- Department of Internal Medicine, The Ohio State University Medical Center, Columbus, OH, USA
Intracellular bacterial pathogens exploit host cells as a part of their lifecycle, and they do so by manipulating host cell signaling events. Many such bacteria are known to produce effector proteins that promote cell invasion, alter membrane trafficking, and disrupt signaling cascades. This review highlights recent advances in our understanding of signaling pathways involved in host cell responses to Francisella tularensis, a facultative Gram-negative intracellular pathogen that causes tularemia. We highlight several key pathways that are targeted by Francisella, with a focus on the phosphatidylinositol 3-kinase/Akt pathway. Lastly, we discuss the emerging role of microRNAs (miRs), specifically miR-155, as a key regulator of host signaling and defense.
Introduction
Successful intracellular pathogens must have mechanisms to usurp their hosts’ normal functions and defenses, thereby creating a favorable environment. For example, Legionella pneumophila secrete type IV effectors that form a permissive niche (Berger et al., 1994) and Yersinia pestis disrupts host nuclear factor kappa B (NF-κB) signaling via the Yop J proteins (Zhou et al., 2005). However, each pathogen can use slightly – or vastly – different mechanisms to conquer the complex signaling pathways that orchestrate the host response. A better understanding of these responses and the methods by which pathogens defeat them will fuel the generation of novel therapeutics.
Francisella tularensis is a Gram-negative bacterial pathogen and is the causative agent of tularemia. The highest-virulence F. tularensis subspecies tularensis requires as few as 10 colony forming units to cause disease and death in humans (Sjostedt, 2007). Because of this it has been classified as a category A select agent by the United States Centers for Disease Control. However, there are also lower-virulence subspecies and strains, such as F. tularensis subspecies novicida and the live vaccine strains (LVS) of F. tularensis subspecies holarctica. These subspecies are used extensively for research because they lead to disease in mice that resembles human tularemia, their intracellular lifecycles are similar to that of F. tularensis tularensis, and they present minimal risk to humans. Since 2001 there has been a renewed interest in the study of this pathogen for biodefense purposes, and tremendous advances have been made in recent years (Oyston et al., 2004; Elkins et al., 2007; Santic et al., 2010).
One of the most interesting aspects of infection with F. tularensis is that there is a lack or delay of inflammatory response during the early stages of infection (Andersson et al., 2006; Bosio et al., 2007). Even though this pathogen is Gram-negative, the lipopolysaccharide (LPS) is modified in such a way that it does not activate toll-like receptor (TLR) 4 (Hajjar et al., 2006; Gunn and Ernst, 2007). Additionally, F. tularensis can suppress the host’s ability to respond to pro-inflammatory signals (Telepnev et al., 2003, 2005; Bosio et al., 2007), thus demonstrating that it does not merely evade host cell immune responses. Indeed, genomewide studies on the effect of F. tularensis on human phagocytes shows a broad suppression of numerous signaling pathways including TLR signaling, autophagy, major histocompatibility complex (MHC) presentation, interferon signaling, and phosphatidylinositol 3-kinase (PI3K) signaling (Butchar et al., 2008).
Bacterial Entry into Phagocytes
Internalization of Francisella by human phagocytes occurs though a unique process termed “looping phagocytosis” (Clemens and Horwitz, 2007). Optimal phagocytosis of Francisella requires an intact complement pathway, as internalization is dramatically reduced in the absence of complement (Lofgren et al., 1983; Balagopal et al., 2006; Clemens and Horwitz, 2007). However, the bacterium itself is resistant to complement-mediated lysis though its LPS O antigen (Clay et al., 2008). Other receptors such as the scavenger receptor (Pierini, 2006) and mannose receptor (Balagopal et al., 2006; Schulert and Allen, 2006) have been implicated in promoting bacterial uptake. Fc receptors also play a role, aiding both internalization of bacteria (Balagopal et al., 2006) and host protection (Kirimanjeswara et al., 2007).
Downstream signaling events implicated in the phagocytosis of F. novicida include the splenic tyrosine kinase (Syk) and extracellular signal-regulated kinase (ERK) pathways. Genetic and pharmacologic manipulation of these two kinases established that both pathways are required for macrophage phagocytosis of bacteria under heat-inactivated fetal bovine serum culture conditions (Parsa et al., 2008b). Conflicting reports exist on the role of PI3K signaling during phagocytosis. Phagocytosis of F. tularensis in human monocyte derived macrophages cultured in AB serum is wortmannin sensitive (Clemens and Horwitz, 2007). However, PI3K or downstream Akt signaling in murine macrophages cultured with heat-inactivated fetal bovine serum does not have any effect on bacterial internalization (Parsa et al., 2008b; Rajaram et al., 2009).
Intracellular Replication
Intracellular replication requires escape from the phagosome to gain access to the cytosol. Numerous escape/replication deficient mutants of F. novicida have been generated (Lauriano et al., 2004; Santic et al., 2005, 2007; Mohapatra et al., 2008). Studies with these mutants have shown that Francisella manipulates host signaling in at least two distinct ways to promote intracellular proliferation. Firstly, Francisella inhibits protective responses that would promote bacterial clearance. For example, it has been shown that F. novicida induces suppressor of cytokine signaling 3 (SOCS3) expression to interfere with IFNγ-mediated activation of STAT1, which contributes to the intracellular killing of bacteria (Parsa et al., 2008a). Hence, although IFNγ itself is upregulated following Francisella infection (Butchar et al., 2007, 2008), its autocrine/paracrine activity would be minimal. Along with manipulating these downstream mediators, it was shown that Francisella can regulate expression of the interferon gamma receptor (IFNγR) itself. Infection with the novicida and tularensis subspecies (Butchar et al., 2008) as well as with holarctica (unpublished observations) leads to downregulation of the IFNγR in human monocytes, and F. novicida infection of RAW264.7 murine macrophages induces downregulation of the IFNγR alpha chain (Roth et al., 2009).
