- 1Department of Earth and Environment, Boston University, Boston, MA, USA
- 2Department of Biology, Boston University, Boston, MA, USA
- 3Department of Chemistry and Physics, King’s College, Wilkes-Barre, PA, USA
- 4Department of Marine Sciences, University of Georgia, Athens, GA, USA
- 5Department of Earth and Environmental Sciences, Wright State University, Dayton, OH, USA
- 6Department of Microbiology, University of Tennessee, Knoxville, TN, USA
Here we examined the impact of a commonly employed method used to measure nitrogen fixation, the acetylene reduction assay (ARA), on a marine sediment community. Historically, the ARA technique has been broadly employed for its ease of use, in spite of numerous known artifacts. To gauge the severity of these effects in a natural environment, we employed high-throughput 16S rRNA gene sequencing to detect differences in acetylene-treated sediments vs. non-treated control sediments after a 7 h incubation. Within this short time period, significant differences were seen across all activity of microbes identified in the sediment, implying that the changes induced by acetylene occur quickly. The results have important implications for our understanding of marine nitrogen budgets. Moreover, because the ARA technique has been widely used in terrestrial and freshwater habitats, these results may be applicable to other ecosystems.
Introduction
Every sampling effort and each experimental design impacts the environment and the processes we wish to measure. Quantifying the nature and extent of that effect is a necessary step in our broader understanding of microbial processes and for the interpretation of the data we collect. High-throughput 16S rRNA gene-based bacterial community surveys provide a powerful mechanism for us to examine the impact of commonly employed geochemical methods on the microbial population.
Of particular interest to many aquatic biogeochemists is the microbially-mediated process of nitrogen fixation (N-fixation), which converts inert dinitrogen (N2) gas to biologically reactive nitrogen (N). While Earth’s atmosphere is comprised primarily of N2 gas, most organisms cannot tap into this reservoir. Thus, N-fixation provides critical links between biological organisms, this unreactive N pool, and N concentrations in the environment. Indeed, the ability to fix N2 provides a significant advantage to organisms with this capability. Once N is fixed, various processes transform reactive N until it is returned to the atmosphere through denitrification. Because N is an essential and often limiting nutrient for primary productivity, its availability, at least in part, constrains global primary productivity (Tyrrell, 1999; Elser et al., 2007).
Direct measurements of N-fixation rates from decreases in N2 concentration are difficult, as they require detection of small changes in a large reservoir of N2 gas. Equally challenging is the measurement of in situ total N increases to quantify N fixation, because this method also requires detection of a small amount of N against a much larger background of environmental organic nitrogen (Seitzinger and Garber, 1987). In recent years, our ability to measure such small changes has increased substantially, but they are time consuming and expensive. As such, for the last five decades we have used the methodologically more simple acetylene reduction assay (ARA) to measure N-fixation (Dilworth, 1966; Hardy et al., 1968; Stewart et al., 1968). The ARA relies on the flexibility of nitrogenase, the enzyme responsible for N2 reduction, as it can reduce acetylene (C2H2) to ethylene (C2H4), which can then be directly quantified. Theoretically, every three moles of ethylene produced corresponds to the reduction of 1 mole of N2 to ammonia.
However, the ARA is known to have numerous experimental artifacts. Some of these are newly discovered and related to the addition of acetylene and subsequent incubation of the sample (Mohr et al., 2010; Wilson et al., 2012). Other factors compromising this technique have been known for much longer and are related to shifts in the activity and ability of the microbial community to process nutrients and carbon in the presence of acetylene. For example, acetylene irreversibly inhibits methanogenesis in cultures and sediments (Oremland and Taylor, 1975). Acetylene also reversibly blocks aerobic nitrification (Hynes and Knowles, 1982) and nitrous oxide reduction in anaerobic denitrifiers (Balderston et al., 1976). Almost thirty years ago, acetylene was shown to partially or completely inhibit carbon dioxide production and growth of two sulfate reducing bacteria, Desulfovibrio desulfuricans and Desulfovibrio gigas although another species, Desulfotomaculum ruminis, was not effected at all (Payne and Grant, 1982). This is particularly relevant for marine ecosystems where sulfur- and sulfate-reducing bacteria are often significant contributors to the N-fixing community (Nielsen et al., 2001; Bertics et al., 2013; Brown and Jenkins, 2014).
To examine the broader applicability of the foundational efforts of Payne and Grant (1982), we used sediments from a temperate New England estuary to investigate the influence of the ARA on the total active microbial community of marine sediment. We directly measured net N2 fluxes and then measured N-fixation using ARA. Following the ARA, we conducted a 16S rRNA bacterial gene community survey to examine the change in active community activity after exposure to acetylene. The results are provided in the context of how the ARA technique alters processes within the community it is intended to unobtrusively query.
Materials and Methods
Study Site and Sample Collection
Samples were collected in July 2011 from a station in Narragansett Bay, RI, USA (41°35.3′, 071°22.3′) where the water column depth was 8 m. The in situ temperature was 17°C and the salinity was 31.3 psu. Intact triplicate sediment cores were hand collected by SCUBA divers with the vertical architecture of the sediment maintained during core collection and handling (Fulweiler and Heiss, 2014). Capped cores were transported with in situ water headspace in coolers filled with site water to an environmental chamber set to ambient temperature at the Graduate School of Oceanography at the University of Rhode Island. The sediment cores were placed in a 17°C water bath in the dark with air gently bubbling through the surface water until the incubation began (∼8 h).
Net N2 Measurements
We first completed an N2/Ar incubation to measure the net flux of N2 across the sediment-water interface. These methods have been documented previously (Fulweiler and Nixon, 2009, 2012). Briefly, before the incubation we carefully replaced the overlying water with filtered (0.2-μm) site water, the cores were sealed with a gas tight lid (no air headspace), gently stirred (∼40 rpm), and replicate samples taken for N2/Ar analysis at five points over the course of an incubation. Each sample was preserved with 20 μl of saturated ZnCl solution. All incubations took place in the dark and lasted ∼ 11 h until we observed an overlying water oxygen concentration drop of at least 2 mg L-1 (62.5 μM), but at no point did the cores approach hypoxia. Dissolved N2 and Ar gas concentrations were analyzed on a quadrupole membrane inlet mass spectrometer with a precision of ± 0.03% (Sisler and ZoBell, 1951; Heip, 1995; Kana et al., 1998). N2 change for each of the triplicate cores was determined from a five-point linear regression. Rates were then prorated for the volume of water overlying the core and the sediment area of the core. The rates calculated from the N2/Ar technique actually present a measure of net N2 flux (gross denitrification – gross N fixation).
