- 1Institute of Disease Control and Prevention, Academy of Military Medical Sciences, Beijing, China
- 2Department of Laboratory Medicine, The General Hospital of Chinese People's Armed Police Forces, Beijing, China
- 3State Key Laboratory of Pathogen and Biosecurity, Institute of Microbiology and Epidemiology, Beijing, China
Klebsiella pneumoniae is a wide-spread nosocomial pathogen. A rapid and sensitive molecular method for the detection of K. pneumoniae in clinical samples is needed to guide therapeutic treatment. In this study, we first described a loop-mediated isothermal amplification (LAMP) method for the rapid detection of capsular polysaccharide synthesis regulating gene rcsA from K. pneumoniaein clinical samples by using two methods including real-time turbidity monitoring and fluorescence detection to assess the reaction. Then dissemination of K. pneumoniae strains was investigated from ICU patients in three top hospitals in Beijing, China. The results showed that the detection limit of the LAMP method was 0.115 pg/μl DNA within 60 min under isothermal conditions (61°C), a 100-fold increase in sensitivity compared with conventional PCR. All 30 non- K. pneumoniae strains tested were negative for LAMP detection, indicating the high specificity of the LAMP reaction. To evaluate the application of the LAMP assay to clinical diagnosis, of 110 clinical sputum samples collected from ICU patients with clinically suspected multi-resistant infections in China, a total of 32 K. pneumoniae isolates were identified for LAMP-based surveillance of rcsA. All isolates belonged to nine different K. pneumoniae multilocus sequence typing (MLST) groups. Strikingly, of the 32 K. pneumoniae strains, 18 contained the Klebsiella pneumoniae Carbapenemase (KPC)-encoding gene blaKPC-2 and had high resistance to β-lactam antibiotics. Moreover, K. pneumoniae WJ-64 was discovered to contain blaKPC-2 and blaNDM-1genes simultaneously in the isolate. Our data showed the high prevalence of blaKPC-2 among K. pneumoniae and co-occurrence of many resistant genes in the clinical strains signal a rapid and continuing evolution of K. pneumoniae. In conclusion, we have developed a rapid and sensitive visual K. pneumoniae detection LAMP assay, which could be a useful tool for clinical screening, on-site diagnosis and primary quarantine purposes.
Introduction
As a Gram-negative bacterium, Klebsiella pneumoniae has been identified as a major nosocomial pathogen (Fukigai et al., 2007), which can cause pneumonia, bronchitis, urinary tract and wound infections, especially in infants, diabetics, tumor patients, antibiotic users, and elderly people (Podschun and Ullmann, 1998) in the clinical context. Moreover, antibiotic-resistant Klebsiella pneumoniae emerged in recent years has become a serious problem in clinics (Ali Abdel Rahim and Ali Mohamed, 2014). Thus, the rapid and sensitive detection of this pathogen is required if the appropriate therapy is to be administered and outbreaks controlled.
Conventional methods used to detect K. pneumoniae based on the phenotypic system include microscopic examination, biochemical identification, and the use of newly developed automatic bacterial identification instruments, such as the VITEK®2 system (Hay et al., 2007). However, they are time-consuming, with low sensitivity, usually requiring several days of incubation. Recently, a number of molecular biological techniques have been used to detect K. pneumoniae. PCR based on the 16S-23S internal transcribed spacer was carried out to detect K. pneumoniae in infant formula (Liu et al., 2008). Triplex PCR (Jeong et al., 2013) and real-time PCR systems based on SYBR Green (Sun et al., 2010a) have also been used for its specific identification. However, these methods are relatively complex and require specialized, expensive instruments. Moreover, Taq DNA polymerase can be inactivated in PCR assays by inhibitors present in crude biological samples (de Franchis et al., 1988).
Therefore, another rapid, simple, and cost-effective assay is required to complement current PCR methods. The loop-mediated isothermal amplification (LAMP) method is a novel nucleic acid detection method, based on auto cycling strand displacement DNA synthesis using Bst DNA polymerase under isothermal conditions within 1 h (Notomi et al., 2000). The LAMP method has high specificity because four or six specific primers are used that recognize six or eight different sequences on the target gene. The technique has been widely used in the clinical detection of pathogens, including bacteria (Wei et al., 2008; Hanaki et al., 2011), viruses (Wang et al., 2011), parasites (Chen et al., 2011) and fungi (Sun et al., 2010b), and even in fetal sex determination (Hirayama et al., 2006).
