Diagnostic Test Accuracy of Serum Anti-PLA2R Autoantibodies and Glomerular PLA2R Antigen for Diagnosing Idiopathic Membranous Nephropathy: An Updated Meta-Analysis
- Division of Nephrology, West China Hospital, Sichuan University, Chengdu, Sichuan, China
Background: M-type phospholipase A2 receptor (PLA2R) is known as a major antigen on podocytes, which is involved with the pathogenesis of idiopathic membranous nephropathy (iMN). Many studies have shown that serum anti-PLA2R autoantibodies (sPLA2R) are prevalent in patients with iMN but are rarely detected in secondary membranous nephropathy (SMN) or other glomerulonephritis. The anti-PLA2R is considered as a promising serum biomarker in iMN but reports about its diagnostic value are variable and inconsistent.
Objective: To evaluate the diagnostic test accuracy (DTA) of anti-PLA2R and glomerular PLA2R antigen (gPLA2R) for diagnosing iMN.
Method: MEDLINE, EMBASE, WEB OF SCIENCE, and COCHRANE LIBRARY were searched from 2009 January to February 2018. Heterogeneity was evaluated by Q test and I2. Source of heterogeneity was explored by subgroup analysis and meta-regression. Meta-analysis was executed and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement.
Results: Totally, 35 studies were retrieved under the pre-set study eligibility criteria. Twenty-eight studies were included to evaluate the DTA of anti-PLA2R for differentiating iMN from non-iMN. They indicated a pooled sensitivity of 65% (63–67%), specificity of 97% (97–98%), positive likelihood ratio of 15.65 (9.95–24.62), and negative likelihood ratio of 0.37 (0.32–0.42) with a diagnostic OR (sDOR) of 50.41 (31.56 to 80.52) and AUC of 0.9393. No threshold effect was detected. The heterogeneity analysis for sDOR showed that I2 = 50.3% and Cochran-Q = 54.29, df = 27 (p = 0.0014). Heterogeneity was significant. Meta-regression revealed that sample size might be the potential source of heterogeneity. Subgroup analysis demonstrated that method type and ratio of patients with nephrotic-range proteinuria at baseline might be the source of heterogeneity. Sixteen studies reported the diagnostic value of glomerular PLA2R antigen for differentiating iMN from non-iMN. The pooled sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, sDOR, and AUC were 79% (76–81%), 90% (88–92%), 8.17 (5.60–11.93), 0.25 (0.19–0.33), 39.37 (22.18–60.13), and 0.9278. Heterogeneity analysis showed that Cochran-Q = 35.36; df = 15 (p = 0.002), and I2 for sDOR was 57.6%.
Conclusion: sPLA2R and gPLA2R demonstrated a good diagnostic accuracy in differentiating iMN and non-iMN.
Introduction
Membranous nephropathy (MN) is a common cause of massive proteinuria, accounting for 20–30% of nephrotic syndrome in Caucasian adults (1). The pathologic findings are featured by diffuse thickening of glomerular basement membrane in the absence of significant hypercellularity and subepithelial deposits of immunoglobulin G (IgG) and complement 3. MN is often idiopathic, and the diagnosis of secondary membranous nephropathy (SMN) is mainly established by the evidence of secondary causes such as systemic lupus erythematosus, hepatitis B infection, malignancies, or the use of certain drugs and characteristic changes in histology. However, the differential diagnosis of idiopathic membranous nephropathy (iMN) and SMN, which is of great clinical significance, could still be challenging in some clinical scenario.
M-type phospholipase A2 receptor (PLA2R) was identified as a major target antigen on glomerular podocytes in iMN. Beck et al. found that serum anti-PLA2R autoantibodies (sPLA2R) were detected in 70% of patients with iMN, and the IgG eluted from renal biopsy tissues of patients with iMN was reactive with PLA2R antigen (2). Emerging evidence from studies about recurrent MN in post-transplantation patients suggested a higher recurrence rate of MN in patients with positive pre-transplantation anti-PLA2R, which gave us more insight into the role of anti-PLA2R in the occurrence of MN (3). Discovery of anti-PLA2R has contributed to substantial advances in our understanding of the pathogenesis of MN. However, the pathogenic effect of anti-PLA2R in iMN has not been verified experimentally yet, and the exact pathogenic role remains to be elucidated. Nevertheless, the significance of sPLA2R and glomerular PLA2R antigen (gPLA2R) in diagnosing and monitoring the disease activity of iMN should not be ignored (4). In this study, we aimed to investigate the diagnostic performance and clinical value of anti-PLA2R autoantibodies as serum biomarkers for diagnosing iMN, which is helpful in assisting the differentiation between iMN and SMN and execution of etiology-based treatment.
Methods
Eligibility Criteria
Study eligibility criteria included the following: (1) both cohorts with iMN and non-iMN were enrolled in studies. (2) Index test results for sPLA2R or gPLA2R were reported in cases of iMN and non-iMN. (3) Renal biopsy was used as reference test for diagnosing MN. Identified secondary etiology of MN confirmed that the diagnosis of SMN and exclusion of secondary causes in patients with MN was considered as iMN. (4) Absolute number of true positive, false positive, true negative, and false negative was reported or could be derived. Different methods were used to test the positivity of sPLA2R in studies including ELISA, Western blot (WB), indirect immunofluorescence test (IFFT), and time-resolved fluoroimmunoassay (FIA). All of the studies meeting the eligibility criteria above were included in our meta-analysis, even though different methods for index test might be used.
Information Sources and Search Strategies
Literature was searched in MEDLINE, EMBASE, WEB OF SCIENCE, and COCHRANE LIBRARY from January 2009 to February 2018. Search strategy in MEDLINE: {[Glomerulonephritis, Membranous (Mesh) OR Membranous Glomerulonephritides (tiab) OR Membranous Glomerulonephritis (tiab) OR Membranous Glomerulopathy (tiab) OR Membranous Nephropathy (tiab) OR Heymann Nephritis (tiab)] AND [Receptors, Phospholipase A2 (Mesh) OR PLA(2) Receptor OR Phospholipase A2 Receptor OR Anti-Phospholipase A2 Receptor OR M-type phospholipase A2 receptor OR anti-PLA2R OR aPLA2R OR PLA2R] AND [Sensitivity and Specificity (Mesh) OR Sensitiv* OR Specific*]}. Search strategy in EMBASE: (“membranous glomerulonephritides” OR “membranous glomerulonephritis”/exp OR “membranous glomerulonephritis” OR “membranous glomerulopathy” OR “membranous nephropathy” OR “heymann nephritis”) AND (“pla2 receptor” OR “phospholipase a2 receptor”/exp OR “phospholipase a2 receptor” OR “anti-phospholipase a2 receptor” OR “m-type phospholipase a2 receptor” OR “anti-pla2r” OR “apla2r” OR “pla2r”) AND (“sensitivity and specificity”/exp OR “sensitivity and specificity” OR “diagnostic value”/exp OR “diagnostic value” OR sensitiv* OR specific* OR “diagnos*”). Search strategy in Cochrane library: {Membranous Glomerulonephritis OR Membranous Glomerulopathy OR Membranous Nephropathy OR [MeSH descriptor: (Glomerulonephritis, Membranous) explode all trees]} AND {PLA (2) Receptor OR Phospholipase A2 Receptor OR M-type phospholipase A2 receptor OR anti-PLA2R OR PLA2R OR [MeSH descriptor: (Receptors, Phospholipase A2) explode all tree]}. The reference lists of the identified articles were also reviewed manually to identify additional articles.
Study Selection
Two reviewers (MD and MD) screened studies and determined eligibility independently. Disagreements were resolved by discussion and common consensus. Reviewers initially screened the titles and abstracts to detect the potential relevant papers, and then the shortlisted studies were screened again to evaluate their adherence to the eligibility criteria. There was no language restriction for the search but during selection language was restricted to English. Conference abstracts were scrutinized and excluded because they lacked data for quality assessment.
Data Extraction
Data from all studies were extracted by two reviewers independently (MD and MD) and combined to develop a definitive data collection sheet. Discrepancies during data extraction and methodological quality assessment process were resolved by discussion and global consensus. The extracted information included author, year, country, mean age, gender, study design, sample size, gold standard, method of sPLA2R measurement, time interval between biopsy and antibody measurement, cutoff value, mean serum creatinine, mean albumin, mean 24 h proteinuria, percentage of included patients receiving immunosuppressive therapy (IST) at baseline, percentage of included patients with nephrotic range proteinuria (NRP). Absolute number of true-positive, true-negative, false-positive, and false-negative were retrieved or calculated to develop 2 × 2 contingency table.
Risk of Bias and Applicability
All the included studies were assessed for their methodological quality and potential sources of bias using Quality Assessment of Diagnostic Accuracy Studies-2 criteria provided by Review Manager (RevMan) Version 5.3.
Statistical Synthesis and Data Analysis
Sensitivity, specificity, likelihood ratios, diagnostic odds ratios, and receiver operating characteristic curves (sROC) with 95% confidence intervals were pooled using the DerSimonian and Laird method (random-effects model). Studies were categorized according to method type used as an index test and subgroup analysis was conducted to assess the effect of method type on pooled diagnostic performance and study heterogeneity. The level of proteinuria and interference of IST are correlated with the immunologic activity and clinical status, and therefore more subgroup analysis was conducted according to the ratio of patients with NRP at baseline and ratio of patients with IST at baseline. Spearman correlation coefficient of sensitivity and 1 − specificity were calculated to analyze the threshold effect. Publication bias was explored by funnel plot. Asymmetry of the funnel plot was considered to be significant in publication bias. Study heterogeneity was assessed by Q test and I2. I2 > 50% and p value < 0.05 in the Q test were interpreted as the presence of significant heterogeneity. Meta-regression and subgroup analysis were conducted to search for the source of heterogeneity. Meta-analysis was executed and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement. All the analysis was performed by Meta-DiSc 1.4 and RevMan Version 5.3. Statistical significance was defined as p value < 0.05.