ARA Measurements
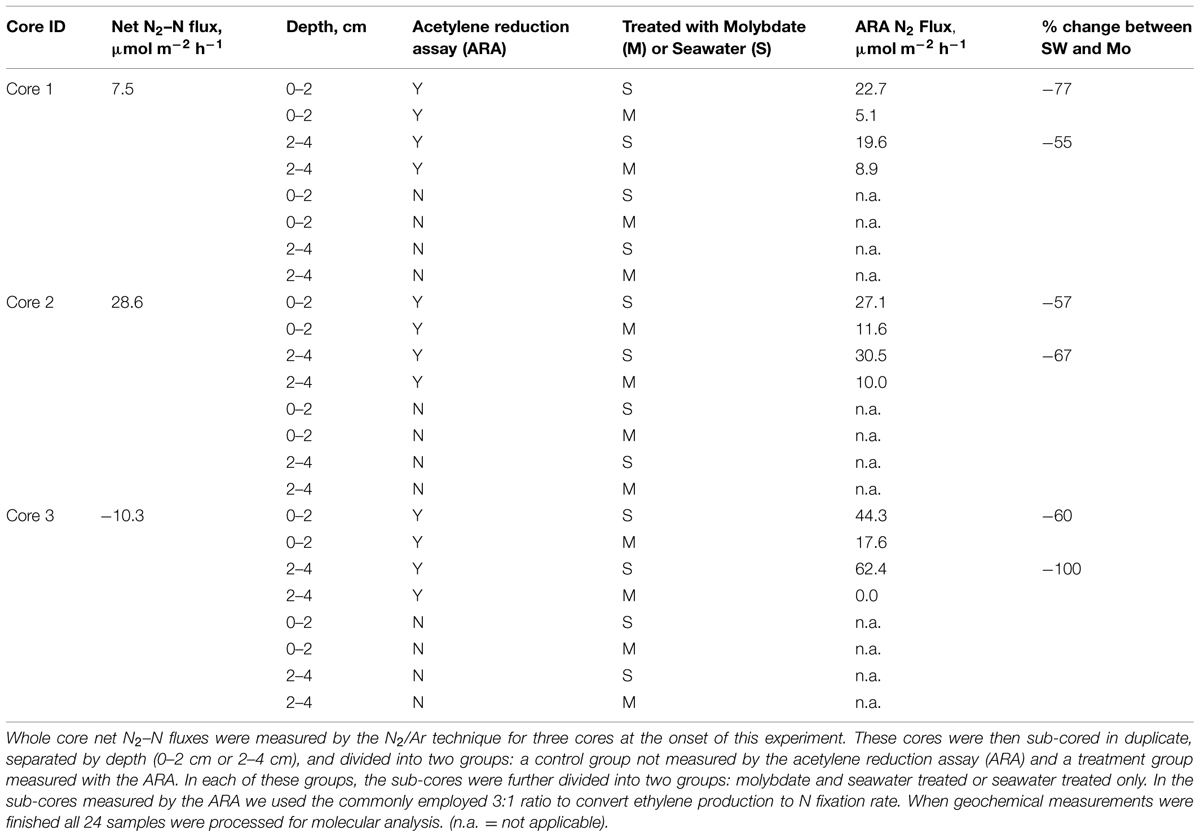
After net N2 measurements were made, we quantified the reduction of acetylene (C2H2) to ethylene (C2H4) as a proxy for N-fixation (Hardy et al., 1968). Duplicate sub-cores (∼2.5 cm i.d.) were collected from each core and sectioned into 0–2m and 2–4 cm increments, halved, and placed in 40 mL glass vials. For each depth, each core duplicate sample halves were separated into the following treatments: acetylene plus filtered seawater, acetylene plus 40 mM sodium molybdate (Na2MoO4), seawater alone, and 40 mM Na2MoO4 alone. Molybdate is an established inhibitor of sulfate-reducing bacteria and used as an indicator of N-fixation by sulfate reducing bacteria (Postgate, 1949; Oremland and Taylor, 1978). Samples were placed in sealed vials, the air headspace was evacuated, and replaced with argon gas after which 10 mL of C2H2 was injected into the vials (Hamilton et al., 2011; Cole and McGlathery, 2012). The ARA incubation lasted 7 h. Gas samples were run on a Shimadzu gas chromatograph equipped with a flame ionization detector using a Porapak N column, mesh size 80/100 at the Graduate School of Oceanography at the University of Rhode Island. The halved sub-core samples were then extrapolated to area units and rates of sediment acetylene reduction were converted to N-fixed using the common 3:1 molar conversion (Seitzinger and Garber, 1987). All samples were then frozen at -80°C and shipped overnight to the University of Tennessee for molecular analysis.
Molecular Analysis
RNA was extracted from all 24 sediment samples using the MoBio PowerSoilTM RNA isolation kit (MoBio, Carlsbad, CA, USA) according to manufacturer’s protocols and checked for contaminating DNA by PCR (below). cDNA was produced from extracted RNA using 16S rRNA gene-specific primers (9F and 1522R, E. coli numbering) using the Invitrogen Superscript III First-Strand Synthesis SuperMix kit according the manufacturer’s protocols. We PCR amplified bacterial 16S rRNA using primers targeting bases 338–926 of the 16S rRNA gene (E. coli numbering), which contains the V3–V5 region, with the following PCR protocol: 95°C for 5 min, followed by 30 rounds of (95°C for 30 s, 55°C for 30 s, 72°C for 30 s) and then a final extension step at 72°C for 10 min. Product amplification was verified on a 1% agarose gel stained with ethidium bromide and viewed on a UV transilluminator. Individual samples were processed to remove unincorporated primers and nucleotides using the Qiaquick PCR cleanup kit (Qiagen, Valencia, CA, USA). Amplicon concentrations were determined using a NanoDrop ND-1000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA).
Individual sample amplicons were barcoded (six additional PCR cycles : 95°C for 30 s, 55°C for 30 s, 72°C for 30 s) with primers that contained a unique 8-bp barcode attached to the 454 fusion primers (LeCleir et al., 2014; Wilhelm et al., 2014). The barcoding primers were designed for unidirectional sequencing on a 454 GSFLX sequencer (454 Life Sciences, Branford, CT, USA). This strategy required the use of the Lib-L kit (see Roche application brief 001-2009). We opted for unidirectional sequencing as our PCR product was larger than the average read length of the available 454 Titanium sequencing chemistry. This approach ensured sequences would overlap for the longest length possible. All barcoding reactions were prepared to have 0.5 ng/μl of amplicon DNA per reaction. After the barcoding reaction, we again verified our amplicons on an agarose gel before pooling all barcoded amplicons. Barcoded amplicons were processed to remove unincorporated primers and nucleotides using a single Qiagen Qiaquick column. Sequencing was completed at the University of Tennessee/Oak Ridge National Laboratory Joint institute of Biological Sciences. Sequence information has been deposited in the NCBI short-read archive in bioproject PRJNA271790.
We used the Mothur software package (version 1.27.0; Schloss et al., 2009) to process our sequences for sufficient length and quality. We processed our sequences similar to the Schloss SOP1 with some modifications of the shhh.flows command; we changed the number of flows value in the shhh.flows command to 360–720 from 450 (Quince et al., 2009). Mothur was also used to cluster sequences into operational taxonomic units (OTUs) and for phylogenetic classification. A 0.03 cutoff (97% identity) was chosen for OTU determination. To visualize the influence of acetylene on OTU abundance, proportional abundances for each OTU were compared for samples collected in the presence vs. absence of acetylene. Outputs were visualized after clustering (using the Euclidean distance method) with Cluster 3.02 using Java TreeView (Saldanha, 2004).