In K. pneumoniae, the ability to synthesize large amount of capsular polysaccharide (CPS) is an important correlate of virulence (Goncalves et al., 2014). RcsA, a gene specific to K. pneumoniae that regulates the synthesis of CPS (Lin et al., 2013), was selected as the target gene in this study. We designed five sets of primers and optimized the LAMP assay to detect K. pneumoniae. The specificity and sensitivity of the LAMP method for the detection of K. pneumoniae were determined. Finally, clinical isolates of K. pneumoniae were identified with LAMP.
Materials and Methods
Bacterial Isolates, Identification, MLST Typing, Antimicrobial Susceptibility Testing and Preparation of Templates
A total of 64 bacterial strains were used in this work to develop the LAMP assay (Supplemental Materials). K. pneumoniae ATCC BAA-2146 carrying blaNDM−1 and K. pneumoniae ATCC BAA-1705 carrying blaKPC−2 were used as the positive control. Twenty-one non- K. pneumoniae bacterial species maintained in our microorganism center, including common clinical infectious and opportunistic pathogens, were included to evaluate the specificity of the LAMP assay. One hundred and ten clinical sputum samples containing suspected K. pneumoniae strains and multi-resistant infections were collected from ICU hospitalized patients with cough or pneumonia in the Wujing hospital, 307 hospital and 301 hospital in Beijing, China. The species identification was carried out using an automated system (Phoenix and BD systems) and matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS). The sequences of 16S ribosomal DNA (rDNA) and rcsA were validated by PCR-based sequencing and showed 100% identity with the sequences of previously reported genes.
Seven housekeeper genes including gapA, infB, mdh, pgi, phoE, ropB, and tonB were detected by PCR. The allele number for each gene was assigned to the MLST database (http://bigsdb.web.pasteur.fr/klebsiella/klebsiella.html). A combination of the allelic sequences of the 7 genes yielded the allelic profile. Antimicrobial susceptibility testing was performed by microbroth dilution VITECaccording to the Clinical and Laboratory Standards Institute (CLSI, Performance standards for antimicrobial susceptibility testing; Twenty-third informational supplement CLSI Document M100-S23. Wayne, PA, USA 2013) and Etest strips (bioMérieux) for carbapenems.
The strains were screened for the presence of known MBL and other β-lactamase genes (blaNDM−1, blaKPC−2, blaTEM, blaVIM, blaIMP, blaCTX, blaSIM−1, blaAIM−1, and blaOXA−48) by PCR with primers as reported previously (Poirel et al., 2007; Patzer et al., 2009).
The bacterial strains and clinical samples were cultured in brain heart infusion (BHI) broth at 37°C according to a standard protocol. Genomic DNA was extracted using the Wizard Genomic DNA Purification Kit (Promega Co. USA).
Primer Design
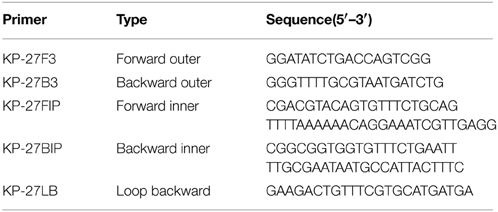
The sequence of the rcsA gene (http://www.ncbi.nlm.nih.gov/gene/7946097) was downloaded from NCBI GenBank database and further analyzed by Primer Explorer (Version 4, http://primerexplorer.jp/elamp4.0.0/index.html). Five primer sets were designed (Table 1 and Table S2). To compare the sensitivity of the LAMP and conventional PCR assay, PCR was conducted with the KP-27F3 and KP-27B3 primer pair (Table 1), which amplifies a 176-bp fragment. All primers were synthesized commercially (Sangon Biotech Co., Ltd, Shanghai, China).