Results
Search Results
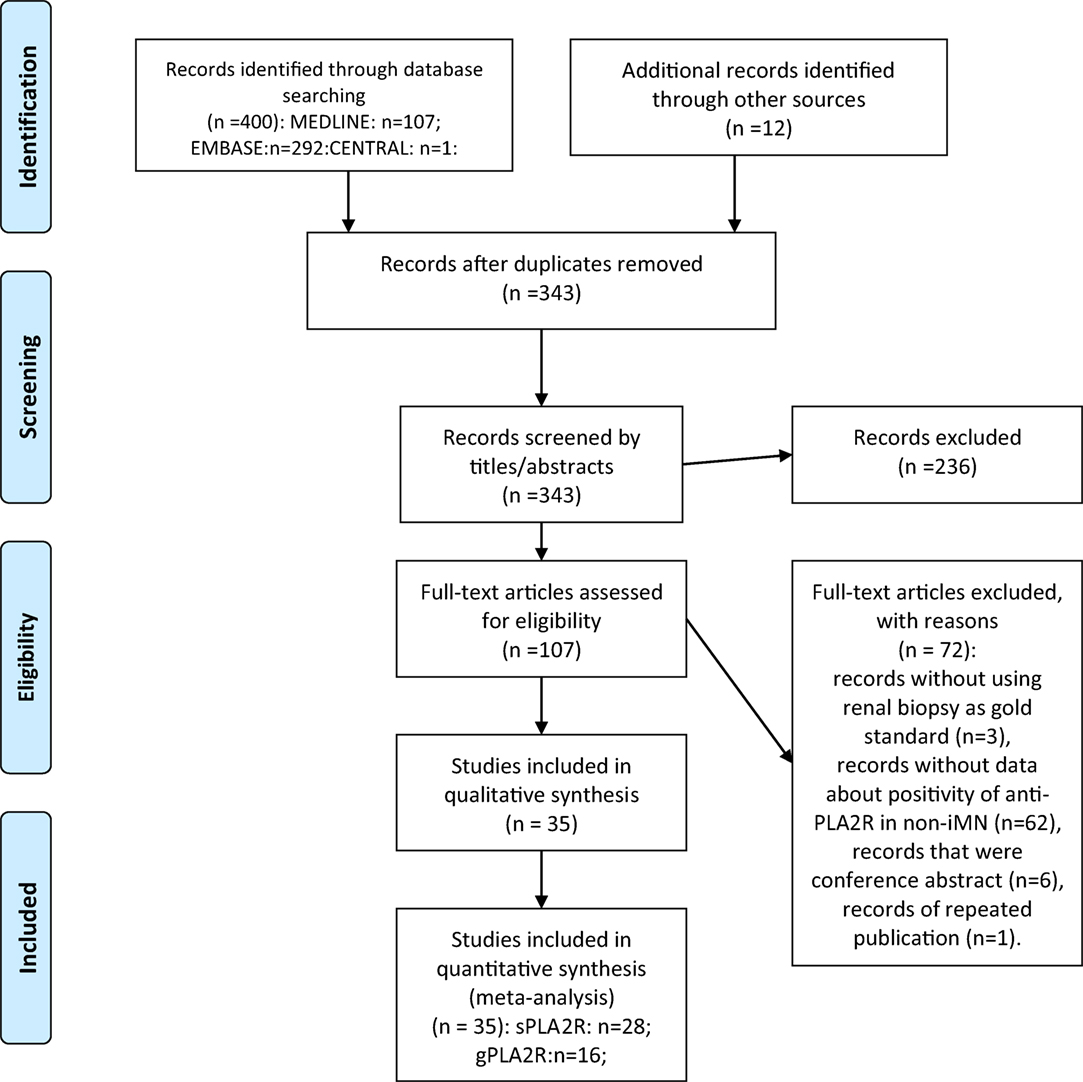
Overall, 400 records were found from the search strategy, 12 additional records were identified from reference lists of the included records. 343 records were identified after duplicate removal and were screened by titles and abstracts. By reviewing titles and abstracts, 107 records were retrieved for full-text assessment. The following records were excluded: records without using renal biopsy as gold standard (n = 3), records with no data about the positive ratio of anti-PLA2R in non-iMN (n = 62), records that were conference abstracts (n = 6), records of repeated publication (n = 1). Thirty-five records were retrieved for quantitative synthesis. Twenty-eight of them reported data about the diagnostic performance of serum anti-PLA2R autoantibodies in differentiating iMN and non-iMN. Sixteen studies provided data about evaluating the value of glomerular PLA2R antigen in diagnosing iMN. The results of the search strategy are presented in the flowchart (Figure 1).
Study Characteristics
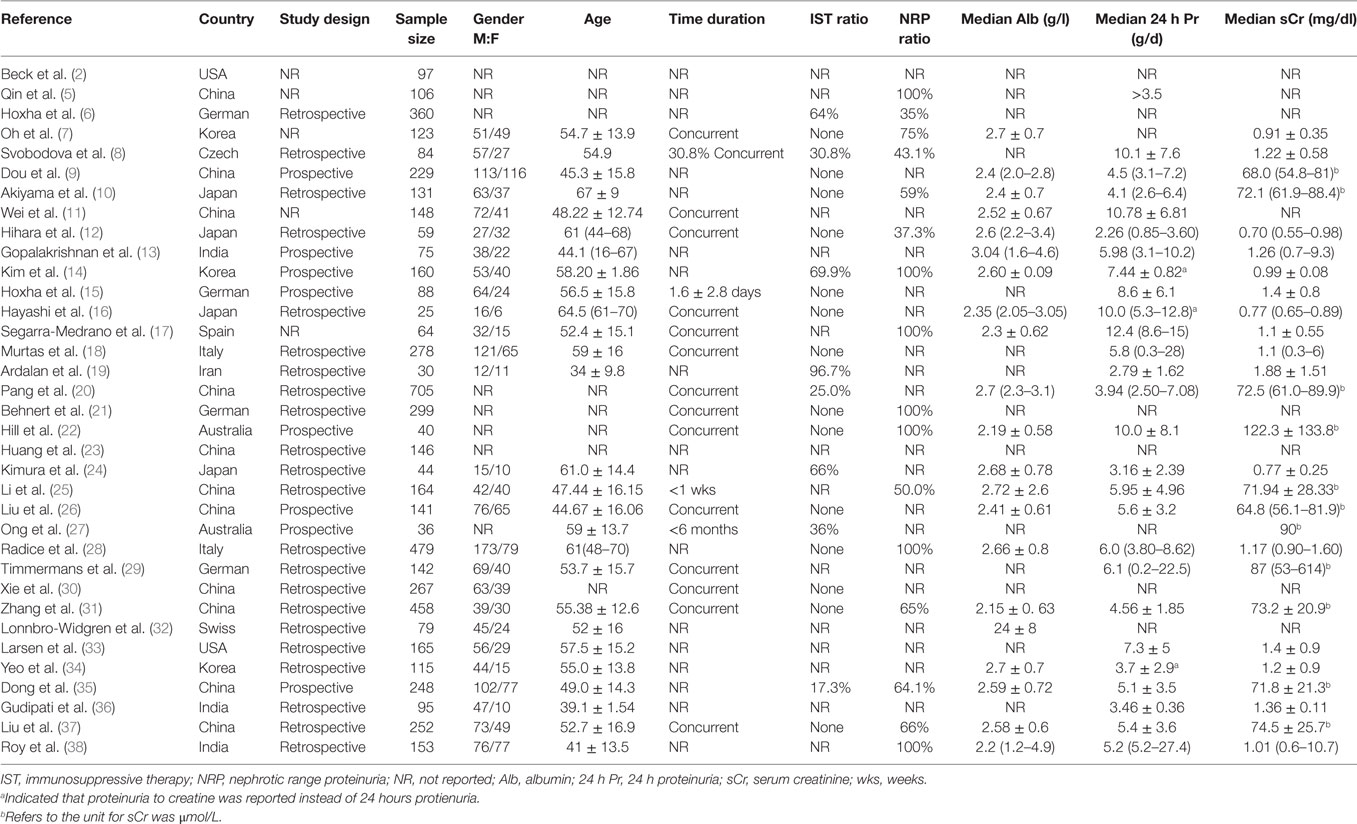
Thirty-five records and 6,085 subjects were enrolled in our analysis. All the patients enrolled in studies had renal biopsy for the pathological diagnosis for MN. SMN was discriminated from iMN by searches for the evidence of secondary causes by clinical evaluation and lab tests. Included studies covered a wide spectrum of country and ethnicity including Caucasian, Asian, Indian and Spanish. Only 20.0% of included studies declared a prospective study design. 51.4% of studies reported that all included patients were sampled at the time of biopsy. 40.0% of studies only included patients whose serum samples were collected before IST. 20.0% of studies only included patients who were presented with nephrotic-range proteinuria at baseline. The baseline characteristics of included studies are presented in Table 1.