A second way by which Francisella manipulates host signaling is to activate pathways that benefit bacterial replication. Recently it was shown that host signaling through Ras, SOS2, GrB2, PKCα, and PKCβI promotes host cell survival and maintenance of the replicative niche for F. novicida (Al Khodor and Abu Kwaik, 2010). In another study, a forward genetics screen identified USP22, CDC27, and PI4KCA as host factors required for intracellular replication of F. novicida (Akimana et al., 2010). Hence, Francisella is capable not only of suppressing host responses, but also of manipulating the host environment to facilitate its own survival and replication. Without either of these general mechanisms, it is unlikely that Francisella would succeed as an intracellular pathogen.
Autophagy: Re-Entry into the Endocytic Pathway
At the late stages of infection, cytosolic F. tularensis LVS becomes enclosed within vacuoles that resemble autophagosomes. This has been conclusively shown in murine macrophages (Checroun et al., 2006), and there is some evidence that it also occurs in human monocytic cells (Mohapatra et al., 2008). Detailed functional consequences of autophagy have yet to be elucidated, but it has been shown that human monocytes treated with an autophagy-inducing agent carried a reduced bacterial burden (Chiu et al., 2009). Hence, it is likely that autophagy is beneficial to the host and serves to promote intracellular control of bacteria by sequestering them into vacuoles after escape. It is important to note that many autophagy-related genes are downregulated during F. novicida and F. tularensis infection (Butchar et al., 2008; Cremer et al., 2009a), raising the possibility that Francisella is also antagonizing this means of host defense.
Recognition of Francisella and Triggering of the Inflammatory Response
During the infection cycle of Francisella there is activation of extracellular and cytosolic pattern recognition receptors. At the cell surface and within the phagosome, TLR2/myeloid differentiation primary response gene (88) (MyD88) signaling is essential for activating NF-κB and for producing pro-inflammatory mediators (Katz et al., 2006). CD14 is also required for effective TLR signaling upon F. tularensis infection (Chase and Bosio, 2010). After escape from the phagosome the bacteria gain access to the cytosol and activate the inflammasome (Mariathasan et al., 2005; Gavrilin et al., 2006). Only recently were the cytosolic sensors of F. tularensis identified. First it was shown that pyrin was essential for F. novicida-induced inflammasome activation within human monocytes (Gavrilin et al., 2009). Later studies with murine macrophages led to two concurrently published reports showing that absent in melanoma 2 (AIM-2) was essential for inflammasome activation and that this was responsible for caspase-1 and IL-1β processing (Fernandes-Alnemri et al., 2010; Rathinam et al., 2010). These findings were later supported by another report (Jones et al., 2010). However, human monocytes, which are robust producers of IL-1β during F. novicida infection (Gavrilin et al., 2006) do not express AIM-2 (Rathinam et al., 2010). Hence, it is likely that more than one cytosolic receptor is capable of responding to Francisella, and that these receptors may be cell- or species-specific.
The PI3K Signaling Pathway Regulates Host Responses
PI3K signaling is known to regulate numerous cellular processes such as autophagy (Petiot et al., 2000), phagocytosis (Araki et al., 1996), cell survival, oxidative burst (Chen et al., 2003; Hoyal et al., 2003), and inflammatory cytokine production (Parsa et al., 2006). There are three classes of PI3K, which differ in the products that they produce and thereby drive different cellular functions. Class I PI3K convert PI(4,5)P2 to PI(3,4,5)P3, which then serves as a second messenger and can activate protein kinase B/Akt (Vanhaesebroeck et al., 1997). This signaling pathway can be activated by a variety of cell surface receptors such as Fc receptors (Marshall et al., 2001), TLR (Laird et al., 2009), and the insulin receptor (Lavan et al., 1992). Our laboratory has shown that PI3K (Parsa et al., 2006) and Akt (Rajaram et al., 2006) positively regulate transcriptional activity of NF-κB in response to F. novicida within macrophages. Furthermore, Akt promotes the production of pro-inflammatory cytokines such as TNFα, IL-6, and IL-12, while inhibiting the production of the anti-inflammatory cytokine IL-10. Studies in vivo have demonstrated that PI3K/Akt activation is host-protective. Compared to wild-type mice, mice expressing a macrophage-specific form of constitutively active Akt (MyrAkt) showed a survival advantage when challenged with a lethal dose of F. novicida (Rajaram et al., 2006). There was also a reduced bacterial burden in the MyrAkt mice. This activated Akt was found to promote phagosome maturation, thus inhibiting bacterial escape and replication. Related to this, we found that macrophages from MyrAkt mice showed reduced Fas-induced caspase-3 death after infection. Because phagosomal escape mutants of Francisella do not induce Fas nor activate caspase-3 (Rajaram et al., 2009), it is likely that bacterial escape or perhaps stress from bacterial replication triggers apoptosis. Caspase-3-mediated death appears to be of major importance, as the highly virulent subspecies F. tularensis activates caspase-3 and not caspase-1 (Wickstrum et al., 2009). Interestingly, the virulent F. tularensis specifically downregulates host cell TLR2, PI3K p85α, and Akt expression (Butchar et al., 2008), which should result in suboptimal activation of the host-protective PI3K/Akt pathway. This is in line with earlier work showing that F. tularensis infection elicits minimal to no observable response in vivo during early stages of infection (Bosio et al., 2007). This ability to infect without eliciting an immune response confers a stealth-like nature to Francisella (Sjostedt, 2006).
