- Molecular Microbial Biochemistry Laboratory, Faculty of Science, University of Ontario Institute of Technology, Oshawa, ON, Canada
Komagataeibacter (formerly Gluconacetobacter) xylinus ATCC 53582 is a plant-associated model organism for bacterial cellulose (BC) biosynthesis. This bacterium inhabits the carposphere where it interacts with fruit through the bi-directional transfer of phytohormones. The majority of research regarding K. xylinus has been focused on identifying and characterizing structural and regulatory factors that control BC biosynthesis, but its ecophysiology has been generally overlooked. Ethylene is a phytohormone that regulates plant development in a variety of ways, but is most commonly known for its positive role on fruit ripening. In this study, we utilized ethephon (2-chloroethylphosphonic acid) to produce in situ ethylene to investigate the effects of this phytohormone on BC production and the expression of genes known to be involved in K. xylinus BC biosynthesis (bcsA, bcsB, bcsC, bcsD, cmcAx, ccpAx and bglAx). Using pellicle assays and reverse transcription quantitative polymerase chain reaction (RT-qPCR), we demonstrate that ethephon-derived ethylene enhances BC directly in K. xylinus by up-regulating the expression of bcsA and bcsB, and indirectly though the up-regulation of cmcAx, ccpAx, and bglAx. We confirm that IAA directly decreases BC biosynthesis by showing that IAA down-regulates bcsA expression. Similarly, we confirm that ABA indirectly influences BC biosynthesis by showing it does not affect the expression of bcs operon genes. In addition, we are the first to report the ethylene and indole-3-acetic acid (IAA) induced differential expression of genes within the bacterial cellulose synthesis (bcs) operon. Using bioinformatics we have identified a novel phytohormone-regulated CRP/FNRKx transcription factor and provide evidence that it influences BC biosynthesis in K. xylinus. Lastly, utilizing current and previous data, we propose a model for the phytohormone-mediated fruit-bacteria interactions that K. xylinus experiences in nature.
Introduction
Komagataeibacter (formerly Gluconacetobacter) xylinus ATCC 53582 is an acetic acid bacterium (Yamada et al., 2012; Mamlouk and Gullo, 2013) studied for its ability to synthesize and secrete large quantities of crystalline cellulose at the air-liquid interface of static cultures (Schramm and Hestrin, 1954). Bacterial cellulose (BC) produced by K. xylinus is of higher purity compared to plant cellulose since it is devoid of hemicellulose, pectin and lignin (Schramm and Hestrin, 1954; Kudlicka and Brown, 1996), allowing for a highly ordered, crystalline, and recalcitrant cellulose I matrix.
Komagataeibacter xylinus and related species can be isolated from fruit (Park et al., 2003; Dellaglio et al., 2005; Jahan et al., 2012; Neera et al., 2015) and spoiled wine (Bartowsky and Henschke, 2008). These are sugar-rich environments that provide ideal conditions for K. xylinus growth and BC biosynthesis. In the environment, K. xylinus synthesizes a BC biofilm to facilitate adherence and subsequent colonization of its fruit substrate. A biofilm is defined as a surface-associated bacterial community embedded within an extracellular matrix consisting of polysaccharides, proteins and extracellular DNA (Geesey et al., 1978; Costerton et al., 1995; Flemming and Wingender, 2010). The BC matrix also provides protection from environmental stresses and provides a competitive advantage over other microorganisms (Williams and Cannon, 1989). This phenomenon is also observed with Enterobacter amnigenus GH-1, which synthesizes BC to adhere to fruits and abiotic materials (Kim et al., 2006; Hungund and Gupta, 2010). Numerous other bacteria also synthesize biofilms containing BC and other polysaccharides to facilitate interactions with plants and animals. The role of BC in facilitating the diverse environmental interactions of various biofilm-producing bacteria has recently been reviewed (Augimeri et al., 2015).
Bacterial cellulose is synthesized on the cytoplasmic side of the inner membrane (Bureau and Brown, 1987) and is subsequently transported through the periplasmic space before it is released into the extracellular environment (Figure 1A; Morgan et al., 2013). BC synthesis terminal complexes are found along the longitudinal axis of the rod-shaped K. xylinus cell (Brown et al., 1976; Kimura et al., 2001; Sunagawa et al., 2013) and are responsible for the synthesis and export of BC. This arrangement allows adjacent glucan chains to be hydrogen bonded as they are being exported, and thus couples elongation, translocation, and crystallization of BC (Morgan et al., 2013).
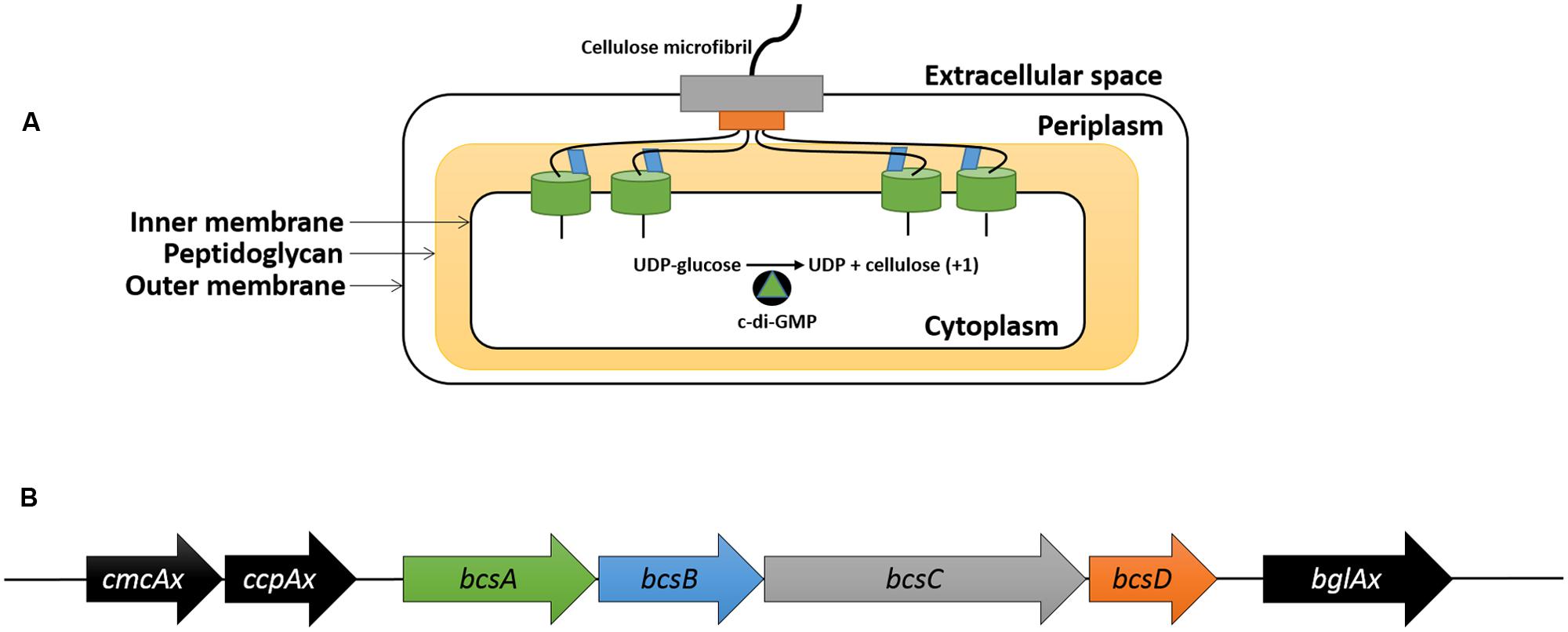
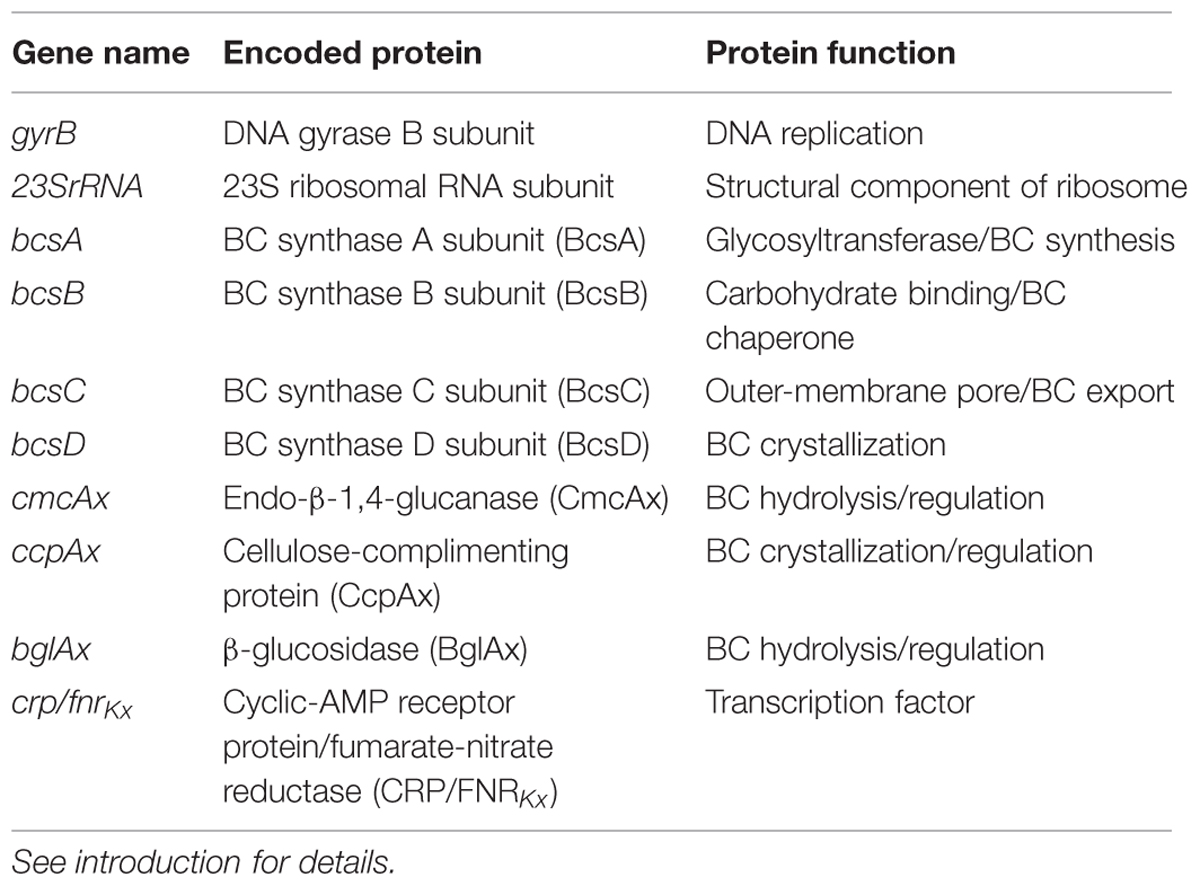
FIGURE 1. Structural and genetic organization of the bacterial cellulose synthesis complex and the bcs operon. BcsA (green), activated by c-di-GMP, adds a glucose unit to the cellulose chain using UDP-glucose as a substrate in the cytoplasm; BcsB (blue) guides the glucan chain through the periplasm; BcsD (orange) crystallizes four glucan chains in the periplasm; BcsC (gray) exports the BC microfibril into the extracellular space (A). The genetic organization of the bcs operon that encodes the bacterial cellulose synthesis complex and the genes up- and downstream (B). The genes in the bcs operon (B) are color coordinated with their protein products (A).
The BC synthesis terminal complexes in K. xylinus ATCC 53582 consist of four protein subunits (BcsA, BcsB, BcsC, and BcsD) that are encoded within the BC synthesis (bcsABCD) operon (Figure 1B). The BcsA subunit, located on the cytoplasmic face of the inner membrane (Omadjela et al., 2013) possesses a catalytic β-1,4-glycosyltransferase domain containing the DDDQ(Q/R)XRW motif (Richmond and Somerville, 2000) responsible for polymerizing monomers of uridine diphosphoglucose (UDP-glucose) into β-1,4-glucan chains of cellulose (Figure 1A; Lin et al., 1990). The activity of the catalytic domain is regulated at the C-terminal PilZ domain (Fujiwara et al., 2013; Omadjela et al., 2013; Morgan et al., 2014) by the allosteric activator of BC synthesis, bis-(3′→5′)-cyclic diguanylate (c-di-GMP; Ross et al., 1987). The levels of c-di-GMP are modulated by three cyclic diguanylate (cdg) operons (Tal et al., 1998) that encode diguanylate cyclases (DGCs) and phosphodiesterases (PDEs) that synthesize (Qi et al., 2009) and degrade (Chang et al., 2001) c-di-GMP, respectively. Per-Arnt-Sim (PAS) sensory domains on these enzymes control the catalytic domains, c-di-GMP levels and thus BC biosynthesis, by perceiving oxygen levels and cellular redox status (Chang et al., 2001; Qi et al., 2009; Henry and Crosson, 2011). BcsB binds to BcsA in the periplasm by a single C-terminal transmembrane helix (Morgan et al., 2013), where it stabilizes BcsA and guides glucan chains through the periplasmic space using two carbohydrate-binding domains (CBD1 and CBD2; Figure 1A).
Extrusion of BC from the periplasm to the extracellular environment is believed to be facilitated through the action of BcsC (Figure 1A), which is predicted to form a pore in the outer membrane of K. xylinus based on its structure. Consistent with the view that BcsC is an outer membrane porin, is the observation that BcsC is essential for in vivo, but not in vitro BC biosynthesis (Saxena et al., 1994).
Crystallization of BC is achieved through the action of BcsD, a cylindrical octameric periplasmic protein (Iyer et al., 2011) that contains four spiral channels that facilitates hydrogen bonding of four glucan chains during export through BcsC (Figure 1A; Hu et al., 2010). In Komagataeibacter hansenii ATCC 23769, bcsD is regulated independently of the other bcs genes (Deng et al., 2013), as a 321 base-pair untranslated region (UTR) separates bcsC and bcsD, leaving room for a functional promoter (Deng et al., 2013). The coding regions of bcsC and bcsD in K. xylinus have a 1 base-pair overlap, suggesting that bcsD is regulated from the same promoter as the rest of the bcs genes in this strain.
Located upstream of the bcs operon, is cmcAx (Figure 1B), which encodes an endo-β-1,4-glucanase that has cellulose-hydrolyzing activity in vitro on cellopentose or longer oligosaccharide substrates (Kawano et al., 2002a). In small amounts, exogenous CmcAx enhances BC production of K. xylinus (Kawano et al., 2002a); while endogenous overexpression of cmcAx increases BC yield (Kawano et al., 2002a). This suggests that the cellulose hydrolyzing activity of CmcAx may exert a regulatory effect on BC biosynthesis.
In the same upstream operon as cmcAx, is ccpAx (Figure 1B), which encodes the cellulose-complementing protein (CcpAx) that is required for in vivo BC biosynthesis (Deng et al., 2013; Standal et al., 1994), particularly its crystallization (Nakai et al., 2002). CcpAx is co-localized with BcsD longitudinally on the extracellular side of the cell membrane, suggesting that CcpAx is a critical part of the BC synthesis terminal complex (Sunagawa et al., 2013). Since this protein is of low molecular weight with predicted secondary structures rich in α-helices, CcpAx may facilitate protein-protein interactions for the spatial assembly of BC synthesis complexes (Sunagawa et al., 2013). Disruption of ccpAx results in a significant reduction in the levels of BcsB and BcsC (Deng et al., 2013), indicating CcpAx also plays a regulatory role in BC biosynthesis.
Downstream of the BC synthesis operon is bglAx (Figure 1B), which encodes a β-glucosidase (BglAx; Tajima et al., 2001). This monomeric enzyme is secreted (Tahara et al., 1998) and has the ability to hydrolyze oligosaccharides larger than three residues into single β-D-glucose units (Tahara et al., 1998; Tajima et al., 2001). In K. hansenii ATCC 23769, the expression of bglAx is transcriptionally regulated by CRP/FNRKh, a cyclic-AMP receptor/fumarate nitrate reductase protein (Deng et al., 2013). Transposon insertion into crp/fnrKh (GXY_00863) completely abolished production of BC and BglAx, providing evidence that CRP/FNRKh controls BC biosynthesis at the transcriptional level. Since bglAx deletion results in reduced BC synthesis but not the absence of BC production observed with crp/fnrKh deletion, it is probable that CRP/FNRKh controls the expression of additional genes that are essential for BC biosynthesis (Deng et al., 2013).