Methodological Quality Assessment and Risk of Bias
Generally included studies showed a low concern on applicability of reference standard; studies that were obscure in the setting or interpretation of reference standard were excluded in our analysis. 31.4% of studies did not exclude patients who received IST before serum test or whose proteinuria was below the nephrotic range at baseline, which led to relatively high concerns on applicability of patients’ selection because the interference from IST would influence the immunological activity of patients. Three out of 35 studies did not report a pre-set cutoff value, two of 35 studies tested sPLA2R titer by a new type of method time-resolved FIA, compared to other widely accepted test methods such as ELISA, WB, and IFFT. The unauthorized test methods in these two studies might bring certain concern on the applicability of index test. The risk of bias mainly came from a case–control design in most of the studies. Furthermore, only two studies reported that blinding was applied for the interpretation of index test. 60.0% of studies reported a long time interval between biopsy and serum test or did not provide the related information, which introduced relatively high risk of bias to flow and timing. The methodological quality assessment and risk of bias are summarized in Figure 2.
Quantitative Synthesis
Twenty-eight studies reported the diagnostic value of serum anti-PLA2R for differentiating iMN from non-iMN (Table 2). They indicated a pooled sensitivity of 65% (63–67%), specificity of 97% (97–98%), positive likelihood ratio of 15.65 (9.95–24.62), and negative likelihood ratio of 0.37 (0.32–0.42) with sDOR of 50.41 (31.56–80.52) and AUC of 0.9393. The heterogeneity analysis indicated that Cochran-Q = 54.29, df = 27 (p = 0.0014), and I2 for diagnostic OR was 50.3% (Figures 3 and 4). The Spearman correlation coefficient = 0.299 (p-value = 0.122), suggesting that there was no threshold effect. SROC plot did not show a curve in the top left corner of the plot, further indicating the lack of threshold effect. Funnel plot showed the existence of asymmetry, which suggested publication bias existing in the studies of serological tests (Figure S1 in Supplementary Material). Meta-regression analyzed covariates including sample size, method types, time interval between biopsy and serum test, whether receiving IST at baseline, and whether excluding patients who were not in nephrotic-range proteinuria for the potential source of heterogeneity, and the result showed that sample size might contribute to the heterogeneity. A subgroup was made with all the studies in which sample size for patients with non-iMN was more than 50, and it showed a diagnostic value of 67.25 (38.56–117.28), I2 = 47% (Figure S2 in Supplementary Material). Therefore, the heterogeneity was lessened but did not disappear when the restriction for the sample size of included patients with non-iMN was applied.
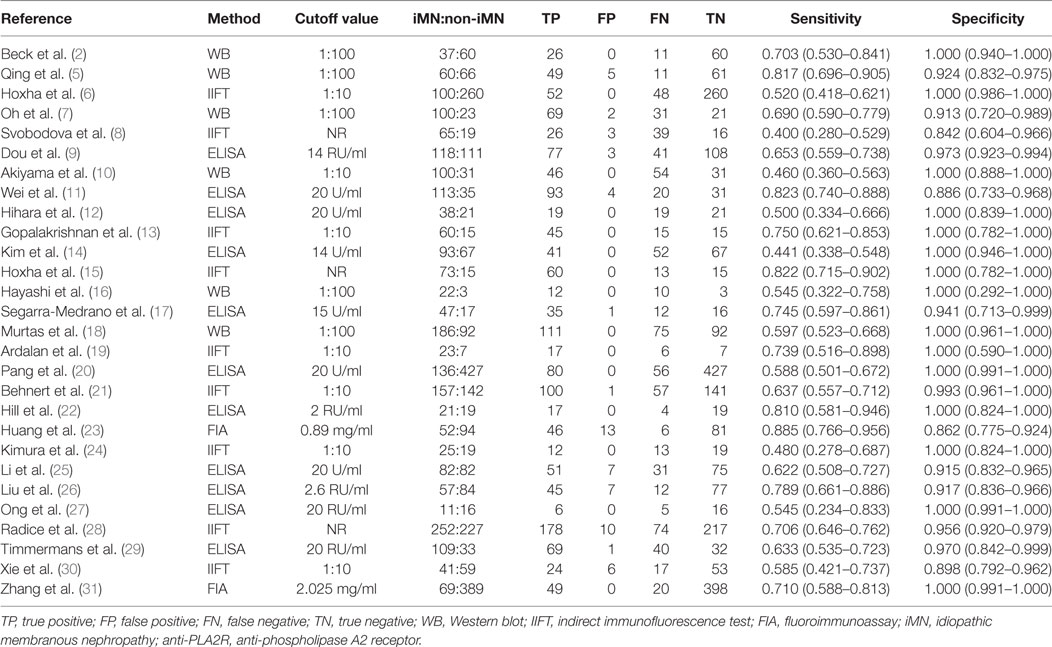
Table 2. Sensitivity and specificity for differentiating iMN with non-iMN by serum anti-PLA2R autoantibodies.
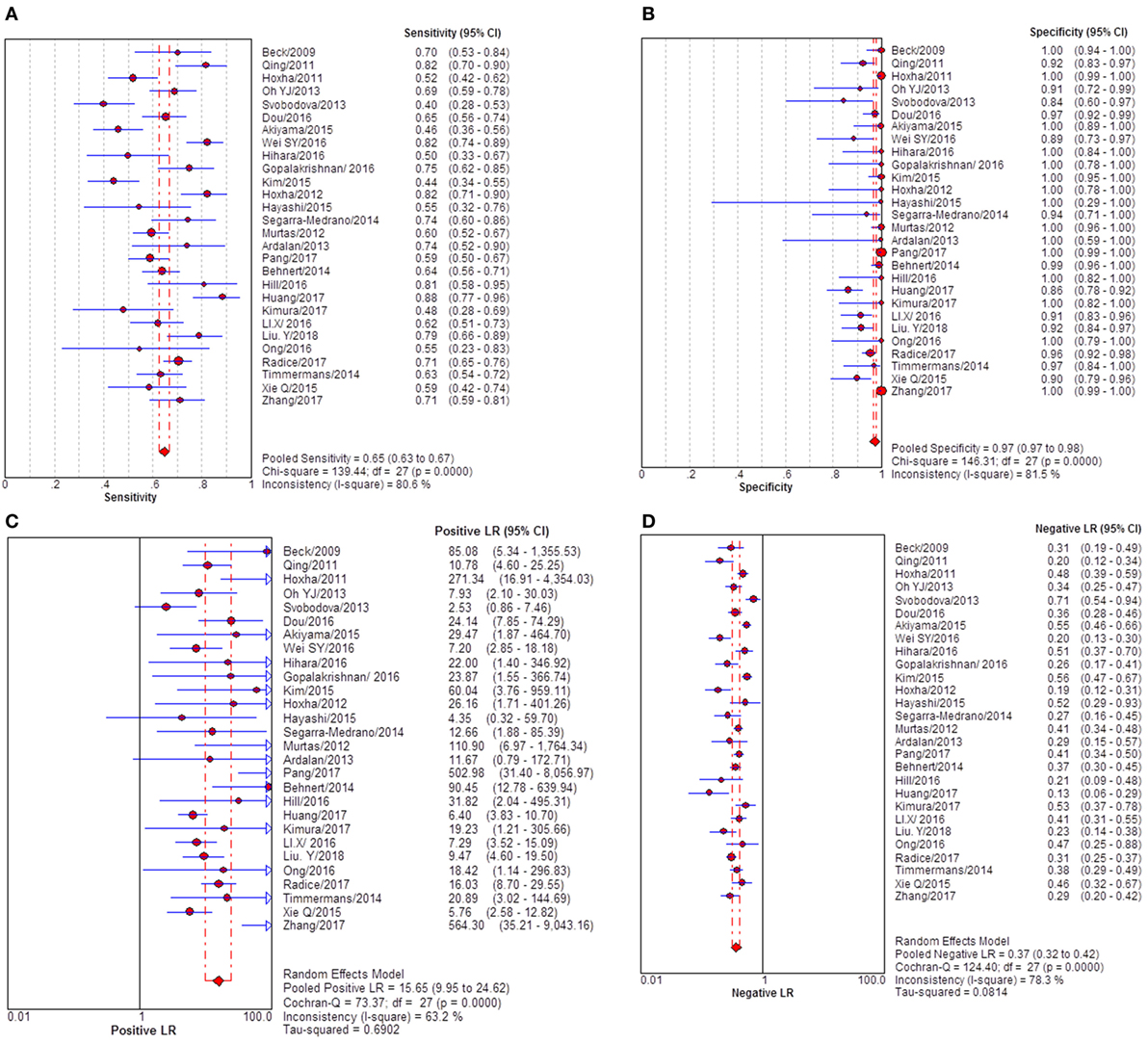
Figure 3. Pooled sensitivity, specificity, PLR, and NLR of serum anti-phospholipase A2 receptor (sPLA2R) for differentiating idiopathic membranous nephropathy (iMN) from non-iMN. (A) The pooled sensitivity of sPLA2R for differentiating iMN from non-iMN was 65% (63–67%). (B) The pooled specificity of sPLA2R for differentiating iMN from non-iMN was 97% (97–98%). (C) The pooled PLR of sPLA2R for differentiating iMN from non-iMN was 15.65 (9.95–24.62). (D) The pooled NLR of sPLA2R for differentiating iMN from non-iMN was 0.37 (0.32–0.42). Abbreviations: PLR, positive likelihood ratio, NLR, negative likelihood ratio.