The Primer-E software package (Version 6.0; Clarke and Gorley, 2006) was used to more deeply interrogate relationships between OTUs across samples and to also look for correlations between OTU presence/abundance and environmental parameters. The “shared” file (a matrix file containing OTU abundances for each sample) created by Mothur was imported directly into the Primer-E software package. All OTUs with an abundance (sum of counts across all libraries) >25 sequences were used for further analyses. OTUs meeting these criteria were standardized to the total number of sequences per barcoded library (proportional abundances). These standardized abundances were then square-root transformed to partially deemphasize more highly abundant OTUs. A Bray-Curtis similarity matrix was constructed and used to perform non-metric multidimensional scaling analysis (NMDS) for visualization of community structure relationships between the different samples. The ANOSIM and PERMANOVA programs within Primer-E were used to document the statistical significance of our findings. Both tests were performed on our Bray-Curtis similarity matrix of OTU abundances. A principal components analysis (PCA) was performed on the square-root transformed data to visualize community structure differences and identify OTUs driving the differences in the bacterial communities. Phylogenetic identities of OTUs were determined using the Ribosomal Database Project (RDP) classification within Mothur. We also employed SIMPER to define discriminating species between treatment and control (Clarke, 1993) as well as the software package LEfSe (Segata et al., 2011) to statistically compare differentially abundant OTUs.
Results
Net N2 Fluxes and N-Fixation Estimates
The net N2 flux across the sediment water-interface was quantified to determine if the sediments were net denitrifying (positive N2-flux dominated) or net nitrogen fixing (negative N2-flux dominated). Net N2 fluxes across the sediment-water interface for the individuals cores ranged from -10 to 28.6 μmol N2-N m-2 h-1, suggesting that both N-fixation and denitrification were occurring (Table 1). N-fixation rates measured with the ARA, similarly, revealed high variability among the cores and, in some cases rates differed by depth (Table 1). After the addition of molybdate, N-fixation rates were reduced on average by almost 70%, indicating that sulfate reducers were responsible for a significant component of the observed N-fixation (Gandy and Yoch, 1988).
Activity of the Microbial Community
After screening sequences to ensure sufficient quality and length as well as removal of chimeric sequences, 115,319 16S rRNA gene sequences remained across the 24 different samples. Total sequence abundances (per library) ranged from a minimum of 1,379 sequences to a maximum of 10,569 sequences, with an average of 4,805 sequences per library. After clustering, 9,979 OTUs at 0.03 cut-off (97% similarity) remained.
To determine how acetylene influenced total community structure, we compared total community 16S rRNA expression by principle component analyses (Figure 1; Table 2). Importantly, we performed PCA analysis only for those samples treated with acetylene and the control groups. Samples treated with molybdate (with or without acetylene) were excluded, as we found no impact of molybdate on the active microbial community (Table 3). The PCA highlights two clear patterns. First, there is distinct separation between those samples treated with acetylene and the control along principal component one (PC1). And secondly, depth appears to also be a key driver separating samples along principal component two (PC2). Similarly, the ANOSIM and PERMANOVA analyses show the significant impact of acetylene and depth with the latter being the most significant driver (Table 3).
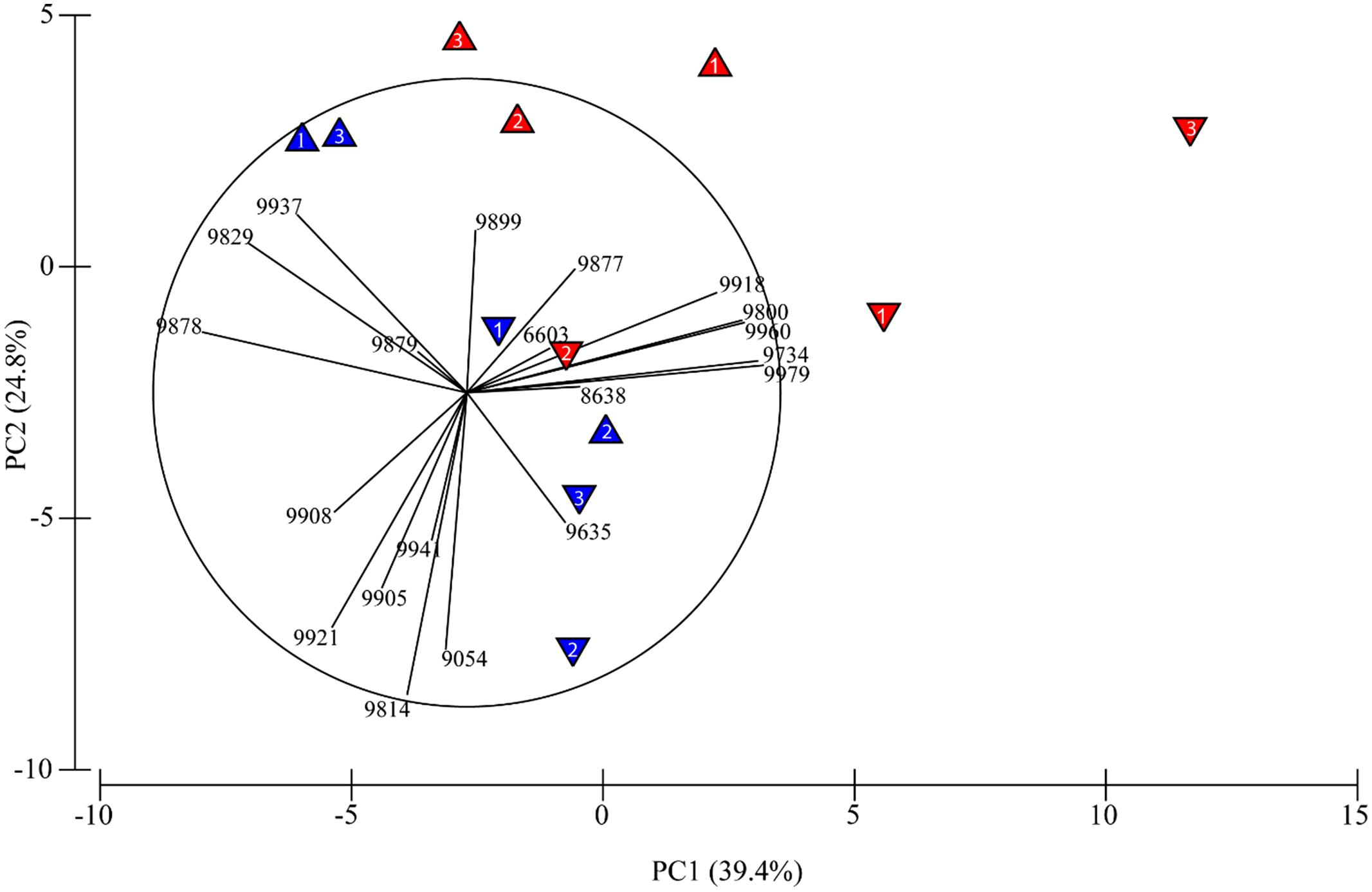
FIGURE 1. Results of principal components analysis (PCA) of operational taxonomic units (OTUs) and how they separate between acetylene treated (red triangles) and untreated (blue triangles) and by depth (0–2 cm: upward facing triangles and 2–4 cm: downward facing triangles). The number in each triangle indicates which core the sample came from. No molybdate treated samples are included here, as molybdate did not significantly alter the active sediment microbial community. Principal component (PC1 and PC2) together, account for 64.2% of the variance in these data.