A downstream target of Akt that has also been investigated within the context of Francisella is glycogen synthase kinase 3 beta (GSK3β). This molecule is normally active in the cell until inactivated by Akt (Sutherland et al., 1993; Sutherland and Cohen, 1994). Thus, increased Akt activity results in decreased GSK3β activity. It has been found that inhibiting GSK3β in vivo results in increased host survival in mice. This is consistent with the role of Akt as host-protective. However, published data on GSK3β indicates that this molecule is required for pro-inflammatory response and concurrently inhibits anti-inflammatory IL-10 production (Zhang et al., 2009). This might be expected because GSK3β has been shown to be required for NF-κB activation (Hoeflich et al., 2000). Other work, however, has shown that GSK3β can repress NF-κB activity and nuclear translocation as well as cytokine production (Vines et al., 2006; Escribano et al., 2009; Saijo et al., 2009; Beurel et al., 2010). Taken together, these results suggest that the function of GSK3β may be highly context-specific.
Although these results suggest that PI3K activity is beneficial to the host during Francisella infection, there is also evidence that it may be detrimental. Medina et al. (2010) reported that treatment with wortmannin, a PI3K inhibitor, led to increased ERK and p38 phosphorylation as well as enhanced TNFα and IL-6 production in murine bone marrow-derived macrophages following infection with the LVS of Francisella. They also found a PI3K-mediated upregulation of the MAPK inhibitor MKP-1, which likely led to much of the MAPK inhibition (Medina et al., 2010). MKP-1 is upregulated in human monocytes following infection with the novicida, holarctica, and tularensis subspecies of Francisella (Butchar et al., 2008 and unpublished observations), so may not fully explain the observed discrepancies. However, as pointed out by Medina et al. (2010), differences in subspecies and/or culture conditions of this facultative bacterium might account for this. For example, the LPS of F. novicida elicits a greater immune response than that of LVS (Kieffer et al., 2003). More broadly, differences in cellular context (e.g., maturation state) as well as in both strength and type of stimulus can determine whether and to what extent PI3K activity can inhibit ERK (Rommel et al., 1999; Zimmermann and Moelling, 1999; Moelling et al., 2002).
Phosphatase Regulation of Host Response to Francisella
PIP3 levels are antagonized by the regulatory phosphatases PTEN that converts PI(3,4,5)P3 back to PI(4,5)P2, and SH2 domain- containing inositol 5′-phosphatase 1 (SHIP) that converts PI(3,4,5)P3 to PI(3,4)2. By these actions, both phosphatases negatively regulate Akt activation. Our laboratory has shown that SHIP is phosphorylated in response to Francisella. Consequently, SHIP represses the production of pro-inflammatory cytokines (Parsa et al., 2006), phagosome maturation, and macrophage survival (Rajaram et al., 2009). This is consistent with to role of Akt in promoting these functions within the host. We also found that SHIP expression is strongly downregulated during infection with the low-virulence F. novicida subspecies but not with the more virulent F. tularensis subspecies (Cremer et al., 2009b). This is likely a contributing factor to the lack of inflammatory response during F. tularensis infection (Bosio et al., 2007; Butchar et al., 2008; Cremer et al., 2009b).
Recently it has been reported that infection with the virulent F. tularensis SCHU S4 increases expression and activation of phosphatase and tensin homolog (PTEN), an effect brought about by the production of antioxidants from the bacterium (Melillo et al., 2010). Hence, F. tularensis appears to use both phosphatases – PTEN and SHIP – to dampen PI3K signaling. The idea that F. tularensis does so through presumably different mechanisms (upregulation of PTEN versus inhibiting downregulation of SHIP) further highlights the intricacies and complexities of this pathogen.
Bacterial Disruption of Host Signaling
Many bacterial pathogens are known to secrete factors that disrupt host signaling proteins. Because of the numerous examples showing that microbes inhibit Akt activation (Celli et al., 2001; Wiles et al., 2008; Popova et al., 2009), components of the PI3K/Akt pathway may soon come to light as potential therapeutic targets. During the infection cycle of Francisella there is initial contact with the host cell and containment within a phagosome, then escape of the bacteria into the cytosol. Our laboratory has seen that there is initial phosphorylation/activation of Akt followed by decline to a more inactivated state, but infection with mutants of Francisella that cannot escape from phagosome results in sustained Akt activation (Rajaram et al., 2006). This is specific in the sense that the same activation – inactivation phenomenon is not seen with phosphorylation/activation of the MAPKs (ERK, JNK, and p38). Therefore, it appears that Francisella specifically targets the PI3K/Akt pathway.
Along with PI3K/Akt regulation, Francisella dampens other signaling pathways such as MAPK and the inflammasome. Recently it has been shown that ripA of Francisella inhibits MAPK activation, which subsequently inhibits the production of TLR-dependent and inflammasome-dependent pro-inflammatory cytokines (Huang et al., 2010). This bacterial factor also promotes intracellular replication, presumably though its inhibition of host response. Another recent report shows that the bacterial protein mviN inhibits AIM-2 inflammasome activation in murine macrophages infected with LVS (Ulland et al., 2010). Hence, Francisella dampens host response on many fronts, and further work will undoubtedly uncover other such mechanisms.