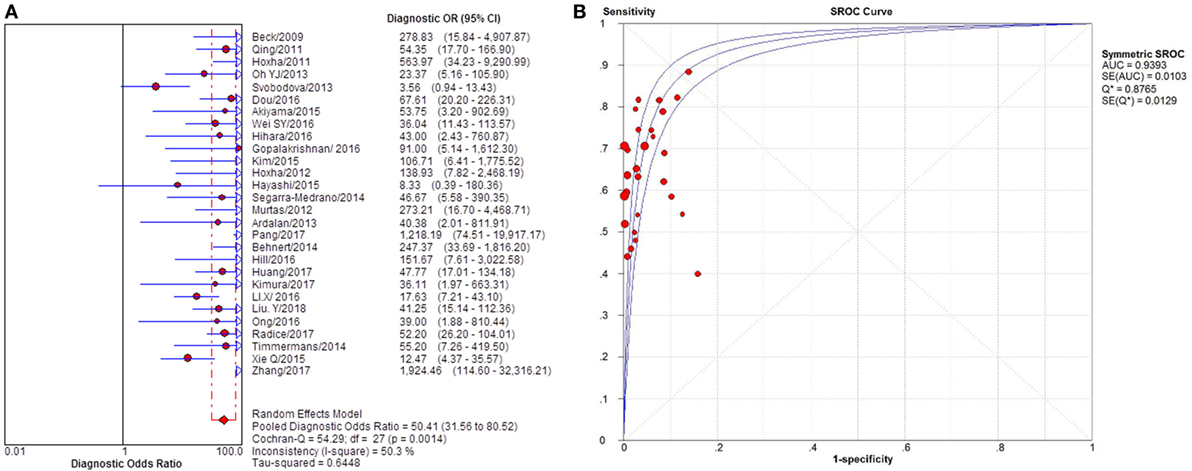
Figure 4. Pooled diagnostic OR and SROC curve of anti-phospholipase A2 receptor (sPLA2R) for differentiating idiopathic membranous nephropathy (iMN) from non-iMN. (A) The pooled diagnostic OR of sPLA2R for differentiating iMN from non-iMN was 50.41 (31.56–80.52). (B) SROC curve showed that AUC was 0.9393. SROC, summary receiver operating characteristic.
Sixteen studies reported the diagnostic value of glomerular PLA2R antigen for differentiating iMN from non-iMN (Table 3). The pooled sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, sDOR, and AUC were 79% (76–81%), 90% (88–92%), 8.17 (5.60–11.93), 0.25 (0.19–0.33), 39.37 (22.18–60.13), and 0.9278 (Figures 5 and 6). Heterogeneity analysis showed that Cochran-Q = 35.36; df = 15 (p = 0.002), and I2 for sDOR was 57.6%. The Spearman correlation coefficient = −0.060 (p-value = 0.824), suggesting that there is no threshold effect. SROC plot did not show a curve in the top left corner of the plot, further indicating the lack of threshold effect. Funnel plot showed the existence of asymmetry, which suggested publication bias existing in the studies of gPLA2R tests (Figure S3 in Supplementary Material).
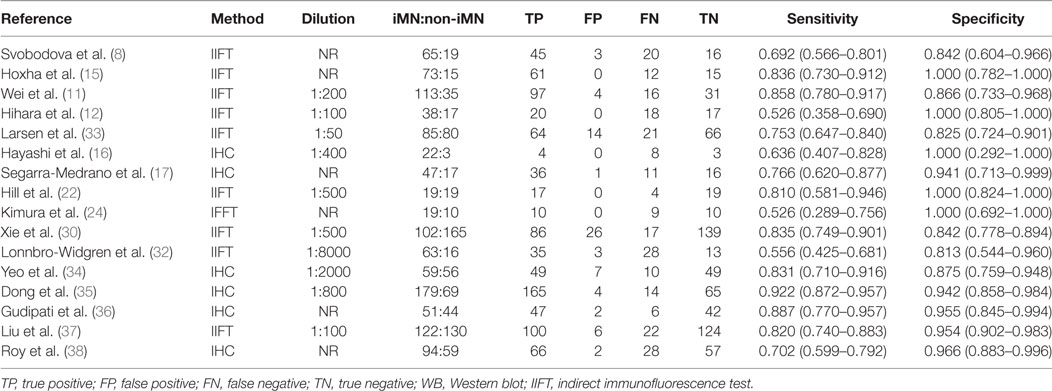
Table 3. Sensitivity and specificity for differentiating iMN with non-iMN by glomerular deposit of PLA2R.
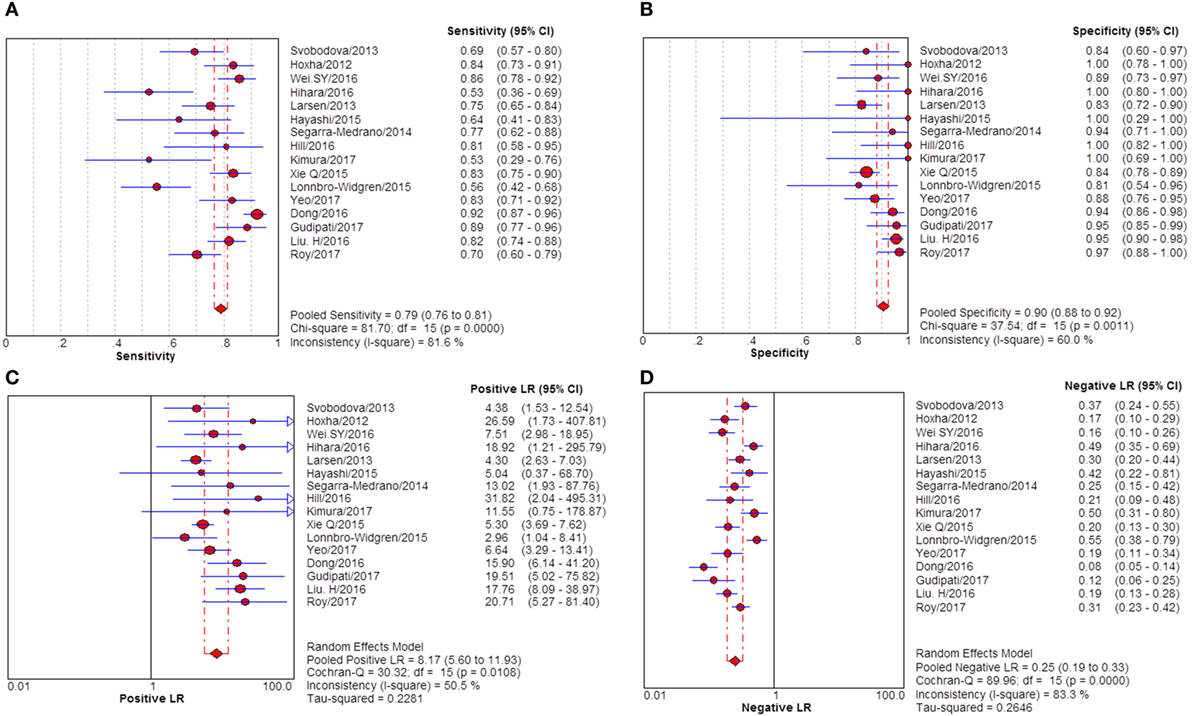
Figure 5. Pooled sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR) of glomerular PLA2R (gPLA2R) antigen for differentiating idiopathic membranous nephropathy (iMN) from non-iMN. (A) The pooled sensitivity of gPLA2R for differentiating iMN from non-iMN was 79% (76–81%). (B) The pooled specificity of gPLA2R for differentiating iMN from non-iMN was 90% (88–92%). (C) The pooled PLR of gPLA2R for differentiating iMN from non-iMN was 8.17 (5.60–11.93). (D) The pooled NLR of gPLA2R for differentiating iMN from non-iMN was 0.25 (0.19–0.33).
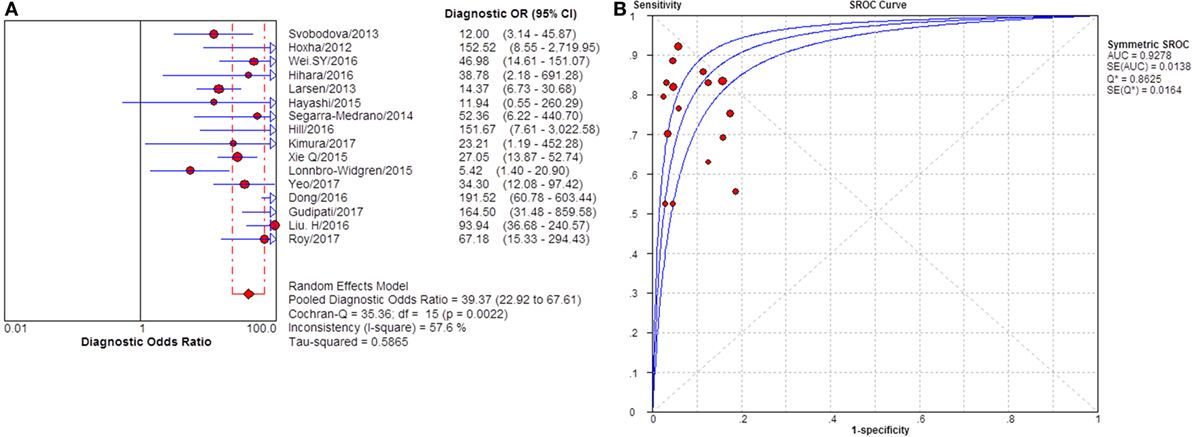
Figure 6. Pooled diagnostic OR and summary receiver operating characteristic (SROC) curve of glomerular PLA2R (gPLA2R) for differentiating idiopathic membranous nephropathy (iMN) from non-iMN. (A) The pooled diagnostic OR of gPLA2R for differentiating iMN from non-iMN was 39.37 (22.92–67.61). (B) SROC showed that AUC was 0.9278.
