Anti-High-Density Lipoprotein Antibodies and Antioxidant Dysfunction in Immune-Driven Diseases
- 1Area of Immunology, Department of Functional Biology, University of Oviedo, Oviedo, Spain
- 2Instituto de Investigación Sanitaria del Principado de Asturias (ISPA), Oviedo, Spain
- 3Department of Immunology, Hospital Universitario Central de Asturias, Oviedo, Spain
- 4Academic Rheumatology Department, King’s College London, London, United Kingdom
- 5Rheumatology Department, Whittington Hospital, London, United Kingdom
Introduction: Impaired high-density lipoprotein (HDL) levels and antioxidant functionality of HDL, mainly attributed to a decreased paraoxonase-1 (PON1) functionality, have been described in autoimmune conditions. In this setting, a role for humoral response in cardiovascular disease is emerging. This study evaluates the role of immunoglobulin G (IgG) antibodies against HDL and disease-related autoantibodies on HDL dysfunction in immune-driven diseases.
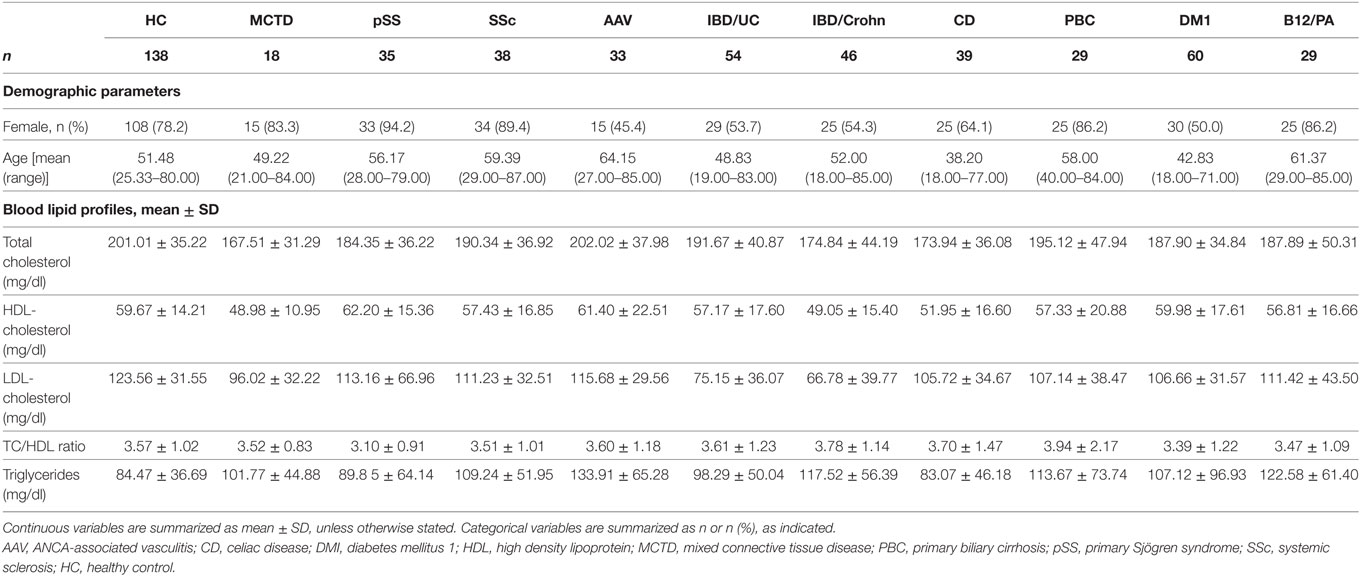
Methods: Serum IgG anti-HDL antibodies, PON1 activity, and total antioxidant capacity (TAC) were quantified in 381 patients with different immune-driven diseases [18 mixed connective tissue disease (MCTD), 35 primary Sjögren syndrome (pSS), 38 systemic sclerosis (SSc), 33 ANCA-associated vasculitis (AAV), 60 diabetes mellitus 1, 29 autoimmune B12 deficiency/pernicious anemia, 29 primary biliary cirrhosis, 46 IBD/Crohn, 54 IBD/UC, and 39 celiac disease (CD)] and 138 healthy controls.
Results: IgG anti-HDL antibodies were increased in MCTD, pSS, AAV, and inflammatory bowel disease (IBD) [Crohn and ulcerative colitis (UC)], even after correcting for total IgG levels, but not in organ-specific autoimmune diseases. Anti-HDL antibodies were negatively associated with PON1 activity in MCTD (r = −0.767, p < 0.001) and AAV (r = −0.478, p = 0.005), whereas both anti-HDL and anti-neutrophil cytoplasm antibod levels were related to an impaired PON1 activity and TAC in IBD/UC. In SSc, anti-centromere antibodies correlated PON1 activity. anti-Saccharomyces cerevisiae antibodies levels were negatively associated with PON1 activity (r = −0.257, p = 0.012) and PON1/TAC ratio (r = −0.261, p = 0.009) in IBD/Crohn. HDL dysfunction in CD was only related to anti-transglutaminase levels.
Conclusion: IgG anti-HDL antibodies and HDL dysfunction are common hallmarks of systemic autoimmunity. Anti-HDL and disease-related autoantibodies account for the HDL antioxidant dysfunction in immune-driven conditions, mainly in systemic autoimmune disorders.
Introduction
Systemic autoimmune disorders are commonly associated with an increased risk of cardiovascular disease (CVD) occurrence compared to the general population (1, 2). Different studies showed that traditional CV risk factors fail to fully account for this increased CV morbidity (3, 4), whereas chronic inflammation and immune dysregulation are thought to play a role (5, 6). However, the actual mediators remain unknown.
High-density lipoproteins (HDL) are pivotal in the prevention of atherosclerosis. HDL promote endothelial homeostasis by their role in the reverse cholesterol transport as well as by their antioxidant and anti-inflammatory activities (7). Altered levels of HDL and blood lipids have been described in systemic autoimmune diseases, and a paradoxical effect on CV risk (the so-called “lipid paradox,” whereby low lipid levels are associated with increased CV risk) is widely accepted (8, 9), although poorly understood. Similarly, impaired HDL functionality, mainly due to a decreased enzymatic activity of the calcium-dependent esterase paraoxonase 1 (PON1), has been reported in these conditions (10–12). Due to their association with CVD development, understanding changes in lipid levels and/or functionality as well as the underlying mechanisms, represents a major research topic.
In recent years, the hypothesis that autoantibodies can have a role in the pathogenesis of CVD has emerged from clinical studies (13–17). However, contradictory results (18, 19) suggest that although the humoral immune response can be linked to CVD development, the specific autoantibodies and their targets are yet to be elucidated.
Recent findings point to a detrimental interplay between autoantibodies and lipid profiles. Autoantibodies targeting HDL lipoproteins [immunoglobulin G (IgG) anti-HDL antibodies] and their components have been described in systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA). Initially described in association with disease activity and antioxidant features (20, 21), our group has recently revealed an association among anti-HDL antibodies, lipid profiles, and lipoprotein functionality in SLE and RA (22–24). Interestingly, these antibodies were not related to traditional CV risk factors, hence confirming its role as non-traditional CV risk factors in this setting. Thus, these autoantibodies may be considered as orchestrators of the interactions between immunity and altered lipid profiles. However, with anti-HDL being documented in RA and SLE with similar clinical outcomes, these findings pose the question as whether these antibodies may be also detected in other immune-driven conditions.