MicroRNA Regulation
MicroRNAs (miRs) have been shown to be key regulators of host functions that influence viral persistence (Gottwein and Cullen, 2008). However, only a few studies address miRs within the field of bacterial pathogenesis, leaving open a potentially fruitful avenue of investigation. Early studies showed that pathogen associated molecular patterns such as LPS could induce the expression of selected miRs such as miR-155, miR-146, and miR-132 (Taganov et al., 2006; O’Connell et al., 2007). MiR-155 has been of considerable interest since it was shown that this miR is required for B-cell maturation, normal T-cell function, dendritic cell antigen presentation, and inflammatory response (Rodriguez et al., 2007; Thai et al., 2007; O’Connell et al., 2010).
MiR-155 is of relevance to Francisella infection because this miR negatively regulates SHIP expression, which in turn regulates Akt and multiple host responses. Our laboratory found that miR-155 expression is highly induced in monocytes/macrophages infected with the low-virulence F. novicida. This was also found to occur in vivo within 48 h in mice given a lethal dose of F. novcidia. The induction of this miR required TLR signaling and NF-κB activation. Genetic manipulation showed that miR-155 modulates the inflammatory response by promoting TNFα and IL-6 production (Cremer et al., 2009b). This is logical given that miR-155 represses SHIP, which represses Akt activation and the production of pro-inflammatory mediators in response to F. novicida (Parsa et al., 2006). Importantly, although miR-155 is highly induced upon infection with low-virulence F. novicida it is minimally induced with high-virulence F. tularensis. These results support the consensus that virulent forms of Francisella subvert and suppress host immune responses, as the lack of miR-155 with the virulent F. tularensis will result in higher levels of the inhibitory SHIP. Hence, host cell Akt activation is impaired through at least three mechanisms: downregulation of PI3K/Akt pathway members, the bacterial antioxidant system, and miR-155. A model summarizing these findings is shown in Figure 1.
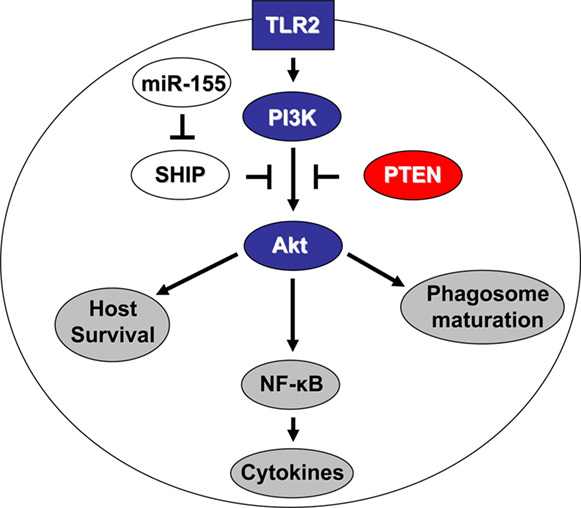
Figure 1. Francisella tularensis tularensis targets the PI3K/Akt pathway at multiple levels. Blue represents downregulated genes, red upregulated, white unchanged, and gray the resulting cellular outcomes. TLR2 is required for full PI3K activation by Francisella, which in turn activates Akt. Active Akt promotes NF-κB activation and cytokine production, as well as phagosome maturation and host cell survival. However, the phosphatases SHIP and PTEN antagonize this Akt activation. SHIP expression is inhibited by miR-155, which is induced by the low-virulence but not high-virulence subspecies of Francisella. This lack of miR-155 induction by the high-virulence subspecies leads to maintenance of SHIP levels. The other phosphatase PTEN is upregulated and maintained in a highly active state upon infection with high-virulence Francisella.
Conclusion
This review has highlighted recent advances in our understanding of host response to Francisella. Research on this pathogen has been at an all time high in the recent years and great progress has been made. Not only is there interest in studying this pathogen due to its highly virulent nature, it has also become a useful tool for studying various aspects of the immune system. Here, we have discussed several critical host signaling pathways that Francisella manipulates in order to subvert normal pro-inflammatory responses. Importantly, the idea that miRs play key roles – as mediators of host response and as targets of Francisella – has begun to surface, and this may open an avenue for a new class of therapeutics. It is almost certain that even more exciting findings await us in the future, as our research tools become increasingly sophisticated and as pathogen and host continue to evolve against each other.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was sponsored by the National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases (NIAID) Regional Center of Excellence for Biodefense and Emerging Infectious Diseases Research (RCE) Program. The authors wish to acknowledge membership within and support from the Region V “Great Lakes” RCE (NIH award 1-U54-AI-057153). This work was also sponsored by NIH/NIAID award # 1-T32-AI-065411, a NRSA training grant administered by the Center for Microbial Interface Biology (CMIB), at The Ohio State University, supporting Thomas John Cremer, Jonathan P. Butchar is supported by an American Cancer Society Ohio Division Postdoctoral Fellowship.
References
Akimana, C., Al Khodor, S., and Abu Kwaik, Y. (2010). Host factors required for modulation of phagosome biogenesis and proliferation of Francisella tularensis within the cytosol. PLoS ONE 5, e11025. doi: 10.1371/journal.pone.0011025
Al Khodor, S., and Abu Kwaik, Y. (2010). Triggering Ras signalling by intracellular Francisella tularensis through recruitment of PKCalpha and betaI to the SOS2/GrB2 complex is essential for bacterial proliferation in the cytosol. Cell. Microbiol. 12, 1604–1621.
Andersson, H., Hartmanova, B., Kuolee, R., Ryden, P., Conlan, W., Chen, W., and Sjostedt, A. (2006). Transcriptional profiling of host responses in mouse lungs following aerosol infection with type A Francisella tularensis. J. Med. Microbiol. 55, 263–271.