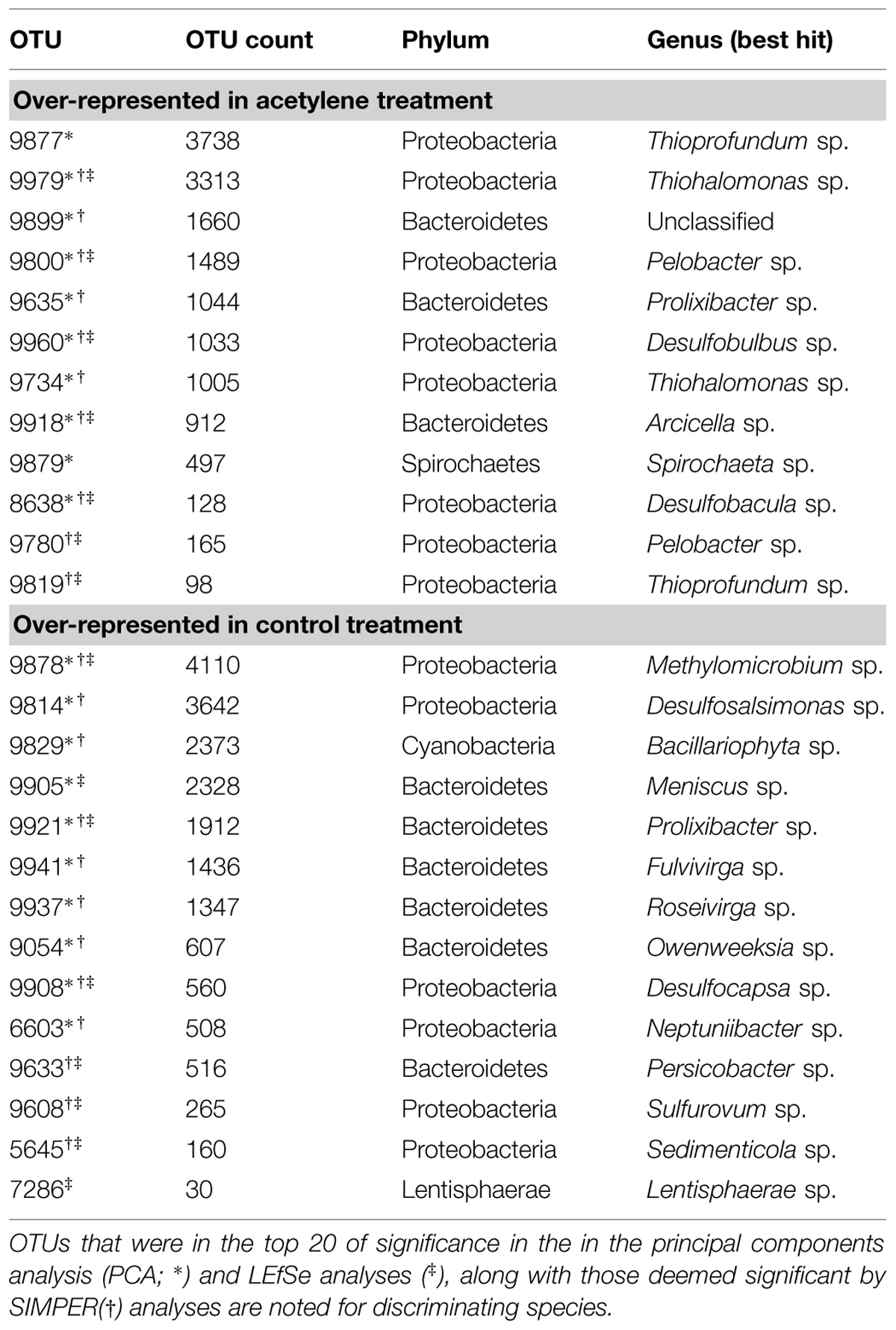
TABLE 2. Dominant operational taxonomic units (OTUs) that drive the separation of the acetylene treated sediments versus the control sediments.
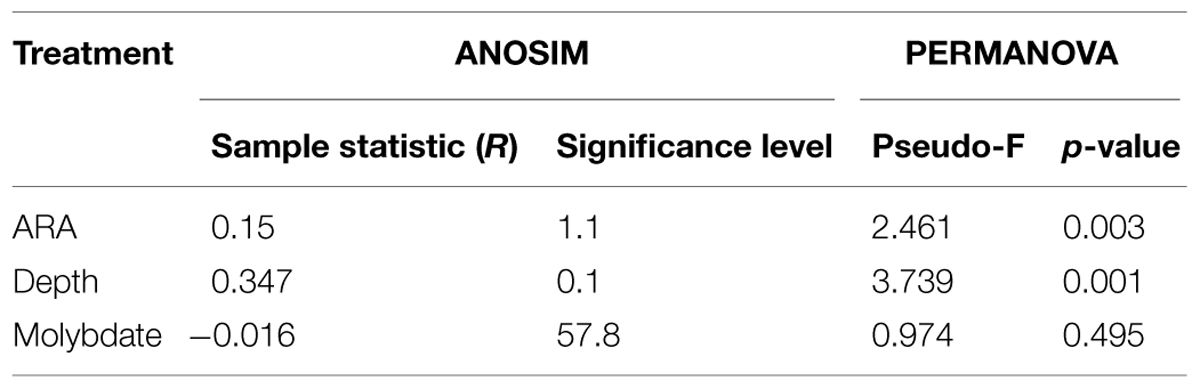
TABLE 3. Results from the ANOSIM and PERMANOVA analyses describing the impact acetylene, depth, or molybdate has on the active sediment microbial community.
Together, the first and second components of the PCA explained just over 64% of the variation in the samples (Figure 1). The treatments separated primarily along the first component which showed a positive relationship with OTU 9979 (Thiohalomonas) and a negative relationship with OTU 9878 (Methylomicrobium). The samples also appear to separate by depth along PC2 which is dominated by a negative relationship with OTU 9814 (Desulfosalsimonas) and a positive relationship with OTU 9829 (Bacillariophyta). In some cases, treatment with acetylene led to increased representation of a number of OTUs within the active community (Table 2). Concomitantly, we also observed a number of OTUs that appear to be underrepresented in the acetylene treated versus control groups suggesting that the acetylene may inhibit or alter their activity (Table 2). Overall, nine OTUs were identified by all three approaches (PCA, SIMPER, and LEfSe) as significant drivers of difference in the communities while other combinations of approaches (different by SIMPER and LEfSe, 4; different by SIMPER and PCA 9; different by LEfSe and PCA, 1) revealed another 14 OTUs of interest.