LAMP Reaction
The LAMP reactions were performed in a final volume of 25 μl containing 12.5 μl reaction mixture, 1 μl Bst DNA polymerase, 2 μl template by using the Loopamp DNA Amplification kit (Loopamp DNA Amplification Kit; Eiken Chemical Co., Ltd, Tochigi, Japan) for real-time turbidimeter and another 1 μl calcein/Mn2+ solution (Eiken Chemical Co., Ltd) for visual detection. Primers were used at a concentration of 40 pmol for FIP and BIP, 20 pmol for LB and LF, and 5 pmol for F3 and B3. Finally, the reaction mix was overlaid with the protectant to prevent cross-contamination of samples by aerosol and the reactions were performed in the reaction tubes (Eiken Chemical Co. Ltd.) for 60 min at 61°C. During the amplification, the protectant could melted to liquid without disturbing the reaction and solidified as the temperature in the tubes decreased to room time (Patent: ZL201210371448.5 in china).
Two different methods, based on sample turbidity or fluorescence, were used to detect the LAMP products. Real-time changes in turbidity were monitored with spectrophotometric analysis by recording the optical density (650 nm) every 6 s with a Loopamp Realtime Turbidimeter (LA-320c; Eiken Chemical Co., Ltd). For direct visual detection, 1 μl of calcein/Mn2+ fluorescent detection reagent was added to the reaction. LAMP amplification results in a green fluorescent emission as a result of magnesium ions forming a complex with calcein. The color change from orange to green when samples are positive is visible to the naked eye under natural light or with the aid of UV light (Tomita et al., 2008). Each experiment was performed at least three times.
PCR Detection
A 25 μl reaction volume was used for all PCRs, with mixtures that contained the following components: 12.5 μl of PCR MasterMix reagents (Tiangen Biotech Co., Ltd, Beijing, China), 9.5 μl of double-distilled water, 1 μM KP-27F3 and KP-27B3 primers, and the same amount of DNA template as was used in the LAMP reaction. The PCR cycling parameters were: initial PCR activation, 95°C for 5 min; amplification, 30 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 30 s; final extension, 72°C for 10 min. The products were separated with 1% agarose gel (Amresco) electrophoresis and stained with ethidium bromide. Images were documented with a Gel Doc EQ imaging system (Bio-Rad).
Results
Optimization of LAMP Assay
Five sets of primers were initially tested to detect K. pneumoniae. All five sets amplified the target sequence under the same reaction conditions. The KP-27 primer set began to amplify the target gene in the shortest time (Figure 1) and was therefore chosen as the optimal primer set for K. pneumoniae detection with LAMP (Table 1).
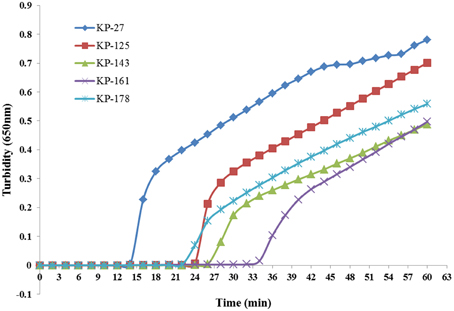
Figure 1. Five sets of primers were used to amplify the target gene under the same conditions. Turbidity was monitored every 6 s with a Loopamp Realtime Turbidimeter at 650 nm.
Reaction temperatures ranging from 59°C to 69°C at 2°C intervals were compared for optimal amplification. As shown in Figure 2, the most suitable reaction temperature range was 59–63°C. Finally, we chose 61°C as the optimal reaction temperature.
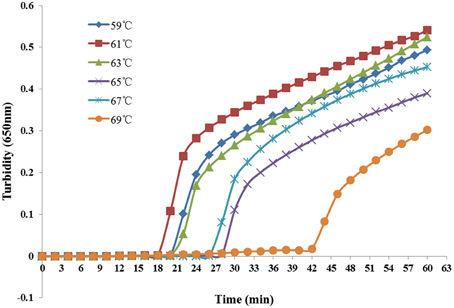
Figure 2. Different temperatures at which the LAMP reaction detected K. pneumoniae. Turbidity was monitored every 6 s with a Loopamp Realtime Turbidimeter at 650 nm.
Specificity of the LAMP Assay
K. pneumoniae ATCC BAA-2146 was used as the positive control and double-distilled water as the negative control when evaluating the specificity of LAMP for the detection of K. pneumoniae. Twenty-one other non- K. pneumoniae bacterial strains (Supplemental Materials) were also tested. As shown in Figure 3, both methods of analysis positively identified the K. pneumoniae. All other strains, including the blank control, tested negative, indicating that the LAMP assay was specific to K. pneumoniae.
