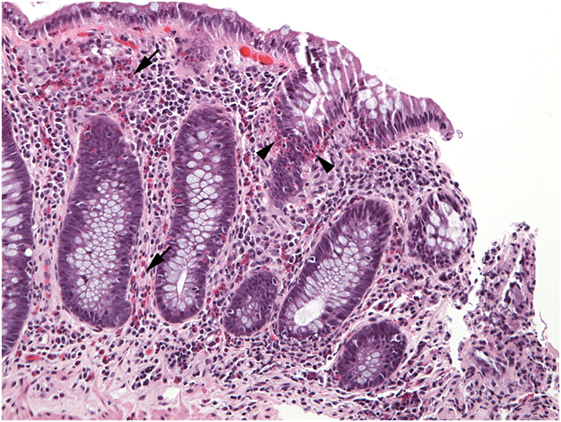
Figure 7. Numerous eosinophils populate the lamina propria (arrows) in this well-oriented section of colonic mucosa and also invade crypt epithelium (arrowheads).
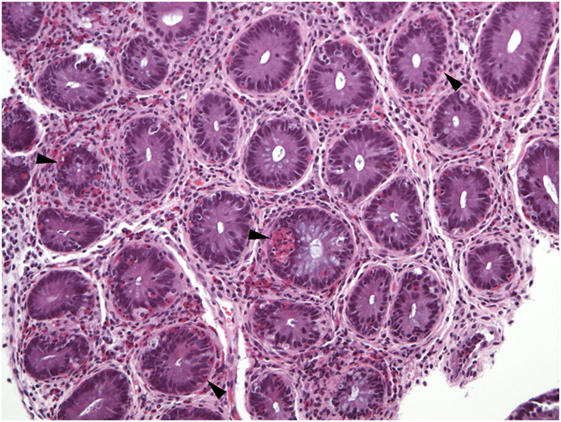
Figure 8. Virtually all crypts in this field of a colon biopsy display increased numbers of intraepithelial eosinophils (arrowheads).
The prevalence of EC is difficult to ascertain, partly, because guidelines for clinical or pathologic diagnosis do not exist. Nevertheless, a reasonable approach is that EC should be a clinicopathologic diagnosis, akin to EoE, requiring both symptoms referable to colonic dysfunction and colon biopsies showing excess eosinophils. Colon biopsies that displayed eosinophil density greater than normal for site plus six SDs from 194 patients were identified in a pathology database of 1.2 million patients (prevalence 1:6,000) (57). Most of those patients had symptoms, mainly diarrhea and abdominal pain, but approximately one-third were asymptomatic. Reported endoscopic abnormalities included erythema, erosions, whitish elevated lesions, pale granular mucosa, and aphthous ulcers.
Several studies have documented that excess eosinophils may be found in mucosal biopsies from patients who have IBD (57, 61, 64, 65). Indeed eosinophils may be more numerous in biopsies from children who have IBD compared to biopsies from children with allergic conditions (66), and elevated eotaxin-1 levels are reported in rectosigmoid biopsies from children with ulcerative colitis (64). The presence of acute inflammatory cells in colon biopsies showing chronic changes and excess eosinophils, especially those lacking sheets of eosinophils, should raise suspicion for IBD with excess eosinophils as the correct diagnosis. More abundant co-localization of IgE deposits with tryptase deposits in perineural locations may distinguish EC biopsies from biopsies of patients who have IBD with excess eosinophils (67).
The role of eosinophils in IBD is not clear. Recently, however, increased mucosal expression of genes that mediate type 2 and type 17 immune responses were shown to distinguish UC at baseline from colon-only Crohn disease at diagnosis, and high IL-13 expression was found in patients who subsequently exhibited improved clinical outcome (68).
Increased numbers of eosinophils may be found in colon biopsies of immunosuppressed patients, especially those receiving tacrolimus, who have had an organ transplant (69–71) which may resolve with food restriction.
Few studies quantify mast cells in the colon (56, 72), but colonic biopsies that show apparently increased numbers of mast cells, either as part of mastocytic enterocolitis (73, 74) or as part of systemic mastocytosis (75, 76), also show increased numbers of eosinophils.