Araki, N., Johnson, M. T., and Swanson, J. A. (1996). A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J. Cell Biol. 135, 1249–1260.
Balagopal, A., MacFarlane, A. S., Mohapatra, N., Soni, S., Gunn, J. S., and Schlesinger, L. S. (2006). Characterization of the receptor-ligand pathways important for entry and survival of Francisella tularensis in human macrophages. Infect. Immun. 74, 5114–5125.
Berger, K. H., Merriam, J. J., and Isberg. R. R. (1994). Altered intracellular targeting properties associated with mutations in the Legionella pneumophila dotA gene. Mol. Microbiol. 14, 809–822.
Beurel, E., Michalek, S. M., and Jope, R. S. (2010). Innate and adaptive immune responses regulated by glycogen synthase kinase-3 (GSK3). Trends Immunol. 31, 24–31.
Bosio, C. M., Bielefeldt-Ohmann, H., and Belisle, J. T. (2007). Active suppression of the pulmonary immune response by Francisella tularensis Schu4. J. Immunol. 178, 4538–4547.
Butchar, J. P., Cremer, T. J., Clay, C. D., Gavrilin, M. A., Wewers, M. D., Marsh, C. B., Schlesinger, L. S., and Tridandapani, S. (2008). Microarray analysis of human monocytes infected with Francisella tularensis identifies new targets of host response subversion. PLoS ONE 3, e2924. doi: 10.1371/journal.pone.0002924
Butchar, J. P., Rajaram, M. V., Ganesan, L. P., Parsa, K. V., Clay, C. D., Schlesinger, L. S., and Tridandapani, S. (2007). Francisella tularensis induces IL-23 production in human monocytes. J. Immunol. 178, 4445–4454.
Celli, J., Olivier, M., and Finlay, B. B. (2001). Enteropathogenic Escherichia coli mediates antiphagocytosis through the inhibition of PI 3-kinase-dependent pathways. EMBO J. 20, 1245–1258.
Chase, J. C., and Bosio, C. M. (2010). The presence of CD14 overcomes evasion of innate immune responses by virulent Francisella tularensis in human dendritic cells in vitro and pulmonary cells in vivo. Infect. Immun. 78, 154–167.
Checroun, C., Wehrly, T. D., Fischer, E. R., Hayes, S. F., and Celli, J. (2006). Autophagy-mediated reentry of Francisella tularensis into the endocytic compartment after cytoplasmic replication. Proc. Natl. Acad. Sci. U.S.A. 103, 14578–14583.
Chen, Q., Powell, D. W., Rane, M. J., Singh, S., Butt, W., Klein, J. B., and McLeish, K. R. (2003). Akt phosphorylates p47phox and mediates respiratory burst activity in human neutrophils. J. Immunol. 170, 5302–5308.
Chiu, H. C., Soni, S., Kulp, S. K., Curry, H., Wang, D., Gunn, J. S., Schlesinger, L. S., and Chen, C. S. (2009). Eradication of intracellular Francisella tularensis in THP-1 human macrophages with a novel autophagy inducing agent. J. Biomed. Sci. 16, 110.
Clay, C. D., Soni, S., Gunn, J. S., and Schlesinger, L. S. (2008). Evasion of complement-mediated lysis and complement C3 deposition are regulated by Francisella tularensis lipopolysaccharide O antigen. J. Immunol. 181, 5568–5578.
Clemens, D. L., and Horwitz, M. A. (2007). Uptake and intracellular fate of Francisella tularensis in human macrophages. Ann. N. Y. Acad. Sci. 1105, 160–186.
Cremer, T. J., Amer, A., Tridandapani, S., and Butchar, J. P. (2009a). Francisella tularensis regulates autophagy-related host cell signaling pathways. Autophagy 5, 125–128.
Cremer, T. J., Ravneberg, D. H., Clay, C. D., Piper-Hunter, M. G., Marsh, C. B., Elton, T. S., Gunn, J. S., Amer, A., Kanneganti, T. D., Schlesinger, L. S., Butchar, J. P., and Tridandapani, S. (2009b). MiR-155 induction by F. novicida but not the virulent F. tularensis results in SHIP down-regulation and enhanced pro-inflammatory cytokine response. PLoS ONE 4, e8508. doi: 10.1371/journal.pone.0008508
Elkins, K. L., Cowley, S. C., and Bosio, C. M. (2007). Innate and adaptive immunity to Francisella. Ann. N. Y. Acad. Sci. 1105, 284–324.
Escribano, C., Delgado-Martin, C., and Rodriguez-Fernandez, J. L. (2009). CCR7-dependent stimulation of survival in dendritic cells involves inhibition of GSK3beta. J. Immunol. 183, 6282–6295.
Fernandes-Alnemri, T., Yu, J. W., Juliana, C., Solorzano, L., Kang, S., Wu, J., Datta, P., McCormick, M., Huang, L., McDermott, E., Eisenlohr, L., Landel, C. P., and Alnemri. E. S. (2010). The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat. Immunol. 11, 385–393.
Gavrilin, M. A., Bouakl, I. J., Knatz, N. L., Duncan, M. D., Hall, M. W., Gunn, J. S., and Wewers, M. D. (2006). Internalization and phagosome escape required for Francisella to induce human monocyte IL-1beta processing and release. Proc. Natl. Acad. Sci. U.S.A. 103, 141–146.
Gavrilin, M. A., Mitra, S., Seshadri, S., Nateri, J., Berhe, F., Hall, M. W., and Wewers, M. D. (2009). Pyrin critical to macrophage IL-1beta response to Francisella challenge. J. Immunol. 182, 7982–7989.