We hypothesize that the HDL dysfunction associated with anti-HDL antibodies can be a common feature in immune-mediated diseases rather than a specific phenomenon of a single disease. Thus, the overarching aim of this study is to analyze the levels of anti-HDL antibodies in a broad range of different immune-mediated conditions. The specific objectives are (i) to analyze the levels of IgG anti-HDL antibodies in different immune-driven diseases, from systemic rheumatic to organ-specific autoimmune conditions, (ii) to study the associations between anti-HDL antibodies, lipid profiles, and HDL functionality, and (iii) to analyze the interactions between the IgG anti-HDL–HDL axis with clinical features and disease-related autoantibodies among diseases.
Materials and Methods
Patients and Controls
This study involved a cross-sectional sample of 381 individuals with different immune-mediated diseases recruited from the department of Clinical Immunology at the Hospital Universitario Central de Asturias (Asturias, Spain). Patients were recruited after a clinical appointment in the respective clinical departments (Internal Medicine, Rheumatology, Gastroenterology and Nephrology) at the same institution. Fasting blood samples, taken during the routine clinical examination at the clinical departments, were sent to the department of Clinical Immunology to be immediately processed and stored at −80°C until further analyses. A fresh aliquot was used for the determination of the panel of the disease-related autoantibodies for each condition (Table 1).
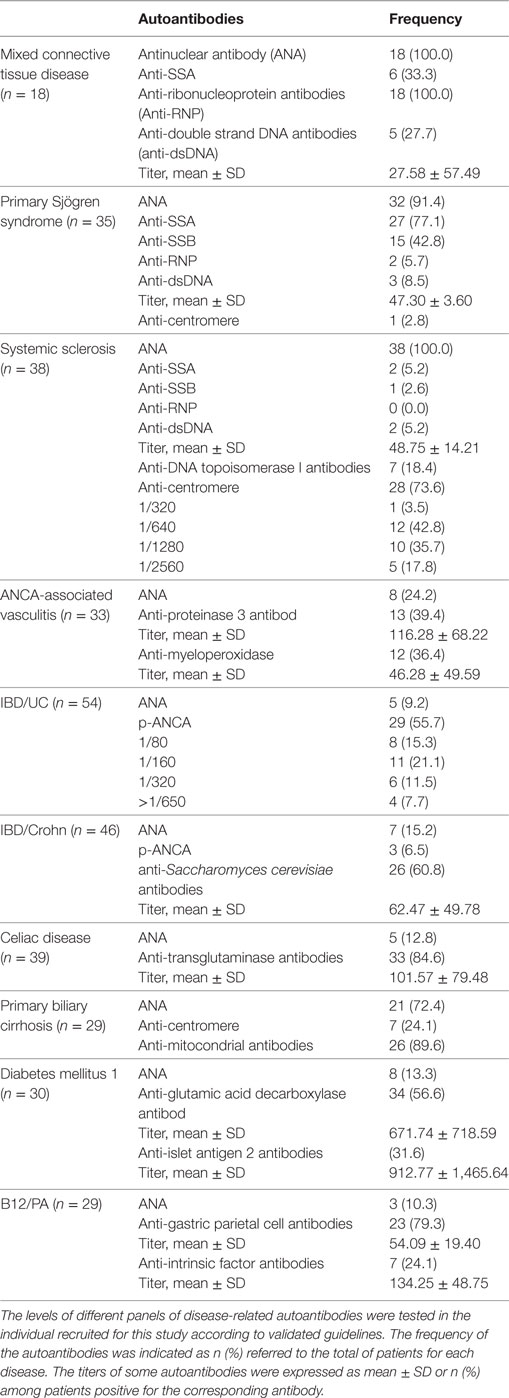
Disease diagnoses were confirmed by the corresponding consultant from each clinical department according to validated criteria: Alarcón-Segovia criteria for mixed connective tissue disease (MCTD) (25), 2002 EULAR classification criteria for primary Sjögren Syndrome (pSS) (26), 1980 ACR criteria for Systemic Sclerosis (SSc) (27), 1990 ACR criteria for the classification of vasculitis (28), criteria for primary biliary cirrhosis (29), American Diabetes Association criteria for type 1 diabetes mellitus (DM1) (30), classification criteria for gastritis and pernicious anemia (B12/PA) (31), Vienna/Montreal criteria for Inflammatory Bowel Diseases (IBD) (32, 33), and World Gastroenterology Organization guidelines for celiac disease (CD) (34).
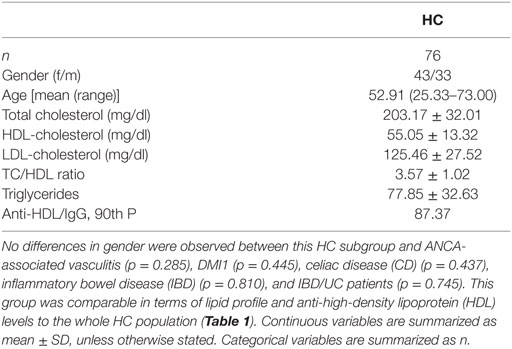
A group of 138 matched healthy individuals (without any disease or treatment at the time of sampling) from the same population was simultaneously recruited as the healthy control (HC) group. Automated analysis of serum lipids was performed on fresh blood samples from all of the participants. Study approval was obtained from the Institutional Review Board (Comité de Ética Regional de Investigación Clínica) in compliance with the Declaration of Helsinki. All participants gave written informed consent prior to their inclusion in the study.
Analysis of Autoantibodies
Antinuclear, anti-neutrophil cytoplasm, and anti-mitochondria antibodies [antinuclear antibodies (ANA), anti-neutrophil cytoplasm antibodies (ANCA), and anti-mitocondrial antibodies] were determined by indirect immunofluorescence on Hep-2 cells, ethanol/formalin fixed neutrophils, and rat liver/stomach/kidney (INOVA Diagnostics, San Diego, CA, USA), respectively. Anti-double strand DNA antibodies, myeloperoxidase, proteinase 3 (PR3), and transglutaminase IgA (TGA) antibodies were quantified in a chemiluminescent analyzer (ZENIT RA, Menarini Diagnostics, Firenze, Italy). ENA specificities (SSA/Ro, SSB/La, RNP, Sm, and Scl-70) were identified by line blot analysis (Euroimmun, Lübeck, Germany). An ELISA technique was used to quantify antibodies against gastric parietal cells, intrinsic factor (Menarini Diagnostics), glutamic decarboxylase (GAD), tyrosine phosphatase-related islet antigen 2 (IA2) (RSR Ltd., Cardiff, UK), and Saccharomyces cerevisiae [anti-Saccharomyces cerevisiae antibodies (ASCA)] (Wieslab, Malmö, Sweden).
Determination of IgG anti-HDL Antibodies
Serum levels of IgG anti-HDL antibodies were measured by ELISA techniques as previously described (23, 35). Briefly, 96-well NUNC Maxisorp plates were overnight coated with 20 µg/ml human HDL (Sigma Aldrich, Germany) in 70% ethanol (test half) or ethanol alone (control half). After a blocking for 1 h (PBS 1% BSA), plates were washed and 1:50-diluted serum samples were incubated for 2 h at room temperature. Then, plates were washed with TBS (three times) and an alkaline phosphatase-conjugated anti-human IgG (1:1,000) (Immunostep, Spain) was added. One-hour later, the plates were washed twice with TBS and 1 mg/ml p-nitrophenylphosphate (Sigma) in dietanolamine buffer was added as substrate. Absorbance at 405 nm was recorded and signal from the control half of the plate was subtracted to that of the test half. Anti-HDL arbitrary units (AU) were calculated for each sample according to the standard curves (from pooled sera diluted 1:16 to 1:512).
Total IgG was quantified by conventional ELISA techniques and AU values obtained from the anti-HDL ELISA were corrected using total IgG levels (anti-HDL/IgG). To evaluate the burden of IgG anti-HDL positivity, the cutoff for an individual to be classified as “positive” was set using the 90th percentile found in the HC population (=90.78) (23, 35).