The CRP/FNR family of transcription factors are ubiquitous in bacteria (Matsui et al., 2013) and regulate processes that are critical to bacterial growth and survival. Some of these processes include catabolite repression, aerobic growth, nitrogen fixation, oxidative stress responses, stationary phase survival, arginine catabolism, and pathogenicity (Körner et al., 2003).
Komagataeibacter xylinus is believed to participate in various fruit-bacteria interactions when inhabiting the carposphere. We previously showed that K. xylinus ATCC 53582 endogenously synthesized and secreted the phytohormones, zeatin, gibberellic acid (GA3), and abscisic acid (ABA; Qureshi et al., 2013), all of which are involved in fruit growth and ripening (McAtee et al., 2013). Unlike Gluconacetobacter diazotrophicus (Lee and Kennedy, 2000), K. xylinus does not produce indole-3-acetic acid (IAA; Qureshi et al., 2013), a phytohormone known to inhibit fruit ripening and senescence when applied exogenously to plants (Davies et al., 1997). K. xylinus cultures grown in the presence of exogenous zeatin, GA3, and ABA grew faster and had greater BC yields than untreated cultures (Qureshi et al., 2013). Exogenous IAA, however, increased growth but decreased the BC yield (Qureshi et al., 2013). The effect of IAA on BC production is therefore direct, while ABA, zeatin, and GA3 indirectly increase BC yield due to an enhanced growth rate. These results led us to investigate ethylene, a fruit ripening phytohormone directly affected by the concentrations of IAA and ABA in plants, for its effects on BC biosynthesis.
Ethylene is a gaseous phytohormone important to many aspects of plant growth and development, including having a positive role on ripening, senescence and rotting of climacteric fruit (Lieberman, 1979). Decreased IAA levels with a concomitant increase in ABA concentration during fruit growth, triggers the biosynthesis of ethylene (Zhang et al., 2009) which induces expression of genes whose protein products are required for the enzymatic degradation of fruit polysaccharides into soluble monosaccharides (Zegzouti et al., 1999). Numerous studies have investigated the effect of bacterially produced ethylene on plants (Weingart and Völksch, 1997; Baca and Elmerich, 2007), as well as the effect of lowering plant-produced ethylene levels by bacterially produced 1-aminocyclopropane-1-carboxylate (ACC) deaminase enzymes (Glick, 2005). However, there is a paucity of literature regarding the effect of plant-produced ethylene on bacteria. This may be because ethylene is a gas and thus difficult to control in a laboratory setting without specialized equipment. Ethephon (2-chloroethylphosphonic acid) is an ethylene-releasing compound that produces in situ ethylene at a 1:1 molar ratio above pH 3.5 (Zhang and Wen, 2010). Base-catalyzed chemical degradation of ethephon results in the production of ethylene, chloride, and phosphate through a first-order reaction (Warner and Leopold, 1969; Biddle et al., 1976; Klein et al., 1979). The rate of ethephon decomposition is positively correlated with pH and temperature (Biddle et al., 1976; Klein et al., 1979). Like ethylene, numerous studies have shown that application of ethephon induces and accelerates ripening of various fruits (Jolliffe, 1975; Hardie et al., 1981; Szyjewicz et al., 1984; Ban et al., 2007; Dhillon and Mahajan, 2011; Zhang et al., 2012; Dhall and Singh, 2013), allowing ethephon to be used in agriculture as a chemical replacement for gaseous ethylene during pre- and post-harvest fruit ripening (Singh and Janes, 2001; Khorshidi and Davarynejad, 2010; Gill et al., 2014).
In plants, ethylene binds to ethylene-binding domains (EBDs) that belong to a family of ethylene receptors that subsequently activate intracellular signal cascades that leads to ethylene-dependent phenotypes (Lacey and Binder, 2014). A bioinformatics study has shown that EBDs are common in plants and cyanobacteria, occur in fungi and green algae, but are generally absent in archeabacteria and eubacteria (Wang et al., 2006). However, Kim et al. (2007) demonstrated that various Pseudomonas species, including plant-associated P. syringae and P. putida, were chemotactic towards ethylene and that a mutant with the deletion of a methyl-accepting chemotaxis protein (MCP) gene (cheR) was unable to respond to ethylene, suggesting that ethylene chemotaxis involved one or more MCPs. The CheR protein contains two domains: the CheR domain, which is involved in chemotaxis signaling and sensing environmental signals and an AdoMet_MTase domain that use S-adenosyl-L-methionine as a substrate for methyl group transfers (i e., methyltransferase) establishing that bacteria have the ability to respond to exogenous ethylene.
Protein alignments comparing CheR of P. aeruginosa PAO1 to the K. xylinus E25 genome reveals that K. xylinus contains a putitive MCP (27% identities; E = 1e-27; 90% query coverage; Gene: H845_2135). A search of the K. xylinus E25 genome showed that this putitive MCP is co-located on the chromosome with a methyl-accepting sensory transducer gene (H845_2133), various genes involved in chemotaxis, such as cheW (H845_2134 and H845_2136), cheA (H845_2132), and cheB (H845_2131), a histidine kinase signal transduction response regulator (H845_2130), and a PAS/PAC hybrid histidine kinase sensor (H845_2129). The cheB gene encodes a methyltransferase, cheW encodes a small regulator protein unique to bacterial chemotaxis, and cheA encodes a histidine kinase. In addition to its role in chemotaxis, cheA affects biofilm formation in Pseudomonas (Tremaroli et al., 2011).
In this study, we used ethephon to analyze the effects of exogenous ethylene on K. xylinus ATCC 53582 growth, BC production and pellicle properties. Release of ethylene from ethephon decomposition in a pH 7 Schramm-Hestrin (SH) medium was verified using the Arabidopsis thaliana triple response assay. Ethylene increased the crystallinity of K. xylinus BC pellicles, which resulted in decreased pellicle hydration. K. xylinus grown in the presence of ethylene on solid medium and in broth cultures produced more BC than controls, while the growth rate in agitated broth cultures was not affected, indicating that the positive effect of ethylene on BC biosynthesis was direct. These results suggest that ethylene produced by ripening fruit may aid in K. xylinus fruit colonization by enhancing the production and recalcitrance of its BC, thereby providing an advantage against competing microorganisms in nature. Reverse transcription quantitative polymerase chain reaction (RT-qPCR) assays revealed that ethylene, IAA and ABA affect the expression of genes known to be involved in K. xylinus BC biosynthesis. We report for the first time, the differential expression of genes within the bcs operon, as well as the identification of a phytohormone-regulated CRP/FNR transcription factor gene in K. xylinus (crp/fnrKx). In addition, we are the first to use ethephon to study the effects of ethylene on bacteria. This study elaborates on the putative fruit-bacteria interactions of K. xylinus and gives new insights into the transcriptional regulation of the bcs operon.
Materials and Methods
Chemicals
Stocks of ethephon (2-chloroethylphosphonic acid; Sigma), NaCl (BioBasic), and NaH2PO4⋅H2O (BioBasic) were dissolved in ultra-pure water (pH 2.5), while indole-3-acetic acid (IAA; BioShop) and abscisic acid (ABA; BioShop) were prepared in 100% dimethyl sulfoxide (DMSO; BioBasic). All stock solutions were filter-sterilized and frozen at -20oC until used.
Bacteria and Culture Conditions
Komagataeibacter xylinus ATCC 53582 was maintained as frozen glycerol stocks at -80°C. All starter cultures were grown by inoculating a single rough colony of K. xylinus from an SH agar plate streaked from glycerol stock, in 5 mL of SH broth (pH 5) supplemented with 0.2% (v/v) filter-sterilized cellulase. Cultures were grown in triplicate and incubated at 30°C with agitation at 150 rpm until the cells reached an OD600 of 0.3-0.4. Cultures were harvested by centrifugation, washed twice and suspended in sterile 0.85% (w/v) NaCl and quantified with a Petroff-Hauser counting chamber. All experiments employed an inoculum level of 105 cells/mL, incubation at 30°C, and shaking at 150 rpm if grown with agitation.
Triple Response Assay
Ethylene production from ethephon decomposition on SH medium (pH 7) was verified using the Arabidopsis thaliana triple response assay (Guzmán and Ecker, 1990). Seeds of A. thaliana ecotype Columbia were surface-sterilized in a sealed plastic container using chlorine gas produced from the addition of 3 mL concentrated HCl into 100 mL of bleach. The assay was performed using glass petri dishes that were divided into four quadrants. Growth medium for seeds was added in the quadrants adjacent to those containing SH agar at pH 7 (Supplementary Figure S1). Negative controls consisted of sterile seeds plated on 1X Murashige and Skoog salts medium (MS; pH 6.0; 0.8% agar) containing 1% (w/v) sucrose. Positive controls consisted of seeds plated on the MS-sucrose medium supplemented with 10 μM 1-aminocyclopropane carboxylic acid (ACC), the precursor for ethylene biosynthesis in plants. Ethephon (1 mM) was spread on pH 7 SH agar (1.5%) with seeds plated on the adjacent MS-sucrose medium. Plates were sealed with Parafilm, wrapped in foil and incubated in the dark for 4 days at 4°C before being exposed to light for 2 h. Stratified seeds were germinated in the dark for 72 h at 23°C. The experiment was performed with three biological replicates. Seedlings were photographed using a Cannon digital SLR camera or a USB 2.0 USB Digital Microscope (Plugable Technologies). The hypocotyl length of 60 seedlings per biological replicate (180 seedlings per treatment) was measured using ImageJ software (Schneider et al., 2012). Statistics were performed using a one-way ANOVA with a Tukey’s multiple comparison test. Differences were considered statistically significant if p < 0.05.
Time-course pH Analysis
The pH change during growth of K. xylinus cultures in the presence of ethephon was assessed. Starter cultures were used to inoculate 150 mL of SH medium (pH 7) supplemented with 0.2 % (v/v) cellulase and were incubated with agitation for 14 days. Ethephon was tested at concentrations of 0.01, 0.1, and 1.0 mM, while the untreated control culture was supplemented with an equal volume of acidified ultra-pure water (pH 2.5). An identical experiment using phosphate and chloride as the test compounds was run in parallel. All flasks were covered with foil and sealed tightly with tape to prevent the escape of released ethylene. Each day, an aliquot of culture was removed and the pH was measured. Three biological replicates were tested with three technical replicates each. Statistical analysis was completed using a one-way ANOVA with Tukey’s multiple comparisons test. Differences were considered significant if p < 0.05.
Minimum Inhibitory Concentration (MIC) Assay
Minimum inhibitory concentration assays to determine the lowest concentration of ethephon that prevented visible growth of K. xylinus were performed in sterile 96-well plates using the two-fold serial broth dilution MIC method (Witebsky et al., 1979). Ethephon was tested using a concentration range of 0.195-100 mM and was diluted in SH broth (pH 7). Wells were inoculated with K. xylinus starter cultures in a final volume of 150 μL. Growth controls lacking ethephon and sterile controls were included. Plates were sealed with Parafilm and incubated statically for 5 days. Clear wells were an indication of growth inhibition. To ensure the results were caused by ethylene and not by chloride or phosphate, the two by-products that result from ethephon decomposition, the same experiment was performed using phosphate and chloride as the test compounds. Three biological replicates were tested with one technical replicate each.
Growth Kinetics
The effect of ethephon on the growth of agitated K. xylinus cultures was determined in 96-well plates that were inoculated, in triplicate, with K. xylinus starter cultures in a final volume of 200 μL of SH medium (pH 7) supplemented with 0.4% (v/v) cellulase. Three biological replicates and sterile controls, each with six technical replicates, were included for each treatment. Ethephon concentrations of 0.01, 0.1, and 1.0 mM, along with an untreated control plate that was supplemented with sterile ultra-pure water (pH 2.5), were all tested using separate plates. All unused wells contained sterile water. Two experiments were conducted; one in which ethephon or ultra-pure water (pH 2.5) was added only at the beginning of the experiment, while the second included the addition of ethephon or ultra-pure water (pH 2.5) every two days. All plates were sealed with Parafilm and incubated with agitation as described above. Optical density (OD) was recorded at 600 nm using a Bio-Rad xMarkTM Microplate Absorbance Spectrophotometer (Bio-Rad). Growth was followed for 335 h.
Pellicle Assays and Analysis
Komagataeibacter xylinus pellicles grown in the presence of ethephon were characterized to determine the effect of ethylene on BC production under static conditions. Pellicle assays were conducted in sterile 24-well plates using a final well volume of 2 mL of SH broth (pH 7). Wells were inoculated with K. xylinus starter cultures using three biological replicates, each with six technical replicates. Ethephon stock solution was added to obtain final ethephon concentrations of 0.01, 0.1, and 1.0 mM. Each plate contained a row of sterile control wells that consisted of only medium and ethephon to control for contamination. All plates were sealed with Parafilm and incubated statically for 7 days. Untreated control plates, as well as phosphate-chloride (0.01, 0.1, and 1.0 mM) control plates were run in parallel. Each treatment was run in its own plate to prevent crossover of ethylene. Additionally, control and ethephon-treated plates were spatially separated by growing them in different incubators.
The thickness of K. xylinus pellicles was measured without the removal of water at the time of harvest. All pellicles, aligned with a ruler, were photographed from the side and measured at three different positions using ImageJ software (Schneider et al., 2012). These values were averaged to get one value for each technical replicate, which were averaged to obtain one value for each biological replicate.
Pellicle water-holding capacity and BC yield was determined by measuring the wet weights and dry weights, respectively. Pellicle wet weights were determined by removing pellicles from the plates and then holding them on paper towel for three seconds to remove excess medium before weighing. BC yield was determined by treating the pellicles with 0.1 N NaOH at 80°C for 20 min to lyse cells. Pellicles were neutralized by shaking in ultra-pure water for 24 h with two water changes and then dried in microcentrifuge tubes at 50°C to constant weight before being weighed for pellicle dry weight. Pellicle hydration was calculated by subtracting the dry weight from the wet weight.
The crystallinity index, CI(IR), of untreated K. xylinus pellicles as well as those formed in the presence of 0.01, 0.1, and 1.0 mM ethephon and phosphate-chloride, were assessed using Fourier-transform infrared spectroscopy (FTIR). Pellicles from each treatment that had been NaOH-treated, washed and dried were used for FTIR analysis on a Perkin Elmer Precisely Spectrum 100 FTIR spectrometer with a horizontal attenuated total reflectance sampling accessory. For each treatment, three technical replicates for each of the three biological replicates were analyzed using 32 scans with a resolution of 4 cm-1 in the range of 4000 to 650 cm-1. Background correction was performed prior to collecting sample data. CI(IR) was calculated using A1437/A895 as previously described (Czaja et al., 2004).
Values for technical replicates of pellicles were averaged to get one value for each biological replicate, which was used for statistical analysis. The means of ethephon-treated cultures were normalized to the mean of the control cultures and presented as the percent of the control. A one-way ANOVA with a Tukey’s multiple comparison test was used. Differences were considered statistically significant if p < 0.05.
Colony Morphology
The effect of ethephon-derived ethylene on the morphology of K. xylinus colonies was assessed using an agar plate assay. Plates were made with 25 mL of SH agar (pH 7) and ethephon or phosphate-chloride were spread onto plates at concentrations of 0.01, 0.1, and 1.0 mM. Untreated and solvent control plates which consisted of no spreading and the spreading of ultra-pure water (pH 2.5), respectively, were also performed. Triplicate K. xylinus starter cultures were grown, washed and inoculated onto agar plates that were sealed with Parafilm and incubated statically for 5 days. Colonies were photographed with a USB 2.0 USB Digital Microscope (Plugable Technologies). All treatments included three biological replicates.