Allergic colitis of infancy has been diagnosed if >20 eosinophils/HPF are found in rectal biopsies (77) which may be in a patchy distribution (78), which resolves clinically following removal of the offending antigens, typically cow’s milk, from an affected infant’s diet. Since this condition is so easily, and apparently permanently, treated by withdrawal of a single food substance from the diet, classification as an EGID is considered inappropriate by some experts.
The differential diagnosis for increased eosinophil density in colon mucosa is more extensive than discussed above, and includes parasitic infections, hypereosinophilic syndrome, etc. Primary EC is a diagnosis made only after all known causes for increased mucosal colon eosinophils have been eliminated (58) (Table 2).
Eosinophilic Gastroenteritis (EGE)
This term is used in multiple ways and unfortunately may indicate excess eosinophils in one or more than one part of the GI tract. Use of site-specific terminology, such as EG for eosinophilia restricted to the stomach, would help to bring greater clarity than using a term that is less specific.
In children with allergic EGE defined as excess eosinophils in either gastric or duodenal mucosa, significantly greater numbers of mast cells were found in duodenal but not gastric biopsies from patients who had associated protein-losing enteropathy compared to those who did not (79). All patients responded well to amino-acid-based formula, but food hypersensitivities did not completely resolve.
Future Directions
Many aspects of EGID diagnosis, pathogenesis, therapy, etc., remain to be determined. Recently, the NIH funded the Consortium of Eosinophilic Gastrointestinal Researchers (CEGIR) to facilitate studies of these rare diseases. CEGIR conducts observational and interventional studies of EGID subjects that include correlative studies of clinical, pathological, molecular, genetic, and microbiomic components of these diseases. These studies will hopefully yield basic science and clinical knowledge that will lead to novel and effective therapies.
Author Contributions
MC, KC, and G-YY: substantial contribution to conception and design of work; drafting and revising for intellectual content; final approval of version to be published; and agreement to be accountable for all aspects of the work.
Conflict of Interest Statement
MC has received funding for central pathology review in clinical trials to treat eosinophilic esophagitis from Meritage, Shire, Receptos, and Regeneron. The remaining coauthors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This work was supported by the Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR, U54 AI117804), which is part of the Rare Disease Clinical Research Network (RDCRN), an initiative of the Office of Rare Disease Research (ORDR), NCATS, and is funded through collaboration between NCATS, NIAID, and NIDDK. It was also supported by R01 AI124355, the Food Allergy Research and Initiative (FARE), the Campaign Urging Research for Eosinophilic Disease (CURED), and the Buckeye Foundation.
References
1. Klein NC, Hargrove RL, Sleisenger MH, Jeffries GH. Eosinophilic gastroenteritis. Medicine (1970) 49:299–319. doi:10.1097/00005792-197007000-00003
2. Rothenberg ME, Mishra A, Brandt EB, Hogan SP. Gastrointestinal eosinophils in health and disease. Adv Immunol (2001) 78:291–328. doi:10.1016/S0065-2776(01)78007-8
3. Rothenberg ME. Eosinophilic gastrointestinal disorders (EGID). J Allergy Clin Immunol (2004) 113(1):11–28. doi:10.1016/j.jaci.2003.10.047
4. Travers J, Rothenberg ME. Eosinophils in mucosal immune responses. Mucosal Immunol (2015) 8(3):464–75. doi:10.1038/mi.2015.2
5. Jung Y, Rothenberg ME. Roles and regulation of gastrointestinal eosinophils in immunity and disease. J Immunol (2014) 193(3):999–1005. doi:10.4049/jimmunol.1400413
6. Talley NJ, Shorter RJ, Phillips SF, Zinsmeister AR. Eosinophilic gastroenteritis: a clinicopathological study of patients with disease of the mucosa, muscle layer and subserosal tissues. Gut (1990) 31:54–8. doi:10.1136/gut.31.1.54
7. Collins MH. Histopathologic features of eosinophilic esophagitis and eosinophilic gastrointestinal diseases. Gastroenterol Clin North Am (2014) 43:257–68. doi:10.1016/j.gtc.2014.02.007
8. Attwood SEA, Smyrk TC, Demeester TR, Jones JB. Esophageal eosinophilia with dysphagia: a distinct clinicopathologic syndrome. Dig Dis Sci (1993) 38:109–16. doi:10.1007/BF01296781
9. Straumann A, Spichtin HP, Bernoulli R, Loosli J, Voegtlin J. Idiopathic eosinophilic esophagitis: a frequently overlooked disease with typical clinical aspects and discrete endoscopic findings (in German with English abstract). Schweiz Med Wochenschr (1994) 124:1419–29.
