- 1Department of Civil and Environmental Engineering, University of Massachusetts Amherst, Amherst, MA, USA
- 2Department of Environmental Health Sciences, University of South Carolina, Columbia, SC, USA
Bacteria are essential components of all natural and many engineered systems. The most active fractions of bacteria are now recognized to occur as biofilms, where cells are attached and surrounded by a secreted matrix of “sticky” extracellular polymeric substances. Recent investigations have established that significant accumulation of nanoparticles (NPs) occurs in aquatic biofilms. These studies point to the emerging roles of biofilms for influencing partitioning and possibly transformations of NPs in both natural and engineered systems. While attached biofilms are efficient “sponges” for NPs, efforts to elucidate the fundamental mechanisms guiding interactions between NPs and biofilms have just begun. In this mini review, special attention is focused on NP–biofilm interactions within the aquatic environment. We highlight key physical, chemical, and biological processes that affect interactions and accumulation of NPs by bacterial biofilms. We posit that these biofilm processes present the likely possibility for unique biological and chemical transformations of NPs. Ultimately, the environmental fate of NPs is influenced by biofilms, and therefore requires a more in-depth understanding of their fundamental properties.
Introduction: Important Properties of the Biofilm Microenvironment in the Bigger Picture
Microbial biofilms are an omnipresent component in many environments supporting life. Hence, the multifaceted roles of biofilms in the sequestration, accumulation, transformation, and trophic transfer of environmental contaminants have been a subject of much study and controversy. A biofilm is, in its simplest form, a collection of surface-attached microbial cells that are surrounded by a matrix of extracellular polymers. The inherent properties and physical structure of biofilms resemble that of a sorptive sponge capable of capturing various chemical and biological components in their vicinity. Natural and engineered systems that are significantly impacted by biofilms include soil mineral surfaces, microbial mats, wastewater treatment, and biofouling of ships and pipes. These will not be addressed further here. Rather, this review focuses on the mechanistic interactions of bacterial biofilm with natural and synthetic nanoparticles (NPs); an emerging concern in both the environment and health. Such NP–biofilm interactions within the aquatic environment are highlighted.
Nanoparticle–Biofilm Interactions
It is now recognized that environmental biofilms are efficient binding matrices for NPs (Battin et al., 2009; Ferry et al., 2009; Nevius et al., 2012; Kroll et al., 2014), and this can be attributed largely to the extracellular polymeric substances (EPS) that hold biofilms together (Flemming and Wingender, 2010; Nevius et al., 2012). Recent studies have shown that significant accumulations of NPs occurred in biofilms of riverine- and marine-mesocosms (Battin et al., 2009; Ferry et al., 2009). When gold nanorods (65 nm × 15 nm) were added to a marine/estuarine mesocosm containing sediments, seagrass, bivalves, shrimp, and plankton, the nanorods were most strongly bioconcentrated by microbial biofilms with their bioconcentration accounting for greater than 60% of the added nanorods (Ferry et al., 2009). Similar bioconcentration was found in riverine mesocosms using 20 nm TiO2 NPs (Battin et al., 2009). These initial studies point to an important role of biofilms for influencing environmental partitioning of NPs within natural systems. In retrospect, this is not surprising since biofilms are efficient chelators for physical-trapping and binding of dissolved and colloidal forms of metals and organic matter in a wide range of systems such as wastewater treatment (Wuertz et al., 2000; Hu et al., 2005; Hawari and Mulligan, 2006), drinking-water filtration (Lehtola et al., 2004; Berry et al., 2006), and marine and freshwater systems (Schlekat et al., 1998; Decho, 2000; Battin et al., 2009). As the research focus on NP–biofilm interactions is still in its early stages, this mini review is designed to provide a brief overview of published studies and some insights into future directions to improve our understanding of the mechanisms and the bigger-picture implications of these interactions.
The interactions between NPs and the biofilm can be viewed as a three-step process: (1) transport of NPs to the vicinity of the biofilm; (2) attachment to the biofilm surface; and (3) migration within the biofilm (Figure 1). At each of these steps, the interactions are a complex interplay of a myriad of factors including, but not limited to, NP characteristics, the physicochemical and biological makeup of the biofilm matrix, and environmental parameters such as water chemistry, flow, and temperature. The effects of various environmental parameters on the fate of NPs have been extensively studied and reviewed in detail (e.g., Petosa et al., 2010; Levard et al., 2012). Similarly, many biofilm researchers have studied how environmental parameters influence biofilms (as reviewed by, e.g., Sutherland, 2001; Karatan and Watnick, 2009). For example, the effects of ionic strength of the aqueous environment on both NPs (e.g., NPs aggregate as ionic strength increases) and biofilms (e.g., pore sizes change with different ionic strengths) are well documented. While such parameters are likely to have direct or indirect impacts, studies that thoroughly examine their influence on NP–biofilm interactions are currently lacking. Rather, the following sections aim to highlight the currently known NP and biofilm factors that have a critical impact on their interactions.