Reverse-transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
Growth Conditions
Komagataeibacter xylinus was grown in the presence of ethephon, ABA, and IAA to assess the effect these hormones had on the expression of genes involved with BC biosynthesis. For ethephon- and phosphate-chloride-treated cultures, triplicate K. xylinus starter cultures were grown, pooled, washed, and quantified. A 2 L Erlenmeyer flask, containing 1 L of SH broth (pH 5) with 0.2 % (v/v) cellulase was inoculated and incubated until an OD600 of 0.4-0.5 was reached. Cells were harvested by centrifugation, washed twice with room temperature SH broth (pH 7), and suspended in 1 L of SH broth (pH 7). This synchronized culture was then separated into 25 mL cultures, which were supplemented with 10 μM ethephon, 10 μM phosphate-chloride, or an equal volume of ultra-pure water (pH 2.5) for the control. These cultures were incubated for 24 h to allow for the decomposition of ethephon, and subsequent release of ethylene before being harvested. Flasks were covered with foil and tightly sealed with tape to trap released ethylene. Cultures treated with IAA and ABA were inoculated similarly to those treated with ethephon. After the OD600 reached 0.4-0.5, the culture was separated into triplicate 25 mL cultures and supplemented with IAA or ABA at a concentration of 10 μM. A control experiment consisting of DMSO-treated cultures was performed in parallel. Cultures were harvested 1 hour after being treated with hormone. At the time of harvest, for the ethephon, IAA, and ABA experiments, medium-free cell pellets were flash-frozen in liquid nitrogen and stored at -80oC until RNA extraction could be completed (no longer than 7 days). All treatments included three biological replicates.
RNA Purification, Quality Control, and First-strand cDNA Synthesis
Flash-frozen cell pellets (109 cells) were subject to total RNA extraction and purification using the Norgen Biotek RNA Purification Plus Kit following manufacturer instructions. Cells were not allowed to thaw prior to treatment with lysis buffer to ensure no change in mRNA levels occurred. Genomic DNA (gDNA) was removed by passing the crude RNA through a gDNA-removal column provided in the kit.
Quality control of RNA was completed on the same day of cDNA synthesis. RNA quality was determined by agarose gel electrophoresis. RNA concentration and purity was determined spectrophotometrically on a Cary 50 UV-Vis Spectrophotometer (Varian), by measuring the A260 and A260/A280 values, respectively. Only samples with A260/A280 values within 1.9-2.1 were used for cDNA synthesis. All samples were diluted to the same concentration using sterile diethylpyrocarbonate (DEPC)-treated water. In a reaction volume of 40 μL, 2 μg of RNA from each sample was converted to first-strand cDNA using the Bio-Rad iScript Select cDNA Synthesis Kit using random hexamer primers according to manufacturer instructions. Three samples were chosen at random from each experiment, and were subject to a mock-cDNA reaction that contained all components except reverse transcriptase, to assess RNA samples for gDNA contamination using RT-qPCR. All cDNA samples were stored at -20oC.
Primer Design
RT-qPCR primers were designed and validated for eight genes involved in K. xylinus BC production (bcsA, bcsB, bcsC, bcsD, cmcAx, ccpAx, bglAx, and crp/fnrKx; Table 1), and also two reference genes (23SrRNA and gyrB; Table 1). BlastN analysis comparing the nucleotide sequence of crp/fnrKh (GXY_00863) to the K. xylinus E25 genome sequence (accession: CP004360.1) revealed the presence of a crp/fnrKx gene (H845_3156) that is 79% similar (E = 4e-175, 97% query coverage) to crp/fnrKh. BlastX analysis showed that the encoded protein sequences have 81% identity (E = 1e-121, 94% query coverage). This high level of similarity led us to hypothesize that CRP/FNRKx may regulate BC biosynthesis in K. xylinus ATCC 53582, similar to the results of Deng et al. (2013) that showed CRP/FNRKh regulates BC biosynthesis in K. hansenii ATCC 23769. Since the K. xylinus ATCC 53582 genome sequence is not published, primers for 23SrRNA and gyrB were also designed using the nucleotide sequence of identical genes from other K. xylinus strains (Supplemental Table S1). End-point PCR was conducted using the crp/fnrKx, 23SrRNA and gyrB primer sets and K. xylinus ATCC 53582 gDNA as the template to ensure the expected amplicons were produced. Primer specificity was assessed empirically by end-point PCR using K. xylinus ATCC 53582 gDNA as the template and by RT-qPCR melt-curve analysis. All primer sets were designed using Primer3Plus (Untergasser et al., 2007) to be 20-27 base-pairs in length, have a GC content of 45-55%, a melting temperature (Tm) of 55-65°C, and to produce an amplicon of 90-300 base-pairs. Sequences were checked to be specific in silico using the Primer-Blast program1 to compare primer sequences to the genome sequences of various K. xylinus strains. The mfold web server2 was used to check primers and amplicons for potential secondary structures. Validated primer sequences are given (Supplemental Table S1).
RT-qPCR
The relative expression levels of these genes were determined after treatment with 10 μM ethephon, IAA, and ABA using the CFX Connect Real-Time PCR Detection System (Bio-Rad). RT-qPCR reactions (10 μL) included the SsoFastTM EvaGreen® Supermix (Bio-Rad), 300 or 500 nM primers (Supplemental Table S2), and 4 μL of the appropriate cDNA sample. Annealing temperature gradients were performed to empirically determine the optimal annealing temperature (Ta,opt.) for each primer set. The template for annealing temperature gradients consisted of aliquots of cDNA from each sample that were pooled and diluted 1/20 in 10 mM Tris-HCl (pH 8). The Ta,opt. was chosen as the annealing temperature that resulted in the lowest Ct value and caused specific amplification as shown by melt-curve analysis. Standard curves were done to ensure the PCR amplification efficiency of each primer set at their optimal annealing temperature was between 90 and 110% (Taylor et al., 2010). The same pooled and 1/20-diluted cDNA were subject to a minimum of four serial dilutions and used as the template for standard curves. The fold-dilution depended on the expression level of each gene, as determined from annealing temperature gradients. The cDNA template concentration used for expression analysis (Supplemental Table S2) was determined by the linear dynamic range (LDR) of each primer set, as determined from the standard curves. Samples to be compared were diluted to the middle range of the LDR of each primer set. Every run included a no template control (NTC) containing 10 mM Tris-HCl (pH 8) as the template to check for reagent contamination and a no reverse-transcriptase (NRT) control containing the pooled mock cDNA reaction as the template to check for gDNA contamination. The qPCR reaction conditions were as follows: 2 min at 95.0°C, 40 cycles of 95.0°C for 5 s, the Ta,opt. (Supplemental Table S2) for 15 s, and 72.0°C for 5 s. Melt-curve analysis was carried out at the end of every run with an initial denaturation at 95.0°C for 10 s, and then a temperature gradient from 65.0 to 95.0°C by steps of 0.5°C every 5 s. RT-qPCR plates (96-well) were loaded using the sample maximization strategy as previously described (Hellemans et al., 2007) by analyzing all samples with a particular gene on one plate. Each RT-qPCR was run in triplicate for three biological replicates per treatment.
RT-qPCR Quality Control, Data Analysis, and Statistics
Quality control of raw Ct values was completed after every RT-qPCR run. Technical replicates were excluded if the standard deviation of their Ct values was over 0.2, but the mean of at least two technical replicates for each sample was used for comparison. Data analysis was carried out using the ΔΔCt method (Livak and Schmittgen, 2001) employed in the Bio-Rad CFX Manager 3.1 Gene Study software using efficiency-corrected Ct values. Ct values from ethephon and phosphate-chloride RT-qPCR were made relative to Ct values obtained from the ultra-pure water (pH 2.5) control samples, while Ct values from IAA and ABA RT-qPCR were made relative to Ct values from their respective DMSO-treated controls. All RT-qPCR data was then normalized to the geometric mean of the efficiency-corrected expression data for the 23SrRNA and gyrB reference genes. The stability of the reference genes was assessed using the geNorm algorithm (Vandesompele et al., 2002) within the Bio-Rad CFX Manager 3.1 Gene Study software, which uses a pair-wise comparison approach to calculate a gene stability value (M). Reference genes are considered stable if the M value is below 0.5 (Hellemans et al., 2007). Expression differences between hormone-treated and control samples were tested for statistical significance in the Bio-Rad CFX Manager 3.1 Gene Study software using a two-tailed, unpaired t-test. Differences were considered statistically significant if p < 0.05.
Results
Decomposition of Ethephon into Ethylene Occurs in SH Medium (pH 7)
The production of ethylene from ethephon decomposition on SH medium (pH 7) was verified using the triple response assay. When grown in the presence of ethylene, dark-grown A. thaliana seedlings display reduced hypocotyl elongation, increased hypocotyl thickness and an exaggerated apical hook (Guzmán and Ecker, 1990). Growing seedlings in medium containing ACC, the precursor for ethylene biosynthesis in plants, allows for the production of ethylene and induction of the triple response phenotype. Ethephon decomposition was performed on SH agar (pH 7) in a sectored petri dish containing A. thaliana seeds on MS-sucrose agar. The hypocotyl thickness was increased and the apical hook was exaggerated for seedlings grown in the presence of ACC and ethephon compared to the untreated control (Figure 2A). The apical hook of seedlings grown in the presence of ethephon-derived ethylene was more exaggerated than those grown with ACC. In addition, the hypocotyl length of seedlings grown in the presence of ACC and ethephon were significantly (p < 0.0001) shorter than the untreated control (Figure 2B). This result confirms that ethephon decomposition occurs in SH medium (pH 7) and that sufficient ethylene is released to induce a biological response.
FIGURE 2. Ethylene is released via ethephon decomposition in SH medium (pH 7). Dark-grown Arabidopsis thaliana seedlings display the triple response phenotype (shorter and thicker hypocotyl with exaggerated apical hook) when grown in the presence of ACC and ethephon-derived ethylene compared to the untreated control (A). The hypocotyl length of seedlings grown in the presence of ACC and ethephon-derived ethylene were significantly shorter than the untreated control (B). Scale bar represents 1 mm. ∗∗∗∗p < 0.0001. Error bars show SD (n = 3).
K. xylinus Culture pH Allows for Ethephon Decomposition
Komagataeibacter xylinus is an acetic acid bacterium and is known to decrease the pH of its culture medium (Kawano et al., 2002b). Therefore, we performed a time-course pH analysis over 14 days to ensure the culture pH remained above 3.5 so that ethephon decomposition into ethylene would proceed unimpaired. The pH of all cultures decreased from pH 7 to approximately pH 5.5 for the first 7 days, wherein the pH increased back to pH 7 (Figure 3). Thus, acidification of the culture medium by K. xylinus would not have significantly affected ethephon decomposition and consequently, ethylene evolution. In addition, all cultures containing ethephon had a higher final pH than the untreated control culture (Supplementary Figure S2). The final pH of phosphate-chloride cultures were not different from the untreated control (data not shown), confirming the higher pH observed with ethephon treatment was due to the presence of ethylene.
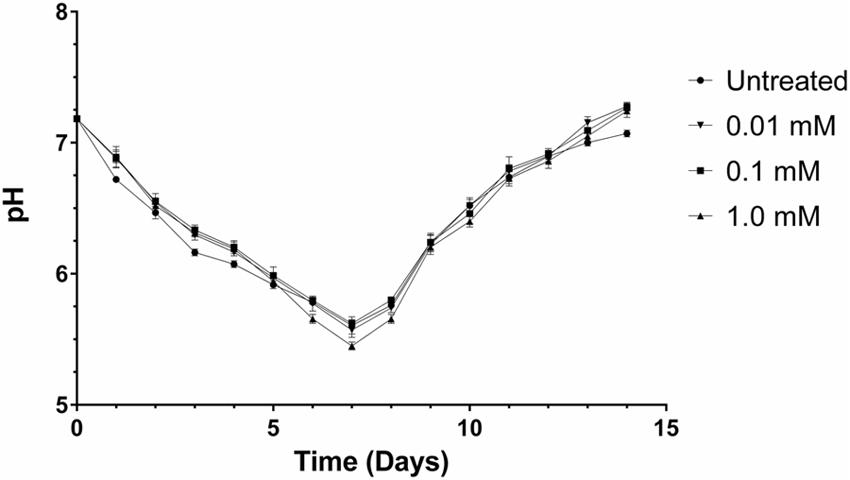
FIGURE 3. The pH of K. xylinus cultures stays above 3.5, allowing for efficient decomposition of ethephon into ethylene. K. xylinus was grown in SH medium (pH 7) that was supplemented with ethephon and 0.2% (v/v) cellulase. The change in culture pH was monitored for 14 days. Note that the y-axis begins at pH 5, and that the final pH of all ethephon-treated cultures is higher than the untreated control. Error bars show SD (n = 3).
Ethylene is Relatively Non-toxic to K. xylinus
Minimum inhibitory concentration assays were performed to assess whether ethephon inhibited the growth of K. xylinus. Ethephon concentrations of 0.195 to 100 mM were tested in SH (pH 7). Concentrations equal to or greater than 50 mM inhibited growth of K. xylinus (data not shown). This effect was not observed when chloride and phosphate were tested at the same concentration, suggesting that ethephon-derived ethylene was responsible for K. xylinus growth inhibition. Growth inhibition was observed for very high, physiologically irrelevant levels of chloride-phosphate. In this case, we cannot rule out the possibility that the observed growth inhibition in the 100 mM chloride-phosphate treatment may be due to the Na+ counter-ion. Since NaCl and NaH2PO4⋅H2O were both added at 100 mM, the [Na+] would have been 200 mM, which could have prevented the growth of K. xylinus due to osmolarity effects.
Ethylene does not Affect the Growth of K. xylinus in Agitated Broth Cultures
The effect of ethephon-derived ethylene on the growth of agitated K. xylinus broth cultures grown in SH medium (pH 7) was investigated. Since ethylene is a gas and the 96-well plates had to be opened to read the optical density, some ethylene was inevitably lost during this process. To determine if this loss was a factor, we either added ethephon only on the day of inoculation or on the day of inoculation and every two days thereafter. When compared to controls, no significant difference in growth was observed when ethephon was added to cultures at the time of inoculation (Supplementary Figure S3A) or when ethephon was added every 2 days (Supplementary Figure S3B). The same result was obtained when comparing the ethephon-treated cultures to phosphate-chloride controls. Therefore, ethylene does not influence K. xylinus growth in agitated culture.
Ethylene Increases BC Yield and Decreases K. xylinus Pellicle Hydration Due to an Increase in Crystallinity
Pellicles formed by K. xylinus grown in the presence of ethephon were weighed at the time of harvest without the removal of water and after drying to assess their hydration. The wet weight of pellicles was affected by ethephon when cultured in SH medium at pH 7 (Figure 4A). The wet weight of pellicles produced in the presence of ethephon was decreased by 10% with 0.01 mM ethephon, 14% in the presence of 0.1 mM ethephon, and 9% with 1.0 mM ethephon, in comparison to the untreated control. The relationship between ethephon concentration and wet weight was not linear, and differences between ethephon treatments were not significant. Ethephon did not influence the thickness of K. xylinus pellicles (data not shown).