Assessment of PON1 Activity
Serum PON1 activity was assessed according to the method described by Eckerson et al. with little modifications as previously described (23). Briefly, 300 µl of freshly prepared 1 mM paraoxon (Sigma) in 50 mM glycine buffer containing 1 mM CaCl2 (pH 10.5) was incubated with 7.5 µl of serum samples in 96-well NUNC Maxisorp plates for 20 min at 37°C. Production of p-nitrophenol was monitored at 405 nm. A unit of PON1 activity was expressed as micromoles of p-nitrophenol produced per minute and per milliliter of serum. Quality controls were included in each plate to correct for interassay variations.
Analysis of Total Antioxidant Capacity (TAC)
Serum TAC was measured by means of a colorimetric assay based on the cupric reducing antioxidant capacity (CUPRAC method). A commercial kit (TAC Assay Kit, Sciencell Research Laboratories) was used following the instructions provided by the manufacturer. Serum TAC was expressed as millimolar Trolox equivalent units (mM T-Eq).
Statistical Analysis
Continuous variables were summarized as median (interquartile range) or mean ± SD, whereas n (%) was used for categorical ones. Differences among groups were analyzed by Kruskal–Wallis (with Dunn–Bonferroni correction for multiple comparisons tests) or Mann–Whitney U tests, as appropriated. Spearman’s rank test was used for correlations, whereas χ2 tests were performed to analyze the distribution of categorical variables. Multiple regression analyses were carried out to evaluate the potential simultaneous effect of different independent factors on a dependent variable. Variables were log-transformed to achieve normal distribution prior to multiple regression analyses and B coefficients and 95% confidence intervals (CI) were computed. Significance was assumed at a p-value of <0.050. All statistical analyses were performed under SPSS 22.0 and GraphPad Prism 5.0 for Windows.
Results
Levels of IgG anti-HDL Antibodies in Immune-Mediated Diseases
The levels of IgG anti-HDL antibodies were quantified in serum samples from 138 HC and 381 patients with different immune-driven diseases (Table 1). Levels of IgG anti-HDL antibodies were found to be increased in MCTD [40.98 (164.65) AU, p < 0.001], pSS [19.72 (51.38) AU, p = 0.012], AAV [23.90 (42.26) AU, p = 0.030], CD [11.23 (31.63) AU, p = 0.050], IDB/Crohn [12.16 (53.03) AU, p = 0.020], and IBD/UC [17.34 (79.79) AU, p < 0.001] patients compared to those in HC [2.44 (12.92) AU]. Equivalent results were obtained when anti-HDL levels were corrected for total IgG levels (anti-HDL/IgG) (Figure 1A). According to the established cutoff, increased prevalence of anti-HDL positivity was found in MCTD, SS, AAV, IBD/Crohn, and IBD/UC (Figure 1B). IgG anti-HDL antibodies were not associated with age (r = −0.027, p = 0.765) nor with gender (p = 0.136) in HC or patients (both p > 0.050). Since a differential distribution of gender was observed in AAV, DM1, CD, IBD/C, and IBD/UC, a subgroup of HC with paired gender distribution was selected (Table 2). Differences in anti-HDL levels using this control population remained statistically significant (Figure S1 in Supplementary Material), therefore excluding the possibility that gender distribution could be a confounding factor.
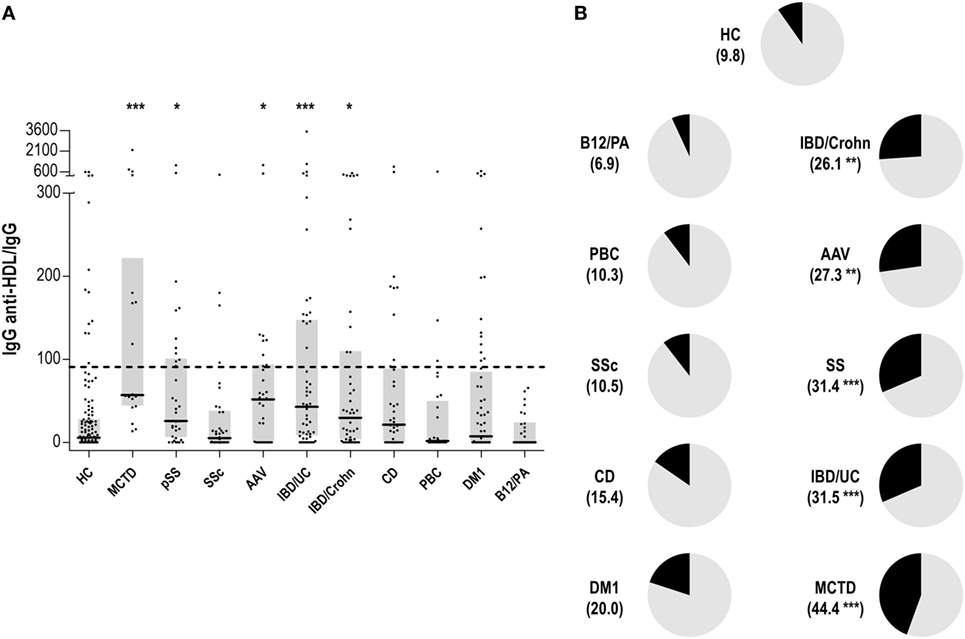
Figure 1. Immunoglobulin G (IgG) anti-high-density lipoprotein (HDL) antibodies in different autoimmune conditions. (A) Serum levels of anti-HDL/IgG antibodies in different autoimmune conditions and healthy control (HC). Each dot represents one subject, whereas horizontal bars represent the median value and gray boxes delimit 25th and 75th percentiles. Horizonal dashed lines represents the value of 90th percentile of anti-HDL/IgG in the HC group (=90.78). Differences against HC group were assessed by Kruskal–Wallis test and Dunnett correction for multiple comparisons test. (B) Positivity of anti-HDL antibodies in autoimmune conditions. The frequency of individuals classified as anti-HDL positive (using the 90th percentile found in the HC group as reference) is shown for each condition between parenthesis and represented as the black section of each graph. Differences compared to the HC were assessed by χ2 tests. Statistical significance is indicated as *p < 0.05, **p < 0.010, and ***p < 0.001.
Finally, the potential associations between anti-HDL/IgG levels and the blood lipid profile were studied. However, anti-HDL/IgG was not correlated with total cholesterol (r = −0.023, p = 0.801), nor with HDL (r = −0.002, p = 0.979) or LDL fractions (r = −0.035, p = 0.703) in HC, neither among immune-mediated diseases (all p < 0.050).
All these results revealed a specific increase in IgG anti-HDL antibodies in patients with systemic immune-mediated disorders, but not in organ-specific conditions.
IgG Anti-HDL Antibodies and PON1 Activity
We wondered whether IgG anti-HDL antibodies could be associated to a decreased HDL functionality. To test this hypothesis, serum PON1 activity and TAC were measured.
Serum PON1 activity was decreased in MCTD, SSc, AAV, CD, IBD/Crohn, and IBD/UC patients (Figure 2A), suggesting an overlap between impaired PON1 activity and increased anti-HDL positivity. Interestingly, anti-HDL/IgG antibodies were negatively correlated with PON1 activity in MCTD, AAV, and IBD/UC (Figure 2B).