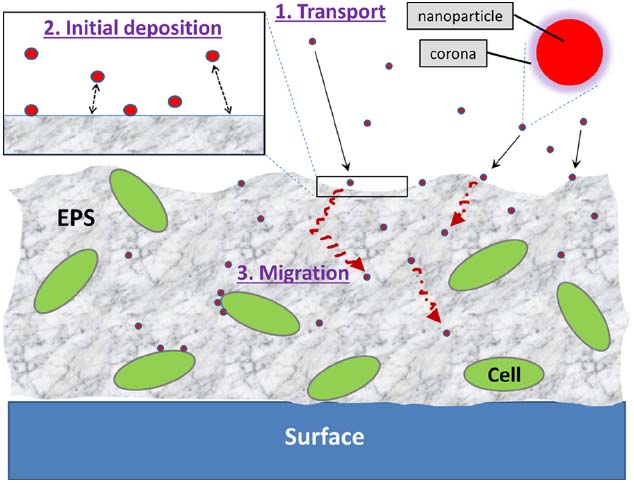
Figure 1. The three steps involved in NP–biofilm interactions: (1) transport of NPs to the vicinity of the biofilm, (2) initial deposition of NPs onto the biofilm surface, and (3) migration of NPs into deeper areas of the biofilm. NPs may also interact directly with cell surfaces within the biofilm matrix.
Impact of NP Characteristics on NP–Biofilm Interactions
Engineered and naturally forming NPs can vary widely in their physicochemical characteristics such as shape, size, and charge (Hochella et al., 2008; Petosa et al., 2010). As discussed in the following section, these NP characteristics have been reported to impact their interactions with biofilm-coated surfaces at all three steps of the interactions. NP transport through the water column has been studied extensively (Lecoanet and Wiesner, 2004; Jaisi et al., 2008; Phenrat et al., 2009) and is primarily a function of various NP characteristics and water chemistry conditions; these bulk-phase transport phenomena will not be discussed in length here. Instead, this section will focus on the NP characteristics that influence the small-scale interactions in the proximity of biofilm surfaces including transport and attachment.
Surface modification of NPs, through ligand capping during synthesis or post-synthesis passive sorption of organic molecules, plays a critical role in NP–biofilm interactions. In fact, pure and single-component NPs are rare or non-existent in the environment. Engineered NPs are typically functionalized by specific organic ligands for a variety of target applications. When these capped NPs are being used or released in various environments, they are often subjected to further modifications in an uncontrollable manner by passive sorption of different organic molecules (e.g., proteins and polysaccharides). Dawson and colleagues introduced the concept of the “protein corona” as an important entity for NPs interacting with the external environment (Cedervall et al., 2007; Lynch et al., 2009; Walczyk et al., 2010). The corona is a temporally evolving collection of organic molecules that associate with NPs. Though these initial studies focused on proteins, recent investigations have expanded the concept of the “corona” to include other biomolecules as well as proteins (Monopoli et al., 2012). When a NP with an organic corona approaches a surface, proteins and other biomolecules that reside long enough on the NP surface will mediate subsequent interactions. These biomolecule–NP interactions are highlighted particularly in nanomedicine as a biofunctionalization mechanism. In environmental systems, a corona-like coating is also likely to form around NPs; in this case, the organic molecules in the corona are expected to primarily consist of components of natural organic matter (NOM). The organic corona as well as other organic coatings of NPs are likely to have significant impacts on NP–biofilm interactions. For example, cadmium selenide quantum dots (QDs) conjugated with polyethylene glycol were found to penetrate more easily into Pseudomonas aeruginosa PAO1 biofilms than QDs having surface carboxyl (–COOH) groups (Morrow et al., 2010). In another study, incubation of Pseudomonas fluorescens biofilms with silver NPs pre-exposed to NOM was shown to result in greater cell viability compared to silver NPs without NOM exposure (Wirth et al., 2012), suggesting that the NOM-based corona associated with the NPs had a mitigating effect on silver toxicity.