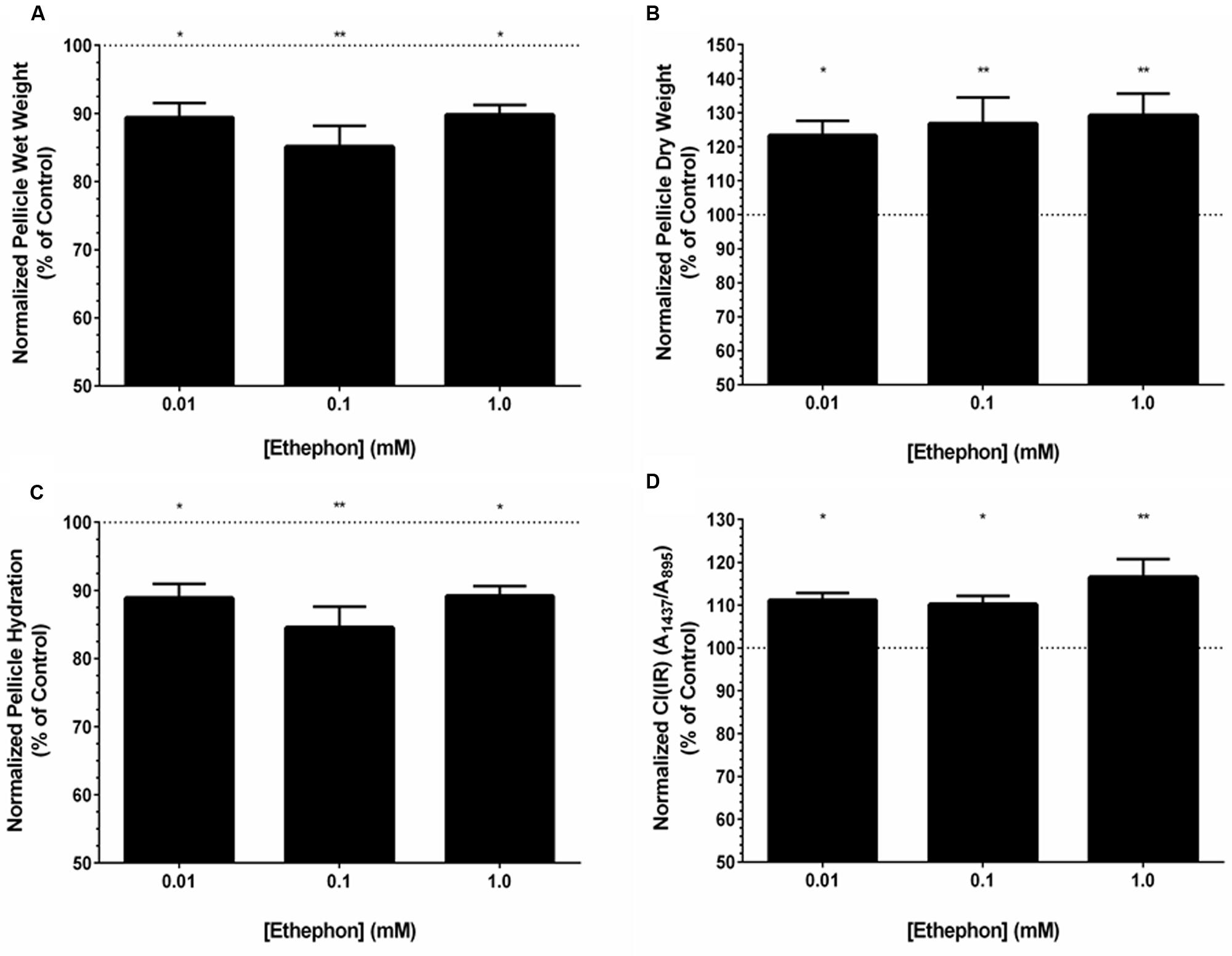
FIGURE 4. Ethephon-derived ethylene influences the properties and yield of K. xylinus BC pellicles. Cultures were grown statically in SH broth (pH 7) in 24-well plates, and incubated at 30°C for 7 days before pellicles were harvested and analyzed. Ethephon-derived ethylene decreases the wet weight (A), increases the dry weight (B), reduces pellicle hydration (C), and increases the crystallinity (D) of K. xylinus pellicles compared to the untreated control. The different ethephon treatments were not significantly different from each other. Data was normalized to and expressed as percent of the untreated control. Note the y-axis begins at 50%. Error bars show SD (n = 3). ∗p < 0.05; ∗∗p < 0.01.
Similar to the wet weights, pellicle dry weight was affected by ethephon. All concentrations of ethephon resulted in a significant increase in pellicle dry weight (Figure 4B). Dry weights increased linearly with ethephon concentration in a dose-dependent manner, although differences between ethephon treatments were not statistically significant. In comparison to the untreated control, a 23% increase in BC yield was observed when K. xylinus was grown in the presence of 0.01 mM ethephon, while a 27 and 29% increase was observed after ethephon treatment at concentrations of 0.1 and 1.0 mM, respectively.
Ethephon significantly decreased pellicle hydration compared to the untreated control (Figure 4C). We hypothesized that the decrease in pellicle hydration was related to differences in crystallinity. To test this, the crystallinity index, CI(IR), of K. xylinus pellicles produced in the presence of ethephon was determined. Compared to the untreated control, pellicles grown in the presence of all concentrations of ethephon had a higher crystallinity (Figure 4D). The CI(IR) of untreated pellicles was 0.62, while pellicles grown with ethephon treatments of 0.01, 0.1, and 1.0 mM had a significantly increased CI(IR) of 0.69, 0.68, and 0.73, respectively. Therefore pellicle crystallinity increased by 11, 10, and 16% when synthesized in the presence of 0.01, 0.1, and 1.0 mM ethephon, respectively (Figure 4D). This is consistent with the observed decrease in pellicle hydration after ethephon treatment. In the presence of ethephon, the pellicles were more crystalline and therefore less able to retain water. Control experiments with chloride and phosphate yielded results comparable to untreated cultures, indicating the observed phenotypes were caused by ethylene.
Ethylene Increases K. xylinus BC Production on Solid Medium
In order to assess how ethylene affected K. xylinus colony morphology and BC production on solid medium, agar plates were pre-treated with ethephon, acidified ultra-pure water (pH 2.5), or phosphate-chloride, then streaked for isolated colonies. Figure 5 shows representative colonies from the various treatments. Colonies formed under control conditions (untreated) were about 1 mm in diameter, convex in elevation, irregular in form, and slightly orange in color (Figure 5A). All treatments, including the acidified water (Figure 5B) and phosphate-chloride (Figures 5C-E) controls influenced BC production of agar-grown cultures. Colonies grown on untreated plates produced some BC, as shown by the hazy material surrounding the central part of the colony (Figure 5A). Interestingly, when plates were pre-treated with acidified ultra-pure water (Figure 5B) or phosphate-chloride (Figures 5C-E), K. xylinus colonies produced minimal BC. Colonies grown in the presence of ethephon (Figures 5F-H) produced more BC than all of the controls, with the largest increase observed for the 0.01 mM ethephon treatment (Figure 5F). Colonies grown on the phosphate-chloride control plates were similar to those grown with acidified ultra-pure water, demonstrating that the ethephon-induced BC over-production phenotype was caused by ethylene. This result is reinforced by the increased BC yield observed for statically grown liquid cultures exposed to ethylene (Figure 4B).
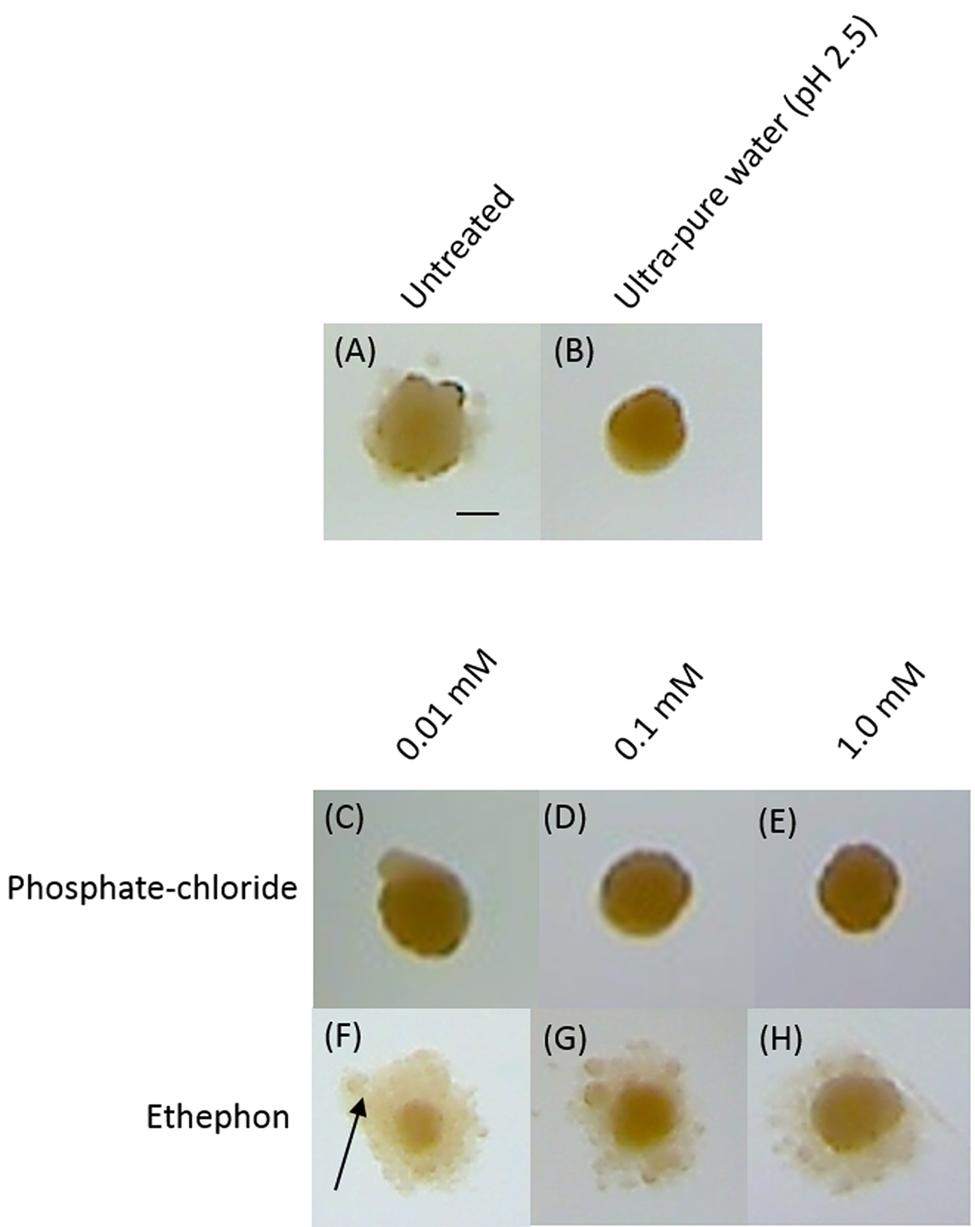
FIGURE 5. Ethephon enhances K. xylinus BC production when grown on solid medium. K. xylinus was streaked onto SH agar plates (pH 7) that were untreated (A), or pre-treated with ultra-pure water (pH 2.5); (B) phosphate and chloride (C-E) or ethephon (F-H). Plates were incubated at 30°C for 7 days. Representative colonies are shown. The arrow points to BC, seen as the hazy substance surrounding the colony. Scale bar represents 0.5 mm.
Ethylene and IAA Cause Differential Expression of bcs Operon Genes
All primer sets produced amplification efficiencies between 90–110%, as shown by the standard curves (Supplemental Figures S4–S13; Supplemental Table S2), indicating all assays were robust. The expression of the bcs operon genes (Figure 1B), bcsA, bcsB, bcsC, and bcsD, encoding proteins that form the BC synthase complex responsible for BC synthesis (Table 1; Figure 1A), were analyzed from K. xylinus cultures grown in the presence of 10 μM ethephon, 10 μM IAA, and 10 μM ABA. When cultures were treated with ethephon, bcsA, and bcsB were up-regulated by 34% (p < 0.001) and 16% (p < 0.05), respectively, compared to the untreated control (Figure 6A). Interestingly, bcsC and bcsD were not affected. The phosphate-chloride control treatment caused no differences in the expression of the bcs operon genes (data not shown), supporting the conclusion that the observed differential expression of these genes was caused by ethephon-derived ethylene. IAA treatment down-regulated bcsA by 23% (p < 0.01) compared to the untreated control, while bcsB, bcsC, and bcsD were not affected (Figure 6A). The expression of all bcs operon genes were unaffected by ABA treatment (Figure 6A). Ethylene and IAA therefore uniquely cause differential expression of the genes within the K. xylinus bcs operon. Ethylene up-regulates bcsA and bcsB which is consistent with the increase in BC yield (Figures 4B and 5F-H). IAA down regulates bcsA while ABA has no effect on the expression of bcs genes which is consistent with our previous observation that IAA directly decreases BC production while ABA had an indirect effect on BC yield in K. xylinus (Qureshi et al., 2013).
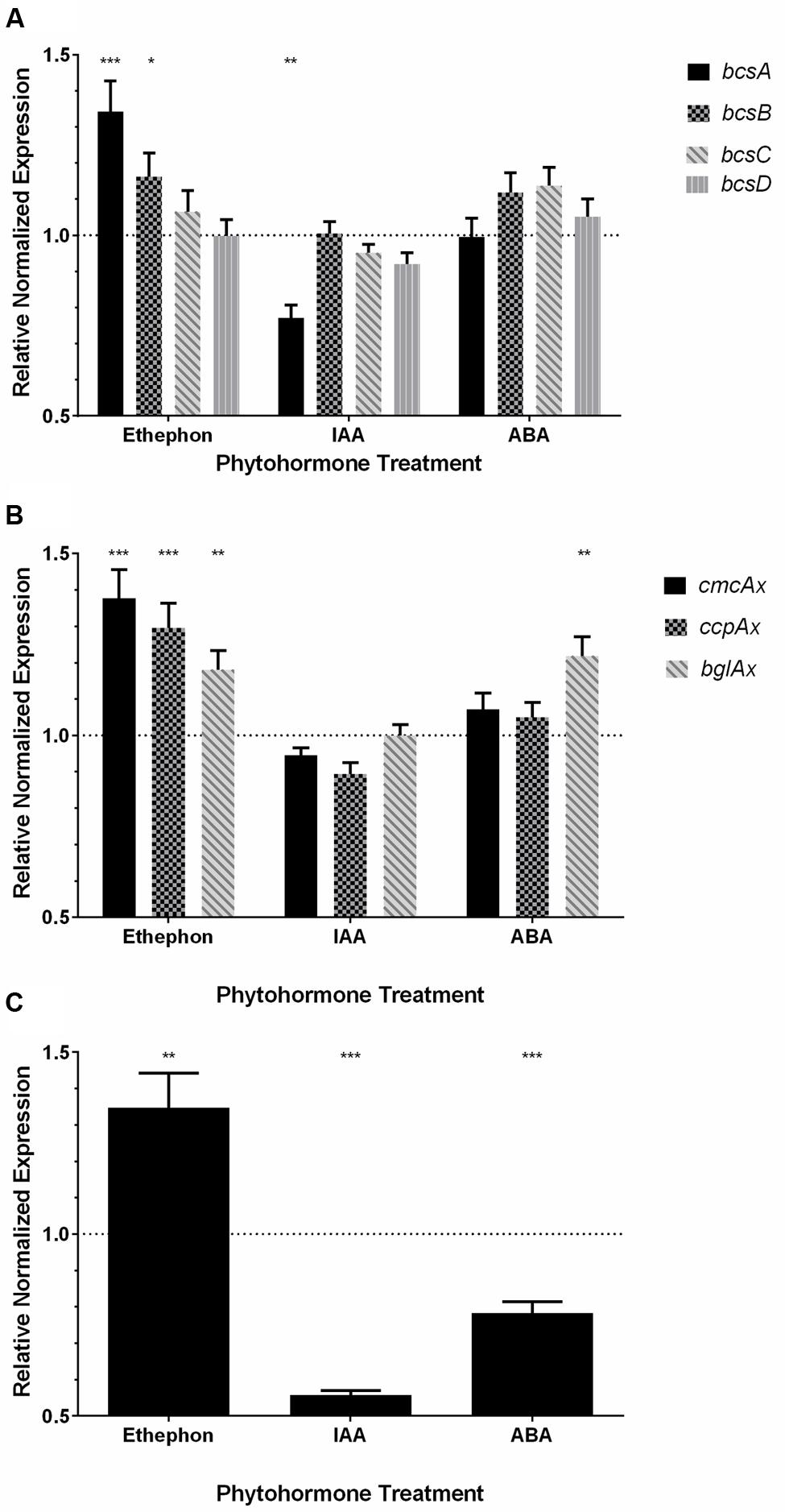
FIGURE 6. The expression of genes involved in K. xylinus BC biosynthesis are regulated by phytohormones. Ethephon-derived ethylene and IAA induce differential expression of the genes within the bcs operon (A). Ethephon-derived ethylene and ABA influence the expression of genes flanking the K. xylinus bcs operon (B). Ethephon-derived ethylene, IAA and ABA regulate the expression of crp/fnrKx (C). Gene expression was quantified using RT-qPCR after treatment with 10 μM ethephon, 10 μM IAA, or 10 μM ABA. Expression values were made relative to the respective untreated controls and normalized using the expression values of reference genes, 23SrRNA and gyrB. The dotted line indicates the relative normalized expression value for the untreated control. Error bars show the SD (n = 3). ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
Ethylene and ABA Affect the Expression of Genes Flanking the bcs Operon
Three other genes known to be involved in K. xylinus BC biosynthesis were also analyzed via RT-qPCR. The ccpAx and cmcAx genes form an operon upstream, while bglAx is located downstream of the bcs operon (Figure 1B; Table 1). Ethephon-derived ethylene caused significant up-regulation of ccpAx (30%, p < 0.001), cmcAx (38%, p < 0.001) and bglAx (18%, p < 0.01) compared to the untreated control (Figure 6B). The expression of bglAx was significantly up-regulated 22% (p < 0.01) compared to the control after treatment with ABA, while IAA treatment had no effect on these genes (Figure 6B).