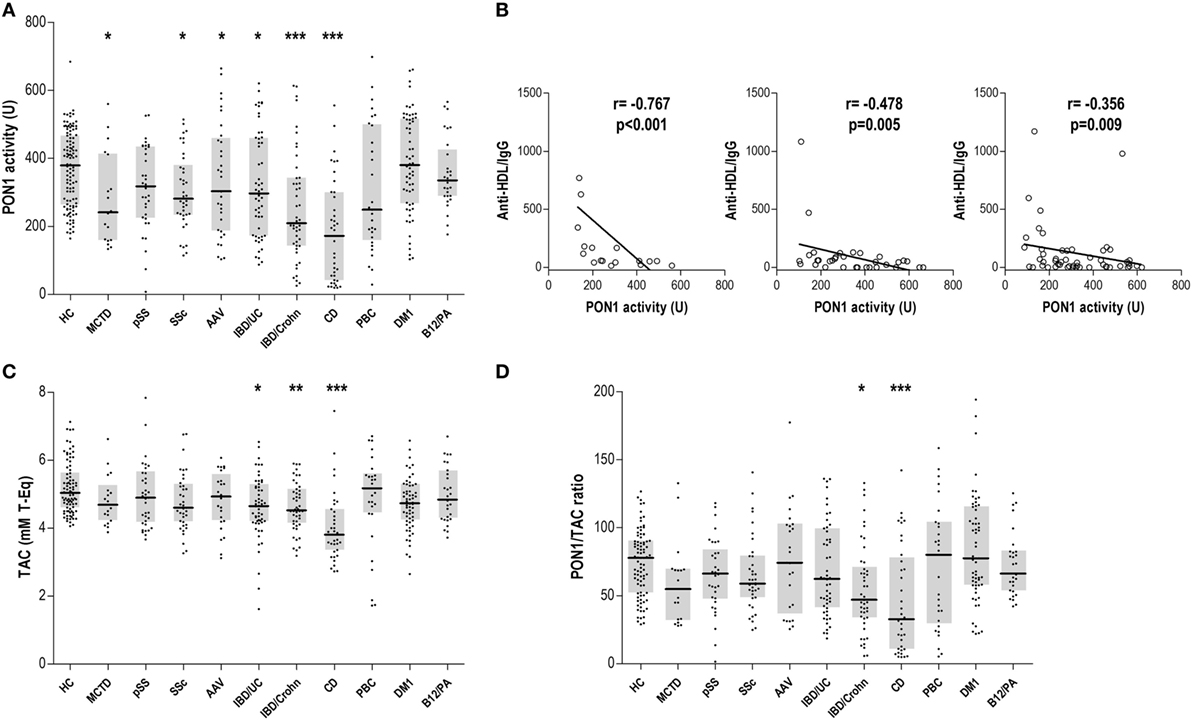
Figure 2. Paraoxonase 1 (PON1) activity, total antioxidant capacity (TAC), and PON1/TAC ratio in autoimmune conditions. (A) Serum PON1 activity, (C) serum TAC, and (D) PON1/TAC ratio were evaluated in autoimmune patients and healthy control (HC). Each dot represents one subject, whereas horizontal bar represents the median value and gray boxes delimit 25th and 75th percentiles. Differences against HC group were assessed by Kruskal–Wallis test and Dunnett correction for multiple comparisons test. Statistical significance is indicated as *p < 0.05, **p < 0.010, and ***p < 0.001. (B) Association between anti-high-density lipoprotein/IgG and serum PON1 activity in mixed connective tissue disease (MCTD), ANCA-associated vasculitis (AAV), and IBD/UC patients. Correlations were assessed by Spearman rank’s test.
Total antioxidant capacity was decreased in patients with gut-related immune conditions (Figure 2C). Remarkably, anti-HDL levels were only associated with TAC in IBD/UC (r = −0.343, p = 0.011). However, a positive correlation between TAC and PON1 activity was observed in IBD/Crohn patients (r = 0.360, p = 0.014). The analysis of the PON1/TAC ratio (Figure 2D) revealed a different contribution of the PON1 activity to the total antioxidant status, being lower in CD.
Therefore, our findings demonstrate an antioxidant dysfunction in some immune-driven diseases. IgG anti-HDL antibodies may account for the impaired PON1 activity in some systemic conditions.
IgG Anti-HDL Antibodies–PON1 Axis in Systemic Immune-Driven Diseases: Involvement of Disease-Related Autoantibodies
Since a notable heterogeneity was found among immune-driven diseases not only concerning the IgG anti-HDL levels observed but also regarding the association between the latter and serum PON1 activity, we aimed to evaluate whether disease-related autoantibodies (Table 3) can explain these discrepancies.
Anti-HDL/IgG antibodies nor PON1 activity were associated with disease-related autoantibodies in MCTD, pSS, or AAV. Interestingly, a different picture was observed in SSc patients. No differences in IgG anti-HDL/IgG antibodies (p = 0.334) or PON1 activity (p = 0.253) were found between limited (n = 29) or diffuse SSc (n = 6) patients. However, ANA titer was negatively correlated with serum PON1 activity (r = −0.404, p = 0.012), this correlation being restricted to patients with a centromere pattern (Figure 3A). Additionally, SSc patients positive for anti-DNA Topoisomerase I antibodies exhibited diminished TAC compared to their negative counterparts [4.21 (0.60) vs 5.47 (1.39) mM T-Eq, p = 0.042].
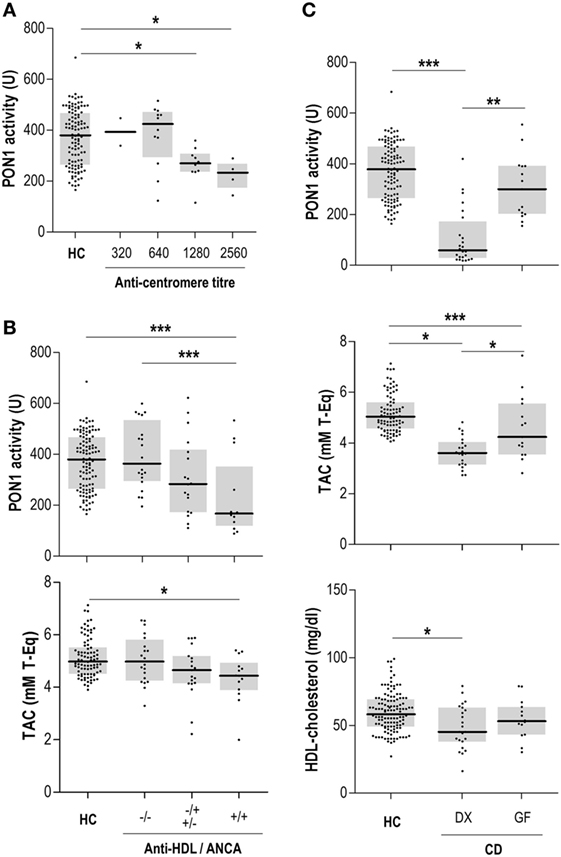
Figure 3. Anti-high-density lipoprotein (HDL) antibodies, disease-related autoantibodies, and paraoxonase 1 (PON1) activity. (A) Association between anti-centromere titer and serum PON1 activity in systemic sclerosis patients. (B) Association between anti-HDL and anti-neutrophil cytoplasm antibodies (ANCA) positivity and PON1 activity and serum total antioxidant capacity (TAC) in IBD/UC patients. (C) PON1 activity, serum TAC, and HDL levels in celiac disease (CD) patients recruited at diagnosis (DX) or those under a strict gluten-free diet (GF). Each dot represents one subject, whereas horizontal bars represent the median value and gray boxes delimit 25th and 75th percentiles. Differences were assessed by Kruskal–Wallis test and Dunn–Bonferroni correction for multiple comparisons test. Statistical significance is indicated as *p < 0.05, **p < 0.010, and ***p < 0.001.