The charge and size of NPs also affect NP–biofilm interactions. In the case of fluorescent polystyrene NPs, surface sulfate (SO4–) groups on NPs resulted in greater sorption onto Alteromonas macleodii biofilms compared to NPs functionalized with amine (–NH) or carboxyl groups (Nevius et al., 2012). Both the size and charge of silver NPs were reported to be important in modulating their transport within P. fluorescens biofilms with their self-diffusion coefficients decreasing with increasing size and negative charge (Peulen and Wilkinson, 2011). This effect of NP size was also dependent on the density of the biofilm with NP self-diffusion becoming severely limited when the size was larger than 50 nm only in dense biofilms. Electrostatic (not steric) forces were shown to control the diffusion of positively and negatively charged latex beads (∼28 nm) in biofilms having relatively low (Lactococcus lactis) and high (Stenotrophomonas maltophilia) EPS contents (Guiot et al., 2002). Furthermore, particle size, charge, and particle surface chemistry may collectively affect the fate and transport of NPs in biofilm-coated porous media (Tripathi et al., 2012). While it is clear that NP characteristics influence NP–biofilm interactions, these interactions involve a highly complex interplay of such characteristics as well as biofilm features.
Impact of Biofilm Matrix Chemistry on NP–Biofilm Interactions
The EPS matrix is the primary emergent property of the biofilm (Flemming and Wingender, 2010). Once NPs reach the water–biofilm interface, the physicochemical matrix of EPS has direct implications on both the initial attachment of NPs onto the biofilm surface and their subsequent movement into the biofilm matrix. The EPS matrix is physicochemically complex and extremely heterogeneous over small spatial scales (e.g., micrometers; Lawrence et al., 2007). It can be thought of as a 3D filter, which surrounds biofilm cells and forms a dynamic trapping network for organic molecules and ions, and NPs, and consists of an interlinked network of polymer molecules, many of which are charged.
The density of EPS depends upon its local concentration, but also the charges and number of linkages between adjacent polymer chains. Although the EPS matrix is usually highly hydrated (often 99 % wt/wt), most of the water is not bound to EPS but rather is localized in pore spaces between adjacent polymer chains (Schmitt et al., 1995). These physicochemical features of EPS significantly impact NP–biofilm interactions. Of particular interest is how different molecules within the EPS matrix influences the initial deposition and continued accumulation of NPs. EPS are a complex array of polysaccharides, proteins, lipids, and even nucleic acids (Whitchurch et al., 2002; Flemming and Wingender, 2010). Functional group moieties on individual EPS molecules have varying potentials to bind ions, charged molecules, and NPs (Braissant et al., 2009). The nature of linkages between an EPS functional group(s) and the sorbed moiety (e.g., NPs), therefore, can result in binding with different affinities during initial deposition and subsequent accumulation.
While the initial attachment of NPs onto the outermost surface of biofilms may be influenced by a variety of physicochemical interactions, the specifics of these interfacial NP–biofilm interactions are largely unknown. A recent study by our laboratory showed that surfaces coated with polysaccharides, a major component of EPS, significantly affected the deposition of iron oxide NPs (hematite, α-Fe2O3) NPs (Ikuma et al., 2014). Different physicochemical features of surface-adsorbed polysaccharides, particularly surface charge heterogeneity, resulted in varying degrees of NP deposition due to changes in electrostatic interactions (see Liang et al., 2007 for a review of intermolecular forces). These observations strongly indicate that, unsurprisingly, not all polysaccharides (or any other group of EPS components) are equal for NPs, and thus, simple chemical characterization of the biofilm matrix into groups such as polysaccharides and proteins may not provide the necessary information for assessing the likelihood of the occurrence of NP–biofilm interactions. Electrostatic forces were also implicated as an important mechanism for deposition of TiO2 NPs onto synthetic biofilms (Sahle-Demessie and Tadesse, 2011) and for fullerene (C60) NPs onto surfaces coated with EPS extracted from Escherichia coli (Tong et al., 2010). On the other hand, polymer-mediated steric interactions were suggested as a dominant force for the attachment of poly(acrylic acid)-stabilized zerovalent iron NPs onto biofilm-coated porous media (Lerner et al., 2012). While this observation was based on NPs with polymer coatings, steric interactions are likely to be important in NP–biofilm interactions in the aquatic environment due to the polymeric natures of biofilms, and NPs coated with an organic corona. In addition, other potential interaction forces involved in NP deposition onto biofilm surfaces can be inferred from recent studies showing NP attachment to surfaces coated with organic compounds. For example, NOM has been extensively documented to adsorb to various NPs through combinations of many forces such as electrostatic, steric, and hydrophobic interactions (e.g., Hyung et al., 2007; Pelley and Tufenkji, 2008; Stankus et al., 2011).