The CRP/FNRKx Transcription Factor Gene is Hormonally Regulated
The expression of the crp/fnrKx gene (H845_3156), bioinformatically identified in the genome of K. xylinus E25, was assessed by RT-qPCR to determine if it is regulated by ethylene, IAA, or ABA. This gene was studied since it has high similarity to a crp/fnrKh gene in K. hansenii ATCC 23769 (GXY_00863) that has recently been shown to be essential for BC biosynthesis (Deng et al., 2013). RT-qPCR primers were designed based on the nucleotide sequence of a crp/fnrKx gene (H845_3156) from the K. xylinus E25 genome. Primers were validated using K. xylinus ATCC 53582 gDNA as the template (data not shown). The expression of crp/fnrKx is regulated by ethylene, IAA, and ABA (Figure 6C). Compared to the untreated controls, ethephon-derived ethylene significantly up-regulated crp/fnrKx by 35% (p < 0.01), while IAA and ABA down-regulated its expression by 45% (p < 0.001) and 22% (p < 0.001), respectively. Taken together, these results indicate that crp/fnrKx is phytohormonally regulated. Ethylene directly increases BC production (Figures 4B and 5F-H) and up-regulates crp/fnrKx expression (Figure 6C), while IAA directly decreases BC production (Qureshi et al., 2013) and down-regulates crp/fnrKx expression (Figure 6C). This suggests that like CRP/FNRKh in K. hansenii (Deng et al., 2013), CRP/FNRKx may directly regulate BC biosynthesis in K. xylinus.
Discussion
Ethephon, an ethylene-releasing compound, was used to investigate the effect of ethylene on K. xylinus ATCC 53582 growth, BC production, pellicle properties and gene expression. K. xylinus is a plant-associated carposphere bacterium (Park et al., 2003; Dellaglio et al., 2005; Jahan et al., 2012; Neera et al., 2015). This close association with fruit exposes K. xylinus to a plethora of plant-derived compounds, including phytohormones that regulate plant growth and development when present at low concentrations (Davies, 2010). Ethylene is the main ripening hormone in climacteric fruit and is released in high concentrations during the ripening stage (Pech et al., 2008; McAtee et al., 2013). In non-climacteric fruit, ABA is believed to be the most important ripening hormone (Jia et al., 2011; Li et al., 2011; McAtee et al., 2013). However, recent data has shown that even non-climacteric fruit, such as strawberry, grapes, and citrus respond to ethylene and experience a spike in ethylene production during ripening, though the magnitude of response and production is cultivar-dependent (Chervin et al., 2004; Trainotti et al., 2005; Iannetta et al., 2006; Paul et al., 2012). Nevertheless, K. xylinus would be exposed to exogenous ethylene when it inhabits both climacteric and non-climacteric fruit in the environment.
The ripening stage of fruit development is characterized by modulation of endogenous hormone levels (McAtee et al., 2013). Numerous metabolic changes occur that result in the fruit being sweeter, due to starch hydrolysis by amylase (Agravante et al., 1990) and softer, due to the activity of pectinase and cellulase enzymes that degrade the fruit cell wall (Ahmed and Labavitch, 1980; Sitrit and Bennett, 1998; Lohani et al., 2004). Due to the intense turgor pressure in plant cells, weakening of the cell wall results in the release of exudate onto the fruit surface (Brummell and Harpster, 2001; Brummell, 2006), providing an attractive nutrient environment for microorganisms. The levels of ethylene are typically low during fruit growth and maturation, but spike at the onset of ripening (McAtee et al., 2013). Ethylene, due to its positive role in fruit ripening, would therefore be a signal indicating an ideal nutrient environment for K. xylinus as fruit at this developmental stage contain high levels of soluble monosaccharides that can be used for growth and BC production.
Investigations using ethephon in plants have shown ethylene-dependent phenotypes even though the dosage and magnitude of ethephon decomposition was not precisely controlled (Zhang and Wen, 2010; Zhang et al., 2010). In this study, ethephon was used as a convenient vehicle to produce in situ ethylene when added to K. xylinus cultures growing in SH medium. Ethephon decomposition is linear, in that the amount of ethylene produced is proportional to the amount of ethephon used (Klein et al., 1979; Zhang and Wen, 2010). The half-life in aqueous medium at 30°C is 5.1 ± 0.9 h at pH 7 and 6.0 ± 0.9 h at pH 6 (Klein et al., 1979). Biddle et al. (1976) demonstrated that ethephon decomposition below pH 5 is extremely slow, while the rate is greatly increased at pH 7. The A. thaliana triple response assay verified that ethephon decomposition and ethylene production occurred in SH medium (pH 7) and time-course pH analysis showed that the decrease in culture pH would not have hampered this process. Ethephon decomposition results in the production of chloride and phosphate ions in addition to the desired ethylene. Therefore, control experiments using chloride and phosphate were completed alongside the ethephon experiments. In all cases, the effects of phosphate and chloride were insignificant, supporting our conclusion that the observed results were due to ethylene.
Minimum inhibitory concentration assays were performed to ensure ethephon was not toxic to K. xylinus. Ethephon concentrations of 50 and 100 mM prevented K. xylinus growth. Control assays revealed that the bacterium could grow when chloride and phosphate concentrations were at 50 mM but not 100 mM, suggesting that ethylene was responsible for K. xylinus growth inhibition when the medium was supplemented with 50 mM ethephon. To our knowledge, this is the first time ethephon has been used to treat bacteria with ethylene. However, there are numerous literature reports that describe treating plants with millimolar concentrations of ethephon (Denney and Martin, 1994; Khan, 2004; Kong et al., 2009; Dhillon and Mahajan, 2011; Wang et al., 2013). Burg and Burg (1962) showed that ethylene production levels in fruit vary depending on the cultivar. The internal ethylene concentration of various fruit varieties is in the micromolar range, with the exception of apples and passion fruit, which produce ethylene in the high mircomolar to low millimolar range. Furthermore, Wheeler et al. (2004) demonstrated that the peak ethylene concentration in ripening tomatoes is approximately 10 μM. Based on this, an ethylene concentration of 50 mM is not physiologically relevant, and therefore the non-toxic ethephon-derived ethylene concentrations of 0.01, 0.1, and 1.0 mM were utilized in our study.
Ethylene reduced pellicle hydration by increasing pellicle crystallinity, suggesting a higher degree of hydrogen bonding between adjacent glucan chains that resulted in a reduced ability to hydrogen bond to water. Qureshi et al. (2013) showed that exogenous IAA, ABA, GA3, and zeatin influenced the hydration and crystallinity of K. xylinus pellicles in a concentration-dependant manner. In contrast, all concentrations of ethylene decreased hydration and increased the crystallinty of the pellicles in this study. Increased crystallinity enhances the recalcitrance of BC, which provides a survival advantage to K. xylinus in the environment since it would be less susceptible to cellulase-producing microorganisms.
An increase in BC yield by K. xylinus is a direct result of exposure to ethylene in both liquid and solid media; growth is not affected. We observed an increase in BC production after ethylene treatment, further supporting the conclusion that it has a direct effect on BC biosynthesis. This is in contrast to ABA, zeatin, and GA3 which indirectly enhance BC production due to an increased growth rate (Qureshi et al., 2013). Hu and Catchmark (2010) investigated the effect of 1-methylcyclopropene (1-MCP), a known inhibitor of ethylene-dependant responses in plants (Tassoni et al., 2006), on the growth and BC production of K. xylinus. This compound is thought to antagonistically bind ethylene receptors and blocks the effects of ethylene in plants (Sisler, 1991). Though an ethylene receptor has not been reported for K. xylinus, Hu and Catchmark (2010) showed that 1-MCP decreased K. xylinus growth rate and increased BC yield, indicating that similar to ethylene, 1-MCP has a direct effect on BC biosynthesis. This suggests that an ethylene receptor exists in K. xylinus and that ethylene and 1-MCP bind the same receptor since they induce similar phenotypes. This is in contrast to their effects on fruit, since treatment with 1-MCP inhibits ethylene-dependent ripening phenotypes (Tassoni et al., 2006). The direct target of ethylene and 1-MCP in K. xylinus has not yet been identified.
The analysis of pH changes over the course of K. xylinus growth revealed that the final culture pH is higher after treatment with ethylene. We observed a decrease in pH during exponential growth and then an increase in pH during stationary growth phase. This was also observed by Kawano et al. (2002b), who correlated this trend in pH alteration to glucose oxidation into gluconic acid, and subsequent resorption of gluconic acid. They also showed that unlike K. hansenii ATCC 23769, K. xylinus ATCC 53582 is able to synthesize BC after glucose depletion by using gluconic acid as carbon source. The higher final pH and increased production of BC observed in this study may therefore be attributed in part to increased gluconic acid metabolism in the presence of ethylene. However, this requires further investigation.
We report for the first time, the differential expression of genes within the K. xylinus bcs operon. This operon encodes proteins that comprise the complex responsible for BC production in K. xylinus. The bcs operon in K. xylinus encodes four genes (bcsABCD) which are believed to form a polycistronic mRNA. However, little is known about how the bcs operon is regulated, or how relative gene expression is affected by external signals. Ethylene up-regulated the expression of bcsA and bcsB, which encode enzymes responsible for BC biosynthesis, but not bcsC and bcsD, which encode enzymes for BC export and crystallization. Therefore, an increase in BC production only requires additional synthesis proteins (BcsA and BcsB), but not increased levels of export/crystallization proteins (BcsC and BcsD). Similarly, IAA down-regulated bcsA but did not affect the other bcs genes. Since bcsA encodes the BC synthase (BcsA) that is responsible for synthesis of BC, it follows that its down-regulation results in decreased BC production as observed with IAA, and that its up-regulation results in increased BC production as observed with ethylene. This data indicates that ethylene and IAA directly influence BC biosynthesis at a transcriptional level. In order to increase BC biosynthesis, both bcsA and bcsB must be up-regulated, since functional BcsA is stabilized by BcsB in the periplasm (Morgan et al., 2013). However, decreasing BC biosynthesis only requires the down-regulation of bcsA, since its protein product directly synthesizes BC. It is possible that K. xylinus preserves cellular energy by maintaining constitutive levels of bcsB transcription so that the transcript is readily available for translation into BcsB once bcsA repression is relieved.
The differential relative expression of genes within operons is especially useful for regulating encoded proteins that serve different functions, such as the protein products of the bcs operon genes. BcsA synthesizes BC, BcsB chaperones BC chains through the periplasm, BcsC facilitates export of BC into the extracellular environment and BcsD is responsible for crystallization of BC. In some cases, differential relative expression of genes within an operon can be attributed to different translation efficiencies of operon-encoded mRNA due to variations in ribosome-binding sites (Vellanoweth, 1993). Inhibition of individual genes in a polycistronic mRNA by anti-sense RNA causes differential relative gene expression of the E. coli galactose operon (Møller et al., 2002) and various other operons, including those regulated by excludons (Sesto et al., 2013; Lasa and Villanueva, 2014). Lastly, polycistronic mRNA can undergo RNase cleavage and produce individual mRNAs that differ in stability, leading to differential protein expression. For example, the genes within the Escherichia coli atp operon that encode proteins that make up the ATP synthase (Gay and Walker, 1981) are differentially regulated (McCarthy et al., 1991). Like the BC synthesis complex, the ATP synthase is made up of numerous protein subunits and is embedded in the cell membrane of Gram-negative bacteria. The differential expression of the atpIBEFHAGDC operon genes has been attributed to segmental differences in mRNA stability (McCarthy et al., 1991; Lagoni et al., 1993); the mRNA of the first two genes of the operon are rapidly degraded by RNase enzymes (Ziemke and McCarthy, 1992), while the remaining seven genes are more stable (McCarthy et al., 1991). In addition, the translational efficiencies of mRNA from the atp genes vary (Brusilow et al., 1982) so that the differential expression of the operon is controlled at two post-transcriptional levels. This type of regulation has also been observed for the malEFG operon in E. coli (Newbury et al., 1987), the gap-pgk operon of Zymomonas mobilis (Eddy et al., 1991; Burchhardt et al., 1993) and Clostridium acetobutylicum (Schreiber and Dürre, 2000), the dnaK operon of B. subtilis (Homuth et al., 1999), the puf operon in Rhodobacter capsulatus (Belasco et al., 1985; Klug et al., 1987) and the uropathogenic E. coli pap operon (Båga et al., 1988; Nilsson et al., 1996). It is therefore possible that the bcs operon in K. xylinus is differentially regulated through one of these mechanisms.
The deletion of ccpAx in K. hansenii ATCC 23769 significantly decreased levels of BcsB and BcsC, but not BcsA, which are controlled by the same promoter (Deng et al., 2013). The expression of bcsD is regulated by a different promoter (Deng et al., 2013) supporting the notion that differential expression of the genes that make up the BC synthesis complex does occur. Therefore, it is possible that in K. hansenii, ccpAx plays a role in regulating differential relative expression of the bcsABC operon leading to the differential protein expression observed in the study by Deng et al. (2013).
Based on the results of the current study, as well as data provided by Qureshi et al. (2013), we propose a model for the phytohormone-mediated fruit-bacteria interactions of K. xylinus (Figure 7). On unripe fruit (Figure 7A), K. xylinus is exposed to high concentrations of IAA, zeatin (Z), and GA3. As a fruit ripening inhibitor (Frenkel and Dyck, 1973; Davies et al., 1997; Symons et al., 2012; Ziliotto et al., 2012), IAA directly inhibits energetically costly BC biosynthesis by down-regulating bcsA, since carbon source is limited on unripe fruit. Exogenous IAA, zeatin and GA3 increase K. xylinus growth, enhancing bacterial production of zeatin and GA3. These two hormones increase fruit size by controlling cytokinesis (zeatin) and cell enlargement (GA3; McAtee et al., 2013). Previous studies showed that rhizosphere bacteria that produce endogenous zeatin and GA3 can enhance plant growth (Bottini et al., 2004; Arkhipova et al., 2005) and that application of GA3 increases the size of grape berries (Casanova et al., 2009) and tomatoes (Serrani et al., 2007). Therefore, we postulate that endogenous production of zeatin and GA3 by K. xylinus may contribute to the pool of endogenous hormone levels in the fruit so that there is more biomass to colonize once ripening begins. Endogenous production of ABA is also increased as a result of enhanced cell growth. Exposing fruit to exogenous ABA increases endogenous ABA levels in fruit tissues, triggers ethylene biosynthesis and induces ripening (Zhang et al., 2009; McAtee et al., 2013; Leng et al., 2014). ABA production by K. xylinus may therefore play a role in triggering ripening, resulting in a preferred growth environment compared to unripe fruit. Furthermore, IAA-, zeatin-, and GA3-mediated growth enhancement ensures that cell density is at its peak when ripening begins. On ripe fruit (Figure 7B), K. xylinus would be exposed to high concentrations of ABA and ethylene (McAtee et al., 2013). Fruit-produced ABA increases K. xylinus growth (Qureshi et al., 2013), increasing bacterial production of ABA, which in turn up-regulates plant-produced ethylene and accelerates ripening (Zhang et al., 2009). Our hypothesis that ABA does not directly influence BC biosynthesis is supported by the observation that it did not affect the expression of genes within the bcs operon. In contrast, exogenous ethylene directly enhances BC biosynthesis in K. xylinus by up-regulating the expression of bcsA and bcsB. Therefore, ABA and ethylene act together as environmental signals to promote colonization of ripe fruit by K. xylinus through increased cell growth and direct enhancement of BC biosynthesis. Over production of BC provides a competitive advantage to K. xylinus as shown by apple colonization studies (Williams and Cannon, 1989). Enhancement of BC production and crystallization by ethylene therefore increases the environmental fitness of K. xylinus within the carposphere.