10. Kelly KJ, Lazenby AJ, Rowe PC, Yardley JH, Perman JA, Sampson HA. Eosinophilic esophagitis attributed to gastroesophageal reflux: improvement with an amino-acid based formula. Gastroenterology (1995) 109:1503–12. doi:10.1016/0016-5085(95)90637-1
11. Gupta SK, Fitzgerald JF, Chong SKF, Croffie JM, Collins MH. Vertical lines in the distal esophageal mucosa (VLEM): a true endoscopic manifestation of esophagitis in children? Gastrointest Endosc (1997) 45:485–9. doi:10.1016/S0016-5107(97)70178-0
12. Vanderheyden AD, Petras RE, DeYoung BR, Mitros FA. Emerging eosinophilic (allergic) esophagitis: increased incidence or increased recognition? Arch Pathol Lab Med (2007) 131:777–9. doi:10.1043/1543-2165(2007)131[777:EEAEII]2.0.CO;2
13. Whitney-Miller CL, Katzka D, Furth EE. Eosinophilic esophagitis: a retrospective review of esophageal biopsy specimens from 1992 to 2004 at an adult academic medical center. Am J Clin Pathol (2009) 131:788–92. doi:10.1309/AJCPOMPXJFP7EB4P
14. DeBrosse CW, Collins MH, Buckmeier Butz BK, Allen CL, King EC, Assa’ad AH, et al. Identification, epidemiology, and chronicity of pediatric esophageal eosinophilia, 1982–1999. J Allergy Clin Immunol (2010) 126:112–9. doi:10.1016/j.jaci.2010.05.027
15. DeBrosse CW, Franciosi JP, King EC, Butz BK, Greenberg AB, Collins MH, et al. Long-term outcomes in pediatric-onset esophageal eosinophilia. J Allergy Clin Immunol (2011) 128:132–8. doi:10.1016/j.jaci.2011.05.006
16. Furuta GT, Liacouras CA, Collins MH, Gupta SK, Justinich C, Putnam PE, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology (2007) 133:1342–63. doi:10.1053/j.gastro.2007.08.017
17. Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol (2011) 128:3–20. doi:10.1016/j.jaci.2011.02.040
18. Dellon ES, Gonsalves N, Hirano H, Furuta GT, Liacouras CA, Katzka DA. ACG clinical guideline: evidence-based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis. Am J Gastroenterol (2013) 108:679–92. doi:10.1038/ajg.2013.71
19. Lucendo AJ, Molina-Infante J, Arias Á, von Arnim U, Bredenoord AJ, Bussmann C, et al. Guidelines on eosinophilic esophagitis: evidence-based statements and recommendations for diagnosis and management in children and adults. United European Gastroenterol J (2017) 5(3):335–58. doi:10.1177/2050640616689525
20. Collins MH, Martin LJ, Alexander ES, Boyd JT, Sheridan R, He H, et al. Newly developed and validated eosinophilic esophagitis histology scoring system and evidence that it outperforms peak eosinophil count for disease diagnosis and monitoring. Dis Esophagus (2016) 30(3):1–8. doi:10.1111/dote.12470
21. Hirano I, Moy N, Heckman MG, Thomas CS, Gonsalves N, Achem SR. Endoscopic assessment of oesophageal features of eosinophilic esophagitis: validation of a novel classification and grading system. Gut (2013) 62:489–95. doi:10.1136/gutjnl-2011-301817