Once a NP binds to EPS, it can subsequently migrate deeper into the EPS matrix. NP penetration into and movement within the biofilm is considered to be driven primarily by diffusion (Peulen and Wilkinson, 2011). In this case, diffusion of NPs into the biofilm may depend on the pore sizes of the biofilm (Sahle-Demessie and Tadesse, 2011), the charge of both the NPs and the biofilm matrix (Peulen and Wilkinson, 2011), hydrophobicity of the surrounding environment (Habimana et al., 2011), and the chemical gradient within the matrix. The EPS matrix pore-spaces (containing water) between adjacent molecules can vary in size. Ions and organic molecules diffuse and penetrate into a biofilm by moving (i.e., diffusing) through these pore-spaces. This presents the likely possibility that the EPS pore-spacing will be especially important in this process. This nanoscale variability, however, is poorly characterized and understood.
While accumulation of NPs within biofilms results from initial attachment and migration of NPs, these two processes need not always occur sequentially. It is likely that more NPs are depositing onto the outermost surface simultaneously as other NPs are penetrating into the biofilm matrix and vice versa. Another possible mechanism that contributes to NP accumulation within the matrix is the active outgrowth of the biofilm, forming new layers above the surface-deposited NPs. In such cases, penetration of NPs into the biofilm matrix is not necessary to occur for accumulation to take place. However, differentiation between these different processes would be a difficult task in practice. All three are likely to occur in the complex and dynamic environments where biofilms naturally occur. Most recent studies on NP–biofilm interactions have examined either the combined effects of initial surface NP deposition and penetration into the biofilm (Morrow et al., 2010; Habimana et al., 2011; Peulen and Wilkinson, 2011) or all three steps of NP–biofilm interactions outlined above in Section “Nanoparticle–Biofilm Interactions” (transport, attachment, migration; Fabrega et al., 2009; Choi et al., 2010). Overall, the migration of NPs into the 3D matrix of the biofilm is the least understood step of NP–biofilm interactions.
Fate of Nanoparticles Within Biofilms
Accumulation of NPs within biofilms has been previously documented (Ferry et al., 2009; Fabrega et al., 2011). Is the biofilm a “sink” for concentrating NPs from the overlying water? This is not likely to be the case. A final point here is that since biofilms and their associated EPS are readily consumed by grazing animals (see Decho, 1990, 2000, for reviews), the biofilm presents a potentially efficient vehicle for the trophic-transfer of NPs to food webs. However, the exact fate of the NPs within biofilms is not clear.
Behavior of Nanoparticles Upon Accumulation
One question we could ask is whether the NPs stay nano-sized and as particles. Given the right conditions, NPs will easily aggregate to form micro-size agglomerates. As shown by Choi et al. (2010), nanosilver that is introduced into E. coli biofilms were shown to aggregate to a larger degree than in planktonic cultures, possibly due to differences in ionic strength used in the experiments. On the other hand, organic ligands, including some that are found in EPS, typically stabilize NPs against aggregation, suggesting that NPs that enter the biofilm matrix as monomers may indeed stay as such within the biofilm. Another potential outcome of NP accumulation is NP dissolution as the NPs may be surrounded by enzymes and other organic matter that speed up those processes. This may especially be an important outcome for metallic NPs. Carbon-based NPs may undergo different changes in biofilms compared to metallic NPs. For example, microbial transformation of carbon-based NPs has been documented (Chae et al., 2014), which may also occur in the biofilm if the NPs are in contact with cells.
The EPS matrix is the primary emergent- and adaptive-property of the microbial cells forming a biofilm (Flemming and Wingender, 2010). Therefore, the matrix may potentially change in response to the presence of NPs. NP effects on biofilm microorganisms are likely to be dependent on the type of NP that is accumulated. For example, in the case of nanosilver or other NPs with antibacterial effects, cells could be severely stressed or directly killed as the concentration of NPs embedded in the biofilm increase. However, these antibacterial effects of nanosilver appeared to be considerably lower on cells in biofilms compared to planktonic cells (Choi et al., 2010). Other antibacterial NPs are designed to overcome such barriers; for example, Hetrick et al. (2009) developed nitric oxide-releasing silica NPs that do not rely on direct NP–cell contact, and hence, appeared to be highly effective at controlling pathogenic biofilm growth. The targeted use of NPs for biofilm control has been studied extensively by medical and dental as well as biofouling researchers and has been reviewed in detail (Allaker, 2010; Sousa et al., 2011; Natalio et al., 2012). Furthermore, nanosilver has been shown to affect the microbial community in wastewater biofilms (Sheng and Liu, 2011). These changes in the microbial community structure were mainly attributed to the antibacterial effects of nanosilver and the differences in tolerance levels across bacterial species. On the other hand, even NPs that have no antibacterial effects may induce shifts in the biofilm microbial community possibly due to changes in nutrient availability or chemical gradient within the biofilm matrix (Fabrega et al., 2011).