Concerning organ-specific conditions, anti-HDL/IgG antibodies paralleled those of anti-glutamic acid decarboxylase antibodies (anti-GAD) (r = 0.246, p = 0.068) in DM1. Thus, anti-HDL positivity was slightly higher in anti-GAD positive DM1 patients (3/34, 26.4%) compared to their anti-GAD negative counterparts (3/22, 13.6%). Moreover, serum PON1 activity exhibited a non-significant negative trend with IA2 titer (r = −0.237, p = 0.079). No differences between DM1 recruited at onset (n = 6) and those recruited in later stages were found.
Gut-related conditions exhibited disease-specific patterns regarding the associations between the IgG anti-HDL-PON1 axis and disease-related antibodies. In Crohn patients, ASCA levels were associated with PON1 activity (r = −0.257, p = 0.012) and PON1/TAC ratio (r = −0.261, p = 0.009). On the other hand, PON1 activity was negatively correlated to ANCA titer (r = −0.382, p = 0.006) in IBD/UC. A clear additive effect on PON1 impairment was observed when both ANCA and anti-HDL autoantibodies were present (Figure 3B). This negative effect on PON1 activity was confirmed by multiple regression analysis: ANCA (B[95% CI], p: −0.326 [−0.284, −0.023], p = 0.022) and anti-HDL (−0.273 [−0.280, −0.002], p < 0.050). A similar detrimental effect was also observed for TAC (Figure 3B). Finally, PON1 activity and TAC were strongly decreased in CD, an association among anti-transglutaminase antibodies (anti-TGA) antibodies, HDL levels and antioxidant features being disclosed. Patients recruited at disease diagnosis (DX, n = 24) exhibited notable differences in serum PON1, TAC, and HDL levels compared with those with a longer disease duration following a strict gluten-free diet (GF, n = 15) (Figure 3C). These differences paralleled those found with anti-TGA levels [DX: 125.00 (178.78) vs GF: 15.80 (90.52) IU, p = 0.019].
Overall, anti-HDL IgG were not associated with disease-related autoantibodies, thus reinforcing the relevance of anti-HDL as novel biomarkers. Interestingly, anti-HDL and disease-related autoantibodies exhibited different associations with PON1 activity, HDL and PON1 among the different conditions.
Discussion
Although a growing body of evidence supports a role for humoral immunity in CVD, the underlying mediators remain unclear. The results presented in this study point to the autoantibodies against HDL as the missing link between humoral response, lipoprotein dysfunction and oxidative stress in immune-driven diseases. Anti-HDL antibodies can be proposed as an explanation for the elusive hypothesis of the “lipid paradox.” Although initially described as a consequence of the inflammatory burden, further studies revealed that inflammation itself cannot totally account for the alteration in the lipid profile (9), thus suggesting the involvement of additional mediators. More recently, the concept of “lipid paradox” has been expanded to “HDL dysfunction,” since not only the levels but also the HDL functionality has been found altered in inflammatory diseases (36, 37). Thus, anti-HDL antibodies emerge as potential orchestrators of this phenomenon. Because of the role of lipoprotein dysfunction in CVD, a role for anti-HDL antibodies as biomarkers may be expected.
Anti-HDL Antibodies in Immune-Mediated Diseases: Systemic vs Organ-Specific Conditions
A remarkable finding from our study is the difference in anti-HDL levels among different immune-driven diseases. The highest anti-HDL levels were found in systemic autoimmune rheumatic conditions (MCTD, pSS, and AAV) as well as in IBD, whereas increased anti-HDL levels were not observed in organ-specific autoimmune diseases. These results are in line with previous studies from our group and others, revealing a similar prevalence in RA and SLE (23, 24, 35).
Among systemic diseases, MCTD exhibited the highest prevalence of anti-HDL positivity. Interestingly, although CVD occurrence is increased in MCTD (38), the responsible mechanisms are unknown. Increased levels of disease-related autoantibodies have been found in MCTD patients with CVD (39, 40), thereby suggesting a role for humoral immune response in the CVD development in MCTD. However, these studies failed to disclose an association between these autoantibodies and markers of CVD. Instead, decreased apolipoprotein (Apo) A1, but not Apo B, was associated to impaired vascular homeostasis (39), stressing the relevance of HDL particles. Our results show a clear association between anti-HDL antibodies and the impaired PON1 activity in MCTD, thus strengthening the role of these autoantibodies in this condition. Moreover, MCTD exhibited greater alterations in surrogate markers of CVD than other connective tissue disorders, such as pSS or SSc (41) and different mechanisms of vascular damage between MCTD and SSc have been reported (42), which is in accordance with the differences in the association between IgG anti-HDL-PON1 axis and disease-related autoantibodies herein reported.
Another interesting finding from our study is the emergence of anti-HDL antibodies in gut-related diseases. Increased levels of anti-HDL antibodies were found in IBD, comparable to those in systemic autoimmune diseases. An increased CVD risk has been documented in IBD (43), especially during disease flares (44), hence suggesting the involvement of immune-driven mechanisms. However, although impaired lipid levels (45) and PON1 activity (46) have been reported in IBD, the underlying mediators are yet to be defined. Our findings expand the current knowledge in this scenario demonstrating a connection between anti-HDL and PON1 functionality in IBD. The differences in anti-HDL levels between ulcerative colitis (UC) and Crohn disease mirror that of the prevalence of subclinical atherosclerosis burden (47) and may be attributed to the shift toward Th2-response and humoral immunity present in UC compared to Crohn (48). Interestingly, HDL dysfunction in Crohn was similar to that of CD, in accordance with shared immunopathological features between these disorders (49). CD patients exhibited no increased anti-HDL levels, despite showing a notable impairment in PON1 activity and an increased pro-oxidant status. However, the alterations in PON1, TAC, and HDL levels were counteracted by a strict gluten-free diet, in parallel with a reduction of anti-TGA titers. Taken together, these findings may underlie the beneficial effects of a gluten-free diet on CVR factors in CD (50), something worth keeping in mind in clinical practice.
Anti-HDL Antibodies and Lipoprotein Dysfunction: A Translational Approach for the Clinical Setting
CVR stratification and management in immune-driven diseases is suboptimal (51). A major criticism in the management approach over time has been the lack of a more holistic approach to patient care and a more index disease-centric care, with less attention to comorbid conditions or CVR. Moreover, the lack of a strong interphase between primary and secondary care, clinical pressures and health service infrastructures that challenge the management of factors and conditions beyond the index disease, along with routine unavailability of advanced imaging techniques for CVR stratification, all negatively influence the management of these conditions. Furthermore, available tools for CVR assessment have been demonstrated to be insufficient in systemic diseases (1, 52), as they are solely based on traditional CVR factors, hence emphasizing the urgent need of additional biomarkers. Taking into account our findings, anti-HDL antibodies may potentially represent a useful biomarker for addressing this unmet clinical need. Anti-HDL antibodies may improve the CVR management by identifying patients where lipoprotein dysfunction is observed and thus, capturing this pathological feature. Importantly, anti-HDL levels can be measured through conventional, operator-independent, and automatized laboratory techniques, hence representing a cost-effective option.
Overall, our findings delineate four different scenarios related to four different associations between the role of autoantibodies with the lipid profiles and HDL dysfunction. One scenario is composed by those diseases where lipoprotein dysfunction is solely associated with anti-HDL antibodies, such as MCTD or AAV. Previous results from our group and others suggest similar conclusions in SLE and RA (20, 22–24), with anti-HDL but not disease-related autoantibodies being associated with HDL dysfunction, indicating that this scenario may be restricted to systemic autoimmune diseases. Another group can be considered for diseases where blood lipid outcomes are determined by both anti-HDL and disease-related autoantibodies, such as IBD/UC or SSc. Moreover, some conditions exhibit alterations in the blood lipids that are only associated with disease-related autoantibodies, like IBD/Crohn or DM1. Finally, an independent group can be considered for those diseases where other (non-autoimmune) mechanisms are linked to HDL dysfunction, such as CD.