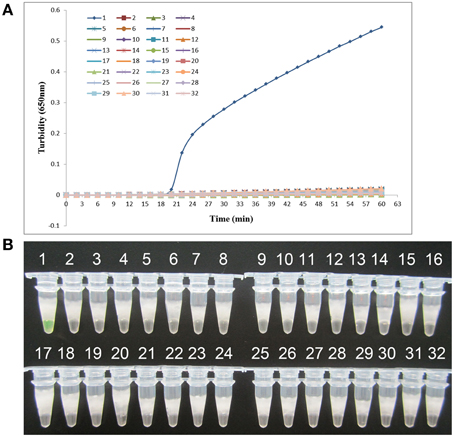
Figure 3. Specificity of the LAMP reaction in detecting K. pneumoniae. (A) Turbidity was monitored every 6 s using a Loopamp Realtime Turbidimeter at 650 nm. (B) The results were visualized by the addition of 1 μl of fluorescent detection reagent to the 25 μl LAMP reaction mixture before the LAMP reaction. Amplification was performed at 61°C for 60 min. 1, positive control (K. pneumoniae ATCC BAA-2146); 2, negative control (double-distilled water); 3, Klebsiella oxytoca ATCC 700324; 4, Klebsiella rhinoscleromatis CMCC 46111; 5, Citrobacter freundii CMCC 48001; 6, Enterobacter aerogenes ATCC 13048; 7, Enterobacter cloacae ATCC 13047; 8, Proteus mirabilis CMCC 49005; 9, Proteus vulgaris CMCC 49027; 10, Serratia marcescens ATCC 14756; 11, Morganella morganii ATCC 25830; 12, Streptococcus pneumoniae 112-07; 13, Mycobacterium tuberculosis 005; 14, Pseudomonas aeruginosa D104; 15, Haemophilus influenza ATCC 49247; 16, Yersinia enterocolitica 027; 17, Yersinia pestis 2638; 18, Bacillus tularense 3450; 19, Vibrio cholera 3802; 20, Salmonella aberdeen 9264; 21, Neisseria meningitides CMCC 29022; 22, Staphylococcus aureus 2740; 23, Pseudomonas pseudomallei 029; 24, Salmonella typhimurium 4030; 25, Corynebacterium diphtheriae CMCC 38001; 26, Bacillus megatherium 4623; 27, Stenotrophomonas maltophilia K279a; 28, Legionella pneumophila 9135; 29, Acinetobacter baumannii 12101; 30, enteroinvasive E. coli 44825; 31, enterotoxigenic E. coli 44824; 32, enteropathogenic E. coli 2348.
Sensitivity of the LAMP Assay vs. PCR for K. pneumoniae Detection
To compare the detection limit of LAMP using either real-time turbidity measurements or visual color change with traditional PCR, pure genomic DNA was extracted from K. pneumoniae ATCC BAA-2146 and subjected to serial 10-fold dilution, from 115.0 ng/μl to 0.0115 pg/μl. As shown in Figure 4, the detection limit of the real-time turbidity and visual detection was both 0.115 pg/μl, which was 100-fold more sensitive than traditional PCR assay.
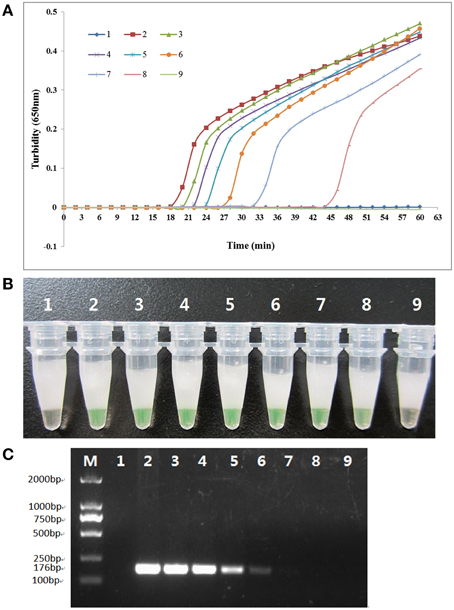
Figure 4. Comparison of the sensitivities of the LAMP reaction and conventional PCR in detecting the rcsAgene of K. pneumoniae. Pure genomic DNA extracted from K. pneumoniae ATCC BAA-2146 was serially diluted 10-fold. (A) Turbidity was monitored every 6 s with a Loopamp Realtime Turbidimeter at 650 nm. (B) The reaction result was detected visually by the addition of 1 μl of fluorescent detection reagent to the 25 μl LAMP reaction mixture before the LAMP reaction. (C) PCR products were separated by 2% agarose gel electrophoresis and stained with ethidium bromide. Amplification was performed at 61°C for 60 min. 1, negative control (double-distilled water); 2, 115.0 ng/μl; 3, 11.5 ng/μl; 4, 1.15 ng/μl; 5, 115.0 pg/μl; 6, 11.5 pg/μl; 7, 1.15 pg/μl; 8, 0.115 pg/μl; 9, 0.0115 pg/μl.