Formation of NPs Within Biofilms
The potential of biofilms to act as a factory for NP production has been increasingly recognized in both natural and engineered systems. Natural biofilms play a critical role in the biogeochemical cycling of elements which can lead to NP formation. For example, microbes precipitate metals in the form of NPs as a detoxification mechanism. Reith et al. (2006, 2010) have shown that gold dissolution and re-precipitation of nanoparticulate gold is directly coupled with biofilms on gold grain surfaces. Biofilms dominated by sulfate reducing bacteria were found to be responsible for the formation of zinc sulfide NPs (Labrenz et al., 2000; Labrenz and Banfield, 2004). As the number of such biofilm-mediated NP formation studies increases, the use of biofilms in the synthesis of nanomaterials is becoming popular for its relatively clean, non-toxic, and environmentally benign procedures (Mandal et al., 2006). For example, an electrochemically active biofilm was being utilized as a catalyst for extracellular production of monodispersed crystalline silver NPs (Kalathil et al., 2011).
Conclusions and Future Directions—Environmental Implications
There has been significant progress in understanding the micro- and nano-scale complexity within biofilms. Despite their complexity, it is now possible to experimentally examine the nature of NP interactions and penetration into biofilms. The ability to understand these fundamental and decisive processes can be approached using carefully controlled laboratory manipulations of EPS and NPs. Recent development of surface-sensitive techniques, high-resolution microscopies, and synchrotron-based spectroscopies provide powerful tools to promote an integrated approach to understanding NP–biofilm interactions. Multi-scale computational modeling efforts will be useful in complementing empirical data and enhancing the predictability of NP behavior within the biofilm matrix. Ultimately, improving our mechanistic understanding of NP–biofilm interactions will enable better risk assessment of nanotechnology as well as sustainable design of NPs.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported, in part, by grants from the US National Science Foundation (BME-1032579) and the NanoCenter at the University of South Carolina.
References
Allaker, R. P. (2010). The use of nanoparticles to control oral biofilm formation. J. Dent. Res. 89, 1175–1186. doi: 10.1177/0022034510377794
Battin, T. J., Von Der Kammer, F., Weilhartner, A., Ottofuelling, S., and Hofmann, T. (2009). Nanostructured TiO2: transport behavior and effects on aquatic microbial communities under environmental conditions. Environ. Sci. Technol. 43, 8098–8104. doi: 10.1021/es9017046
Berry, D., Xi, C., and Raskin, L. (2006). Microbial ecology of drinking water distribution systems. Curr. Opin. Biotechnol. 17, 297–302. doi: 10.1016/j.copbio.2006.05.007
Braissant, O., Decho, A. W., Przekop, K. M., Gallagher, K. L., Glunk, C., Dupraz, C., et al. (2009). Characteristics and turnover of exopolymeric substances in a hypersaline microbial mat. FEMS Microbiol. Ecol. 67, 293–307. doi: 10.1111/j.1574-6941.2008.00614.x
Cedervall, T., Lynch, I., Lindman, S., Berggard, T., Thulin, E., Nilsson, H., et al. (2007). Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc. Natl. Acad. Sci. U.S.A. 104, 2050–2055. doi: 10.1073/pnas.0608582104
Chae, S.-R., Hunt, D. E., Ikuma, K., Yang, S., Cho, J., Gunsch, C. K., et al. (2014). Aging of fullerene C60 nanoparticle suspensions in the presence of microbes. Water Res. 65, 282–289. doi: 10.1016/j.watres.2014.07.038
Choi, O., Yu, C.-P., Fernandez, G. E., and Hu, Z. (2010). Interactions of nanosilver with Escherichia coli cells in planktonic and biofilm cultures. Water Res. 44, 6095–6103. doi: 10.1016/j.watres.2010.06.069
Decho, A. W. (1990). Microbial exopolymer secretions in ocean environments: their role(s) in food webs and marine processes. Oceanogr. Mar. Biol. Ann. Rev. 28, 73–154.
Decho, A. W. (2000). “Exopolymer-mediated microdomains as a structuring agent for microbial activities,” in Microbial Sediments, ed. R. Riding (Berlin: Springer), 9–15.