Gottwein, E., and Cullen, B. R. (2008). Viral and cellular microRNAs as determinants of viral pathogenesis and immunity. Cell Host Microbe 3, 375–387.
Gunn, J. S., and Ernst, R. K. (2007). The structure and function of Francisella lipopolysaccharide. Ann. N. Y. Acad. Sci. 1105, 202–218.
Hajjar, A. M., Harvey, M. D., Shaffer, S. A., Goodlett, D. R., Sjostedt, A., Edebro, H., Forsman, M., Bystrom, M., Pelletier, M., Wilson, C. B., Miller, S. I., Skerrett, S. J., and Ernst, R. K. (2006). Lack of in vitro and in vivo recognition of Francisella tularensis subspecies lipopolysaccharide by Toll-like receptors. Infect. Immun. 74, 6730–6738.
Hoeflich, K. P., Luo, J., Rubie, E. A., Tsao, M. S., Jin, O., and Woodgett, J. R. (2000). Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature 406, 86–90.
Hoyal, C. R., Gutierrez, A., Young, B. M., Catz, S. D., Lin, J. H., Tsichlis, P. N., and Babior, B. M. (2003). Modulation of p47PHOX activity by site-specific phosphorylation: Akt-dependent activation of the NADPH oxidase. Proc. Natl. Acad. Sci. U.S.A. 100, 5130–5135.
Huang, M. T., Mortensen, B. L., Taxman, D. J., Craven, R. R., Taft-Benz, S., Kijek, T. M., Fuller, J. R., Davis, B. K., Allen, I. C., Brickey, W. J., Gris, D., Wen, H., Kawula, T. H., and Ting, J. P. (2010). Deletion of ripA alleviates suppression of the inflammasome and MAPK by Francisella tularensis. J. Immunol. 185, 5476–5485.
Jones, J. W., Kayagaki, N., Broz, P., Henry, T., Newton, K., O’Rourke, K., Chan, S., Dong, J., Qu, Y., Roose-Girma, M., Dixit, V. M., and Monack, D. M. (2010). Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proc. Natl. Acad. Sci. U.S.A. 107, 9771–9776.
Katz, J., Zhang, P., Martin, M., Vogel, S. N., and Michalek, S. M. (2006). Toll-like receptor 2 is required for inflammatory responses to Francisella tularensis LVS. Infect. Immun. 74, 2809–2816.
Kieffer, T. L., Cowley, S., Nano, F. E., and Elkins, K. L. (2003). Francisella novicida LPS has greater immunobiological activity in mice than F. tularensis LPS, and contributes to F. novicida murine pathogenesis. Microbes Infect. 5, 397–403.
Kirimanjeswara, G. S., Golden, J. M., Bakshi, C. S., and Metzger, D. W. (2007). Prophylactic and therapeutic use of antibodies for protection against respiratory infection with Francisella tularensis. J. Immunol. 179, 532–539.
Laird, M. H., Rhee, S. H., Perkins, D. J., Medvedev, A. E., Piao, W., Fenton, M. J., and Vogel, S. N. (2009). TLR4/MyD88/PI3K interactions regulate TLR4 signaling. J. Leukoc. Biol. 85, 966–977.
Lauriano, C. M., Barker, J. R., Yoon, S. S., Nano, F. E., Arulanandam, B. P., Hassett, D. J., and Klose, K. E. (2004). MglA regulates transcription of virulence factors necessary for Francisella tularensis intraamoebae and intramacrophage survival. Proc. Natl. Acad. Sci. U.S.A. 101, 4246–4249.
Lavan, B. E., Kuhne, M. R., Garner, C. W., Anderson, D., Reedijk, M., Pawson, T., and Lienhard, G. E. (1992). The association of insulin-elicited phosphotyrosine proteins with src homology 2 domains. J. Biol. Chem. 267, 11631–11636.
Lofgren, S., Tarnvik, A., Bloom, G. D., and Sjoberg, W. (1983). Phagocytosis and killing of Francisella tularensis by human polymorphonuclear leukocytes. Infect. Immun. 39, 715–720.
Mariathasan, S., Weiss, D. S., Dixit, V. M., and Monack, D. M. (2005). Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J. Exp. Med. 202, 1043–1049.
Marshall, J. G., Booth, J. W., Stambolic, V., Mak, T., Balla, T., Schreiber, A. D., Meyer, T., and Grinstein, S. (2001). Restricted accumulation of phosphatidylinositol 3-kinase products in a plasmalemmal subdomain during Fc gamma receptor-mediated phagocytosis. J. Cell Biol. 153, 1369–1380.
Medina, E. A., Morris, I. R., and Berton, M. T. (2010). Phosphatidylinositol 3-kinase activation attenuates the TLR2-mediated macrophage proinflammatory cytokine response to Francisella tularensis live vaccine strain. J. Immunol. 185, 7562–7572.
Melillo, A. A., Bakshi, C. S., and Melendez, J. A. (2010). Francisella tularensis antioxidants harness reactive oxygen species to restrict macrophage signaling and cytokine production. J. Biol. Chem. 285, 27553–27560.
Moelling, K., Schad, K., Bosse, M., Zimmermann, S., and Schweneker, M. (2002). Regulation of Raf-Akt cross-talk. J. Biol. Chem. 277, 31099–31106.
Mohapatra, N. P., Soni, S., Reilly, T. J., Liu, J., Klose, K. E., and Gunn, J. S. (2008). Combined deletion of four Francisella novicida acid phosphatases attenuates virulence and macrophage vacuolar escape. Infect. Immun. 76, 3690–3699.