To visualize which OTUs were driving observed relationships, we clustered OTU expression based on differences between control and acetylene-treated replicates (Figure 2). We then identified the dominant OTUs that were over-represented under acetylene (Figure 2B) and those that were under-represented (Figure 2C). Within these groups, changes were subtle: the most upregulated groups (OTUs most closely identified as Thiohalamonas, Thioprofundum, and Pelobacter) demonstrated increases in 16S rRNA representation of up to 9.5%. In parallel, the most down-regulated OTUs in any sample showed decreases in representation of ∼5.0% (Paraferrimonas and Desulfosalimonas).
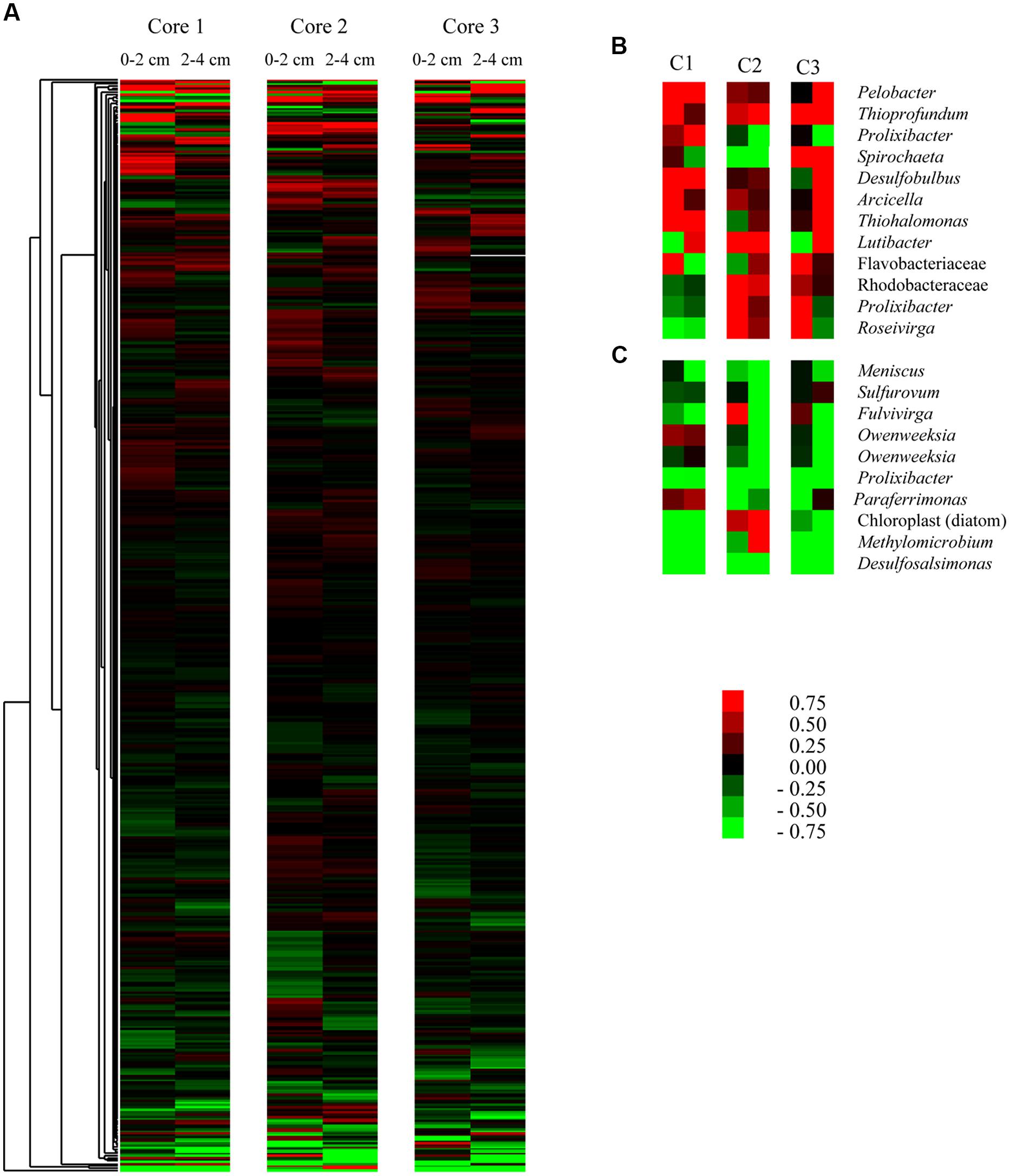
FIGURE 2. Influence of acetylene on the distribution of individual (OTUs) from the sediment cores used in this study. Heat map (A) of all OTUs with greater than 25 representative signatures in the complete data set. Data within libraries were normalized to percent of total reads and then expressed as differences between treatment and control [values above zero (red) signify over-representation in the treatment where values below zero (green) represent underrepresentation in the treatment]. Data are shown for three replicate cores and for 0–2 cm and 2–4 cm depth fractions and for seawater treated only (no molybdate). Insets show the 10 most over-represented (B) and underrepresented (C) groups with cores (C1–C3) and depths (0–2 or 2–4 cm depth) presented in the same order as in (A).
Discussion
Geochemistry of the Samples
We directly quantified the flux of N2 gas across the sediment-water interface using the N2/Ar technique. We chose this technique because it is the least intrusive and yet direct measurement of N2 flux. Unfortunately, it only provides a net measurement and thus only reports the balance between sediment N-fixation and its opposite process, denitrification (the microbial conversion of nitrate to N2). At the time of this experiment, the sediments exhibited net positive N2 fluxes (or net denitrification) as well as net negative N2 fluxes (or N-fixation; Table 1). It is not unusual for sediments at this site to show such variability. In fact, the sediments at this site routinely alternate between net denitrification and net N-fixation and the month following these measurements the sediments were dominated by net N-fixation (Fulweiler and Heiss, 2014). Additionally, we did observe the conversion of acetylene to ethylene for each core and at every depth. The conversion of acetylene to ethylene was considerably reduced when exposed to molybdate suggesting sulfate-reducing bacteria are driving the N-fixation in these sediments. These results are consistent with previous research at this site where N-fixation was observed in core incubations and in large experimental mesocosm studies and, in all cases, the active N-fixing community was dominated by anaerobic sulfate reducers and sulfur/iron reducers (Brown, 2013; Fulweiler et al., 2013; Brown and Jenkins, 2014).
ARA Impacts on Sulfate Reducing Bacteria and N-Fixation
Several dominant OTUs related to sulfur (S) cycling were observed in this study (Table 2). Specifically, we observed OTUs closely related to both sulfur and sulfate reducers (e.g., Desulfosalsimonas, Desulfobulbus, etc.) and those related to sulfur oxidizers (e.g., Thioprofundum and Thiohalomonas). Sulfur and sulfate reducing bacteria, specifically, Desulfovibrio spp. and Desulfobacter sp. have long been known to fix N in culture (Sisler and ZoBell, 1951; Widdel, 1987). A rich literature in sea grass beds (Welsh et al., 1996; McGlathery et al., 1998) and salt marshes (Gandy and Yoch, 1988; Piceno and Lovell, 2000) have credited sulfate reducers as the primary N-fixers. More recently, in Baltic Sea sediments, acetylene reduction, and sulfate reduction rates showed similar seasonal patterns and molecular analysis revealed the presence of two sulfate reducing bacteria, Desulfovibrio vulgaris, and Desulfonema limicola (Bertics and Ziebis, 2010). As the work that motivated this research highlighted, over three decades ago Payne and Grant (1982) reported that acetylene partially or completely inhibited the growth of two sulfate reducing bacteria, Desulfovibrio desulfuricans, and Desulfovibrio gigas while a third species, Desulfotomaculum ruminis, was completely unaffected. In this study, we find a similar species or closely related groups of bacteria responding in different ways to the acetylene (Figure 2). The different responses may have important consequences for our understanding of heterotrophic N cycling in marine sediments. Sulfur- and sulfate-reducers are important N-fixers in a range of marine environments, and they experience up or down regulation in the presence of acetylene, one of the most commonly used techniques to measure N-fixation. Thus, we have likely been under- or over-estimating N-fixation rates in these environments. Complicating the picture further is that response appears to be species specific, thus we cannot apply a correction across measurements leaving us with a methodologically introduced unknown amount of error.