Subgroup Analysis
Twenty-two studies reported the diagnostic value of sPLA2R for differentiating iMN from SMN. They indicated a pooled sensitivity of 65% (62–67%), specificity of 91% (88–94%), positive likelihood ratio of 5.91 (3.81–9.16), and negative likelihood ratio of 0.39 (0.33–0.46) with sDOR of 17.59 (10.38–29.81) and AUC of 0.8770 (Figure 7). I2 for diagnostic OR was 41.4%, Cochran-Q = 35.82, df = 21 (p-value = 0.023).
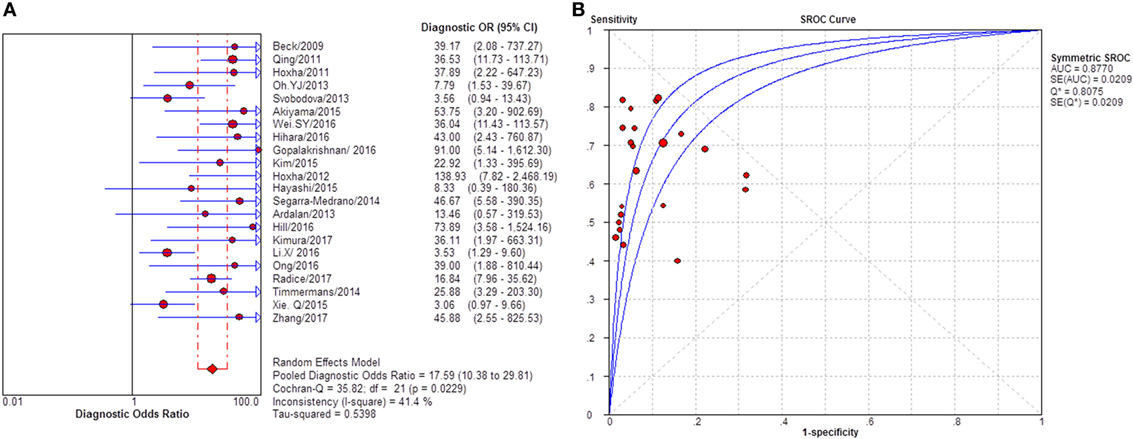
Figure 7. Pooled diagnostic OR and summary receiver operating characteristic (SROC) curve of serum anti-phospholipase A2 receptor (sPLA2R) for differentiating idiopathic membranous nephropathy (iMN) from SMN. (A) The pooled diagnostic OR of sPLA2R for differentiating iMN from secondary membranous nephropathy (SMN) was 17.59 (10.38–29.81). (B) SROC showed that AUC was 0.8770.
Severity of proteinuria and interference of IST are factors that would affect the immunological activity and disease status. In the assessment of applicability, both severity of proteinuria and interference from IST were the factors that might bring risk of bias on the applicability of patient selection, and therefore the effect from each factor was explored by subgroup analysis separately. First, a subgroup included studies that set a specific criteria on patient selection and in which only patients with nephrotic-range proteinuria at the time of sampling were enrolled (Table S1 in Supplementary Material). A pooled diagnostic OR for studies only including patients with nephrotic-range proteinuria was 56.40 (33.81–94.08). The heterogeneity analysis showed that I2 = 0.0%, Cochran-Q = 3.88, df = 7 (p = 0.794) (Figure 8). The heterogeneity disappeared after considering the covariate of ratio of patients with nephrotic-range proteinuria at baseline, which suggested that it might be the source of heterogeneity. Moreover, the interference of IST might cover up the real level of sPLA2R and gPLA2R deposit resulting from the disease-related pathological process. Studies that specified that all the serums were collected from patients before receiving any IST were analyzed as a subgroup (Table S2 in Supplementary Material) and the result for heterogeneity analysis indicated that I2 = 62.4%, Cochran-Q = 31.9, df = 12 (p = 0.0014) (Figure 9). The consistency of heterogeneity suggested that the ratio of patients who received IST before serum collection did not contribute to the heterogeneity in this analysis. Finally, a subgroup analysis for the test method showed that the use of different assay methods for testing serum level of anti-PLA2R was one of the causes of heterogeneity (Figures 10–12). After pooling all the results of studies that using ELISA as the test method, the heterogeneity turned to be I2 = 16.2%, p = 0.2851 (Table S3 in Supplementary Material). For all the studies that used WB as the single test method, the heterogeneity analysis showed that I2 = 0.0%, p = 0.5524 (Table S4 in Supplementary Material). The heterogeneity of the subgroup analysis for ELISA and WB disappeared, which suggested that the test method might be the source of heterogeneity.
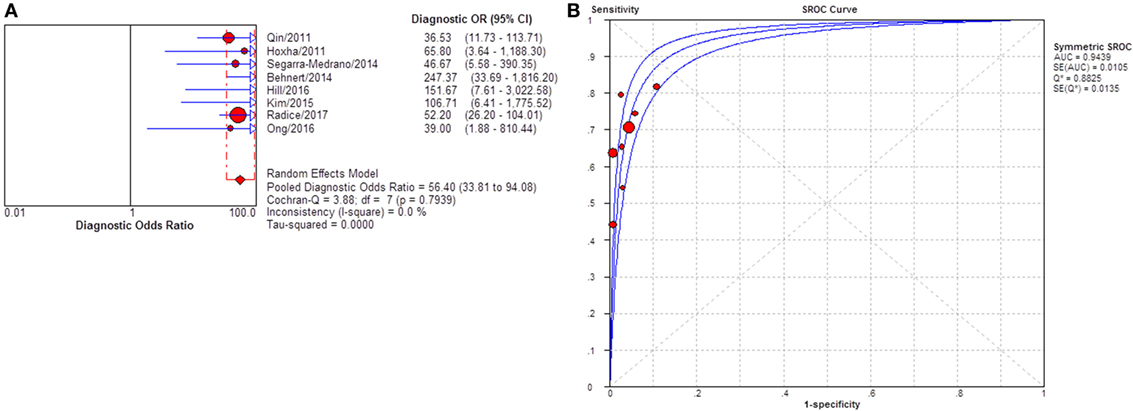
Figure 8. Pooled diagnostic OR and summary receiver operating characteristic (SROC) curve of serum anti-phospholipase A2 receptor (sPLA2R) for studies that only enrolled patients with NRP at baseline. (A) The pooled diagnostic OR of sPLA2R for studies that only enrolled patients with NRP at baseline was 56.40 (33.81–94.08). No heterogeneity was detected. (B) SROC showed that AUC was 0.9439. NRP, nephrotic range proteinuria.
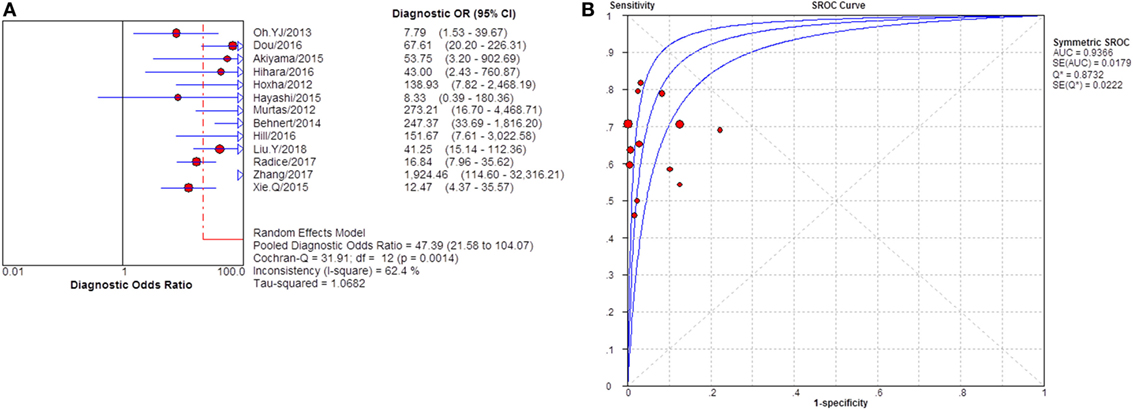
Figure 9. Pooled diagnostic OR and summary receiver operating characteristic (SROC) curve of serum anti-phospholipase A2 receptor (sPLA2R) for studies that only enrolled patients with no IST at baseline. (A) The pooled diagnostic OR of sPLA2R for studies that only enrolled patients with no IST at baseline was 47.39 (21.58–104.07). The heterogeneity was 62.4%. (B) SROC showed that AUC was 0.9366. IST, immunosuppressive therapy.