Fabrega, J., Renshaw, J. C., and Lead, J. R. (2009). Interactions of silver nanoparticles with Pseudomonas putida biofilms. Environ. Sci. Technol. 43, 9004–9009. doi: 10.1021/es901706j
Fabrega, J., Zhang, R., Renshaw, J. C., Liu, W.-T., and Lead, J. R. (2011). Impact of silver nanoparticles on natural marine biofilm bacteria. Chemosphere 85, 961–966. doi: 10.1016/j.chemosphere.2011.06.066
Ferry, J. L., Craig, P., Hexel, C., Sisco, P., Frey, R., Pennington, P. L., et al. (2009). Transfer of gold nanoparticles from the water column to the estuarine food web. Nat. Nanotechnol. 4, 441–444. doi: 10.1038/nnano.2009.157
Flemming, H.-C., and Wingender, J. (2010). The biofilm matrix. Nat. Rev. Microbiol. 8, 623–633. doi: 10.1038/nrmicro2415
Guiot, E., Georges, P., Brun, A., Fontaine-Aupart, M. P., Bellon-Fontaine, M. N., and Briandet, R. (2002). Heterogeneity of diffusion inside microbial biofilms determined by fluorescence correlation spectroscopy under two-photon excitation. Photochem. Photobiol. 75, 570–578. doi: 10.1562/0031-8655(2002)075<0570:hodimb>2.0.co;2
Habimana, O., Steenkeste, K., Fontaine-Aupart, M.-P., Bellon-Fontaine, M.-N., and Briandet, R. (2011). Diffusion of nanoparticles in biofilms is altered by bacterial cell wall hydrophobicity. Appl. Environ. Microbiol. 77, 367–368. doi: 10.1128/AEM.02163-10
Hawari, A. H., and Mulligan, C. N. (2006). Biosorption of lead(II), cadmium(II), copper(II) and nickel (II) by anaerobic granular biomass. Bioresour. Technol. 97, 692–700. doi: 10.1016/j.biortech.2005.03.033
Hetrick, E. M., Shin, J. H., Paul, H. S., and Schoenfisch, M. H. (2009). Anti-biofilm efficacy of nitric oxide-releasing silica nanoparticles. Biomaterials 30, 2782–2789. doi: 10.1016/j.biomaterials.2009.01.052
Hochella, M. F., Lower, S. K., Maurice, P. A., Penn, R. L., Sahai, N., Sparks, D. L., et al. (2008). Nanominerals, mineral nanoparticles, and earth systems. Science 319, 1631–1635. doi: 10.1126/science.1141134
Hu, Z., Hidalgo, G., Houston, P. L., Hay, A. G., Shuler, M. L., Abruna, H. D., et al. (2005). Determination of spatial distributions of zinc and active biomass in microbial biofilms by two-photon laser scanning microscopy. Appl. Environ. Microbiol. 71, 4014–4021. doi: 10.1128/AEM.71.7.4014-4021.2005
Hyung, H., Fortner, J. D., Hughes, J. B., and Kim, J.-H. (2007). Natural organic matter stabilizes carbon nanotubes in the aqueous phase. Environ. Sci. Technol. 41, 179–184. doi: 10.1021/es061817g
Ikuma, K., Madden, A. S., Decho, A. W., and Lau, B. L. T. (2014). Deposition of nanoparticles onto polysaccharide-coated surfaces: implications for nanoparticle–biofilm interactions. Environ. Sci. Nano 1, 117–122. doi: 10.1039/c3en00075c
Jaisi, D. P., Saleh, N. B., Blake, R. E., and Elimelech, M. (2008). Transport of single-walled carbon nanotubes in porous media: filtration mechanisms and reversibility. Environ. Sci. Technol. 42, 8317–8323. doi: 10.1021/es801641v
Kalathil, S., Lee, J., and Cho, M. H. (2011). Electrochemically active biofilm-mediated synthesis of silver nanoparticles in water. Green Chem. 13, 1482–1485. doi: 10.1039/c1gc15309a
Karatan, E., and Watnick, P. (2009). Signals, regulatory networks, and materials that build and break bacterial biofilms. Microbiol. Mol. Biol. Rev. 73, 310–347. doi: 10.1128/MMBR.00041-08
Kroll, A., Behra, R., Kaegi, R., and Sigg, L. (2014). Extracellular polymeric substances (EPS) of freshwater biofilms stabilize and modify CeO2 and Ag nanoparticles. PLoS ONE 9:e110709. doi: 10.1371/journal.pone.0110709
Labrenz, M., and Banfield, J. F. (2004). Sulfate-reducing bacteria-dominated biofilms that precipitate ZnS in a subsurface circumneutral-pH mine drainage system. Microb. Ecol. 47, 205–217. doi: 10.1007/s00248-003-1025-8
Labrenz, M., Druschel, G. K., Thomsen-Ebert, T., Gilbert, B., Welch, S. A., Kemner, K. M., et al. (2000). Formation of sphalerite (ZnS) deposits in natural biofilms of sulfate-reducing bacteria. Science 290, 1744–1747. doi: 10.1126/science.290.5497.1744
Lawrence, J. R., Swerhone, G. D., Kuhlicke, U., and Neu, T. R. (2007). In situ evidence for microdomains in the polymer matrix of bacterial microcolonies. Can. J. Microbiol. 53, 450–458. doi: 10.1139/W06-146