22. Blanchard C, Wang N, Stringer KF, Mishra A, Fulkerson PC, Abonia JP, et al. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest (2006) 116:536–47. doi:10.1172/JCI26679
23. Wen T, Stucke EM, Grotjan TM, Kemme KA, Abonia JP, Putnam PE, et al. Molecular diagnosis of eosinophilic esophagitis by gene expression profiling. Gastroenterology (2013) 145:1289–99. doi:10.1053/j.gastro.2013.08.046
24. Noel RJ, Putnam PE, Rothenberg ME. Eosinophilic esophagitis. N Engl J Med (2003) 351:940–1. doi:10.1056/NEJM200408263510924
25. Konikoff MR, Noel RJ, Blanchard C, Kirby C, Jameson SC, Buckmeier BK, et al. A randomized, double-blind, placebo-controlled trial of fluticasone propionate for pediatric eosinophilic esophagitis. Gastroenterology (2006) 131:1381–91. doi:10.1053/j.gastro.2006.08.033
26. Dohil R, Newbury R, Fox L, Bastian J, Aceves S. Oral viscous budesonide is effective in children with eosinophilic esophagitis in a randomized, placebo-controlled trial. Gastroenterology (2010) 139:418–29. doi:10.1053/j.gastro.2010.05.001
27. Straumann A, Conus S, Degen L, Felder S, Kummer M, Engel H, et al. Budesonide is effective in adolescent and adult patients with active eosinophilic esophagitis. Gastroenterology (2010) 139:1526–37. doi:10.1053/j.gastro.2010.07.048
28. Butz BK, Wen T, Gleich GJ, Furuta GT, Spergel J, King E, et al. Efficacy, dose reduction, and resistance to high-dose fluticasone in patients with eosinophilic esophagitis. Gastroenterology (2014) 147:324–33. doi:10.1053/j.gastro.2014.04.019
29. Gupta SK, Vitanza JM, Collins MH. Efficacy and safety of oral budesonide suspension in pediatric patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol (2015) 13:66–76. doi:10.1016/j.cgh.2014.05.021
30. Dellon ES, Katzka DA, Collins MH, Hamdani M, Gupta SK, Hirano I, et al. Budesonide oral suspension improves symptomatic, endoscopic, and histologic parameters compared with placebo in patients with eosinophilic esophagitis. Gastroenterology (2017) 152:776–86. doi:10.1053/j.gastro.2016.11.021
31. Kagalwalla AF, Sentongo TA, Ritz S, Hess T, Nelson SP, Emerick KM, et al. Effect of six-food elimination diet on clinical and histologic outcomes in eosinophilic esophagitis. Clin Gastroenterol Hepatol (2006) 4:1097–102. doi:10.1016/j.cgh.2006.05.026
32. Gonsalves N, Yang GY, Doerfler B, Ritz S, Ditto AM, Hirano I. Elimination diet effectively treats eosinophilic esophagitis in adults; food reintroduction identifies causative factors. Gastroenterology (2012) 142:1451–9. doi:10.1053/j.gastro.2012.03.001
33. Blanchard C, Stucke EM, Rodriguez-Jimenez B, Burwinkel K, Collins MH, Ahrens A, et al. A striking local esophageal cytokine expression profile in eosinophilic esophagitis. J Allergy Clin Immunol (2011) 127:208–17. doi:10.1016/j.jaci.2010.10.039
34. Stein ML, Collins MH, Villaneuva JM, Kushner JP, Putnam PE, Buckmeier BK, et al. Anti-IL-5 (mepolizumab) therapy for eosinophilic esophagitis. J Allergy Clin Immunol (2006) 118:1312–9. doi:10.1016/j.jaci.2006.09.007