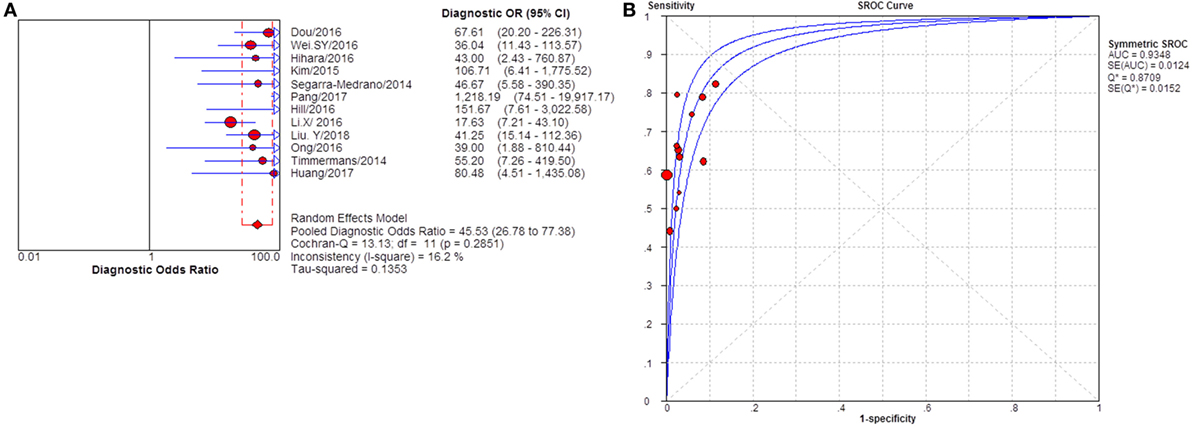
Figure 10. Pooled diagnostic OR and summary receiver operating characteristic (SROC) curve of serum anti-phospholipase A2 receptor (sPLA2R) for studies that used ELISA as test method. (A) The pooled diagnostic OR of sPLA2R for studies that used ELISA as test method was 45.53 (26.78–77.38). The heterogeneity was insignificant. (B) SROC showed that AUC was 0.9348. ELISA, enzyme-linked immunosorbent assay.
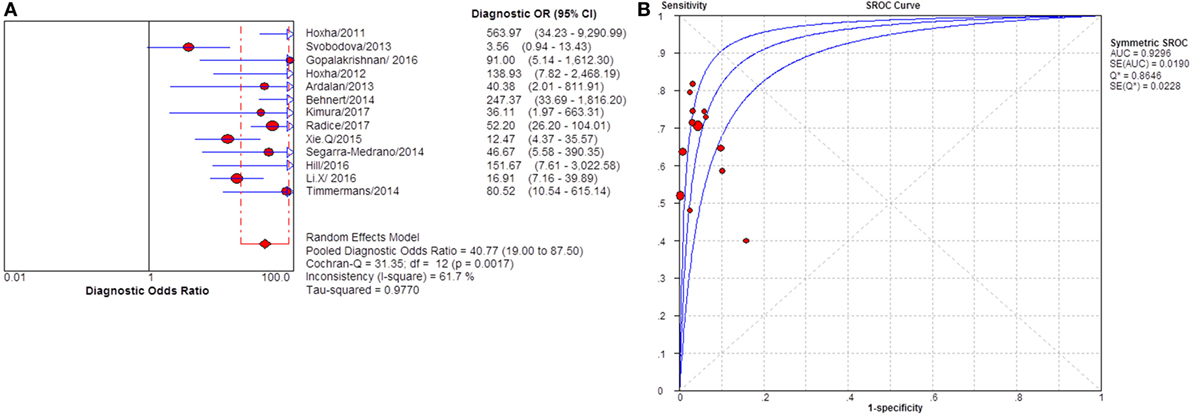
Figure 11. Pooled diagnostic OR and summary receiver operating characteristic (SROC) curve of serum anti-phospholipase A2 receptor (sPLA2R) for studies that used IIFT as test method. (A) The pooled diagnostic OR of sPLA2R for studies that used IIFT as test method was 40.77 (19.00–87.50). The heterogeneity was 61.7% (B) SROC showed that AUC was 0.9296. IIFT, indirect immunofluorescence test.
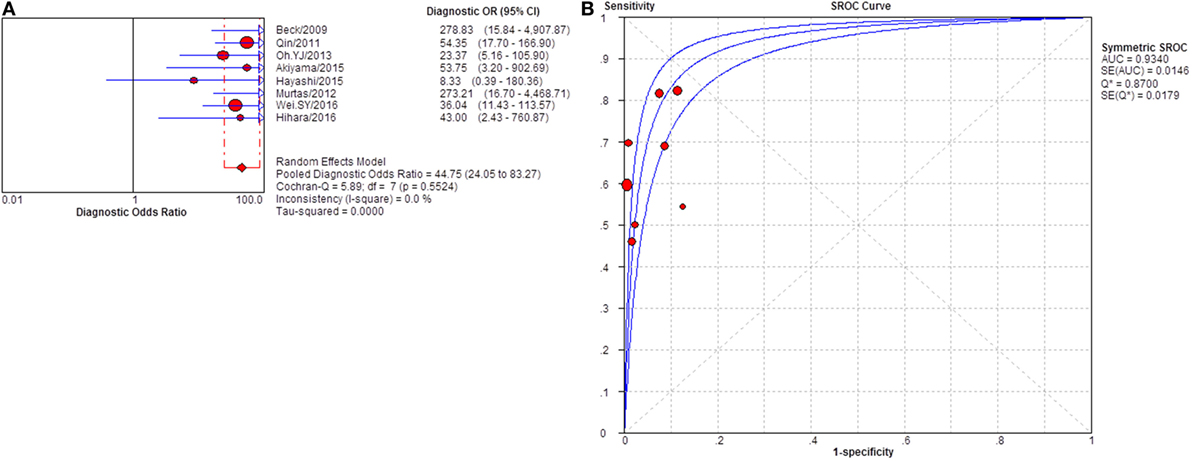
Figure 12. Pooled diagnostic OR and summary receiver operating characteristic (SROC) curve of serum anti-phospholipase A2 receptor (sPLA2R) for studies that used western blot as test method. (A) The pooled diagnostic OR of sPLA2R for studies that used western blot as test method was 44.75 (24.05–83.27). The heterogeneity was 61.7% (B) SROC showed that AUC was 0.9340.
Discussion
Few meta-analyses evaluating diagnostic test accuracy of anti-PLA2R have been reported previously: Dai et al. performed a diagnostic accuracy meta-analysis for sPLA2R and gPLA2R in 2015; however, they included eight meeting abstracts for statistical synthesis, which compromised the fidelity of the analysis because of the low standard for study inclusion (39). Besides, two studies that did not focus on the diagnostic performance of anti-PLA2R and also lacked sufficient data for quantitative synthesis were also included. Yeo et al. also reported the result of diagnostic test for anti-PLA2R; however, only eight studies were included in total and literature searches were only updated until the year of 2016 (34). In this study, we did a comprehensive literature search in common databases without language restriction, and the database search was updated until February 2018. As a result, 35 records were identified under a strict inclusion criterion. Twenty-three more records were included compared with the latest available meta-analysis for the diagnostic performance of anti-PLA2R. Also, we excluded conference abstracts and repeated publication on the same cohort to avoid unnecessary bias and to improve the overall quality of the studies included. The source of heterogeneity was fully explored by meta-regression and subgroup analysis. Our analysis showed that both anti-PLA2R autoantibodies and glomerular deposit of PLA2R antigen demonstrated a good diagnostic accuracy in differentiating iMN from non-iMN or SMN. Heterogeneity mainly came from the test method and ratio of patients with NRP at baseline. The prevalence of serum PLA2R reported by studies in different area varies from 57 to 82% (5, 6, 10, 13, 25, 40, 41). In this study, pooled sensitivity of serum anti-PLA2R and gPLA2R for differentiating iMN from non-iMN are 65 and 79%, respectively. The relatively low sensitivity might bring limitations on the interpretation of test result especially with a negative result. Apparently, serological test for anti-PLA2R is not a suitable option for screening test, and a negative test result leads to the need for renal biopsy and further search for potential secondary causes. However, both test for serum PLA2R and PLA2R antigen has high specificity, which means that a positive result indicates a high likely diagnosis of iMN. Its high positive predictive value helps the exclusion of non-MN diseases with a positive test result.
The limitations of this study are discussed subsequently. First of all, the heterogeneity of included studies was significant although attempts were made to find the source of heterogeneity by subgroup analysis and meta-regression. Besides, asymmetry was observed in the funnel plot with more studies appeared toward right (indicating higher odds ratio) in the bottom of the graph, which indicated the possibility that some studies might be missing on the left. The exclusion of conference abstracts and gray literature in study selection might account for the asymmetry; however this exclusion criterion was set to improve the overall quality of the included studies. Furthermore, concern on the applicability of patient selection might be relatively high because of the prevalent case–control design in included studies. A proportion of studies also did not set a strict enrollment criterion for patient selection which failed to minimize the interference from the severity of proteinuria and the use of IST before index test. But subgroup analysis was conducted according to the severity of proteinuria and whether or not receiving IST to specify the feature of target patients.