Dissemination of K. pneumoniae in Clinic
One hundred and ten clinical sputum samples were collected for LAMP-based surveillance of K. pneumoniae from ICU patients with clinically suspected multi-resistant infections in three top hospitals of Beijing. Ten sputum samples from healthy people were collected as controls. Both LAMP and PCR assay were involved to analyze the clinical samples. As shown in Figure 5, of the 110 clinical samples, LAMP detected 32 positive samples while 25 were detected by PCR. Then, 32 K. pneumoniae strains were successfully cultured from these positive samples. The healthy control samples all tested negative in each of the assays. The sequence analysis of the rcsA genes from K. pneumoniae isolates confirmed conservation with the nucleotide sequences of reported gene.

Figure 5. LAMP results for K. pneumoniae isolates from clinical samples. (A) Turbidity was monitored every 6 s using a Loopamp Realtime Turbidimeter at 650 nm. (B) The results were visualized by the addition of 1 μl of fluorescent detection reagent to the 25 μl LAMP reaction mixture before the LAMP reaction. 1, Negative control (double-distilled water); 2, positive control (K. pneumoniae ATCC BAA-2146); 3, K. pneumoniae WJ-48; 4, K. pneumoniae WJ-50; 5, K. pneumoniae WJ-51; 6, K. pneumoniae WJ-52; 7, K. pneumoniae WJ-53; 8, K. pneumoniae WJ-57; 9, K. pneumoniae WJ-58; 10, K. pneumoniae WJ-60; 11, K. pneumoniae WJ-61; 12, K. pneumoniae WJ-64; 13, K. pneumoniae WJ-65; 14, K. pneumoniae WJ-66; 15, K. pneumoniae WJ-68; 16, K. pneumoniae 301-052; 17, K. pneumoniae 301-207; 18, K. pneumoniae 301-263; 19, K. pneumoniae 301-432; 20, K. pneumoniae 301-416; 21, K. pneumoniae 301-323; 22, K. pneumoniae 301-365; 23, K. pneumoniae 301-282; 24, K. pneumoniae 301-406; 25, K. pneumoniae 301-158; 26, K. pneumoniae 307-206; 27, K. pneumoniae 307-082; 28, K. pneumoniae 307-429; 29, K. pneumoniae 307-095; 30, K. pneumoniae 307-003; 31, K. pneumoniae 307-030; 32, K. pneumoniae 307-194; 33, K. pneumoniae 307-356; 34, K. pneumoniae 307-235.
MLST analysis showed that the 32 K. pneumoniae strains belonged to different sequence type (ST) including ST11, ST21, ST30, ST37, ST40, ST84, ST104, ST147 or ST322, respectively (Supplemental Materials). To further characterize the clinical K. pneumoniae isolates, PCR screening of MBL and other β-lactamase genes was performed and antimicrobial susceptibility tested, the results showed a co-occurrence of resistance genes in most of the K. pneumoniae clinical isolates. The positive PCR products were sequenced and the result showed 100% identities with previously reported genes. It is interesting to note that 18 of the 32 K. pneumoniae strains contain the blaKPC−2 gene, resulting in increased resistance to β-lactams antibiotics (Supplemental Materials). Moreover, the isolate named K. pneumoniae WJ-64 simultaneously contained blaKPC−2 and blaNDM−1which has emerged in recent years and attracted wide attention, presented increased resistance to all β-lactams (MIC > 128μg/ml for meropenem and imipenem), cephalosporins and aminoglycosides, and was only susceptible to tigecycline. The isolate was also positive for imipenem-EDTA double-disk synergy test (DDST) and modified Hodge test (MHT).