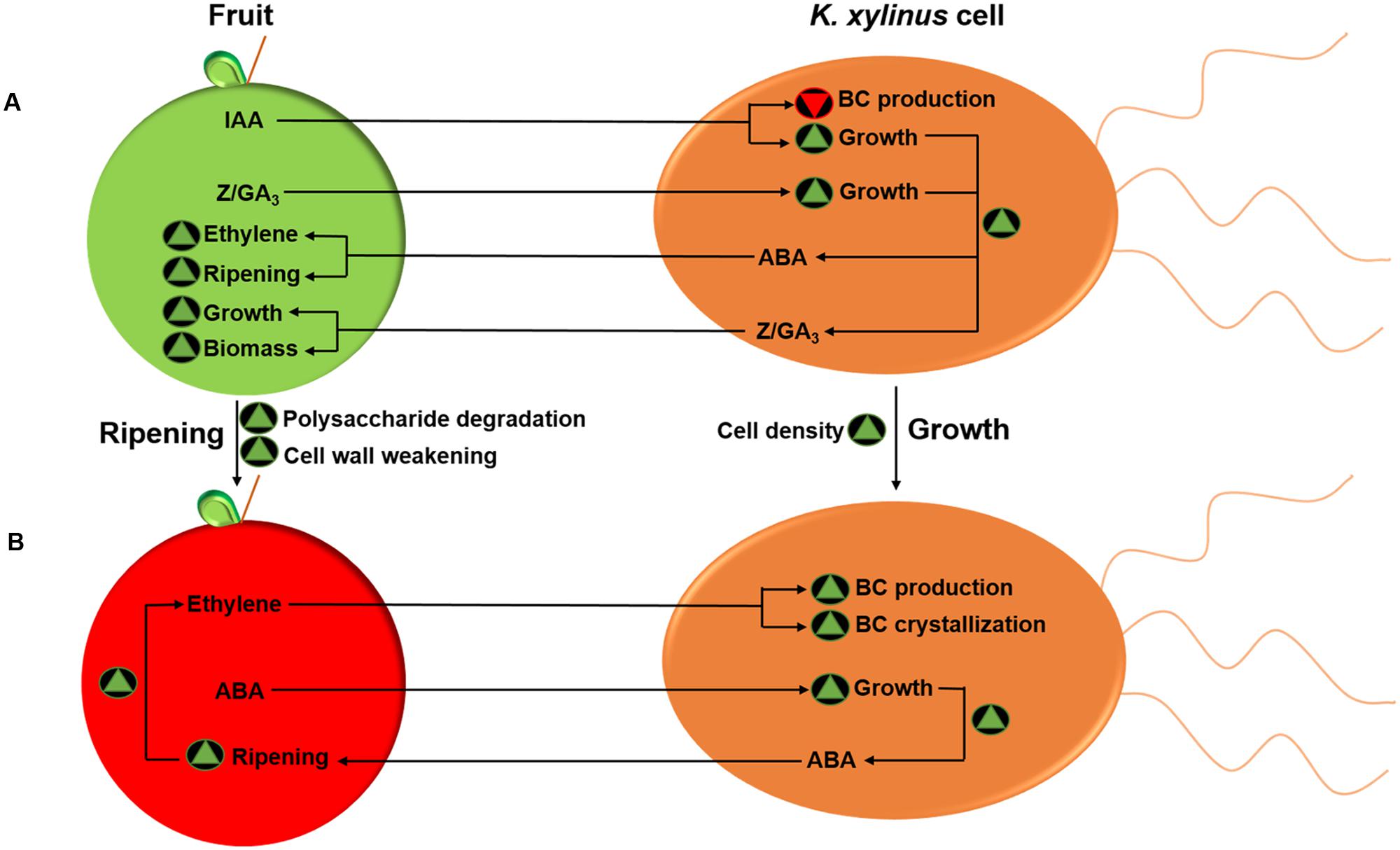
FIGURE 7. Model for the phytohormone-mediated fruit-bacteria interactions of K. xylinus. Unripe fruit (A) contain high concentrations of IAA, zeatin (Z) and GA3. IAA decreases K. xylinus BC production, while IAA, zeatin, and GA3 increase bacterial cell growth enhancing endogenous production of ABA, zeatin, and GA3 by K. xylinus. These hormones increase fruit size and induce ripening, characterized by degradation of polysaccharides and weakening of the fruit cell wall. On ripe fruit (B), plant-produced ethylene up-regulates biosynthesis and crystallization of BC. Exogenous ABA increases K. xylinus growth, allowing it to accelerate the fruit ripening process, which facilitates colonization. Green triangles and inverted red triangles indicate a process is enhanced or repressed, respectively. Only direct effects are shown.
Ethylene also up-regulated cmcAx and ccpAx which form their own operon upstream of the bcs operon (Kawano et al., 2002b; Sunagawa et al., 2013). CcpAx is essential for BC biosynthesis (Standal et al., 1994) and is believed to be involved in crystallizing BC due to its localization with BcsD (Sunagawa et al., 2013). The precise function and transcriptional regulation of CcpAx is not known. Similarly, the exact function of CmcAx is not known. However, it is required for BC biosynthesis as CmcAx inhibition by antibodies results in decreased BC production (Koo et al., 1998) and addition of minute amounts of CmcAx, or its overexpression increases BC production (Tonouchi et al., 1995; Kawano et al., 2002a, 2008). CmcAx is believed to play a role in editing distorted glucan chains since cmcAx deletion results in the formation of highly twisted ribbons (Nakai et al., 2013). Expression of cmcAx is induced by gentiobiose produced by BglAx (Kawano et al., 2008). The expression of bglAx is induced by the CRP/FNRKh transcription factor in K. hansenii ATCC 23769 (Deng et al., 2013). We demonstrated that ethylene and ABA up-regulate the expression of bglAx. The ethylene-dependant up-regulation of all three genes may be required to cope with the enhancement of BC biosynthesis. In addition, up-regulation of cmcAx and bglAx may lead to increased levels of secreted CmcAx and BglAx which have cellulose-hydrolyzing activity (Tahara et al., 1998; Tajima et al., 2001; Kawano et al., 2002b). These proteins may aid in the degradation of plant cellulose in order to weaken the fruit cell wall and provide more glucose for BC production.
The CRP/FNR family of transcription factors regulate the expression of genes critical to bacterial growth and survival in response to changing environmental conditions (Körner et al., 2003). Through a bioinformatics approach, we identified a novel phytohormone-regulated CRP/FNR transcription factor (CRP/FNRKx) in K. xylinus ATCC 53582. Ethylene up-regulated crp/fnrKx expression and increased BC production. IAA down-regulated crp/fnrKx expression and decreased BC production. Together, these results suggest that CRP/FNRKx directly regulates BC biosynthesis in K. xylinus at a transcriptional level, similar to how CRP/FNRKh regulates BC biosynthesis in K. hansenii (Deng et al., 2013). Positive regulation of biofilm formation by CRP/FNR family protein, CRP, has also been observed in E. coli (Jackson et al., 2002) and Shewanella oneidensis (Thormann et al., 2005) when bound to cAMP. In contrast, CRP-cAMP negatively regulates transcription of genes involved in the biosynthesis of Vibrio polysaccharide (VPS) in Vibrio cholerae (Fong and Yildiz, 2008). While CRP/FNRKx positively regulates BC production in K. xylinus, ABA down-regulates crp/fnrKx but does not decrease BC yield (Qureshi et al., 2013). Since this study focused on a small subset of genes, it is likely that ABA regulates a yet to be identified gene whose protein product counteracts the down-regulation of crp/fnrkx underscoring the complexity of the regulatory pathway involved in controlling BC synthesis.
The CRP/FNR family transcription factor, Bcam1349, binds c-di-GMP and regulates biofilm formation by enhancing the production of BC and curli fimbriae in Burkholderia cenocepacia (Fazli et al., 2011). Binding of c-di-GMP enhanced the ability of Bcam1349 to bind the promoter region and increase the expression of bcs operon genes (Fazli et al., 2011) along with the Bcam1330-Bcam1341 gene cluster involved in exopolysaccharide production (Fazli et al., 2013). Interestingly, various diguanylate cyclases and phosphodiesterases involved with c-di-GMP metabolism are regulated by the CRP/FNR family protein, CRP, in V. cholerae (Fong and Yildiz, 2008). Whether CRP/FNRKx binds c-di-GMP, or regulates the expression of diguanylate cyclases and phosphodiesterases remains to be investigated.
CRP/FNRKh regulates BC biosynthesis in K. hansenii ATCC 23769 by positively regulating the expression of bglAx and other genes required for BC production that have yet to be identified (Deng et al., 2013). Ethylene up-regulates both crp/fnrKx and bglAx. CRP/FNRKx may positively regulate bglAx transcription in K. xylinus. In contrast, ABA induces inverse expression of crp/fnrKx and bglAx, while IAA down-regulates crp/fnrKx, but has no effect on bglAx suggesting that the mechanisms involved in CRP/FNRKx-mediated transcriptional regulation are influenced differently by ethylene, IAA and ABA. This may be explained by the complex regulatory role that CRP/FNR family transcription factors have in bacteria, due to their ability to regulate numerous target genes depending on activation by a ligand. For example, the E. coli CRP transcription factor activates the transcription of over 100 promoters (Kolb et al., 1993; Harman, 2001; Brown and Callan, 2004). The activation of CRP is dependent on allosteric binding of cAMP (Harman, 2001). Apo-CRP has low DNA binding affinity and displays minimal sequence specificity. Binding of cAMP results in conformational changes that result in high affinity, sequence-specific DNA binding and interaction with RNA polymerase (Won et al., 2009). Sigma factors produced under the specific conditions then coordinate sequence-specific gene expression mediated by active CRP and RNA polymerase (Colland et al., 2000). Therefore, it is possible that ethylene, IAA and ABA alter the DNA binding specificity of CRP/FNRKx resulting in differential regulation of bglAx. To our knowledge, this is the first report of a bacterial CRP/FNR family transcription factor that is regulated by phytohormones.
Conclusion
We have demonstrated that ethylene produced through the in situ decomposition of ethephon can be used to study the effects of this hormone on bacteria. To our knowledge, we are the first group to utilize this approach. Ethylene caused an increase in K. xylinus BC biosynthesis directly by up-regulating the expression of bcsA and bcsB and indirectly by up-regulating the expression of cmcAx, ccpAx and bglAx. IAA decreases BC biosynthesis directly by down-regulating bcsA expression. We also showed that the bcs operon in K. xylinus is differentially regulated by ethylene and IAA. Altogether, we have expanded on the putative fruit-bacteria interactions of K. xylinus, provided new insights into the transcriptional regulation of the bcs operon and identified a new phytohormone-regulated CRP/FNRKx transcription factor that plays a role in BC biosynthesis in K. xylinus ATCC 53582.
Author Contributions
JS conceived the research topic, contributed to the experimental design, and co-wrote the manuscript. RA carried out the experimental work, contributed to the experimental design, and co-wrote the manuscript.
Funding
This work was supported by a Natural Sciences and Engineering Research Council of Canada Discovery Grant (NSERC-DG) to JS, and an Ontario Graduate Scholarship (OGS) to RA.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Andrew Varley for useful discussions and for assistance in editing this manuscript; Dr. Dario Bonetta for providing the Arabidopsis thaliana seeds and for assistance with the triple response assay; and Simone Quaranta for assistance with FTIR.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2015.01459
Footnotes
- ^ http://www.ncbi.nlm.nih.gov/tools/primer-blast/
- ^ http://mfold.rna.albany.edu/?q=mfold/dna-folding-form
References
Agravante, J. U., Matsui, T., and Kitagawa, H. (1990). Starch breakdown and changes in amylase activity during ripening of ethylene and ethanol treated bananas. Acta Hort. 269, 133–140. doi: 10.17660/ActaHortic.1990.269.18
Ahmed, A. E., and Labavitch, J. M. (1980). Cell wall metabolism in ripening fruit: II. Changes in carbohydrate-degrading enzymes in ripening Bartlett pears. Plant Physiol. 65, 1014–1016. doi: 10.1104/pp.65.5.1014
Arkhipova, T. N., Veselov, S. U., Melentiev, A. I., Martynenko, E. V., and Kudoyarova, G. R. (2005). Ability of bacterium Bacillus subtilis to produce cytokinins and to influence the growth and endogenous hormone content of lettuce plants. Plant Soil 272, 201–209. doi: 10.1007/s11104-004-5047-x
Augimeri, R. V., Varley, A. J., and Strap, J. L. (2015). Establishing a role for bacterial cellulose in environmental interactions: lessons learned from diverse biofilm-producing Proteobacteria. Front. Microbiol. 6:1282. doi: 10.3389/fmicb.2015.01282
Baca, B. E., and Elmerich, C. (2007). “Microbial production of plant hormones,” in Associative and Endophytic Nitrogen-Fixing Bacteria and Cyanobacterial Associations, eds C. Elmerich and W. E. Newton (Amsterdam: Springer), 113–143. doi: 10.1007/1-4020-3546-2
Båga, M., Göransson, M., Normark, S., and Uhlin, B. E. (1988). Processed mRNA with differential stability in the regulation of E. coli pilin gene expression. Cell 52, 197–206. doi: 10.1016/0092-8674(88)90508-9
Ban, T., Kugishima, M., Ogata, T., Shiozaki, S., Horiuchi, S., and Ueda, H. (2007). Effect of ethephon (2-chloroethylphosphonic acid) on the fruit ripening characters of rabbiteye blueberry. Sci. Hortic. (Amsterdam) 112, 278–281. doi: 10.1016/j.scienta.2006.12.027
Bartowsky, E. J., and Henschke, P. A. (2008). Acetic acid bacteria spoilage of bottled red wine- a review. Int. J. Food Microbiol. 125, 60–70. doi: 10.1016/j.ijfoodmicro.2007.10.016
Belasco, J. G., Beatty, J. T., Adams, C. W., von Gabain, A., and Cohen, S. N. (1985). Differential expression of photosynthesis genes in R. capsulata results from segmental differences in stability within the polycistronic rxcA transcript. Cell 40, 171–181. doi: 10.1016/0092-8674(85)90320-4
Biddle, E., Kerfoot, D. G. S., Kho, Y. H., and Russell, K. E. (1976). Kinetic studies of the thermal decomposition of 2-chloroethylphosphonic acid in aqueous solution. Plant Physiol. 58, 700–702. doi: 10.1104/pp.58.5.700
Bottini, R., Cassán, F., and Piccoli, P. (2004). Gibberellin production by bacteria and its involvement in plant growth promotion and yield increase. Appl. Microbiol. Biotechnol. 65, 497–503. doi: 10.1007/s00253-004-1696-1
Brown, C. T., and Callan, C. G. (2004). Evolutionary comparisons suggest many novel cAMP response protein binding sites in Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 101, 2404–2409. doi: 10.1073/pnas.0308628100
Brown, R. M., Willison, J. H., and Richardson, C. L. (1976). Cellulose biosynthesis in Acetobacter xylinum: visualization of the site of synthesis and direct measurement of the in vivo process. Proc. Natl. Acad. Sci. U.S.A. 73, 4565–4569. doi: 10.1073/pnas.73.12.4565
Brummell, D. A. (2006). Cell wall disassembly in ripening fruit. Funct. Plant Biol. 33, 103–119. doi: 10.1071/FP05234
Brummell, D. A., and Harpster, M. H. (2001). Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Mol. Biol. 47, 311–340. doi: 10.1023/A:1010656104304
Brusilow, W. S., Klionsky, D. J., and Simoni, R. D. (1982). Differential polypeptide synthesis of the proton-translocating ATPase of Escherichia coli. J. Bacteriol. 151, 1363–1371.
Burchhardt, G., Keshav, K. F., Yomano, L., and Ingram, L. O. (1993). Mutational analysis of segmental stabilization of transcripts from the Zymomonas mobilis gap-pgk operon. J. Bacteriol. 175, 2327–2333.
Bureau, T. E., and Brown, R. M. (1987). In vitro synthesis of cellulose II from a cytoplasmic membrane fraction of Acetobacter xylinum. Proc. Natl. Acad. Sci. U.S.A. 84, 6985–6989. doi: 10.1073/pnas.84.20.6985
Burg, S. P., and Burg, E. A. (1962). Role of ethylene in fruit ripening. Plant Physiol. 37, 179–189. doi: 10.1104/pp.37.2.179
Casanova, L., Casanova, R., Moret, A., and Agustí, M. (2009). The application of gibberellic acid increases berry size of “Emperatriz” seedless grape. Spanish J. Agric. Res. 7, 919–927. doi: 10.5424/sjar/2009074-1105
Chang, A. L., Tuckerman, J. R., Gonzalez, G., Mayer, R., Weinhouse, H., Volman, G., et al. (2001). Phosphodiesterase A1, a regulator of cellulose synthesis in Acetobacter xylinum, is a heme-based sensor. Biochemistry 40, 3420–3426. doi: 10.1021/bi0100236
Chervin, C., El-Kereamy, A., Roustan, J. P., Latché, A., Lamon, J., and Bouzayen, M. (2004). Ethylene seems required for the berry development and ripening in grape, a non-climacteric fruit. Plant Sci. 167, 1301–1305. doi: 10.1016/j.plantsci.2004.06.026
Colland, F., Barth, M., Hengge-Aronis, R., and Kolb, A. (2000). σ factor selectivity of Escherichia coli RNA polymerase: role for CRP, IHF and Lrp transcription factors. EMBO J. 19, 3028–3037. doi: 10.1093/emboj/19.12.3028
Costerton, J. W., Lewandowski, Z., Caldwell, D. E., Korber, D. R., and Lappin-Scott, H. M. (1995). Microbial biofilms. Annu. Rev. Microbiol. 49, 711–745. doi: 10.1146/annurev.mi.49.100195.003431
Czaja, W., Romanovicz, D., and Brown, R. M. (2004). Structural investigations of microbial cellulose produced in stationary and agitated culture. Cellulose 11, 403–411. doi: 10.1023/b:cell.0000046412.11983.61
Davies, C., Boss, P. K., and Robinson, S. P. (1997). Treatment of grape berries, a nonclimacteric fruit with a synthetic auxin, retards ripening and alters the expression of developmentally regulated genes. Plant Physiol. 115, 1155–1161.