O’Connell, R. M., Kahn, D., Gibson, W. S., Round, J. L., Scholz, R. L., Chaudhuri, A. A., Kahn, M. E., Rao, D. S., and Baltimore, D. (2010). MicroRNA-155 promotes autoimmune inflammation by enhancing inflammatory T cell development. Immunity 33, 607–619.
O’Connell, R. M., Taganov, K. D., Boldin, M. P., Cheng, G. H., and Baltimore, D. (2007). MicroRNA-155 is induced during the macrophage inflammatory response. Proc. Natl. Acad. Sci. U.S.A. 104, 1604–1609.
Oyston, P. C., Sjostedt, A., and Titball, R. W. (2004). Tularaemia: bioterrorism defence renews interest in Francisella tularensis. Nat. Rev. Microbiol. 2, 967–978.
Parsa, K. V., Butchar, J. P., Rajaram, M. V., Cremer, T. J., Gunn, J. S., Schlesinger, L. S., and Tridandapani, S. (2008a). Francisella gains a survival advantage within mononuclear phagocytes by suppressing the host IFNgamma response. Mol. Immunol. 45, 3428–3437.
Parsa, K. V., Butchar, J. P., Rajaram, M. V., Cremer, T. J., and Tridandapani, S. (2008b). The tyrosine kinase Syk promotes phagocytosis of Francisella through the activation of Erk. Mol. Immunol. 45, 3012–3021.
Parsa, K. V., Ganesan, L. P., Rajaram, M. V., Gavrilin, M. A., Balagopal, A., Mohapatra, N. P., Wewers, M. D., Schlesinger, L. S., Gunn, J. S., and Tridandapani, S. (2006). Macrophage pro-inflammatory response to Francisella novicida infection is regulated by SHIP. PLoS Pathog. 2:e71. doi: 10.1371/journal.ppat.0020071
Petiot, A., Ogier-Denis, E., Blommaart, E. F., Meijer, A. J., and Codogno, P. (2000). Distinct classes of phosphatidylinositol 3′-kinases are involved in signaling pathways that control macroautophagy in HT-29 cells. J. Biol. Chem. 275, 992–998.
Pierini, L. M. (2006). Uptake of serum-opsonized Francisella tularensis by macrophages can be mediated by class A scavenger receptors. Cell. Microbiol. 8, 1361–1370.
Popova, T., Espina, V., Bailey, C., Liotta, L., Petricoin, E., and Popov, S. (2009). Anthrax infection inhibits the AKT signaling involved in the E-cadherin-mediated adhesion of lung epithelial cells. FEMS Immunol. Med. Microbiol. 56, 129–142.
Rajaram, M. V., Butchar, J. P., Parsa, K. V., Cremer, T. J., Amer, A., Schlesinger, L. S., and Tridandapani, S. (2009). Akt and SHIP modulate Francisella escape from the phagosome and induction of the Fas-mediated death pathway. PLoS ONE 4, e7919. doi: 10.1371/journal.pone.0007919
Rajaram, M. V., Ganesan, L. P., Parsa, K. V., Butchar, J. P., Gunn, J. S., and Tridandapani, S. (2006). Akt/Protein kinase B modulates macrophage inflammatory response to Francisella infection and confers a survival advantage in mice. J. Immunol. 177, 6317–6324.
Rathinam, V. A., Jiang, Z., Waggoner, S. N., Sharma, S., Cole, L. E., Waggoner, L., Vanaja, S. K., Monks, B. G., Ganesan, S., Latz, E., Hornung, V., Vogel, S. N., Szomolanyi-Tsuda, E., and Fitzgerald, K. A. (2010). The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat. Immunol. 11, 395–402.
Rodriguez, A., Vigorito, E., Clare, S., Warren, M. V., Couttet, P., Soond, D. R., van Dongen, S., Grocock, R. J., Das, P. P., Miska, E. A., Vetrie, D., Okkenhaug, K., Enright, A. J., Dougan, G., Turner, M., and Bradley, A. (2007). Requirement of bic/microRNA-155 for normal immune function. Science 316, 608–611.
Rommel, C., Clarke, B. A., Zimmermann, S., Nunez, L., Rossman, R., Reid, K., Moelling, K., Yancopoulos, G. D., and Glass, D. J. (1999). Differentiation stage-specific inhibition of the Raf-MEK-ERK pathway by Akt. Science 286, 1738–1741.
Roth, K. M., Gunn, J. S., Lafuse, W., and Satoskar, A. R. (2009). Francisella inhibits STAT1-mediated signaling in macrophages and prevents activation of antigen-specific T cells. Int. Immunol. 21, 19–28.
Saijo, K., Winner, B., Carson, C. T., Collier, J. G., Boyer, L., Rosenfeld, M. G., Gage, F. H., and Glass, C. K. (2009). A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell 137, 47–59.
Santic, M., Al Khodor, S., and Abu Kwaik, Y. (2010). Cell biology and molecular ecology of Francisella tularensis. Cell. Microbiol. 12, 129–139.
Santic, M., Molmeret, M., Barker, J. R., Klose, K. E., Dekanic, A., Doric, M., and Abu Kwaik, Y. (2007). A Francisella tularensis pathogenicity island protein essential for bacterial proliferation within the host cell cytosol. Cell. Microbiol. 9, 2391–2403.
Santic, M., Molmeret, M., Klose, K. E., Jones, S., and Abu Kwaik, Y. (2005). The Francisella tularensis pathogenicity island protein IglC and its regulator MglA are essential for modulating phagosome biogenesis and subsequent bacterial escape into the cytoplasm. Cell. Microbiol. 7, 969–979.