Discussion
Klebsiella pneumoniae is a widespread nosocomial pathogen and the most significant member of the genus Klebsiella in the family Enterobacteriaceae. Researchers have confirmed that the bacteremia caused by K. pneumoniae can greatly increase in-patient mortality (Chetcuti Zammit et al., 2014). In the past two decades, K. pneumoniae has surpassed Escherichia coli as the predominant species isolated from patients with pyogenic liver abscess globally (Liu et al., 2013). With the acquisition of antibiotic-resistant genes (such as those encoding blaKPC−2, blaNDM−1, and blaTEM), it is increasingly difficult to cure carbapenem-resistant K. pneumoniae (de Sanctis et al., 2014). Therefore, the early diagnosis of this pathogen has become increasingly important.
To meet this challenge, we have established a novel detection assay using the LAMP method, which can be completed within 60 min. We selected the capsular polysaccharide synthesis regulating gene rcsA as the target gene because it is more stable. Other specific genes of K. pneumoniae, such as phoE and tyrB, always have some mutants in their sequences. As far as we know, this is the first study to apply the LAMP method to the detection of K. pneumoniae. The results of sensitivity and specificity experiments show that the LAMP assay is 100-fold more sensitive than the conventional PCR assay, and is specific for K. pneumoniae. Furthermore, the LAMP reaction requires only a constant-temperature environment, so a temperature-controlled water bath that can be stably heated is sufficient, whereas PCR must be performed under temperature-cycling conditions. Importantly, Kaneko found that the LAMP reaction is not susceptible to the influence of the different components often present in clinical samples. Thus, the purification of DNA from a sample is not necessary (Kaneko et al., 2007). Although the LAMP assay has a complex amplification principle, it is rapid, easy to operate, highly sensitive and specific. Therefore, it is appropriate for the detection of K. pneumoniae, especially for routine diagnosis and infection control purposes.
A drawback of the LAMP method is that it has a relatively high rate of false-positive results. This is because the amplification efficiency of the LAMP assay is extremely high, and 20 μg of specific DNA can be synthesized in a 25 μl reaction mixture within 60 min (Mori et al., 2001). Strict spatial separation between the reagent preparation and the performance of the test is also very important in avoiding contamination. In this study, a sealing agent was added to the reaction tube after the reaction reagents were prepared to prevent the spread of the amplification products, and proved useful in precluding contamination.
Application of LAMP assay to samples taken from hospital admissions of cough or pneumonia indicated that K. pneumoniae was prevalent with nearly 30% of samples tested positive. Moreover, there was a high prevalence of blaKPC−2 among K. pneumoniae. Diverse MLST types of K. pneumoniae carrying blaKPC−2 and co-occurrence of many resistance genes in the clinical strains signal a rapid and continuing evolution of K. pneumoniae resulting from their wide spread in clinical infections, and there would be a lot of difficulties to control.
In conclusion, a specific, sensitive, rapid, and effective method for the detection of K. pneumoniae with the loop-mediated isothermal amplification method was established. We anticipate its routine use in hospital and point-of-care testing regimes, particularly for rapid clinical diagnoses where time and resources are limited.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are indebted to the Wujing hospital, 301 hospital, and 307 hospital for kindly providing K. pneumoniae and helpful information. This work was supported by a grant from the National Natural Science Foundation of China (31370093) to JY, Mega-projects of Science and Technology Research of China (Grant 2011ZX10004-001 and 2011ZX10004-205), and the Special Grant for the Prevention and Control of Infectious Diseases of China (Grant 2013ZX10004-203).