Davies, P. J. (2010). “The plant hormones: their nature, occurrence, and functions,” in Plant Hormones, ed. P. J. Davies (Amsterdam: Springer), 1–15.
Dellaglio, F., Cleenwerck, I., Felis, G. E., Engelbeen, K., Janssens, D., and Marzotto, M. (2005). Description of Gluconacetobacter swingsii sp. nov. and Gluconacetobacter rhaeticus sp. nov., isolated from Italian apple fruit. Int. J. Syst. Evol. Microbiol. 55, 2365–2370. doi: 10.1099/ijs.0.63301-0
Deng, Y., Nagachar, N., Xiao, C., Tien, M., and Kao, T. H. (2013). Identification and characterization of non-cellulose-producing mutants of Gluconacetobacter hansenii generated by Tn5 transposon mutagenesis. J. Bacteriol. 195, 5072–5083. doi: 10.1128/JB.00767-13
Denney, J. O., and Martin, G. C. (1994). Ethephon tissue penetration and harvest effectiveness in olive as a function of solution pH, application time, and BA or NAA addition. J. Am. Soc. Hortic. Sci. 119, 1185–1192.
Dhall, R. K., and Singh, P. (2013). Effect of ethephon and ethylene gas on ripening and quality of tomato (Solanum lycopersicum L.) during cold storage. J. Nutr. Food Sci. 3:244.
Dhillon, W. S., and Mahajan, B. V. C. (2011). Ethylene and ethephon induced fruit ripening in pear. J. Stored Prod. Postharvest Res. 2, 45–51.
Eddy, C. K., Keshav, K. F., An, H., Utt, E. A., Mejia, J. P., and Ingram, L. O. (1991). Segmental message stabilization as a mechanism for differential expression from the Zymomonas mobilis gap operon. J. Bacteriol. 173, 245–254.
Fazli, M., McCarthy, Y., Givskov, M., Ryan, R. P., and Tolker-Nielsen, T. (2013). The exopolysaccharide gene cluster Bcam1330–Bcam1341 is involved in Burkholderia cenocepacia biofilm formation, and its expression is regulated by c-di-GMP and Bcam1349. Microbiologyopen 2, 105–122. doi: 10.1002/mbo3.61
Fazli, M., O’Connell, A., Nilsson, M., Niehaus, K., Dow, J. M., Givskov, M., et al. (2011). The CRP/FNR family protein Bcam1349 is a c-di-GMP effector that regulates biofilm formation in the respiratory pathogen Burkholderia cenocepacia. Mol. Microbiol. 82, 327–341. doi: 10.1111/j.1365-2958.2011.07814.x
Flemming, H.-C., and Wingender, J. (2010). The biofilm matrix. Nat. Rev. Microbiol. 8, 623–633. doi: 10.1080/0892701031000072190
Fong, J. C. N., and Yildiz, F. H. (2008). Interplay between cyclic AMP-cyclic AMP receptor protein and cyclic di-GMP signaling in Vibrio cholerae biofilm formation. J. Bacteriol. 190, 6646–6659. doi: 10.1128/JB.00466-08
Frenkel, C., and Dyck, R. (1973). Auxin inhibition of ripening in Bartlett pears. Plant Physiol. 51, 6–9. doi: 10.1104/pp.51.1.6
Fujiwara, T., Komoda, K., Sakurai, N., Tajima, K., Tanaka, I., and Yao, M. (2013). The c-di-GMP recognition mechanism of the PilZ domain of bacterial cellulose synthase subunit A. Biochem. Biophys. Res. Commun. 431, 802–807. doi: 10.1016/j.bbrc.2012.12.103
Gay, N. J., and Walker, J. E. (1981). The atp operon: nucleotide sequence of the promoter and the genes for the membrane proteins, and the δ subunit of Escherichia coli ATP-synthase. Nucleic Acids Res. 9, 3919–3926. doi: 10.1093/nar/9.16.3919
Geesey, G. G., Mutch, R., Costerton, J. W., and Green, R. B. (1978). Sessile bacteria: an important component of the microbial population in small mountain streams. Limnol. Oceanogr. 23, 1214–1223. doi: 10.4319/lo.1978.23.6.1214
Gill, P. S., Jawandha, S. K., Singh, N., Kaur, N., and Verma, A. (2014). Influence of postharvest applications of ethephon on fruit ripening in mango. Int. J. Adv. Biol. Res. 4, 438–441.
Glick, B. R. (2005). Modulation of plant ethylene levels by the bacterial enzyme ACC deaminase. FEMS Microbiol. Lett. 251, 1–7. doi: 10.1016/j.femsle.2005.07.030
Guzmán, P., and Ecker, J. R. (1990). Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell 2, 513–523. doi: 10.1105/tpc.2.6.513
Hardie, W. J., Johnson, J. O., and Weaver, R. J. (1981). The influence of vine water regime on ethephon-enhanced ripening of Zinfandel. Am. J. Enol. Vitic. 32, 115–121.
Harman, J. G. (2001). Allosteric regulation of the cAMP receptor protein. Biochim. Biophys. Acta (BBA)-Protein Struct. Mol. Enzymol. 1547, 1–17. doi: 10.1016/S0167-4838(01)00187-X
Hellemans, J., Mortier, G., De Paepe, A., Speleman, F., and Vandesompele, J. (2007). qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 8:R19. doi: 10.1186/gb-2007-8-2-r19
Henry, J. T., and Crosson, S. (2011). Ligand-binding PAS domains in a genomic, cellular, and structural context. Annu. Rev. Microbiol. 65, 261–286. doi: 10.1146/annurev-micro-121809-151631
Homuth, G., Mogk, A., and Schumann, W. (1999). Post-transcriptional regulation of the Bacillus subtilis dnaK operon. Mol. Microbiol. 32, 1183–1197. doi: 10.1046/j.1365-2958.1999.01428.x
Hu, S. Q., Gao, Y. G., Tajima, K., Sunagawa, N., Zhou, Y., Kawano, S., et al. (2010). Structure of bacterial cellulose synthase subunit D octamer with four inner passageways. Proc. Natl. Acad. Sci. U.S.A. 107, 17957–17961. doi: 10.1073/pnas.1000601107
Hu, Y., and Catchmark, J. M. (2010). Influence of 1-methylcyclopropene (1-MCP) on the production of bacterial cellulose biosynthesized by Acetobacter xylinum under the agitated culture. Lett. Appl. Microbiol. 51, 109–113. doi: 10.1111/j.1472-765X.2010.02866.x
Hungund, B. S., and Gupta, S. G. (2010). Production of bacterial cellulose from Enterobacter amnigenus GH-1 isolated from rotten apple. World J. Microbiol. Biotechnol. 26, 1823–1828. doi: 10.1007/s11274-010-0363-1
Iannetta, P. P. M., Laarhoven, L., Medina-Escobar, N., James, E. K., McManus, M. T., Davies, H. V., et al. (2006). Ethylene and carbon dioxide production by developing strawberries show a correlative pattern that is indicative of ripening climacteric fruit. Physiol. Plant 127, 247–259. doi: 10.1111/j.1399-3054.2006.00656.x
Iyer, P. R., Catchmark, J., Brown, N. R., and Tien, M. (2011). Biochemical localization of a protein involved in synthesis of Gluconacetobacter hansenii cellulose. Cellulose 18, 739–747. doi: 10.1007/s10570-011-9504-4
Jackson, D. W., Simecka, J. W., and Romeo, T. (2002). Catabolite repression of Escherichia coli biofilm formation. J. Bacteriol. 184, 3406–3410. doi: 10.1128/JB.184.12.3406-3410.2002
Jahan, F., Kumar, V., Rawat, G., and Saxena, R. K. (2012). Production of microbial cellulose by a bacterium isolated from fruit. Appl. Biochem. Biotechnol. 167, 1157–1171. doi: 10.1007/s12010-012-9595-x
Jia, H. F., Chai, Y. M., Li, C. L., Lu, D., Luo, J. J., Qin, L., et al. (2011). Abscisic acid plays an important role in the regulation of strawberry fruit ripening. Plant Physiol. 157, 188–199. doi: 10.1104/pp.111.177311
Jolliffe, P. A. (1975). Effects of ethephon on raspberry fruit ripeness, fruit weight and fruit removal. Can. J. Plant Sci. 55, 429–437. doi: 10.4141/cjps75-067
Kawano, S., Tajima, K., Kono, H., Erata, T., Munekata, M., and Takai, M. (2002a). Effects of endogenous endo-beta-1,4-glucanase on cellulose biosynthesis in Acetobacter xylinum ATCC23769. J. Biosci. Bioeng. 94, 275–281. doi: 10.1016/S1389-1723(02)80162-1
Kawano, S., Tajima, K., Uemori, Y., Yamashita, H., Erata, T., Munekata, M., et al. (2002b). Cloning of cellulose synthesis related genes from Acetobacter xylinum ATCC23769 and ATCC53582: comparison of cellulose synthetic ability between strains. DNA Res. 9, 149–156. doi: 10.1093/dnares/9.5.149
Kawano, S., Tajima, K., Kono, H., Numata, Y., Yamashita, H., Satoh, Y., et al. (2008). Regulation of endoglucanase gene (cmcax) expression in Acetobacter xylinum. J. Biosci. Bioeng. 106, 88–94. doi: 10.1263/jbb.106.88
Khan, N. A. (2004). An evaluation of the effects of exogenous ethephon, an ethylene releasing compound, on photosynthesis of mustard (Brassica juncea) cultivars that differ in photosynthetic capacity. BMC Plant Biol. 4:21. doi: 10.1186/1471-2229-4-21
Khorshidi, S., and Davarynejad, G. (2010). Influence of preharvest ethephon spray on fruit quality and chemical attributes of “Cigany” sour cherry cultivar. Fruits 4, 133–141.
Kim, H., Ryu, J.-H., and Beuchat, L. R. (2006). Attachment of and biofilm formation by Enterobacter sakazakii on stainless steel and enteral feeding tubes. Appl. Environ. Microbiol. 72, 5846–5856. doi: 10.1128/AEM.00654-06
Kim, H.-E., Shitashiro, M., Kuroda, A., Takiguchi, N., and Kato, J. (2007). Ethylene chemotaxis in Pseudomonas aeruginosa and other Pseudomonas species. Microbes Environ. 22, 186–189. doi: 10.1264/jsme2.22.186
Kimura, S., Chen, H. P., Saxena, I. M., Brown, R. M., and Itoh, T. (2001). Localization of c-di-GMP-binding protein with the linear terminal complexes of Acetobacter xylinum. J. Bacteriol. 183, 5668–5674. doi: 10.1128/JB.183.19.5668-5674.2001
Klein, I., Lavee, S., and Ben-Tal, Y. (1979). Effect of water vapor pressure on the thermal decomposition of 2-chloroethylphosphonic acid. Plant Physiol. 63, 474–477. doi: 10.1104/pp.63.3.474
Klug, G., Adams, C. W., Belasco, J., Doerge, B., and Cohen, S. N. (1987). Biological consequences of segmental alterations in mRNA stability: effects of deletion of the intercistronic hairpin loop region of the Rhodobacter capsulatus puf operon. EMBO J. 6, 3515.
Kolb, A., Busby, S., Buc, I. I., Garges, S., and Adhya, S. (1993). Transcriptional regulation by cAMP and its receptor protein. Annu. Rev. Biochem. 62, 749–797. doi: 10.1146/annurev.bi.62.070193.003533
Kong, Y., Wang, Z., Chen, J., Pan, X. T., Liu, D. T., and Zhang, E. J. (2009). Effects of ethephon on aerenchyma formation in rice roots. Rice Sci. 16, 210–216. doi: 10.1016/S1672-6308(08)60081-5
Koo, H. M., Song, S. H., Pyun, Y. R., and Kim, Y. S. (1998). Evidence that a beta-1,4-endoglucanase secreted by Acetobacter xylinum plays an essential role for the formation of cellulose fiber. Biosci. Biotechnol. Biochem. 62, 2257–2259. doi: 10.1271/bbb.62.2257
Körner, H., Sofia, H. J., and Zumft, W. G. (2003). Phylogeny of the bacterial superfamily of CRP-FNR transcription regulators: exploiting the metabolic spectrum by controlling alternative gene programs. FEMS Microbiol. Rev. 27, 559–592. doi: 10.1016/S0168-6445(03)00066-4
Kudlicka, K., and Brown, R. M. (1996). Cellulose biosynthesis in higher plants. Acta Soc. Bot. Pol. 65, 17–24. doi: 10.1016/S1360-1385(96)80050-1
Lacey, R. F., and Binder, B. M. (2014). How plants sense ethylene gas - the ethylene receptors. J. Inorg. Biochem. 133, 58–62. doi: 10.1016/j.jinorgbio.2014.01.006
Lagoni, O. R., von Meyenburg, K., and Michelsen, O. (1993). Limited differential mRNA inactivation in the atp (unc) operon of Escherichia coli. J. Bacteriol. 175, 5791–5797.
Lasa, I., and Villanueva, M. (2014). Overlapping transcription and bacterial RNA removal. Proc. Natl. Acad. Sci. U.S.A. 111, 2868–2869. doi: 10.1073/pnas.1324236111
Lee, S., and Kennedy, C. (2000). “Characterization of indole-3-acetic acid (IAA) produced by the sugarcane endophyte Acetobacter diazotrophicus, in sugarcane growth,” in Nitrogen Fixation: From Molecules to Crop Productivity, eds F. O. Pedrosa, M. Hungria, G. Yates, and W. E. Newton (Houten: Springer), 421.
Leng, P., Yuan, B., Guo, Y., and Chen, P. (2014). The role of abscisic acid in fruit ripening and esponses to abiotic stress. J. Exp. Bot. 65, 4577–4578. doi: 10.1093/jxb/eru204
Li, C., Jia, H., Chai, Y., and Shen, Y. (2011). Abscisic acid perception and signaling transduction in strawberry: a model for non-climacteric fruit ripening. Plant Signal. Behav. 6, 1950–1953. doi: 10.4161/psb.6.12.18024
Lieberman, M. (1979). Biosynthesis and action of ethylene. Annu. Rev. Plant Physiol. 30, 533–591. doi: 10.1146/annurev.pp.30.060179.002533
Lin, F. C., Brown, R. M., Drake, R. R., and Haley, B. E. (1990). Identification of the uridine 5’-diphosphoglucose (UDP-Glc) binding subunit of cellulose synthase in Acetobacter xylinum using the photoaffinity probe 5-azido-UDP-Glc. J. Biol. Chem. 265, 4782–4784.