The fact that different scenarios can be delineated paves the ground for the implementation of tailored strategies to complement the currently available CVR stratification strategies (Figure 4). Including a screening of anti-HDL antibodies in systemic diseases (first group) would be advisable to improve CVR stratification. Similarly, anti-HDL assessment together with disease-related autoantibodies should be performed in the second group of patients. On the contrary, a tight control of disease features, including disease-related autoantibodies screening, should be performed in the third group, since anti-HDL antibodies seem to not play a significant role. These different scenarios are in line with the concept of personalized medicine which is particularly relevant in those with complex clinical needs (Figure 4).
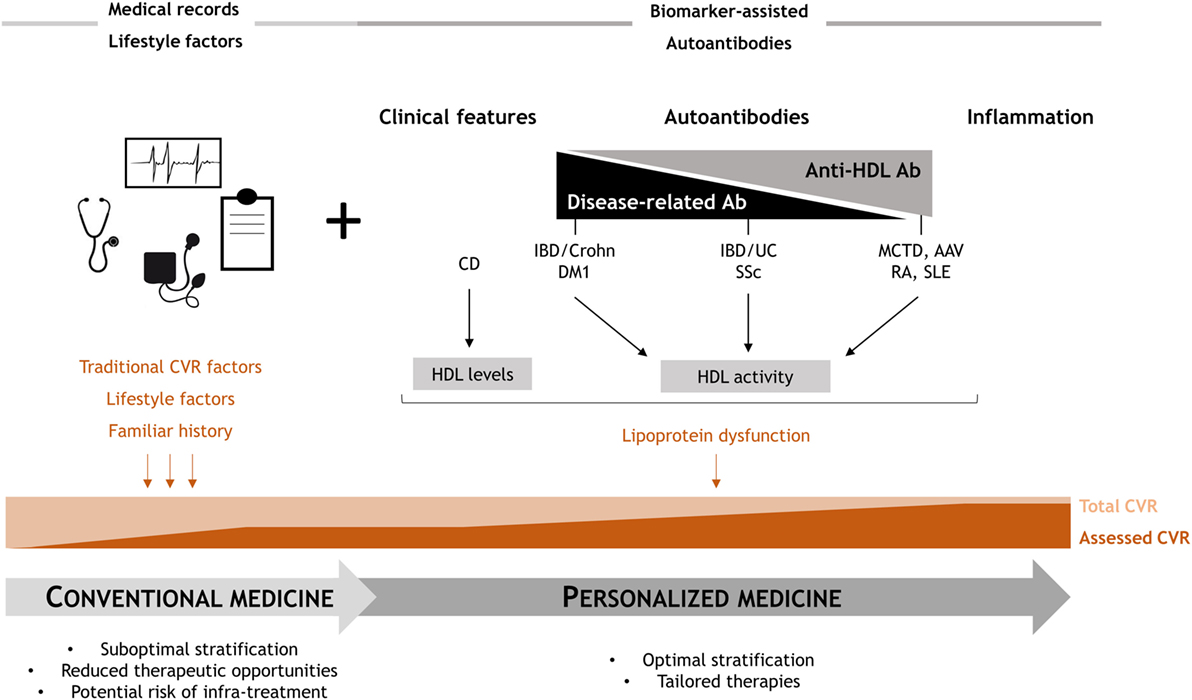
Figure 4. Overview of the study findings: clinical relevance. Conventional clinical CVR management provides a suboptimal risk stratification, since only a partial estimation of the actual risk is achieved. Anti-high-density lipoprotein (HDL) antibodies may cover this unmet clinical need, as they are able to account, at least in part, for the lipoprotein dysfunction. Depending on the role of anti-HDL and disease-related autoantibodies, four different scenarios can be proposed, hence providing a rationale for personalized medicine approaches to improve the current standards of care in these conditions.
A number of limitations of the present report must be remarked. This study was designed as a proof of concept study to analyze the prevalence of anti-HDL antibodies in a wide range of immune-driven conditions beyond SLE and RA. Consequently, an in-depth analysis of the anti-HDL levels and specific clinical features or co-existing traditional CV risk factors in each condition was not conceived and surrogate markers of CVD were not evaluated in our analyses. Finally, the data did not allow for analysis of the treatments received in each group of patients. However, although this may be considered as a limitation, early studies by our group and others showed no major effects of immunomodulatory treatments (22–24, 53).
In conclusion, our findings revealed the presence of IgG anti-HDL antibodies in different immune-mediated diseases, with a strong connection to HDL dysfunction. Furthermore, higher prevalence of anti-HDL antibodies was observed in systemic rheumatic diseases. To the best of our knowledge, the emergence of anti-HDL antibodies is reported by our study for the first time in MCTD, AAV, and IBD. Our findings expand the current knowledge on the association between humoral immunity and CVD, demonstrating that anti-HDL antibodies represent a promising tool to account for the HDL dysfunction in immune-driven diseases. As such, their use as potential biomarkers in conjunction with traditional CV risk factor assessment may help better stratify CVR in a personalized medicine approach.
Ethics Statement
Study approval was obtained from the Institutional Review Board (Comité de ética Regional de Investigación Clínica, Asturias, Spain) in compliance with the Declaration of Helsinki. Experimental and clinical procedures were performed in accordance with the recommendations of the Institutional Review Board. All participants gave written informed consent prior to their inclusion in the study.
Author Contributions
JR-C performed most of the experimental procedures, carried out the statistical analyses, and drafted and edited the manuscript. LM was in charge of patients’ recruitment and clinical data collection. PL performed some experimental procedures and contributed to the interpretation of the data. EN contributed in the analyses, interpretation, and discussion of the results. AS conceived the study, designed the protocols, and drafted and edited the manuscript.
Conflict of Interest Statement
The authors declare that the funders have no role in the study design, data collection, analysis, and interpretation of data nor in the writing of the report or in the decision to publish.
Funding
This work was supported by European Union FEDER funds and “Fondo de Investigación Sanitaria” (FIS, PI12/00523 and PI16/0011; ISCIII, Spain). JR-C is supported by a postdoctoral contract from the “Juan de la Cierva” program (FJCI-2015-23849; MICINN, Spain).
Supplementary Material
The Supplementary Material for this article can be found online at https://www.frontiersin.org/articles/10.3389/fmed.2018.00114/full#supplementary-material.
Abbreviations
AAV, ANCA-associated vasculitis; AMA, anti-mitocondrial antibodies; ANA, antinuclear antibodies; ANCA, anti-neutrophil cytoplasm antibodies; anti-dsDNA, anti-double strand DNA antibodies; anti-GAD, anti-glutamic acid decarboxylase antibodies; anti-GPC, anti-gastric parietal cell antibodies; anti-IA2, anti-islet antigen 2 antibodies; anti-IF, anti-intrinsic factor antibodies; anti-MPO, anti-myeloperoxidase; anti-PR3, anti-proteinase 3 antibodies; anti-RNP, anti-ribonucleoprotein antibodies; anti-Scl70, anti-DNA Topoisomerase I antibodies; anti-TGA, anti-transglutaminase antibodies; ASCA, anti-Saccharomyces cerevisiae antibodies; AU, arbitrary units; CD, celiac disease; B12, autoimmune B12 deficiency; CV, cardiovascular; DM1, diabetes mellitus 1; HDL, high-density lipoprotein; IBD, inflammatory bowel disease; IgG, immunoglobulin G; MCTD, mixed connective tissue disease; PA, pernicious anemia; PBC, primary biliary cirrhosis; PON1, paraoxonase 1; pSS, primary Sjögren syndrome; SSc, systemic sclerosis; TAC, total antioxidant capacity; UC, ulcerative colitis.