Lecoanet, H. F., and Wiesner, M. R. (2004). Velocity effects on fullerene and oxide nanoparticle deposition in porous media. Environ. Sci. Technol. 38, 4377–4382. doi: 10.1021/es035354f
Lehtola, M. J., Miettinen, I. T., Keinanen, M. M., Kekki, T. K., Laine, O., Hirvonen, A., et al. (2004). Microbiology, chemistry and biofilm development in a pilot drinking water distribution system with copper and plastic pipes. Water Res. 38, 3769–3779. doi: 10.1016/j.watres.2004.06.024
Lerner, R. N., Lu, Q., Zeng, H., and Liu, Y. (2012). The effects of biofilm on the transport of stabilized zerovalent iron nanoparticles in saturated porous media. Water Res. 46, 975–985. doi: 10.1016/j.watres.2011.11.070
Levard, C., Hotze, M., Lowry, G. V., and Brown, G. E. (2012). Environmental transformations of silver nanoparticles: impact on stability and toxicity. Environ. Sci. Technol. 46, 6900–6914. doi: 10.1021/es2037405
Liang, Y., Hilal, N., Langston, P., and Starov, V. (2007). Interaction forces between colloidal particles in liquid: theory and experiment. Adv. Colloid Interface Sci. 134–135, 151–166. doi: 10.1016/j.cis.2007.04.003
Lynch, I., Salvati, A., and Dawson, K. A. (2009). Protein–nanoparticle interactions: what does the cell see? Nat. Nanotechnol. 4, 546–547. doi: 10.1038/nnano.2009.248
Mandal, D., Bolander, M. E., Mukhopadhyay, D., Sarkar, G., and Mukherjee, P. (2006). The use of microorganisms for the formation of metal nanoparticles and their application. Appl. Microbiol. Biotechnol. 69, 485–492. doi: 10.1007/s00253-005-0179-3
Monopoli, M. P., Aberg, C., Salvati, A., and Dawson, K. A. (2012). Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotechnol. 7, 779–786. doi: 10.1038/nnano.2012.207
Morrow, J. B., Arango, P. C., and Holbrook, R. D. (2010). Association of quantum dot nanoparticles with Pseudomonas aeruginosa biofilm. J. Environ. Qual. 39, 1934–1941. doi: 10.2134/jeq2009.0455
Natalio, F., Andre, R., Hartog, A. F., Stoll, B., Jochum, K. P., Wever, R., et al. (2012). Vanadium pentoxide nanoparticles mimic vanadium haloperoxidases and thwart biofilm formation. Nat. Nanotechnol. 7, 530–535. doi: 10.1038/nnano.2012.91
Nevius, B. A., Chen, Y. P., Ferry, J. L., and Decho, A. W. (2012). Surface-functionalization effects on uptake of fluorescent polystyrene nanoparticles by model biofilms. Ecotoxicology 21, 2205–2213. doi: 10.1007/s10646-012-0975-3
Pelley, A. J., and Tufenkji, N. (2008). Effect of particle size and natural organic matter on the migration of nano- and microscale latex particles in saturated porous media. J. Colliod Interface Sci. 321, 74–83. doi: 10.1016/j.jcis.2008.01.046
Petosa, A. R., Jaisi, D. P., Quevedo, I. R., Elimelech, M., and Tufenkji, N. (2010). Aggregation and deposition of engineered nanomaterials in aquatic environments: role of physicochemical interactions. Environ. Sci. Technol. 44, 6532–6549. doi: 10.1021/es100598h
Peulen, T.-O., and Wilkinson, K. J. (2011). Diffusion of nanoparticles in a biofilm. Environ. Sci. Technol. 45, 3367–3373. doi: 10.1021/es103450g
Phenrat, T., Kim, H. J., Fagerlund, F., Illagasekare, T., Tilton, R. D., and Lowry, G. V. (2009). Particle size distribution, concentration, and magnetic attraction affect transport of polymer-modified FeO nanoparticles in sand columns. Environ. Sci. Technol. 43, 5079–5085. doi: 10.1021/es900171v
Reith, F., Fairbrother, L., Nolze, G., Wilhelmi, O., Clode, P. L., Gregg, A., et al. (2010). Nanoparticle factories: biofilms hold the key to gold dispersion and nugget formation. Geology 38, 843–846. doi: 10.1130/G31052.1
Reith, F., Rogers, S. L., Mcphail, D. C., and Webb, D. (2006). Biomineralization of gold: biofilms on bacterioform gold. Science 313, 233–236. doi: 10.1126/science.1125878
Sahle-Demessie, E., and Tadesse, H. (2011). Kinetics and equilibrium adsorption of nano-TiO2 particles on synthetic biofilm. Surf. Sci. 605, 1177–1184. doi: 10.1016/j.susc.2011.03.022
Schlekat, C. E., Decho, A. W., and Chandler, G. T. (1998). Sorption of cadmium to bacterial extracellular polymeric sediment coatings under estuarine conditions. Environ. Toxicol. Chem. 17, 1867–1874. doi: 10.1002/etc.5620170930
Schmitt, J., Nivens, D., White, D. C., and Flemming, H.-C. (1995). Changes of biofilm properties in response to sorbed substances—an FTIR-ATR study. Water Sci. Technol. 32, 149–155.