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-(ΔΔC(T)) method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Lohani, S., Trivedi, P. K., and Nath, P. (2004). Changes in activities of cell wall hydrolases during ethylene-induced ripening in banana: effect of 1-MCP, ABA and IAA. Postharvest Biol. Technol. 31, 119–126. doi: 10.1016/j.postharvbio.2003.08.001
Mamlouk, D., and Gullo, M. (2013). Acetic acid bacteria: physiology and carbon sources oxidation. Indian J. Microbiol. 53, 377–384. doi: 10.1007/s12088-013-0414-z
Matsui, M., Tomita, M., and Kanai, A. (2013). Comprehensive computational analysis of bacterial CRP/FNR superfamily and its target motifs reveals stepwise evolution of transcriptional networks. Genome Biol. Evol. 5, 267–282. doi: 10.1093/gbe/evt004
McAtee, P., Karim, S., Schaffer, R., and David, K. (2013). A dynamic interplay between phytohormones is required for fruit development, maturation, and ripening. Front. Plant Sci. 4:79. doi: 10.3389/fpls.2013.00079
McCarthy, J. E. G., Gerstel, B., Surin, B., Wiedemann, U., and Ziemke, P. (1991). Differential gene expression from the Escherichia coli atp operon mediated by segmental defferences in mRNA stability. Mol. Microbiol. 5, 2447–2458. doi: 10.1111/j.1365-2958.1991.tb02090.x
Møller, T., Franch, T., Udesen, C., Gerdes, K., and Valentin-Hansen, P. (2002). Spot 42 RNA mediates discoordinate expression of the E. coli galactose operon. Genes Dev. 16, 1696–1706. doi: 10.1101/gad.231702
Morgan, J. L. W., McNamara, J. T., and Zimmer, J. (2014). Mechanism of activation of bacterial cellulose synthase by cyclic di-GMP. Nat. Struct. Mol. Biol. 21, 489–496. doi: 10.1038/nsmb.2803
Morgan, J. L. W., Strumillo, J., and Zimmer, J. (2013). Crystallographic snapshot of cellulose synthesis and membrane translocation. Nature 493, 181–186. doi: 10.1038/nature11744
Nakai, T., Nishiyama, Y., Kuga, S., Sugano, Y., and Shoda, M. (2002). ORF2 gene involves in the construction of high-order structure of bacterial cellulose. Biochem. Biophys. Res. Commun. 295, 458–462. doi: 10.1016/S0006-291X(02)00696-4
Nakai, T., Sugano, Y., Shoda, M., Sakakibara, H., Oiwa, K., Tuzi, S., et al. (2013). Formation of highly twisted ribbons in a carboxymethylcellulase gene-disrupted strain of a cellulose-producing bacterium. J. Bacteriol. 195, 958–964. doi: 10.1128/JB.01473-12
Neera, Ramana, K. V., and Batra, H. V. (2015). Occurrence of cellulose-producing Gluconacetobacter spp. in fruit samples and kombucha tea, and production of the biopolymer. Appl. Biochem. Biotechnol. 176, 1162–1173. doi: 10.1007/s12010-015-1637-8
Newbury, S. F., Smith, N. H., and Higgins, C. F. (1987). Differential mRNA stability controls relative gene expression within a polycistronic operon. Cell 51, 1131–1143. doi: 10.1016/0092-8674(87)90599-X
Nilsson, P., Naureckiene, S., and Uhlin, B. E. (1996). Mutations affecting mRNA processing and fimbrial biogenesis in the Escherichia coli pa operon. J. Bacteriol. 178, 683–690.
Omadjela, O., Narahari, A., Strumillo, J., Mélida, H., Mazur, O., Bulone, V., et al. (2013). BcsA and BcsB form the catalytically active core of bacterial cellulose synthase sufficient for in vitro cellulose synthesis. Proc. Natl. Acad. Sci. U.S.A. 110, 17856–17861. doi: 10.1073/pnas.1314063110
Park, J. K., Park, Y. H., and Jung, J. Y. (2003). Production of bacterial cellulose by Gluconacetobacter hansenii PJK isolated from rotten apple. Biotechnol. Bioprocess Eng. 8, 83–88. doi: 10.1007/BF02940261
Paul, V., Pandey, R., and Srivastava, G. C. (2012). The fading distinctions between classical patterns of ripening in climacteric and non-climacteric fruit and the ubiquity of ethylene-An overview. J. Food Sci. Technol. 49, 1–21. doi: 10.1007/s13197-011-0293-4
Pech, J. C., Bouzayen, M., and Latché, A. (2008). Climacteric fruit ripening: ethylene-dependent and independent regulation of ripening pathways in melon fruit. Plant Sci. 175, 114–120. doi: 10.1016/j.plantsci.2008.01.003
Qi, Y., Rao, F., Luo, Z., and Liang, Z. X. (2009). A flavin cofactor-binding PAS domain regulates c-di-GMP synthesis in AxDGC2 from Acetobacter xylinum. Biochemistry 48, 10275–10285. doi: 10.1021/bi901121w
Qureshi, O., Sohail, H., Latos, A., and Strap, J. L. (2013). The effect of phytohormones on the growth, cellulose production and pellicle properties of Gluconacetobacter xylinus ATCC 53582. Acetic Acid Bact. 2, 39–46. doi: 10.4081/aab.2013.s1.e7
Richmond, T. A., and Somerville, C. R. (2000). The cellulose synthase superfamily. Plant Physiol. 124, 495–498. doi: 10.1104/pp.124.2.495
Ross, P., Weinhouse, H., Aloni, Y., Michaeli, D., Weinberger-Ohana, P., Mayer, R., et al. (1987). Regulation of cellulose synthesis in Acetobacter xylinum by cyclic diguanylic acid. Nature 325, 279–281. doi: 10.1038/325279a0
Saxena, I. M., Kudlicka, K., Okuda, K., and Brown, R. M. (1994). Characterization of genes in the cellulose-synthesizing operon (acs operon) of Acetobacter xylinum: implications for cellulose crystallization. J. Bacteriol. 176, 5735–5752.
Schneider, C. A., Rasband, W. S., and Eliceiri, K. W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675. doi: 10.1038/nmeth.2089
Schramm, M., and Hestrin, S. (1954). Factors affecting production of cellulose at the air/ liquid interface of a culture of Acetobacter xylinum. J. Gen. Microbiol. 11, 123–129.
Schreiber, W., and Dürre, P. (2000). Differential expression of genes within the gap operon of Clostridium acetobutylicum. Anaerobe 6, 291–297. doi: 10.1006/anae.2000.0352
Serrani, J. C., Sanjuán, R., Ruiz-Rivero, O., Fos, M., and García-Martínez, J. L. (2007). Gibberellin regulation of fruit set and growth in tomato. Plant Physiol. 145, 246–257. doi: 10.1104/pp.107.098335
Sesto, N., Wurtzel, O., Archambaud, C., Sorek, R., and Cossart, P. (2013). The excludon: a new concept in bacterial antisense RNA-mediated gene regulation. Nat. Rev. Microbiol. 11, 75–82. doi: 10.1038/nrmicro2934
Singh, Z., and Janes, J. (2001). Effects of postharvest application of ethephon on fruit ripening, quality and shelf life of mango under modified atmosphere packaging. Acta Hortic. 553, 599–602. doi: 10.17660/ActaHortic.2001.553.141
Sisler, E. C. (1991). “Ethylene binding components in plants,” in The Plant Hormone Ethylene, eds A. Mattoo and J. Suttle (Boca Raton, FL: CRC Press), 81–99.
Sitrit, Y., and Bennett, A. B. (1998). Regulation of tomato fruit polygalacturonase mRNA accumulation by ethylene: a re-examination. Plant Physiol. 116, 1145–1150. doi: 10.1104/pp.116.3.1145
Standal, R., Iversen, T. G., Coucheron, D. H., Fjaervik, E., Blatny, J. M., and Valla, S. (1994). A new gene required for cellulose production and a gene encoding cellulolytic activity in Acetobacter xylinum are colocalized with the bcs operon. J. Bacteriol. 176, 665–672.
Sunagawa, N., Fujiwara, T., Yoda, T., Kawano, S., Satoh, Y., Yao, M., et al. (2013). Cellulose complementing factor (Ccp) is a new member of the cellulose synthase complex (terminal complex) in Acetobacter xylinum. J. Biosci. Bioeng. 115, 607–612. doi: 10.1016/j.jbiosc.2012.12.021
Symons, G. M., Chua, Y. J., Ross, J. J., Quittenden, L. J., Davies, N. W., and Reid, J. B. (2012). Hormonal changes during non-climacteric ripening in strawberry. J. Exp. Bot. 63, 4741–4750. doi: 10.1093/jxb/ers147
Szyjewicz, E., Rosner, N., and Kliewer, W. M. (1984). Ethephon ((2-chloroethyl) phosphonic acid, Ethrel, CEPA) in viticulture-a review. Am. J. Enol. Vitic. 35, 117–123.
Tahara, N., Tonouchi, N., and Yano, H. (1998). Purification and characterization of exo-1, 4-glucosidase from Acetobacter xylinum BPR2001. J. Ferment. 85, 589–594. doi: 10.1016/S0922-338X(98)80010-X
Tajima, K., Nakajima, K., Yamashita, H., Shiba, T., Munekata, M., and Takai, M. (2001). Cloning and sequencing of the beta-glucosidase gene from Acetobacter xylinum ATCC 23769. DNA Res. 8, 263–269. doi: 10.1093/dnares/8.6.263
Tal, R., Wong, H. C., Calhoon, R., Gelfand, D., Fear, A. L., Volman, G., et al. (1998). Three cdg operons control cellular turnover of cyclic di-GMP in Acetobacter xylinum: genetic organization and occurrence of conserved domains in isoenzymes. J. Bacteriol. 180, 4416–4425.
Tassoni, A., Watkins, C. B., and Davies, P. J. (2006). Inhibition of the ethylene response by 1-MCP in tomato suggests that polyamines are not involved in delaying ripening, but may moderate the rate of ripening or over-ripening. J. Exp. Bot. 57, 3313–3325. doi: 10.1093/jxb/erl092
Taylor, S., Wakem, M., Dijkman, G., Alsarraj, M., and Nguyen, M. (2010). A practical approach to RT-qPCR-Publishing data that conform to the MIQE guidelines. Methods 50, S1–S5. doi: 10.1016/j.ymeth.2010.01.005
Thormann, K. M., Saville, R. M., Shukla, S., and Spormann, A. M. (2005). Induction of rapid detachment in Shewanella oneidensis MR-1 biofilms. J. Bacteriol. 187, 1014–1021. doi: 10.1128/JB.187.3.1014-1021.2005
Tonouchi, N., Tahara, N., Tsuchida, T., Yoshinaga, F., Beppu, T., and Horinouchi, S. (1995). Addition of a small amount of an endoglucanase enhances cellulose production by Acetobacter xylinum. Biosci. Biotechnol. Biochem. 59, 805–808. doi: 10.1271/bbb.59.805
Trainotti, L., Pavanello, A., and Casadoro, G. (2005). Different ethylene receptors show an increased expression during the ripening of strawberries: does such an increment imply a role for ethylene in the ripening of these non-climacteric fruits? J. Exp. Bot. 56, 2037–2046. doi: 10.1093/jxb/eri202
Tremaroli, V., Fedi, S., Tamburini, S., Viti, C., Tatti, E., Ceri, H., et al. (2011). A histidine-kinase cheA gene of Pseudomonas pseudoalcaligens KF707 not only has a key role in chemotaxis but also affects biofilm formation and cell metabolism. Biofouling 27, 33–46. doi: 10.1080/08927014.2010.537099
Untergasser, A., Nijveen, H., Rao, X., Bisseling, T., Geurts, R., and Leunissen, J. A. M. (2007). Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res. 35, W71–W74. doi: 10.1093/nar/gkm306
Vandesompele, J., De Preter, K., Pattyn, F., Poppe, B., Van Roy, N., De Paepe, A., et al. (2002). Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3, 1–11. doi: 10.1186/gb-2002-3-7-research0034
Vellanoweth, R. L. (1993). “Translation and its regulation in Gram-positive bacteria,” in Bacillus subtilis and Other Gram-positive Bacteria: Physiology, Biochemistry and Molecular Biology, eds J. Hoch, R. Losick, and A. Sonenshein (Washington, DC: ASM), 699–711.
Wang, A. Q., Huang, W. J., Niu, J. Q., Liu, M., Yang, L. T., and Li, Y. R. (2013). Effects of ethephon on key enzymes of sucrose metabolism in relation to sucrose accumulation in sugarcane. Sugar Tech. 15, 177–186. doi: 10.1007/s12355-012-0202-9
Wang, W., Esch, J. J., Shiu, S.-H., Agula, H., Binder, B. M., Chang, C., et al. (2006). Identification of important regions for ethylene binding and signaling in the transmembrane domain of the ETR1 ethylene receptor of Arabidopsis. Plant Cell 18, 3429–3442. doi: 10.1105/tpc.106.044537
Warner, H. L., and Leopold, A. C. (1969). Ethylene evolution from 2-chloroethylphosphonic acid. Plant Physiol. 44, 156–158. doi: 10.1104/pp.44.1.156
Weingart, H., and Völksch, B. (1997). Ethylene production by Pseudomonas syringae pathovars in vitro and in planta. Appl. Environ. Microbiol. 63, 156–161.
Wheeler, R. M., Peterson, B. V., and Stutte, G. W. (2004). Ethylene production throughout growth and development of plants. HortScience 39, 1541–1545.
Williams, W. S., and Cannon, R. E. (1989). Alternative environmental roles for cellulose produced by Acetobacter xylinum. Appl. Environ. Microbiol. 55, 2448–2452.
Witebsky, F. G., Maclowry, J. D., and French, S. S. (1979). Broth dilution minimum inhibitory concentrations?: rationale for use of selected antimicrobial concentrations. J. Clin. Microbiol. 9, 589–595.
Won, H. S., Lee, Y. S., Lee, S. H., and Lee, B. J. (2009). Structural overview on the allosteric activation of cyclic AMP receptor protein. Biochim. Biophys. Acta (BBA)-Proteins Proteomics 1794, 1299–1308. doi: 10.1016/j.bbapap.2009.04.015
Yamada, Y., Yukphan, P., Lan, Vu, H. T., Muramatsu, Y., Ochaikul, D., et al. (2012). Description of Komagataeibacter gen. nov., with proposals of new combinations (Acetobacteraceae). J. Gen. Appl. Microbiol. 58, 397–404. doi: 10.2323/jgam.58.397
Zegzouti, H., Jones, B., Frasse, P., Marty, C., and Maitre, B. (1999). Ethylene-regulated gene expression in tomato fruit: characterization of novel ethylene-responsive and ripening-related genes isolated by differential display. Plant J. 18, 589–600. doi: 10.1046/j.1365-313x.1999.00483.x
Zhang, L., Li, S., Liu, X., Song, C., and Liu, X. (2012). Effects of ethephon on physicochemical and quality properties of kiwifruit during ripening. Postharvest Biol. Technol. 65, 69–75. doi: 10.1016/j.postharvbio.2011.11.004
Zhang, M., Yuan, B., and Leng, P. (2009). The role of ABA in triggering ethylene biosynthesis and ripening of tomato fruit. J. Exp. Bot. 60, 1579–1588. doi: 10.1093/jxb/erp026
Zhang, W., Hu, W., and Wen, C. K. (2010). Ethylene preparation and its application to physiological experiments. Plant Signal. Behav. 5, 453–457. doi: 10.4161/psb.5.4.10875
Zhang, W., and Wen, C. K. (2010). Preparation of ethylene gas and comparison of ethylene responses induced by ethylene, ACC, and ethephon. Plant Physiol. Biochem. 48, 45–53. doi: 10.1016/j.plaphy.2009.10.002
Ziemke, P., and McCarthy, J. E. G. (1992). The control of mRNA stability in Escherichia coli: manipulation of the degradation pathway of the polycistronic atp mRNA. Biochim. Biophys. Acta (BBA)-Gene Struct. Expr. 1130, 297–306. doi: 10.1016/0167-4781(92)90442-3
Keywords: ethylene, Komagataeibacter (Gluconacetobacter) xylinus, bacterial cellulose, CRP/FNR, indole-3-acetic acid (IAA), abscisic acid (ABA), plant-microbe interaction, fruit-bacteria interaction
Citation: Augimeri RV and Strap JL (2015) The Phytohormone Ethylene Enhances Cellulose Production, Regulates CRP/FNRKx Transcription and Causes Differential Gene Expression within the Bacterial Cellulose Synthesis Operon of Komagataeibacter (Gluconacetobacter) xylinus ATCC 53582. Front. Microbiol. 6:1459. doi: 10.3389/fmicb.2015.01459
Received: 21 September 2015; Accepted: 04 December 2015;
Published: 22 December 2015.
Edited by:
Cheng Zhong, Tianjin University of Science and Technology, ChinaReviewed by:
Paul Richard Himes, University of Louisville, USAJustin Joseph Donato, University of St. Thomas, USA
Copyright © 2015 Augimeri and Strap. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Janice L. Strap, janice.strap@uoit.ca
 Richard V. Augimeri
Richard V. Augimeri Janice L. Strap
Janice L. Strap