35. Assa’ad AH, Gupta SK, Collins MH, Thomson M, Heath AT, Smith DA, et al. An antibody against IL-5 reduces numbers of esophageal intraepithelial eosinophils in children with eosinophilic esophagitis. Gastroenterology (2011) 141:1593–604. doi:10.1053/j.gastro.2011.07.044
36. Spergel JM, Rothenberg ME, Collins MH, Furuta GT, Markowitz JE, Fuchs G, et al. Reslizumab in children and adolescents with eosinophilic esophagitis: results of a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol (2012) 129:456–63. doi:10.1016/j.jaci.2011.11.044
37. Rothenberg ME, Wen T, Greenberg A, Alpan O, Enav B, Hirano I, et al. Intravenous IL-13 mAb QAX576 for the treatment of eosinophilic esophagitis. J Allergy Clin Immunol (2015) 135:500–7. doi:10.1016/j.jaci.2014.07.049
38. Rothenberg ME. Molecular, genetic, and cellular bases for treating eosinophilic esophagitis. Gastroenterology (2015) 148(6):1143–57. doi:10.1053/j.gastro.2015.02.002
39. Molina-Infante J, Bredenoord AJ, Cheng E, Dellon ES, Furuta GT, Gupta SK, et al. Proton pump inhibitor-responsive oesophageal eosinophilia: an entity challenging current diagnostic criteria for eosinophilic oesophagitis. Gut (2016) 65:524–31. doi:10.1136/gutjnl-2015-310991
40. Wen T, Dellon ES, Moawad FJ, Furuta GT, Aceves SS, Rothenberg ME. Transcriptome analysis of proton pump inhibitor-responsive esophageal eosinophilia reveals proton-pump inhibitor-reversible allergic inflammation. J Allergy Clin Immunol (2015) 135:187–97. doi:10.1016/j.jaci.2014.08.043
41. Dellon ES. Epidemiology of eosinophilic esophagitis. Gastroenterol Clin North Am (2014) 43(2):201–18. doi:10.1016/j.gtc.2014.02.002
42. Hruz P, Straumann A, Bussmann C, Heer P, Simon HU, Zwahlen M, et al. Escalating incidence of eosinophilic esophagitis: a 20-year prospective, population-based study in Olten County. Switzerland. J Allergy Clin Immunol (2011) 128:1349.e–50.e. doi:10.1016/j.jaci.2011.09.013
43. Dellon ES, Jensen ET, Martin CF, Shaheen NJ, Kappelman MD. Prevalence of eosinophilic esophagitis in the United States. Clin Gastroenterol Hepatol (2014) 12:589.e–96.e. doi:10.1016/j.cgh.2013.09.008
44. Sperry SL, Crockett SD, Miller CB, Shaheen NJ, Dellon ES. Esophageal foreign-body impactions: epidemiology, time trends, and the impact of the increasing prevalence of eosinophilic esophagitis. Gastrointest Endosc (2011) 74:985–91. doi:10.1016/j.gie.2011.06.029
45. Gawron AJ, Hirano I. Eosinophilic esophagitis – emerging epidemic or misdiagnosed malady? Clin Gastroenterol Hepatol (2014) 12(4):597–8. doi:10.1016/j.cgh.2013.10.036
46. Jensen ET, Kappelman MD, Martin CF, Dellon ES. Health-care utilization, costs, and the burden of disease related to eosinophilic esophagitis in the United States. Am J Gastroenterol (2015) 110:626–32. doi:10.1038/ajg.2014.316
47. Lowichik A, Weinberg AG. A quantitative evaluation of mucosal eosinophils in the pediatric gastrointestinal tract. Mod Pathol (1996) 9:110–4.