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2015.00519/abstract
References
Ali Abdel Rahim, K. A, and Ali Mohamed, A. M. (2014). Prevalence of extended spectrum β-lactamase-producing Klebsiella pneumoniae in clinical isolates. Jundishapur J. Microbiol. 11:e17114. doi: 10.5812/jjm.17114
Chen, R., Tong, Q., Zhang, Y., Lou, D., Kong, Q., Lv, S., et al. (2011). Loop-mediated isothermal amplification: rapid detection of Angiostrongylus cantonensis infection in Pomacea canaliculata. Parasit. Vectors 4, 204. doi: 10.1007/s00436-010-2162-x
Chetcuti Zammit, S., Azzopardi, N., and Sant, J. (2014). Mortality risk score for Klebsiella pneumoniae bacteraemia. Eur. J. Intern. Med. 25, 571–576. doi: 10.1016/j.ejim.2014.04.008
de Franchis, R., Cross, N.C., Foulkes, N.S., and Cox, T.M. (1988). A potent inhibitor of Taq polymerase copurifies with human genomic DNA. Nucleic Acids Res. 16, 10355. doi: 10.1093/nar/16.21.10355
de Sanctis, J., Teixeira, L., van Duin, D., Odio, C., Hall, G., Tomford, J.W., et al. (2014). Complex prosthetic joint infections due to carbapenemase-producing Klebsiella pneumoniae: a unique challenge in the era of untreatable infections. Int. J. Infect. Dis. 25, 73–78. doi: 10.1016/j.ijid.2014.01.028
Fukigai, S., Alba, J., Kimura, S., Iida, T., Nishikura, N., Ishii, Y., et al. (2007). Nosocomial outbreak of genetically related IMP-1 β-lactamase-producing Klebsiella pneumoniae in a general hospital in Japan. Int. J. Antimicrob. Agents 29, 306–310. doi: 10.1016/j.ijantimicag.2006.10.011
Goncalves, M. S., Delattre, C., Balestrino, D., Charbonnel, N., Elboutachfaiti, R., Wadouachi, A., et al. (2014). Anti-biofilm activity: a function of Klebsiella pneumoniae capsular polysaccharide. PLoS ONE 9:e99995. doi: 10.1371/journal.pone.0099995.
Hanaki, K., Sekiguchi, J., Shimada, K., Sato, A., Watari, H., Kojima, T., et al. (2011). Loop-mediated isothermal amplification assays for identification of antiseptic- and methicillin- resistant Staphylococcus aureus. J. Microbiol. Methods 84, 251–254. doi: 10.1016/j.mimet.2010.12.004
Hay, A., Macdonald, E., Evans, R., and Davidson, M. (2007). Use of VITEK for surveillance of antibiotic resistance in Escherichia coli in the Scottish Highlands: results over 15 years. J. Infection 55, e87–e88. doi: 10.1016/j.jinf.2007.04.132
Hirayama, H., Kageyama, S., Takahashi, Y., Moriyasu, S., Sawai, K., Onoe, S., et al. (2006). Rapid sexing of water buffalo (Bubalus bubalis) embryos using loop-mediated isothermal amplification. Theriogenology 66, 1249–1256. doi: 10.1016/j.theriogenology.2006.03.036
Jeong, E. S., Lee, K. S., Heo, S. H., Seo, J. H., and Choi, Y. K. (2013). Rapid identification of Klebsiella pneumonia, Corynebacterium kutscheri, and Streptococcus pneumoniae using triplex polymerase chain reaction in rodents. Exp. Anim. 62, 35–40. doi: 10.1538/expanim.62.35
Kaneko, H., Kawana, T., Fukushima, E., and Suzutani, T. (2007). Tolerance of loop-mediated isothermal amplification to a culture medium and biological substances. J. Biochem. Biophys. Methods 70, 499–501. doi: 10.1016/j.jbbm.2006.08.008
Lin, C. T., Chen, Y. C., Jinn, T. R., Wu, C. C., Hong, Y. M., and Wu, W. H. (2013). Role of the cAMP-dependent carbon catabolite repression in capsular polysaccharide biosynthesis in Klebsiella pneumoniae. PLoS ONE 8:e54430. doi: 10.1371/journal.pone.0054430
Liu, Y., Liu, C., Zheng, W., Zhang, X., Yu, J., Gao, Q., et al. (2008). PCR detection of Klebsiella pneumoniae in infant formula based on 16S-23S internal transcribed spacer. Int. J. Food Microbiol. 125, 230–235. doi: 10.1016/j.ijfoodmicro.2008.03.005
Liu, Y., Wang, J. Y., and Jiang, W. (2013). An increasing prominent disease of Klebsiella pneumoniae Liver Abscess: Etiology, Diagnosis, and Treatment. Gastroenterol. Res. Pract. 9, 1–12. doi: 10.1155/2013/258514
Mori, Y., Nagamine, K., Tomita, N., and Notomi, T. (2001). Detection of loop-mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochem. Biophys. Res. Commun. 289, 150–154. doi: 10.1006/bbrc.2001.5921
Notomi, T., Okayama, H., Masubuchi, H., Yonekawa, T., Watanabe, K., Amino, N., et al. (2000). Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 28, e63 doi: 10.1093/nar/28.12.e63
Patzer, J. A., Walsh, T. R., Weeks, J., Dzierzanowska, D., and Toleman, M. A. (2009). Emergence and persistence of integron structures harbouring VIM genes in the Children's Memorial Health Institute, Warsaw, Poland, 1998-2006. J. Antimicrob. Chemother. 63, 269–273. doi: 10.1093/jac/dkn512
Podschun, R., and Ullmann, U. (1998). Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin. Microbiol. Rev. 11, 589–603.