These findings are significant because ARA has been and is still widely used in both terrestrial and aquatic ecosystem studies of N-fixation. This may be particularly important for marine systems, where debate exists concerning whether the modern N budget is balanced. Although some estimates have suggested that the oceanic fixed N budget is balanced, they are plagued with gross uncertainties (Gruber, 2004). More recent estimates indicate that the budget is unbalanced with a substantial N deficit, although these budgets also contain considerable uncertainty (Codispoti et al., 1992; Brandes and Devol, 2002; Deutsch et al., 2007). This deficit is driven mainly by larger denitrification rates, as denitrification is thought to be a dominant process in the N cycle, while sediment N fixation is traditionally considered to be inconsequential (Howarth et al., 1988). Thus, to balance the modern marine N budget, rates of denitrification need to be decreased and/or rates of N-fixation increased. Several recent lines of evidence argue for the latter as it appears N-fixation may be more important than originally anticipated (open ocean: Zehr et al., 2001; Davis and McGillicuddy, 2006; shallow coastal systems: Gardner et al., 2006; Fulweiler et al., 2007; Mortazavi et al., 2012). Importantly, these more recent studies have used other techniques besides ARA to measure rates of N-fixation (e.g., N2/Ar technique, nifH expression, etc.). We propose one important driver delaying our thorough understanding of the role of N-fixation in marine environments is the widespread historic and current use of ARA. Furthermore, because ARA is also used in terrestrial and freshwater ecosystems rates of N-fixation rates may also be inaccurate by varying degrees across these environments.
ARA Impacts on the Active Microbial Community
We expected to see significant changes in the active microbial community and this was observed in our 16S rRNA bacterial community survey data. We found a significant difference in sediments treated with acetylene versus those in the control samples (Table 3). However, the impact of acetylene was not uniform across phylum or genera (Table 2). Instead, we observed the up or down regulation of different OTUs associated with Proteobacteria, Spirochaetes, and Bacteroidetes. OTUs dominant under the acetylene treatment most closely identified with a range of bacteria, including Pelobacter and Arcicella. In contrast, other OTUs were more active in the control samples such as Methylomicrobium, Roseiviriga, and Bacillariophyta.
The data also highlight the heterogenic nature of the sediment microbial community and provide hints about how this heterogeneity alters the community response to disturbance. In this experiment, sediment cores were hand collected by divers within close distance to each other. Despite the careful nature of the collection and the proximity of the sediments, sediment core 2 appears dramatically different to sediments cores 1 and 3 (Table 1; Figure 2). In core 2 we measured the highest rate of net denitrification (29 μmol N2-N m-2 h-1) and a different microbial response to acetylene as compared to the other cores. The difference in active community response is seen most clearly in the PCA where the 2–4 cm depth sample in core 2 did not change across PC 1. In addition, depth was the most significant driver of differences in the microbial community (Table 3). This makes sense as the sediment microbial community was exposed to acetylene for only 7 h and presumably they had much more time to acclimate to there location within the sediments.
Our observations are not surprising as the ARA technique has long been known to inhibit various organisms (Oremland and Taylor, 1975; Schink, 1985; Knowles, 1990). In fact, it is this inhibitory power that has been harnessed to measure denitrification rates in the acetylene block technique (Joye et al., 1996; Welsh et al., 2001) and the importance of methane production and consumption processes (Prior and Dalton, 1985). Thus, as expected we observed repression in the activity of an OTU identifying as Methylomicrobium, a strictly aerobic group of methanotrophs (Hanson and Hanson, 1996; Vuilleumier et al., 2012). However, to our knowledge this is the first study to show total active community changes driven by acetylene using 16S rRNA data. These data also allowed us to observe a rapid change in active community metabolism as incubations only lasted 7 h. Overall, our observations concerning the rapid effects of acetylene on microbial community activity give pause concerning the robustness of the technique, adding yet another cautionary note when interpreting ARA data.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This material is based on work funded through a development grant (2011-R/RC-124-PD) to RWF from the MIT Sea Grant College Program (NOAA). Additionally, this work was supported through grants from the Rhode Island Sea Grant College Program (NOAA) and NSF (OCE 0926859) to RWF, and OCE-1030518 and OCE-1061352 to SWW. We thank the Sloan Foundation for additional support to RWF. We thank Sarah Foster, Lindsey Fields, Matt Horn, Jason Krumholz, Connor McManus, and Jeff Mercer for field help. We thank Luke Cole and Melanie Hayn for method development help long ago. Additionally, we thank Leanna Heffner and Dennis Graham for GC instruction and troubleshooting and David Smith, Art Spivack, and Steve D’Hondt for allowing us to use the GC at the Graduate School of Oceanography at the University of Rhode Island.
Footnotes
- ^ http://www.mothur.org/wiki/Schloss_SOP
- ^ http://bonsai.hgc.jp/~mdehoon/software/cluster/software.htm#ctv
References
Balderston, W. L., Sherr, B., and Payne, W. (1976). Blockage by acetylene of nitrous oxide reduction in Pseudomonas perfectomarinus. Appl. Environ. Microbiol. 31, 504–508.
Bertics, V. J., Loscher, C. R., Salonen, I., Dale, A. W., Gier, J., Schmitz, R. A., et al. (2013). Occurrence of benthic microbial nitrogen fixation coupled to sulfate reduction in the seasonally hypoxic Eckernforde Bay, Baltic Sea. Biogeosciences 10, 1243–1258. doi: 10.5194/bg-10-1243-2013
Bertics, V. J., and Ziebis, W. (2010). Bioturbation and the role of microniches for sulfate reduction in coastal marine sediments. Environ. Microbiol. 12, 3022–3034. doi: 10.1111/j.1462-2920.2010.02279.x
Brandes, J. A., and Devol, A. H. (2002). A global marine-fixed nitrogen isotopic budget: implications for Holocene nitrogen cycling. Global Biogeochem. Cycles 146, 67-61–67-14. doi: 10.1029/2001GB001856
Brown, S. M. (2013). Using Molecular Tools to Elucidate Controls on Microbes Driving the Nitrogen Cycle in Marine Sediments. Kingston, RI: University of Rhode Island.