48. DeBrosse CW, Case JW, Putnam PE, Collins MH, Rothenberg ME. Quantity and distribution of eosinophils in the gastrointestinal tract of children. Pediatr Dev Pathol (2006) 9:210–8. doi:10.2350/11-05-0130.1
49. Lwin T, Melton SD, Genta RM. Eosinophilic gastritis: histopathological characterization and quantification of the normal gastric eosinophil count. Mod Pathol (2011) 24:556–63. doi:10.1038/modpathol.2010.221
50. Ko HM, Morotti R, Yershov O, Chehade M. Eosinophilic gastritis in children: clinicopathological correlation, disease course, and response to therapy. Am J Gastroenterol (2014) 109:1277–85. doi:10.1038/ajg.2014.166
51. Caldwell JM, Collins MH, Stucke EM, Putnam PE, Franciosi JP, Kushner JP, et al. Histologic eosinophilic gastritis is a systemic disorder associated with blood and extragastric eosinophilia, Th2 immunity, and a unique gastric transcriptome. J Allergy Clin Immunol (2014) 134:1114–24. doi:10.1016/j.jaci.2014.07.026
52. Caldwell JM, Collins MH, Kemme KA, Sherrill JD, Wen T, Rochman M, et al. Cadherin 26 is an alpha integrin-binding epithelial receptor regulated during allergic inflammation. Mucosal Immunol (2017) 10(5):1190–201. doi:10.1038/mi.2016.120
53. Talley NJ, Walker MM, Aro P, Ronkainen J, Storskrubb T, Hindley LA, et al. Non-ulcer dyspepsia and duodenal eosinophilia: an adult endoscopic population-based case-control study. Clin Gastroenterol Hepatol (2007) 5:1175–83. doi:10.1016/j.cgh.2007.05.015
54. Robert M. Gluten sensitive enteropathy and other causes of small intestinal lymphocytosis. Semin Diagn Pathol (2005) 22:284–94. doi:10.1053/j.semdp.2006.04.004
55. Xavier RJ, Gala MK, Bronzo BD, Kelly PJ. Case 23-2012: a 59-year-old man with abdominal pain and weight loss. N Engl J Med (2012) 367(4):363–73. doi:10.1056/NEJMcpc1109275
56. Saad AG. Normal quantity and distribution of mast cells and eosinophils in the pediatric colon. Pediatr Dev Pathol (2011) 14:294–300. doi:10.2350/10-07-0878-OA.1
57. Turner KO, Sinkre RA, Neumann WL, Genta RM. Primary colonic eosinophilia and eosinophilic colitis in adults. Am J Surg Pathol (2017) 41:225–33. doi:10.1097/PAS.0000000000000760
58. Brandon JL, Schroeder S, Furuta GT, Capocelli K, Masterson JC, Fenton LZ. CT imaging features of eosinophilic colitis in children. Pediatr Radiol (2013) 43(6):697–702. doi:10.1007/s00247-012-2615-8
59. Bates AWH. Diagnosing eosinophilic colitis: histopathological pattern or nosologic entity? Scientifica (Cairo) (2012) 2012:9. doi:10.6064/2012/682576
60. Collins MH. Histopathology associated with eosinophilic gastrointestinal diseases. Immunol Allergy Clin North Am (2009) 29:109–17. doi:10.1016/j.iac.2008.10.005
61. Hurrell JM, Genta RM, Melton SD. Histopathologic diagnosis of eosinophilic conditions in the gastrointestinal tract. Adv Anat Pathol (2011) 18:335–48. doi:10.1097/PAP.0b013e318229bfe2
62. Alfadda AA, Storr MA, Shaffer EA. Eosinophilic colitis: an update on pathophysiology and treatment. Br Med Bull (2011) 100:59–72. doi:10.1093/bmb/ldr045
63. Gaertner WB, MacDonald JE, Kwaan MR, Shepela C, Madoff R, Jessurun J, et al. Eosinophilic colitis: University of Minnesota experience and literature review. Gastroenterol Res Prac (2011) 2011:6. doi:10.1155/2011/857508
64. Ahrens R, Waddell A, Seidu L, Blanchard C, Carey R, Forbes E, et al. Intestinal macrophage/epithelial-cell derived CCL11/eotaxin-1 mediates eosinophil recruitment and function in pediatric ulcerative colitis. J Immunol (2008) 181:7390–9. doi:10.4049/jimmunol.181.10.7390
65. Morgenstern S, Brook E, Rinawi F, Shamir R, Assa A. Tissue and peripheral eosinophilia as predictors for disease outcome in children with ulcerative colitis. Dig Liver Dis (2017) 49:170–4. doi:10.1016/j.dld.2016.11.007