Renal biopsy is considered as the gold standard for diagnosing MN for a long time. However, our current standard to discriminate iMN from SMN might be confused by some subjective factors. Searching for secondary causes could be omitted or there may be a delayed appearance or detection of clinical presentation or evidence of secondary etiology. Under these circumstances, looking for a better marker to discriminate iMN from SMN that could be interpreted objectively is of great clinical significance (42). The discovery and developing insights toward the role of anti-PLA2R in MN is a milestone in our understanding toward the pathological mechanism of iMN (4). It is noteworthy that sPLA2R and gPLA2R demonstrated a high specificity in iMN, in contrast with in SMN or other non-MN GN, which indicated that anti-PLA2R antibodies were a highly possible cause of glomerular pathology rather than the consequence of proteinuria or glomerular injury. The potential etiological role of anti-PLA2R in iMN and the development of serological assay for anti-PLA2R bring the possibility of non-invasive diagnosis for iMN (43). Nevertheless, question remained about a non-negligible percentage of patients with iMN showing negative test result for serum anti-PLA2R. The negative cases in iMN could be explained by possible existence of other pathogenesis-related auto-antigens such as thrombospondin type1 domain-containing 7A and neutral endopeptidase (44). Besides, the recognition of antigen is strictly configuration-dependent and only reacts with reduction-sensitive epitope (2). Therefore, it is possible that there are other undiscovered cryptic epitopes. Due to a relatively low sensitivity, routine serum test for the anti-PLA2R level is recommended for patient with proteinuria or nephrotic syndrome of unknown etiology in our clinical practice, but a negative result is not able to exclude iMN, which warrants further evaluation by renal biopsy. The sensitivity of gPLA2R was 79%, which was a litter higher than sPLA2R. The combined measurement and interpretation of sPLA2R and gPLA2R might boost the overall performance of the diagnostic value, which worth further exploration. On the other aspect, serological test for anti-PLA2R is known to be highly specific, even though there were still reports about positive results in the test for anti-PLA2R in SMN (1), which is consistent with a pooled specificity of 91% reported in this study. However, there were also studies reporting that the positive test for serum anti-PLA2R were proved to be iMN with superimposed but unrelated hepatitis virus infection or cancer (5, 45), and some scholars advocated the value of anti-PLA2R in excluding non-MN diseases, because no positive result in the test for serum anti-PLA2R has been found in patients with proteinuric condition other than MN (46). More evidence and understanding toward the exact pathogenic role of PLA2R in MN need to be accumulated to provide a thorough rationale for the applicability of PLA2R related test (5).
Based on the result of this meta-analysis and existing evidence, we recommended that the interpretation should be combined with renal biopsy and clinical findings before the next milestone of our comprehension toward PLA2R is achieved. Studies with stricter enrollment criteria to reduce the interference from IST and to ensure the enrolled patients are in an immunologically active stage of the disease would be helpful in giving us a more accurate view on the prevalence of anti-PLA2R in iMN, although the post-treatment measurement of sPLA2R might have its potential value in monitoring disease activity and serve as a guide for therapeutic strategy (47, 48). Future research should focus on evaluating the diagnostic value of anti-PLA2R IgG 4 subtype, which is the prominent subtype of immunoglobulin in iMN and the significance of the combining serum anti-PLA2R and gPLA2R deposit in diagnosing iMN (40).
Conclusion
Both sPLA2R and gPLA2R demonstrated a good diagnostic accuracy in differentiating iMN and non-iMN. The positive test for sPLA2R is highly indicative for the diagnosis of iMN. We recommend that the conduction and interpretation of test for anti-PLA2R and gPLA2R should be combined with renal biopsy and calibrated according to specific clinical scenario.
Author Contributions
WL took the major in conceptualization, literature search and review, article drafting, and writing. YZ contributed by editing and reviewing. PF contributed by general supervision, reviewing, and validation.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Mr. Nicholas Shelhamer for editing this article and picture layout and also Dongmei Chi for helping with the search strategy.
Funding
National Natural Science Foundation of China (81700588), Science Foundation of Health and Family Planning Commission of Sichuan Province (16PJ278), and Young Scholar Foundation of Sichuan University (20826041A4210).
Supplementary Material
The Supplementary Material for this article can be found online at https://www.frontiersin.org/articles/10.3389/fmed.2018.00101/full#supplementary-material.
References
1. Cattran DC, Brenchley PE. Membranous nephropathy: integrating basic science into improved clinical management. Kidney Int (2017) 91(3):566–74. doi:10.1016/j.kint.2016.09.048
2. Beck LH Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med (2009) 361(1):11–21. doi:10.1056/NEJMoa0810457
3. Kattah A, Ayalon R, Beck LH Jr, Sethi S, Sandor DG, Cosio FG, et al. Anti-phospholipase A(2) receptor antibodies in recurrent membranous nephropathy. Am J Transplant (2015) 15(5):1349–59. doi:10.1111/ajt.13133
4. Obrisca B, Ismail G, Jurubita R, Baston C, Andronesi A, Mircescu G. Antiphospholipase A2 receptor autoantibodies: a step forward in the management of primary membranous nephropathy. Biomed Res Int (2015) 2015:249740. doi:10.1155/2015/249740
5. Qin W, Beck LH Jr, Zeng C, Chen Z, Li S, Zuo K, et al. Anti-phospholipase A2 receptor antibody in membranous nephropathy. J Am Soc Nephrol (2011) 22(6):1137–43. doi:10.1681/ASN.2010090967
6. Hoxha E, Harendza S, Zahner G, Panzer U, Steinmetz O, Fechner K, et al. An immunofluorescence test for phospholipase-A(2)-receptor antibodies and its clinical usefulness in patients with membranous glomerulonephritis. Nephrol Dial Transplant (2011) 26(8):2526–32. doi:10.1093/ndt/gfr247
7. Oh YJ, Yang SH, Kim DK, Kang SW, Kim YS. Autoantibodies against phospholipase A2 receptor in Korean patients with membranous nephropathy. PLoS One (2013) 8(4):e62151. doi:10.1371/journal.pone.0062151
8. Svobodova B, Honsova E, Ronco P, Tesar V, Debiec H. Kidney biopsy is a sensitive tool for retrospective diagnosis of PLA2R-related membranous nephropathy. Nephrol Dial Transplant (2013) 28(7):1839–44. doi:10.1093/ndt/gfs439
9. Dou Y, Zhang L, Liu D, Wang C, Quan S, Ma S, et al. The accuracy of the anti-phospholipase A2 receptor antibody in the diagnosis of idiopathic membranous nephropathy: a comparison of different cutoff values as measured by the ELISA method. Int Urol Nephrol (2016) 48(6):845–9. doi:10.1007/s11255-016-1263-6
10. Akiyama S, Akiyama M, Imai E, Ozaki T, Matsuo S, Maruyama S. Prevalence of anti-phospholipase A2 receptor antibodies in Japanese patients with membranous nephropathy. Clin Exp Nephrol (2015) 19(4):653–60. doi:10.1007/s10157-014-1054-2
11. Wei SY, Wang YX, Li JS, Zhao SL, Diao TT, Wang Y, et al. Serum anti-PLA2R antibody predicts treatment outcome in idiopathic membranous nephropathy. Am J Nephrol (2016) 43(2):129–40. doi:10.1159/000445361
12. Hihara K, Iyoda M, Tachibana S, Iseri K, Saito T, Yamamoto Y, et al. Anti-phospholipase A2 receptor (PLA2R) antibody and glomerular PLA2R expression in Japanese patients with membranous nephropathy. PLoS One (2016) 11(6):e0158154. doi:10.1371/journal.pone.0158154
13. Gopalakrishnan N, Abeesh P, Dineshkumar T, Murugananth S, Sakthirajan R, Raman GS, et al. Prevalence of serum anti M-type phospholipase A2 receptor antibody in primary membranous nephropathy: a single center experience. Indian J Nephrol (2016) 26(4):257–61. doi:10.4103/0971-4065.160334
14. Kim YG, Choi YW, Kim SY, Moon JY, Ihm CG, Lee TW, et al. Anti-phospholipase A2 receptor antibody as prognostic indicator in idiopathic membranous nephropathy. Am J Nephrol (2015) 42(3):250–7. doi:10.1159/000440983
15. Hoxha E, Kneissler U, Stege G, Zahner G, Thiele I, Panzer U, et al. Enhanced expression of the M-type phospholipase A2 receptor in glomeruli correlates with serum receptor antibodies in primary membranous nephropathy. Kidney Int (2012) 82(7):797–804. doi:10.1038/ki.2012.209