Schulert, G. S., and Allen, L. A. (2006). Differential infection of mononuclear phagocytes by Francisella tularensis: role of the macrophage mannose receptor. J. Leukoc. Biol. 80, 563–571.
Sjostedt, A. (2006). Intracellular survival mechanisms of Francisella tularensis, a stealth pathogen. Microbes Infect. 8, 561–567.
Sjostedt, A. (2007). Tularemia: history, epidemiology, pathogen physiology, and clinical manifestations. Ann. N. Y. Acad. Sci. 1105, 1–29.
Sutherland, C., and Cohen, P. (1994). The alpha-isoform of glycogen synthase kinase-3 from rabbit skeletal muscle is inactivated by p70 S6 kinase or MAP kinase-activated protein kinase-1 in vitro. FEBS Lett. 338, 37–42.
Sutherland, C., Leighton, I. A., and Cohen, P. (1993). Inactivation of glycogen synthase kinase-3 beta by phosphorylation: new kinase connections in insulin and growth-factor signalling. Biochem. J. 296(Pt 1), 15–19.
Taganov, K. D., Boldin, M. P., Chang, K. J., and Baltimore, D. (2006). NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. U.S.A. 103, 12481–12486.
Telepnev, M., Golovliov, I., Grundstrom, T., Tarnvik, A., and Sjostedt, A. (2003). Francisella tularensis inhibits Toll-like receptor-mediated activation of intracellular signalling and secretion of TNF-alpha and IL-1 from murine macrophages. Cell. Microbiol. 5, 41–51.
Telepnev, M., Golovliov, I., and Sjostedt, A. (2005). Francisella tularensis LVS initially activates but subsequently down-regulates intracellular signaling and cytokine secretion in mouse monocytic and human peripheral blood mononuclear cells. Microb. Pathog. 38, 239–247.
Thai, T. H., Calado, D. P., Casola, S., Ansel, K. M., Xiao, C., Xue, Y., Murphy, A., Frendewey, D., Valenzuela, D., Kutok, J. L., Schmidt-Supprian, M., Rajewsky, N., Yancopoulos, G., Rao, A., and Rajewsky, K. (2007). Regulation of the germinal center response by microRNA-155. Science 316, 604–608.
Ulland, T. K., Buchan, B. W., Ketterer, M. R., Fernandes-Alnemri, T., Meyerholz, D. K., Apicella, M. A., Alnemri, E. S., Jones, B. D., Nauseef, W. M., and Sutterwala, F. S. (2010). Cutting edge: mutation of Francisella tularensis mviN leads to increased macrophage absent in melanoma 2 inflammasome activation and a loss of virulence. J. Immunol. 185, 2670–2674.
Vanhaesebroeck, B., Leevers, S. J., Panayotou, G., and Waterfield, M. D. (1997). Phosphoinositide 3-kinases: a conserved family of signal transducers. Trends Biochem. Sci. 22, 267–272.
Vines, A., Cahoon, S., Goldberg, I., Saxena, U., and Pillarisetti, S. (2006). Novel anti-inflammatory role for glycogen synthase kinase-3beta in the inhibition of tumor necrosis factor-alpha- and interleukin-1beta-induced inflammatory gene expression. J. Biol. Chem. 281, 16985–16990.
Wickstrum, J. R., Bokhari, S. M., Fischer, J. L., Pinson, D. M., Yeh, H. W., Horvat, R. T., and Parmely, M. J. (2009). Francisella tularensis induces extensive caspase-3 activation and apoptotic cell death in the tissues of infected mice. Infect. Immun. 77, 4827–4836.
Wiles, T. J., Dhakal, B. K., Eto, D. S., and Mulvey, M. A. (2008). Inactivation of host Akt/protein kinase B signaling by bacterial pore-forming toxins. Mol. Biol. Cell 19, 1427–1438.
Zhang, P., Katz, J., and Michalek, S. M. (2009). Glycogen synthase kinase-3beta (GSK3beta) inhibition suppresses the inflammatory response to Francisella infection and protects against tularemia in mice. Mol. Immunol. 46, 677–687.
Zhou, H., Monack, D. M., Kayagaki, N., Wertz, I., Yin, J., Wolf, B., and Dixit, V. M. (2005). Yersinia virulence factor YopJ acts as a deubiquitinase to inhibit NF-kappa B activation. J. Exp. Med. 202, 1327–1332.
Keywords: PI3K, Akt, SHIP, miR-155, host response, Francisella
Citation: Cremer TJ, Butchar JP and Tridandapani S (2011) Francisella subverts innate immune signaling: focus on PI3K/Akt. Front. Microbio. 2:13. doi: 10.3389/fmicb.2011.00013
Received: 03 November 2010;
Accepted: 19 January 2011;
Published online: 07 February 2011.
Edited by:
Anders Sjostedt, Umeå University, SwedenReviewed by:
Brian Coombes, McMaster University, CanadaJohn T. Belisle, Colorado State University, USA
Copyright: © 2011 Cremer, Butchar and Tridandapani. This is an open-access article subject to an exclusive license agreement between the authors and Frontiers Media SA, which permits unrestricted use, distribution, and reproduction in any medium, provided the original authors and source are credited.
*Correspondence: Susheela Tridandapani, Department of Internal Medicine, Davis Heart and Lung Research Institute, The Ohio State University Medical Center, 415, 473 West, 12th Avenue, Columbus, OH 43210, USA. e-mail: tridandapani.2@osu.edu