66. Pensabene L, Brundler MA, Bank JM, Di Lorenzo C. Evaluation of mucosal eosinophils in the pediatric colon. Dig Dis Sci (2005) 50:221–9. doi:10.1007/s10620-005-1586-0
67. Torrente F, Barabino A, Bellini T, Murch SH. Intraepithelial lymphocyte eotaxin-2 expression and perineural mast cell degranulation differentiate allergic/eosinophilic colitis from classic IBD. J Pediatr Gastroenterol Nutr (2014) 59:300–7. doi:10.1097/MPG.0000000000000432
68. Rosen MJ, Karns R, Vallance JE, Bezold R, Waddell A, Collins MH, et al. Mucosal expression of type 2 and type 17 immune response genes distinguishes ulcerative colitis from colon-only Crohn’s disease in treatment-naïve pediatric patients. Gastroenterology (2017) 152(6):1345.e–57.e. doi:10.1053/j.gastro.2017.01.016
69. Saeed SA, Integlia MJ, Pleskow RG, Calenda KA, Rohrer RJ, Dayal Y, et al. Tacrolimus-associated eosinophilic gastroenterocolitis in pediatric liver transplant recipients: role of potential food allergies in pathogenesis. Pediatr Transplant (2006) 10:730–5. doi:10.1111/j.1399-3046.2006.00538.x
70. Lee JH, Park HY, Choe YH, Lee SK, Lee SI. The development of eosinophilic colitis after liver transplantation in children. Pediatr Transplant (2007) 11:518–23. doi:10.1111/j.1399-3046.2007.00693.x
71. Bush JW, Mohammad S, Melin-Aldana H, Kagalwalla AF, Arva NC. Eosinophilic density in graft biopsies positive for rejection and blood eosinophil count can predict development of post-transplant digestive tract eosinophilia. Pediatr Transplant (2016) 20:540–51. doi:10.1111/petr.12693
72. Tison BE, Debrosse CW, Rainey HF, Collins MH, Putnam PE, Abonia JP, et al. Number and distribution of mast cells in the pediatric gastrointestinal tract. J Allergy Clin Immunol (2010) 2:AB182. doi:10.1016/j.jaci.2009.12.713
73. Jakate S, Demeo M, John R, Tobin M, Keshavarzian A. Mastocytic enterocolitis. Increased mucosal mast cells in chronic intractable diarrhea. Arch Pathol Lab Med (2006) 130:362–7. doi:10.1043/1543-2165(2006)130[362:MEIMMC]2.0.CO;2
74. Akhavein MA, Patel NR, Muniyappa PK, Glover SC. Allergic mastocytic gastroenteritis and colitis: an unexplained etiology in chronic abdominal pain and gastrointestinal dysmotility. Gastroenterol Res Pract (2012) 2012:950582. doi:10.1155/2012/950582
75. Hahn HP, Hornick JL. Immunoreactivity for CD25 in gastrointestinal mucosal mast cells is specific for systemic mastocytosis. Am J Surg Pathol (2007) 31:1669–76. doi:10.1097/PAS.0b013e318078ce7a
76. Kirsch R, Geboes K, Shepherd NA, de Hertogh G, Di Nicola N, Lebel S, et al. Systemic mastocytosis involving the gastrointestinal tract: clinicopathologic and molecular study of five cases. Mod Pathol (2008) 21:1508–16. doi:10.1038/modpathol.2008.158
77. Machida HM, Catto Smith AG, Gall DG, Trevenen C, Scott RB. Allergic colitis in infancy: clinical and pathologic aspects. J Pediatr Gastroenterol Nutr (1994) 19:22–6. doi:10.1097/00005176-199407000-00004
78. Odze RD, Bines J, Leichtner AM, Goldman H, Antonioli DA. Allergic proctocolitis in infants: a prospective clinicopathologic biopsy study. Hum Pathol (1993) 24:668–74. doi:10.1016/0046-8177(93)90248-F
Keywords: esophagitis, colitis, inflammatory bowel disease, allergy, genome
Citation: Collins MH, Capocelli K and Yang G-Y (2018) Eosinophilic Gastrointestinal Disorders Pathology. Front. Med. 4:261. doi: 10.3389/fmed.2017.00261
Received: 03 August 2017; Accepted: 26 December 2017;
Published: 15 January 2018
Edited by:
Florence Emmanuelle Roufosse, Université libre de Bruxelles, BelgiumReviewed by:
Laurence De Leval, Centre Hospitalier Universitaire Vaudois (CHUV), SwitzerlandOwen McCarty, Oregon Health & Science University, United States
Copyright: © 2018 Collins, Capocelli and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Margaret H. Collins, margaret.collins@cchmc.org
 Margaret H. Collins
Margaret H. Collins Kelley Capocelli3,4
Kelley Capocelli3,4