References
1. Symmons DPM, Gabriel SE. Epidemiology of CVD in rheumatic disease, with a focus on RA and SLE. Nat Rev Rheumatol (2011) 7(7):399–408. doi:10.1038/nrrheum.2011.75
2. Riboldi P, Gerosa M, Luzzana C, Catelli L. Cardiac involvement in systemic autoimmune diseases. Clin Rev Allergy Immunol (2002) 23(3):247–61. doi:10.1385/CRIAI:23:3:247
3. Esdaile JM, Abrahamowicz M, Grodzicky T, Li Y, Panaritis C, Berger R, et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum (2001) 44(10):2331–7. doi:10.1002/1529-0131(200110)44:10<2331::AID-ART395>3.0.CO;2-I
4. del Rincón ID, Williams K, Stern MP, Freeman GL, Escalante A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum (2001) 44(12):2737–45. doi:10.1002/1529-0131(200112)44:12<2737::AID-ART460>3.0.CO;2-#
5. Sherer Y, Shoenfeld Y. Mechanisms of disease: atherosclerosis in autoimmune diseases. Nat Clin Pract Rheumatol (2006) 2(2):99–106. doi:10.1038/ncprheum0092
6. Bartoloni E, Shoenfeld Y, Gerli R. Inflammatory and autoimmune mechanisms in the induction of atherosclerotic damage in systemic rheumatic diseases: two faces of the same coin. Arthritis Care Res (Hoboken) (2011) 63(2):178–83. doi:10.1002/acr.20322
7. Nofer J, Kehrel B, Fobker M, Levkau B, Assmann G, von Eckardstein A. Review article HDL and arteriosclerosis : beyond reverse cholesterol transport. Atherosclerosis (2002) 161:1–16. doi:10.1016/S0021-9150(01)00651-7
8. González-Gay MA, González-Juanatey C. Inflammation and lipid profile in rheumatoid arthritis: bridging an apparent paradox. Ann Rheum Dis (2014) 73(7):1281–3. doi:10.1136/annrheumdis-2013-204933
9. Robertson J, Peters MJ, McInnes IB, Sattar N. Changes in lipid levels with inflammation and therapy in RA: a maturing paradigm. Nat Rev Rheumatol (2013) 9(9):513–23. doi:10.1038/nrrheum.2013.91
10. Tripi LM, Manzi S, Chen Q, Kenney M, Shaw P, Kao A, et al. Relationship of serum paraoxonase 1 activity and paraoxonase 1 genotype to risk of systemic lupus erythematosus. Arthritis Rheum (2006) 54(6):1928–39. doi:10.1002/art.21889
11. Szántó A, Harangi M, Seres I, Paragh G, Zeher M. Decreased human paraoxonase-1 activity in patients with Sjögren’s syndrome. Int Immunol (2010) 22(7):605–9. doi:10.1093/intimm/dxq045
12. Hahn BH, Grossman J, Chen W, McMahon M. The pathogenesis of atherosclerosis in autoimmune rheumatic diseases: roles of inflammation and dyslipidemia. J Autoimmun (2007) 28(2–3):69–75. doi:10.1016/j.jaut.2007.02.004
13. Garcia ABA, Dardin LP, Minali PA, Czapkowsky A, Ajzen SA, Trevisani VFM. Asymptomatic atherosclerosis in primary Sjögren syndrome: correlation between low ankle brachial index and autoantibodies positivity. J Clin Rheumatol (2016) 22(6):295–8. doi:10.1097/RHU.0000000000000413
14. Mantel Ä, Holmqvist M, Nyberg F, Tornling G, Frisell T, Alfredsson L, et al. Risk factors for the rapid increase in risk of acute coronary events in patients with new-onset rheumatoid arthritis: a nested case-control study. Arthritis Rheumatol (Hoboken) (2015) 67(11):2845–54. doi:10.1002/art.39267
15. López-Longo FJ, Oliver-Miñarro D, de la Torre I, González-Díaz de Rábago E, Sánchez-Ramón S, Rodríguez-Mahou M, et al. Association between anti-cyclic citrullinated peptide antibodies and ischemic heart disease in patients with rheumatoid arthritis. Arthritis Rheum (2009) 61(4):419–24. doi:10.1002/art.24390
16. Liang KP, Kremers HM, Crowson CS, Snyder MR, Therneau TM, Roger VL, et al. Autoantibodies and the risk of cardiovascular events. J Rheumatol (2009) 36(11):2462–9. doi:10.3899/jrheum.090188
17. Cambridge G, Acharya J, Cooper JA, Edwards JC, Humphries SE. Antibodies to citrullinated peptides and risk of coronary heart disease. Atherosclerosis (2013) 228(1):243–6. doi:10.1016/j.atherosclerosis.2013.02.009
18. Mackey RH, Kuller LH, Deane KD, Walitt BT, Chang Y-F, Holers VM, et al. Rheumatoid arthritis, anti-cyclic citrullinated peptide positivity, and cardiovascular disease risk in the women’s health initiative. Arthritis Rheumatol (Hoboken) (2015) 67(9):2311–22. doi:10.1002/art.39198
19. Montes A, Corrales A, Calaza M, Lopez-Mejias R, Parra JA, González-Gay MA, et al. Brief report: lack of replication of an association between anti-citrullinated fibrinogen and subclinical atherosclerosis in patients with rheumatoid arthritis. Arthritis Rheumatol (Hoboken) (2015) 67(11):2861–5. doi:10.1002/art.39302
20. Delgado Alves J, Ames PRJ, Donohue S, Stanyer L, Nourooz-Zadeh J, Ravirajan C, et al. Antibodies to high-density lipoprotein and beta2-glycoprotein I are inversely correlated with paraoxonase activity in systemic lupus erythematosus and primary antiphospholipid syndrome. Arthritis Rheum (2002) 46(10):2686–94. doi:10.1002/art.10542
21. Batuca JR, Ames PRJ, Amaral M, Favas C, Isenberg DA, Delgado Alves J. Anti-atherogenic and anti-inflammatory properties of high-density lipoprotein are affected by specific antibodies in systemic lupus erythematosus. Rheumatology (2009) 48(1):26–31. doi:10.1093/rheumatology/ken397
22. Rodríguez-Carrio J, Alperi-López M, López P, Ballina-García FJ, Abal F, Suárez A. Antibodies to high-density lipoproteins are associated with inflammation and cardiovascular disease in rheumatoid arthritis patients. Transl Res (2015) 166(6):529–39. doi:10.1016/j.trsl.2015.07.004
23. Rodríguez-Carrio J, López-Mejías R, Alperi-López M, López P, Ballina-García FJ, González-Gay MÁ, et al. PON activity is modulated by rs662 polymorphism and IgG anti-HDL antibodies in rheumatoid arthritis patients: potential implications for CV disease. Arthritis Rheumatol (2016) 68(6):1367–76. doi:10.1002/art.39609
24. López P, Rodríguez-Carrio J, Martínez-Zapico A, Pérez-Álvarez ÁI, López-Mejías R, Benavente L, et al. Serum levels of anti-PON1 and anti-HDL antibodies as potential biomarkers of premature atherosclerosis in systemic lupus erythematosus. Thromb Haemost (2017) 117(11):2194–206. doi:10.1160/TH17-03-0221
25. Alarcón-Segovia D, Villarreal M. Classification and diagnostic criteria for mixed connective tissue disease. In: Kasukawa R, GC S, editors. Mixed Connective Tissue Disease and Anti-Nuclear Antibodies. Amsterdam: Excerpta Medica (1987). p. 33–40.
26. Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis (2002) 61(6):554–8. doi:10.1136/ard.61.6.554
27. van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum (2013) 65(11):2737–47. doi:10.1002/art.38098
28. Hunder GG, Arend WP, Bloch DA, Calabrese LH, Fauci AS, Fries JF, et al. The American College of Rheumatology 1990 criteria for the classification of vasculitis. Introduction. Arthritis Rheum (1990) 33(8):1065–7. doi:10.1002/art.1780330802
29. Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med (2005) 353(12):1261–73. doi:10.1056/NEJMra043898
30. American Diabetes Association. Standards of medical care in diabetes – 2013. Diabetes Care (2013) 36(Suppl 1):S11–66. doi:10.2337/dc13-S011
31. Bizzaro N, Antico A. Diagnosis and classification of pernicious anemia. Autoimmun Rev (2014) 13(4–5):565–8. doi:10.1016/j.autrev.2014.01.042
32. Satsangi J, Silverberg MS, Vermeire S, Colombel J-F. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut (2006) 55(6):749–53. doi:10.1136/gut.2005.082909
33. Lennard-Jones J. Classification of inflammatory bowel disease. Scand J Gastroenterol (1989) 170S:2–6. doi:10.3109/00365528909091339
34. Bai JC, Fried M, Corazza GR, Schuppan D, Farthing M, Catassi C, et al. World Gastroenterology Organisation global guidelines on celiac disease. J Clin Gastroenterol (2013) 47(2):121–6. doi:10.1097/MCG.0b013e31827a6f83
35. Rodríguez-Carrio J, Alperi-López M, López P, Alonso-Castro S, Ballina-García FJ, Suárez A. Red cell distribution width is associated with cardiovascular risk and disease parameters in rheumatoid arthritis. Rheumatology (Oxford) (2015) 54(4):641–6. doi:10.1093/rheumatology/keu345
36. Fisher EA, Feig JE, Hewing B, Hazen SL, Smith JD. High-density lipoprotein function, dysfunction, and reverse cholesterol transport. Arterioscler Thromb Vasc Biol (2012) 32(12):2813–20. doi:10.1161/ATVBAHA.112.300133
37. Eren E, Yilmaz N, Aydin O. High density lipoprotein and it’s dysfunction. Open Biochem J (2012) 6:78–93. doi:10.2174/1874091X01206010078
38. Ortega-Hernandez O-D, Shoenfeld Y. Mixed connective tissue disease: an overview of clinical manifestations, diagnosis and treatment. Best Pract Res Clin Rheumatol (2012) 26(1):61–72. doi:10.1016/j.berh.2012.01.009
39. Soltesz P, Bereczki D, Szodoray P, Magyar MT, Der H, Csipo I, et al. Endothelial cell markers reflecting endothelial cell dysfunction in patients with mixed connective tissue disease. Arthritis Res Ther (2010) 12(3):R78. doi:10.1186/ar2999
40. Bodolay E, Prohászka Z, Paragh G, Csipő I, Nagy G, Laczik R, et al. Increased levels of anti-heat-shock protein 60 (anti-Hsp60) indicate endothelial dysfunction, atherosclerosis and cardiovascular diseases in patients with mixed connective tissue disease. Immunol Res (2014) 60(1):50–9. doi:10.1007/s12026-014-8552-x
41. Soltész P, Kerekes G, Dér H, Szücs G, Szántó S, Kiss E, et al. Comparative assessment of vascular function in autoimmune rheumatic diseases: considerations of prevention and treatment. Autoimmun Rev (2011) 10(7):416–25. doi:10.1016/j.autrev.2011.01.004
42. Reiseter S, Molberg Ø, Gunnarsson R, Lund MB, Aalokken TM, Aukrust P, et al. Associations between circulating endostatin levels and vascular organ damage in systemic sclerosis and mixed connective tissue disease: an observational study. Arthritis Res Ther (2015) 17:231. doi:10.1186/s13075-015-0756-5
43. Singh S, Kullo IJ, Pardi DS, Loftus EV. Epidemiology, risk factors and management of cardiovascular diseases in IBD. Nat Rev Gastroenterol Hepatol (2015) 12(1):26–35. doi:10.1038/nrgastro.2014.202
44. Kristensen SL, Ahlehoff O, Lindhardsen J, Erichsen R, Jensen GV, Torp-Pedersen C, et al. Disease activity in inflammatory bowel disease is associated with increased risk of myocardial infarction, stroke and cardiovascular death – a Danish nationwide cohort study. PLoS One (2013) 8(2):e56944. doi:10.1371/journal.pone.0056944
45. Sappati Biyyani RSR, Putka BS, Mullen KD. Dyslipidemia and lipoprotein profiles in patients with inflammatory bowel disease. J Clin Lipidol (2010) 4(6):478–82. doi:10.1016/j.jacl.2010.08.021
46. Boehm D, Krzystek-Korpacka M, Neubauer K, Matusiewicz M, Berdowska I, Zielinski B, et al. Paraoxonase-1 status in Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis (2009) 15(1):93–9. doi:10.1002/ibd.20582
47. Wu G-C, Leng R-X, Lu Q, Fan Y-G, Wang D-G, Ye D-Q. Subclinical atherosclerosis in patients with inflammatory bowel diseases: a systematic review and meta-analysis. Angiology (2016) 68(5):447–61. doi:10.1177/0003319716652031
48. Bamias G, Cominelli F. Role of type 2 immunity in intestinal inflammation. Curr Opin Gastroenterol (2015) 31(6):471–6. doi:10.1097/MOG.0000000000000212
49. Pascual V, Dieli-Crimi R, López-Palacios N, Bodas A, Medrano LM, Núñez C. Inflammatory bowel disease and celiac disease: overlaps and differences. World J Gastroenterol (2014) 20(17):4846–56. doi:10.3748/wjg.v20.i17.4846
50. Zanini B, Mazzoncini E, Lanzarotto F, Ricci C, Cesana BM, Villanacci V, et al. Impact of gluten-free diet on cardiovascular risk factors. A retrospective analysis in a large cohort of coeliac patients. Dig Liver Dis (2013) 45(10):810–5. doi:10.1016/j.dld.2013.04.001
51. Emanuel G, Charlton J, Ashworth M, Gulliford MC, Dregan A. Cardiovascular risk assessment and treatment in chronic inflammatory disorders in primary care. Heart (2016) 102(24):1957–62. doi:10.1136/heartjnl-2016-310111
52. O’Sullivan M, Bruce IN, Symmons DPM. Cardiovascular risk and its modification in patients with connective tissue diseases. Best Pract Res Clin Rheumatol (2016) 30(1):81–94. doi:10.1016/j.berh.2016.03.003
Keywords: anti-HDL, autoantibodies, high-density lipoprotein, antioxidant, autoimmunity, cardiovascular
Citation: Rodríguez-Carrio J, Mozo L, López P, Nikiphorou E and Suárez A (2018) Anti-High-Density Lipoprotein Antibodies and Antioxidant Dysfunction in Immune-Driven Diseases. Front. Med. 5:114. doi: 10.3389/fmed.2018.00114
Received: 25 December 2017; Accepted: 06 April 2018;
Published: 23 April 2018
Edited by:
Anne Grete Semb, Diakonhjemmet Hospital, NorwayCopyright: © 2018 Rodríguez-Carrio, Mozo, López, Nikiphorou and Suárez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ana Suárez, anasua@uniovi.es
 Javier Rodríguez-Carrio
Javier Rodríguez-Carrio Lourdes Mozo2,3
Lourdes Mozo2,3
 Elena Nikiphorou
Elena Nikiphorou