16. Hayashi N, Akiyama S, Okuyama H, Matsui Y, Adachi H, Yamaya H, et al. Clinicopathological characteristics of M-type phospholipase A2 receptor (PLA2R)-related membranous nephropathy in Japanese. Clin Exp Nephrol (2015) 19(5):797–803. doi:10.1007/s10157-014-1064-0
17. Segarra-Medrano A, Jatem-Escalante E, Quiles-Perez MT, Salcedo MT, Arbos-Via MA, Ostos H, et al. Prevalence, diagnostic value and clinical characteristics associated with the presence of circulating levels and renal deposits of antibodies against the M-type phospholipase A2 receptor in idiopathic membranous nephropathy. Nefrologia (2014) 34(3):353–9. doi:10.3265/Nefrologia.pre2013.Dec.12291
18. Murtas C, Bruschi M, Candiano G, Moroni G, Magistroni R, Magnano A, et al. Coexistence of different circulating anti-podocyte antibodies in membranous nephropathy. Clin J Am Soc Nephrol (2012) 7(9):1394–400. doi:10.2215/CJN.02170312
19. Ardalan M, Ghafari A, Hamzavi F, Nasri H, Baradaran B, Majidi J, et al. Anti-phospholipase A2 receptor antibody in idiopathic membranous nephropathy: a report from Iranian population. J Nephropathol (2013) 2(4):241–8. doi:10.12860/JNP.2013.38
20. Pang L, Zhang A-M, Li H-X, Du J-L, Jiao L-L, Duan N, et al. Serum anti-PLA2R antibody and glomerular PLA2R deposition in Chinese patients with membranous nephropathy. Medicine (2017) 96(24):e7218. doi:10.1097/MD.0000000000007218
21. Behnert A, Schiffer M, Muller-Deile J, Beck LH Jr, Mahler M, Fritzler MJ. Antiphospholipase A(2) receptor autoantibodies: a comparison of three different immunoassays for the diagnosis of idiopathic membranous nephropathy. J Immunol Res (2014) 2014:143274. doi:10.1155/2014/143274
22. Hill PA, McRae JL, Dwyer KM. PLA2R and membranous nephropathy: a 3 year prospective Australian study. Nephrology (Carlton) (2016) 21(5):397–403. doi:10.1111/nep.12624
23. Huang B, Wang L, Zhang Y, Zhang J, Zhang Q, Xiao H, et al. A novel time-resolved fluoroimmunoassay for the quantitative detection of antibodies against the phospholipase A2 receptor. Sci Rep (2017) 7:46096. doi:10.1038/srep46096
24. Kimura Y, Miura N, Debiec H, Morita H, Yamada H, Banno S, et al. Circulating antibodies to alpha-enolase and phospholipase A(2) receptor and composition of glomerular deposits in Japanese patients with primary or secondary membranous nephropathy. Clin Exp Nephrol (2017) 21(1):117–26. doi:10.1007/s10157-016-1235-2
25. Li X, Wei D, Zhou Z, Wang B, Xu Y, Pan J, et al. Anti-PLA2R antibodies in Chinese patients with membranous nephropathy. Med Sci Monit (2016) 22:1630–6. doi:10.12659/MSM.896090
26. Liu Y, Li X, Ma C, Wang P, Liu J, Su H, et al. Serum anti-PLA2R antibody as a diagnostic biomarker of idiopathic membranous nephropathy: the optimal cut-off value for Chinese patients. Clin Chim Acta (2018) 476:9–14. doi:10.1016/j.cca.2017.11.006
27. Ong L, Silvestrini R, Chapman J, Fulcher DA, Lin MW. Validation of a phospholipase A2 receptor antibody ELISA in an Australian cohort with membranous glomerulonephritis. Pathology (2016) 48(3):242–6. doi:10.1016/j.pathol.2016.02.001
28. Radice A, Pieruzzi F, Trezzi B, Ghiggeri G, Napodano P, D’Amico M, et al. Diagnostic specificity of autoantibodies to M-type phospholipase A2 receptor (PLA2R) in differentiating idiopathic membranous nephropathy (IMN) from secondary forms and other glomerular diseases. J Nephrol (2018) 31(2):271–8. doi:10.1007/s40620-017-0451-5
29. Timmermans SA, Damoiseaux JG, Heerings-Rewinkel PT, Ayalon R, Beck LH Jr, Schlumberger W, et al. Evaluation of anti-PLA2R1 as measured by a novel ELISA in patients with idiopathic membranous nephropathy: a cohort study. Am J Clin Pathol (2014) 142(1):29–34. doi:10.1309/AJCP8QMOY5GLRSFP
30. Xie Q, Li Y, Xue J, Xiong Z, Wang L, Sun Z, et al. Renal phospholipase A2 receptor in hepatitis B virus-associated membranous nephropathy. Am J Nephrol (2015) 41(4–5):345–53. doi:10.1159/000431331
31. Zhang Q, Huang B, Liu X, Liu B, Zhang Y, Zhang Z, et al. Ultrasensitive quantitation of anti-phospholipase A2 receptor antibody as A diagnostic and prognostic indicator of idiopathic membranous nephropathy. Sci Rep (2017) 7(1):12049. doi:10.1038/s41598-017-12014-1
32. Lonnbro-Widgren J, Ebefors K, Molne J, Nystrom J, Haraldsson B. Glomerular IgG subclasses in idiopathic and malignancy-associated membranous nephropathy. Clin Kidney J (2015) 8(4):433–9. doi:10.1093/ckj/sfv049
33. Larsen CP, Messias NC, Silva FG, Messias E, Walker PD. Determination of primary versus secondary membranous glomerulopathy utilizing phospholipase A2 receptor staining in renal biopsies. Mod Pathol (2013) 26(5):709–15. doi:10.1038/modpathol.2012.207
34. Yeo MK, Kim YH, Choi DE, Choi SY, Kim KH, Suh KS. The Usefulness of phospholipase A2 receptor and IgG4 detection in differentiation primary membranous nephropathy from secondary membranous nephropathy in renal biopsy. Appl Immunohistochem Mol Morphol (2017). doi:10.1097/PAI.0000000000000460
35. Dong HR, Wang YY, Cheng XH, Wang GQ, Sun LJ, Cheng H, et al. Retrospective study of phospholipase A2 receptor and IgG subclasses in glomerular deposits in Chinese patients with membranous nephropathy. PLoS One (2016) 11(5):e0156263. doi:10.1371/journal.pone.0156263
36. Gudipati A, Uppin MS, Kalidindi RK, Swarnalatha G, Das U, Taduri G, et al. Immunohistochemical analysis of anti-phospholipase A2 receptor antibody on renal biopsies: a single tertiary care center Study. Indian J Nephrol (2017) 27(5):353–8. doi:10.4103/ijn.IJN_79_17
37. Liu H, Luo W, Gong S, Ding X. Detection and clinical significance of glomerular M-type phospholipase A2 receptor in patients with idiopathic membranous nephropathy. Intern Med J (2016) 46(11):1318–22. doi:10.1111/imj.13233
38. Roy S, Korula A, Basu G, Jacob S, Varughese S, Tamilarasi V. Immunohistochemical glomerular expression of phospholipase A2 receptor in primary and secondary membranous nephropathy: a retrospective study in an Indian cohort with clinicopathological correlations. Nephron Extra (2017) 7(1):1–9. doi:10.1159/000453675
39. Dai H, Zhang H, He Y. Diagnostic accuracy of PLA2R autoantibodies and glomerular staining for the differentiation of idiopathic and secondary membranous nephropathy: an updated meta-analysis. Sci Rep (2015) 5:8803. doi:10.1038/srep08803
40. Debiec H, Ronco P. PLA2R autoantibodies and PLA2R glomerular deposits in membranous nephropathy. N Engl J Med (2011) 364(7):689–90. doi:10.1056/NEJMc1011678
41. Hofstra JM, Debiec H, Short CD, Pelle T, Kleta R, Mathieson PW, et al. Antiphospholipase A2 receptor antibody titer and subclass in idiopathic membranous nephropathy. J Am Soc Nephrol (2012) 23(10):1735–43. doi:10.1681/ASN.2012030242
42. Kalantari S, Nafar M. A comprehensive narrative review of diagnostic biomarkers in human primary membranous nephropathy. Biomark Med (2017) 11:9. doi:10.2217/bmm-2017-0081
43. Brenchley PE. Anti-phospholipase A2 receptor antibody and immunosuppression in membranous nephropathy: more evidence for pathogenicity of anti-phospholipase A2 receptor autoantibodies. J Am Soc Nephrol (2015) 26(10):2308–11. doi:10.1681/ASN.2015020181
44. Beck LH Jr. PLA2R and THSD7A: disparate paths to the same disease? J Am Soc Nephrol (2017) 28(9):2579–89. doi:10.1681/ASN.2017020178
45. Timmermans SAMEG, Ayalon R, van Paassen P, Beck LH Jr, van Rie H, Wirtz JJJM, et al. Anti–phospholipase A2 receptor antibodies and malignancy in membranous nephropathy. Am J Kidney Dis (2013) 62(6):1223–5. doi:10.1053/j.ajkd.2013.07.019
46. Hofstra JM, Wetzels JF. Phospholipase A2 receptor antibodies in membranous nephropathy: unresolved issues. J Am Soc Nephrol (2014) 25(6):1137–9. doi:10.1681/ASN.2014010091
47. Beck LH Jr, Salant DJ. Membranous nephropathy: recent travels and new roads ahead. Kidney Int (2010) 77(9):765–70. doi:10.1038/ki.2010.34
Keywords: membranous nephropathy, M-type phospholipase A2 receptor, anti-phospholipase A2 receptor, diagnostic test accuracy, secondary membranous nephropathy, sPLA2R, gPLA2R
Citation: Li W, Zhao Y and Fu P (2018) Diagnostic Test Accuracy of Serum Anti-PLA2R Autoantibodies and Glomerular PLA2R Antigen for Diagnosing Idiopathic Membranous Nephropathy: An Updated Meta-Analysis. Front. Med. 5:101. doi: 10.3389/fmed.2018.00101
Received: 15 February 2018; Accepted: 28 March 2018;
Published: 26 April 2018
Edited by:
Christos Argyropoulos, University of New Mexico, United StatesReviewed by:
Swapnil Hiremath, University of Ottawa, CanadaTetsuhiro Tanaka, The University of Tokyo, Japan
Copyright: © 2018 Li, Zhao and Fu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping Fu, fupinghx@163.com
 Weiying Li
Weiying Li Yuliang Zhao
Yuliang Zhao  Ping Fu
Ping Fu