Brown, S., and Jenkins, B. (2014). Profiling gene expression to distinguish the likely active diazotrophs from a sea of genetic potential in marine sediments. Environ. Microbiol. 16, 3128–3142. doi: 10.1111/1462-2920.12403
Clarke, K. R. (1993). Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 18, 17–143. doi: 10.1111/j.1442-9993.1993.tb00438.x
Clarke, K., and Gorley, R. (2006). PRIMER v6: User Manual/Tutorial. Plymouth: Plymouth Marine Laboratory.
Codispoti, L. A., Elkins, J. W., Yoshinari T., Friederich, G. E., Sakamoto, C. M., and Packard, T. T. (1992). “On the nitrous-oxide flux from productive regions that contain low oxygen waters,” in Oceanography of the Indian Ocean, ed. B. N. Desai (Rotterdam: A. A. Balkema), 271–284.
Cole, L. W., and McGlathery, K. J. (2012). Nitrogen fixation in restored eelgrass meadows. Mar. Ecol. Progr. Ser. 448, 235–246. doi: 10.3354/Meps09512
Davis, C. S., and McGillicuddy, D. J. (2006). Transatlantic abundance of the N2-fixing colonial cyanobacterium Trichodesmium. Science 312, 1517–1520. doi: 10.1126/science.1123570
Deutsch, C., Sarmiento, J. L., Sigman, D. M., Gruber, N., and Dunne, J. P. (2007). Spatial coupling of nitrogen inputs and losses in the ocean. Nature 445, 163–167. doi: 10.1038/nature05392
Dilworth, M. (1966). Acetylene reduction by nitrogen-fixing preparations from Clostridium pasteurianum. Biochim. Biophys. Acta Gen. Subj. 127, 285–294. doi: 10.1016/0304-4165(66)90383-7
Elser, J. J., Bracken, M. E. S., Cleland, E. E., Gruner, D. S., Harpole, W. S., Hillebrand, H., et al. (2007). Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 10, 1135–1142. doi: 10.1111/j.1461-0248.2007.01113.x
Fulweiler, R. W., Brown, S. M., Nixon, S. W., and Jenkins, B. D. (2013). Evidence and a conceptual model for the co-occurrence of nitrogen fixation and denitrification in heterotrophic marine sediments. Mar. Ecol. Progr. Ser. 482, 57–68. doi: 10.3354/Meps10240
Fulweiler, R., and Heiss, E. (2014). (Nearly) a decade of directly measured sediment N2 fluxes: what can Narragansett Bay tell us about the global ocean nitrogen budget. Oceanography 27, 184–195. doi: 10.5670/oceanog.2014.22
Fulweiler, R. W., and Nixon, S. W. (2009). Responses of benthic-pelagic coupling to climate change in a temperate estuary. Hydrobiologia 629, 147–156. doi: 10.1007/s10750-009-9766-0
Fulweiler, R. W., and Nixon, S. W. (2012). Net Sediment N2 fluxes in a southern new england estuary - variations in space and time. Biogeochemistry 111, 111–124. doi: 10.1007/s10533-011-9660-5
Fulweiler, R. W., Nixon, S. W., Buckley, B. A., and Granger, S. L. (2007). Reversal of the net dinitrogen gas flux in coastal marine sediments. Nature 448, 180–182. doi: 10.1038/Nature05963
Gandy, E. L., and Yoch, D. C. (1988). Relationship between nitrogen-fixing sulfate reducers and fermenters in salt-marsh sediments and roots of Spartina-Alterniflora. Appl. Environ. Microbiol. 54, 2031–2036.
Gardner, W. S., Mccarthy, M. J., An, S. M., Sobolev, D., Sell, K. S., and Brock, D. (2006). Nitrogen fixation and dissimilatory nitrate reduction to ammonium (DNRA) support nitrogen dynamics in Texas estuaries. Limnol. Oceanogr. 51, 558–568. doi: 10.4319/lo.2006.51.1_part_2.0558
Gruber, N. (2004). “The dynamics of the marine nitrogen cycle and its influence on atmospheric CO2 variations,” in The Ocean Carbon Cycle and Climate, eds M. Follows and T. Oguz (Berlin: Springer), 97–148.
Hamilton, T. L., Lange, R. K., Boyd, E. S., and Peters, J. W. (2011). Biological nitrogen fixation in acidic high-temperature geothermal springs in Yellowstone National Park, Wyoming. Environ. Microbiol. 13, 2204–2215. doi: 10.1111/j.1462-2920.2011.02475.x
Hardy, R. W., Holsten, R., Jackson, E., and Burns, R. (1968). The acetylene-ethylene assay for N2 fixation: laboratory and field evaluation. Plant Physiol. 43, 1185–1207. doi: 10.1104/pp.43.8.1185
Heip, C. (1995). Eutrophication and zoobenthos dynamics. Ophelia 41, 113–136. doi: 10.1080/00785236.1995.10422040
Howarth, R. W., Marino, R., Lane, J., and Cole, J. J. (1988). Nitrogen-fixation in fresh-water, estuarine, and marine ecosystems.1. Rates and importance. Limnol. Oceanogr. 33, 669–687. doi: 10.4319/lo.1988.33.4_part_2.0669
Hynes, R., and Knowles, R. (1982). Effect of acetylene on autotrophic and heterotrophic nitrification. Can. J. Microbiol. 28, 334–340. doi: 10.1139/m82-049
Joye, S. B., Smith, S. V., Hollibaugh, J. T., and Paerl, H. W. (1996). Estimating denitrification rates in estuarine sediments: a comparison of stoichiometric and acetylene based methods. Biogeochemistry 33, 197–215. doi: 10.1007/BF02181072
Kana, T. M., Sullivan, M. B., Cornwell, J. C., and Groszkowski, K. M. (1998). Denitrification in estuarine sediments determined by membrane inlet mass spectrometry. Limnol. Oceanogr. 43, 334–339. doi: 10.4319/lo.1998.43.2.0334
Knowles, R. (1990). “Acetylene inhibition technique: development, advantages, and potential problems,” in Denitrification in Soil and Sediment, eds N. P. Revsbech and J. Sørensen (Berlin: Springer), 151–166.
LeCleir, G. R., Debruyn, J. M., Maas, E. W., Boyd, P. W., and Wilhelm, S. W. (2014). Temporal changes in particle-associated microbial communities after interception by non-lethal sediment traps. FEMS Microbiol. Ecol. 87, 153–163. doi: 10.1111/1574-6941.12213
McGlathery, K. J., Risgaard-Petersen, N., and Christensen, P. B. (1998). Temporal and spatial variation in nitrogen fixation activity in the eelgrass Zostera marina rhizosphere. Mar. Ecol. Progr. Ser. 168, 245–258. doi: 10.3354/Meps168245
Mohr, W., Grosskopf, T., Wallace, D. W. R., and Laroche, J. (2010). Methodological underestimation of oceanic nitrogen fixation rates. PLoS ONE 5:e12583. doi: 10.1371/journal.pone.0012583
Mortazavi, B., Riggs, A. A., Caffrey, J. M., Genet, H., and Phipps, S. W. (2012). The contribution of benthic nutrient regeneration to primary production in a shallow eutrophic estuary, Weeks Bay, Alabama. Estuaries Coasts 35, 862–877. doi: 10.1007/s12237-012-9478-y
Nielsen, L. B., Finster, K., Welsh, D. T., Donelly, A., Herbert, R. A., De Wit, R., et al. (2001). Sulphate reduction and nitrogen fixation rates associated with roots, rhizomes and sediments from Zostera noltii and Spartina maritima meadows. Environ. Microbiol. 3, 63–71. doi: 10.1046/j.1462-2920.2001.00160.x
Oremland, R. S., and Taylor, B. F. (1975). Inhibition of methanogenesis in marine sediments by acetylene and ethylene: validity of the acetylene reduction assay for anaerobic microcosms. Appl. Microbiol. 30, 707–709.