Poirel, L., Pitout, J. D., and Nordmann, P. (2007). Carbapenemases: molec-ular diversity and clinical consequences. Future Microbiol. 2, 501–512. doi: 10.2217/17460913.2.5.501
Sun, F. L., Wu, D. C., Qiu, Z. G., Jin, M., Wang, X. W., and Li, J. W. (2010a). Development of real-time PCR systems based on SYBR Green for the specific detection and quantification of Klebsiella pneumoniae in infant formula. Food Control 21, 487–491. doi: 10.1016/j.foodcont.2009.07.014
Sun, J., Najafzadeh, M. J., Vicente, V., Xi, L., and de, Hoog, G. S. (2010b). Rapid detection of pathogenic fungi using loop-mediated isothermal amplification, exemplified by Fonsecaea agents of chromoblastomycosis. J. Microbiol. Methods 80, 19–24. doi: 10.1016/j.mimet.2009.10.002
Tomita, N., Mori, Y., Kanda, H., and Notomi, T. (2008). Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat. Protoc. 3, 877–882. doi: 10.1038/nprot.2008.57
Wang, B., Wang, Y., Tian, Z. J., An, T. Q., Peng, J. M., and Tong, G. Z. (2011). Development of a reverse transcription loop-mediated isothermal amplification assay for detection of Porcine teschovirus. J. Vet. Diagn. Invest. 23, 516–518. doi: 10.1177/1040638711403427
Keywords: Klebsiella pneumoniae, loop-mediated isothermal amplification, LAMP, rapid detection
Citation: Dong D, Liu W, Li H, Wang Y, Li X, Zou D, Yang Z, Huang S, Zhou D, Huang L and Yuan J (2015) Survey and rapid detection of Klebsiella pneumoniae in clinical samples targeting the rcsA gene in Beijing, China. Front. Microbiol. 6:519. doi: 10.3389/fmicb.2015.00519
Received: 18 March 2015; Accepted: 09 May 2015;
Published: 22 May 2015.
Edited by:
Tzi Bun Ng, The Chinese University of Hong Kong, ChinaReviewed by:
Dmitri Debabov, NovaBay Pharmaceuticals, USAYuji Morita, Aichi Gakuin University, Japan
Copyright © 2015 Dong, Liu, Li, Wang, Li, Zou, Yang, Huang, Zhou, Huang and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dongsheng Zhou, State Key Laboratory of Pathogen and Biosecurity, Institute of Microbiology and Epidemiology, No.20 Dongda Street, Fengtai District, Beijing 100071, China, dongshengzhou1977@gmail.com
Liuyu Huang and Jing Yuan, Institute of Disease Control and Prevention, Academy of Military Medical Sciences, No.20 Dongda Street, Fengtai District, Beijing 100071, China, huangliuyuly@163.com; yuanjing6216@163.com
†These authors have contributed equally to this work.
 Derong Dong
Derong Dong Wei Liu
Wei Liu Huan Li
Huan Li Yufei Wang2
Yufei Wang2 Zhan Yang
Zhan Yang Simo Huang
Simo Huang Dongsheng Zhou
Dongsheng Zhou Liuyu Huang
Liuyu Huang Jing Yuan
Jing Yuan