Sheng, Z., and Liu, Y. (2011). Effects of silver nanoparticles on wastewater biofilms. Water Res. 45, 6039–6050. doi: 10.1016/j.watres.2011.08.065
Sousa, C., Botelho, C., and Oliveira, R. (2011). “Nanotechnology applied to medical biofilms control,” in Science Against Microbial Pathogens: Communicating Current Research and Technological Advances, ed. A. Mendez-Vilas (Badajoz: Formatex Research Center), 878–888.
Stankus, D. P., Lohse, S. E., Hutchison, J. E., and Nason, J. A. (2011). Interactions between natural organic matter and gold nanoparticles stabilized with different organic capping agents. Environ. Sci. Technol. 45, 3238–3244. doi: 10.1021/es102603p
Sutherland, I. W. (2001). Biofilm exopolysaccharides: a strong and sticky framework. Microbiology 147, 3–9.
Tong, M., Ding, J., Shen, Y., and Zhu, P. (2010). Influence of biofilm on the transport of fullerene (C60) nanoparticles in porous media. Water Res. 44, 1094–1103. doi: 10.1016/j.watres.2009.09.040
Tripathi, S., Champagne, D., and Tufenkji, N. (2012). Transport behavior of selected nanoparticles with different surface coatings in granular porous media coated with Pseudomonas aeruginosa biofilm. Environ. Sci. Technol. 46, 6942–6949. doi: 10.1021/es202833k
Walczyk, D., Bombelli, F. B., Monopoli, M. P., Lynch, I., and Dawson, K. A. (2010). What the cell “sees” in bionanoscience. J. Am. Chem. Soc. 132, 5761–5768. doi: 10.1021/ja910675v
Whitchurch, C. B., Tolker-Nielsen, T., Ragas, P. C., and Mattick, J. S. (2002). Extracellular DNA required for bacterial biofilm formation. Science 295, 1487. doi: 10.1126/science.295.5559.1487
Wirth, S. M., Lowry, G. V., and Tilton, R. D. (2012). Natural organic matter alters biofilm tolerance to silver nanoparticles and dissolved silver. Environ. Sci. Technol. 46, 12687–12696. doi: 10.1021/es301521p
Keywords: nanoparticle–biofilm interactions, extracellular polymeric substances, protein corona, pore space, surface forces, biofilm matrix
Citation: Ikuma K, Decho AW and Lau BLT (2015) When nanoparticles meet biofilms—interactions guiding the environmental fate and accumulation of nanoparticles. Front. Microbiol. 6:591. doi: 10.3389/fmicb.2015.00591
Received: 13 January 2015; Accepted: 29 May 2015;
Published: 16 June 2015.
Edited by:
David Emerson, Bigelow Laboratory for Ocean Sciences, USAReviewed by:
Thomas R. Neu, Helmholtz Centre for Environmental Research, GermanyJohn J. Kelly, Loyola University Chicago, USA
Copyright © 2015 Ikuma, Decho and Lau. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Boris L. T. Lau, Department of Civil and Environmental Engineering, University of Massachusetts Amherst, 18B Marston Hall, 130 Natural Resources Road, Amherst, MA 01003-9293, USA, borislau@engin.umass.edu
 Kaoru Ikuma
Kaoru Ikuma