Oremland, R. S., and Taylor, B. F. (1978). Sulfate reduction and methanogenesis in marine sediments. Geochim. Cosmochim. Acta 42, 209–214. doi: 10.1016/0016-7037(78)90133-3
Payne, W. J., and Grant, M. A. (1982). Influence of acetylene on growth of sulfate-respiring bacteria. Appl. Environ. Microbiol. 43, 727–730.
Piceno, Y. M., and Lovell, C. R. (2000). Stability in natural bacterial communities: II. Plant resource allocation effects on rhizosphere diazotroph assemblage composition. Microbial. Ecol. 39, 41–48. doi: 10.1007/s002489900191
Postgate, J. (1949). Competitive inhibition of sulphate reduction by selenate. Nature 164, 670–671. doi: 10.1038/164670b0
Prior, S., and Dalton, H. (1985). Acetylene as a suicide substrate and active site probe for methane monooxygenase from Methylococcus capsulatus (Bath). FEMS Microbiol. Lett. 29, 105–109. doi: 10.1111/j.1574-6968.1985.tb00843.x
Quince, C., Lanzen, A., Curtis, T. P., Davenport, R. J., Hall, N., Head, I. M., et al. (2009). Accurate determination of microbial diversity from 454 pyrosequencing data. Nat. Methods 6, 639–627. doi: 10.1038/nmeth.1361
Saldanha, A. J. (2004). Java Treeview – extensible visualization of microarray data. Bioinformatics 20, 3246–3248. doi: 10.1093/bioinformatics/bth349
Schink, B. (1985). Inhibition of methanogenesis by ethylene and other unsaturated hydrocarbons. FEMS Microbiol. Lett. 31, 63–68. doi: 10.1111/j.1574-6968.1985.tb01132.x
Schloss, P. D., Westcott, S. L., Ryabin, T., Hall, J. R., Hartmann, M., Hollister, E. B., et al. (2009). Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75, 7537–7541. doi: 10.1128/aem.01541
Segata, N., Izard, J., Waldron, L., Gevers, D., Miropolsky, L., Garrett, W. S., et al. (2011). Metagenomic biomarker discovery and explanation. Genome Biol. 12, R60. doi: 10.1186/gb-2011-12-6-r60
Seitzinger, S. P., and Garber, J. H. (1987). Nitrogen-fixation and N-15(2) calibration of the acetylene-reduction assay in coastal marine-sediments. Mar. Ecol. Progr. Ser. 37, 65–73. doi: 10.3354/Meps037065
Sisler, F. D., and ZoBell, C. E. (1951). Nitrogen fixation by sulfate reducing bacteria indicated by Nitrogen/Argon ratios. Science 113, 511. doi: 10.1126/science.113.2940.511
Stewart, W., Fitzgerald, G., and Burris, R. (1968). Acetylene reduction by nitrogen-fixing blue-green algae. Archiv für Mikrobiol. 62, 336–348. doi: 10.1007/BF00425639
Tyrrell, T. (1999). The relative influences of nitrogen and phosphorus on oceanic primary production. Nature 400, 525–531. doi: 10.1038/22941
Vuilleumier, S., Khmelenina, V. N., Bringel, F., Reshetnikov, A. S., Lajus, A., Mangenot, S., et al. (2012). Genome sequence of the haloalkaliphilic methanotrophic bacterium Methylomicrobium alcaliphilum 20Z. J. Bacteriol. 194, 551–552. doi: 10.1128/JB.06392-11
Welsh, D. T., Bourgues, S., Dewit, R., and Herbert, R. A. (1996). Seasonal variation in rates of heterotrophic nitrogen fixation (acetylene reduction) in Zostera noltii meadows and uncolonised sediments of the Bassin d’Arcachon, south-west France. Hydrobiologia 329, 161–174. doi: 10.1007/Bf00034555
Welsh, D., Castadelli, G., Bartoli, M., Poli, D., Careri, M., De Wit, R., et al. (2001). Denitrification in an intertidal seagrass meadow, a comparison of 15N-isotope and acetylene-block techniques: dissimilatory nitrate reduction to ammonia as a source of N2O? Mar. Biol. 139, 1029–1036. doi: 10.1007/s002270100672
Widdel, F. (1987). New types of acetate-oxidizing, sulfate-reducing desulfobacter species, D-hydrogenophilus Sp-Nov, D-latus Sp-Nov, and D-curvatus Sp-Nov. Arch. Microbiol. 148, 286–291. doi: 10.1007/BF00456706
Wilhelm, S. W., Lecleir, G. R., Bullerjahn, G. S., Mckay, R. M., Saxton, M. A., Twiss, M. R., et al. (2014). Seasonal changes in microbial community structure and activity imply winter production is linked to summer hypoxia in a large lake. FEMS Microbiol. Ecol. 87, 475–485. doi: 10.1111/1574-6941.12238
Wilson, S. T., Bottjer, D., Church, M. J., and Karl, D. M. (2012). Comparative assessment of nitrogen fixation methodologies, conducted in the Oligotrophic North Pacific Ocean. Appl. Environ. Microbiol. 78, 6516–6523. doi: 10.1128/Aem.01146-12
Keywords: heterotrophic nitrogen fixation, acetylene reduction assay, sediments, sulfate-reducing bacteria, high throughput sequencing
Citation: Fulweiler RW, Heiss EM, Rogener MK, Newell SE, LeCleir GR, Kortebein SM and Wilhelm SW (2015) Examining the impact of acetylene on N-fixation and the active sediment microbial community. Front. Microbiol. 6:418. doi: 10.3389/fmicb.2015.00418
Received: 16 February 2015; Accepted: 20 April 2015;
Published online: 12 May 2015
Edited by:
Rex Malmstrom, Department of Energy Joint Genome Institute, USAReviewed by:
Jennifer F. Biddle, University of Delaware, USARobert Michael Bowers, Department of Energy Joint Genome Institute, USA
Copyright © 2015 Fulweiler, Heiss, Rogener, Newell, LeCleir, Kortebein and Wilhelm. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Robinson W. Fulweiler, Department of Earth and Environment, 685 Commonwealth Avenue, Boston University, Boston, MA 02215, USA, rwf@bu.edu
 Robinson W. Fulweiler
Robinson W. Fulweiler Elise M. Heiss
Elise M. Heiss Mary Kate Rogener
Mary Kate Rogener Silvia E. Newell1,5
Silvia E. Newell1,5 Gary R. LeCleir
Gary R. LeCleir Steven W. Wilhelm
Steven W. Wilhelm